Abstract
An ideal inducible system should be cell specific and have absolutely no background recombination without induction (i.e., no leakiness), a high recombination rate after induction, and complete fidelity in cell specificity (i.e., restricted recombination exclusively in cells where the driver gene is expressed). However, such an ideal mouse model remains unavailable for collecting duct research. Here, we report a mouse model that meets these criteria. In this model, a cassette expressing ERT2CreERT2 (ECE) is inserted at the ATG of the endogenous Aqp2 locus to disrupt Aqp2 function and to express ECE under the control of the Aqp2 promoter. The resulting allele is named Aqp2ECE. There was no indication of a significant impact of disruption of a copy of Aqp2 on renal function and blood pressure control in adult Aqp2ECE/+ heterozygotes. Without tamoxifen, Aqp2ECE did not activate a Cre-dependent red fluorescence protein (RFP) reporter in adult kidneys. A single injection of tamoxifen (2 mg) to adult mice enabled Aqp2ECE to induce robust RFP expression in the whole kidney 24 h postinjection, with the highest recombination efficiency of 95% in the inner medulla. All RFP-labeled cells expressed principal cell markers (Aqp2 and Aqp3), but not intercalated cell markers (V-ATPase B1B2, and carbonic anhydrase II). Hence, Aqp2ECE confers principal cell-specific tamoxifen-inducible recombination with absolutely no leakiness, high inducibility, and complete fidelity in cell specificity, which should be an important tool for temporospatial control of target genes in the principal cells and for Aqp2+ lineage tracing in adult mice.
Keywords: Aqp2, background recombination, ERT2CreERT2, faithfulness, recombination rate
INTRODUCTION
Tamoxifen-dependent CreER recombinases have been developed for disruption or activation of target genes at will in a temporospatially controlled manner (4). They consist of Cre fused to a mutated ligand-binding domain (LBD) of the estrogen receptor (ER). The mutated LBD binds with high affinity the synthetic ER ligand tamoxifen and its active metabolite 4-hydroxytamoxifen (4OHT), but not the endogenous estrogens. In the absence of the ligand, the CreER fusion protein remains inactive in the cytoplasm. In the presence of tamoxifen, the CreER fusion binds tamoxifen and moves into the nucleus, where it recombines its loxP-flanked DNA substrate. Currently, the most successful CreER version is CreERT2, which harbors the human ER LBD with a G400V/M543A/L544A triple mutation. By breeding the CreERT2 driver with mice containing floxed essential exon(s) of target genes, gene targeting can be temporally regulated. To achieve spatial control, the expression of CreERT2 is placed under the control of the various tissue/cell-specific promoters.
The kidney contains many functionally and structurally different cells. Several segment-specific CreERT2 transgenic or knock-in mouse lines have been reported, using promoters of Gamma glutamyl transpeptidase (GGT) (7), Podocin (23), Cited1 (3), Lgr5 (2), Six2 (9), Zfyve27 (16), Foxd1 (8), and Ksp-cadherin (11, 17). In addition, transgenic or knock-in mice expressing Tet-off-eGFPCre and eGFPCreERT2 driven by the promoters of Foxd1 (8), Six2 (9), and SLC34a1 (10), respectively, are also available. Many of these inducible Cre lines are active in multiple segments, particularly in proximal tubules.
In Aqp2Cre (15, 20) and Aqp2CreERT2 (21) mice, Aqp2 serves as the driver gene since its promoter drives Cre and CreERT2 expression, specifically in connecting tubule/collecting duct (CNT/CD). These models have been used by others (1, 18, 20, 21, 27) and us (25) to create CNT/CD-specific knockout mice. However, the constitutive nature of the Aqp2Cre (15, 20) and the high background recombination in the absence of tamoxifen (i.e., leakiness) of the Aqp2CreERT2 (21) do not offer temporal control, limiting their use in studying the role of CNT/CD in pathological conditions of the adult kidney.
Electroporation studies demonstrated that ERT2CreERT2 (ECE) had no leakiness (13). To our knowledge, the tight control of ECE has not been strictly tested in vivo, since ECE transgenic or knock-in mice have not been reported. Here, we report a new inducible mouse model, in which an ECE cassette is inserted into mouse genome at the ATG of the endogenous Aqp2 locus. The resulting allele is referred to as Aqp2ECE. We demonstrate that Aqp2ECE has very mild or no effect on renal function, absolutely no leaky Cre activity in the absence of tamoxifen, high recombination efficiency upon induction, and specific recombination exclusively in the cells where Aqp2 expression occurs (i.e., complete fidelity or faithfulness in cell specificity). Our study defines Aqp2ECE as a powerful new system for evaluating gene function specifically in the principal cells at any time and for identifying Aqp2+ progenitor cells in adult kidney. Our ECE knock-in strategy can also be applied to develop various tissue/cell-specific ECE drivers that may also have high inducibility, complete faithfulness, and no leakiness.
MATERIALS AND METHODS
Reagents.
Two mouse antibodies specific for carbonic anhydrase II (CAII, sc-48351) and V-ATPase B1 B2 (sc-55544), one rabbit Aqp2 antibody (sc-28629), and two goat antibodies against Aqp2 (sc-9882) and Pendrin (sc-16894) were obtained from Santa Cruz Biotechnology (Dallas, TX). Rabbit-anti RFP (632496; Clontech, Mountain View, CA), and chicken anti-GFP (ab13970; Abcam, Cambridge, MA) were also used. The secondary antibodies were purchased either from Invitrogen (Carlsbad, CA) or Jackson ImmunoResearch (West Grove, PA). They were Alexa 647 donkey anti-goat IgG (A21447), Alexa 568 donkey anti-rabbit IgG (A10042), Alexa 488 donkey anti-goat IgG (A11055), Alexa 488 donkey anti-chicken IgG (703-545-155), and Alexa 488 donkey anti-mouse IgG (715-545-150). Tamoxifen (T5648), sunflower oil (S5007), and all primers (Table 1) were ordered from Sigma-Aldrich (St. Louis, MO). pCAG-ERT2CreERT2 was obtained from Addgene (Cambridge, MA). An Arg8-Vasopressin ELISA kit (ADI-900-017A) was purchased from Enzo (Farmingdale, NY).
Table 1.
Primer list
| Primers | Note | Sequence (5′ to 3′) |
|---|---|---|
| WZ1026 | Aqp2-COM forward | AAGTGCCCACAGTCTAGCCTCT |
| WZ1028 | Aqp2-WT reverse | GGAGAACGCTATGGACCGGAGT |
| WZ1094 | Aqp2 forward | ATGTGGGAACTCCGGTCCATA |
| WZ1095 | Aqp2 reverse | ACGGCAATCTGGAGCACAG |
| WZ1106 | GAPDH forward | AGGTCGGTGTGAACGGATTTG |
| WZ1107 | GAPDH reverse | TGTAGACCATGTAGTTGAGGTCA |
| WZ1631 | Aqp2-ECE reverse | TCGCCGCTCCCGATTCGCAG |
| WZ1494 | 5′ arm reverse | AAGGTTGGCAGCTCTCATGTCTCCAGCCATGCTGCTCGGCCTTCTGAGCGCTGGCCAGTGGTCT |
| WZ1495 | 5′ arm ECE junction forward | AGACCACTGGCCAGCGCTCAGAAGGCCGAGCAGCATGGCTGGAGACATGAGAGCTGCCAACCTT |
| WZ1496 | Cre reverse | TACACCAGAGACGGAAATCCATCGCTCG |
| WZ1499 | 3′ arm forward | ATCTGTGTATACTGGGAACTCCGGTCCATAG |
| WZ1500 | 3′ arm reverse | CAGACTACATGTTAGGTCATACTGATACAACAGGATGC |
| WZ1502 | 5′ arm forward | ATCTGTGAATTCCTGGCTCCCTAAACCTCCTATG |
| WZ1643 | Nnt-COM forward | GTAGGGCCAACTGTTTCTGCATGA |
| WZ1644 | Nnt-WT reverse | GGGCATAGGAAGCAAATACCAAGTTG |
| WZ1645 | Nnt-MT reverse | GTGGAATTCCGCTGAGAGAACTCTT |
| TMF241 | 5′ probe forward | CCGCACCGTGGAAATGTGGATG |
| TMF242 | 5′ probe reverse | TCAACCATAGCACACCCAGAAAGGGT |
| TMF571 | 3′ probe forward | TCGGTTCCCAGTGCAGGGTAGCTAG |
| TMF572 | 3′ probe reverse | TCCAGGACTCCCTTGCGAAACCCGA |
Aqp2, aquaporin 2; ECE, cassette expressing estrogen receptor (ER)T2CreERT2; Nnt, nicotinamide nucleotide transhydrogenase.
