Abstract
The metabolism of nutrient substrates, including glucose, glutamine, and fatty acids, provides acetyl-CoA for the tricarboxylic acid cycle to generate energy, as well as metabolites for the biosynthesis of biomolecules, including nucleotides, proteins, and lipids. It has been shown that metabolism of glucose, fatty acid, and glutamine plays important roles in modulating cellular proliferation, differentiation, apoptosis, autophagy, senescence, and inflammatory responses. All of these cellular processes contribute to the pathogenesis of chronic lung diseases, including bronchopulmonary dysplasia, chronic obstructive pulmonary disease, and pulmonary fibrosis. Recent studies demonstrate that metabolic reprogramming occurs in patients with and animal models of chronic lung diseases, suggesting that metabolic dysregulation may participate in the pathogenesis and progression of these diseases. In this review, we briefly discuss the catabolic pathways for glucose, glutamine, and fatty acids, and focus on how metabolic reprogramming of these pathways impacts cellular functions and leads to the development of these chronic lung diseases. We also highlight how targeting metabolic pathways can be utilized in the prevention and treatment of these diseases.
Keywords: bronchopulmonary dysplasia, chronic obstructive pulmonary disease, metabolic flexibility, metabolic reprogramming, pulmonary fibrosis
INTRODUCTION
The catabolism of major nutrient substrates, including glucose, glutamine, and fatty acids (FAs) provides acetyl-CoA for the tricarboxylic acid (TCA) cycle to generate energy. It also generates a variety of metabolites for the biosynthesis of biomolecules, including nucleotides, proteins, and lipids (anabolism). In catabolism, these nutrient substrates are oxidized into CO2 and H2O, which requires the mitochondrial electron transport chain (ETC) using oxygen as a terminal electron acceptor. This couples to ATP production and is termed oxidative phosphorylation.
Cellular bioenergetics is very important for maintaining cell function under physiological and pathological conditions. It has been shown that metabolic homeostasis plays important roles in regulating cell proliferation and differentiation (3). Metabolic reprogramming or dysregulation also affects autophagy, apoptosis, senescence, and inflammatory responses (8, 31, 43, 95). All of these cellular processes have been shown to participate in the pathogenesis of chronic lung diseases, including bronchopulmonary dysplasia (BPD), chronic obstructive pulmonary disease (COPD) and lung fibrosis (9, 20, 23, 50, 99). Interestingly, metabolic reprogramming is observed in the lungs or cells of patients with BPD, COPD, and lung fibrosis (51–53, 105). However, the roles of this metabolic reprograming and the molecular mechanisms underlying it in these diseases are not fully understood.
In this review, we will briefly discuss glucose, fatty acid, and glutamine catabolism, and focus on their metabolic reprogramming in the pathogenesis of BPD, COPD and pulmonary fibrosis.
OVERVIEW OF GLUCOSE, FATTY ACID, AND GLUTAMINE CATABOLISM
Glucose Catabolism
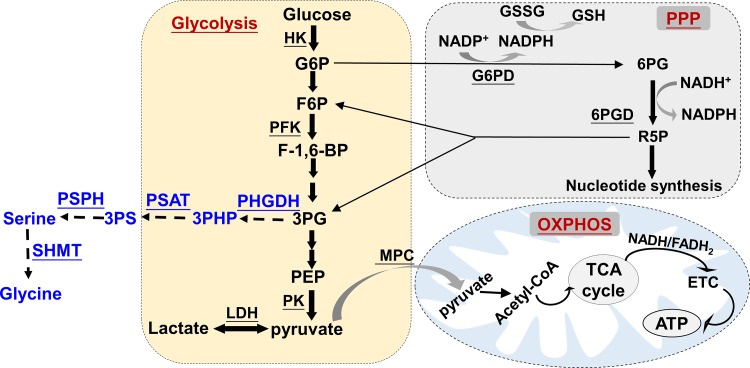
Upon transport into the cytosol by glucose transporters, glucose is converted into pyruvate through a sequence of 10 biochemical reactions termed glycolysis (25, 26, 28, 56, 94). There are three rate-limiting enzymatic reactions catalyzed by hexokinase (HK), phosphofructokinase (PFK), and pyruvate kinase, which control the glycolytic flux rate (25, 26, 28, 56, 94). In conditions of sufficient oxygen, pyruvate is commonly transported into mitochondria by the mitochondrial pyruvate carrier (MPC), and then converted to acetyl-CoA. This molecule, derived from glucose and FA β-oxidation, enters the TCA cycle to produce NADH and FADH2, the electron carriers, that donate electrons to the ETC, thereby resulting in ATP generation via ATP synthase (25, 26, 56) (Fig. 1). In the setting of oxygen deprivation, pyruvate is metabolized to lactate in the cytosol (25, 26, 56). Nevertheless, under aerobic conditions, some cells, including cancer cells and endothelial cells, rely on glycolysis for bioenergetics. This is referred to as the “Warburg effect” (25, 26, 28, 56, 94). A recent study in the mouse demonstrated that glucose can provide substrates for the TCA cycle via circulating lactate, suggesting that glycolysis and the TCA cycle are uncoupled at the level of lactate, and lactate is a primary circulating TCA substrate (46).
Fig. 1.
Glucose catabolism. Upon transport into the cell, glucose is subject to the following two major pathways for metabolism: glycolysis and the pentose phosphate pathway (PPP), in which G6P appears at the crossroads of these pathways. There are three rate-limiting steps, catalyzed by hexokinase (HK), phosphofructokinase (PFK), and pyruvate kinase (PK), which converts glucose into pyruvate. Glycolysis is a major pathway for glucose metabolism, in which pyruvate can be converted into lactate by lactate dehydrogenase (LDH) or be transported into mitochondria by mitochondrial pyruvate carrier (MPC) to fuel ATP production by oxidative phosphorylation through the electron transport chain (ETC). The PPP divides into the oxidative and nonoxidative branches, which the oxidative branch generates reducing equivalents in the form of NAPDH, while the nonoxidative branch produces ribose-5-phosphate (R5P). The PPP can feed back glycolysis at the levels of glyceraldehyde 3-phosphate (3PG) and fructose-6-phosphate (F6P) (solid arrows). The 3PG metabolite can be used for the de novo synthesis of serine and glycine, which provides the essential precursors for the synthesis of proteins, nucleic acids, and lipids (dashed arrows). 6PG, 6-phosphogluconate; 3PHP, 3-phosphohydroxypyruvate; 3PS, 3-phosphoserine; F-1,6-BP, fructose-1,6-biphosphate; PEP, phosphoenolpyruvate; PHGDH, phosphoglycerate dehydrogenase; PSAT, phosphoserine aminotransferase; SHMT, serine hydroxymethyltransferase; TCA, tricarboxylic acid; G6PD, glucose-6-phosphate dehydrogenase; 6PGD, 6-phosphogluconate dehydrogenase.
Pentose Phosphate Pathway
Glucose can be shuttled to the pentose phosphate pathway (PPP) rather than proceed to glycolysis under certain circumstances. The PPP is important for maintaining carbon homeostasis by generating precursors for nucleotide and amino acid biosynthesis and for protecting against oxidative stress by generating NADPH (Fig. 1). The PPP utilizes glucose-6-phosphate (G6P), which is oxidized by glucose-6-phosphate dehydrogenase (G6PD) to form 6-phosphogluconolactone. This is the rate-limiting step of the PPP. The 6-phosphogluconate is then converted into ribulose 5-phosphate through oxidative decarboxylation. The above steps are the oxidative reactions of the PPP. Together with other NADPH-regenerating systems, such as NADP-dependent isocitrate dehydrogenase (IDH) and malic enzyme, NADPH is generated and used for anabolic pathways and for maintaining reduced glutathione as antioxidant functions. The second phase of the PPP is the nonoxidative pathway, which produces five-carbon sugars [i.e., ribose 5-phosphate, (R5P)], providing precursors for the synthesis of nucleic acids and amino acids. The nonoxidative reactions of the PPP can also provide metabolic intermediates for glycolysis (i.e., glyceraldehyde 3-phosphate and fructose 6-phosphate), depending on the biochemical demand. In general, the oxidative function of the PPP is to produce NADPH to prevent oxidative stress and for synthesizing biomass, while the nonoxidative effect of the PPP is to produce R5P for nucleotide synthesis. The glycolytic pathway and the PPP cannot be distinguished when one just measures glucose uptake and lactate production, unless a metabolic flux assay is used to measure carbon incorporation from glucose into five-carbon-atom sugars (17).
