Abstract
Previously, we and others have demonstrated that activation of peroxisome proliferator-activated receptor γ (PPARγ) by specific pharmacological agonists inhibits the pathogenesis of chronic hypoxia-induced pulmonary hypertension (CHPH) by suppressing the proliferation and migration in distal pulmonary arterial smooth muscle cells (PASMCs). Moreover, these beneficial effects of PPARγ are mediated by targeting the intracellular calcium homeostasis and store-operated calcium channel (SOCC) proteins, including the main caveolae component caveolin-1. However, other than the caveolin-1 targeted mechanism, in this study, we further uncovered a caveolin-1 dependent mechanism within the activation of PPARγ by the specific agonist GW1929. First, effective knockdown of caveolin-1 by small-interfering RNA (siRNA) markedly abolished the upregulation of GW1929 on PPARγ expression at both mRNA and protein levels; Then, in HEK293T, which has previously been reported with low endogenous caveolin-1 expression, exogenous expression of caveolin-1 significantly enhanced the upregulation of GW1929 on PPARγ expression compared with nontransfection control. In addition, inhibition of PPARγ by either siRNA or pharmacological inhibitor T0070907 led to increased phosphorylation of cellular mitogen-activated protein kinases ERK1/2 and p38. In parallel, GW1929 dramatically decreased the expression of the proliferative regulators (cyclin D1 and PCNA), whereas it increased the apoptotic factors (p21, p53, and mdm2) in hypoxic PASMCs. Furthermore, these effects of GW1929 could be partially reversed by recovery of the drug treatment. In combination, PPARγ activation by GW1929 reversibly drove the cell toward an antiproliferative and proapoptotic phenotype in a caveolin-1-dependent and -targeted mechanism.
Keywords: caveolin-1, extracellular signal-regulated kinase 1/2, p38, peroxisome proliferator-activated receptor-γ, pulmonary arterial smooth muscle cells
INTRODUCTION
Pulmonary hypertension (PH) is a fatal disease characterized by a list of physiological and pathological features, including sustained increase in the pulmonary arterial pressure and the state of excessive media wall thickening and remodeling in the distal pulmonary arteries (PAs), progressively leading to right ventricular hypertrophy, heart failure, and death (40). During the disease development progression, it is well accepted that the excessive proliferation and migration of the distal intralobar pulmonary arterial smooth muscle cells (PASMCs) play a central role in promoting the abnormally PA thickening and remodeling (14).
The transcriptional factor peroxisome proliferator-activated receptor- γ (PPARγ) belongs to the nuclear ligand-activated hormone receptor superfamily and is ubiquitously expressed in numerous lung cell types, including the pulmonary vascular endothelial cells and smooth muscle cells (37). In experimental PH animal models, PPARγ expression is downregulated in the lungs and distal PAs (1, 10), and moreover, transgenic mice with deletion of PPARγ in either smooth muscle cells (SMCs) (12) or endothelial cells (ECs) (11) develop pulmonary arterial hypertension (PAH), suggesting a protective role of PPARγ in PAH. Activation of PPARγ by specific pharmacological agonists [e.g., thiazolidinedione (TZD)] have been widely proven as promising treatment reagents against PH in experimental animal models (17, 32, 45). In mechanism, TZD can protect PH by normalizing the elevated pulmonary arterial pressure and remodeling in distal PAs in part by the inhibition of the increased proliferation and migration of PASMCs (45). In our previous study, we observed that activation of PPARγ by either the TZD class agonist rosiglitazone (45) or a more specific nonthiazolidinedione agonist, GW1929 (44), could all effectively suppress the hypoxia-elevated basal intracellular free calcium concentration ([Ca2+]i) and the store-operated calcium entry (SOCE) through store-operated calcium channel (SOCC) in PASMCs. As has been shown before, besides the core SOCC component transient receptor potential cation channels (TRPCs), the membrane scaffolding protein caveolin-1 also plays critical role in regulating SOCE and contributes to the development of PH (34, 47). Moreover, GW1929 activation of PPARγ could inhibit caveolin-1 expression by promoting its protein degradation through the lysosome system (47), indicating that a caveolin-1 targeted mechanism is involved in GW1929-mediated therapeutic consequences in PH pathogenesis.
Caveolae are flask-like invaginations that locate at the cell plasma membrane and play essential roles in numerous membrane events, including the extracellular signal transduction, calcium homeostasis regulation, and intracellular vesicle trafficking, etc. Notably, caveolae can also act as the key pharmacologically relevant signal transduction molecules that mediate the signal transduction of many important extracellular cytokines and pharmacological chemical compounds and regulate the activation of a number of cellular kinases, including the activation of mitogen-activated protein kinase (MAPK) ERK1/2 and p38 (33). In our previous studies, we demonstrated that in hypoxic PASMCs, hypoxia increases the expression of BMP4 in a HIF-1α-dependent mechanism (42), activates the BMP4 signal transduction, leading to phosphorylation of ERK1/2 and p38, which upregulate the expression of store-operated calcium channel components TRPC1 and TRPC6, triggers the SOCE, and induces the increase in proliferation and migration of PASMCs (20). Based on these background, in this study, we further investigated the regulation among PPARγ, caveolin-1, and cellular MAPK activation in PASMCs.
MATERIALS AND METHODS
Isolation and culture of primary rat distal pulmonary arterial smooth muscle cells.
All animal experiment procedures were approved by the Animal Care and Use Committee of The Johns Hopkins University School of Medicine and The First Affiliated Hospital of Guangzhou Medical University. The surgical procedure on adult male Wistar rats (150–250 g; Harlan, Frederick, MD) was performed under anesthesia with pentobarbital sodium (65 mg/kg ip), and all efforts were made to minimize animal suffering. Rats were euthanized according to previously described protocols. First, the heart and lung tissues were harvested, and the distal (>4th generation) intrapulmonary arteries were carefully dissected by using forceps and scissors. Second, the adventitia was carefully removed from the isolated PAs, the vessel was then longitudinally opened, and the luminal endothelium was eliminated by rubbing the with a cotton swab. The primary distal PASMCs were enzymatically digested from the vascular smooth muscle layer and cultured in smooth muscle growth medium-2 (Lonza, Walkersville, MD), as previously described (43). Prior to the treatments in each experiment, the purity of cultured PASMCs were assessed by >90% positive from the following two methods: 1) calcium influx for >50 nM in response to 60 mM KCl and 2) immunocytochemistry staining by antibodies of smooth muscle cell-specific markers, smooth muscle α-actin (Sigma, St. Louis, MO), and myosin heavy chain (Abcam, Cambridge, MA).
Hypoxic exposure of primary cultured PASMCs.
Primary cultured PASMCs grown to 50–70% confluence will be serum starved in smooth muscle basal medium (Lonza) supplemented with 0.3% FBS for 24 h before hypoxic exposure. Afterward, the growth-arrested cells were then put in a clamped chamber fulfilled with 4% O2-5% CO2 in the presence of siRNA knockdown of caveolin-1 and/or GW1929 treatment for 48 and 72 h, respectively. Cells exposed to normoxia serve as controls.
Real-time quantitative polymerase chain reaction.
Total RNA was extracted from cultured rat distal PASMCs under different treatment conditions by using RNeasy column and RNase-free DNase (Qiagen, Valencia, CA). RNA was reverse transcribed by iScript cDNA synthesis kit (Bio-Rad, Hercules, CA) with a reaction mixture containing 500 ng of total RNA in a 20-µl volume. Real-time quantitative polymerase chain reaction was performed by using QuantiTect SYBR Green PCR Master Mix (Qiagen, Valencia, CA) in an iCyclerIQ detection system (Bio-Rad) to amplify specific genes in the cDNA template. The protocol consisted of initial enzyme activation at 95°C for 3 min, followed by 40 cycles at 95°C for 5 s, and at 60°C for 15 s (23). Primer sequences were designed using the NCBI primer designing tool. The specific primer pairs for quantitative PCR were caveolin-1 (sense 5′-CGCACACCAAGGAGATTGAT-3′, anti-sense 5′-ACTGTGTGTCCCTTCTGGTT-3′), PPARγ (sense 5′-CCTGAAGCTCCAAGAATACCAAA-3′, anti-sense 5′-AGAGTTGGGTTTTTTCAGAATAATAAGG-3′), and 18S (sense 5′-GCAATTATTCCCCATGAACG-3′, anti-sense 5′-GGCCTCACTAAACCATCCAA-3′).