Generation of the targeting vector, ES cells, and chimeras.
A 2.5-kb PCR fragment of Aqp2 gene as the 3′ arm was amplified using primers WZ1499/1500 with mouse tail DNA as template. The fragment was cloned into pcDNA 3.1 (+) at PciI and BstZ171 to create p692. A 2.9-kb fragment containing ERT2CreERT2 was released from pCAG-ERT2CreERT2 and inserted into p692 at EcoRI and NotI to generate p693. The 5′ arm was created through overlapping PCR. The first, a 4.1-kb fragment of the Aqp2 gene, was amplified from mouse genomic DNA with primers WZ1494/1502. The second, a 1.6-kb fragment, was synthesized with primers WZ1495/1496 using pCAG-ERT2CreERT2 as the template. The final 5.7-kb 5′ arm, obtained via PCR using primers WZ1496/1502 with the two fragments as the template, was then cloned into p693 at EcoRI and AgeI to generate the final target vector p694. The authenticity of the whole insert sequence in the final target was confirmed by sequencing.
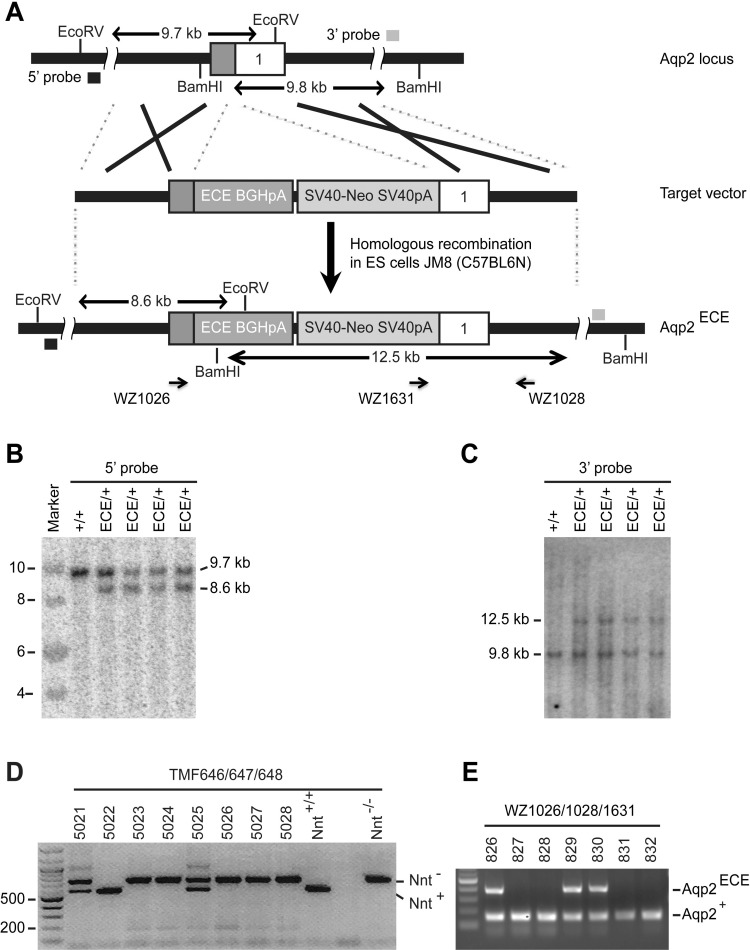
After linearization with KpnI, which trimmed the 5′ and 3′ arms to 1.8 kb and 2.3 kb, respectively, the targeting vector was electroporated into C57BL/6N JM8.N4 embryonic stem (ES) cells. The C57BL/6N background of the ES cells offers a unique advantage for identification of chimeras (see below). A total of 384 G418-resistant clones were picked, duplicated, and expanded, and total DNA was extracted for Southern analyses. Probes to screen for homologous recombination in Southern analyses were selected that were outside the region of homology used for the arms of the targeting vector. Probes were generated using PCR. The 3′ probe (893 bp) was amplified using primers TMF571/572. The 5′ probe (474 bp) was amplified using primers TMF241/242. DNA fragments were labeled using [α-32P]dCTP and hybridized and washed to digested ES cell genomic DNA using standard methods. To detect recombination at the 3′ arm, DNA was digested with BamHI, with a 9.8-kb hybridizing band indicating a wild-type (WT) and a 12.5-kb band allele denoting targeted allele. For the 5′ arm, DNA was digested with EcoRV with band sizes of 9.7 kb (WT) and 8.6 kb (targeted) (Fig. 1A). Chromosome counting was carried out to verify the genome integrity of six correctly targeted ES clones. Two of these clones with >80% euploid cells were injected into C57BL/6J blastocysts to produce chimeras. Since the ES cells and the blastocysts are homozygous for the WT and mutant nicotinamide nucleotide transhydrogenase gene (Nnt+/+ and Nnt−/−), respectively (19), PCR analyses were done to identify the chimeras and to evaluate the relative contribution of the ES cells. The expected products are 576 bp for the WT allele and 743 bp for the mutant allele.
Fig. 1.
Generation of Aqp2ECE/+ mice. Aqp, aquaporin; ECE, cassette expressing estrogen receptor (ER)T2CreERT2. A: schematic illustration of targeting strategy for the Aqp2ECE knock-in allele. B and C: Southern blotting analyses of embryonic stem (ES) cell clones confirming the targeted Aqp2ECE knock-in allele. D: PCR-based genotyping of chimeras showing male 5022 with almost 100% contribution of ES cells, as evidenced by the presence of the ES cell-derived WT allele coupled with barely detectable host blastocyst mutant allele of nicotinamide nucleotide transhydrogenase gene (Nnt). E: PCR-based genotyping showing that male 5022 had a high rate in germline transmission of Aqp2ECE allele to offspring.
Generation and genotyping of ECE/+ RFP/+ and ECE/+ RFP/RFP mice.
Male chimeras founders were bred with R26R-RFP [Jackson Laboratory stocks 007914 (12)] for germline transmission and for introduction of the Cre-dependent reporter. The resulting mice from successful germline transmission were heterozygous for both the ECE and RFP alleles. They were referred as ECE/+ RFP/+ mice. ECE/+RFP/RFP mice were heterozygous for the ECE allele, but homozygous for the RFP allele. They were created through crossing ECE/+RFP/+ with the R26R-RFP strain. PCR-based genotyping was conducted to identify the genotypes. Primers used for the WT (150 bp) and Aqp2ECE (380 bp) alleles were WZ1026/1028/1631. The R26R-RFP allele was genotyped according to the instructions listed on the Jackson Laboratory website. All mice were generated in a highly pure C57BL/6 background, and examined at the age of 2–3 mo.
RT-qPCR and Western blot analyses.
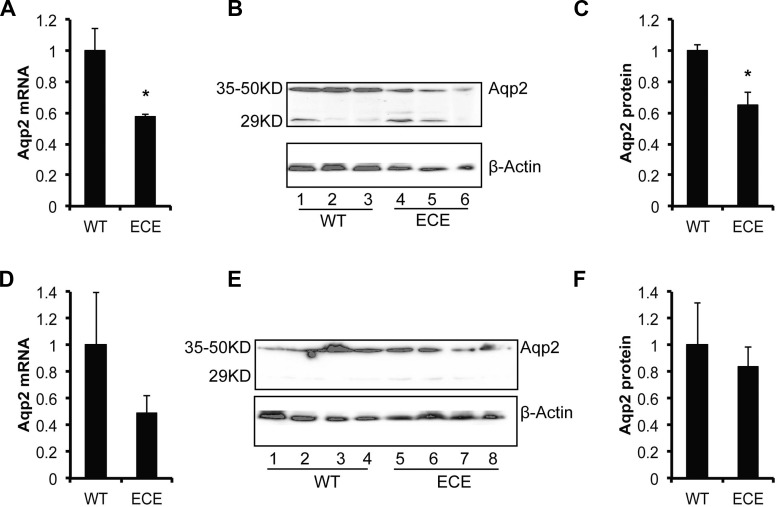
These assays were conducted as we reported (6, 25). Briefly, total RNAs and proteins were isolated from kidneys of uninduced Aqp2+/+ and Aqp2ECE/+ kidneys with free water access (n = 3 mice/genotype) and under water deprivation for 24 h (n = 4 mice/genotype). Each of the RNA samples was analyzed via real-time RT-qPCR in triplicate, with GAPDH as the internal control. The PCR primers were WZ1094/1095 for Aqp2 (137 bp) and WZ1106/1107 for GAPDH (123 bp). Western blotting analyses were performed using a rabbit anti-Aqp2 antibody (Santa Cruz, sc-28629). The 35- to 50-kDa band corresponding to the glycosylated Aqp2 was quantified and normalized to β-actin. In all cases, the relative Aqp2 mRNA and protein levels in WT were set to 1.
