Abstract
Cardiovascular disease is a leading cause of death, and translational research is needed to understand better mechanisms whereby the left ventricle responds to injury. Mouse models of heart disease have provided valuable insights into mechanisms that occur during cardiac aging and in response to a variety of pathologies. The assessment of cardiovascular physiological responses to injury or insult is an important and necessary component of this research. With increasing consideration for rigor and reproducibility, the goal of this guidelines review is to provide best-practice information regarding how to measure accurately cardiac physiology in animal models. In this article, we define guidelines for the measurement of cardiac physiology in mice, as the most commonly used animal model in cardiovascular research.
Listen to this article’s corresponding podcast at http://ajpheart.podbean.com/e/guidelines-for-measuring-cardiac-physiology-in-mice/.
Keywords: cardiac physiology, echocardiography, hemodynamics, magnetic resonance imaging, rigor and reproducibility
INTRODUCTION
The measurement of cardiac physiology is the foundation for assessing changes in anatomic and physiological features that occur within the myocardium during aging, in response to genetic alterations, and after a variety of experimentally induced pathologies. Cardiac physiology measurements also provide a means to examine the effects of therapeutic interventions.
With increasing concerns over data rigor and reproducibility, best-practice information is needed regarding measurements of cardiac anatomy and physiology in experimental settings. The focus of this review is to provide comprehensive guidelines on how to assess cardiac physiology in mice. We will discuss the importance of having a complete and rigorous physiological assessment when evaluating a number of cardiac pathologies. We will clarify what needs to be measured in different cardiac pathologies and discuss parameters to establish consistency both within and among laboratories. This guidelines article is a good companion for the article “Guidelines for animal models of myocardial ischemia and infarction” (106).
The sections in this article are divided by approach and include echocardiography, MRI, and hemodynamics measurements. Within each section, we discuss how each specific approach can be used to assess alterations in cardiac structure and function under different conditions, including studies of aging, cardiomyopathies such as postchemotherapy, diabetes, or sepsis, myocardial infarction (MI), and pressure overload cardiac hypertrophy induced by transverse aortic constriction (TAC), angiotensin II infusion, or isoproterenol infusion. Table 1 shows a list of suggested variables for assessment of cardiac physiology under these different conditions. The reference list serves as an additional resource to investigators new to the field.
Table 1.
Suggested variables for assessing cardiac physiology under different conditions
| Condition | Variables |
|---|---|
| Aging |
|
| Chemotherapy |
|
| Diabetes |
|
| Myocardial infarction (MI)/ischemia-reperfusion (IR) |
|
| Hypertrophy and dilated cardiomyopathy (with or without hypertrophy) |
|
FS, fractional shortening; EF, ejection fraction; LV, left ventricular; E/A, E wave-to-A wave ratio; EDD, end-diastolic dimension; EDV, end-diastolic volume; ESD, end-systolic dimension; ESV, end-systolic volume. Dimension-based calculations are as follows: EF = [(EDV − ESV)/EDV] × 100, FS = [(EDD − ESD)/EDD] × 100, LV hypertrophy index = EDD/wall thickness (at diastole), LV remodeling index = EDV/LV mass, and LV sphericity index = EDV/volume of a sphere with a diameter equal to EDD.
ECHOCARDIOGRAPHY
Echocardiographic imaging is a widely used, noninvasive means to assess cardiac physiology and architecture in rodent models of heart disease and aging and allows for repeated assessment of heart function over the course of disease progression (8, 15–18, 35, 41, 50, 61, 74, 79, 84, 86, 88, 91, 112, 113, 123, 125, 126, 136, 142, 143, 150–153, 163, 164, 168, 171, 175, 177, 191, 202, 205, 212–214). One reason for its appeal is that echocardiographic ultrasound imaging provides a comprehensive array of information on cardiac anatomy, physiology, and mechanical properties. While ultrasound can also be used to obtain information from the vasculature (arteries and veins), we will focus on the application of echocardiography in analyzing cardiac structure and function. To obtain reliable and reproducible information from echocardiography that can be compared across laboratories, a number of factors need to be considered. These criteria are outlined in terms of the type and depth of anesthesia, the mode of recording, and the (space-time) variables most informative for accurate and precise assessment of various models of heart disease and related interventions.
Echocardiographic imaging uses high-frequency sound beams that penetrate the thoracic cavity and are reflected back to the ultrasound transducer when they reach an interface among moieties of different acoustic impedance, such as the myocardium, blood, valves, or vessel wall. This reverberated signal is then processed by the instrument software to produce a real-time image of the heart (or vessel). Echocardiography uses four main principal imaging formats: two-dimensional (2-D) brightness mode (B-mode), motion mode (M-mode), Doppler imaging, and three-dimensional (3-D) imaging. Unique challenges facing echocardiography in mice include the small heart size (5- to 8-mm length in an adult mouse) coupled with high heart rates (400–650 beats/min in an unanesthetized mouse, depending on the strain). Recent technological developments include high-frequency transducers (up to 70 MHz) and enhanced imaging frame rates to provide high spatial and temporal resolutions to view structural and physiological changes in the left ventricle (LV). In addition, integration of respiration, heart rate, and ECG monitoring during ultrasound recordings allows for additional quality control (and potential normalization) during recording. Echocardiography is now commonly used to measure different aspects of cardiac architecture (wall thickness and chamber dilation) and physiology (systolic and diastolic) in rodents.
Cardiac hypertrophy and dilated cardiomyopathy are generally associated with a uniform structural remodeling of the LV, and, as such, M-mode and B-mode imaging are suitable approaches to measure LV wall thickness (septal and free wall) and LV chamber size. MI (permanent occlusion of the left anterior descending artery), on the other hand, is associated with nonuniform remodeling of the LV chamber due to the scar (infarct) formation that replaces a major fraction of the LV free wall (and sometimes the septum). In this scenario, M-mode imaging is limited in its assessment capacity; global changes in the structure of an infarcted, remodeled LV are better assessed using 2-D or even 3-D images. ECG-gated kilohertz visualization (EKV) is a 3-D reconstruction of the heart using imaging gated to the ECG (20). With EKV, one can obtain serial M-mode images of the LV (from the apex to base), which are then spatially and temporally reconstructed into an ultrasound B-mode image data set for one cardiac cycle. The high temporal resolution allows for better tracking of myocardial borders and can be used to measure LV chamber size and LV contractility (121).
Anesthesia
The first step in preparing for echocardiographic imaging is to decide what, if any, anesthetic to use. Some studies have reported that ultrasound recordings can be performed on conscious, carefully restrained mice to avoid an anesthesia-induced decrease in heart rate that can influence cardiac function (203). Mice can be imaged free hand or restrained on a platform using elastic cord or tape. In the latter setting, ECG electrodes would be taped to the paws (46). It is important to note that the acquirement of reliable results from ultrasound recordings in conscious animals requires that the mouse be sufficiently acclimatized to the imaging environment (e.g., the platform, restraining devices, use of warm gel) and to the individual capturing the images. This will significantly influence the demeanor of the animal, as attempts should be made to avoid a stress-related rise in heart rate that can subsequently have an impact on cardiac performance. While the two schools of thought (anesthetized vs. awake) continue to exist and evolve, it is critical to recognize that either approach should be done under conditions that minimize procedure-related changes to cardiac function and that echocardiography data acquired from anesthetized mice should not be compared with those obtained from conscious mice.
The anesthetics that have been most commonly used for echocardiography in mice are isoflurane and ketamine-xylazine, while other anesthetics, such as tribromoethanol (Avertin), medetomidine, pentobarbital sodium, and ketamine, in combination with other anesthetics (including midazolam and fentanyl), have also been occasionally used in mice (48, 90). Ketamine alone and avertin have both been shown to keep heart rates in the range of 550−beats/min and have little cardiodepressant effects (195, 212).
A mixture of ketamine (an N-methyl-d-aspartate receptor antagonist, 100 mg/kg) and xylazine (an α2-adrenoceptor agonist, 10 mg/kg) has been previously and prevalently used as the anesthetic of choice for surgeries as well as for echocardiographic imaging. However, in the past two decades, numerous studies have demonstrated cardiodepressant effects of ketamine-xylazine, which have made this choice of anesthetic unacceptable for physiological measurements. This is mainly due to the cardiodepressant effects of xylazine, which can significantly reduce heart rate and LV function compared with isoflurane (135, 146). Isoflurane (1–2%) has become the most popular anesthetic for echocardiography in mice (78, 86, 88, 107). As the hemodynamic effects of anesthesia vary over time, care must be taken to acquire echocardiograms at the same time after induction and at a similar heart rate. For example, a 10-min wait period after the mouse is placed on the board provides a uniform acclimation period. A heart rate of >400 beats/min is advised to be within the physiological range of murine heart rate under anesthesia. In a recent study (135), the effects of different anesthetics on LV systolic function were evaluated. While it is important to sustain a high heart rate during assessment of cardiac function in rodents, heart rates of >650 beats/min suggest activation of the autonomic nervous system, and, therefore, results with heart rates of <400 and >650 beats/min should be interpreted with caution (116, 199). A reduction in the heart rate (<400 beats/min), secondary to the type or depth of anesthesia, suppresses LV systolic and diastolic function, whereas an excess rise in heart rate (>650 beats/min, e.g., due to stress or activation of the autonomic nervous system) can result in insufficient LV filling. As discussed later, heart rates of >500–600 beats/min are also accompanied with fusion of LV filling waves [early (E wave) and atrial (late, A wave)] and the inability to assess diastolic function in mice.
In choosing an anesthetic for echocardiography, additional criteria, beyond heart rate and LV systolic function, need to be considered. Table 2 shows a list of common anesthetics used for echocardiography in mice, with reported advantages and limitations. Some anesthetics have been reported to have protective effects on cardiac recovery from surgery under certain dosage and duration conditions (49, 109, 183). Important factors in choosing an anesthetic include ease of handling, amount of stress induced, ability to adjust level and duration, impact on physiological parameters (blood pressure, heart rate, and cardiac function), and recovery time. Moreover, while the decision of choosing an anesthetic (or none at all) is often based on the effects on heart rate, it is important to note that if cardiac diastolic function is also to be assessed by echocardiography, at very high heart rates, then the E and A waves (or the tissue Doppler equivalents, E′ and A′ waves) can become fused (merged), therefore preventing accurate measurement of diastolic function. A rapid assessment using free-hand scanning can be done in <5 min, while for a more thorough echocardiogram acquired over 10–20 min, stable anesthetic conditions are advised.
Table 2.
| Anesthetic | Dosage range | Advantages | Limitations |
|---|---|---|---|
| Isoflurane | 1–3% (1 l/min) |
|
|
| Barbiturates | 30–90 mg/kg |
|
|
| Ketamine | 80–100 mg/kg |
|
|
| Ketamine/xylazine | 80–100 mg/kg; 5–15 mg/kg |
|
|
| 2,2,2-Tribromoethanol (avertin) or 2-methyl-2-butanol | 240 mg/kg |
|
|
B-Mode Imaging
B-mode produces 2-D views of the heart (short or long axis) and allows for assessment of cardiac chamber dimensions, cardiac physiology, and visualization of cardiac anatomic structures, such as papillary muscle and valves. In cine loops obtained from B-mode, the LV endocardium can be traced in diastole and systole to calculate cardiac ejection fraction (EF). Similarly, LV mass can be estimated by tracing the epicardium and endocardium in a midventricular parasternal short-axis view. B-mode also serves as an orientation guideline for regions of the heart that require further assessment by other imaging modes, such as M-mode or color Doppler imaging. Imaging of the right ventricle (RV) in the short-axis view in the mouse is challenging, due to interference from the sternum not allowing proper positioning of the probe for RV imaging; detailed protocols have been developed for using echocardiography to assess RV structure and physiology (25, 96, 122).
Among the currently available imaging modes, EKV provides maximum frame rate imaging with maximum resolution, allowing for increased temporal and spatial resolution imaging (20, 121). As a comparison, with B-mode imaging, acquisition occurs at frame rates of 300 frames/s compared with ~1,000 frames/s with EKV recording. This mode of imaging can be useful in assessing cardiac structure and physiology, where the LV undergoes wall thinning (e.g., in MI) when high-resolution images are required to visualize the infarcted thin and dyskinetic LV wall (87, 171).
M-Mode Imaging
M-mode images are obtained by a rapid succession of B-mode scans along a single axis displayed over time. M-mode images appear as a continuous tracing, showing the motion of the myocardial walls as they contract during systole and relax during diastole. This mode provides a very high temporal resolution of LV wall motion to assess LV contractile patterns and chamber size. Parameters that can be obtained from M-mode imaging include LV internal diameter (LVID) at end systole (LVIDs) and end diastole (LVIDd), which can then be used to calculate fractional shortening (FS) as a measure of systolic function and cardiac contractility [FS = (LVIDd − LVIDs)/LVIDd × 100]. M-mode images can also be used to derive additional functional parameters such as calculated EF, cardiac output (CO), and stroke volume (SV) or structural parameters such as LV mass. Calculations of LV volumes and EF from M-mode, however, have a number of geometric assumptions that must be taken into account. In calculations derived from M-mode dimensions, the LV is assumed to be of a spherical (symmetric) rather than an oval (asymmetric) shape. This is not accurate and leads to even greater errors in pathological cases, such as post-MI remodeling. Similarly, while the calculated LV mass is often comparable with actual mass, the gravimetric measurement of LV mass remains the gold standard.
Doppler Imaging
This imaging mode uses the Doppler shift principle, reflected by the moving target, to determine blood flow velocity and direction, as evidenced by color differential. In the case of color Doppler, the moving target is blood cells (moving parallel to the beam); in the case of tissue Doppler, the moving target is the myocardium. An increase in blood flow will be reflected as an increase in Doppler shift. In pulsed-wave Doppler, the transducer transmits and receives the sound waves, whereby the blood flow-velocity profile is determined from a precise location (determined by 2-D image guidance). Pulsed-wave Doppler can be used to measure transvalvular flow-velocity profiles, which are particularly useful in assessing cardiac diastolic function, including isovolumic relaxation time (IVRT), E wave and A wave ventricular filling velocities, and deceleration of the E wave. More details on accurate assessment of diastolic function are provided in later sections. Pulsed-wave Doppler can also measure systolic parameters, such as ejection time (ET), isovolumic contraction time (IVCT), and IVRT. These parameters provide information on the kinetics of LV systole (ET and IVCT) and cardiac diastole (IVRT). For instance, prolonged ET or IVCT may reflect a reduced rate of contraction, whereas an increase in IVRT often reflects impaired diastolic function.
Color Doppler imaging uses a color-encoded map of flow velocity and direction superimposed on the 2-D image. Blood flowing toward the ultrasound transducer is identified in red (increase in frequency), blood flowing away from the transducer is depicted in blue (decrease in frequency), and blood flowing horizontally is not detected. Doppler evaluation of blood flow and velocity can also be obtained in any vascular bed. One of the possible applications of Doppler imaging to measure blood flow and velocity is in determining the severity of pressure overload in the TAC surgical model to induce cardiac hypertrophy (88, 136). Presence of a turbulent flow results in a mosaic of colors at the site of constriction, where pressure and flow gradient can be obtained through pulsed-wave Doppler. Pulsed-wave Doppler can also be used to measure the pressure gradient, which can be particularly useful in TAC to ensure that all mice in a study are subjected to the same degree of aortic constriction. It must be noted, however, that a limitation of pulsed Doppler is that it cannot measure high velocities; the maximum pressure gradient that can be measured is ~60 mmHg.
Tissue Doppler imaging is used to assess global and regional cardiac function. Mitral annulus measurements of motion velocity are used to assess E′, A′, and S waves (LV diastolic parameters of relaxation). Myocardial velocities can also be measured and strain (deformation) rates derived from these measures. Peak systolic myocardial strain rate is a relatively load-independent measure that can identify subtle changes in systolic function. One limitation of the Tissue Doppler imaging method is that it only measures velocities parallel to the beam; therefore, analyses of radial function are limited to the anterior and posterior walls, and the circumferential function is limited to the septal and lateral walls.
3-D Images
M-mode and B-mode images represent a grayscale of amplitude in one and two dimensions. 3-D echocardiography in mice is based on reconstruction from multiple 2-D images. The images can be digitally stacked as a series of short- or long-axis views. Alternatively, a semi-automated 3-D acquisition image, obtained from varying transducer positions at the same points in the cardiac cycle (gating), measures LV chamber volume during end systole (LVESv) and end diastole (LVEDv), which are used to calculate EF [EF = (LVEDv − LVESv)/LVEDv × 100]. Whereas 2-D echocardiography is based on the symmetric LV shape and structure assumption, 3-D imaging avoids LV shape assumptions, a particularly useful feature in assessing LV dilation and dysfunction in a model of MI (40). It is important to emphasize that EF, whether in 2-D or 3-D space, is highly dependent on loading conditions (preload or afterload).
