Abstract
In pulmonary fibrosis (PF), fibroblasts and myofibroblasts proliferate and deposit excessive extracellular matrix in the interstitium, impairing normal lung function. Because most forms of PF have a poor prognosis and limited treatment options, PF represents an urgent unmet need for novel, effective therapeutics. Although the role of immune cells in lung fibrosis is unclear, recent studies suggest that T lymphocyte (T cell) activation may be impaired in PF patients. Furthermore, we have previously shown that activated T cells can produce prostaglandins with anti-scarring potential. Here, we test the hypothesis that activated T cells directly inhibit myofibroblast differentiation using a coculture system. Coculture with activated primary blood-derived T cells, from both healthy human donors and PF patients, inhibited transforming growth factor β-induced myofibroblast differentiation in primary human lung fibroblasts isolated from either normal or PF lung tissue. Coculture supernatants contained anti-fibrotic prostaglandins D2 and E2, and the inhibitory effect of coculture on myofibroblast differentiation was largely reversed when prostaglandin production was abrogated either by resting the T cells before coculture or via specific pharmacological inhibitors. Moreover, coculture conditions induced COX-2 in HLFs but not in T cells, suggesting that T cells deliver an activating signal to HLFs, which in turn produce anti-fibrotic prostaglandins. We show for the first time that coculture with activated primary human T lymphocytes strongly inhibits myofibroblast differentiation, revealing a novel cell-to-cell communication network with therapeutic implications for fibrotic lung diseases.
INTRODUCTION
Pathological scarring, or fibrosis, is an exaggerated wound-healing response that can result in impaired tissue function in any organ. Pulmonary fibrosis (PF) is a spectrum of related disorders with a common pathology involving interstitial fibrosis and destruction of the lung’s gas exchange function. Idiopathic pulmonary fibrosis (IPF), identified histologically as usual interstitial pneumonia, is the most common and severe form of idiopathic interstitial pneumonia, with a median survival time of just 2.9 yr following diagnosis (61). Estimating the global disease burden of IPF has been complicated, but a recent, carefully designed study in Canada reported an incidence rate of 9.0–18.7 per 100,000 population, with prevalence in the same study estimated at 20.0–41.8 per 100,000 population (31). Increased age and male sex are associated with higher disease incidence, and recent epidemiological studies suggest that, whereas IPF incidence rates are likely remaining stable, overall cumulative prevalence is steadily rising, reflecting an ever-growing IPF patient population (60).
Although the pathogenesis of lung fibrosing diseases remains largely enigmatic, vigorous research in recent decades has unveiled a complex network of profibrotic signaling pathways, with fibroblasts and myofibroblasts known to play a central role in the disease (22). Myofibroblasts are scar-forming mesenchymal cells that maintain many features of their fibroblast precursors while also acquiring smooth muscle cell characteristics, including contractility and expression of α-smooth muscle actin (αSMA) and calponin (26, 27). In PF, myofibroblasts proliferate and accumulate in the lung interstitium, where they deposit excessive extracellular matrix (ECM), destroying normal lung architecture and impeding gas exchange (41, 74). Fibroblast to myofibroblast differentiation can be induced by a variety of profibrotic cytokines, chiefly transforming growth factor-β (TGFβ), a pleiotropic cytokine that plays key roles in both normal wound healing and fibrosis (4, 5, 18, 38). TGFβ drives the expression of many ECM proteins, including collagen, fibronectin, and the tissue inhibitors of matrix metalloproteinases that are upregulated during wound healing and in fibrotic diseases (7, 39, 69). Moreover, TGFβ potently prevents myofibroblast apoptosis and may thereby prevent resolution of normal wound healing (78).
Despite substantial progress in elucidating the pathophysiology of PF, clinical trials using novel therapeutics for PF have yielded mostly disappointing results, reflecting the complex, redundant, and often self-propagating nature of the profibrotic signaling pathways involved in the disease (67). Only two drugs, pirfenidone and nintedanib, are currently approved by the US Food and Drug Administration for the treatment of IPF (40, 47). Furthermore, there is little evidence that these drugs substantially alter the fatal course of the disease, underscoring an enormous unmet need for effective treatments (61).
The role of immune cells in lung fibrosis is unclear, but recent evidence suggests that activated T cells may be key anti-fibrotic regulators and that a lack of T cell activation may contribute to the progression of PF. Birjandi et al. (3) reported a lymphocyte-dependent increase in bleomycin-induced lung fibrosis in mice treated with an IL-2/IL-2 antibody complex to expand CD4+CD25hiFoxp3+ regulatory T cells (Tregs) in vivo. This result is consistent with the finding of an imbalanced Treg/Th17 axis in IPF patients, wherein the proportion and absolute number of Tregs in circulation is markedly elevated, whereas the frequency of circulating Th17 cells is significantly decreased (21). Interestingly, gene expression profiling of peripheral blood mononuclear cells (PBMCs) in IPF patients has shown that decreased expression of genes associated with T cell activation, including CD28, inducible T cell costimulator (ICOS), lymphocyte-specific protein tyrosine kinase (LCK), and interleukin-2-inducible T cell kinase (ITK), is significantly associated with reduced transplant-free survival (23, 25). Taken together, these studies strongly suggest a potential role for activated T cells as anti-fibrotic effectors.
Our group previously demonstrated that in coculture with orbital fibroblasts, activated human T cells can produce D-series prostaglandins, lipid mediators with potent proadipogenic, anti-scarring effects that drive human orbital fibroblast differentiation to adipocytes (14). A dehydration product of PGD2, 15-deoxy-Δ12,14 prostaglandin J2 (15d-PGJ2), is a natural ligand for peroxisome proliferator-activated receptor-γ (PPARγ), a nuclear transcription factor that regulates adipogenesis (20, 42). We and others have shown that naturally occurring and synthetic PPARγ agonists inhibit TGFβ-induced myofibroblast differentiation in human lung fibroblasts (5, 16, 35, 45, 50, 57). Thus far, no one has directly investigated the role of human T lymphocytes in TGFβ-driven lung myofibroblast differentiation.
Here, we test the hypothesis that activated T cells inhibit TGFβ-induced lung myofibroblast differentiation through production of anti-fibrotic prostaglandins. To accomplish this, we cocultured activated human PBMC-derived T cells with primary human lung fibroblasts (HLFs) in the presence of TGFβ. We report the novel finding that T cells potently inhibit TGFβ-induced HLF differentiation to myofibroblasts via an unknown signal that prompts cyclooxygenase (COX)-2 expression by the HLFs and subsequent production of anti-fibrotic prostaglandins.
MATERIALS AND METHODS
Reagents.
Recombinant human TGFβ1 and IL-2 were purchased from R & D Systems (Minneapolis, MN). The hematopoietic PGD synthase (hPGDS) inhibitor HQL-79 (Cayman Chemical, Ann Arbor, MI) and microsomal PGE synthase (mPGES)-1 inhibitor Arzanol (Sigma-Aldrich, St. Louis, MO) were prepared as per the manufacturers’ instructions in DMSO and used at the final concentrations indicated in the figure legends. Puromycin was purchased from Sigma-Aldrich.
Cell culture studies.
All patient samples were obtained with written, informed consent under the approval of the University of Rochester Institutional Review Board. Primary HLFs were derived and grown from control subjects as well as subjects with IPF, as previously described (2, 17). Briefly, fresh human lung tissue was washed with sterile PBS and minced in 100-mm dishes with scissors to <1 mm size. Minimum essential medium (MEM; GIBCO-Life Technologies, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS; Hyclone/GE Healthcare Life Sciences, Logan, UT), 2 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 0.25 μg/ml amphotericin B (GIBCO-Life Technologies) was added to the minced tissue, which was then cultured at 37°C in humidified 7% CO2. Following visible growth of fibroblasts, cells were removed from the 100-mm dishes using Trypsin-EDTA (GIBCO-Life Technologies) and then passaged and cultured in supplemented MEM before being used for experimentation. These cells are morphologically consistent with fibroblasts and express collagen and vimentin. They do not express CD45, factor VIII, or cytokeratin. Cells were used between passages 4 and 10.
T lymphocytes were isolated from peripheral blood and cultured as previously described (15). Briefly, whole blood was separated on a Ficoll-Plaque Plus (GE Healthcare, Pittsburgh, PA) gradient to obtain PBMCs. Adherent cells were depleted by overnight adhesion in MEM supplemented with 10% FBS, 2 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 0.25 μg/ml amphotericin B. T lymphocytes were cultured in RPMI 1640 medium (GIBCO-Life Technologies) supplemented with 50 μM 2-mercaptoethanol, 2 mM l-glutamine, 10 mM HEPES buffer, 0.1 mM nonessential amino acids, 1 mM sodium pyruvate, 0.05 mg/ml gentamicin, and 10% FBS. T lymphocytes were activated and expanded using CD3/CD28 Dynabeads (GIBCO-Life Technologies) and 25 U/ml IL-2 in supplemented RPMI 1640 medium.
Enriched T cell preparations were stained with anti-CD3-Alexa Fluor 647 (Biolegend, San Diego, CA), anti-CD4-Alexa Fluor 488 (Biolegend), and anti-CD8-PE-Cy5 (BD Biosciences, San Jose, CA) antibodies and analyzed on an LSRII flow cytometer (BD Biosciences) to determine purity. Subsequent analysis was performed using FlowJo software (Ashland, OR). For some experiments, the expanded, enriched T cell population was purified using CD4+ (Miltenyi Biotec, San Diego, CA) and CD8+ (R & D Systems, Minneapolis, MN) negative selection columns according to the manufacturer’s instructions. Jurkat T leukemia cells (Jurkat T cells) were maintained in RPMI 1640 medium supplemented with 5% FBS. Jurkat T cells were irradiated with 15 Gray (Gy) from a 137Cs γ-ray source at ∼270 cGy/min dose rate before coculture.
Fibroblast-T lymphocyte coculture.
On day 1, HLFs were plated in 12-well culture plates at 25,000 cells/well in supplemented MEM. On day 2, activated and expanded T cells were harvested from culture and resuspended in supplemented RPMI 1640 medium and added directly to HLFs at HLF/T cell ratios of 1:20, 1:10, and 1:5. Cocultures were then treated with 5 ng/ml TGFβ and maintained at 37°C in humidified 7% CO2 for 72 h unless otherwise indicated.
In some experiments, HLFs were cultured as described above, but T cells were cocultured with physical separation from HLFs using Millicell hanging inserts (EMD Millipore, Billerica, MA). These inserts consisted of a semipermeable polyethylene terephthalate film punctuated with 1.0-µm pores at a density of 2 × 106 pores/cm2, enabling the free passage of soluble mediators between the apical (T cell) and basolateral (HLF) compartments. In some experiments, T cells were removed from activating media and “rested” in culture media without CD3/CD28 activating beads or IL-2 for 18 h before coculture with HLFs. In some experiments, pharmacological inhibitors of prostaglandin synthesis were added daily at the concentrations indicated in the figure legends.
Western blot analysis.
At the end of the 3-day coculture, T cells were removed from the coculture by repeated washes with PBS, and microscopic evaluation was used to confirm complete removal of T cells before harvesting and preparing HLF whole cell lysates using lysis buffer (60 mM Tris·HCl, pH 6.8, 2% SDS) supplemented with protease inhibitor cocktail (Sigma-Aldrich), as previously described (45). Total protein (5–10 μg/lane) was separated by denaturing SDS-PAGE and then electrotransferred to PVDF membrane (Millipore). Antibodies were as follows: α-smooth muscle actin (α-SMA; A2547, detects a band of 42-kDa size; Sigma-Aldrich; see Ref. 65), calponin (EP798Y, detects a band of 34 kDa; Abcam; see Ref. 30), COX-2 (160112, detects a band of ∼70 kDa; Cayman Chemical; see Ref. 52), and cleaved PARP (no. 9541, detects a band of ∼90 kDa; Cell Signaling Technology; see Ref. 77). β-Tubulin (Abcam ab6046) or GAPDH (Abcam ab8245) was used as a loading control. Protein expression was detected using horseradish peroxidase-conjugated secondary antibodies (Jackson ImmunoResearch, West Grove, PA) and visualized by enhanced chemiluminescence substrate (Millipore). Image capture was performed by film (BioBlot BXR; Laboratory Products Sales, Rochester, NY) or by using a c-DiGit blot scanner (LI-COR, Lincoln, NE), and densitometry analysis was performed with Image Studio (version 5.2) software (LI-COR).