Metabolic balance study.
Aqp2+/+ and their Aqp2ECE/+ littermates without tamoxifen injection were given a normal Na+ diet (LabDiet, 5P76). Mice either had free water access or were under water deprivation for 24 h. They were placed in metabolic cages (Tecniplast, 3700M022) with modified feeders to improve food delivery. Urine was obtained via the metabolic cage and used to estimate all urinary parameters. Na+ excretion was estimated as the product of urine [Na+] × 24-h urine volume and normalized to body weight BW. K+ excretion was calculated similarly. For the water deprivation study, residual urine was eliminated at the start of the water deprivation study. Systolic and diastolic blood pressure with the CODA tail cuff blood pressure system (Kent Scientific, Torrington, CT) was measured as we previously reported (6, 25, 29). Each mouse received at least one cycle of measurement containing 20–30 individual readings for each parameter each day. To minimize circadian effects and daily variation, water intake, food intake, urine collection, and blood presure measurements were performed around 3 PM each day for three running days. For each mouse, data from multiple days were pooled to calculate the final average to represent that mouse and counted as 1 (n = 1).
Blood and urine measurements.
Measurements of blood and urine parameters were conducted as we reported before (6, 25). Briefly, urine [Na+] and [K+] were measured with a flame photometer (PFP7, Jenway). Urine osmolarity was recorded with a vapor pressure osmometer (Wescor Vapro Vapor Pressure Osmometer 5520; Scimetrics, Houston, TX). Urine [AVP] was determined using an ELISA kit as reported (28). Blood was analyzed using VetScan i-STAT (ABAXIS, Union City, CA) according to the manufacturer’s instruction. Blood [glucose] was also measured via a tail snip with a normal glucose meter.
All animal studies were approved by The Institutional Animal Care and Use Committee, Albany Medical College.
Induction with tamoxifen.
Tamoxifen was dissolved in sunflower oil by sonication at 10 mg/ml, aliquoted, and stored at −80°C before use. To minimize the change in tamoxifen activity due to repeated freezing and thawing, each aliquot was used only once. Adult ECE/+RFP/+ and ECE/+RFP/RFP mice were given a single intraperitoneal injection of 2 or 4 mg of tamoxifen or an equal volume of sunflower oil. Mice were euthanized for tissue harvest 24 h or 2 mo postinjection.
Immunofluorescence and confocal microscopy studies.
Immunofluorescence staining and analyses of epifluorescence and confocal images were conducted as we reported (24–26), with one exception. Confocal images were taken using a Zeiss LSM 880 NLO confocal microscope with Airyscan at The Albany Medical Center Imaging Center. At least ten fields were taken from the whole kidney in each of ECE/+RFP/+ and ECE/+RFP/RFP mice. Cell counting was restricted to tubules consisting of at least one Aqp2+ cell or at least one RFP+ cell. DAPI staining was added to aid cell counting. Recombination efficiency was defined as the number of RFP+Aqp2+ cells divided by the sum of the number of RFP+Aqp2+ cells and the number of RFP−Aqp2+ cells.
Statistical analyses.
Quantitative data are presented as means ± SD. Student's t-test was conducted to determine the statistical significance, which was set at P < 0.05.
RESULTS
Generation of a new inducible Aqp2ECE/+ mouse model.
A PAC transgene (Aqp2CreERT2) with CreERT2 inserted into the Aqp2 locus at the position of the Aqp2 initiation codon caused tamoxifen-independent recombination in mice (21), indicating leakiness. Similarly, Cre fusions (CreERT2, ERT2Cre, and FlpeERT2) caused high background recombination without 4OHT when temporally expressed from electroporated plasmids (13). In contrast, ERT2CreERT2 (ECE) tested in parallel was 4OHT dependent (13). To create a principal cell-specific, tightly controlled, inducible system, we intended to create an Aqp2ECE (or ECE in short) knock-in allele. To this end, a targeting vector with an ECE cassette inserted into the Aqp2 locus at the position of the Aqp2 ATG was constructed (Fig. 1A and Ref. 5), and electroporated into C57BL/6N-derived ES cells to knock in ECE at the endogenous Aqp2 locus and to completely disrupt the function of Aqp2. Southern analysis revealed an extremely efficient targeting frequency of > 90% (Fig. 1, B and C, and data not shown). Injection of two correctly targeted ES clones yielded four male chimeras. As shown in Fig. 1D, male chimera 5022 had almost 100% contribution from the ES cells, as indicated by the presence of an ES cell-derived WT Nnt band coupled with an undetectable mutant Nnt band. Consistently, it exhibited a high rate in germline transfer of the ECE allele to its offspring (Fig. 1E). Two of the other three male founders with 50–90% ES contribution also showed germline transmission. The resulting heterozygotes were termed Aqp2ECE/+ or ECE/+. Since all ECE/+ mice derived from the three male founders did not show a particular phenotype and were healthy, viable, and fertile, they were characterized further.
ECE/+ mice apparently have normal renal function and blood pressure.
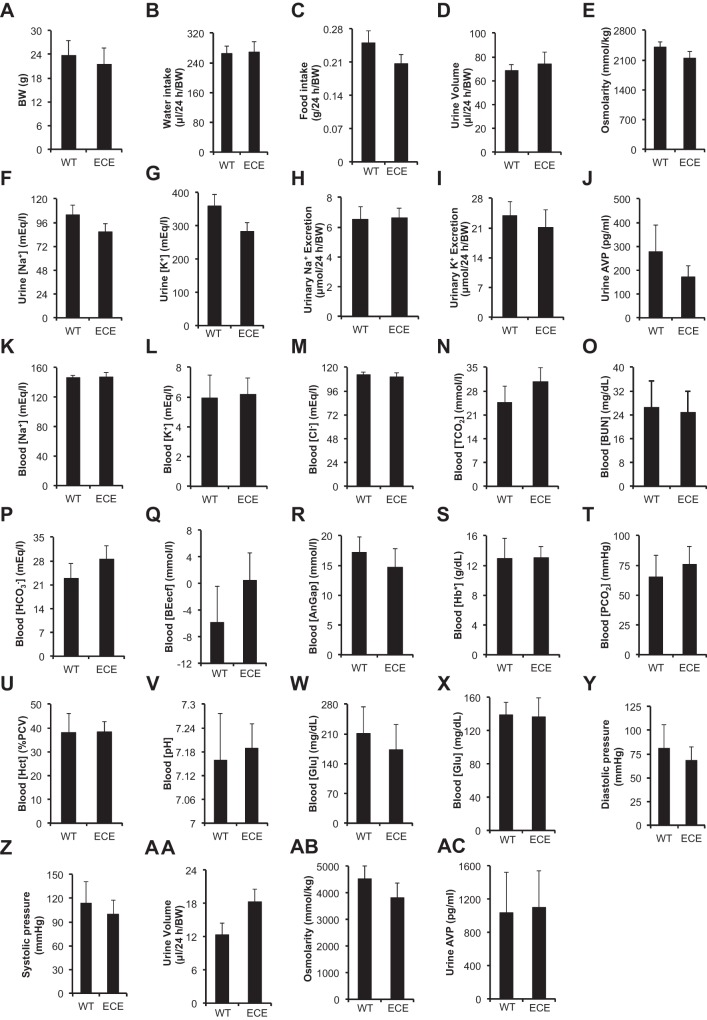
To determine whether disruption of one copy of Aqp2 by the ECE insertion would cause a significant effect on Aqp2 mRNA expression, renal function and blood pressure (BP) control, we performed real-time RT-qPCR, Western blotting, metabolic analyses, and BP measurements. In these experiments, uninduced adult mice was given a normal Na+ diet either with free access to water or under water deprivation for 24 h. Aqp2 mRNA and protein levels were significantly decreased in ECE/+ vs. WT (n = 3–4 mice/genotype; Fig. 2, A–C) when they had free access to water. However, such differences were not observed when they were challenged with the water deprivation (n = 4 mice/genotype; Fig. 2, D–F). Analyses of 10–16 WT and 11–22 ECE/+ mice revealed no significant difference in body weight, water intake, food intake, urine parameters (urine volume, osmolarity, [Na+], [K+], excretion of Na+ and K+, and [AVP]), blood parameters ([Na+], [K+], [Cl−], [TCO2], [BUN], [], [BEecf], [AnGap], [Hb], [Pco2], [Hct], pH, and [glucose]), and diastolic and systolic pressure between the two genotypes (Fig. 3, A–Z). It should be noted that [glucose] measured with iSTAT was substantially higher than [glucose] measured via tail snip with a normal glucose meter for both genotypes (Fig. 3, W and X).
Fig. 2.