Speckle-Tracking Imaging
Speckle tracking is a novel, non-Doppler-based technique used to detect myocardial displacement, wall motion velocity, and myocardial deformation indexes, such as strain (fractional change in length of myocardial segment) and strain rate (the rate of change in strain). Strain and strain rate detect early LV dysfunction before changes in EF occur (7). Similar to EF, strain is also load dependent, whereas strain rate appears to be less load dependent. In speckle-tracking imaging, LV endocardial and epicardial borders are traced to form a region of interest, and speckle patterns are identified inside this region of interest. The group of speckles that correspond to each region of the myocardium can be tracked from frame to frame using a speckle-tracking algorithm. The resulting geometric shift during a cardiac cycle is used to calculate displacement, regional velocity (displacement per unit time), strain, and strain rate along the radial, circumferential, and longitudinal planes of the heart. The advantage of speckle tracking over Doppler imaging is that quantitative speckle-tracking assessment of myocardial performance is not angle dependent. The frame rate used to measure strain rate needs to be high (at least 350–500 frames/s depending on heart rate) to capture the maximum strain rate, which is a brief event.
Displacement can be measured along the radial and longitudinal axes as the distance traveled by the speckles from peak systole to peak diastole. In addition, strain and strain rate can be used to assess regional myocardial function. Radial strain (percent change in myocardial wall thickness) can be measured along the short or the long axis and is depicted as a positive curve during systole (reflecting increasing wall thickness) and a negative curve during diastole (reflecting decreasing LV wall thickness). Circumferential strain is obtained from a short-axis view and represents the percent change in myocardial circumference, whereas longitudinal strain detects the percent change in LV length (from the apex to base). Time to peak analysis is another index measured by speckle tracking that is used to assess dyssynchronous contraction among different myocardial regions (21). In this analysis, the LV myocardium (short- or long-axis image) is divided into six segments. In a healthy LV, all segments should be synchronized (similar velocities), peaking at similar times. Under pathological conditions, certain segments may move at a different velocity (e.g., due to the presence of fibrotic lesions) and therefore peak at different times, resulting in dyssynchrony. Peak regional strain values can be reported for specific LV segments, whereas averaging peak strain and strain rate measurements across all six segments can be reported as global strain (19, 144).
Regional strain per LV segment (rather than global strain) can provide useful information in a post-MI model, since there is a clear regional difference in LV structural remodeling and function. As such, summation of all segments could mask regional differences. In hypertrophic or dilated cardiomyopathy associated with focal fibrosis, enhanced stiffness of a fibrotic segment reduces regional velocity, resulting in dyssynchrony. Therefore, speckle-track imaging can provide very useful information regarding the alterations within the myocardium that may be too subtle to be detected by conventional imaging modes. One important caveat with speckle tracking is that different ultrasound machines use their own proprietary software to acquire and track the speckles, resulting in a lack of standardization of strain and strain rate values. Care must be taken to acquire serial echocardiograms on the same equipment.
Assessment of LV Systolic Physiology
Systole is the contraction phase of the heart that ensures normal ejection of blood from the chambers into the arteries to supply the body with oxygen. The most commonly used variables to evaluate systolic function are FS and EF, both provided as percentage values during systole compared with diastole. FS indicates the percentage change in LV chamber size and is an index of myocyte contraction, whereas EF indicates the percentage change in LV volumes and is an index of LV function. Other systolic physiology variables, such as SV and CO, can be calculated from measurements of LV wall thickness and chamber dimensions. The volumes and EF derived from M-mode are generally calculated using the cubed formula (diameter3), which assumes the heart is a sphere. Volumes and EF derived from 2-D measurements assume a symmetrical LV geometry, which, of course, will not be the case in cardiac pathologies, such as MI. FS and EF are calculated based on LV chamber diameter and volume. Heart rate positively correlates with FS and EF, and, therefore, a drop (or rise) in heart rate, due to anesthetic, can lead to altered systolic function assessment. For instance, low heart rate associated with deep anesthesia is accompanied with LV dilation and overall cardiac depression. Therefore, the maintenance of a stable heart rate throughout the echocardiographic recording is particularly important, especially when assessing cardiac function in postinjury hearts that exhibit LV remodeling.
After pressure overload, LV volume first decreases, due to thickening of the LV wall (compensatory hypertrophy), followed by LV dilation and enlarged LV volume. Since the LV undergoes a uniform remodeling (LV wall thickness is altered similarly in the free wall and the septum), M-mode imaging can be used to measure LV chamber diameter, from which FS can be calculated as FS = [(LVIDd − LVIDs)/LVIDd × 100].
After MI or ischemia-reperfusion (I/R), there is nonuniform LV remodeling that includes thinning of the LV free wall and thickening of the septal nonischemic LV wall. Therefore, M-mode imaging in these cases provides an incomplete and sometimes false evaluation of LV remodeling and function. Additionally, M-mode could provide a biased view of the LV if the probe is not placed in the same location across all of the mice examined, particularly across control and MI groups. Therefore, it is our recommendation that M-mode measurements not be used as end points in this model. We recommend that images are acquired and analyzed according to American Society of Echocardiography guidelines for humans, tracing from leading edge to leading edge or trailing edge to trailing edge. While 2-D imaging provides clear views of wall motion abnormalities, several planes need to be imaged to delineate the extent and geometry of the abnormality. For this reason, small wall motion abnormalities can be missed. To circumvent this issue, 3-D imaging to evaluate LV volume (rather than LVID) and to calculate EF [EF = (LVEDv − LVESv)/LVEDv × 100] is superior and more accurate compared with FS measurements. LV volumes can be measured from several short-axis views using the Simpsons method (153), which represents the LV cavity as a stack of disks, and LV volume is calculated as a summation of all disks.
LV function post-MI or post-I/R can also be assessed by determination of the wall motion score index (WMSI) (207), which is calculated based on a 10- to 16-segment model on short-axis views and is scored as 1 for normal, 2 for hypokinetic, 3 for akinetic, 4 for dyskinetic, and 5 for aneurysmal. WMSI is calculated as the sum of scores divided by the total number of segments that were analyzed. A higher WMSI value corresponds to a greater degree of LV dysfunction. This parameter, along with EF, provides information on LV systolic function post-MI or post-IR (86, 145, 171). As EKV yields a higher temporal and spatial resolution compared with B-mode imaging, the use of EKV recording to assess LV volumes and WMSI in post-MI hearts can provide a more accurate assessment of these parameters.
As in humans, an injection of contrast microbubbles can improve the definition of the endocardium. Additionally, the RV can be visualized. This approach, however, necessitates an intravenous injection. Care must be taken to inject a small volume of fluid (<20 µl) so that hemodynamic conditions are not disrupted by the injection.
The Tei index or myocardial performance index provides an overall, combined assessment of both the systolic and diastolic function of the heart: (IVCT + IVRT)/ET. While the Tei index was originally and erroneously used to report diastolic dysfunction, it is an index of cardiac performance during a complete cardiac cycle (systole and diastole) (173). It has been previously reported in mice but is not widely used, because the Tei index does not correctly reflect changes in heart function in some disease models (159).
LV Diastolic Physiology
Diastole is the relaxation phase of the heart that ensures normal filling of the ventricles during rest and provides adequate blood volume to maintain normal CO. Diastolic dysfunction is defined as an impairment in active relaxation, passive stiffness, or the combination. Assessment of diastolic dysfunction in animal models of heart disease has received significantly more attention, since heart failure with preserved EF has become increasingly recognized in patients (133, 154). Assessment of LV diastolic function in mice includes evaluation of LV filling velocity measured by the magnitude of E and A waves as well as E′ and A′ waves (tissue Doppler imaging), IVRT, E wave deceleration time (DT), and left atrial (LA) size. These parameters have been used to assess reliably diastolic dysfunction in mice (17, 123, 171).
It is important to note that the size of the mouse is a critical factor in selecting appropriate parameters to assess diastolic dysfunction accurately. A word of caution: a number of parameters, such as pulmonary venous flow and transmitral flow propagation velocity, which have been proven to be useful in patients, cannot be used in rodent models, due to the small size of the heart and fast heart rate in these animals, which restrict the spatial and temporal resolution beyond the detection limits of currently available technologies. Furthermore, the variability of these indexes among individual mice contributes to the difficulty and inaccuracy/inconsistency of diastolic assessment in this species.
Mitral Flow Profile
The mitral flow profile comprises two waveforms, the early filling (E wave) and late or atrial filling (A wave), and provides information about the LV filling dynamics (155). The E wave represents the blood flow through the mitral valve during the early filling phase of the LV and can be affected by the rate of relaxation and compliance of the LV. The A wave represents the blood flow (through the valve) during the atrial contraction phase and can be altered by LA contractility or compliance (100). The E wave to A wave ratio (E/A) can be used as a measurement of diastolic dysfunction (if E/A < 1), however, not in isolation; as outlined by the new diastolic guidelines from the American Society of Echocardiography, E and A must be analyzed in the context of myocardial disease, annular tissue velocities, and LA size (127).
Mitral flow profile also provides information on DT and IVRT. DT is the time required for pressure equilibration between LV and LA and is measured as the time from the peak of the E wave to the baseline. IVRT is the time from closure of the aortic valve to the opening of the mitral valve. Increased IVRT represents prolonged LV relaxation. Although these measurements provide valuable information on LV filling kinetics, these parameters can be influenced by a number of factors, such as preload, arrhythmia, or very high heart rate as well as diseases that cause a hyperdynamic state. Furthermore, normal values of DT in mice are not well reported, and IVRT−a very short time interval−can be difficult to measure.
LA Size
LA size can be used as a marker for chronic elevation of LV filling pressure and is often used to assess diastolic dysfunction in humans (83, 139) and animal models (17, 50, 123, 171). Given the small LA size in mice, strict attention, with respect to probe location, is required for accurate measurements. M-mode, in the parasternal long-axis view, can be used to measure the maximal anteroposterior LA diameter in rodents (17). We recommend normalization of the LA diameter by the tibial length to avoid variability due to individual animal size. Other indexes, such as the use of pulmonary venous flow and transmitral flow propagation velocity, have been reported to correlate with elevated LV filling pressures in mice but are not routinely used (33, 68, 179).
Other Specific Echocardiographic Techniques
Assessment of cardiac physiology by echocardiography would not be complete without mentioning the possibility to assess ischemia and coronary reserve using stress echocardiography, Doppler-derived coronary reserve and myocardial perfusion using contrast echocardiography (5, 62, 142, 143, 190). Additionally, in pulmonary and RV pathologies, the measurement of pulmonary acceleration time, which has been used extensively in humans as a measure of pulmonary pressure, has been validated in mice (175). On the Vevo 3100, there is a noncontrast power Doppler mode, which provides information on vascularity that has mostly been used for oncology or muscle studies and color Doppler. It is possible for vessels to be studied using high-frequency probes, yielding information on their size and distensibility, thus providing additional information on the ventricular–arterial coupling. In our experience, the power Doppler mode has been difficult to interpret in the heart.
Literature Analysis of Articles Published in American Journal of Physiology Journals Using Echocardiography
To evaluate the current reporting of echocardiographic results, we accessed PubMed on July 21, 2017, and searched for “mouse and American Journal of Physiology and (echocardiography or echocardiographic or ultrasound).” This search resulted in 437 articles, of which 52 articles were published between January 1, 2016, and present as well as 14 articles that were excluded as false positives (two kidney, one liver, one lung, one bone, one RV, two rat not mouse, one fetal not adult, and five with no cardiac echocardiography results). The remaining 38 articles were analyzed both for completeness of information provided and for quality assessment of the echocardiography values reported (11, 13, 22, 27, 30, 36, 38, 39, 42, 45, 52, 54, 57, 59, 65, 70, 75, 76, 78, 85, 102, 114, 117–119, 132, 147, 148, 158, 160, 165, 170, 172, 182, 187, 193, 201, 206).
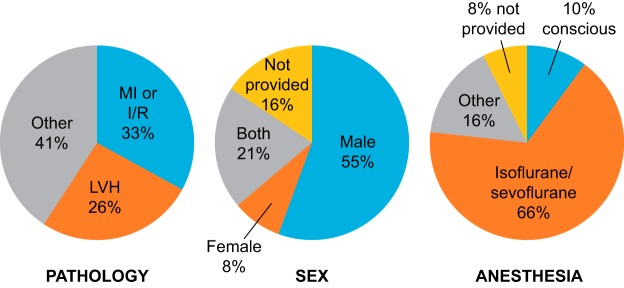
A summary of the pathologies examined, mouse characteristics, anesthesia used, and results reported is shown in Fig. 1. The pathology assessed was approximately equally split between MI and LV hypertrophy models. Over one-half of the studies used male animals only; of note, 21% of the studies used both sexes. Isoflurane was the most common anesthetic used, with very few studies using conscious animals. A total of 68% of the articles used the C57 strain, although this was inconsistently provided as C57, C57BL/6, or C57BL/6J; while it is assumed all of these mean C57BL/6J, only 8 of 26 articles (30.8%) in this category specified the exact strain details. There was an even split in how results were presented, with 44% using tables and 56% using graphs. Most reported statistical analysis details, although not all specified which test was used for each evaluation. A surprising 58% did not report heart rates, and 82% did not report details on whether acquisition and analysis were blinded. While 92% provided some details on what instrument and probe were used, what views were acquired, and how the probe was positioned, very few provided sufficient details to assess completely quality of acquisition or analysis.
Fig. 1.
Results of a literature analysis of articles published in the American Journal of Physiology since January 1, 2016. The pie charts show the percentages of articles that covered different pathologies (left), divided by the sex of the mice (middle), and separated by the anesthesia used (right). LV hypertrophy (LVH) included genetic models as well as angiotensin II infusion or transverse aortic constriction models of pressure overload.
There was a wide range of values reported for each parameter, even for healthy control mice; this is likely due, in part, to the wide range of conditions under which imaging was performed. A total of six studies in healthy wild-type mice, using similar conditions (C57BL/6J, one of the Vevo instruments, isoflurane), provided results in tables so that we could obtain mean values and had results that the committee considered to be of high quality. These results were combined and are shown in Table 3. Of note, awake mice or mice anesthetized using different anesthetic regimens (e.g., ketamine alone) would be expected to have smaller LV dimensions and higher FS and EF. Based on this analysis, we developed a checklist for authors and reviewers on the minimum information that should be provided when reporting echocardiographic experiments (Table 4).
Table 3.
Compilation of echocardiography results in healthy male C57BL/6J mice from 6 studies using similar instrument and anesthesia protocols (13, 45, 78, 117, 170, 201)
| Left Ventricular Internal Diameter at End Diastole, mm | Left Ventricular Internal Diameter at End Systole, mm | Fractional Shortening, % | Ejection Fraction, % | |
|---|---|---|---|---|
| Number of mice | 5 | 6 | 6 | 3 |
| Low | 2.8 | 1.7 | 34 | 60 |
| High | 3.8 | 2.6 | 45 | 75 |
| Mean | 3.4 | 2.1 | 40 | 69 |
| SD | 0.4 | 0.3 | 4.1 | 8.2 |
| Coefficient of variation | 12 | 15 | 10 | 12 |
Table 4.
Checklist for authors and reviewers: minimum details needed for cardiac physiology methods and results
Methods:
|
Results:
|
MAGNETIC RESONANCE IMAGING
MRI is a noninvasive, high-resolution imaging technique that can be used to assess myocardial anatomy, perfusion, wall motion and contractility, and physiology in mice (58, 192). Myocardial molecular imaging/tagging is not discussed here to maintain the focus of this discussion on cardiac physiological measurements; please refer to several excellent articles regarding its use (140, 161, 185, 189, 198). The use of MRI in mice has two major challenges: the small size of the heart (spatial resolution) and elevated heart rates (typically 400–650 beats/min depending on the strain) that can introduce motion artifacts. These challenges, in addition to respiration and signal-to-noise ratio limitations, can severely deteriorate image quality. While MRI is the gold standard in the clinic, its use in mice is still in development. Unlike human imaging, which can use single slice acquisitions, imaging of the mouse heart requires stacking images obtained at a particular time in the cardiac cycle to generate the slice image (26). Therefore, in thinned tissue, the imaging quality may not be as robust. Tailored imaging protocols and dedicated cardiac hardware and software are essential to achieve appropriate temporal and spatial resolution for cardiac MRI in rodents. Below, we provide guidelines for cardiac MRI in mice.
Hardware
Dynamic imaging of the myocardium requires high ventricular blood-to-myocardium contrast, full coverage of the cardiac cycle, and high temporal and spatial resolution. For this reason, gradient echocardiography-based imaging techniques are used (97). MRI systems for mice have small bore magnets imaging at very high field strengths (>7 T) (188). Increasing field strength for MRI increases the signal-to-noise ratio, resulting in higher resolution. The increase of field strength has the trade offs of also increased lack of field homogeneity, susceptibility to artifacts, and higher radiofrequency power deposition (124). While the full range of field strengths uses a span from 1.5 to 3 T, using optimized coils at the low end (58, 67) to 17.6 T at the high end (66), the range of 7–11.7 T offers the best compromise between resolution and presence of artifacts for mice imaging (47).
