Abstract
An intact blood-brain barrier (BBB) limits entry of proinflammatory and neurotoxic blood-derived factors into the brain parenchyma. The BBB is damaged in Alzheimer’s disease (AD), which contributes significantly to the progression of AD pathologies and cognitive decline. However, the mechanisms underlying BBB breakdown in AD remain elusive, and no interventions are available for treatment or prevention. We and others recently established that inhibition of the mammalian/mechanistic target of rapamycin (mTOR) pathway with rapamycin yields significant neuroprotective effects, improving cerebrovascular and cognitive function in mouse models of AD. To test whether mTOR inhibition protects the BBB in neurological diseases of aging, we treated hAPP(J20) mice modeling AD and low-density lipoprotein receptor-null (LDLR−/−) mice modeling vascular cognitive impairment with rapamycin. We found that inhibition of mTOR abrogates BBB breakdown in hAPP(J20) and LDLR−/− mice. Experiments using an in vitro BBB model indicated that mTOR attenuation preserves BBB integrity through upregulation of specific tight junction proteins and downregulation of matrix metalloproteinase-9 activity. Together, our data establish mTOR activity as a critical mediator of BBB breakdown in AD and, potentially, vascular cognitive impairment and suggest that rapamycin and/or rapalogs could be used for the restoration of BBB integrity.
NEW & NOTEWORTHY This report establishes mammalian/mechanistic target of rapamycin as a critical mediator of blood-brain barrier breakdown in models of Alzheimer's disease and vascular cognitive impairment and suggests that drugs targeting the target of rapamycin pathway could be used for the restoration of blood-brain barrier integrity in disease states.
Keywords: blood-brain barrier, brain endothelium, cerebrovasculature, mammalian/mechanistic target of rapamycin, rapamycin
INTRODUCTION
The blood-brain barrier (BBB) is a highly selective permeable barrier that dynamically regulates the exchange of molecules between the peripheral circulation and central nervous system. Cerebral capillaries are enclosed by a single layer of vascular endothelial cells connected by tight junctions, which prevent paracellular diffusion of various solutes and lipophilic compounds from the blood into the cerebrospinal fluid. Clinical and experimental studies have suggested that disruption of the BBB has a critical role in the pathogenesis of Alzheimer’s disease (AD) (6, 54, 65, 78, 89, 90). Through the damaged BBB, plasma-derived neurotoxic and proinflammatory factors, including immunoglobulins, fibrinogen, bacterial breakdown products, thrombin, hemoglobin, and iron, can enter the brain parenchyma, disrupting normal functioning of the central nervous system and contributing to microglial activation, neuroinflammation, and neurodegeneration (6, 29, 59, 90). Recent studies have supported the hypothesis that BBB disruption may be one of the earliest changes in the transition to mild cognitive impairment in AD pathogenesis (78), offering potential targets for intervention. Despite these advances, clinically relevant strategies for the prevention of BBB disruption in AD are not available.
Recent studies from our laboratories (38, 39, 70) and others (7, 43) have demonstrated that pharmacological inhibition of the mammalian/mechanistic target of rapamycin (mTOR) pathway with rapamycin, a United States Food and Drug Administration-approved drug that inhibits mTOR complex 1 (mTORC1), exerts significant, multifaceted protective effects in preclinical models of AD. Rapamycin treatment was shown to improve cognitive function, increase cerebral blood flow (CBF), and restore microvascular endothelial function in mouse models of AD (38, 39). Inhibition of mTOR has also been proposed to inhibit cellular processes of neurodegeneration (8, 61, 70).
The present study was designed to test the hypothesis that mTOR has a role in BBB dysfunction in AD. To test our hypothesis, we assessed rapamycin-induced changes in biomarkers of BBB breakdown (measured as in vivo extravasation of circulating fluorescently labeled dextran and leakage of fibrinogen into the brain parenchyma) as well as expression of specific tight junction proteins and matrix metalloproteinase (MMP)-9 in the hAPP(J20) mouse model of AD. To determine the direct effect of mTOR on endothelial barrier function, the effects of mTORC1 attenuation with rapamycin were assessed in cultured endothelial cells treated with amyloid-β (Aβ). Because recent studies have shown that BBB disruption may also contribute to the pathogenesis of non-AD-associated neurological dysfunction and cognitive impairments (33, 54, 77), we used high-fat diet (HFD)-fed low-density lipoprotein receptor-null (LDLR−/−) mice to assess whether mTOR attenuation protects BBB function in a novel mouse model of vascular cognitive impairment (VCI).
MATERIALS AND METHODS
Animals.
All experiments were performed under approval of the University of Texas Health Science Center at San Antonio Institutional Animal Care and Use Committee (Animal Welfare Assurance Number A3345-01) and in compliance with Animal Research: Reporting In Vivo Experiments guidelines for reporting animal experiments. hAPP(J20) mice, used for in vivo two-photon experiments, were maintained as previously described (39) by heterozygous crosses with C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME). Nontransgenic littermates were used as controls. Animals of both genotypes were randomly assigned to receive chow supplemented with either encapsulated rapamycin (14 ppm), prepared as described elsewhere (28), or vehicle chow containing the inactive Eudragit encapsulation material. Animals were 4 mo old when diets were started and 9–12 mo old when they were subjected to two-photon imaging.
Male and female LDLR−/− (B6.129S7-LdldrtmlHer/J) mice (Jackson Laboratories) were randomly assigned to a maintenance diet (AIN-76A, BioServ) or HFD (AIN-76A diet supplemented with 21% saturated milk fat and 0.2% cholesterol). Encapsulated rapamycin (14 ppm), prepared as previously described (28), was added to the HFD as indicated, and a high-fat vehicle diet containing only the encapsulation material for rapamycin (Eudragit) was used as a control. Diets were started when the mice were 5–6 mo of age and were continued for 10 mo. Mice were group housed and maintained on a 12:12-h light-dark cycle. Animals were euthanized by isoflurane overdose followed by cervical dislocation. Experimenters identified mice by strain [hAPP(J20) or LDL] and animal identification number but remained blinded to the treatment condition.
Surgical thinning of mouse skulls for visualization of cortical vessels.
Nine- to twelve-mo-old male hAPP(J20) mice or wild-type (WT) littermates were anesthetized under 1–2% isoflurane gas (Butler Schein, Dublin OH), hair was removed from the back of the head, and the exposed skin was swabbed with povidone-iodine (Betadine) and isopropanol. A water-warmed heating pad was used to maintain body temperature. A 1- to 1.5-cm incision was made above the midline of the skull, and a custom metal halo was securely glued directly to the dry skull using Vetbond (3M, St. Paul, MN) and stabilized by fastening to an ear bar holder mounted on a custom frame adapted for use with microscopy. The skull over the somatosensory cortex (1–3 mm posterior to bregma, 2–4 mm lateral) was first thinned using a variable-speed electric drill (Fine Science Tools, Foster City, CA) and further thinned to ~50 μm using a surgical razor blade. Care was taken to protect the skull from heat generated during thinning by wetting the field with room temperature sterile saline every 10–20 s. Once the skull was sufficiently thinned, clear cyanoacrylate glue was used to attach a no. 1.5 round glass coverslip directly to the skull. Any exposed skull not covered by glass was also covered with glue and allowed to dry. Mice were given 300–500 µl subcutaneous saline to prevent dehydration and were imaged.
Two-photon imaging and leakage analysis of the cortical vasculature.
Imaging was performed using a Prairie two-photon system equipped with a Nikon Eclipse SN1 upright microscope and acoustic optical deflection mode. An APO ×25 water-immersion objective with a 1.10-numerical aperture lens was used. The titanium-sapphire laser was tuned at each use to 750–900 nm. Mice were injected with 100 µl of filtered FITC-tagged 150-kDa dextran (TdB Consultancy, Uppsala, Sweden) in sterile saline (10 mg/ml) via the tail vein. Images were captured within 5 min of the initial injection as well as 30 min postinjection. The images represent a 50-µm-depth z projection (50 slices, 1 µm each) and were within 200 µm of the cortical surface. For measurement of dye leakage, the same 50-µm-depth field was compared at 5 and 30 min postinjection for each individual mouse. ImageJ (National Institutes of Health, Bethesda, MD) was used to subtract bright vasculature via thresholding, and the overall gray value of the background was determined. Percent increase was calculated by expression of background intensity at the 30-min time point as a percentage of the 5-min value.
Fibrinogen immunohistochemistry in LDLR−/− mice.
Cryosections (10 μm thick) were mounted on charged slides and fixed in ice-cold 4% paraformaldehyde in PBS for 30 min on a rocker. Slides were washed four times for 10 min in PBS and subsequently blocked in 5% BSA-5% goat serum in Tris-buffered saline (TBS) on a rocker for 1 h at room temperature. Rabbit anti-fibrinogen (catalog no. A0080, Dako, Glostrup, Denmark) and an Alexa 488-tagged tomato lectin (Dylight DL1174, Vector Laboratories, Burlingame, CA) were applied to sections at a 1:750 dilution in the aforementioned blocking solution overnight in a light-protected, humidified container on a rocker at 4°C. On the following day, slides were washed three times for 10 min in TBS. Alexa 594-tagged anti-rabbit secondary antibody was applied at 1:500 dilution in blocking solution for 1 h at room temperature on a rocker protected from light. Slides were again washed three times for 10 min in TBS, and coverslips were mounted using ProLong Gold antifade reagent (Life Technologies, Carlsbad, CA). A secondary antibody-only control section was included for the calculation of background values. Images of cortical vasculature were obtained on a Zeiss fluorescence microscope and processed in Adobe Photoshop while identical gain and contrast ratios were maintained. For each brain section, the raw grayscale images containing fibrinogen and corresponding tomato lectin staining were analyzed using ImageJ. The positively stained areas were identified using the threshold function, and the area of positive staining was quantified for both channels. The area of fibrinogen staining was divided by the area of tomato lectin staining to correct for vascular area within each section; therefore, a higher ratio indicates increased fibrinogen leakage from the vasculature.