Analyses of Aqp2 expression. Total RNAs and proteins were isolated from kidneys of uninduced Aqp2+/+ and Aqp2ECE/+ kidneys with free water access (n = 3 mice/genotype, A–C) and under water deprivation for 24 h (n = 4 mice/genotype, D–F). Each RNA sample was analyzed by real-time RT-qPCR in triplicates, using GAPDH as internal control. For Western blotting analyses, rabbit Aqp2 (sc-28629) was used. The 35- to 50-kDa band corresponding to glycosylated Aqp2 was quantified, normalized to β-actin, and presented in C and F, respectively. In all case, relative Aqp2 mRNA and protein levels in WT were set to 1. *P < 0.05 vs. WT.
Fig. 3.
ECE/+ mice apparently have normal renal function and blood pressure. Uninduced adult WT (n = 10–16) and ECE/+ mice (ECE; n = 11–22) were given a normal Na+ diet with free water access (A–Z) or were under water deprivation for 24 h. AA–AC were analyzed for parameters as indicated. K–W were done with iSTAT. X was measured via tail snip with a normal glucose meter. Significance of difference at P < 0.05 in each of the parameters between the two genotypes was not reached.
ECE/+ vs. WT mice were also indistinguishable in the urine volume, osmolarity and [AVP] after 24-h water deprivation (Fig. 3, AA–AC). Hence, there is no indication of a significant effect of inactivation of one copy of Aqp2 by the ECE knock-in on renal function and BP maintentance under the conditions tested.
Aqp2ECE possesses absolutely no leaky activity.
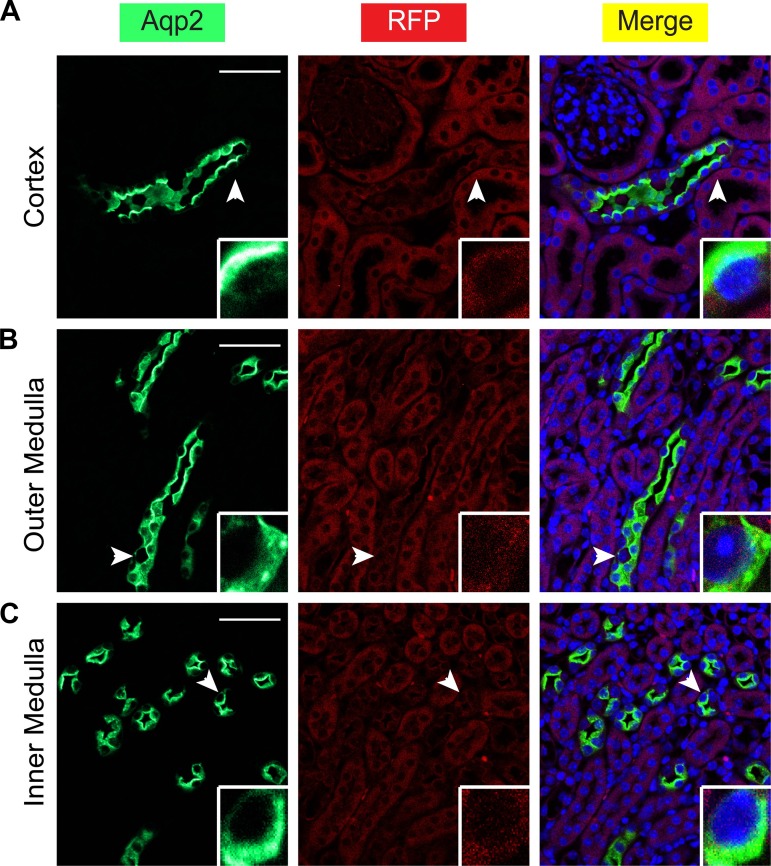
ECE had no detectable recombination activity without 4OHT in electroporation studies (13). However, to our knowledge, the tight control of ECE has not been rigorously evaluated when it is permanently expressed in vivo, since no ECE transgenic or knock-in animals have been reported. Accordingly, we bred each of the three male chimera founders with R26RtdTomato/+ (referred as RFP) reporter (Jackson Laboratory stock 007914). RFP is expressed after removal of the floxed stop cassette through Cre-mediated recombination. Immunofluorescence staining with anti-RFP and anti-Aqp2 antibodies verified the lack of RFP expression in the whole kidney in oil-injected 2-mo-old double heterozygotes ECE/+RFP/+ and ECE/+RFP/RFP mice that were heterozygous for ECE but homozygous for RFP (Fig. 4 and data not shown). Therefore, our data demonstrate absolutely no “leaky” activity of ECE in the absence of tamoxifen induction. In addition, our data also indicate that ECE transmission can be carried out through males. There are two possibilities: the male germline activity of the Aqp2 promoter (15) did not cause ECE-mediated background recombination in the sperm; alternatively, Aqp2 promoter is inactive in the male germline. The latter is consistent with undetectable Aqp2 and RFP expression in the testis in the induced ECE/+ mice (see Fig. 10B).
Fig. 4.
Aqp2ECE possesses absolute no leaky activity. A–C: immunofluorescence staining for Aqp2 (green) and red fluorescent protein (RFP; red) showing no RFP expression in whole kidney of an oil-injected adult ECE/+RFP/RFP mouse. Cells indicated by arrowheads were magnified in the insets. Scale bar, 50 μm, 8.3 μm for inset.
Fig. 10.
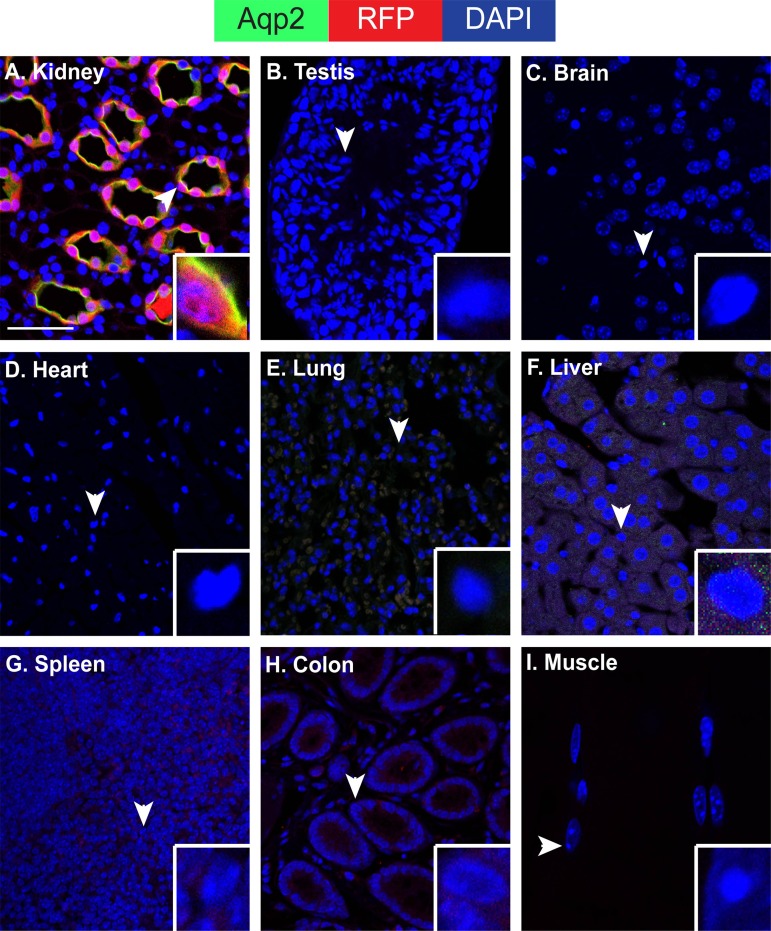
There is no indication of extrarenal Aqp2 expression and ECE-mediated recombination. A–I: immunofluorescence staining for Aqp2 (green) and RFP (red) showing lack of their expression in all organs except kidney, as indicated in adult ECE/+RFP/RFP mice treated with a single dose of tamoxifen (2 mg) for 24 h. Cells indicated by arrowheads are magnified in the insets. DAPI staining (blue) for nuclei was added. Scale bar, 50 μm, 8.3 μm for inset for each panel.
Aqp2ECE confers high inducibility and complete fidelity in cell specificity in adult mice.
