Abstract
Age-related macular degeneration (AMD) is the leading cause of vision loss among the elderly population, and is associated with severe macular degeneration and choroidal neovascularization (CNV). Although the pathogenesis of AMD is associated with choroidal dysfunction and CNV, the detailed underlying mechanisms remain unresolved. Altered production of pigment epithelium-derived factor (PEDF), a neuroprotective and antiangiogenic factor, contributes to CNV. Furthermore, exogenous PEDF mitigates angiogenesis in preclinical CNV models. How PEDF expression affects choroidal endothelial cell (ChEC) function is unknown. Here we isolated ChECs from PEDF+/+ and PEDF-deficient (PEDF−/−) mice and determined the impact of PEDF expression on the proangiogenic and pro-inflammatory properties of ChECs. We showed that PEDF expression significantly affects the proliferation, migration, adhesion, and oxidative and inflammatory state of ChECs. The PEDF−/− ChECs were, however, more sensitive to H2O2 challenge and exhibited increased rate of apoptosis and oxidative stress. We also observed a significant increase in production of cytokines with a primary role in inflammation and angiogenesis including vascular endothelial growth factor (VEGF) and osteopontin, and a reprograming of chemokines and cytokines expression profiles in PEDF−/− ChECs. Collectively, our results indicate that PEDF expression has a significant impact on oxidative and inflammatory properties of ChECs, whose alteration could contribute to pathogenesis of chronic inflammatory diseases including exudative AMD.
Keywords: cell adhesion, extracellular matrix proteins, inflammation, oxidative stress, thrombospondins
INTRODUCTION
Age-related macular degeneration (AMD) is associated with progressive macular degeneration and severe irreversible vision loss (11, 37, 54). AMD is characterized in two major forms. The dry form, which involves degeneration of retinal pigment epithelial (RPE) cells, and the exudative or wet form, which is associated with choroidal neovascularization (CNV) (2, 34). Choriocapillaris is the most distinguished anatomical feature of the choroid that is located beneath the RPE and supplies nutrients to photoreceptor and RPE cells. Morphometric changes in choroidal vascular density may play an important role in the development and progression of AMD. Reduced density and diameter of choriocapillaris is observed with aging and during the early phases of AMD (8). Although various factors are identified as contributors to choroidal dysfunction in AMD, there is not much known about the detailed molecular and cellular mechanisms that impact the choroid and pathogenesis of AMD.
Alterations in the choroid and peripheral vasculature in AMD imply abnormal endothelial cell (EC) function. Unlike retinal vasculature, fenestrated ECs are observed in the inner surface of choriocapillaris, and its basement membrane is involved in transporting nutrients to the RPE and photoreceptors (8, 13, 51, 75). Choroidal ECs (ChECs) also display different responses to various growth factors, including insulin-like growth factor-I (IGF-I), fibroblast growth factor-2 (FGF2), and vascular endothelial growth factor (VEGF) when compared with retinal ECs (12, 78). In addition, increased production of VEGF is crucial in the development of clinically relevant AMD changes that are associated with CNV. Furthermore, decreased production or loss of endogenous inhibitors of angiogenesis, including pigment epithelium-derived factor (PEDF), are suggested as important factors favoring CNV. Decreased level of PEDF is detected in vitreous and/or serum samples from patients with AMD. Clinical studies also indicated decreased levels of PEDF in Bruch’s membrane and choriocapillaris complex during AMD (8).
PEDF is a 50-kDa glycoprotein and a member of serine protease inhibitor (serpins) family that lacks activity (5, 6, 69). It acts as a multifunctional protein with major roles in different physiological and pathophysiological mechanisms, including neuroprotection, angiogenesis, inflammatory responses, and fibrosis (31). Although endogenous expression of PEDF minimally affects ocular vascular development and neovascularization during oxygen-induced ischemic retinopathy (OIR) or laser-induced CNV (25, 35), exogenous PEDF inhibits angiogenesis by blocking the activity of proangiogenic factors including VEGF and FGF2 (19). PEDF induces apoptosis of ECs by activating FAS/FAS-L signal transduction cascade (4, 71). In addition, overexpression of PEDF in the eye leads to dramatic inhibition of neovascularization during OIR (56). Similarly, intravitreal injection of adeno-associated virus-encoding PEDF significantly inhibited retinal and choroidal neovascularization during OIR and CNV, respectively (53). However, the cell autonomous effect of PEDF expression on ChECs remains unknown.
The ChEC cultured from bovine and human eyes have provided some insight into their physiology. However, these cells have limited proliferation capacity and/or require special conditions that restrict extensive biochemical studies that require large number of cells (30, 33). We recently described a method for culturing ChECs from wild-type and transgenic mice that can be readily manipulated in culture and maintained for many passages without a significant impact on the expression of their specific markers (26). Here, using ChECs prepared from PEDF+/+ and PEDF−/− mice, we show for the first time that PEDF expression has a significant impact on ChEC function. PEDF−/− ChECs exhibited increased proliferation, decreased adhesion and migration, failed to undergo capillary morphogenesis, and produced increased levels of pro-inflammatory cytokines VEGF and osteopontin, along with a reprogrammed chemokine and cytokine expression profile. PEDF−/− ChECs also exhibited increased oxidative stress and were more susceptible to oxidative challenge. Thus expression of PEDF plays an important role in oxidative and inflammatory properties of ChECs.
MATERIALS AND METHODS
Experimental animals.
All experiments were conducted in accordance with the Association for Research in Vision and Ophthalmology statement for the use of Animals in Ophthalmic and Vision Research and were approved by the Institutional Animal Care and Use Committee of the University of Wisconsin School of Medicine and Public Health. Immorto mice expressing a temperature-sensitive simian virus 40 large T antigen were obtained from Charles Rivers (Wilmington, MA) and backcrossed to C57BL/6j for at least 10 generations. PEDF−/− mice on a C57BL/6J background were generated and maintained, as previously described, and crossed with Immorto mice (42, 68). The isolated DNA from tail biopsies was used for screening of various transgenes, as previously described (62, 68).
Isolation and culture of ChECs.
ChECs were isolated form one litter (6 or 7 pups) of 4-wk-old PEDF+/+ and PEDF−/− Immorto mice (all on C57BL/6J background), as previously described (26). A mixture of male and female mice were used. The isolated ChECs were plated into a single well of a 24-well plate precoated with 2 µg/ml of human fibronectin (BD Biosciences, Bedford, MA). ChECs were grown in DMEM containing 10% FBS, 2 mM l-glutamine, 2 mM sodium pyruvate, 20 mM HEPES, 1% nonessential amino acids, 100 µg/ml streptomycin, 100 U/ml penicillin, freshly added heparin at 55 U/ml (Sigma, St. Louis, MO), endothelial growth supplement 100 µg/ml (Sigma), and murine recombinant interferon-γ (IFN-γ; R & D, Minneapolis, MN) at 44 units/ml and incubated in a tissue culture incubator at 33°C with 5% CO2. Cells were progressively passaged to larger plates and maintained in 60-mm tissue culture plates coated with 1% gelatin. For all experiments, cells were incubated with EC growth medium containing IFN-γ in a tissue culture incubator at 33°C with 5% CO2 unless stated otherwise. To confirm the observed results were specifically due to PEDF deficiency and independent of large T antigen expression, cells were incubated with EC growth medium without IFN-γ in a tissue culture incubator at 37°C with 5% CO2 for at least 48 h (44). For all experiments, cells were used at 70–80% confluence. For some experiments, cells were allowed to reach confluence, including junctional localization and conditioned medium studies. Two different isolations of ChECs were used in these studies, and all experiments were repeated at least once (n ≥ 4).
FACS analysis.
ChECs from 60-mm culture plates were rinsed with PBS containing 0.04% EDTA and incubated with 1.5 ml of cell dissociation solution [2 mM EDTA, 0.05% BSA in Tris-buffered saline (TBS); 25 mM Tris·HCl, 150 mM NaCl, pH 7.6]. Cells were then washed, collected from plates with DMEM containing 10% FBS, centrifuged, and blocked in 0.5 ml of TBS with 1% goat serum for 20 min on ice. Cells were then pelleted and incubated in 0.5 ml TBS with 1% BSA containing a specific primary antibody on ice for 30 min. The following antibodies were used at dilutions as recommended by the supplier: anti-VE-cadherin (catalog no. ALX-210-232-C100; Enzo Life Sciences, Farmingdale, NY); anti-VCAM-1 (CBL1300), anti-endoglin (CBL1358), anti-β1 (MAB 2000), anti-β2 (MABT42), anti-β3 (MAB 1957), anti-α5β1 (MAB 1999), anti-αvβ3 (MAB 1976Z), anti-α2 (AB1936), anti-α3 (AB1920), anti-α5 (AB1921), anti-αV integrins (MAB 1930) (Millopore, Billerica, MA); anti-ICAM-1 (SC-1511), anti-β5 (SC-5401), anti-β8 (SC-25714) integrins (SC-10817), and HARE-Y20 (stabilin-2) (sc-27751) (Santa Cruz Biotechnology, Santa Cruz, CA); anti-ICAM-2, anti-α1-integrin, anti-β4, anti-PV-1, and anti-platelet EC adhesion molecule-1 (PECAM-1) (BD Biosciences); anti-VEGF receptor-1 (VEGFR-1), anti-VEGFR-2, and α7 integrin (R&D Systems); anti-PDGF-Rα and anti-PDGF-Rβ (eBioscience, San Diego, CA); and anti-FAS and anti-FAS-L (Enzo Life Sciences). After incubation, cells were then washed twice with TBS containing 1% BSA and incubated with appropriate FITC-conjugated secondary antibody (Jackson ImmunoResearch, West Grove, PA) prepared in TBS containing 1% BSA for 30 min on ice. After incubation, cells were rinsed twice with TBS containing 1% BSA, resuspended in 0.5 ml of TBS with 1% BSA and analyzed by FACScan caliber flow cytometer (Becton Dickinson, Franklin Lakes, NJ). These experiments were repeated twice using two different isolations of ChECs with similar results. The representative mean fluorescent intensities are shown for each antibody in each panel.
Cell proliferation assays.
Cell proliferation was evaluated by counting the number of cells for 2 wk. Cells (7 × 103) were plated in multiple sets of gelatin-coated 60-mm tissue culture plates, fed every other day for the duration of experiment. The number of cells was determined by counting every other day, on days not fed, in triplicates. The rate of DNA synthesis was also assessed using Click-It EdU Alexa Flour 488 as recommended by the supplier (Life Technologies, Grand Island, NY). The assay quantifies the rate of DNA synthesis using 5-ethynyl-2′-deoxyuridine (EdU), a nucleoside analog of thymidine. The percentage of cells undergoing active DNA synthesis was determined by FACScan caliber flow cytometry (Becton Dickinson).
TdT-dUPT terminal nick-end labeling (TUNEL) was used to assess rates of apoptotic cell death. TUNEL staining was performed using Click-iT-TUNEL Alexa Flour imaging assay as recommended by the supplier (Life Technologies). A similar experiment was performed in the presence of 200 µM H2O2 (Fisher Scientific). This concentration was determined based on moderate effect on cell viability after 24–48 h. Positive apoptotic cells were counted in 10 high-power fields (×200) and calculated as percentage of total cell number. All samples were prepared in duplicate and repeated twice.
Indirect immunofluorescence studies.
Cells (7 × 104) were plated on fibronectin-coated 4-well chamber slides (5 µg/ml in DMEM for 2 h in the tissue culture incubator) and allowed to reach confluence (2–3 days). Cells were washed with PBS, fixed with cold acetone for 10 min on ice, permeabilized with TBS containing 0.1% Triton X-100 for 12 min at room temperature, and then blocked with TBS containing 1% BSA at 37°C for 30 min. After incubation, slides were rinsed once with TBS and incubated with specific primary antibodies for 2 h at room temperature. The primary antibodies were anti-ZO1 (catalog no. MABT11; Thermofisher), anti-β-catenin (catalog no. 610154), anti-P120 catenin (catalog no. 610133); anti-N-cadherin (catalog no. 610920; BD Bioscience); and anti-VE-cadherin (catalog no. 141441; eBioscience) prepared in TBS (1:200) containing 1% BSA. After incubation, slides were rinsed with TBS and incubated with specific Cy3-conjugated secondary antibodies (1:800; Jackson ImmunoResearch) in TBS containing 1% BSA for 1 h at room temperature. After incubation, slides were washed four times for 10 min with TBS and evaluated using a fluorescence microscope (Carl Zeiss Optical, Germany), and images were obtained in digital format.
Scratch wound assays.
Cells (1 × 106) were plated in 60-mm tissue culture dishes and allowed to reach confluence (1–2 days). Plates were wounded using a 1-ml micropipette tip, washed with growth medium twice to remove detached cells, and fed with growth medium containing 1 µM 5-fluorouracil (Sigma) to block cell proliferation. Wound closure was monitored by phase microscopy at different time points (0, 24, 48 h) and images were captured in digital format. The migrated distance as percentage of total distance was determined for quantitative assessment of data, as previously described (20).
Transwell migration assays.
Cell migration was also determined using a transwell migration assay. Costar transwell inserts (8-µm pore size, 6.5-mm membrane; Lowell, MA) were coated with DMEM containing fibronectin (5 µg/ml) on the bottom side at 4°C overnight. After washing with PBS, inserts were blocked in PBS containing 1% BSA for 1 h at room temperature and rinsed with PBS. Cells were trypsinized and resuspended in serum-free medium, and 1 × 105 cells/0.1 ml was added to the top of inserts. The inserts were placed in a 24-well plate containing 0.5 ml of serum-free medium and incubated for 4 h at 33°C. After incubation, cells were fixed with 4% paraformaldehyde for 12 min at room temperature, stained with hematoxylin and eosin, and the inserts were mounted on a slide with the cell side facing down. The number of cells that migrated through the membrane was determined by counting 10 high-power fields (×200). Each experiment was done in triplicate and repeated with two different isolation of cells (n ≥ 6).
