Abstract
The production of endogenous adenosine during secretagogue stimulation of CFTR leads to feedback inhibition limiting further chloride secretion in the rectal gland of the dogfish shark (Squalus acanthias). In the present study, we examined the role of AMP-kinase (AMPK) as an energy sensor also modulating chloride secretion through CFTR. We found that glands perfused with forskolin and isobutylmethylxanthine (F + I), potent stimulators of chloride secretion in this ancient model, caused significant phosphorylation of the catalytic subunit Thr172 of AMPK. These findings indicate that AMPK is activated during energy-requiring stimulated chloride secretion. In molecular studies, we confirmed that the activating Thr172 site is indeed present in the α-catalytic subunit of AMPK in this ancient gland, which reveals striking homology to AMPKα subunits sequenced in other vertebrates. When perfused rectal glands stimulated with F + I were subjected to severe hypoxic stress or perfused with pharmacologic inhibitors of metabolism (FCCP or oligomycin), phosphorylation of AMPK Thr172 was further increased and chloride secretion was dramatically diminished. The pharmacologic activation of AMPK with AICAR-inhibited chloride secretion, as measured by short-circuit current, when applied to the apical side of shark rectal gland monolayers in primary culture. These results indicate that that activated AMPK, similar to adenosine, transmits an inhibitory signal from metabolism, that limits chloride secretion in the shark rectal gland.
Keywords: adenosine, AMPK, CFTR, chloride secretion, shark rectal gland, Squalus acanthias
INTRODUCTION
Adenosine, derived from adenine nucleotide breakdown, is secreted and has an extracellular autocrine-paracrine role during energetic stress in the rectal gland. Adenosine monophosphate-activated protein kinase (AMPK) is an intracellular kinase that is part of a mechanism that is responsive to increases in AMP and ADP concentrations that accompany ATP breakdown (38). Both are part of metabolic mechanisms that are responsive to cellular energetic stress and regulate function in multiple mammalian tissues, including skeletal muscle (14, 15), cardiac muscle (1, 23, 24, 39), brain (3, 8), gut (40), and kidney (2, 12, 25, 28, 29).
The cystic fibrosis transmembrane conductance regulator (CFTR) is a chloride channel in epithelia that secretes or reabsorbs NaCl. Mutations in CFTR that reduce its membrane expression or activity cause the hereditary disease cystic fibrosis (CF) (5, 31). CFTR also contributes to the secretion of cyst fluid in polycystic kidney disease (31) and plays a crucial role in secretory diarrhea (21, 33).
CFTR is a member of the superfamily of ATP-binding cassette (ABC) transporters and is localized predominantly in the apical membrane of epithelial cells (26, 31). CFTR contains 12 transmembrane helices, five cytoplasmic domains, including two nucleotide-binding domains; regulatory (R) domain containing protein kinase A (PKA), protein kinase C (PKC), and protein kinase G (PKG) phosphorylation sites (35); and NH2- and COOH-terminal cytoplasmic tails (18, 26).
In epithelial tissues, the transcellular movement of ions accounts for a high percentage of the expenditure of cellular energy (20). In a previous study from our laboratory (17), we defined the role of endogenously produced adenosine in the perfused rectal gland of the dogfish shark, a 400-million-year-old model epithelia of hormone-stimulated chloride transport (4, 7). Membranes of the rectal gland of Squalus acanthias contain record amounts of the Na-K-ATPase pump (7), the Na-K-2Cl cotransporter (NKCC1) (6), and CFTR (7). The rectal gland of the shark has a high oxygen consumption when stimulated to secrete chloride and thus is an ideal model to establish inhibitory signals from metabolism.
In the rectal gland, as chloride secretion increases in response to forskolin and other secretagogues, venous adenosine and inosine concentrations increase in parallel, rising a thousand fold, from low nanomolar-to-millimolar range (17). Inhibition of chloride transport with bumetanide, an inhibitor of the Na-K-2Cl cotransporter, or ouabain, an inhibitor of the Na-K-ATPase pump, reduced venous adenosine and inosine to basal nanomolar values. Additionally, when the interaction of endogenous adenosine with extracellular A1 receptors was prevented, chloride transport in response to secretagogues increased by up to 1.7–2.3-fold (17). These studies established that endogenous adenosine is a “retaliatory metabolite” that is released in response to hormone-stimulated cellular energy consuming transport and acts at A1 adenosine receptors as a feedback inhibitor of chloride transport (17).
The energy sensor AMP-activated protein kinase (AMPK) has been shown to be a regulator of energy-generating and energy-consuming pathways in many mammalian tissues (12). The activity of AMPK increases during conditions of cellular stress and responds to elevated intracellular AMP/ATP and ADP/ATP ratios (38). AMPK has been linked to CFTR-mediated chloride secretion in several mammalian tissues (10, 11, 13). Regulation of CFTR by AMPK was first discovered by a yeast two-hybrid screen using the cloned human COOH-terminal tail of CFTR and a human testis cDNA library (13). The 407–550 region of the AMPKα subunit binds to the COOH-terminal tail of CFTR at residues 1420–1457, leading to the phosphorylation of the R domain at Ser768 (18, 27). As a result, AMPK-dependent phosphorylation of CFTR blunts PKA stimulation of CFTR, thereby diminishing the PKA responsiveness of CFTR when human Calu-3 cells are subjected to hypoxia or metabolic stress (18).
Although AMPK is known to inhibit CFTR-mediated chloride secretion in mammalian cells (12), this pathway has not been identified in the ancient elasmobranch shark rectal gland. Furthermore, if AMPK activation is present, it is unknown which cellular sensor (AMPK or adenosine) is employed by epithelia during stimulation of NaCl secretion or if both mechanisms act to limit cellular work during periods of stimulation or stress.
The objectives of the present experiments in the shark rectal gland were 1) to determine whether the phosphorylation of AMPK increases when chloride secretion is increased by secretagogues; 2) to determine whether the cDNA for AMPKα is detected in shark rectal glands; 3) to examine the effects of hypoxia and perfusion with metabolic inhibitors (FCCP or oligomycin) on both chloride secretion and activation of AMPK in the rectal gland; and 4) to examine whether ion transport is inhibited when a stimulator of AMPK phosphorylation, AICAR, is added apically to primary cultures of rectal gland tubules. Finally, we integrate the roles of extracellular adenosine and intracellular activated AMPK and propose a model for both as cellular mechanisms activated by energy requiring ion transport in epithelia.
MATERIALS AND METHODS
In vitro perfusion of shark rectal glands.
Rectal glands were obtained from dogfish sharks (Squalus acanthias), weighing 1–3 kg, caught by gill nets in Frenchman Bay, ME, and kept in 5,000 gallon tanks with flow-through seawater until use, usually within 3 days of capture. Dissolved oxygen in the seawater tank averaged 102 ± 0.4% (n = 79 in measurements by Mount Desert Island Biological Laboratory staff. Sharks were euthanized by pithing the spinal cord and brain using a protocol approved by the Mount Desert Island Biological Laboratory Animal Care and Use Committee (approval no. 10-03). Rectal glands were excised, and cannulas were placed in the artery, vein, and duct as previously described (4). For perfusion studies, rectal glands were placed in a glass perfusion chamber, maintained at 15°C with running sea water and perfused with shark Ringer’s solution containing (in mM) 270 NaCl, 4 KCl, 3 MgCl2, 2.5 CaCl2, 1 KH2PO4, 8 NaHCO3, 350 urea, 5 glucose, and 0.5 Na2SO4 (all from Sigma). Ringer’s perfusate was bubbled with either 99% O2 and 1% CO2 (similar to the oxygen content of the tank where sharks are kept) or 99% N2 and 1% CO2. All glands were initially perfused for 30 min with Ringer’s alone to achieve basal rates of chloride secretion, and measurements were made every 10 min. After 30 min, forskolin (1 µM) and IBMX (100 µM) were added to the perfusate and 1-min measurements of chloride secretion were made until the end of the experiment. Chloride secretion was calculated by determining the product of duct flow (in μl), the chloride concentration of the ductal fluid (in mEq/l), and the time of collection and dividing this value by the wet weight of the gland. Results are expressed as mean microequivalents of chloride secreted per hour per gram wet weight (μEq·h−1·g−1).