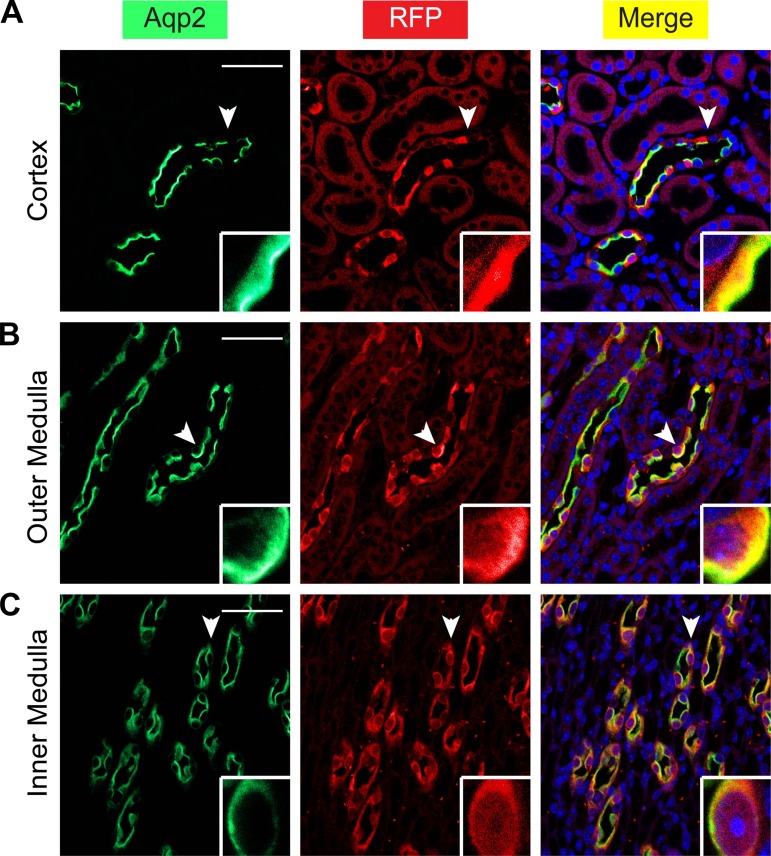
To test inducibility and fidelity, we treated 2-mo-old ECE/+RFP/+ and ECE/+RFP/RFP mice with tamoxifen (2 mg) for 24 h. Analyses of frozen sections revealed many RFP+ cells in cortex and outer and inner medulla of both genotypes (Fig. 5 and data not shown). Immunofluorescence staining for RFP and Aqp2 showed that all RFP-labeled cells expressed Aqp2 (Fig. 6) regardless of the regions (cortex and outer and inner medulla) examined. We categorized all Aqp2+ cells into Aqp2+ RFP+ and Aqp2+ RFP− cells and defined the recombination rate as the number of Aqp2+ RFP+ cells divided by all Aqp2+ cells in each region. For the induced ECE/+RFP/+ mice (n = 3), the recombination rates were 46% (354/765) in the cortex, 68% (671/994) in the outer medulla, and 71% (777/1,093) in the inner medulla. For the induced ECE/+RFP/RFP mice (n = 3), the recombination rates were 46% (252/547) in the cortex, 91% (1,632/1,799) in the outer medulla, and 95% (1,327/1,401) in the inner medulla. The recombination rates in ECE/+RFP/+ mice were increased by either increasing the dose of tamoxifen or examination at a longer time point post induction. A single injection of tamoxifen (4 mg) increased the recombination rates to 52% (405/786), 86% (884/1,026), and 90% (1,062/1,177) in the cortex, outer medulla, and inner medulla in adult ECE/+ RFP/+ mice 24 h postinjection. About 72% (2,680/3,728), 93% (5,696/6,142), and 93% (5,823/6,231) Aqp2+ cells in cortex, outer medulla, and inner medulla expressed RFP when adult ECE/+RFP/+ mice (n = 3) were given a single injection of tamoxifen (2 mg) and analyzed 2 mo later.
Fig. 5.
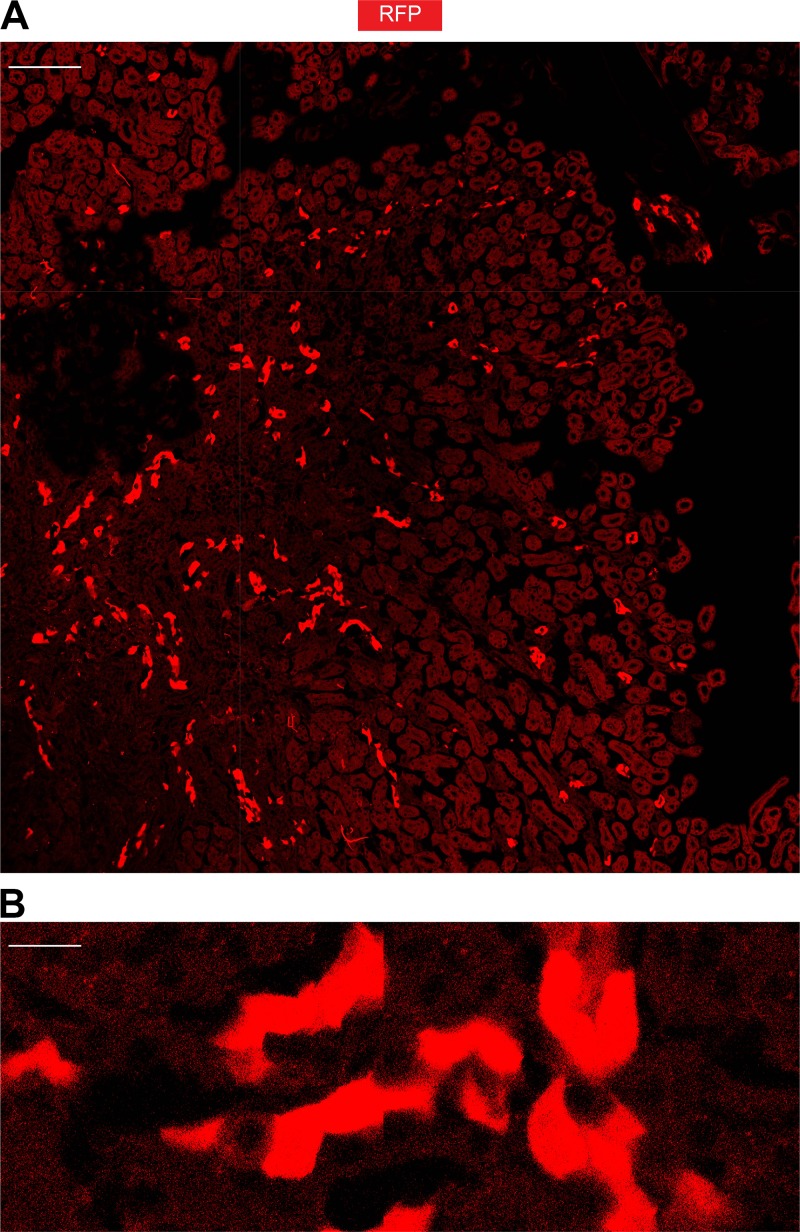
Aqp2ECE is highly inducible. Representative frozen kidney section of an adult ECE/+RFP/RFP mouse treated with a single dose of tamoxifen (2 mg) for 24 h showing robust RFP expression in whole kidney. Boxed area is magnified in B. Scale bar, 200 μm for A, 20 μm for B.
Fig. 6.
Aqp2ECE has complete fidelity in recapitulating the cell-specific expression pattern of Aqp2. A–C: immunofluorescence staining for Aqp2 (green) and RFP (red) showing all RFP+ cells were also Aqp2+ in whole kidney of adult ECE/+RFP/RFP mice treated with a single dose of tamoxifen (2 mg) for 24 h. Cells indicated by arrowheads are magnified in the insets. Scale bar, 50 μm, 8.3 μm for inset.