Gating Strategies
To limit motion artifacts, it is essential to perform cardiac gating to acquire quality images. Images should be acquired over several cardiac cycles and simultaneously with an ECG for synchronization, a method commonly designated as prospective gating (54). Prospective gating involves MRI acquisition at a predefined portion of the cardiac cycle (for example, at diastole) after detection of the upslope of an R wave in the ECG (97). While there are some variations in mouse MRI protocols across laboratories, basic principles are commonly followed (shown in Table 5). First, with few exceptions, mice are under anesthesia and allowed to breathe freely. Isoflurane inhalation is currently the preferred approach, due to quick anesthesia induction and fast awakening, minimal hemodynamic depression, and easy regulation of anesthesia depth (98). Mice usually receive 1.0–2.0% isoflurane, 30–50% oxygen, and 50–70% air (69). Second, temperature (by rectal probe), heart rate (400–650 beats/min, depending on the strain), and breathing activity (~50–100 cycles/min) are closely monitored and controlled (29). Third, the animal’s position has to be reproducible; this is a critical step and can affect data quality and the degree to which motion artifacts affect the imaging. The animal is placed in a prone position (fixed with tape or plastic pins to an animal sleigh or other fixation device), with the anesthetic agent supplied through a nose cone, the breathing sensor normally attached around the abdomen, and the temperature measured by a fixed rectal probe. Fourth, prospective gating synchronized to the ECG has to be performed. Finally, a multiphase gradient echocardiography-based cine cardiac imaging is acquired and the data are analyzed.
Table 5.
Basic principles followed during MRI in mice
| Anesthesia |
|
| Animal position |
|
| Hemodynamic parameters monitored |
|
| Prospective gating |
|
| Echocardiography |
|
While currently less widely available, more resource intensive, and with lower temporal resolution than echocardiography, advantages of using cardiac MRI in mouse models of heart failure include high accuracy and versatility (6, 167). Cardiac MRI in mice uses pulse sequences that not only allow assessment of volumes, mass, and systolic and diastolic physiology but also myocardial perfusion, flow, viability, and myocardial strain and anatomy.
Cardiac Volumes, Mass, and Physiology
Cardiac MRI benefits from unrestricted spatial access to the myocardium, allowing long-axis imaging of two, three, or four chambers and continuous stacks of serial short-axis images for reproducible measurements of ventricular volumes and masses (9, 167, 184). Current instruments allow for a temporal resolution of 10–20 phases/heart beat (i.e., 5–10 ms) and a spatial resolution of 0.1–0.2 mm (188). A stack of six to eight serial, 1-mm-thick, short-axis slices is recommended to cover the entire LV and RV (184, 197).
Assessment of LV diastolic physiology by MRI in mice is more challenging. Nevertheless, there have been a few successful reports. For example, in a mouse model of dilated cardiomyopathy, Chłopicki and colleagues used images of the midventricular short-axis plane at the level of the papillary muscle to measure filling rates derived from time-area curves (44, 180). Other laboratories have also successfully used the same approach at higher fields of 7–9.4 T (1, 14, 196).
Myocardial Perfusion and Blood Flow
MRI is a powerful tool for the assessment of myocardial perfusion (i.e., myocardial blood flow per gram of tissue, in ml·g−1·min−1), providing a means to characterize the relationship between blood flow oxygen delivery and cardiac contraction. There are currently two cardiac MRI approaches to evaluate myocardial perfusion: 1) Gd-enhanced, first-pass perfusion after an intravenous bolus injection of an exogen contrast agent and 2) arterial spin labeling using the intrinsic properties of water protons in blood as an endogenous tracer not requiring contrast injections (95). The Epstein laboratory (129) is a leader in the MRI field and has compared both techniques in mice. During low myocardial blood flow conditions, such as post-MI, the use of first-pass perfusion MRI is preferred, due to better reproducibility and lower variability. At high myocardial blood flow during vasodilation, arterial spin labeling may be more suitable, due to superior image quality and lower user variability. First-pass perfusion MRI has a substantial speed advantage and has successfully been applied in mouse MI models (34, 178), cardiac hypertrophy (186), and obesity (128, 130).
Myocardial Viability
Similar to echocardiography, MRI can be used noninvasively to assess changes serially in the same mouse over time. One advantage for MRI is the ability to assess nonviable versus stunned myocardium and, therefore, to measure infarct area over time. This is achieved by late Gd-enhancement imaging after injection of a contrast agent (92). The technique is based on the principle that Gd chelates have an extravascular distribution volume (188). Late Gd enhancement images are acquired at least 10 min after an intravenous or intraperitoneal injection of the contrast agent. Infarcted or fibrotic myocardium presents decreased cellular volume and increased extracellular volume, which result in higher contrast concentrations at equilibrium, translating to shorter LV longitudinal relaxation times (T1) (81). Gd-based contrast dyes are low-molecular-weight extracellular agents that are small enough to move across the vascular wall into the extracellular space yet are large enough that they do not infiltrate cells with intact membranes (137). To obtain optimal T1 time between normal and ischemic myocardium, postcontrast imaging is performed with inversion recovery to subtract the signal intensity for normal myocardium with a look-locker sequence (138). For late Gd enhancement imaging in mice, field strengths of 4.7 T (176), 7 T (141), or 9.4 T are typically used (23), with segmented gradient-recalled echocardiography or fast low-angle shot sequences, both using multiechocardiographic acquisition. In 2011, Price et al. (138) proposed a faster late Gd enhancement imaging protocol using multislice acquisition that allows for higher flip angles and, therefore, higher signal-to-noise rate efficiency. This technique has been used for imaging of cardiac ischemia (131) and coronary artery plaques (77). Data can be confounded, however, in the presence of pathologies, where increased water is a symptom, such as edema and amyloidosis, which can be detected by an increase in transverse relaxation (T2 signal) (137). In these cases, cardiac T1 mapping without the use of a Gd-based contrast agent has been shown to be a sensitive approach in both human and mouse models (51, 89, 101, 174, 181).
Myocardial Strain Mapping
Cardiovascular magnetic resonance tagging is an established technique for measuring regional myocardial function. It allows quantification of myocardial motion measures, such as strain and strain rate, by visualizing transmural myocardial movement without having to insert physical markers (73). This technique opened the door for a series of developments and technical improvements that continue to develop. Methods used for myocardial strain mapping in mice include the following: displacement encoding via stimulated echoes (DENSE), spatial modulation of magnetization (SPAMM)-tagged imaging, and harmonic phase (HARP) MRI. DENSE has the ability to extract myocardial motion data at high spatial density over segments of the cardiac cycle (4). DENSE MRI is particularly useful in the quantification of LV volumes and mass in mice (60) and to quantify cardiac displacement directly (56). In SPAMM-tagged MRI, the magnetization is modulated using radiofrequency pulses and magnetic field gradients (120). This results in saturated bands in the magnetization distribution and, as a result, contrasting patterns in the image data. Tissue tagging by SPAMM has often been used to access myocardial strain in humans and in animal models (37, 43, 72, 208). Zhou and colleagues (211) used SPAMM MRI in combination with a cine protocol to map myocardial strains and displacements in mice. HARP MRI allows for automated and fast analysis of high-tagging resolution images in mice (64, 104, 209, 210). HARP MRI has also proven useful to compare cardiac displacement and strain between cardiomyocyte-specific genotypes and wild-type mice (32). More recently, the introduction and coupling of the feature-tracking technique with MRI have allowed for the assessment of 3-D regional and global (longitudinal, circumferential, and radial strains) myocardial dysfunction (110, 194). While FT MRI has become a popular choice in the clinic for assessment of myocardial strain (12, 111), it is still in its infancy in animal models. Nonetheless, a recent study (99) evaluated FT MRI reproducibility in mice and reported good to excellent inter- and intraobserver reproducibility, suggesting that FT MRI shows analytic potential for experimental cardiac research. For a more complete list of myocardial strain-mapping techniques, see Ibrahim (73) and Jiang and Yu (82).
Myocardial Anatomy
Cardiac muscle architecture directly correlates to the mechanical and electrical properties of the myocardium, and changes in fiber structure and orientation are of prime importance in post-MI remodeling assessment (149). Diffusion-encoded or diffusion-tensor MRI can be used to examine myocardial fiber orientation. Microstructural myocardial imaging, at the scale of individual myofiber tracts and sheets, has the potential to provide a mechanistic bridge between cellular and molecular events and the whole organ physiology (71). While in vivo cardiac diffusion tensor MRI is still in the early stages of development, ex vivo microscopic structural imaging is commonly used to assess myocardial structural remodeling after MI or aging (31, 103, 166, 200).
While cardiac MRI is a powerful, noninvasive, and versatile technique that provides high-quality cardiac images and allows for reproducible study of cardiac anatomy and physiology, there are some limitations. Major limitations with the use of MRI include cost (instrument and contrast agents), longer and more resource-intensive protocols, lower temporal resolution, signal-to-noise ratio limitations, and reduced availability (see Table 6 for a detailed list of advantages and limitations of MRI). While many clinical studies have compared data obtained from both echocardiography and MRI in the same patients (93, 94), experimental models comparing both modalities are limited. Experimental mouse models comparing the use of echocardiography with MRI noted good correlation in measures of volume and physiology (108). With the comparison of changes in cardiac structure, MRI was better at detecting moderate to severe diffuse myocardial fibrosis. A study by Li et al. (105) reported good correlation between radial strain and circumferential strain between 2-D echo and MRI short-axis views. Thus, echocardiography and MRI deliver comparable cardiac physiological measurements, with MRI offering increased versatility and potential benefit on studies focused on myocardial fibrosis and assessment of tissue viability.
Table 6.
Recommended methods for cardiac physiological measurements: advantages and limitations of each methodology
| Technique | Advantages | Limitations |
|---|---|---|
| Echocardiography |
|
|
| Cardiac MRI |
|
|
| Hemodynamics |
|
|
HEMODYNAMICS
Invasive hemodynamic measurements can refine the information on cardiac physiology provided by echocardiography. This approach is not routinely used in mice as a firstline method, however, because this approach is technically challenging and is a nonsurvival procedure precluding this from being a serial assessment, and the results can be difficult to interpret. A micromanometer-tip catheter is inserted into the LV chamber (through the carotid artery, retrogradely or through the apex), where it can directly measure the changes in pressure (and volume when a conductance catheter is used) of the LV over time. Illustrations of pressure-volume loops and how they are altered in cardiac disease are shown in Fig. 2.
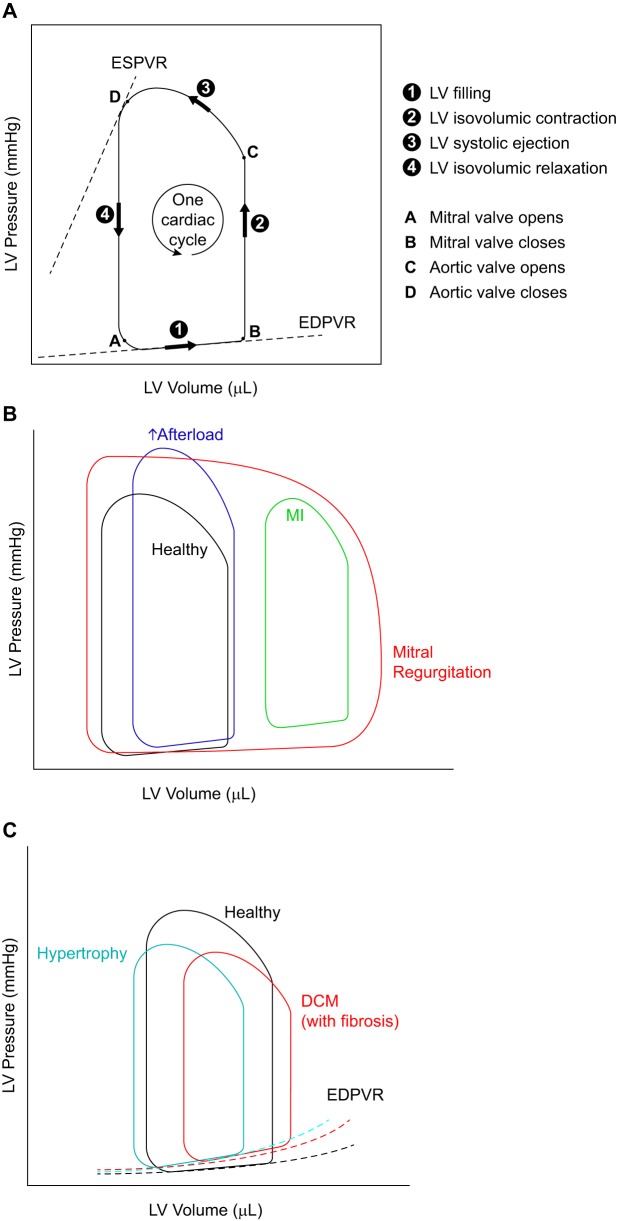
Fig. 2.
Illustrations of pressure-volume (P-V) loops and how they are altered in heart disease. A: P-V loops representing the changes in pressure and volume of the left ventricle (LV) during one cardiac cycle. Information in LV pressure and volume during different phases of a cardiac cycle can be obtained from this loop as indicated. B: the shape and relative location of the P-V loop are affected differently in various types of heart diseases. In mitral regurgitation, the width of the P-V loop does not represent the stroke volume, because not all of the blood is pumped out of the LV, due to the regurgitant mitral valve. The mitral regurgitation is also responsible for the absence of a true isovolumic relaxation or contraction. C: end-diastolic P-V relationship (EDPVR) can serve as a measure of myocardial stiffness (of the LV). A steeper slope for this curve correlates with increased stiffness (reduced compliance) of the LV myocardium. ESPVR, end-systolic pressure-volume relationship; DCM, dilated cardiomyopathy.
Parameters derived from LV pressure loops can be helpful, especially for the study of diastolic physiology. LV minimum pressure rates (dP/dtmin) and the isovolumic relaxation constant (τ), although load dependent, are easier to interpret than echocardiographic mitral inflows in mice, which can be fused at high heart rates. RV pressure measurements are invaluable for a direct measure of pulmonary arterial systolic pressure. Pressure-volume loops are considered the gold standard for hemodynamic assessment of ventricular performance and are mostly obtained from the LV, although reports on RV performance have been made (169). The advantage of pressure-volume hemodynamic measurements over echocardiography is that some of the indexes, such as the end-systolic pressure-volume relationship (ESPVR or Ees, described below), are considered to be relatively load independent (24, 134).
A number of pressure catheters and pressure-volume catheters are commercially available that range in size and sensitivity. A comparison of pressure catheters from Millar (Houston, TX), Scisense (Ithaca, NY), and RADI Medical Systems (Uppsala, Sweden) showed that pressure measurements by all catheters were stable, with a drift of ±2 mmHg within the 0- to 300-mmHg range. Of the three, the Millar amplifier had the shortest delay (0.2 ms) compared with Scisense (3.2 ms) and RADI Medical Systems (10.6 ms) amplifiers (63). For a heart rate of 500 beats/min in a mouse, a 10.6-ms delay translates to 8.3 beats/s, which should not impact recording frequency. The Scisense catheter can detect lateral forces with high sensitivity, which allows the catheter to register pressure from all surrounding regions rather than only the force exerted directly at the tip.
In addition, the Scisense catheter has the advantage of reporting the absolute LV volume without a need for calibration, while Millar pressure-volume catheters require volume calibration. This is achieved in the Scisense pressure-volume catheter, because when inserted into the LV, it measures the overall conductance as well as the parallel conductance coming from the surrounding myocardium (which changes during the cardiac cycle). Parallel conductance is automatically quantified and removed from the total conductance, thereby providing direct information on LV volume (blood volume in the LV chamber). With Millar pressure-volume catheters, the conductance catheter signal is proportional to volume (of blood in the LV) and must be appropriately calibrated to provide accurate absolute volume measurements. It must be emphasized that the calibration is a crucial and often technically difficult step in the use of conductance catheters. One step that we would suggest, in addition to the calibration proposed by the manufacturers (calibration using cuvettes of different sizes), is to validate the conductance-derived volumes using the echocardiography-derived volumes.
Anesthesia and LV Catheterization
Similar to echocardiographic imaging, choice of anesthesia is important for reliable and reproducible assessment of hemodynamics. Isoflurane has become the anesthetic of choice in hemodynamic measurements in mice because of its minimal cardiosuppressant effects compared with ketamine-xylazine and pentobarbital sodium (80), although ketamine-fentanyl has been reported also to lack cardiosupressant effects (74). As mentioned earlier, an additional advantage of an inhalant anesthetic (isoflurane) over injectable options is that its levels can be monitored, and, therefore, the depth of anesthesia can be controlled during the procedure.
