Abstract
Regulation of acid-base metabolism maintains the pH of body fluids within a tight range. Urine pH (UpH) is also regulated under normal conditions. Median pH of 24-h urines is ~6, but others have noted that UpH in women is higher than men, which has been attributed to differences in diet. If true, it would help to explain the fact that calcium phosphate stones, which form at higher urine pH, are much more common in women than in men. We studied 14 normal subjects (7 men and 7 women) fed identical meals in a Clinical Research Center. Urine and blood samples were collected during fasting and after meals. UpH of women (6.74 ± 0.11) exceeded that of men (6.07 ± 0.17) fed, but not fasting, and UpH rose significantly with meals in women but not men. Serum and urine total CO2 rose with meals in women but not men, and in women net acid excretion fell to zero during the fed period. In a general linear model adjusted for age, sex, and weight, net gastrointestinal anion uptake was the main predictor of UpH and was significantly higher in women (3.9 ± 0.6) than men (1.8 ± 0.7) in the fed period. Urine citrate, an anion absorbed by the gastrointestinal tract, was higher in women than men in the fed state, and fractional excretion of citrate was higher in women than men. The higher fed UpH in women is related to a greater absorption of food anions and raises 24-h UpH.
Keywords: net acid excretion, net gastrointestinal anion, urinary acidification, urine pH
INTRODUCTION
The regulation of total body acid-base metabolism is intended primarily to maintain the pH of body fluids within a tight range to permit optimal conditions for cellular functions. However, urine pH is also regulated under normal conditions (11), albeit not as tightly or by the same mechanisms as serum pH. The median pH of human 24-h urines is ∼6 (8). At this pH, solubility of both uric acid and phosphate salts is optimized, which protects against stone formation in the urinary tract. At lower pH, uric acid solubility falls, increasing the risk for uric acid stones, whereas as pH rises above 6 the solubility of calcium phosphate decreases, increasing the risk for brushite and apatite kidney stones (8). Buffering of acid excretion is achieved by use of titratable buffers, primarily phosphate, as well as production of ammonia. Control of excess alkali excretion is less well understood but appears to involve excretion of organic anions such as citrate, allowing loss of alkali without an increase in urine bicarbonate and thus urine pH (7). Under conditions of alkali loading, bicarbonate can be secreted into the urine via pendrin (30), an important pathway for maintaining acid base homeostasis under such conditions.
It was noted by Waters et al. (31) in a community study done in Wales that the first morning urine pH of women was higher than that of men on a free-choice diet. These authors speculated that this was due to higher protein intakes in the men, but the issue was not investigated further. Several recent large epidemiological cohorts (9, 16, 28) have also demonstrated higher 24-h urine pH in women than in men of similar age, again on a free-choice diet.
If urine pH is higher in women than men, it would help to explain the disparity in stone types formed by men and women; calcium phosphate stones, which form at higher urine pH, are much more common in women than in men (24). We have used our group of normal men and women studied in a General Clinical Research Center (GCRC) to discover whether higher urine pH is seen in women compared with men when they are eating identical diets and, if so, what mechanisms are responsible for the difference.
MATERIALS AND METHODS
Subjects
We studied 14 healthy subjects (7 male) without a personal or family history of kidney stones (Table 1). Age and body mass index (BMI) were well matched by sex (Table 1). These subjects have appeared in other reports from our laboratory, as noted in the table (4, 5, 33, 34). The study was approved by the University of Chicago Institutional Review Board.
Table 1.
Subjects
| Subject No. | Age, yr | Weight, kg | Height, cm | BMI, kg/m2 | BSA, m2 |
|---|---|---|---|---|---|
| Females | |||||
| 1 *†#‡ | 47 | 86 | 160 | 33 | 1.94 |
| 2 *#‡ | 44 | 55 | 158 | 22 | 1.55 |
| 3 *†#‡ | 26 | 68 | 168 | 24 | 1.79 |
| 4 *†#‡ | 55 | 67 | 169 | 24 | 1.78 |
| 5 †‡ | 38 | 71 | 167 | 22 | 1.67 |
| 6 *†‡ | 24 | 71 | 178 | 22 | 1.87 |
| 7 *†‡ | 29 | 62 | 161 | 24 | 1.66 |
| Mean ± SE | 38 ± 5 | 67 ± 4+ | 166 ± 3+ | 24 ± 2 | 1.75 ± 0.05+ |
| Males | |||||
| 8 *†#‡ | 28 | 66 | 163 | 25 | 1.73 |
| 9 †#‡ | 48 | 80 | 175 | 26 | 1.97 |
| 10 *†#‡ | 55 | 88 | 181 | 27 | 2.11 |
| 11 *†#‡ | 44 | 85 | 178 | 27 | 2.05 |
| 12 †‡ | 36 | 77 | 170 | 26 | 1.87 |
| 13 *†‡ | 50 | 76 | 183 | 23 | 1.97 |
| 14 *†‡ | 37 | 90 | 189 | 25 | 2.17 |
| Mean ± SE | 43 ± 4 | 80 ± 3 | 177 ± 3 | 25 ± 1 | 1.98 ± 0.06 |
Protocol
Subjects were studied in the GCRC at the University of Chicago over 14 h, as previously described (35). Prior to the study day, a nutritionist interviewed each subject and instructed each on a prestudy diet that mirrored the study diet, which they were asked to follow for 5 days before the study. On the study day, at admission between 6 and 7 AM, two 1-h fasting urine samples were collected. Subsequently, subjects ate three study meals, with hourly urine collections until 3 h after the last meal (14 urine samples total). Matching blood samples were collected hourly and also every half-hour in the 2 h following meals for a total of 21 samples. Finally, an overnight (ON) urine sample was collected (∼10-h) for a total of 15 urines.