Immunofluorescence for αSMA.
Lung fibroblasts were fixed with ice-cold methanol for 10 min and stained with anti-αSMA antibody, followed by AlexaFluor 568-conjugated secondary antibody (Invitrogen). Coverslips were then mounted with Prolong Gold containing DAPI (Invitrogen) to allow visualization of nuclei. Slides were imaged on a Zeiss Axio Imager Z.1 Microscope using Axio Imaging software (Zeiss).
Fibroblast proliferation.
HLFs were cultured on 96-well plates for 24 h and then washed with PBS and cocultured with irradiated Jurkat T cells (15Gy) in RPMI 1640 medium with or without TGFβ for 72 h. [3H]thymidine (1 μCi/well) was added to cells for the final 18 h of the coculture. [3H]thymidine incorporation was determined as previously described (15).
DiOC6 assay.
Cultured HLFs were incubated with 40 nM DiOC6 (3,3′-dihexyloxacarbocyanine iodide; Invitrogen) for 15 min at 37°C and then harvested from the culture dish using trypsin-EDTA, washed, and suspended in PBS. Mitochondrial membrane potential of cells was determined by flow cytometry analysis using a FACS Canto II cytometer (BD Biosciences) using the Blue E detector channel (488 nm laser; 530/30-nm filter). Subsequent analysis was performed using FlowJo software (Ashland, OR).
Enzyme immunoassay for PGD2 and PGE2.
PGD2 and PGE2 in cell culture media were measured by competitive enzyme immunoassay (EIA) using the PGD2-MOX EIA Kit and PGE2 Express EIA kit, respectively, according to the manufacturer’s instructions (Cayman Chemical).
Statistics.
Results are reported as means ± SE as indicated. One-way ANOVA with Tukey posttest was performed using GraphPad Prism software (version 7, La Jolla, CA). P values < 0.05 were considered significant.
RESULTS
Jurkat T cells inhibit TGFβ-induced α-SMA expression and proliferation in human lung fibroblasts without inducing cell death.
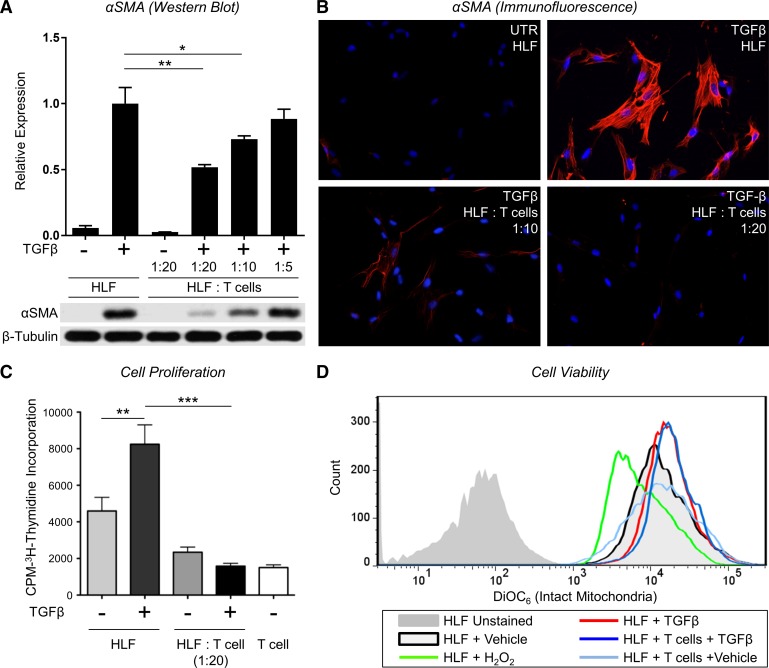
Recent gene expression profiling of PBMCs in IPF patients showed a decreased expression of genes associated with T cell activation associated with reduced transplant-free survival, suggesting that activated T cells may be important anti-fibrotic regulators. To examine whether or not T lymphocytes exert anti-fibrotic effects, we first used a human T leukemia cell line (i.e., Jurkat) in coculture with primary HLFs. Jurkat T cells are rapidly proliferating cells that originated from the peripheral blood of a T cell leukemia patient; therefore, we subjected the Jurkat T cells to 15 Gy ionizing radiation before coculture to limit their proliferation (15). To induce myofibroblast differentiation, primary HLFs were treated with TGFβ (5 ng/ml) in the presence or absence of irradiated Jurkat T cells in a direct contact coculture system. After 72 h, we assessed α-SMA expression as a marker of myofibroblast differentiation by both Western blot (Fig. 1A) and immunofluorescence (Fig. 1B). Fibroblasts treated with TGFβ differentiated to myofibroblasts and expressed α-SMA, and this effect was potently and dose-dependently inhibited by coculturing the HLFs with irradiated Jurkat T cells.
Fig. 1.
Jurkat T lymphocytes inhibit transforming growth factor-β (TGFβ)-induced α-smooth muscle actin (α-SMA) expression and proliferation in human lung fibroblasts without inducing cell death. A: human lung fibroblasts (HLFs) were treated with 5 ng/ml TGFβ in the presence or absence of irradiated Jurkat T cells at indicated ratios. After 72 h, T cells were removed by repeated PBS washes, and α-SMA protein expression in the HLFs was analyzed by Western blot and densitometric analysis. Densitometry of n = 3 replicates per cell strain normalized to TGFβ alone. Data shown are means ± SE. *P < 0.05 and **P < 0.01 by ANOVA. B: HLFs were grown in chamber slides and treated with 5 ng/ml TGFβ in the presence or absence of irradiated Jurkat T cells at indicated ratios. After 72 h, T cells were removed by repeated PBS washes, and HLFs were stained with an antibody to α-SMA (red) and DAPI (blue). C: HLFs were treated with 5 ng/ml TGFβ in the presence or absence of irradiated Jurkat T cells for 48 h. Cells were allowed to incorporate [3H]thymidine for another 18 h; n = 6/group. Data shown are means ± SE. **P < 0.01 and ***P < 0.001 by ANOVA. D: HLFs were treated with 5 ng/ml TGFβ in the presence or absence irradiated Jurkat T cells for 72 h. During the last 15 min, T cells were removed by washing, and HLFs were labeled with 40 nM DiOC6. HLFs with reduced mitochondrial membrane potential were detected by FACS Canto II cytometer using Blue E detector (488 laser + 530/30 filter).
We next investigated the effects of Jurkat T cells on fibroblast proliferation and viability. First, to assess the effect of irradiated Jurkat T cells on TGFβ-induced fibroblast proliferation, we used a [3H]thymidine incorporation assay. Jurkat T cells were irradiated before coculture and added to HLF cultures in the presence or absence of TGFβ for 72 h. [3H]thymidine incorporation was assessed during the final 18 h of incubation (Fig. 1C). A moderate baseline level of proliferation was detected in untreated HLFs in monoculture, and the addition TGFβ significantly promoted HLF proliferation. Addition of irradiated Jurkat T cells significantly attenuated HLF proliferation in both untreated and TGFβ-treated conditions. Irradiated Jurkat T cells alone exhibited negligible proliferation.
Next, we used flow cytometry to determine whether or not Jurkat T cell-induced reduction in HLF proliferation was attributable to loss of HLF viability. We measured mitochondrial membrane potential using DiOC6 to evaluate cell viability in HLFs cocultured with irradiated Jurkat T cells for 72 h. DiOC6 (3,3′-dihexyloxacarbocyanine iodide) is a cell-permeable, lipophilic fluorescent dye that when used at low concentrations selectively accumulates in intact mitochondria of live cells. Loss of fluorescence indicates damaged mitochondria that have lost membrane potential and membrane integrity, reflecting the initiation of proapoptotic pathways (8). As a positive control for reduced mitochondrial membrane potential and induction of apoptosis, cells were incubated with 10 mM H2O2, which caused a considerable reduction in DiOC6 fluorescence (Fig. 1D). No significant differences in fluorescence intensities were detected between the HLFs alone vs. coculture groups, indicating that irradiated Jurkat T cells do not affect HLF viability (Fig. 1D).
Primary T lymphocytes from healthy human donors inhibit TGFβ-induced myofibroblast differentiation in both normal and fibrotic human lung fibroblasts.
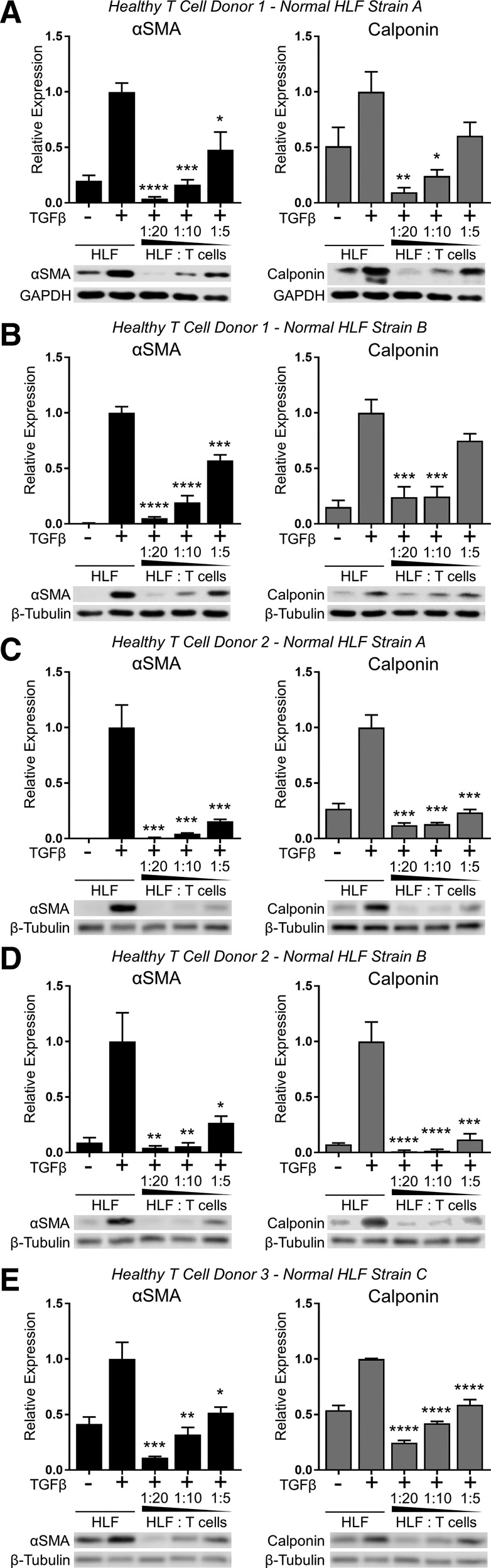
We next investigated whether or not primary human T lymphocytes, like Jurkat T cells, would exert an anti-fibrotic effect on HLFs. Fresh PBMC-derived primary T lymphocytes were purified from three healthy donors and expanded and activated using CD3/CD28 beads in media supplemented with IL-2. Activated T cells were then cocultured in direct contact with primary HLFs isolated from the normal lung tissue of three human subjects without IPF. Cocultures were established using HLF/T cell ratios ranging from 1:5 to 1:20 and maintained for 72 h in the presence or absence of TGFβ. Like Jurkat T cells, primary human T cells potently and dose-dependently inhibited TGFβ-induced myofibroblast differentiation, as determined by calponin and α-SMA expression (Fig. 2).
Fig. 2.
Primary T lymphocytes from healthy donors inhibit TGFβ-induced myofibroblast differentiation in lung fibroblasts isolated from nonfibrotic lung tissue. Three strains of HLFs isolated from normal lung tissue of donors without idiopathic pulmonary fibrosis (IPF; normal HLF strains A, B, and C) were cultured with activated peripheral blood mononuclear cell (PBMC)-derived primary T cells from 3 healthy donors (donors 1, 2, and 3) at indicated ratios and treated with 5 ng/ml TGFβ for 72 h. Expression of α-SMA and calponin was analyzed by Western blot and densitometric analysis. A–E: different combinations of T cell and HLF donors as labeled at top. Data shown are means ± SE of n = 3 replicates/condition, protein expression relative to loading control, normalized to TGFβ alone. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 by ANOVA compared with HLF + TGFβ control.