FA Catabolism
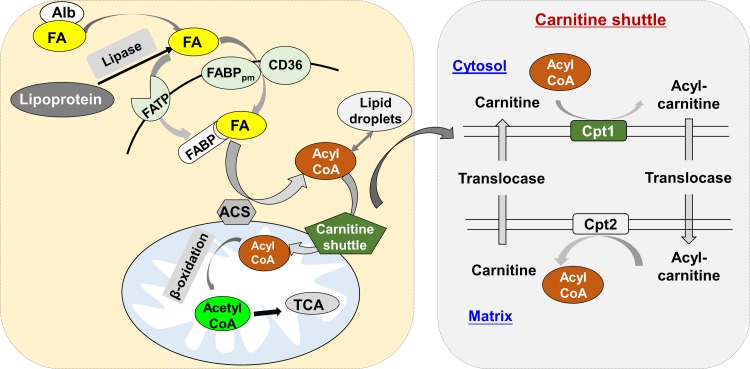
Long-chain FAs (LCFAs) are the most abundant FA species in mammalian cells. LCFAs disassociated from albumin or lipoproteins can enter cells via specific transport proteins (e.g., FATP and CD36/FABPpm). Once inside the cell, LCFAs are catalyzed by fatty acyl-CoA synthetase to form fatty acyl-CoA, which is then ready for storage in lipid droplets (LDs) as neutral lipids (e.g., triglycerides). Under metabolic stress or nutrient deprivation, FAs are released from LDs through lipolysis or lipophagy for energy generation via β-oxidation and the TCA cycle in the mitochondria. Unlike short- and medium-chain FAs, LCFAs require the carnitine shuttle system to be transported into mitochondria for β-oxidation. In every cycle, two carbons are cleaved from LCFAs to form acetyl-CoA. Mitochondria are unable to handle very long-chain FAs (>22 carbons) and branched FAs, which are usually oxidized in peroxisomes to generate H2O2. The latter is further converted into H2O and oxygen by catalase. Once the very long-chain FAs are cleaved to LCFAs, these can be further oxidized in the mitochondria. Carnitine palmitoyltransferase 1 (Cpt1, mainly Cpt1a) is the first and rate-limiting step of the carnitine shuttle system in mitochondria, catalyzing the transfer of the acyl group of LCFAs from CoA to carnitine so as to form acylcarnitine (Fig. 2).
Fig. 2.
Fatty acid uptake, activation, and oxidation. Long-chain fatty acids (FAs) disassociated from albumin (Alb) or lipoproteins can enter cells via specific transport proteins (e.g., FATP and CD36/FABPpm). Once inside the cell, long-chain FAs are catalyzed and activated by fatty acyl-CoA synthetases (ACS) to form fatty acyl-CoA, which is then ready for storage in lipid droplets (LDs) as neutral lipids (e.g., triglycerides). Under metabolic stress or nutrient deprivation, FAs are released from LDs through lipolysis or lipophagy for energy generation by β-oxidation and tricarboxylic acid (TCA) cycle in the mitochondria. Unlike short- and medium-chain FAs, long-chain FAs require the carnitine shuttle system to be transported into mitochondrion for β-oxidation. Cpt1 (mainly Cpt1a) is the first component and rate-limiting step of the carnitine shuttle system, which catalyzes the transfer of the acyl group of LCFAs from coenzyme A (CoA) to carnitine to form acylcarnitine.
Glutamine Metabolism
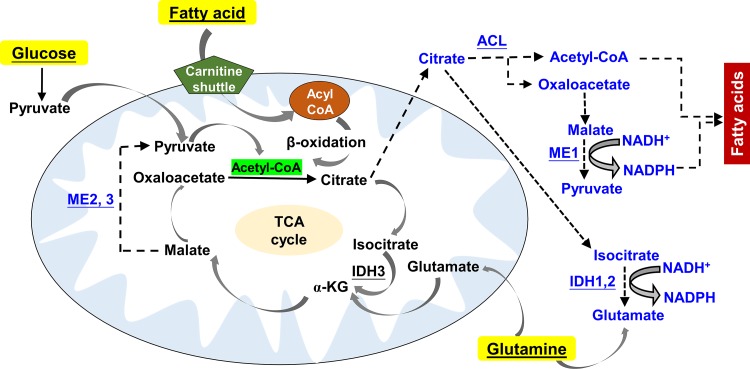
Glutamine is the most abundant free amino acid in plasma. Glutamine catabolism begins with its conversion to glutamate by glutaminase, which is further catalyzed by glutamate dehydrogenase into α-ketoglutarate, a TCA cycle intermediate (42) (Fig. 3). Glutamine/glutamate metabolism can interact with the purine nucleotide cycle and the hepatic urea cycle through aspartate. Glutamate is also a precursor of glutathione, a major cellular antioxidant. In addition, it is also the source of amino groups for nonessential amino acids, which are required for macromolecular synthesis.
Fig. 3.
Simplified schematic of TCA cycle and its metabolites producing NADPH. TCA cycle is composed of a series of chemical reactions used to release stored energy in the form of ATP from major nutrient substrates, including glucose, glutamine, and fatty acids. Acetyl-CoA is the starting point of TCA cycle, which can be generated by pyruvate dehydrogenase from pyruvate or by fatty acid β-oxidation. Glutamate generated from glutaminolysis can be converted into α-ketoglutarate (α-KG) and then participates in the TCA cycle. TCA-derived citrate can be transported into cytosol for producing pyruvate and glutamate with the generation of NADPH. Cytosol citrate can be catalyzed by acetyl-CoA ligase (ACL) to generate acetyl CoA, which is utilized for the creation of fatty acids along with NADPH. Solid arrows denote the steps of TCA cycle, whereas dashed arrows denote the generation of fatty acids and glutamine from glucose-derived citrate. ME, malic enzyme; IDH, isocitrate dehydrogenase.
TCA Cycle and Metabolite Transport into the Cytosol
The TCA cycle is an essential metabolic pathway for the generation of chemical energy (i.e., ATP) by oxidation of acetyl-CoA derived from nutrients. Once pyruvate is transported into mitochondria by MPC, it is decarboxylated by the enzyme pyruvate dehydrogenase generating acetyl-CoA. The TCA cycle contains eight chemical reactions, which produce NADH and FADH2 as electron donors to feed the ETC, leading to ATP production (Fig. 3).
The TCA cycle is largely modulated by product inhibition and substrate availability. Intermediates can serve as substrates for biosynthetic processes. Malate is one of the TCA cycle intermediates exported from the mitochondria to the cytosol, where malic enzyme can then regenerate NADPH and pyruvate from malate for cycling back to the mitochondria (Fig. 3). Citrate can cross from the mitochondrial membrane to the cytosol, where it is cleaved by ATP citrate lyase (ACL) to produce cytosolic acetyl-CoA. The latter is used for fatty acid synthesis through acetyl-CoA carboxylase (ACC)-mediated malonyl-CoA generation.
In humans, there are three isoforms of IDH, which catalyze the conversion of isocitrate to α-ketoglutarate and CO2 in a two-step reaction. While IDH3 catalyzes the conversion of NAD+ to NADH in the mitochondria, the isoforms IDH1 and IDH2 catalyze the same reaction outside of the context of the TCA cycle but utilize NADP+ as a cofactor to produce NADPH (Fig. 3).
Glucose-Fatty Acid (Randle) Cycle and Metabolic Flexibility
Cells have metabolic flexibility to use specific fuels depending on nutrient and oxygen availability. In general, increased plasma FAs suppress glucose oxidation, while excess carbohydrate inhibits FA β-oxidation via the glucose-FA cycle or Randle cycle (45). An increase in FA or ketone body oxidation augments the levels acetyl-CoA and NADH, which inhibit glucose metabolism via pyruvate dehydrogenase. Increased glucose oxidation results in increased mitochondrial and cytosolic concentrations of citrate, which reduces FA oxidation by inhibiting Cpt1 via malonyl-CoA. Increased cytosolic citrate also inhibits glycolysis at the level of PFK, but enhances glucose incorporation into glycogen. Therefore, in response to substrate availability, the cell can dynamically adapt its mitochondrial metabolic pathways.
METABOLIC REGULATION IN CELL PROCESSES
Metabolism of fuel substrates provides energy, reducing equivalents, and metabolites, which play important roles in modulating cell processes, including proliferation. Excessive production of mitochondrial ROS (mtROS) due to dysfunctional mitochondrial respiration also causes inflammatory responses and senescence. The roles of mitochondrial dysfunction per se in modulating these cell processes have been discussed by us and others (24, 102). In this section, we focus on metabolic reprogramming during proliferation, differentiation, apoptosis, autophagy, senescence, and inflammation.
Impact of Metabolism on Cell Proliferation
Proliferative cells utilize and metabolize available nutrients to produce ATP and generate all of the components required for duplication of cell biomass and division. Aerobic glycolysis is the main characteristic of normal proliferating cells (Table 1), because R5P and NADPH, which are available from the PPP, are required for biomass synthesis. Compared with mitochondrial oxidative phosphorylation, glycolysis is not efficient in producing ATP, but it allows more rapid ATP production in proliferating cells. Although the proliferating cells do require more ATP than quiescent cells to maintain homeostasis, ATP is not produced in excess of demand. This is due to allosteric inhibition of PFK and other rate-limiting steps in glycolysis by a high ATP/AMP ratio (88).
Table 1.
Metabolic reprogramming in cell processes
| Cell Process | Glycolysis | PPP | Oxidative Phosphorylation |
|---|---|---|---|
| Proliferation | Increased glycolysis | Increased PPP | Glutamine and FA-derived carbons are able to incorporate into DNA. |
| Differentiation | Glycolysis inhibition causes differentiation. | The PPP shunt is turned off during differentiation | Mitochondrial respiration drives differentiation. |
| Apoptosis | Cancer cells are glycolytic, and glycolytic inhibition results in apoptosis. | Reduced PPP-derived NADPH activates cytochrome c and apoptosis. | Defective mitochondrial respiration triggers apoptosis by mtROS, cytochrome c, and ceramide. |
| Autophagy | Hexokinase II positively modulates glucose starvation-induced autophagy. | Ribose 5-phosphate isomerase inhibits autophagy. | Deletion of acetyl-CoA stimulates autophagy. |
| Senescence | Increased glycolysis during senescence | Reduced PPP causes susceptibility to develop senescence | Reduced mitochondrial respiration during senescence |
| Inflammation | Glycolysis supports M1 polarization of macrophage. | The PPP-derived NADPH reduces oxidative stress-induced inflammation. | M2 macrophage exploits mitochondrial oxidative phosphorylation. |
PPP, pentose phosphate pathway; FA, fatty acid; mtROS, mitochondrial reactive oxygen species; CoA, coenzyme A.