Western blot analysis of shark rectal gland lysates.
Lysates were prepared from rectal glands snap frozen at the end of perfusion using Wollenberg clamps, precooled in liquid nitrogen. One milliliter of lysis buffer (20 mM HEPES pH 7.4, 50 mM β-glycerol phosphate, 2 mM EGTA, 1 mM DTT, 10 mM NaF, 1 mM NaVO4, 1% Triton, 10% glycerol, and 1 Protease Inhibitor Cocktail Tablet per 10 ml lysis buffer) was added to 20 mg of frozen rectal gland tissue. Samples were homogenized on ice three times for 30 s using a homogenizer (IKA-Werke T-25 Basic). Cellular debris was removed by 10 min of centrifugation at 13,000 rpm at 4°C. Protein concentrations were measured using ultraviolet absorption spectrophotometry at 215 and 225 µm and samples were diluted to equivalent protein concentrations. LDS sample buffer (NuPage, Novex; Invitrogen, Life Technologies) was added, and 15–20 µg protein per sample were loaded onto either Novex 10% Tris-Glycine Gel Mini 1.5 mm or 3–8% gradient gels. Samples were transferred onto PVDF membranes (Millipore) and blocked with 5% skim milk dissolved in TBST (50 mM Tris, 150 mM NaCl, and 0.05% Tween 20). Membranes were incubated overnight at 4°C with primary antibodies: rabbit anti AMPKα or rabbit anti pThr172 AMPKα. All primary antibodies were diluted at 1:1000. Membranes were washed three times in TBST and incubated for 1 h with goat anti-rabbit IgG, horseradish peroxidase-linked (Cell Signaling) at a dilution of 1:2,000. Proteins were detected with enhanced chemiluminescence in the ChemiDoc XRS+ Imager (Bio-Rad) and densitometry was performed using ImageJ. Membranes were then treated with Restore Western Blot Stripping Buffer (Thermo Scientific) to remove primary and secondary antibodies and incubated overnight with primary antibodies against GAPDH or β-actin (Cell Signaling; 1:1,000) to assess protein loading of the samples.
Preparation of total RNA from shark rectal gland tissue, degenerate primer design, PCR, and cloning of PCR fragments.
Since the genome of the dogfish shark (Squalus acanthias) is incompletely sequenced, we sought to clone the AMPKα gene (PRKAA) in the shark rectal gland by preparing total RNA from rectal gland tissue, and designing degenerate primers for use in PCR reactions, and 5′- and 3′-rapid amplification of cDNA ends (RACE) (32). Total RNA was extracted with TRIzol reagents (Invitrogen) from 100 mg of shark tissue that had been excised from the animal and immediately snap frozen in liquid nitrogen. First-strand cDNA was synthesized using Invitrogen SuperScript synthesis system for RT-PCR. ClustalW was used to identify regions of high amino acid homology in PRKAA1, the AMPKα1 gene, aligning Homo sapiens, Rattus norvegicus, Sus scrofa, Gallus gallus, Xenopus laevis, and Danio rerio (alignment one) as well as aligning Homo sapiens, Bos taurus, Rattus norvegicus, Sus scrofa, Gallus gallus, and Xenopus laevis (alignment two). Three degenerate primer pairs were designed from both alignment one and two using Consensus-Degenerate Hybrid Oligonucleotide Primers (CODEHOP) targeting different regions of PRKAA1. Of the six primer pairs, one successfully amplified a target sequence in the shark rectal gland. This primer pair was designed from regions of high conservation of the six species of alignment two. The successful primer pair consisted of a 24-fold degenerate forward oligonucleotide primer [5′-CGGAGGAGAACTATTCGACTACAT(A/C/T)TG(C/T)AA(A/G)(A/C)A-3′] that codes for the Gly-Gly-Glu-Leu-Phe-Asp-Tyr-Ile-Cys-Lys-Asn sequence and a 16-fold degenerate reverse oligonucleotide primer [5′-ATATCTTTTTGAATAGTGTTGGTACGTG(A/G)TC(A/G)TC(A/G)TC(A/G)AA-3′] that codes for the Phe-Asp-Asp-Asp-His-Val-Pro-Thr-Leu-Phe-Lys-Lys-Ile-Cys sequence. PCR amplifications were performed with cDNA template using Taq DNA Polymerase, recombinant (Invitrogen). Touch-down PCR amplifications were performed in a Dyad Peltier Thermal Cycler with the following parameters: hot start for 3 min at 94°C; followed by 40 cycles of 95°C for 45 s, 65°C annealing for 1 min that was lowered by 0.5°C each cycle, and 72°C for 1.5 min; and an extension period of 72°C for 10 min. The resulting 389-bp PCR product was purified from a 2% agarose gel and ligated into the pCR II TOPO TA cloning vector (Invitrogen). Positive transformants were blue/white screened on LB plates with 100 µg/ml ampicillin. Ten positive colonies per plate were cultured overnight in LB medium containing 100 µg/ml ampicillin. Plasmid DNA was then isolated with QIAprep Spin Miniprep Kit (Qiagen) followed by screening for insert size with PCR. Two positive clones were sent to the Keck Biotechnology Resource Laboratory at Yale for bidirectional sequencing. The 389-bp sequence was translated into amino acid sequences using ExPASy Bioinformatics Resource Portal. The amino acid sequence with the longest open reading frame was analyzed against the GenBank database with a BLAST alignment and identified the fragment as having 93% similarity with human AMPKα1 and 91% similarity with human AMPKα2.
Rapid amplification of cDNA ends-PCR to obtain 5′- and 3′-ends of shark AMPKα1 and cloning of RACE-PCR fragments.
Shark rectal gland total RNA was adaptor ligated according to manufacturer’s protocol (SMARTer RACE cDNA Amplification Kit, Clontech). For 3′-rapid amplification of cDNA ends (RACE)-PCR, four AMPK1-specific primers were designed and used in subsequent PCRs. The AMPKα1-specific primer and external cassette-specific primer (Universal Primer A Mix, Clontech) were used in a PCR using Advantage 2 PCR Kit (Clontech) with adaptor-ligated shark rectal gland 3′-SMART RACE-ready cDNA template. Fifty-microliter reaction mixtures were cycled in a Dyad Peltier Thermal Cycler with the following parameters: 5 cycles of 94°C for 30 s and 72°C for 3 min, 5 cycles of 94°C for 30 s, 70°C for 30 s, and 72°C for 3 min followed by 22 cycles of 94°C for 30 s, 68°C for 30 s, and 72°C for 3 min. For 5′-RACE-PCR, four AMPKα1-specific primers were designed and used in subsequent PCRs. The AMPKα1-specific primer and external cassette-specific primer (Universal Primer A Mix, Clontech) were used in a PCR using Advantage 2 PCR Kit (Clontech) with adaptor ligated shark rectal gland 5′-SMART RACE-ready cDNA template. Fifty-microliter reaction mixtures were cycled in a Dyad Peltier Thermal Cycler with the following parameters: five cycles of 94°C for 30 s and 72°C for 3 min, five cycles of 94°C for 30 s, 70°C for 30 s, and 72°C for 3 min followed by 22 cycles of 94°C for 30 s, 68°C for 30 s, and 72°C for 3 min. The 3′- and 5′-products were extracted from 2% agarose gels of the RACE-PCR amplifications. These PCR products were then cloned in pCR II TOPO TA cloning vectors (Invitrogen) as previously described (32). Clones were sequenced bidirectionally at the Keck Biotechnology Resource Laboratory at Yale. The cloned sequences were translated into amino acid sequences using ExPASy Bioinformatics Resource Portal. All amino acid sequences were analyzed against the GenBank database with a BLAST alignment. Phylogenetic trees were generated by ClustalW alignment with MEGA6.06 phylogenetic tree software (Fig. 5). A search within the National Center for Biotechnology Information (NCBI) protein database was performed to determine in which animal species the AMPKα sequence was present. Zebrafish (Osteichthyans, ~340 million years old), elephant shark, and dogfish shark (both Chondrichthyes, the oldest living group of vertebrates, ~400 million years old) were the only marine species in which AMPKα was found. Frog (~360 million years old) was the only species from the Amphibia group in which AMPKα was found. Additionally, Lizard (~300–200 million years old) was the only species from the Reptilia group. The 10 species from the Mammalia group (~200 million years old) were chosen randomly.
