Abstract
Platelet-derived growth factor receptor (PDGFR) signaling plays an important role in the fundamental biological activities of many cells that compose musculoskeletal tissues. However, little is known about the role of PDGFR signaling during tendon growth and remodeling in adult animals. Using the hindlimb synergist ablation model of tendon growth, our objectives were to determine the role of PDGFR signaling in the adaptation of tendons subjected to a mechanical growth stimulus, as well as to investigate the biological mechanisms behind this response. We demonstrate that both PDGFRs, PDGFRα and PDGFRβ, are expressed in tendon fibroblasts and that the inhibition of PDGFR signaling suppresses the normal growth of tendon tissue in response to mechanical growth cues due to defects in fibroblast proliferation and migration. We also identify membrane type-1 matrix metalloproteinase (MT1-MMP) as an essential proteinase for the migration of tendon fibroblasts through their extracellular matrix. Furthermore, we report that MT1-MMP translation is regulated by phosphoinositide 3-kinase/Akt signaling, while ERK1/2 controls posttranslational trafficking of MT1-MMP to the plasma membrane of tendon fibroblasts. Taken together, these findings demonstrate that PDGFR signaling is necessary for postnatal tendon growth and remodeling and that MT1-MMP is a critical mediator of tendon fibroblast migration and a potential target for the treatment of tendon injuries and diseases.
Keywords: extracellular matrix, fibroblast, matrix metalloproteinase, platelet-derived growth factor receptor, tenocyte
INTRODUCTION
Tendon is an integral component of the musculoskeletal system. Anatomically situated between skeletal muscle and bone, tendon transmits and stores force which allows for efficient locomotion. The function of tendon is determined by the biochemical composition and macromolecular structural organization of its extracellular matrix (ECM), which consists of a dense network of cross-linked type I collagen and smaller amounts of type III collagen, elastin, and various proteoglycans (31). This collagen-rich tissue provides structural support to the tendon, selectively binds and releases growth factors that regulate multiple cellular functions, and organizes the compartments that contain various cell populations (10). Tendon fibroblasts are the predominant cell type in tendon and are responsible for the production, maintenance, modification, and repair of matrix proteins (31). Despite the importance of tendon to the overall function of the musculoskeletal system, relatively little is known about the cellular and molecular mechanisms that regulate tendon growth and remodeling in adult animals.
Tendon adapts to increased mechanical signals by undergoing hypertrophy, as evidenced by increases in tendon cross-sectional area (CSA), cell density, peak stress, peak strain, and type I collagen content in response to loading (7, 15, 38). While the specific growth factors and signaling pathways required for this process are largely unknown, one family of growth factors that are upregulated following tendon injury and are active at multiple stages of the healing process are the platelet-derived growth factors (PDGFs) (39). PDGFs typically function as secreted hetero- or homodimers of disulfide-linked polypeptide chains (PDGF-AA, PDGF-BB, PDGF-CC, PDGF-DD and PDGF-AB) (1), with PDGF-BB among the most frequent PDGF isoforms used to enhance regeneration in various animal models of tendon injury (33, 61). PDGFs bind to and activate a class of structurally related receptor tyrosine kinase transmembrane proteins known as PDGF receptors-α (PDGFRα) and -β (PDGFRβ) (1). Upon binding to their ligands, PDGFRs dimerize and undergo autophosphorylation of conserved cytoplasmic tyrosine residues that initiate multiple signal transduction cascades, including the phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt), and extracellular signal-related kinases 1 and 2 (ERK1/2) pathways (1, 56). PDGFs are potent mitogens, and their signaling events control key biological functions in many cell types of mesenchymal origin, including proliferation, differentiation, migration, and ECM synthesis and remodeling (54). While PDGFs are expressed in tendon tissue and are active at multiple stages of the healing process in injured tendons (39), the identity and tissue localization of PDGFRα+ and PDGFRβ+ cells in tendon have not been clearly defined, and the overall importance of PDGFR signaling to the growth response of mechanically loaded tendons is presently unknown.
During periods of tendon growth and remodeling, fibroblasts express matrix metalloproteinases (MMPs), a family of zinc-dependent endopeptidases that collectively degrade multiple ECM components, including type I collagen (10, 52). Membrane-type MMPs (MT-MMPs) are a subclass of MMPs that allow cells to migrate through their respective ECMs, by constraining type I collagen degradation to the leading edge of the cell membrane (22, 49). Within the MT-MMP subgroup, membrane type-1 MMP (MT1-MMP) is required for proper tendon development, as the deletion of MT1-MMP in embryonic tendon fibroblasts results in the failure of normal tendon formation (59). MT1-MMP expression has also been shown to correlate with pivotal events in the growth response of mechanically loaded tendons of adult animals (52). In other cell types, MT1-MMP expression is regulated by receptor tyrosine kinase signaling (20, 56), but the signal transduction pathways that regulate MT1-MMP expression in tendon fibroblasts are not known.
Given that PDGFs are expressed in tendon, and that MT1-MMP is important for tendon development, we sought to determine the role of PDGFR signaling in postnatal tendon growth and remodeling. We hypothesized that inhibition of PDGFR signaling would suppress the normal growth of tendon tissue during mechanical overload due to defects in cell proliferation and migration. To test this hypothesis, we induced tendon growth in adult mice via mechanical overload using the hindlimb synergist ablation model which has been previously used to study muscle and tendon growth, and blocked PDGFRα and PDGFRβ signaling through the use of a highly specific pharmacological inhibitor, CP-673,451. We also conducted a series of in vitro experiments to determine the molecular mechanisms that underlie the PDGFR-dependent growth of tendons in adult animals.
METHODS
Mice.
All animal studies were approved by the University of Michigan Institutional Animal Care and Use Committee. Wild-type (WT) C57BL/6 mice and transgenic PDGFRαeGFP/+ mice were obtained from the Jackson Laboratory (Bar Harbor, ME). PDGFRαeGFP/+ mice express the nuclear-localized H2B-eGFP reporter gene from the endogenous PDGFRa locus (18). Four-month-old male mice were used in all experiments. Sample sizes for each study are provided in the figure legends.
Synergist ablation.
Mice were randomized to 3- and 10-day groups. Bilateral synergist ablation procedures were performed under isoflurane anesthesia as described previously (15, 54). An overview is presented in Fig. 1A. The Achilles tendon was surgically excised to prevent the gastrocnemius and soleus muscles from plantarflexing the talocrural joint, resulting in compensatory hypertrophy of the synergist plantaris muscle-tendon unit. Buprenorphine was administered for postoperative analgesia, and ad libitum weight-bearing and cage activity were allowed in the postoperative period. Mice were closely monitored during the postoperative period for any adverse reactions. At tissue harvest, the left plantaris tendons were collected for gene expression analysis, while the right plantaris tendons were used for histological examination. After the tendons were removed, mice were euthanized by cervical dislocation and induction of bilateral pneumothorax. Plantaris tendons from additional nonoverloaded mice were obtained as described above for gene expression analysis.
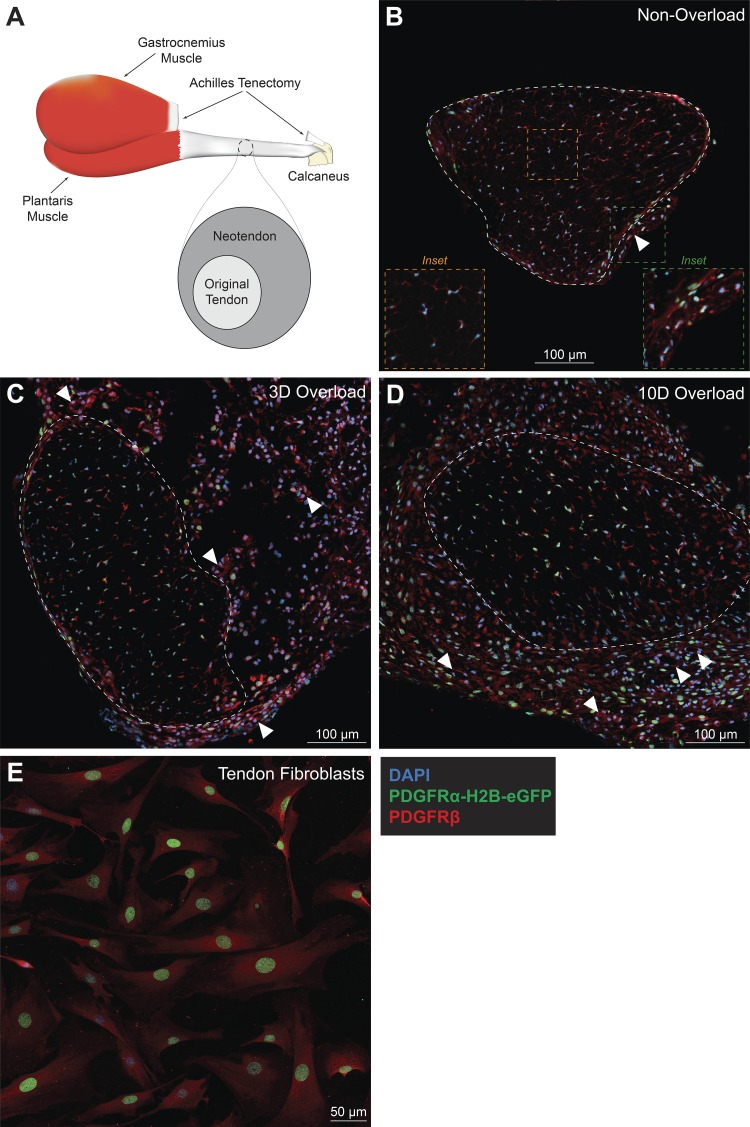
Fig. 1.
Tendon fibroblasts express both PDGFRα and PDGFRβ. A: schematic of the synergist ablation procedure. All histological sections were taken from the midportion of the plantaris tendon. B–D: representative images of nonoverloaded (B), 3-day (3D) overloaded (C), and 10-day (10D) overloaded (D) plantaris tendons. Dashed white line indicates the boundary between the original tendon and neotendon. A dashed yellow line is a high-magnification inset of the core of the tendon, and a dashed green line is a high magnification inset of the neurovascular bundle region (B). White arrowheads indicate blood vessels composed of PDGFRα-/PDGFRβ+ cells. Scale bars are 100 μm. E: tail tendon fibroblasts were isolated from PDGFRαeGFP/+ mice and immunostained for PDGFRβ. Scale bar is 50 μm. DAPI, blue; PDGFRα-H2B-eGFP, green; PDGFRβ, red.
Inhibition of PDGFR signaling.
Within each group of mice, half of the mice were treated with vehicle and half with CP-673,451 (Biorbyt, Cambridge, UK), a specific inhibitor of PDGFRα and PDGFRβ (11, 47). CP-673,451 was dissolved first in 2 parts dimethyl sulfoxide and then in 8 parts phosphate-buffered saline. CP-673,451 was administered by intraperitoneal injection at 15 mg/kg twice daily, with a total daily dose of 30 mg/kg, starting the day before synergist ablation and continued each day until tissue harvest. This dose has previously been shown to be effective at inhibiting PDGFR phosphorylation in mice in vivo (21, 28, 47, 54). An equal volume of DMSO and PBS was administered to the vehicle control groups.