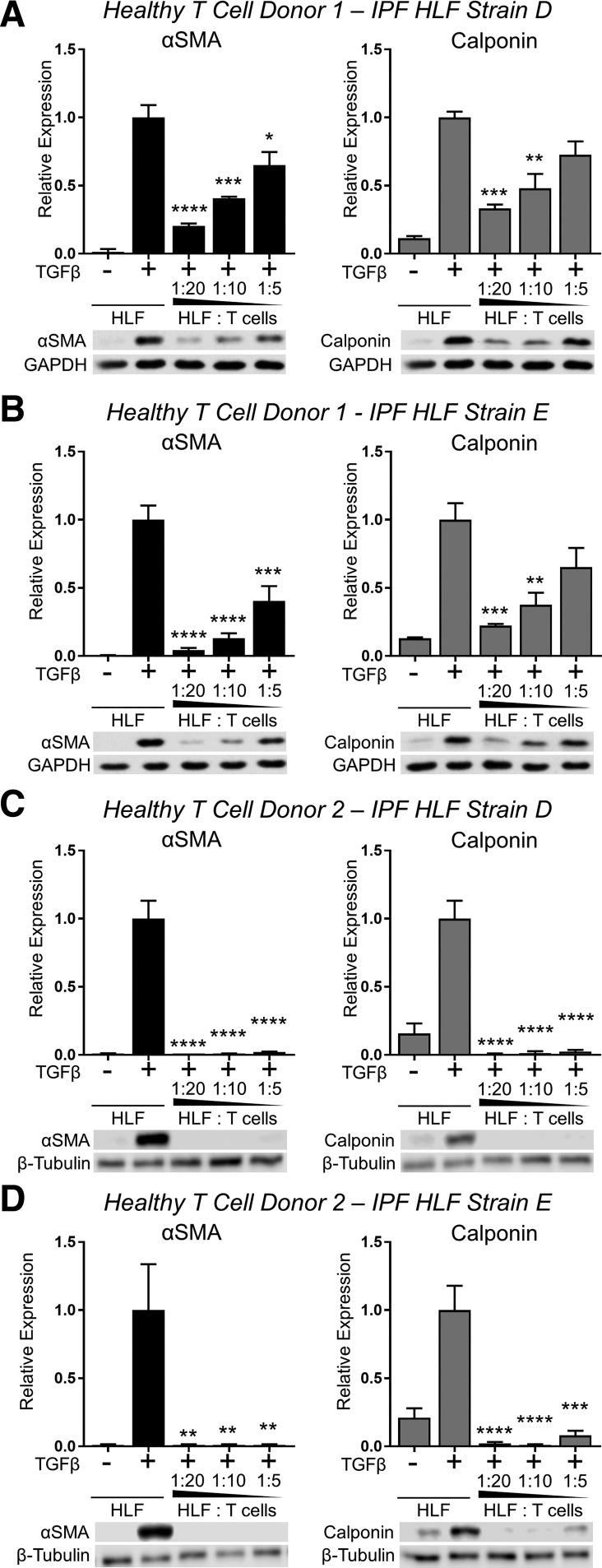
Some studies have reported that HLFs isolated from IPF patients differ fundamentally from those isolated from healthy lung tissue (33, 51, 62, 75, 76). Therefore, we also investigated whether or not HLFs isolated from IPF patients would be susceptible to the inhibitory effects of activated T cells on TGFβ-induced myofibroblast differentiation. We cocultured two primary IPF HLF strains in contact with activated T cells isolated from two healthy blood donors in the presence of TGFβ for 72 h. Like HLFs isolated from normal lung tissue, both strains of IPF HLFs exhibited significantly reduced TGFβ-induced myofibroblast differentiation in the presence of activated T cells (Fig. 3).
Fig. 3.
Primary T lymphocytes from healthy donors inhibit TGFβ-induced myofibroblast differentiation in lung fibroblasts isolated from IPF lung tissue. Two strains of HLFs isolated from the fibrotic lung tissue of donors with IPF (IPF HLF strains D and E) were cultured with activated PBMC-derived primary T cells from 2 healthy donors (donors 1 and 2) at indicated ratios and treated with 5 ng/ml TGFβ for 72 h. Expression of α-SMA and calponin were analyzed by Western blot and densitometric analysis. A–D: different combinations of T cell and HLF donors as labeled at top. Data shown are means ± SE of n = 3 replicates/condition, protein expression relative to loading control, normalized to TGFβ alone. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 by ANOVA compared with HLF + TGFβ control.
Primary T lymphocytes from IPF patients inhibit TGFβ-induced myofibroblast differentiation in both normal and fibrotic human lung fibroblasts.
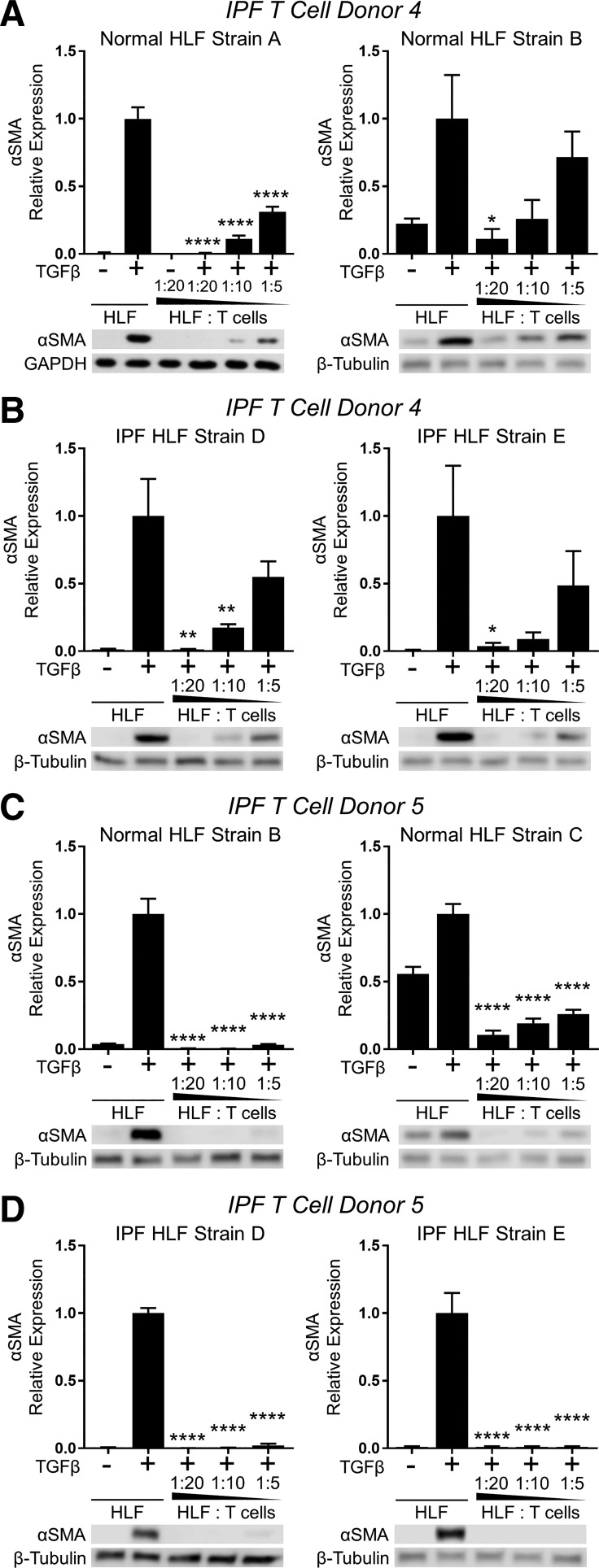
We next determined whether or not primary T cells isolated from IPF patients would have anti-fibrotic effects similar to those of healthy donors. Activated T cells from each of two IPF patient donors were cultured with two normal and two IPF HLF strains in the presence of TGFβ for 72 h. Like irradiated Jurkat T cells and primary T cells isolated from healthy donors, activated T cells isolated from IPF patients inhibited TGFβ-induced myofibroblast differentiation in both normal and IPF strains of HLFs (Fig. 4).
Fig. 4.
Primary T lymphocytes from IPF patients inhibit TGFβ-induced myofibroblast differentiation in both normal and fibrotic human lung fibroblasts. PBMC-derived primary T cells were purified, expanded, and activated from 2 IPF patient donors (IPF donors 4 and 5). T cells from each donor were used in coculture with 2 normal HLF strains (normal HLF strains A and B or B and C) and 2 IPF HLF strains (IPF HLF strains D and E) at indicated ratios in the presence of 5 ng/ml TGFβ for 72 h. Expression of α-SMA was analyzed by Western blot and densitometric analysis. A–D: different combinations of T cell and HLF donors as labeled at top. Data shown are means ± SE of n = 3 replicates/condition, protein expression relative to loading control, normalized to TGFβ alone. *P < 0.05, **P < 0.01, and ****P < 0.0001 by ANOVA compared with HLF + TGFβ control.
Inhibitory effects of coculture with primary T lymphocytes on HLF myofibroblast differentiation are not attributable to HLF cell death or spillover of IL-2 from T cell activation media.
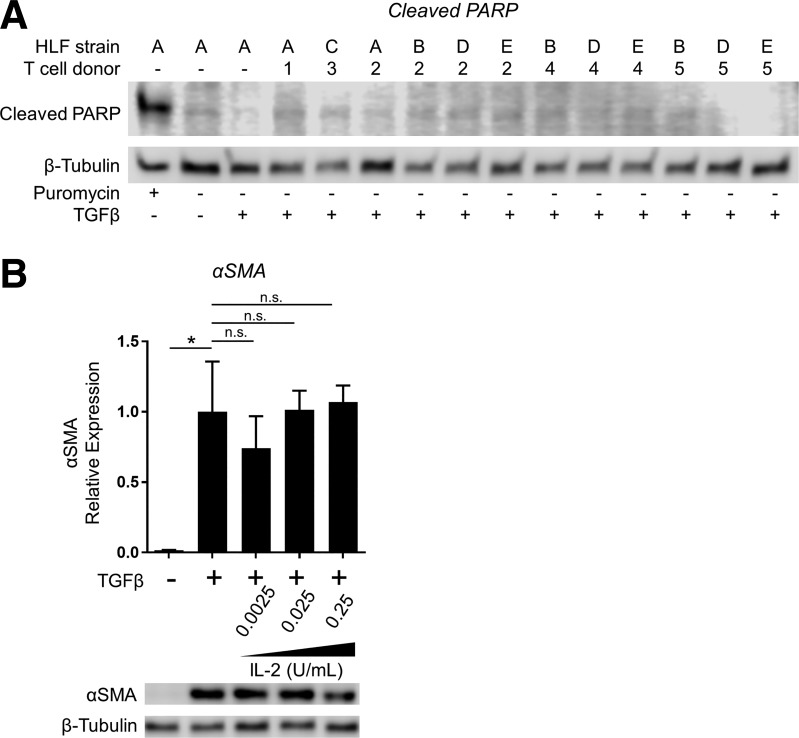
As in our initial cocultures with irradiated Jurkat T cells, cocultures with activated primary T cells did not cause any overt effects on HLF cell viability detectable at the light microscopic level. However, to exclude induction of HLF cell death as a contributor to the anti-fibrotic effects of coculture, we tested HLFs cocultured with activated T cells for expression of cleaved PARP, a sensitive marker of apoptosis, by Western blot. Cell lysates from HLFs treated for 24 h with puromycin were used as a positive control (Fig. 5A). No HLF expression of cleaved PARP was detected in any of the 12 tested HLF-T cell coculture combinations, indicating that coculture conditions did not induce HLF apoptosis, consistent with our earlier findings.
Fig. 5.