In vitro BBB model.
An immortalized mouse brain endothelial cell line, bEnd.3 (American Type Culture Collection, Manassas, VA), was used for all in vitro experiments, as these cells display barrier properties comparable to those of primary brain endothelial cells (80). Endothelial cells were seeded onto Corning Transwell inserts with 0.4 μm pores (no. 3470) at a density of 5 × 104 cells/well in 300 μl DMEM (Life Technologies) containing 10% cosmic calf serum (CCS; HyClone/GE Healthcare, Little Chalfont, UK) and 1% penicillin-streptomycin (Life Technologies). The lower chamber of the Transwell system was supplied with 600 μl of the same medium. Cells were incubated at 37°C with 5% CO2 for 7 days; medium in both chambers was changed every other day. Once cells had achieved >95% confluence, they were treated with rapamycin (5.5 nM, LC Laboratories, Woburn, MA), excess (+1.2 mM) leucine (Fisher Scientific, Hampton, NH), or control medium for 24 h. At 3 h into the treatment period, FITC-tagged 150-kDa dextran (TdB Consultancy) was added to the upper (luminal) chamber at a final concentration of 0.5 mg/ml. Relative fluorescence (485-nm excitation/535-nm emission) was determined at the end of the 24-h incubation period by collection of 100-μl aliquots in triplicate from each lower (abluminal) chamber and measurement of fluorescence using the Victor plate reader (Perkin-Elmer, Waltham, MA). In experiments where Aβ was used to disrupt BBB function, 3 μM human Aβ-(1–40) (Abcam, Cambridge, MA) was added to the lower chamber and read from the upper chamber of each well to simulate brain-to-vessel transport. All Aβ experiments also used DMEM with only 0.1% CCS and 1% penicillin-streptomycin during the final 24-h treatment period. In experiments where drugs targeting the mTOR pathway were used, Aβ was applied concurrently with rapamycin, leucine, or control medium. MMP-9 was inhibited by treatment with a MMP-2/MMP-9 inhibitor (250 nM, Calbiochem, Temecula, CA) for 24 h.
Isolation of the mouse brain microvasculature.
Methods for isolation of the mouse brain microvasculature are based on those previously described by Wu et al. (83). Mouse brains were dissected, large pial vessels were removed, and the brain was minced into small pieces. The tissue was gently homogenized in MCDB131 medium (Life Technologies) with 2% CCS using a Dounce tissue grinder with a large-clearance pestle. The homogenate was centrifuged at 1,000 g for 5 min at 4°C. The pellet was resuspended in 15% dextran (~70,000 molecular weight, Sigma) in MCDB131 medium with 2% FBS and centrifuged at 10,000 rpm for 15 min at 4°C in a swing-bucket rotor (SW 40 TI, Beckman). The pellet containing brain microvessels was resuspended in 1× lysis buffer (Cell Signaling Technology) with protease inhibitors (cOmplete Mini, Roche). The lysate was sonicated briefly and centrifuged at 12,000 g for 15 min at 4°C to remove cellular debris.
Immunoblot analysis of tight junction proteins.
Samples were resolved by SDS-PAGE (10% Mini-PROTEAN TGX, Bio-Rad) and electroblotted onto nitrocellulose membrane (GE Healthcare). Each membrane was blocked (2% Advanced ECL blocking solution, GE Healthcare) and incubated with the respective primary and secondary antibodies. Primary antibodies were junctional adhesion molecule A (JAM-A; catalog no. 361700, Life Technologies), claudin-5 (catalog no. 352500, Life Technologies), zonula occludens-1 (ZO-1; catalog no. 339100, Life Technologies), and MMP-9 (catalog no. PA5-13199, ThermoFisher). Samples were visualized via chemiluminescence using the GE Healthcare ECL Western blotting reagent system. Band intensities within the linear range of the antibody combinations were obtained by exposure of the samples to GE Healthcare Hyperfilm ECL. Films were scanned at 300 dpi, and numeric band density and background values were acquired using ImageJ.
Statistical analyses.
Statistical analyses were performed using GraphPad Prism (GraphPad, San Diego, CA) and included three-way ANOVA, two-way ANOVA, or one-way ANOVA as appropriate. Values are means ± SE. P < 0.05 was considered significant.
Power analysis for animal experiments.
Group sizes were calculated using predicted effect sizes obtained from previous research or preliminary data generated in our laboratory. Power calculations were made using G*Power 3.1 (18). For in vivo two-photon experiments of BBB integrity in WT and hAPP(J20) mice, n = 4 mice/group was determined to be sufficient to provide 0.8 power at α = 0.05 because of the large effect size observed in preliminary data. Similarly, for ex vivo analysis of BBB integrity, n = 5 mice/group was sufficient to provide 0.8 power at α = 0.05. For protein expression analyses, we determined that, given an effect size of 0.6, n = 7 mice/group would be sufficient to provide 0.8 power at α = 0.05.
RESULTS
Chronic mTOR inhibition with rapamycin restores BBB integrity in the hAPP(J20) mouse model of AD.
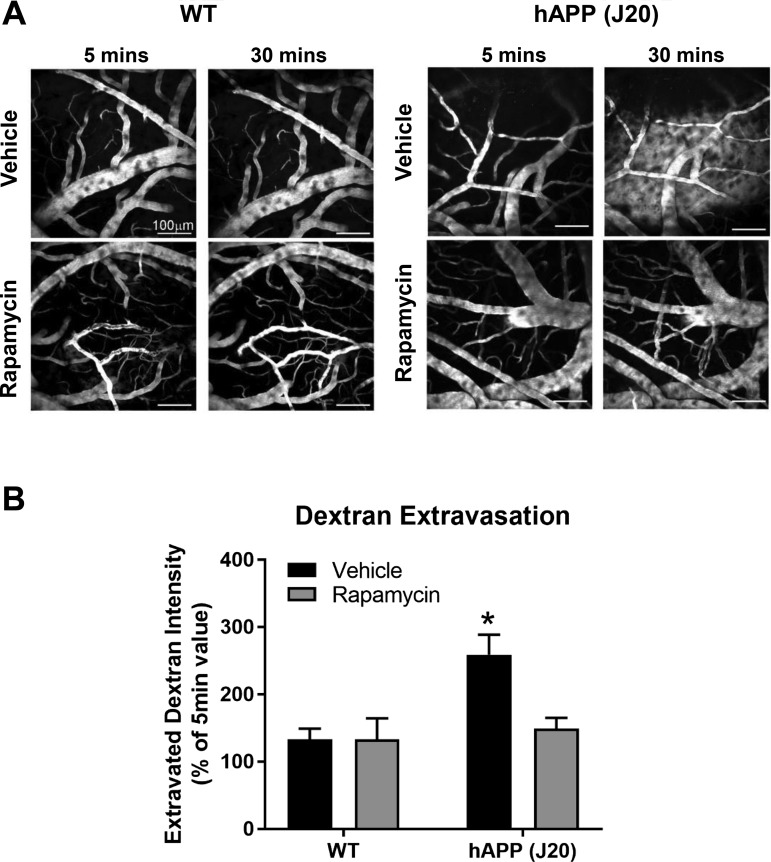
We have previously shown that chronic attenuation of mTOR restores cerebrovascular integrity and function in several mouse models of AD (39). To determine the role of mTOR in AD-induced BBB breakdown, we used two-photon optical imaging to measure BBB integrity in hAPP(J20) mice that had been treated with rapamycin for 16 wk starting at 8 mo of age. Untreated hAPP(J20) mice exhibited significant extravasation of intravenously injected FITC-dextran in layer 1 of the somatosensory cortex at 12 mo of age (Fig. 1), indicating BBB disruption. In contrast, levels of extravasated parenchymal FITC-dextran in rapamycin-treated hAPP(J20) animals were indistinguishable from those in littermate controls (Fig. 1). These data indicate that inhibition of mTOR activity protects against BBB breakdown induced by AD-like pathology.
Fig. 1.
Mammalian/mechanistic target of rapamycin (mTOR) inhibition prevents blood-brain barrier (BBB) breakdown in hAPP(J20) mice. A: representative two-photon images from transgenic hAPP(J20) mice and nontransgenic [wild-type (WT)] littermates treated with vehicle or rapamycin-supplemented diet showing extravasation of intravenous FITC-dextran at 5−30 min after injection to indicate BBB breakdown. Scale bar = 100 μm. B: quantitative analyses of rhodamine-dextran extravasation, calculated as background intensity at 30 min postinjection as a percentage of background intensity at 5 min postinjection. *Different from WT + vehicle by Tukey’s multiple-comparison post hoc test [q(10) = 5.01, P = 0.023] applied to a genotype × treatment interaction [F(1,10) = 4.80, P = 0.05]. Values are means ± SE; n = 3–4 mice/group.
Chronic mTOR inhibition by rapamycin restores BBB integrity in the LDLR−/− mouse model of VCI.