Relative concentration of each transcript was calculated using the Pfaffl method (36).
Western blotting.
PASMCs were lysed using T-PER sample buffer (Pierce, Rockford, IL) containing 5% protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO) for total protein extraction. For the experiments, including protein phosphorylation, phosphatase inhibitor cocktail 2 and 3 (Sigma-Aldrich, St. Louis, MO) were added in the T-PER lysis buffer. Total protein concentration in the whole cell lysates was determined by bicinchoninic acid protein assay (Pierce), using bovine serum albumin as a standard. Homogenates were denatured by adding 150 mM dithiothreitol (DTT) and heating at 95°C for 3 min. Whole cell lysates were resolved on SDS-PAGE calibrated with Precision Plus protein dual-color molecular weight markers (Bio-Rad). The proteins were then transferred onto 0.45-µM polyvinylidene difluoride membranes (Bio-Rad), which were blocked with 5% nonfat dry milk (Bio-Rad) in Tris-buffered saline containing 0.2% Tween 20 and blotted with affinity-purified polyclonal antibodies against PPARγ (Santa Cruz Biotechnology, Dallas, TX), p-p38, t-p38, p-ERK1/2, t-ERK1/2, and cyclin D1 (Cell Signaling Technology, Beverly, MA) or monoclonal antibody against caveolin-1, p53, p21, and mdm2 (BD Biosciences, San Jose, CA), PCNA, GAPDH, and β-tubulin (Sigma-Aldrich). The membranes were then washed for 10 min three times and incubated with horseradish peroxidase-conjugated goat anti-rabbit or anti-mouse IgG (Kirkegaard and Perry Laboratories, Gaithersburg, MD). Bound antibodies were detected using an enhanced chemiluminescence system (ECL; GE Healthcare, Piscataway, NJ).
Small-interfering RNA silencing.
The small-interfering RNA (siRNA) SMARTpool against PPARγ was designed and purchased from Thermo Scientific (Lafayette, CO), and the siRNA against caveolin-1 was synthesized by Invitrogen (Carlsbad, CA). Nontargeting siRNA treatment cells served as controls. Cultured rat distal PASMCs grown to 50–70% confluence were serum starved in 0.3% FBS smooth muscle basal medium (Lonza) for 24 h before the transfection with each specific siRNA using the Gene Silencer kit (Genlantis, San Diego, CA) according to the manufacturer’s instructions. Knockdown efficiency was determined by real-time quantitative PCR and Western blot thereafter.
Plasmid transfection.
Plasmid vectors of pEGFP-C1 and pEGFP-caveolin-1 were obtained from Addgene (Cambridge, MA). Cultured HEK293T cells grown to 50–70% confluence were transfected with either pEGFP-C1 or pEGFP-caveolin-1 with Lipofectamine 2000 (Thermo Fisher Scientific, Lafayette, CO) that was prepared in Opti-Mem media (GIBCO; Thermo Fisher Scientific) according to the manufacturer’s instructions. Cells were harvested 48 h posttransfection to extract whole cell lysates for the Western blot experiment.
Cell proliferation assay.
Cell proliferation was assessed with an EdU Alexa Fluor 488 Imaging Kit (Ribobio). Cultured PASMCs were incubated with 10 mM EdU for 8–10 h. The cells were harvested afterward to flow tubes and washed with ice-cold phosphate-buffered saline (PBS) and pelleted by centrifugation. The supernatant was then removed. Then, 100 μl of 4% paraformaldehyde was added to the tube and mixed well. The cells were incubated at room temperature for 15 min, neutralized with 2 mg/ml glycine for 5 min, and then washed with PBS for one time. The cells were incubated with 0.5% Triton X-100 for 10 min and then washed in PBS. One-hundred microliters of 1 × Apollo staining solution was added to each tube and resuspended, and the cells were incubated at room temperature for 10 min, protected from light. The cells were recentrifuged, and the staining solution was removed. The cells were washed with 0.5%Triton X-100 three times, and then the cells were resuspended in 500 µl of PBS. The stained cells were then analyzed by flow cytometry (BD), using 488-nm excitation with a green emission filter (530/30 nm or similar).
Cell apoptosis assay.
Cell apoptosis was assessed with an Annexin V-FITC/PI Cell Apoptosis Detection Kit (TransGen Biotech). After the treatment with caveolin-1 knockdown and GW1929 under normoxia and hypoxia, the cultured PASMCs were harvested and washed twice in cold PBS. The washed cells were recentrifuged, the supernatant was discarded, and the cells were resuspended in 100 µl of cold 1 × Annexin V binding buffer. Five microliters of Annexin V-FITC and 5 µl of PI working solution were added to each 100 µl of cell suspension and mixed gently. The cells were incubated at room temperature (20–25°C) for 15 min and protected from light. After the incubation, 400 µl of ice-cold 1 × Annexin V binding buffer was added and mixed gently, and the samples were kept on ice. The stained cells were analyzed by flow cytometry (BD), with the fluorescence emission measured at 530 and >575 nm. Annexin V- and PI-double-positive cells were scored as late-phase apoptotic cells.
Statistical analysis.
All data are shown as means ± SE, and n represents the number of experiments. Statistical analyses for pairwise comparison were performed using Student’s t-test, whereas for multiple group comparisons, analyses were performed using one-way ANOVA. Differences were considered significant when P < 0.05.
RESULTS
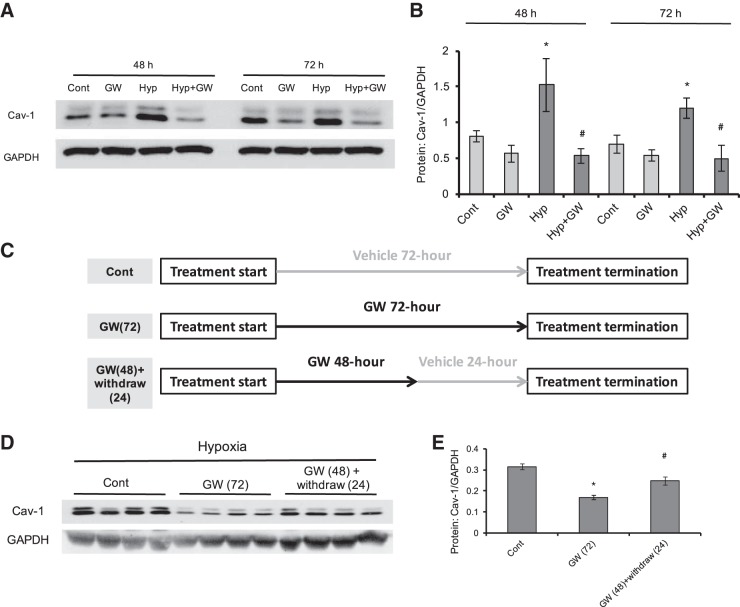
PPARγ agonist GW1929 inhibited prolonged hypoxia (4% O2)-induced upregulation of caveolin-1 expression in a reversible manner.
Consistent with our previous study (47), we first found that PPARγ activation by treatment of GW1929 (10 μM) dramatically decreased the hypoxic upregulation of caveolin-1 expression at protein level under either 48- or 72-h treatment (Fig. 1, A and B). Moreover, we further found that under 72-h treatment, withdrawal of GW1929 treatment for 24 h (48-h treatment of GW1929, following by 24-h recovery in fresh medium) can partially reverse the downregulation of caveolin-1 protein (Fig. 1, D and E), suggesting that GW1929 altered caveolin-1 level in a reversible manner.