Histology.
Histological examination of the tendon tissue was performed as described previously (52, 54). Plantaris tendons were placed in 30% sucrose solution for one hour, snap frozen in Tissue-Tek OCT Compound (Sakura Finetek, Torrance, CA) and stored at −80°C until use. Tendons were sectioned at a thickness of 10 µm in a cryostat and stained with hematoxylin and eosin to determine tendon CSA and cell density. For immunohistochemistry, tendon sections were fixed in 4% paraformaldehyde, permeabilized with 0.2% Triton X-100, and blocked with 5% goat serum. To identify cell types in nonoverloaded and overloaded tendons, slides from PDGFRαEGFP/+ mice were incubated with rabbit anti-PDGFRβ (1:100; sc-339, Santa Cruz Biotechnology, Santa Cruz, CA) primary antibodies. Proliferating cells were identified in overloaded tendons treated with vehicle or PDGFR inhibitor, by incubating slides from WT mice subjected to synergist ablation with rabbit anti-Ki67 (1:100; ab16667, Abcam, Cambridge, MA) primary antibodies. Primary rabbit antibodies against p-PDGFRαY849/PDGFRβY857 (1:100; no. 3170, Cell Signaling Technology, Danvers, MA) were also used on slides from C57BL/6 mice to determine differences in the level of PDGFR phosphorylation in response to vehicle or PDGFR inhibitor treatment. The ECM was identified with wheat germ agglutinin (WGA) lectin conjugated to Alexa Fluor 488 (AF488) (1:200; W11261, Thermo Fisher Scientific, Carlsbad, CA). Secondary antibodies conjugated to AF555 (1:300; A-21429, Thermo Fisher Scientific) were used to detect primary antibodies. Nuclei were stained with DAPI (1:500; D9542, Sigma Aldrich, St. Louis, MO). High-resolution digital images were captured with an Olympus BX-51 microscope and camera (Olympus, Center Valley, PA) for the hematoxylin and eosin and Ki67 slides, while a Nikon A1 confocal laser microscope (Nikon Instruments, Tokyo, Japan) was used for the PDGFR slides. Quantification was performed in a blinded fashion using ImageJ software (NIH, Bethesda, MD).
Cell culture.
Fibroblasts were isolated from the tail tendons of mice as described previously (24). Tail tendons are useful for obtaining a large number of early passage cells, they arise from the same population of somitic progenitor cells as limb tendons during development (40), and the behavior of fibroblasts from the non-synovial tail and hindlimb tendons is anticipated to be similar. Briefly, mice were anesthetized with isoflurane as described above, the tail was removed, and animals were euthanized by cervical dislocation. Fascicles were isolated from tail tendons, and then were finely minced with scissors and placed in low-glucose Dulbecco’s modified Eagle’s medium (DMEM; GIBCO, Carlsbad, CA) containing 0.2% type II collagenase (GIBCO) for 1 h at 37°C with constant agitation. An equal volume of growth medium (GM) that consists of low-glucose DMEM with 10% fetal bovine serum (FBS; GIBCO) and 1% antibiotic-antimycotic (GIBCO) was added to the digested tissue to inactivate the collagenase. Cells were pelleted by centrifugation at 300 g for 10 min, resuspended in GM, and plated on 100-mm type I collagen-coated dishes (Corning, Corning, NY). All cells were maintained in humidified incubators at 37°C and 5% CO2. Passage 2–3 cells were used in all experiments. To block PDGFR signaling, cells were incubated with 1 μM CP-673,451, prepared as described above. This dose has previously been shown to selectively block PDGFR activation in vitro (25, 47, 64, 65).
Migration assay.
Type I collagen was acid-extracted from cadaver rat tail tendons as described (22). Briefly, tendons were excised and placed in 0.2% acetic acid for 5 days at 4°C. The collagen solution was then centrifuged at 24,000 g for 30 min, and the supernatant was collected, lyophilized, and dissolved again in 0.2% acetic acid to a final concentration of 2.7 mg/ml. Collagen gels were prepared in the upper chambers of Transwell dishes (12-mm diameter, 3-μm pore size; Corning) by combining the collagen solution with 10× minimum essential medium (MEM, Thermo Fisher Scientific) and 0.34 N NaOH in an 8:1:1 ratio. After 45 min at 37°C, the gelling process was complete and 1 × 105 tendon fibroblasts were seeded on top of the collagen gels. Growth factors, either PDGF-BB (20 ng/ml; R&D Systems, Minneapolis, MN) or transforming growth factor-β1 (TGF-β1; 10 ng/ml; PeproTech, Rocky Hill, NJ), were added to the lower chambers. Where indicated, the medium was supplemented with the synthetic broad spectrum MMP inhibitor BB-94 (5 μM; Tocris Bioscience, Bristol, UK), the mitogen-activated protein kinase kinases 1 and 2 (MEK1/2) inhibitor PD98059 (50 μM; InvivoGen, San Diego, CA), or the PI3K inhibitor wortmannin (10 μM; InvivoGen). All media, including growth factors and inhibitors, were replaced every 2 days. Migratory activity was monitored by phase-contrast microscopy, and the cells were allowed to migrate for 6 days. At the completion of the experiment, gels were fixed with 10% neutral buffered formalin, embedded in paraffin, sectioned at a thickness of 10 µm, and stained with hematoxylin and eosin. The number of migrating cells and the maximum distance migrated were quantified in five randomly selected fields of a single experiment from three or more independent experiments performed.
Proliferation assay.
Uptake of bromodeoxyuridine (BrdU) by proliferating tendon fibroblasts was measured as described previously (37). Tendon fibroblasts were incubated overnight with or without 20 ng/ml of PDGF-BB in media containing 0.5% FBS. After overnight incubation, fresh medium was added along with 20 μM BrdU (Sigma Aldrich) for 1 h. Cells were then rinsed with phosphate-buffered saline, fixed in ice-cold methanol, and permeabilized with 0.5% Triton X-100. The BrdU epitope was exposed by denaturing DNA with 2 M HCl. BrdU was visualized with an anti-BrdU antibody (1:50; G3G4, Developmental Studies Hybridoma Bank, Iowa City, IA) and a secondary antibody was conjugated to AF555 (1:200; A-21127, Thermo Fisher Scientific). Nuclei were counterstained with DAPI (1:500; D9542, Sigma Aldrich) to determine total cell number. The number of proliferating cells were quantified in five randomly selected fields of a single experiment from six independent experiments performed.
Quantitative RT-PCR.
Gene expression analysis was performed as described previously (24, 55). Plantaris tendons were homogenized in QIAzol (Qiagen, Valencia, CA) and RNA was purified using a miRNeasy Micro Kit (Qiagen) supplemented with DNase I (Qiagen). RNA was reverse transcribed into cDNA with iScript Reverse Transcription Supermix (Bio-Rad, Hercules, CA). Amplification of cDNA was performed in a CFX96 real-time thermal cycler (Bio-Rad) using iTaq Universal SYBR Green Supermix (Bio-Rad). Target gene expression was normalized to the stable housekeeping gene peptidylprolyl isomerase D (PPID), and further normalized to tendons that were not subjected to synergist ablation using the 2−ΔΔCt method. PPID was selected as a housekeeping gene from microarray data and validated with qPCR. For cell culture experiments, relative transcript copy number was calculated using the linear regression of efficiency method (50). Primer sequences are provided in Supplemental Material Table S1 (Supplemental Material is available online at the Journal website).
Microarray.
Microarray measurements were performed by the University of Michigan DNA Sequencing Core as described previously (24, 54). Equal amounts of RNA isolated from four individual tendons were pooled into a single sample for microarray analysis, and two pooled samples from each group were analyzed. RNA was pooled because gene expression from a pooled sample is similar to the average of the individual samples composing the pooled sample (6, 29). Biotinylated cDNA was prepared using the GeneChip WT PLUS Reagent Kit (Affymetrix, Santa Clara, CA) and hybridized to Mouse Gene 2.1 ST Array Strips (Affymetrix). Raw microarray data were loaded into ArrayStar (version 12.1, DNASTAR, Madison, WI) to calculate fold changes in gene expression. The microarray data set has been deposited to the NIH Gene Expression Omnibus database (accession no. GSE95794).
siRNA transfection.
Predesigned fluorescent-labeled siRNAs directed against mouse MT1-MMP (NM_008608; SI02733822, Qiagen) were transfected into tendon fibroblasts using Lipofectamine RNAiMAX (Thermo Fisher Scientific) at a final concentration of 10 nM. AllStars Negative Control siRNA (Qiagen) was used as a negative control.
Immunoblots.
Immunoblotting was performed as described (24, 54). Whole tendons and cell pellets were homogenized in ice-cold RIPA buffer (Sigma Aldrich) supplemented with 1% protease and phosphatase inhibitor cocktail (Thermo Fisher Scientific). Protein homogenates were diluted in Laemmli sample buffer, boiled for 2 min and 20 μg of protein was separated on either 6% or 12% SDS-PAGE gels depending on the protein of interest. Proteins were transferred to 0.45-µm nitrocellulose membranes (Bio-Rad) using the Trans-Blot SD semidry transfer apparatus (Bio-Rad), blocked with 5% nonfat powdered milk in TBST solution, and incubated with primary rabbit antibodies (1:1,000; Cell Signaling Technology) against p-PDGFRαY849/PDGFRβY857 (no. 3170), PDGFRα (no. 3174), PDGFRβ (no. 3169), p-ERK1/2T202/Y204 (no. 4370), ERK1/2 (no. 4695), p-AktT308 (no. 13038), Akt (no. 4691), p-p70S6KT389 (no. 9234) and p70S6K (no. 2708), primary rabbit antibodies (1:1,000; Abcam) against MT1-MMP (ab51074) or primary rabbit antibodies (1:1,000; Santa Cruz Biotechnology) against procollagen type I (Pro-Col1a1; sc-30136). β-Tubulin (ab6046, Abcam) or Coomassie staining was used to determine equal protein loading. Following primary antibody incubation, membranes were rinsed and incubated with horseradish peroxidase-conjugated goat anti-rabbit secondary antibodies (1:10,000; ab97051, Abcam). Proteins were detected using enhanced chemiluminescent reagents (Bio-Rad) and visualized using a digital chemiluminescent documentation system (Bio-Rad), and expressed as arbitrary densitometry units.
Cell surface biotinylation.
Cell surface proteins from tendon fibroblasts were biotinylated and purified using the Cell Surface Protein Isolation Kit (Thermo Fisher Scientific). Briefly, cells in monolayer were washed with ice-cold phosphate-buffered saline and incubated with 0.25 mg/ml of Sulfo-NHS-SS-Biotin for 30 min at 4°C with constant agitation. Adherent cells were then lysed and the biotinylated proteins were affinity-purified using streptavidin agarose beads. Coomassie staining was used to determine equal protein loading for immunoblots. Biotinylated proteins were analyzed by immunoblotting as described above with anti-MT1-MMP (ab51074, Abcam) antibodies, while GAPDH (MA5-15738, Thermo Fisher Scientific) served as the control for cytosolic proteins.