LV catheterization can be performed by a closed- or open-chest approach. In the open-chest approach, the catheter is inserted into the LV (or RV) through the apex, whereas in the closed-chest approach, the catheter is inserted in the carotid artery and extended into the ascending aorta and LV. The closed-chest approach has a number of advantages: arterial blood pressure can be recorded from the carotid at the beginning or end of the procedure, intubation is not required, and animals can remain stable for a longer time, which is ideal for prolonged procedures, such as drug testing. This approach is also preferred for hemodynamic measurements post-MI, since the LV infarction often extends to the apex, and insertion of the catheter through the scarred myocardium can be problematic. Open-chest LV catheterization also has advantages, since proper placement of the catheter in the LV is easier to confirm and is a better approach if the carotid artery is severely atherosclerotic (e.g., high-fat diet-fed apolipoprotein E or LDL receptor-deficient mice) or in cases of aortic valve calcification. In all cases, we would recommend the use of the 1-Fr catheter to ease the introduction of the catheter in the LV and to decrease the risk of obstruction of the LV cavity around the catheter with elevated and inaccurate systolic pressure recordings.
Hemodynamic Data Analysis and Interpretation
dP/dtmax and dP/dtmin rise and decline can be derived from LV pressure traces (first derivative). LV τ can be calculated by the Weiss method, expressed as the regression of the pressure versus time logarithm, or by the Glantz method, expressed as the regression of dP/dt versus pressure (204). τ increases as LV relaxation decreases (115). Systolic dysfunction is noted by a decrease in dP/dtmax, and diastolic dysfunction is detected by an increase in LV end-diastolic pressure and τ and a decrease in dP/dtmin. Due to the small values of some parameters (e.g., LV end-diastolic pressure), calibration is crucial to minimize error. All parameters are load dependent.
With the use of a conductance catheter, parallel changes in LV pressure and volume can be recorded over consecutive cardiac cycles. A number of parameters can be obtained from the conductance catheters, including SV (SV = EDV – ESV), EF (EF = SV/EDV), CO (SV × heart rate), and arterial elastance (arterial elastance = end-systolic pressure/SV), where EDV is end-diastolic volume and ESV is end-systolic volume. Volume measurements rely on geometrica assumptions, which can make results difficult to interpret in asymmetrical volumes and for RV measurements (134). The pressure-volume relationship is presented as loops along the y-axis (pressure) and x-axis (volume).
In addition, with the adjustment of preload, i.e., by temporary occlusion and slow release of the inferior vena cava, the ESPVR and end-diastolic pressure-volume relationship (EDPVR) can be determined. ESPVR represents the maximal pressure developed by the LV at any given volume and is a measure of cardiac contractility. The slope of ESPVR, also referred to as Ees, is an index of end-systolic elastance, an index that provides information on contractile function (134). The degree to which Ees is load dependent is under discussion (3). Ventriculoarterial coupling, which reflects the interaction of the heart and its afterload, can also be assessed using the ratio of Ees/arterial elastance. Regarding the diastolic properties of the heart, EDPVR represents the passive filling properties of the LV, and the slope of this curve is a measure of myocardial compliance (reverse of stiffness). An increase in this slope will indicate increased myocardial stiffness or decreased compliance (162). Both Ees and EDPVR are dependent on chamber size and remodeling.
In hypertrophic cardiomyopathy, the LV walls become thicker and LV chamber size decreases or remains unchanged. The increased LV wall thickness can reduce compliance, resulting in an increase in EDPVR slope. During compensatory hypertrophy when systolic pressure is not suppressed, no change in end-systolic pressure (y-axis) will be observed. In dilated cardiomyopathy, ESV and EDV increase, but LV pressure can remain unchanged; therefore, the ESPVR and EDPVR are shifted to the right. If dilated cardiomyopathy is associated with fibrosis and diastolic dysfunction, then an upward shift in the EDPVR will be observed. After MI or other instances of volume overload, ESV and EDV increase, end-systolic pressure decreases, and end-diastolic pressure increases; as a result, the pressure-volume loop will look smaller (shorter) with a rightward shift (see Fig. 2).
OVERALL DISCUSSION AND CONCLUSIONS
As highlighted throughout these guidelines, the measurement of cardiac physiology is a critical component of cardiovascular research. Methods for the accomplishment of this include echocardiography, MRI, and hemodynamic evaluation using pressure-volume catheters. A summary of overall recommendations, including strengths and limitations of each technique, is shown in Table 6. The approach used will vary depending on the questions being addressed; as such, all of the approaches described above may be considered an appropriate approach if they answer the target hypothesis. While echocardiography is the most frequently used technique, due to its availability, technical ease of use, capacity for serial imaging, and cost−all methods discussed in this guidelines article−provide excellent means to evaluate cardiac physiology and can be complementary to each other. A combination of methods is frequently used to overlap limitations of one approach with strengths of the other (10).
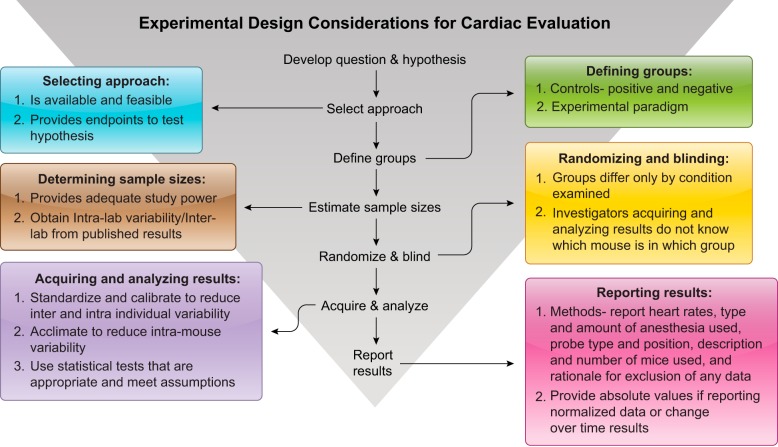
Prospective planning of study design (i.e., randomization for appropriate control vs. treatment, blinding, and adequate statistics) is mandatory for reproducibility of all experimental approaches, and cardiac physiology experiments are no exception (Fig. 3). The absolute values of the results and the components entering into a calculation should be reported for any normalized measurements. For example, studies that only report changes in calculated measurements, such as EF, do not enable the field to know whether the altered EF resulted from a change in systolic volume, diastolic volume, or both, and this lack of details confounds our ability to understand the underlying mechanisms of altered physiology. Additionally, there are an increasing number of computational models and tools being developed to understand cardiac physiology, predict disease-related remodeling, and design novel interventional therapies. The accuracy of these models are dependent on comprehensive measurements of cardiac dimensions and function under various circumstances. Reporting only percent change without showing absolute values forces model developers to make assumptions for the missing data.
Fig. 3.
Experimental design considerations for studies measuring cardiac physiology indexes.
The ultimate validation is confirmation of results across individuals and across laboratories. As larger data sets are being acquired, consideration for how to harness big data and evaluate cardiac physiology results across laboratories should be given (156, 157). This will be a particular challenge, as values obtained from imaging approaches can be affected by both acquisition and analysis conditions. The compilation of databases to incorporate results from across studies and across laboratories will provide a means to use epidemiological approaches or big data tools to validate published findings, generate novel hypotheses, and assess individual variability in cardiac structure and function. In conclusion, these guidelines provide recommendations to help the investigator plan and execute a full range of studies involving cardiac physiology.
GRANTS
Support from the following funding agencies is acknowledged by the authors: National Heart, Lung, and Blood Institute Grants HL-075360, HL-129823, HL-051971, and HL-131613; National Institute of General Medical Science Grants GM-104357 and GM-114833; American Heart Association Grant 14SDG18860050; Biomedical Laboratory Research and Development Service of the Veterans Affairs Office of Research and Development Grant 5I01BX000505; Heart and Stroke Foundation (Canada); and Canadian Institute of Health Research.
DISCLOSURES
The content is solely the responsibility of the authors and does not necessarily represent the official views of any of the funding agencies listed. No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.L.L. conceived and designed research; M.L.L. prepared figures; M.L.L., Z.K., J.A.I.V., L.E.d.C.B., and M.S-C. drafted manuscript; M.L.L., Z.K., J.A.I.V., L.E.d.C.B., and M.S-C. edited and revised manuscript; M.L.L., Z.K., J.A.I.V., L.E.d.C.B., and M.S-C. approved final version of manuscript.
REFERENCES
- 1.Abdurrachim D, Ciapaite J, Wessels B, Nabben M, Luiken JJ, Nicolay K, Prompers JJ. Cardiac diastolic dysfunction in high-fat diet fed mice is associated with lipotoxicity without impairment of cardiac energetics in vivo. Biochim Biophys Acta 1842: 1525–1537, 2014. doi: 10.1016/j.bbalip.2014.07.016. [DOI] [PubMed] [Google Scholar]
- 2.Adams S, Pacharinsak C. Mouse anesthesia and analgesia. Curr Protoc Mouse Biol 5: 51–63, 2015. doi: 10.1002/9780470942390.mo140179. [DOI] [PubMed] [Google Scholar]
- 3.Aghajani E, Muller S, Kjørstad KE, Korvald C, Nordhaug D, Revhaugand A, Myrmel T. The pressure-volume loop revisited: is the search for a cardiac contractility index a futile cycle? Shock 25: 370–376, 2006. doi: 10.1097/01.shk.0000209521.20496.7a. [DOI] [PubMed] [Google Scholar]
- 4.Aletras AH, Ding S, Balaban RS, Wen H. DENSE: displacement encoding with stimulated echoes in cardiac functional MRI. J Magn Reson 137: 247–252, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alvarez E, Dalton ND, Gu Y, Smith D, Luong A, Hoshijima M, Peterson KL, Rychak J. A novel method for quantitative myocardial contrast echocardiography in mice. Am J Physiol Heart Circ Physiol 314: H370−H379, 2018. doi: 10.1152/ajpheart.00568.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amundsen BH, Ericsson M, Seland JG, Pavlin T, Ellingsen Ø, Brekken C. A comparison of retrospectively self-gated magnetic resonance imaging and high-frequency echocardiography for characterization of left ventricular function in mice. Lab Anim 45: 31–37, 2011. doi: 10.1258/la.2010.010094. [DOI] [PubMed] [Google Scholar]
- 7.An X, Wang J, Li H, Lu Z, Bai Y, Xiao H, Zhang Y, Song Y. Speckle tracking based strain analysis is sensitive for early detection of pathological cardiac hypertrophy. PLoS One 11: e0149155, 2016. doi: 10.1371/journal.pone.0149155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aoyagi T, Kusakari Y, Xiao CY, Inouye BT, Takahashi M, Scherrer-Crosbie M, Rosenzweig A, Hara K, Matsui T. Cardiac mTOR protects the heart against ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 303: H75–H85, 2012. doi: 10.1152/ajpheart.00241.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arias T, Chen J, Fayad ZA, Fuster V, Hajjar RJ, Chemaly ER. Comparison of echocardiographic measurements of left ventricular volumes to full volume magnetic resonance imaging in normal and diseased rats. J Am Soc Echocardiogr 26: 910–918, 2013. doi: 10.1016/j.echo.2013.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aronsen JM, Espe EKS, Skårdal K, Hasic A, Zhang L, Sjaastad I. Noninvasive stratification of postinfarction rats based on the degree of cardiac dysfunction using magnetic resonance imaging and echocardiography. Am J Physiol Heart Circ Physiol 312: H932–H942, 2017. doi: 10.1152/ajpheart.00668.2016. [DOI] [PubMed] [Google Scholar]
- 11.Asson-Batres MA, Ryzhov S, Tikhomirov O, Duarte CW, Congdon CB, Lessard CR, McFarland S, Rochette-Egly C, Tran TL, Galindo CL, Favreau-Lessard AJ, Sawyer DB. Effects of vitamin A deficiency in the postnatal mouse heart: role of hepatic retinoid stores. Am J Physiol Heart Circ Physiol 310: H1773–H1789, 2016. doi: 10.1152/ajpheart.00887.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baessler B, Schaarschmidt F, Dick A, Michels G, Maintz D, Bunck AC. Diagnostic implications of magnetic resonance feature tracking derived myocardial strain parameters in acute myocarditis. Eur J Radiol 85: 218–227, 2016. doi: 10.1016/j.ejrad.2015.11.023. [DOI] [PubMed] [Google Scholar]
- 13.Bai T, Hu X, Zheng Y, Wang S, Kong J, Cai L. Resveratrol protects against lipopolysaccharide-induced cardiac dysfunction by enhancing SERCA2a activity through promoting the phospholamban oligomerization. Am J Physiol Heart Circ Physiol 311: H1051–H1062, 2016. doi: 10.1152/ajpheart.00296.2016. [DOI] [PubMed] [Google Scholar]
- 14.Bakermans AJ, Geraedts TR, van Weeghel M, Denis S, João Ferraz M, Aerts JM, Aten J, Nicolay K, Houten SM, Prompers JJ. Fasting-induced myocardial lipid accumulation in long-chain acyl-CoA dehydrogenase knockout mice is accompanied by impaired left ventricular function. Circ Cardiovasc Imaging 4: 558–565, 2011. doi: 10.1161/CIRCIMAGING.111.963751. [DOI] [PubMed] [Google Scholar]
- 15.Barlow SC, Doviak H, Jacobs J, Freeburg LA, Perreault PE, Zellars KN, Moreau K, Villacreses CF, Smith S, Khakoo AY, Lee T, Spinale FG. Intracoronary delivery of recombinant TIMP-3 after myocardial infarction: effects on myocardial remodeling and function. Am J Physiol Heart Circ Physiol 313: H690–H699, 2017. doi: 10.1152/ajpheart.00114.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Basu R, Lee J, Wang Z, Patel VB, Fan D, Das SK, Liu GC, John R, Scholey JW, Oudit GY, Kassiri Z. Loss of TIMP3 selectively exacerbates diabetic nephropathy. Am J Physiol Renal Physiol 303: F1341–F1352, 2012. doi: 10.1152/ajprenal.00349.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Basu R, Oudit GY, Wang X, Zhang L, Ussher JR, Lopaschuk GD, Kassiri Z. Type 1 diabetic cardiomyopathy in the Akita (Ins2WT/C96Y) mouse model is characterized by lipotoxicity and diastolic dysfunction with preserved systolic function. Am J Physiol Heart Circ Physiol 297: H2096–H2108, 2009. doi: 10.1152/ajpheart.00452.2009. [DOI] [PubMed] [Google Scholar]