Cell adhesion assays.
Cell adhesion assays were conducted using 96-well plates (Maxisorb Nunc Immunoplate, Fisher Scientific) coated with different concentrations of collagen I, collagen IV, vitronectin, and fibronectin (BD Biosciences), and diluted in TBS (50 µl/well) containing 2 mM CaCl2, 2 mM MgCl2 (Ca/Mg) overnight at 4°C. Plates were rinsed four times with TBS containing Ca/Mg (200 µl/well) and blocked using TBS with Ca/Mg containing 1% BSA (200 µl/well) at room temperature for 1 h. Cells were collected from tissue culture plates using 2 ml of dissociation buffer (2 mM EDTA, 0.05% BSA in TBS), rinsed with TBS, and resuspended in cell binding buffer (150 mM NaCl, 20 mM HEPES, 4 mg/ml BSA, pH 7.4) at ~5 × 105 cells/ml. The coated plates were washed with TBS containing Ca/Mg and incubated with equal amount (50 µl/well) of cell suspension and TBS with Ca/Mg for 2 h at 37°C. After incubation, plates were washed with TBS with Ca/Mg to remove nonadherent cells. The number of adherent cells was quantified by measuring intracellular acid phosphatase activity, as previously described (59). These assays were performed in triplicate and repeated with two different isolations of ChECs (n ≥ 6).
Western blot analysis.
Cells (1 × 106) were plated in 60-mm tissue culture dishes until confluence (~90%). Cells were then washed with serum-free DMEM medium and incubated in growth medium without serum (conditioned medium, CM) for 2 days. The CM was centrifuged at 400 g for 5 min to remove cell debris and stored at −80°C for further analysis. Cell lysates were also prepared using 200 µl of lysis buffer (50 mM HEPES, pH 7.5, 1 mM MgCl2, 1 mM CaCl2, 100 mM NaCl and 0.1 mM EDTA with 1% NP-40, 1% Triton X-100, and protease inhibitor cocktail; Roche Biochemicals, Mannheim, Germany). BCA protein assay (Bio-Rad, Hercules, CA) was used to determine protein concentration. Samples were mixed with appropriate amount of 6X SDS sample buffer and analyzed by 4–20% SDS-PAGE (Invitrogen). Proteins were transferred to nitrocellulose membrane and blocked in TBS containing 0.05% Tween 20 (TBST) with 5% skim milk for 1 h at room temperature. Membranes were incubated with primary antibody for 2 h at room temperature, washed with TBST, and incubated with appropriate horseradish peroxidase-conjugated secondary antibody (1:10,000; Jackson ImmunoResearch) for 2 h at room temperature. The following antibodies were used at dilutions recommended by the supplier: anti-fibronectin (catalog no. SC-9068), anti-eNOS (catalog no. SC-654), and anti-c-Src (catalog no. SC-8056) (Santa Cruz Biotechnology); anti-tenascin C (catalog no. AB19013) and anti-TSP1 (catalog no. MA5-13398; Thermofisher); anti-iNOS (catalog no. 610332), anti-nNOS (catalog no. 610310), anti-N-Cadherin (catalog no. 610920), anti-β-Catenin (catalog no. 610154), anti-p120 (catalog no. 610133), and anti-TSP2 (catalog no. 611150; BD Bioscience); anti-SPARC (catalog no. AF942), anti-opticin (catalog no. AF3547), anti-periostin (catalog no. MAB348), anti-osteopontin (catalog no. AF808), and anti-PEDF (catalog no.MAB1149; R&D Systems); anti-collagen IV (catalog no. AB756P; Millipore); anti-p-Src (catalog no. 2101, Tyr416), anti-p-P38 (catalog no. 9211), anti-P38 (catalog no. 9212), anti-p-Akt (catalog no. 9271), anti-Akt (catalog no. 9272), anti-p-ERK (catalog no. 9106), and anti-ERK (catalog no. 9102; Cell Signaling); and anti-β-actin (catalog no. A2066, Sigma). The protein bands were visualized using enhanced chemiluminscence reagent (GE Bioscience, Piscataway, NJ). The mean band intensities were determined using Image J 1.46a (National Institute of Health, Bethesda, MD) and normalized against β-actin band as loading control.
Secreted VEGF levels.
The level of VEGF produced by PEDF+/+ and PEDF−/− ChECs were determined using the Mouse VEGF Immunoassay Kit (R&D Systems). Cells (1 × 106) were plated in 60-mm tissue culture dishes and allowed to reach 90% confluence. The cells were washed with serum-free DMEM and incubated with 2 ml of growth medium without serum for 2 days. The CM were collected, centrifuged at 400 g for 5 min to remove cell debris, and used for VEGF measurements as recommended by the supplier.
Capillary morphogenesis assays.
Tissue culture plates (35 mm) were coated with 0.5 ml Matrigel (10 mg/ml; BD Biosciences) and incubated at 37°C for at least 30 min to allow harden. The ChECs were removed by trypsin-EDTA and washed with DMEM containing 10% FBS. Cells (1.5 × 105 cells/ml) were resuspended in serum-free medium. Cells (2 ml) were applied to the Matrigel-coated plates, incubated at 37°C, and photographed after 14–18 h with a Nikon microscope in a digital format. For quantitative assessment of the data, the mean number of branch points, was determined by counting the branch points in five high-power fields (×100).
PEDF expression studies in PEDF−/− ChECs.
To express PEDF in PEDF−/− ChECs, 1 × 105 cells were plated in 35-mm tissue culture dishes. The next day, 40 μl of lenti booster (Sirion Biotech, Martinsried, Germany) including 20 μl each of 1:100 dilution of solution A and B in DMEM were mixed with 2 ml of EC growth medium and added to plates and incubated overnight. The next day, lenti viruses encoding human PEDF or GFP (control) (25 pfu/cell, 12.5 μl) were mixed and diluted in 750 μl of opti-MEM (Life Technologies) and incubated for 15 min at room temperature. After incubation, the tissue culture plates were washed twice with serum-free medium and incubated with 750 µl of lenti virus mixture overnight. The next day, medium containing lenti virus mixture was removed, and fresh growth medium was added to the plates and incubated for 1 day. The next day, puromycin (1 µg/ml), as a selection reagent in growth medium, was added to the plates and incubated as needed to selectively grow and expand infected cells for further analysis.
RT2 profiler PCR array analysis.
Changes in expression of inflammatory cytokines and chemokines were detected by using RT2 Profiler Mouse inflammatory response and autoimmunity PCR array (catalog no. PAMM-077Z, QIAGEN). Total RNA was extracted as described in the previous section. The cDNA was synthesized using RT2 First Strand Kit (catalog no. 330404, QIAGEN). RT2 SYBR Green qPCR Master Mix (catalog no. 330521, QIAGEN) was used for the reaction, following manufacturer’s instructions. Amplification and real-time analysis were performed using an Eppendorf Realplex PCR machine. Gene expression analysis was performed as recommended by the supplier, and the genes whose expression was increased or decreased by at least twofold are summarized in Tables 1 and 2.
Table 1.
Genes overexpressed in PEDF−/− vs. PEDF+/+ ChECs
| Position | Gene Symbol | Fold Regulation | Comments | RT2 Catalog |
|---|---|---|---|---|
| H12 | PPC | 21.69 | ||
| F04 | Ltb | 5.44 | PPM03119A | |
| C02 | Cd40 | 5.27 | PPM03426C | |
| D04 | Cxcr4 | 5.16 | PPM03149E | |
| F08 | Nos2 | 3.07 | PPM02928B | |
| E04 | Il1rn | 2.98 | PPM03547B | |
| C07 | Cxcl1 | 2.97 | A | PPM03058C |
| G09 | Tlr9 | 2.74 | PPM04221A | |
| F01 | Itgb2 | 2.72 | PPM03592F | |
| A11 | Ccl20 | 2.61 | PPM03142B | |
| C10 | Cxcl2 | 2.42 | PPM02969F | |
| D08 | Il10 | 2.25 | PPM03017C | |
| H07 | RTC | 2.23 | PPX63340A | |
| A01 | Bcl6 | 2.22 | PPM04965C | |
| H09 | RTC | 2.21 | PPX63340A | |
| B10 | Ccr3 | 2.19 | PPM03173A | |
| A12 | Ccl22 | 2.02 | PPM02950B | |
| H08 | RTC | 2.02 | PPX63340A | |
| B11 | Ccr4 | 2.02 | PPM03147A |
Fold change threshold is 2. Fold change values >1 imply a positive or an increase, and the fold regulation is equal to fold change. Fold change values <1 imply a negative or decrease, and the fold regulation is the negative inverse of the fold change. A: the gene’s average threshold cycle is relatively high (>30) in either the control or test sample, and is reasonably low in other sample (<30). These data mean that the gene expression is relatively low in one sample and reasonably detected in the other sample, suggesting that the actual fold change value is at least as large as the calculated and reported fold change result.
Table 2.
Genes underexpressed in PEDF−/− vs. PEDF+/+ ChECs
| Position | Gene Symbol | Fold Regulation | Comments | RT2 Catalog |
|---|---|---|---|---|
| C11 | Cxcl3 | −28.47 | A | PPM34590C |
| B04 | Ccl4 | −13.65 | A | PPM02948F |
| C09 | Cxcl11 | −7.76 | A | PPM03192C |
| E09 | Il6 | −7.14 | A | PPM03015A |
| A03 | C3ar1 | −5.92 | PPM04821A | |
| A07 | Ccl12 | −5.36 | A | PPM02977E |
| B07 | Ccl8 | −5.08 | PPM03165A | |
| A06 | Ccl11 | −4.60 | PPM02967G | |
| F10 | Ptgs2 | −2.81 | PPM03647E | |
| B05 | Ccl5 | −2.10 | PPM02960F | |
| C06 | Csf1 | −2.08 | PPM03116C | |
| B12 | Ccr7 | −2.07 | PPM03156G | |
| G03 | Tlr2 | −2.04 | PPM04220B |
Fold change threshold is 2. Fold change values >1 imply a positive or an increase, and the fold regulation is equal to fold change. Fold change values <1 imply a negative or decrease, and the fold regulation is the negative inverse of the fold change. A: the gene’s average threshold cycle is relatively high (>30) in either the control or the test sample, and is reasonably low in the other sample (<30). These data mean that the gene expression is relatively low in one sample and reasonably detected in the other sample, suggesting that the actual fold change value is at least as large as the calculated and reported fold change result.
RNA purification and qPCR analysis.
The total RNA from ChECs was extracted using mirVana PARIS Kit (Invitrogen). cDNA synthesis was performed from 1 µg of total RNA using Sprint RT Complete-Double PrePrimed Kit (Clontech, Mountain View, CA). One microliter of each cDNA was used as template for qPCR performed in triplicates of three biological replicates using a Mastercycler Realplex (Eppendorf, Hauppauge, NY) and SYBR Green qPCR Premix (Clontech). Amplification parameters were as follows: 95°C for 2 min followed by 40 cycles of amplification (95°C for 15 s, 60°C for 40 s), and the dissociation curve step (95°C for 15 s, 60°C for 15 s, 95°C for 15 s). Primer sequences for different inflammatory cytokines were TNF-α 5′-ACCGTCAGCCGATTTGCTAT-3′ (forward) and TNF-α 5′-TTGACGGCAGAGAGGAG GTT-3′ (reverse), IL-18 5′-AAGAA AATGGAGACC TGGAATCAG-3′ (forward) and IL-18 5′-ATTCCGTATTACTGCGGTT GTACA-3′ (reverse), MCP-1 5′-GTCTGTGCTGACCCC AAGAAG-3′ (forward) and MCP-1 5′-TGG TTCCGATCCAGGTTTTTA-3′ (reverse), RANTES 5′-GCCCACGTC AAGGAGTA TTTCT-3′ (forward) and RANTES 5′-CAAACA CGACTGCAAGATTGGA-3′ (reverse), IL-1β 5′-GTTCCCATTAGACAACTGCACTACA-3′ (forward) and 5′-CCGACA GCACGAGGCTTTT-3′(reverse), VEGF 5′-GGAGAGCAGAAGTCCCATGA-3′ (forward) and 5′-ACT CCAGGGCTTCATCGTTA-3′ (reveres), IL-6 5′-CAACCACGGCCTTCCC TACT-3′ (forward) and 5′-TTGGGAGTGGTA TCCTCTGTGA-3′(reverse), PEDFR 5′-AATCTCTACCGCCTCTCGAA-3′ (forward) and 5′-TTGGTTCAGT AGGCCATTCC-3′ (reverse), PEDF laminin R 5′-CCATCGAGAATCCT GCTGAC-3′ (forward) and 5′-GGCTGCTTGG ATCTGGTTAG-3′(reverse), human PEDF 5′-CTGTTTTACGCTAT GGCTTGG-3′ (forward) and 5′-GATACTCATGCTTCCGGTCAA-3′ (reverse), and mouse PEDF 5′-GCCCAGATGAAAGGGAAGATT-3′ (forward) and 5′-TGAGGGCACTGGGCATTT-3′ (reverse).
The linear regression line for nanograms of DNA was determined from relative fluorescent units at a threshold fluorescence value (Ct) to quantify gene targets from cell extracts and normalized by the simultaneous amplification of RpL13A, a housekeeping gene. The following primers for RpL13A were used: 5′-TCTCAAGGTTGTTCGGCTGAA-3′ (forward) and 5′-CCAGACG CCCCAGGTA-3′ (reverse). Mean and standard deviation of all experiments performed were calculated after normalization to RpL13A.