Statistics.
Results are presented as means ± SD. Prism version 7.0 (GraphPad Software, La Jolla, CA) was used to conduct statistical analyses. A two-way ANOVA (α = 0.05) followed by Tukey’s post hoc sorting evaluated the interaction between time after synergist ablation and PDGFR inhibitor treatment. For cell culture experiments, differences between groups were tested with an unpaired Student’s t-test or a one-way ANOVA followed by Tukey’s post hoc sorting where indicated with α = 0.05.
RESULTS AND DISCUSSION
PDGFRα and PDGFRβ are expressed in tendon fibroblasts.
We first identified which cells express the PDGFRs in tendon tissue (Fig. 1, A–D). Tendon fibroblasts, which are the major cell population in tendon, express either PDGFRα or PDGFRβ, and in some cases both receptors (Fig. 1B). We then performed mechanical overload of the plantaris muscle-tendon unit by removal of the tendon that transmits the load from the synergist gastrocnemius and soleus muscles. This techniques has been used to study muscle hypertrophy in numerous studies (12, 17, 36, 54), and we and others have also previously used this to study tendon hypertrophy (15, 43, 52). In this model, the neotendon tissue that forms around the existing tendon is populated by proliferating tendon fibroblasts, which mature and generate a mature ECM that resembles the original tendon matrix (15, 43, 52). After mechanical overload, we also observed a substantial number of PDGFRα+/PDGFRβ+ cells in the neotendon tissue (Fig. 1, C and D). In vitro, tendon fibroblasts express both PDGFR subtypes (PDGFRα+/PDGFRβ+) (Fig. 1E). That most of the fibroblasts in tendon express PDGFRα and PDGFRβ, with some cells demonstrating expression of both receptors, differs from what is observed in the extracellular matrix of skeletal muscle where populations of PDGFR-expressing cells can be separated into two distinct groups. PDGFRα is expressed by fibroadipogenic precursor cells or fibroblasts in the skeletal muscle interstitium, and PDGFRβ is expressed by perivascular cells or pericytes that often behave as pluripotent stem cells, with almost no cells demonstrating expression of both PDGFR isoforms (27, 54, 62). While tendon has PDGFRα−/PDGFRβ+-expressing cells around blood vessels in the neurovascular bundle that are likely pericytes, even though the ECM of skeletal muscle and tendon are intimately linked, the finding that most tendon fibroblasts express PDGFRα and PDGFRβ adds further support to observations from the developmental biology literature that tendon fibroblasts are a distinct cell type from muscle fibroblasts (53).
PDGFR inhibition prevents growth of tendon tissue after mechanical overload.
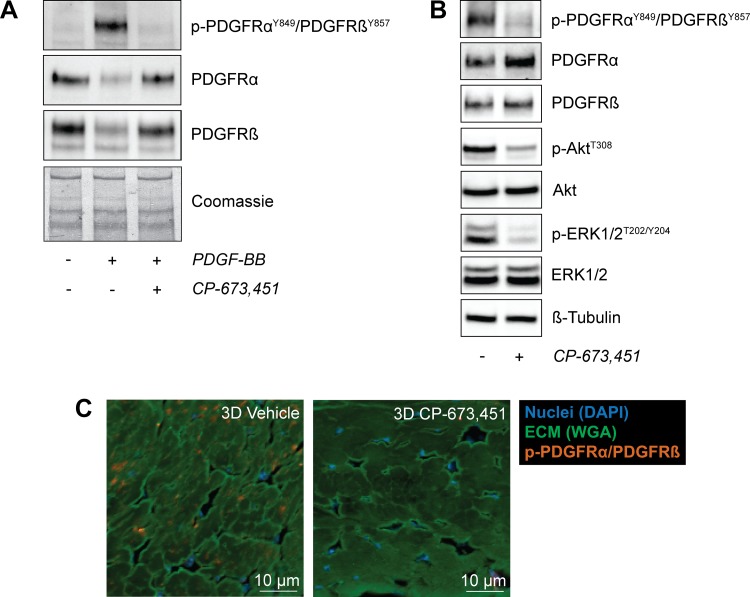
We next determined whether inhibition of PDGFR signaling would impact the growth of tendons subjected to mechanical overload. To accomplish this, we used the small molecule CP-673,451 to block phosphorylation of both PDGFR subtypes in cultured tendon fibroblasts and in whole tendon tissue. CP-673,451 inhibits both PDGFRα and PDGFRβ kinases with greater than 450-fold selectivity compared with other structurally related receptor tyrosine kinases, including vascular endothelial growth factor receptor (VEGFR), fibroblast growth factor receptor (FGFR), epidermal growth factor receptor (EGFR), and insulin-like growth factor I receptor (IGFIR) (47). Treatment of tendon fibroblasts with PDGF-BB resulted in phosphorylation of PDGFRα and PDGFRβ, and the addition of CP-673,451 blocked PDGFR phosphorylation (Fig. 2A). In whole tendon tissue, mice treated with CP-673,451 demonstrated reduced levels of p-PDGFRαY849/PDGFRβY857 after mechanical overload compared with vehicle-treated controls (Fig. 2B). We also evaluated whether kinases known to be downstream of PDGFR activation were impacted by CP-673,451 (42, 54), and we observed significant attenuation of p-AktT308 and p-ERK1/2T202/Y204 compared with controls (Fig. 2B). These findings were also supported with histology, which demonstrated that CP-673,451 reduced PDGFR phosphorylation in vivo (Fig. 2C).
Fig. 2.
CP-673,451 inhibits the phosphorylation of PDGFRα and PDGFRβ in tendon fibroblasts in vitro and in vivo. A: representative immunoblots of serum-starved tail tendon fibroblasts treated with 20 ng/ml of PDGF-BB for 30 min with or without 1 μM of the PDGFR inhibitor CP-673,451. A Coomassie stained membrane is shown as a loading control. B: representative immunoblots of 3-day overloaded plantaris tendons demonstrating the ability of CP-673,451 to inhibit phosphorylation of PDGFRα and PDGFRβ in vivo. Phosphorylation of Akt and ERK1/2 was also inhibited by PDGFR inhibitor treatment. β-Tubulin is shown as a loading control. C: immunohistochemistry of 3-day overloaded plantaris tendons treated with vehicle or PDGFR inhibitor showing a decrease in the abundance of p-PDGFRα/PDGFRβ-expressing cells in the overloaded plantaris tendons treated with CP-673,451 relative to vehicle-treated controls. Scale bars are 10 μm. DAPI, blue; WGA, green; p-PDGFRαY849/PDGFRβY857, red.
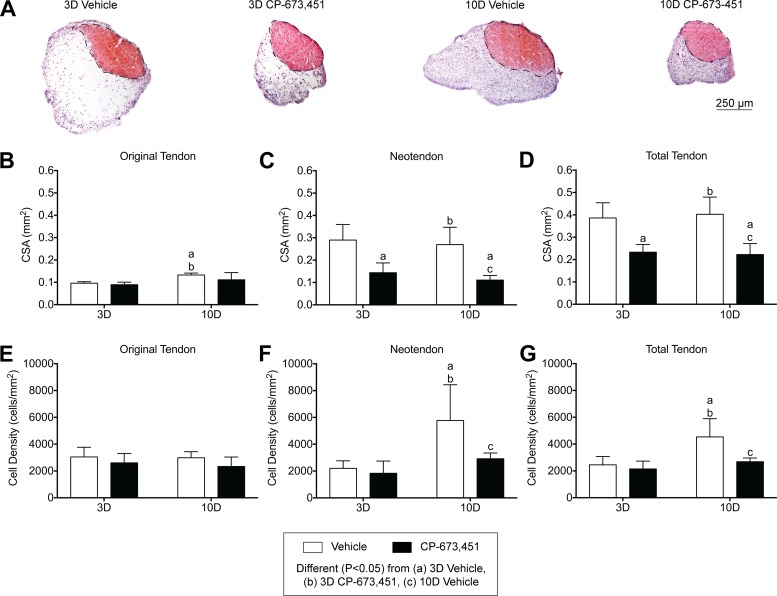
We then explored morphological effects of PDGFR signaling on tendon growth. Mechanical overload of plantaris tendons resulted in outward growth of a neotendon matrix from the most superficial layers of the original tendon (Fig. 3A), consistent with previous studies (15, 52). Overall, PDGFR inhibition did not impact the general morphological features of the plantaris tendons, but noticeable differences in tendon CSA and cell density were observed (Fig. 3, B–G). For the original tendon, the CSA of the 10-day vehicle group was slightly larger than both the 3-day vehicle and PDGFR inhibitor groups, but otherwise the CSA of the original tendon did not change in response to time after overload or treatment with PDGFR inhibitor (Fig. 3B). In contrast, the CSA of the neotendon in the 3- and 10-day PDGFR inhibitor groups was 50% and 59% smaller compared with their respective vehicle-treated controls (Fig. 3C). Changes in total tendon CSA generally followed the same trends observed in the neotendon data (Fig. 3D). For the original tendon, the cell density of all groups was similar and did not change in response to time after overload or treatment with PDGFR inhibitor (Fig. 3E). However, the cell density of the neotendon in the 10-day vehicle group was 60% higher compared with both the 3-day vehicle and PDGFR inhibitor groups, and this increase was not observed in response to PDGFR inhibition (Fig. 3F). Similar to the total tendon CSA results, changes in total tendon cell density generally followed the same trends observed in the neotendon data (Fig. 3G).
Fig. 3.
Inhibition of PDGFR signaling prevents growth of plantaris tendons subjected to mechanical overload. A: representative cross sections of 3- and 10-day overloaded plantaris tendons, treated with vehicle or PDGFR inhibitor, and stained with hematoxylin and eosin. Dashed black line indicates the boundary between the original tendon and neotendon. Scale bar is 250 μm. B–G: quantitative analysis of cross-sectional area (CSA, in mm2) (B–D) and cell density (cells/mm2) (E–G) for the original tendon, neotendon, and total tendon. Values are mean ± SD; n = 5 tendons for each group. Differences between groups were tested using a two-way ANOVA (α = 0.05) followed by Tukey’s post hoc sorting: different (P < 0.05) from a, 3D vehicle; b, 3D PDGFR inhibitor; c, 10D vehicle.