Glutamine/glutamate metabolism not only generates energy but also provides intermediates for macromolecule biosynthesis. Indeed, glutamine is indispensable for proliferation because it provides nitrogen for the biosynthesis of purines, pyrimidines, and proteins (42). If mitochondrial respiration is limited, glutamine can provide carbons for lipogenic acetyl-CoA via reductive glutamine metabolism. This depends on the cytosolic or mitochondrial IDH reactions (7).
A recent study has shown that FA carbon is essential for dNTP synthesis in the endothelial cells (79). Isotope labeling experiments in endothelial cells show that FA carbons substantially replenish the TCA cycle and are incorporated into aspartate (a nucleotide precursor), uridine monophosphate (a precursor of pyrimidine nucleoside triphosphates), and DNA (79). Inhibition of FA oxidation by Cpt1 blockade reduces endothelial cell proliferation and subsequent angiogenesis (79). In addition to its direct role, β-oxidation-derived acetyl-CoA promotes endothelial cell proliferation through epigenetic mechanisms (79, 96). Therefore, all nutrient substrates can support the synthesis of biomass for proliferation. However, some highly proliferating cells, such as cancer cells, rely on substrate availability, but are independent of metabolic demand per se for their proliferation (40).
How Metabolism Modulates Cell Differentiation
In general, inhibition of glycolysis with increased mitochondrial respiration favors cell differentiation (44) (Table 1). Bracha et al. (18) reported that silencing phosphoglycerate kinase and hexose-6-phosphate dehydrogenase induced differentiation of mouse C2C12 myoblasts. Similarly, inhibition of aerobic glycolysis is required for neuronal differentiation (106). Mitochondrial oxidative phosphorylation drives intestine epithelial cell differentiation and crypt formation (78). Furthermore, Treg cells favor FA catabolism via β-oxidation for their differentiation (63, 81). This is also corroborated by the findings that genetic ablation of Cpt2 impairs osteoclast differentiation (54). Synthesis of acetyl-CoA via ACL is required for nuclear histone acetylation and cytoplasmic cholesterol biosynthesis. In addition to myoblasts, ACL inhibition also causes cancer cell differentiation but prevents cancer cell proliferation and tumor growth (18, 39). Overall, these findings suggest that carbon metabolism, including glycolysis, the PPP, and the cholesterol biosynthetic pathway, plays important roles in regulating differentiation (62). A recent study has shown that glutamine regulates the pluripotency transcription factor OCT4, which orchestrates stem cell differentiation, through glutathione (60). This provides a potential metabolic approach to force cancer stem cells to differentiate and lose their self-renewal property, thereby rendering them more vulnerable to chemotherapy.
Role of Metabolism in Apoptosis
Mitochondria are the main organelles for apoptotic signaling. A variety of death stimuli, including DNA damage and death receptor ligands, can converge on the mitochondria to trigger the release of cytochrome c from the mitochondrial intermembrane space. The released cytochrome c could cause the formation of the apoptosome via oligomerization of apaf-1 with the cysteine-aspartic protease (caspase)-9, thereby activating the executioner caspases, caspase-3, and caspase-7, leading to apoptosis. Interestingly, cells including cancer cells, which heavily rely on glucose as a mitochondrial fuel, are refractory to cytochrome c-induced caspase activation. This is due to inactivation of cytochrome c with reduced state by enhanced levels of NADPH via the PPP (90) (Table 1). In addition, acetyl-CoA-dependent protein N-α-acetylation (e.g., caspase 2 acetylation) promotes apoptosis resistance (101). Identifying key apoptotic proteins that are susceptible to acetylation could uncover mechanisms underlying apoptosis resistance.
Inhibition of FA oxidation by a specific Cpt1 inhibitor, etomoxir, enhanced apoptosis, which is associated with increased de novo synthesis of ceramide, a known mediator of apoptosis and autophagy (41, 72). Interestingly, inhibition of FA oxidation by etomoxir attenuated cigarette smoke-induced cell death in bronchial epithelial cells by reducing mtROS production during oxidative phosphorylation (50). Therefore, the balance among FA accumulation-derived synthesis, lipid peroxidation, and mtROS production plays an important role in modulating apoptosis and cell death by FA oxidation.
Metabolism and Autophagy
Autophagy is a process that engulfs cytoplasmic proteins and organelles, including mitochondria, and degrades them in lysosomes (37). In general, autophagy releases sugars, fatty acids, amino acids, and nucleosides from the degradation of carbohydrates, lipids, proteins, and nucleic acids, respectively. These can be reutilized for metabolic processes and nucleic acid synthesis. Once mitochondria are depolarized, they can be targeted for autophagy (mitophagy) to limit mtROS production and oxidative damage so as to maintain a functional mitochondrial pool.
Metabolic inputs, such as AMP/ATP levels, have been found to regulate autophagy through AMPK (38). Other metabolic processes such as glycolysis, acetyl-CoA synthesis, and FA oxidation also play an important role in the regulation of autophagy (29, 30, 74, 77) (Table 1). In general, it is thought that catabolic processes increase autophagic flux, whereas anabolic processes reduce autophagy. For instance, inhibition of hexokinase II (HKII) reduced, whereas overexpression of HKII augmented glucose deprivation-induced autophagy (77). The modulation of autophagy by metabolic processes can be achieved through specific metabolic intermediates as well as indirect mechanisms, such as the oxidative status of the cell. For example, α-ketoglutarate derived from glutamine by glutaminolysis inhibits autophagy by activating mTORC1 (84). Ammonium, a product of glutaminolysis, activates autophagy at low concentrations, but inhibits this process at high concentrations (32).
ROS and reducing equivalents are pivotal in the regulation of autophagy because the proteins encoded by autophagy-related genes are redox sensitive. This also contributes to glutaminolysis-mediated inhibition of autophagy by increasing the levels of GSH and NADPH, which counteract the effects of ROS (92). Therefore, modulating autophagy by metabolic regulators could provide potential therapeutic options for diseases in which autophagy is impaired.
How Metabolism Regulates Senescence
Cellular senescence refers to the irreversible arrest of proliferation, which is characterized by a senescence-associated secretory phenotype and apoptosis resistance. A general metabolic feature of senescent cells is a significant shift to increased glycolytic flux but reduced energetic status (47, 107) (Table 1). This metabolic shift from mitochondrial oxidative phosphorylation toward glycolysis results in an increase in the ADP/ATP and AMP/ATP ratio. The molecular mechanisms underlying metabolic shift-driven senescence are associated with AMPK activation due to increased AMP/ATP ratio, leading to activation of p53/p21 and pRB/p16 pathways. It is interesting to note that certain types of senescence (e.g., senescence during embryonic development) are independent of p53 activation (66), and p53 has been shown to inhibit glycolysis (49, 55, 61, 80, 104). These findings suggest that p53 is a key factor in fine-tuning glycolysis in senescent cells during tissue remolding.
GAPDH-depleted human lung carcinoma cells establish a senescence phenotype, which cannot be rescued by pyruvate supplementation, despite ATP levels returning to normal (73). In contrast, GAPDH can interact and inhibit telomerase RNA component, leading to telomere shortening and senescence in breast cancer cells (67). These findings suggest that GAPDH affects senescence in a cell-specific manner, independent of its role in glycolysis. Glycolytic cancer cells lacking 6-phosphogluconate dehydrogenase (6PGD) metabolize glucose to induce senescence without affecting the steady-state levels of NADPH or the PPP (83). Indeed, the increased PPP in senescent cells may contribute to their high-energy consuming function, e.g., synthesizing and secreting larger amounts of senescence-associated secretory phenotype mediators and factors.
It has been shown that key metabolites, including pyruvate and malate, modulate the development of senescence through the generation of mtROS and through the NAD+/NADH ratio. For instance, supraphysiological levels of pyruvate cause senescence likely due to increased mtROS production through oxidative phosphorylation (100). Malate is another key metabolite that regulates senescence, and inhibition of malate enzymes 1 and 2 induces senescence due to increased NAD+/NADH and NADP+/NADPH ratios (48).
Role of Metabolism in Inflammation
The monocyte-macrophage lineage has the diversity and plasticity to acquire the distinct functional phenotypes M1 and M2. The M1 phenotype generates proinflammatory cytokines, reactive nitrogen and/or oxygen intermediates; promotes Th1 response; and exhibits strong microbicidal and tumoricidal activity. In contrast, M2 macrophages are involved in tissue repair and remodeling. In general, glycolysis supports M1 polarization, whereas M2 macrophage uses mitochondrial oxidative phosphorylation (59, 65, 85, 89) (Table 1). In fact, monocytes and macrophages from patients with atherosclerotic coronary disease showed increased glucose uptake and glycolytic flux, leading to pyruvate kinase M2 (PKM2) nuclear translocation. Nuclear PKM2 can phosphorylate the transcription factor STAT3, resulting IL-6 and IL-1β production (82). Thus, promotion of a metabolic shift from glycolysis toward oxidative phosphorylation could render an inflammatory into a proresolution phenotype. This suggests that there may be new therapeutic approaches to reduce inflammation based on metabolism.