Nitric oxide measurements.
The intracellular nitric oxide (NO) levels produced by PEDF+/+ and PEDF−/− ChECs were determined using 4-amino-5-methylamino-2 and 7-difluoroflourescein diacetate (DAF-FM diacetate; Invitrogen). DAF-FM is produced after deacetylation of DAF-FM diacetate by intracellular esterases. The elevated fluorescence intensity of DAF-FM due to the reaction with NO is measured with a fluorescein filter. Cells (5 × 103 cells/0.1 ml) were plated on gelatin-coated 96-well black/clear bottom plates (BD Biosciences) and incubated overnight. The next day, medium was removed ,and 0.1 ml EC growth medium containing 30 μM DAF-FM was added to each well and incubated for 40 min. The medium was replaced with fresh EC medium and incubated for an additional 30 min. After incubation, the cells were washed with TBS (0.1 ml, twice), and fluorescence intensity was measured at an excitation of 458 nm and an emission of 535 nm using a fluorescent microplate reader (CLARIOstar, BMG LABTECH). This assay was conducted in triplicate and repeated with two different isolations of ChECs.
Assessments of reactive oxygen species.
The level of cellular reactive oxygen species (ROS) was assessed with dihydroethidium staining (DHE). DHE is a weak blue fluorescent dye that binds to DNA on oxidation to red fluorescent ethidium by O2·−. Cells (3 × 104) were plated on fibronectin-coated chamber slides (5 µg/ml; BD Bioscience) and incubated for 24 h in growth medium. After incubation, the cells were exposed to 1 µM DHE for 15–20 min, washed with growth medium and incubated twice with growth medium each for 30 min. Digital images were captured at the same exposure time for all samples. For quantitative analysis, images were analyzed with ImageJ software (National Institute of Health, Bethesda, MD). The mean fluorescent intensity was determined from intensities of multiple cells (100 cells). A similar experiment was performed using cells treated with 100 µM H2O2 (Fisher Scientific) for 24 h, and three independent experiments with two different isolations of ChECs were performed.
Statistical analysis.
Statistical differences between control and treated samples were evaluated using GraphPad Prism software (La Jolla, CA) according to the Tukey multiple comparison test with a P value <0.05 considered significant.
RESULTS
Isolation and characterization of PEDF−/− ChECs.
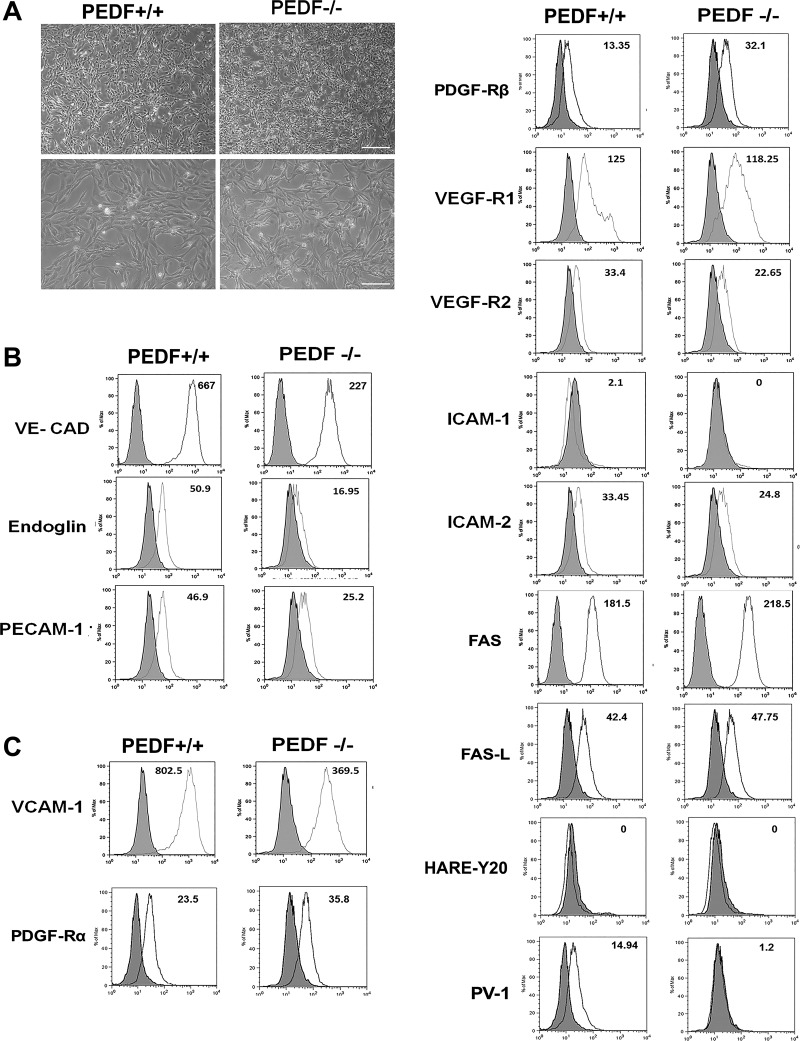
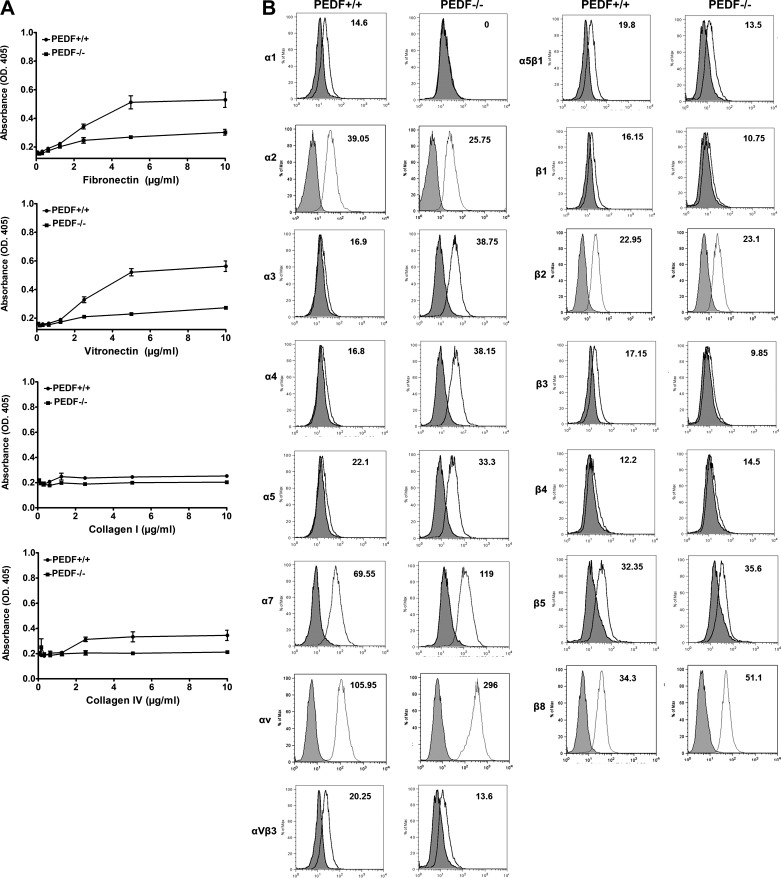
ChECs were prepared from 4-wk-old PEDF+/+ and PEDF−/− Immorto mice as described in materials and methods. We isolated a homogeneous population of ChECs with greater than 98% purity determined by FACS analysis and immunofluorescence staining. Figure 1A shows the morphology of ChECs prepared from PEDF+/+ and PEDF−/− mice. PEDF−/− ChECs exhibited a similar morphology to PEDF+/+ cells when plated on gelatin-coated plates. The expression of ChEC markers was evaluated by FACS analysis (Fig. 1B), with all cells expressing VE-cadherin and PECAM-1 as expected. The results are representative of sample mean fluorescence intensity from two different isolations. The PEDF−/− ChECs exhibited a dramatic decrease in the VE-cadherin and PECAM-1 expression compared with PEDF+/+ cells. In addition, PEDF deficiency resulted in decreased expression of endoglin, a gene whose increased expression is associated with angiogenesis (57).
Fig. 1.
Isolation and characterization of mouse ChECs. PEDF+/+ and PEDF−/− ChECs were prepared as described in materials and methods and propagated in 60-mm tissue culture dishes coated with 1% gelatin. A: phase micrographs of ChECs. Images were captured in digital format at scale bar = 100 µm and magnifications at scale bar = 20 µm. B: expression of specific ChEC markers. ChECs were examined for expression of VE-cadherin, endoglin, and PECAM-1 by FACS analysis. C: expression of other RPE cell markers. ChECs were examined for expression of VCAM-1, PDGF-Rα, PDGF-Rβ, VEGF-R1, VEGF-R2, ICAM-1, ICAM-2, FAS, FAS-L, PV-1, and HARE-Y20 by FACS analysis. The shaded area shows control IgG staining. These experiments were repeated using two different isolations of ChECs with similar results (n ≥ 3). The representative mean fluorescence intensities are indicated in each graph.
We next examined the expression of other EC markers by FACS analysis (Fig. 1C). Altered expression of VCAM-1, VEGF receptors, and ICAM-1 are considered as underlying factors in the progression of exudative AMD. Decreased VCAM-1 expression was observed in PEDF−/− ChECs compared with PEDF+/+ cells. We did not detect ICAM-1 expression in ChECs. However, ICAM-2 expression was moderately decreased in the absence of PEDF. The expression of VEGF-R1 and VEGF-R2 was observed in ChECs with modest variation between the genotypes (Fig. 1C). The role of PDGF-Rα and -Rβ in regulation of EC function is not well established. Studies indicate that not all ECs express PDGF-Rα and -Rβ. Here we observed that both PDGF-Rα and -Rβ are expressed by ChECs. In addition, the PEDF−/− ChECs exhibited an increase in expression of PDGF-Rα and -Rβ levels compared with PEDF+/+ cells (Fig. 1C). Furthermore, PEDF−/− ChECs displayed increased expression of FAS compared with PEDF+/+ cells, whereas FAS-L expression was similar in ChECs. We also examined markers of fenestration HARE-Y20 (stabilin-2) and PV-1 expression in ChECs. Only PEDF+/+ cells expressed detectable levels of PV-1 (Fig. 1C). These results suggest that PEDF deficiency could impact the proliferative and apoptotic properties of ChECs.
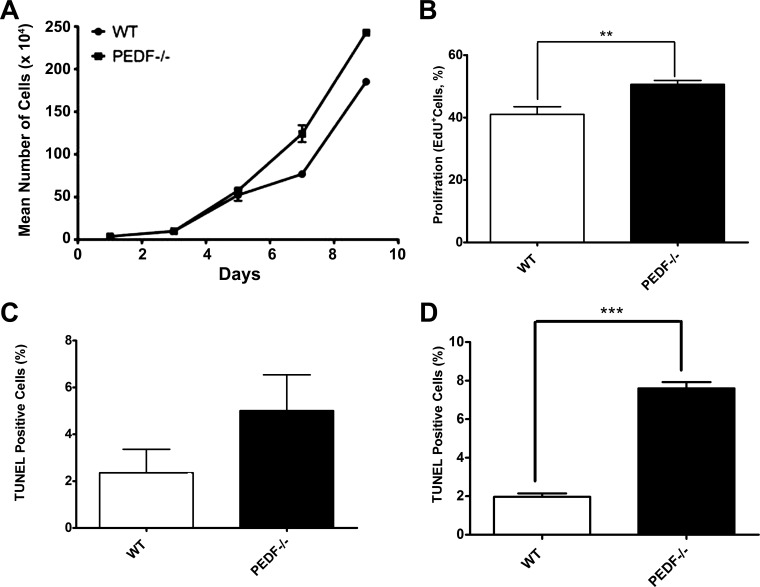
PEDF−/− ChECs exhibit increased proliferation.
The effect of PEDF deficiency on cell proliferation was determined by counting the number of cells for 2 wk. PEDF−/− ChECs showed an increase in the rate of proliferation compared with the PEDF+/+ cells (Fig. 2A). We next determined whether the increase in proliferation was the result of enhanced DNA synthesis. The percentage of cells undergoing active DNA synthesis was evaluated by EdU labeling, a synthetic nucleoside analog. We observed significant increase in DNA synthesis in PEDF−/− ChECs compared with PEDF+/+ cells, indicating PEDF deficiency was associated with enhanced ChECs proliferation (Fig. 2B).
Fig. 2.
Alterations in proliferation and apoptosis of ChECs. PEDF deficiency resulted in decreased proliferation of ChECs. A: the rate of cell proliferation was determined by counting the number of cells. A significant increase in the proliferation of PEDF−/− ChECs was observed compared with PEDF +/+ cells. B: an increase in the rate of DNA synthesis was observed in PEDF−/− ChECs compared with the PEDF+/+ cells. C: the rate of apoptosis was determined using TdT-dUTP terminal nick-end labeling (TUNEL). A similar experiment was carried out using H2O2 (200 μΜ) as an inducer of apoptosis (positive control). No significant differences were observed in the basal rate of apoptosis in these cells. D: a significant increase in the rate of apoptosis was observed in PEDF−/− ChECs when challenged with H2O2 (200 μΜ) compared with PEDF+/+ cells (**P < 0.01, ***P < 0.001, n = 3).
We next determined whether lack of PEDF in ChECs affected their apoptosis. TUNEL staining was performed to assess the level of apoptosis in ChECs under basal and challenged conditions. No significant difference in the basal level of apoptosis was observed in PEDF+/+ and PEDF−/− cells (Fig. 2C). However, under stress conditions (200 µM H2O2) PEDF−/− ChECs displayed significantly higher rate of apoptosis compared with PEDF+/+ cells (Fig. 2D; P < 0.05). Thus lack of PEDF expression resulted in increased proliferation and apoptosis of ChECs in response oxidative challenge.