Inhibition of myofibroblast differentiation in coculture with primary T cells is not attributable to IL-2 spillover or HLF cell death. A: HLFs from normal lung tissue (strains A, B, and C) and IPF lung tissue (strains D and E) were cocultured with PBMC-derived primary T cells from healthy donors (donors 1, 2, and 3) and IPF patient donors (donors 4 and 5) at 1:10 HLF/T cell ratio in the presence of 5 ng/ml TGFβ for 72 h. Expression of cleaved PARP was analyzed by Western blot. As a positive control (lane 1), HLFs were treated with puromycin (5 ug/ml) for 24 h to induce apoptosis. B: HLFs were treated with 5 ng/ml TGFβ in the absence or presence of IL-2 at the doses indicated. Expression of α-SMA was analyzed by Western blot with densitometry. Data shown are means ± SE of n = 3 replicates/condition, protein expression relative to loading control, normalized to TGFβ alone. *P < 0.05 by ANOVA. NS, not significant.
As detailed in materials and methods, primary T cells were grown and expanded in an activation medium containing CD3/CD28 beads and supplemental IL-2. Prior to placement into cocultures, beads were completely removed using a magnet, and activation medium was removed by centrifugation and aspiration before resuspension in fresh media without IL-2. However, to ascertain whether or not spillover of IL-2 from the T cell activation medium could be responsible for the inhibitory effects of coculture on HLF myofibroblast differentiation, we treated HLFs with TGFβ in the presence or absence of recombinant IL-2 at final concentrations of 1/100th, 1/1,000th, and 1/10,000th of that present in T cell activation medium. As shown in Fig. 5B, IL-2 did not significantly inhibit TGFβ-induced HLF myofibroblast differentiation, indicating that the inhibitory effect of coculture was not attributable to IL-2 spillover from T cell activation medium.
Purified CD4+ and CD8+ primary T lymphocytes inhibit myofibroblast differentiation.
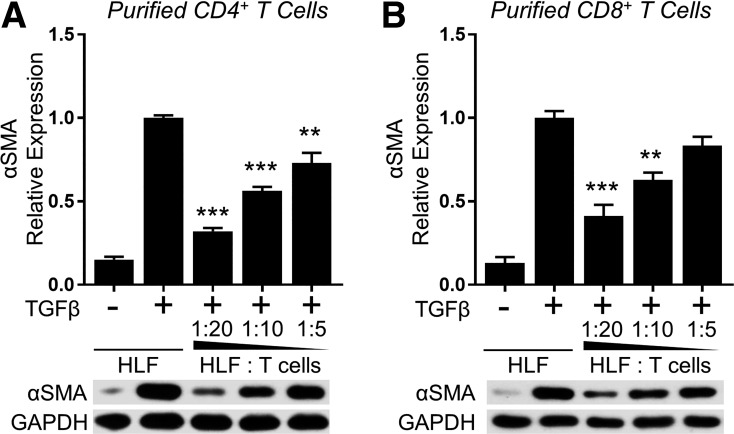
Both CD4+ and CD8+ T cells have been found in the bronchoalveolar lavage fluid of IPF patients, and the ratio generally skews toward high CD8+ T cell numbers (19, 34, 48). Therefore, we examined whether the CD4+ or CD8+ subset of T cells plays a more dominant role in inhibiting TGFβ-induced myofibroblast differentiation. Prior to coculture with HLFs, we separated highly enriched primary human T cells into CD4+ and CD8+ subsets using negative selection magnetic beads or T cell columns, respectively. Interestingly, both CD4+ and CD8+ cells potently and dose-dependently inhibited α-SMA expression in TGFβ-treated HLFs similarly to total T cells (Fig. 6).
Fig. 6.
Purified CD4+ and CD8+ primary T lymphocytes inhibit myofibroblast differentiation. CD4+ (A) and CD8+ (B) T cells were purified from the expanded, enriched T cell population using negative selection. HLFs (normal HLF strain A) were cultured with the purified T cell subsets and treated with 5 ng/ml TGFβ for 72 h. Expression of α-SMA was analyzed by Western blot and densitometric analysis. Data shown are means ± SE of n = 3 replicates/condition, protein expression relative to loading control, normalized to TGFβ alone. **P < 0.01 and ***P < 0.001 by ANOVA compared with HLF + TGFβ control.
Primary T lymphocyte-mediated inhibition of myofibroblast differentiation is contact independent.
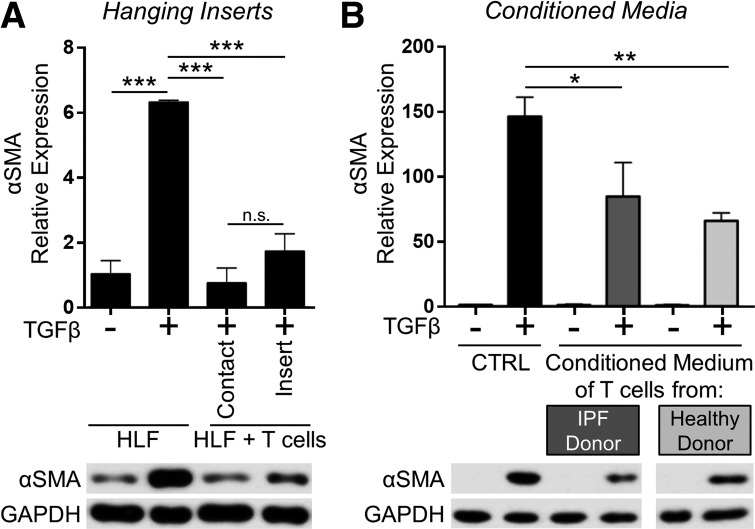
Our laboratory has previously shown that HLFs can engage with T cells via CD40/CD40L interactions, and T cells express other surface molecules, such as the T cell receptor, which could engage with HLF MHC to facilitate cell-to-cell signaling (53, 68, 79). To determine whether the anti-fibrotic effects of coculture on HLFs required direct contact with T cells or were due to the production of soluble mediators such as cytokines or prostaglandins, we used hanging cell culture inserts to physically separate T cells from HLFs in coculture while permitting the passage of soluble mediators through a porous membrane. As in our previous experiments, TGFβ-induced α-SMA expression in HLFs was strongly inhibited by direct contact coculture with T cells. Additionally, TGFβ-induced HLF α-SMA expression was also inhibited in the noncontact coculture system, indicating that direct contact is not necessary for coculture with activated T cells to inhibit HLF myofibroblast differentiation and suggesting that one or more soluble factors may mediate the anti-fibrotic effect (Fig. 7A). To further explore this finding, we treated HLFs with the conditioned culture medium of activated T cells in the presence of TGFβ for 72 h. The conditioned medium of activated T cells also inhibited TGFβ-induced myofibroblast differentiation, confirming the presence of one or more soluble anti-fibrotic mediators (Fig. 7B).
Fig. 7.
Primary T-lymphocyte-mediated inhibition of myofibroblast differentiation is contact independent. A: HLFs were cocultured with primary T cells (1:20 ratio) either in contact or separated by a semipermeable hanging insert and treated with 5 ng/ml TGFβ for 72 h. B: conditioned media from activated normal T cells and IPF T cells were collected. HLFs were treated with 5 ng/ml TGF-β in the presence of T cell-conditioned medium for 72 h. α-SMA protein expression was analyzed by Western blot and densitometric analysis. Data shown are means ± SE of n = 3 replicates/condition, protein expression relative to loading control, normalized to untreated control. *P < 0.05, **P < 0.01, and ***P < 0.001 by ANOVA. NS, not significant. Note that the indicated samples were resolved on the same gel, and intervening irrelevant lanes are not shown.
Anti-fibrotic prostaglandins are present in the conditioned medium of HLF-primary T cell cocultures but not monocultures.
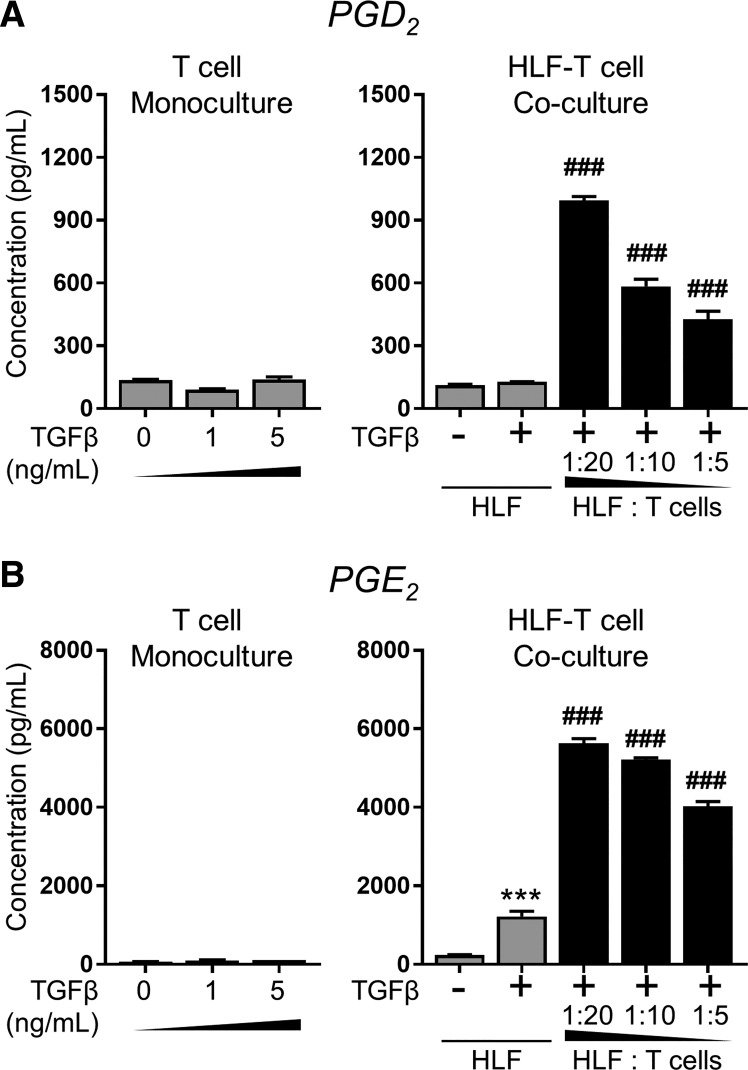
Our group has previously reported that activated primary human T cells can produce eicosanoids, including D2 and E2 series prostaglandins, and that the PGD2 derivative 15d-PGJ2 is a potent endogenous antifibrotic mediator (5, 14, 16). We have also recently reported that healthy human lung epithelial cells inhibit TGFβ-induced myofibroblast differentiation via PGE2, an eiscosanoid well documented to exert anti-fibrotic effects in the lung (13, 32, 58, 73). Therefore, we used enzyme immunoassays specific for PGD2 and PGE2 to determine whether or not these anti-fibrotic mediators were present in the conditioned medium of either T cell or HLF monocultures or HLF-T cell cocultures. The conditioned media of monocultured activated primary T cells did not contain significant levels of PGD2 or PGE2, regardless of treatment with TGFβ. Untreated monocultured HLFs produced low basal levels of PGD2 and PGE2, and treatment with TGFβ induced a small increase in PGE2 production. In contrast, the conditioned media of HLF-T cell cocultures contained elevated levels of both prostaglandins, with concentrations dependent partly on T cell density in the coculture (Fig. 8).
Fig. 8.
Antifibrotic prostaglandins are present in the conditioned medium of HLF primary T cell cocultures, but not monocultures. For T cell monocultures, PBMC-derived primary T cells from healthy donor 1 were plated at the same density as that used in 1:20 HLF-T cell cocultures and treated with TGFβ at the indicated doses for 48 h. For HLF monocultures and HLF-T cell cocultures, HLFs (normal strain A) were cultured either alone (gray) or with activated PBMC-derived primary T cells from healthy donor 1 at indicated ratios and treated with 5 ng/ml TGFβ for 72 h. Supernatants were collected and analyzed for prostaglandins D2 (PGD2; A) and E2 (PGE2; B) content by enzyme immunoassay (EIA). Data shown are means ± SE of n = 3 biological replicates/condition. ***P < 0.001 by ANOVA, compared with HLF − TGFβ, or HLF alone, or HLF without TGFβ; ###P < 0.001 by ANOVA compared with HLF + TGFβ.