Diet
The study diet consisting of three isocaloric meals was designed to contain calcium, phosphorus, and sodium evenly distributed among the meals. The diet was planned by a nutritionist in the GCRC using Nutritionist IV software version 4.1 (N-Squared Computing, San Bruno, CA). The 1,800-kcal base diet provided 1,160 mg of calcium and 1,240 mg of phosphorus daily, as determined by laboratory analysis of homogenates of the three meals (Covance Laboratories, Madison, WI). The base diet provided 2,141 mg/day sodium, 2,427 mg/day potassium, and 218 mg/day magnesium. Subjects were stratified to one of three caloric levels (1,800, 2,100, or 2,400 kcal/day) according to an estimate of individual energy needs using the Schofield equation (25).
Laboratory Measurements
Urine sodium, potassium, total CO2 (TCO2), calcium, magnesium, phosphorus, ammonia, and creatinine were measured in our laboratory on a Beckman DxC 600 analyzer (3). Citrate was measured on the Beckman DxC 600 by citrate lyase enzyme assay using an adaptation of the citric acid test kit from R-Biopharm (Darmstadt, Germany). Urine sulfate concentration was assayed by measuring turbidity (OD630) after barium precipitation (17). Urine pH was measured by a glass electrode on a Beckman f390 pH meter. Serum samples were ultrafiltered using Amicon Ultra-4 filter tubes with a 10-kDa MW cutoff membrane (Millipore, Bedford, MA); TCO2 and citrate were measured in the ultrafiltrate (UF TCO2 and UF Citrate) using the same methods as for urine. Serum calcium was measured by atomic absorption on a PerkinElmer AAnalyst 100 spectrometer (6).
Analysis of Data
Calculations.
Urine titratible acidity (TA) and net acid excretion (NAE) and all fractional excretions were calculated conventionally (12, 14, 15, 29). Net gastrointestinal (GI) anion uptake was calculated as the sum of noncombustible cations (Na+ + K+ + Ca+ + Mg+) minus the sum of noncombustible anions (Cl− + P−) all in meq/l and presented in meq/h (20, 21). The phosphate divalent/trivalent anion concentration was calculated using a pKa of 6.8 and the pH of the sample. All excretions are expressed per 1.73 m2 body surface area.
Statistical analysis.
ANOVA and general linear modeling (GLM) were performed conventionally. A priori adjustment for age, sex, and weight were included in all regression models as potential confounding factors. Forward stepwise modeling was used to assess the potential associations between urine and serum biomarkers with each response. Multiple measurements were made on study subjects throughout the day, resulting in within-subject correlated measurement. Accordingly, statistical methods for correlated data were used to analyze the mean difference in each laboratory measurement of interest between the sexes during fasting, fed, and ON periods. Generalized estimating equations (GEE) (10) utilizing an identity link function and independence working covariance structures were used to compare laboratory values between males and females. To correct inference for within-subject correlation, robust variance estimates clustering on patients were used (37). To determine whether the mean difference in each laboratory value comparing male and female changed with respect to meal period state, multiplicative interactions between the indicator of sex and meal period were modeled. Simultaneous tests of regression model coefficients were conducted using multivariate Wald tests (1). All statistical calculations were performed using R and Systat 13 software (Systat Software, Chicago, IL) (13).
RESULTS
Urine pH and Acid Excretion
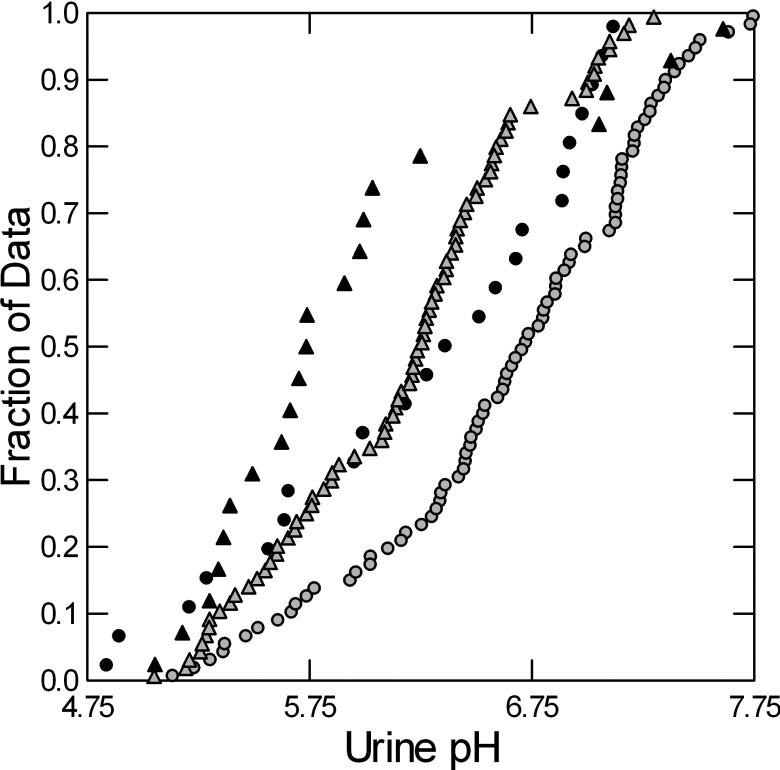
Urine pH of women exceeded that of men in the fed state (Fig. 1) but not fasting or overnight (Table 2). Urine pH rose significantly with meals in women but not in men. Serum total CO2 (TCO2) content rose significantly in women with meals (Table 2). Urine TA increased with food in men but not women, and TA and NAE of men exceeded that of women fed but not fasting. Urine TCO2 excretion of women rose with food and exceeded that of men in the fed state (Table 2). Urine phosphate excretion increased with food in men but not women. In other words, the lower urine pH of men was accompanied by a higher acid excretion. In women, NAE fell with food essentially to zero.
Fig. 1.