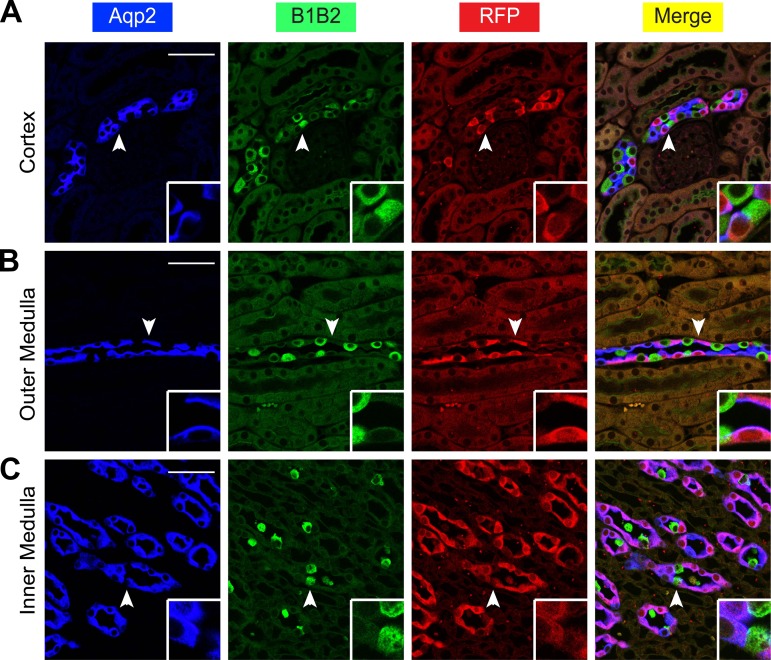
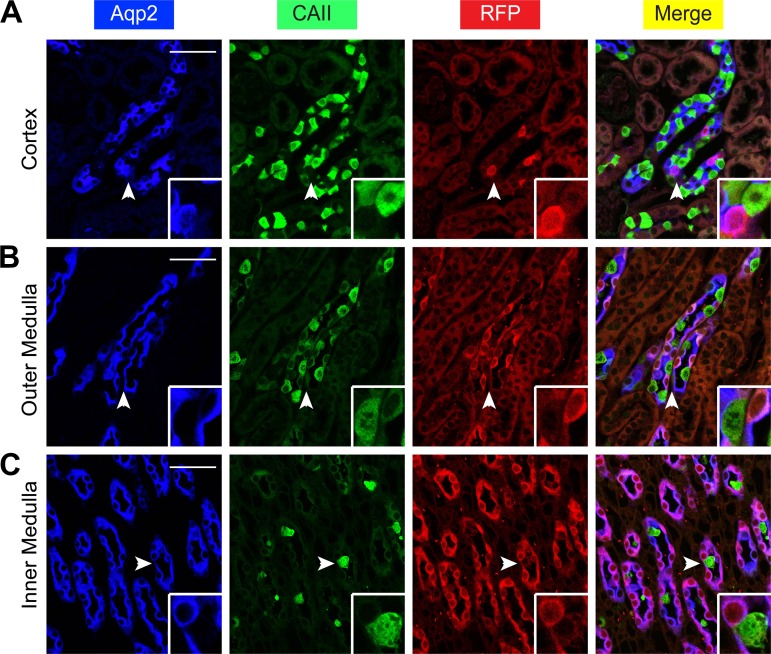
To determine whether RFP was coexpressed with IC markers, we first stained the kidneys of the ECE/+RFP/RFP mice with three antibodies specific for RFP, Aqp2, and V-ATPase subunits B1 and B2 (B1B2) as the IC marker. We did not find a single RFP+ B1B2+ cell in the whole kidney (Fig. 7). We repeated the experiment by replacing the B1B2 antibody with an antibody specific for carbonic anhydrase II (CAII), another well-established IC marker. No RFP+ CAII+ cells were detected (Fig. 8). In brief, our data demonstrate that the Aqp2ECE allele is highly inducible and mediates recombination exclusively in the Aqp2+ cells in adult mice.
Fig. 7.
Aqp2ECE is inactive in intercalated cells marked by V-ATPase subunits B1 and B2 (B1B2). A–C: immunofluorescence staining for Aqp2 (blue) and B1B2 (green) to mark intercalated cells and RFP (red) showing no RFP coexpression with B1B2 in whole kidney of adult ECE/+RFP/RFP mice treated with a single dose of tamoxifen (2 mg) for 24 h. Cells indicated by arrowheads are magnified in the insets. Scale bar, 50 μm, 8.3 μm for inset.
Fig. 8.
Aqp2ECE is inactive in intercalated cells marked by carbonic anhydrase II (CAII). A–C: immunofluorescence staining for Aqp2 (blue) and CAII (green) to mark intercalated cells and RFP (red) showing no RFP coexpression with CAII in whole kidney of adult ECE/+RFP/RFP mice treated with a single dose of tamoxifen (2 mg) for 24 h. Cells indicated by arrowheads are magnified in the insets. Scale bar, 50 μm, 8.3 μm for inset.
ECE confers principal cell-specific recombination.
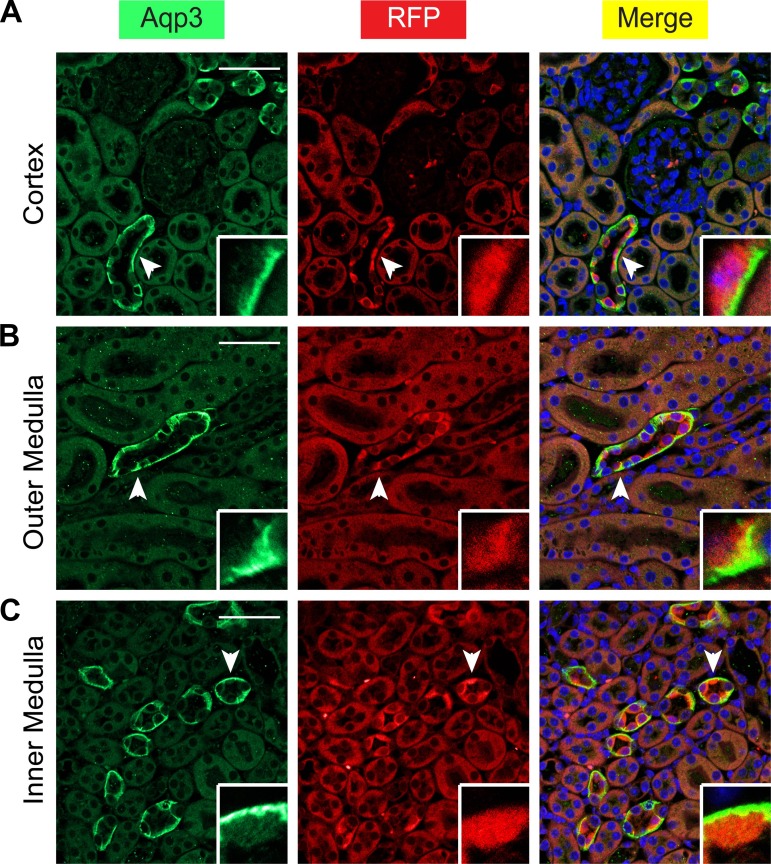
In addition to Aqp2 itself, Aqp3 is also a well-established principal cell marker. To further confirm the cell specificity of ECE-mediated recombination, coexpression of RFP with Aqp3 was investigated in the kidneys of the induced ECE/+RFP/RFP mice by immunofluorescence staining. As expected, all RFP+ cells were also positive for Aqp3 (Fig. 9).
Fig. 9.
Aqp2ECE is principal-cell specific. A–C: immunofluorescence staining for Aqp3 to mark principal cells (blue) and RFP (red) showing that all RFP-expressing cells also expressed Aqp3 in whole kidney of adult ECE/+RFP/RFP mice treated with a single dose of tamoxifen (2 mg) for 24 h. Cells indicated by arrowheads are magnified in the insets. Scale bar, 50 μm, 8.3 μm for inset.
There is no indication of extrarenal Aqp2 expression and ECE-mediated recombination.
To determine whether ECE-mediated recombination outside of kidney, we performed immunofluorescence staining of Aqp2 and RFP in eight major organs (testis, brain, heart, lung, liver, spleen, colon, and muscle), with kidney as a positive control. All of these tissues were isolated from ECE/+RFP/RFP mice induced with tamoxifen (2 mg) for 24 h. Whereas Aqp2 and RFP were readily observed in the kidney (Fig. 10A), they were undetectable in each of all the other organs tested (Fig. 10, B–I).
DISCUSSION
To dissect the function of target genes at a particular developmental stage in a particular cell type, an ideal inducible system is required. It should strictly meet four criteria: 1) cell specific, 2) no background recombination in the absence of induction, 3) high recombination rate after induction, and 4) complete fidelity in cell specificity to restrict recombination exclusively in cells where the endogenous gene whose promoter controls the recombinase production is expressed. Our data presented in this study demonstrate that Aqp2ECE is indeed an ideal inducible system by these criteria. Our study also for the first time offers solid evidence suggesting that the activity of ECE is absolutely dependent on tamoxifen even when ECE is expressed permanently and constantly under the control of a strong promoter such as the Aqp2 promoter, which was used in this study.
Many tamoxifen-based inducible mouse models have been developed in which CreERT2 is placed under the control of a ubiquitous or a tissue/cell-specific promoter. These inducible Cre drivers are essential reagents for temporal and spatial control of gene modifications. However, in many cases, little or no information is available with respect to leakage Cre activity in the absence of tamoxifen, recombination efficiency, and/or the faithfulness in replicating the tissue/cell-specific expression pattern of the endogenous gene whose promoter drives the CreERT2 recombinase. Without these data, it is a challenge for investigators to select a driver line, design experiments, and evaluate Cre recombination-induced phenotypes of their flox mice.