Fig. 1.
PPARγ agonist GW1929 inhibits prolonged hypoxia (4% O2)-induced upregulation of caveolin-1 expression in a reversible manner in cultured rat distal pulmonary arterial smooth muscle cells (PASMCs). A and B: Western blot (A) and bar graph (B) showing the protein expression level of caveolin-1 upon GW1929 (GW; 10 μM) treatment in rat distal PASMCs under normoxic and hypoxic conditions. C: treatment strategy of straight GW1929 and of GW1929 plus drug withdrawal. D and E: Western blot (D) and bar graph (E) showing the protein expression level of caveolin-1 under GW1929 treatment (72 h) and GW1929 treatment (48 h) plus drug withdrawal (24 h) in hypoxic PASMCs. GAPDH serves as housekeeping protein. Bar graph represents means ± SE; n = 4 in each group. *P < 0.05 vs. control (B, 48 or 72 h) or control (E); #P < 0.05 vs. hypoxia (48 or 72 h) or GW 72 h (E).
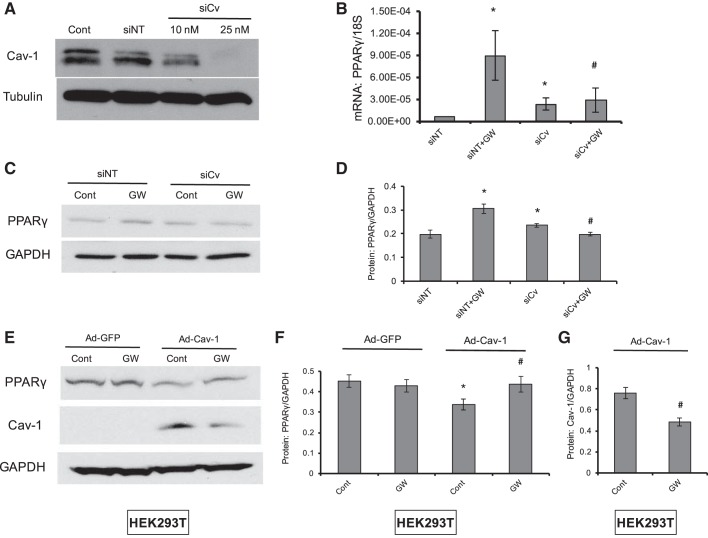
GW1929 activated PPARγ via a caveolin-1-dependent mechanism.
During our observation that PPARγ activation by GW1929 markedly inhibited hypoxia-induced caveolin-1 protein expression in PASMCs, we occasionally found that caveolin-1 not only could act as a molecular target of PPARγ activation but also participated in the process of pharmacological activation of PPARγ. First, siRNA against caveolin-1 reduced caveolin-1 protein in a dose-dependent manner, indicating effective knockdown (Fig. 2A). Second, knockdown of caveolin-1 could dominantly block the GW1929 activation of PPARγ level at both mRNA (Fig. 2B) and protein (Fig. 2, C and D) levels. Then, in an endogenous low caveolin-1 expression cell line (HEK293T), overexpression of caveolin-1 enhanced the GW1929 activation of PPARγ expression (Fig. 2, E and F). Moreover, compared with normal HEK293T cells, GW1929 also led to reduced expression of caveolin-1 in caveolin-1 overexpression of HEK293T (Fig. 3G). Collectively, all these data indicated that caveolin-1 acted as both a molecular target and a mediator of pharmacological activation of PPARγ.
Fig. 2.
GW1929 activates PPARγ via a caveolin-1-dependent mechanism. A–D: knockdown of caveolin-1 (siCv) eliminated GW1929-upregulated PPARγ expression in PASMCs. Western blot (A) shows the siRNA knockdown efficiency of caveolin-1 as relevant to β-tubulin. Bar graph (B and D) and Western blot (C) show the mRNA (normalized to 18S) and protein expression (normalized to GAPDH) levels of PPARγ under treatment of caveolin-1 knockdown and GW1929 in rat distal PASMCs. E–G: overexpression of caveolin-1 in HEK293T enhanced the GW1929-mediated upregulation of PPARγ and downregulation of caveolin-1. Western blot (E) and bar graph (F and G) represent the protein expression levels of PPARγ and caveolin-1 under treatment of caveolin-1 overexpression and GW1929 in HEK293T. Bar graph represents means ± SE; n = 6 in each group. *P < 0.05 vs. nontargeted siRNA (siNT; B and D) or Ad-GFP control (F and G); #P < 0.05 vs. siNT + GW (B and D) or Ad-Cav-1 control (F and G).
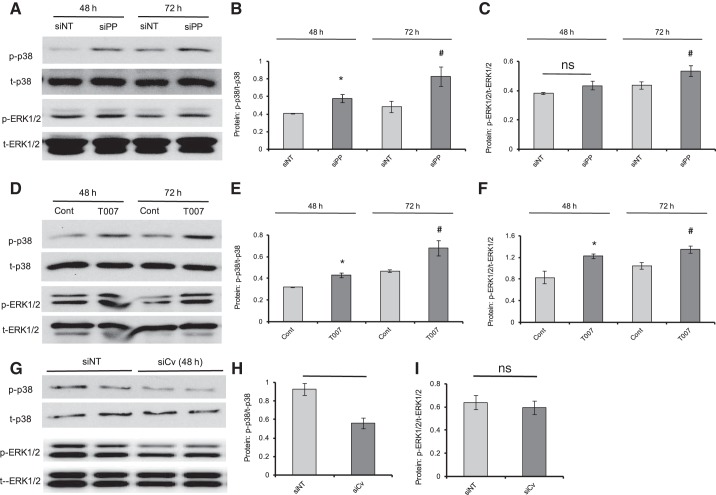
Fig. 3.
Inhibition of PPARγ and caveolin-1 altered the basal phosphorylation of p38 and ERK1/2 in PASMCs. A–F: Western blots (A and D) showing the protein expression levels of p-p38, t-p38, p-ERK1/2, and t-ERK1/2 upon treatment of either PPARγ knockdown (siPP) or PPARγ antagonist T0070907 (T007; 10 μM) for 48 h and 72 h in rat distal PASMCs. Bar graphs (B, C, E, and F) represent the phosphorylation rates of p-p38 (B and E) and p-ERK1/2 (C and F) as normalized to t-p38 and t-ERK1/2. G–I: Western blots (G) showing the protein expression levels of p-p38, t-p38, p-ERK1/2, and t-ERK1/2 upon treatment of either nontargeted siRNA (siNT) or caveolin-1 (siCv) knockdown for 48 h in rat distal PASMCs. Bar graphs represent the phosphorylation rates of p-p38 (H) and p-ERK1/2 (I) as normalized to t-p38 and t-ERK1/2, respectively. Bar graph represents means ± SE; n = 4–6 in each group. *P < 0.05 vs. siNT or Cont (48 h); #P < 0.05 vs. siNT or Cont (72 h); ns, no significant difference.
Inhibition of PPARγ and caveolin-1 altered the basal phosphorylation pattern of p38 and ERK1/2 in PASMCs.
As is well known, p38 and ERK1/2 belong to the important kinases to mediate the cellular pro-proliferation subsequences. A number of previous studies have well indicated that the p38 and ERK1/2-MAPK signaling axis could mediate the proliferation of smooth muscle cells upon activation from upstream stimulation. In our previous study, we found that the elevated phosphorylation of p38 and ERK1/2 due to activated HIF-1-BMP4 signaling axis contributes largely to the excessive proliferation and migration in hypoxic PASMCs (20, 42). Therefore, we further tested the role of PPARγ and caveolin-1 on the phosphorylation of p38 and ERK1/2 in PASMCs by using siRNA knockdown and pharmacological inhibition. Our results revealed that inhibition of PPARγ by either knockdown (Fig. 3 A–C) or pharmacological inhibitor T0070907 (Fig. 3, D–F) led to significant increases in the phosphorylation of p38 and ERK1/2 under both 48- and 72-h treatment duration, whereas siRNA knockdown of caveolin-1 resulted in significantly reduced levels of basal p38 phosphorylation (Fig. 3, G and H), but not basal ERK1/2 phosphorylation (Fig. 3, G and I), in cultured rat distal PASMCs.