- 18.Baudouy D, Michiels JF, Vukolic A, Wagner KD, Wagner N. Echocardiographic and histological examination of cardiac morphology in the mouse. J Vis Exp. In press. doi: 10.3791/55843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bauer M, Cheng S, Jain M, Ngoy S, Theodoropoulos C, Trujillo A, Lin FC, Liao R. Echocardiographic speckle-tracking based strain imaging for rapid cardiovascular phenotyping in mice. Circ Res 108: 908–916, 2011. doi: 10.1161/CIRCRESAHA.110.239574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benavides-Vallve C, Corbacho D, Iglesias-Garcia O, Pelacho B, Albiasu E, Castaño S, Muñoz-Barrutia A, Prosper F, Ortiz-de-Solorzano C. New strategies for echocardiographic evaluation of left ventricular function in a mouse model of long-term myocardial infarction. PLoS One 7: e41691, 2012. doi: 10.1371/journal.pone.0041691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beyhoff N, Brix S, Betz IR, Klopfleisch R, Foryst-Ludwig A, Krannich A, Stawowy P, Knebel F, Grune J, Kintscher U. Application of speckle-tracking echocardiography in an experimental model of isolated subendocardial damage. J Am Soc Echocardiogr 30: 1239–1250.e2, 2017. doi: 10.1016/j.echo.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 22.Birch CL, Behunin SM, Lopez-Pier MA, Danilo C, Lipovka Y, Saripalli C, Granzier H, Konhilas JP. Sex dimorphisms of crossbridge cycling kinetics in transgenic hypertrophic cardiomyopathy mice. Am J Physiol Heart Circ Physiol 311: H125–H136, 2016. doi: 10.1152/ajpheart.00592.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bohl S, Lygate CA, Barnes H, Medway D, Stork LA, Schulz-Menger J, Neubauer S, Schneider JE. Advanced methods for quantification of infarct size in mice using three-dimensional high-field late gadolinium enhancement MRI. Am J Physiol Heart Circ Physiol 296: H1200–H1208, 2009. doi: 10.1152/ajpheart.01294.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borlaug BA, Kass DA. Invasive hemodynamic assessment in heart failure. Heart Fail Clin 5: 217–228, 2009. doi: 10.1016/j.hfc.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brittain E, Penner NL, West J, Hemnes A. Echocardiographic assessment of the right heart in mice. J Vis Exp. In press. doi: 10.3791/50912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buonincontri G, Methner C, Carpenter TA, Hawkes RC, Sawiak SJ, Krieg T. MRI and PET in mouse models of myocardial infarction. J Vis Exp 2013: e50806, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cao J, Singh SP, McClung J, Joseph G, Vanella L, Barbagallo I, Jiang H, Falck JR, Arad M, Shapiro JI, Abraham NG. EET intervention on Wnt1, NOV, and HO-1 signaling prevents obesity-induced cardiomyopathy in obese mice. Am J Physiol Heart Circ Physiol 313: H368−H380, 2017. doi: 10.1152/ajpheart.00093.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carbone L, Austin J. Pain and laboratory animals: publication practices for better data reproducibility and better animal welfare. PLoS One 11: e0155001, 2016. doi: 10.1371/journal.pone.0155001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chacko VP, Aresta F, Chacko SM, Weiss RG. MRI/MRS assessment of in vivo murine cardiac metabolism, morphology, and function at physiological heart rates. Am J Physiol Heart Circ Physiol 279: H2218–H2224, 2000. doi: 10.1152/ajpheart.2000.279.5.H2218. [DOI] [PubMed] [Google Scholar]
- 30.Chen J, Ceholski DK, Liang L, Fish K, Hajjar RJ. Variability in coronary artery anatomy affects consistency of cardiac damage after myocardial infarction in mice. Am J Physiol Heart Circ Physiol 313: H275−H282, 2017. doi: 10.1152/ajpheart.00127.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen J, Song SK, Liu W, McLean M, Allen JS, Tan J, Wickline SA, Yu X. Remodeling of cardiac fiber structure after infarction in rats quantified with diffusion tensor MRI. Am J Physiol Heart Circ Physiol 285: H946–H954, 2003. doi: 10.1152/ajpheart.00889.2002. [DOI] [PubMed] [Google Scholar]
- 32.Chuang JS, Zemljic-Harpf A, Ross RS, Frank LR, McCulloch AD, Omens JH. Determination of three-dimensional ventricular strain distributions in gene-targeted mice using tagged MRI. Magn Reson Med 64: 1281–1288, 2010. doi: 10.1002/mrm.22547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Colazzo F, Castiglioni L, Sironi L, Fontana L, Nobili E, Franzosi M, Guerrini U. Murine left atrium and left atrial appendage structure and function: echocardiographic and morphologic evaluation. PLoS One 10: e0125541, 2015. doi: 10.1371/journal.pone.0125541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coolen BF, Moonen RP, Paulis LE, Geelen T, Nicolay K, Strijkers GJ. Mouse myocardial first-pass perfusion MR imaging. Magn Reson Med 64: 1658–1663, 2010. doi: 10.1002/mrm.22588. [DOI] [PubMed] [Google Scholar]
- 35.Creemers EE, Davis JN, Parkhurst AM, Leenders P, Dowdy KB, Hapke E, Hauet AM, Escobar PG, Cleutjens JP, Smits JF, Daemen MJ, Zile MR, Spinale FG. Deficiency of TIMP-1 exacerbates LV remodeling after myocardial infarction in mice. Am J Physiol Heart Circ Physiol 284: H364–H371, 2003. doi: 10.1152/ajpheart.00511.2002. [DOI] [PubMed] [Google Scholar]
- 36.Crnkovic S, Schmidt A, Egemnazarov B, Wilhelm J, Marsh LM, Ghanim B, Klepetko W, Olschewski A, Olschewski H, Kwapiszewska G. Functional and molecular factors associated with TAPSE in hypoxic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 311: L59–L73, 2016. doi: 10.1152/ajplung.00381.2015. [DOI] [PubMed] [Google Scholar]
- 37.Daire JL, Jacob JP, Hyacinthe JN, Croisille P, Montet-Abou K, Richter S, Botsikas D, Lepetit-Coiffé M, Morel D, Vallée JP. Cine and tagged cardiovascular magnetic resonance imaging in normal rat at 1.5 T: a rest and stress study. J Cardiovasc Magn Reson 10: 48, 2008. doi: 10.1186/1532-429X-10-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dal-Secco D, DalBó S, Lautherbach NE, Gava FN, Celes MR, Benedet PO, Souza AH, Akinaga J, Lima V, Silva KP, Kiguti LRA, Rossi MA, Kettelhut IC, Pupo AS, Cunha FQ, Assreuy J. Cardiac hyporesponsiveness in severe sepsis is associated with nitric oxide-dependent activation of G protein receptor kinase. Am J Physiol Heart Circ Physiol 313: H149–H163, 2017. doi: 10.1152/ajpheart.00052.2016. [DOI] [PubMed] [Google Scholar]
- 39.Daniel LL, Scofield SL, Thrasher P, Dalal S, Daniels CR, Foster CR, Singh M, Singh K. Ataxia telangiectasia-mutated kinase deficiency exacerbates left ventricular dysfunction and remodeling late after myocardial infarction. Am J Physiol Heart Circ Physiol 311: H445–H452, 2016. doi: 10.1152/ajpheart.00338.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dawson D, Lygate CA, Saunders J, Schneider JE, Ye X, Hulbert K, Noble JA, Neubauer S. Quantitative 3-dimensional echocardiography for accurate and rapid cardiac phenotype characterization in mice. Circulation 110: 1632–1637, 2004. doi: 10.1161/01.CIR.0000142049.14227.AD. [DOI] [PubMed] [Google Scholar]
- 41.DeLeon-Pennell KY, Tian Y, Zhang B, Cates CA, Iyer RP, Cannon P, Shah P, Aiyetan P, Halade GV, Ma Y, Flynn E, Zhang Z, Jin YF, Zhang H, Lindsey ML. CD36 is a matrix metalloproteinase-9 substrate that stimulates neutrophil apoptosis and removal during cardiac remodeling. Circ Cardiovasc Genet 9: 14–25, 2016. doi: 10.1161/CIRCGENETICS.115.001249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Devine RD, Bicer S, Reiser PJ, Wold LE. Increased hypoxia-inducible factor-1α in striated muscle of tumor-bearing mice. Am J Physiol Heart Circ Physiol 312: H1154–H1162, 2017. doi: 10.1152/ajpheart.00090.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dorsey SM, McGarvey JR, Wang H, Nikou A, Arama L, Koomalsingh KJ, Kondo N, Gorman JH III, Pilla JJ, Gorman RC, Wenk JF, Burdick JA. MRI evaluation of injectable hyaluronic acid-based hydrogel therapy to limit ventricular remodeling after myocardial infarction. Biomaterials 69: 65–75, 2015. doi: 10.1016/j.biomaterials.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Drelicharz Ł, Woźniak M, Skórka T, Tyrankiewicz U, Heinze-Paluchowska S, Jabłońska M, Gebska A, Chłopicki S. Application of magnetic resonance imaging in vivo for the assessment of the progression of systolic and diastolic dysfunction in a mouse model of dilated cardiomyopathy. Kardiol Pol 67: 386–395, 2009. [PubMed] [Google Scholar]
- 45.Du J, Zhang L, Wang Z, Yano N, Zhao YT, Wei L, Dubielecka-Szczerba P, Liu PY, Zhuang S, Qin G, Zhao TC. Exendin-4 induces myocardial protection through MKK3 and Akt-1 in infarcted hearts. Am J Physiol Cell Physiol 310: C270–C283, 2016. doi: 10.1152/ajpcell.00194.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.DuSablon A, Kent S, Coburn A, Virag J. EphA2-receptor deficiency exacerbates myocardial infarction and reduces survival in hyperglycemic mice. Cardiovasc Diabetol 13: 114, 2014. doi: 10.1186/s12933-014-0114-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Epstein FH. MR in mouse models of cardiac disease. NMR Biomed 20: 238–255, 2007. doi: 10.1002/nbm.1152. [DOI] [PubMed] [Google Scholar]
- 48.Erhardt W, Hebestedt A, Aschenbrenner G, Pichotka B, Blümel G. A comparative study with various anesthetics in mice (pentobarbitone, ketamine-xylazine, carfentanyl-etomidate). Res Exp Med (Berl) 184: 159–169, 1984. doi: 10.1007/BF01852390. [DOI] [PubMed] [Google Scholar]
- 49.Eroglu A. The effect of intravenous anesthetics on ischemia-reperfusion injury. BioMed Res Int 2014: 821513, 2014. doi: 10.1155/2014/821513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fan D, Takawale A, Basu R, Patel V, Lee J, Kandalam V, Wang X, Oudit GY, Kassiri Z. Differential role of TIMP2 and TIMP3 in cardiac hypertrophy, fibrosis, and diastolic dysfunction. Cardiovasc Res 103: 268–280, 2014. doi: 10.1093/cvr/cvu072. [DOI] [PubMed] [Google Scholar]
- 51.Ferreira VM, Piechnik SK, Dall’Armellina E, Karamitsos TD, Francis JM, Choudhury RP, Friedrich MG, Robson MD, Neubauer S. Non-contrast T1-mapping detects acute myocardial edema with high diagnostic accuracy: a comparison to T2-weighted cardiovascular magnetic resonance. J Cardiovasc Magn Reson 14: 42–42, 2012. doi: 10.1186/1532-429X-14-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Franklin S, Kimball T, Rasmussen TL, Rosa-Garrido M, Chen H, Tran T, Miller MR, Gray R, Jiang S, Ren S, Wang Y, Tucker HO, Vondriska TM. The chromatin-binding protein Smyd1 restricts adult mammalian heart growth. Am J Physiol Heart Circ Physiol 311: H1234–H1247, 2016. doi: 10.1152/ajpheart.00235.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gao XM, Wu QZ, Kiriazis H, Su Y, Han LP, Pearson JT, Taylor AJ, Du XJ. Microvascular leakage in acute myocardial infarction: characterization by histology, biochemistry, and magnetic resonance imaging. Am J Physiol Heart Circ Physiol 312: H1068–H1075, 2017. doi: 10.1152/ajpheart.00073.2017. [DOI] [PubMed] [Google Scholar]
- 55.Gargiulo S, Greco A, Gramanzini M, Esposito S, Affuso A, Brunetti A, Vesce G. Mice anesthesia, analgesia, and care, part I: anesthetic considerations in preclinical research. ILAR J 53: E55–E69, 2012. doi: 10.1093/ilar.53.1.55. [DOI] [PubMed] [Google Scholar]
- 56.Gilliam AD, Epstein FH, Acton ST. Cardiac motion recovery via active trajectory field models. IEEE Trans Inf Technol Biomed 13: 226–235, 2009. doi: 10.1109/TITB.2008.2009221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gillis TE, Klaiman JM, Foster A, Platt MJ, Huber JS, Corso MY, Simpson JA. Dissecting the role of the myofilament in diaphragm dysfunction during the development of heart failure in mice. Am J Physiol Heart Circ Physiol 310: H572–H586, 2016. doi: 10.1152/ajpheart.00773.2015. [DOI] [PubMed] [Google Scholar]
- 58.Gilson WD, Kraitchman DL. Cardiac magnetic resonance imaging in small rodents using clinical 1.5 T and 3.0 T scanners. Methods 43: 35–45, 2007. doi: 10.1016/j.ymeth.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.González GE, Rhaleb NE, D’Ambrosio MA, Nakagawa P, Liao TD, Peterson EL, Leung P, Dai X, Janic B, Liu YH, Yang XP, Carretero OA. Cardiac-deleterious role of galectin-3 in chronic angiotensin II-induced hypertension. Am J Physiol Heart Circ Physiol 311: H1287–H1296, 2016. doi: 10.1152/ajpheart.00096.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Haggerty CM, Kramer SP, Skrinjar O, Binkley CM, Powell DK, Mattingly AC, Epstein FH, Fornwalt BK. Quantification of left ventricular volumes, mass, and ejection fraction using cine displacement encoding with stimulated echoes (DENSE) MRI. J Magn Reson Imaging 40: 398–406, 2014. doi: 10.1002/jmri.24350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Halade GV, Kain V, Ingle KA. Heart functional and structural compendium of cardiosplenic and cardiorenal networks in acute and chronic heart failure pathology. Am J Physiol Heart Circ Physiol 314: H255−H267, 2018. doi: 10.1152/ajpheart.00528.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hartley CJ, Reddy AK, Madala S, Michael LH, Entman ML, Taffet GE. Doppler estimation of reduced coronary flow reserve in mice with pressure overload cardiac hypertrophy. Ultrasound Med Biol 34: 892–901, 2008. doi: 10.1016/j.ultrasmedbio.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hartley CJ, Reddy AK, Taffet GE. In-vitro evaluation of sensors and amplifiers to measure left ventricular pressure in mice. Conf Proc IEEE Eng Med Biol Soc 2008: 965–968, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Heijman E, Strijkers GJ, Habets J, Janssen B, Nicolay K. Magnetic resonance imaging of regional cardiac function in the mouse. MAGMA 17: 170–178, 2004. doi: 10.1007/s10334-004-0082-4. [DOI] [PubMed] [Google Scholar]
- 65.Hellström M, Ericsson M, Johansson B, Faraz M, Anderson F, Henriksson R, Nilsson SK, Hedman H. Cardiac hypertrophy and decreased high-density lipoprotein cholesterol in Lrig3-deficient mice. Am J Physiol Regul Integr Comp Physiol 310: R1045–R1052, 2016. doi: 10.1152/ajpregu.00309.2015. [DOI] [PubMed] [Google Scholar]
- 66.Herold V, Parczyk M, Mörchel P, Ziener CH, Klug G, Bauer WR, Rommel E, Jakob PM. In vivo measurement of local aortic pulse-wave velocity in mice with MR microscopy at 17.6 tesla. Magn Reson Med 61: 1293–1299, 2009. doi: 10.1002/mrm.21957. [DOI] [PubMed] [Google Scholar]
- 67.Herrmann K-H, Schmidt S, Kretz A, Haenold R, Krumbein I, Metzler M, Gaser C, Witte OW, Reichenbach JR. Possibilities and limitations for high resolution small animal MRI on a clinical whole-body 3T scanner. MAGMA 25: 233–244, 2012. doi: 10.1007/s10334-011-0284-5. [DOI] [PubMed] [Google Scholar]
- 68.Hoit BD. Spectral and color M-mode Doppler in genetically altered mice. Assessment of diastolic function. Minerva Cardioangiol 51: 609–618, 2003. [PubMed] [Google Scholar]
- 69.Hoyer C, Gass N, Weber-Fahr W, Sartorius A. Advantages and challenges of small animal magnetic resonance imaging as a translational tool. Neuropsychobiology 69: 187–201, 2014. doi: 10.1159/000360859. [DOI] [PubMed] [Google Scholar]
- 70.Huang J, Wu J, Wang S, You J, Ye Y, Ding Z, Yang F, Wang X, Guo J, Ma L, Yuan J, Shen Y, Yang X, Sun A, Jiang H, Bu L, Backx PH, Ge J, Zou Y. Ultrasound biomicroscopy validation of a murine model of cardiac hypertrophic preconditioning: comparison with a hemodynamic assessment. Am J Physiol Heart Circ Physiol 313: H138–H148, 2017. doi: 10.1152/ajpheart.00004.2017. [DOI] [PubMed] [Google Scholar]
- 71.Huang S, Sosnovik DE. Molecular and microstructural imaging of the myocardium. Curr Cardiovasc Imaging Rep 3: 26–33, 2010. doi: 10.1007/s12410-010-9007-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hyacinthe JN, Ivancevic MK, Daire JL, Vallée JP. Feasibility of complementary spatial modulation of magnetization tagging in the rat heart after manganese injection. NMR Biomed 21: 15–21, 2008. doi: 10.1002/nbm.1144. [DOI] [PubMed] [Google Scholar]