To determine whether rapamycin can exert protective effects on the BBB by inhibiting mTOR-dependent mechanisms common to other age-associated neurological diseases, we also tested the consequences of mTOR attenuation in LDLR−/− mice. LDLR−/− mice exhibit hypercholesterolemia and proatherosclerotic vascular alterations, which are associated with significant BBB disruption and cerebromicrovascular dysfunction (32), mimicking important aspects of the pathogenesis of vascular cognitive impairment in human patients. To determine the effects of rapamycin treatment on BBB integrity in LDLR−/− mice, we used immunohistochemistry to assess the extravasation of fibrinogen (19, 75), which is a well-defined and widely used marker of BBB breakdown in postmortem tissues (4, 5, 10, 26, 30, 62, 65, 66, 82). This approach was preferred to in vivo two-photon imaging, which involves 3- to 5-h surgeries, because of increased morbidity in HFD-fed LDLR−/− mice (25, 32, 41, 42, 50, 51).
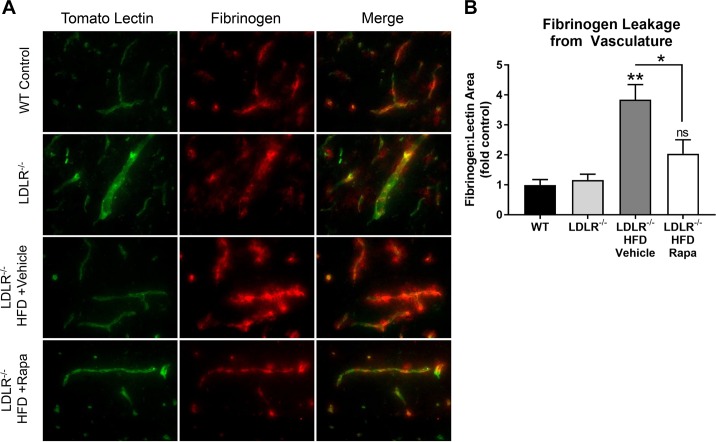
In the brains of control animals, fibrinogen was present only in the lectin-labeled microvessels, whereas in LDLR−/− mice, significant extravasation of fibrinogen was present, indicative of BBB disruption (Fig. 2). Rapamycin treatment prevented extravasation of fibrinogen in the brains of LDLR−/− mice, indicating rescue of BBB integrity (Fig. 2). Together, these data suggest that mTOR attenuation, in addition to reducing Aβ (70), protects the BBB through mechanisms that are common to AD- and VCI-like pathogenesis in mouse models.
Fig. 2.
Mammalian/mechanistic target of rapamycin (mTOR) inhibition attenuates blood-brain barrier (BBB) breakdown in high-fat diet (HFD)-fed low-density lipoprotein receptor-null (LDLR−/−) mice modeling atherosclerosis and vascular cognitive impairment. A: representative double-immunofluorescence microscopy images of cortical vasculature (illuminated with tomato lectin and fibrinogen, a BBB-impermeable serum protein) from wild-type (WT) and LDLR−/− mouse brains. Rapa, rapamycin. Magnification: ×40. B: quantitative analyses of fibrinogen extravasation, measured as increased fibrinogen-positive area relative to tomato lectin-positive area. Results were normalized to WT controls. **Different from WT and LDLR−/− by Tukey’s multiple-comparison post hoc test [q(15) > 6.14, P < 0.003 for both comparisons]. ns, Not significant. *Significant difference between LDLR−/− + HFD + vehicle (Veh) and LDLR−/− + HFD + rapamycin by Tukey’s multiple-comparison post hoc test [q(15) = 4.55, P = 0.03]. Post hoc tests were applied to a significant omnibus ANOVA [F(3,15) = 10.12, P = 0.0007]. Values are means ± SE; n = 4–6 mice/group.
mTOR regulates expression of tight junction proteins.
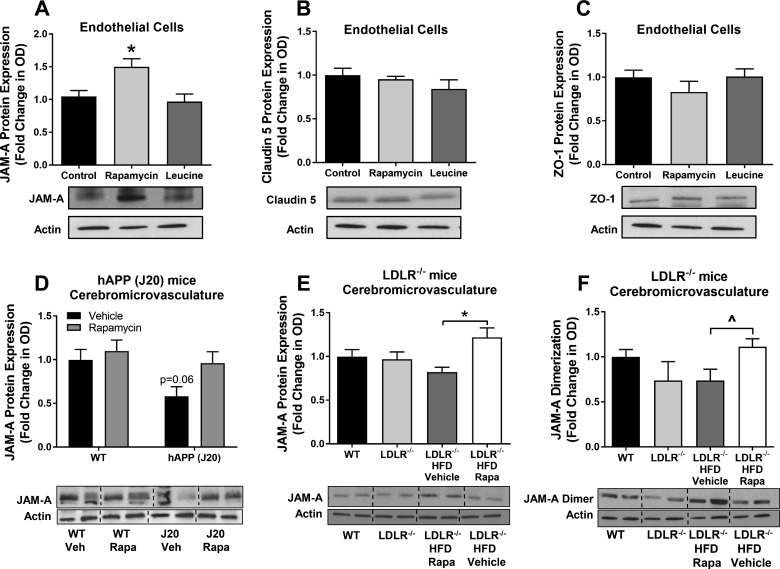
Tight junctions have a critical role in regulation of BBB permeability through the paracellular transport route (71). Thus, we sought to determine whether mTOR regulates BBB function by regulating the expression of key tight junction proteins in brain endothelial cells. In cultured mouse brain vascular endothelial cells, we found that rapamycin increased expression of JAM-A (Fig. 3A), a cell adhesion molecule involved in the formation and regulation of tight junctions (48, 86). In contrast, rapamycin did not alter expression of the tight junction proteins claudin-5 (Fig. 3B) and ZO-1 (Fig. 3C). Hyperactivation of mTOR by leucine also did not affect levels of JAM-A, claudin-5, or ZO-1 in cultured mouse brain vascular endothelial cells (Fig. 3, A–C). We next determined whether mTOR regulates tight junction protein levels in cerebral microvessels isolated from hAPP(J20) and LDLR−/− mice. A trend toward a decrease in JAM-A expression, which was prevented by rapamycin treatment (Fig. 3D), was observed in microvessels of hAPP(J20) mice. Rapamycin treatment also significantly increased expression of JAM-A in the brain microvasculature of LDLR−/− mice compared with control mice (Fig. 3E). Consistent with our in vitro data, microvascular expression of claudin-5 and ZO-1 was unaffected by rapamycin treatment in hAPP(J20) and LDLR−/− mice (data not shown).
Fig. 3.
Mammalian/mechanistic target of rapamycin (mTOR) attenuation increases junctional adhesion molecule A (JAM-A) in cultured endothelial cells and preserves JAM-A expression in cerebromicrovasculature isolated from hAPP(J20) and low-density lipoprotein receptor-null (LDLR−/−) mice. A–C: increased expression of JAM-A, but not claudin-5 or zonula occludens-1 (ZO-1), in confluent cultures of endothelial cells treated for 24 h with rapamycin. Representative Western blots are shown below graphs. *Significantly different from control [q(50) = 4.13, P = 0.014] by Tukey’s post hoc test applied to a significant one-way ANOVA [F(2,50) = 6.82, P = 0.002]. Values are means ± SE; n = 17–18 mice/group. D: trend toward reduced JAM-A expression in the brain microvasculature of hAPP(J20) mice treated with vehicle (Veh) [P = 0.06, t(26) = 2.45] indicated by Sidak’s post hoc comparisons with a control [wild-type (WT) + vehicle] group. Values are means ± SE; n = 7–8 mice/group. E: increased cerebromicrovascular JAM-A expression in brains of rapamycin (Rapa)-treated compared with vehicle-treated high-fat diet (HFD)-fed LDLR−/− mice. *q(27) = 4.59, P = 0.016 by Tukey’s post hoc test applied to a significant one-way ANOVA [F(3,27) = 3.65, P = 0.025]. Values are means ± SE; n = 7–8 mice/group. F: JAM-A dimerization may be restored by rapamycin treatment in HFD-fed LDLR−/− mice compared with vehicle treatment. ^t(10) = 2.45, P = 0.034 by Student’s t-test for comparison of contrasts of interest; however, one-way ANOVA failed to reach significance [F(3.20) = 1.94, P = 0.16]. Data were normalized to the control group. Values are means ± SE; n = 6 mice/group. In representative Western blots, the last two conditions are reversed compared with the graphs above.
Previous studies have demonstrated that JAM-A function depends on its homodimerization (46, 68). Thus, we next sought to determine how mTOR activation may impact JAM-A dimerization. To determine JAM-A dimerization, we immunoprecipitated JAM-A from lysed microvascular samples and used native gels to measure the presence of JAM-A dimers in the immunoprecipitated complexes in immunoblot experiments. We found that JAM-A dimers were significantly decreased in HFD-fed vehicle-treated compared with rapamycin-treated HFD-fed LDLR−/− mice (Fig. 3F), suggesting that, in addition to regulating JAM-A levels, mTOR may also regulate JAM-A dimerization. Levels of JAM-A dimers in the brain microvasculature of hAPP(J20) mice could not be measured because of low yields of brain microvascular fractions purified from mice from these groups.
mTOR attenuation by rapamycin protects barrier integrity in an in vitro model of BBB.