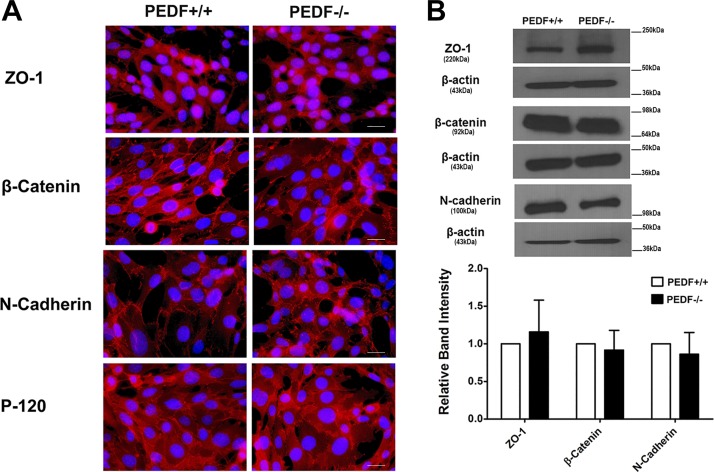
PEDF deficiency minimally affected the expression and organization of junctional proteins.
Adherens junctions facilitate intercellular adhesions, a process with major roles in maintaining vascular integrity. VE-cadherin plays an important role in maintaining vascular integrity by modulation of cell-cell interactions through formation of adherens junctions (15). Although we observed significant expression of VE-cadherin by FACS analysis in ChECs, we did not detect any junctional localization of VE-cadherin in both PEDF−/− and PEDF+/+ ChECs (not shown). This is in contrast to other types of ECs in which VE-cadherin is the major junctional cadherin and participates in junctional localization (59, 68). This suggests that another cadherin may take part in the formation and maintenance of adherent junctions in ChECs. An important cadherin in vascular stabilization and angiogenesis is N-cadherin, which generally competes with VE-cadherin for localization to the sites of cell-cell contact (46, 55, 73). However, PEDF deficiency did not affect N-cadherin expression or localization in PEDF−/− ChECs compared with PEDF+/+ cell (Fig. 3, A and B).
Fig. 3.
Cellular localization and expression of junctional proteins. A: the localization of ZO-1, N-cadherin, β-catenin, and P-120 catenin was determined by immunofluorescence staining. The PEDF+/+ and PEDF−/− ChECs were plated on fibronectin-coated chamber slides and stained with specific antibodies as detailed in materials and methods. No staining was observed in the absence of primary antibody (not shown). No significant difference was observed in localization of these proteins (scale bar = 20 µm). B: Western blot analysis of junctional proteins. Total cell lysates were prepared from PEDF+/+ and PEDF−/− ChECs and analyzed for expression of ZO-1, N-cadherin, β-catenin, P-120 catenin, and β-actin. β-Actin was used for loading control. The quantification of data is shown in the panel at bottom. No significant differences were observed in the levels of these proteins. These experiments were repeated using two different isolations of ChECs with similar results (n ≥ 3).
We also examined the effect of PEDF expression on β-catenin expression and junctional localization. No significant changes were observed in the expression level and junctional localization of β-catenin in PEDF−/− ChECs (Fig. 3, A and B). Similar results were observed for p120 localization in ChEC. ZO-1 has a crucial role in the formation and stabilization of tight junctions. Junctional localization of ZO-1 in ECs is dependent on VE-cadherin expression (26). Although PEDF deficiency in ChECs resulted in a dramatic decrease in VE-cadherin expression, we observed similar expression and junctional localization of ZO-1 in both PEDF+/+ and PEDF−/− ChECs (Fig. 3, A and B). To rule out any potential effects of large T antigen and IFN-γ present in growth medium on the junctional localization of ZO-1, all experiments were repeated in the absence of IFN-γ at 37°C with similar results (not shown). Thus PEDF deficiency minimally affected the localization of junctional proteins in ChECs.
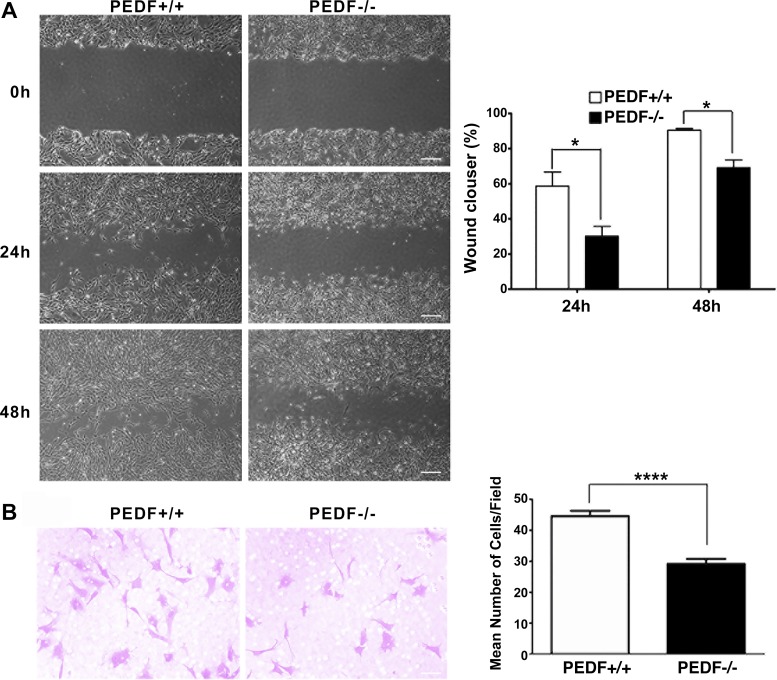
PEDF−/− ChECs were less migratory.
Cell migration is a crucial characteristic of ECs during angiogenesis. We next assessed the impact of PEDF expression on migration of ChECs using a scratch wound assay. PEDF−/− ChECs exhibited a significant delay in wound closure compared with PEDF+/+ cells (Fig. 4A). Similar results were observed using a transwell migration assay (Fig. 4B). Thus PEDF expression affects ChEC migration.
Fig. 4.
Attenuation of migration in PEDF−/− ChECs. A: scratch wound assay of ChEC monolayers on gelatin-coated plates was used to determine cell migration. Wound closure was observed by phase microscopy at indicated time points (scale bar = 100 µm). The quantitative assessment of wound migration is shown on the right (*P < 0.05; n = 3). B: migration of ChECs in transwell assays. Note the significant decrease in the migration of PEDF−/− ChECs compared with PEDF+/+ cells (*P < 0.05, ****P < 0.0001, n = 6; scale bar = 20 µm).
PEDF−/− ChECs were less adherent.
Altered migration of PEDF−/− ChECs suggested changes in their adhesion properties may exist. We next evaluated the adhesion of ChECs to various ECM proteins. PEDF−/− ChECs showed reduced adhesion on fibronectin, vitronectin, collagen I, and collagen IV compared with PEDF+/+ cells (Fig. 5A). To determine if altered adhesion was mediated through changes in integrin expression, we next examined the expression level of various integrins by FACS analysis. Similar expression levels for β2, β4, and β5 integrins were observed in both PEDF+/+ and PEDF−/− ChECs (Fig. 5B). However, PEDF−/− ChECs exhibited a modest decrease in expression of α2, αvβ3, α5β1, β1, and β3 compared with the PEDF+/+ cells (Fig. 5B). These results are consistent with reduced adhesion observed in PEDF−/− ChECs. PEDF−/− ChECs had undetectable levels of α1 integrin compared with PEDF+/+ cells. Furthermore, increased expression of α3, α4, α5, α7, αV, and β8 integrins was observed in PEDF−/− ChEC compared with PEDF+/+ cells (Fig. 5B). Although there were dramatic changes in the expression of integrins, the possibility of alteration in affinity and avidity of these integrins cannot be excluded.
Fig. 5.
Altered adhesion and expression of integrins in ChECs. A: adhesion of ChECs to fibronectin, vitronectin, collagen I, and collagen IV was determined as described in materials and methods. PEDF−/− ChECs adhered less to various extracellular matrix (ECM) proteins compared with PEDF+/+ cells. B: expression of integrins in ChECs; α1, α2, α3, α4, α5, α7, αv, αvβ3, α5β1, β1, β2, β3, β4, β5, and β8 integrin expression was determined by FACS analysis as described in materials and methods. Note lack of α1 detection in PEDF−/− ChECs. The representative mean fluorescence intensities are indicated for each graph. These experiments were repeated using two different isolations of ChECs with similar results (n ≥ 3).
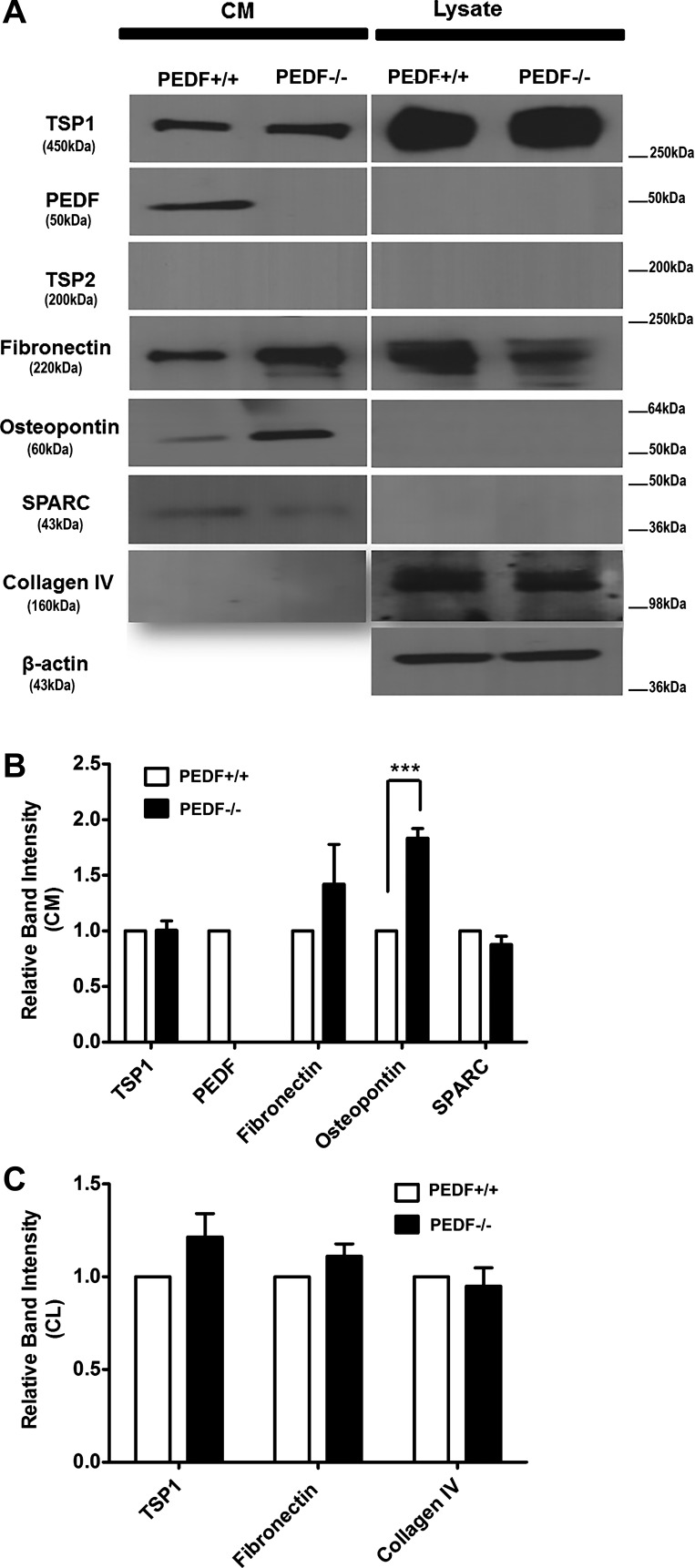
Altered production of different ECM proteins can influence different biological events, including cell adhesion and migration, diverse developmental processes, inflammatory responses, wound closure, and angiogenesis. We next determined ECM protein levels from ChECs, including fibronectin, collagen IV, PEDF, TSP1, TSP2, tenascin C, SPARC, opticin, periostin, and osteopontin (Fig. 6A). Although ChECs expressed significant amounts of TSP1, no detectable level of TSP2 was observed in these cells, regardless of PEDF status. PEDF deficiency resulted in increased expression of osteopontin and decreased expression of SPARC and collagen IV. We did not detect tenascin C, periostin, and opticin in ChECs. The quantitative assessment of the data is shown in Fig. 6B. An important role for osteopontin in chronic inflammation and vascular disease has been established (61). Thus the significant increase in production of osteopontin in PEDF−/− ChECs is consistent with the proposed anti-inflammatory activity of PEDF (1, 45, 61, 64).
Fig. 6.
Altered expression of ECM proteins in ChECs. A: PEDF+/+ and PEDF−/− ChECs were plated on gelatin-coated 60-mm dishes and incubated for 48 h in serum-free growth medium. The conditioned medium and cell lysates were collected for analysis of ECM proteins by Western blot analysis as described in materials and methods. The expression of TSP1, PEDF, TSP2, fibronectin, osteopontin, SPARC, and collagen IV was determined using specific antibodies. Note the increased expression of osteopontin in PEDF−/− ChECs compared with PEDF+/+ cells. β-Actin was used as a loading control for cell lysates (bottom). B and C: quantitative assessment of the data (***P < 0.001; n = 4). CM, conditioned medium; CL, cell lysate.
Increased production of VEGF in PEDF−/− ChECs.