- 73.Ibrahim SH. Myocardial tagging by cardiovascular magnetic resonance: evolution of techniques–pulse sequences, analysis algorithms, and applications. J Cardiovasc Magn Reson 13: 36, 2011. doi: 10.1186/1532-429X-13-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ichinose F, Bloch KD, Wu JC, Hataishi R, Aretz HT, Picard MH, Scherrer-Crosbie M. Pressure overload-induced LV hypertrophy and dysfunction in mice are exacerbated by congenital NOS3 deficiency. Am J Physiol Heart Circ Physiol 286: H1070–H1075, 2004. doi: 10.1152/ajpheart.00940.2003. [DOI] [PubMed] [Google Scholar]
- 75.Ikeda M, Wakasaki R, Schenning KJ, Swide T, Lee JH, Miller MB, Choi HS, Anderson S, Hutchens MP. Determination of renal function and injury using near-infrared fluorimetry in experimental cardiorenal syndrome. Am J Physiol Renal Physiol 312: F629–F639, 2017. doi: 10.1152/ajprenal.00573.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Inoue T, Ikeda M, Ide T, Fujino T, Matsuo Y, Arai S, Saku K, Sunagawa K. Twinkle overexpression prevents cardiac rupture after myocardial infarction by alleviating impaired mitochondrial biogenesis. Am J Physiol Heart Circ Physiol 311: H509–H519, 2016. doi: 10.1152/ajpheart.00044.2016. [DOI] [PubMed] [Google Scholar]
- 77.Ishimoto T, Taniguchi Y, Miyati T, Kawakami M, Ishihara M. Non-contrast coronary artery wall and plaque imaging using inversion-recovery prepared steady-state free precession. BMC Med Imaging 15: 26, 2015. doi: 10.1186/s12880-015-0071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Iyer RP, de Castro Brás LE, Cannon PL, Ma Y, DeLeon-Pennell KY, Jung M, Flynn ER, Henry JB, Bratton DR, White JA, Fulton LK, Grady AW, Lindsey ML. Defining the sham environment for post-myocardial infarction studies in mice. Am J Physiol Heart Circ Physiol 311: H822–H836, 2016. doi: 10.1152/ajpheart.00067.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Iyer RP, de Castro Brás LE, Patterson NL, Bhowmick M, Flynn ER, Asher M, Cannon PL, Deleon-Pennell KY, Fields GB, Lindsey ML. Early matrix metalloproteinase-9 inhibition post-myocardial infarction worsens cardiac dysfunction by delaying inflammation resolution. J Mol Cell Cardiol 100: 109–117, 2016. doi: 10.1016/j.yjmcc.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Janssen BJ, De Celle T, Debets JJ, Brouns AE, Callahan MF, Smith TL. Effects of anesthetics on systemic hemodynamics in mice. Am J Physiol Heart Circ Physiol 287: H1618–H1624, 2004. doi: 10.1152/ajpheart.01192.2003. [DOI] [PubMed] [Google Scholar]
- 81.Jellis CL, Yingchoncharoen T, Gai N, Kusunose K, Popović ZB, Flamm S, Kwon D. Correlation between right ventricular T1 mapping and right ventricular dysfunction in non-ischemic cardiomyopathy. Int J Cardiovasc Imaging 34: 1–11, 2017. doi: 10.1007/s10554-017-1113-3. [DOI] [PubMed] [Google Scholar]
- 82.Jiang K, Yu X. Quantification of regional myocardial wall motion by cardiovascular magnetic resonance. Quant Imaging Med Surg 4: 345–357, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jorge AJ, Ribeiro ML, Rosa ML, Licio FV, Fernandes LC, Lanzieri PG, Jorge BA, Brito FO, Mesquita ET. Left atrium measurement in patients suspected of having heart failure with preserved ejection fraction. Arq Bras Cardiol 98: 175−181, 2012. [DOI] [PubMed] [Google Scholar]
- 84.Jung M, Ma Y, Iyer RP, DeLeon-Pennell KY, Yabluchanskiy A, Garrett MR, Lindsey ML. IL-10 improves cardiac remodeling after myocardial infarction by stimulating M2 macrophage polarization and fibroblast activation. Basic Res Cardiol 112: 33, 2017. doi: 10.1007/s00395-017-0622-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kaimoto S, Hoshino A, Ariyoshi M, Okawa Y, Tateishi S, Ono K, Uchihashi M, Fukai K, Iwai-Kanai E, Matoba S. Activation of PPAR-α in the early stage of heart failure maintained myocardial function and energetics in pressure-overload heart failure. Am J Physiol Heart Circ Physiol 312: H305–H313, 2017. doi: 10.1152/ajpheart.00553.2016. [DOI] [PubMed] [Google Scholar]
- 86.Kandalam V, Basu R, Abraham T, Wang X, Awad A, Wang W, Lopaschuk GD, Maeda N, Oudit GY, Kassiri Z. Early activation of matrix metalloproteinases underlies the exacerbated systolic and diastolic dysfunction in mice lacking TIMP3 following myocardial infarction. Am J Physiol Heart Circ Physiol 299: H1012–H1023, 2010. doi: 10.1152/ajpheart.00246.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kandalam V, Basu R, Abraham T, Wang X, Soloway PD, Jaworski DM, Oudit GY, Kassiri Z. TIMP2 deficiency accelerates adverse post-myocardial infarction remodeling because of enhanced MT1-MMP activity despite lack of MMP2 activation. Circ Res 106: 796–808, 2010. doi: 10.1161/CIRCRESAHA.109.209189. [DOI] [PubMed] [Google Scholar]
- 88.Kandalam V, Basu R, Moore L, Fan D, Wang X, Jaworski DM, Oudit GY, Kassiri Z. Lack of tissue inhibitor of metalloproteinases 2 leads to exacerbated left ventricular dysfunction and adverse extracellular matrix remodeling in response to biomechanical stress. Circulation 124: 2094–2105, 2011. doi: 10.1161/CIRCULATIONAHA.111.030338. [DOI] [PubMed] [Google Scholar]
- 89.Karamitsos TD, Piechnik SK, Banypersad SM, Fontana M, Ntusi NB, Ferreira VM, Whelan CJ, Myerson SG, Robson MD, Hawkins PN, Neubauer S, Moon JC. Noncontrast T1 mapping for the diagnosis of cardiac amyloidosis. JACC Cardiovasc Imaging 6: 488–497, 2013. doi: 10.1016/j.jcmg.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 90.Kawai S, Takagi Y, Kaneko S, Kurosawa T. Effect of three types of mixed anesthetic agents alternate to ketamine in mice. Exp Anim 60: 481–487, 2011. doi: 10.1538/expanim.60.481. [DOI] [PubMed] [Google Scholar]
- 91.Kholmukhamedov A, Logdon C, Hu J, McKinney RA, Spinale FG, Lemasters JJ, Mukherjee R. Cyclosporin A in left ventricular remodeling after myocardial infarction. Am J Physiol Heart Circ Physiol 306: H53–H59, 2014. doi: 10.1152/ajpheart.00079.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kim RJ, Albert TS, Wible JH, Elliott MD, Allen JC, Lee JC, Parker M, Napoli A, Judd RM; Gadoversetamide Myocardial Infarction Imaging Investigators . Performance of delayed-enhancement magnetic resonance imaging with gadoversetamide contrast for the detection and assessment of myocardial infarction: an international, multicenter, double-blinded, randomized trial. Circulation 117: 629–637, 2008. doi: 10.1161/CIRCULATIONAHA.107.723262. [DOI] [PubMed] [Google Scholar]
- 93.Kinno M, Nagpal P, Horgan S, Waller AH. Comparison of echocardiography, cardiac magnetic resonance, and computed tomographic imaging for the evaluation of left ventricular myocardial function: part 1 (global assessment). Curr Cardiol Rep 19: 9, 2017. doi: 10.1007/s11886-017-0815-4. [DOI] [PubMed] [Google Scholar]
- 94.Kinno M, Nagpal P, Horgan S, Waller AH. Comparison of echocardiography, cardiac magnetic resonance, and computed tomographic imaging for the evaluation of left ventricular myocardial function: part 2 (diastolic and regional assessment). Curr Cardiol Rep 19: 6, 2017. doi: 10.1007/s11886-017-0816-3. [DOI] [PubMed] [Google Scholar]
- 95.Kober F, Iltis I, Cozzone PJ, Bernard M. Myocardial blood flow mapping in mice using high-resolution spin labeling magnetic resonance imaging: influence of ketamine/xylazine and isoflurane anesthesia. Magn Reson Med 53: 601–606, 2005. doi: 10.1002/mrm.20373. [DOI] [PubMed] [Google Scholar]
- 96.Kohut A, Patel N, Singh H. Comprehensive echocardiographic assessment of the right ventricle in murine models. J Cardiovasc Ultrasound 24: 229–238, 2016. doi: 10.4250/jcu.2016.24.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Krishnamurthy R, Cheong B, Muthupillai R. Tools for cardiovascular magnetic resonance imaging. Cardiovasc Diagn Ther 4: 104–125, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lairez O, Lonjaret L, Ruiz S, Marchal P, Franchitto N, Calise D, Fourcade O, Mialet-Perez J, Parini A, Minville V. Anesthetic regimen for cardiac function evaluation by echocardiography in mice: comparison between ketamine, etomidate and isoflurane versus conscious state. Lab Anim 47: 284–290, 2013. doi: 10.1177/0023677213496236. [DOI] [PubMed] [Google Scholar]
- 99.Lapinskas T, Grune J, Zamani SM, Jeuthe S, Messroghli D, Gebker R, Meyborg H, Kintscher U, Zaliunas R, Pieske B, Stawowy P, Kelle S. Cardiovascular magnetic resonance feature tracking in small animals−a preliminary study on reproducibility and sample size calculation. BMC Med Imaging 17: 51, 2017. doi: 10.1186/s12880-017-0223-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lester SJ, Tajik AJ, Nishimura RA, Oh JK, Khandheria BK, Seward JB. Unlocking the mysteries of diastolic function: deciphering the Rosetta Stone 10 years later. J Am Coll Cardiol 51: 679–689, 2008. doi: 10.1016/j.jacc.2007.09.061. [DOI] [PubMed] [Google Scholar]
- 101.Li M, Akhavan-Sharif RM, Friedlander RM, Du R, Thiex R. What sequences on high-field MR best depict temporal resolution of experimental ICH and edema formation in mice? J Biomed Biotechnol 2012: 961461, 2012. doi: 10.1155/2012/961461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li W, Jin D, Hata M, Takai S, Yamanishi K, Shen W, El-Darawish Y, Yamanishi H, Okamura H. Dysfunction of mitochondria and deformed gap junctions in the heart of IL-18-deficient mice. Am J Physiol Heart Circ Physiol 311: H313–H325, 2016. doi: 10.1152/ajpheart.00927.2015. [DOI] [PubMed] [Google Scholar]
- 103.Li W, Lu M, Banerjee S, Zhong J, Ye A, Molter J, Yu X. Ex vivo diffusion tensor MRI reflects microscopic structural remodeling associated with aging and disease progression in normal and cardiomyopathic Syrian hamsters. NMR Biomed 22: 819–825, 2009. doi: 10.1002/nbm.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li W, Yu X. Quantification of myocardial strain at early systole in mouse heart: restoration of undeformed tagging grid with single-point HARP. J Magn Reson Imaging 32: 608–614, 2010. doi: 10.1002/jmri.22256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li Y, Garson CD, Xu Y, Beyers RJ, Epstein FH, French BA, Hossack JA. Quantification and MRI validation of regional contractile dysfunction in mice post myocardial infarction using high resolution ultrasound. Ultrasound Med Biol 33: 894–904, 2007. doi: 10.1016/j.ultrasmedbio.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lindsey ML, Bolli R, Canty J, Du X-J, Frangogiannis NG, Frantz S, Gourdie R, Holmes JW, Jones SP, Kloner RA, Lefer D, Liao R, Murphy E, Ping P, Przyklenk K, Recchia F, Schwartz Longacre L, Ripplinger CM, Van Eyk J, Heusch G. Guidelines for animal models of myocardial ischemia and infarction. Am J Physiol Heart Circ Physiol. doi: 10.1152/ajpheart.00335.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lindsey ML, Iyer RP, Zamilpa R, Yabluchanskiy A, DeLeon-Pennell KY, Hall ME, Kaplan A, Zouein FA, Bratton D, Flynn ER, Cannon PL, Tian Y, Jin YF, Lange RA, Tokmina-Roszyk D, Fields GB, de Castro Brás LE. A novel collagen matricryptin reduces left ventricular dilation post-myocardial infarction by promoting scar formation and angiogenesis. J Am Coll Cardiol 66: 1364–1374, 2015. doi: 10.1016/j.jacc.2015.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lottonen-Raikaslehto L, Rissanen R, Gurzeler E, Merentie M, Huusko J, Schneider JE, Liimatainen T, Ylä-Herttuala S. Left ventricular remodeling leads to heart failure in mice with cardiac-specific overexpression of VEGF-B167: echocardiography and magnetic resonance imaging study. Physiol Rep 5: e13096, 2017. doi: 10.14814/phy2.13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lucchinetti E, Jamnicki M, Fischer G, Zaugg M. Preconditioning by isoflurane retains its protection against ischemia-reperfusion injury in postinfarct remodeled rat hearts. Anesth Analg 106: 17–23, table of contents, 2008. doi: 10.1213/01.ane.0000289527.70545.ed. [DOI] [PubMed] [Google Scholar]
- 110.Luetkens JA, Homsi R, Sprinkart AM, Doerner J, Dabir D, Kuetting DL, Block W, Andrié R, Stehning C, Fimmers R, Gieseke J, Thomas DK, Schild HH, Naehle CP. Incremental value of quantitative CMR including parametric mapping for the diagnosis of acute myocarditis. Eur Heart J Cardiovasc Imaging 17: 154–161, 2016. doi: 10.1093/ehjci/jev246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Luetkens JA, Schlesinger-Irsch U, Kuetting DL, Dabir D, Homsi R, Doerner J, Schmeel FC, Fimmers R, Sprinkart AM, Naehle CP, Schild HH, Thomas D. Feature-tracking myocardial strain analysis in acute myocarditis: diagnostic value and association with myocardial oedema. Eur Radiol 27: 4661–4671, 2017. doi: 10.1007/s00330-017-4854-4. [DOI] [PubMed] [Google Scholar]
- 112.Ma Y, Chiao YA, Clark R, Flynn ER, Yabluchanskiy A, Ghasemi O, Zouein F, Lindsey ML, Jin YF. Deriving a cardiac ageing signature to reveal MMP-9-dependent inflammatory signalling in senescence. Cardiovasc Res 106: 421–431, 2015. doi: 10.1093/cvr/cvv128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mani SK, Balasubramanian S, Zavadzkas JA, Jeffords LB, Rivers WT, Zile MR, Mukherjee R, Spinale FG, Kuppuswamy D. Calpain inhibition preserves myocardial structure and function following myocardial infarction. Am J Physiol Heart Circ Physiol 297: H1744–H1751, 2009. doi: 10.1152/ajpheart.00338.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Martherus R, Jain R, Takagi K, Mendsaikhan U, Turdi S, Osinska H, James JF, Kramer K, Purevjav E, Towbin JA. Accelerated cardiac remodeling in desmoplakin transgenic mice in response to endurance exercise is associated with perturbed Wnt/β-catenin signaling. Am J Physiol Heart Circ Physiol 310: H174–H187, 2016. doi: 10.1152/ajpheart.00295.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Masuyama T, Yamamoto K, Sakata Y, Doi R, Nishikawa N, Kondo H, Ono K, Kuzuya T, Sugawara M, Hori M. Evolving changes in Doppler mitral flow velocity pattern in rats with hypertensive hypertrophy. J Am Coll Cardiol 36: 2333–2338, 2000. doi: 10.1016/S0735-1097(00)01000-7. [DOI] [PubMed] [Google Scholar]
- 116.Mattson DL. Comparison of arterial blood pressure in different strains of mice. Am J Hypertens 14: 405–408, 2001. doi: 10.1016/S0895-7061(00)01285-1. [DOI] [PubMed] [Google Scholar]
- 117.Matyas C, Varga ZV, Mukhopadhyay P, Paloczi J, Lajtos T, Erdelyi K, Nemeth BT, Nan M, Hasko G, Gao B, Pacher P. Chronic plus binge ethanol feeding induces myocardial oxidative stress, mitochondrial and cardiovascular dysfunction, and steatosis. Am J Physiol Heart Circ Physiol 310: H1658–H1670, 2016. doi: 10.1152/ajpheart.00214.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mayorga M, Kiedrowski M, Shamhart P, Forudi F, Weber K, Chilian WM, Penn MS, Dong F. Early upregulation of myocardial CXCR4 expression is critical for dimethyloxalylglycine-induced cardiac improvement in acute myocardial infarction. Am J Physiol Heart Circ Physiol 310: H20–H28, 2016. doi: 10.1152/ajpheart.00449.2015. [DOI] [PubMed] [Google Scholar]
- 119.Miyawaki A, Mitsuhara Y, Orimoto A, Nakayasu Y, Tsunoda S, Obana M, Maeda M, Nakayama H, Yoshioka Y, Tsutsumi Y, Fujio Y. Moesin is activated in cardiomyocytes in experimental autoimmune myocarditis and mediates cytoskeletal reorganization with protrusion formation. Am J Physiol Heart Circ Physiol 311: H476–H486, 2016. doi: 10.1152/ajpheart.00180.2016. [DOI] [PubMed] [Google Scholar]