METABOLIC REPROGRAMMING IN CHRONIC LUNG DISEASES
Metabolic Dysregulation in BPD
BPD is characterized by alveolar dysplasia and impaired vascularization along with inflammatory responses and fibrogenesis. This disease is defined clinically by continued dependency on supplemental oxygen beyond 36-wk corrected gestation in premature infants. However, oxygen supplementation can blunt normal lung development and the growth of the pulmonary microvasculature. Although most BPD survivors eventually can be weaned from supplemental oxygen, there can be residual pulmonary dysfunction and cardiovascular sequelae that persist into adolescence and adulthood (19, 36, 97).
It has been reported that the resting metabolic expenditure was elevated in BPD patients with growth failure (58), suggesting impaired substrate utilization. This is corroborated by the findings that the sets of genes characteristic of oxidative phosphorylation were reduced in BPD infants compared with control infants (21). This is in agreement with the findings that L-type amino acid transporter-1 was reduced in BPD patients (12), suggesting abnormal metabolism of amino acids. Human umbilical venous endothelial cells from patients with BPD had lower mitochondrial respiration compared with cells from infants who survived without BPD. This suggests that maximal oxygen consumption is a significant predictor of BPD (51) (Table 2). Indeed, hyperoxic exposure, a model of BPD in rodents, causes reduction of mitochondrial respiration and complex I activity in neonatal mice (75). Furthermore, administration of the complex I inhibitor, pyridaben, caused significantly delayed alveolarization compared with control mice (75), suggesting that dysfunction of mitochondrial respiration contributes to hyperoxic lung injury.
Table 2.
Metabolic reprogramming in chronic lung diseases
| Cell process | BPD | COPD | Lung Fibrosis |
|---|---|---|---|
| Glycolysis | Hyperoxia increased glucose uptake but reduced glycolytic capacity and reserve in MLE-12 (23). | Cigarette smoke reduced glycolysis in type II cells (1). | Increased glycolysis during lung fibrosis (20, 35, 99) |
| PPP | Hyperoxia increased PPP in lipofibroblasts (15). | Compensatory increase in PPP (2) | Trend in increased PPP |
| Mitochondrial respiration | Reduced mitochondrial respiration in BPD and hyperoxia-exposed MLE-12 cells (23, 51) | Disrupted lipid metabolism (5, 57, 69, 86, 91) | Reduced mitochondrial respiration during lung fibrosis (105) |
| FA oxidation | FABP4 and FABP5 were increased (34). | Cigarette smoke enhances FA uptake and oxidation (1). | Reduced FA oxidation (105) |
| Amino acid metabolism | L-type amino acid transporter-1 was reduced BPD patients (12). | Increased glutamine, serine, histidine, arginine, proline, asparagine, aspartic acid, glycine-proline and lysine in patients with emphysema (87) | Increased serine, glycine, and proline synthesis (13) |
| Metabolite | Urine lactate was increased in BPD patients (33). | Acetyl CoA, succinate, NADH, and FADH2 reduction in smokers (27) | Overall reduction of TCA cycle metabolites and enzymes (105) |
BPD, bronchopulmonary dysplasia; COPD, chronic obstructive pulmonary disease.
The resident lipofibroblasts supply neutral lipids to type II pneumocytes for surfactant phospholipid synthesis in the immature fetal lungs. Exposure to hyperoxia causes the lipo-to-myofibroblast transdifferentiation. This is accompanied by an increase in the synthesis of nucleic acid ribose from glucose through the PPP and a decrease in de novo lipid synthesis after hyperoxic exposure. This may explain fibrogenesis and reduced surfactant protein synthesized by type II cells in BPD patients (15).
The main function of fatty acid-binding protein (FABP) family members is the regulation of FA binding, transport, and activation. Ghelfi et al. (34) observed that FABP4 was significantly increased in a subset of macrophages in lungs and endothelial cells of peribronchial blood vessels in BPD patients. The expression of lung FABP3 and FABP4 was also increased in a rat model of BPD induced by intrauterine growth restriction (103). This may cause expansion of the peribronchial vasculature, leading to vessel leakiness, mucosal edema, and inflammatory responses. Nevertheless, the role of FA oxidation in the simplified alveolarization and impaired vascularization, as well as inflammatory responses observed in BPD, is not clear.
Hyperoxic exposure has been shown to induce high glucose uptake in A549 cells (6). Interestingly, no significant change in glycolysis, reduced glycolytic capacity, and reserve were observed in MLE-12 cells exposed to hyperoxia (23). This may be due to the cell-specific characteristic. For example, A549 is a cancer cell line, whereas MLE-12 is an immortalized cell line. It remains elusive whether hyperoxia alters glycolysis and mitochondrial fuel utilization in primary lung type II cells.
Endothelial cells rely on glycolysis, whereas epithelial cells rely on mitochondrial glucose oxidation for bioenergetics. Therefore, it would be important to determine cell-specific changes and roles in metabolism during the development of BPD. Lipid droplets have been considered as a cargo of tunneling nanotubes (10). Therefore, the dysregulated FA metabolism in lipofibroblasts or endothelial cells by hyperoxia or ventilation would be unable to provide alveolar type II cells for synthesis of surfactant phospholipids and surfactant proteins, which may contribute to lung collapse in BPD (76).
Some BPD survivors show evidence of pulmonary and cardiovascular sequelae (e.g., COPD and pulmonary hypertension) in adolescence and adulthood, which suggests the early-life origins of chronic lung diseases (11, 16). Therefore, it is intriguing to determine whether the metabolic reprogramming during the lung development in neonates has long-term effects on the susceptibility to develop other lung diseases in adults.
Dysregulated Metabolisms in COPD
COPD is a leading cause of chronic morbidity and mortality worldwide. Smoking, biofuels, and air pollution are the main risk factors for this disease. COPD is characterized by the excess mucus production, chronic bronchitis, small airway destruction, leading to the decline in lung function. These pathological changes are associated with oxidative stress, inflammatory responses, accelerated senescence, and an apoptosis/proliferation imbalance.
Recent studies have shown that metabolic dysregulation occurs in COPD patients (53, 71, 93) (Table 2). For example, disrupted lipid metabolism was detected in human COPD patients (5, 57, 69, 86), as well as in cigarette smoke-induced emphysema in mice (91), suggesting dysregulation of lipid metabolism during the onset of COPD (50). This is corroborated by the findings that l-carnitine, which serves to carry activated LCFAs into mitochondria, was reduced in elastase-induced emphysema in mice (22). In this study, enhancing FA oxidation by l-carnitine protected against elastase-induced lung function decline and apoptosis in mice. This suggests that impaired FA oxidation is important in the development of COPD/emphysema. In contrast, cigarette smoke exposure (for 8 wk) increased FA uptake and oxidation through upregulation of CD36 and Cpt1 gene expression (1). Pharmacological inhibition of FA oxidation by etomoxir blunted cigarette smoke-induced cell death in bronchial epithelial cells in vitro by reducing mtROS production (50). The discrepancies between these findings may be interpreted as follows: short-term cigarette smoke exposure increases Cpt1 so as to oxidize increased FAs in cytosol, thereby protecting against lipotoxicity. In COPD/emphysema, the carnitine shuttle system is impaired, which leads to accumulation of FAs in the cytosol and subsequent lipotoxicity. The role of FA oxidation in cigarette smoke-induced emphysema will be further elucidated via dynamic analysis of FA uptake, accumulation, and oxidation, as well as genetic approaches using Cpt1a knockout mice.
Metabolomic analysis of primary basal stem/progenitor cells in the airways of healthy long-term smokers revealed reduced metabolites and cofactors compared with nonsmokers (13, 27). For instance, acetyl-CoA levels were reduced in smokers, which may explain the deficit in succinate NADH and FADH2, compared with healthy nonsmokers (27). These deficiencies are indicative of reduced TCA cycle activity, suggesting a bioenergetic crisis. It has been shown that perturbations in amino acid metabolism can subclassify patients with COPD (87). Hence, measurement of metabolites may serve as potential biomarkers for COPD.
Cigarette smoke exposure has been shown to reduce glycolysis in type II cells, which is associated with inhibition of GAPDH via S-glutathionylation, a key enzyme for glycolysis (1). However, COPD patients showed faster whole body glucose metabolism with increased glycolysis (53). Further study is required to investigate these differences.
Metabolic Reprogramming in Lung Fibrosis
Idiopathic pulmonary fibrosis is a progressive lung disease characterized by alveolar epithelial cell injury, differentiation of fibroblasts into myofibroblasts, and extracellular matrix accumulation/remodeling, leading to irreversible distortion of the lung parenchyma. Profiling analysis demonstrates abnormalities in metabolic pathways, including glycolysis, mitochondrial β-oxidation, glutamate/aspartate metabolism, and TCA cycle, in patients with lung fibrosis (52, 105) (Table 2). This is confirmed by increased glycolysis in alveolar macrophages isolated from mice with lung fibrosis, suggesting that glycolysis contributes to the profibrotic phenotype (98). Interestingly, FA oxidation and glutaminolysis are dispensable for the profibrotic M2-like phenotype of macrophages (98). Nevertheless, confirmation of the metabolic changes in a large human cohort is required.
