Abstract
Myocardial infarction is a prevalent major cardiovascular event that arises from myocardial ischemia with or without reperfusion, and basic and translational research is needed to better understand its underlying mechanisms and consequences for cardiac structure and function. Ischemia underlies a broad range of clinical scenarios ranging from angina to hibernation to permanent occlusion, and while reperfusion is mandatory for salvage from ischemic injury, reperfusion also inflicts injury on its own. In this consensus statement, we present recommendations for animal models of myocardial ischemia and infarction. With increasing awareness of the need for rigor and reproducibility in designing and performing scientific research to ensure validation of results, the goal of this review is to provide best practice information regarding myocardial ischemia-reperfusion and infarction models.
Listen to this article’s corresponding podcast at ajpheart.podbean.com/e/guidelines-for-experimental-models-of-myocardial-ischemia-and-infarction/.
Keywords: animal models, cardiac remodeling, heart failure, myocardial infarction, reperfusion, rigor and reproducibility
INTRODUCTION
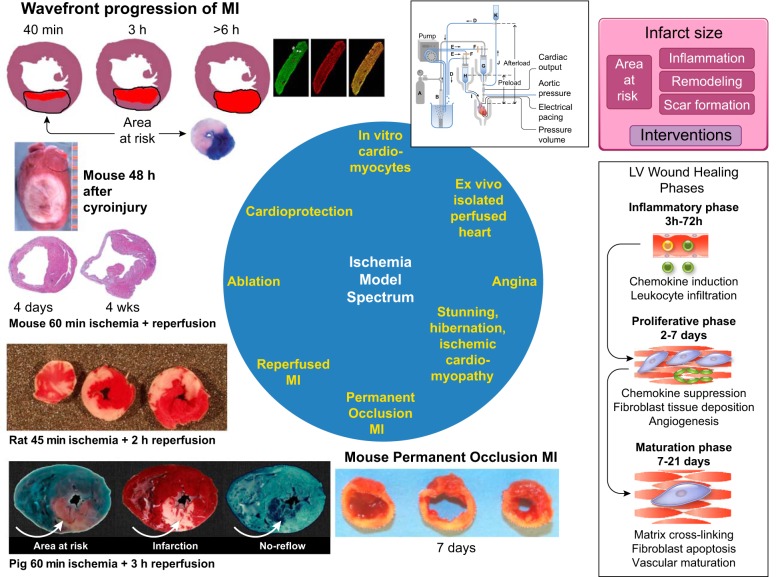
Ischemia occurs when blood flow to the myocardium is reduced (129). Ischemia of prolonged duration induces myocardial infarction (MI), and MI is a common cause of heart failure (295). Ischemic cardiomyopathy is the most common cause of heart failure and can arise from remodeling after an acute ST segment elevation myocardial infarction (STEMI) from multiple small nontransmural infarctions or from chronic repetitive ischemia in the absence of infarction (15). Ischemia can range in its extent from low flow to total coronary occlusion, can be of short to long duration, can be successfully reversed by reperfusion in a timely manner or not reperfused at all, and can induce injury or provide cardioprotection. Likewise, there is a diverse variety of animal models to address each type of ischemia within this spectrum. Figure 1 shows the range of models that reflect the scale of ischemia and variety of models available to better understand how the heart responds to ischemia and the mechanisms whereby the heart can either adapt to ischemia or progress to failure.
Fig. 1.
The spectrum of ischemia encapsulates in vitro, ex vivo, and in vivo models of ischemia that range from transient to prolonged in duration with acute to chronic consequences. The pig section (bottom left) is modified from Heusch (126). MI, myocardial infarction; LV, left ventricular;
Experimental models of myocardial ischemia serve two nearly opposing aims, both worthy of investigation. The first aim is to provide better mechanistic insight that cannot be obtained from a clinical situation. To achieve this aim, experimental studies may be reductionist with low direct applicability to the clinical situation (e.g., when using temporally induced cell specific over- or underexpression of a gene). The second aim is to provide mechanistic insight from an experimental study for translation to the clinical situation, and for this aim experimental models must replicate the clinical setting as closely as possible (127).
For cardiovascular science to continue advancing, experimental results should be reproducible and replicable, and rigorous experimental design is a fundamental element of reproducibility. Reproducibility refers to results that can be repeated by multiple scientists and is a means of validation across laboratories. Rigor refers to robust and unbiased experimental design, methodology, analysis, interpretation, and reporting of results. With increasing awareness by journals and granting agencies of the need for reproducibility and rigor in designing and performing scientific research in preclinical studies, the goal of this consensus article is to provide best practice information regarding myocardial ischemia and infarction models. The strengths and limitations of the different models are discussed, with a summary shown in Table 1. We also address ways to incorporate Animals in Research: Reporting In Vivo Experiments (ARRIVE) guidelines and similar standard operating procedures (168). The extensive reference list provided also serves as a resource for researchers new to the field.
Table 1.
Comparison of different approaches with strengths and limitations for each method
| Approach | End-Point Measurements | Strengths | Limitations and Pitfalls |
|---|---|---|---|
| In vitro cardiomyocytes | Cell viability (live-dead assay) | High throughput | Reductionist |
| Type of cell death (i.e., apoptosis) | Isolate effects of hypoxia/reoxygenation on cardiomyocytes without other cell types or circulating factors | Cardiomyocyte viability may not predict changes in infarct size in vivo | |
| Adult cardiomyocyte culture technically challenging | |||
| Isolated perfused hearts | Infarct size per area at risk | Relatively easy and reproducible | Tissue edema |
| Left ventricular function | Can study ischemia and reperfusion | May not fully represent the in vivo response | |
| Assessment of cardiac troponin I as a secondary cardiac injury index | Accurate measure of infarct size | Glucose as the sole substrate | |
| Ample sample for biochemistry | Limited stability | ||
| Compatible with NMR studies | Excessive coronary flow | ||
| Capacity for high throughput | Reductionist | ||
| Neurohormonal factor independent | |||
| Eliminates confounding effect of intervention on systemic blood vessels or circulating factors | |||
| Angina | Regional flow and function | Close to clinical situation | Technically complex; time and cost intensive |
| Metabolism, morphology, molecular biology, nerve activity, rhythm | |||
| Hibernation/stunning | Regional flow and function | Close to clinical situation | Technically complex; time and cost intensive |
| Metabolism, morphology, molecular biology, rhythm | |||
| Permanent occlusion MI | Inflammation, wound healing, scar formation, remote region myocytes, electrical activity | In the era of percutaneous coronary intervention, ~15–25% patients are not successfully reperfused in a timely manner (53, 104) | Does not reflect the reperfused MI patient response |
| Robust remodeling response; large effect size | |||
| Ischemia-reperfusion MI | Inflammation, wound healing, scar formation, myocyte viability, electrical activity | Close to clinical scenario | More technically challenging surgery |
| Reperfusion injury can expand area of damage | |||
| Ablation | Inflammation, wound healing, scar formation, myocyte electrical activity | Geometrically defined lesion | Nonischemic lethal injury |
| Infarct size/location independent of coronary anatomy | Mechanisms of cell death different from ischemia | ||
| Cardioprotection | Infarct size per area at riskLeft ventricular geometry and function; no reflow; circulating biomarkers such as cardiac troponin I | Mouse, rat, rabbit, and pig: models of low collateral flow (measurement of regional myocardial blood flow not required)Rat and rabbit: reliable infarct production; relatively high survival ratePig: mimics humans with low collateral flowDog: large amount of historical data; shows effect of intervention in the setting of variable collateral flow; mimics humans with high collateral flow | Mouse: small size; substantial variability requiring large n valuesRat, rabbit, pig, and dog: not high throughput; higher costRabbit, pig, and dog: potential for lethal arrhythmiasDog: variability in collateral perfusion (regional myocardial blood flow must be measured) |
MI, myocardial infarction.
IN VITRO AND EX VIVO MODELS
Myocyte Cell Culture
Model rationale.
Isolated fresh or cultured cardiomyocytes can be used as a powerful in vitro model of ischemia-reperfusion (I/R), whereby ischemia is simulated with hypoxia and reperfusion with reoxygenation (H/R). This system allows precise control of the cellular and extracellular environment, notably the specific impact of hypoxia and reoxygenation on cardiomyocytes without confounding influences of other cell types (e.g., fibroblasts, endothelial cells, inflammatory/immune cells, and platelets) or circulating factors (e.g., hormones, neurotransmitters, and cytokines).
Variables measured.
The most common use of this model system is for in vitro testing of specific factors proposed to be involved in I/R injury or cardioprotection (31, 166, 227, 240, 244, 330). After H/R, cultured cardiomyocytes undergo apoptosis, accompanied by cytochrome c release and caspase activation or necrosis (200, 296). Thus, assays of cell viability are often performed to assess the role of a particular genetic or pharmacological intervention in exacerbating or protecting the cell from H/R-induced cell death. Viability may be measured with a variety of assays, including lactate dehydrogenase (LDH) release or propidium iodide exclusion as an indicator of membrane integrity (24, 31, 58). Apoptosis is assessed with TUNEL or annexin V staining (24, 58, 166). Mitochondrial damage, including disruption of mitochondrial membrane potential, is also a key component of cellular injury after H/R and may be assessed using fluorescent dyes, such as tetramethylrhodamine methyl ester (TMRM). The loss of mitochondrial membrane potential causes TMRM to leak from the mitochondria, decreasing fluorescence (24, 31). In addition, reactive oxygen species (ROS) have been implicated in H/R injury (275); thus, ROS production is another common assessment (24, 58, 124, 125, 227).
More detailed analyses of cardiomyocyte responses to H/R include assessments of morphology, contractile function (i.e., cell shortening), intracellular Ca2+ handling, and action potentials (124, 166, 207). Contractile function is an important but often overlooked variable in H/R assays, since contraction requires ~70% of total energy utilization within a myocyte (182). Many H/R studies use quiescent myocytes; however, markedly impaired recovery of myocyte function and increased cell death result when cells are stimulated to contract throughout the H/R protocol (207). For all variables assessed, technical replicates on the same sample should be performed to establish the variability of the measurement technique. Biological replicates often include measurements on plates or myocytes from the same heart/harvest. If these are to be treated as independent samples, n values for both the plate/myocyte number as well as heart/harvest number should be fully reported.
There is currently no standardized protocol for H/R in cultured cardiomyocytes, but cardiomyocyte source and H/R conditions must be carefully considered. Theoretically, freshly isolated adult cardiomyocytes are the ideal gold standard for H/R experiments (166, 207, 244), although neonatal cardiomyocytes (58, 227, 296), cardiac progenitor cells (12), induced pluripotent stem cell-derived cardiomyocytes (iPSC-CMs) (24, 31), and various cell lines such as H9c2 and HL-1 have been used (330). Of these, neonatal cardiomyocytes are most commonly used, due to their relative ease of isolation and robust viability for several days in culture; however, neonatal cardiomyocytes are more resistant to hypoxia than adult myocytes (236, 255), and the mechanisms of this resistance remain incompletely resolved, which may limit interpretation of such results when designing more translational studies (235). Adult myocytes are preferred, because ischemic heart disease almost exclusively occurs in adults, and fresh isolation eliminates the potential confounding factor of phenotypic transformation in culture. The caveat is that adult cardiomyocytes are more difficult to isolate and do not survive long in culture, impeding longer H/R protocols or those requiring pre-H/R transfection. Therefore, many investigators turn to neonatal cardiomyocytes or cell lines (e.g., H9c2 or HL-1) if genetic modifications are necessary, in which case these genetically engineered cells may be an important complement to in vivo or adult cardiomyocyte studies. In addition, adult myocytes do not form monolayers as seen with neonatal cells, limiting their use for studies on gap junctions and electrical conductivity. Thus, authors should carefully consider experimental end points in the context of overall study design, because either neonatal or adult cell sources will be the best choice depending on the question posed. For both neonatal and adult primary cells, isolation protocols must assure for a cardiomyocyte-enriched population (i.e., free of fibroblasts and other cell types). For neonatal cells, the differential attachment technique is often used, whereas gravity separation is typical for adult cardiomyocytes (161, 287). Regardless of the isolation and enrichment method, manual or automated cell counting, expression of cardiomyocyte-specific markers, and visualization or quantification of T-tubular structure should be performed to verify purity and phenotype. Use of iPSC-CMs may overcome some of these primary cell limitations, but the response of iPSC-CMs to H/R has not yet been fully characterized and may depend on their maturation state (256).
The most common in vitro conditions used to simulate in vivo ischemia are anoxia (<1% O2, 5% CO2, 94+% N2) and complete substrate depletion (serum-free, glucose-free medium). There are many variations to the protocol with additional conditions that more closely mimic the ischemic heart, such as partial hypoxia, partial or no substrate depletion, hyperkalemia, acidosis, and use of electrical stimulation (207). The cell type needs to be carefully considered when determining optimal control and ischemic conditions. Neonatal cardiomyocytes and cell lines (H9c2 and HL-1) favor glucose metabolism and under control conditions are often cultured in hyperglycemic medium, which is known to induce ROS production and cell death in adult cardiomyocytes. Furthermore, neonatal cardiomyocytes are insulin resistant and supraphysiological concentrations of insulin are required to increase glucose uptake in these cells compared with adult cardiomyocytes (201). Thus, studies focusing on metabolism with H/R and/or metabolic pathology need to carefully consider the cellular environment in both control and ischemic conditions. In addition to the cellular environment, the duration of hypoxia and reoxygenation is also an important consideration, as myocyte viability not only depends on the duration of hypoxia but also the duration of reoxygenation (166, 244).
To summarize, the major strengths and limitations of studies using isolated cardiomyocytes are shown in Table 1. The major strength of H/R experiments in cultured cardiomyocytes is the ability to control precisely the cellular and extracellular environment, each factor present in ischemic conditions (e.g., hypoxia, metabolic inhibition, or acidosis) can be tested alone and in combination to determine individual contributions to cellular injury. Even with the most carefully designed experimental protocol, in vitro conditions can never fully recapitulate the full spectrum of I/R injury in vivo. Thus, although in vitro experiments can be mechanistically informative and identify new targets for intervention, it is imperative that the results are later validated in an appropriate intact animal model. Nonetheless, even if there is a discrepancy between in vivo I/R and in vitro H/R experiments, important insight is to be gained from parallel studies. As an example, angiopoietin-like protein 4 (ANGPTL4) reduces infarct size after I/R in vivo but does not prevent cardiomyocyte death in vitro (90). These findings indicate that other cell types (e.g., endothelial cells, fibroblasts, or immune cells) are key to the cardioprotective effect of ANGPTL4. Likewise, factors that prevent myocyte cell death in culture may not reduce infarct size in vivo, suggesting that the cardioprotective effect may be outweighed by noncardiomyocyte factors.
Isolated Perfused Hearts
Model rationale.
The isolated perfused heart is a convenient and reproducible model to test mechanisms of myocardial injury and cardioprotection (14, 192). The heart is removed from the animal and perfused, typically with a physiological saline solution such as Krebs-Henseleit buffer. For screening drugs or interventions for protective properties, this model is ideal, because the isolated perfused heart is studied independently of circulating factors or neuroendocrine inputs from other organs but retains the function, composition, and architecture of the intact heart. This approach is also easily amenable to biochemistry or imaging studies in a nuclear magnetic resonance (NMR) magnet, which can provide useful information to decipher mechanisms of cardioprotection.
Perfused hearts can be studied in a working heart mode or in a nonworking Langendorff mode. In the Langendorff mode, the perfusate enters the coronary arteries to perfuse and oxygenate the heart, which continues to beat for several hours (292). Heart rate and left ventricular (LV) developed pressure are measured with a fluid-filled balloon placed in the cavity of the LV and connected to a pressure transducer as indexes of cardiac function (physiology). The heart can be perfused at constant pressure, in which case the flow rate can vary, or flow can be set with a pump, in which case the perfusion pressure can vary. In the Langendorff mode, the heart does not pump against a gradient and does not perform external work (226). In the working heart mode, the perfusate enters the atrium at a filling pressure set by the operator, and the heart pumps perfusate against a hydrostatic pressure set to different levels (30). A heart model performing external work is technically more challenging, particularly with smaller hearts.
In a model of global ischemia, perfusate flow to the entire heart is stopped, whereas in a regional ischemic model, a suture is tied around a single coronary artery for occlusion. After the ischemic period (typically 20–40 min for rodent models), perfusion is restarted and the heart will usually beat and develop a lower LV developed pressure than at baseline, reflecting postischemic contractile dysfunction or stunning. Contractile dysfunction is a measure of ischemic injury but is not synonymous with cell death. Both contractile dysfunction and cell death often result from the same mechanisms. It is therefore important not to infer that protection against contractile dysfunction is the same as protection against infarct size.
Variables measured.
To measure cell death or infarct size, it is necessary to reperfuse the heart for a sufficient duration (at least 60–120 min) to wash out reductive equivalents (79, 271). Triphenyltetrazolium chloride (TTC) is then added to the perfusate or hearts are cut into transverse slices and incubated in TTC solution. TTC is a dye that stains viable myocardium red due to a formazan reaction with NADH and NADPH, which are washed out from irreversibly injured myocardium (81, 172), whereas necrotic tissue remains unstained and thus appears white. Necrotic tissue area is normalized to the total ventricular area (global ischemia) or the ischemic area at risk for infarction (regional ischemia). For regional ischemia preparations, the area at risk is measured after coronary reocclusion and staining of the nonischemic myocardium with a dye such as Evans blue.
The susceptibility of the heart to arrhythmias during I/R is readily assessed through recording of an electrocardiogram (313). Updated guidelines exist for the quantification of such arrhythmias (56). The isolated heart is amenable to monitoring of intracellular ions by optical methods using fluorescent indicators (where the signal originates from a thin layer of epicardial cells) or by NMR spectroscopy (where the signal is a global average from the whole heart). Intracellular Na+ and Ca2+ concentrations have been monitored and intracellular H+ concentration (i.e., intracellular pH) has been estimated in isolated rodent hearts perfused and subjected to I/R within the vertical bore of the NMR magnet (288). Intracellular high-energy phosphate (ATP and creatine phosphate) have also been monitored by this method, and recent developments using hyperpolarized substrates now also allow real-time analysis of metabolic flux through distinct pathways (167).
The rate of occurrence of cell death is determined by the work that the heart performs at the time it becomes ischemic. When global flow is stopped completely, the heart will continue to beat for a short period of time and continue to consume ATP. Reducing work at the start of ischemia is cardioprotective, and this is the basis of cardioplegic solutions. Therefore, it is important to assure that work is similar between control and experimental hearts, which means that heart rate and temperature must be controlled and held constant in the different treatment groups (281, 292). Due to the lack of neurohumoral factor influences on the perfused heart, heart rate is typically lower than in an intact animal, and it can be controlled by pacing. A slight (<1°C) difference in temperature can result in a large difference of infarct size. Temperature is usually measured by a probe in the heart and controlled by immersing the heart in a fluid bath.
In the absence of blood, the reduction in the oxygen-carrying ability of the saline perfusate results in edema and an increase in flow rate. Because the mouse has a high heart rate, it is likely that oxygen delivery is on the edge of oxygen demand under baseline perfusion conditions. Furthermore, Krebs-Henseleit buffer typically contains only glucose as a substrate, whereas the heart normally uses fatty acids as its prime substrate. Fatty acids can be given as substrates but require the addition of a vehicle such as BSA and also require specialized methods for oxygenating the buffer. An advantage of the perfused heart model is that it allows one to examine the impact of different substrates either alone or in combinations. The effect of differences associated with perfusion with long-chain versus short-chain fatty acids also can be studied. Ex vivo hearts can be readily perfused with radioactive- or stable isotope-labeled substrates, allowing evaluation of substrate selection and metabolism (167, 211, 245).
The no-reflow phenomenon (179) can also impact infarct size and its measurement in isolated hearts. During ischemia and early reflow, the heart goes into contracture, which restricts flow to the subendocardium. The severity of no reflow depends on the severity of ischemic injury and can vary between control and protected hearts. In isolated hearts, no-reflow issues can be reduced by deflating the balloon in the LV for a few minutes right at the start of reperfusion (94).
To summarize, the major strengths and limitations of the isolated perfused heart are shown in Table 1. In consideration for all of the above factors, it is imperative that control and treated hearts are studied under identical conditions. Although ischemic pre- and postconditioning were originally described in an in vivo dog model, much of the information about the molecular signaling pathways responsible for protection was established in perfused heart models (128).
IN VIVO MODELS
Chronic Coronary Artery Disease
Coronary stenosis and stress-induced myocardial ischemia: model rationale and variables measured.
Reversible episodes of ischemia can lead to contractile dysfunction in the absence of significant myocyte necrosis (42). Because ischemic entities are frequently encountered in clinical practice, the task of understanding their pathophysiology and testing therapeutic interventions has stimulated the development of animal models in which acute and chronic adaptations to ischemia and the ensuing functional recovery can be evaluated over time. Most of these entities are consequences of either brief or chronic episodes of ischemia, and the models developed to study them will be discussed separately below.
Chronic stable angina is a clinical condition whereby a patient has one or more coronary stenoses that have largely compromised or even exhausted autoregulatory coronary reserve. Frequently, these limitations are partially compensated by collateral blood flow from adjacent less-compromised coronary arteries such that myocardial blood flow and contractile function remain normal at rest. Stress situations such as exercise, emotions, or pain, however, can precipitate acute myocardial ischemia with or without typical chest pain. Chronic stable angina in patients does not usually inflict global myocardial ischemia but is a regional event. The acute precipitation of myocardial ischemia requires an in vivo model where an acute coronary stenosis can be produced to reduce coronary blood flow. Alternatively, a stable stenosis must be created where blood flow is maintained at baseline but acute ischemia is elicited, e.g., by pacing or adrenergic activation in anesthetized animals or by exercise in conscious animals (9, 93, 131).
To reflect the regional character of chronic stable angina, regional myocardial blood flow and regional contractile function must be measured. The standard approach to monitor regional blood flow is to use microspheres (142), which have traditionally been labeled with radioactive isotopes (66) and, more recently, nonradioactive colored dyes or fluorescent dyes (107, 184). Analysis of regional myocardial blood flow during acute ischemia reveals an inability of perfusion to increase distal to a stenosis compared with normal remote myocardium (33, 39). As coronary vasodilator reserve is exhausted, there is a major redistribution of blood flow away from the ischemic region toward the nonischemic myocardium where metabolic vasodilation prevails. In addition, there is a transmural blood flow redistribution from the ischemic subendocardium toward the subepicardium (93).
The gold standard for experimental regional contractile function measurements is sonomicrometry (265). Simultaneous measurements of regional myocardial blood flow and regional contractile function provide a means to determine the quantitative relationship between regional blood flow (as a surrogate for oxygen/energy supply) and regional contractile function (as a surrogate for oxygen/energy demand). The relation between regional contractile function and subendocardial perfusion (flow-function relation) demonstrates close coupling during steady-state ischemia at rest as well as over a wide range of cardiac workloads (33, 35, 37, 91, 309). During steady-state acute myocardial ischemia, there appears to be no imbalance between supply (blood flow) and demand (function); rather, there is a matched reduction in both parameters (129, 130). Such matching between regional blood flow and contractile function also persists during major changes in heart rate, when both blood flow and contractile function are normalized for a single cardiac cycle (92). This matching can persist for several hours and contribute to the maintenance of myocardial viability and full recovery of contractile function after eventual reperfusion (212). More specifically, the hallmarks of short-term myocardial hibernation are a perfusion-contraction match (259), together with metabolic signs of adaptation to ischemia (210, 267) and the potential to recruit an inotropic reserve in the dysfunctional myocardium (267). Also, all pharmacological interventions to attenuate acute myocardial ischemia (e.g., by nitrates, β-blockers, Ca2+ antagonists, or their combinations) operate along a fixed flow-function relationship (213–215). The two major mechanisms that precipitate myocardial ischemia and must therefore be pharmacologically addressed are tachycardia (112, 113) and coronary vasoconstriction (134, 273).
Studies evaluating brief total coronary occlusions can be conducted in a variety of species. In contrast, to study coronary artery stenosis, the animal under study must be large enough that coronary artery instrumentation along with regional flow and function measurements is feasible (i.e., in dogs and pigs that can be instrumented with a hydraulic occluder on the coronary artery). Acutely anesthetized animals have provided insight into short-term coronary flow regulation over minutes to hours but cannot evaluate the effects of chronic coronary stenosis on long-term microvascular remodeling and collateral growth over days to weeks (285). Acute experiments have the advantage that sequential myocardial biopsies can be taken for the analysis of metabolic and molecular analyses (101, 174, 210, 283). Microdialysis probes can be implanted to evaluate interstitial mediators (209, 270), and the activity of the cardiac innervation can be measured (132).