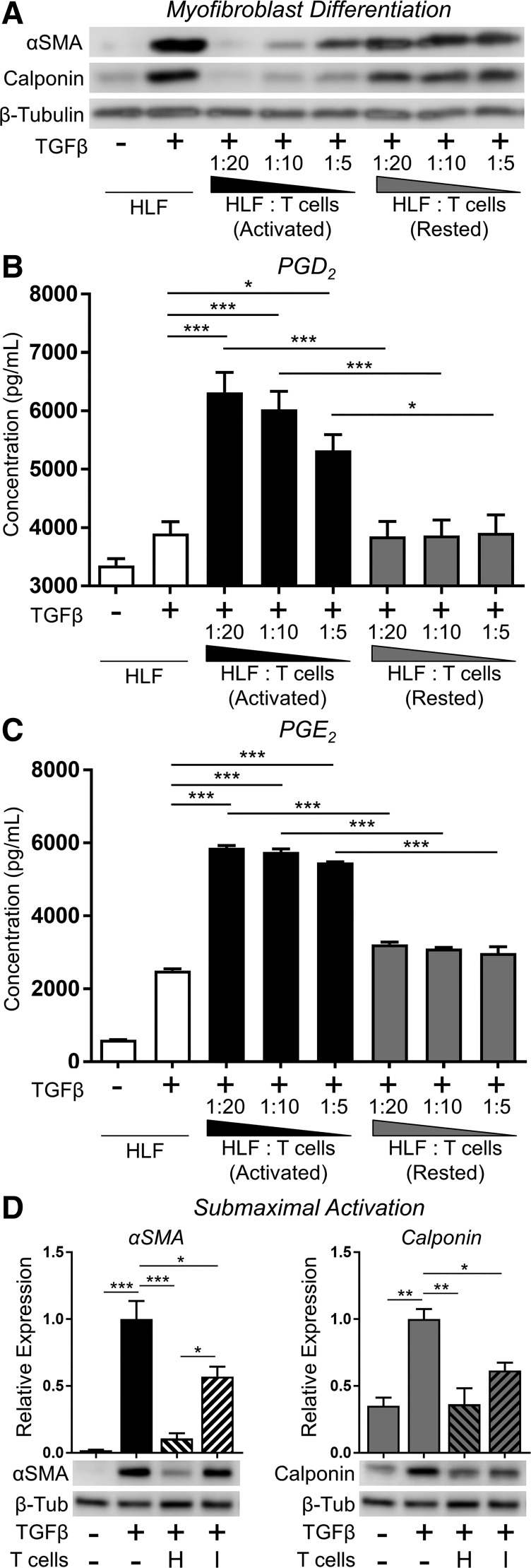
Prostaglandin production and anti-fibrotic function are abrogated in cocultures with rested and submaximally activated primary human T cells.
Some recent reports suggest that impaired T cell activation may be associated with IPF disease progression (23, 25). Therefore, we investigated whether resting the T cells before coculture with HLFs would reduce their inhibitory efficacy. T cells were activated with CD3/CD28 beads and IL-2, as described in materials and methods, and then removed to fresh culture medium lacking activators and rested for 18 h before being put in coculture with fibroblasts. As a positive control, an equal population of T cells was placed in fresh complete activation medium. Prior to placement in coculture, both rested and normally activated T cells were assessed for viability by trypan blue dye exclusion, with no difference between treatments. T cells were then counted and placed into direct contact cocultures with HLFs at the ratios previously described, in the presence of TGFβ, for 72 h. As shown in Fig. 9A, normally activated T cells potently inhibited TGFβ-induced HLF myofibroblast differentiation, whereas rested T cells were markedly less effective. Importantly, the supernatants of HLFs cocultured with activated, but not rested, T cells contained anti-fibrotic PGD2 and PGE2, suggesting that these mediators are essential for the anti-fibrotic effects of T cells on HLFs in coculture (Fig. 9, B and C). We also evaluated a “submaximal” activation strategy. Primary donor T cells were cultured in submaximal activation conditions by reducing IL-2 and CD3/CD28 beads to one-tenth that of normal activation medium. After 24 h, cocultures were established and treated with TGFβ, and myofibroblast differentiation was assessed by Western blot after 72 h. Although T cells from a healthy donor were still very effective at inhibiting α-SMA and calponin expression when submaximally activated, T cells from an IPF donor were much less potent albeit still able to significantly inhibit expression of differentiation markers (Fig. 9D).
Fig. 9.
Prostaglandin production and antifibrotic function are abrogated in rested and submaximally activated primary human T cells. A–C: HLFs were cocultured with PBMC-derived primary T cells that were activated with CD3/CD28 beads and IL-2 (activated) or removed from activating media and rested for 18 h before coculture (rested). Cocultures were established at the indicated HLF/T cell ratios and treated with 5 ng/ml TGFβ for 72 h. Expression of α-SMA and calponin were analyzed by Western blot (A), and supernatants were collected and analyzed for PGD2 (B) and PGE2 (C) content by EIA. D: PBMC-derived primary T cells from healthy donor 1 (H) and IPF patient donor 4 (I) were cultured in “submaximal activation” conditions by reducing IL-2 and CD3/CD28 beads to one-tenth that of normal activation concentrations (i.e., 2.5 U/ml IL-2 and bead/T cell ratio of 1:10). After 24 h, cocultures were established at a 1:10 ratio of HLFs (normal strain A) to submaximally activated T cells, and cocultures were treated with TGFβ (5 ng/ml); after 72 h, T cells were removed and HLF lysates prepared for Western blot and densitometric analysis of markers of myofibroblast differentiation. Data shown are means ± SE of n = 3 biological replicates per condition. *P < 0.05, **P < 0.01, and ***P < 0.001 by ANOVA.
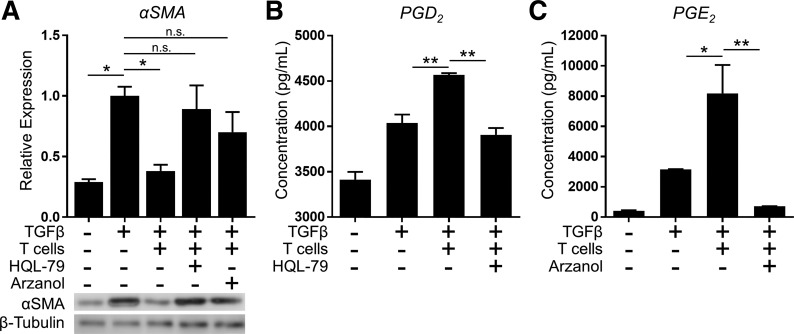
Inhibition of myofibroblast differentiation by T cells is dependent on hPGDS and mPGES-1.
To specifically explore the relative contributions of PGD2 and PGE2 to the anti-fibrotic effect of T cells on HLFs in coculture, we used pharmacological inhibitors of the enzymes most proximately responsible for their synthesis: HQL-79 to specifically inhibit hPGDS and Arzanol to inhibit mPGES-1. T cells and HLFs were pretreated with enzyme inhibitors separately in monoculture for 18 h before coculture and then daily throughout the 72-h coculture period. As shown in Fig. 10, HQL-79 and Arzanol abrogated the production of PGD2 and PGE2, respectively. Most importantly, inhibition of either prostaglandin significantly blunted the inhibitory effect of T cells on TGFβ-induced HLF myofibroblast differentiation. Taken together, these findings reveal that anti-fibrotic PGD2 and PGE2 each mediate the inhibitory effect of T cells on TGFβ-induced myofibroblast differentiation.
Fig. 10.
Inhibition of myofibroblast differentiation by T cells is dependent on hematopoietic PGD synthase (hPGDS) and microsomal PGE synthase-1 (mPGES-1). HLFs were cocultured with activated PBMC-derived primary T cells at a HLF/T cell ratio of 1:10 and treated with 5 ng/ml TGFβ for 72 h. Pharmacological inhibitors of hPGDS (HQL-79, 25 µM) or mPGES-1 (Arzanol, 2.5 μM) were added to the separate monocultures for 18 h before coculture and daily throughout the 3-day coculture experiment. A: expression of α-SMA was analyzed by Western blot. B and C: supernatants were collected and analyzed for PGD2 (B) and PGE2 (C) content by EIA. Data shown are means ± SE of n = 3 biological replicates/condition. *P < 0.05 and **P < 0.01 by ANOVA. NS, not significant.
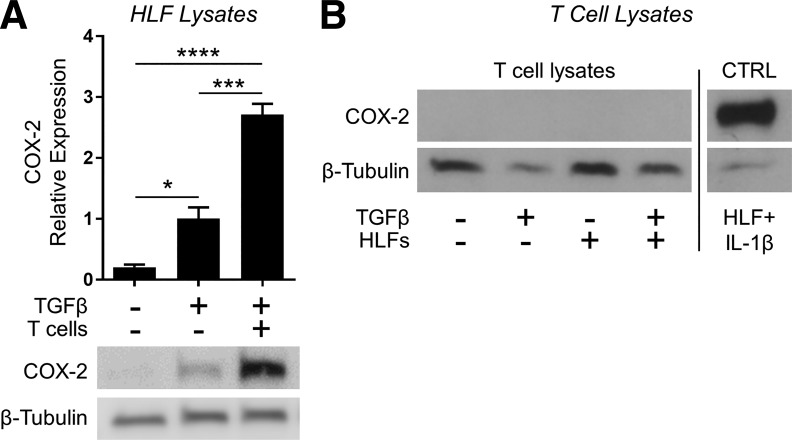
Coculture conditions cause COX-2 expression in human lung fibroblasts but not T cells.
Our work up to this point indicated that in coculture with activated T cells, HLFs resisted TGFβ-induced myofibroblast differentiation, and this effect required the presence of anti-fibrotic prostaglandins in the coculture. Both T cells and HLFs are capable of producing prostaglandins, but our coculture system precluded us from specifically treating one cell type with an inhibitor without the possibility of carryover that would affect the other cell type. Therefore, we instead examined expression of COX-2, the rate-limiting enzyme in prostaglandin biosynthesis. Here, we used noncontact cocultures with the fibroblasts on the bottom of the well and the activated T cells in hanging inserts. After 24 h of coculture with TGFβ, we prepared lysates from each compartment for Western blot analysis. As shown in Fig. 11A, COX-2 expression was absent in untreated HLFs, as expected. Treatment with TGFβ alone induced a small increase in COX-2 expression, consistent with the modest production of PGD2 and PGE2 we saw in Fig. 10, B and C, in HLF monocultures treated with TGFβ. Importantly, COX-2 expression in the fibroblast compartment was markedly enhanced in coculture conditions. On the other hand, T cells did not express COX-2, either in monoculture or coculture conditions (Fig. 11B). Taken together, these data suggest that activated T cells produce a soluble factor that stimulates HLFs to produce prostaglandins, which in turn exert an anti-fibrotic effect in an autocrine fashion.
Fig. 11.
Coculture conditions cause cyclooxygenase-2 (COX-2) expression in human lung fibroblasts but not T cells. HLFs were cocultured with Jurkat T cells (1:20 ratio of HLFs to T cells) separated by a porous hanging insert and treated with 5 ng/ml TGFβ for 24 h. Expression of COX-2 in both populations was analyzed by Western blot. A: data shown are means ± SE of n = 3 biological replicates/condition, protein expression relative to loading control, normalized to TGFβ alone. *P < 0.05, ***P < 0.001, and ****P < 0.0001 by ANOVA. B: all samples, including positive control (lysate from HLFs treated with 1 ng/ml IL-1β), were resolved on the same gel, and irrelevant intervening lanes are not shown.