Glycolysis can enhance the differentiation of lung fibroblast into myofibroblasts. Inhibition of glycolysis by targeting glut1, PFKFB3, and pyruvate protects against the development of lung fibrosis in rodents (20, 35, 99). De novo serine synthesis is mediated by the phosphoglycerate dehydrogenase, phosphoserine aminotransferase 1, and phosphoserine phosphatase, which is then converted to glycine via serine hydroxymethyltransferase (Fig. 1). Furthermore, the de novo synthesis of serine and glycine is required for collagen synthesis in lung fibroblasts treated with TGF-β (68). Therefore, one of the mechanisms for lung fibrogenesis involves increased glycolysis leading to the synthesis of glycine, which is then incorporated into collagen.
A recent study has shown that TGF-β1 augments the expression of glutaminase 1, resulting in enhanced glutaminolysis in myofibroblasts (14). Furthermore, blockade of glutaminolysis using glutamine-free culture media or silencing of glutaminase 1 reduces TGFβ1-induced myofibroblast differentiation. This study demonstrates that glutaminolysis plays an important role in promoting myofibroblast differentiation. Nevertheless, it remains elusive whether glutaminolysis contributes to TGF-β1-induced lung fibrosis in vivo. Given TGF-β1 increases both glycolysis and oxidative phosphorylation, it would be important to trace and differentiate the glucose, glutamine, and fatty acid oxidation during TGF-β1-mediated myofibroblast differentiation and fibrosis.
CONCLUSIONS AND PERSPECTIVES
Metabolic homeostasis plays important roles in regulating physiological and pathological processes, including proliferation, differentiation, autophagy, apoptosis, senescence, and inflammatory responses. All of these cell processes contribute to the development of chronic lung diseases, including BPD, COPD, and lung fibrosis. Metabolic dysregulation could contribute to the development of these diseases. Nevertheless, a combination of integrative approaches, including metabolomics, metabolic flux analysis, and positron emission tomography, could reveal the panorama of metabolic alterations in these chronic lung diseases.
Currently, it remains unclear how pharmacologically modulating metabolism of glucose, glutamine, and fatty acids will be of clinical benefit for chronic lung diseases. The first-in-human trial of a mitochondria-targeting drug shows that inhibition of pyruvate dehydrogenase kinase by dichloroacetate improves pulmonary arterial hypertension in genetically susceptible patients (64). Furthermore, enhancing FA oxidation by l-carnitine through nutritional supplementation is beneficial for patients with BPD and asthma (4, 70). Therefore, continued granular dissection of the metabolic abnormalities in these chronic lung diseases could result in the development of targeted therapeutic strategies by targeting metabolic enzymes and pathways.
GRANTS
This work was supported by an Institutional Development Award from the National Institute of General Medical Sciences of the National Institutes of Health under Grant P20GM103652, the National Natural Science Foundation of China (Grants 81472984 and 81001245), and the Science and Technology Innovation Fund of Shanxi Medical University (01201301).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
H.Y. conceived and designed research; H.Z. and H.Y. prepared figures and tables; H.Z. and H.Y. drafted manuscript; H.Z., P.A.D., and H.Y. approved final version of manuscript; P.A.D. and H.Y. edited and revised manuscript.
REFERENCES
- 1.Agarwal AR, Yin F, Cadenas E. Short-term cigarette smoke exposure leads to metabolic alterations in lung alveolar cells. Am J Respir Cell Mol Biol 51: 284–293, 2014. doi: 10.1165/rcmb.2013-0523OC. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal AR, Zhao L, Sancheti H, Sundar IK, Rahman I, Cadenas E. Short-term cigarette smoke exposure induces reversible changes in energy metabolism and cellular redox status independent of inflammatory responses in mouse lungs. Am J Physiol Lung Cell Mol Physiol 303: L889–L898, 2012. doi: 10.1152/ajplung.00219.2012. [DOI] [PubMed] [Google Scholar]
- 3.Agathocleous M, Harris WA. Metabolism in physiological cell proliferation and differentiation. Trends Cell Biol 23: 484–492, 2013. doi: 10.1016/j.tcb.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Al-Biltagi M, Isa M, Bediwy AS, Helaly N, El Lebedy DD. l-carnitine improves the asthma control in children with moderate persistent asthma. J Allergy (Cairo) 2012: 509730, 2012. doi: 10.1155/2012/509730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ali Assad N, Sood A. Leptin, adiponectin and pulmonary diseases. Biochimie 94: 2180–2189, 2012. doi: 10.1016/j.biochi.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allen CB, White CW. Glucose modulates cell death due to normobaric hyperoxia by maintaining cellular ATP. Am J Physiol Lung Cell Mol Physiol 274: L159–L164, 1998. doi: 10.1152/ajplung.1998.274.1.L159. [DOI] [PubMed] [Google Scholar]
- 7.Anastasiou D, Cantley LC. Breathless cancer cells get fat on glutamine. Cell Res 22: 443–446, 2012. doi: 10.1038/cr.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andersen JL, Kornbluth S. The tangled circuitry of metabolism and apoptosis. Mol Cell 49: 399–410, 2013. doi: 10.1016/j.molcel.2012.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ao X, Lubman DM, Davis MA, Xing X, Kong FM, Lawrence TS, Zhang M. Comparative proteomic analysis of radiation-induced changes in mouse lung: fibrosis-sensitive and -resistant strains. Radiat Res 169: 417–425, 2008. doi: 10.1667/RR1173.1. [DOI] [PubMed] [Google Scholar]
- 10.Astanina K, Koch M, Jüngst C, Zumbusch A, Kiemer AK. Lipid droplets as a novel cargo of tunnelling nanotubes in endothelial cells. Sci Rep 5: 11453, 2015. doi: 10.1038/srep11453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baker CD, Abman SH, Mourani PM. Pulmonary hypertension in preterm infants with bronchopulmonary dysplasia. Pediatr Allergy Immunol Pulmonol 27: 8–16, 2014. doi: 10.1089/ped.2013.0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bao EL, Chystsiakova A, Brahmajothi MV, Sunday ME, Pavlisko EN, Wempe MF, Auten RL. Bronchopulmonary dysplasia impairs L-type amino acid transporter-1 expression in human and baboon lung. Pediatr Pulmonol 51: 1048–1056, 2016. doi: 10.1002/ppul.23402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barupal DK, Pinkerton KE, Hood C, Kind T, Fiehn O. Environmental tobacco smoke alters metabolic systems in adult rats. Chem Res Toxicol 29: 1818–1827, 2016. doi: 10.1021/acs.chemrestox.6b00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernard K, Logsdon NJ, Benavides GA, Sanders Y, Zhang J, Darley-Usmar VM, Thannickal VJ. Glutaminolysis is required for TGF-β1-induced myofibroblast differentiation and activation. J Biol Chem 293: 1218–1228, 2017. doi: 10.1074/jbc.RA117.000444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boros LG, Torday JS, Paul Lee WN, Rehan VK. Oxygen-induced metabolic changes and transdifferentiation in immature fetal rat lung lipofibroblasts. Mol Genet Metab 77: 230–236, 2002. doi: 10.1016/S1096-7192(02)00140-3. [DOI] [PubMed] [Google Scholar]
- 16.Boucherat O, Morissette MC, Provencher S, Bonnet S, Maltais F. Bridging lung development with chronic obstructive pulmonary disease. Relevance of developmental pathways in chronic obstructive pulmonary disease pathogenesis. Am J Respir Crit Care Med 193: 362–375, 2016. doi: 10.1164/rccm.201508-1518PP. [DOI] [PubMed] [Google Scholar]
- 17.Bouzier-Sore AK, Bolaños JP. Uncertainties in pentose-phosphate pathway flux assessment underestimate its contribution to neuronal glucose consumption: relevance for neurodegeneration and aging. Front Aging Neurosci 7: 89, 2015. doi: 10.3389/fnagi.2015.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bracha AL, Ramanathan A, Huang S, Ingber DE, Schreiber SL. Carbon metabolism-mediated myogenic differentiation. Nat Chem Biol 6: 202–204, 2010. doi: 10.1038/nchembio.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Check J, Gotteiner N, Liu X, Su E, Porta N, Steinhorn R, Mestan KK. Fetal growth restriction and pulmonary hypertension in premature infants with bronchopulmonary dysplasia. J Perinatol 33: 553–557, 2013. doi: 10.1038/jp.2012.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cho SJ, Moon JS, Lee CM, Choi AM, Stout-Delgado HW. Glucose Transporter 1-dependent glycolysis is increased during aging-related lung fibrosis, and phloretin inhibits lung fibrosis. Am J Respir Cell Mol Biol 56: 521–531, 2017. doi: 10.1165/rcmb.2016-0225OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen J, Van Marter LJ, Sun Y, Allred E, Leviton A, Kohane IS. Perturbation of gene expression of the chromatin remodeling pathway in premature newborns at risk for bronchopulmonary dysplasia. Genome Biol 8: R210, 2007. doi: 10.1186/gb-2007-8-10-r210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Conlon TM, Bartel J, Ballweg K, Günter S, Prehn C, Krumsiek J, Meiners S, Theis FJ, Adamski J, Eickelberg O, Yildirim AO. Metabolomics screening identifies reduced l-carnitine to be associated with progressive emphysema. Clin Sci (Lond) 130: 273–287, 2016. doi: 10.1042/CS20150438. [DOI] [PubMed] [Google Scholar]