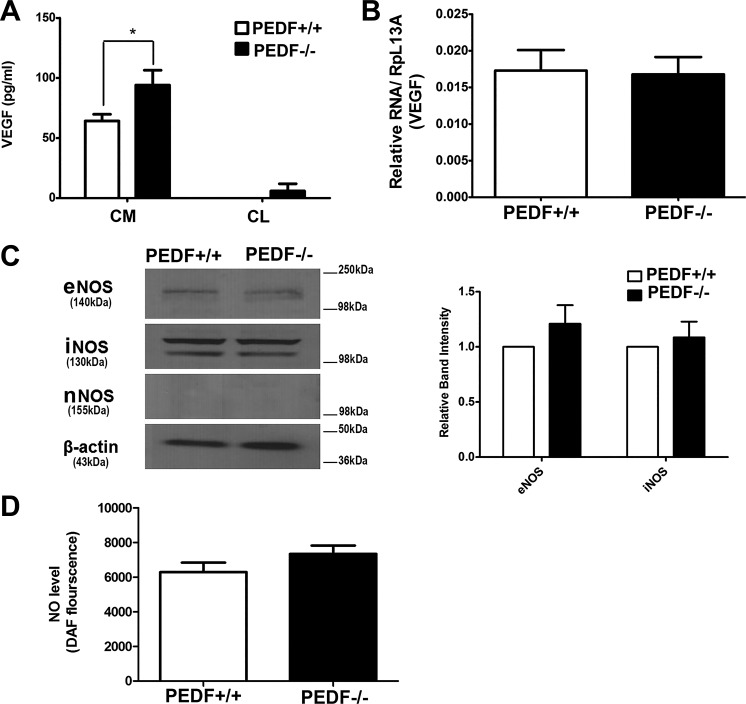
The increased production of VEGF, a cytokine with a primary role in inflammation and angiogenesis, has been indicated as a crucial factor in the development and progression of AMD and CNV (41, 76). To examine the impact of PEDF deficiency on VEGF production, we measured VEGF levels in conditioned medium collected form PEDF+/+ and PEDF−/− ChECs. A significant increase in VEGF levels was observed in PEDF−/− ChECs compared with PEDF+/+ cells (Fig. 7A). Very limited amounts of VEGF were cell associated. However, VEGF mRNA levels were not affected.
Fig. 7.
Alterations in VEGF and NOS expression. A: the level of VEGF was determined in conditioned medium (CM) or cell lysates (CL) collected from PEDF+/+ and PEDF−/− ChECs using an ELISA as detailed in materials and methods. Note the significant increase in the level of VEGF (CM) produced by PEDF−/− ChECs compared with PEDF+/+ cells (*P < 0.05, n = 3). B: the level of VEGF mRNA was determined by qPCR using RNA prepared from ChECs. There was no significant difference in the amount of VEGF mRNA detected in ChECs (P > 0.05, n = 3). C: the levels of eNOS, iNOS, and nNOS were determined by Western blot analysis of cell lysates. There was no significant difference in the level of eNOS and iNOS in ChECs. We did not detect nNOS expression in ChECs. β-Actin was used as a loading control. D: the level of intracellular NO was determined using DAF-FM as detailed in materials and methods. Note similar NO levels in all cells (P > 0.05, n = 3).
Nitric oxide (NO) modulates endothelium-derived vascular relaxation and plays a key role in VEGF-mediated angiogenesis (39–42). In the choroid, NO acts as vasodilator where it is colocalized with vasointestinal polypeptide (VIP) (43–45). Altered expression of nitric oxide synthase (NOS) isoforms has been reported in AMD (46). We next examined the expression of various NOS isoforms in PEDF−/− ChECs by Western blot analysis. In the choroid, endothelial NOS (eNOS) is associated with blood vessels. However, neuronal NOS (nNOS) was found in perivascular neurons, in scattered cells throughout the choroid, and in the RPE (9). We did not detect a significant difference in the expression of the inducible NOS (iNOS) and eNOS in PEDF−/− and PEDF+/+ ChECs. nNOS expression was undetectable in ChECs (Fig. 7C). These observations were consistent with similar NO levels detected in PEDF−/− ChECs compared with PEDF+/+ cells (Fig. 7D).
PEDF−/− ChECs failed to undergo capillary morphogenesis.
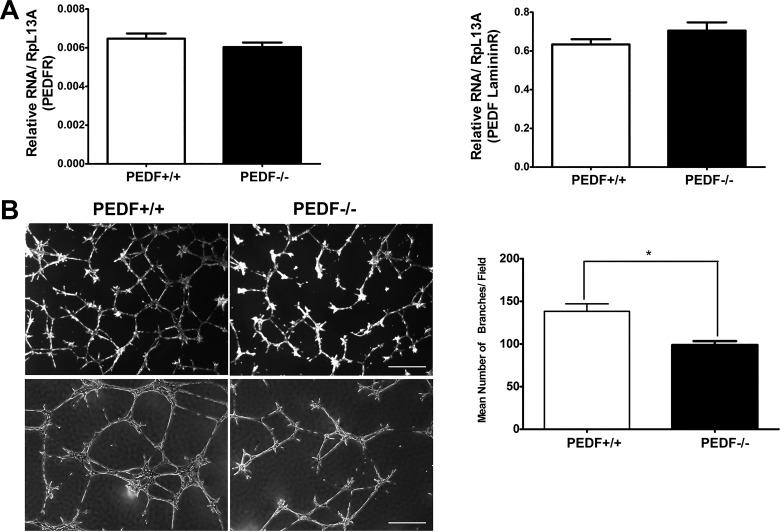
Formation of CNV under the retina results in leaking blood and serous fluid followed by blinding disciform scar in and under the retina, which ultimately leads to severe vision loss in patients with exudative AMD (17). PEDF conveys its biological activities through its receptors. Here we showed that ChECs express both major PEDF receptors, PEDFR and PEDF laminin R (Fig. 8A). The PEDF laminin R was expressed at nearly 100 times higher than PEDFR. PEDF deficiency did not affect PEDF receptors expression in ChECs (Fig. 8A).
Fig. 8.
Attenuation of capillary morphogenesis of PEDF−/− ChECs. A: ChECs express PEDF receptors. ChECs expressed similar levels of PEDF receptors, PEDFR and laminin B receptor, independent of PEDF expression. B: PEDF−/− ChECs fail to undergo capillary morphogenesis in Matrigel. Images were captured in digital format after 14 h in culture (scale bar = 50 µm in upper panels and 100 µm in lower panels). There was a significant decrease in capillary morphogenesis of PEDF−/− ChECs compared with PEDF+/+ cells (*P < 0.05, n = 3).
We next examined the ability of PEDF+/+ and PEDF−/− ChECs to undergo capillary morphogenesis. Endothelial cells undergo capillary morphogenesis forming a capillary-like network when plated on Matrigel, which mimics the late stages of angiogenesis (41). Capillary morphogenesis of ChECs was analyzed and quantified as described in materials and methods. PEDF−/− ChECs exhibited a significant decrease in capillary morphogenesis compared with PEDF+/+ cells (Fig. 8B). These results are consistent with decreased migratory and adhesive properties of PEDF−/− ChECs (Figs. 4 and 5). Representative images of capillary morphogenesis are shown in Fig. 8B.
Expression of PEDF is sufficient to restore capillary morphogenesis of PEDF−/− ChECs.
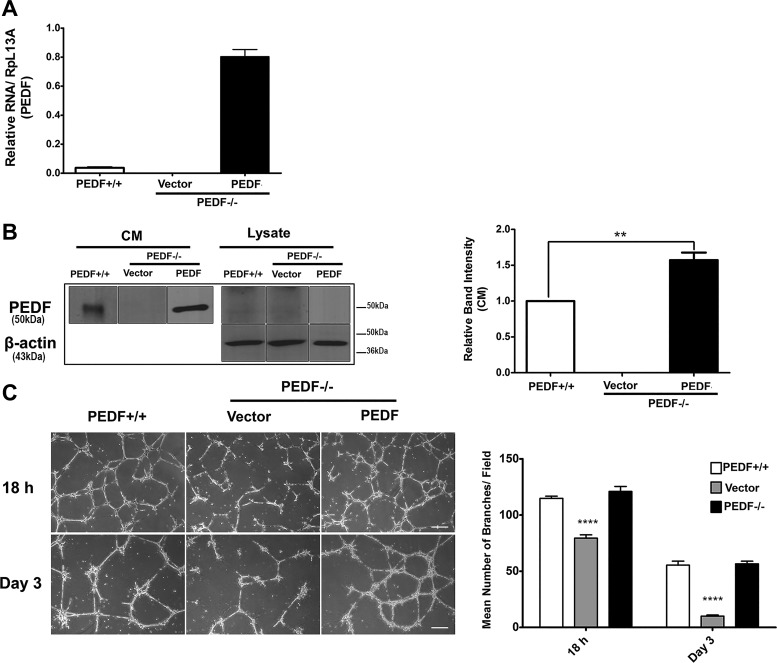
PEDF−/− ChECs showed attenuated capillary morphogenesis compared with PEDF+/+ cells. To determine if reexpression of PEDF is sufficient to restore capillary morphogenesis of PEDF−/− ChECs, we expressed human PEDF in ChECs. The expression of PEDF was confirmed by qPCR and Western blot analysis of cell lysates and conditioned medium (Fig. 9, A and B). A significant improvement in capillary morphogenesis was observed in PEDF−/− ChECs expressing human PEDF (Fig. 9C). In addition, reexpression of PEDF enhanced the stability of the capillary network formed by PEDF−/− ChECs in Matrigel (Fig. 9C).
Fig. 9.
PEDF expression restored capillary morphogenesis of PEDF−/− ChECs. PEDF−/− ChECs were infected with lenti virus-encoding human PEDF as described in materials and methods. A: expression of PEDF was quantified using PCR. Note expression of human PEDF in the transfected cells compared with the mouse PEDF in the PEDF+/+ cells. The human primers do not work well with mouse PEDF. B: the expression of PEDF was also confirmed by Western blot analysis. C: the capillary morphogenesis of PEDF−/− ChECs expressing human PEDF in Matrigel. Note the restored capillary morphogenesis and increased capillary network stability of PEDF−/− ChECs expressing PEDF (**P < 0.01, ****P < 0.0001, n = 3; scale bar = 100 µm).
PEDF−/− ChECs exhibited increased ROS generation.
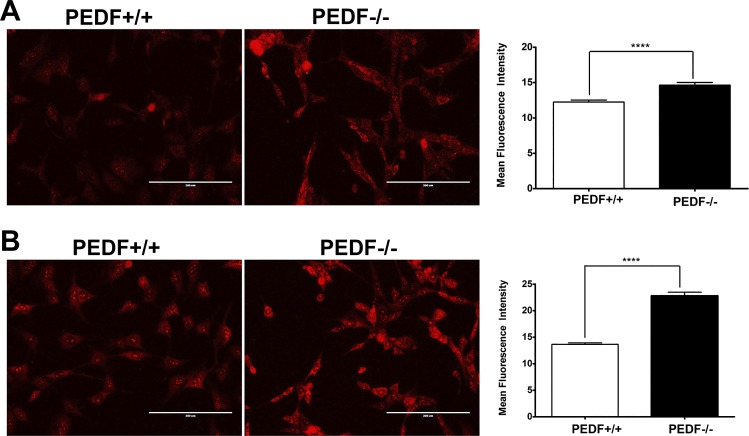
The majority of vascular diseases and their pathologies are associated with changes in the cellular oxidative state. Oxidative stress plays a key role in inflammatory responses and has been identified as a crucial element for the pathogenesis of AMD (80). Using dihydroethidium (DHE) staining, we determined ROS production at basal level and following incubation with 200 µM H2O2. PEDF−/− ChECs exhibited more fluorescent intensity compared with PEDF+/+ cells suggesting increased ROS production under both basal level and stress conditions (Fig. 10, A and B). Thus PEDF deficiency was associated with increased oxidative stress in ChECs, and is consistent with the reported reduced PEDF levels in the vitreous samples collected from AMD patients (49).
Fig. 10.
Increased oxidative stress in PEDF−/− ChECs. The level of oxidative stress in ChECs was assessed by dihydroethidium (DHE) staining under basal (A) or challenged conditions (200 μM H2O2) (B). A significant increase in the level of ROS was detected under basal and challenged conditions in PEDF−/− ChECs compared with PEDF+/+ cells (****P < 0.0001, n = 3; scale bar = 200 µm).
Reprograming of inflammatory chemokine and cytokine expression profiles in PEDF−/− ChECs.