Fig. 5.
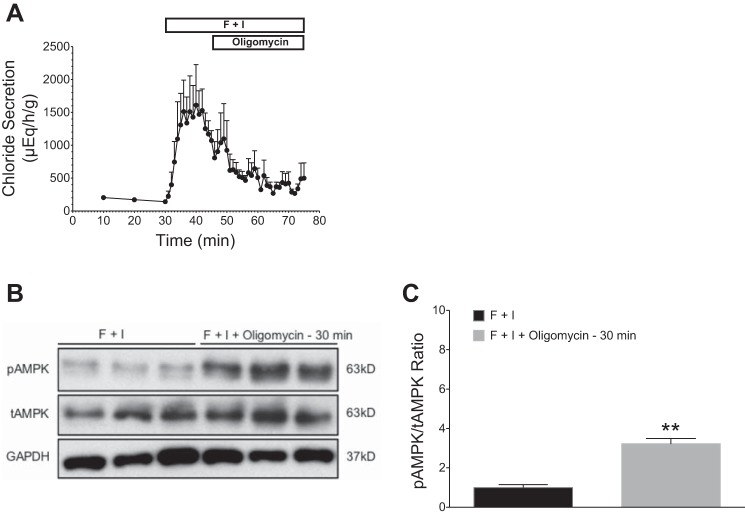
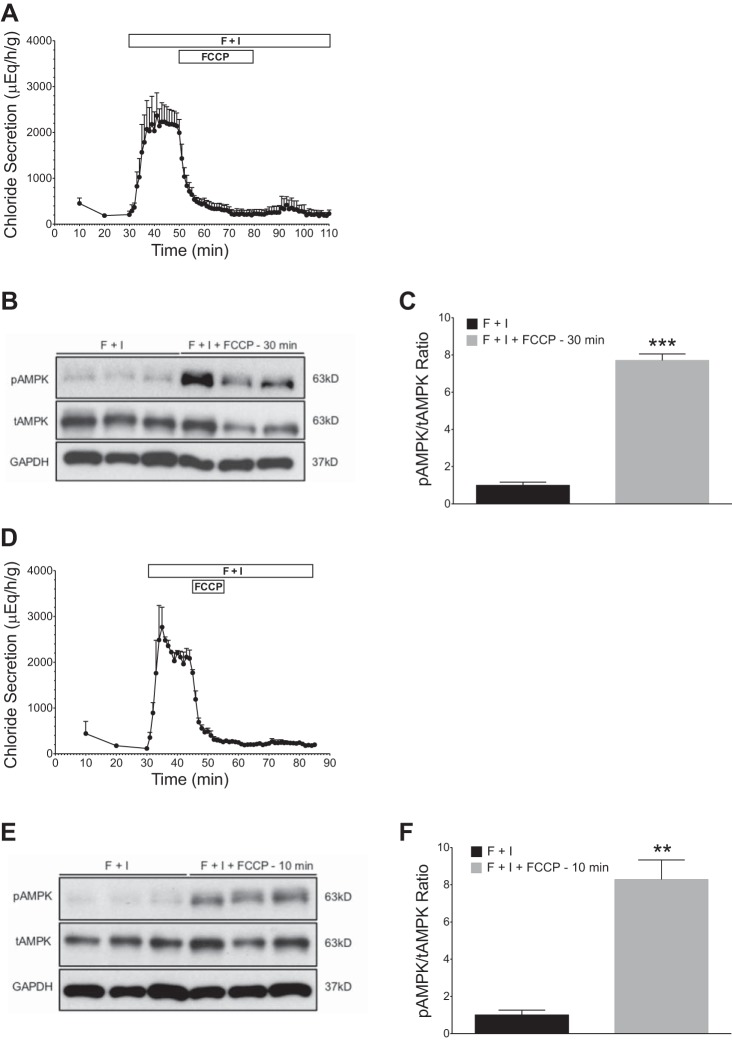
Effects of oligomycin on stimulated chloride secretion (forskolin + IBMX) and AMPK activation in perfused shark rectal glands. All glands were perfused with elasmobranch Ringer’s bubbled with 99% O2 and 1% CO2. Baseline secretion levels were established for 30 min before the addition of forskolin (1 µM) and IBMX (100 µM). Stimulation with F + I was carried out for 45 min (A). Oligomycin (2 μM) was added at 45 min, and perfusion was continued to 75 min. Oligomycin treatment markedly inhibited chloride secretion. B: glands were snap frozen in liquid N2, and rectal gland homogenates were immunoblotted with specific antibodies against AMPKα (tAMPK), pThr172AMPKα (pAMPK), and GAPDH. C: immunoblots were quantified with densitometry and pAMPK/tAMPK ratios were normalized to F + I-perfused controls (n = 3, **P < 0.01, Student’s t-test).
Measurements of transepithelial chloride transport as short-circuit current in primary culture monolayers of shark rectal gland tissue.
Isolated tubules of shark rectal gland epithelial cells were prepared under sterile conditions as previously described (36) and rectal gland tubules were plated onto Corning Transwell polyester membrane inserts (pore size: 0.4 um; membrane diameter: 6.5 mm; catalog no. CLS3470; Sigma Aldrich, St. Louis, MO) (30). Confluent primary culture monolayers were mounted in a modified Ussing chamber and bathed with a solution containing (in mM) 268 NaCl, 6 KCl, 3 MgCl2, 2.5 CaCl2, 20 NaHCO3, 350 urea, and 5 glucose at pH 7.5. The chamber was kept at 20°C and was constantly gassed with 95% O2-5% CO2. The voltage clamp and data acquisition equipment was designed and constructed by W. Van Driessche (Catholic University, Louvain, Belgium) and has been described in detail previously (14). After stabilization of the short-circuit current (Isc) and chloride current, apical CFTR channels were maximally stimulated by the addition of forskolin (10 μM) to the basolateral side. Following stimulation, AICAR (AMPK activator; 1 mM) was applied to the apical membrane of the monolayer.
Statistics.
Data were analyzed using Student’s t-test to identify differences between groups. Significance was determined as P < 0.05. Results are represented as means ± SE.
RESULTS
Effects of F + I on chloride secretion and AMPK activation under normal oxygen perfusion conditions.