- 23.Das KC. Hyperoxia decreases glycolytic capacity, glycolytic reserve and oxidative phosphorylation in MLE-12 cells and inhibits complex I and II function, but not complex IV in isolated mouse lung mitochondria. PLoS One 8: e73358, 2013. doi: 10.1371/journal.pone.0073358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de la Mata M, Cotán D, Villanueva-Paz M, de Lavera I, Álvarez-Córdoba M, Luzón-Hidalgo R, Suárez-Rivero JM, Tiscornia G, Oropesa-Ávila M. Mitochondrial dysfunction in lysosomal storage disorders. Diseases 4: E31, 2016. doi: 10.3390/diseases4040031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab 7: 11–20, 2008. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 26.DeBerardinis RJ, Thompson CB. Cellular metabolism and disease: what do metabolic outliers teach us? Cell 148: 1132–1144, 2012. doi: 10.1016/j.cell.2012.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deeb RS, Walters MS, Strulovici-Barel Y, Chen Q, Gross SS, Crystal RG. Smoking-associated disordering of the airway basal stem/progenitor cell metabotype. Am J Respir Cell Mol Biol 54: 231–240, 2016. doi: 10.1165/rcmb.2015-0055OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doherty JR, Cleveland JL. Targeting lactate metabolism for cancer therapeutics. J Clin Invest 123: 3685–3692, 2013. doi: 10.1172/JCI69741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duan L, Perez RE, Davaadelger B, Dedkova EN, Blatter LA, Maki CG. p53-regulated autophagy is controlled by glycolysis and determines cell fate. Oncotarget 6: 23135–23156, 2015. doi: 10.18632/oncotarget.5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eisenberg T, Schroeder S, Andryushkova A, Pendl T, Küttner V, Bhukel A, Mariño G, Pietrocola F, Harger A, Zimmermann A, Moustafa T, Sprenger A, Jany E, Büttner S, Carmona-Gutierrez D, Ruckenstuhl C, Ring J, Reichelt W, Schimmel K, Leeb T, Moser C, Schatz S, Kamolz LP, Magnes C, Sinner F, Sedej S, Fröhlich KU, Juhasz G, Pieber TR, Dengjel J, Sigrist SJ, Kroemer G, Madeo F. Nucleocytosolic depletion of the energy metabolite acetyl-coenzyme a stimulates autophagy and prolongs lifespan. Cell Metab 19: 431–444, 2014. doi: 10.1016/j.cmet.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eming SA, Wynn TA, Martin P. Inflammation and metabolism in tissue repair and regeneration. Science 356: 1026–1030, 2017. doi: 10.1126/science.aam7928. [DOI] [PubMed] [Google Scholar]
- 32.Eng CH, Yu K, Lucas J, White E, Abraham RT. Ammonia derived from glutaminolysis is a diffusible regulator of autophagy. Sci Signal 3: ra31, 2010. doi: 10.1126/scisignal.2000911. [DOI] [PubMed] [Google Scholar]
- 33.Fanos V, Pintus MC, Lussu M, Atzori L, Noto A, Stronati M, Guimaraes H, Marcialis MA, Rocha G, Moretti C, Papoff P, Lacerenza S, Puddu S, Giuffrè M, Serraino F, Mussap M, Corsello G. Urinary metabolomics of bronchopulmonary dysplasia (BPD): preliminary data at birth suggest it is a congenital disease. J Matern Fetal Neonatal Med 27, Suppl 2: 39–45, 2014. doi: 10.3109/14767058.2014.955966. [DOI] [PubMed] [Google Scholar]
- 34.Ghelfi E, Karaaslan C, Berkelhamer S, Akar S, Kozakewich H, Cataltepe S. Fatty acid-binding proteins and peribronchial angiogenesis in bronchopulmonary dysplasia. Am J Respir Cell Mol Biol 45: 550–556, 2011. doi: 10.1165/rcmb.2010-0376OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goodwin J, Choi H, Hsieh MH, Neugent ML, Ahn JM, Hayenga HN, Singh PK, Shackelford DB, Lee IK, Shulaev V, Dhar S, Takeda N, Kim JW. Targeting HIF-1α/PDK1 axis by dichloroacetate (DCA) suppresses bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol 58: 216–231, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gough A, Linden M, Spence D, Patterson CC, Halliday HL, McGarvey LP. Impaired lung function and health status in adult survivors of bronchopulmonary dysplasia. Eur Respir J 43: 808–816, 2014. doi: 10.1183/09031936.00039513. [DOI] [PubMed] [Google Scholar]
- 37.Guo JY, White E. Autophagy, metabolism, and cancer. Cold Spring Harb Symp Quant Biol 81: 73–78, 2016. doi: 10.1101/sqb.2016.81.030981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ha S, Jeong SH, Yi K, Chung KM, Hong CJ, Kim SW, Kim EK, Yu SW. Phosphorylation of p62 by AMP-activated protein kinase mediates autophagic cell death in adult hippocampal neural stem cells. J Biol Chem 292: 13795–13808, 2017. doi: 10.1074/jbc.M117.780874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hatzivassiliou G, Zhao F, Bauer DE, Andreadis C, Shaw AN, Dhanak D, Hingorani SR, Tuveson DA, Thompson CB. ATP citrate lyase inhibition can suppress tumor cell growth. Cancer Cell 8: 311–321, 2005. doi: 10.1016/j.ccr.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 40.Helmlinger G, Sckell A, Dellian M, Forbes NS, Jain RK. Acid production in glycolysis-impaired tumors provides new insights into tumor metabolism. Clin Cancer Res 8: 1284–1291, 2002. [PubMed] [Google Scholar]
- 41.Henique C, Mansouri A, Fumey G, Lenoir V, Girard J, Bouillaud F, Prip-Buus C, Cohen I. Increased mitochondrial fatty acid oxidation is sufficient to protect skeletal muscle cells from palmitate-induced apoptosis. J Biol Chem 285: 36,818–36,827, 2010. doi: 10.1074/jbc.M110.170431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hensley CT, Wasti AT, DeBerardinis RJ. Glutamine and cancer: cell biology, physiology, and clinical opportunities. J Clin Invest 123: 3678–3684, 2013. doi: 10.1172/JCI69600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ho TT, Warr MR, Adelman ER, Lansinger OM, Flach J, Verovskaya EV, Figueroa ME, Passegué E. Autophagy maintains the metabolism and function of young and old stem cells. Nature 543: 205–210, 2017. doi: 10.1038/nature21388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hu C, Fan L, Cen P, Chen E, Jiang Z, Li L. Energy metabolism plays a critical role in stem cell maintenance and differentiation. Int J Mol Sci 17: 253, 2016. doi: 10.3390/ijms17020253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hue L, Taegtmeyer H. The Randle cycle revisited: a new head for an old hat. Am J Physiol Endocrinol Metab 297: E578–E591, 2009. doi: 10.1152/ajpendo.00093.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hui S, Ghergurovich JM, Morscher RJ, Jang C, Teng X, Lu W, Esparza LA, Reya T, Le Zhan, Yanxiang Guo J, White E, Rabinowitz JD. Glucose feeds the TCA cycle via circulating lactate. Nature 551: 115–118, 2017. doi: 10.1038/nature24057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.James EL, Michalek RD, Pitiyage GN, de Castro AM, Vignola KS, Jones J, Mohney RP, Karoly ED, Prime SS, Parkinson EK. Senescent human fibroblasts show increased glycolysis and redox homeostasis with extracellular metabolomes that overlap with those of irreparable DNA damage, aging, and disease. J Proteome Res 14: 1854–1871, 2015. doi: 10.1021/pr501221g. [DOI] [PubMed] [Google Scholar]
- 48.Jiang P, Du W, Mancuso A, Wellen KE, Yang X. Reciprocal regulation of p53 and malic enzymes modulates metabolism and senescence. Nature 493: 689–693, 2013. doi: 10.1038/nature11776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jiang P, Du W, Wang X, Mancuso A, Gao X, Wu M, Yang X. p53 regulates biosynthesis through direct inactivation of glucose-6-phosphate dehydrogenase. Nat Cell Biol 13: 310–316, 2011. doi: 10.1038/ncb2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jiang Z, Knudsen NH, Wang G, Qiu W, Naing ZZC, Bai Y, Ai X, Lee CH, Zhou X. Genetic control of fatty acid β-oxidation in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol 56: 738–748, 2017. doi: 10.1165/rcmb.2016-0282OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kandasamy J, Olave N, Ballinger SW, Ambalavanan N. Vascular endothelial mitochondrial function predicts death or pulmonary outcomes in preterm infants. Am J Respir Crit Care Med 196: 1040–1049, 2017. doi: 10.1164/rccm.201702-0353OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kang YP, Lee SB, Lee JM, Kim HM, Hong JY, Lee WJ, Choi CW, Shin HK, Kim DJ, Koh ES, Park CS, Kwon SW, Park SW. Metabolic profiling regarding pathogenesis of idiopathic pulmonary fibrosis. J Proteome Res 15: 1717–1724, 2016. doi: 10.1021/acs.jproteome.6b00156. [DOI] [PubMed] [Google Scholar]