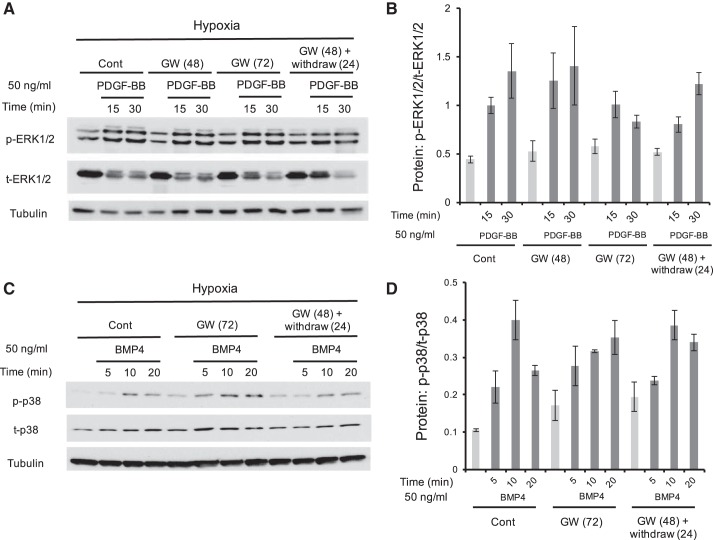
Neither PDGF-BB- nor BMP4-induced phosphorylation of p38 and ERK1/2 was affected by GW1929 treatment in hypoxic PASMCs.
In previous studies, caveolin-1 has long been reported to mediate a list of cellular kinases, including both the Smad and MAPK signaling cascade. Activation of caveolin-1 sequesters BMPRII to the plasma membrane and in doing so prevents the protein degradation of BMPRII through the lysosome system and facilitates the signal transduction of BMPs involving the antiproliferative consequences mediated by the Smad and inhibitor of the DNA-binding protein (ID) signaling axis (16). Because our data indicated that both PPARγ and caveolin-1 could alter the basal phosphorylation of p38 and ERK1/2 in PASMCs (Fig. 3), therefore, we further evaluated whether loss of caveolin-1 by GW1929-mediated PPARγ activation can also regulate the MAPK phosphorylation that is induced by cytokines like PDGF-BB or BMP4. As is shown in Fig. 4, PDGF-BB (50 ng/ml) or BMP4 (50 ng/ml) time-dependently activated the phosphorylation of p38 and ERK1/2, which were not affected by either GW1929 treatment (10 μM, 72 h) or GW1929 recovery (48-h treatment plus 24-h withdrawal), suggesting that although PPARγ and caveolin-1 are important for the basal activation of ERK1/2 and p38, they are indispensable for PDGF-BB- or BMP4-induced cellular MAPK activation in PASMCs.
Fig. 4.
Neither PDGF-BB- nor BMP4-induced phosphorylation of p38 and ERK1/2 was affected by GW1929 treatment in hypoxic PASMCs. Western blots (A and C) show the protein expression levels of p-p38, t-p38, p-ERK1/2, and t-ERK1/2 in cultured rat distal PASMCs upon treatment of either PDGF-BB (50 ng/ml; A) or BMP4 (50 ng/ml; C) for different time courses. Bar graphs (B and D) represent the phosphorylation rates of p-ERK1/2 as normalized to t-p38 and t-ERK1/2, respectively. Bar graph represents means ± SE; n = 3 in each group.
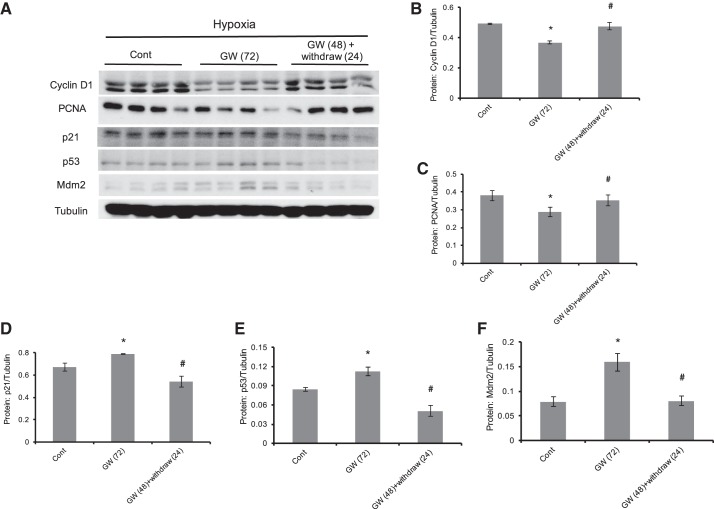
GW1929 activation of PPARγ reversibly inhibited cellular proliferative markers and increased apoptotic markers in hypoxic PASMCs.
Given the fact that PPARγ activation normalized hypoxia-induced proliferation and migration by driving the cell into an antiproliferation and proapototic phenotype (45, 47), we next examined the roles of GW1929 on the group of well-known proliferative and apoptotic proteins. In detail, on the one hand, GW1929 treatment (10 μM, 72 h) brought marked decreases in the protein levels of cyclin D1 and PCNA, two classic proliferative regulators. On the other hand, GW1929 treatment (10 μM, 72 h) significantly activated the apoptotic p53 signaling, including p53, and the two known p53 downstream regulators mdm2 and p21. Notably, removal of GW1929 treatment could normalize all these alterations, suggesting that the protective role of GW1929 is reversible.
Knockdown of caveolin-1 failed to mediate the functional consequences of pharmacological PPARγ activation on cell proliferation and apoptosis in hypoxic PASMCs.
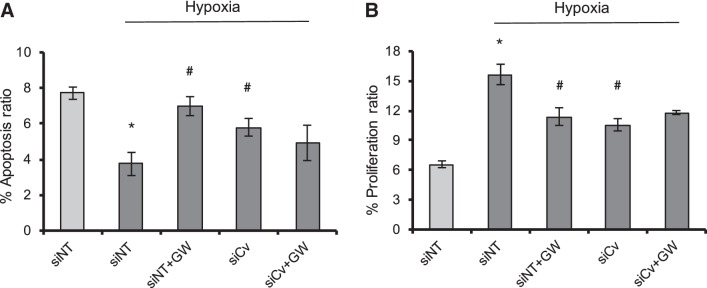
To further confirm our conclusion that the hypoxic upregulation of caveolin-1 could enhance the functional outputs of PPARγ agonists by mediating the PPARγ activation, we then measured the cell proliferation and apoptosis in PASMCs under the treatment of caveolin-1 knockdown and/or GW1929 (10 μM, 72 h) treatment. As seen in Fig. 5, first, prolonged hypoxia (4% O2) brought in significantly decreased apoptosis ratio (Fig. 5A) and increased proliferation ratio (Fig. 5B). Then, either PPARγ activation by GW1929 or knockdown of caveolin-1 markedly promoted the apoptosis and inhibited proliferation. However, dual treatment by both GW1929 and caveolin-1 failed to induce greater changes in both proliferation and apoptosis, suggesting that the roles of these two treatments were mediated through the same signaling pathway, which indicated that the caveolin-1 expression is essential in mediating the PPARγ consequences in PASMCs.
Fig. 5.
Knockdown of caveolin-1 failed to mediate the functional consequences of pharmacological PPARγ activation on cell proliferation and apoptosis in hypoxic PASMCs. A: cell apoptosis assay showing the effects of hypoxia (4% O2, 72 h), GW1929 (10 μM, 48 h), and/or siRNA knockdown of caveolin-1 (25 nM, 48 h) on cell apoptosis of PASMCs. B: cell proliferation assay showing the effects of hypoxia (4% O2, 72 h), GW1929 (10 μM, 48 h), and/or siRNA knockdown of caveolin-1 (25 nM, 48 h) on cell proliferation property of PASMCs. Bar graph represents means ± SE; n = 6 in each group. *P < 0.05 vs. siNT; #P < 0.05 vs. hypoxia + siNT.