A significant experimental challenge is maintaining a fixed degree of coronary artery narrowing throughout a study using a hydraulic occluder. This limitation can by circumvented by perfusing the coronary artery at constant pressure from a reservoir, controlling flow with an extracorporeal pump or perfusion of the region of interest with an extracorporeal pressure control system (38). A major limitation of studies in acutely anesthetized animals are the substantial confounding effects of anesthesia and neurohormonal activation on hemodynamics and flow, which alter coronary autoregulation and produce varying degrees of coronary vasodilation and vasoconstriction that modulate autoregulatory responses (33, 36, 39). The limitations of acute studies can be circumvented by studying conscious, chronically instrumented animals. While strict control of hydraulic occluders and coronary collaterals stimulated by repetitive ischemia and chronic stenosis at first seems a limitation, these factors can be capitalized upon by provoking coronary collateral development to the point where the artery can be totally occluded without reducing resting myocardial perfusion. This is typically accomplished in dogs using ameroid occluders, which are hygroscopic and gradually swell to produce a progressive stenosis resulting in a total occlusion within 3–4 wk.
Collateral blood vessel growth can also be stimulated in dogs by repetitive brief coronary occlusions using a hydraulic occluder (303, 320). Once collaterals are developed, variability in the hydraulic stenosis severity is no longer a problem, and intervention effects on stress-induced ischemia can be studied under multiple conditions. While pigs can also develop collateral-dependent myocardium after ameroid occluder placement (260), collaterals grow more slowly than in dogs, and pigs frequently develop a subendocardial infarction (230). The admixture of infarcted and normal myocardium greatly complicates measurements of perfusion and function. Infarction can largely be circumvented by employing a fixed diameter stenosis on the coronary artery of farm-bred swine, resulting in a much more severe limitation of subendocardial flow reserve than in dogs, and there is usually contractile dysfunction at rest (74, 77). While this is an extremely useful model to study chronic vascular adaptations and interventions to promote angiogenesis, alterations in myocardial physiology can complicate the interpretation of flow changes. A major drawback of chronic large animal models of coronary stenosis and collateral-dependent myocardium is their expense and the labor-intensive nature of the animal care and handling. The guinea pig has a very well-developed collateral circulation that prevents infarction from occurring following occlusion of a single main coronary artery; to obtain infarction, multiple coronary arteries need to be ligated (216). Thus, guinea pigs are not suitable for in vivo ligation studies but can be used for heart perfusion with global ischemia experiments. These issues have motivated studies to assess flow regulation using repetitive coronary occlusions in rats and mice, including genetically altered animals (303).
Coronary microembolization: model rationale and variables measured.
Subclinical atherosclerotic plaque rupture or erosion that does not result in complete thrombotic occlusion of the coronary artery but leaves a residual blood flow into the distal coronary microcirculation occurs spontaneously, with or without clinical symptoms. Coronary microembolization is also induced iatrogenically during percutaneous coronary interventions. Atherosclerotic debris from the culprit lesion, together with thrombotic material, soluble vasoconstrictors, as well as thrombogenic and inflammatory substances, is washed into the coronary microcirculation where it causes microvascular obstruction with resulting patchy microinfarcts and an inflammatory reaction (135, 173). The inflammatory response includes increased expression of tumor necrosis factor-α associated with profound contractile dysfunction and upregulation of signal transduction pathways involving nitric oxide, sphingosine, and ROS, which contribute to impaired excitation-contraction coupling (32, 299). Repetitive coronary microembolization can result in global LV dysfunction and, even in the absence of overt infarction, in heart failure (261). Coronary microembolization can be simulated experimentally by intracoronary infusion of inert particles of various diameter (67) and also by intracoronary infusion of autologous microthrombi (191). When the target under study is ischemic heart failure, repeated coronary microembolization can be used in both small and large animal models. When the target under study is a spontaneous or periprocedural minor infarction, the animal must be large enough such that regional myocardial measurements of flow, contractile function, metabolism, and morphology are possible (i.e., dog or pig models are preferable).
The major strengths and limitations of angina models are shown in Table 1.
Stunning, Hibernation, and Ischemic Cardiomyopathy
Stunning: model rationale and variables measured.
When ischemia caused by a total coronary occlusion is brief (e.g., as may be experienced from coronary vasospasm), regional contractile dysfunction persists for hours after reperfusion but then completely normalizes within 24 h. This phenomenon was first demonstrated after a 15-min circumflex coronary artery occlusion in chronically instrumented dogs, was subsequently called stunned myocardium, and is common in patients with acute coronary syndrome (17, 19, 143). Most investigators assume that the complete normalization of function, lack of evidence of infarction by TTC staining, and lack of sarcolemmal disruption on electron microscopy indicate that no cardiomyocyte death is associated with stunning. While pathological evidence of myocyte necrosis is indeed absent, TUNEL staining performed 1 h after reperfusion demonstrates that programmed cell death or myocyte apoptosis develops in rare isolated cardiac myocytes and circulating cardiac troponin I is increased (314). Thus, while there is no evidence of infarction in stunned myocardium, regional myocyte loss can develop when stunning becomes repetitive.
Because the essence of stunned myocardium consists of relatively rapid (24–48 h) reversibility of contractile dysfunction in the absence of TTC or pathological evidence of infarction, most studies use chronic large animal models in which serial measurements of function can be performed. In addition, regional ischemia is the preferred model to allow assessment of the remote nonischemic regions of the heart as an internal control. While stunned myocardium occurs after demand-induced ischemia distal to a coronary stenosis (144), most studies have used transient total coronary occlusion. Animals are instrumented with a hydraulic occluder to produce brief ischemia 1–2 wk after recovery from surgical instrumentation.
To assess regional function, most studies have used sonomicrometry for direct measurements of subendocardial segment length shortening or wall thickening. Recent studies have used transient occlusion of the left anterior descending coronary artery (LAD) using a balloon angioplasty catheter in closed-chest sedated animals where regional function can be assessed with imaging approaches such as echocardiography (314). The latter approach circumvents the need for chronic surgical instrumentation through a prior thoracotomy. Echocardiography can also be employed to assess stunning in mice chronically instrumented with an occluder to produce transient ischemia (63).
The dog (20, 143), pig (291, 314), and rabbit (18) are the most commonly used species to study myocardial hibernation. Pigs and rabbits offer the advantage of having little or no collateral circulation, so that the severity of the ischemic insult and of the subsequent contractile dysfunction are more uniform. In contrast, dogs exhibit a highly variable degree of collateral circulation resulting in widely different degrees of myocardial stunning (20). There are also species differences in the time course of recovery despite similar occlusion durations (277). Many studies have also used open-chest animal models, although the severity of myocardial stunning in these preparations is significantly exacerbated versus conscious animal models (21, 304). Most experimental approaches to assess stunning are quite straightforward, although ventricular fibrillation can develop. This is more common in swine as opposed to canine models, because pigs have little innate coronary collateral flow (164). Because myocardial function assessed using segment shortening and wall thickening is load dependent, it is important to ensure that heart rate, systolic blood pressure, and LV end-diastolic pressure remain reasonably constant over the time frame of the measurements. An advantage of chronic models using regional ischemia is that each animal can potentially serve as its own control; hence, it is possible to use the same animals to study the effects of pharmacological interventions on physiological end points.
Short-term hibernation: model rationale and variables measured.
A prolonged episode of moderately severe ischemia can be sustained for a period of hours in the absence of pathological evidence of infarction. This phenomenon is termed short-term hibernation (139, 212). An approximate 50% reduction in perfusion leads to reduced function and perfusion-contraction matching, which largely prevents irreversible myocyte injury. If the heart is reperfused within a few hours, contractile dysfunction persists in a fashion similar to stunned myocardium but with a more protracted time course of recovery (i.e., lasting in the timeframe of days rather than hours) as is typically seen with stunning after a brief total occlusion. This in part appears to relate to reversible myofibrillar disassembly and myolysis in the absence of sarcolemmal disruption (279). Unfortunately, when the initial adaptive response to moderate ischemia in short-term hibernation is present for longer than 12 h, progressive myocardial necrosis begins to develop, resulting in some degree of myocardial infarction usually confined to the subendocardium (48, 185, 269). While imposition of acute moderate ischemia was initially proposed as a mechanism of chronic hibernating myocardium, the development of progressive infarction when flow reductions last longer than 12 h leads to a pathological entity with myofibrillar disassembly and cardiac biomarker release that can no longer be defined as hibernation but, rather, is more in line with subendocardial infarction (48, 279).
Studies of short-term hibernation usually require closed-chest animal models, although considerable insight about adjustments between flow and function has been gleaned from open-chest studies of myocardial metabolism (137, 210, 239). The latter studies usually use a cannulated branch of the left coronary artery perfused at constant pressure. Closed-chest animal studies usually employ chronically instrumented dogs or pigs. In these studies, a hydraulic occluder is placed around a coronary to reduce flow or coronary pressure to a fixed level, which is released after several hours.
Chronic hibernation and stunning: model rationale and variables measured.
While both stunning and short-term hibernation are characterized by complete functional recovery, chronic contractile dysfunction can develop when recurrent ischemia develops before functional normalization (21). Chronic contractile dysfunction from repetitive ischemia develops in the absence of histological infarction, and both hibernation and stunning involve the loss of myocytes via apoptosis in a fashion similar to what happens after brief episodes of ischemia (193, 314). Unlike stunning, which was initially an experimental observation that later became associated with multiple clinical correlates, chronic hibernating myocardium was first characterized in patients with chronic ischemic heart disease displaying regional contractile dysfunction in the absence of manifest ischemia (23, 139). Only later were animal models used to identify cellular and molecular mechanisms responsible for the adaptive responses to chronic ischemia (77).
While it was originally controversial whether or not flow was reduced or normal at rest (34), it is now clear that chronic repetitive ischemia initially results in contractile dysfunction with normal resting flow or chronic stunning (73, 278). When this situation persists, the reduction in function leads to a secondary reduction in regional energy utilization accompanied by reduction in resting flow (76). Thus, the reduction in resting flow characteristic of chronic hibernating myocardium is a result, rather than cause, of regional dysfunction.
In contrast to models of short-term ischemia, animal models of hibernating myocardium are based on chronic coronary stenoses that frequently progress to total occlusion and collateral-dependent myocardium. In studies using ameroid occluders that gradually swell to produce chronic stenosis, dogs usually do not develop contractile dysfunction at rest but can do so when preexisting epicardial collaterals are ligated at the time of instrumentation (36). Swine ameroid occluder models frequently have contractile dysfunction in collateral-dependent myocardium, and this is usually associated with some degree of subendocardial infarction (230).
A more consistent model of hibernating myocardium can be produced by instrumenting juvenile swine with a fixed diameter stenosis (1.5-mm diameter) on the proximal LAD (73, 77). As the animals grow over the subsequent 3 mo, there is a slowly progressive limitation in coronary flow reserve, because the LAD stenosis limits maximal myocardial perfusion, while the mass of myocardium distal to the stenosis increases in parallel with cardiac growth. As a result, there is a more prolonged and gradual stimulus for coronary collateral development so that LAD occlusion almost always develops in the absence of infarction.
After 3 mo, regional contractile dysfunction with mild reductions of resting flow in the absence of infarction is consistently manifest and is similar to the changes seen in humans with hibernating myocardium caused by a chronic LAD occlusion (308). Serial studies of this animal model have demonstrated that the heart progressively adapts from a state of contractile dysfunction with normal resting flow (chronic stunning) to a state where resting flow decreases, consistent with hibernating myocardium (41). Such chronic hibernation is associated with a downregulation in mitochondrial metabolism and regional myocyte hypertrophy that maintains myocardial wall thickness constant in the setting of regional apoptosis-induced myocyte loss.
Over longer periods of time (up to 6 mo) the adaptive response of hibernating myocardium persists unchanged (75), and the downregulation in metabolism and upregulation of proteins involved in cellular survival and cytoprotection prevent cell death and, hence, further myocyte loss (62). While infarction does not develop in this model, revascularization only partially reverses myocardial dysfunction and does so over a much longer time frame than seen with either myocardial stunning or short-term hibernation (237). Chronic contractile dysfunction in the absence of infarction can also be induced using a hydraulic occluder to produce an acute stenosis in chronically instrumented animals. Chronic stunning can develop in swine subjected to daily episodes of short-term hibernation (169). A more rapid transition from chronic stunning to hibernating myocardium than the one observed in the fixed diameter stenosis model can be achieved by acutely imposing a critical stenosis on the LAD (301). The latter model can develop reductions in flow with regional contractile dysfunction after 2 wk of a stenosis sufficient to prevent reactive hyperemia.
The fixed diameter chronic stenosis model is advantageous in that hibernating myocardium develops reproducibly in a predictable time frame. A limitation of the fixed stenosis porcine model is that it requires cardiac growth to produce a progressive physiological impairment in maximum myocardial perfusion, and the 3- or 4-mo period required to develop hibernating myocardium is viewed as cost prohibitive. This model has so far only been studied in juvenile farm bred swine and may produce variable results if cardiac growth is attenuated by limiting feeding. It is not clear whether the model can be effected in purpose-bred swine and, particularly, in mini-swine, where growth rates are substantially attenuated. An additional disadvantage is that the chronic stenosis model is associated with a high rate of spontaneous ventricular fibrillation (40). This has provided insight into the mechanisms of sudden cardiac arrest in chronic coronary disease but reduced the success of studying chronic adaptations to ischemia in survivors. Finally, because of the long duration of the studies in the presence of animal growth, it is not feasible to chronically instrument animals. Nevertheless, it is feasible to use telemetry to assess chronically LV pressure and arrhythmias in untethered conscious animals (242).
Ischemic cardiomyopathy: model rationale and variables measured.
Ischemic cardiomyopathy is the underlying cause of LV dysfunction in two out of every three patients with heart failure (105). Ischemic cardiomyopathy in humans can arise from LV remodeling after a large myocardial infarction but, more commonly, is the result of extensive multivessel coronary artery disease with modest amounts of diffuse fibrosis and patchy infarction in multiple coronary artery distributions (15). Along these lines, preclinical studies have established that chronic coronary artery stenosis can induce significant myocyte loss with modest global replacement fibrosis that leads to global LV dysfunction and varying degrees of congestive heart failure when the area at risk is large. Conceptually, the stenosis does not limit blood flow at rest. Rather, by reducing maximal perfusion in response to stress, it sets the stage for repetitive episodes of subendocardial ischemia. A key feature of all animal models of ischemic cardiomyopathy is that the myocardium at risk of repetitive ischemia represents a large portion of the LV (>70% of LV mass). This has been achieved using stenosis of the left main coronary artery in rodents or multivessel coronary artery stenoses in large animals. As a result of the large area at risk, myocyte cell death arises from both ischemia and myocyte stretch and slippage from increased LV end-diastolic pressure (possibly also reflecting transient ischemia).
In rats, ischemic cardiomyopathy can be induced by producing a fixed coronary stenosis of ~50−60% diameter reduction on the proximal left coronary artery, which causes variable degrees of LV dysfunction (44, 45). While replacement fibrosis occurs in these animals, it is patchy and modest, only increasing twofold over control for an average of <10% of LV cross-sectional area. Interestingly, the degree of LV dysfunction is primarily related to myocyte cell loss (necrosis and apoptosis) and the elevation in LV end-diastolic pressure related to fibrosis. A similar model of ischemic cardiomyopathy has also been obtained in mice (189). While rodent models afford the ability to perform higher throughput studies and use transgenic animals to study molecular mechanisms, they have relatively high surgical and postoperative mortality. In addition, there is considerable variability in physiological outcomes, such that frequently animals are retrospectively categorized into mild, moderate, and severe heart failure groups. Reproducibility of ischemic cardiomyopathy models, therefore, is indeed a concern.
While left main coronary stenosis is not feasible in large animals, multivessel coronary stenoses can produce a large ischemic risk area and recapitulate ischemic cardiomyopathy. When fixed diameter occluders are placed on both the proximal LAD and circumflex arteries in growing farm-bred swine, LV ejection fraction declines with elevated resting LV end-diastolic pressure (74), consistent with compensated LV dysfunction and no overt evidence of heart failure. These animals also exhibit primary myocyte loss with only an approximately twofold increase in extracellular matrix accumulation. A similar condition has been produced using multivessel ameroid occluders in dogs (80). Aside from requiring survival surgery, the major disadvantage of these approaches arises from the development of sudden cardiac arrest, which in swine is related to both ventricular fibrillation and to a lesser extent bradyarrhythmias. In mice, a state of ischemic cardiomyopathy can be induced using repetitive brief coronary occlusions, and this model is associated with substantial but reversible fibrosis of the myocardial region subjected to repetitive ischemia (63).
Noninvasive imaging.
Noninvasive cardiac imaging technologies such as echocardiography, magnetic resonance imaging (MRI), and computed tomography can measure regional and global contractile function and are increasingly available for preclinical studies, particularly in larger animals. NMR spectroscopy can provide information on cardiac energetics (114). More sophisticated imaging technologies such as positron emission tomography can measure regional myocardial perfusion and regional myocardial metabolism and sympathetic innervation and are increasingly used in preclinical studies (77, 187, 268). MRI can serially measure myocardial perfusion (264) and can provide reliable measurements of infarct size and microvascular obstruction. MRI-derived edema, however, is time dependent and sensitive to cardioprotective interventions (141, 148). Therefore, MRI-derived edema can be used to stratify an ischemic/reperfused myocardial region for protocol assignment but not for quantitative normalization of infarct size to area at risk.
To summarize, the major strengths and limitations of stunning, hibernation, and ischemic cardiomyopathy models are shown in Table 1.
Myocardial Infarction Models: Permanent Coronary Artery Occlusion with Nonreperfused and Reperfused Myocardial Infarction
MI: general considerations.
Coronary occlusion causes immediate cessation of aerobic metabolism in the ischemic myocardium, leading to rapid ATP depletion and metabolite accumulation and resulting in severe systolic dysfunction within seconds (86). If the duration of the ischemic insult is <15 min in larger mammals such as dog and pig, restoration of flow reverses the early ischemic cardiomyocyte changes (transient mitochondrial swelling or glycogen depletion) and all cardiomyocytes in the ischemic area can survive (158). Longer periods of ischemia cause death of an increasing number of cardiomyocytes. A 20- to 30-min interval of severe ischemia is sufficient to induce irreversible changes in some cardiomyocytes of the subendocardial area, inducing sarcolemmal disruption and striking perturbations in mitochondrial architecture, such as ultrastructural evidence of amorphous matrix densities and severe mitochondrial swelling (156). These early ultrastructural alterations mark cardiomyocytes that cannot be salvaged and will ultimately die in the infarct environment (157).
Experimental studies in the canine model of MI demonstrate a transmural heterogeneity in the myocardial response to ischemia, suggesting that the subendocardium, where myocardial oxygen demand is greatest, is more susceptible to ischemic injury than the midmyocardium and subepicardium (2). Thus, the prevailing paradigm suggests a wavefront of cardiomyocyte death that progresses from the more susceptible subendocardium to the less vulnerable subepicardium as the duration of the ischemic insult increases (159, 252). Experimental studies in large animal models have demonstrated that ischemic myocardium cannot be salvaged by reperfusion after 6 h of coronary occlusion (251). The increased vulnerability of subendocardial regions to coronary occlusion may reflect a greater reduction of the subendocardial blood flow due to transmural differences in vascularization (2, 25) and extravascular compression (68, 286). The wavefront concept of ischemia developing into infarction was derived from experimental studies in dogs, where a substantial coronary collateral circulation influences the time course of cardiomyocyte necrosis (86).
The major species difference in the MI response lies in the temporal and spatial kinetics of events and differences due to myocardial size. In mice, durations of coronary occlusion exceeding 60–90 min are considered irreversible, and inflammation and wound healing processes are accelerated (64, 88, 221, 222). In mouse and rat models, reperfused infarcts are typically midmyocardial, and subepicardial and subendocardial regions are relatively spared (50, 69, 325). Studies in a sheep model of reperfused infarction also suggest that the midmyocardium may be most vulnerable to ischemic injury; in contrast, the subendocardium is relatively resistant (263). The pig model of coronary occlusion-reperfusion comes closest to human STEMI in its temporal and spatial development, but other models are nevertheless useful to study fundamental mechanisms of MI (140).
MI: technical considerations.
Extensive protocols providing technical details for performing permanent occlusion MI and reperfused MI in mice and rats are available (221, 222, 228, 317, 327). While MI is most commonly performed in rodent models, protocols in other animal models are also available (151, 183, 218, 331). For mice, the quality of open-chest surgery to induce coronary occlusion directly impacts study outcomes (152, 221, 222). Minimizing the size of the thoracotomy and limiting bleeding by entering the thorax through intercostal muscles are recommended.
Biomarkers that have been used to evaluate the presence of MI include cardiac troponins and creatine kinase, and plasma proteins such as macrophage migration inhibitory factor can also be measured as indices of injury (47, 55). Infarct size is widely measured as a key variable for testing genetic or therapeutic intervention efficacy, and infarct size measurements taken serially at both early and late time points can evaluate the extent of infarct expansion (22). Echocardiography can also be used for infarct sizing, with the caveat that echocardiography does not distinguish between reversible ischemic dysfunction (stunning) and irreversible loss of function and therefore a secondary method is needed for confirmation of infarct size at early time points. For more details on measuring cardiac function in mice, the reader is advised to consult the article Guidelines for measuring cardiac physiology in mice (196).
Permanent occlusion MI: model rationale and variables measured.
Permanent coronary occlusion is a relevant animal model of acute STEMI reflective of patients who, due to contraindications or logistic issues, do not receive timely or successful reperfusion (53, 104). Permanent coronary occlusion yields acute ST segment elevation infarction with robust myocardial inflammation and long-term remodeling, thus providing a large effect size that reduces the sample size needed to detect differences between groups. Infarction assessed in the first 1–14 days after coronary ligation is histologically characterized by coagulation band necrosis with a fulminant inflammatory infiltrate in the infarct and border zone regions. Infarction is geometrically and physiologically characterized by wall thinning, increases in LV dimensions and volumes, and decreases in fractional shortening and ejection fraction.
Changes that occur over the first week provide information on myocyte cell death and infarct development, inflammation and leukocyte physiology, extracellular matrix (ECM) turnover and fibroblast activation, and the role of endothelial cells in neovascularization (83, 154, 165, 194, 205). Chronic evaluation at time points 4–8 wk post-MI provides information on long-term remodeling. Whether the infarct region or remote region is the focus of investigation depends on the question asked. Examining the infarct region provides details on active inflammation and scar formation, while examining the remote region provides details on still-viable myocytes within the myocardium and remote inflammatory and ECM processes.
Perioperative and postoperative survival should be assessed, and the time point of delineation between these two phases should be defined. For some laboratories, the perioperative phase includes the time until the animal recovers and becomes ambulatory (usually within 1–3 h for mice). For other laboratories, the perioperative phase includes the first 24 h after surgery. Perioperative death within 24 h post-MI in mice is usually due to surgical errors (or very large infarct sizes), and, in established laboratories, the 24 h surgical mortality rate due to technical issues is <10%. In the permanent occlusion MI model in mice, postoperative death (deaths at >24 h time point) typically occurs during days 3–7 post-MI and is due to rupture, acute heart failure, or arrhythmias (59, 98, 233). Autopsy is strongly recommended for all mice that die prematurely, to evaluate early deaths due to technical issues and later deaths due to complications of MI. Seven-day postoperative mortality rates are ~10–25% (75–90% survival) for female young mice and 50–70% (30–50% survival) for male young mice (47, 61, 89, 98, 152, 170, 195, 202, 206, 234, 310–312, 319, 323). Immediate survival from the surgery can also be affected by baseline characteristics such as obesity, diabetes, or high levels of circulating inflammatory cells, which, in turn, determine the response to anesthesia and surgery (60, 123, 202, 203). While there is no difference in infarct tolerance between young and middle-aged mice (323), older mice may survive better than younger mice (202, 319).