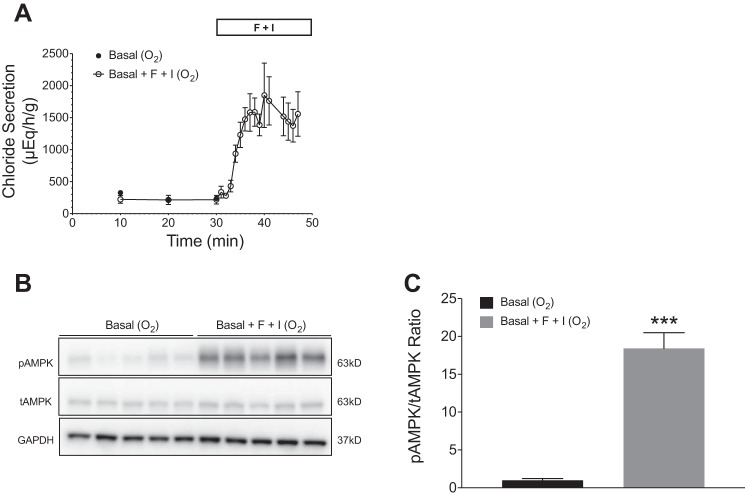
Rectal glands were perfused with shark Ringer’s for 30 min to achieve basal levels of secretion (100–200 μEq·h−1·g−1) (Fig. 1A). After addition of F + I at 30 min under oxygen perfusion conditions (99% O2-1% CO2), chloride secretion increased ~10-fold to a mean of 1,849 ± 503 μEq·h−1·g−1 (Fig. 1A). Western blot analysis showed a significant increase in phosphorylated AMPK (pAMPK) in glands perfused with F + I (Fig. 1B). Additionally, the ratio of pAMPK to total AMPK (tAMPK) was significantly greater in glands perfused with F + I when compared with glands without F + I stimulation (P < 0.001; Fig. 1C).
Fig. 1.
Effects of forskolin and IBMX (F + I) on chloride secretion and AMPK activation in perfused shark rectal glands. All glands were perfused with elasmobranch Ringer’s bubbled with 99% O2 and 1% CO2. Baseline secretion levels were established for 30 min, before the addition of forskolin (1 µM) and IBMX (100 µM). Stimulation with F + I was carried out for 15 min. A: perfusion with F + I resulted in a rapid and sustained increase in chloride secretion (n = 5). Values are means ± SE; n = 5. P < 0.001 for all values from 30 to 45 min. B: glands were snap frozen in liquid N2 and rectal gland homogenates were immunoblotted with specific antibodies against AMPKα (tAMPK), pThr172AMPKα (pAMPK), and GAPDH. C: immunoblots were quantified with densitometry and pAMPK/tAMPK ratios were normalized to controls perfused with elasmobranch Ringer’s only (n = 5, ***P < 0.001, Student’s t-test).
Cloning of a 389-bp sequence of the kinase domain of the AMPKα subunit.
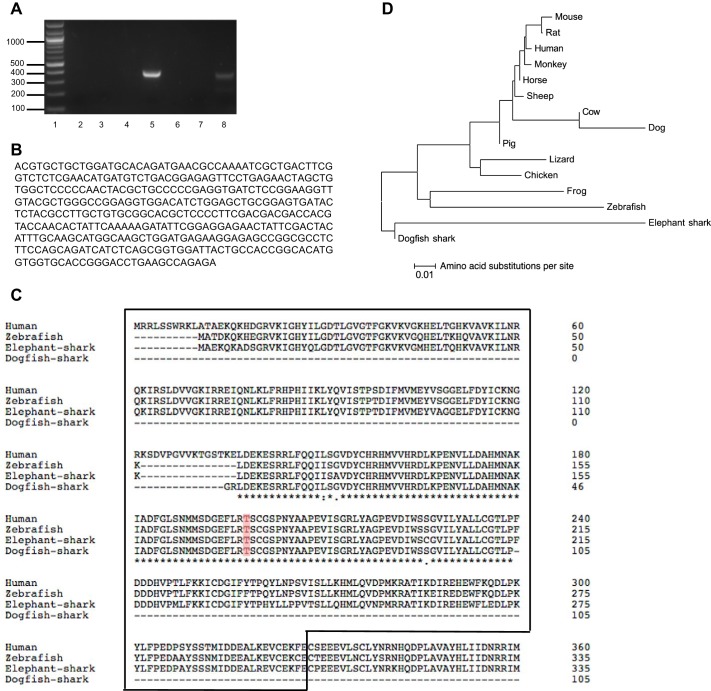
We cloned the AMPKα subunit from the dogfish shark rectal gland, starting with CODEHOP designed degenerate primers from conserved regions of human, cow, rat, pig, chicken, and frog AMPKα1. A 389-bp PCR product was amplified from shark rectal gland cDNA (Fig. 2A, lane 5). A protein-protein BLAST search of the 389-bp product against the human genome (NCBI) showed 93% similarity with human AMPKα1 and 91% similarity with human AMPKα2. Within this sequence were the active site, the ATP-binding site, the substrate binding site, and the activation loop containing Thr172 (Fig. 2, A and B). Figure 2B shows the nucleotide sequence of the cloned 389-bp of the catalytic kinase domain of the AMPKα subunit in the shark rectal gland. 3′- and 5′- RACE-PCR products were extracted from 2% agarose gels and sequenced, but no similarities with AMPKα1 were found. AMPKα amino acid alignment of human, zebrafish, elephant shark, and the dogfish shark fragment showed a majority of identical amino acids (Fig. 2C). Phylogenetic analysis revealed that the cloned shark fragment is most closely related to elephant shark AMPKα (Fig. 2D). The elephant shark, Callorhinchus milii, a holocephalian living in Australia and New Zealand, was chosen for sequencing because it has a much smaller genome (910 Mb) than the dogfish shark (Squalus acanthias) or other elasmobranchs in which physiological studies have been done (genome sizes range from 3,500 to 7,000 Mb; Ref. 37).
Fig. 2.
Cloning of a 389-bp DNA sequence of the AMPKα subunit in the dogfish shark rectal gland. A: PCR of shark rectal gland cDNA yielded a 389-bp product of AMPKα1 using degenerate primers designed from conserved regions of human, cow, rat, pig, chicken, and frog AMPKα1 (lane 5). Shown are a 100-bp DNA ladder (lane 1), PCR with different pairs of AMPKα1 degenerate primers against regions of the gene (lanes 2–7), and a positive control using TASK-1 potassium channel degenerate primers (394-bp PCR product, lane 8). B: cloned 389-bp DNA sequence of the catalytic kinase domain of the AMPKα subunit in the shark rectal gland. C: amino acid alignment of the dogfish shark fragment and of AMPKα from human, zebrafish, and elephant shark. Asterisk (*) indicates the amino acids are identical; colon (:) indicates that conserved substitutions are observed; and period (.) indicates that semiconserved substitutions are observed. The catalytic domain of the protein is enclosed in a box. The phosphorylation residue Thr172 is marked in color. D: phylogenetic tree showing the relationship of vertebrate and invertebrate AMPKα. Protein alignments were performed with ClustalW. The evolutionary history was inferred using the Neighbor-Joining method. The optimal tree with a sum of branch length of 0.36877630 is shown. The tree is drawn to scale 0.01, with branch lengths and evolutionary distances both in amino acid substitutions per site. The evolutionary distances were computed using the Poisson correction method. Rate variation among sites was modeled with a gamma distribution (shape parameter = 1). The analysis involved 15 amino acid sequences. All ambiguous positions were removed for each sequence pair. There were a total of 620 positions in the final data set. Evolutionary analyses were conducted in MEGA6.
Effects of severe hypoxia (N2 perfusion) compared with normoxia (O2 perfusion) on F + I-stimulated chloride secretion and AMPK activation.