Urine pH in normal men (triangles) and women (circles) during the fasted (black) and fed (gray) periods. Urine pH rises with meals (compare black and gray, same symbols), but the increase is significant only in women. Urine pH of women is significantly higher than men in the fed state (Table 2).
Table 2.
Selected components of urine pH metabolism
| Women |
Men |
|||||
|---|---|---|---|---|---|---|
| Fasted | Fed | ON | Fasted | Fed | ON | |
| Urine pH | 6.31 ± 0.18 | 6.74 ± 0.11* | 6.0 ± 0.19 | 5.84 ± 0.24 | 6.07 ± 0.17# | 5.81 ± 0.17 |
| Serum K, mmol/l | 3.96 ± 0.07 | 3.96 ± 0.07 | 3.88 ± 0.15 | 3.85 ± 0.10 | ||
| Serum TCO2, mmol/l | 24.4 ± 0.60 | 26.1 ± 0.70* | 24.5 ± 0.52 | 24.9 ± 0.71 | ||
| FL TCO2 | 126 ± 12 | 140 ± 6 | 156 ± 11 | 168 ± 6# | ||
| Urine TCO2 | 0.60 ± 0.16 | 1.4 ± 0.25* | 0.21 ± 0.11 | 0.60 ± 0.36 | 0.55 ± 0.25# | 0.09 ± 0.13 |
| FE TCO2 (×102) | 0.56 ± 0.18 | 0.96 ± 0.1* | 0.41 ± 0.17 | 0.31 ± 0.1# | ||
| Urine titratable acidity | 0.36 ± 0.09 | 0.35 ± 0.06 | 0.44 ± 0.08 | 0.46 ± 0.11 | 0.65 ± 0.08*# | 0.62 ± 0.07 |
| Urine ammonia | 0.93 ± 0.15 | 0.92 ± 0.13 | 1.1 ± 0.16 | 1.1 ± 0.18 | 1.3 ± 0.31 | 1.1 ± 0.17 |
| Urine net acid excretion | 0.70 ± 0.27 | −0.14 ± 0.39* | 1.4 ± 0.29 | 0.97 ± 0.56 | 1.4 ± 0.49# | 1.6 ± 0.25 |
| Urine phosphorus | 0.74 ± 0.11 | 0.96 ± 0.08 | 0.73 ± 0.07 | 0.70 ± 0.13 | 1.13 ± 0.11* | 0.88 ± 0.07 |
| Urine sulfate | 1.7 ± 0.10 | 1.8 ± 0.20 | 1.3 ± 0.17 | 1.9 ± 0.17 | 1.8 ± 0.11 | 1.8 ± 0.15 |
| Urine gastrointestinal anion | 2.5 ± 0.46 | 3.9 ± 0.55* | 0.95 ± 0.44 | 1.9 ± 0.88 | 1.8 ± 0.73# | 0.64 ± 0.37 |
| UF citrate, mmol/l | 0.24 ± 0.01 | 0.27 ± 0.01 | 0.21 ± 0.02 | 0.27 ± 0.01* | ||
| FL citrate | 1.5 ± 0.1 | 1.7 ± 0.1 | 1.5 ± 0.1 | 2.0 ± 0.1* | ||
| Urine citrate | 0.17 ± 0.02 | 0.21 ± 0.02 | 0.10 ± 0.02 | 0.12 ± 0.03 | 0.15 ± 0.02# | 0.10 ± 0.03 |
| FE citrate | 0.07 ± 0.04 | 0.09 ± 0.04 | 0.03 ± 0.04# | 0.03 ± 0.03# | ||
| Adjusted urine citrate | 0.14 ± 0.02 | 0.15 ± 0.01 | 0.09 ± 0.01# | 0.10 ± 0.01# | ||
Values are means ± SE. ON, overnight; TCO2, total CO2; FL, filtered load; FE, fractional excretion; UF, ultrafilterable. All urine excretions and filtered loads are mmol·h−1·1.73 m−2, except for gastrointestinal (GI) anion (meq·h−1·1.73 m−2). Serum K, serum TCO2, and UF citrate (mmol/l). All values are adjusted for age and weight. Adjusted urine citrate is adjusted for filtered load of citrate in addition.
Differs from fasted same sex, P < 0.05;
differs from female same food period, P < 0.05.
Mechanisms of increased urine TCO2 appear to involve tubule bicarbonate reabsorption. Fractional excretion (FE) TCO2 rises with food in women but not men (Table 2), and values for women exceed those of men in the fed state. Urine TCO2 of men is below that of women in the fed state even though filtered load of TCO2 in men exceeds that of women (Table 2).
Urine Sulfate and GI Anion Excretion
Men and women did not differ in urine sulfate excretion fasted, fed, or overnight (Table 2). However, GI anion excretion (materials and methods) of women exceeded men in the fed state and rose with meals in women but not in men (Table 2).
Citrate Filtration and Excretion
With food, UF citrate and filtered load of citrate rose in men (Table 2) but not women. Urine citrate of women exceeded that of men in the fed period (Table 2), and FE citrate of women exceeded men fasted and fed. Urine citrate of women exceeded that of men even when adjusted for filtered load of citrate both fasted and fed; this is an alternative form of analysis to the use of FE citrate. In summary, although UF and filtered load of citrate were the same in men and women, urine citrate excretion was higher in women, speaking to a difference in renal citrate transport between the sexes.
Determinants of Urine pH
To investigate potential factors responsible for the higher urine pH in women, we performed regression analysis of those factors known to affect urine pH through established pathways (12). These included serum potassium and TCO2, urine sulfate, TCO2, phosphate, and GI anion. Age, sex, and weight were considered in all models as potential confounding factors. Sex and food period were used as categorical variables. We excluded urine ammonia, TA, and NAE because all three could affect and be affected by urine pH, and their inclusion could create circularity.