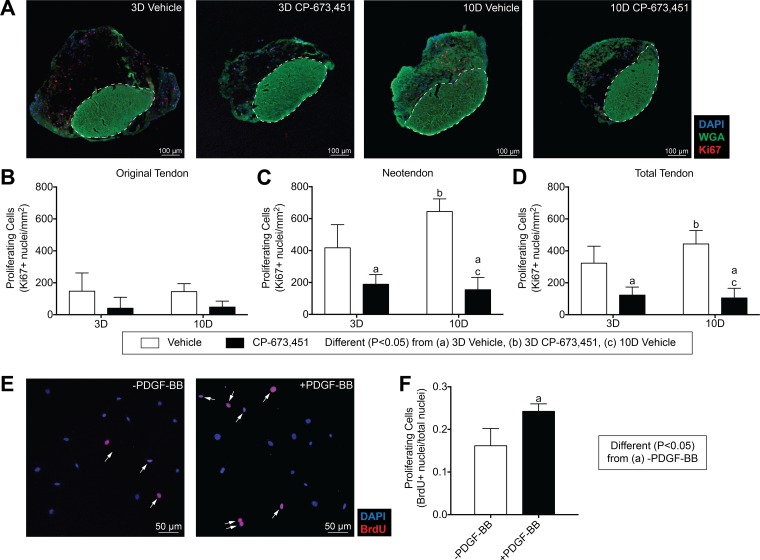
For cell proliferation, very few cells expressing the proliferation marker Ki67 were observed in the original tendon of mechanically overload tissue, and no differences between vehicle or PDGFR inhibitor groups were observed (Fig. 4, A and B). This is generally consistent with previous reports of the synergist ablation model in tendon and supports the notion that tendon fibroblasts are largely a terminally differentiated cell population in adult tendons (15, 52). In contrast, the number of proliferating cells in the neotendon of the 3- and 10-day PDGFR inhibitor groups was 55% and 76% less than their respective vehicle-treated controls (Fig. 4C). Changes in the number of proliferating cells in the total tendon generally followed the same trends observed in the neotendon (Fig. 4D). Treatment of cultured tendon fibroblasts with PDGF-BB also increased cell proliferation by 33% (Fig. 4, E and F). Taken together, these results indicate that PDGFR inhibition suppresses the normal growth of tendon tissue after mechanical overload, and this is partly explained by defects in cell proliferation. The generally positive effects observed from the therapeutic use of PDGF in animal models of tendon injury may in part be due to the positive impact of PDGFR activation on tendon fibroblast proliferation (8, 33, 60).
Fig. 4.
Inhibition of PDGFR signaling decreases tendon fibroblast proliferation. A: representative Ki67 immunostaining of 3- and 10-day overloaded plantaris tendons, treated with vehicle or PDGFR inhibitor. Dashed white line indicates the boundary between the original tendon and neotendon. Scale bars are 100 μm. DAPI, blue; WGA, green; Ki67, red. B–D: quantitative analysis of proliferating cells (Ki67+ nuclei/mm2) for the original tendon, neotendon, and total tendon. Values are means ± SD; n ≥ 4 tendons for each group. Differences between groups were tested using a two-way ANOVA (α = 0.05) followed by Tukey’s post hoc sorting: different (P < 0.05) from a, 3D vehicle; b, 3D PDGFR inhibitor; c, 10D vehicle. E: in vitro proliferative activity of tail tendon fibroblasts was measured by BrdU uptake in the presence of 0.5% FBS with or without 20 ng/ml of PDGF-BB treatment. Proliferating cells were double-labeled for DAPI (blue) and BrdU (red) and are marked by white arrows. F: proliferative activity was quantified as means ± SD in 5 randomly selected fields of a single experiment from 6 independent experiments performed. Differences between groups were tested using an unpaired t-test; a, P < 0.05.
PDGFR-inhibited tendons demonstrate deficits in angiogenesis, ECM synthesis and remodeling, and cell specification and proliferation.
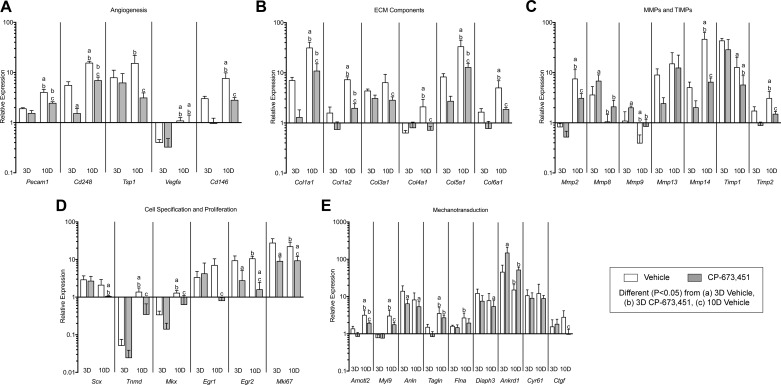
Since inhibition of PDGFR signaling resulted in reduced tendon growth, we next sought to further explore the mechanisms behind this response. We initially used microarray experiments and gene ontology (GO) analysis to identify genes to measure with qPCR. For markers of angiogenesis (Fig. 5A), multiple genes demonstrated reduced expression in the 10-day PDGFR inhibitor compared with vehicle-treated controls, including platelet/endothelial cell adhesion molecule 1 (PECAM1), also known as CD31, which is a membrane glycoprotein involved in cell adhesion of endothelial cells, and CD146, another adhesion molecule that is highly expressed by pericytes (13). CD248, which is a C-type lectin domain protein important for angiogenesis (41), was reduced at 3 and 10 days in response to PDGFR inhibition. Thrombospondin 1 (TSP1), which is a secreted glycoprotein that inhibits angiogenesis through direct effects on endothelial cell proliferation and migration (19), was also downregulated at 10 days in the PDGFR inhibitor group compared with vehicle-treated controls, while slight changes in the expression of the angiogenic growth factor vascular endothelial growth factor A (VEGFA) were noted as a result of time after overload. These results are in general agreement with previous observations in skeletal muscle, where inhibition of PDGFR signaling prevented angiogenesis in mechanically overloaded muscles (54). Activation of PDGFRβ in pericytes appears important in the process of angiogenesis (46), and while we blocked both PDGFRs in this study and were not able to target PDGFRβ specifically, the reduction in angiogenesis-associated genes may be due to diminished pericyte activity.
Fig. 5.
Inhibition of PDGFR signaling prevents the expression of angiogenesis, ECM synthesis and remodeling, cell specification and proliferation, and cell migration and mechanotransduction genes in plantaris tendons subjected to mechanical overload. A–E: quantitative expression of angiogenesis (A), ECM synthesis (B), MMPs and TIMPs (C), cell specification and proliferation (D), and mechanotransduction (E) genes in 3- and 10-day overloaded plantaris tendons, treated with vehicle or PDGFR inhibitor. Target gene expression was normalized to the stable housekeeping gene peptidylprolyl isomerase D (PPID), and further normalized to plantaris tendons that were not subjected to synergist ablation. Values are means ± SD; n ≥ 5 tendons for each group. Differences between groups were tested using a two-way ANOVA (α = 0.05) followed by Tukey’s post hoc sorting: different (P < 0.05) from a, 3D vehicle; b, 3D PDGFR inhibitor; c, 10D vehicle.
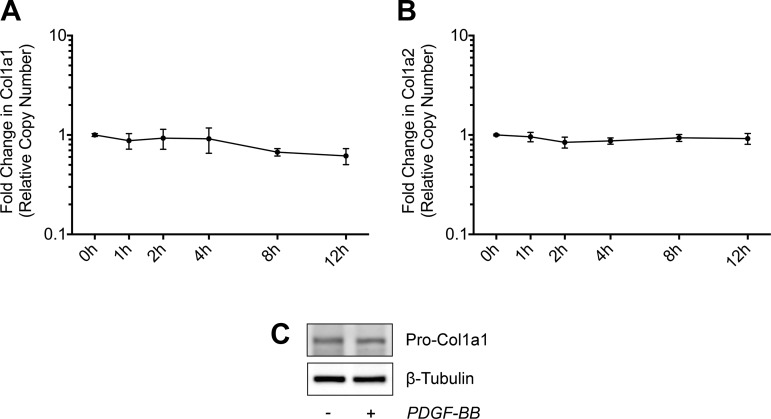
In addition to the changes observed in markers of angiogenesis, numerous ECM synthesis and remodeling genes were also affected by PDGFR inhibition (Fig. 5B). Expression of the fibrillar types I, III, and V collagens as well as the network types IV and VI collagens was not different at 3 days, but was reduced at 10 days in response to PDGFR inhibition. Given that type I collagen is the main structural protein of tendon, we sought to determine whether the reduction in type I collagen transcripts at the whole tissue level was a direct effect of PDGFR inhibition on tendon fibroblasts, or an indirect effect related to a decrease in cell number. When cultured tendon fibroblasts were treated with PDGF-BB over a 12-h time course, PDGF-BB stimulation had no significant effect on Col1a1 and Col1a2 transcript levels or pro-collagen 1a1 protein abundance (Fig. 6, A–C). This is consistent with findings from mechanically overloaded skeletal muscles, where reduced fibrillar collagen expression in PDGFR-inhibited animals occurred along with a reduction in markers of muscle fibroblast abundance (54). Combined, these results indicate that PDGF-BB does not regulate the expression of type I collagen at the mRNA and protein levels in tendon fibroblasts, and hence the reduction in type I collagen transcripts at the whole tissue level is likely an indirect effect on other signaling molecules that regulate type I collagen expression, or related to a decrease in cell number.
Fig. 6.
Effect of PDGF-BB treatment on collagen expression. A and B: quantitative expression of Col1a1 (A) and Col1a2 (B) transcript levels in tail tendon fibroblasts treated with 20 ng/ml of PDGF-BB for 1, 2, 4, 8, and 12 h. Values are means ± SD; n ≥ 3 replicates. Differences between groups were tested using one-way ANOVA (P < 0.05). No significant differences were found between groups. C: representative immunoblots of Pro-Col1a1 protein levels in tail tendon fibroblasts treated with 20 ng/ml of PDGF-BB for 24 h in low-serum conditions. β-Tubulin is shown as a loading control. Differences between groups were tested using a one-way ANOVA (α = 0.05) followed by Tukey’s post hoc sorting.
Accompanying the changes observed in ECM synthesis and remodeling genes, PDGFR inhibition also resulted in marked changes in the expression of MMPs and tissue inhibitors of metalloproteinases (TIMPs; Fig. 5C). For the gelatinases, MMP2 expression was 59% lower at 10 days in the PDGFR inhibitor group compared with vehicle-treated controls, while MMP9 was 83% higher at 3 days in response to PDGFR inhibition. This is consistent with findings from other cell types that demonstrated a reduction in MMP9 expression in response to PDGFR activation (4). Similar to MMP9, the collagenase MMP8 was 89% higher at 3 days in response to PDGFR inhibition, whereas no differences in the expression of collagenase MMP13 were demonstrated in response to time after overload or treatment with PDGFR inhibitor. MT1-MMP, also known as MMP14, is a membrane-tethered MMP that demonstrated the most profound upregulation in response to mechanical overload and was also most substantially impacted by PDGFR inhibition. MT1-MMP was upregulated by greater than 5- and 46-fold at 3 and 10 days after mechanical overload, respectively, and treatment with PDGFR inhibitor prevented the increase in transcript levels at 10 days compared with vehicle-treated controls. Compared with the 3-day time point, TIMP1 transcript levels were lower in both 10-day groups, while TIMP2 transcript levels did not increase at 10 days in response to PDGFR inhibition. These findings are consistent with other studies that demonstrated PDGFR signaling was associated with increased expression and activation of MMPs, in particular MT1-MMP, as well as TIMPs, in other cell types (32, 49, 54, 56).