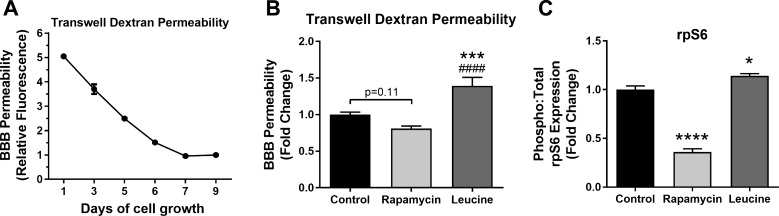
To determine the mechanisms by which mTOR drives BBB breakdown, we used an in vitro BBB model in which cultured mouse brain endothelial cells are grown to confluence on Transwell inserts to establish a barrier between two compartments in a culture system (1). Permeability through the established in vitro endothelial cell barrier was measured by testing movement of FITC-dextran from the upper chamber, which models the vascular compartment, into the lower chamber, which models the brain tissue compartment. Initial experiments determined that endothelial cells reached maximal barrier integrity by day 7 in culture (Fig. 4A). Thus, all experiments were conducted in cultured endothelial cells on day 7. We used FITC-labeled 150-kDa dextran to avoid confounding by artifactual paracellular diffusion arising from incomplete junction formation along the edges of the inner wall of the luminal chamber [i.e., “edge effect” (58)], which was a concern with lower-molecular-weight dextrans. The use of FITC-labeled 150-kDa dextran also allowed us to model BBB breakdown in vivo (Fig. 1). Edge effects (58) also precluded the use of mixtures of different-sized dextrans to determine the relative contribution of changes in transcellular transport (in which relatively homogeneous transport of different-sized molecules is expected) from changes in paracellular permeability (in which greater transport of smaller molecules is expected) in the permeability changes that were measured (49).
Fig. 4.
Hyperactivation of mammalian/mechanistic target of rapamycin (mTOR) induces blood-brain barrier (BBB) dysfunction in vitro. A: in vitro barrier function is established at day 7 in culture. B: in vitro endothelial cell barrier disruption is induced by overactivation of mTOR with excess leucine. ***Significantly different from control [q(92) = 6.39, P = 0.0001]. ####Significantly different from rapamycin [q(92) = 9.24, P < 0.0001]. Attenuation of mTOR activity with rapamycin produced a trend toward improved barrier function compared with control, as indicated by P = 0.11, with q(92) = 3.21. Tukey’s multiple-comparison post hoc tests were applied to a significant omnibus ANOVA [F(3.92) = 14.66, P < 0.0001]. Values are means ± SE; n = 21–27 mice/group. C: mTOR activity modulates ribosomal protein S6 (rpS6) phosphorylation. ****Significant reduction of rpS6 phosphorylation by mTOR inhibition with rapamycin vs. control [q(12) = 20.59, P < 0.0001]. *Significant increase in rpS6 phosphorylation by mTOR activation with leucine vs. control [q(12) = 4.60, P = 0.18]. Tukey’s post hoc tests were applied to a significant ANOVA [F(2,12) = 180.0, P < 0.0001]. Values are means ± SE; n = 5 mice/group.
We established in vivo that mTOR drives BBB disruption (Figs. 1 and 2), and we confirmed in our in vitro endothelial cell barrier model that overactivation of mTOR with excess leucine significantly increases barrier permeability (Fig. 4B). However, mTOR inhibition with rapamycin was not associated with improved barrier function, although a trend toward improvement was noted (Fig. 4B).
Consistent with its known inhibitory effect on mTORC1, treatment with rapamycin significantly reduced phosphorylation of ribosomal protein S6 (rpS6), a downstream target of mTORC1 (Fig. 4D). In contrast, overactivation of mTORC1 by treatment with leucine significantly increased phosphorylation of rpS6 (Fig. 4D). Together, these in vitro results provide additional evidence that increased mTOR activity disrupts endothelial barrier integrity and that barrier disruption is exacerbated by high Aβ, suggesting that mTOR attenuation may preserve in vitro barrier integrity when Aβ is present. Although the contribution of transcellular transport was not assessed in the present study, permeability to a relatively low-molecular-weight (150-kDa) dextran suggests that mTOR may drive increased in vitro BBB permeability by increasing paracellular transport (57). Future studies may directly address this hypothesis (57). Paracellular permeability is regulated by tight junctions; therefore, we next sought to determine the potential role of tight junction protein breakdown in Aβ-triggered in vitro barrier dysfunction and ascertain the role of mTOR.
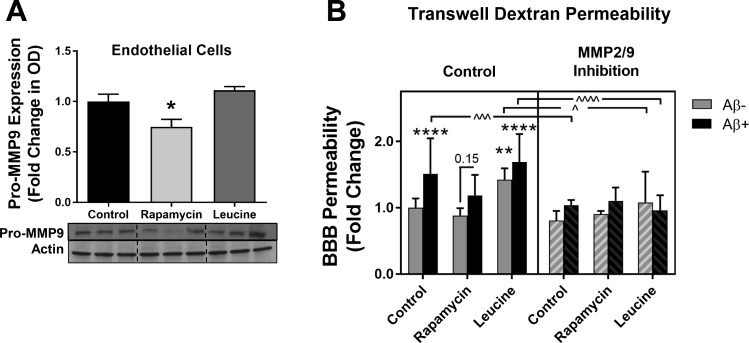
Inhibition of mTOR downregulates MMP-9: potential role in protection of endothelial barrier function.
Several studies have suggested that MMP-9 is involved in the breakdown of tight junction proteins that form the BBB (72, 85). It has also been reported that MMP-9 is downstream of mTOR (53). We found that inhibition of mTOR with rapamycin significantly decreased MMP-9 expression in endothelial cells (Fig. 5A). It is well established that Aβ disrupts BBB integrity. Therefore, we investigated the interaction of Aβ and MMP-9 at different levels of mTOR activation (Fig. 5B). We found that treatment with Aβ significantly increased barrier permeability, which was prevented by MMP-2/MMP-9 inhibition (250 nM, Calbiochem). Additionally, attenuation of mTOR with rapamycin prevented Aβ-induced increases in in vitro BBB permeability, suggesting that Aβ-induced breakdown of endothelial barrier function may, at least partially, be mitigated by mTOR attenuation in vitro (Fig. 5B). Furthermore, overactivation of mTOR by excess leucine was sufficient to induce endothelial barrier breakdown and also exacerbated Aβ-induced BBB permeability (Fig. 5B). Inhibition of MMP-2/MMP-9 was generally protective, as it prevented the barrier disruption induced by Aβ and mTOR overactivation with leucine (Fig. 5B). However, MMP-2/MMP-9 inhibition in rapamycin-treated cells did not afford additional protection (Fig. 5B), suggesting that MMPs are downstream of mTOR. Consistent with this notion, MMP inhibition prevented the exacerbation of BBB disruption by Aβ application during mTOR overactivation by leucine (Fig. 5B).
Fig. 5.
Matrix metalloproteinase (MMP)-9 activity is implicated in amyloid-β (Aβ)- and leucine-induced in vitro blood-brain barrier (BBB) breakdown. A: rapamycin-induced attenuation of mammalian/mechanistic target of rapamycin (mTOR) decreases pro-MMP-9 expression in cultured endothelial cells. OD, optical density. *Significant decrease vs. control [q(15) = 3.95, P = 0.034] by Tukey’s post hoc test on a significant one-way ANOVA [F(2,15) = 8.58, P = 0.003]. Values are means ± SE; n = 6 mice/condition. B: MMP-9 mediates Aβ-induced BBB breakdown in vitro as assessed by FITC-dextran leakage. Three-way ANOVA revealed significant main effects: MMP-9 [F(1,168) = 50.5, P < 0.0001], Aβ [F(1,168) = 30.2, P < 0.0001], and mTOR [F(2,168) = 14.6, P < 0.0001] activity. Holm-Sidak’s multiple-comparison post hoc test revealed that Aβ induced BBB permeability in control cells (****t = 4.96, P < 0.0001). mTOR overactivation with leucine was sufficient to induce barrier disruption (**t = 4.1, P = 0.0029) and exacerbate Aβ-induced barrier permeability (****t = 6.67, P < 0.0001). Attenuation of mTOR with rapamycin treatment prevented Aβ-induced disruption of barrier function (t = 2.92, P = 0.15). Furthermore, MMP-2/MMP-9 inhibition prevented barrier disruption by Aβ in control cells (^^^t = 4.58, P = 0.0005), barrier permeability induced by mTOR overactivation in leucine-treated cells (^t = 3.33, P = 0.044), and exacerbation of barrier permeability by Aβ in the presence of hyperactive mTOR (^^^^t = 7.06, P < 0.0001). Data were normalized to the control group and are expressed as fold change. Values are means ± SE; n = 15 mice/condition.
DISCUSSION
This is the first study to demonstrate that inhibition of mTOR with rapamycin protects the functional integrity of the BBB in a preclinical mouse model of AD. The BBB is critically dependent on the integrity and function of the microvascular endothelium (71), and our study provides strong evidence that inhibition of mTOR exerts significant protective effects directly in cerebromicrovascular endothelial cells in models of AD (Fig. 1) and VCI (Fig. 2). In support of this concept, we have previously demonstrated that mTOR attenuation improves microvascular endothelial vasodilator function and increases cerebromicrovascular density, rescuing CBF and cognitive impairments in the same mouse model of AD (39, 70). Collectively, these studies suggest that mTOR attenuation blocks the multifaceted phenotypic and functional alterations in cerebromicrovascular endothelial cells associated with AD and VCI pathologies.
Our findings have translational relevance, as increased BBB permeability likely represents a key mechanism in the early stages of AD (16, 54, 78). Rapamycin (sirolimus) is a United States Food and Drug Administration-approved drug originally used to prevent organ transplant rejection (64). The toxicity profiles of rapamycin and its derivatives are well characterized. Since the discovery that inhibition of mTOR with rapamycin extends the lifespan of mice (28, 52, 81), not by decreasing specific age-associated pathologies such as cancer but by delaying the aging process, there have been intensive research efforts to define the potential of mTOR attenuation by rapamycin to treat/prevent diseases, including, but not limited to, AD, in which increasing age contributes >90% of risk (64). Indeed, a recent study showed that a 6-wk course of rapamycin was well tolerated, improved the response to influenza vaccination in older (>65 yr old) individuals by ~20%, and reduced the percentage of CD4 and CD8 T lymphocytes expressing the programmed death-1 receptor, which inhibits T cell signaling and is increased with age, raising the possibility that mTOR attenuation may have beneficial effects on immunosenescence in the elderly.