After adjustment for age, sex, and weight, we found that urine GI anion, urine sulfate, and serum TCO2 were significantly associated with urine pH (Table 3). Because the sex difference in urine pH was more marked in the fed than in the fasting period (Table 2), we also performed a general linear model on the same variables using only the fed period. Again, stepwise model building retained urine GI anion and sulfate and serum TCO2 (Table 4), a finding that is identical to the model in the fasted and fed periods combined (Table 3). Overall, GI anion, urine sulfate, and serum TCO2 are consistent across all models. However, in both models (Tables 3 and 4), sex remains a significant variable, suggesting that GI anion, urine sulfate, and serum TCO2 do not fully explain the higher urine pH in women.
Table 3.
General linear model of urine pH (fasted and fed)
| Factor | Estimated Difference in Mean Urine pH (95% CI) | P Value |
|---|---|---|
| Urine GI anion, meq·h−1·1.73 m−2 | 0.212 (0.175, 0.249) | <0.0001 |
| Serum TCO2, mmol/l | 0.057 (0.011, 0.103) | 0.014 |
| Urine sulfate, mmol·h−1·1.73 m−2 | −0.219 (−0.387, −0.05) | 0.011 |
| Sex (female vs. male) | −0.213 (−0.379, −0.047) | 0.012 |
| Weight, kg | 0.008 (−0.005, 0.021) | 0.241 |
| Age, yr | 0.002 (−0.008, 0.013) | 0.644 |
CI, confidence interval.
Table 4.
General linear model of urine pH (fed state)
| Factor | Estimated Difference in Mean Urine pH (95% CI) | P Value |
|---|---|---|
| Urine GI anion, meq·h−1·1.73 m−2 | 0.215 (0.186, 0.245) | <0.0001 |
| Serum TCO2, mmol/l | 0.053 (0.012, 0.094) | 0.012 |
| Urine sulfate, mmol·h−1·1.73 m−2 | −0.228 (−0.409, −0.047) | 0.013 |
| Sex (female vs. male) | −0.192 (−0.337, −0.047) | 0.009 |
| Weight, kg | 0.009 (−0.001, 0.020) | 0.081 |
| Age, yr | 0.002 (−0.006, 0.010) | 0.654 |
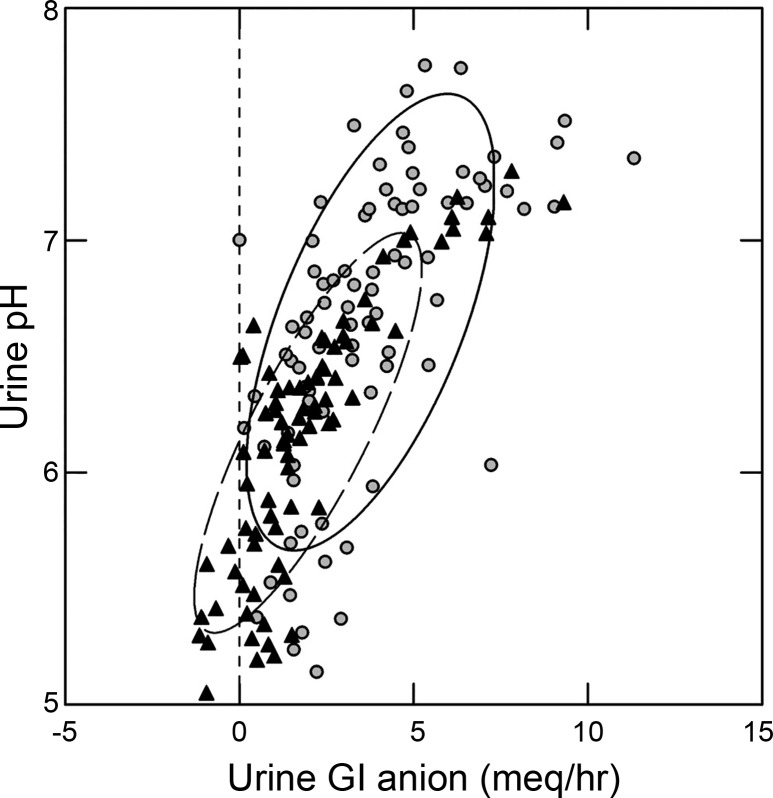
A simple plot of urine pH vs. GI anion (Fig. 2) visualizes their marked correlation. The regressions for men and women overlap, and in a general linear model the cross-product of sex by GI anion is not significant. The cross-product is calculated to assess the effect of a categorical variable, in this case sex, on the interaction between two variables, in this case urine pH and GI anion.
Fig. 2.
Relationship between urine pH and urine gastrointestinal (GI) anion excretion in men (black triangles) and women (gray circles) during the fed period. Nonparametric ellipses (female, solid line; and male, broken line) contain 68% of points in each group. Dashed vertical line is a visual marker for zero on the x-axis. Note that female subjects rise above males on both axes.
Determinants of Urine Citrate
Citrate is among the anions the GI tract can absorb from food; however, we wished to investigate what other factors besides GI anion uptake might influence urine citrate excretion differently between men and women. We performed regression analysis of those factors known to affect urine citrate excretion through established pathways (22). These included serum TCO2 and potassium, filtered load of citrate, GI anion, and urine pH. Sex and food period were used as categorical variables.