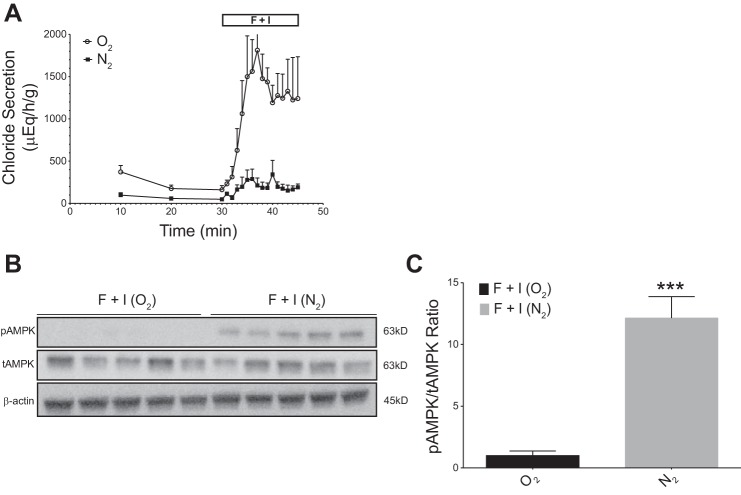
When rectal glands were perfused with shark Ringer’s for 30 min to achieve basal levels of secretion in both groups (100–200 μEq·h−1·g−1), there was a trend for lower chloride secretion with nitrogen vs. oxygen perfusion (Fig. 3A). Addition of F + I at the 30-min time point, however, under oxygen perfusion conditions increased chloride secretion to a mean value of 1813 ± 334 μEq·h−1·g−1, a 10-fold increase from baseline. In glands rendered severely hypoxic by nitrogen perfusion, baseline secretion levels were ~40–50 μEq·h−1·g−1 by 30 min of perfusion. Stimulation of chloride secretion with F + I was markedly inhibited during hypoxic conditions, reaching only 343 ± 166 μEq·h−1·g−1 (P < 0.01 compared with oxygen perfusion; Fig. 3A).
Fig. 3.
Effects of severe hypoxia on stimulated chloride secretion (with forskolin + IBMX) and AMPK activation in perfused shark rectal glands. Glands were perfused with elasmobranch Ringer’s bubbled with either 99% O2 and 1% CO2 or 99% N2 and 1% CO2 as indicated. Baseline secretion levels were established for 30 min before the addition of forskolin (1 µM) and IBMX (100 µM) to stimulate chloride secretion. Stimulation with F + I was carried out for 15 min. A: Comparison of glands perfused with 99% O2 and 1% CO2 (control, “O2”) vs. 99% N2-1% CO2 (severe hypoxia, “N2”). N2 perfusion significantly inhibited chloride secretion when compared with O2 controls. Values are means ± SE (n = 5, P < 0.01 for all values from 35 to 45 min, Student’s t-test). B: glands were snap frozen in liquid nitrogen and rectal gland homogenates were immunoblotted with specific antibodies against AMPK (tAMPK), pThr172AMPKα (pAMPK) and β-actin. C: immunoblots were quantified by densitometry and pAMPK/tAMPK ratios were normalized to O2-perfused controls. A 10-fold increase in the pAMPK/tAMPK ratio was observed in hypoxic, N2-perfused glands (n = 5; ***P < 0.001, Student’s t-test).
Western blot analysis showed an increase in pAMPK in glands perfused with nitrogen (Fig. 3B). Additionally, the ratio of pAMPK to tAMPK was significantly greater in F + I-stimulated glands perfused under nitrogen conditions when compared with stimulated glands perfused under oxygen conditions (P < 0.001; Fig. 3C). This blot shows an ~13 fold increase in pAMPK in hypoxic F + I-treated glands relative to O2 F + I-treated glands.
Response to FCCP on chloride secretion and AMPK activation in the perfused shark rectal gland.
We next examined the action of two metabolic inhibitors (FCCP and oligomycin) on chloride secretion and AMPK phosphorylation in the intact, in vitro perfused rectal gland. The hydrophobic acid FCCP is an uncoupler of oxidative phosphorylation in mitochondria that dissipates the electrochemical proton gradient, increasing mitochondrial oxygen consumption. After stimulation with F + I, FCCP (2 µM) was added to the perfusate for either 10 min or 30 min. FCCP was then removed and perfusion with F + I was continued for 30 min. Figure 4A depicts results of the addition of FCCP for 30 min (between 50 and 80 min of perfusion). Mean chloride secretion levels were 1,994 ± 290 μEq·h−1·g−1 at 50 min, before the addition of FCCP, and were 228 ± 92 μEq·h−1·g−1 after 30 min of exposure (at 80 min). Removal of FCCP from the perfusate failed to elicit a functional recovery, and secretion levels remained low despite the continued presence of F + I for an additional 30 min of perfusion. We therefore added FCCP for a shorter time (10 min). When FCCP was added for 10 min only (Fig. 4D), there was an abrupt decrease in chloride secretion, which again persisted after the withdrawal of FCCP. As illustrated in Fig. 4, B, C, E, and F, Western blotting revealed significantly greater pAMPK in glands exposed to FCCP (2 μM) when compared with glands perfused with F + I alone (P < 0.001 for 30 min in Fig. 4C, and P < 0.01 for 10 min, Fig. 4F).
Fig. 4.
Effects of FCCP on stimulated chloride secretion (forskolin + IBMX) and AMPK activation in perfused shark rectal glands. All glands were perfused with elasmobranch Ringer’s bubbled with 99% O2 and 1% CO2. Baseline secretion levels were established for 30 min, before the addition of forskolin (1 µM) and IBMX (100 µM). Stimulation with F + I was carried out for 80 min (A) or 60 min (D). FCCP (2 µM) was added for 30 min (A) or 10 min (D) and then removed. A and D: FCCP (2 µM) dramatically inhibited chloride secretion with no recovery after removal. B and E: glands from A and D were snap frozen in liquid N2, and rectal gland homogenates were immunoblotted with specific antibodies against AMPKα (tAMPK), pThr172 AMPKα (pAMPK), and GAPDH. C and F: immunoblots were quantified with densitometry and pAMPK/tAMPK ratios were normalized to F + I-perfused controls (n = 3; **P < 0.01, ***P < 0.001, Student’s t-test).
Response to oligomycin on chloride secretion and AMPK activation in the perfused shark rectal gland.
Oligomycin is an inhibitor of ATP synthase and prevents state 3 respiration by inhibiting the proton channel necessary for oxidative phosphorylation of ADP to ATP. After stimulation with F + I, oligomycin (2 μM) was added to the perfusate for 30 min (Fig. 5A). Marked inhibition of chloride secretion was observed, as mean stimulated chloride secretion declined from 1,072 ± 150 at 45 min to 508 ± 95 μEq·h−1·g−1 at 55 min. Western blotting showed a significant increase in pAMPK in glands treated with oligomycin when compared with glands treated with F + I alone (P < 0.01; Fig. 5, B and C).
Response to AICAR: chloride secretion measured as Isc in shark rectal gland polarized cell culture monolayers.
In shark rectal gland cell primary culture monolayers, net chloride secretion via CFTR is responsible for the ionic conductance of the apical membrane. Net basolateral transport of chloride occurs via NKCC cotransporters, acting in concert with the Na-K-ATPase and K-channels to recycle Na and K across the basolateral membrane. Ussing chamber experiments were carried out (n = 5) by measuring chloride secretion as Isc in cultured monolayers in which Isc is equal to net chloride flux (36).