Transcription factors that play crucial roles in different stages of tendon development and specification (23) were upregulated after mechanical overload (Fig. 5D). The basic helix-loop-helix transcription factor scleraxis (Scx) was upregulated greater than 2-fold at both 3 and 10 days and was not affected by PDGFR inhibitor treatment. After an initial downregulation of the atypical homeodomain transcription factor mohawk (Mkx) and the type II transmembrane glycoprotein tenomodulin (Tnmd) at 3 days, their transcript levels increased by 10 days compared with nonoverloaded controls. The zinc finger transcription factors early growth response 1 and 2 (Egr1 and Egr2) did not increase at 10 days in response to PDGFR inhibition, while Egr2 expression was also 71% lower at 3 days in the PDGFR inhibitor group compared with vehicle-treated controls. Ki67 (Mki67) was reduced at 3 and 10 days in response to PDGFR inhibition. While pericytes are able to differentiate into cells that form blood vessels in a process dependent on PDGFRβ activation, they are also able to differentiate into other cell lineages, including fibroblasts (13). Lineage tracing studies have not yet been performed, but there is compelling indirect evidence that pericytes are the progenitor cells of fibroblasts in adult tendon tissue (34, 52, 57), and it is possible that inhibiting PDGFR signaling resulted in reduced activity of fibroblast progenitor cells, which then decreased the expression of genes involved with tendon specification, proliferation, and differentiation.
There has been an association between the PDGF pathway and activation of the yes-associated protein (YAP) signal transduction network in hepatic stellate cells (35), and we sought to evaluate whether PDGFR inhibition would impact the expression of YAP-regulated genes (Fig. 5E) involved in mechanotransduction (5). Angiomotin-like protein 2 (AmotL2), which is a membrane-associated scaffold protein that localizes to lamellipodia during cell migration, and myosin light chain 9 (Myl9), which regulates contractility and cytoskeletal tension during cell migration, were downregulated at 10 days in response to PDGFR inhibition. The cytokinetic scaffold protein anillin (Anln), which binds F-actin and can recruit Rho GTPases to the leading edge of the cell membrane during cell migration, was reduced at 3 days in response to PDGFR inhibition. Other actin-binding proteins that help reorganize the actin cytoskeleton during cell migration include transgelin (Tagln), filamin A (Flna), and diaphanous related formin 3 (Diaph3), were not different between vehicle- and inhibitor-treated groups at each time point. In contrast, ankyrin repeat domain 1 (Ankrd1), which is a transcriptional repressor of MMP13, increased by greater than 3-fold in the 3-day PDGFR inhibitor group compared with vehicle-treated controls. The matricellular proteins cysteine-rich angiogenic inducer 61 (Cyr61) and connective tissue growth factor (CTGF) are key mediators of cell migration through their interaction with cell surface integrins, and while Cyr61 was not affected by PDGFR inhibition, CTGF expression was 65% lower at 10 days in the PDGFR inhibitor group compared with vehicle-treated controls. Overall, while several YAP-regulated genes were induced in overloaded tendons, only a few genes were differentially regulated between vehicle and PDGFR treated animals at a given time point. Although mechanotransduction mediated by YAP is likely important in controlling tendon growth, there does not appear to be marked interaction between the PDGFR and YAP pathways in mechanically overloaded tendons.
MT1-MMP is an essential proteinase for fibroblast migration through reconstituted tendon ECM.
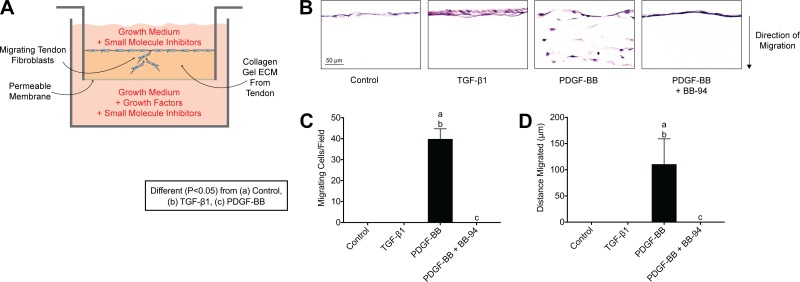
As MMPs were highly induced after mechanical overload and differentially regulated by PDGFR inhibitor treatment (Fig. 5C), we sought to explore the role of PDGF-BB and MMPs in tendon fibroblast migration in vitro. Tendon fibroblasts were placed on top of a collagen-rich gel reconstituted from tendon ECM, exposed to growth factors, and underwent manipulation of different kinases and effector proteins (Fig. 7A). In the absence of specific growth factors, fibroblasts remained on the surface of the gel (Fig. 7, B–D). Treatment with TGF-β1 did not cause fibroblasts to enter the gel but did appear to increase proliferation as evidenced by a thicker layer of cells on the surface of the gel (Fig. 7, B–D). However, upon treatment with PDGF-BB, fibroblasts did migrate into the gel (Fig. 7, B–D). This process was prevented using the broad-spectrum MMP inhibitor BB-94 (Fig. 7, B–D), indicating that migration was dependent on MMP activity, which is in agreement with observations from other types of nontendon cells (32, 49, 51, 56).
Fig. 7.
PDGF-BB stimulates tendon fibroblast migration in vitro. A: schematic representation of the in vitro migration assay. B: representative images of tail tendon fibroblasts obtained 6 days after being placed on the surface of a tendon ECM Transwell system, treated with no additional growth factors (control), TGF-β1, PDGF-BB, and PDGF-BB + BB-94 (a synthetic broad spectrum MMP inhibitor). C and D: quantification of the number of migrating cells per field (C) and maximum distance migrated (D). Sections were stained with hematoxylin and eosin. Values are means ± SD; n = 4 replicates per group. Differences between groups were tested using a one-way ANOVA (α = 0.05) followed by Tukey’s post hoc sorting: different (P < 0.05) from a, control; b, TGF-β1; c, PDGF-BB.
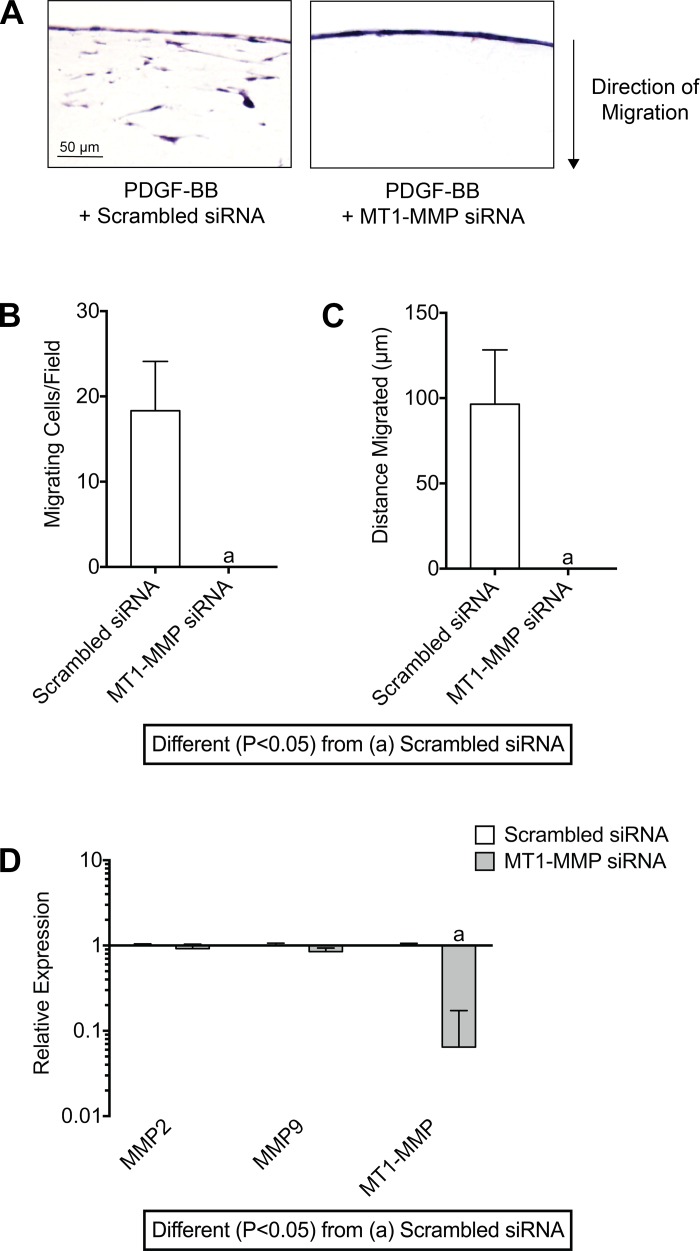
As MT1-MMP was the most highly induced MMP after mechanical overload, and the expression of MT1-MMP was markedly attenuated in response to PDGFR inhibition at 10 days (Fig. 5C), we then focused on the specific role of MT1-MMP in tendon fibroblast migration. Knocking down MT1-MMP expression with siRNA prevented the migration of fibroblasts into the tendon gel (Fig. 8, A–C). Taken together, these results indicate that MT1-MMP is required for the migration of tendon fibroblasts through their ECM.
Fig. 8.
MT1-MMP is required for PDGF-BB-mediated fibroblast migration through tendon ECM. A: representative images of tail tendon fibroblasts transfected with scrambled siRNA or MT1-MMP siRNA, and treated with PDGF-BB, measured after 6 days. B and C: quantification of the number of migrating cells per field (B) and maximum distance migrated of the MT1-MMP-silenced tendon fibroblasts (C). Sections were stained with hematoxylin and eosin. D: quantitative expression of MMP2, MMP9, and MT1-MMP mRNA in tendon fibroblasts transfected with scrambled or MT1-MMP siRNA for 48 h demonstrating specificity and efficiency of MT1-MMP mRNA knockdown. Values are means ± SD; n = 4 replicates per group. Differences between groups were tested using an unpaired Student’s t-test; a, P < 0.05.
PDGF-BB controls the translation, membrane targeting, and activation of MT1-MMP in tendon fibroblasts.
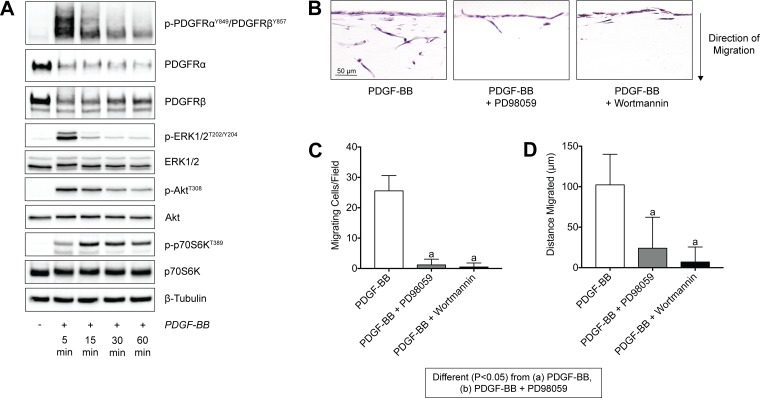
As PDGF signaling directed tendon fibroblast migration in a MT1-MMP-dependent fashion, we next sought to identify the signaling pathways that regulate this process by evaluating the phosphorylation of various kinases that have previously reported to be involved in migration and proliferation of other cell types (58). In response to activation of the PDGFRs, there was a rapid phosphorylation of ERK1/2 and Akt, followed soon thereafter by p70S6K, which is downstream from Akt (Fig. 9A). We then stimulated tendon fibroblasts in Transwell systems with PDGF-BB and either PD98059 to block ERK1/2 phosphorylation through inhibition of its upstream kinase MEK1 (44), or wortmannin to block Akt phosphorylation through inhibition of its upstream kinase PI3K (2). In response to treatment with either of these inhibitors, there was a greater than 90% reduction in the number of migrating cells per field, and more than 80% in the distance migrated by these cells (Fig. 9, B and C).