Preclinical studies conducted in mouse models of AD have demonstrated that rapamycin confers significant cognitive benefits, which may be attributed, at least in part, to its protective effects on the cerebromicrovasculature (39). The mechanisms of BBB disruption in AD are likely multifaceted and may involve increased endothelial oxidative stress (9, 31), inflammation (24), and dysregulation of nitric oxide (NO) (14), among other mechanisms. Specifically, we demonstrated that rapamycin-induced restoration of cerebromicrovascular endothelial function in the hAPP(J20) model involved activation of endothelial NO synthase (eNOS) (39). Dysregulation of eNOS and decreased NO bioavailability have also been linked to development of amyloid plaques (34) and neurovascular uncoupling in AD (74), both of which are also mitigated by mTOR attenuation. Further studies are warranted to determine the role of increased NO bioavailability in the effects of rapamycin on the BBB. A common mechanism underlying endothelial dysfunction and BBB breakdown may involve eNOS uncoupling, whereby eNOS generates superoxide, rather than NO, during pathological conditions (37). Reactive oxygen species (ROS), including superoxides, are well known to disrupt BBB integrity (35, 67, 87), and mTOR regulates both BBB disruption (as these studies have demonstrated) and eNOS uncoupling. Specifically, inhibition of the downstream effector of mTOR, rpS6, has been shown to reduce superoxide production and enhance NO production in senescent endothelial cells (63). Therefore, mTOR may promote endothelial cell dysfunction, including eNOS uncoupling and BBB breakdown, through the production of ROS. In conditions of acute injury such as stroke, however, activation of the Akt-mTOR pathway was shown to promote neuronal survival in vivo (12, 15, 47, 79, 84) and in vitro (15). In particular, it was shown that mitochondrial depolarization with unchanged ROS production (indicating increased energy production vs. ROS generation) induced by treatment of neurons with diazoxide, a drug that can open mitochondrial ATP-dependent K+ channels, affords significant neuronal protection in an in vitro model of stroke involving oxygen and glucose deprivation and that this protective effect was dependent on the coactivation of the Akt-mTOR axis as a prosurvival pathway (15).
Although mTOR attenuation reduces Aβ accumulation in AD transgenic mice (70) and the present study demonstrates that mTOR partially mediates the deleterious effects of Aβ on endothelial barrier function in vitro (Fig. 5B), its protective effects on the endothelium are not unique to AD-like pathogenesis. In agreement with this notion, mTOR attenuation with rapamycin rescued BBB integrity in a non-Aβ-dependent model of VCI in LDLR−/− mice (32). LDLR−/− mice show significant cognitive dysfunction and CBF deficits (13, 32, 56) in the absence of Aβ pathology and, thus, may model VCI. Our recent studies (32, 38, 39) have indicated that mTOR attenuation restores cerebromicrovascular integrity and function, including CBF, and preserves learning and memory in the LDLR−/− mouse model of VCI. These observations are consistent with the hypothesis that mTOR-dependent cerebromicrovascular endothelial alterations may be common to the pathogeneses of several different neurological diseases of aging.
Our study suggests that the mechanisms by which inhibition of mTOR exerts protective effects on BBB function include upregulation of the tight junction scaffold-like protein JAM-A (Fig. 3, A, D, and E). There is growing evidence that JAM-A promotes recruitment and localization of proteins to tight junctions (3, 55), regulating barrier function (55, 68, 76), and that its decreased expression is associated with BBB breakdown (86). Thus, pharmacological upregulation of JAM-A may be critical to preservation of tight junction assembly and stability and, thereby, to restoration of BBB function (48, 86) in AD and non-AD types of dementias. JAM-A homodimerization has been shown to be integral to the maintenance of BBB function (2). Thus, treatments that promote JAM-A dimerization will likely confer additional benefits (Fig. 3F). While our study only examined the role of mTOR in regulation of the paracellular component of transport across the BBB, recent experimental evidence has pointed out an important role of mTOR in the regulation of transcellular transport pathways in epithelial cells of the renal tubule (22, 23, 91). However, evidence for a role of mTOR in the regulation of transcellular transport in endothelial cells is, at this point, restricted to its involvement in the control of autophagy (20, 21, 27, 88).
Activation of MMP-9, an enzyme that facilitates breakdown of the extracellular matrix, has been linked to disruption of the BBB (69, 73, 85). Our results extend these findings, demonstrating that Aβ- and mTOR-induced endothelial barrier dysfunction involves MMP activation. MMP-9 is downstream of mTOR (11, 36) and is regulated through the mTORC1 effector rpS6 (53). Consistent with this concept, our results show that, in brain endothelial cells, MMP-9 expression is downregulated by mTOR inhibition with rapamycin, which decreases rpS6 phosphorylation. Importantly, attenuation of mTOR with rapamycin and the subsequent downregulation of MMP-9 abolished the MMP-dependent component of Aβ-induced endothelial barrier dysfunction (Fig. 5B). Our present data, along with previous research (11, 36), suggest that mTOR activates MMP-9 to induce BBB disruption. Thus, we propose that MMP-9 activation may mechanistically link hyperactive mTOR activity to BBB breakdown through modulation of the extracellular matrix during progression of AD and that this pathway could be targeted by rapamycin. In addition to regulating expression of tight junction components and downregulating MMPs, attenuation of mTOR may also impact other pathways involved in cerebromicrovascular dysfunction during the initiation and/or progression of AD, including pathways regulating proteostasis and inflammation. Further studies are needed to explore the mechanistic roles of these pathways and their contribution to the protective effects of rapamycin treatment. Interestingly, mTOR1 has been shown to regulate the blood-testis barrier through rpS6, subsequent induction of MMP-9 activation, and MMP-9-mediated downregulation of tight junction proteins (53). Additionally, there is evidence that mTOR is involved in the regulation of tight junction-mediated intestinal barrier permeability, since inhibition of mTOR enhances intestinal barrier function in Caco-2 human epithelial colorectal cells (60) and maintains intestinal barrier function in aging Drosophila melanogaster (17). Some reports, however, have suggested that very high doses of sirolimus may disrupt gastrointestinal barrier function (40, 44, 45). This evidence suggests that mTOR1 may reduce the abundance of tight junction proteins at several blood-tissue barriers, possibly through MMP-9 activation. Therefore, mTOR attenuation via rapamycin or other rapalogs may be efficacious in restoring blood-tissue barrier function through the modulation of MMP-9 activity and tight junction proteins in multiple organ systems.
Our study suggests that mTOR drives BBB breakdown in AD and VCI and, therefore, that rapamycin or other inhibitors of the mTOR pathway could be beneficial in promoting BBB integrity in different neurological diseases of aging that share brain microvascular endothelial alterations as part of their pathology. We provide evidence that rapamycin may exert these beneficial BBB effects by altering various tight junction proteins and by preventing MMP-9 elevation under pathological conditions. Attenuation of mTOR-dependent cerebromicrovascular endothelial dysfunction may thus be an important novel target for pharmacological intervention in AD as well as mixed-type dementias and VCI (54).
GRANTS
This work supported by a grant from the William & Ella Owens Medical Research Foundation and an award from the National Institutes of Health (NIH) Institute for Integration of Medicine and Science Award (to V. Galvan) and, in part, by US Department of Veterans Affairs Biomedical Laboratory Research and Development Service Merit Review Award I01 BX002211-01A2 (to V. Galvan). C. E. Van Skike is funded by Alzheimer’s Association Research Fellowship Grant AARF-17-504221 and National Institute on Aging (NIA) Grant T32 AG-021890. J. B. J. and A. B. O. are supported by NIA Grant T32 AG-021890. S. A. Hussong is funded by the United States Department of Veterans Affairs Biomedical Laboratory Research and Development Career Development Award (CDA-2) IK2 BX003798-01A1. Further support was provided by the San Antonio Nathan Shock Center of Excellence in the Biology of Aging (NIA Grant 2-P30 AG-013319-21), the San Antonio Medical Foundation (to V. Galvan), the JMR Barker Foundation (to V. Galvan), generous support from the Robert L. Bailey and daughter Lisa K. Bailey Alzheimer’s Fund in memory of Jo Nell Bailey (to V. Galvan), NIH Grants R01 AG-047879, R01 AG-038747, T32 AG-052363, 3-P30 AG-050911-02S1, and R01-NS-056218, and the Oklahoma Center for the Advancement of Science and Technology (to Z. Ungvari).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.E.V.S., J.B.J., A.B.O., and N.L.S. analyzed data; C.E.V.S., J.B.J., N.L.S., Z.I.U., J.D.L., and V.G. interpreted results of experiments; C.E.V.S. and J.B.J. prepared figures; C.E.V.S., J.B.J., and V.G. drafted manuscript; C.E.V.S., S.A.H., Z.I.U., and V.G. edited and revised manuscript; C.E.V.S., A.B.O., N.L.S., S.A.H., Z.I.U., J.D.L., and V.G. approved final version of manuscript; J.B.J. and V.G. conceived and designed research; J.B.J., A.B.O., and N.L.S. performed experiments.
ACKNOWLEDGMENTS
In vivo images were generated in the University of Texas Health San Antonio (UTHSA) Core Optical Imaging Facility, which is supported by the UTHSA and National Institutes of Health Grants P30 CA-54174 (Cancer Therapy and Research Center at UTHSA) and P01 AG-19316.