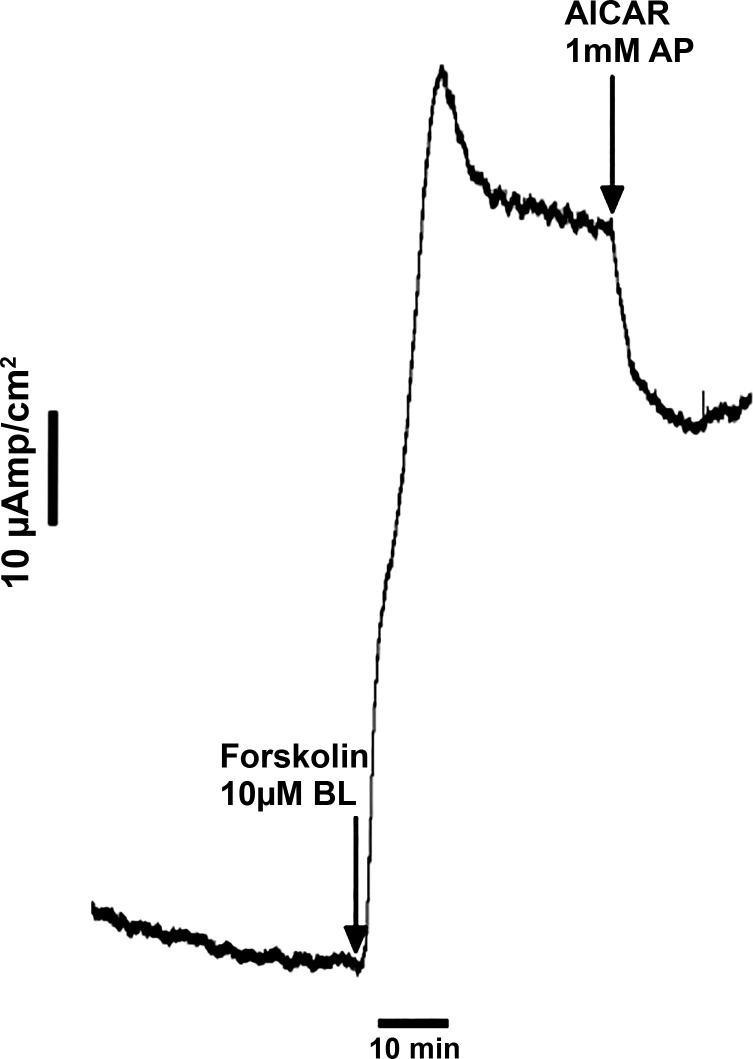
We used the compound AICAR (5-aminoimidazole-4-carboxamide ribonucleotide), a stimulator of AMPK, to examine the extent to which AMPK regulates CFTR mediated chloride secretion. AICAR is an adenosine analog that, once taken up by cells, is converted to ZMP (5-aminoimidazole-4-carboxamide ribonucleotide). ZMP acts as an AMP mimetic and activates AMPK (11). After steady-state basal currents were measured, 10 μM forskolin were added basolaterally to stimulate CFTR-mediated chloride conductance at the apical membrane (Fig. 6). AICAR inhibited the forskolin-stimulated Isc by 27 ± 5.0% when applied to the apical side of the monolayers (n = 5, P < 0.05). Western blotting wase attempted on lysates of shark rectal gland cell primary culture monolayers (pore size: 0.4 um; membrane diameter: 6.5 mm), but the amount of protein was too small for reproducible Western blots.
Fig. 6.
Representative experiment of application of AICAR to the apical membrane (AP) of shark rectal gland primary cell cultures inhibits short-circuit current (Isc). This experiment was repeated 5 times. Transepithelial chloride transport was measured as Isc input across voltage clamped, confluent monolayers of shark rectal gland primary cell cultures. Inhibition of chloride secretion is reflected as a decrease in Isc. Mean inhibition after AP addition of 1 mM AICAR was 27 ± 5.0% (n = 5). BL, basolateral.
DISCUSSION
The new findings of the present study are 1) AMPK is present in the ancient dogfish shark rectal gland and contains a fairly homologous α-catalytic subunit to mammalian species, including the Thr172 critical for posttranslational activation of the complex; the sequence of this catalytic subunit forms the root of the phylogenetic tree of known species in which AMPK has been cloned; 2) phosphorylated AMPK is present during basal oxygenated conditions of in vitro perfused shark rectal glands; 3) combination forskolin and IBMX treatment to stimulate chloride transport under oxygen conditions elicits a significant increase in AMPK Thr172 phosphorylation; 4) perfusion under nitrogen conditions amplifies AMPK Thr172 phosphorylation to a level that is greater than that observed in glands perfused under oxygen conditions and chloride transport is more greatly inhibited; 5) glands perfused with metabolic inhibitors (FCCP and oligomycin) and stimulated with forskolin and IBMX show significantly decreased chloride secretion and greater levels of AMPK phosphorylation than controls under oxygen conditions; 6) AICAR, an activator of AMPK, when applied to the apical membrane of primary cultured rectal gland cells, reduces chloride secretion through CFTR as measured by short-circuit current.
This study demonstrates the presence of AMPK in the shark rectal gland and implicates pAMPK as a second regulator of hormone-stimulated chloride secretion. We present the first data in an intact epithelia that increases in the activated form of AMPK occur with increases in cellular work that accompanies ion transport after hormonal stimulation. We emphasize that we have established a direct link in oxygen-perfused glands between AMPK activation and forskolin+and IBMX stimulation of chloride secretion.
Additionally, AMPK phosphorylation was increased during different conditions (metabolic inhibitors and severe hypoxia) and was associated with a functional decline of chloride secretion. These results are consistent with a graded suppression of chloride transport in parallel with the degree of AMPK activation.
In shark rectal glands rendered severely hypoxic by N2 perfusion, additional hypoxia activated signaling pathways may be involved. Activation of hypoxia-inducible factor-1 has been shown to selectively downregulate Na-K-2Cl cotransporter (NKCC1) in chloride-secreting epithelia (16). AMPK has been shown in selected tissues to play a critical role in the regulation of hypoxia-inducible factor-1 as part of the adaptive response to hypoxia (19). Hallows (9) has shown that AMPK directly downregulates NKCC1 and Na-K-ATPase in different models. It is possible that these additional adaptive mechanisms to hypoxia also contribute to CFTR regulation in the primitive dogfish shark.
AMPK-independent mechanisms that limit chloride secretion during hormonal stimulation are well described. Our laboratory previously characterized the role of adenosine as an energy-sensing “retaliatory metabolite” in the shark rectal gland. Stimulation of chloride secretion by forskolin was shown to release endogenous adenosine, which then acted to diminish chloride secretion via interaction with the A1 adenosine receptor (17). The present study identifies that AMPK functions to regulate ion transport in the shark rectal gland. Thus, in this model tissue, there are two well-defined metabolically sensitive signals that act together as feedback inhibitors of cellular work in CFTR-mediated chloride transport.
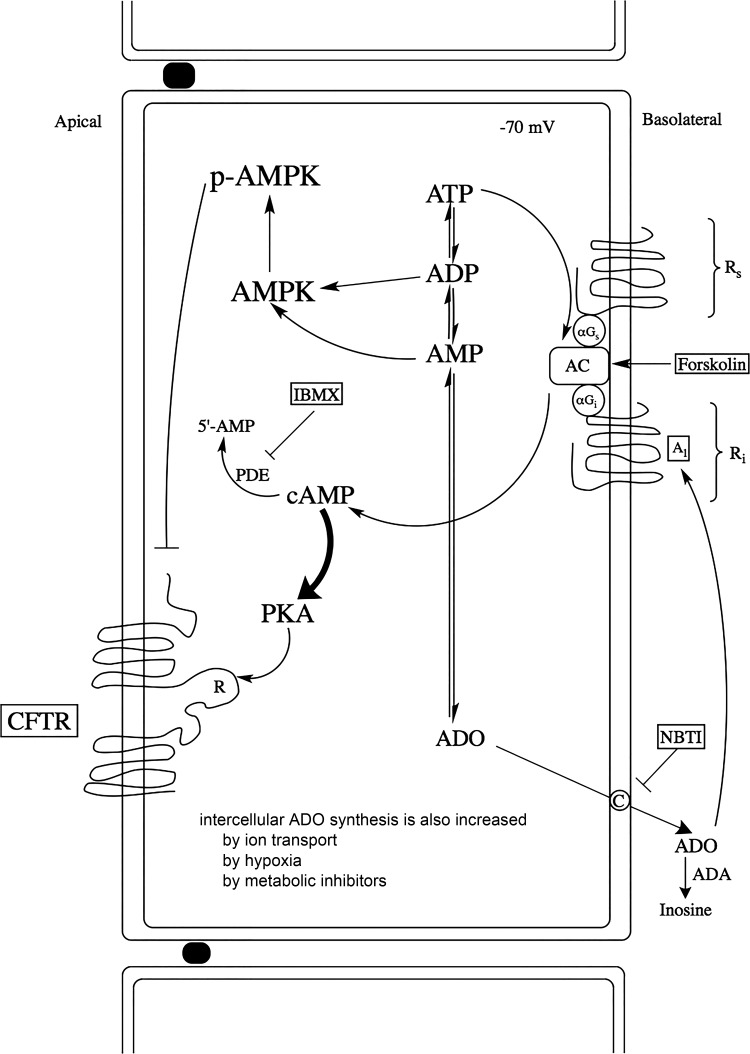
In Fig. 7, we present a new model of both AMPK and adenosine in ion-transporting epithelia. As hormones and secretagogues (CNP, VIP, forskolin, and IBMX) increase chloride transport, adenosine is released into the extracellular space through a nitrobenzylthioinosine (NBTI)-sensitive transporter. This extracellular adenosine then feeds back to inhibit chloride secretion via the A1 adenosine receptor, reducing the cAMP-PKA phosphorylation of the R domain of CFTR. At the same time, as ion transport and energy utilization increase and ATP, AMP, and ADP levels fall, AMPK is activated by rising AMP:ATP and ADP:ATP ratios. Severe hypoxia and metabolic inhibitors (electron transport chain poisons) do similar things. It has been shown recently that AMPK mediates mitochondrial fission through mitochondrial fission factor in response to energy stress (34).