DISCUSSION
Recent work in our laboratory indicates that cellular cross-talk between human lung epithelial cells and fibroblasts is important for maintaining homeostasis and that healthy lung epithelial cells exert an anti-fibrotic effect on fibroblasts (13). We also recently published the targeted lipidome of activated HLFs, which showed that activated HLFs produce anti-inflammatory and anti-fibrotic prostaglandins (46). Here, we investigated the role of activated T lymphocytes on TGFβ-induced HLF to myofibroblast differentiation. Our data show that both a T cell clone and primary human T lymphocytes potently inhibit TGFβ-induced myofibroblast differentiation of HLFs from both normal lung tissue and IPF patients. Inhibition does not require direct cell-cell contact but does require that T cells be activated and not rested. Most intriguingly, we show that activated T cells stimulate HLFs via an unknown signal to upregulate COX-2 expression and produce their own antifibrotic prostaglandins, which act in an autocrine fashion to maintain homeostasis and confer resistance to a profibrotic stimulus. This is the first report of T cells exerting a direct antifibrotic effect on primary HLFs.
It is important to acknowledge that our coculture system is artificial and may not reflect the activation state of T cells or the ratio of T cells to HLFs that would be present in fibrotic lesions in vivo. However, our results are, at the very least, proof of principle that interactions with T cells can enable fibroblasts to resist profibrotic stimuli. Several studies have reported that mononuclear cells, including lymphocytes, exert anti-fibrotic effects (9–12, 36, 43, 63). In extrapulmonary tissues, including the heart (28) and skin (71), T cell interactions with local cells are important in maintaining normal tissue homeostasis and facilitating wound healing (24). In the lung, the role of T cells in pulmonary fibrosis is still unclear. Although biopsies, particularly of end-stage lesions, often have only mild mononuclear cell infiltrates (61), a few studies have specifically characterized the distribution of T cells in IPF tissue and suggested that T cells may be numerous and in close proximity to fibroblasts, in some stages of the disease (54, 70). One study found significantly elevated numbers of T cells in regions of active disease (i.e., “epithelial dominant” areas characterized by epithelial cell regeneration and active fibrosis) but markedly decreased T cell numbers in more advanced fibrotic (i.e., “stromal dominant”) regions of the lung (54), suggesting that loss of T cell aggregates may coincide with lesion progression to an irreversible fibrotic phenotype. Moreover, the dynamics of T cell interactions with HLFs in vivo are unknown, and it is possible that even intermittent communication between normal activated T cells and HLFs could result in important homeostatic and antifibrotic signaling, even if T cells are not a predominant feature histologically.
It should be noted that, although we used T cells isolated from circulating PBMCs rather than from lung tissue, there is support in the literature for the idea that circulating T cells have similar properties to pulmonary T cells. For example, gene profiling studies in circulating PBMCs have shown that reduced expression of genes involved in T cell activation is associated with shortened transplant-free survival in IPF patients (23, 25), whereas downregulated CD28 expression on circulating CD4+ T cells is reflective of CD4+CD28null T cell infiltrates in IPF lung tissue and correlates with poorer prognosis (23). In sum, there is reasonable evidence to suggest that, rather than driving disease progression, activated T cells play a role in patient survival and disease inhibition. Our results are congruent with this view in that coculture with activated T cells inhibits myofibroblast differentiation in HLFs.
As CD4+ T cells have been reported to be associated with better patient outcomes in IPF (1), and with reduced fibrosis in the bleomycin mouse model (37), we tested whether or not the ability to inhibit myofibroblast differentiation was greater in CD4+ cells. Somewhat surprisingly, we found that activated CD4+ and CD8+ T cells were equally effective inhibitors of TGFβ-induced myofibroblast differentiation (Fig. 6). However, the role of specific helper T cell subsets in PF remains unclear. Several studies have reported deficiency or impairment of FOXP3+ regulatory T cells (Tregs) in PF, suggesting that Tregs may be antifibrotic regulators (44, 64). In direct contrast, however, others have shown that IPF patients have significantly increased numbers of Tregs in the lung, with concomitant impairment of Th17 cells (21), and Tregs have been reported to enhance bleomycin-induced pulmonary fibrosis in a mouse model (3). T cells in PF patients may be unable to reach the site of action due to vascular remodeling or destruction of the capillary beds (51, 52), depriving fibroblasts of an important homeostatic signal. Our model may be a useful tool in future studies to clarify these conflicting data and address how specific T cell subsets differentially affect myofibroblast differentiation. Given our novel finding that activated T cells produce an unknown soluble factor that stimulates HLFs to produce antifibrotic prostaglandins, identification of that factor will be a prerequisite to determining which T cells produce it and under which conditions.
One important unanswered question is whether there are differences in this response in PF patients. Are fibrotic T cells impaired in their ability to activate an antifibrotic program in HLFs? Are fibrotic HLFs impaired in their ability to respond to activation by T cells and/or respond to their own autocrine signals? Previous reports have indicated that fibroblasts from IPF patients exhibit impaired upregulation of COX-2 and PGE2 when stimulated with LPS, PMA, or IL-1β (75), and other studies have reported reduced COX-2 expression in IPF lung tissue (59) and in the rodent bleomycin model (56). Therefore, it was unexpected that IPF fibroblasts would respond similarly to T cell stimulation as normal fibroblasts. On the other hand, we have previously reported that HLFs from IPF patients respond to PGE2 released by epithelial cells in coculture (13). A number of hypotheses can be suggested to explain these results. Most simply, the soluble factor produced by activated T cells that stimulates prostaglandin production in fibroblasts may be more effective at stimulating IPF fibroblasts than LPS or IL-1β. Additionally, pulmonary fibrosis is a heterogeneous disorder, and fibroblasts from different donors, or even from different regions of the same donor, may respond differently to stimulation by different activators. Coculture systems are inherently more complex than monocultures, although they are also a step closer to full organ culture, and the T cells here might produce multiple factors that work together to upregulate both COX-2 and prostaglandin production and overcome any resistance to stimulation that is part of an IPF fibroblast phenotype. Our study is not sufficiently powered to claim that all IPF fibroblasts can respond to activated T cells by producing PGs, but neither can we rule out that T cells might produce one or more factors that not only stimulate PG production,but also help IPF fibroblasts overcome this acquired defect.
We also note that the T cell stimulation conditions and T cell/fibroblast ratios may not reflect physiological conditions, and indeed, T cells subject to reduced stimulation were less effective at blocking myofibroblast differentiation (Fig. 9D). This is a common issue encountered when trying to translate complex physiological processes to comparatively simple in vitro models. Fibroblasts in situ are exposed to T cells and other lung resident and transient cells over months or years, in contrast to an in vitro assay that uses pure TGFβ to drive essentially 100% myofibroblast differentiation in less than 3 days. Here, we show proof of concept that activated T cells can promote homeostasis in lung fibroblasts by making them resistant to TGFβ stimulation. Whether this is a significant contributor to homeostasis in vivo, and whether the loss of such interactions is a contributor to the pathogenesis of pulmonary fibrosis, requires further study.
The role of eicosanoids and other lipid-derived molecules as key mediators of endogenous homeostatic and antifibrotic pathways in the lung is increasingly recognized. Although its functions are complex and context dependent, PGE2 is well-reported to exert potent antifibrotic effects in the lung (6, 58, 72). Recent work in our laboratory has shown that lung epithelial cell-derived PGE2 strongly attenuates TGFβ-induced HLF to myofibroblast differentiation (13). Likewise, PGD2 and several of its metabolites, including 15d-PGJ2, are known natural ligands for PPARγ, a transcription factor that we have previously shown to mediate potent anti-inflammatory and antifibrotic effects in the lung (17, 29, 45). Although we have reported that activated HLFs upregulate COX-2 and produce a wide variety of prostaglandins, some with anti-inflammatory or antifibrotic properties (46, 49, 55, 66), the ability of HLFs to produce their own autocrine antifibrotic mediators represents a novel paradigm with significant translational implications. Instead of supplying antifibrotic mediators exogenously, a new therapeutic goal may be to identify the signals produced by T cells, epithelial cells, and other lung cells that drive HLFs to produce their own antifibrotic mediators to reinforce beneficial cell-cell communication and resist disease progression.
In summary, we studied directly the effects of coculture with primary human T cells on TGFβ-induced differentiation of primary HLFs to myofibroblasts. Our finding that T cells exert a potent activation status- and cell number-dependent antifibrotic effect is consistent with recent studies suggesting that impaired T cell activation may contribute to disease progression in PF (21, 23, 25). To our knowledge, this is the first report of a direct antifibrotic effect of primary human T cells on primary HLFs. This endogenous regulatory pathway may represent a novel therapeutic opportunity for treating pulmonary fibrosis.
GRANTS
This work was supported in part by National Institutes of Health grants R01-HL-120908, T32-HL-066988, and P30-ES-001247, the Greg Chandler and Guy F. Solimano Fibrosis Research Fund, the Doran Family Endowment, and the C. Jane Davis and C. Robert Davis Professorship.
S. H. Lacy was funded in part by the US Army Medical Department.
DISCLAIMERS
The views expressed herein are those of the author and do not reflect the official policy or position of the Department of the Army, Department of Defense, or the US Government.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.H.L., A.P.E., C.F.W., T.H.T., R.P.P., and P.J.S. conceived and designed research; S.H.L., A.P.E., and S.J.P. performed experiments; S.H.L., A.P.E., S.J.P., C.F.W., T.H.T., R.P.P., and P.J.S. analyzed data; S.H.L., A.P.E., C.F.W., T.H.T., R.P.P., and P.J.S. interpreted results of experiments; S.H.L., A.P.E., and S.J.P. prepared figures; S.H.L. and A.P.E. drafted manuscript; S.H.L., A.P.E., S.J.P., C.F.W., T.H.T., R.P.P., and P.J.S. edited and revised manuscript; S.H.L., A.P.E., S.J.P., C.F.W., T.H.T., R.P.P., and P.J.S. approved final version of manuscript.