DISCUSSION
In early 2000, Razani et al. (38) and Zhao et al. (50) first reported that mice lacking caveolin-1 could spontaneously establish PH, which is characterized by a hyperproliferative phenotype, vascular abnormalities, persistent increase in PA pressure, and right ventricle hypertrophy. Ever since then, studies have linked caveolin-1 well with the disease progression of PH and uncovered essential function of caveolin-1 in both PASMC and PAEC physiology underlying PH development (13), which are, however, extremely cell type specific (24). 1) Caveolin-1 expression was markedly downregulated in endothelial cells isolated from monocrotaline-induced PH (MCT-PH) rats (25). Jasmin et al. (15) found that short-term administration of a cell-permeable caveolin-1 peptide could prevent the development of MCT-PH and right ventricular hypertrophy. Moreover, Murata et al. (28) reported that re-expression of caveolin-1 in the endothelium effectively rescues the vascular, cardiac, and pulmonary defects in global caveolin-1-knockout PH mice. Caveolin-1 shares tight coupling with eNOS, acting as a natural inhibitor that controls the activity of eNOS (19, 30). In hypoxic endothelium, the inhibited caveolin-1 failed to antagonize eNOS, resulting in eNOS uncoupling and persistent activation, leading to oxidative and nitrosative stress and vascular contraction and remodeling (29, 51). 2) In PA smooth muscle cells, Studies from Patel et al. (34) and our group (47) reported significant upregulation of caveolin-1 expression in the PASMCs isolated from either IPAH patients or chronic hypoxia-induced PH rats. The increased caveolin-1 level contributes to elevated SOCE and intracellular calcium homeostasis, which promotes the cell proliferation in PASMCs.
Interestingly, the effects of PPARγ on caveolin-1 are also contrary in different cell types. Reports have shown that PPARγ ligands or active components upregulate caveolin-1 expression in a list of human carcinoma cell lines (3), do not alter caveolin-1 in human colon cancer cell line HCT-116 (4), and downregulate caveolin-1 in prostate cancer cell lines (5). However, by using an ovine shunt model, which mimics PAH associated with congenital heart defects, Tian et al. (41) reported that a downregulation of PPARγ is associated with upregulation of caveolin-1 in the lung tissues. Pharmacological inhibition of PPARγ leads to increased caveolin-1 gene transcription, suggesting that PPARγ inhibits caveolin-1 in the lung tissue. In our previous study, we demonstrated that the hypoxic upregulation of caveolin-1 expression contributes to a large part of the hypoxia-enhanced SOCE and proliferation in PASMCs, whereas PPARγ activation by the specific pharmacological agonist GW1929 significantly attenuated the hypoxia-elevated [Ca2+]i and SOCE by normalizing the hypoxia-increased caveolin-1 level in PASMCs (47).
In this study, we occasionally observed that caveolin-1 could not only act as a molecular therapeutic target of PPARγ but also act as an intermediate regulator that mediates the activation of GW1929 on PPARγ. First, knockdown of caveolin-1 by using a specific siRNA markedly attenuated the upregulation of GW1929 on PPARγ expression at both mRNA and protein levels (Fig. 2, B–D). Then, in a previously reported cell line (HEK293T) with low level of endogenous caveolin-1 expression, overexpression of caveolin-1 remarkably enhanced the upregulation of GW1929 on PPARγ protein (Fig. 2, E and F).
BMPs are a group of factors that belong to the TGF-β superfamily, which play a critical role in regulating cell proliferation, apoptosis, and differentiation in different cell and tissue types. BMP ligands exert their signals by binding with type II transmembrane serine/threonine kinase receptors, recruiting type I receptors, initiating the activation of cellular Smad-dependent and Smad-independent (e.g., ERK, JNK, and p38MAPK) signals, regulating different gene expression, and inducing the multiple downstream cell fate (27). To date, three type II receptors (BMPRII, ActRIIa, and ActRIIb) and three type I receptors (ALK2, ALK3, and ALK6) have been identified to participate in the BMP signal transduction (27). In the development of PH, the dysregulated BMPs signaling has been linked to the PH pathogenesis in both experimental animal models and clinical patients. The first landmark finding of dysregulated BMP signal in PH is the observation of BMPRII mutation in numerous idiopathic and familial PAH patients (6, 13a), which has been confirmed as reduced BMPRII expression in several PH animal models (21). Caveolin-1 is reported to sequester BMPRII to the plasma membrane and in doing so prevents the protein degradation of BMPRII through lysosome system and facilitates the signal transduction of BMPs, involving the classic Smad-ID signaling axis-mediated antiproliferative consequences (16). Treatment of specific lysosomal inhibitor chloroquine can increase the cell surface BMPRII level, restore BMP9 signaling in endothelial cells harboring BMPRII mutations (7), and reverse the disease pathogenesis of experimental PH in vivo (22). Besides the classic Smad-dependent downstream signaling, the caveolae has also been reported to affect the baseline activation of other Smad-independent kinases, such as the ERK1/2 and p38 (2, 9). However, within the cellular activation of ERK1/2 and p38 that is induced by numerous different stimuli, the role of caveolae becomes complicated. Several studies have shown that caveolae does not participate in the PDGF-induced activation of ERK1/2 and p38 (26, 35, 39). Forrester et al. (8) reported that caveolin-1 acts as a regulator of angiotensin II-induced remodeling in cardiovascular smooth muscle. However, Zeidan et al. (48) reported that caveolae mediates the activation of ERK1/2 that is induced by ET-1 but not angiotensin II in vascular smooth muscle cells. By using specific knockdown of caveolin-1, Nickel et al. (31) indicated that loss of caveolin-1 brought in a significant reduction of cellular activation of both Smad and ERK1/2 that is induced by BMP7 and BMP9 in PASMCs and PAECs, which is thought to be caused by the reduced level of BMPRII in the plasma membrane caveolae. We have previously demonstrated that hypoxia induces stabilization of HIF-1α, which transcriptionally activates the expression of BMP4 (42), induces the activation of cellular kinases ERK1/2 and p38 to mediate the BMP4-induced upregulation of the principle SOCC components TRPC1 and TRPC6, triggers the SOCE, and increases the proliferation and migration in PASMCs (20), whereas targeted block of the BMP4 signal by either knockdown (42) or specific antagonist (46) is proven as a powerful strategy to normalize the hypoxia-induced proliferation in PASMCs. By using specific siRNA knockdown strategies, we further found that neither Smad1 (42) nor BMPRII (49) was required for the BMP4-induced TRPC-SOCE signaling axis, suggesting the BMPRII-SMAD signaling is dispensable for BMP4-accelerated PASMC proliferation and migration. Based on this evidence, we further identified whether caveolin-1 and PPARγ could influence the baseline phosphorylation and stimuli (e.g., BMP4)-induced activation of p38 and ERK1/2 in PASMCs. Our results showed that specific siRNA knockdown of caveolin-1 resulted in decreased baseline phosphorylation of p38 (Fig. 3, G and H); moreover, knockdown of PPARγ led to increased phosphorylation of ERK1/2 and p38MAPK (Fig. 3, A–F). However, GW1929 activation of PPARγ decreased caveolin-1 expression but does not affect the activation of ERK1/2 or p38 that is induced by either PDGF-BB or BMP4 (Fig. 4). These results indicated that, on the one hand, loss-of-caveolin-1 does not affect the function of PDGF receptors, consistent with previous reports (26, 35, 39), but on the other hand, the loss of caveolin-1-mediated loss of BMPRII does not affect the BMP4-induced MAPK phosphorylation, suggesting that other BMP receptors, but not BMPRII, might account for the cellular MAPK signal transduction, which is also similar to our previous hypothesis (49).