Fig. 7.
Integrated model of metabolic feedback during chloride secretion: actions of adenosine (ADO) and AMPK in the shark rectal gland. When chloride secretion is increased by hormones and secretagogues (forskolin and IBMX, VIP, CNP), intracellular adenosine production increases. Adenosine then passes through a basolateral, nitrobenzylthioinosine (NBTI)-sensitive nucleoside transporter. Adenosine accumulation in the extracellular space acts on an A1 (inhibitory) adenosine receptor. In parallel, as AMP:ATP and ADP:ATP ratios increase during conditions of cellular stress, AMPK is phosphorylated at Thr172. In other ion-transporting tissues, hormones and secretogogues such as vasopressin may indirectly inhibit the activity of AMPK (22).
GRANTS
This work was supported by National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-34208 and National Institute of Environmental Health Sciences Grant 5-P30 ES-03828 (Center for Membrane Toxicology Studies) to J. N. Forrest and by National Science Foundation Grant DBI-0139190 (Research Experience for Undergraduates site at Mount Desert Island Biological Laboratory).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.I.N., J.A.v.K., D.J.P., L.H.Y., and J.N.F. conceived and designed research; R.I.N., J.A.v.K., D.J.P., D.M.M., and J.N.F. performed experiments; R.I.N., J.A.v.K., D.J.P., D.M.M., L.H.Y., and J.N.F. analyzed data; R.I.N., J.A.v.K., D.J.P., D.M.M., L.H.Y., and J.N.F. interpreted results of experiments; R.I.N., J.A.v.K., D.J.P., and J.N.F. prepared figures; R.I.N., J.A.v.K., D.J.P., D.M.M., L.H.Y., and J.N.F. drafted manuscript; R.I.N., J.A.v.K., D.J.P., D.M.M., L.H.Y., and J.N.F. edited and revised manuscript; R.I.N., J.A.v.K., D.J.P., L.H.Y., and J.N.F. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank and acknowledge the help of August Melita, Jonah Ury, Montana Morris, and Ryan Viola, who carried out certain shark rectal gland perfusions.
REFERENCES
- 1.Alesutan I, Voelkl J, Stöckigt F, Mia S, Feger M, Primessnig U, Sopjani M, Munoz C, Borst O, Gawaz M, Pieske B, Metzler B, Heinzel F, Schrickel JW, Lang F. AMP-activated protein kinase α1 regulates cardiac gap junction protein connexin 43 and electrical remodeling following pressure overload. Cell Physiol Biochem 35: 406–418, 2015. doi: 10.1159/000369706. [DOI] [PubMed] [Google Scholar]
- 2.Arch JR, Newsholme EA. The control of the metabolism and the hormonal role of adenosine. Essays Biochem 14: 82–123, 1978. [PubMed] [Google Scholar]
- 3.Ashabi G, Khodagholi F, Khalaj L, Goudarzvand M, Nasiri M. Activation of AMP-activated protein kinase by metformin protects against global cerebral ischemia in male rats: interference of AMPK/PGC-1α pathway. Metab Brain Dis 29: 47–58, 2014. doi: 10.1007/s11011-013-9475-2. [DOI] [PubMed] [Google Scholar]
- 4.Bewley MS, Pena JT, Plesch FN, Decker SE, Weber GJ, Forrest JN Jr. Shark rectal gland vasoactive intestinal peptide receptor: cloning, functional expression, and regulation of CFTR chloride channels. Am J Physiol Regul Integr Comp Physiol 291: R1157–R1164, 2006. doi: 10.1152/ajpregu.00078.2006. [DOI] [PubMed] [Google Scholar]
- 5.Collins FS. Cystic fibrosis: molecular biology and therapeutic implications. Science 256: 774–779, 1992. doi: 10.1126/science.1375392. [DOI] [PubMed] [Google Scholar]
- 6.Forbush B 3rd, Lytle C, Xu JC, Payne JA, Biemesderfer D. The Na, K, C cotransporter of shark rectal gland. Ren Physiol Biochem 17: 201–204, 1994. [DOI] [PubMed] [Google Scholar]
- 7.Forrest JN., Jr Cellular and molecular biology of chloride secretion in the shark rectal gland: regulation by adenosine receptors. Kidney Int 49: 1557–1562, 1996. doi: 10.1038/ki.1996.224. [DOI] [PubMed] [Google Scholar]
- 8.Fredholm BB, Jonzon B, Lindgren E, Lindström K. Adenosine receptors mediating cyclic AMP production in the rat hippocampus. J Neurochem 39: 165–175, 1982. doi: 10.1111/j.1471-4159.1982.tb04715.x. [DOI] [PubMed] [Google Scholar]
- 9.Hallows KR. Emerging role of AMP-activated protein kinase in coupling membrane transport to cellular metabolism. Curr Opin Nephrol Hypertens 14: 464–471, 2005. doi: 10.1097/01.mnh.0000174145.14798.64. [DOI] [PubMed] [Google Scholar]
- 10.Hallows KR, Kobinger GP, Wilson JM, Witters LA, Foskett JK. Physiological modulation of CFTR activity by AMP-activated protein kinase in polarized T84 cells. Am J Physiol Cell Physiol 284: C1297–C1308, 2003. doi: 10.1152/ajpcell.00227.2002. [DOI] [PubMed] [Google Scholar]
- 11.Hallows KR, McCane JE, Kemp BE, Witters LA, Foskett JK. Regulation of channel gating by AMP-activated protein kinase modulates cystic fibrosis transmembrane conductance regulator activity in lung submucosal cells. J Biol Chem 278: 998–1004, 2003. doi: 10.1074/jbc.M210621200. [DOI] [PubMed] [Google Scholar]
- 12.Hallows KR, Mount PF, Pastor-Soler NM, Power DA. Role of the energy sensor AMP-activated protein kinase in renal physiology and disease. Am J Physiol Renal Physiol 298: F1067–F1077, 2010. doi: 10.1152/ajprenal.00005.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hallows KR, Raghuram V, Kemp BE, Witters LA, Foskett JK. Inhibition of cystic fibrosis transmembrane conductance regulator by novel interaction with the metabolic sensor AMP-activated protein kinase. J Clin Invest 105: 1711–1721, 2000. doi: 10.1172/JCI9622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hardie DG. Targeting an energy sensor to treat diabetes. Science 357: 455–456, 2017. doi: 10.1126/science.aao1913. [DOI] [PubMed] [Google Scholar]
- 15.Honig CR, Frierson JL. Role of adenosine in exercise vasodilation in dog gracilis muscle. Am J Physiol Heart Circ Physiol 238: H703–H715, 1980. [DOI] [PubMed] [Google Scholar]
- 16.Ibla JC, Khoury J, Kong T, Robinson A, Colgan SP. Transcriptional repression of Na-K-2Cl cotransporter NKCC1 by hypoxia-inducible factor-1. Am J Physiol Cell Physiol 291: C282–C289, 2006. doi: 10.1152/ajpcell.00564.2005. [DOI] [PubMed] [Google Scholar]