An argument for a much tighter control of ECE than all the other three Cre fusions including CreERT2 has been proposed based on electroporation studies (13). Although the conclusion reached in this study agrees with our own, our case is more compelling. In our study, both the Cre-dependent RFP reporter and the ECE cassette were permanently inserted into the mouse genome in the defined loci. In contrast, previous studies used electroporated plasmids encoding Cre reporters and Cre fusions. The possibility that the lack of reporter expression simply resulted from the loss of the plasmids or the position effect of the integration site(s), although very low, cannot be completely ruled out.
Another line of evidence in support of the notion that ECE is more tightly controlled by tamoxifen than CreERT2 comes from the substantial background recombination of the PAC Aqp2CreERT2 transgene (21). The multiple copies of the transgene, the strong Aqp2 promoter, and possibly the integration site of the transgene result in high levels of CreERT2 production. It can be speculated that the highly abundant CreERT2 plus the lack of its absolute dependence on tamoxifen led to nuclear translocation of the Cre fusion to catalyze recombination in the absence of tamoxifen. Moreover, leaky activity has been reported in multiple cases including female KspCad-CreERT2 (17), Col2a1-CreERT2 (22), UBC-CreERT2 (22), and CAG-CreERT2 (14). Accumulation of the effect of even a minor leakiness over time may complicate data analyses because of the irreversibility of every recombination event; hence, the usefulness of the leaky drivers is very limited. The limitations include that they cannot be employed to analyze the effect of disruption of a gene of interest at a particular time point and to perform in vivo lineage tracing in adult animals.
The ECE knock-in strategy we used in this study may be applied to other genes of interest, even with strong promoters for development of various tissue/cell-specific drivers, particularly, for those having shown a leaky activity of CreERT2. Knock-in permits insertion of the ECE at a predefined genomic locus and ensures the presence of only a single copy of the ECE allele in the heterozygotes, excluding the possible overproduction of the Cre fusion due to an increased copy number.
Three mechanisms can be speculated on for a tighter control of the ECE than CreERT2 (13). Compared with CreERT2, ECE may form a tighter inactive complex with Hsp90 in the cytoplasm, remain inactive even after losing one ER domain, and be less active. However, the high recombination rate of Aqp2ECE after a single dose of tamoxifen administration argues against the lesser activity of ECE.
One injection of tamoxifen was sufficient to induce robust RFP expression after only 24 h in ECE/+RFP/+ and ECE/+RFP/RFP mice. The recombination efficiency can reach up to 95%. Many Cre drivers exhibited substantially lower recombination rates even after three to five daily injections of tamoxifen. For example, KspCad-CreERT2 yielded a recombination efficiency of only 40–50% even after administration of tamoxifen at a much higher dose (5 × 5 mg/day) (11). SLC34a1GCE induced with five doses of 3 mg of tamoxifen mediated 58% recombination in the cortical S3 segment (10). Injection of tamoxifen in adult Podocin-iCreERT2-containing mice once resulted in only 10% excision (23).
Since the ECE is inserted at the ATG of the endogenous Aqp2 locus, it is under the control of the same regulatory elements that govern Aqp2 expression. As expected, RFP signals as the output of the ECE-mediated recombination recapitulated the principal cell-specific expression of the endogenous Aqp2 in the CD with complete fidelity in cell specificity, although some Aqp2+ cells were RFP−. We verified the fidelity with a high resolution at single cells by costaining Aqp2 and RFP with or without IC markers on the same slides. A previous report using RT-PCR and immunohistochemistry demonstrated Aqp2 expression in the male reproductive system of C57BL/CBA mice (15). However, we did not detect Aqp2 and thus RFP expression in the testis of the induced ECE/+RFP/RFP mice with a highly pure C57BL/6 background. Whether this inconsistency stems from the difference in the genetic background of the mouse models used or something else requires further investigation.
The cell-specific property distinguishes the Aqp2ECE/+ from the vast majority of the existing kidney-specific inducible mouse models, which are not cell specific. For instance, KspCad-CreERT2 transgene activates R26R-EYFP reporter in proximal tubules, thick ascending limbs of loops of Henle, distal convoluted tubule, and collecting duct (17). The sodium-dependent inorganic phosphate transporter SLC34a1 and thus SLC34a1-GFPCreERT2 are expressed in differentiated proximal tubule, which consists of S1, S2, and S3 segments (10). Protrudin, which is encoded by Zfyve27, is expressed in interstitial cells, collecting duct cells, and thin limbs of Henle’s loop in the kidney papilla (16). Faithful recapitulation of protrudin is expected to confer Zfyve27-CreERT2 being active in these cell types. Hence, Aqp2ECE/+ mice should be very powerful and complementary to the increasing list of inducible mouse model systems for renal research.
The most obvious application of the Aqp2ECE/+ mice is to inactivate floxed targeted genes temporally and specifically in the principal cells in CD and presumably in CNT. A typical example is deletion of Pkd1 and Pkd2 in adult mice. Like many other disorders such as various types of cancer, polycystic kidney disease (PKD) is a focal disease in nature and involves somatic mutations of target genes. In this adult-onset disease, cysts develop in <1% of the nephrons. While renal cysts in autosomal dominant PKD can arise from cells throughout the nephron (18), most cysts stain positive for markers of collecting duct cells in advanced autosomal dominant PKD kidneys. Therefore, CD/CNT-specific inactivation of Pkd1 and Pkd2 in adult mice using Aqp2ECE/+ may offer critical insights into the molecular and pathological basis of PKD.
Aqp2ECE/+ can also be employed to temporally activate genes of interest by removing a “STOP” cassette that prevents their expression within the CD. This is clearly demonstrated by the expression of RFP in this study. Such applications will undoubtedly enhance our ability to genetically dissect the role of putative disease-causing genes within either developing or adult kidney.
Given the absolutely no leakiness and complete fidelity in cell specificity, Aqp2ECE/+ mice will provide a unique advantage to trace Aqp2+ lineage. With the abolished histone H3 K79 dimethylation as a tracing marker, we have shown that the principal cells and most intercalated cells are derived from a new pool of progenitor cells expressing Aqp2 in Dot1lAC mice during development (25). However, whether Aqp2+ progenitor cells exist in adult kidney remains completely unknown. Aqp2ECE/+ mice will become a unique tool to address this question.
Like all other constitutive or inducible Cre drivers, Aqp2ECE did not yield a 100% recombination rate under three conditions tested for ECE/+RFP/+ mice (2 and 4 mg tamoxifen for 24 h, and 2 mg tamoxifen for 2 mo) and one condition for ECE/+RFP/RFP mice (2 mg tamoxifen for 24 h). The recombination rates in the cortex and outer and inner medulla may be further increased via a variety of methods including repeated tamoxifen injections, higher doses of tamoxifen, examination at even longer time points postinjection, and water deprivation or restriction to increase the Aqp2 promoter activity. It is also possible that the rates may not be significantly changed due to the very high recombination rates that we have already observed, the potential intrinsic limitation of the Aqp2ECE model system, or both. Nevertheless, these possibilities have not been completely addressed, a limitation of the current study. Aqp2ECE can presumably be used for genetic modifications in the CNT. However, immunofluorescence staining experiments with CNT-specific markers are required to conclusively demonstrate ECE-mediated recombination in the CNT.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants 2R01 DK-080236 06A1 and R21 DK-70834 (both to W. Zhang.)
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.C. and W.Z. conceived and designed research; L.C., C.G., L.Z., Y.Z., and E.C. performed experiments; L.C., C.G., L.Z., Y.Z., and W.Z. analyzed data; L.C., C.G., L.Z., Y.Z., E.C., and W.Z. interpreted results of experiments; C.G. and W.Z. prepared figures; W.Z. drafted manuscript; L.C., C.G., and W.Z. edited and revised manuscript; L.C., C.G., L.Z., Y.Z., E.C., and W.Z. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the UCI Transgenic Mouse Facility (TMF) for ES targeting and production of chimeric mice. The UCI TMF is a shared resource funded in part by the Chao Family Comprehensive Cancer Center Support Grant (P30 CA-062203) from the National Cancer Institute. This study was started when Dr. Lihe Chen pursued his Ph.D. in Dr. W. Zhang’s laboratory in the University of Texas Health Science Center at Houston.