REFERENCES
- 1.Adegunsoye A, Hrusch CL, Bonham CA, Jaffery MR, Blaine KM, Sullivan M, Churpek MM, Strek ME, Noth I, Sperling AI. Skewed Lung CCR4 to CCR6 CD4+T Cell Ratio in Idiopathic Pulmonary Fibrosis Is Associated with Pulmonary Function. Front Immunol 7: 516, 2016. doi: 10.3389/fimmu.2016.00516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baglole CJ, Reddy SY, Pollock SJ, Feldon SE, Sime PJ, Smith TJ, Phipps RP. Isolation and phenotypic characterization of lung fibroblasts. Methods Mol Med 117: 115–127, 2005. doi: 10.1385/1-59259-940-0:115. [DOI] [PubMed] [Google Scholar]
- 3.Birjandi SZ, Palchevskiy V, Xue YY, Nunez S, Kern R, Weigt SS, Lynch JP III, Chatila TA, Belperio JA. CD4(+)CD25(hi)Foxp3(+) cells exacerbate bleomycin-induced pulmonary fibrosis. Am J Pathol 186: 2008–2020, 2016. doi: 10.1016/j.ajpath.2016.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Broekelmann TJ, Limper AH, Colby TV, McDonald JA. Transforming growth factor beta 1 is present at sites of extracellular matrix gene expression in human pulmonary fibrosis. Proc Natl Acad Sci USA 88: 6642–6646, 1991. doi: 10.1073/pnas.88.15.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burgess HA, Daugherty LE, Thatcher TH, Lakatos HF, Ray DM, Redonnet M, Phipps RP, Sime PJ. PPARgamma agonists inhibit TGF-beta induced pulmonary myofibroblast differentiation and collagen production: implications for therapy of lung fibrosis. Am J Physiol Lung Cell Mol Physiol 288: L1146–L1153, 2005. doi: 10.1152/ajplung.00383.2004. [DOI] [PubMed] [Google Scholar]
- 6.Choung J, Taylor L, Thomas K, Zhou X, Kagan H, Yang X, Polgar P. Role of EP2 receptors and cAMP in prostaglandin E2 regulated expression of type I collagen alpha1, lysyl oxidase, and cyclooxygenase-1 genes in human embryo lung fibroblasts. J Cell Biochem 71: 254–263, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- 7.Clarke DL, Carruthers AM, Mustelin T, Murray LA. Matrix regulation of idiopathic pulmonary fibrosis: the role of enzymes. Fibrogenesis Tissue Repair 6: 20, 2013. doi: 10.1186/1755-1536-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cottet-Rousselle C, Ronot X, Leverve X, Mayol JF. Cytometric assessment of mitochondria using fluorescent probes. Cytometry A 79A: 405–425, 2011. doi: 10.1002/cyto.a.21061. [DOI] [PubMed] [Google Scholar]
- 9.Elias JA, Rossman MD, Daniele RP. Inhibition of human lung fibroblast growth by mononuclear cells. Am Rev Respir Dis 125: 701–705, 1982. doi: 10.1164/arrd.1982.125.6.701. [DOI] [PubMed] [Google Scholar]
- 10.Elias JA, Rossman MD, Zurier RB, Daniele RP. Human alveolar macrophage inhibition of lung fibroblast growth. A prostaglandin-dependent process. Am Rev Respir Dis 131: 94–99, 1985. doi: 10.1164/arrd.1985.131.1.94. [DOI] [PubMed] [Google Scholar]
- 11.Elias JA, Zurier RB, Rossman MD, Berube ML, Daniele RP. Inhibition of lung fibroblast growth by human lung mononuclear cells. Am Rev Respir Dis 130: 810–816, 1984. doi: 10.1164/arrd.1984.130.5.810. [DOI] [PubMed] [Google Scholar]
- 12.Elias JA, Zurier RB, Schreiber AD, Left JA, Daniele RP. Monocyte inhibition of lung fibroblast growth: relationship to fibroblast prostaglandin production and density-defined monocyte subpopulations. J Leukoc Biol 37: 15–28, 1985. doi: 10.1002/jlb.37.1.15. [DOI] [PubMed] [Google Scholar]
- 13.Epa AP, Thatcher TH, Pollock SJ, Wahl LA, Lyda E, Kottmann RM, Phipps RP, Sime PJ. Normal human lung epithelial cells inhibit transforming growth factor-β induced myofibroblast differentiation via prostaglandin E2. PLoS One 10: e0135266, 2015. doi: 10.1371/journal.pone.0135266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feldon SE, O’loughlin CW, Ray DM, Landskroner-Eiger S, Seweryniak KE, Phipps RP. Activated human T lymphocytes express cyclooxygenase-2 and produce proadipogenic prostaglandins that drive human orbital fibroblast differentiation to adipocytes. Am J Pathol 169: 1183–1193, 2006. doi: 10.2353/ajpath.2006.060434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feldon SE, Park DJ, O’Loughlin CW, Nguyen VT, Landskroner-Eiger S, Chang D, Thatcher TH, Phipps RP. Autologous T-lymphocytes stimulate proliferation of orbital fibroblasts derived from patients with Graves’ ophthalmopathy. Invest Ophthalmol Vis Sci 46: 3913–3921, 2005. doi: 10.1167/iovs.05-0605. [DOI] [PubMed] [Google Scholar]
- 16.Ferguson HE, Kulkarni A, Lehmann GM, Garcia-Bates TM, Thatcher TH, Huxlin KR, Phipps RP, Sime PJ. Electrophilic peroxisome proliferator-activated receptor-gamma ligands have potent antifibrotic effects in human lung fibroblasts. Am J Respir Cell Mol Biol 41: 722–730, 2009. doi: 10.1165/rcmb.2009-0006OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferguson HE, Thatcher TH, Olsen KC, Garcia-Bates TM, Baglole CJ, Kottmann RM, Strong ER, Phipps RP, Sime PJ. Peroxisome proliferator-activated receptor-gamma ligands induce heme oxygenase-1 in lung fibroblasts by a PPARgamma-independent, glutathione-dependent mechanism. Am J Physiol Lung Cell Mol Physiol 297: L912–L919, 2009. doi: 10.1152/ajplung.00148.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fernandez IE, Eickelberg O. The impact of TGF-β on lung fibrosis: from targeting to biomarkers. Proc Am Thorac Soc 9: 111–116, 2012. doi: 10.1513/pats.201203-023AW. [DOI] [PubMed] [Google Scholar]
- 19.Fireman E, Vardinon N, Burke M, Spizer S, Levin S, Endler A, Stav D, Topilsky M, Mann A, Schwarz Y, Kivity S, Greif J. Predictive value of response to treatment of T-lymphocyte subpopulations in idiopathic pulmonary fibrosis. Eur Respir J 11: 706–711, 1998. [PubMed] [Google Scholar]
- 20.Forman BM, Tontonoz P, Chen J, Brun RP, Spiegelman BM, Evans RM. 15-Deoxy-delta 12, 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell 83: 803–812, 1995. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- 21.Galati D, De Martino M, Trotta A, Rea G, Bruzzese D, Cicchitto G, Stanziola AA, Napolitano M, Sanduzzi A, Bocchino M. Peripheral depletion of NK cells and imbalance of the Treg/Th17 axis in idiopathic pulmonary fibrosis patients. Cytokine 66: 119–126, 2014. doi: 10.1016/j.cyto.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Gharaee-Kermani M, Hu B, Phan SH, Gyetko MR. Recent advances in molecular targets and treatment of idiopathic pulmonary fibrosis: focus on TGFbeta signaling and the myofibroblast. Curr Med Chem 16: 1400–1417, 2009. doi: 10.2174/092986709787846497. [DOI] [PubMed] [Google Scholar]
- 23.Gilani SR, Vuga LJ, Lindell KO, Gibson KF, Xue J, Kaminski N, Valentine VG, Lindsay EK, George MP, Steele C, Duncan SR. CD28 down-regulation on circulating CD4 T-cells is associated with poor prognoses of patients with idiopathic pulmonary fibrosis. PLoS One 5: e8959, 2010. doi: 10.1371/journal.pone.0008959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res 89: 219–229, 2010. doi: 10.1177/0022034509359125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herazo-Maya JD, Noth I, Duncan SR, Kim S, Ma SF, Tseng GC, Feingold E, Juan-Guardela BM, Richards TJ, Lussier Y, Huang Y, Vij R, Lindell KO, Xue J, Gibson KF, Shapiro SD, Garcia JG, Kaminski N. Peripheral blood mononuclear cell gene expression profiles predict poor outcome in idiopathic pulmonary fibrosis. Sci Transl Med 5: 205ra136, 2013. doi: 10.1126/scitranslmed.3005964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hinz B. The myofibroblast: paradigm for a mechanically active cell. J Biomech 43: 146–155, 2010. doi: 10.1016/j.jbiomech.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 27.Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol 170: 1807–1816, 2007. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hofmann U, Beyersdorf N, Weirather J, Podolskaya A, Bauersachs J, Ertl G, Kerkau T, Frantz S. Activation of CD4+ T lymphocytes improves wound healing and survival after experimental myocardial infarction in mice. Circulation 125: 1652–1663, 2012. doi: 10.1161/CIRCULATIONAHA.111.044164. [DOI] [PubMed] [Google Scholar]
- 29.Hogan CM, Thatcher TH, Sapinoro RE, Gurell MN, Ferguson HE, Pollock SJ, Jones C, Phipps RP, Sime PJ. Electrophilic PPARγ ligands attenuate IL-1β and silica-induced inflammatory mediator production in human lung fibroblasts via a PPARγ-independent mechanism. PPAR Res 2011: 318134, 2011. doi: 10.1155/2011/318134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hong X, Margariti A, Le Bras A, Jacquet L, Kong W, Hu Y, Xu Q. Transdifferentiated Human Vascular Smooth Muscle Cells are a New Potential Cell Source for Endothelial Regeneration. Sci Rep 7: 5590, 2017. doi: 10.1038/s41598-017-05665-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hopkins RB, Burke N, Fell C, Dion G, Kolb M. Epidemiology and survival of idiopathic pulmonary fibrosis from national data in Canada. Eur Respir J 48: 187–195, 2016. doi: 10.1183/13993003.01504-2015. [DOI] [PubMed] [Google Scholar]
- 32.Huang S, Wettlaufer SH, Hogaboam C, Aronoff DM, Peters-Golden M. Prostaglandin E(2) inhibits collagen expression and proliferation in patient-derived normal lung fibroblasts via E prostanoid 2 receptor and cAMP signaling. Am J Physiol Lung Cell Mol Physiol 292: L405–L413, 2007. doi: 10.1152/ajplung.00232.2006. [DOI] [PubMed] [Google Scholar]
- 33.Huang SK, Scruggs AM, McEachin RC, White ES, Peters-Golden M. Lung fibroblasts from patients with idiopathic pulmonary fibrosis exhibit genome-wide differences in DNA methylation compared to fibroblasts from nonfibrotic lung. PLoS One 9: e107055, 2014. doi: 10.1371/journal.pone.0107055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jara-Palomares L, Martín-Juan J, Gómez-Izquierdo L, Cayuela-Domínguez A, Rodríguez-Becerra E, Rodríguez-Panadero F. Hallazgos en el lavado broncoalveolar de pacientes con enfermedad pulmonar intersticial difusa. Estudio de una cohorte prospectiva de 562 pacientes. Arch Bronconeumol 45: 111–117, 2009. doi: 10.1016/j.arbres.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 35.Jeon KI, Kulkarni A, Woeller CF, Phipps RP, Sime PJ, Hindman HB, Huxlin KR. Inhibitory effects of PPARγ ligands on TGF-β1-induced corneal myofibroblast transformation. Am J Pathol 184: 1429–1445, 2014. doi: 10.1016/j.ajpath.2014.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jimenez SA, McArthur W, Rosenbloom J. Inhibition of collagen synthesis by mononuclear cell supernates. J Exp Med 150: 1421–1431, 1979. doi: 10.1084/jem.150.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kass DJ, Yu G, Loh KS, Savir A, Borczuk A, Kahloon R, Juan-Guardela B, Deiuliis G, Tedrow J, Choi J, Richards T, Kaminski N, Greenberg SM. Cytokine-like factor 1 gene expression is enriched in idiopathic pulmonary fibrosis and drives the accumulation of CD4+ T cells in murine lungs: evidence for an antifibrotic role in bleomycin injury. Am J Pathol 180: 1963–1978, 2012. doi: 10.1016/j.ajpath.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khalil N, O’Connor RN, Unruh HW, Warren PW, Flanders KC, Kemp A, Bereznay OH, Greenberg AH. Increased production and immunohistochemical localization of transforming growth factor-beta in idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol 5: 155–162, 1991. doi: 10.1165/ajrcmb/5.2.155. [DOI] [PubMed] [Google Scholar]
- 39.Kim ES, Kim MS, Moon A. TGF-beta-induced upregulation of MMP-2 and MMP-9 depends on p38 MAPK, but not ERK signaling in MCF10A human breast epithelial cells. Int J Oncol 25: 1375–1382, 2004. [PubMed] [Google Scholar]
- 40.King TE Jr, Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, Gorina E, Hopkins PM, Kardatzke D, Lancaster L, Lederer DJ, Nathan SD, Pereira CA, Sahn SA, Sussman R, Swigris JJ, Noble PW; ASCEND Study Group . A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med 370: 2083–2092, 2014. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 41.King TE Jr, Schwarz MI, Brown K, Tooze JA, Colby TV, Waldron JA Jr, Flint A, Thurlbeck W, Cherniack RM. Idiopathic pulmonary fibrosis: relationship between histopathologic features and mortality. Am J Respir Crit Care Med 164: 1025–1032, 2001. doi: 10.1164/ajrccm.164.6.2001056. [DOI] [PubMed] [Google Scholar]