For permanent occlusion MI models, infarct size must be measured in fresh LV slices at the time of necropsy by TTC staining and typically ranges from 30% to 60% of the total LV (47, 48, 61, 89, 98, 152, 195, 202, 206, 223, 310–312, 319, 323). The method for calculating infarct size varies across laboratories. Some laboratories use area calculations, other laboratories measure length in the midmyocardium, and other laboratories measure and average lengths in the subendocardium and subepicardium. There is no need to use Evans blue for area at risk assessment in permanent coronary occlusion models that pass the point from ischemia to infarction, as the entire area at risk is infarcted. It is important that MI surgical success is confirmed and that the initial infarct injury is comparable across groups, to assess remodeling differences at later stages. In mice, ligating the coronary artery at the same anatomical location across groups is important; 1 mm distal to the left atrium is the recommended site to generate large infarcts (35–60% of total LV). Failure to induce MI can occur, usually due to missing the coronary artery during the ligation step. Monitoring the electrocardiogram for ST segment elevation during the procedure reduces this possibility. Echocardiography at 3 h after coronary occlusion can be used to exclude animals with excessively small or large MI before randomizing groups (153, 155, 195). Late gadolinium-enhanced MRI is also useful for selecting animals with consistent infarct sizes (262). When assessing effects of treatments initiated post-MI, it is important to show that infarct size is not different between groups before treatment. Plasma sampling at 24 h post-MI can be used to assay cardiac biomarkers, such as troponins and inflammatory cytokines, with the caveat that these measurements can indicate presence or absence of infarct and not extent of injury. After coronary occlusion, care should be taken in performing these assessments to minimize disturbing animals at times when cardiac rupture may be triggered by stress, particularly at days 3–7 post-MI in untreated controls (96, 98). Small infarcts may reflect technical issues in missing the coronary artery, resulting in damage from the suture rather than reflecting the intended myocardial ischemia and infarction. Infarct sizes <30% are typically excluded. If included, small and large infarcts may need to be grouped separately to reduce possible type II statistical errors.
Cardiac wound healing and remodeling, typically assessed days to weeks post-MI, can be examined using a wide variety of approaches, including echocardiography, histology, biochemistry, and cell biology (5, 217, 328). Serial measurements of cardiac geometry and function by echocardiography are useful for defining phenotypes. Cardiac dimensions vary depending on heart rate and depth of anesthesia, and these parameters must be carefully controlled and matched across groups. Cardiac functional reserve can be assessed by measuring the contractile response to inotropic drugs or volume overload. Cardiac MRI and hemodynamic assessment by pressure-volume catheterization are other ways to measure cardiac physiology parameters. It is feasible to quantify infarct size noninvasively and serially by using cardiac MRI (181). Hemodynamic evaluation in mice is a terminal procedure, which prevents its use in serial assessments.
Hematoxylin and eosin staining provides information on areas of necrosis and inflammation, while picrosirius red staining provides information on total collagen accumulation both in the scar and remote regions (316). Immunohistochemistry for neutrophils, macrophages, lymphocytes, fibroblasts, and endothelial cells provides information on the extent of inflammation, scar formation, and neovascularization. Isolating individual cell types and assessment of ex vivo phenotypes in culture can further aid in understanding mechanisms. Studies have revealed that inflammation evoked by acute myocardial infarction also occurs systemically and that the spleen and liver are important sources of cells and factors that influence LV remodeling (71, 72, 95, 116, 198, 199, 293).
I/R MI: model rationale and variables measured.
Implementation of myocardial reperfusion strategies has significantly reduced mortality in acute STEMI. Reperfusion has contributed to the growing pool of patients who survive the acute event and are at risk for adverse remodeling and subsequent development of heart failure (133, 136). In addition to salvaging cardiomyocytes, reperfusion has profound effects on cellular events responsible for repair and remodeling.
Although timely reperfusion is essential to salvage viable cardiomyocytes from ischemic death, extensive preclinical and clinical evidence suggests that reperfusion itself causes injury (119, 121, 147). Reperfusion-induced arrhythmias and myocardial stunning are self-limited and reversible forms of reperfusion injury, while microvascular obstruction and lethal cardiomyocyte injury are irreversible and extend damage, thus contributing to adverse outcomes following MI (13, 126, 177, 241, 318). In patients, no reflow during reperfusion may be exacerbated due to the generation of microemboli composed of atherosclerotic debris and thrombi during percutaneous coronary interventions (135, 253).
MI both with or without reperfusion shares many of the same technical guidelines, and this information is provided above. The one technical difference is whether the ligation is removed at 45–60 min after the occlusion to reperfuse the myocardium. Similar to permanent occlusion MI, studies investigating the inflammatory and reparative response following MI with reperfusion need to take into account the dynamic sequence of cellular events involved in repair. Common measurements shared by the two MI models are shown in Table 2. For studies aimed at investigating acute myocardial injury using a reperfusion strategy, the duration of coronary occlusion needs to be sufficient for the induction of significant MI but not overly prolonged to cause irreversible injury in the entire area at risk. From the cell physiology perspective, the reparative response after MI can be divided into three distinct but overlapping phases: inflammation, proliferation, and maturation (26, 65). In the infarcted myocardium, dying cardiomyocytes release damage-associated molecular patterns and induce cytokines and chemokines to recruit leukocytes into the infarcted region, thus triggering an intense inflammatory reaction that serves to clear the infarct from dead cells and ECM debris, while initiating a reparative response (84). Early reperfusion after irreversible cardiomyocyte injury accelerates and accentuates the inflammatory reaction and has profound effects on the pathological features of the infarct. Microvascular hyperpermeability is evident in the myocardium with acute I/R (97). Rapid extravasation of blood cells through the hyperpermeable vessels may result in hemorrhagic changes (98, 178). Influx of phagocytotic macrophages is accelerated, resulting in more rapid removal of dead cardiomyocytes compared with permanent occlusion MI. In reperfused infarcts, dying cardiomyocytes often exhibit large contraction bands, comprised of hypercontracted sarcomeres. Subsarcolemmal blebs and granular mitochondrial densities, which are already present in irreversibly injured cardiomyocytes before restoration of blood flow, become more prominent upon reperfusion.
Table 2.
Common output measurements for in vivo MI and MI/reperfusion studies
| Measurement | Information Provided |
|---|---|
| Infarct size | Infarct size (MI) |
| Infarct size per area at risk (MI/reperfusion) | |
| Initial ischemic stimulus | |
| Final area of damage | |
| Effect of therapy or intervention | |
| Plasma biomarkers | Ischemia: creatine kinase, troponins |
| Inflammation: cytokines and chemokines | |
| Scar formation: growth factors and the ECM | |
| Neovascularization: angiogenic factors | |
| Left ventricular physiology (echocardiography, MRI, positron emission tomography imaging) | Geometry and function: dimensions, wall thickness, left ventricular dimensions and volumes, fractional shortening, ejection fraction, remodeling index |
| Electrophysiological function: PR, QRS, and QT intervals/morphology; spontaneous and inducible arrhythmias | |
| Inflammation | Immunohistochemistry and immunoblot analysis for cell numbers and inflammatory protein expression |
| Flow cytometry analysis of the digested myocardium for individual cell phenotypes | |
| Gene expression | |
| Systemic and circulating inflammation | |
| ECM scar | Picrosirius red for collagen deposition |
| Immunohistochemistry and immunoblot analysis for ECM proteins and cross-linking enzymes | |
| Gene expression | |
| Scar strength assessment | |
| Neovascularization | Blood vessel numbers |
| Vessel type and quality | |
| Microvascular damage | Microvascular plugging |
| Hyperpermeability/edema | |
| Hemorrhage |
MI, myocardial infarction; ECM, extracellular matrix; MRI, magnetic resonance imaging.
Phagocytosis of dead cells by activated macrophages results in the activation of endogenous anti-inflammatory pathways, ultimately leading to resolution of the inflammatory infiltrate. Suppression of inflammation is followed by recruitment of activated myofibroblasts that deposit large amounts of ECM proteins and by activation of angiogenesis (145). As the scar matures, fibroblasts become quiescent and infarct neovessels acquire a coat of mural cells (332). Compared with large mammals, rodents exhibit an accelerated time course of infiltration with inflammatory and reparative cells (64).
Leukocyte infiltration during the inflammatory phase of infarct healing and myofibroblast activation and accumulation during the proliferative phase are predominantly localized in the infarct region and border zone (87, 122, 280). During scar maturation, the cellular content in the infarcted region is reduced. At the same time, however, the number of activated macrophages and fibroblasts in the remote remodeling myocardium increases. Therefore, study of inflammatory and reparative cell infiltration and assessment of ECM protein deposition should include systematic assessment of each end point in the infarcted region, the peri-infarct area, and the remote remodeling myocardium.
Sympathetic nerves are damaged by permanent coronary occlusion but can regenerate after injury (220). In the setting of chronic MI, regional hyperinnervation around the infarcted region has been observed, and activation of cardiac sympathetic nerves is important in triggering ventricular arrhythmias, and such proarrhythmic action is dependent on the extent of infarction (1, 70, 315, 326). In contrast, after I/R, chondroitin sulfate proteoglycans prevent reinnervation (99, 100). Thus, the model selected for sympathetic nerve evaluation should be taken into consideration and depends on what question is being asked.
MI: intervention considerations.
The effects of interventions on post-MI remodeling can be studied using both nonreperfused MI and reperfused MI/R models (11, 219, 232, 294, 322). Typically, nonreperfused MI yields accentuated dilative remodeling and exacerbated dysfunction compared with a reperfused infarct involving the same vascular territory, reflecting a combination of more extensive infarct and less effective repair. In the reperfused MI/R model, the effects of genetic or pharmacologic interventions implemented early after reperfusion may reflect differences in the extent of acute cardiomyocyte injury rather than differences in wound healing responses. With permanent occlusion MI (assuming a standardized area at risk) or very late reperfusion models, differences in geometry and function of the remodeling heart are independent of acute cardiomyocyte injury and reflect effects on inflammatory, reparative, or fibrotic cascades. In the presence of an occluded coronary artery, the delivery of systemically administered pharmacologic agents to the infarcted region of large animal models may be dependent on formation of collaterals.
While the development of genetically targeted animals (mice, rats, and rabbits) resulted in an explosion of studies dissecting cell biological mechanisms and molecular pathways, large animal models are considered closer to the clinical situation for translational studies to test safety and effectiveness. Optimal study of molecular, cellular, and LV functional end points and interpretation of the findings require understanding of the underlying pathophysiology. Assessment of infarct size is typically the primary end point for investigations examining the mechanisms of cardioprotection. Assessment of chamber dimensions using echocardiography or MRI is crucial to study the progression of adverse remodeling. Systolic and diastolic cardiac geometry and function can be assessed noninvasively using echocardiography (including Doppler ultrasound and speckle tracking), MRI, and hemodynamic assessment. Mechanistic dissection of specific pathways may require inclusion of additional cell physiology and molecular or proteomic end points. In experimental models of MI, understanding the time course of the cellular and molecular events is critical for optimal study design. The effects of varying ischemic intervals on survival and activation of noncardiomyocyte cellular and acellular (e.g., ECM) compartments are poorly understood. Longer coronary occlusion times have distinct effects on cardiac repair, by extending infarct size and by influencing susceptible noncardiomyocyte populations, such as endothelial cells, fibroblasts, pericytes, and immune cells (85). Most studies characterizing responses to myocardial injury have so far been performed in healthy young animals. Comorbid conditions, such as aging, diabetes, and metabolic dysfunction, affect the pattern of ischemic injury and modify the time course and qualitative characteristics of the inflammatory and reparative responses (27, 106, 202, 238, 298, 319, 323). These comorbidities are relevant in the clinical context and must be considered in translation of experimental findings to the clinic.
To summarize, the major strengths and limitations of the nonreperfused and reperfused MI models are shown in Table 1.
Ablation
Model rationale and variables measured.
The primary advantages of ablative injury techniques such as cryo-, thermal-, and radio-frequency ablation are rigid and reproducible control over the size, shape, and location of the region of damage. With such methods, a wound can be stamped on the target myocardial tissue with consistent dimensions, shape, and transmural depth. Because the size of the damaged region is independent of animal-to-animal variations in coronary anatomy (223), the resultant ablation scar is also more reproducible than ligation-induced injury (52, 160, 307), aiding studies of long-term structural and functional remodeling and providing better power to detect the effects of an experimental drug or cell therapy. Infarct location can thus be controlled independently from coronary anatomy and infarct transmurality can be controlled (52, 160, 290, 305, 307). There are, however, important differences in the modes of cell death in ablative vs. occlusion injuries. For example, cryoinjury results in necrosis due to the generation of ice crystals and disruption of the cell membrane rather than direct ischemia. Furthermore, ablative injuries are typically generated from the epicardial surface inward, whereas ischemic infarcts tend to be propagated outward from the inner myocardial layers (52, 160).
Unlike MI or MI with reperfusion, cryoinjury kills all (or nearly all) cells within the core of the damaged region and creates distinct wound margins. Thus, a number of studies have used cryoinjury to avoid confounding effects of resident surviving cells when testing stem cell and other related therapies (6, 7, 258, 302). Ablation procedures typically apply a cooled/heated probe to either the epicardial or endocardial surface of the heart. The extent and depth of the lesion depend on both the temperature of the probe and the time it remains in contact with the tissue; damage can be extended by generating multiple adjacent lesions or by repeat application at the same location. Because these physical factors are central to injury formation, investigators should report probe size and material, temperature, method and duration of preheating/cooling, precise anatomic location and duration of probe application, and interlesion time and number of lesions (if applicable). Cryoablation has been used to generate reproducible wounds and scar tissue for the study of myocardial injury response in various species including dogs (160, 171, 297), rabbits (6, 7, 302), rats (49, 82, 149, 190), and mice (109, 204, 257, 289, 305, 307). In mice, survival rate after cryoinjury was nearly twice that of permanent coronary ligation over an 8-wk period (307), whereas dysfunction was similar. Lower mortality may be a consequence of smaller infarct size. Ablative methodologies have also been used in nonmammalian species such as zebrafish, to probe the response of cardiac electrical properties to injury, regeneration and scar formation (43, 46, 108). The ability to destroy all cells within the cryoinfarct has provided interesting clues regarding regeneration of fetal myocardium following injury. In neonatal mice, mechanical or ischemic injuries to the ventricular apex typically trigger regeneration, producing recovery of myocardial structure and function without scarring (243, 276). Nontransmural cryoinfarcts in neonatal mice similarly heal with minimal evidence of scarring and full functional recovery with ongoing postnatal growth, while injuries spanning the full thickness of the ventricular wall do not regenerate muscle (57). Because neonatal mice can regenerate myocardium during the first postnatal week, models of myocardial ischemia in neonatal mice may be used to identify pathways involved in cardiac regeneration (8, 208).
As with coronary ligation models, evaluation of LV geometry and function with echocardiography and assessment of electrophysiological remodeling and arrhythmia risk are routinely performed in cryoinjury models. Given the early time course and different mechanisms of necrotic injury in cryoinjury versus ligation models, cryoinjury studies are typically more focused on long-term myocardial regeneration or mechanical/electrophysiological remodeling rather than mechanisms of acute postinjury cell death, inflammation, and scar formation (52, 160). Ablation procedures are now used commonly in clinical electrophysiology, and it is therefore not surprising that a number of experimental electrophysiology studies have taken advantage of geometric control provided by this model (54, 231). Cryoinjury and ischemic injury differ in their transmural localization and the amount of surviving myocardium (52, 109, 257, 258, 305). Finally, methods for ablative targeting of cardiac neural tissues have proven useful in studies of the role of autonomic inputs in normal and arrhythmic hearts (254).
To summarize, the major strengths and limitations of the cryoinjury model are shown in Table 1.
Cardioprotection
Model rationale and variables measured.
An intervention that is cardioprotective is broadly defined as serving to protect the heart (https://www.merriam-webster.com/dictionary/cardioprotective), thereby in theory encompassing all of the aforementioned aspects of cardiac damage and dysfunction. To date, the only clinically established cardioprotective intervention is early reperfusion (119, 133). The archetypal additive cardioprotective intervention is, without question, ischemic conditioning, encompassing the phenomena of ischemic preconditioning, postconditioning, and remote conditioning (118, 175, 224, 247, 248, 329). Despite differences in the timing of the protective stimulus (with preconditioning applied in a prophylactic manner and postconditioning administered at the time of reperfusion) and the site of the protective trigger (either locally, or, for remote conditioning, in a tissue or organ distant from the at-risk myocardium), all three forms of conditioning share a common theme: there is overwhelming agreement that ischemic preconditioning, postconditioning, and remote conditioning render the heart resistant to lethal I/R-induced injury (78, 118, 128, 163, 247).
This consensus with regard to ischemic conditioning and cardioprotection is, however, a notable exception in the field. Indeed, for the vast majority of the innumerable cardioprotective strategies that have been investigated, the current preclinical literature on the topic of cardioprotection is fraught with controversies and a lack of reproducibility among investigators and laboratories (163, 188). The ensuing confusion in the field may be attributed in part to two confounding factors: an overly broad use of the term cardioprotection in some studies, together with false positive and false negative outcomes derived from protocols executed in a suboptimal manner (162, 163, 188).
In some instances, a broad and suitably framed definition of cardioprotection incorporating, for example, endothelial integrity and vascular function, is appropriate (126, 225). A generally acknowledged and more narrowly focused hallmark of cardioprotection is defined as an agent or intervention that, when administered in the setting of ischemia/reperfusion, augments myocardial salvage and reduces myocardial infarct size beyond that achieved by reperfusion alone (128). Accordingly, for our purposes, we focus on cardiomyocyte viability and define cardioprotection as a strategy that attenuates cardiomyocyte death. Cardiomyocyte viability can be assessed in a full spectrum of models, ranging from cardiomyocytes in culture to isolated buffer-perfused hearts to in vivo studies in rodents (mice and rats) or larger animals (including rabbits, dogs, pigs, sheep, and, in a small number of studies, primates). There is no ideal model that completely mirrors the clinical scenario to fully ensure absolute translational relevance. Rather, each model has merits and disadvantages (as shown in Table 3).
Table 3.
Recommendations for cardioprotection studies
| Model | Gold Standard Primary End Point | Required Covariates | Potential Secondary End points | Advantages | Limitations |
|---|---|---|---|---|---|
| Cultured cardiomyocytes | Cell viability (live-dead assay) | None | Types of cell death (i.e., apoptosis), mitochondrial function; ROS production | Capacity for high throughput | Reductionist |
| Isolated buffer-perfused hearts (mouse, rat, and rabbit) | Infarct size (TTC) | For models of regional ischemia: area at risk | Measures of LV function and coronary flow; cTn as a secondary index of cardiac injury | Throughput higher than in vivo; eliminates confounding effect of intervention on systemic blood vessels | Reductionist |
| Mouse | Infarct size (TTC) | Area at risk; hemodynamics | Measures of LV function; measures of no reflow; CK or cTn as secondary indexes of cardiac injury | Availability of genetically modified strains; model of low collateral flow (measurement of RMBF not required) | Small size; differences between strains; substantial variability requiring large n values |
| Rat and rabbit | Infarct size (TTC) | Area at risk; hemodynamics | Measures of LV function; measures of no reflow; CK or cTn as secondary index of cardiac injury | Reliable infarct production; relatively high survival rate in experienced hands; commercial availability of strains that have comorbidities; model of low collateral flow (measurement of RMBF not required) | Not high throughput |
| Dog | Infarct size (TTC) | Collateral blood flow; area at risk; hemodynamics | Measures of LV function; measures of no reflow; CK or cTn as secondary indexes of cardiac injury | Large amount of historical data; shows effect of intervention in the setting of variable collateral flow; mimics humans with high collateral flow | High cost; variability in collateral perfusion (RMBF must be measured); potential for lethal arrhythmias; not high throughput |
| Pig | Infarct size (TTC) | Area at risk; hemodynamics | Measures of LV function; measures of no reflow; CK or cTn as secondary indexes of cardiac injury | Model of low collateral flow (measurement of RMBF not required); mimics humans with low collateral flow | High cost; high incidence of lethal arrhythmias; not high throughput |
TTC, triphenyltetrazolium chloride; LV, left ventricular; CK, creatine kinase; cTn, cardiac troponin; RMBF, regional myocardial blood flow.
The overwhelming strength of the mouse species is the availability of genetically modified strains to elucidate molecular mechanisms once candidate cardioprotective strategies have been identified, a benefit that is balanced by inherent variability and resultant requirement for large sample sizes. An additional problem of the mouse is the atypical geometry of nontransmural infarcts, in which the subendocardium is spared from death by diffusion of oxygen from the LV cavity and occupies an inordinate proportion of the total LV wall thickness. In all rodents, heart rate is much higher and therefore infarct development is much faster than in larger mammals and humans. Studies conducted in large animals (in particular, the pig) are considered to have the greatest potential for preclinical relevance (103, 140). This advantage is accompanied by substantial costs incurred and, particularly in pigs, the well-documented high incidence of lethal ventricular arrhythmias (282).
In all studies focused on cardioprotection, the primary end point must be a quantitative assessment of cardiomyocyte viability. In cell culture models, these data may be obtained using a live-dead assay such as trypan blue exclusion, propidium iodide exclusion, or other commercially available assays. In intact hearts, including isolated buffer-perfused hearts and all in vivo models, the gold standard end point is myocardial infarct size by TTC staining and, at later time points, histopathologic analysis (79, 81). For models involving reperfusion, the area at risk must be quantified and infarct size must be expressed as a proportion of the risk region. With global rather than regional ischemia/reperfusion, the entire heart is rendered at risk and thus infarct size is appropriately expressed as a proportion of the total ventricular area. The duration of ischemia must be sufficient to cause significant infarction but not complete death of at-risk cardiomyocytes in the control cohort. That is, if the duration of ischemia is selected such that infarct size in controls is either inordinately small or excessively large, the scope for salvage and probability of achieving cardioprotection with any intervention is negligible. Irrespective of the model used, the protocol must involve I/R (or, in cell culture models, H/R) rather than ischemia (or hypoxia) alone. This requirement reflects the fact that even the most powerful and well-established cardioprotective strategies such as ischemic preconditioning simply delay, rather than prevent, the progression to cardiomyocyte death and infarction (224, 324). The duration of reperfusion must be sufficient to allow for the accurate and unambiguous delineation of necrotic and viable myocardium. This is of particular importance when infarct size is quantified using TTC staining: a minimum of 1–2 h of reperfusion is considered mandatory in rodent hearts, while longer periods of at least 3 h are standard in large animal models (283).
Attention must be paid to essential covariates and possible cofounders. Important considerations for all in vivo models include body temperature, the choice of anesthetics and analgesics, and changes in the determinants of myocardial oxygen supply and demand, all of which are well recognized to have profound effects on infarct size (111, 247). Particular care must be taken to avoid the possibility of inadvertent preconditioning: examples include triggering a protective phenotype by unintentionally subjecting the myocardium to brief periods of hypoxia or ischemia during surgical preparation, or intentionally imposing a period of ischemia in an effort to identify the extent of the at-risk myocardium (180). Additional covariates and confounders are model specific. In rodents, age, sex, and strain of the animals have all been implicated or identified to influence myocardial infarct size (10, 111, 306) Circadian variation, the time of day at which experiments are performed, may also be important (16, 28, 110, 266). The canine model is known for its variability in the magnitude of collateral blood flow. Accordingly, when using this model, measurement of regional myocardial blood flow during coronary artery occlusion and incorporation of collateral flow as a covariate in the analysis of infarct size are mandatory (64, 284).
Finally, the overwhelming majority of studies conducted to date have assessed the efficacy of candidate cardioprotective strategies using healthy, juvenile, or adult animals. There is evidence that the infarct-sparing effect of these purportedly protective interventions may be lost or attenuated in the setting of clinically relevant comorbidities, including aging, type 1 and type 2 diabetes, hypercholesterolemia, and hypertension, and may be influenced by diet or exercise (3, 4, 51, 78, 115, 117, 146, 150, 186, 197, 246, 249, 321). Accordingly, once proof of principle is established, it is imperative that promising cardioprotective therapies be reevaluated, adhering to the essential elements of rigor described above, in comorbid models (127). All protocols must include concurrent and appropriate control cohorts. For example, when potential cardioprotective drugs are evaluated, controls must receive matched volumes of vehicle administered in an identical manner.