- 53.Kao CC, Hsu JW, Bandi V, Hanania NA, Kheradmand F, Jahoor F. Glucose and pyruvate metabolism in severe chronic obstructive pulmonary disease. J Appl Physiol (1985) 112: 42–47, 2012. doi: 10.1152/japplphysiol.00599.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim SP, Li Z, Zoch ML, Frey JL, Bowman CE, Kushwaha P, Ryan KA, Goh BC, Scafidi S, Pickett JE, Faugere MC, Kershaw EE, Thorek DLJ, Clemens TL, Wolfgang MJ, Riddle RC. Fatty acid oxidation by the osteoblast is required for normal bone acquisition in a sex- and diet-dependent manner. JCI Insight 2: 92704, 2017. doi: 10.1172/jci.insight.92704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kondoh H, Lleonart ME, Gil J, Wang J, Degan P, Peters G, Martinez D, Carnero A, Beach D. Glycolytic enzymes can modulate cellular life span. Cancer Res 65: 177–185, 2005. [PubMed] [Google Scholar]
- 56.Koppenol WH, Bounds PL, Dang CV. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat Rev Cancer 11: 325–337, 2011. doi: 10.1038/nrc3038. [DOI] [PubMed] [Google Scholar]
- 57.Koršić M, Kušec V. Serum leptin and skeletal differences between obese and non-obese patients with chronic obstructive pulmonary disease. Obes Facts 7: 399–407, 2014. doi: 10.1159/000369990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kurzner SI, Garg M, Bautista DB, Bader D, Merritt RJ, Warburton D, Keens TG. Growth failure in infants with bronchopulmonary dysplasia: nutrition and elevated resting metabolic expenditure. Pediatrics 81: 379–384, 1988. [PubMed] [Google Scholar]
- 59.Lampropoulou V, Sergushichev A, Bambouskova M, Nair S, Vincent EE, Loginicheva E, Cervantes-Barragan L, Ma X, Huang SC, Griss T, Weinheimer CJ, Khader S, Randolph GJ, Pearce EJ, Jones RG, Diwan A, Diamond MS, Artyomov MN. Itaconate links inhibition of succinate dehydrogenase with macrophage metabolic remodeling and regulation of inflammation. Cell Metab 24: 158–166, 2016. doi: 10.1016/j.cmet.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marsboom G, Zhang GF, Pohl-Avila N, Zhang Y, Yuan Y, Kang H, Hao B, Brunengraber H, Malik AB, Rehman J. Glutamine metabolism regulates the pluripotency transcription factor OCT4. Cell Reports 16: 323–332, 2016. doi: 10.1016/j.celrep.2016.05.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Matoba S, Kang JG, Patino WD, Wragg A, Boehm M, Gavrilova O, Hurley PJ, Bunz F, Hwang PM. p53 regulates mitochondrial respiration. Science 312: 1650–1653, 2006. doi: 10.1126/science.1126863. [DOI] [PubMed] [Google Scholar]
- 62.McGraw TE, Mittal V. Stem cells: metabolism regulates differentiation. Nat Chem Biol 6: 176–177, 2010. doi: 10.1038/nchembio.324. [DOI] [PubMed] [Google Scholar]
- 63.Michalek RD, Gerriets VA, Jacobs SR, Macintyre AN, MacIver NJ, Mason EF, Sullivan SA, Nichols AG, Rathmell JC. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J Immunol 186: 3299–3303, 2011. doi: 10.4049/jimmunol.1003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Michelakis ED, Gurtu V, Webster L, Barnes G, Watson G, Howard L, Cupitt J, Paterson I, Thompson RB, Chow K, O’Regan DP, Zhao L, Wharton J, Kiely DG, Kinnaird A, Boukouris AE, White C, Nagendran J, Freed DH, Wort SJ, Gibbs JSR, Wilkins MR. Inhibition of pyruvate dehydrogenase kinase improves pulmonary arterial hypertension in genetically susceptible patients. Sci Transl Med 9: eaao4583, 2017. doi: 10.1126/scitranslmed.aao4583. [DOI] [PubMed] [Google Scholar]
- 65.Mills EL, Kelly B, Logan A, Costa ASH, Varma M, Bryant CE, Tourlomousis P, Däbritz JHM, Gottlieb E, Latorre I, Corr SC, McManus G, Ryan D, Jacobs HT, Szibor M, Xavier RJ, Braun T, Frezza C, Murphy MP, O’Neill LA. Succinate dehydrogenase supports metabolic repurposing of mitochondria to drive inflammatory macrophages. Cell 167: 457–470.e13, 2016. doi: 10.1016/j.cell.2016.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Muñoz-Espín D, Cañamero M, Maraver A, Gómez-López G, Contreras J, Murillo-Cuesta S, Rodríguez-Baeza A, Varela-Nieto I, Ruberte J, Collado M, Serrano M. Programmed cell senescence during mammalian embryonic development. Cell 155: 1104–1118, 2013. doi: 10.1016/j.cell.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 67.Nicholls C, Pinto AR, Li H, Li L, Wang L, Simpson R, Liu JP. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) induces cancer cell senescence by interacting with telomerase RNA component. Proc Natl Acad Sci USA 109: 13308–13313, 2012. doi: 10.1073/pnas.1206672109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nigdelioglu R, Hamanaka RB, Meliton AY, O’Leary E, Witt LJ, Cho T, Sun K, Bonham C, Wu D, Woods PS, Husain AN, Wolfgeher D, Dulin NO, Chandel NS, Mutlu GM. Transforming growth factor (TGF)-β promotes de novo serine synthesis for collagen production. J Biol Chem 291: 27239–27251, 2016. doi: 10.1074/jbc.M116.756247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Oh YM, Jeong BH, Woo SY, Kim SY, Kim H, Lee JH, Lim SY, Rhee CK, Yoo KH, Lee JH, Yoon HK, Sin DD, Lee SD, Kim EK, Park HY; KOLD Study Group . Association of plasma adipokines with chronic obstructive pulmonary disease severity and progression. Ann Am Thorac Soc 12: 1005–1012, 2015. doi: 10.1513/AnnalsATS.201501-005OC. [DOI] [PubMed] [Google Scholar]
- 70.Ozturk MA, Kardas Z, Kardas F, Gunes T, Kurtoglu S. Effects of l-carnitine supplementation on respiratory distress syndrome development and prognosis in premature infants: A single blind randomized controlled trial. Exp Ther Med 11: 1123–1127, 2016. doi: 10.3892/etm.2015.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Paige M, Burdick MD, Kim S, Xu J, Lee JK, Shim YM. Pilot analysis of the plasma metabolite profiles associated with emphysematous chronic obstructive pulmonary disease phenotype. Biochem Biophys Res Commun 413: 588–593, 2011. doi: 10.1016/j.bbrc.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Paumen MB, Ishida Y, Muramatsu M, Yamamoto M, Honjo T. Inhibition of carnitine palmitoyltransferase I augments sphingolipid synthesis and palmitate-induced apoptosis. J Biol Chem 272: 3324–3329, 1997. doi: 10.1074/jbc.272.6.3324. [DOI] [PubMed] [Google Scholar]
- 73.Phadke M, Krynetskaia N, Mishra A, Krynetskiy E. Accelerated cellular senescence phenotype of GAPDH-depleted human lung carcinoma cells. Biochem Biophys Res Commun 411: 409–415, 2011. doi: 10.1016/j.bbrc.2011.06.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rambold AS, Cohen S, Lippincott-Schwartz J. Fatty acid trafficking in starved cells: regulation by lipid droplet lipolysis, autophagy, and mitochondrial fusion dynamics. Dev Cell 32: 678–692, 2015. doi: 10.1016/j.devcel.2015.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ratner V, Starkov A, Matsiukevich D, Polin RA, Ten VS. Mitochondrial dysfunction contributes to alveolar developmental arrest in hyperoxia-exposed mice. Am J Respir Cell Mol Biol 40: 511–518, 2009. doi: 10.1165/rcmb.2008-0341RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rehan VK, Torday JS. The lung alveolar lipofibroblast: an evolutionary strategy against neonatal hyperoxic lung injury. Antioxid Redox Signal 21: 1893–1904, 2014. doi: 10.1089/ars.2013.5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Roberts DJ, Tan-Sah VP, Ding EY, Smith JM, Miyamoto S. Hexokinase-II positively regulates glucose starvation-induced autophagy through TORC1 inhibition. Mol Cell 53: 521–533, 2014. doi: 10.1016/j.molcel.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rodríguez-Colman MJ, Schewe M, Meerlo M, Stigter E, Gerrits J, Pras-Raves M, Sacchetti A, Hornsveld M, Oost KC, Snippert HJ, Verhoeven-Duif N, Fodde R, Burgering BM. Interplay between metabolic identities in the intestinal crypt supports stem cell function. Nature 543: 424–427, 2017. doi: 10.1038/nature21673. [DOI] [PubMed] [Google Scholar]