Fig. 9.
PDGF-BB stimulation of tendon fibroblasts activates PI3K/Akt and ERK1/2 pathways, which both in turn mediate tendon fibroblast migration through tendon ECM. A: representative immunoblots of total and phospho PDGFRα, PDGFRβ, ERK1/2, Akt, and p70S6K from serum-starved tail tendon fibroblasts treated with PDGF-BB for 5, 15, 30, and 60 min. β-Tubulin is shown as a loading control. B: representative images of tail tendon fibroblasts obtained 6 days after being placed on the surface of a tendon ECM Transwell system, treated with PDGF-BB, PDGF-BB + PD98059, or PDGF-BB + wortmannin. C and D: quantification of the number of migrating cells per field (C) and maximum distance migrated (D). Sections were stained with hematoxylin and eosin. Values are means ± SD; n = 4 replicates per group. Differences between groups were tested using a one-way ANOVA (α = 0.05) followed by Tukey’s post hoc sorting: different (P < 0.05) from a, PDGF-BB; b, PDGF-BB + PD98059.
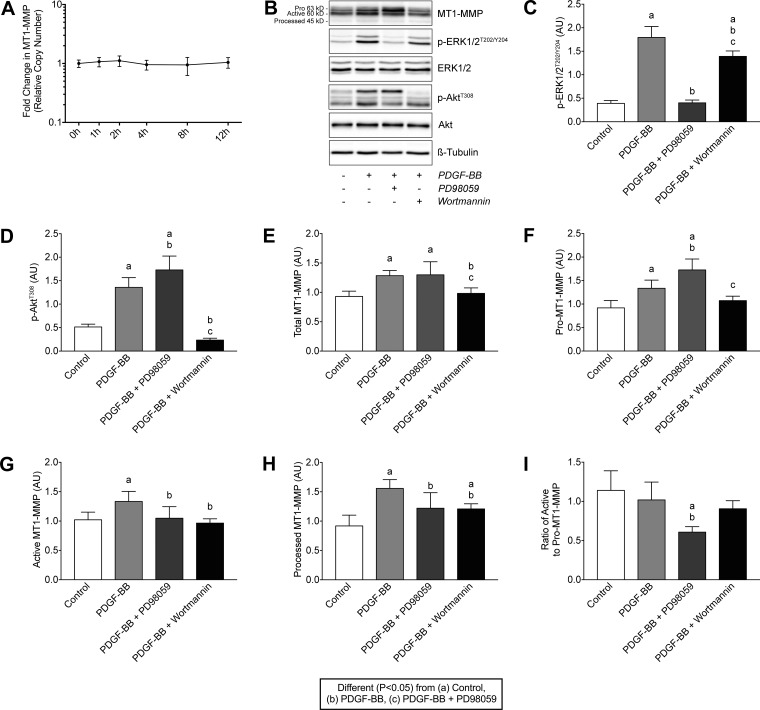
PDGF-BB did not change the transcript levels of MT1-MMP in cultured tendon fibroblasts (Fig. 10A), and we therefore evaluated how PDGF-BB signaling regulated MT1-MMP in a posttranscriptional fashion. The specificity and efficacy of PD98059 and wortmannin to block ERK1/2 and Akt signaling, respectively, were verified (Fig. 10, B–D). MT1-MMP is produced in an inactive 63-kDa pro-form, that is eventually activated as a 60-kDa form, and after some time is cleaved to a 45-kDa inactive, processed form (9). The treatment of tendon fibroblasts with PDGF-BB increased total MT1-MMP protein levels, including pro-MT1-MMP, active MT1-MMP, and processed MT1-MMP (Fig. 10, E–H). Inhibiting of ERK1/2 resulted in a further increase in pro-MT1-MMP, but it reduced active and processed MT1-MMP (Fig. 10, E–H). Blocking Akt reduced total MT1-MMP levels, which is consistent with the role of the downstream kinase p70S6K in phosphorylating the ribosomal protein S6 that initiates protein synthesis by ribosomes (14). Additionally, inhibiting Akt did not change pro-MT1-MMP levels compared with PDGF-BB stimulation alone, but it did decrease active and processed MT1-MMP (Fig. 10, E–H). When the ratio of active to pro-MT1-MMP was calculated, inhibition of ERK1/2 resulted in a reduction in active MT1-MMP relative to pro-MT1-MMP compared with other treatments (Fig. 10I).
Fig. 10.
PDGF-BB does not regulate MT1-MMP mRNA expression but does increase MT1-MMP protein levels through a PI3K/Akt-dependent mechanism. A: quantification of MT1-MMP transcript levels in tail tendon fibroblasts treated with 20 ng/ml of PDGF-BB for 1, 2, 4, 8, and 12 h. Values are means ± SD; n ≥ 3 replicates. Differences between groups were tested using one-way ANOVA (P < 0.05). B: representative immunoblots of Pro-MT1-MMP (63 kDa), active MT1-MMP (60 kDa), processed MT1-MMP (45 kDa), and phospho and total ERK1/2 and Akt are shown, from tendon fibroblasts incubated alone or with PDGF-BB in the presence or absence of PD98059 or wortmannin for 24 h. β-Tubulin is shown as a loading control. C–H: quantification of p-ERK1/2T202/Y204 (C), p-AktT308 (D), total MT1-MMP (E), pro-MT1-MMP (F), active MT1-MMP (G) and processed MT1-MMP (H) protein levels. AU, arbitrary units. I: ratio of the pro- to active form of MT1-MMP. Values are means ± SD; n = 6 replicates. Differences between groups were tested using a one-way ANOVA (α = 0.05) followed by Tukey’s post hoc sorting: different (P < 0.05) from a, control; b, PDGF-BB; c, PDGF-BB + PD98059.
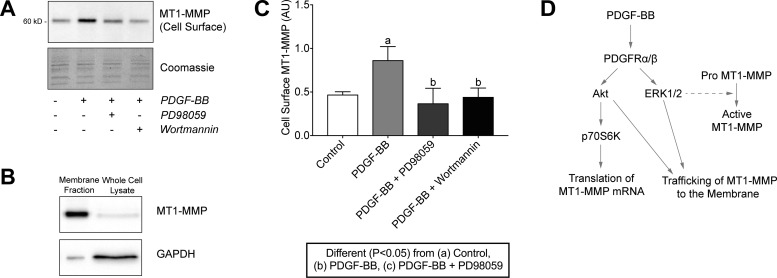
Finally, because MT1-MMP exerts its effects in the extracellular matrix, we determined whether ERK1/2 or Akt regulated the trafficking of MT1-MMP to the plasma membrane. Inhibition of both kinases prevented the PDGF-BB-induced localization of MT1-MMP to the plasma membrane (Fig. 11, A–C). Taken together, these results indicate that in tendon fibroblasts, PDGF-BB controls the translation of MT1-MMP mRNA through the Akt/p70S6K pathway, ERK1/2 is important for the activation of MT1-MMP, and that the trafficking of MT1-MMP to the plasma membrane is dependent on both ERK1/2 and Akt signaling pathways (Fig. 11D). These findings differ from previous reports in bone marrow-derived mesenchymal stem cells that indicated that treatment with PDGF-BB increased MT1-MMP mRNA expression and protein levels in a PI3K/Akt- and ERK1/2-dependent manner (56), suggesting that the regulation of MT1-MMP expression in response to PDGF-BB stimulation is cell type and tissue specific.
Fig. 11.
PDGF-BB-dependent membrane trafficking of MT1-MMP in tendon fibroblasts is regulated by the PI3K/Akt and ERK1/2 pathways. (A) Representative immunoblots of cell surface MT1-MMP protein levels in tail tendon fibroblasts after incubation alone or with PDGF-BB in the presence or absence of PD98059 or wortmannin for 24 h in low-serum conditions. Coomassie staining is shown as a total protein loading control. (B) Representative immunoblots of the membrane fraction and whole cell lysates of tendon fibroblasts using MT1-MMP and GAPDH as typical membrane and cytosolic proteins, respectively. Due to the isolation detergents, membrane fraction lanes run in a narrow fashion. (C) Quantification of cell surface MT1-MMP protein levels. Values are means ± SD; n ≥ 3 replicates. Differences between groups were tested using a one-way ANOVA (α = 0.05) followed by Tukey’s post hoc sorting: different (P < 0.05) from a, control; b, PDGF-BB; c, PDGF-BB + PD98059. D: diagram of PDGF-BB-dependent regulation of MT1-MMP expression and membrane trafficking in tendon fibroblasts.
Limitations.
There are several limitations to the current study. We used a specific inhibitor of PDGFRα and PDGFRβ (47) and were not able to selectively inhibit each receptor to determine its individual function. Although the selectivity of CP-673,451 is over 450-fold more for PDGFRs than other related receptor tyrosine kinases in cultured glioblastoma cells (47), as with nearly all small molecule kinase inhibitors, there is the potential for off-target effects. Although more than 20 MMPs have been described, we only evaluated MT1-MMP during our migration assays, and it remains possible that other MMPs are also important for tendon fibroblast migration. Additionally, we measured differences in tendon morphology after mechanical overload due to PDGFR inhibition, but tendon mechanics and other functional assays were not performed. The synergist ablation procedure is reliable, reproducible and is well tolerated by mice, but the net mechanical load placed on the plantaris tendon is greater than that experienced during normal locomotion or as a result of treadmill training. We also focused in vitro studies on cells from nonoverloaded tendons, and it is possible that cells from overloaded tendons could behave differently than those from nonoverloaded tendons. Further, the cells were isolated from tail tendons to obtain an abundant number of low-passage cells for in vitro studies, but it is possible that cells from the tail tendon do not behave in the same capacity as other limb tendons. Furthermore, while our in vivo studies focused only on the plantaris tendon, the observed differences might not be reflected in other trunk or limb tendons. We measured the expression of many transcripts using microarray and qPCR, but changes in the transcriptome might not follow changes in the proteome. There are differences between some in vitro and in vivo data, which reflects the complexity of the in vivo environment and the need to conduct whole animal experiments along with in vitro basic, mechanism-driven experiments. Finally, we collected data at only two time points after mechanical overload, and additional time points are likely to provide further mechanistic insight. Despite these limitations, we feel this work provides an important contribution to our understanding of how PDGFR signaling controls tendon growth in adult animals.
Summary and future directions.