- 120.Moerman KM, Sprengers AM, Simms CK, Lamerichs RM, Stoker J, Nederveen AJ. Validation of SPAMM tagged MRI based measurement of 3D soft tissue deformation. Med Phys 38: 1248–1260, 2011. doi: 10.1118/1.3533942. [DOI] [PubMed] [Google Scholar]
- 121.Moran CM, Thomson AJ, Rog-Zielinska E, Gray GA. High-resolution echocardiography in the assessment of cardiac physiology and disease in preclinical models. Exp Physiol 98: 629–644, 2013. doi: 10.1113/expphysiol.2012.068577. [DOI] [PubMed] [Google Scholar]
- 122.Moreth K, Afonso LC, Fuchs H, Gailus-Durner V, Katus HA, Bekeredjian R, Lehman L, Hrabě de Angelis M. High throughput phenotyping of left and right ventricular cardiomyopathy in calcineurin transgene mice. Int J Cardiovasc Imaging 31: 669–679, 2015. doi: 10.1007/s10554-015-0596-z. [DOI] [PubMed] [Google Scholar]
- 123.Mori J, Patel VB, Abo Alrob O, Basu R, Altamimi T, Desaulniers J, Wagg CS, Kassiri Z, Lopaschuk GD, Oudit GY. Angiotensin 1−7 ameliorates diabetic cardiomyopathy and diastolic dysfunction in db/db mice by reducing lipotoxicity and inflammation. Circ Heart Fail 7: 327–339, 2014. doi: 10.1161/CIRCHEARTFAILURE.113.000672. [DOI] [PubMed] [Google Scholar]
- 124.Moser E, Stahlberg F, Ladd ME, Trattnig S. 7-T MR–from research to clinical applications? NMR Biomed 25: 695–716, 2012. doi: 10.1002/nbm.1794. [DOI] [PubMed] [Google Scholar]
- 125.Mukherjee R, McQuinn TC, Dugan MA, Saul JP, Spinale FG. Cardiac function and circulating cytokines after endotoxin exposure in neonatal mice. Pediatr Res 68: 381–386, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mukherjee R, Mingoia JT, Bruce JA, Austin JS, Stroud RE, Escobar GP, McClister DM Jr, Allen CM, Alfonso-Jaume MA, Fini ME, Lovett DH, Spinale FG. Selective spatiotemporal induction of matrix metalloproteinase-2 and matrix metalloproteinase-9 transcription after myocardial infarction. Am J Physiol Heart Circ Physiol 291: H2216–H2228, 2006. doi: 10.1152/ajpheart.01343.2005. [DOI] [PubMed] [Google Scholar]
- 127.Nagueh SF, Smiseth OA, Appleton CP, Byrd BF III, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Popescu BA, Waggoner AD. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 29: 277–314, 2016. doi: 10.1016/j.echo.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 128.Naresh NK, Butcher JT, Lye RJ, Chen X, Isakson BE, Gan LM, Kramer CM, Annex BH, Epstein FH. Cardiovascular magnetic resonance detects the progression of impaired myocardial perfusion reserve and increased left-ventricular mass in mice fed a high-fat diet. J Cardiovasc Magn Reson 18: 53, 2016. doi: 10.1186/s12968-016-0273-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Naresh NK, Chen X, Moran E, Tian Y, French BA, Epstein FH. Repeatability and variability of myocardial perfusion imaging techniques in mice: comparison of arterial spin labeling and first-pass contrast-enhanced MRI. Magn Reson Med 75: 2394–2405, 2016. doi: 10.1002/mrm.25769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Naresh NK, Chen X, Roy RJ, Antkowiak PF, Annex BH, Epstein FH. Accelerated dual-contrast first-pass perfusion MRI of the mouse heart: development and application to diet-induced obese mice. Magn Reson Med 73: 1237–1245, 2015. doi: 10.1002/mrm.25238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Noseda M, Harada M, McSweeney S, Leja T, Belian E, Stuckey DJ, Abreu Paiva MS, Habib J, Macaulay I, de Smith AJ, al-Beidh F, Sampson R, Lumbers RT, Rao P, Harding SE, Blakemore AIF, Jacobsen SE, Barahona M, Schneider MD. PDGFRα demarcates the cardiogenic clonogenic Sca1+ stem/progenitor cell in adult murine myocardium. Nat Commun 6: 6930, 2015. doi: 10.1038/ncomms7930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Omiya S, Omori Y, Taneike M, Protti A, Yamaguchi O, Akira S, Shah AM, Nishida K, Otsu K. Toll-like receptor 9 prevents cardiac rupture after myocardial infarction in mice independently of inflammation. Am J Physiol Heart Circ Physiol 311: H1485–H1497, 2016. doi: 10.1152/ajpheart.00481.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ouzounian M, Lee DS, Liu PP. Diastolic heart failure: mechanisms and controversies. Nat Clin Pract Cardiovasc Med 5: 375–386, 2008. doi: 10.1038/ncpcardio1245. [DOI] [PubMed] [Google Scholar]
- 134.Pacher P, Nagayama T, Mukhopadhyay P, Bátkai S, Kass DA. Measurement of cardiac function using pressure-volume conductance catheter technique in mice and rats. Nat Protoc 3: 1422–1434, 2008. doi: 10.1038/nprot.2008.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pachon RE, Scharf BA, Vatner DE, Vatner SF. Best anesthetics for assessing left ventricular systolic function by echocardiography in mice. Am J Physiol Heart Circ Physiol 308: H1525–H1529, 2015. doi: 10.1152/ajpheart.00890.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Patel VB, Wang Z, Fan D, Zhabyeyev P, Basu R, Das SK, Wang W, Desaulniers J, Holland SM, Kassiri Z, Oudit GY. Loss of p47phox subunit enhances susceptibility to biomechanical stress and heart failure because of dysregulation of cortactin and actin filaments. Circ Res 112: 1542–1556, 2013. doi: 10.1161/CIRCRESAHA.111.300299. [DOI] [PubMed] [Google Scholar]
- 137.Perea RJ, Ortiz-Perez JT, Sole M, Cibeira MT, de Caralt TM, Prat-Gonzalez S, Bosch X, Berruezo A, Sanchez M, Blade J. T1 mapping: characterisation of myocardial interstitial space. Insights Imaging 6: 189–202, 2015. doi: 10.1007/s13244-014-0366-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Price AN, Cheung KK, Lim SY, Yellon DM, Hausenloy DJ, Lythgoe MF. Rapid assessment of myocardial infarct size in rodents using multi-slice inversion recovery late gadolinium enhancement CMR at 9.4T. J Cardiovasc Magn Reson 13: 44, 2011. doi: 10.1186/1532-429X-13-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pritchett AM, Mahoney DW, Jacobsen SJ, Rodeheffer RJ, Karon BL, Redfield MM. Diastolic dysfunction and left atrial volume: a population-based study. J Am Coll Cardiol 45: 87–92, 2005. doi: 10.1016/j.jacc.2004.09.054. [DOI] [PubMed] [Google Scholar]
- 140.Protti A, Lavin B, Dong X, Lorrio S, Robinson S, Onthank D, Shah AM, Botnar RM. Assessment of myocardial remodeling using an elastin/tropoelastin specific agent with high field magnetic resonance imaging (MRI). J Am Heart Assoc 4: e001851, 2015. doi: 10.1161/JAHA.115.001851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Protti A, Sirker A, Shah AM, Botnar R. Late gadolinium enhancement of acute myocardial infarction in mice at 7T: cine-FLASH versus inversion recovery. J Magn Reson Imaging 32: 878–886, 2010. doi: 10.1002/jmri.22325. [DOI] [PubMed] [Google Scholar]
- 142.Raher MJ, Thibault H, Poh KK, Liu R, Halpern EF, Derumeaux G, Ichinose F, Zapol WM, Bloch KD, Picard MH, Scherrer-Crosbie M. In vivo characterization of murine myocardial perfusion with myocardial contrast echocardiography: validation and application in nitric oxide synthase 3 deficient mice. Circulation 116: 1250–1257, 2007. doi: 10.1161/CIRCULATIONAHA.107.707737. [DOI] [PubMed] [Google Scholar]
- 143.Raher MJ, Thibault HB, Buys ES, Kuruppu D, Shimizu N, Brownell AL, Blake SL, Rieusset J, Kaneki M, Derumeaux G, Picard MH, Bloch KD, Scherrer-Crosbie M. A short duration of high-fat diet induces insulin resistance and predisposes to adverse left ventricular remodeling after pressure overload. Am J Physiol Heart Circ Physiol 295: H2495–H2502, 2008. doi: 10.1152/ajpheart.00139.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ram R, Mickelsen DM, Theodoropoulos C, Blaxall BC. New approaches in small animal echocardiography: imaging the sounds of silence. Am J Physiol Heart Circ Physiol 301: H1765–H1780, 2011. doi: 10.1152/ajpheart.00559.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Richardson WJ, Clarke SA, Quinn TA, Holmes JW. Physiological implications of myocardial scar structure. Compr Physiol 5: 1877–1909, 2015. doi: 10.1002/cphy.c140067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Roth DM, Swaney JS, Dalton ND, Gilpin EA, Ross J Jr. Impact of anesthesia on cardiac function during echocardiography in mice. Am J Physiol Heart Circ Physiol 282: H2134–H2140, 2002. doi: 10.1152/ajpheart.00845.2001. [DOI] [PubMed] [Google Scholar]
- 147.Rowe GC, Asimaki A, Graham EL, Martin KD, Margulies KB, Das S, Saffitz J, Arany Z. Development of dilated cardiomyopathy and impaired calcium homeostasis with cardiac-specific deletion of ESRRβ. Am J Physiol Heart Circ Physiol 312: H662–H671, 2017. doi: 10.1152/ajpheart.00446.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sakai M, Suzuki T, Tomita K, Yamashita S, Palikhe S, Hattori K, Yoshimura N, Matsuda N, Hattori Y. Diminished responsiveness to dobutamine as an inotrope in mice with cecal ligation and puncture-induced sepsis: attribution to phosphodiesterase 4 upregulation. Am J Physiol Heart Circ Physiol 312: H1224–H1237, 2017. doi: 10.1152/ajpheart.00828.2016. [DOI] [PubMed] [Google Scholar]
- 149.Savadjiev P, Strijkers GJ, Bakermans AJ, Piuze E, Zucker SW, Siddiqi K. Heart wall myofibers are arranged in minimal surfaces to optimize organ function. Proc Natl Acad Sci USA 109: 9248–9253, 2012. doi: 10.1073/pnas.1120785109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Scherrer-Crosbie M. Role of echocardiography in studies of murine models of cardiac diseases. Arch Mal Coeur Vaiss 99: 237–241, 2006. [PubMed] [Google Scholar]
- 151.Scherrer-Crosbie M, Kurtz B. Ventricular remodeling and function: insights using murine echocardiography. J Mol Cell Cardiol 48: 512–517, 2010. doi: 10.1016/j.yjmcc.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Scherrer-Crosbie M, Thibault HB. Echocardiography in translational research: of mice and men. J Am Soc Echocardiogr 21: 1083–1092, 2008. doi: 10.1016/j.echo.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Scherrer-Crosbie M, Ullrich R, Bloch KD, Nakajima H, Nasseri B, Aretz HT, Lindsey ML, Vançon AC, Huang PL, Lee RT, Zapol WM, Picard MH. Endothelial nitric oxide synthase limits left ventricular remodeling after myocardial infarction in mice. Circulation 104: 1286–1291, 2001. doi: 10.1161/hc3601.094298. [DOI] [PubMed] [Google Scholar]
- 154.Schnelle M, Catibog N, Zhang M, Nabeebaccus AA, Anderson G, Richards DA, Sawyer G, Zhang X, Toischer K, Hasenfuss G, Monaghan MJ, Shah AM. Echocardiographic evaluation of diastolic function in mouse models of heart disease. J Mol Cell Cardiol 114: 20–28, 2018. doi: 10.1016/j.yjmcc.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Schumacher A, Khojeini E, Larson D. ECHO parameters of diastolic dysfunction. Perfusion 23: 291–296, 2008. doi: 10.1177/0267659109102485. [DOI] [PubMed] [Google Scholar]
- 156.Scruggs SB, Watson K, Su AI, Hermjakob H, Yates JR III, Lindsey ML, Ping P. Harnessing the heart of big data. Circ Res 116: 1115–1119, 2015. doi: 10.1161/CIRCRESAHA.115.306013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Seldin MM, Kim ED, Romay MC, Li S, Rau CD, Wang JJ, Krishnan KC, Wang Y, Deb A, Lusis AJ. A systems genetics approach identifies Trp53inp2 as a link between cardiomyocyte glucose utilization and hypertrophic response. Am J Physiol Heart Circ Physiol 312: H728–H741, 2017. doi: 10.1152/ajpheart.00068.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Shahid M, Spagnolli E, Ernande L, Thoonen R, Kolodziej SA, Leyton PA, Cheng J, Tainsh RE, Mayeur C, Rhee DK, Wu MX, Scherrer-Crosbie M, Buys ES, Zapol WM, Bloch KD, Bloch DB. BMP type I receptor ALK2 is required for angiotensin II-induced cardiac hypertrophy. Am J Physiol Heart Circ Physiol 310: H984–H994, 2016. doi: 10.1152/ajpheart.00879.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Shingu Y, Amorim P, Nguyen TD, Mohr FW, Schwarzer M, Doenst T. Myocardial performance (Tei) index is normal in diastolic and systolic heart failure induced by pressure overload in rats. Eur J Echocardiogr 11: 829–833, 2010. doi: 10.1093/ejechocard/jeq077. [DOI] [PubMed] [Google Scholar]
- 160.Sorrentino A, Borghetti G, Zhou Y, Cannata A, Meo M, Signore S, Anversa P, Leri A, Goichberg P, Qanud K, Jacobson JT, Hintze TH, Rota M. Hyperglycemia induces defective Ca2+ homeostasis in cardiomyocytes. Am J Physiol Heart Circ Physiol 312: H150–H161, 2017. doi: 10.1152/ajpheart.00737.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sosnovik DE, Garanger E, Aikawa E, Nahrendorf M, Figuiredo JL, Dai G, Reynolds F, Rosenzweig A, Weissleder R, Josephson L. Molecular MRI of cardiomyocyte apoptosis with simultaneous delayed-enhancement MRI distinguishes apoptotic and necrotic myocytes in vivo: potential for midmyocardial salvage in acute ischemia. Circ Cardiovasc Imaging 2: 460–467, 2009. doi: 10.1161/CIRCIMAGING.109.859678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Spinale FG. Assessment of cardiac function–basic principles and approaches. Compr Physiol 5: 1911–1946, 2015. doi: 10.1002/cphy.c140054. [DOI] [PubMed] [Google Scholar]
- 163.Spinale FG, Escobar GP, Hendrick JW, Clark LL, Camens SS, Mingoia JP, Squires CG, Stroud RE, Ikonomidis JS. Chronic matrix metalloproteinase inhibition following myocardial infarction in mice: differential effects on short and long-term survival. J Pharmacol Exp Ther 318: 966–973, 2006. doi: 10.1124/jpet.106.104455. [DOI] [PubMed] [Google Scholar]
- 164.Spinale FG, Escobar GP, Mukherjee R, Zavadzkas JA, Saunders SM, Jeffords LB, Leone AM, Beck C, Bouges S, Stroud RE. Cardiac-restricted overexpression of membrane type-1 matrix metalloproteinase in mice: effects on myocardial remodeling with aging. Circ Heart Fail 2: 351–360, 2009. doi: 10.1161/CIRCHEARTFAILURE.108.844845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Standage SW, Bennion BG, Knowles TO, Ledee DR, Portman MA, McGuire JK, Liles WC, Olson AK. PPARα augments heart function and cardiac fatty acid oxidation in early experimental polymicrobial sepsis. Am J Physiol Heart Circ Physiol 312: H239–H249, 2017. doi: 10.1152/ajpheart.00457.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Strijkers GJ, Bouts A, Blankesteijn WM, Peeters TH, Vilanova A, van Prooijen MC, Sanders HM, Heijman E, Nicolay K. Diffusion tensor imaging of left ventricular remodeling in response to myocardial infarction in the mouse. NMR Biomed 22: 182–190, 2009. doi: 10.1002/nbm.1299. [DOI] [PubMed] [Google Scholar]
- 167.Stuckey DJ, Carr CA, Tyler DJ, Clarke K. Cine-MRI versus two-dimensional echocardiography to measure in vivo left ventricular function in rat heart. NMR Biomed 21: 765–772, 2008. doi: 10.1002/nbm.1268. [DOI] [PubMed] [Google Scholar]
- 168.Suzuki K, Satoh K, Ikeda S, Sunamura S, Otsuki T, Satoh T, Kikuchi N, Omura J, Kurosawa R, Nogi M, Numano K, Sugimura K, Aoki T, Tatebe S, Miyata S, Mukherjee R, Spinale FG, Kadomatsu K, Shimokawa H. Basigin promotes cardiac fibrosis and failure in response to chronic pressure overload in mice. Arterioscler Thromb Vasc Biol 36: 636–646, 2016. doi: 10.1161/ATVBAHA.115.306686. [DOI] [PubMed] [Google Scholar]