REFERENCES
- 1.Bachmeier C, Mullan M, Paris D. Characterization and use of human brain microvascular endothelial cells to examine β-amyloid exchange in the blood-brain barrier. Cytotechnology 62: 519–529, 2010. doi: 10.1007/s10616-010-9313-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bazzoni G. Pathobiology of junctional adhesion molecules. Antioxid Redox Signal 15: 1221–1234, 2011. doi: 10.1089/ars.2010.3867. [DOI] [PubMed] [Google Scholar]
- 3.Bazzoni G, Martinez-Estrada OM, Orsenigo F, Cordenonsi M, Citi S, Dejana E. Interaction of junctional adhesion molecule with the tight junction components ZO-1, cingulin, and occludin. J Biol Chem 275: 20520–20526, 2000. doi: 10.1074/jbc.M905251199. [DOI] [PubMed] [Google Scholar]
- 4.Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, Zlokovic BV. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron 68: 409–427, 2010. doi: 10.1016/j.neuron.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bell RD, Winkler EA, Singh I, Sagare AP, Deane R, Wu Z, Holtzman DM, Betsholtz C, Armulik A, Sallstrom J, Berk BC, Zlokovic BV. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature 485: 512–516, 2012. doi: 10.1038/nature11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bell RD, Zlokovic BV. Neurovascular mechanisms and blood-brain barrier disorder in Alzheimer’s disease. Acta Neuropathol 118: 103–113, 2009. doi: 10.1007/s00401-009-0522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caccamo A, De Pinto V, Messina A, Branca C, Oddo S. Genetic reduction of mammalian target of rapamycin ameliorates Alzheimer’s disease-like cognitive and pathological deficits by restoring hippocampal gene expression signature. J Neurosci 34: 7988–7998, 2014. doi: 10.1523/JNEUROSCI.0777-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caccamo A, Majumder S, Richardson A, Strong R, Oddo S. Molecular interplay between mammalian target of rapamycin (mTOR), amyloid-β, and τ: effects on cognitive impairments. J Biol Chem 285: 13107–13120, 2010. doi: 10.1074/jbc.M110.100420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cai Z, Zhao B, Ratka A. Oxidative stress and β-amyloid protein in Alzheimer’s disease. Neuromolecular Med 13: 223–250, 2011. doi: 10.1007/s12017-011-8155-9. [DOI] [PubMed] [Google Scholar]
- 10.Carrano A, Hoozemans JJ, van der Vies SM, van Horssen J, de Vries HE, Rozemuller AJ. Neuroinflammation and blood-brain barrier changes in capillary amyloid angiopathy. Neurodegener Dis 10: 329–331, 2012. doi: 10.1159/000334916. [DOI] [PubMed] [Google Scholar]
- 11.Chandrika G, Natesh K, Ranade D, Chugh A, Shastry P. Suppression of the invasive potential of glioblastoma cells by mTOR inhibitors involves modulation of NFκB and PKC-α signaling. Sci Rep 6: 22455, 2016. doi: 10.1038/srep22455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen H, Qu Y, Tang B, Xiong T, Mu D. Role of mammalian target of rapamycin in hypoxic or ischemic brain injury: potential neuroprotection and limitations. Rev Neurosci 23: 279–287, 2012. doi: 10.1515/revneuro-2012-0001. [DOI] [PubMed] [Google Scholar]
- 13.de Oliveira J, Hort MA, Moreira EL, Glaser V, Ribeiro-do-Valle RM, Prediger RD, Farina M, Latini A, de Bem AF. Positive correlation between elevated plasma cholesterol levels and cognitive impairments in LDL receptor knockout mice: relevance of cortico-cerebral mitochondrial dysfunction and oxidative stress. Neuroscience 197: 99–106, 2011. doi: 10.1016/j.neuroscience.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 14.Di Marco LY, Venneri A, Farkas E, Evans PC, Marzo A, Frangi AF. Vascular dysfunction in the pathogenesis of Alzheimer’s disease—a review of endothelium-mediated mechanisms and ensuing vicious circles. Neurobiol Dis 82: 593–606, 2015. doi: 10.1016/j.nbd.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 15.Dutta S, Rutkai I, Katakam PV, Busija DW. The mechanistic target of rapamycin (mTOR) pathway and S6 kinase mediate diazoxide preconditioning in primary rat cortical neurons. J Neurochem 134: 845–856, 2015. doi: 10.1111/jnc.13181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erickson MA, Banks WA. Blood-brain barrier dysfunction as a cause and consequence of Alzheimer’s disease. J Cereb Blood Flow Metab 33: 1500–1513, 2013. doi: 10.1038/jcbfm.2013.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fan X, Liang Q, Lian T, Wu Q, Gaur U, Li D, Yang D, Mao X, Jin Z, Li Y, Yang M. Rapamycin preserves gut homeostasis during Drosophila aging. Oncotarget 6: 35274–35283, 2015. doi: 10.18632/oncotarget.5895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39: 175–191, 2007. doi: 10.3758/BF03193146. [DOI] [PubMed] [Google Scholar]
- 19.Fiala M, Liu QN, Sayre J, Pop V, Brahmandam V, Graves MC, Vinters HV. Cyclooxygenase-2-positive macrophages infiltrate the Alzheimer’s disease brain and damage the blood-brain barrier. Eur J Clin Invest 32: 360–371, 2002. doi: 10.1046/j.1365-2362.2002.00994.x. [DOI] [PubMed] [Google Scholar]
- 20.Ge D, Han L, Huang S, Peng N, Wang P, Jiang Z, Zhao J, Su L, Zhang S, Zhang Y, Kung H, Zhao B, Miao J. Identification of a novel mTOR activator and discovery of a competing endogenous RNA regulating autophagy in vascular endothelial cells. Autophagy 10: 957–971, 2014. doi: 10.4161/auto.28363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grahammer F, Haenisch N, Steinhardt F, Sandner L, Roerden M, Arnold F, Cordts T, Wanner N, Reichardt W, Kerjaschki D, Ruegg MA, Hall MN, Moulin P, Busch H, Boerries M, Walz G, Artunc F, Huber TB. mTORC1 maintains renal tubular homeostasis and is essential in response to ischemic stress. Proc Natl Acad Sci USA 111: E2817–E2826, 2014. doi: 10.1073/pnas.1402352111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grahammer F, Ramakrishnan SK, Rinschen MM, Larionov AA, Syed M, Khatib H, Roerden M, Sass JO, Helmstaedter M, Osenberg D, Kühne L, Kretz O, Wanner N, Jouret F, Benzing T, Artunc F, Huber TB, Theilig F. mTOR regulates endocytosis and nutrient transport in proximal tubular cells. J Am Soc Nephrol 28: 230–241, 2017. doi: 10.1681/ASN.2015111224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grammas P. Neurovascular dysfunction, inflammation and endothelial activation: implications for the pathogenesis of Alzheimer’s disease. J Neuroinflammation 8: 26, 2011. doi: 10.1186/1742-2094-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gruben N, Funke A, Kloosterhuis NJ, Schreurs M, Sheedfar F, Havinga R, Houten SM, Shiri-Sverdlov R, van de Sluis B, Kuivenhoven JA, Koonen DP, Hofker MH. Cholesterol-induced hepatic inflammation does not underlie the predisposition to insulin resistance in dyslipidemic female LDL receptor knockout mice. J Diabetes Res 2015: 956854, 2015. doi: 10.1155/2015/956854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Halliday MR, Rege SV, Ma Q, Zhao Z, Miller CA, Winkler EA, Zlokovic BV. Accelerated pericyte degeneration and blood-brain barrier breakdown in apolipoprotein E4 carriers with Alzheimer’s disease. J Cereb Blood Flow Metab 36: 216–227, 2016. doi: 10.1038/jcbfm.2015.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han J, Pan XY, Xu Y, Xiao Y, An Y, Tie L, Pan Y, Li XJ. Curcumin induces autophagy to protect vascular endothelial cell survival from oxidative stress damage. Autophagy 8: 812–825, 2012. [DOI] [PubMed] [Google Scholar]
- 28.Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460: 392–395, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev 57: 173–185, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Hay JR, Johnson VE, Young AM, Smith DH, Stewart W. Blood-brain barrier disruption is an early event that may persist for many years after traumatic brain injury in humans. J Neuropathol Exp Neurol 74: 1147–1157, 2015. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26574669&dopt=Abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Islam MT. Oxidative stress and mitochondrial dysfunction-linked neurodegenerative disorders. Neurol Res 39: 73–82, 2017. doi: 10.1080/01616412.2016.1251711. [DOI] [PubMed] [Google Scholar]
- 32.Jahrling JB, Lin AL, DeRosa N, Hussong SA, Van Skike CE, Girotti M, Javors M, Zhao Q, Maslin LA, Asmis R, Galvan V. mTOR drives cerebral blood flow and memory deficits in LDLR−/− mice modeling atherosclerosis and vascular cognitive impairment. J Cereb Blood Flow Metab 38: 58−74, 2018. doi: 10.1177/0271678X17705973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Janelidze S, Hertze J, Nägga K, Nilsson K, Nilsson C, Wennström M, van Westen D, Blennow K, Zetterberg H, Hansson O; Swedish BioFINDER Study Group . Increased blood-brain barrier permeability is associated with dementia and diabetes but not amyloid pathology or APOE genotype. Neurobiol Aging 51: 104–112, 2017. doi: 10.1016/j.neurobiolaging.2016.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jeynes B, Provias J. Significant negative correlations between capillary expressed eNOS and Alzheimer lesion burden. Neurosci Lett 463: 244–248, 2009. doi: 10.1016/j.neulet.2009.07.091. [DOI] [PubMed] [Google Scholar]