- 79.Schoors S, Bruning U, Missiaen R, Queiroz KC, Borgers G, Elia I, Zecchin A, Cantelmo AR, Christen S, Goveia J, Heggermont W, Goddé L, Vinckier S, Van Veldhoven PP, Eelen G, Schoonjans L, Gerhardt H, Dewerchin M, Baes M, De Bock K, Ghesquière B, Lunt SY, Fendt SM, Carmeliet P. Fatty acid carbon is essential for dNTP synthesis in endothelial cells. Nature 520: 192–197, 2015. doi: 10.1038/nature14362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schwartzenberg-Bar-Yoseph F, Armoni M, Karnieli E. The tumor suppressor p53 down-regulates glucose transporters GLUT1 and GLUT4 gene expression. Cancer Res 64: 2627–2633, 2004. doi: 10.1158/0008-5472.CAN-03-0846. [DOI] [PubMed] [Google Scholar]
- 81.Shi LZ, Wang R, Huang G, Vogel P, Neale G, Green DR, Chi H. HIF1α-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J Exp Med 208: 1367–1376, 2011. doi: 10.1084/jem.20110278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shirai T, Nazarewicz RR, Wallis BB, Yanes RE, Watanabe R, Hilhorst M, Tian L, Harrison DG, Giacomini JC, Assimes TL, Goronzy JJ, Weyand CM. The glycolytic enzyme PKM2 bridges metabolic and inflammatory dysfunction in coronary artery disease. J Exp Med 213: 337–354, 2016. doi: 10.1084/jem.20150900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sukhatme VP, Chan B. Glycolytic cancer cells lacking 6-phosphogluconate dehydrogenase metabolize glucose to induce senescence. FEBS Lett 586: 2389–2395, 2012. doi: 10.1016/j.febslet.2012.05.052. [DOI] [PubMed] [Google Scholar]
- 84.Tan HWS, Sim AYL, Long YC. Glutamine metabolism regulates autophagy-dependent mTORC1 reactivation during amino acid starvation. Nat Commun 8: 338, 2017. doi: 10.1038/s41467-017-00369-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G, Frezza C, Bernard NJ, Kelly B, Foley NH, Zheng L, Gardet A, Tong Z, Jany SS, Corr SC, Haneklaus M, Caffrey BE, Pierce K, Walmsley S, Beasley FC, Cummins E, Nizet V, Whyte M, Taylor CT, Lin H, Masters SL, Gottlieb E, Kelly VP, Clish C, Auron PE, Xavier RJ, O’Neill LA. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 496: 238–242, 2013. doi: 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Telenga ED, Hoffmann RF, t’Kind R, Hoonhorst SJ, Willemse BW, van Oosterhout AJ, Heijink IH, van den Berge M, Jorge L, Sandra P, Postma DS, Sandra K, ten Hacken NH. Untargeted lipidomic analysis in chronic obstructive pulmonary disease. Uncovering sphingolipids. Am J Respir Crit Care Med 190: 155–164, 2014. doi: 10.1164/rccm.201312-2210OC. [DOI] [PubMed] [Google Scholar]
- 87.Ubhi BK, Cheng KK, Dong J, Janowitz T, Jodrell D, Tal-Singer R, MacNee W, Lomas DA, Riley JH, Griffin JL, Connor SC. Targeted metabolomics identifies perturbations in amino acid metabolism that sub-classify patients with COPD. Mol Biosyst 8: 3125–3133, 2012. doi: 10.1039/c2mb25194a. [DOI] [PubMed] [Google Scholar]
- 88.Vander Heiden MG, Locasale JW, Swanson KD, Sharfi H, Heffron GJ, Amador-Noguez D, Christofk HR, Wagner G, Rabinowitz JD, Asara JM, Cantley LC. Evidence for an alternative glycolytic pathway in rapidly proliferating cells. Science 329: 1492–1499, 2010. doi: 10.1126/science.1188015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vats D, Mukundan L, Odegaard JI, Zhang L, Smith KL, Morel CR, Wagner G, Greaves DR, Murray PJ, Chawla A. Oxidative metabolism and PGC-1β attenuate macrophage-mediated inflammation. Cell Metab 4: 13–24, 2006. doi: 10.1016/j.cmet.2006.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vaughn AE, Deshmukh M. Glucose metabolism inhibits apoptosis in neurons and cancer cells by redox inactivation of cytochrome c. Nat Cell Biol 10: 1477–1483, 2008. doi: 10.1038/ncb1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vernooy JH, Bracke KR, Drummen NE, Pauwels NS, Zabeau L, van Suylen RJ, Tavernier J, Joos GF, Wouters EF, Brusselle GG. Leptin modulates innate and adaptive immune cell recruitment after cigarette smoke exposure in mice. J Immunol 184: 7169–7177, 2010. doi: 10.4049/jimmunol.0900963. [DOI] [PubMed] [Google Scholar]
- 92.Villar VH, Merhi F, Djavaheri-Mergny M, Durán RV. Glutaminolysis and autophagy in cancer. Autophagy 11: 1198–1208, 2015. doi: 10.1080/15548627.2015.1053680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang L, Tang Y, Liu S, Mao S, Ling Y, Liu D, He X, Wang X. Metabonomic profiling of serum and urine by (1)H NMR-based spectroscopy discriminates patients with chronic obstructive pulmonary disease and healthy individuals. PLoS One 8: e65675, 2013. doi: 10.1371/journal.pone.0065675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ward PS, Thompson CB. Metabolic reprogramming: a cancer hallmark even Warburg did not anticipate. Cancer Cell 21: 297–308, 2012. doi: 10.1016/j.ccr.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wiley CD, Campisi J. From ancient pathways to aging cells-connecting metabolism and cellular senescence. Cell Metab 23: 1013–1021, 2016. doi: 10.1016/j.cmet.2016.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wong BW, Wang X, Zecchin A, Thienpont B, Cornelissen I, Kalucka J, García-Caballero M, Missiaen R, Huang H, Brüning U, Blacher S, Vinckier S, Goveia J, Knobloch M, Zhao H, Dierkes C, Shi C, Hägerling R, Moral-Dardé V, Wyns S, Lippens M, Jessberger S, Fendt SM, Luttun A, Noel A, Kiefer F, Ghesquière B, Moons L, Schoonjans L, Dewerchin M, Eelen G, Lambrechts D, Carmeliet P. The role of fatty acid β-oxidation in lymphangiogenesis. Nature 542: 49–54, 2017. doi: 10.1038/nature21028. [DOI] [PubMed] [Google Scholar]
- 97.Wong PM, Lees AN, Louw J, Lee FY, French N, Gain K, Murray CP, Wilson A, Chambers DC. Emphysema in young adult survivors of moderate-to-severe bronchopulmonary dysplasia. Eur Respir J 32: 321–328, 2008. doi: 10.1183/09031936.00127107. [DOI] [PubMed] [Google Scholar]
- 98.Xie N, Cui H, Ge J, Banerjee S, Guo S, Dubey S, Abraham E, Liu RM, Liu G. Metabolic characterization and RNA profiling reveal glycolytic dependence of profibrotic phenotype of alveolar macrophages in lung fibrosis. Am J Physiol Lung Cell Mol Physiol 313: L834–L844, 2017. doi: 10.1152/ajplung.00235.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xie N, Tan Z, Banerjee S, Cui H, Ge J, Liu RM, Bernard K, Thannickal VJ, Liu G. Glycolytic reprogramming in myofibroblast differentiation and lung fibrosis. Am J Respir Crit Care Med 192: 1462–1474, 2015. doi: 10.1164/rccm.201504-0780OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xu D, Finkel T. A role for mitochondria as potential regulators of cellular life span. Biochem Biophys Res Commun 294: 245–248, 2002. doi: 10.1016/S0006-291X(02)00464-3. [DOI] [PubMed] [Google Scholar]
- 101.Yi CH, Pan H, Seebacher J, Jang IH, Hyberts SG, Heffron GJ, Vander Heiden MG, Yang R, Li F, Locasale JW, Sharfi H, Zhai B, Rodriguez-Mias R, Luithardt H, Cantley LC, Daley GQ, Asara JM, Gygi SP, Wagner G, Liu CF, Yuan J. Metabolic regulation of protein N-α-acetylation by Bcl-xL promotes cell survival. Cell 146: 607–620, 2011. doi: 10.1016/j.cell.2011.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yue L, Yao H. Mitochondrial dysfunction in inflammatory responses and cellular senescence: pathogenesis and pharmacological targets for chronic lung diseases. Br J Pharmacol 173: 2305–2318, 2016. doi: 10.1111/bph.13518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zana-Taieb E, Pham H, Franco-Montoya ML, Jacques S, Letourneur F, Baud O, Jarreau PH, Vaiman D. Impaired alveolarization and intra-uterine growth restriction in rats: a postnatal genome-wide analysis. J Pathol 235: 420–430, 2015. doi: 10.1002/path.4470. [DOI] [PubMed] [Google Scholar]
- 104.Zhang C, Lin M, Wu R, Wang X, Yang B, Levine AJ, Hu W, Feng Z. Parkin, a p53 target gene, mediates the role of p53 in glucose metabolism and the Warburg effect. Proc Natl Acad Sci USA 108: 16259–16264, 2011. doi: 10.1073/pnas.1113884108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhao YD, Yin L, Archer S, Lu C, Zhao G, Yao Y, Wu L, Hsin M, Waddell TK, Keshavjee S, Granton J, de Perrot M. Metabolic heterogeneity of idiopathic pulmonary fibrosis: a metabolomic study. BMJ Open Respir Res 4: e000183, 2017. doi: 10.1136/bmjresp-2017-000183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zheng X, Boyer L, Jin M, Mertens J, Kim Y, Ma L, Ma L, Hamm M, Gage FH, Hunter T. Metabolic reprogramming during neuronal differentiation from aerobic glycolysis to neuronal oxidative phosphorylation. eLife 5: 5, 2016. doi: 10.7554/eLife.13374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zwerschke W, Mazurek S, Stöckl P, Hütter E, Eigenbrodt E, Jansen-Dürr P. Metabolic analysis of senescent human fibroblasts reveals a role for AMP in cellular senescence. Biochem J 376: 403–411, 2003. doi: 10.1042/bj20030816. [DOI] [PMC free article] [PubMed] [Google Scholar]