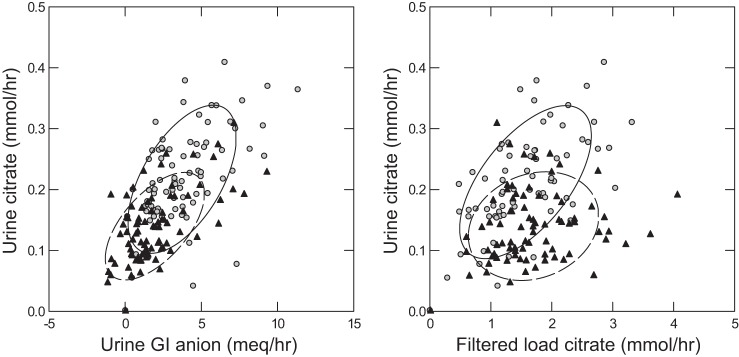
Of these, filtered load of citrate, GI anion, sex, weight, and age were all significantly associated with urine citrate (Table 5). These relationships are visualized in Fig. 3. Urine citrate is strongly associated with GI anion in both sexes (Fig. 3, left). Urine citrate appears visually more strongly correlated with filtered load of citrate in women (Fig. 3, right). In the full regression analysis, the cross-product of filtered load by sex was not significant. However, in a simple regression of the unadjusted data, the slopes of the regressions differed (Fig. 3 caption). In males, urine citrate excretion does not rise significantly with filtered load, whereas in women it does.
Table 5.
General linear model of urine citrate
| Factor | Estimated Difference in Mean Urine pH (95% CI) | P Value |
|---|---|---|
| FL citrate, mmol·h−1·1.73 m−2 | 0.047 (0.037, 0.056) | <0.0001 |
| Urine GI anion, meq·h−1·1.73 m−2 | 0.019 (0.015, 0.023) | <0.0001 |
| Sex (female vs. male) | −0.033 (−0.051, −0.015) | <0.0001 |
| Weight, kg | −0.002 (−0.002, −0.001) | <0.0001 |
| Age, yr | 0.002 (0.001, 0.003) | <0.0001 |
Fig. 3.
Relationship between urine citrate excretion and GI anion excretion (left) and filtered load of citrate (right) during the fed period. Regression of urine citrate (mmol/h) on urine GI anion (meq/h): 0.016 (0.012, 0.021), men (black triangles); 0.020 (0.013, 0.026), women (gray circles). The 95% confidence intervals (CI) overlap, indicating that the regressions do not differ. Regression of urine citrate (mmol/h) on filtered load of citrate (mmol/h): 0.015 (−0.003, 0.034), men; 0.071 (0.049, 0.093), women. Note that the 95% CI of these regressions do not overlap and that the regression for men is not significant.
Determinants of Urine Ammonia
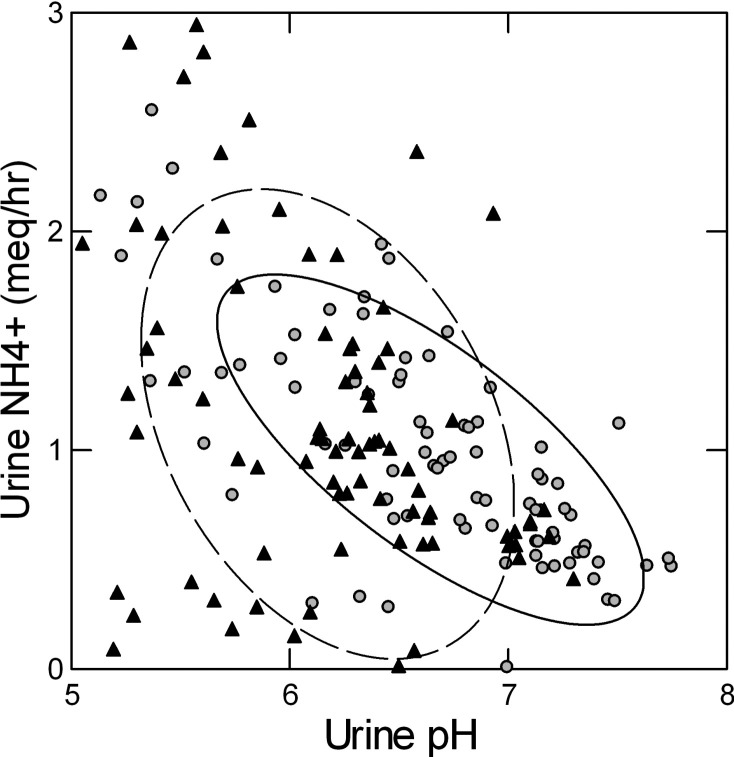
To clarify possible sex differences in ammonia excretion adjusting for pH, we considered a model containing serum potassium, serum TCO2, and urine sulfate and pH, all established determinants of urine ammonia excretion. To determine which of these have independent relationships with urine ammonia in this population, we performed a stepwise model that included these four factors as well as sex, age, and weight as potential confounding factors (Table 6). In this model, we found that only urine pH was significantly associated with urine ammonia excretion. No significant association between urine ammonia excretion and age, sex, or weight was found. The relationship between urine ammonia and urine pH for the two sexes is shown in Fig. 4.
Table 6.
General linear model of urine
| Factor | Estimated Difference in Mean Urine pH (95% CI) | P Value |
|---|---|---|
| Urine pH | −0.464 (−0.673, −0.256) | <0.0001 |
| Sex (female vs. male) | 0.030 (−0.532, 0.591) | 0.918 |
| Weight, kg | −0.005 (−0.024, 0.015) | 0.650 |
| Age, yr | −0.008 (−0.025, 0.008) | 0.316 |
Fig. 4.
Relationship between urine ammonia excretion and urine pH during the fed period in men (black triangles) and women (gray circles). Nonparametric ellipses (female, solid line; male, dashed line) contain 68% of points in each group. Regression of urine ammonia (meq/h) on urine pH: −0.443 (−0.704, −0.181; F = 11.3), men; −0.598 (−0.721, −0.475; F = 93.6), women. The slopes do not differ.
DISCUSSION
Urine pH
We have shown that women have higher urine pH than men during the fed period while eating an identical diet, and at least one important mechanism appears to be an increase in their GI anion absorption. We recognize that association does not prove causality, but in this case it is strongly suggestive. Prior work using 24-h urines demonstrated that average urine pH was higher in women than in men on free-choice diets (9, 16, 28). Our work makes it clear that the pH difference is not due to differences in the diets of men and women but is related to differential absorption of food anions.