In addition, we also found that PPARγ activation by GW1929 treatment induced remarkable downregulation of the proliferative markers, including cyclin D1 and PCNA, but upregulation of the apoptotic markers such as p53, p21, and mdm2 in a reversible manner (Fig. 6), indicating a mechanism that modulation of PPARγ expression could guide a switch between a proliferative phenotype and an apoptotic phenotype in PASMCs. This observation added additional knowledge in explaining how PPARγ activation protects the dysregulated physiology of PASMCs during PH pathogenesis.
Fig. 6.
GW1929 activation of PPARγ reversibly inhibited cellular proliferative markers and increased apoptotic markers in hypoxic PASMCs. A: Western blots showing the protein expression levels of cyclin D1, PCNA, p53, mdm2, p21, and β-tubulin under treatment of GW1929 (10 μM, 72-h) and GW1929 treatment (48 h) plus drug withdrawal (24 h) in hypoxic PASMCs. B–F: bar graphs representing the expression levels of cyclin D1 (B), PCNA (C), p21 (D), p53 (E), and mdm2 (F) as normalized to β-tubulin. Bar graph represents means ± SE; n = 4 in each group. *P < 0.05 vs. control; #P < 0.05 vs. GW(72) -treated groups (72 h).
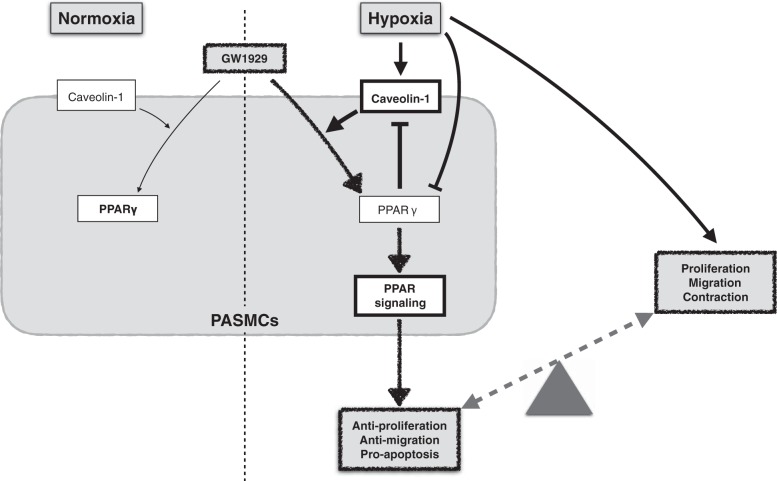
Taken together, as summarized in Fig. 7, we conclude that 1) in normoxic PASMCs, physiological level of caveolin-1 facilitates a mild activation of PPARγ upon treatment with a specific pharmacological agonist, retains a fairly unstimulated downstream PPARγ signal, and maintains a normal phenotype; 2) in hypoxic PASMCs, the downregulated PPARγ and upregulated caveolin-1 levels comprehensively cause a sustained elevation of SOCE and [Ca2+]i, which leads to a proliferative phenotype in PASMCs; 3) in hypoxic PASMCs, the hyperphysiological level of caveolin-1 results in enhanced activation of PPARγ upon agonist treatment, induces a dominant active PPARγ signal, and guides the cell into an antiproliferative and proapoptotic phenotype; and 4) activated PPARγ inhibits the expression level of caveolin-1, minimizes the agonist-induced activation of PPARγ, and maintains a balanced phenotype in PASMCs. This mechanism explains why pharmacological agonist-mediated PPARγ activation can selectively normalize hypoxia-induced proliferation in cultured PASMCs and hypoxia-induced PH pathogenesis in experimental animal models without affecting the normal cells and animals. This study sheds light on the novel molecular mechanism that PPARγ inhibits the proliferation in not only a caveolin-1-targeted but also a caveolin-1-dependent mechanism, which has clinical significance in guiding the PPARγ activation-based strategies against PH therapy and as well provides new insights in identifying novel therapeutic targets in the treatment of PH.
Fig. 7.
Schematic working model. Pharmacological activation of PPARγ leads to an antiproliferative and proapoptotic phenotype, which relies on a caveolin-1-targeted and -dependent mechanism. Activation of PPARγ contributes to a dominant beneficial consequence in PASMCs and leads to a gateway for the treatment of pulmonary hypertension.
GRANTS
This work was supported by the National Heart, Lung, and Blood Institute (R01-HL-093020), the National Natural Science Foundation of China (81173112, 81470246, 81170052, and 81220108001), the Guangzhou Department of Education Yangcheng Scholarship (12A001S), the Guangzhou Department of Natural Science (2014Y2-00167), and the Guangdong Province Universities and Colleges Pearl River Scholar Funded Scheme (2014; W. Lu).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.Y., M.Z., and J.W. conceived and designed research; K.Y., M.Z., J.H., C.Z., Q.Z., and H.J. performed experiments; K.Y., J.H., and Y.C. analyzed data; K.Y., Y.C., and W.L. interpreted results of experiments; K.Y. and M.Z. prepared figures; K.Y. drafted manuscript; K.Y., M.Z., W.L., and J.W. edited and revised manuscript; K.Y., M.Z., J.H., C.Z., Q.Z. Y.C., H.J., W.L., and J.W. approved final version of manuscript.
REFERENCES
- 1.Ameshima S, Golpon H, Cool CD, Chan D, Vandivier RW, Gardai SJ, Wick M, Nemenoff RA, Geraci MW, Voelkel NF. Peroxisome proliferator-activated receptor gamma (PPARgamma) expression is decreased in pulmonary hypertension and affects endothelial cell growth. Circ Res 92: 1162–1169, 2003. doi: 10.1161/01.RES.0000073585.50092.14. [DOI] [PubMed] [Google Scholar]
- 2.Ballard-Croft C, Locklar AC, Kristo G, Lasley RD. Regional myocardial ischemia-induced activation of MAPKs is associated with subcellular redistribution of caveolin and cholesterol. Am J Physiol Heart Circ Physiol 291: H658–H667, 2006. doi: 10.1152/ajpheart.01354.2005. [DOI] [PubMed] [Google Scholar]
- 3.Burgermeister E, Tencer L, Liscovitch M. Peroxisome proliferator-activated receptor-gamma upregulates caveolin-1 and caveolin-2 expression in human carcinoma cells. Oncogene 22: 3888–3900, 2003. doi: 10.1038/sj.onc.1206625. [DOI] [PubMed] [Google Scholar]
- 4.Chintharlapalli S, Papineni S, Baek SJ, Liu S, Safe S. 1,1-Bis(3′-indolyl)-1-(p-substitutedphenyl)methanes are peroxisome proliferator-activated receptor gamma agonists but decrease HCT-116 colon cancer cell survival through receptor-independent activation of early growth response-1 and nonsteroidal anti-inflammatory drug-activated gene-1. Mol Pharmacol 68: 1782–1792, 2005. doi: 10.1124/mol.105.017046. [DOI] [PubMed] [Google Scholar]
- 5.Chintharlapalli S, Papineni S, Safe S. 1,1-bis(3′-indolyl)-1-(p-substitutedphenyl)methanes inhibit growth, induce apoptosis, and decrease the androgen receptor in LNCaP prostate cancer cells through peroxisome proliferator-activated receptor gamma-independent pathways. Mol Pharmacol 71: 558–569, 2007. doi: 10.1124/mol.106.028696. [DOI] [PubMed] [Google Scholar]