- 17.Kelley GG, Aassar OS, Forrest JN Jr. Endogenous adenosine is an autacoid feedback inhibitor of chloride transport in the shark rectal gland. J Clin Invest 88: 1933–1939, 1991. doi: 10.1172/JCI115517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.King JD Jr, Fitch AC, Lee JK, McCane JE, Mak DO, Foskett JK, Hallows KR. AMP-activated protein kinase phosphorylation of the R domain inhibits PKA stimulation of CFTR. Am J Physiol Cell Physiol 297: C94–C101, 2009. doi: 10.1152/ajpcell.00677.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee M, Hwang JT, Lee HJ, Jung SN, Kang I, Chi SG, Kim SS, Ha J. AMP-activated protein kinase activity is critical for hypoxia-inducible factor-1 transcriptional activity and its target gene expression under hypoxic conditions in DU145 cells. J Biol Chem 278: 39653–39661, 2003. doi: 10.1074/jbc.M306104200. [DOI] [PubMed] [Google Scholar]
- 20.Mandel LJ, Balaban RS. Stoichiometry and coupling of active transport to oxidative metabolism in epithelial tissues. Am J Physiol Renal Physiol 240: F357–F371, 1981. [DOI] [PubMed] [Google Scholar]
- 21.Noël S, Wilke M, Bot AG, De Jonge HR, Becq F. Parallel improvement of sodium and chloride transport defects by miglustat (n-butyldeoxynojyrimicin) in cystic fibrosis epithelial cells. J Pharmacol Exp Ther 325: 1016–1023, 2008. doi: 10.1124/jpet.107.135582. [DOI] [PubMed] [Google Scholar]
- 22.Nofziger C, Kalsi K, West TA, Baines D, Blazer-Yost BL. Vasopressin regulates the phosphorylation state of AMP-activated protein kinase (AMPK) in MDCK-C7 cells. Cell Physiol Biochem 22: 487–496, 2008. doi: 10.1159/000185505. [DOI] [PubMed] [Google Scholar]
- 23.Olsson RA. Changes in content of purine nucleoside in canine myocardium during coronary occlusion. Circ Res 26: 301–306, 1970. doi: 10.1161/01.RES.26.3.301. [DOI] [PubMed] [Google Scholar]
- 24.Olsson RA, Pearson JD. Cardiovascular purinoceptors. Physiol Rev 70: 761–845, 1990. doi: 10.1152/physrev.1990.70.3.761. [DOI] [PubMed] [Google Scholar]
- 25.Osswald H, Hermes HH, Nabakowski G. Role of adenosine in signal transmission of tubuloglomerular feedback. Kidney Int Suppl 12: S136–S142, 1982. [PubMed] [Google Scholar]
- 26.Sheppard DN, Welsh MJ. Structure and function of the CFTR chloride channel. Physiol Rev 79, Suppl: S23–S45, 1999. doi: 10.1152/physrev.1999.79.1.S23. [DOI] [PubMed] [Google Scholar]
- 27.Siwiak M, Edelman A, Zielenkiewicz P. Structural models of CFTR-AMPK and CFTR-PKA interactions: R-domain flexibility is a key factor in CFTR regulation. J Mol Model 18: 83–90, 2012. doi: 10.1007/s00894-011-1029-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spielman WS, Arend LJ. Adenosine receptors and signaling in the kidney. Hypertension 17: 117–130, 1991. doi: 10.1161/01.HYP.17.2.117. [DOI] [PubMed] [Google Scholar]
- 29.Spielman WS, Thompson CI. A proposed role for adenosine in the regulation of renal hemodynamics and renin release. Am J Physiol Renal Physiol 242: F423–F435, 1982. [DOI] [PubMed] [Google Scholar]
- 30.Stahl M, Stahl K, Brubacher MB, Forrest JN Jr. Divergent CFTR orthologs respond differently to the channel inhibitors CFTRinh-172, glibenclamide, and GlyH-101. Am J Physiol Cell Physiol 302: C67–C76, 2012. doi: 10.1152/ajpcell.00225.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takiar V, Nishio S, Seo-Mayer P, King JD Jr, Li H, Zhang L, Karihaloo A, Hallows KR, Somlo S, Caplan MJ. Activating AMP-activated protein kinase (AMPK) slows renal cystogenesis. Proc Natl Acad Sci USA 108: 2462–2467, 2011. doi: 10.1073/pnas.1011498108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Telles CJ, Decker SE, Motley WW, Peters AW, Mehr AP, Frizzell RA, Forrest JN Jr. Functional and molecular identification of a TASK-1 potassium channel regulating chloride secretion through CFTR channels in the shark rectal gland: implications for cystic fibrosis. Am J Physiol Cell Physiol 311: C884–C894, 2016. doi: 10.1152/ajpcell.00030.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thiagarajah JR, Verkman AS. CFTR inhibitors for treating diarrheal disease. Clin Pharmacol Ther 92: 287–290, 2012. doi: 10.1038/clpt.2012.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toyama EQ, Herzig S, Courchet J, Lewis TL Jr, Losón OC, Hellberg K, Young NP, Chen H, Polleux F, Chan DC, Shaw RJ. Metabolism. AMP-activated protein kinase mediates mitochondrial fission in response to energy stress. Science 351: 275–281, 2016. doi: 10.1126/science.aab4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vaandrager AB, Tilly BC, Smolenski A, Schneider-Rasp S, Bot AG, Edixhoven M, Scholte BJ, Jarchau T, Walter U, Lohmann SM, Poller WC, de Jonge HR. cGMP stimulation of cystic fibrosis transmembrane conductance regulator Cl− channels co-expressed with cGMP-dependent protein kinase type II but not type Ibeta. J Biol Chem 272: 4195–4200, 1997. doi: 10.1074/jbc.272.7.4195. [DOI] [PubMed] [Google Scholar]
- 36.Valentich JD, Forrest JN Jr. Cl− secretion by cultured shark rectal gland cells. I. Transepithelial transport. Am J Physiol 260: C813–C823, 1991. doi: 10.1152/ajpcell.1991.260.4.C813. [DOI] [PubMed] [Google Scholar]
- 37.Venkatesh B, Kirkness EF, Loh YH, Halpern AL, Lee AP, Johnson J, Dandona N, Viswanathan LD, Tay A, Venter JC, Strausberg RL, Brenner S. Survey sequencing and comparative analysis of the elephant shark (Callorhinchus milii) genome. PLoS Biol 5: e101, 2007. doi: 10.1371/journal.pbio.0050101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiao B, Sanders MJ, Underwood E, Heath R, Mayer FV, Carmena D, Jing C, Walker PA, Eccleston JF, Haire LF, Saiu P, Howell SA, Aasland R, Martin SR, Carling D, Gamblin SJ. Structure of mammalian AMPK and its regulation by ADP. Nature 472: 230–233, 2011. doi: 10.1038/nature09932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zaha VG, Young LH. AMP-activated protein kinase regulation and biological actions in the heart. Circ Res 111: 800–814, 2012. doi: 10.1161/CIRCRESAHA.111.255505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng L, Kelly CJ, Colgan SP. Physiologic hypoxia and oxygen homeostasis in the healthy intestine. A Review in the Theme: Cellular Responses to Hypoxia. Am J Physiol Cell Physiol 309: C350–C360, 2015. doi: 10.1152/ajpcell.00191.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]