In response to changes in mechanical load, tendon actively remodels its ECM to meet the new demands placed on it, which is typically accompanied by increases in tendon CSA, cell density, and type I collagen content (15, 30, 38). Tendon fibroblasts constantly sense and respond to biomechanical and biochemical cues in their environment, and an increase in mechanical load placed on the tendon is often a signal for growth (16, 63). In the current study, we report that tendon fibroblasts express PDGFRα and PDGFRβ, that PDGFR signaling is required for the load-induced growth of tendons in adult animals, and that MT1-MMP is an essential proteinase for the migration of tendon fibroblasts through their ECM via Akt and ERK1/2 dependent mechanisms. While PDGFR inhibition resulted in reduced MT1-MMP expression at the whole tissue level, treatment of tendon fibroblasts with PDGF-BB directly did not alter MT1-MMP expression, suggesting that the results observed at the whole tissue level are likely due to indirect effects. These combined findings help to provide mechanistic insights into the generally positive outcomes in applied studies that have used PDGF-BB to stimulate tendon regeneration (8, 33, 60), and the disordered ECM observed in the tendons of mice that lack MT1-MMP in tendon fibroblasts (ScxCre:MT1-MMPf/f) (59). Increased PDGFRβ expression and greater proliferation have been observed in biopsies of patients with patellar tendinopathy compared with healthy controls (48), and it is possible that the targeted manipulation of PDGFR signaling may be useful in the treatment of chronic tendon disorders.
Apart from its role in cell migration, MT1-MMP also regulates other key cellular processes including proliferation, differentiation, and cell survival, by either changing the tissue architecture or converting labile matrix proteins into active signaling molecules (26). Although the mechanisms underlying the ERK1/2-mediated activation of MT1-MMP are not known, furin is a protease that is a member of the subtilisin-like proprotein convertase (SPC) family and is known to activate MT1-MMP (26). Members of the SPC family are known to be regulated by PDGF-BB (3), and furin expression has been shown to be regulated by ERK1/2 activation (45). Additional studies of furin and other SPC proteases are likely to provide further insight into the molecular and biochemical mediators of tendon growth in adult animals.
GRANTS
This work was supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases Grants R01-AR063649 and F32-AR067086.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.B.S. and C.L.M. conceived and designed research; K.B.S., J.F.M., N.P.D., A.M.R., J.R.T., D.C.S., and C.L.M. performed experiments; K.B.S., J.F.M., N.P.D., A.M.R., J.R.T., D.C.S., S.V.B., and C.L.M. analyzed data; K.B.S., S.V.B., and C.L.M. interpreted results of experiments; K.B.S., J.F.M., N.P.D., A.M.R., J.R.T., and C.L.M. prepared figures; K.B.S. and C.L.M. drafted manuscript; K.B.S., S.V.B., and C.L.M. edited and revised manuscript; K.B.S., J.F.M., N.P.D., A.M.R., D.C.S., S.V.B., and C.L.M. approved final version of manuscript.
Supplemental Data
Table S1. Primer sequences used for quantitative PCR. - .docx (29 KB)
ACKNOWLEDGMENTS
The authors acknowledge helpful discussions and technical support by Dr. Stephen Weiss and Dr. Farideh Sabeh.
REFERENCES
- 1.Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev 22: 1276–1312, 2008. doi: 10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arcaro A, Wymann MP. Wortmannin is a potent phosphatidylinositol 3-kinase inhibitor: the role of phosphatidylinositol 3,4,5-trisphosphate in neutrophil responses. Biochem J 296: 297–301, 1993. doi: 10.1042/bj2960297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bando M, Matsuoka A, Tsuji A, Matsuda Y. The proprotein convertase PACE4 is upregulated by PDGF-BB in megakaryocytes: gene expression of PACE4 and furin is regulated differently in Dami cells. J Biochem 132: 127–134, 2002. doi: 10.1093/oxfordjournals.jbchem.a003189. [DOI] [PubMed] [Google Scholar]
- 4.Borkham-Kamphorst E, Alexi P, Tihaa L, Haas U, Weiskirchen R. Platelet-derived growth factor-D modulates extracellular matrix homeostasis and remodeling through TIMP-1 induction and attenuation of MMP-2 and MMP-9 gelatinase activities. Biochem Biophys Res Commun 457: 307–313, 2015. doi: 10.1016/j.bbrc.2014.12.106. [DOI] [PubMed] [Google Scholar]
- 5.Calvo F, Ege N, Grande-Garcia A, Hooper S, Jenkins RP, Chaudhry SI, Harrington K, Williamson P, Moeendarbary E, Charras G, Sahai E. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat Cell Biol 15: 637–646, 2013. doi: 10.1038/ncb2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaillou T, Jackson JR, England JH, Kirby TJ, Richards-White J, Esser KA, Dupont-Versteegden EE, McCarthy JJ. Identification of a conserved set of upregulated genes in mouse skeletal muscle hypertrophy and regrowth. J Appl Physiol (1985) 118: 86–97, 2015. doi: 10.1152/japplphysiol.00351.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Couppé C, Kongsgaard M, Aagaard P, Hansen P, Bojsen-Moller J, Kjaer M, Magnusson SP. Habitual loading results in tendon hypertrophy and increased stiffness of the human patellar tendon. J Appl Physiol (1985) 105: 805–810, 2008. doi: 10.1152/japplphysiol.90361.2008. [DOI] [PubMed] [Google Scholar]
- 8.Cummings SH, Grande DA, Hee CK, Kestler HK, Roden CM, Shah NV, Razzano P, Dines DM, Chahine NO, Dines JS. Effect of recombinant human platelet-derived growth factor-BB-coated sutures on Achilles tendon healing in a rat model: A histological and biomechanical study. J Tissue Eng 3: 2041731412453577, 2012. doi: 10.1177/2041731412453577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daja MM, Niu X, Zhao Z, Brown JM, Russell PJ. Characterization of expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in prostate cancer cell lines. Prostate Cancer Prostatic Dis 6: 15–26, 2003. doi: 10.1038/sj.pcan.4500609. [DOI] [PubMed] [Google Scholar]
- 10.Davis ME, Gumucio JP, Sugg KB, Bedi A, Mendias CL. MMP inhibition as a potential method to augment the healing of skeletal muscle and tendon extracellular matrix. J Appl Physiol (1985) 115: 884–891, 2013. doi: 10.1152/japplphysiol.00137.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ehnman M, Missiaglia E, Folestad E, Selfe J, Strell C, Thway K, Brodin B, Pietras K, Shipley J, Östman A, Eriksson U. Distinct effects of ligand-induced PDGFRα and PDGFRβ signaling in the human rhabdomyosarcoma tumor cell and stroma cell compartments. Cancer Res 73: 2139–2149, 2013. doi: 10.1158/0008-5472.CAN-12-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodman CA, Dietz JM, Jacobs BL, McNally RM, You J-S, Hornberger TA. Yes-Associated Protein is up-regulated by mechanical overload and is sufficient to induce skeletal muscle hypertrophy. FEBS Lett 589: 1491–1497, 2015. doi: 10.1016/j.febslet.2015.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gökçinar-Yagci B, Uçkan-Çetinkaya D, Çelebi-Saltik B. Pericytes: properties, functions and applications in tissue engineering. Stem Cell Rev 11: 549–559, 2015. doi: 10.1007/s12015-015-9590-z. [DOI] [PubMed] [Google Scholar]
- 14.Gumucio JP, Mendias CL. Atrogin-1, MuRF-1, and sarcopenia. Endocrine 43: 12–21, 2013. doi: 10.1007/s12020-012-9751-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gumucio JP, Phan AC, Ruehlmann DG, Noah AC, Mendias CL. Synergist ablation induces rapid tendon growth through the synthesis of a neotendon matrix. J Appl Physiol (1985) 117: 1287–1291, 2014. doi: 10.1152/japplphysiol.00720.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gumucio JP, Sugg KB, Mendias CL. TGF-β superfamily signaling in muscle and tendon adaptation to resistance exercise. Exerc Sport Sci Rev 43: 93–99, 2015. doi: 10.1249/JES.0000000000000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamilton DL, Philp A, MacKenzie MG, Patton A, Towler MC, Gallagher IJ, Bodine SC, Baar K. Molecular brakes regulating mTORC1 activation in skeletal muscle following synergist ablation. Am J Physiol Endocrinol Metab 307: E365–E373, 2014. doi: 10.1152/ajpendo.00674.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamilton TG, Klinghoffer RA, Corrin PD, Soriano P. Evolutionary divergence of platelet-derived growth factor alpha receptor signaling mechanisms. Mol Cell Biol 23: 4013–4025, 2003. doi: 10.1128/MCB.23.11.4013-4025.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hellsten Y, Hoier B. Capillary growth in human skeletal muscle: physiological factors and the balance between pro-angiogenic and angiostatic factors. Biochem Soc Trans 42: 1616–1622, 2014. doi: 10.1042/BST20140197. [DOI] [PubMed] [Google Scholar]
- 20.Hiden U, Lassance L, Tabrizi NG, Miedl H, Tam-Amersdorfer C, Cetin I, Lang U, Desoye G. Fetal insulin and IGF-II contribute to gestational diabetes mellitus (GDM)-associated up-regulation of membrane-type matrix metalloproteinase 1 (MT1-MMP) in the human feto-placental endothelium. J Clin Endocrinol Metab 97: 3613–3621, 2012. doi: 10.1210/jc.2012-1212. [DOI] [PubMed] [Google Scholar]
- 21.Hojjat-Farsangi M. Small-molecule inhibitors of the receptor tyrosine kinases: promising tools for targeted cancer therapies. Int J Mol Sci 15: 13768–13801, 2014. doi: 10.3390/ijms150813768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hotary K, Allen E, Punturieri A, Yana I, Weiss SJ. Regulation of cell invasion and morphogenesis in a three-dimensional type I collagen matrix by membrane-type matrix metalloproteinases 1, 2, and 3. J Cell Biol 149: 1309–1323, 2000. doi: 10.1083/jcb.149.6.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang AH, Lu HH, Schweitzer R. Molecular regulation of tendon cell fate during development. J Orthop Res 33: 800–812, 2015. doi: 10.1002/jor.22834. [DOI] [PubMed] [Google Scholar]
- 24.Hudgens JL, Sugg KB, Grekin JA, Gumucio JP, Bedi A, Mendias CL. Platelet-rich plasma activates proinflammatory signaling pathways and induces oxidative stress in tendon fibroblasts. Am J Sports Med 44: 1931–1940, 2016. doi: 10.1177/0363546516637176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hye Kim J, Gyu Park S, Kim WK, Song SU, Sung JH. Functional regulation of adipose-derived stem cells by PDGF-D. Stem Cells 33: 542–556, 2015. doi: 10.1002/stem.1865. [DOI] [PubMed] [Google Scholar]
- 26.Itoh Y, Seiki M. MT1-MMP: a potent modifier of pericellular microenvironment. J Cell Physiol 206: 1–8, 2006. doi: 10.1002/jcp.20431. [DOI] [PubMed] [Google Scholar]