GI Anion Absorption
The diets that were eaten by subjects in the study contain alkali in the form of molecules such as lactate, malate, citrate, and so forth. This alkali can be absorbed and can be excreted unchanged in the urine or metabolized to produce bicarbonate in the Krebs cycle. The amount of this anion in the urine is detected as the difference between measured urine cations and the nonmetabolizable anions (Cl and phosphate). The unmeasured anions reflect those absorbed from the GI tract that have escaped metabolism to bicarbonate. We recognize that GI anion calculated using charge difference (materials and methods) has a limited capacity to mark acid base balance. However, an extensive review of the problem points out that urine GI anion as we calculate is almost perfectly correlated with true net GI anion absorption, as directly measured from analysis of diet and stool cation and anion content (14, 21).
As far as we know, the fate of the absorbed anions must be either excretion in the urine unchanged or metabolism to produce bicarbonate. The new bicarbonate from metabolism can be detected by changes in serum TCO2 and urine pH and TCO2. However, because some of the bicarbonate may be neutralized by protons stored outside the extracellular fluid (32), one cannot quantitatively measure anion metabolism by appearance of new urine TCO2. In our comparison between men and women, we must make the assumption that the fraction of absorbed anion that is metabolized to bicarbonate is roughly the same in both sexes.
In our present experiment, increasing excretion of GI anion was accompanied by increased excretion of urine TCO2 and rising urine pH in women, meaning that a component of what was absorbed was metabolized, of which some was excreted as urine TCO2 and some was retained as shown by the increase in serum TCO2. Because GI anion absorption in the fed state is greater in women, and urine pH, serum TCO2, and urine TCO2 are higher in women than in men, we presume their higher anion absorption has led to higher bicarbonate production via metabolism of intermediates. Being related to anion absorption from food, the phenomenon is most marked in the fed state. Because the fed period represents a large portion of the day, we would expect to find a higher urine pH in the 24-h urines of women compared with men, which may account for what others have found before us.
The mechanism for the increased excretion of TCO2 in women does not appear to be via increased filtered load of TCO2, which does not differ significantly between the fasted and fed states; in addition, filtered load of TCO2 is higher in men than women. Instead, the fractional excretion of TCO2 increases in women and is significantly higher in women than in men during the fed state. We might speculate that this is mediated via receptor protein tyrosine phosphatase-γ, which appears to sense extracellular CO2 and HCO3, leading to excretion of alkali (38). It is unclear in what way this phenomenon is related to the so-called alkaline tide following meals, which has been attributed to generation of alkali in the process of proton secretion in the stomach (19). However, in our patients, no rise in urine TCO2 or pH was seen in men who ate the same diet as the women, and generation of alkali in the gastric mucosa would not account for the increase in gastrointestinal anion excretion that we have found.
Our key finding is that men and women appear to differ remarkably with respect to GI anion absorption. In women, urine GI anion excretion and urine TCO2 rise with meals to such an extent that NAE becomes indistinguishable from zero. Urine pH accordingly rises along with urine and serum TCO2. Men have no such increases, and therefore, they do not show this specific pattern of what amounts to alkali loading sufficient to abrogate the need for acid excretion. Given that all subjects ate the same diet, the differences in GI anion uptake must be ascribed to a true biological difference between the sexes.
One might be surprised at the very high urine TCO2 of normal women during the fed period. A careful reader of the 12th figure in Ref. 14, for example, would find that, at the urine pH of those women, others have recorded a significantly lower TCO2 level. We believe that the answer lies exactly in the main point of theatpaper. Unlike the 24-h studies that pool fasting, fed, and overnight periods together and constitute the basis for the figure, we have pulled these periods apart by sex. For example, overnight values for TCO2 are very low compared even with fasting (0.2 and 0.09 vs. 0.6; overnight females and males vs. fasting) and even more from fed, as Table 2 shows. When you put the sexes together as in Ref. 14 and pool the three food periods, fasted, fed, and overnight, the combined mean will deviate markedly and not predictably from the individual six means we present: two sexes and three food periods. In other words, the 12th figure in Ref. 14 is correct, and we would expect our data to deviate markedly from it.
On top of the pooling vs. individuation issues, we adjusted for age and weight and, as we mentioned, divided data into six groups by two sexes and three food periods. In a simple ANOVA used here merely for illustration, the effects model predicts that for every 10 yr of age urine TCO2 will fall by 0.139 mmol/h and rise by 0.185 mmol/h for every 10 kg body wt. This must further deviate our results from any based on simple 24-h urine collections without such corrections. Finally, we have scaled our excretion rates per 1.73 m2, creating yet another source of deviation.
Urine Citrate and Citrate Reabsorption
Our findings also bear on the well-known higher citrate excretion of women (23). In our study, we were able to estimate tubule citrate reabsorption and document that it was lower in women than in men. Because of this, although filtered load of citrate was the same in men and women, their lower tubule reabsorption permitted women to excrete more citrate.
It has been shown that alkali feeding leads to increased citrate excretion without a direct effect on NaDC-1 activity (2). This may be due to an increase in proximal tubule pH, which increases trivalent citrate and decreases the abundance of divalent citrate, which is the species that is transported (36). In addition, alkali loading affects intracellular citrate metabolism via decreasing the activity of mitochondrial aconitase (18), increasing intracellular citrate concentration and decreasing the gradient for citrate uptake across the membrane (27). The decreased citrate reabsorption seen in women is compatible with the possibility that increased GI anion absorption, which is associated with an increase in serum TCO2, raised proximal tubule bicarbonate concentration and, therefore, pH, decreasing citrate uptake. Our present data do not allow further investigation of this phenomenon.