THE ISSUE OF TRANSLATION: TOWARD A RANDOMIZED CONTROLLED STUDY OR TRIAL DESIGN
There is currently no established intervention, aside from timely reperfusion, that limits damage to hearts of patients experiencing myocardial ischemia to the extent that clinical outcome is improved (133, 138). Discussions of prior failures and hope for future successes have been reviewed elsewhere (120, 127, 176). Several elements missing from preclinical studies of infarct size reduction have been identified and include absence of critical investigator blinding, statistical weaknesses (underpowered studies), and insufficient methodological detail. These deficiencies explain in part the failure to translate preclinical results into effective infarct-sparing treatments in patients. Thus, many have questioned the reproducibility of interventions to protect from MI (i.e., reduce infarct size).
Much of the lack of reproducibility has been ascribed to limited or lacking scientific rigor (29, 250). In response to these and other concerns, the United States National Institutes of Health now includes explicit requirements for applicants to show, and reviewers to evaluate, the level of scientific rigor in grant applications. Whether suboptimal rigor fully explains and underlies the reproducibility crisis is unclear (162). Nonetheless, advocating for reproducibility and scientific rigor is welcome.
Appropriate statistical issues should be considered before initiating an infarct-sparing intervention. Investigators should know the standard deviation of their primary, prespecified end point, such as infarct size, chamber dimension, or ejection fraction. A power analysis for the primary end point will determine and justify the number of subjects to be enrolled in each group. This may not be feasible when investigating an entirely innovative strategy as there may be no basis for an estimation of the expected effect size. The standard deviation of the investigator’s most recent blinded study can be used to determine group size (229). The choice of statistical analyses must be appropriate for the study design (300). For two-group studies, t-tests (or nonparametric equivalent) may be used; for protocols involving multiple cohorts, ANOVA (or nonparametric equivalent) is mandatory. Analysis of covariance, assessing the effects including variations in risk region and collateral blood flow, may also be applied.
Blinded assignment of animals and randomization to control or treated groups is mandatory whenever possible. Incorporation of randomization with blinding is an easy and logical approach. For testing classical drug-based interventions or when comparing mutant mice, individuals responsible for blinding can use block randomization and label tubes or mice with a simple unique alphanumeric code. The surgeon performing the protocol should have no knowledge of the intervention or genotype. We recommend that the same surgeon perform all surgeries; if multiple surgeons are used, equal numbers from all groups should be matched across the surgeon pool. It is imperative that neither the individual analyzing infarct size, nor the individual performing any other secondary analyses, knows the intervention or genotype until all data are compiled. If the potential for excluding animals exists, this should be done based on previously declared exclusion and inclusion criteria, and all decisions should be made before disclosure of group assignment; details of such exclusions should be made clear in any publications. Table 4 shows ARRIVE guidelines for manuscript submission, modified to focus on ischemia studies.
Table 4.
Checklist of considerations for rigor and reproducibility, modified from the ARRIVE Guidelines (168)
| Item | Details |
|---|---|
| Ethical statements | Institutional Animal Care and Use Committee approval, Guide for the Care and Use of Laboratory Animals, welfare assessments and interventions |
| Animal description, housing, husbandry | Species, strain, source, age (mean and range), sex, genotypes, body weight (mean and range), health/immune status, housing type (specific pathogen free), cage type, bedding material, number of cage companions, light-dark cycle, room temperature and humidity, food type, food and water access |
| Study design | Define model used; define groups; matching, randomization, and blinding protocols; order of treatment and assessment of groups; method to confirm model success; inclusion/exclusion criteria (e.g., lower limit for infarct size); daily monitor to record time and cause of death; define timing of perioperative and postoperative phases for survival analysis, flow chart for complex designs |
| Experimental procedures | Define area examined (e.g., infarct, remote, both regions); positive and negative controls; for drugs: formulation, dose, site, and route of administration; anesthesia and analgesic use and pain monitoring; surgical procedure details and monitoring records (e.g., electrocardiogram, heart rate, and anesthesia depth); method of euthanasia; time of day performed |
| Sample sizes | Number of animals used per group for each experiment; sample size calculation; number of independent replicates for cell culture studies |
| Variables measured | Primary and secondary end points, list any animals or samples removed from analysis with reason |
| Statistics | Methods used for each analysis; test for assumptions |
ARRIVE, Animals in Research: Reporting In Vivo Experiments.
The Consortium for Preclinical Assessment of Cardioprotective Therapies (CAESAR) endeavored to address the primary issues related to reproducibility in cardioprotection studies (163). Performing the same protocol at multiple sites and in multiple species was extraordinarily challenging; the most notable were challenges in identifying the underlying explanations for differences in infarct size (or other variables) between centers while they were implementing consonant protocols. Interestingly, there were several instances of mice being ordered simultaneously from the same vendor, only to have significantly different body weights at the time of study (despite being fed the same chow). To be clear, the weight differences were relatively small but on occasion were significantly different for reasons unknown. It is likely that small differences, such as this, could theoretically affect the results (or the perception of not being able to reproduce studies) of published studies. During the CAESAR experience, numerous seemingly extraneous factors were considered, such as differences in municipal water source, room temperature, relative humidity, type of lighting, traffic in the room, and other seemingly innocuous details that may or may not affect the responsiveness of mice to an infarct-sparing regimen. All of these variables reflect inherent challenges in performing in vivo studies.
More important than slight differences in body weight or other such ancillary factors were initial challenges in generating equivalent infarct sizes at different institutions, despite using the same protocol. Such occasional variations emphasize the unequivocal requirement in all protocols for concurrent and appropriate controls (see Cardioprotection).
OVERALL DISCUSSION AND CONCLUSIONS
As highlighted throughout these guidelines, ischemia and I/R have multiple consequences that show temporal variation in terms of both incidence and influence on outcomes and may be model dependent. Examples range from acute effects (including biochemical perturbations in cardiomyocytes and other cardiac cell types, disruption in cardiac conduction and development of arrhythmias, contractile dysfunction, abnormalities in endothelial, and vascular reactivity) to longer term outcomes (such as cardiomyocyte death, microvascular obstruction and no-reflow, scar healing, and LV remodeling) and, ultimately, major adverse cardiac events, including heart failure and death (136). Figure 1 shows the diversity in models available to assess ischemia across its spectrum, and Tables 4 and 5 show general recommendations for rigor and reproducibility in ischemia studies.
Table 5.
Recommendations for ischemia studies
| Common | Experimental design should follow ARRIVE guidelines (see Table 4) |
| Cardiomyocytes | When comparing groups, geometry and function end points by echocardiography assessment may not change. This does not necessarily indicate no effect of the intervention. |
| Control groups can be shared across studies to reduce animal use, as long as the samples are collected within the same timeframe, under identical conditions, and details are provided in the methods. Previously collected historical controls should be avoided or clearly indicated. | |
| For time-course studies, sham surgery can be replaced by day 0 negative controls if minimally invasive procedures are used, which greatly reduces animal use. | |
| Use to discern direct cardiomyocyte effects and responses | |
| Isolated perfused hearts | Use to discern cardiac effects and responses |
| Angina, stunning, hibernation, and ischemic cardiomyopathy | Use to reflect a particular clinical scenario |
| MI | Use nonreperfused or reperfused MI to study repair and remodeling |
| Use nonreperfused MI to test interventions in a robust remodeling model and to test interventions in a model clinically relevant to the nonreperfused patient | |
| Use reperfused MI to test interventions in a model clinically relevant to the reperfused patient | |
| Use reperfused MI to study cardioprotection | |
| Essential to measure infarct size for nonreperfused MI and infarct size and area at risk for reperfused MI | |
| Ablation | Use to control size, shape, or location |
| Use to achieve maximal and uniform cell death in the target region | |
| Use to investigate mechanisms of action of corresponding to clinical ablation technique | |
| While not suited to study MI pathophysiology, is well suited to study repair and regeneration | |
| Essential to quantify transmural extent of damage, to assess transmural variation | |
| Essential to recognize that transmural extent of the lesion may evolve over time | |
| Essential to standardize experimental protocol (e.g., probe temperature and contact time) to achieve consistent lesions | |
| Cardioprotection | Use to evaluate potentially protective strategies in the ischemia-reperfusion model |
| Essential to measure infarct size and area at risk |
ARRIVE, Animals in Research: Reporting In Vivo Experiments; MI, myocardial infarction.
While writing these guidelines, the authors discussed whether an algorithm to define the choice of model for a given scientific question would be helpful. The consensus was that the topic of myocardial ischemia and infarction and the many clinical manifestations of coronary artery disease resulting in and from myocardial ischemia or infarction are so broad and so complex that we find ourselves unable to provide an algorithm that truly covers all potential scientific approaches. Indeed, such an algorithm may be used by regulatory and funding agencies to actually limit research in the field, which would be counterproductive to the goals of this document.
The approach used will vary depending on the questions being addressed; as such, all of the approaches described above may be considered good and a gold standard if appropriate to address the target hypothesis. In vitro studies using isolated cardiomyocytes or even isolated organelles (e.g., mitochondria) are well suited to identify single molecular targets of injury and protection (102). Isolated, buffer-perfused heart models can be used to study the acute biochemical and functional mechanisms of myocardial I/R injury and cardioprotection. Due to the need for stable stenosis and the required spatial resolution of regional myocardial blood flow and contractile function measurements, large animal preparations are recommended as models for the clinical manifestations of chronic stable angina and coronary microembolization. In vivo preparations are required to study more long-term effects of myocardial I/R and respective therapeutic interventions. We distinguish between permanent occlusion MI and reperfused MI models and also highlight measurements in common. Permanent coronary occlusion MI and reperfused MI models are both well suited to study repair and remodeling. I/R models are mandatory to study cardioprotection, and the ischemia must be of sufficient severity and duration to cause some infarction. Cryo-/thermoinjury is not suited to study the pathophysiology of MI but well suited to study repair and regeneration processes.
Prospective planning of study design, i.e., randomization for appropriate control versus treatment, blinding of investigators (as much as possible for a given experimental protocol and the subsequent data analysis), and adequate statistics, is mandatory for reproducibility of all experimental models of myocardial I/R and infarction. As larger data sets are being acquired, consideration for how to harness big data should be given (272, 274). Compiling databases to incorporate results from across studies and across laboratories will provide a means to use epidemiological approaches or big data tools to validate published findings, generate novel hypotheses, and assess individual variability in response to ischemia.
In conclusion, these guidelines provide recommendations to help the investigator plan and execute a full range of studies involving myocardial ischemia and infarction.
GRANTS
We acknowledge support from the following: National Institutes of Health Grants HL-002066, HL-051971, HL-056728, HL-061610, HL-075360, HL-078825, HL-088533, HL-092141, HL-093579, HL-107153, HL-111600, HL-112730, HL-112831, HL-113452, HL-113530, HL-116449, HL-128135, HL-129120, HL-129823, HL-130266, HL-131647, HL-132075, HL-135772, GM-103492, HL-131647, HL-76246, HL-85440, GM-104357, GM-114833, GM-115428, and UL1-TR-001412; American Heart Association Grants 16GRNT30960054 and 16CSA28880004; Grand Challenge Award; Department of Defense Grants 16W81XWH-16–1-0592, PR151051, PR151134, and PR151029; Biomedical Laboratory Research and Development Service of the Veterans Affairs Office of Research and Development Awards 1IO1BX002659 and I01BX000505; Australian National Health and Medical Research Council Research Fellowship APP1043026; Bundesministerium für Bildung und Forschung Grant BMBF01 EO1004; and German Research Foundation Grants DFG He 1320/18-3 and SFB 1116 B8. R. A. Kloner reports grant support from Stealth Biotherapeutics, Servier, Inc., and Faraday to test experimental compounds in experimental myocardial infarction models.
DISCLAIMERS
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, American Heart Association, United States Department of Defense, United States Veterans Administration, National Health and Medical Research Council, or German Research Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.L.L. and G.H. conceived and designed research; M.L.L., R.G.G., K.P., C.M.R., and G.H. prepared figures; M.L.L., R.B., J.M.C., X.-J.D., N.G.F., S.F., R.G.G., J.W.H., S.P.J., R.K., D.J.L., R.L., E.M., P.P., K.P., F.A.R., L.S.L., C.M.R., J.E.V.E., and G.H. drafted manuscript; M.L.L., R.B., J.M.C., X.-J.D., N.G.F., S.F., R.G.G., J.W.H., S.P.J., R.K., D.J.L., R.L., E.M., P.P., K.P., F.A.R., L.S.L., C.M.R., J.E.V.E., and G.H. edited and revised manuscript; M.L.L., R.B., J.M.C., X.-J.D., N.G.F., S.F., R.G.G., J.W.H., S.P.J., R.K., D.J.L., R.L., E.M., P.P., K.P., F.A.R., L.S.L., C.M.R., J.E.V.E., and G.H. approved final version of manuscript.
REFERENCES
- 1.Ajijola OA, Lux RL, Khahera A, Kwon O, Aliotta E, Ennis DB, Fishbein MC, Ardell JL, Shivkumar K. Sympathetic modulation of electrical activation in normal and infarcted myocardium: implications for arrhythmogenesis. Am J Physiol Heart Circ Physiol 312: H608–H621, 2017. doi: 10.1152/ajpheart.00575.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Algranati D, Kassab GS, Lanir Y. Why is the subendocardium more vulnerable to ischemia? A new paradigm. Am J Physiol Heart Circ Physiol 300: H1090–H1100, 2011. doi: 10.1152/ajpheart.00473.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alleman RJ, Tsang AM, Ryan TE, Patteson DJ, McClung JM, Spangenburg EE, Shaikh SR, Neufer PD, Brown DA. Exercise-induced protection against reperfusion arrhythmia involves stabilization of mitochondrial energetics. Am J Physiol Heart Circ Physiol 310: H1360–H1370, 2016. doi: 10.1152/ajpheart.00858.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andres AM, Kooren JA, Parker SJ, Tucker KC, Ravindran N, Ito BR, Huang C, Venkatraman V, Van Eyk JE, Gottlieb RA, Mentzer RM Jr. Discordant signaling and autophagy response to fasting in hearts of obese mice: implications for ischemia tolerance. Am J Physiol Heart Circ Physiol 311: H219–H228, 2016. doi: 10.1152/ajpheart.00041.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aronsen JM, Espe EK, Skårdal K, Hasic A, Zhang L, Sjaastad I. Noninvasive stratification of postinfarction rats based on the degree of cardiac dysfunction using magnetic resonance imaging and echocardiography. Am J Physiol Heart Circ Physiol 312: H932–H942, 2017. doi: 10.1152/ajpheart.00668.2016. [DOI] [PubMed] [Google Scholar]
- 6.Atkins BZ, Hueman MT, Meuchel J, Hutcheson KA, Glower DD, Taylor DA. Cellular cardiomyoplasty improves diastolic properties of injured heart. J Surg Res 85: 234–242, 1999. doi: 10.1006/jsre.1999.5681. [DOI] [PubMed] [Google Scholar]
- 7.Atkins BZ, Hueman MT, Meuchel JM, Cottman MJ, Hutcheson KA, Taylor DA. Myogenic cell transplantation improves in vivo regional performance in infarcted rabbit myocardium. J Heart Lung Transplant 18: 1173–1180, 1999. doi: 10.1016/S1053-2498(99)00096-0. [DOI] [PubMed] [Google Scholar]
- 8.Aurora AB, Porrello ER, Tan W, Mahmoud AI, Hill JA, Bassel-Duby R, Sadek HA, Olson EN. Macrophages are required for neonatal heart regeneration. J Clin Invest 124: 1382–1392, 2014. doi: 10.1172/JCI72181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bache RJ, Arentzen CE, Simon AB, Vrobel TR. Abnormalities in myocardial perfusion during tachycardia in dogs with left ventricular hypertrophy: metabolic evidence for myocardial ischemia. Circulation 69: 409–417, 1984. doi: 10.1161/01.CIR.69.2.409. [DOI] [PubMed] [Google Scholar]
- 10.Baker JE, Konorev EA, Gross GJ, Chilian WM, Jacob HJ. Resistance to myocardial ischemia in five rat strains: is there a genetic component of cardioprotection? Am J Physiol Heart Circ Physiol 278: H1395–H1400, 2000. doi: 10.1152/ajpheart.2000.278.4.H1395. [DOI] [PubMed] [Google Scholar]
- 11.Barlow SC, Doviak H, Jacobs J, Freeburg LA, Perreault PE, Zellars KN, Moreau K, Villacreses CF, Smith S, Khakoo AY, Lee T, Spinale FG. Intracoronary delivery of recombinant TIMP-3 after myocardial infarction: effects on myocardial remodeling and function. Am J Physiol Heart Circ Physiol 313: H690–H699, 2017. doi: 10.1152/ajpheart.00114.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bauer M, Kang L, Qiu Y, Wu J, Peng M, Chen HH, Camci-Unal G, Bayomy AF, Sosnovik DE, Khademhosseini A, Liao R. Adult cardiac progenitor cell aggregates exhibit survival benefit both in vitro and in vivo. PLoS One 7: e50491, 2012. doi: 10.1371/journal.pone.0050491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Becerra R, Román B, Di Carlo MN, Mariangelo JI, Salas M, Sanchez G, Donoso P, Schinella GR, Vittone L, Wehrens XH, Mundiña-Weilenmann C, Said M. Reversible redox modifications of ryanodine receptor ameliorate ventricular arrhythmias in the ischemic-reperfused heart. Am J Physiol Heart Circ Physiol 311: H713–H724, 2016. doi: 10.1152/ajpheart.00142.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bell RM, Mocanu MM, Yellon DM. Retrograde heart perfusion: the Langendorff technique of isolated heart perfusion. J Mol Cell Cardiol 50: 940–950, 2011. doi: 10.1016/j.yjmcc.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 15.Beltrami CA, Finato N, Rocco M, Feruglio GA, Puricelli C, Cigola E, Quaini F, Sonnenblick EH, Olivetti G, Anversa P. Structural basis of end-stage failure in ischemic cardiomyopathy in humans. Circulation 89: 151–163, 1994. doi: 10.1161/01.CIR.89.1.151. [DOI] [PubMed] [Google Scholar]
- 16.Bennardo M, Alibhai F, Tsimakouridze E, Chinnappareddy N, Podobed P, Reitz C, Pyle WG, Simpson J, Martino TA. Day-night dependence of gene expression and inflammatory responses in the remodeling murine heart post-myocardial infarction. Am J Physiol Regul Integr Comp Physiol 311: R1243–R1254, 2016. doi: 10.1152/ajpregu.00200.2016. [DOI] [PubMed] [Google Scholar]
- 17.Bolli R. Mechanism of myocardial “stunning”. Circulation 82: 723–738, 1990. doi: 10.1161/01.CIR.82.3.723. [DOI] [PubMed] [Google Scholar]
- 18.Bolli R, Bhatti ZA, Tang XL, Qiu Y, Zhang Q, Guo Y, Jadoon AK. Evidence that late preconditioning against myocardial stunning in conscious rabbits is triggered by the generation of nitric oxide. Circ Res 81: 42–52, 1997. doi: 10.1161/01.RES.81.1.42. [DOI] [PubMed] [Google Scholar]
- 19.Bolli R, Marbán E. Molecular and cellular mechanisms of myocardial stunning. Physiol Rev 79: 609–634, 1999. doi: 10.1152/physrev.1999.79.2.609. [DOI] [PubMed] [Google Scholar]
- 20.Bolli R, Zhu WX, Thornby JI, O’Neill PG, Roberts R. Time course and determinants of recovery of function after reversible ischemia in conscious dogs. Am J Physiol Heart Circ Physiol 254: H102–H114, 1988. [DOI] [PubMed] [Google Scholar]
- 21.Bolli R, Zughaib M, Li XY, Tang XL, Sun JZ, Triana JF, McCay PB. Recurrent ischemia in the canine heart causes recurrent bursts of free radical production that have a cumulative effect on contractile function. A pathophysiological basis for chronic myocardial “stunning”. J Clin Invest 96: 1066–1084, 1995. doi: 10.1172/JCI118093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boyle MP, Weisman HF. Limitation of infarct expansion and ventricular remodeling by late reperfusion. Study of time course and mechanism in a rat model. Circulation 88: 2872–2883, 1993. doi: 10.1161/01.CIR.88.6.2872. [DOI] [PubMed] [Google Scholar]