- 27.Joe AW, Yi L, Natarajan A, Le Grand F, So L, Wang J, Rudnicki MA, Rossi FM. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol 12: 153–163, 2010. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson JR, Folestad E, Rowley JE, Noll EM, Walker SA, Lloyd CM, Rankin SM, Pietras K, Eriksson U, Fuxe J. Pericytes contribute to airway remodeling in a mouse model of chronic allergic asthma. Am J Physiol Lung Cell Mol Physiol 308: L658–L671, 2015. doi: 10.1152/ajplung.00286.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kendziorski C, Irizarry RA, Chen KS, Haag JD, Gould MN. On the utility of pooling biological samples in microarray experiments. Proc Natl Acad Sci USA 102: 4252–4257, 2005. doi: 10.1073/pnas.0500607102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kjaer M, Magnusson P, Krogsgaard M, Boysen Møller J, Olesen J, Heinemeier K, Hansen M, Haraldsson B, Koskinen S, Esmarck B, Langberg H. Extracellular matrix adaptation of tendon and skeletal muscle to exercise. J Anat 208: 445–450, 2006. doi: 10.1111/j.1469-7580.2006.00549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kjaer M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev 84: 649–698, 2004. doi: 10.1152/physrev.00031.2003. [DOI] [PubMed] [Google Scholar]
- 32.Koenig GC, Rowe RG, Day SM, Sabeh F, Atkinson JJ, Cooke KR, Weiss SJ. MT1-MMP-dependent remodeling of cardiac extracellular matrix structure and function following myocardial infarction. Am J Pathol 180: 1863–1878, 2012. doi: 10.1016/j.ajpath.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kovacevic D, Gulotta LV, Ying L, Ehteshami JR, Deng XH, Rodeo SA. rhPDGF-BB promotes early healing in a rat rotator cuff repair model. Clin Orthop Relat Res 473: 1644–1654, 2015. doi: 10.1007/s11999-014-4020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee CH, Lee FY, Tarafder S, Kao K, Jun Y, Yang G, Mao JJ. Harnessing endogenous stem/progenitor cells for tendon regeneration. J Clin Invest 125: 2690–2701, 2015. doi: 10.1172/JCI81589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mannaerts I, Leite SB, Verhulst S, Claerhout S, Eysackers N, Thoen LFR, Hoorens A, Reynaert H, Halder G, van Grunsven LA. The Hippo pathway effector YAP controls mouse hepatic stellate cell activation. J Hepatol 63: 679–688, 2015. doi: 10.1016/j.jhep.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 36.McCarthy JJ, Mula J, Miyazaki M, Erfani R, Garrison K, Farooqui AB, Srikuea R, Lawson BA, Grimes B, Keller C, Van Zant G, Campbell KS, Esser KA, Dupont-Versteegden EE, Peterson CA. Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development 138: 3657–3666, 2011. doi: 10.1242/dev.068858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mendias CL, Bakhurin KI, Faulkner JA. Tendons of myostatin-deficient mice are small, brittle, and hypocellular. Proc Natl Acad Sci USA 105: 388–393, 2008. doi: 10.1073/pnas.0707069105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mendias CL, Gumucio JP, Bakhurin KI, Lynch EB, Brooks SV. Physiological loading of tendons induces scleraxis expression in epitenon fibroblasts. J Orthop Res 30: 606–612, 2012. doi: 10.1002/jor.21550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Molloy T, Wang Y, Murrell G. The roles of growth factors in tendon and ligament healing. Sports Med 33: 381–394, 2003. doi: 10.2165/00007256-200333050-00004. [DOI] [PubMed] [Google Scholar]
- 40.Murchison ND, Price BA, Conner DA, Keene DR, Olson EN, Tabin CJ, Schweitzer R. Regulation of tendon differentiation by scleraxis distinguishes force-transmitting tendons from muscle-anchoring tendons. Development 134: 2697–2708, 2007. doi: 10.1242/dev.001933. [DOI] [PubMed] [Google Scholar]
- 41.Naylor AJ, McGettrick HM, Maynard WD, May P, Barone F, Croft AP, Egginton S, Buckley CD. A differential role for CD248 (Endosialin) in PDGF-mediated skeletal muscle angiogenesis. PLoS One 9: e107146, 2014. doi: 10.1371/journal.pone.0107146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nunes-Xavier CE, Martín-Pérez J, Elson A, Pulido R. Protein tyrosine phosphatases as novel targets in breast cancer therapy. Biochim Biophys Acta 1836: 211–226, 2013. [DOI] [PubMed] [Google Scholar]
- 43.Olesen JL, Heinemeier KM, Haddad F, Langberg H, Flyvbjerg A, Kjaer M, Baldwin KM. Expression of insulin-like growth factor I, insulin-like growth factor binding proteins, and collagen mRNA in mechanically loaded plantaris tendon. J Appl Physiol (1985) 101: 183–188, 2006. doi: 10.1152/japplphysiol.00636.2005. [DOI] [PubMed] [Google Scholar]
- 44.Pang L, Sawada T, Decker SJ, Saltiel AR. Inhibition of MAP kinase kinase blocks the differentiation of PC-12 cells induced by nerve growth factor. J Biol Chem 270: 13585–13588, 1995. doi: 10.1074/jbc.270.23.13585. [DOI] [PubMed] [Google Scholar]
- 45.Poli M, Luscieti S, Gandini V, Maccarinelli F, Finazzi D, Silvestri L, Roetto A, Arosio P. Transferrin receptor 2 and HFE regulate furin expression via mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/Erk) signaling. Implications for transferrin-dependent hepcidin regulation. Haematologica 95: 1832–1840, 2010. doi: 10.3324/haematol.2010.027003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raica M, Cimpean AM. Platelet-derived growth factor (PDGF)/PDGF receptors (PDGFR) axis as target for antitumor and antiangiogenic therapy. Pharmaceuticals (Basel) 3: 572–599, 2010. doi: 10.3390/ph3030572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roberts WG, Whalen PM, Soderstrom E, Moraski G, Lyssikatos JP, Wang H-F, Cooper B, Baker DA, Savage D, Dalvie D, Atherton JA, Ralston S, Szewc R, Kath JC, Lin J, Soderstrom C, Tkalcevic G, Cohen BD, Pollack V, Barth W, Hungerford W, Ung E. Antiangiogenic and antitumor activity of a selective PDGFR tyrosine kinase inhibitor, CP-673,451. Cancer Res 65: 957–966, 2005. [PubMed] [Google Scholar]
- 48.Rolf CG, Fu BS, Pau A, Wang W, Chan B. Increased cell proliferation and associated expression of PDGFRbeta causing hypercellularity in patellar tendinosis. Rheumatology (Oxford) 40: 256–261, 2001. doi: 10.1093/rheumatology/40.3.256. [DOI] [PubMed] [Google Scholar]
- 49.Rowe RG, Keena D, Sabeh F, Willis AL, Weiss SJ. Pulmonary fibroblasts mobilize the membrane-tethered matrix metalloprotease, MT1-MMP, to destructively remodel and invade interstitial type I collagen barriers. Am J Physiol Lung Cell Mol Physiol 301: L683–L692, 2011. doi: 10.1152/ajplung.00187.2011. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rutledge RG, Stewart D. Assessing the performance capabilities of LRE-based assays for absolute quantitative real-time PCR. PLoS One 5: e9731, 2010. doi: 10.1371/journal.pone.0009731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sabeh F, Li XY, Saunders TL, Rowe RG, Weiss SJ. Secreted versus membrane-anchored collagenases: relative roles in fibroblast-dependent collagenolysis and invasion. J Biol Chem 284: 23001–23011, 2009. doi: 10.1074/jbc.M109.002808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schwartz AJ, Sarver DC, Sugg KB, Dzierzawski JT, Gumucio JP, Mendias CL. p38 MAPK signaling in postnatal tendon growth and remodeling. PLoS One 10: e0120044, 2015. doi: 10.1371/journal.pone.0120044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schweitzer R, Zelzer E, Volk T. Connecting muscles to tendons: tendons and musculoskeletal development in flies and vertebrates. Development 137: 2807–2817, 2010. doi: 10.1242/dev.047498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sugg KB, Korn MA, Sarver DC, Markworth JF, Mendias CL. Inhibition of platelet-derived growth factor signaling prevents muscle fiber growth during skeletal muscle hypertrophy. FEBS Lett 591: 801–809, 2017. doi: 10.1002/1873-3468.12571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sugg KB, Lubardic J, Gumucio JP, Mendias CL. Changes in macrophage phenotype and induction of epithelial-to-mesenchymal transition genes following acute Achilles tenotomy and repair. J Orthop Res 32: 944–951, 2014. doi: 10.1002/jor.22624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sun X, Gao X, Zhou L, Sun L, Lu C. PDGF-BB-induced MT1-MMP expression regulates proliferation and invasion of mesenchymal stem cells in 3-dimensional collagen via MEK/ERK1/2 and PI3K/AKT signaling. Cell Signal 25: 1279–1287, 2013. doi: 10.1016/j.cellsig.2013.01.029. [DOI] [PubMed] [Google Scholar]
- 57.Tan Q, Lui PPY, Lee YW. In vivo identity of tendon stem cells and the roles of stem cells in tendon healing. Stem Cells Dev 22: 3128–3140, 2013. doi: 10.1089/scd.2013.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tavares MR, Pavan IC, Amaral CL, Meneguello L, Luchessi AD, Simabuco FM. The S6K protein family in health and disease. Life Sci 131: 1–10, 2015. doi: 10.1016/j.lfs.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 59.Taylor SH, Yeung CY, Kalson NS, Lu Y, Zigrino P, Starborg T, Warwood S, Holmes DF, Canty-Laird EG, Mauch C, Kadler KE. Matrix metalloproteinase 14 is required for fibrous tissue expansion. eLife 4: e09345, 2015. doi: 10.7554/eLife.09345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thomopoulos S, Das R, Silva MJ, Sakiyama-Elbert S, Harwood FL, Zampiakis E, Kim HM, Amiel D, Gelberman RH. Enhanced flexor tendon healing through controlled delivery of PDGF-BB. J Orthop Res 27: 1209–1215, 2009. doi: 10.1002/jor.20875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thomopoulos S, Parks WC, Rifkin DB, Derwin KA. Mechanisms of tendon injury and repair. J Orthop Res 33: 832–839, 2015. doi: 10.1002/jor.22806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Uezumi A, Fukada S, Yamamoto N, Ikemoto-Uezumi M, Nakatani M, Morita M, Yamaguchi A, Yamada H, Nishino I, Hamada Y, Tsuchida K. Identification and characterization of PDGFRα+ mesenchymal progenitors in human skeletal muscle. Cell Death Dis 5: e1186, 2014. doi: 10.1038/cddis.2014.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang JH-C. Mechanobiology of tendon. J Biomech 39: 1563–1582, 2006. doi: 10.1016/j.jbiomech.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 64.Wheeler DB, Zoncu R, Root DE, Sabatini DM, Sawyers CL. Identification of an oncogenic RAB protein. Science 350: 211–217, 2015. doi: 10.1126/science.aaa4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xi Y, Chen M, Liu X, Lu Z, Ding Y, Li D. CP-673451, a platelet-derived growth-factor receptor inhibitor, suppresses lung cancer cell proliferation and migration. Onco Targets Ther 7: 1215–1221, 2014. doi: 10.2147/OTT.S62946. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Primer sequences used for quantitative PCR. - .docx (29 KB)