- 6.Deng Z, Morse JH, Slager SL, Cuervo N, Moore KJ, Venetos G, Kalachikov S, Cayanis E, Fischer SG, Barst RJ, Hodge SE, Knowles JA. Familial primary pulmonary hypertension (gene PPH1) is caused by mutations in the bone morphogenetic protein receptor-II gene. Am J Hum Genet 67: 737–744, 2000. doi: 10.1086/303059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunmore BJ, Drake KM, Upton PD, Toshner MR, Aldred MA, Morrell NW. The lysosomal inhibitor, chloroquine, increases cell surface BMPR-II levels and restores BMP9 signalling in endothelial cells harbouring BMPR-II mutations. Hum Mol Genet 22: 3667–3679, 2013. doi: 10.1093/hmg/ddt216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forrester SJ, Elliott KJ, Kawai T, Obama T, Boyer MJ, Preston KJ, Yan Z, Eguchi S, Rizzo V. Caveolin-1 deletion prevents hypertensive vascular remodeling induced by angiotensin II. Hypertension 69: 79–86, 2017. doi: 10.1161/HYPERTENSIONAHA.116.08278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galbiati F, Volonte D, Engelman JA, Watanabe G, Burk R, Pestell RG, Lisanti MP. Targeted downregulation of caveolin-1 is sufficient to drive cell transformation and hyperactivate the p42/44 MAP kinase cascade. EMBO J 17: 6633–6648, 1998. doi: 10.1093/emboj/17.22.6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gong K, Xing D, Li P, Aksut B, Ambalavanan N, Yang Q, Nozell SE, Oparil S, Chen YF. Hypoxia induces downregulation of PPAR-γ in isolated pulmonary arterial smooth muscle cells and in rat lung via transforming growth factor-β signaling. Am J Physiol Lung Cell Mol Physiol 301: L899–L907, 2011. doi: 10.1152/ajplung.00062.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guignabert C, Alvira CM, Alastalo TP, Sawada H, Hansmann G, Zhao M, Wang L, El-Bizri N, Rabinovitch M. Tie2-mediated loss of peroxisome proliferator-activated receptor-γ in mice causes PDGF receptor-β-dependent pulmonary arterial muscularization. Am J Physiol Lung Cell Mol Physiol 297: L1082–L1090, 2009. doi: 10.1152/ajplung.00199.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hansmann G, de Jesus Perez VA, Alastalo TP, Alvira CM, Guignabert C, Bekker JM, Schellong S, Urashima T, Wang L, Morrell NW, Rabinovitch M. An antiproliferative BMP-2/PPARgamma/apoE axis in human and murine SMCs and its role in pulmonary hypertension. J Clin Invest 118: 1846–1857, 2008. doi: 10.1172/JCI32503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang J, Wolk JH, Gewitz MH, Loyd JE, West J, Austin ED, Mathew R. Enhanced caveolin-1 expression in smooth muscle cells: possible prelude to neointima formation. World J Cardiol 7: 671–684, 2015. doi: 10.4330/wjc.v7.i10.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13a.International PPH Consortium; Lane KB, Machado RD, Pauciulo MW, Thomson JR, Phillips JA 3rd, Loyd JE, Nichols WC, Trembath RC;. Heterozygous germline mutations in BMPR2, encoding a TGF-beta receptor, cause familial primary pulmonary hypertension. Nat Genet 26: 81–84, 2000. doi: 10.1038/79226. [DOI] [PubMed] [Google Scholar]
- 14.Huetsch JC, Suresh K, Bernier M, Shimoda LA. Update on novel targets and potential treatment avenues in pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 311: L811–L831, 2016. doi: 10.1152/ajplung.00302.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jasmin JF, Mercier I, Dupuis J, Tanowitz HB, Lisanti MP. Short-term administration of a cell-permeable caveolin-1 peptide prevents the development of monocrotaline-induced pulmonary hypertension and right ventricular hypertrophy. Circulation 114: 912–920, 2006. doi: 10.1161/CIRCULATIONAHA.106.634709. [DOI] [PubMed] [Google Scholar]
- 16.Jiang Y, Nohe A, Bragdon B, Tian C, Rudarakanchana N, Morrell NW, Petersen NO. Trapping of BMP receptors in distinct membrane domains inhibits their function in pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 301: L218–L227, 2011. doi: 10.1152/ajplung.00300.2010. [DOI] [PubMed] [Google Scholar]
- 17.Kim EK, Lee JH, Oh YM, Lee YS, Lee SD. Rosiglitazone attenuates hypoxia-induced pulmonary arterial hypertension in rats. Respirology 15: 659–668, 2010. doi: 10.1111/j.1440-1843.2010.01756.x. [DOI] [PubMed] [Google Scholar]
- 19.Li S, Couet J, Lisanti MP. Src tyrosine kinases, Gα subunits, and H-Ras share a common membrane-anchored scaffolding protein, caveolin. J Biol Chem 271: 29182–29190, 1996. doi: 10.1074/jbc.271.46.29182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li X, Lu W, Fu X, Zhang Y, Yang K, Zhong N, Ran P, Wang J. BMP4 increases canonical transient receptor potential protein expression by activating p38 MAPK and ERK1/2 signaling pathways in pulmonary arterial smooth muscle cells. Am J Respir Cell Mol Biol 49: 212–220, 2013. doi: 10.1165/rcmb.2012-0051OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Long L, Crosby A, Yang X, Southwood M, Upton PD, Kim DK, Morrell NW. Altered bone morphogenetic protein and transforming growth factor-beta signaling in rat models of pulmonary hypertension: potential for activin receptor-like kinase-5 inhibition in prevention and progression of disease. Circulation 119: 566–576, 2009. doi: 10.1161/CIRCULATIONAHA.108.821504. [DOI] [PubMed] [Google Scholar]
- 22.Long L, Yang X, Southwood M, Lu J, Marciniak SJ, Dunmore BJ, Morrell NW. Chloroquine prevents progression of experimental pulmonary hypertension via inhibition of autophagy and lysosomal bone morphogenetic protein type II receptor degradation. Circ Res 112: 1159–1170, 2013. doi: 10.1161/CIRCRESAHA.111.300483. [DOI] [PubMed] [Google Scholar]
- 23.Lu W, Wang J, Shimoda LA, Sylvester JT. Differences in STIM1 and TRPC expression in proximal and distal pulmonary arterial smooth muscle are associated with differences in Ca2+ responses to hypoxia. Am J Physiol Lung Cell Mol Physiol 295: L104–L113, 2008. doi: 10.1152/ajplung.00058.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mathew R. Cell-specific dual role of caveolin-1 in pulmonary hypertension. Pulm Med 2011: 573432, 2011. doi: 10.1155/2011/573432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mathew R, Huang J, Shah M, Patel K, Gewitz M, Sehgal PB. Disruption of endothelial-cell caveolin-1alpha/raft scaffolding during development of monocrotaline-induced pulmonary hypertension. Circulation 110: 1499–1506, 2004. doi: 10.1161/01.CIR.0000141576.39579.23. [DOI] [PubMed] [Google Scholar]
- 26.Mattsson CL, Andersson ER, Nedergaard J. Differential involvement of caveolin-1 in brown adipocyte signaling: impaired beta3-adrenergic, but unaffected LPA, PDGF and EGF receptor signaling. Biochim Biophys Acta 1803: 983–989, 2010. doi: 10.1016/j.bbamcr.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 27.Miyazono K, Kamiya Y, Morikawa M. Bone morphogenetic protein receptors and signal transduction. J Biochem 147: 35–51, 2010. doi: 10.1093/jb/mvp148. [DOI] [PubMed] [Google Scholar]
- 28.Murata T, Lin MI, Huang Y, Yu J, Bauer PM, Giordano FJ, Sessa WC. Reexpression of caveolin-1 in endothelium rescues the vascular, cardiac, and pulmonary defects in global caveolin-1 knockout mice. J Exp Med 204: 2373–2382, 2007. doi: 10.1084/jem.20062340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murata T, Lin MI, Stan RV, Bauer PM, Yu J, Sessa WC. Genetic evidence supporting caveolae microdomain regulation of calcium entry in endothelial cells. J Biol Chem 282: 16631–16643, 2007. doi: 10.1074/jbc.M607948200. [DOI] [PubMed] [Google Scholar]
- 30.Murata T, Sato K, Hori M, Ozaki H, Karaki H. Decreased endothelial nitric-oxide synthase (eNOS) activity resulting from abnormal interaction between eNOS and its regulatory proteins in hypoxia-induced pulmonary hypertension. J Biol Chem 277: 44085–44092, 2002. doi: 10.1074/jbc.M205934200. [DOI] [PubMed] [Google Scholar]