Urine Ammonia
We recognized that differences in ammonia production could have played a role in the pH differences between the sexes and explored this issue using multivariate analysis. We could find no effects on urine ammonia apart from urine pH, and no significant interactions between sex and the pH effect on urine ammonia. Put another way, despite the importance and complexity of ammonia regulation and the contribution it makes to NAE, we did not find any independent contribution of ammonia regulation to the sex difference that we present here.
Implications of Our Work
We have no immediate hypothesis that explains the differential absorption of alkali between men and women, but we speculate that it may have its roots in the need to preserve bone mineral in women, which may be depleted during pregnancy. The higher serum bicarbonate we find may benefit bone mineralization as acidosis demineralizes bone, and alkali may preserve bone mineral (26). This could be tested in subsequent investigation beyond the scope of the present study.
Our findings may help explain why females are more subject than males to calcium stones with a high phosphate content. A higher urine pH will increase urinary supersaturation with respect to calcium phosphate, predicting formation of calcium phosphate in preference to calcium oxalate stones (24). We and others have identified urine pH as the primary driver for calcium phosphate supersaturation and calcium phosphate content of stones (24).
Our work immediately raises a number of potential questions for the future. Especially important would be the relationship of GI anion to menarche, menopause, and hormone changes during the menstrual cycle. As already noted, this lies far from our present state of knowledge.
GRANTS
The research reported in this publication was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant P01-DK-056788 and by the National Center For Advancing Translational Sciences of the National Institutes of Health under award no. 4-UL1-TR-000430-10.
DISCLAIMERS
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
E.M.W., D.L.G., and F.L.C. conceived and designed research; E.M.W., K.J.B., D.L.G., and F.L.C. analyzed data; E.M.W. and F.L.C. interpreted results of experiments; E.M.W., K.J.B., D.L.G., and F.L.C. drafted manuscript; K.J.B. performed experiments; F.L.C. prepared figures.
REFERENCES
- 1.Akaike H. A new look at the statistical model identification. IEEE Trans Automat Contr 19: 716–723, 1974. doi: 10.1109/TAC.1974.1100705. [DOI] [Google Scholar]
- 2.Aruga S, Wehrli S, Kaissling B, Moe OW, Preisig PA, Pajor AM, Alpern RJ. Chronic metabolic acidosis increases NaDC-1 mRNA and protein abundance in rat kidney. Kidney Int 58: 206–215, 2000. doi: 10.1046/j.1523-1755.2000.00155.x. [DOI] [PubMed] [Google Scholar]
- 3.Asplin JR, Parks JH, Coe FL. Dependence of upper limit of metastability on supersaturation in nephrolithiasis. Kidney Int 52: 1602–1608, 1997. doi: 10.1038/ki.1997.491. [DOI] [PubMed] [Google Scholar]
- 4.Bergsland KJ, Coe FL, Gillen DL, Worcester EM. A test of the hypothesis that the collecting duct calcium-sensing receptor limits rise of urine calcium molarity in hypercalciuric calcium kidney stone formers. Am J Physiol Renal Physiol 297: F1017–F1023, 2009. doi: 10.1152/ajprenal.00223.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergsland KJ, Zisman AL, Asplin JR, Worcester EM, Coe FL. Evidence for net renal tubule oxalate secretion in patients with calcium kidney stones. Am J Physiol Renal Physiol 300: F311–F318, 2011. doi: 10.1152/ajprenal.00411.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bowers GN Jr, Rains TC. Measurement of total calcium in biological fluids: flame atomic absorption spectrometry. Methods Enzymol 158: 302–319, 1988. doi: 10.1016/0076-6879(88)58062-X. [DOI] [PubMed] [Google Scholar]
- 7.Cheema-Dhadli S, Lin SH, Halperin ML. Mechanisms used to dispose of progressively increasing alkali load in rats. Am J Physiol Renal Physiol 282: F1049–F1055, 2002. doi: 10.1152/ajprenal.00006.2001. [DOI] [PubMed] [Google Scholar]
- 8.Coe FL, Evan AP, Worcester E. Pathogenesis and treatment of nephrolithiasis. In: Seldin and Giebisch: The Kidney, edited by Alpern RJ, Caplan MJ, and Moe OW. Amsterdam: Academic, 2013. doi: 10.1016/B978-0-12-381462-3.00067-7. [DOI] [Google Scholar]
- 9.Curhan GC, Taylor EN. 24-h uric acid excretion and the risk of kidney stones. Kidney Int 73: 489–496, 2008. doi: 10.1038/sj.ki.5002708. [DOI] [PubMed] [Google Scholar]
- 10.Diggle PJ, Heagerty P, Liang K-Y, Zeger SL. Analysis of Longitudinal Data. Oxford, UK: Oxford University, 2002. [Google Scholar]
- 11.Halperin ML, Cheema Dhadli S, Kamel KS. Physiology of acid-base balance: links with kidney stone prevention. Semin Nephrol 26: 441–446, 2006. doi: 10.1016/j.semnephrol.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Hamm LL, Nakhoul N, Hering-Smith KS. Acid-Base Homeostasis. Clin J Am Soc Nephrol 10: 2232–2242, 2015. doi: 10.2215/CJN.07400715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ihaka R, Gentleman R. R: A language for data analysis and graphics. J Comput Graph Stat 5: 299–314, 1996. [Google Scholar]