- 23.Braunwald E, Rutherford JD. Reversible ischemic left ventricular dysfunction: evidence for the “hibernating myocardium”. J Am Coll Cardiol 8: 1467–1470, 1986. doi: 10.1016/S0735-1097(86)80325-4. [DOI] [PubMed] [Google Scholar]
- 24.Brodarac A, Šarić T, Oberwallner B, Mahmoodzadeh S, Neef K, Albrecht J, Burkert K, Oliverio M, Nguemo F, Choi YH, Neiss WF, Morano I, Hescheler J, Stamm C. Susceptibility of murine induced pluripotent stem cell-derived cardiomyocytes to hypoxia and nutrient deprivation. Stem Cell Res Ther 6: 83, 2015. doi: 10.1186/s13287-015-0057-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buckberg GD, Fixler DE, Archie JP, Hoffman JI. Experimental subendocardial ischemia in dogs with normal coronary arteries. Circ Res 30: 67–81, 1972. doi: 10.1161/01.RES.30.1.67. [DOI] [PubMed] [Google Scholar]
- 26.Bujak M, Dobaczewski M, Chatila K, Mendoza LH, Li N, Reddy A, Frangogiannis NG. Interleukin-1 receptor type I signaling critically regulates infarct healing and cardiac remodeling. Am J Pathol 173: 57–67, 2008. doi: 10.2353/ajpath.2008.070974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bujak M, Kweon HJ, Chatila K, Li N, Taffet G, Frangogiannis NG. Aging-related defects are associated with adverse cardiac remodeling in a mouse model of reperfused myocardial infarction. J Am Coll Cardiol 51: 1384–1392, 2008. doi: 10.1016/j.jacc.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bulluck H, Nicholas J, Crimi G, White SK, Ludman AJ, Pica S, Raineri C, Cabrera-Fuentes HA, Yellon D, Rodriguez-Palomares J, Garcia-Dorado D, Hausenloy DJ. Circadian variation in acute myocardial infarct size assessed by cardiovascular magnetic resonance in reperfused STEMI patients. Int J Cardiol 230: 149–154, 2017. doi: 10.1016/j.ijcard.2016.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bustin SA, Huggett JF. Reproducibility of biomedical research–the importance of editorial vigilance. Biomol Detect Quantif 11: 1–3, 2017. doi: 10.1016/j.bdq.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bux AS, Lindsey ML, Vasquez HG, Taegtmeyer H, Harmancey R. Glucose regulates the intrinsic inflammatory response of the heart to surgically induced hypothermic ischemic arrest and reperfusion. Physiol Genomics 49: 37–52, 2017. doi: 10.1152/physiolgenomics.00102.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Canfield SG, Sepac A, Sedlic F, Muravyeva MY, Bai X, Bosnjak ZJ. Marked hyperglycemia attenuates anesthetic preconditioning in human-induced pluripotent stem cell-derived cardiomyocytes. Anesthesiology 117: 735–744, 2012. doi: 10.1097/ALN.0b013e3182655e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Canton M, Skyschally A, Menabò R, Boengler K, Gres P, Schulz R, Haude M, Erbel R, Di Lisa F, Heusch G. Oxidative modification of tropomyosin and myocardial dysfunction following coronary microembolization. Eur Heart J 27: 875–881, 2006. doi: 10.1093/eurheartj/ehi751. [DOI] [PubMed] [Google Scholar]
- 33.Canty JM., Jr Coronary pressure-function and steady-state pressure-flow relations during autoregulation in the unanesthetized dog. Circ Res 63: 821–836, 1988. doi: 10.1161/01.RES.63.4.821. [DOI] [PubMed] [Google Scholar]
- 34.Canty JM Jr, Fallavollita JA. Resting myocardial flow in hibernating myocardium: validating animal models of human pathophysiology. Am J Physiol Heart Circ Physiol 277: H417–H422, 1999. [DOI] [PubMed] [Google Scholar]
- 35.Canty JM Jr, Giglia J, Kandath D. Effect of tachycardia on regional function and transmural myocardial perfusion during graded coronary pressure reduction in conscious dogs. Circulation 82: 1815–1825, 1990. doi: 10.1161/01.CIR.82.5.1815. [DOI] [PubMed] [Google Scholar]
- 36.Canty JM Jr, Klocke FJ. Reduced regional myocardial perfusion in the presence of pharmacologic vasodilator reserve. Circulation 71: 370–377, 1985. doi: 10.1161/01.CIR.71.2.370. [DOI] [PubMed] [Google Scholar]
- 37.Canty JM Jr, Klocke FJ. Reductions in regional myocardial function at rest in conscious dogs with chronically reduced regional coronary artery pressure. Circ Res 61: II107–II116, 1987. [PubMed] [Google Scholar]
- 38.Canty JM Jr, Mates RE. A programmable pressure control system for coronary flow studies. Am J Physiol Heart Circ Physiol 243: H796–H802, 1982. [DOI] [PubMed] [Google Scholar]
- 39.Canty JM Jr, Smith TP Jr. Adenosine-recruitable flow reserve is absent during myocardial ischemia in unanesthetized dogs studied in the basal state. Circ Res 76: 1079–1087, 1995. doi: 10.1161/01.RES.76.6.1079. [DOI] [PubMed] [Google Scholar]
- 40.Canty JM Jr, Suzuki G, Banas MD, Verheyen F, Borgers M, Fallavollita JA. Hibernating myocardium: chronically adapted to ischemia but vulnerable to sudden death. Circ Res 94: 1142–1149, 2004. doi: 10.1161/01.RES.0000125628.57672.CF. [DOI] [PubMed] [Google Scholar]
- 41.Canty JM Jr, Duncker DJ. Coronary blood flow and myocardial ischemia. In: Braunwald’s Heart Disease, edited by Mann DL, Zipes DP, Libby P, Bonow RO. Philadelphia, PA: Elsevier, 2014, p. 1029–1056. [Google Scholar]
- 42.Canty JM Jr, Suzuki G. Myocardial perfusion and contraction in acute ischemia and chronic ischemic heart disease. J Mol Cell Cardiol 52: 822–831, 2012. doi: 10.1016/j.yjmcc.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cao H, Yu F, Zhao Y, Zhang X, Tai J, Lee J, Darehzereshki A, Bersohn M, Lien CL, Chi NC, Tai YC, Hsiai TK. Wearable multi-channel microelectrode membranes for elucidating electrophysiological phenotypes of injured myocardium. Integr Biol 6: 789–795, 2014. doi: 10.1039/C4IB00052H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Capasso JM, Li P, Anversa P. Nonischemic myocardial damage induced by nonocclusive constriction of coronary artery in rats. Am J Physiol Heart Circ Physiol 260: H651–H661, 1991. [DOI] [PubMed] [Google Scholar]
- 45.Capasso JM, Malhotra A, Li P, Zhang X, Scheuer J, Anversa P. Chronic nonocclusive coronary artery constriction impairs ventricular function, myocardial structure, and cardiac contractile protein enzyme activity in rats. Circ Res 70: 148–162, 1992. doi: 10.1161/01.RES.70.1.148. [DOI] [PubMed] [Google Scholar]
- 46.Chablais F, Jaźwińska A. Induction of myocardial infarction in adult zebrafish using cryoinjury. J Vis Exp 2012: 3666, 2012. doi: 10.3791/3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chan W, White DA, Wang XY, Bai RF, Liu Y, Yu HY, Zhang YY, Fan F, Schneider HG, Duffy SJ, Taylor AJ, Du XJ, Gao W, Gao XM, Dart AM. Macrophage migration inhibitory factor for the early prediction of infarct size. J Am Heart Assoc 2: e000226, 2013. doi: 10.1161/JAHA.113.000226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen C, Chen L, Fallon JT, Ma L, Li L, Bow L, Knibbs D, McKay R, Gillam LD, Waters DD. Functional and structural alterations with 24-hour myocardial hibernation and recovery after reperfusion. A pig model of myocardial hibernation. Circulation 94: 507–516, 1996. doi: 10.1161/01.CIR.94.3.507. [DOI] [PubMed] [Google Scholar]
- 49.Cho SW, Gwak SJ, Kim IK, Cho MC, Hwang KK, Kwon JS, Choi CY, Yoo KJ, Kim BS. Granulocyte colony-stimulating factor treatment enhances the efficacy of cellular cardiomyoplasty with transplantation of embryonic stem cell-derived cardiomyocytes in infarcted myocardium. Biochem Biophys Res Commun 340: 573–582, 2006. doi: 10.1016/j.bbrc.2005.12.044. [DOI] [PubMed] [Google Scholar]
- 50.Christia P, Bujak M, Gonzalez-Quesada C, Chen W, Dobaczewski M, Reddy A, Frangogiannis NG. Systematic characterization of myocardial inflammation, repair, and remodeling in a mouse model of reperfused myocardial infarction. J Histochem Cytochem 61: 555–570, 2013. doi: 10.1369/0022155413493912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chung Y. Myocardial Po2 does not limit aerobic metabolism in the postischemic heart. Am J Physiol Heart Circ Physiol 310: H226–H238, 2016. doi: 10.1152/ajpheart.00335.2015. [DOI] [PubMed] [Google Scholar]
- 52.Ciulla MM, Paliotti R, Ferrero S, Braidotti P, Esposito A, Gianelli U, Busca G, Cioffi U, Bulfamante G, Magrini F. Left ventricular remodeling after experimental myocardial cryoinjury in rats. J Surg Res 116: 91–97, 2004. doi: 10.1016/j.jss.2003.08.238. [DOI] [PubMed] [Google Scholar]
- 53.Cohen M, Boiangiu C, Abidi M. Therapy for ST-segment elevation myocardial infarction patients who present late or are ineligible for reperfusion therapy. J Am Coll Cardiol 55: 1895–1906, 2010. doi: 10.1016/j.jacc.2009.11.087. [DOI] [PubMed] [Google Scholar]
- 54.Costa AR, Panda NC, Yong S, Mayorga ME, Pawlowski GP, Fan K, Penn MS, Laurita KR. Optical mapping of cryoinjured rat myocardium grafted with mesenchymal stem cells. Am J Physiol Heart Circ Physiol 302: H270–H277, 2012. doi: 10.1152/ajpheart.00019.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cullen LA, Mills NL, Mahler S, Body R. Early rule-out and rule-in strategies for myocardial infarction. Clin Chem 63: 129–139, 2017. doi: 10.1373/clinchem.2016.254730. [DOI] [PubMed] [Google Scholar]
- 56.Curtis MJ, Hancox JC, Farkas A, Wainwright CL, Stables CL, Saint DA, Clements-Jewery H, Lambiase PD, Billman GE, Janse MJ, Pugsley MK, Ng GA, Roden DM, Camm AJ, Walker MJ. The Lambeth Conventions (II): guidelines for the study of animal and human ventricular and supraventricular arrhythmias. Pharmacol Ther 139: 213–248, 2013. doi: 10.1016/j.pharmthera.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 57.Darehzereshki A, Rubin N, Gamba L, Kim J, Fraser J, Huang Y, Billings J, Mohammadzadeh R, Wood J, Warburton D, Kaartinen V, Lien CL. Differential regenerative capacity of neonatal mouse hearts after cryoinjury. Dev Biol 399: 91–99, 2015. doi: 10.1016/j.ydbio.2014.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Date T, Belanger AJ, Mochizuki S, Sullivan JA, Liu LX, Scaria A, Cheng SH, Gregory RJ, Jiang C. Adenovirus-mediated expression of p35 prevents hypoxia/reoxygenation injury by reducing reactive oxygen species and caspase activity. Cardiovasc Res 55: 309–319, 2002. doi: 10.1016/S0008-6363(02)00412-1. [DOI] [PubMed] [Google Scholar]
- 59.De Jesus NM, Wang L, Lai J, Rigor RR, Francis Stuart SD, Bers DM, Lindsey ML, Ripplinger CM. Anti-arrhythmic effects of interleukin-1 inhibition following myocardial infarction. Heart Rhythm 14: 727–736, 2017. doi: 10.1016/j.hrthm.2017.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.DeLeon-Pennell KY, de Castro Brás LE, Iyer RP, Bratton DR, Jin YF, Ripplinger CM, Lindsey ML. P. gingivalis lipopolysaccharide intensifies inflammation post-myocardial infarction through matrix metalloproteinase-9. J Mol Cell Cardiol 76: 218–226, 2014. doi: 10.1016/j.yjmcc.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.DeLeon-Pennell KY, Tian Y, Zhang B, Cates CA, Iyer RP, Cannon P, Shah P, Aiyetan P, Halade GV, Ma Y, Flynn E, Zhang Z, Jin YF, Zhang H, Lindsey ML. CD36 is a matrix metalloproteinase-9 substrate that stimulates neutrophil apoptosis and removal during cardiac remodeling. Circ Cardiovasc Genet 9: 14–25, 2016. doi: 10.1161/CIRCGENETICS.115.001249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Depre C, Kim SJ, John AS, Huang Y, Rimoldi OE, Pepper JR, Dreyfus GD, Gaussin V, Pennell DJ, Vatner DE, Camici PG, Vatner SF. Program of cell survival underlying human and experimental hibernating myocardium. Circ Res 95: 433–440, 2004. doi: 10.1161/01.RES.0000138301.42713.18. [DOI] [PubMed] [Google Scholar]
- 63.Dewald O, Frangogiannis NG, Zoerlein M, Duerr GD, Klemm C, Knuefermann P, Taffet G, Michael LH, Crapo JD, Welz A, Entman ML. Development of murine ischemic cardiomyopathy is associated with a transient inflammatory reaction and depends on reactive oxygen species. Proc Natl Acad Sci USA 100: 2700–2705, 2003. doi: 10.1073/pnas.0438035100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dewald O, Ren G, Duerr GD, Zoerlein M, Klemm C, Gersch C, Tincey S, Michael LH, Entman ML, Frangogiannis NG. Of mice and dogs: species-specific differences in the inflammatory response following myocardial infarction. Am J Pathol 164: 665–677, 2004. doi: 10.1016/S0002-9440(10)63154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dewald O, Zymek P, Winkelmann K, Koerting A, Ren G, Abou-Khamis T, Michael LH, Rollins BJ, Entman ML, Frangogiannis NG. CCL2/monocyte chemoattractant protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ Res 96: 881–889, 2005. doi: 10.1161/01.RES.0000163017.13772.3a. [DOI] [PubMed] [Google Scholar]
- 66.Domenech RJ, Hoffman JI, Noble MI, Saunders KB, Henson JR, Subijanto S. Total and regional coronary blood flow measured by radioactive microspheres in conscious and anesthetized dogs. Circ Res 25: 581–596, 1969. doi: 10.1161/01.RES.25.5.581. [DOI] [PubMed] [Google Scholar]
- 67.Dörge H, Neumann T, Behrends M, Skyschally A, Schulz R, Kasper C, Erbel R, Heusch G. Perfusion-contraction mismatch with coronary microvascular obstruction: role of inflammation. Am J Physiol Heart Circ Physiol 279: H2587–H2592, 2000. doi: 10.1152/ajpheart.2000.279.6.H2587. [DOI] [PubMed] [Google Scholar]
- 68.Downey JM, Kirk ES. Inhibition of coronary blood flow by a vascular waterfall mechanism. Circ Res 36: 753–760, 1975. doi: 10.1161/01.RES.36.6.753. [DOI] [PubMed] [Google Scholar]
- 69.Du CK, Zhan DY, Akiyama T, Inagaki T, Shishido T, Shirai M, Pearson JT. Myocardial interstitial levels of serotonin and its major metabolite 5-hydroxyindole acetic acid during ischemia-reperfusion. Am J Physiol Heart Circ Physiol 312: H60–H67, 2017. doi: 10.1152/ajpheart.00471.2016. [DOI] [PubMed] [Google Scholar]
- 70.Du XJ, Cox HS, Dart AM, Esler MD. Sympathetic activation triggers ventricular arrhythmias in rat heart with chronic infarction and failure. Cardiovasc Res 43: 919–929, 1999. doi: 10.1016/S0008-6363(99)00139-X. [DOI] [PubMed] [Google Scholar]
- 71.Dutta P, Hoyer FF, Grigoryeva LS, Sager HB, Leuschner F, Courties G, Borodovsky A, Novobrantseva T, Ruda VM, Fitzgerald K, Iwamoto Y, Wojtkiewicz G, Sun Y, Da Silva N, Libby P, Anderson DG, Swirski FK, Weissleder R, Nahrendorf M. Macrophages retain hematopoietic stem cells in the spleen via VCAM-1. J Exp Med 212: 497–512, 2015. doi: 10.1084/jem.20141642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dutta P, Hoyer FF, Sun Y, Iwamoto Y, Tricot B, Weissleder R, Magnani JL, Swirski FK, Nahrendorf M. E-selectin inhibition mitigates splenic HSC activation and myelopoiesis in hypercholesterolemic mice with myocardial infarction. Arterioscler Thromb Vasc Biol 36: 1802–1808, 2016. doi: 10.1161/ATVBAHA.116.307519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fallavollita JA, Canty JM Jr. Differential 18F-2-deoxyglucose uptake in viable dysfunctional myocardium with normal resting perfusion: evidence for chronic stunning in pigs. Circulation 99: 2798–2805, 1999. doi: 10.1161/01.CIR.99.21.2798. [DOI] [PubMed] [Google Scholar]
- 74.Fallavollita JA, Canty JM Jr. Ischemic cardiomyopathy in pigs with two-vessel occlusion and viable, chronically dysfunctional myocardium. Am J Physiol Heart Circ Physiol 282: H1370–H1379, 2002. doi: 10.1152/ajpheart.00138.2001. [DOI] [PubMed] [Google Scholar]
- 75.Fallavollita JA, Logue M, Canty JM Jr. Stability of hibernating myocardium in pigs with a chronic left anterior descending coronary artery stenosis: absence of progressive fibrosis in the setting of stable reductions in flow, function and coronary flow reserve. J Am Coll Cardiol 37: 1989–1995, 2001. doi: 10.1016/S0735-1097(01)01250-5. [DOI] [PubMed] [Google Scholar]
- 76.Fallavollita JA, Malm BJ, Canty JM Jr. Hibernating myocardium retains metabolic and contractile reserve despite regional reductions in flow, function, and oxygen consumption at rest. Circ Res 92: 48–55, 2003. doi: 10.1161/01.RES.0000049104.57549.03. [DOI] [PubMed] [Google Scholar]
- 77.Fallavollita JA, Perry BJ, Canty JM Jr. 18F-2-deoxyglucose deposition and regional flow in pigs with chronically dysfunctional myocardium. Evidence for transmural variations in chronic hibernating myocardium. Circulation 95: 1900–1909, 1997. doi: 10.1161/01.CIR.95.7.1900. [DOI] [PubMed] [Google Scholar]
- 78.Ferdinandy P, Hausenloy DJ, Heusch G, Baxter GF, Schulz R. Interaction of risk factors, comorbidities, and comedications with ischemia/reperfusion injury and cardioprotection by preconditioning, postconditioning, and remote conditioning. Pharmacol Rev 66: 1142–1174, 2014. doi: 10.1124/pr.113.008300. [DOI] [PubMed] [Google Scholar]
- 79.Ferrera R, Benhabbouche S, Bopassa JC, Li B, Ovize M. One hour reperfusion is enough to assess function and infarct size with TTC staining in Langendorff rat model. Cardiovasc Drugs Ther 23: 327–331, 2009. doi: 10.1007/s10557-009-6176-5. [DOI] [PubMed] [Google Scholar]
- 80.Firoozan S, Wei K, Linka A, Skyba D, Goodman NC, Kaul S. A canine model of chronic ischemic cardiomyopathy: characterization of regional flow-function relations. Am J Physiol Heart Circ Physiol 276: H446–H455, 1999. [DOI] [PubMed] [Google Scholar]
- 81.Fishbein MC, Meerbaum S, Rit J, Lando U, Kanmatsuse K, Mercier JC, Corday E, Ganz W. Early phase acute myocardial infarct size quantification: validation of the triphenyl tetrazolium chloride tissue enzyme staining technique. Am Heart J 101: 593–600, 1981. doi: 10.1016/0002-8703(81)90226-X. [DOI] [PubMed] [Google Scholar]
- 82.Fomovsky GM, Rouillard AD, Holmes JW. Regional mechanics determine collagen fiber structure in healing myocardial infarcts. J Mol Cell Cardiol 52: 1083–1090, 2012. doi: 10.1016/j.yjmcc.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Francis Stuart SD, De Jesus NM, Lindsey ML, Ripplinger CM. The crossroads of inflammation, fibrosis, and arrhythmia following myocardial infarction. J Mol Cell Cardiol 91: 114–122, 2016. doi: 10.1016/j.yjmcc.2015.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Frangogiannis NG. The immune system and the remodeling infarcted heart: cell biological insights and therapeutic opportunities. J Cardiovasc Pharmacol 63: 185–195, 2014. doi: 10.1097/FJC.0000000000000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Frangogiannis NG. Inflammation in cardiac injury, repair and regeneration. Curr Opin Cardiol 30: 240–245, 2015. doi: 10.1097/HCO.0000000000000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Frangogiannis NG. Pathophysiology of myocardial infarction. Compr Physiol 5: 1841–1875, 2015. doi: 10.1002/cphy.c150006. [DOI] [PubMed] [Google Scholar]
- 87.Frangogiannis NG, Michael LH, Entman ML. Myofibroblasts in reperfused myocardial infarcts express the embryonic form of smooth muscle myosin heavy chain (SMemb). Cardiovasc Res 48: 89–100, 2000. doi: 10.1016/S0008-6363(00)00158-9. [DOI] [PubMed] [Google Scholar]
- 88.Frantz S, Bauersachs J, Ertl G. Post-infarct remodelling: contribution of wound healing and inflammation. Cardiovasc Res 81: 474–481, 2009. doi: 10.1093/cvr/cvn292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Frey A, Saxon VM, Popp S, Lehmann M, Mathes D, Pachel C, Hofmann U, Ertl G, Lesch KP, Frantz S. Early citalopram treatment increases mortality due to left ventricular rupture in mice after myocardial infarction. J Mol Cell Cardiol 98: 28–36, 2016. doi: 10.1016/j.yjmcc.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 90.Galaup A, Gomez E, Souktani R, Durand M, Cazes A, Monnot C, Teillon J, Le Jan S, Bouleti C, Briois G, Philippe J, Pons S, Martin V, Assaly R, Bonnin P, Ratajczak P, Janin A, Thurston G, Valenzuela DM, Murphy AJ, Yancopoulos GD, Tissier R, Berdeaux A, Ghaleh B, Germain S. Protection against myocardial infarction and no-reflow through preservation of vascular integrity by angiopoietin-like 4. Circulation 125: 140–149, 2012. doi: 10.1161/CIRCULATIONAHA.111.049072. [DOI] [PubMed] [Google Scholar]
- 91.Gallagher KP, Matsuzaki M, Koziol JA, Kemper WS, Ross J Jr. Regional myocardial perfusion and wall thickening during ischemia in conscious dogs. Am J Physiol Heart Circ Physiol 247: H727–H738, 1984. [DOI] [PubMed] [Google Scholar]
- 92.Gallagher KP, Matsuzaki M, Osakada G, Kemper WS, Ross J Jr. Effect of exercise on the relationship between myocardial blood flow and systolic wall thickening in dogs with acute coronary stenosis. Circ Res 52: 716–729, 1983. doi: 10.1161/01.RES.52.6.716. [DOI] [PubMed] [Google Scholar]
- 93.Gallagher KP, Osakada G, Matsuzaki M, Kemper WS, Ross J Jr. Myocardial blood flow and function with critical coronary stenosis in exercising dogs. Am J Physiol Heart Circ Physiol 243: H698–H707, 1982. [DOI] [PubMed] [Google Scholar]
- 94.Ganote CE, Humphrey SM. Effects of anoxic or oxygenated reperfusion in globally ischemic, isovolumic, perfused rat hearts. Am J Pathol 120: 129–145, 1985. [PMC free article] [PubMed] [Google Scholar]
- 95.Gao XM, Moore XL, Liu Y, Wang XY, Han LP, Su Y, Tsai A, Xu Q, Zhang M, Lambert GW, Kiriazis H, Gao W, Dart AM, Du XJ. Splenic release of platelets contributes to increased circulating platelet size and inflammation after myocardial infarction. Clin Sci (Lond) 130: 1089–1104, 2016. doi: 10.1042/CS20160234. [DOI] [PubMed] [Google Scholar]
- 96.Gao XM, White DA, Dart AM, Du XJ. Post-infarct cardiac rupture: recent insights on pathogenesis and therapeutic interventions. Pharmacol Ther 134: 156–179, 2012. doi: 10.1016/j.pharmthera.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 97.Gao XM, Wu QZ, Kiriazis H, Su Y, Han LP, Pearson JT, Taylor AJ, Du XJ. Microvascular leakage in acute myocardial infarction: characterization by histology, biochemistry, and magnetic resonance imaging. Am J Physiol Heart Circ Physiol 312: H1068–H1075, 2017. doi: 10.1152/ajpheart.00073.2017. [DOI] [PubMed] [Google Scholar]