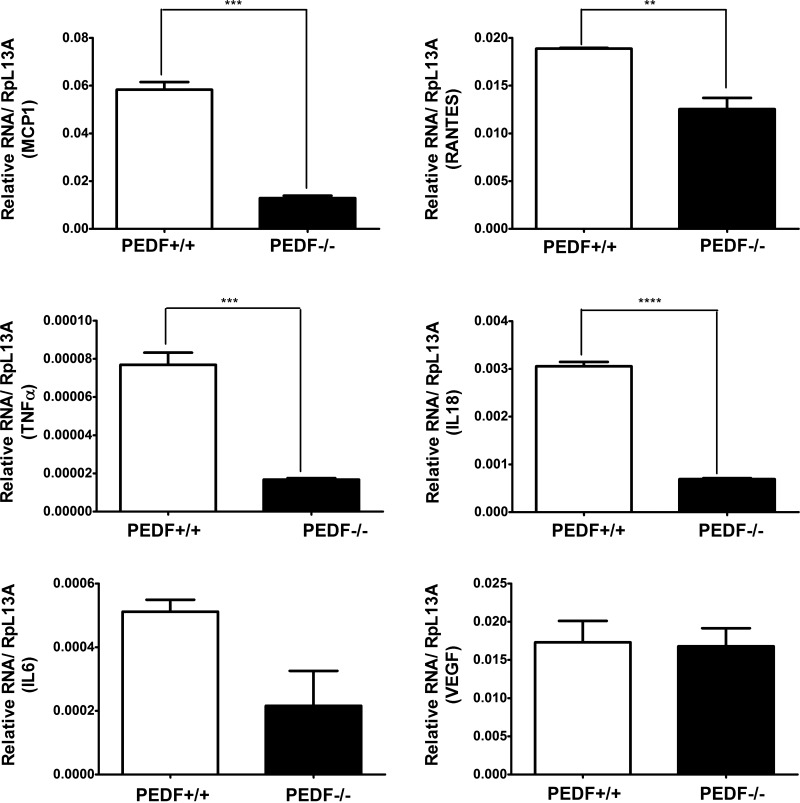
The reported antioxidant and anti-inflammatory activity of PEDF (79) suggested potential changes in the inflammatory responses of PEDF−/− ChECs. This notion is supported by increased levels of VEGF and osteopontin, cytokines with a primary role in inflammation and angiogenesis, detected in PEDF−/− ChECs. To get a broader view of inflammatory changes in PEDF−/− ChECs, we next determined the expression profiles of chemokines and cytokines of ChECs by using RT2 profiler PCR array as described in materials and methods. PEDF deficiency resulted in altered expression of inflammatory chemokines and cytokines in ChECs. Although the absence of PEDF was associated with upregulation of some pro-inflammatory genes, there was a decrease in expression of other inflammatory mediators (Tables 1 and 2). The changes in expression of inflammatory mediators were further confirmed by qPCR analysis. Figure 11 shows decreased expression of IL-18, IL-6, TNF-α, monocyte chemotactic protein-1 (MCP1; CCL2), and RANTES (CCL5) in PEDF−/− ChECs compared with the PEDF+/+ cells. IL-18, TNF-α CCL2, and CCL5 are not presented on the PCR array but are known to be altered in neovascular eye diseases, including CNV (3, 77). Although RT2 profiler PCR array analysis showed increased NOS2 expression in PEDF−/− ChECs, we observed no significant difference in NOS2 protein level (Fig. 7C). We were also unable to detect IL-1β (not present on the PCR array) mRNA expression in ChECs. These results indicated that the absence of PEDF contributed to a distinct inflammatory chemokine and cytokine profile in ChECs.
Fig. 11.
Decreased expression of inflammatory mediators in PEDF−/− ChECs. Expression of various inflammatory mediators (MCP-1, RANTES, TNF-α, IL-18, IL-6, and VEGF) was assessed with quantitative real-time PCR using RNA from ChECs as described in materials and methods. Note decreased expression of inflammatory cytokines in PEDF−/− ChECs compared with PEDF+/+ cells (**P < 0.01, ***P < 0.001, ****P < 0.0001, n = 3).
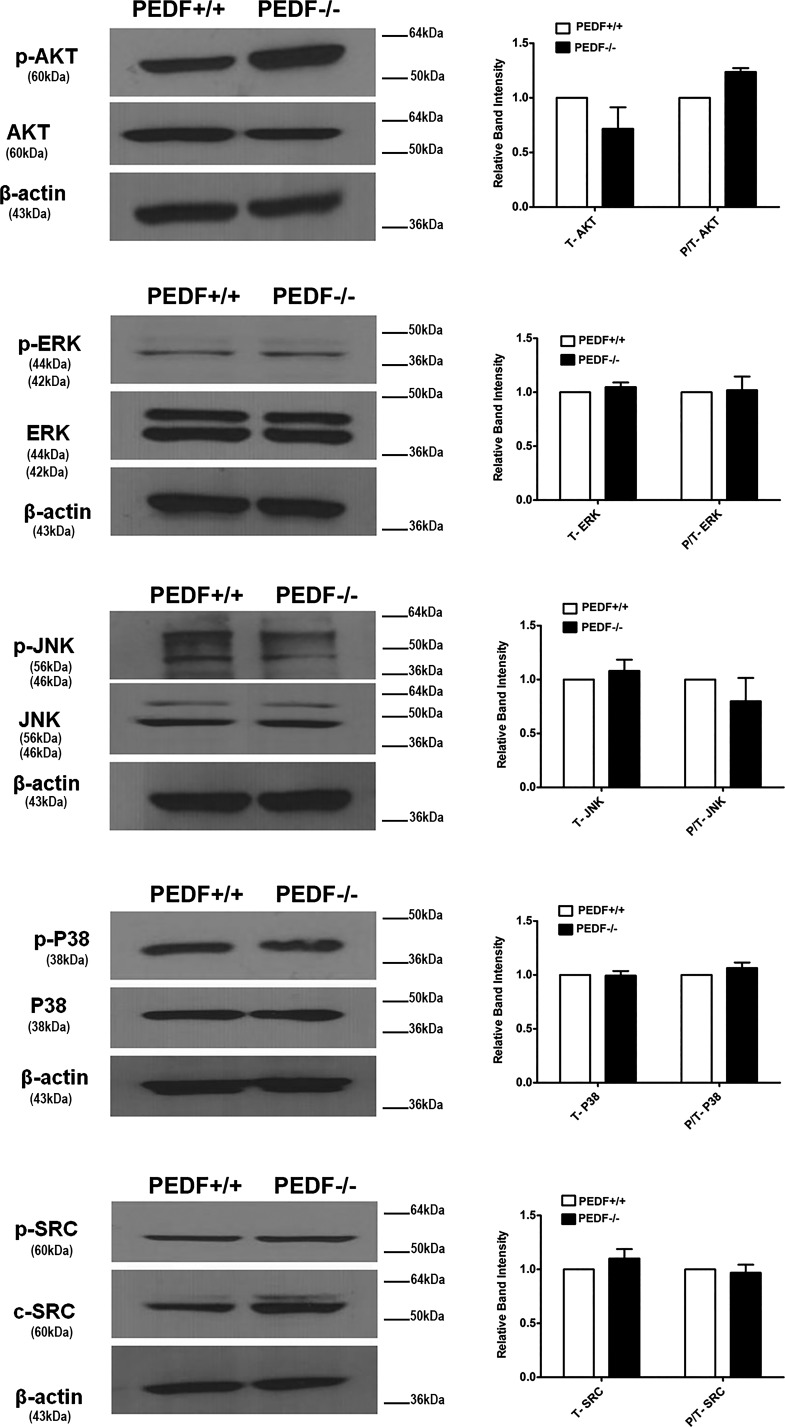
Altered proliferation rate in PEDF−/− ChECs suggested potential changes in Akt activation and/or expression. Akt plays a major role in cell proliferation and survival (40). Here we observed increased Akt activation in PEDF−/− ChECs compared with PEDF+/+ cells. However, expression of total Akt in ChECs was not affected by PEDF deficiency (Fig. 12). A controversial role has been suggested for MAPK pathways during oxidative stress. The MAPK ERK1/2 is involved in cell proliferation and differentiation. However, it may also contribute to an apoptotic response (27). Here we did not observe significant changes in the levels of p-ERK1/2 and total ERK1/2 in PEDF−/− ChECs compared with PEDF+/+ cells (Fig. 12). It has been reported that changes in the levels and/or activation of JNK and p38 under stress conditions might demonstrate a protective, a pro-apoptotic, and or no effects (40). The levels of p-p38 and total p38 were not affected by PEDF deficiency. We also did not observe significant changes in the level of p-JNK and total JNK in PEDF−/− ChECs. Similarly, no changes were observed in the level of p-SRC and total SRC with PEDF deficiency.
Fig. 12.
PEDF deficiency does not affect the downstream Akt and MAPK signaling pathways. The activation of MAPK and Akt pathways were evaluated by Western blot analysis using specific antibodies as detailed in materials and methods. No significant changes in these pathways were detected in PEDF−/− ChECs compared with PEDF+/+ cells. These experiments were repeated with two different isolations of ChECs with similar results (n ≥ 3).
DISCUSSION
Pigment epithelium derived factor is a neuroprotective and antiangiogenic factor whose alterations could contribute to pathogenesis of exudative AMD. Although it is believed that PEDF is mainly produced by RPE cells to keep choroidal vasculature in check and protect photoreceptor cells from degeneration, little is known about PEDF production and/or its cell autonomous function in ChECs. Here we successfully isolated and cultured ChECs from PEDF−/− and PEDF+/+ mice. These cells expressed EC markers including VE-cadherin, PECAM-1, and endoglin, although at lower levels in PEDF−/− ChECs. ChECs were readily passaged and maintained in culture, for up to 6 mo, without significant loss in expression of EC markers. We also detected PV-1, a marker of fenestrated ECs (36, 43), detectable only in PEDF+/+ cells. Thus expression of PEDF may be essential for maintenance of ChEC fenestration and perhaps is linked to alterations in VEGF levels (50), as observed in PEDF−/− ECs. We also showed that absence of PEDF resulted in increased proliferation of ChECs. The PEDF−/− ChECs were also less adherent and less migratory, and showed increased oxidative stress with minimal impact on basal levels of apoptosis. However, the PEDF−/− ChECs were more sensitive to oxidative challenge, showed altered production of ECM proteins and inflammatory mediators, and failed to undergo capillary morphogenesis. Reexpression of PEDF was sufficient to restore capillary morphogenesis of PEDF−/− ChECs. Thus expression of PEDF has a significant impact on ChEC proangiogenic and pro-inflammatory characteristics.
The proliferation and migration of ECs play a major role during angiogenesis. We recently showed that PEDF−/− retinal ECs and RPE cells proliferate significantly faster than wild-type cells (22, 23). Here we also observed a significant increase in proliferation of PEDF−/− ChECs. Our results are consistent with a previous study suggesting PEDF expression contributes to attenuation of cell proliferation by decreasing the number of cells entering S phase of the cell cycle and increasing the number of cells entering G0 (4). The increased proliferation of PEDF−/− ChECs was concomitant with increased expression of the PDGF receptors on the cell surface. This may suggest a PDGF-mediated proliferation of ChECs, which is impacted by PEDF expression. Normally, ECs produce PDGF to stimulate pericyte migration and recruitment to the newly forming vessels (32). Thus, a PDGF autocrine mechanism may exist in ChECs whose activity is regulated by PEDF expression; this needs further investigation.
PEDF−/− ChECs were less adherent on fibronectin, vitronectin, and collagen IV compared with PEDF+/+ cells. The alteration in the adhesion of PEDF−/− cells might be attributed to observed changes in the expression of integrins. We observed a decrease in expression of α5β1 and αvβ3 integrins, the major receptors for fibronectin and vitronectin, with important roles in angiogenesis (67). In addition, oxidative stress-mediated alterations in expression of the matricellular proteins may contribute to altered adhesive properties of these cells. We observed a significant increase in production of osteopontin, a proangiogenic and pro-inflammatory factor, whose expression contributes to the pathogenesis of ocular neovascular disorders, including CNV (1, 45). In addition, increased oxidative stress promotes osteopontin expression along with VEGF, and promotes ischemia-mediated angiogenesis (18, 47, 48). Thus expression of PEDF may reduce oxidative stress and suppress osteopontin and VEGF expression, halting angiogenesis and fibrogenic activities (16).
Alterations in ChEC migration contributes to the development of exudative AMD (28, 29). Here we demonstrated that PEDF deficiency resulted in a less migratory phenotype of ChECs. These results are consistent with mitigation of capillary morphogenesis of ChECs in the absence of PEDF and its restoration by reexpression of PEDF in null cells. Taken together, our results suggest that PEDF may have critical roles in cell-cell and cell-ECM interaction regulating the proproliferative and promigratory signaling pathways of ChECs. Furthermore, changes in ECM composition of ChECs, due to PEDF deficiency and oxidative stress, may contribute to the pro-inflammatory and proangiogenesis in exudative AMD.
VE-cadherin is a specific EC cadherin, which modulates adhesion between ECs and is involved in vascular morphogenesis through intracellular signaling (15). FACS analysis demonstrated a dramatic decrease in the expression of VE-cadherin in PEDF−/− ChECs compared with PEDF+/+ cells. Loss of VE-cadherin leads to defects in normal vascular development through expanding sprouting angiogenesis and remodeling in a branching network of large and small vessels (15). Additionally, ECs with VE-cadherin deficiency fail to develop organized vessel-like patterns in vitro (70). Similarly, decreased VE-cadherin expression may have contributed to reduced capillary morphogenesis in PEDF−/− ChECs observed here. We have also shown PECAM-1 expression is essential for EC migration, capillary morphogenesis, and endoglin expression (21, 57, 59, 60). Thus decreased expression of PECAM-1 could also contribute to migratory and capillary morphogenesis defects observed in PEDF−/− ChECs.
VEGF is a proangiogenic and pro-inflammatory factor, which promotes survival and migratory activity of ECs. Increased production of VEGF, as an essential element in the development and progression of AMD and CNV, has been well established. Here we showed that PEDF−/− cells expressed significantly higher levels of VEGF compared with PEDF+/+ cells. Increased VEGF level in PEDF−/− ChECs is consistent with their enhanced proliferation, and reduced levels of fenestration markers, VE-cadherin, and PECAM-1. However, we have not observed increased CNV in PEDF−/− mice subjected to laser-induced CNV (24). The reason for lack of a significant increase in CNV area in PEDF−/− mice is not clear. This could be attributed to the predominant role of proangiogenic factors during active angiogenesis, whereas negative regulators have a more passive role. The negative regulators of angiogenesis are more effective when angiogenesis is completed and the level of proangiogenic factor decreases to establish the homeostatic state (74). However, our observations are consistent with the reports indicating increased VEGF levels are associated with elevated oxidative stress and decreased levels of PEDF during diabetes (10). We also showed that diabetic mice deficient in PEDF develop more severe retinopathies (66). Although a relationship between decreased PEDF levels and AMD has been previously reported, we did not find a direct association between severity of diabetic retinopathy and decreased PEDF levels in vitreous samples from diabetic patients (72). Thus the action of PEDF on retinal vasculature may be different from that of the choroidal vasculature, and its contribution to the pathogenesis of these diseases deserves further investigation.