- 14.Lemann J Jr, Bushinsky DA, Hamm LL. Bone buffering of acid and base in humans. Am J Physiol Renal Physiol 285: F811–F832, 2003. doi: 10.1152/ajprenal.00115.2003. [DOI] [PubMed] [Google Scholar]
- 15.Lennon EJ, Lemann J Jr, Litzow JR. The effects of diet and stool composition on the net external acid balance of normal subjects. J Clin Invest 45: 1601–1607, 1966. doi: 10.1172/JCI105466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lieske JC, Turner ST, Edeh SN, Smith JA, Kardia SL. Heritability of urinary traits that contribute to nephrolithiasis. Clin J Am Soc Nephrol 9: 943–950, 2014. doi: 10.2215/CJN.08210813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma RSW, Chan JCM. Endogenous sulphuric acid production: a method of measurement by extrapolation. Clin Biochem 6: 82–87, 1973. doi: 10.1016/S0009-9120(73)80016-5. [DOI] [PubMed] [Google Scholar]
- 18.Melnick JZ, Preisig PA, Moe OW, Srere P, Alpern RJ. Renal cortical mitochondrial aconitase is regulated in hypo- and hypercitraturia. Kidney Int 54: 160–165, 1998. doi: 10.1046/j.1523-1755.1998.00974.x. [DOI] [PubMed] [Google Scholar]
- 19.Niv Y, Fraser GM. The alkaline tide phenomenon. J Clin Gastroenterol 35: 5–8, 2002. doi: 10.1097/00004836-200207000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Oh MS. A new method for estimating G-I absorption of alkali. Kidney Int 36: 915–917, 1989. doi: 10.1038/ki.1989.280. [DOI] [PubMed] [Google Scholar]
- 21.Oh MS, Carroll HJ. Whole body acid-base balance. Contrib Nephrol 100: 89–104, 1992. doi: 10.1159/000421453. [DOI] [PubMed] [Google Scholar]
- 22.Pajor AM. Sodium-coupled dicarboxylate and citrate transporters from the SLC13 family. Pflügers Arch 466: 119–130, 2014. doi: 10.1007/s00424-013-1369-y. [DOI] [PubMed] [Google Scholar]
- 23.Parks JH, Coe FL. A urinary calcium-citrate index for the evaluation of nephrolithiasis. Kidney Int 30: 85–90, 1986. doi: 10.1038/ki.1986.155. [DOI] [PubMed] [Google Scholar]
- 24.Parks JH, Worcester EM, Coe FL, Evan AP, Lingeman JE. Clinical implications of abundant calcium phosphate in routinely analyzed kidney stones. Kidney Int 66: 777–785, 2004. doi: 10.1111/j.1523-1755.2004.00803.x. [DOI] [PubMed] [Google Scholar]
- 25.Schofield WN. Predicting basal metabolic rate, new standards and review of previous work. Hum Nutr Clin Nutr 39, Suppl 1: 5–41, 1985. [PubMed] [Google Scholar]
- 26.Sebastian A, Harris ST, Ottaway JH, Todd KM, Morris RC Jr. Improved mineral balance and skeletal metabolism in postmenopausal women treated with potassium bicarbonate. N Engl J Med 330: 1776–1781, 1994. doi: 10.1056/NEJM199406233302502. [DOI] [PubMed] [Google Scholar]
- 27.Simpson DP. Citrate excretion: a window on renal metabolism. Am J Physiol 244: F223–F234, 1983. doi: 10.1152/ajprenal.1983.244.3.F223. [DOI] [PubMed] [Google Scholar]
- 28.Taylor EN, Stampfer MJ, Mount DB, Curhan GC. DASH-style diet and 24-hour urine composition. Clin J Am Soc Nephrol 5: 2315–2322, 2010. doi: 10.2215/CJN.04420510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Ypersele de Strihou C. Importance of endogenous acid production in the regulation of acid-base equilibrium: the role of the digestive tract. Adv Nephrol Necker Hosp 9: 367–385, 1980. [PubMed] [Google Scholar]
- 30.Wall SM, Lazo-Fernandez Y. The role of pendrin in renal physiology. Annu Rev Physiol 77: 363–378, 2015. doi: 10.1146/annurev-physiol-021014-071854. [DOI] [PubMed] [Google Scholar]
- 31.Waters WE, Sussman M, Asscher AW. Community study of urinary pH and osmolality. Br J Prev Soc Med 21: 129–132, 1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wesson DE, Simoni J, Broglio K, Sheather S. Acid retention accompanies reduced GFR in humans and increases plasma levels of endothelin and aldosterone. Am J Physiol Renal Physiol 300: F830–F837, 2011. doi: 10.1152/ajprenal.00587.2010. [DOI] [PubMed] [Google Scholar]
- 33.Worcester EM, Bergsland KJ, Gillen DL, Coe FL. Evidence for increased renal tubule and parathyroid gland sensitivity to serum calcium in human idiopathic hypercalciuria. Am J Physiol Renal Physiol 305: F853–F860, 2013. doi: 10.1152/ajprenal.00124.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Worcester EM, Coe FL, Evan AP, Bergsland KJ, Parks JH, Willis LR, Clark DL, Gillen DL. Evidence for increased postprandial distal nephron calcium delivery in hypercalciuric stone-forming patients. Am J Physiol Renal Physiol 295: F1286–F1294, 2008. doi: 10.1152/ajprenal.90404.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Worcester EM, Gillen DL, Evan AP, Parks JH, Wright K, Trumbore L, Nakagawa Y, Coe FL. Evidence that postprandial reduction of renal calcium reabsorption mediates hypercalciuria of patients with calcium nephrolithiasis. Am J Physiol Renal Physiol 292: F66–F75, 2007. doi: 10.1152/ajprenal.00115.2006. [DOI] [PubMed] [Google Scholar]
- 36.Zacchia M, Preisig P. Low urinary citrate: an overview. J Nephrol 23, Suppl 16: S49–S56, 2010. [PubMed] [Google Scholar]
- 37.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics 42: 121–130, 1986. doi: 10.2307/2531248. [DOI] [PubMed] [Google Scholar]
- 38.Zhou Y, Skelton LA, Xu L, Chandler MP, Berthiaume JM, Boron WF. Role of receptor protein tyrosine phosphatase γ in sensing extracellular CO2 and HCO3−. J Am Soc Nephrol 27: 2616–2621, 2016. doi: 10.1681/ASN.2015040439. [DOI] [PMC free article] [PubMed] [Google Scholar]