- 35.Katsu M, Niizuma K, Yoshioka H, Okami N, Sakata H, Chan PH. Hemoglobin-induced oxidative stress contributes to matrix metalloproteinase activation and blood-brain barrier dysfunction in vivo. J Cereb Blood Flow Metab 30: 1939–1950, 2010. doi: 10.1038/jcbfm.2010.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kou B, Liu W, He W, Zhang Y, Zheng J, Yan Y, Zhang Y, Xu S, Wang H. Tetrandrine suppresses metastatic phenotype of prostate cancer cells by regulating Akt/mTOR/MMP-9 signaling pathway. Oncol Rep 35: 2880–2886, 2016. doi: 10.3892/or.2016.4649. [DOI] [PubMed] [Google Scholar]
- 37.Li H, Förstermann U. Uncoupling of endothelial NO synthase in atherosclerosis and vascular disease. Curr Opin Pharmacol 13: 161–167, 2013. doi: 10.1016/j.coph.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 38.Lin AL, Jahrling JB, Zhang W, DeRosa N, Bakshi V, Romero P, Galvan V, Richardson A. Rapamycin rescues vascular, metabolic and learning deficits in apolipoprotein E4 transgenic mice with pre-symptomatic Alzheimer’s disease. J Cereb Blood Flow Metab 37: 217−226, 2015. doi: 10.1177/0271678X15621575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin AL, Zheng W, Halloran JJ, Burbank RR, Hussong SA, Hart MJ, Javors M, Shih YY, Muir E, Solano Fonseca R, Strong R, Richardson AG, Lechleiter JD, Fox PT, Galvan V. Chronic rapamycin restores brain vascular integrity and function through NO synthase activation and improves memory in symptomatic mice modeling Alzheimer’s disease. J Cereb Blood Flow Metab 33: 1412–1421, 2013. doi: 10.1038/jcbfm.2013.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu SQ, Zhao JP, Fan XX, Liu GH, Jiao HC, Wang XJ, Sun SH, Lin H. Rapamycin, a specific inhibitor of the target of rapamycin complex 1, disrupts intestinal barrier integrity in broiler chicks. J Anim Physiol Anim Nutr (Berl) 100: 323–330, 2016. doi: 10.1111/jpn.12375. [DOI] [PubMed] [Google Scholar]
- 41.Maeda NC, Givens R, Reddick RL. Cardiovascular disease: mouse models of atherosclerosis. In: The Mouse in Biomedical Research (2nd ed.), edited by Fox JG, Davisson MT, Quimby FW, Barthold SW, Newcomer CE, Smith AL. Burlington, MA: Academic, 2007, vol. III, chapt. 16, p. 535-563. [Google Scholar]
- 42.Maedeker JA, Stoka KV, Bhayani SA, Gardner WS, Bennett L, Procknow JD, Staiculescu MC, Walji TA, Craft CS, Wagenseil JE. Hypertension and decreased aortic compliance due to reduced elastin amounts do not increase atherosclerotic plaque accumulation in Ldlr−/− mice. Atherosclerosis 249: 22–29, 2016. doi: 10.1016/j.atherosclerosis.2016.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Majumder S, Caccamo A, Medina DX, Benavides AD, Javors MA, Kraig E, Strong R, Richardson A, Oddo S. Lifelong rapamycin administration ameliorates age-dependent cognitive deficits by reducing IL-1β and enhancing NMDA signaling. Aging Cell 11: 326–335, 2012. doi: 10.1111/j.1474-9726.2011.00791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Malinowski M, Martus P, Lock JF, Neuhaus P, Stockmann M. Systemic influence of immunosuppressive drugs on small and large bowel transport and barrier function. Transpl Int 24: 184–193, 2011. doi: 10.1111/j.1432-2277.2010.01167.x. [DOI] [PubMed] [Google Scholar]
- 45.Malinowski M, Martus P, Neuhaus P, Stockmann M. The influence of commonly used immunosuppressive drugs on the small bowel functions−a comparative experimental study. Ann Transplant 14: 38–44, 2009. [PubMed] [Google Scholar]
- 46.Mandell KJ, McCall IC, Parkos CA. Involvement of the junctional adhesion molecule-1 (JAM1) homodimer interface in regulation of epithelial barrier function. J Biol Chem 279: 16254–16262, 2004. doi: 10.1074/jbc.M309483200. [DOI] [PubMed] [Google Scholar]
- 47.Mao L, Jia J, Zhou X, Xiao Y, Wang Y, Mao X, Zhen X, Guan Y, Alkayed NJ, Cheng J. Delayed administration of a PTEN inhibitor BPV improves functional recovery after experimental stroke. Neuroscience 231: 272–281, 2013. doi: 10.1016/j.neuroscience.2012.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martìn-Padura I, Lostaglio S, Schneemann M, Williams L, Romano M, Fruscella P, Panzeri C, Stoppacciaro A, Ruco L, Villa A, Simmons D, Dejana E. Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J Cell Biol 142: 117–127, 1998. doi: 10.1083/jcb.142.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mayhan WG, Heistad DD. Permeability of blood-brain barrier to various sized molecules. Am J Physiol Heart Circ Physiol 248: H712–H718, 1985. doi: 10.1152/ajpheart.1985.248.5.H712. [DOI] [PubMed] [Google Scholar]
- 50.Meurs I, Lammers B, Zhao Y, Out R, Hildebrand RB, Hoekstra M, Van Berkel TJ, Van Eck M. The effect of ABCG1 deficiency on atherosclerotic lesion development in LDL receptor knockout mice depends on the stage of atherogenesis. Atherosclerosis 221: 41–47, 2012. doi: 10.1016/j.atherosclerosis.2011.11.024. [DOI] [PubMed] [Google Scholar]
- 51.Meydani M, Kwan P, Band M, Knight A, Guo W, Goutis J, Ordovas J. Long-term vitamin E supplementation reduces atherosclerosis and mortality in Ldlr−/− mice, but not when fed Western style diet. Atherosclerosis 233: 196–205, 2014. doi: 10.1016/j.atherosclerosis.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miller RA, Harrison DE, Astle CM, Fernandez E, Flurkey K, Han M, Javors MA, Li X, Nadon NL, Nelson JF, Pletcher S, Salmon AB, Sharp ZD, Van Roekel S, Winkleman L, Strong R. Rapamycin-mediated lifespan increase in mice is dose and sex dependent and metabolically distinct from dietary restriction. Aging Cell 13: 468–477, 2014. doi: 10.1111/acel.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mok KW, Mruk DD, Cheng CY. rpS6 regulates blood-testis barrier dynamics through Akt-mediated effects on MMP-9. J Cell Sci 127: 4870–4882, 2014. doi: 10.1242/jcs.152231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Montagne A, Barnes SR, Sweeney MD, Halliday MR, Sagare AP, Zhao Z, Toga AW, Jacobs RE, Liu CY, Amezcua L, Harrington MG, Chui HC, Law M, Zlokovic BV. Blood-brain barrier breakdown in the aging human hippocampus. Neuron 85: 296–302, 2015. doi: 10.1016/j.neuron.2014.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Monteiro AC, Sumagin R, Rankin CR, Leoni G, Mina MJ, Reiter DM, Stehle T, Dermody TS, Schaefer SA, Hall RA, Nusrat A, Parkos CA. JAM-A associates with ZO-2, afadin, and PDZ-GEF1 to activate Rap2c and regulate epithelial barrier function. Mol Biol Cell 24: 2849–2860, 2013. doi: 10.1091/mbc.E13-06-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mulder M, Jansen PJ, Janssen BJ, van de Berg WD, van der Boom H, Havekes LM, de Kloet RE, Ramaekers FC, Blokland A. Low-density lipoprotein receptor-knockout mice display impaired spatial memory associated with a decreased synaptic density in the hippocampus. Neurobiol Dis 16: 212–219, 2004. doi: 10.1016/j.nbd.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 57.Muradashvili N, Tyagi R, Lominadze D. A dual-tracer method for differentiating transendothelial transport from paracellular leakage in vivo and in vitro. Front Physiol 3: 166, 2012. doi: 10.3389/fphys.2012.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Naik P, Cucullo L. In vitro blood-brain barrier models: current and perspective technologies. J Pharm Sci 101: 1337–1354, 2012. doi: 10.1002/jps.23022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nelson AR, Sweeney MD, Sagare AP, Zlokovic BV. Neurovascular dysfunction and neurodegeneration in dementia and Alzheimer’s disease. Biochim Biophys Acta 1862: 887–900, 2016. doi: 10.1016/j.bbadis.2015.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nighot PK, Hu C-AA, Ma TY. Autophagy enhances intestinal epithelial tight junction barrier function by targeting claudin-2 protein degradation. J Biol Chem 290: 7234–7246, 2015. doi: 10.1074/jbc.M114.597492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Paccalin M, Pain-Barc S, Pluchon C, Paul C, Besson MN, Carret-Rebillat AS, Rioux-Bilan A, Gil R, Hugon J. Activated mTOR and PKR kinases in lymphocytes correlate with memory and cognitive decline in Alzheimer’s disease. Dement Geriatr Cogn Disord 22: 320–326, 2006. doi: 10.1159/000095562. [DOI] [PubMed] [Google Scholar]