PEDF−/− ChECs exhibited altered expression of chemokine ligands and receptors involved in angiogenesis. We observed increased expression of proangiogenic chemokines including CXCL1, CXCL2, CXCL5, and CCL2, and decreased expression of angiostatic chemokines including CXCL9, CXCL10, and CXCL11 in PEDF−/− ChECs (Tables 1 and 2) (38). These results are consistent with the endogenous antiangiogenesis activity of PEDF. However, PEDF−/− ChECs exhibited a dramatic decrease in expression of CXCL3, an angiogenic chemokine, which is involved in the regulation of monocyte migration and adhesion (65). Monocytes play an important role in angiogenesis including tissue repair, neovascularization, and tumor formation. Absence of monocytes lead to a dramatic reduction in angiogenesis, whereas chemoattraction of monocytes significantly enhances angiogenesis (39, 52). Monocyte-macrophages also regulate the severity of CNV (63). These observations suggest a potential link between PEDF expression and the modulation of monocytes recruitment by CXCL3 expression.
Although the level of NOS2 transcript was increased in PEDF−/− ChECs (Table 1), its protein level was not significantly affected compared with PEDF+/+ cells. NOS2 or inducible NOS (iNOS) expression is considered as a response to inflammation, which leads to increased production of NO. The excess NO can react with superoxide anions and generate peroxynitrite, a highly reactive chemical species, which interacts with various substrates causing additional vascular damage (14). We observed no significant differences in the NO levels produced by PEDF+/+ and PEDF−/− ECs. However, we cannot exclude the possibility of increased peroxynitrite production in PEDF−/− ChECs and increased oxidative stress. We also noted that the expression of anti-inflammatory factor IL-10 was increased in PEDF−/− ChECs (Table 1). The reason for this increase in IL-10 level is not known but may be linked to a feedback mechanism to dampen the pro-inflammatory phenotype associated with PEDF deficiency. This notion is supported by decreased levels of IL-18 and undetectable levels of IL-1β in PEDF−/− ChEC.
In summary, we demonstrated that PEDF plays a critical autocrine role in ChEC function and perhaps in AMD pathogenesis. We demonstrated that PEDF deficiency in ChECs was associated with a more proliferative but less migratory phenotype. Other significant changes included increased oxidative stress, alerted expression of inflammatory mediators osteopontin and VEGF, and attenuation of capillary morphogenesis. These results expand our understanding of the PEDF cell autonomous regulatory mechanisms in ChECs and how alteration in PEDF levels may impact the progression of exudative AMD.
GRANTS
This work was supported by an unrestricted award from Research to Prevent Blindness to the Department of Ophthalmology and Visual Sciences, Retina Research Foundation, and Grants P30 EY-016665, P30 CA-014520, EPA 83573701, R24 EY-022883, and R01 EY-026078. C. Sorenson is supported by the RRF/Daniel M. Albert Chair. N. Sheibani is a recipient of the RPB Stein Innovation Award.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.F., C.M.S., and N.S. conceived and designed research; M.F. and C.M.S. performed experiments; M.F. and N.S. analyzed data; M.F., C.M.S., and N.S. interpreted results of experiments; M.F. prepared figures; M.F. drafted manuscript; M.F., C.M.S., and N.S. edited and revised manuscript; M.F., C.M.S., and N.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Juliana Falero-Perez for help with figure preparations.
REFERENCES
- 1.Abu El-Asrar AM, Imtiaz Nawaz M, Kangave D, Siddiquei MM, Geboes K. Osteopontin and other regulators of angiogenesis and fibrogenesis in the vitreous from patients with proliferative vitreoretinal disorders. Mediators Inflamm 2012: 493043, 2012. doi: 10.1155/2012/493043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ambati J, Ambati BK, Yoo SH, Ianchulev S, Adamis AP. Age-related macular degeneration: etiology, pathogenesis, and therapeutic strategies. Surv Ophthalmol 48: 257–293, 2003. doi: 10.1016/S0039-6257(03)00030-4. [DOI] [PubMed] [Google Scholar]
- 3.Ambati J, Anand A, Fernandez S, Sakurai E, Lynn BC, Kuziel WA, Rollins BJ, Ambati BK. An animal model of age-related macular degeneration in senescent Ccl-2- or Ccr-2-deficient mice. Nat Med 9: 1390–1397, 2003. doi: 10.1038/nm950. [DOI] [PubMed] [Google Scholar]
- 4.Barnstable CJ, Tombran-Tink J. Neuroprotective and antiangiogenic actions of PEDF in the eye: molecular targets and therapeutic potential. Prog Retin Eye Res 23: 561–577, 2004. doi: 10.1016/j.preteyeres.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Becerra SP. Structure-function studies on PEDF. Chemistry and Biology of Serpins, edited by Church FC, Cunningham DD, Ginsburg D, Hoffman MR, Stone SR, Tollefsen DM. New York: Springer, 1997, p. 223–237. doi: 10.1007/978-1-4615-5391-5_21. [DOI] [Google Scholar]
- 6.Becerra SP, Sagasti A, Spinella P, Notario V. Pigment epithelium-derived factor behaves like a noninhibitory serpin. Neurotrophic activity does not require the serpin reactive loop. J Biol Chem 270: 25992–25999, 1995. doi: 10.1074/jbc.270.43.25992. [DOI] [PubMed] [Google Scholar]
- 8.Bhutto I, Lutty G. Understanding age-related macular degeneration (AMD): relationships between the photoreceptor/retinal pigment epithelium/Bruch’s membrane/choriocapillaris complex. Mol Aspects Med 33: 295–317, 2012. doi: 10.1016/j.mam.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhutto IA, Baba T, Merges C, McLeod DS, Lutty GA. Low nitric oxide synthases (NOSs) in eyes with age-related macular degeneration (AMD). Exp Eye Res 90: 155–167, 2010. doi: 10.1016/j.exer.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boehm BOLG, Lang G, Volpert O, Jehle PM, Kurkhaus A, Rosinger S, Lang GK, Bouck N. Low content of the natural ocular anti-angiogenic agent pigment epithelium-derived factor (PEDF) in aqueous humor predicts progression of diabetic retinopathy. Diabetologia 46: 394–400, 2003. doi: 10.1007/s00125-003-1040-9. [DOI] [PubMed] [Google Scholar]
- 11.Bressler NM. Age-related macular degeneration is the leading cause of blindness. JAMA 291: 1900–1901, 2004. doi: 10.1001/jama.291.15.1900. [DOI] [PubMed] [Google Scholar]
- 12.Browning AC, Halligan EP, Stewart EA, Swan DC, Dove R, Samaranayake GJ, Amoaku WM. Comparative gene expression profiling of human umbilical vein endothelial cells and ocular vascular endothelial cells. Br J Ophthalmol 96: 128–132, 2012. doi: 10.1136/bjophthalmol-2011-300572. [DOI] [PubMed] [Google Scholar]
- 13.Burns MS, Hartz MJ. The retinal pigment epithelium induces fenestration of endothelial cells in vivo. Curr Eye Res 11: 863–873, 1992. doi: 10.3109/02713689209033484. [DOI] [PubMed] [Google Scholar]
- 14.Burtenshaw D, Hakimjavadi R, Redmond EM, Cahill PA. Nox, reactive oxygen species and regulation of vascular cell fate. Antioxidants (Basel) 6: E90, 2017. doi: 10.3390/antiox6040090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carmeliet P, Lampugnani M-G, Moons L, Breviario F, Compernolle V, Bono F, Balconi G, Spagnuolo R, Oosthuyse B, Dewerchin M, Zanetti A, Angellilo A, Mattot V, Nuyens D, Lutgens E, Clotman F, de Ruiter MC, Gittenberger-de Groot A, Poelmann R, Lupu F, Herbert JM, Collen D, Dejana E. Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell 98: 147–157, 1999. doi: 10.1016/S0092-8674(00)81010-7. [DOI] [PubMed] [Google Scholar]
- 16.Chakraborty G, Jain S, Kale S, Raja R, Kumar S, Mishra R, Kundu GC. Curcumin suppresses breast tumor angiogenesis by abrogating osteopontin-induced VEGF expression. Mol Med Rep 1: 641–646, 2008. doi: 10.3892/mmr_00000005. [DOI] [PubMed] [Google Scholar]
- 17.Ciulla TA, Danis RP, Criswell M, Pratt LM. Changing therapeutic paradigms for exudative age-related macular degeneration: antiangiogenic agents and photodynamic therapy. Expert Opin Investig Drugs 8: 2173–2182, 1999. doi: 10.1517/13543784.8.12.2173. [DOI] [PubMed] [Google Scholar]
- 18.Dai J, Peng L, Fan K, Wang H, Wei R, Ji G, Cai J, Lu B, Li B, Zhang D, Kang Y, Tan M, Qian W, Guo Y. Osteopontin induces angiogenesis through activation of PI3K/AKT and ERK1/2 in endothelial cells. Oncogene 28: 3412–3422, 2009. doi: 10.1038/onc.2009.189. [DOI] [PubMed] [Google Scholar]
- 19.Dawson DW, Volpert OV, Gillis P, Crawford SE, Xu H, Benedict W, Bouck NP. Pigment epithelium-derived factor: a potent inhibitor of angiogenesis. Science 285: 245–248, 1999. doi: 10.1126/science.285.5425.245. [DOI] [PubMed] [Google Scholar]
- 20.DiMaio TA, Sheibani N. PECAM-1 isoform-specific functions in PECAM-1-deficient brain microvascular endothelial cells. Microvasc Res 75: 188–201, 2008. doi: 10.1016/j.mvr.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DiMaio TA, Wang S, Huang Q, Scheef EA, Sorenson CM, Sheibani N. Attenuation of retinal vascular development and neovascularization in PECAM-1-deficient mice. Dev Biol 315: 72–88, 2008. doi: 10.1016/j.ydbio.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Falero-Perez J, Park S, Sorenson CM, Sheibani N. PEDF expression affects retinal endothelial cell proangiogenic properties through alterations in cell adhesive mechanisms. Am J Physiol Cell Physiol 313: C405–C420, 2017. doi: 10.1152/ajpcell.00004.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farnoodian M, Kinter JB, Yadranji Aghdam S, Zaitoun I, Sorenson CM, Sheibani N. Expression of pigment epithelium-derived factor and thrombospondin-1 regulate proliferation and migration of retinal pigment epithelial cells. Physiol Rep 3: e12266, 2015. doi: 10.14814/phy2.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farnoodian M, Wang S, Dietz J, Nickells RW, Sorenson CM, Sheibani N. Negative regulators of angiogenesis: important targets for treatment of exudative AMD. Clin Sci (Lond) 131: 1763–1780, 2017. doi: 10.1042/CS20170066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farnoodian M, Wang S, Dietz J, Nickells RW, Sorenson CM, Sheibani N. Negative regulators of angiogenesis: important targets for treatment of exudative AMD. Clin Sci (Lond) 131: 1763–1780, 2017. doi: 10.1042/CS20170066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fei P, Zaitoun I, Farnoodian M, Fisk DL, Wang S, Sorenson CM, Sheibani N. Expression of thrombospondin-1 modulates the angioinflammatory phenotype of choroidal endothelial cells. PLoS One 9: e116423, 2014. doi: 10.1371/journal.pone.0116423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gilley R, March HN, Cook SJ. ERK1/2, but not ERK5, is necessary and sufficient for phosphorylation and activation of c-Fos. Cell Signal 21: 969–977, 2009. doi: 10.1016/j.cellsig.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 28.Green WR. Histopathology of age-related macular degeneration. Mol Vis 5: 27, 1999. [PubMed] [Google Scholar]
- 29.Green WR, Enger C. Age-related macular degeneration histopathologic studies. The 1992 Lorenz E. Zimmerman Lecture. Ophthalmology 100: 1519–1535, 1993. doi: 10.1016/S0161-6420(93)31466-1. [DOI] [PubMed] [Google Scholar]
- 30.He S, Ding Y, Zhou J, Krasnoperov V, Zozulya S, Kumar SR, Ryan SJ, Gill PS, Hinton DR. Soluble EphB4 regulates choroidal endothelial cell function and inhibits laser-induced choroidal neovascularization. Invest Ophthalmol Vis Sci 46: 4772–4779, 2005. doi: 10.1167/iovs.05-0502. [DOI] [PubMed] [Google Scholar]
- 31.He X, Cheng R, Benyajati S, Ma JX. PEDF and its roles in physiological and pathological conditions: implication in diabetic and hypoxia-induced angiogenic diseases. Clin Sci (Lond) 128: 805–823, 2015. doi: 10.1042/CS20130463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hellström M, Kalén M, Lindahl P, Abramsson A, Betsholtz C. Role of PDGF-B and PDGFR-β in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development 126: 3047–3055, 1999. [DOI] [PubMed] [Google Scholar]
- 33.Hoffmann S, Spee C, Murata T, Cui JZ, Ryan SJ, Hinton DR. Rapid isolation of choriocapillary endothelial cells by Lycopersicon esculentum-coated Dynabeads. Graefes Arch Clin Exp Ophthalmol 236: 779–784, 1998. doi: 10.1007/s004170050158. [DOI] [PubMed] [Google Scholar]