- 42.Kliewer SA, Lenhard JM, Willson TM, Patel I, Morris DC, Lehmann JM. A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor gamma and promotes adipocyte differentiation. Cell 83: 813–819, 1995. doi: 10.1016/0092-8674(95)90194-9. [DOI] [PubMed] [Google Scholar]
- 43.Korn JH, Halushka PV, LeRoy EC. Mononuclear cell modulation of connective tissue function: suppression of fibroblast growth by stimulation of endogenous prostaglandin production. J Clin Invest 65: 543–554, 1980. doi: 10.1172/JCI109698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kotsianidis I, Nakou E, Bouchliou I, Tzouvelekis A, Spanoudakis E, Steiropoulos P, Sotiriou I, Aidinis V, Margaritis D, Tsatalas C, Bouros D. Global impairment of CD4+CD25+FOXP3+ regulatory T cells in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 179: 1121–1130, 2009. doi: 10.1164/rccm.200812-1936OC. [DOI] [PubMed] [Google Scholar]
- 45.Kulkarni AA, Thatcher TH, Olsen KC, Maggirwar SB, Phipps RP, Sime PJ. PPAR-γ ligands repress TGFβ-induced myofibroblast differentiation by targeting the PI3K/Akt pathway: implications for therapy of fibrosis. PLoS One 6: e15909, 2011. doi: 10.1371/journal.pone.0015909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lacy SH, Woeller CF, Thatcher TH, Maddipati KR, Honn KV, Sime PJ, Phipps RP. Human lung fibroblasts produce proresolving peroxisome proliferator-activated receptor-γ ligands in a cyclooxygenase-2-dependent manner. Am J Physiol Lung Cell Mol Physiol 311: L855–L867, 2016. doi: 10.1152/ajplung.00272.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lederer DJ, Bradford WZ, Fagan EA, Glaspole I, Glassberg MK, Glasscock KF, Kardatzke D, King TE Jr, Lancaster LH, Nathan SD, Pereira CA, Sahn SA, Swigris JJ, Noble PW. Sensitivity Analyses of the change in FVC in a phase 3 trial of pirfenidone for idiopathic pulmonary fibrosis. Chest 148: 196–201, 2015. doi: 10.1378/chest.14-2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luzina IG, Todd NW, Iacono AT, Atamas SP. Roles of T lymphocytes in pulmonary fibrosis. J Leukoc Biol 83: 237–244, 2008. doi: 10.1189/jlb.0707504. [DOI] [PubMed] [Google Scholar]
- 49.Martey CA, Pollock SJ, Turner CK, O’Reilly KM, Baglole CJ, Phipps RP, Sime PJ. Cigarette smoke induces cyclooxygenase-2 and microsomal prostaglandin E2 synthase in human lung fibroblasts: implications for lung inflammation and cancer. Am J Physiol Lung Cell Mol Physiol 287: L981–L991, 2004. doi: 10.1152/ajplung.00239.2003. [DOI] [PubMed] [Google Scholar]
- 50.Milam JE, Keshamouni VG, Phan SH, Hu B, Gangireddy SR, Hogaboam CM, Standiford TJ, Thannickal VJ, Reddy RC. PPAR-gamma agonists inhibit profibrotic phenotypes in human lung fibroblasts and bleomycin-induced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 294: L891–L901, 2008. doi: 10.1152/ajplung.00333.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moodley YP, Scaffidi AK, Misso NL, Keerthisingam C, McAnulty RJ, Laurent GJ, Mutsaers SE, Thompson PJ, Knight DA. Fibroblasts isolated from normal lungs and those with idiopathic pulmonary fibrosis differ in interleukin-6/gp130-mediated cell signaling and proliferation. Am J Pathol 163: 345–354, 2003. doi: 10.1016/S0002-9440(10)63658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moore AE, Young LE, Dixon DA. A common single-nucleotide polymorphism in cyclooxygenase-2 disrupts microRNA-mediated regulation. Oncogene 31: 1592–1598, 2012. doi: 10.1038/onc.2011.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Noelle RJ, Roy M, Shepherd DM, Stamenkovic I, Ledbetter JA, Aruffo A. A 39-kDa protein on activated helper T cells binds CD40 and transduces the signal for cognate activation of B cells. Proc Natl Acad Sci USA 89: 6550–6554, 1992. doi: 10.1073/pnas.89.14.6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nuovo GJ, Hagood JS, Magro CM, Chin N, Kapil R, Davis L, Marsh CB, Folcik VA. The distribution of immunomodulatory cells in the lungs of patients with idiopathic pulmonary fibrosis. Mod Pathol 25: 416–433, 2012. doi: 10.1038/modpathol.2011.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O’Reilly KM, Phipps RP, Thatcher TH, Graf BA, Van Kirk J, Sime PJ. Crystalline and amorphous silica differentially regulate the cyclooxygenase-prostaglandin pathway in pulmonary fibroblasts: implications for pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 288: L1010–L1016, 2005. doi: 10.1152/ajplung.00024.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ogushi F, Endo T, Tani K, Asada K, Kawano T, Tada H, Maniwa K, Sone S. Decreased prostaglandin E2 synthesis by lung fibroblasts isolated from rats with bleomycin-induced lung fibrosis. Int J Exp Pathol 80: 41–49, 1999. doi: 10.1046/j.1365-2613.1999.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Olsen KC, Epa AP, Kulkarni AA, Kottmann RM, McCarthy CE, Johnson GV, Thatcher TH, Phipps RP, Sime PJ. Inhibition of transglutaminase 2, a novel target for pulmonary fibrosis, by two small electrophilic molecules. Am J Respir Cell Mol Biol 50: 737–747, 2014. doi: 10.1165/rcmb.2013-0092OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Penke LR, Huang SK, White ES, Peters-Golden M. Prostaglandin E2 inhibits α-smooth muscle actin transcription during myofibroblast differentiation via distinct mechanisms of modulation of serum response factor and myocardin-related transcription factor-A. J Biol Chem 289: 17151–17162, 2014. doi: 10.1074/jbc.M114.558130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Petkova DK, Clelland CA, Ronan JE, Lewis S, Knox AJ. Reduced expression of cyclooxygenase (COX) in idiopathic pulmonary fibrosis and sarcoidosis. Histopathology 43: 381–386, 2003. doi: 10.1046/j.1365-2559.2003.01718.x. [DOI] [PubMed] [Google Scholar]
- 60.Raghu G, Chen SY, Yeh WS, Maroni B, Li Q, Lee YC, Collard HR. Idiopathic pulmonary fibrosis in US Medicare beneficiaries aged 65 years and older: incidence, prevalence, and survival, 2001-11. Lancet Respir Med 2: 566–572, 2014. doi: 10.1016/S2213-2600(14)70101-8. [DOI] [PubMed] [Google Scholar]
- 61.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, Lynch DA, Ryu JH, Swigris JJ, Wells AU, Ancochea J, Bouros D, Carvalho C, Costabel U, Ebina M, Hansell DM, Johkoh T, Kim DS, King TE Jr, Kondoh Y, Myers J, Müller NL, Nicholson AG, Richeldi L, Selman M, Dudden RF, Griss BS, Protzko SL, Schünemann HJ; ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis . An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 183: 788–824, 2011. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ramos C, Montaño M, García-Alvarez J, Ruiz V, Uhal BD, Selman M, Pardo A. Fibroblasts from idiopathic pulmonary fibrosis and normal lungs differ in growth rate, apoptosis, and tissue inhibitor of metalloproteinases expression. Am J Respir Cell Mol Biol 24: 591–598, 2001. doi: 10.1165/ajrcmb.24.5.4333. [DOI] [PubMed] [Google Scholar]
- 63.Rezzonico R, Burger D, Dayer JM. Direct contact between T lymphocytes and human dermal fibroblasts or synoviocytes down-regulates types I and III collagen production via cell-associated cytokines. J Biol Chem 273: 18720–18728, 1998. doi: 10.1074/jbc.273.30.18720. [DOI] [PubMed] [Google Scholar]
- 64.Shimizu Y, Dobashi K, Endou K, Ono A, Yanagitani N, Utsugi M, Sano T, Ishizuka T, Shimizu K, Tanaka S, Mori M. Decreased interstitial FOXP3(+) lymphocytes in usual interstitial pneumonia with discrepancy of CXCL12/CXCR4 axis. Int J Immunopathol Pharmacol 23: 449–461, 2010. doi: 10.1177/039463201002300207. [DOI] [PubMed] [Google Scholar]
- 65.Skalli O, Ropraz P, Trzeciak A, Benzonana G, Gillessen D, Gabbiani G. A monoclonal antibody against alpha-smooth muscle actin: a new probe for smooth muscle differentiation. J Cell Biol 103: 2787–2796, 1986. doi: 10.1083/jcb.103.6.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Smith RS, Kelly R, Iglewski BH, Phipps RP. The Pseudomonas autoinducer N-(3-oxododecanoyl) homoserine lactone induces cyclooxygenase-2 and prostaglandin E2 production in human lung fibroblasts: implications for inflammation. J Immunol 169: 2636–2642, 2002. doi: 10.4049/jimmunol.169.5.2636. [DOI] [PubMed] [Google Scholar]
- 67.Spagnolo P, Maher TM, Richeldi L. Idiopathic pulmonary fibrosis: Recent advances on pharmacological therapy. Pharmacol Ther 152: 18–27, 2015. doi: 10.1016/j.pharmthera.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 68.St-Pierre Y, Nabavi N, Ghogawala Z, Glimcher LH, Watts TH. A functional role for signal transduction via the cytoplasmic domains of MHC class II proteins. J Immunol 143: 808–812, 1989. [PubMed] [Google Scholar]
- 69.Sun L, Diamond ME, Ottaviano AJ, Joseph MJ, Ananthanarayan V, Munshi HG. Transforming growth factor-beta 1 promotes matrix metalloproteinase-9-mediated oral cancer invasion through snail expression. Mol Cancer Res 6: 10–20, 2008. doi: 10.1158/1541-7786.MCR-07-0208. [DOI] [PubMed] [Google Scholar]
- 70.Todd NW, Scheraga RG, Galvin JR, Iacono AT, Britt EJ, Luzina IG, Burke AP, Atamas SP. Lymphocyte aggregates persist and accumulate in the lungs of patients with idiopathic pulmonary fibrosis. J Inflamm Res 6: 63–70, 2013. doi: 10.2147/JIR.S40673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Toulon A, Breton L, Taylor KR, Tenenhaus M, Bhavsar D, Lanigan C, Rudolph R, Jameson J, Havran WL. A role for human skin-resident T cells in wound healing. J Exp Med 206: 743–750, 2009. doi: 10.1084/jem.20081787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Walker NM, Badri LN, Wadhwa A, Wettlaufer S, Peters-Golden M, Lama VN. Prostaglandin E2 as an inhibitory modulator of fibrogenesis in human lung allografts. Am J Respir Crit Care Med 185: 77–84, 2012. doi: 10.1164/rccm.201105-0834OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.White ES, Atrasz RG, Dickie EG, Aronoff DM, Stambolic V, Mak TW, Moore BB, Peters-Golden M. Prostaglandin E(2) inhibits fibroblast migration by E-prostanoid 2 receptor-mediated increase in PTEN activity. Am J Respir Cell Mol Biol 32: 135–141, 2005. doi: 10.1165/rcmb.2004-0126OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.White ES, Lazar MH, Thannickal VJ. Pathogenetic mechanisms in usual interstitial pneumonia/idiopathic pulmonary fibrosis. J Pathol 201: 343–354, 2003. doi: 10.1002/path.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wilborn J, Crofford LJ, Burdick MD, Kunkel SL, Strieter RM, Peters-Golden M. Cultured lung fibroblasts isolated from patients with idiopathic pulmonary fibrosis have a diminished capacity to synthesize prostaglandin E2 and to express cyclooxygenase-2. J Clin Invest 95: 1861–1868, 1995. doi: 10.1172/JCI117866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yanai H, Shteinberg A, Porat Z, Budovsky A, Braiman A, Zeische R, Fraifeld VE. Cellular senescence-like features of lung fibroblasts derived from idiopathic pulmonary fibrosis patients. Aging (Albany NY) 7: 664–672, 2015. doi: 10.18632/aging.100807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yosef R, Pilpel N, Tokarsky-Amiel R, Biran A, Ovadya Y, Cohen S, Vadai E, Dassa L, Shahar E, Condiotti R, Ben-Porath I, Krizhanovsky V. Directed elimination of senescent cells by inhibition of BCL-W and BCL-XL. Nat Commun 7: 11190, 2016. doi: 10.1038/ncomms11190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang HY, Phan SH. Inhibition of myofibroblast apoptosis by transforming growth factor beta(1). Am J Respir Cell Mol Biol 21: 658–665, 1999. doi: 10.1165/ajrcmb.21.6.3720. [DOI] [PubMed] [Google Scholar]
- 79.Zhang Y, Cao HJ, Graf B, Meekins H, Smith TJ, Phipps RP. CD40 engagement up-regulates cyclooxygenase-2 expression and prostaglandin E2 production in human lung fibroblasts. J Immunol 160: 1053–1057, 1998. [PubMed] [Google Scholar]