- 62.Paul J, Strickland S, Melchor JP. Fibrin deposition accelerates neurovascular damage and neuroinflammation in mouse models of Alzheimer’s disease. J Exp Med 204: 1999–2008, 2007. doi: 10.1084/jem.20070304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rajapakse AG, Yepuri G, Carvas JM, Stein S, Matter CM, Scerri I, Ruffieux J, Montani JP, Ming XF, Yang Z. Hyperactive S6K1 mediates oxidative stress and endothelial dysfunction in aging: inhibition by resveratrol. PLoS One 6: e19237, 2011. doi: 10.1371/journal.pone.0019237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Richardson A, Galvan V, Lin AL, Oddo S. How longevity research can lead to therapies for Alzheimer’s disease: the rapamycin story. Exp Gerontol 68: 51–58, 2015. doi: 10.1016/j.exger.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ryu JK, McLarnon JG. A leaky blood-brain barrier, fibrinogen infiltration and microglial reactivity in inflamed Alzheimer’s disease brain. J Cell Mol Med 13: 2911–2925, 2009. doi: 10.1111/j.1582-4934.2008.00434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sagare AP, Bell RD, Zhao Z, Ma Q, Winkler EA, Ramanathan A, Zlokovic BV. Pericyte loss influences Alzheimer-like neurodegeneration in mice. Nat Commun 4: 2932, 2013. doi: 10.1038/ncomms3932. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 67.Schreurs MP, Cipolla MJ. Cerebrovascular dysfunction and blood-brain barrier permeability induced by oxidized LDL are prevented by apocynin and magnesium sulfate in female rats. J Cardiovasc Pharmacol 63: 33–39, 2014. doi: 10.1097/FJC.0000000000000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Severson EA, Jiang L, Ivanov AI, Mandell KJ, Nusrat A, Parkos CA. Cis-dimerization mediates function of junctional adhesion molecule A. Mol Biol Cell 19: 1862–1872, 2008. doi: 10.1091/mbc.E07-09-0869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shigemori Y, Katayama Y, Mori T, Maeda T, Kawamata T. Matrix metalloproteinase-9 is associated with blood-brain barrier opening and brain edema formation after cortical contusion in rats. Acta Neurochir Suppl (Wien) 96: 130–133, 2006. doi: 10.1007/3-211-30714-1_29. [DOI] [PubMed] [Google Scholar]
- 70.Spilman P, Podlutskaya N, Hart MJ, Debnath J, Gorostiza O, Bredesen D, Richardson A, Strong R, Galvan V. Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-β levels in a mouse model of Alzheimer’s disease. PLoS One 5: e9979, 2010. doi: 10.1371/journal.pone.0009979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stamatovic SM, Keep RF, Andjelkovic AV. Brain endothelial cell-cell junctions: how to “open” the blood brain barrier. Curr Neuropharmacol 6: 179–192, 2008. doi: 10.2174/157015908785777210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tada Y, Yagi K, Kitazato KT, Tamura T, Kinouchi T, Shimada K, Matsushita N, Nakajima N, Satomi J, Kageji T, Nagahiro S. Reduction of endothelial tight junction proteins is related to cerebral aneurysm formation in rats. J Hypertens 28: 1883–1891, 2010. doi: 10.1097/HJH;0b013e32833c2273. [DOI] [PubMed] [Google Scholar]
- 73.Takata F, Dohgu S, Matsumoto J, Takahashi H, Machida T, Wakigawa T, Harada E, Miyaji H, Koga M, Nishioku T, Yamauchi A, Kataoka Y. Brain pericytes among cells constituting the blood-brain barrier are highly sensitive to tumor necrosis factor-α, releasing matrix metalloproteinase-9 and migrating in vitro. J Neuroinflammation 8: 106, 2011. doi: 10.1186/1742-2094-8-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tarantini S, Tran CH, Gordon GR, Ungvari Z, Csiszar A. Impaired neurovascular coupling in aging and Alzheimer’s disease: contribution of astrocyte dysfunction and endothelial impairment to cognitive decline. Exp Gerontol 94: 52–58, 2016. doi: 10.1016/j.exger.2016.1011.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tomimoto H, Akiguchi I, Suenaga T, Nishimura M, Wakita H, Nakamura S, Kimura J. Alterations of the blood-brain barrier and glial cells in white-matter lesions in cerebrovascular and Alzheimer’s disease patients. Stroke 27: 2069–2074, 1996. doi: 10.1161/01.STR.27.11.2069. [DOI] [PubMed] [Google Scholar]
- 76.Tornavaca O, Chia M, Dufton N, Almagro LO, Conway DE, Randi AM, Schwartz MA, Matter K, Balda MS. ZO-1 controls endothelial adherens junctions, cell-cell tension, angiogenesis, and barrier formation. J Cell Biol 208: 821–838, 2015. doi: 10.1083/jcb.201404140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Turner RJ, Sharp FR. Implications of MMP9 for blood brain barrier disruption and hemorrhagic transformation following ischemic stroke. Front Cell Neurosci 10: 56, 2016. doi: 10.3389/fncel.2016.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.van de Haar HJ, Burgmans S, Jansen JF, van Osch MJ, van Buchem MA, Muller M, Hofman PA, Verhey FR, Backes WH. Blood-brain barrier leakage in patients with early Alzheimer disease. Radiology 281: 527–535, 2016. doi: 10.1148/radiol.2016152244. [DOI] [PubMed] [Google Scholar]
- 79.Wang C, Wang Z, Zhang X, Zhang X, Dong L, Xing Y, Li Y, Liu Z, Chen L, Qiao H, Wang L, Zhu C. Protection by silibinin against experimental ischemic stroke: up-regulated pAkt, pmTOR, HIF-1α and Bcl-2, down-regulated Bax, NF-κB expression. Neurosci Lett 529: 45–50, 2012. doi: 10.1016/j.neulet.2012.08.078. [DOI] [PubMed] [Google Scholar]
- 80.Watanabe T, Dohgu S, Takata F, Nishioku T, Nakashima A, Futagami K, Yamauchi A, Kataoka Y. Paracellular barrier and tight junction protein expression in the immortalized brain endothelial cell lines bEND.3, bEND.5 and mouse brain endothelial cell 4. Biol Pharm Bull 36: 492–495, 2013. doi: 10.1248/bpb.b12-00915. [DOI] [PubMed] [Google Scholar]
- 81.Wilkinson JE, Burmeister L, Brooks SV, Chan CC, Friedline S, Harrison DE, Hejtmancik JF, Nadon N, Strong R, Wood LK, Woodward MA, Miller RA. Rapamycin slows aging in mice. Aging Cell 11: 675–682, 2012. doi: 10.1111/j.1474-9726.2012.00832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Winkler EA, Nishida Y, Sagare AP, Rege SV, Bell RD, Perlmutter D, Sengillo JD, Hillman S, Kong P, Nelson AR, Sullivan JS, Zhao Z, Meiselman HJ, Wendy RB, Soto J, Abel ED, Makshanoff J, Zuniga E, De Vivo DC, Zlokovic BV. GLUT1 reductions exacerbate Alzheimer’s disease vasculo-neuronal dysfunction and degeneration. Nat Neurosci 18: 521–530, 2015. doi: 10.1038/nn.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wu Z, Hofman FM, Zlokovic BV. A simple method for isolation and characterization of mouse brain microvascular endothelial cells. J Neurosci Methods 130: 53–63, 2003. doi: 10.1016/S0165-0270(03)00206-1. [DOI] [PubMed] [Google Scholar]
- 84.Xie R, Wang P, Ji X, Zhao H. Ischemic post-conditioning facilitates brain recovery after stroke by promoting Akt/mTOR activity in nude rats. J Neurochem 127: 723–732, 2013. doi: 10.1111/jnc.12342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yang Y, Estrada EY, Thompson JF, Liu W, Rosenberg GA. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab 27: 697–709, 2007. doi: 10.1038/sj.jcbfm.9600375. [DOI] [PubMed] [Google Scholar]
- 86.Yeung D, Manias JL, Stewart DJ, Nag S. Decreased junctional adhesion molecule-A expression during blood-brain barrier breakdown. Acta Neuropathol 115: 635–642, 2008. doi: 10.1007/s00401-008-0364-4. [DOI] [PubMed] [Google Scholar]
- 87.Zhao Z, Hu J, Gao X, Liang H, Liu Z. Activation of AMPK attenuates lipopolysaccharide-impaired integrity and function of blood-brain barrier in human brain microvascular endothelial cells. Exp Mol Pathol 97: 386–392, 2014. doi: 10.1016/j.yexmp.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 88.Zhou YD, Cao XQ, Liu ZH, Cao YJ, Liu CF, Zhang YL, Xie Y. Rapamycin inhibits oxidized low density lipoprotein uptake in human umbilical vein endothelial cells via mTOR/NF-κB/LOX-1 pathway. PLoS One 11: e0146777, 2016. doi: 10.1371/journal.pone.0146777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zipser BD, Johanson CE, Gonzalez L, Berzin TM, Tavares R, Hulette CM, Vitek MP, Hovanesian V, Stopa EG. Microvascular injury and blood-brain barrier leakage in Alzheimer’s disease. Neurobiol Aging 28: 977–986, 2007. doi: 10.1016/j.neurobiolaging.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 90.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci 12: 723–738, 2011. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zschiedrich S, Bork T, Liang W, Wanner N, Eulenbruch K, Munder S, Hartleben B, Kretz O, Gerber S, Simons M, Viau A, Burtin M, Wei C, Reiser J, Herbach N, Rastaldi MP, Cohen CD, Tharaux PL, Terzi F, Walz G, Gödel M, Huber TB. Targeting mTOR signaling can prevent the progression of FSGS. J Am Soc Nephrol 28: 2144–2157, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]