- 98.Gao XM, Xu Q, Kiriazis H, Dart AM, Du XJ. Mouse model of post-infarct ventricular rupture: time course, strain- and gender-dependency, tensile strength, and histopathology. Cardiovasc Res 65: 469–477, 2005. doi: 10.1016/j.cardiores.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 99.Gardner RT, Habecker BA. Infarct-derived chondroitin sulfate proteoglycans prevent sympathetic reinnervation after cardiac ischemia-reperfusion injury. J Neurosci 33: 7175–7183, 2013. doi: 10.1523/JNEUROSCI.5866-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gardner RT, Wang L, Lang BT, Cregg JM, Dunbar CL, Woodward WR, Silver J, Ripplinger CM, Habecker BA. Targeting protein tyrosine phosphatase σ after myocardial infarction restores cardiac sympathetic innervation and prevents arrhythmias. Nat Commun 6: 6235, 2015. doi: 10.1038/ncomms7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gedik N, Krüger M, Thielmann M, Kottenberg E, Skyschally A, Frey UH, Cario E, Peters J, Jakob H, Heusch G, Kleinbongard P. Proteomics/phosphoproteomics of left ventricular biopsies from patients with surgical coronary revascularization and pigs with coronary occlusion/reperfusion: remote ischemic preconditioning. Sci Rep 7: 7629, 2017. doi: 10.1038/s41598-017-07883-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gedik N, Maciel L, Schulte C, Skyschally A, Heusch G, Kleinbongard P. Cardiomyocyte mitochondria as targets of humoral factors released by remote ischemic preconditioning. Arch Med Sci 13: 448–458, 2017. doi: 10.5114/aoms.2016.61789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gent S, Skyschally A, Kleinbongard P, Heusch G. lschemic preconditioning in pigs: a causal role for signal transducer and activator of transcription 3. Am J Physiol Heart Circ Physiol 312: H478–H484, 2017. doi: 10.1152/ajpheart.00749.2016. [DOI] [PubMed] [Google Scholar]
- 104.Gharacholou SM, Alexander KP, Chen AY, Wang TY, Melloni C, Gibler WB, Pollack CV Jr, Ohman EM, Peterson ED, Roe MT. Implications and reasons for the lack of use of reperfusion therapy in patients with ST-segment elevation myocardial infarction: findings from the CRUSADE initiative. Am Heart J 159: 757–763, 2010. doi: 10.1016/j.ahj.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 105.Gheorghiade M, Bonow RO. Chronic heart failure in the United States: a manifestation of coronary artery disease. Circulation 97: 282–289, 1998. doi: 10.1161/01.CIR.97.3.282. [DOI] [PubMed] [Google Scholar]
- 106.Girod WG, Jones SP, Sieber N, Aw TY, Lefer DJ. Effects of hypercholesterolemia on myocardial ischemia-reperfusion injury in LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol 19: 2776–2781, 1999. doi: 10.1161/01.ATV.19.11.2776. [DOI] [PubMed] [Google Scholar]
- 107.Glenny RW, Bernard S, Brinkley M. Validation of fluorescent-labeled microspheres for measurement of regional organ perfusion. J Appl Physiol 74: 2585–2597, 1993. doi: 10.1152/jappl.1993.74.5.2585. [DOI] [PubMed] [Google Scholar]
- 108.González-Rosa JM, Martín V, Peralta M, Torres M, Mercader N. Extensive scar formation and regression during heart regeneration after cryoinjury in zebrafish. Development 138: 1663–1674, 2011. doi: 10.1242/dev.060897. [DOI] [PubMed] [Google Scholar]
- 109.Grisel P, Meinhardt A, Lehr HA, Kappenberger L, Barrandon Y, Vassalli G. The MRL mouse repairs both cryogenic and ischemic myocardial infarcts with scar. Cardiovasc Pathol 17: 14–22, 2008. doi: 10.1016/j.carpath.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 110.Gumz ML. Taking into account circadian rhythm when conducting experiments on animals. Am J Physiol Renal Physiol 310: F454–F455, 2016. doi: 10.1152/ajprenal.00549.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Guo Y, Flaherty MP, Wu WJ, Tan W, Zhu X, Li Q, Bolli R. Genetic background, gender, age, body temperature, and arterial blood pH have a major impact on myocardial infarct size in the mouse and need to be carefully measured and/or taken into account: results of a comprehensive analysis of determinants of infarct size in 1,074 mice. Basic Res Cardiol 107: 288, 2012. doi: 10.1007/s00395-012-0288-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Guth BD, Heusch G, Seitelberger R, Ross J Jr. Elimination of exercise-induced regional myocardial dysfunction by a bradycardiac agent in dogs with chronic coronary stenosis. Circulation 75: 661–669, 1987. doi: 10.1161/01.CIR.75.3.661. [DOI] [PubMed] [Google Scholar]
- 113.Guth BD, Indolfi C, Heusch G, Seitelberger R, Ross J Jr. Mechanisms of benefit in the ischemic myocardium due to heart rate reduction. Basic Res Cardiol 85, Suppl 1: 157–166, 1990. [DOI] [PubMed] [Google Scholar]
- 114.Guth BD, Martin JF, Heusch G, Ross J Jr. Regional myocardial blood flow, function and metabolism using phosphorus-31 nuclear magnetic resonance spectroscopy during ischemia and reperfusion in dogs. J Am Coll Cardiol 10: 673–681, 1987. doi: 10.1016/S0735-1097(87)80212-7. [DOI] [PubMed] [Google Scholar]
- 115.Halade GV, Kain V, Black LM, Prabhu SD, Ingle KA. Aging dysregulates D- and E-series resolvins to modulate cardiosplenic and cardiorenal network following myocardial infarction. Aging (Albany NY) 8: 2611–2634, 2016. doi: 10.18632/aging.101077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Halade GV, Kain V, Ingle KA. Heart functional and structural compendium of cardiosplenic and cardiorenal networks in acute and chronic heart failure pathology. Am J Physiol Heart Circ Physiol 314: H255−H267, 2018. doi: 10.1152/ajpheart.00528.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Halade GV, Kain V, Ingle KA, Prabhu SD. Interaction of 12/15-lipoxygenase with fatty acids alters the leukocyte kinetics leading to improved postmyocardial infarction healing. Am J Physiol Heart Circ Physiol 313: H89−H102, 2017. doi: 10.1152/ajpheart.00040.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hausenloy DJ, Barrabes JA, Bøtker HE, Davidson SM, Di Lisa F, Downey J, Engstrom T, Ferdinandy P, Carbrera-Fuentes HA, Heusch G, Ibanez B, Iliodromitis EK, Inserte J, Jennings R, Kalia N, Kharbanda R, Lecour S, Marber M, Miura T, Ovize M, Perez-Pinzon MA, Piper HM, Przyklenk K, Schmidt MR, Redington A, Ruiz-Meana M, Vilahur G, Vinten-Johansen J, Yellon DM, Garcia-Dorado D. Ischaemic conditioning and targeting reperfusion injury: a 30 year voyage of discovery. Basic Res Cardiol 111: 70, 2016. doi: 10.1007/s00395-016-0588-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hausenloy DJ, Bøtker HE, Engstrom T, Erlinge D, Heusch G, Ibanez B, Kloner RA, Ovize M, Yellon DM, Garcia-Dorado D. Targeting reperfusion injury in patients with ST-segment elevation myocardial infarction: trials and tribulations. Eur Heart J 38: 935–941, 2017. doi: 10.1093/eurheartj/ehw145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hausenloy DJ, Garcia-Dorado D, Bøtker HE, Davidson SM, Downey J, Engel FB, Jennings R, Lecour S, Leor J, Madonna R, Ovize M, Perrino C, Prunier F, Schulz R, Sluijter JPG, Van Laake LW, Vinten-Johansen J, Yellon DM, Ytrehus K, Heusch G, Ferdinandy P. Novel targets and future strategies for acute cardioprotection: Position Paper of the European Society of Cardiology Working Group on Cellular Biology of the Heart. Cardiovasc Res 113: 564–585, 2017. doi: 10.1093/cvr/cvx049. [DOI] [PubMed] [Google Scholar]
- 121.Hausenloy DJ, Yellon DM. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J Clin Invest 123: 92–100, 2013. doi: 10.1172/JCI62874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hawkins HK, Entman ML, Zhu JY, Youker KA, Berens K, Doré M, Smith CW. Acute inflammatory reaction after myocardial ischemic injury and reperfusion. Development and use of a neutrophil-specific antibody. Am J Pathol 148: 1957–1969, 1996. [PMC free article] [PubMed] [Google Scholar]
- 123.Heaberlin JR, Ma Y, Zhang J, Ahuja SS, Lindsey ML, Halade GV. Obese and diabetic KKAy mice show increased mortality but improved cardiac function following myocardial infarction. Cardiovasc Pathol 22: 481–487, 2013. doi: 10.1016/j.carpath.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Heinzel FR, Luo Y, Dodoni G, Boengler K, Petrat F, Di Lisa F, de Groot H, Schulz R, Heusch G. Formation of reactive oxygen species at increased contraction frequency in rat cardiomyocytes. Cardiovasc Res 71: 374–382, 2006. doi: 10.1016/j.cardiores.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 125.Heinzel FR, Luo Y, Li X, Boengler K, Buechert A, García-Dorado D, Di Lisa F, Schulz R, Heusch G. Impairment of diazoxide-induced formation of reactive oxygen species and loss of cardioprotection in connexin 43 deficient mice. Circ Res 97: 583–586, 2005. doi: 10.1161/01.RES.0000181171.65293.65. [DOI] [PubMed] [Google Scholar]
- 126.Heusch G. The coronary circulation as a target of cardioprotection. Circ Res 118: 1643–1658, 2016. doi: 10.1161/CIRCRESAHA.116.308640. [DOI] [PubMed] [Google Scholar]
- 127.Heusch G. Critical issues for the translation of cardioprotection. Circ Res 120: 1477–1486, 2017. doi: 10.1161/CIRCRESAHA.117.310820. [DOI] [PubMed] [Google Scholar]
- 128.Heusch G. Molecular basis of cardioprotection: signal transduction in ischemic pre-, post-, and remote conditioning. Circ Res 116: 674–699, 2015. doi: 10.1161/CIRCRESAHA.116.305348. [DOI] [PubMed] [Google Scholar]
- 129.Heusch G. Myocardial ischemia: lack of coronary blood flow or myocardial oxygen supply/demand imbalance? Circ Res 119: 194–196, 2016. doi: 10.1161/CIRCRESAHA.116.308925. [DOI] [PubMed] [Google Scholar]
- 130.Heusch G. The regional myocardial flow-function relationship: a framework for an understanding of acute ischemia, hibernation, stunning and coronary microembolization. 1980. Circ Res 112: 1535–1537, 2013. doi: 10.1161/CIRCRESAHA.113.301446. [DOI] [PubMed] [Google Scholar]
- 131.Heusch G, Deussen A. The effects of cardiac sympathetic nerve stimulation on perfusion of stenotic coronary arteries in the dog. Circ Res 53: 8–15, 1983. doi: 10.1161/01.RES.53.1.8. [DOI] [PubMed] [Google Scholar]
- 132.Heusch G, Deussen A, Thämer V. Cardiac sympathetic nerve activity and progressive vasoconstriction distal to coronary stenoses: feed-back aggravation of myocardial ischemia. J Auton Nerv Syst 13: 311–326, 1985. doi: 10.1016/0165-1838(85)90020-7. [DOI] [PubMed] [Google Scholar]
- 133.Heusch G, Gersh BJ. The pathophysiology of acute myocardial infarction and strategies of protection beyond reperfusion: a continual challenge. Eur Heart J 38: 774–784, 2017. [DOI] [PubMed] [Google Scholar]
- 134.Heusch G, Guth BD, Seitelberger R, Ross J Jr. Attenuation of exercise-induced myocardial ischemia in dogs with recruitment of coronary vasodilator reserve by nifedipine. Circulation 75: 482–490, 1987. doi: 10.1161/01.CIR.75.2.482. [DOI] [PubMed] [Google Scholar]
- 135.Heusch G, Kleinbongard P, Böse D, Levkau B, Haude M, Schulz R, Erbel R. Coronary microembolization: from bedside to bench and back to bedside. Circulation 120: 1822–1836, 2009. doi: 10.1161/CIRCULATIONAHA.109.888784. [DOI] [PubMed] [Google Scholar]
- 136.Heusch G, Libby P, Gersh B, Yellon D, Böhm M, Lopaschuk G, Opie L. Cardiovascular remodelling in coronary artery disease and heart failure. Lancet 383: 1933–1943, 2014. doi: 10.1016/S0140-6736(14)60107-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Heusch G, Post H, Michel MC, Kelm M, Schulz R. Endogenous nitric oxide and myocardial adaptation to ischemia. Circ Res 87: 146–152, 2000. doi: 10.1161/01.RES.87.2.146. [DOI] [PubMed] [Google Scholar]
- 138.Heusch G, Rassaf T. Time to give up on cardioprotection? A critical appraisal of clinical studies on ischemic pre-, post-, and remote conditioning. Circ Res 119: 676–695, 2016. doi: 10.1161/CIRCRESAHA.116.308736. [DOI] [PubMed] [Google Scholar]
- 139.Heusch G, Schulz R, Rahimtoola SH. Myocardial hibernation: a delicate balance. Am J Physiol Heart Circ Physiol 288: H984–H999, 2005. doi: 10.1152/ajpheart.01109.2004. [DOI] [PubMed] [Google Scholar]
- 140.Heusch G, Skyschally A, Schulz R. The in-situ pig heart with regional ischemia/reperfusion–ready for translation. J Mol Cell Cardiol 50: 951–963, 2011. doi: 10.1016/j.yjmcc.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 141.Heusch P, Nensa F, Heusch G. Is MRI really the gold standard for the quantification of salvage from myocardial infarction? Circ Res 117: 222–224, 2015. doi: 10.1161/CIRCRESAHA.117.306929. [DOI] [PubMed] [Google Scholar]
- 142.Heymann MA, Payne BD, Hoffman JIE, Rudolph AM. Blood flow measurements with radionuclide-labeled particles. Prog Cardiovasc Dis 20: 55–79, 1977. doi: 10.1016/S0033-0620(77)80005-4. [DOI] [PubMed] [Google Scholar]
- 143.Heyndrickx GR, Baig H, Nellens P, Leusen I, Fishbein MC, Vatner SF. Depression of regional blood flow and wall thickening after brief coronary occlusions. Am J Physiol Heart Circ Physiol 234: H653–H659, 1978. [DOI] [PubMed] [Google Scholar]
- 144.Homans DC, Sublett E, Dai XZ, Bache RJ. Persistence of regional left ventricular dysfunction after exercise-induced myocardial ischemia. J Clin Invest 77: 66–73, 1986. doi: 10.1172/JCI112303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.House SL, Castro AM, Lupu TS, Weinheimer C, Smith C, Kovacs A, Ornitz DM. Endothelial fibroblast growth factor receptor signaling is required for vascular remodeling following cardiac ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 310: H559–H571, 2016. doi: 10.1152/ajpheart.00758.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hunter I, Soler A, Joseph G, Hutcheson B, Bradford C, Zhang FF, Potter B, Proctor S, Rocic P. Cardiovascular function in male and female JCR:LA-cp rats: effect of high-fat/high-sucrose diet. Am J Physiol Heart Circ Physiol 312: H742–H751, 2017. doi: 10.1152/ajpheart.00535.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ibáñez B, Heusch G, Ovize M, Van de Werf F. Evolving therapies for myocardial ischemia/reperfusion injury. J Am Coll Cardiol 65: 1454–1471, 2015. doi: 10.1016/j.jacc.2015.02.032. [DOI] [PubMed] [Google Scholar]
- 148.Ibanez B, Prat-González S, Speidl WS, Vilahur G, Pinero A, Cimmino G, García MJ, Fuster V, Sanz J, Badimon JJ. Early metoprolol administration before coronary reperfusion results in increased myocardial salvage: analysis of ischemic myocardium at risk using cardiac magnetic resonance. Circulation 115: 2909–2916, 2007. doi: 10.1161/CIRCULATIONAHA.106.679639. [DOI] [PubMed] [Google Scholar]
- 149.Iop L, Chiavegato A, Callegari A, Bollini S, Piccoli M, Pozzobon M, Rossi CA, Calamelli S, Chiavegato D, Gerosa G, De Coppi P, Sartore S. Different cardiovascular potential of adult- and fetal-type mesenchymal stem cells in a rat model of heart cryoinjury. Cell Transplant 17: 679–694, 2008. doi: 10.3727/096368908786092739. [DOI] [PubMed] [Google Scholar]
- 150.Ip WT, McAlindon A, Miller SE, Bell JR, Curl CL, Huggins CE, Mellor KM, Raaijmakers AJ, Bienvenu LA, McLennan PL, Pepe S, Delbridge LM. Dietary omega-6 fatty acid replacement selectively impairs cardiac functional recovery after ischemia in female (but not male) rats. Am J Physiol Heart Circ Physiol 311: H768–H780, 2016. doi: 10.1152/ajpheart.00690.2015. [DOI] [PubMed] [Google Scholar]
- 151.Isorni MA, Casanova A, Piquet J, Bellamy V, Pignon C, Puymirat E, Menasche P. Comparative analysis of methods to induce myocardial infarction in a closed-chest rabbit model. BioMed Res Int 2015: 893051, 2015. doi: 10.1155/2015/893051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Iyer RP, de Castro Brás LE, Cannon PL, Ma Y, DeLeon-Pennell KY, Jung M, Flynn ER, Henry JB, Bratton DR, White JA, Fulton LK, Grady AW, Lindsey ML. Defining the sham environment for post-myocardial infarction studies in mice. Am J Physiol Heart Circ Physiol 311: H822–H836, 2016. doi: 10.1152/ajpheart.00067.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Iyer RP, de Castro Brás LE, Patterson NL, Bhowmick M, Flynn ER, Asher M, Cannon PL, Deleon-Pennell KY, Fields GB, Lindsey ML. Early matrix metalloproteinase-9 inhibition post-myocardial infarction worsens cardiac dysfunction by delaying inflammation resolution. J Mol Cell Cardiol 100: 109–117, 2016. doi: 10.1016/j.yjmcc.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Iyer RP, Jung M, Lindsey ML. MMP-9 signaling in the left ventricle following myocardial infarction. Am J Physiol Heart Circ Physiol 311: H190–H198, 2016. doi: 10.1152/ajpheart.00243.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Iyer RP, Patterson NL, Zouein FA, Ma Y, Dive V, de Castro Brás LE, Lindsey ML. Early matrix metalloproteinase-12 inhibition worsens post-myocardial infarction cardiac dysfunction by delaying inflammation resolution. Int J Cardiol 185: 198–208, 2015. doi: 10.1016/j.ijcard.2015.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Jennings RB, Murry CE, Steenbergen C Jr, Reimer KA. Development of cell injury in sustained acute ischemia. Circulation 82, Suppl: II2–II12, 1990. [PubMed] [Google Scholar]
- 157.Jennings RB, Reimer KA. Lethal myocardial ischemic injury. Am J Pathol 102: 241–255, 1981. [PMC free article] [PubMed] [Google Scholar]
- 158.Jennings RB, Schaper J, Hill ML, Steenbergen C Jr, Reimer KA. Effect of reperfusion late in the phase of reversible ischemic injury. Changes in cell volume, electrolytes, metabolites, and ultrastructure. Circ Res 56: 262–278, 1985. doi: 10.1161/01.RES.56.2.262. [DOI] [PubMed] [Google Scholar]
- 159.Jennings RB, Sommers HM, Smyth GA, Flack HA, Linn H. Myocardial necrosis induced by temporary occlusion of a coronary artery in the dog. Arch Pathol 70: 68–78, 1960. [PubMed] [Google Scholar]
- 160.Jensen JA, Kosek JC, Hunt TK, Goodson WH 3rd, Miller DC. Cardiac cryolesions as an experimental model of myocardial wound healing. Ann Surg 206: 798–803, 1987. doi: 10.1097/00000658-198712000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Jian Z, Chen YJ, Shimkunas R, Jian Y, Jaradeh M, Chavez K, Chiamvimonvat N, Tardiff JC, Izu LT, Ross RS, Chen-Izu Y. In vivo cannulation methods for cardiomyocytes isolation from heart disease models. PLoS One 11: e0160605, 2016. doi: 10.1371/journal.pone.0160605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Jones SP. I’ll have the rigor, but hold the mortis. Circ Res 120: 1852–1854, 2017. doi: 10.1161/CIRCRESAHA.117.311114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Jones SP, Tang XL, Guo Y, Steenbergen C, Lefer DJ, Kukreja RC, Kong M, Li Q, Bhushan S, Zhu X, Du J, Nong Y, Stowers HL, Kondo K, Hunt GN, Goodchild TT, Orr A, Chang CC, Ockaili R, Salloum FN, Bolli R. The NHLBI-sponsored Consortium for preclinicAl assESsment of cARdioprotective therapies (CAESAR): a new paradigm for rigorous, accurate, and reproducible evaluation of putative infarct-sparing interventions in mice, rabbits, and pigs. Circ Res 116: 572–586, 2015. doi: 10.1161/CIRCRESAHA.116.305462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Jorge E, Amorós-Figueras G, García-Sánchez T, Bragós R, Rosell-Ferrer J, Cinca J. Early detection of acute transmural myocardial ischemia by the phasic systolic-diastolic changes of local tissue electrical impedance. Am J Physiol Heart Circ Physiol 310: H436–H443, 2016. doi: 10.1152/ajpheart.00754.2015. [DOI] [PubMed] [Google Scholar]
- 165.Jung M, Ma Y, Iyer RP, DeLeon-Pennell KY, Yabluchanskiy A, Garrett MR, Lindsey ML. IL-10 improves cardiac remodeling after myocardial infarction by stimulating M2 macrophage polarization and fibroblast activation. Basic Res Cardiol 112: 33, 2017. doi: 10.1007/s00395-017-0622-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kang PM, Haunstetter A, Aoki H, Usheva A, Izumo S. Morphological and molecular characterization of adult cardiomyocyte apoptosis during hypoxia and reoxygenation. Circ Res 87: 118–125, 2000. doi: 10.1161/01.RES.87.2.118. [DOI] [PubMed] [Google Scholar]
- 167.Khemtong C, Carpenter NR, Lumata LL, Merritt ME, Moreno KX, Kovacs Z, Malloy CR, Sherry AD. Hyperpolarized 13C NMR detects rapid drug-induced changes in cardiac metabolism. Magn Reson Med 74: 312–319, 2015. doi: 10.1002/mrm.25419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 8: e1000412, 2010. doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kim SJ, Peppas A, Hong SK, Yang G, Huang Y, Diaz G, Sadoshima J, Vatner DE, Vatner SF. Persistent stunning induces myocardial hibernation and protection: flow/function and metabolic mechanisms. Circ Res 92: 1233–1239, 2003. doi: 10.1161/01.RES.0000076892.18394.B6. [DOI] [PubMed] [Google Scholar]
- 170.Kingery JR, Hamid T, Lewis RK, Ismahil MA, Bansal SS, Rokosh G, Townes TM, Ildstad ST, Jones SP, Prabhu SD. Leukocyte iNOS is required for inflammation and pathological remodeling in ischemic heart failure. Basic Res Cardiol 112: 19, 2017. doi: 10.1007/s00395-017-0609-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Klein GJ, Harrison L, Ideker RF, Smith WM, Kasell J, Wallace AG, Gallagher JJ. Reaction of the myocardium to cryosurgery: electrophysiology and arrhythmogenic potential. Circulation 59: 364–372, 1979. doi: 10.1161/01.CIR.59.2.364. [DOI] [PubMed] [Google Scholar]
- 172.Klein HH, Schaper J, Puschmann S, Nienaber C, Kreuzer H, Schaper W. Loss of canine myocardial nicotinamide adenine dinucleotides determines the transition from reversible to irreversible ischemic damage of myocardial cells. Basic Res Cardiol 76: 612–621, 1981. doi: 10.1007/BF01908051. [DOI] [PubMed] [Google Scholar]
- 173.Kleinbongard P, Böse D, Baars T, Möhlenkamp S, Konorza T, Schöner S, Elter-Schulz M, Eggebrecht H, Degen H, Haude M, Levkau B, Schulz R, Erbel R, Heusch G. Vasoconstrictor potential of coronary aspirate from patients undergoing stenting of saphenous vein aortocoronary bypass grafts and its pharmacological attenuation. Circ Res 108: 344–352, 2011. doi: 10.1161/CIRCRESAHA.110.235713. [DOI] [PubMed] [Google Scholar]
- 174.Kleinbongard P, Skyschally A, Gent S, Pesch M, Heusch G. STAT3 as a common signal of ischemic conditioning: a lesson on “rigor and reproducibility” in preclinical studies on cardioprotection. Basic Res Cardiol 113: 3, 2018. doi: 10.1007/s00395-017-0660-z. [DOI] [PubMed] [Google Scholar]
- 175.Kleinbongard P, Skyschally A, Heusch G. Cardioprotection by remote ischemic conditioning and its signal transduction. Pflugers Arch 469: 159–181, 2017. doi: 10.1007/s00424-016-1922-6. [DOI] [PubMed] [Google Scholar]
- 176.Kloner RA. Current state of clinical translation of cardioprotective agents for acute myocardial infarction. Circ Res 113: 451–463, 2013. doi: 10.1161/CIRCRESAHA.112.300627. [DOI] [PubMed] [Google Scholar]
- 177.Kloner RA. No-reflow phenomenon: maintaining vascular integrity. J Cardiovasc Pharmacol Ther 16: 244–250, 2011. doi: 10.1177/1074248411405990. [DOI] [PubMed] [Google Scholar]