REFERENCES
- 1.Ahn D, Ge Y, Stricklett PK, Gill P, Taylor D, Hughes AK, Yanagisawa M, Miller L, Nelson RD, Kohan DE. Collecting duct-specific knockout of endothelin-1 causes hypertension and sodium retention. J Clin Invest 114: 504–511, 2004. doi: 10.1172/JCI200421064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barker N, Rookmaaker MB, Kujala P, Ng A, Leushacke M, Snippert H, van de Wetering M, Tan S, Van Es JH, Huch M, Poulsom R, Verhaar MC, Peters PJ, Clevers H. Lgr5(+ve) stem/progenitor cells contribute to nephron formation during kidney development. Cell Reports 2: 540–552, 2012. doi: 10.1016/j.celrep.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 3.Boyle S, Misfeldt A, Chandler KJ, Deal KK, Southard-Smith EM, Mortlock DP, Baldwin HS, de Caestecker M. Fate mapping using Cited1-CreERT2 mice demonstrates that the cap mesenchyme contains self-renewing progenitor cells and gives rise exclusively to nephronic epithelia. Dev Biol 313: 234–245, 2008. doi: 10.1016/j.ydbio.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Branda CS, Dymecki SM. Talking about a revolution: The impact of site-specific recombinases on genetic analyses in mice. Dev Cell 6: 7–28, 2004. doi: 10.1016/S1534-5807(03)00399-X. [DOI] [PubMed] [Google Scholar]
- 5.Chen L. Developmental Origins of Renal Connecting Tubule and Collecting Duct: Role of Aqp2+ Progenitor Cells (PhD Dissertation). Houston, TX: UTHealth, 2015. http://digitalcommons.library.tmc.edu/utgsbs_dissertations/556/. [Google Scholar]
- 6.Chen L, Wu H, Pochynyuk OM, Reisenauer MR, Zhang Z, Huang L, Zaika OL, Mamenko M, Zhang W, Zhou Q, Liu M, Xia Y, Zhang W. Af17 deficiency increases sodium excretion and decreases blood pressure. J Am Soc Nephrol 22: 1076–1086, 2011. doi: 10.1681/ASN.2010121270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dworniczak B, Skryabin B, Tchinda J, Heuck S, Seesing FJ, Metzger D, Chambon P, Horst J, Pennekamp P. Inducible Cre/loxP recombination in the mouse proximal tubule. Nephron, Exp Nephrol 106: e11–e20, 2007. doi: 10.1159/000100554. [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi A, Mugford JW, Krautzberger AM, Naiman N, Liao J, McMahon AP. Identification of a multipotent self-renewing stromal progenitor population during mammalian kidney organogenesis. Stem Cell Reports 3: 650–662, 2014. doi: 10.1016/j.stemcr.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kobayashi A, Valerius MT, Mugford JW, Carroll TJ, Self M, Oliver G, McMahon AP. Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell 3: 169–181, 2008. doi: 10.1016/j.stem.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kusaba T, Lalli M, Kramann R, Kobayashi A, Humphreys BD. Differentiated kidney epithelial cells repair injured proximal tubule. Proc Natl Acad Sci USA 111: 1527–1532, 2014. [Erratum. Proc Natl Acad Sci USA 111: 5754, 2014.] doi: 10.1073/pnas.1310653110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lantinga-van Leeuwen IS, Leonhard WN, van de Wal A, Breuning MH, Verbeek S, de Heer E, Peters DJ. Transgenic mice expressing tamoxifen-inducible Cre for somatic gene modification in renal epithelial cells. Genesis 44: 225–232, 2006. doi: 10.1002/dvg.20207. [DOI] [PubMed] [Google Scholar]
- 12.Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES, Zeng H. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci 13: 133–140, 2010. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsuda T, Cepko CL. Controlled expression of transgenes introduced by in vivo electroporation. Proc Natl Acad Sci USA 104: 1027–1032, 2007. doi: 10.1073/pnas.0610155104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mirantes C, Eritja N, Dosil MA, Santacana M, Pallares J, Gatius S, Bergadà L, Maiques O, Matias-Guiu X, Dolcet X. An inducible knockout mouse to model the cell-autonomous role of PTEN in initiating endometrial, prostate and thyroid neoplasias. Dis Model Mech 6: 710–720, 2013. doi: 10.1242/dmm.011445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nelson RD, Stricklett P, Gustafson C, Stevens A, Ausiello D, Brown D, Kohan DE. Expression of an AQP2 Cre recombinase transgene in kidney and male reproductive system of transgenic mice. Am J Physiol 275: C216–C226, 1998. doi: 10.1152/ajpcell.1998.275.1.C216. [DOI] [PubMed] [Google Scholar]
- 16.Oliver JA, Sampogna RV, Jalal S, Zhang QY, Dahan A, Wang W, Shen TH, Al-Awqati Q. A subpopulation of label-retaining cells of the kidney papilla regenerates injured kidney medullary tubules. Stem Cell Reports 6: 757–771, 2016. doi: 10.1016/j.stemcr.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel V, Li L, Cobo-Stark P, Shao X, Somlo S, Lin F, Igarashi P. Acute kidney injury and aberrant planar cell polarity induce cyst formation in mice lacking renal cilia. Hum Mol Genet 17: 1578–1590, 2008. doi: 10.1093/hmg/ddn045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raphael KL, Strait KA, Stricklett PK, Miller RL, Nelson RD, Piontek KB, Germino GG, Kohan DE. Inactivation of Pkd1 in principal cells causes a more severe cystic kidney disease than in intercalated cells. Kidney Int 75: 626–633, 2009. doi: 10.1038/ki.2008.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ronchi JA, Figueira TR, Ravagnani FG, Oliveira HC, Vercesi AE, Castilho RF. A spontaneous mutation in the nicotinamide nucleotide transhydrogenase gene of C57BL/6J mice results in mitochondrial redox abnormalities. Free Radic Biol Med 63: 446–456, 2013. doi: 10.1016/j.freeradbiomed.2013.05.049. [DOI] [PubMed] [Google Scholar]
- 20.Ronzaud C, Loffing J, Bleich M, Gretz N, Gröne HJ, Schütz G, Berger S. Impairment of sodium balance in mice deficient in renal principal cell mineralocorticoid receptor. J Am Soc Nephrol 18: 1679–1687, 2007. doi: 10.1681/ASN.2006090975. [DOI] [PubMed] [Google Scholar]
- 21.Ronzaud C, Loffing J, Gretz N, Schütz G, Berger S. Inducible renal principal cell-specific mineralocorticoid receptor gene inactivation in mice. Am J Physiol Renal Physiol 300: F756–F760, 2011. doi: 10.1152/ajprenal.00728.2009. [DOI] [PubMed] [Google Scholar]
- 22.Seime T, Kolind M, Mikulec K, Summers MA, Cantrill L, Little DG, Schindeler A. Inducible cell labeling and lineage tracking during fracture repair. Dev Growth Differ 57: 10–23, 2015. doi: 10.1111/dgd.12184. [DOI] [PubMed] [Google Scholar]
- 23.Wang J, Wang Y, Long J, Chang BH, Wilson MH, Overbeek P, Danesh FR. Tamoxifen-inducible podocyte-specific iCre recombinase transgenic mouse provides a simple approach for modulation of podocytes in vivo. Genesis 48: 446–451, 2010. doi: 10.1002/dvg.20635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu H, Chen L, Zhang X, Zhou Q, Li JM, Berger S, Borok Z, Zhou B, Xiao Z, Yin H, Liu M, Wang Y, Jin J, Blackburn MR, Xia Y, Zhang W. Aqp5 is a new transcriptional target of Dot1a and a regulator of Aqp2. PLoS One 8: e53342, 2013. doi: 10.1371/journal.pone.0053342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu H, Chen L, Zhou Q, Zhang X, Berger S, Bi J, Lewis DE, Xia Y, Zhang W. Aqp2-expressing cells give rise to renal intercalated cells. J Am Soc Nephrol 24: 243–252, 2013. doi: 10.1681/ASN.2012080866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiao Z, Chen L, Zhou Q, Zhang W. Dot1l deficiency leads to increased intercalated cells and upregulation of V-ATPase B1 in mice. Exp Cell Res 344: 167–175, 2016. doi: 10.1016/j.yexcr.2015.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang H, Zhang A, Kohan DE, Nelson RD, Gonzalez FJ, Yang T. Collecting duct-specific deletion of peroxisome proliferator-activated receptor γ blocks thiazolidinedione-induced fluid retention. Proc Natl Acad Sci USA 102: 9406–9411, 2005. doi: 10.1073/pnas.0501744102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y, Peti-Peterdi J, Heiney KM, Riquier-Brison A, Carlson NG, Müller CE, Ecelbarger CM, Kishore BK. Clopidogrel attenuates lithium-induced alterations in renal water and sodium channels/transporters in mice. Purinergic Signal 11: 507–518, 2015. doi: 10.1007/s11302-015-9469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou CC, Zhang Y, Irani RA, Zhang H, Mi T, Popek EJ, Hicks MJ, Ramin SM, Kellems RE, Xia Y. Angiotensin receptor agonistic autoantibodies induce pre-eclampsia in pregnant mice. Nat Med 14: 855–862, 2008. doi: 10.1038/nm.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]