- 31.Nickel NP, Spiekerkoetter E, Gu M, Li CG, Li H, Kaschwich M, Diebold I, Hennigs JK, Kim KY, Miyagawa K, Wang L, Cao A, Sa S, Jiang X, Stockstill RW, Nicolls MR, Zamanian RT, Bland RD, Rabinovitch M. Elafin reverses pulmonary hypertension via caveolin-1-dependent bone morphogenetic protein signaling. Am J Respir Crit Care Med 191: 1273–1286, 2015. doi: 10.1164/rccm.201412-2291OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nisbet RE, Bland JM, Kleinhenz DJ, Mitchell PO, Walp ER, Sutliff RL, Hart CM. Rosiglitazone attenuates chronic hypoxia-induced pulmonary hypertension in a mouse model. Am J Respir Cell Mol Biol 42: 482–490, 2010. doi: 10.1165/rcmb.2008-0132OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patel HH, Murray F, Insel PA. Caveolae as organizers of pharmacologically relevant signal transduction molecules. Annu Rev Pharmacol Toxicol 48: 359–391, 2008. doi: 10.1146/annurev.pharmtox.48.121506.124841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel HH, Zhang S, Murray F, Suda RY, Head BP, Yokoyama U, Swaney JS, Niesman IR, Schermuly RT, Pullamsetti SS, Thistlethwaite PA, Miyanohara A, Farquhar MG, Yuan JX, Insel PA. Increased smooth muscle cell expression of caveolin-1 and caveolae contribute to the pathophysiology of idiopathic pulmonary arterial hypertension. FASEB J 21: 2970–2979, 2007. doi: 10.1096/fj.07-8424com. [DOI] [PubMed] [Google Scholar]
- 35.Peterson TE, Guicciardi ME, Gulati R, Kleppe LS, Mueske CS, Mookadam M, Sowa G, Gores GJ, Sessa WC, Simari RD. Caveolin-1 can regulate vascular smooth muscle cell fate by switching platelet-derived growth factor signaling from a proliferative to an apoptotic pathway. Arterioscler Thromb Vasc Biol 23: 1521–1527, 2003. doi: 10.1161/01.ATV.0000081743.35125.05. [DOI] [PubMed] [Google Scholar]
- 36.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45, 2001. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rabinovitch M. PPARgamma and the pathobiology of pulmonary arterial hypertension. Adv Exp Med Biol 661: 447–458, 2010. doi: 10.1007/978-1-60761-500-2_29. [DOI] [PubMed] [Google Scholar]
- 38.Razani B, Engelman JA, Wang XB, Schubert W, Zhang XL, Marks CB, Macaluso F, Russell RG, Li M, Pestell RG, Di Vizio D, Hou H Jr, Kneitz B, Lagaud G, Christ GJ, Edelmann W, Lisanti MP. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J Biol Chem 276: 38121–38138, 2001. doi: 10.1074/jbc.M105408200. [DOI] [PubMed] [Google Scholar]
- 39.Sundberg C, Friman T, Hecht LE, Kuhl C, Solomon KR. Two different PDGF beta-receptor cohorts in human pericytes mediate distinct biological endpoints. Am J Pathol 175: 171–189, 2009. doi: 10.2353/ajpath.2009.080769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sylvester JT, Shimoda LA, Aaronson PI, Ward JP. Hypoxic pulmonary vasoconstriction. Physiol Rev 92: 367–520, 2012. doi: 10.1152/physrev.00041.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tian J, Smith A, Nechtman J, Podolsky R, Aggarwal S, Snead C, Kumar S, Elgaish M, Oishi P, Göerlach A, Fratz S, Hess J, Catravas JD, Verin AD, Fineman JR, She JX, Black SM. Effect of PPARγ inhibition on pulmonary endothelial cell gene expression: gene profiling in pulmonary hypertension. Physiol Genomics 40: 48–60, 2009. doi: 10.1152/physiolgenomics.00094.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang J, Fu X, Yang K, Jiang Q, Chen Y, Jia J, Duan X, Wang EW, He J, Ran P, Zhong N, Semenza GL, Lu W. Hypoxia inducible factor-1-dependent up-regulation of BMP4 mediates hypoxia-induced increase of TRPC expression in PASMCs. Cardiovasc Res 107: 108–118, 2015. doi: 10.1093/cvr/cvv122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang J, Weigand L, Lu W, Sylvester JT, Semenza GL, Shimoda LA. Hypoxia inducible factor 1 mediates hypoxia-induced TRPC expression and elevated intracellular Ca2+ in pulmonary arterial smooth muscle cells. Circ Res 98: 1528–1537, 2006. doi: 10.1161/01.RES.0000227551.68124.98. [DOI] [PubMed] [Google Scholar]
- 44.Wang J, Yang K, Xu L, Zhang Y, Lai N, Jiang H, Zhang Y, Zhong N, Ran P, Lu W. Sildenafil inhibits hypoxia-induced transient receptor potential canonical protein expression in pulmonary arterial smooth muscle via cGMP-PKG-PPARγ axis. Am J Respir Cell Mol Biol 49: 231–240, 2013. doi: 10.1165/rcmb.2012-0185OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Y, Lu W, Yang K, Wang Y, Zhang J, Jia J, Yun X, Tian L, Chen Y, Jiang Q, Zhang B, Chen X, Wang J. Peroxisome proliferator-activated receptor γ inhibits pulmonary hypertension targeting store-operated calcium entry. J Mol Med (Berl) 93: 327–342, 2015. doi: 10.1007/s00109-014-1216-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang K, Lu W, Jia J, Zhang J, Zhao M, Wang S, Jiang H, Xu L, Wang J. Noggin inhibits hypoxia-induced proliferation by targeting store-operated calcium entry and transient receptor potential cation channels. Am J Physiol Cell Physiol 308: C869–C878, 2015. doi: 10.1152/ajpcell.00349.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang K, Lu W, Jiang Q, Yun X, Zhao M, Jiang H, Wang J. peroxisome proliferator-activated receptor γ-mediated inhibition on hypoxia-triggered store-operated calcium entry. A caveolin-1-dependent mechanism. Am J Respir Cell Mol Biol 53: 882–892, 2015. doi: 10.1165/rcmb.2015-0002OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zeidan A, Broman J, Hellstrand P, Swärd K. Cholesterol dependence of vascular ERK1/2 activation and growth in response to stretch: role of endothelin-1. Arterioscler Thromb Vasc Biol 23: 1528–1534, 2003. doi: 10.1161/01.ATV.0000090129.75275.C2. [DOI] [PubMed] [Google Scholar]
- 49.Zhang Y, Wang Y, Yang K, Tian L, Fu X, Wang Y, Sun Y, Jiang Q, Lu W, Wang J. BMP4 increases the expression of TRPC and basal [Ca2+]i via the p38MAPK and ERK1/2 pathways independent of BMPRII in PASMCs. PLoS One 9: e112695, 2014. doi: 10.1371/journal.pone.0112695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao YY, Liu Y, Stan RV, Fan L, Gu Y, Dalton N, Chu PH, Peterson K, Ross J Jr, Chien KR. Defects in caveolin-1 cause dilated cardiomyopathy and pulmonary hypertension in knockout mice. Proc Natl Acad Sci USA 99: 11375–11380, 2002. doi: 10.1073/pnas.172360799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao YY, Zhao YD, Mirza MK, Huang JH, Potula HH, Vogel SM, Brovkovych V, Yuan JX, Wharton J, Malik AB. Persistent eNOS activation secondary to caveolin-1 deficiency induces pulmonary hypertension in mice and humans through PKG nitration. J Clin Invest 119: 2009–2018, 2009. doi: 10.1172/JCI33338. [DOI] [PMC free article] [PubMed] [Google Scholar]