- 178.Kloner RA, Ellis SG, Lange R, Braunwald E. Studies of experimental coronary artery reperfusion. Effects on infarct size, myocardial function, biochemistry, ultrastructure and microvascular damage. Circulation 68: I8–I15, 1983. [PubMed] [Google Scholar]
- 179.Kloner RA, Ganote CE, Jennings RB. The “no-reflow” phenomenon after temporary coronary occlusion in the dog. J Clin Invest 54: 1496–1508, 1974. doi: 10.1172/JCI107898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kloner RA, Przyklenk K, Whittaker P, Hale S. Preconditioning stimuli and inadvertent preconditioning. J Mol Cell Cardiol 27: 743–747, 1995. doi: 10.1016/0022-2828(95)90079-9. [DOI] [PubMed] [Google Scholar]
- 181.Klotz L, Norman S, Vieira JM, Masters M, Rohling M, Dubé KN, Bollini S, Matsuzaki F, Carr CA, Riley PR. Cardiac lymphatics are heterogeneous in origin and respond to injury. Nature 522: 62–67, 2015. doi: 10.1038/nature14483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kolwicz SC Jr, Purohit S, Tian R. Cardiac metabolism and its interactions with contraction, growth, and survival of cardiomyocytes. Circ Res 113: 603–616, 2013. doi: 10.1161/CIRCRESAHA.113.302095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Koudstaal S, Jansen of Lorkeers S, Gho JM, van Hout GP, Jansen MS, Grundeman PF, Pasterkamp G, Doevendans PA, Hoefer IE, Chamuleau SA. Myocardial infarction and functional outcome assessment in pigs. J Vis Exp 86: e51269, 2014. doi: 10.3791/51269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kowallik P, Schulz R, Guth BD, Schade A, Paffhausen W, Gross R, Heusch G. Measurement of regional myocardial blood flow with multiple colored microspheres. Circulation 83: 974–982, 1991. doi: 10.1161/01.CIR.83.3.974. [DOI] [PubMed] [Google Scholar]
- 185.Kudej RK, Ghaleh B, Sato N, Shen YT, Bishop SP, Vatner SF. Ineffective perfusion-contraction matching in conscious, chronically instrumented pigs with an extended period of coronary stenosis. Circ Res 82: 1199–1205, 1998. doi: 10.1161/01.RES.82.11.1199. [DOI] [PubMed] [Google Scholar]
- 186.Lal N, Chiu AP, Wang F, Zhang D, Jia J, Wan A, Vlodavsky I, Hussein B, Rodrigues B. Loss of VEGFB and its signaling in the diabetic heart is associated with increased cell death signaling. Am J Physiol Heart Circ Physiol 312: H1163–H1175, 2017. doi: 10.1152/ajpheart.00659.2016. [DOI] [PubMed] [Google Scholar]
- 187.Lautamäki R, Schuleri KH, Sasano T, Javadi MS, Youssef A, Merrill J, Nekolla SG, Abraham MR, Lardo AC, Bengel FM. Integration of infarct size, tissue perfusion, and metabolism by hybrid cardiac positron emission tomography/computed tomography: evaluation in a porcine model of myocardial infarction. Circ Cardiovasc Imaging 2: 299–305, 2009. doi: 10.1161/CIRCIMAGING.108.846253. [DOI] [PubMed] [Google Scholar]
- 188.Lefer DJ, Bolli R. Development of an NIH consortium for preclinicAl AssESsment of CARdioprotective therapies (CAESAR): a paradigm shift in studies of infarct size limitation. J Cardiovasc Pharmacol Ther 16: 332–339, 2011. doi: 10.1177/1074248411414155. [DOI] [PubMed] [Google Scholar]
- 189.Li B, Li Q, Wang X, Jana KP, Redaelli G, Kajstura J, Anversa P. Coronary constriction impairs cardiac function and induces myocardial damage and ventricular remodeling in mice. Am J Physiol Heart Circ Physiol 273: H2508–H2519, 1997. [DOI] [PubMed] [Google Scholar]
- 190.Li RK, Jia ZQ, Weisel RD, Mickle DA, Zhang J, Mohabeer MK, Rao V, Ivanov J. Cardiomyocyte transplantation improves heart function. Ann Thorac Surg 62: 654–660, discussion 660–651, 1996. [DOI] [PubMed] [Google Scholar]
- 191.Li S, Zhong S, Zeng K, Luo Y, Zhang F, Sun X, Chen L. Blockade of NF-kappaB by pyrrolidine dithiocarbamate attenuates myocardial inflammatory response and ventricular dysfunction following coronary microembolization induced by homologous microthrombi in rats. Basic Res Cardiol 105: 139–150, 2010. doi: 10.1007/s00395-009-0067-6. [DOI] [PubMed] [Google Scholar]
- 192.Liao R, Podesser BK, Lim CC. The continuing evolution of the Langendorff and ejecting murine heart: new advances in cardiac phenotyping. Am J Physiol Heart Circ Physiol 303: H156–H167, 2012. doi: 10.1152/ajpheart.00333.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lim H, Fallavollita JA, Hard R, Kerr CW, Canty JM Jr. Profound apoptosis-mediated regional myocyte loss and compensatory hypertrophy in pigs with hibernating myocardium. Circulation 100: 2380–2386, 1999. doi: 10.1161/01.CIR.100.23.2380. [DOI] [PubMed] [Google Scholar]
- 194.Lindsey ML, Iyer RP, Jung M, DeLeon-Pennell KY, Ma Y. Matrix metalloproteinases as input and output signals for post-myocardial infarction remodeling. J Mol Cell Cardiol 91: 134–140, 2016. doi: 10.1016/j.yjmcc.2015.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lindsey ML, Iyer RP, Zamilpa R, Yabluchanskiy A, DeLeon-Pennell KY, Hall ME, Kaplan A, Zouein FA, Bratton D, Flynn ER, Cannon PL, Tian Y, Jin YF, Lange RA, Tokmina-Roszyk D, Fields GB, de Castro Brás LE. A novel collagen matricryptin reduces left ventricular dilation post-myocardial infarction by promoting scar formation and angiogenesis. J Am Coll Cardiol 66: 1364–1374, 2015. doi: 10.1016/j.jacc.2015.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Lindsey ML, Kassiri Z, Virag JA, de Castro Brás LE, Scherrer-Crosbie M. Guidelines for measuring cardiac physiology in mice. Am J Physiol Heart Circ Physiol. doi: 10.1152/ajpheart.00339.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Liu J, Wang P, Douglas SL, Tate JM, Sham S, Lloyd SG. Impact of high-fat, low-carbohydrate diet on myocardial substrate oxidation, insulin sensitivity, and cardiac function after ischemia-reperfusion. Am J Physiol Heart Circ Physiol 311: H1–H10, 2016. doi: 10.1152/ajpheart.00809.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Liu SQ, Ma XL, Qin G, Liu Q, Li YC, Wu YH. Trans-system mechanisms against ischemic myocardial injury. Compr Physiol 5: 167–192, 2015. doi: 10.1002/cphy.c140026. [DOI] [PubMed] [Google Scholar]
- 199.Liu SQ, Tefft BJ, Roberts DT, Zhang LQ, Ren Y, Li YC, Huang Y, Zhang D, Phillips HR, Wu YH. Cardioprotective proteins upregulated in the liver in response to experimental myocardial ischemia. Am J Physiol Heart Circ Physiol 303: H1446–H1458, 2012. doi: 10.1152/ajpheart.00362.2012. [DOI] [PubMed] [Google Scholar]
- 200.Long X, Boluyt MO, Hipolito ML, Lundberg MS, Zheng JS, O’Neill L, Cirielli C, Lakatta EG, Crow MT. p53 and the hypoxia-induced apoptosis of cultured neonatal rat cardiac myocytes. J Clin Invest 99: 2635–2643, 1997. doi: 10.1172/JCI119452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Lopaschuk GD, Jaswal JS. Energy metabolic phenotype of the cardiomyocyte during development, differentiation, and postnatal maturation. J Cardiovasc Pharmacol 56: 130–140, 2010. doi: 10.1097/FJC.0b013e3181e74a14. [DOI] [PubMed] [Google Scholar]
- 202.Lopez EF, Kabarowski JH, Ingle KA, Kain V, Barnes S, Crossman DK, Lindsey ML, Halade GV. Obesity superimposed on aging magnifies inflammation and delays the resolving response after myocardial infarction. Am J Physiol Heart Circ Physiol 308: H269–H280, 2015. doi: 10.1152/ajpheart.00604.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Luo M, Guan X, Luczak ED, Lang D, Kutschke W, Gao Z, Yang J, Glynn P, Sossalla S, Swaminathan PD, Weiss RM, Yang B, Rokita AG, Maier LS, Efimov IR, Hund TJ, Anderson ME. Diabetes increases mortality after myocardial infarction by oxidizing CaMKII. J Clin Invest 123: 1262–1274, 2013. doi: 10.1172/JCI65268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Ma N, Ladilov Y, Moebius JM, Ong L, Piechaczek C, Dávid A, Kaminski A, Choi YH, Li W, Egger D, Stamm C, Steinhoff G. Intramyocardial delivery of human CD133+ cells in a SCID mouse cryoinjury model: Bone marrow vs. cord blood-derived cells. Cardiovasc Res 71: 158–169, 2006. doi: 10.1016/j.cardiores.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 205.Ma Y, Iyer RP, Jung M, Czubryt MP, Lindsey ML. Cardiac fibroblast activation post-myocardial infarction: current knowledge gaps. Trends Pharmacol Sci 38: 448–458, 2017. doi: 10.1016/j.tips.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Ma Y, Yabluchanskiy A, Iyer RP, Cannon PL, Flynn ER, Jung M, Henry J, Cates CA, Deleon-Pennell KY, Lindsey ML. Temporal neutrophil polarization following myocardial infarction. Cardiovasc Res 110: 51–61, 2016. doi: 10.1093/cvr/cvw024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Maddaford TG, Hurtado C, Sobrattee S, Czubryt MP, Pierce GN. A model of low-flow ischemia and reperfusion in single, beating adult cardiomyocytes. Am J Physiol 277: H788–H798, 1999. [DOI] [PubMed] [Google Scholar]
- 208.Mahmoud AI, Porrello ER, Kimura W, Olson EN, Sadek HA. Surgical models for cardiac regeneration in neonatal mice. Nat Protoc 9: 305–311, 2014. doi: 10.1038/nprot.2014.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Martin C, Schulz R, Post H, Boengler K, Kelm M, Kleinbongard P, Gres P, Skyschally A, Konietzka I, Heusch G. Microdialysis-based analysis of interstitial NO in situ: NO synthase-independent NO formation during myocardial ischemia. Cardiovasc Res 74: 46–55, 2007. doi: 10.1016/j.cardiores.2006.12.020. [DOI] [PubMed] [Google Scholar]
- 210.Martin C, Schulz R, Rose J, Heusch G. Inorganic phosphate content and free energy change of ATP hydrolysis in regional short-term hibernating myocardium. Cardiovasc Res 39: 318–326, 1998. doi: 10.1016/S0008-6363(98)00086-8. [DOI] [PubMed] [Google Scholar]
- 211.Masoud WG, Ussher JR, Wang W, Jaswal JS, Wagg CS, Dyck JR, Lygate CA, Neubauer S, Clanachan AS, Lopaschuk GD. Failing mouse hearts utilize energy inefficiently and benefit from improved coupling of glycolysis and glucose oxidation. Cardiovasc Res 101: 30–38, 2014. doi: 10.1093/cvr/cvt216. [DOI] [PubMed] [Google Scholar]
- 212.Matsuzaki M, Gallagher KP, Kemper WS, White F, Ross J Jr. Sustained regional dysfunction produced by prolonged coronary stenosis: gradual recovery after reperfusion. Circulation 68: 170–182, 1983. doi: 10.1161/01.CIR.68.1.170. [DOI] [PubMed] [Google Scholar]
- 213.Matsuzaki M, Gallagher KP, Patritti J, Tajimi T, Kemper WS, White FC, Ross J Jr. Effects of a calcium-entry blocker (diltiazem) on regional myocardial flow and function during exercise in conscious dogs. Circulation 69: 801–814, 1984. doi: 10.1161/01.CIR.69.4.801. [DOI] [PubMed] [Google Scholar]
- 214.Matsuzaki M, Guth B, Tajimi T, Kemper WS, Ross J Jr. Effect of the combination of diltiazem and atenolol on exercise-induced regional myocardial ischemia in conscious dogs. Circulation 72: 233–243, 1985. doi: 10.1161/01.CIR.72.1.233. [DOI] [PubMed] [Google Scholar]
- 215.Matsuzaki M, Patritti J, Tajimi T, Miller M, Kemper WS, Ross J Jr. Effects of β-blockade on regional myocardial flow and function during exercise. Am J Physiol Heart Circ Physiol 247: H52–H60, 1984. [DOI] [PubMed] [Google Scholar]
- 216.Maxwell MP, Hearse DJ, Yellon DM. Species variation in the coronary collateral circulation during regional myocardial ischaemia: a critical determinant of the rate of evolution and extent of myocardial infarction. Cardiovasc Res 21: 737–746, 1987. doi: 10.1093/cvr/21.10.737. [DOI] [PubMed] [Google Scholar]
- 217.Mayorga M, Kiedrowski M, Shamhart P, Forudi F, Weber K, Chilian WM, Penn MS, Dong F. Early upregulation of myocardial CXCR4 expression is critical for dimethyloxalylglycine-induced cardiac improvement in acute myocardial infarction. Am J Physiol Heart Circ Physiol 310: H20–H28, 2016. doi: 10.1152/ajpheart.00449.2015. [DOI] [PubMed] [Google Scholar]
- 218.McCall FC, Telukuntla KS, Karantalis V, Suncion VY, Heldman AW, Mushtaq M, Williams AR, Hare JM. Myocardial infarction and intramyocardial injection models in swine. Nat Protoc 7: 1479–1496, 2012. doi: 10.1038/nprot.2012.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Meschiari CA, Jung M, Iyer RP, Yabluchanskiy A, Toba H, Garrett MR, Lindsey ML. Macrophage overexpression of matrix metalloproteinase-9 in aged mice improves diastolic physiology and cardiac wound healing after myocardial infarction. Am J Physiol Heart Circ Physiol 314: H224−H235, 2018. doi: 10.1152/ajpheart.00453.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Meyer C, Scherschel K. Ventricular tachycardia in ischemic heart disease: the sympathetic heart and its scars. Am J Physiol Heart Circ Physiol 312: H549–H551, 2017. doi: 10.1152/ajpheart.00061.2017. [DOI] [PubMed] [Google Scholar]
- 221.Michael LH, Ballantyne CM, Zachariah JP, Gould KE, Pocius JS, Taffet GE, Hartley CJ, Pham TT, Daniel SL, Funk E, Entman ML. Myocardial infarction and remodeling in mice: effect of reperfusion. Am J Physiol Heart Circ Physiol 277: H660–H668, 1999. [DOI] [PubMed] [Google Scholar]
- 222.Michael LH, Entman ML, Hartley CJ, Youker KA, Zhu J, Hall SR, Hawkins HK, Berens K, Ballantyne CM. Myocardial ischemia and reperfusion: a murine model. Am J Physiol Heart Circ Physiol 269: H2147–H2154, 1995. [DOI] [PubMed] [Google Scholar]
- 223.Morrissey PJ, Murphy KR, Daley JM, Schofield L, Turan NN, Arunachalam K, Abbott JD, Koren G. A novel method of standardized myocardial infarction in aged rabbits. Am J Physiol Heart Circ Physiol 312: H959–H967, 2017. doi: 10.1152/ajpheart.00582.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation 74: 1124–1136, 1986. doi: 10.1161/01.CIR.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 225.Nakanishi K, Vinten-Johansen J, Lefer DJ, Zhao Z, Fowler WC 3rd, McGee DS, Johnston WE. Intracoronary l-arginine during reperfusion improves endothelial function and reduces infarct size. Am J Physiol Heart Circ Physiol 263: H1650–H1658, 1992. [DOI] [PubMed] [Google Scholar]
- 226.Neely JR, Liebermeister H, Battersby EJ, Morgan HE. Effect of pressure development on oxygen consumption by isolated rat heart. Am J Physiol Heart Circ Physiol 212: 804–814, 1967. [DOI] [PubMed] [Google Scholar]
- 227.Negoro S, Kunisada K, Fujio Y, Funamoto M, Darville MI, Eizirik DL, Osugi T, Izumi M, Oshima Y, Nakaoka Y, Hirota H, Kishimoto T, Yamauchi-Takihara K. Activation of signal transducer and activator of transcription 3 protects cardiomyocytes from hypoxia/reoxygenation-induced oxidative stress through the upregulation of manganese superoxide dismutase. Circulation 104: 979–981, 2001. doi: 10.1161/hc3401.095947. [DOI] [PubMed] [Google Scholar]
- 228.Nossuli TO, Lakshminarayanan V, Baumgarten G, Taffet GE, Ballantyne CM, Michael LH, Entman ML. A chronic mouse model of myocardial ischemia-reperfusion: essential in cytokine studies. Am J Physiol Heart Circ Physiol 278: H1049–H1055, 2000. doi: 10.1152/ajpheart.2000.278.4.H1049. [DOI] [PubMed] [Google Scholar]
- 229.Nuzzo R. Scientific method: statistical errors. Nature 506: 150–152, 2014. doi: 10.1038/506150a. [DOI] [PubMed] [Google Scholar]
- 230.O’Konski MS, White FC, Longhurst J, Roth D, Bloor CM. Ameroid constriction of the proximal left circumflex coronary artery in swine. A model of limited coronary collateral circulation. Am J Cardiovasc Pathol 1: 69–77, 1987. [PubMed] [Google Scholar]
- 231.O’Quinn MP, Palatinus JA, Harris BS, Hewett KW, Gourdie RG. A peptide mimetic of the connexin43 carboxyl terminus reduces gap junction remodeling and induced arrhythmia following ventricular injury. Circ Res 108: 704–715, 2011. doi: 10.1161/CIRCRESAHA.110.235747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Oduk Y, Zhu W, Kannappan R, Zhao M, Borovjagin AV, Oparil S, Zhang J. VEGF nanoparticles repair the heart after myocardial infarction. Am J Physiol Heart Circ Physiol 314: H278−H284, 2018. doi: 10.1152/ajpheart.00471.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Omiya S, Omori Y, Taneike M, Protti A, Yamaguchi O, Akira S, Shah AM, Nishida K, Otsu K. Toll-like receptor 9 prevents cardiac rupture after myocardial infarction in mice independently of inflammation. Am J Physiol Heart Circ Physiol 311: H1485–H1497, 2016. doi: 10.1152/ajpheart.00481.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Ongstad EL, O’Quinn MP, Ghatnekar GS, Yost MJ, Gourdie RG. A connexin43 mimetic peptide promotes regenerative healing and improves mechanical properties in skin and heart. Adv Wound Care (New Rochelle) 2: 55–62, 2013. doi: 10.1089/wound.2011.0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Ostádal B, Ostádalová I, Kolár F, Charvátová Z, Netuka I. Ontogenetic development of cardiac tolerance to oxygen deprivation–possible mechanisms. Physiol Res 58, Suppl 2: S1–S12, 2009. [DOI] [PubMed] [Google Scholar]
- 236.Ostadalova I, Ostadal B, Kolár F, Parratt JR, Wilson S. Tolerance to ischaemia and ischaemic preconditioning in neonatal rat heart. J Mol Cell Cardiol 30: 857–865, 1998. doi: 10.1006/jmcc.1998.0653. [DOI] [PubMed] [Google Scholar]
- 237.Page BJ, Banas MD, Suzuki G, Weil BR, Young RF, Fallavollita JA, Palka BA, Canty JM Jr. Revascularization of chronic hibernating myocardium stimulates myocyte proliferation and partially reverses chronic adaptations to ischemia. J Am Coll Cardiol 65: 684–697, 2015. doi: 10.1016/j.jacc.2014.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Panizzi P, Swirski FK, Figueiredo JL, Waterman P, Sosnovik DE, Aikawa E, Libby P, Pittet M, Weissleder R, Nahrendorf M. Impaired infarct healing in atherosclerotic mice with Ly-6C(hi) monocytosis. J Am Coll Cardiol 55: 1629–1638, 2010. doi: 10.1016/j.jacc.2009.08.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Pantely GA, Malone SA, Rhen WS, Anselone CG, Arai A, Bristow J, Bristow JD. Regeneration of myocardial phosphocreatine in pigs despite continued moderate ischemia. Circ Res 67: 1481–1493, 1990. doi: 10.1161/01.RES.67.6.1481. [DOI] [PubMed] [Google Scholar]
- 240.Park KM, Teoh JP, Wang Y, Broskova Z, Bayoumi AS, Tang Y, Su H, Weintraub NL, Kim IM. Carvedilol-responsive microRNAs, miR-199a-3p and -214 protect cardiomyocytes from simulated ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 311: H371–H383, 2016. doi: 10.1152/ajpheart.00807.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Parrish DC, Francis Stuart SD, Olivas A, Wang L, Nykjaer A, Ripplinger CM, Habecker BA. Transient denervation of viable myocardium after myocardial infarction does not alter arrhythmia susceptibility. Am J Physiol Heart Circ Physiol 314: H415−H423, 2018. doi: 10.1152/ajpheart.00300.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Pizzuto MF, Suzuki G, Banas MD, Heavey B, Fallavollita JA, Canty JM Jr. Dissociation of hemodynamic and electrocardiographic indexes of myocardial ischemia in pigs with hibernating myocardium and sudden cardiac death. Am J Physiol Heart Circ Physiol 304: H1697–H1707, 2013. doi: 10.1152/ajpheart.00166.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, Sadek HA. Transient regenerative potential of the neonatal mouse heart. Science 331: 1078–1080, 2011. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Portal L, Martin V, Assaly R, d’Anglemont de Tassigny A, Michineau S, Berdeaux A, Ghaleh B, Pons S. A model of hypoxia-reoxygenation on isolated adult mouse cardiomyocytes: characterization, comparison with ischemia-reperfusion, and application to the cardioprotective effect of regular treadmill exercise. J Cardiovasc Pharmacol Ther 18: 367–375, 2013. doi: 10.1177/1074248412475158. [DOI] [PubMed] [Google Scholar]
- 245.Pound KM, Sorokina N, Ballal K, Berkich DA, Fasano M, Lanoue KF, Taegtmeyer H, O’Donnell JM, Lewandowski ED. Substrate-enzyme competition attenuates upregulated anaplerotic flux through malic enzyme in hypertrophied rat heart and restores triacylglyceride content: attenuating upregulated anaplerosis in hypertrophy. Circ Res 104: 805–812, 2009. doi: 10.1161/CIRCRESAHA.108.189951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Przyklenk K. Ischaemic conditioning: pitfalls on the path to clinical translation. Br J Pharmacol 172: 1961–1973, 2015. doi: 10.1111/bph.13064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Przyklenk K. Reduction of myocardial infarct size with ischemic “conditioning”: physiologic and technical considerations. Anesth Analg 117: 891–901, 2013. doi: 10.1213/ANE.0b013e318294fc63. [DOI] [PubMed] [Google Scholar]
- 248.Przyklenk K, Bauer B, Ovize M, Kloner RA, Whittaker P. Regional ischemic “preconditioning” protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation 87: 893–899, 1993. doi: 10.1161/01.CIR.87.3.893. [DOI] [PubMed] [Google Scholar]
- 249.Przyklenk K, Maynard M, Greiner DL, Whittaker P. Cardioprotection with postconditioning: loss of efficacy in murine models of type-2 and type-1 diabetes. Antioxid Redox Signal 14: 781–790, 2011. doi: 10.1089/ars.2010.3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Ramirez FD, Motazedian P, Jung RG, Di Santo P, MacDonald ZD, Moreland R, Simard T, Clancy AA, Russo JJ, Welch VA, Wells GA, Hibbert B. Methodological rigor in preclinical cardiovascular studies: targets to enhance reproducibility and promote research translation. Circ Res 120: 1916–1926, 2017. doi: 10.1161/CIRCRESAHA.117.310628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Reimer KA, Jennings RB, Tatum AH. Pathobiology of acute myocardial ischemia: metabolic, functional and ultrastructural studies. Am J Cardiol 52: 72A–81A, 1983. doi: 10.1016/0002-9149(83)90180-7. [DOI] [PubMed] [Google Scholar]
- 252.Reimer KA, Lowe JE, Rasmussen MM, Jennings RB. The wavefront phenomenon of ischemic cell death. 1. Myocardial infarct size vs duration of coronary occlusion in dogs. Circulation 56: 786–794, 1977. doi: 10.1161/01.CIR.56.5.786. [DOI] [PubMed] [Google Scholar]
- 253.Rezkalla SH, Kloner RA. Coronary no-reflow phenomenon: from the experimental laboratory to the cardiac catheterization laboratory. Catheter Cardiovasc Interv 72: 950–957, 2008. doi: 10.1002/ccd.21715. [DOI] [PubMed] [Google Scholar]
- 254.Ripplinger CM, Noujaim SF, Linz D. The nervous heart. Prog Biophys Mol Biol 120: 199–209, 2016. doi: 10.1016/j.pbiomolbio.2015.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Riva E, Hearse DJ. Age-dependent changes in myocardial susceptibility to ischemic injury. Cardioscience 4: 85–92, 1993. [PubMed] [Google Scholar]