- 34.Hua J, Gross N, Schulze B, Michaelis U, Bohnenkamp H, Guenzi E, Hansen LL, Martin G, Agostini HT. In vivo imaging of choroidal angiogenesis using fluorescence-labeled cationic liposomes. Mol Vis 18: 1045–1054, 2012. [PMC free article] [PubMed] [Google Scholar]
- 35.Huang Q, Wang S, Sorenson CM, Sheibani N. PEDF-deficient mice exhibit an enhanced rate of retinal vascular expansion and are more sensitive to hyperoxia-mediated vessel obliteration. Exp Eye Res 87: 226–241, 2008. doi: 10.1016/j.exer.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ioannidou S, Deinhardt K, Miotla J, Bradley J, Cheung E, Samuelsson S, Ng Y-S, Shima DT. An in vitro assay reveals a role for the diaphragm protein PV-1 in endothelial fenestra morphogenesis. Proc Natl Acad Sci USA 103: 16770–16775, 2006. doi: 10.1073/pnas.0603501103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jonas JB. Global prevalence of age-related macular degeneration. Lancet Glob Health 2: e65–e66, 2014. doi: 10.1016/S2214-109X(13)70163-3. [DOI] [PubMed] [Google Scholar]
- 38.Keeley EC, Mehrad B, Strieter RM. Chemokines as mediators of neovascularization. Arterioscler Thromb Vasc Biol 28: 1928–1936, 2008. doi: 10.1161/ATVBAHA.108.162925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kimura YN, Watari K, Fotovati A, Hosoi F, Yasumoto K, Izumi H, Kohno K, Umezawa K, Iguchi H, Shirouzu K, Takamori S, Kuwano M, Ono M. Inflammatory stimuli from macrophages and cancer cells synergistically promote tumor growth and angiogenesis. Cancer Sci 98: 2009–2018, 2007. doi: 10.1111/j.1349-7006.2007.00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klettner A. Oxidative stress induced cellular signaling in RPE cells. Front Biosci (Schol Ed) 4: 392–411, 2012. doi: 10.2741/s275. [DOI] [PubMed] [Google Scholar]
- 41.Kliffen M, Sharma HS, Mooy CM, Kerkvliet S, de Jong PTVM. Increased expression of angiogenic growth factors in age-related maculopathy. Br J Ophthalmol 81: 154–162, 1997. doi: 10.1136/bjo.81.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lawler J, Sunday M, Thibert V, Duquette M, George EL, Rayburn H, Hynes RO. Thrombospondin-1 is required for normal murine pulmonary homeostasis and its absence causes pneumonia. J Clin Invest 101: 982–992, 1998. doi: 10.1172/JCI1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li R, McCourt P, Schledzewski K, Goerdt S, Moldenhauer G, Liu X, Smedsrød B, Sørensen KK. Endocytosis of advanced glycation end-products in bovine choriocapillaris endothelial cells. Microcirculation 16: 640–655, 2009. doi: 10.1080/10739680903133185. [DOI] [PubMed] [Google Scholar]
- 44.Lidington EA, Rao RM, Marelli-Berg FM, Jat PS, Haskard DO, Mason JC. Conditional immortalization of growth factor-responsive cardiac endothelial cells from H-2Kb-tsA58 mice. Am J Physiol Cell Physiol 282: C67–C74, 2002. doi: 10.1152/ajpcell.2002.282.1.C67. [DOI] [PubMed] [Google Scholar]
- 45.Lund SA, Giachelli CM, Scatena M. The role of osteopontin in inflammatory processes. J Cell Commun Signal 3: 311–322, 2009. doi: 10.1007/s12079-009-0068-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luo Y, Radice GL. N-cadherin acts upstream of VE-cadherin in controlling vascular morphogenesis. J Cell Biol 169: 29–34, 2005. doi: 10.1083/jcb.200411127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lyle AN, Joseph G, Fan AE, Weiss D, Landázuri N, Taylor WR. Reactive oxygen species regulate osteopontin expression in a murine model of postischemic neovascularization. Arterioscler Thromb Vasc Biol 32: 1383–1391, 2012. doi: 10.1161/ATVBAHA.112.248922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ma B, Pan Y, Song Q, Tie L, Zhang Y, Xiao Y, Zhang J, Han J, Xu Y, Xiang Y, Yu HM, Li XJ. The effect of topiramate on tumor-related angiogenesis and on the serum proteome of mice bearing Lewis lung carcinoma. Eur J Pharmacol 663: 9–16, 2011. doi: 10.1016/j.ejphar.2011.04.056. [DOI] [PubMed] [Google Scholar]
- 49.Machalińska A, Safranow K, Mozolewska-Piotrowska K, Dziedziejko V, Karczewicz D. PEDF and VEGF plasma level alterations in patients with dry form of age‐related degeneration—a possible link to the development of the disease. Klin Oczna 114: 115–120, 2012. [PubMed] [Google Scholar]
- 50.Maharaj AS, Walshe TE, Saint-Geniez M, Venkatesha S, Maldonado AE, Himes NC, Matharu KS, Karumanchi SA, D’Amore PA. VEGF and TGF-β are required for the maintenance of the choroid plexus and ependyma. J Exp Med 205: 491–501, 2008. doi: 10.1084/jem.20072041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mancini MA, Frank RN, Keirn RJ, Kennedy A, Khoury JK. Does the retinal pigment epithelium polarize the choriocapillaris? Invest Ophthalmol Vis Sci 27: 336–345, 1986. [PubMed] [Google Scholar]
- 52.Moldovan NI. Role of monocytes and macrophages in adult angiogenesis: a light at the tunnel’s end. J Hematother Stem Cell Res 11: 179–194, 2002. doi: 10.1089/152581602753658394. [DOI] [PubMed] [Google Scholar]
- 53.Mori K, Duh E, Gehlbach P, Ando A, Takahashi K, Pearlman J, Mori K, Yang HS, Zack DJ, Ettyreddy D, Brough DE, Wei LL, Campochiaro PA. Pigment epithelium-derived factor inhibits retinal and choroidal neovascularization. J Cell Physiol 188: 253–263, 2001. doi: 10.1002/jcp.1114. [DOI] [PubMed] [Google Scholar]
- 54.Noble J, Chaudhary V. Age-related macular degeneration. CMAJ 182: 1759, 2010. doi: 10.1503/cmaj.090378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paik J-H, Skoura A, Chae S-S, Cowan AE, Han DK, Proia RL, Hla T. Sphingosine 1-phosphate receptor regulation of N-cadherin mediates vascular stabilization. Genes Dev 18: 2392–2403, 2004. doi: 10.1101/gad.1227804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Park K, Jin J, Hu Y, Zhou K, Ma JX. Overexpression of pigment epithelium-derived factor inhibits retinal inflammation and neovascularization. Am J Pathol 178: 688–698, 2011. doi: 10.1016/j.ajpath.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Park S, Dimaio TA, Liu W, Wang S, Sorenson CM, Sheibani N. Endoglin regulates the activation and quiescence of endothelium by participating in canonical and non-canonical TGF-β signaling pathways. J Cell Sci 126: 1392–1405, 2013. doi: 10.1242/jcs.117275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Park S, DiMaio TA, Scheef EA, Sorenson CM, Sheibani N. PECAM-1 regulates proangiogenic properties of endothelial cells through modulation of cell-cell and cell-matrix interactions. Am J Physiol Cell Physiol 299: C1468–C1484, 2010. doi: 10.1152/ajpcell.00246.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Park S, Sorenson CM, Sheibani N. PECAM-1 isoforms, eNOS and endoglin axis in regulation of angiogenesis. Clin Sci (Lond) 129: 217–234, 2015. doi: 10.1042/CS20140714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Scatena M, Liaw L, Giachelli CM. Osteopontin: a multifunctional molecule regulating chronic inflammation and vascular disease. Arterioscler Thromb Vasc Biol 27: 2302–2309, 2007. doi: 10.1161/ATVBAHA.107.144824. [DOI] [PubMed] [Google Scholar]
- 62.Scheef EA, Sorenson CM, Sheibani N. Attenuation of proliferation and migration of retinal pericytes in the absence of thrombospondin-1. Am J Physiol Cell Physiol 296: C724–C734, 2009. doi: 10.1152/ajpcell.00409.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shi YY, Wang YS, Zhang ZX, Cai Y, Zhou J, Hou HY, van Rooijen N. Monocyte/macrophages promote vasculogenesis in choroidal neovascularization in mice by stimulating SDF-1 expression in RPE cells. Graefes Arch Clin Exp Ophthalmol 249: 1667–1679, 2011. doi: 10.1007/s00417-011-1699-4. [DOI] [PubMed] [Google Scholar]
- 64.Shin ES, Sorenson CM, Sheibani N. PEDF expression regulates the proangiogenic and proinflammatory phenotype of the lung endothelium. Am J Physiol Lung Cell Mol Physiol 306: L620–L634, 2014. doi: 10.1152/ajplung.00188.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Smith DF, Galkina E, Ley K, Huo Y. GRO family chemokines are specialized for monocyte arrest from flow. Am J Physiol Heart Circ Physiol 289: H1976–H1984, 2005. doi: 10.1152/ajpheart.00153.2005. [DOI] [PubMed] [Google Scholar]
- 66.Sorenson CM, Wang S, Gendron R, Paradis H, Sheibani N. Thrombospondin-1 deficiency exacerbates the pathogenesis of diabetic retinopathy. J Diabetes Metab Suppl 12: 2013. doi: 10.4172/2155-6156.S12-005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Strömblad S, Cheresh DA. Integrins, angiogenesis and vascular cell survival. Chem Biol 3: 881–885, 1996. doi: 10.1016/S1074-5521(96)90176-3. [DOI] [PubMed] [Google Scholar]
- 68.Su X, Sorenson CM, Sheibani N. Isolation and characterization of murine retinal endothelial cells. Mol Vis 9: 171–178, 2003. [PubMed] [Google Scholar]
- 69.Tombran-Tink J, Johnson LV. Neuronal differentiation of retinoblastoma cells induced by medium conditioned by human RPE cells. Invest Ophthalmol Vis Sci 30: 1700–1707, 1989. [PubMed] [Google Scholar]
- 70.Vittet D, Buchou T, Schweitzer A, Dejana E, Huber P. Targeted null-mutation in the vascular endothelial-cadherin gene impairs the organization of vascular-like structures in embryoid bodies. Proc Natl Acad Sci USA 94: 6273–6278, 1997. doi: 10.1073/pnas.94.12.6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Volpert OV, Zaichuk T, Zhou W, Reiher F, Ferguson TA, Stuart PM, Amin M, Bouck NP. Inducer-stimulated Fas targets activated endothelium for destruction by anti-angiogenic thrombospondin-1 and pigment epithelium-derived factor. Nat Med 8: 349–357, 2002. doi: 10.1038/nm0402-349. [DOI] [PubMed] [Google Scholar]
- 72.Wang S, Gottlieb JL, Sorenson CM, Sheibani N. Modulation of thrombospondin 1 and pigment epithelium-derived factor levels in vitreous fluid of patients with diabetes. Arch Ophthalmol 127: 507–513, 2009. doi: 10.1001/archophthalmol.2009.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang S, Sorenson CM, Sheibani N. Lack of thrombospondin 1 and exacerbation of choroidal neovascularization. Arch Ophthalmol 130: 615–620, 2012. doi: 10.1001/archopthalmol.2011.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang S, Wu Z, Sorenson CM, Lawler J, Sheibani N. Thrombospondin-1-deficient mice exhibit increased vascular density during retinal vascular development and are less sensitive to hyperoxia-mediated vessel obliteration. Dev Dyn 228: 630–642, 2003. doi: 10.1002/dvdy.10412. [DOI] [PubMed] [Google Scholar]
- 75.Whitmore SS, Sohn EH, Chirco KR, Drack AV, Stone EM, Tucker BA, Mullins RF. Complement activation and choriocapillaris loss in early AMD: implications for pathophysiology and therapy. Prog Retin Eye Res 45: 1–29, 2015. doi: 10.1016/j.preteyeres.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yamagishi S, Amano S, Inagaki Y, Okamoto T, Takeuchi M, Inoue H. Pigment epithelium-derived factor inhibits leptin-induced angiogenesis by suppressing vascular endothelial growth factor gene expression through anti-oxidative properties. Microvasc Res 65: 186–190, 2003. doi: 10.1016/S0026-2862(03)00005-0. [DOI] [PubMed] [Google Scholar]
- 77.Yoshimura T, Sonoda KH, Sugahara M, Mochizuki Y, Enaida H, Oshima Y, Ueno A, Hata Y, Yoshida H, Ishibashi T. Comprehensive analysis of inflammatory immune mediators in vitreoretinal diseases. PLoS One 4: e8158, 2009. doi: 10.1371/journal.pone.0008158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zamora DO, Riviere M, Choi D, Pan Y, Planck SR, Rosenbaum JT, David LL, Smith JR. Proteomic profiling of human retinal and choroidal endothelial cells reveals molecular heterogeneity related to tissue of origin. Mol Vis 13: 2058–2065, 2007. [PubMed] [Google Scholar]
- 79.Zhang SX, Wang JJ, Gao G, Shao C, Mott R, Ma JX. Pigment epithelium-derived factor (PEDF) is an endogenous antiinflammatory factor. FASEB J 20: 323–325, 2006. doi: 10.1096/fj.05-4313fje. [DOI] [PubMed] [Google Scholar]
- 80.Zhou T, Hu Y, Chen Y, Zhou KK, Zhang B, Gao G, Ma JX. The pathogenic role of the canonical Wnt pathway in age-related macular degeneration. Invest Ophthalmol Vis Sci 51: 4371–4379, 2010. doi: 10.1167/iovs.09-4278. [DOI] [PMC free article] [PubMed] [Google Scholar]