- 169.Tabima DM, Hacker TA, Chesler NC. Measuring right ventricular function in the normal and hypertensive mouse hearts using admittance-derived pressure-volume loops. Am J Physiol Heart Circ Physiol 299: H2069–H2075, 2010. doi: 10.1152/ajpheart.00805.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Tae HJ, Petrashevskaya N, Marshall S, Krawczyk M, Talan M. Cardiac remodeling in the mouse model of Marfan syndrome develops into two distinctive phenotypes. Am J Physiol Heart Circ Physiol 310: H290–H299, 2016. doi: 10.1152/ajpheart.00354.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Takawale A, Fan D, Basu R, Shen M, Parajuli N, Wang W, Wang X, Oudit GY, Kassiri Z. Myocardial recovery from ischemia-reperfusion is compromised in the absence of tissue inhibitor of metalloproteinase 4. Circ Heart Fail 7: 652–662, 2014. doi: 10.1161/CIRCHEARTFAILURE.114.001113. [DOI] [PubMed] [Google Scholar]
- 172.Takawale A, Zhang P, Azad A, Wang W, Wang X, Murray AG, Kassiri Z. Myocardial overexpression of TIMP3 after myocardial infarction exerts beneficial effects through promoting angiogenesis and suppressing early proteolysis. Am J Physiol Heart Circ Physiol 313: H224−H236, 2017. doi: 10.1152/ajpheart.00108.2017. [DOI] [PubMed] [Google Scholar]
- 173.Tei C, Ling LH, Hodge DO, Bailey KR, Oh JK, Rodeheffer RJ, Tajik AJ, Seward JB. New index of combined systolic and diastolic myocardial performance: a simple and reproducible measure of cardiac function–a study in normals and dilated cardiomyopathy. J Cardiol 26: 357–366, 1995. [PubMed] [Google Scholar]
- 174.Tewes S, Gueler F, Chen R, Gutberlet M, Jang MS, Meier M, Mengel M, Hartung D, Wacker F, Rong S, Hueper K. Functional MRI for characterization of renal perfusion impairment and edema formation due to acute kidney injury in different mouse strains. PLoS One 12: e0173248, 2017. doi: 10.1371/journal.pone.0173248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Thibault H, Gomez L, Bergerot C, Augeul L, Scherrer-Crosbie M, Ovize M, Derumeaux G. Strain-rate imaging predicts the attenuation of left ventricular remodeling induced by ischemic postconditioning after myocardial infarction in mice. Circ Cardiovasc Imaging 4: 550–557, 2011. doi: 10.1161/CIRCIMAGING.110.962282. [DOI] [PubMed] [Google Scholar]
- 176.Thomas D, Dumont C, Pickup S, Misselwitz B, Zhou R, Horowitz J, Ferrari VA. T1-weighted cine FLASH is superior to IR imaging of post-infarction myocardial viability at 4.7T. J Cardiovasc Magn Reson 8: 345–352, 2006. doi: 10.1080/10976640500451986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Toba H, Cannon PL, Yabluchanskiy A, Iyer RP, D’Armiento J, Lindsey ML. Transgenic overexpression of macrophage matrix metalloproteinase-9 exacerbates age-related cardiac hypertrophy, vessel rarefaction, inflammation, and fibrosis. Am J Physiol Heart Circ Physiol 312: H375–H383, 2017. doi: 10.1152/ajpheart.00633.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Totzeck M, Hendgen-Cotta UB, French BA, Rassaf T. A practical approach to remote ischemic preconditioning and ischemic preconditioning against myocardial ischemia/reperfusion injury. J Biol Methods 3: e57, 2016. doi: 10.14440/jbm.2016.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Tsujita Y, Kato T, Sussman MA. Evaluation of left ventricular function in cardiomyopathic mice by tissue Doppler and color M-mode Doppler echocardiography. Echocardiography 22: 245–253, 2005. doi: 10.1111/j.0742-2822.2005.04014.x. [DOI] [PubMed] [Google Scholar]
- 180.Tyrankiewicz U, Skorka T, Jablonska M, Petkow-Dimitrow P, Chlopicki S. Characterization of the cardiac response to a low and high dose of dobutamine in the mouse model of dilated cardiomyopathy by MRI in vivo. J Magn Reson Imaging 37: 669–677, 2013. doi: 10.1002/jmri.23854. [DOI] [PubMed] [Google Scholar]
- 181.Ugander M, Bagi PS, Oki AJ, Chen B, Hsu L-Y, Aletras AH, Shah S, Greiser A, Kellman P, Arai AE. Myocardial edema as detected by pre-contrast T1 and T2 CMR delineates area at risk associated with acute myocardial infarction. JACC Cardiovasc Imaging 5: 596–603, 2012. doi: 10.1016/j.jcmg.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Vaillant F, Lauzier B, Ruiz M, Shi Y, Lachance D, Rivard ME, Bolduc V, Thorin E, Tardif JC, Des Rosiers C. Ivabradine and metoprolol differentially affect cardiac glucose metabolism despite similar heart rate reduction in a mouse model of dyslipidemia. Am J Physiol Heart Circ Physiol 311: H991–H1003, 2016. doi: 10.1152/ajpheart.00789.2015. [DOI] [PubMed] [Google Scholar]
- 183.Van Allen NR, Krafft PR, Leitzke AS, Applegate RL II, Tang J, Zhang JH. The role of volatile anesthetics in cardioprotection: a systematic review. Med Gas Res 2: 22, 2012. doi: 10.1186/2045-9912-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.van de Weijer T, van Ewijk PA, Zandbergen HR, Slenter JM, Kessels AG, Wildberger JE, Hesselink MK, Schrauwen P, Schrauwen-Hinderling VB, Kooi ME. Geometrical models for cardiac MRI in rodents: comparison of quantification of left ventricular volumes and function by various geometrical models with a full-volume MRI data set in rodents. Am J Physiol Heart Circ Physiol 302: H709–H715, 2012. doi: 10.1152/ajpheart.00710.2011. [DOI] [PubMed] [Google Scholar]
- 185.van den Borne SW, Isobe S, Verjans JW, Petrov A, Lovhaug D, Li P, Zandbergen HR, Ni Y, Frederik P, Zhou J, Arbo B, Rogstad A, Cuthbertson A, Chettibi S, Reutelingsperger C, Blankesteijn WM, Smits JF, Daemen MJ, Zannad F, Vannan MA, Narula N, Pitt B, Hofstra L, Narula J. Molecular imaging of interstitial alterations in remodeling myocardium after myocardial infarction. J Am Coll Cardiol 52: 2017–2028, 2008. doi: 10.1016/j.jacc.2008.07.067. [DOI] [PubMed] [Google Scholar]
- 186.van Nierop BJ, Coolen BF, Bax NA, Dijk WJ, van Deel ED, Duncker DJ, Nicolay K, Strijkers GJ. Myocardial perfusion MRI shows impaired perfusion of the mouse hypertrophic left ventricle. Int J Cardiovasc Imaging 30: 619–628, 2014. doi: 10.1007/s10554-014-0369-0. [DOI] [PubMed] [Google Scholar]
- 187.Vang A, Clements RT, Chichger H, Kue N, Allawzi A, O’Connell K, Jeong EM, Dudley SC Jr, Sakhatskyy P, Lu Q, Zhang P, Rounds S, Choudhary G. Effect of α7 nicotinic acetylcholine receptor activation on cardiac fibroblasts: a mechanism underlying RV fibrosis associated with cigarette smoke exposure. Am J Physiol Lung Cell Mol Physiol 312: L748–L759, 2017. doi: 10.1152/ajplung.00393.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Vanhoutte L, Gerber BL, Gallez B, Po C, Magat J, Jean-Luc B, Feron O, Moniotte S. High field magnetic resonance imaging of rodents in cardiovascular research. Basic Res Cardiol 111: 46, 2016. doi: 10.1007/s00395-016-0565-2. [DOI] [PubMed] [Google Scholar]
- 189.Verjans JW, Lovhaug D, Narula N, Petrov AD, Indrevoll B, Bjurgert E, Krasieva TB, Petersen LB, Kindberg GM, Solbakken M, Cuthbertson A, Vannan MA, Reutelingsperger CP, Tromberg BJ, Hofstra L, Narula J. Noninvasive imaging of angiotensin receptors after myocardial infarction. JACC Cardiovasc Imaging 1: 354–362, 2008. doi: 10.1016/j.jcmg.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Verkaik M, van Poelgeest E, Kwekkeboom RF, Ter Wee PM, van den Brom CE, Vervloet MG, Eringa EC. Myocardial contrast echocardiography in mice: technical and physiological aspects. Am J Physiol Heart Circ Physiol 314: H381−H391, 2018. doi: 10.1152/ajpheart.00242.2017. [DOI] [PubMed] [Google Scholar]
- 191.Voorhees AP, DeLeon-Pennell KY, Ma Y, Halade GV, Yabluchanskiy A, Iyer RP, Flynn E, Cates CA, Lindsey ML, Han HC. Building a better infarct: modulation of collagen cross-linking to increase infarct stiffness and reduce left ventricular dilation post-myocardial infarction. J Mol Cell Cardiol 85: 229–239, 2015. doi: 10.1016/j.yjmcc.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Wagenhaus B, Pohlmann A, Dieringer MA, Els A, Waiczies H, Waiczies S, Schulz-Menger J, Niendorf T. Functional and morphological cardiac magnetic resonance imaging of mice using a cryogenic quadrature radiofrequency coil. PLoS One 7: e42383, 2012. doi: 10.1371/journal.pone.0042383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Wang W, Zhang F, Xia Y, Zhao S, Yan W, Wang H, Lee Y, Li C, Zhang L, Lian K, Gao E, Cheng H, Tao L. Defective branched chain amino acid catabolism contributes to cardiac dysfunction and remodeling following myocardial infarction. Am J Physiol Heart Circ Physiol 311: H1160–H1169, 2016. doi: 10.1152/ajpheart.00114.2016. [DOI] [PubMed] [Google Scholar]
- 194.Weigand J, Nielsen JC, Sengupta PP, Sanz J, Srivastava S, Uppu S. Feature tracking-derived peak systolic strain compared to late gadolinium enhancement in troponin-positive myocarditis: a case-control study. Pediatr Cardiol 37: 696–703, 2016. doi: 10.1007/s00246-015-1333-z. [DOI] [PubMed] [Google Scholar]
- 195.Weinheimer CJ, Lai L, Kelly DP, Kovacs A. Novel mouse model of left ventricular pressure overload and infarction causing predictable ventricular remodelling and progression to heart failure. Clin Exp Pharmacol Physiol 42: 33–40, 2015. doi: 10.1111/1440-1681.12318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wiesmann F, Ruff J, Engelhardt S, Hein L, Dienesch C, Leupold A, Illinger R, Frydrychowicz A, Hiller KH, Rommel E, Haase A, Lohse MJ, Neubauer S. Dobutamine-stress magnetic resonance microimaging in mice: acute changes of cardiac geometry and function in normal and failing murine hearts. Circ Res 88: 563–569, 2001. doi: 10.1161/01.RES.88.6.563. [DOI] [PubMed] [Google Scholar]
- 197.Wiesmann F, Ruff J, Hiller KH, Rommel E, Haase A, Neubauer S. Developmental changes of cardiac function and mass assessed with MRI in neonatal, juvenile, and adult mice. Am J Physiol Heart Circ Physiol 278: H652–H657, 2000. doi: 10.1152/ajpheart.2000.278.2.H652. [DOI] [PubMed] [Google Scholar]
- 198.Wildgruber M, Bielicki I, Aichler M, Kosanke K, Feuchtinger A, Settles M, Onthank DC, Cesati RR, Robinson SP, Huber AM, Rummeny EJ, Walch AK, Botnar RM. Assessment of myocardial infarction and postinfarction scar remodeling with an elastin-specific magnetic resonance agent. Circ Cardiovasc Imaging 7: 321–329, 2014. doi: 10.1161/CIRCIMAGING.113.001270. [DOI] [PubMed] [Google Scholar]
- 199.Wu J, You J, Wang S, Ye Y, Wang X, Jia J, Zou Y. Letter to the editor: When what you see might not be what you get: prudent considerations of anesthetics for murine echocardiography. Am J Physiol Heart Circ Physiol 308: H1612–1613, 2015. doi: 10.1152/ajpheart.00286.2015. [DOI] [PubMed] [Google Scholar]
- 200.Wu Y, Chan CW, Nicholls JM, Liao S, Tse HF, Wu EX. MR study of the effect of infarct size and location on left ventricular functional and microstructural alterations in porcine models. J Magn Reson Imaging 29: 305–312, 2009. doi: 10.1002/jmri.21598. [DOI] [PubMed] [Google Scholar]
- 201.Xu H, van Deel ED, Johnson MR, Opić P, Herbert BR, Moltzer E, Sooranna SR, van Beusekom H, Zang WF, Duncker DJ, Roos-Hesselink JW. Pregnancy mitigates cardiac pathology in a mouse model of left ventricular pressure overload. Am J Physiol Heart Circ Physiol 311: H807–H814, 2016. doi: 10.1152/ajpheart.00056.2016. [DOI] [PubMed] [Google Scholar]
- 202.Yabluchanskiy A, Ma Y, DeLeon-Pennell KY, Altara R, Halade GV, Voorhees AP, Nguyen NT, Jin YF, Winniford MD, Hall ME, Han HC, Lindsey ML. Myocardial infarction superimposed on aging: MMP-9 deletion promotes M2 macrophage polarization. J Gerontol A Biol Sci Med Sci 71: 475–483, 2016. doi: 10.1093/gerona/glv034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Yang XP, Liu YH, Rhaleb NE, Kurihara N, Kim HE, Carretero OA. Echocardiographic assessment of cardiac function in conscious and anesthetized mice. Am J Physiol 277: H1967–H1974, 1999. [DOI] [PubMed] [Google Scholar]
- 204.Yuan L, Wang T, Liu F, Cohen ED, Patel VV. An evaluation of transmitral and pulmonary venous Doppler indices for assessing murine left ventricular diastolic function. J Am Soc Echocardiogr 23: 887–897, 2010. doi: 10.1016/j.echo.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Zavadzkas JA, Plyler RA, Bouges S, Koval CN, Rivers WT, Beck CU, Chang EI, Stroud RE, Mukherjee R, Spinale FG. Cardiac-restricted overexpression of extracellular matrix metalloproteinase inducer causes myocardial remodeling and dysfunction in aging mice. Am J Physiol Heart Circ Physiol 295: H1394–H1402, 2008. doi: 10.1152/ajpheart.00346.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Zhang F, Xia Y, Yan W, Zhang H, Zhou F, Zhao S, Wang W, Zhu D, Xin C, Lee Y, Zhang L, He Y, Gao E, Tao L. Sphingosine 1-phosphate signaling contributes to cardiac inflammation, dysfunction, and remodeling following myocardial infarction. Am J Physiol Heart Circ Physiol 310: H250–H261, 2016. doi: 10.1152/ajpheart.00372.2015. [DOI] [PubMed] [Google Scholar]
- 207.Zhang Y, Takagawa J, Sievers RE, Khan MF, Viswanathan MN, Springer ML, Foster E, Yeghiazarians Y. Validation of the wall motion score and myocardial performance indexes as novel techniques to assess cardiac function in mice after myocardial infarction. Am J Physiol Heart Circ Physiol 292: H1187–H1192, 2007. doi: 10.1152/ajpheart.00895.2006. [DOI] [PubMed] [Google Scholar]
- 208.Zhang Z, Friedman D, Dione DP, Lin BA, Duncan JS, Sinusas AJ, Sampath S. Assessment of left ventricular 2D flow pathlines during early diastole using spatial modulation of magnetization with polarity alternating velocity encoding: a study in normal volunteers and canine animals with myocardial infarction. Magn Reson Med 70: 766–775, 2013. doi: 10.1002/mrm.24517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Zhong J, Liu W, Yu X. Characterization of three-dimensional myocardial deformation in the mouse heart: an MR tagging study. J Magn Reson Imaging 27: 1263–1270, 2008. doi: 10.1002/jmri.21367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Zhong J, Liu W, Yu X. Transmural myocardial strain in mouse: quantification of high-resolution MR tagging using harmonic phase (HARP) analysis. Magn Reson Med 61: 1368–1373, 2009. doi: 10.1002/mrm.21942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Zhou R, Pickup S, Glickson JD, Scott CH, Ferrari VA. Assessment of global and regional myocardial function in the mouse using cine and tagged MRI. Magn Reson Med 49: 760–764, 2003. doi: 10.1002/mrm.10423. [DOI] [PubMed] [Google Scholar]
- 212.Zhou T, Li J, Zhao P, Liu H, Jia D, Jia H, He L, Cang Y, Boast S, Chen YH, Thibault H, Scherrer-Crosbie M, Goff SP, Li B. Palmitoyl acyltransferase Aph2 in cardiac function and the development of cardiomyopathy. Proc Natl Acad Sci U S A 112: 15666–15671, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Zile MR, Baicu CF, Stroud RE, Van Laer A, Arroyo J, Mukherjee R, Jones JA, Spinale FG. Pressure overload-dependent membrane type 1-matrix metalloproteinase induction: relationship to LV remodeling and fibrosis. Am J Physiol Heart Circ Physiol 302: H1429–H1437, 2012. doi: 10.1152/ajpheart.00580.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Zile MR, Baicu CF, Stroud RE, Van Laer AO, Jones JA, Patel R, Mukherjee R, Spinale FG. Mechanistic relationship between membrane type-1 matrix metalloproteinase and the myocardial response to pressure overload. Circ Heart Fail 7: 340–350, 2014. doi: 10.1161/CIRCHEARTFAILURE.113.000984. [DOI] [PMC free article] [PubMed] [Google Scholar]