- 256.Robertson C, Tran DD, George SC. Concise review: maturation phases of human pluripotent stem cell-derived cardiomyocytes. Stem Cells 31: 829–837, 2013. doi: 10.1002/stem.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Robey TE, Murry CE. Absence of regeneration in the MRL/MpJ mouse heart following infarction or cryoinjury. Cardiovasc Pathol 17: 6–13, 2008. doi: 10.1016/j.carpath.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Roell W, Fan Y, Xia Y, Stoecker E, Sasse P, Kolossov E, Bloch W, Metzner H, Schmitz C, Addicks K, Hescheler J, Welz A, Fleischmann BK. Cellular cardiomyoplasty in a transgenic mouse model. Transplantation 73: 462–465, 2002. doi: 10.1097/00007890-200202150-00022. [DOI] [PubMed] [Google Scholar]
- 259.Ross J., Jr Myocardial perfusion-contraction matching. Implications for coronary heart disease and hibernation. Circulation 83: 1076–1083, 1991. doi: 10.1161/01.CIR.83.3.1076. [DOI] [PubMed] [Google Scholar]
- 260.Roth DM, Maruoka Y, Rogers J, White FC, Longhurst JC, Bloor CM. Development of coronary collateral circulation in left circumflex ameroid-occluded swine myocardium. Am J Physiol 253: H1279–H1288, 1987. [DOI] [PubMed] [Google Scholar]
- 261.Sabbah HN, Stein PD, Kono T, Gheorghiade M, Levine TB, Jafri S, Hawkins ET, Goldstein S. A canine model of chronic heart failure produced by multiple sequential coronary microembolizations. Am J Physiol Heart Circ Physiol 260: H1379–H1384, 1991. [DOI] [PubMed] [Google Scholar]
- 262.Saeed M, Watzinger N, Krombach GA, Lund GK, Wendland MF, Chujo M, Higgins CB. Left ventricular remodeling after infarction: sequential MR imaging with oral nicorandil therapy in rat model. Radiology 224: 830–837, 2002. doi: 10.1148/radiol.2243011372. [DOI] [PubMed] [Google Scholar]
- 263.Sakamoto H, Parish LM, Hamamoto H, Ryan LP, Eperjesi TJ, Plappert TJ, Jackson BM, St John-Sutton MG, Gorman JH 3rd, Gorman RC. Effect of reperfusion on left ventricular regional remodeling strains after myocardial infarction. Ann Thorac Surg 84: 1528–1536, 2007. doi: 10.1016/j.athoracsur.2007.05.060. [DOI] [PubMed] [Google Scholar]
- 264.Sánchez-González J, Fernandez-Jiménez R, Nothnagel ND, López-Martín G, Fuster V, Ibañez B. Optimization of dual-saturation single bolus acquisition for quantitative cardiac perfusion and myocardial blood flow maps. J Cardiovasc Magn Reson 17: 21, 2015. doi: 10.1186/s12968-015-0116-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Sasayama S, Franklin D, Ross J Jr, Kemper WS, McKown D. Dynamic changes in left ventricular wall thickness and their use in analyzing cardiac function in the conscious dog. Am J Cardiol 38: 870–879, 1976. doi: 10.1016/0002-9149(76)90800-6. [DOI] [PubMed] [Google Scholar]
- 266.Schloss MJ, Horckmans M, Nitz K, Duchene J, Drechsler M, Bidzhekov K, Scheiermann C, Weber C, Soehnlein O, Steffens S. The time-of-day of myocardial infarction onset affects healing through oscillations in cardiac neutrophil recruitment. EMBO Mol Med 8: 937–948, 2016. doi: 10.15252/emmm.201506083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Schulz R, Guth BD, Pieper K, Martin C, Heusch G. Recruitment of an inotropic reserve in moderately ischemic myocardium at the expense of metabolic recovery. A model of short-term hibernation. Circ Res 70: 1282–1295, 1992. doi: 10.1161/01.RES.70.6.1282. [DOI] [PubMed] [Google Scholar]
- 268.Schulz R, Kappeler C, Coenen H, Bockisch A, Heusch G. Positron emission tomography analysis of [1-11C]acetate kinetics in short-term hibernating myocardium. Circulation 97: 1009–1016, 1998. doi: 10.1161/01.CIR.97.10.1009. [DOI] [PubMed] [Google Scholar]
- 269.Schulz R, Post H, Neumann T, Gres P, Lüss H, Heusch G. Progressive loss of perfusion-contraction matching during sustained moderate ischemia in pigs. Am J Physiol Heart Circ Physiol 280: H1945–H1953, 2001. doi: 10.1152/ajpheart.2001.280.5.H1945. [DOI] [PubMed] [Google Scholar]
- 270.Schulz R, Rose J, Post H, Heusch G. Involvement of endogenous adenosine in ischaemic preconditioning in swine. Pflugers Arch 430: 273–282, 1995. doi: 10.1007/BF00374659. [DOI] [PubMed] [Google Scholar]
- 271.Schwarz ER, Somoano Y, Hale SL, Kloner RA. What is the required reperfusion period for assessment of myocardial infarct size using triphenyltetrazolium chloride staining in the rat? J Thromb Thrombolysis 10: 181–187, 2000. doi: 10.1023/A:1018770711705. [DOI] [PubMed] [Google Scholar]
- 272.Scruggs SB, Watson K, Su AI, Hermjakob H, Yates JR III, Lindsey ML, Ping P. Harnessing the heart of big data. Circ Res 116: 1115–1119, 2015. doi: 10.1161/CIRCRESAHA.115.306013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Seitelberger R, Guth BD, Heusch G, Lee JD, Katayama K, Ross J Jr. Intracoronary alpha 2-adrenergic receptor blockade attenuates ischemia in conscious dogs during exercise. Circ Res 62: 436–442, 1988. doi: 10.1161/01.RES.62.3.436. [DOI] [PubMed] [Google Scholar]
- 274.Seldin MM, Kim ED, Romay MC, Li S, Rau CD, Wang JJ, Krishnan KC, Wang Y, Deb A, Lusis AJ. A systems genetics approach identifiesTrp53inp2as a link between cardiomyocyte glucose utilization and hypertrophic response. Am J Physiol Heart Circ Physiol 312: H728–H741, 2017. doi: 10.1152/ajpheart.00068.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Semenza GL. Cellular and molecular dissection of reperfusion injury: ROS within and without. Circ Res 86: 117–118, 2000. doi: 10.1161/01.RES.86.2.117. [DOI] [PubMed] [Google Scholar]
- 276.Sen S, Sadek HA. Neonatal heart regeneration: mounting support and need for technical standards. J Am Heart Assoc 4: e001727, 2015. doi: 10.1161/JAHA.114.001727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Shen YT, Vatner SF. Differences in myocardial stunning following coronary artery occlusion in conscious dogs, pigs, and baboons. Am J Physiol Heart Circ Physiol 270: H1312–H1322, 1996. [DOI] [PubMed] [Google Scholar]
- 278.Shen YT, Vatner SF. Mechanism of impaired myocardial function during progressive coronary stenosis in conscious pigs. Hibernation versus stunning? Circ Res 76: 479–488, 1995. doi: 10.1161/01.RES.76.3.479. [DOI] [PubMed] [Google Scholar]
- 279.Sherman AJ, Klocke FJ, Decker RS, Decker ML, Kozlowski KA, Harris KR, Hedjbeli S, Yaroshenko Y, Nakamura S, Parker MA, Checchia PA, Evans DB. Myofibrillar disruption in hypocontractile myocardium showing perfusion-contraction matches and mismatches. Am J Physiol Heart Circ Physiol 278: H1320–H1334, 2000. doi: 10.1152/ajpheart.2000.278.4.H1320. [DOI] [PubMed] [Google Scholar]
- 280.Shinde AV, Humeres C, Frangogiannis NG. The role of α-smooth muscle actin in fibroblast-mediated matrix contraction and remodeling. Biochim Biophys Acta 1863: 298–309, 2017. doi: 10.1016/j.bbadis.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Skrzypiec-Spring M, Grotthus B, Szelag A, Schulz R. Isolated heart perfusion according to Langendorff–still viable in the new millennium. J Pharmacol Toxicol Methods 55: 113–126, 2007. doi: 10.1016/j.vascn.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 282.Skyschally A, Amanakis G, Neuhäuser M, Kleinbongard P, Heusch G. Impact of electrical defibrillation on infarct size and no-reflow in pigs subjected to myocardial ischemia-reperfusion without and with ischemic conditioning. Am J Physiol Heart Circ Physiol 313: H871–H878, 2017. doi: 10.1152/ajpheart.00293.2017. [DOI] [PubMed] [Google Scholar]
- 283.Skyschally A, Gent S, Amanakis G, Schulte C, Kleinbongard P, Heusch G. Across-species transfer of protection by remote ischemic preconditioning with species-specific myocardial signal transduction by reperfusion injury salvage kinase and survival activating factor enhancement pathways. Circ Res 117: 279–288, 2015. doi: 10.1161/CIRCRESAHA.117.306878. [DOI] [PubMed] [Google Scholar]
- 284.Skyschally A, Schulz R, Heusch G. Pathophysiology of myocardial infarction: protection by ischemic pre- and postconditioning. Herz 33: 88–100, 2008. doi: 10.1007/s00059-008-3101-9. [DOI] [PubMed] [Google Scholar]
- 285.Sorop O, Merkus D, de Beer VJ, Houweling B, Pistea A, McFalls EO, Boomsma F, van Beusekom HM, van der Giessen WJ, VanBavel E, Duncker DJ. Functional and structural adaptations of coronary microvessels distal to a chronic coronary artery stenosis. Circ Res 102: 795–803, 2008. doi: 10.1161/CIRCRESAHA.108.172528. [DOI] [PubMed] [Google Scholar]
- 286.Spaan JA, Breuls NP, Laird JD. Diastolic-systolic coronary flow differences are caused by intramyocardial pump action in the anesthetized dog. Circ Res 49: 584–593, 1981. doi: 10.1161/01.RES.49.3.584. [DOI] [PubMed] [Google Scholar]
- 287.Sreejit P, Kumar S, Verma RS. An improved protocol for primary culture of cardiomyocyte from neonatal mice. In Vitro Cell Dev Biol Anim 44: 45–50, 2008. doi: 10.1007/s11626-007-9079-4. [DOI] [PubMed] [Google Scholar]
- 288.Steenbergen C, Perlman ME, London RE, Murphy E. Mechanism of preconditioning. Ionic alterations. Circ Res 72: 112–125, 1993. doi: 10.1161/01.RES.72.1.112. [DOI] [PubMed] [Google Scholar]
- 289.Strem BM, Zhu M, Alfonso Z, Daniels EJ, Schreiber R, Beygui R, MacLellan WR, Hedrick MH, Fraser JK. Expression of cardiomyocytic markers on adipose tissue-derived cells in a murine model of acute myocardial injury. Cytotherapy 7: 282–291, 2005. doi: 10.1080/14653240510027226. [DOI] [PubMed] [Google Scholar]
- 290.Strungs EG, Ongstad EL, O’Quinn MP, Palatinus JA, Jourdan LJ, Gourdie RG. Cryoinjury models of the adult and neonatal mouse heart for studies of scarring and regeneration. Methods Mol Biol 1037: 343–353, 2013. doi: 10.1007/978-1-62703-505-7_20. [DOI] [PubMed] [Google Scholar]
- 291.Sun JZ, Tang XL, Knowlton AA, Park SW, Qiu Y, Bolli R. Late preconditioning against myocardial stunning. An endogenous protective mechanism that confers resistance to postischemic dysfunction 24 h after brief ischemia in conscious pigs. J Clin Invest 95: 388–403, 1995. doi: 10.1172/JCI117667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Sutherland FJ, Hearse DJ. The isolated blood and perfusion fluid perfused heart. Pharmacol Res 41: 613–627, 2000. doi: 10.1006/phrs.1999.0653. [DOI] [PubMed] [Google Scholar]
- 293.Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P, Aikawa E, Mempel TR, Libby P, Weissleder R, Pittet MJ. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 325: 612–616, 2009. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Takawale A, Zhang P, Azad A, Wang W, Wang X, Murray AG, Kassiri Z. Myocardial overexpression of TIMP3 after myocardial infarction exerts beneficial effects by promoting angiogenesis and suppressing early proteolysis. Am J Physiol Heart Circ Physiol 313: H224–H236, 2017. doi: 10.1152/ajpheart.00108.2017. [DOI] [PubMed] [Google Scholar]
- 295.Tanai E, Frantz S. Pathophysiology of heart failure. Compr Physiol 6: 187–214, 2015. doi: 10.1002/cphy.c140055. [DOI] [PubMed] [Google Scholar]
- 296.Tanaka M, Ito H, Adachi S, Akimoto H, Nishikawa T, Kasajima T, Marumo F, Hiroe M. Hypoxia induces apoptosis with enhanced expression of Fas antigen messenger RNA in cultured neonatal rat cardiomyocytes. Circ Res 75: 426–433, 1994. doi: 10.1161/01.RES.75.3.426. [DOI] [PubMed] [Google Scholar]
- 297.Taylor CB, Davis CB Jr, Vawter GF, Hass GM. Controlled myocardial injury produced by a hypothermal method. Circulation 3: 239–253, 1951. doi: 10.1161/01.CIR.3.2.239. [DOI] [PubMed] [Google Scholar]
- 298.Thakker GD, Frangogiannis NG, Bujak M, Zymek P, Gaubatz JW, Reddy AK, Taffet G, Michael LH, Entman ML, Ballantyne CM. Effects of diet-induced obesity on inflammation and remodeling after myocardial infarction. Am J Physiol Heart Circ Physiol 291: H2504–H2514, 2006. doi: 10.1152/ajpheart.00322.2006. [DOI] [PubMed] [Google Scholar]
- 299.Thielmann M, Dörge H, Martin C, Belosjorow S, Schwanke U, van De Sand A, Konietzka I, Büchert A, Krüger A, Schulz R, Heusch G. Myocardial dysfunction with coronary microembolization: signal transduction through a sequence of nitric oxide, tumor necrosis factor-alpha, and sphingosine. Circ Res 90: 807–813, 2002. doi: 10.1161/01.RES.0000014451.75415.36. [DOI] [PubMed] [Google Scholar]
- 300.Thiese MS, Arnold ZC, Walker SD. The misuse and abuse of statistics in biomedical research. Biochem Med (Zagreb) 25: 5–11, 2015. doi: 10.11613/BM.2015.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Thomas SA, Fallavollita JA, Suzuki G, Borgers M, Canty JM Jr. Dissociation of regional adaptations to ischemia and global myolysis in an accelerated Swine model of chronic hibernating myocardium. Circ Res 91: 970–977, 2002. doi: 10.1161/01.RES.0000040396.79379.77. [DOI] [PubMed] [Google Scholar]
- 302.Thompson RB, Emani SM, Davis BH, van den Bos EJ, Morimoto Y, Craig D, Glower D, Taylor DA. Comparison of intracardiac cell transplantation: autologous skeletal myoblasts versus bone marrow cells. Circulation 108, Suppl 1: II264–II271, 2003. doi: 10.1161/01.cir.0000087657.29184.9b. [DOI] [PubMed] [Google Scholar]
- 303.Toyota E, Warltier DC, Brock T, Ritman E, Kolz C, O’Malley P, Rocic P, Focardi M, Chilian WM. Vascular endothelial growth factor is required for coronary collateral growth in the rat. Circulation 112: 2108–2113, 2005. doi: 10.1161/CIRCULATIONAHA.104.526954. [DOI] [PubMed] [Google Scholar]
- 304.Triana JF, Li XY, Jamaluddin U, Thornby JI, Bolli R. Postischemic myocardial “stunning”. Identification of major differences between the open-chest and the conscious dog and evaluation of the oxygen radical hypothesis in the conscious dog. Circ Res 69: 731–747, 1991. doi: 10.1161/01.RES.69.3.731. [DOI] [PubMed] [Google Scholar]
- 305.van Amerongen MJ, Harmsen MC, Petersen AH, Popa ER, van Luyn MJ. Cryoinjury: a model of myocardial regeneration. Cardiovasc Pathol 17: 23–31, 2008. doi: 10.1016/j.carpath.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 306.van den Borne SW, van de Schans VA, Strzelecka AE, Vervoort-Peters HT, Lijnen PM, Cleutjens JP, Smits JF, Daemen MJ, Janssen BJ, Blankesteijn WM. Mouse strain determines the outcome of wound healing after myocardial infarction. Cardiovasc Res 84: 273–282, 2009. doi: 10.1093/cvr/cvp207. [DOI] [PubMed] [Google Scholar]
- 307.van den Bos EJ, Mees BM, de Waard MC, de Crom R, Duncker DJ. A novel model of cryoinjury-induced myocardial infarction in the mouse: a comparison with coronary artery ligation. Am J Physiol Heart Circ Physiol 289: H1291–H1300, 2005. doi: 10.1152/ajpheart.00111.2005. [DOI] [PubMed] [Google Scholar]
- 308.Vanoverschelde JL, Wijns W, Depré C, Essamri B, Heyndrickx GR, Borgers M, Bol A, Melin JA. Mechanisms of chronic regional postischemic dysfunction in humans. New insights from the study of noninfarcted collateral-dependent myocardium. Circulation 87: 1513–1523, 1993. doi: 10.1161/01.CIR.87.5.1513. [DOI] [PubMed] [Google Scholar]
- 309.Vatner SF. Correlation between acute reductions in myocardial blood flow and function in conscious dogs. Circ Res 47: 201–207, 1980. doi: 10.1161/01.RES.47.2.201. [DOI] [PubMed] [Google Scholar]
- 310.Vogel B, Shinagawa H, Hofmann U, Ertl G, Frantz S. Acute DNase1 treatment improves left ventricular remodeling after myocardial infarction by disruption of free chromatin. Basic Res Cardiol 110: 15, 2015. doi: 10.1007/s00395-015-0472-y. [DOI] [PubMed] [Google Scholar]
- 311.Voorhees AP, DeLeon-Pennell KY, Ma Y, Halade GV, Yabluchanskiy A, Iyer RP, Flynn E, Cates CA, Lindsey ML, Han HC. Building a better infarct: Modulation of collagen cross-linking to increase infarct stiffness and reduce left ventricular dilation post-myocardial infarction. J Mol Cell Cardiol 85: 229–239, 2015. doi: 10.1016/j.yjmcc.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Wan F, Letavernier E, Le Saux CJ, Houssaini A, Abid S, Czibik G, Sawaki D, Marcos E, Dubois-Rande JL, Baud L, Adnot S, Derumeaux G, Gellen B. Calpastatin overexpression impairs postinfarct scar healing in mice by compromising reparative immune cell recruitment and activation. Am J Physiol Heart Circ Physiol 309: H1883–H1893, 2015. doi: 10.1152/ajpheart.00594.2015. [DOI] [PubMed] [Google Scholar]
- 313.Warren M, Sciuto KJ, Taylor TG, Garg V, Torres NS, Shibayama J, Spitzer KW, Zaitsev AV. Blockade of CaMKII depresses conduction preferentially in the right ventricular outflow tract and promotes ischemic ventricular fibrillation in the rabbit heart. Am J Physiol Heart Circ Physiol 312: H752–H767, 2017. doi: 10.1152/ajpheart.00347.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Weil BR, Young RF, Shen X, Suzuki G, Qu J, Malhotra S, Canty JM Jr. Brief Myocardial ischemia produces cardiac troponin i release and focal myocyte apoptosis in the absence of pathological infarction in swine. JACC Basic Transl Sci 2: 105–114, 2017. doi: 10.1016/j.jacbts.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.White IA, Gordon J, Balkan W, Hare JM. Sympathetic reinnervation is required for mammalian cardiac regeneration. Circ Res 117: 990–994, 2015. doi: 10.1161/CIRCRESAHA.115.307465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Whittaker P, Kloner RA, Boughner DR, Pickering JG. Quantitative assessment of myocardial collagen with picrosirius red staining and circularly polarized light. Basic Res Cardiol 89: 397–410, 1994. doi: 10.1007/BF00788278. [DOI] [PubMed] [Google Scholar]
- 317.Wu Y, Yin X, Wijaya C, Huang MH, McConnell BK. Acute myocardial infarction in rats. J Vis Exp 2011: 2464, 2011. doi: 10.3791/2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Xu Y, Huo Y, Toufektsian MC, Ramos SI, Ma Y, Tejani AD, French BA, Yang Z. Activated platelets contribute importantly to myocardial reperfusion injury. Am J Physiol Heart Circ Physiol 290: H692–H699, 2006. doi: 10.1152/ajpheart.00634.2005. [DOI] [PubMed] [Google Scholar]
- 319.Yabluchanskiy A, Ma Y, DeLeon-Pennell KY, Altara R, Halade GV, Voorhees AP, Nguyen NT, Jin YF, Winniford MD, Hall ME, Han HC, Lindsey ML. Myocardial infarction superimposed on aging: MMP-9 deletion promotes M2 macrophage polarization. J Gerontol A Biol Sci Med Sci 71: 475–483, 2016. doi: 10.1093/gerona/glv034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Yamamoto H, Tomoike H, Shimokawa H, Nabeyama S, Nakamura M. Development of collateral function with repetitive coronary occlusion in a canine model reduces myocardial reactive hyperemia in the absence of significant coronary stenosis. Circ Res 55: 623–632, 1984. doi: 10.1161/01.RES.55.5.623. [DOI] [PubMed] [Google Scholar]
- 321.Yamamoto T, Tamaki K, Shirakawa K, Ito K, Yan X, Katsumata Y, Anzai A, Matsuhashi T, Endo J, Inaba T, Tsubota K, Sano M, Fukuda K, Shinmura K. Cardiac Sirt1 mediates the cardioprotective effect of caloric restriction by suppressing local complement system activation after ischemia-reperfusion. Am J Physiol Heart Circ Physiol 310: H1003–H1014, 2016. doi: 10.1152/ajpheart.00676.2015. [DOI] [PubMed] [Google Scholar]
- 322.Yang J, Brown ME, Zhang H, Martinez M, Zhao Z, Bhutani S, Yin S, Trac D, Xi JJ, Davis ME. High-throughput screening identifies microRNAs that target Nox2 and improve function after acute myocardial infarction. Am J Physiol Heart Circ Physiol 312: H1002–H1012, 2017. doi: 10.1152/ajpheart.00685.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Yang Y, Ma Y, Han W, Li J, Xiang Y, Liu F, Ma X, Zhang J, Fu Z, Su YD, Du XJ, Gao XM. Age-related differences in postinfarct left ventricular rupture and remodeling. Am J Physiol Heart Circ Physiol 294: H1815–H1822, 2008. doi: 10.1152/ajpheart.00831.2007. [DOI] [PubMed] [Google Scholar]
- 324.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med 357: 1121–1135, 2007. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 325.Yoshihara HA, Bastiaansen JA, Berthonneche C, Comment A, Schwitter J. An intact small animal model of myocardial ischemia-reperfusion: characterization of metabolic changes by hyperpolarized 13C MR spectroscopy. Am J Physiol Heart Circ Physiol 309: H2058–H2066, 2015. doi: 10.1152/ajpheart.00376.2015. [DOI] [PubMed] [Google Scholar]
- 326.Yu Y, Wei SG, Zhang ZH, Weiss RM, Felder RB. ERK1/2 MAPK signaling in hypothalamic paraventricular nucleus contributes to sympathetic excitation in rats with heart failure after myocardial infarction. Am J Physiol Heart Circ Physiol 310: H732–H739, 2016. doi: 10.1152/ajpheart.00703.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Zamilpa R, Zhang J, Chiao YA, de Castro Brás LE, Halade GV, Ma Y, Hacker SO, Lindsey ML. Cardiac wound healing post-myocardial infarction: a novel method to target extracellular matrix remodeling in the left ventricle. Methods Mol Biol 1037: 313–324, 2013. doi: 10.1007/978-1-62703-505-7_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Zhang F, Xia Y, Yan W, Zhang H, Zhou F, Zhao S, Wang W, Zhu D, Xin C, Lee Y, Zhang L, He Y, Gao E, Tao L. Sphingosine 1-phosphate signaling contributes to cardiac inflammation, dysfunction, and remodeling following myocardial infarction. Am J Physiol Heart Circ Physiol 310: H250–H261, 2016. doi: 10.1152/ajpheart.00372.2015. [DOI] [PubMed] [Google Scholar]
- 329.Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, Vinten-Johansen J. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol 285: H579–H588, 2003. doi: 10.1152/ajpheart.01064.2002. [DOI] [PubMed] [Google Scholar]
- 330.Zhu HL, Wei X, Qu SL, Zhang C, Zuo XX, Feng YS, Luo Q, Chen GW, Liu MD, Jiang L, Xiao XZ, Wang KK. Ischemic postconditioning protects cardiomyocytes against ischemia/reperfusion injury by inducing MIP2. Exp Mol Med 43: 437–445, 2011. doi: 10.3858/emm.2011.43.8.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Ziv O, Schofield L, Lau E, Chaves L, Patel D, Jeng P, Peng X, Choi BR, Koren G. A novel, minimally invasive, segmental myocardial infarction with a clear healed infarct borderzone in rabbits. Am J Physiol Heart Circ Physiol 302: H2321–H2330, 2012. doi: 10.1152/ajpheart.00031.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Zymek P, Bujak M, Chatila K, Cieslak A, Thakker G, Entman ML, Frangogiannis NG. The role of platelet-derived growth factor signaling in healing myocardial infarcts. J Am Coll Cardiol 48: 2315–2323, 2006. doi: 10.1016/j.jacc.2006.07.060. [DOI] [PubMed] [Google Scholar]