Abstract
RNA-Seq was used to better understand the molecular nature of the biological differences among the three major exocrine salivary glands in mammals. Transcriptional profiling found that the adult murine parotid, submandibular, and sublingual salivary glands express greater than 14,300 protein-coding genes, and nearly 2,000 of these genes were differentially expressed. Principle component analysis of the differentially expressed genes revealed three distinct clusters according to gland type. The three salivary gland transcriptomes were dominated by a relatively few number of highly expressed genes (6.3%) that accounted for more than 90% of transcriptional output. Of the 912 transcription factors expressed in the major salivary glands, greater than 90% of them were detected in all three glands, while expression for ~2% of them was enriched in an individual gland. Expression of these unique transcription factors correlated with sublingual and parotid specific subsets of both highly expressed and differentially expressed genes. Gene ontology analyses revealed that the highly expressed genes common to all glands were associated with global functions, while many of the genes expressed in a single gland play a major role in the function of that gland. In summary, transcriptional profiling of the three murine major salivary glands identified a limited number of highly expressed genes, differentially expressed genes, and unique transcription factors that represent the transcriptional signatures underlying gland-specific biological properties.
Keywords: exocrine gland, gene expression, RNA-Seq, salivary gland, transcription factors
INTRODUCTION
The primary function of salivary glands is to secrete saliva, a fluid mixture of water, electrolytes, and proteins. The saliva secreted by mammals originates largely from three paired exocrine glands, the parotid, submandibular, and sublingual salivary glands. Saliva lubricates and moistens the oral cavity to facilitate speech, swallowing, and taste, initiates digestion, and inhibits dental decay and other opportunistic infections (1, 46). Although the three major salivary glands are similar in many respects, several functional characteristics are gland specific. For example, saliva contains more than a thousand proteins, some of which are highly enriched or exclusively expressed by an individual salivary gland (10, 54). Moreover, although the essential ion and water transport proteins have been identified (16, 33), the differences in the fluid secretion mechanism among the different salivary glands have not been fully elucidated (23).
Salivary glands are mainly composed of two cell types, i.e., acinar cells that are responsible for secreting most of the proteins, fluid, and electrolytes found in saliva and duct cells that primarily reabsorb the NaCl secreted by the acinar cells. Some of the gland-specific functional properties are associated with the distinctive cell types found in each salivary gland (2, 10, 33, 41, 51). In most mammals, parotid acinar cells are exclusively serous in nature, while sublingual acinar cells are primarily mucous cells capped by a few serous cells. The acinar cells of the rodent submandibular gland are seromucous, and their duct cells display sexual dimorphism (41).
The genetic and molecular basis for the functional differences among the three major salivary glands remains unclear but can likely be associated with the regulation of salivary gland-specific gene expression by different groups of transcription factors. Transcription factors are the central regulators of gene expression. Approximately 1,600 transcription factors comprise greater than 7% of the protein-coding genes in the mouse genome, which makes this family the single largest gene family (20). Mammalian genes are typically flanked by distinct binding sites for several transcription factors that work in unison to regulate expression of each gene. While globally expressed transcription factors often regulate housekeeping functions (28), others may control specific biological tasks, like organ development (25, 29, 47). Aberrant expression of transcription factors also frequently correlates with tumorigenesis (6, 31). However, expression data are lacking for the specific transcription factors that maintain the adult gland-specific phenotype for each of the three major salivary glands.
The current study used RNA-Seq to evaluate the transcriptional profiles of the three murine salivary glands to better understand the molecular and genetic nature of their well-recognized biological differences. We found that the expression patterns of gland-specific protein-coding genes agree with the adult male mouse submandibular gland transcriptome (12) and the salivary gland secretion literature, validating our RNA-Seq data. Transcriptome variation across the three murine salivary glands correlated with the differential coexpression of a limited number of transcription factors and salivary gland-specific protein-coding genes.
MATERIALS AND METHODS
General methods.
Chemicals were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise stated. Experiments were performed using 12 wk old male and female C57BL/6J mice (Jackson Laboratory) that were housed in pathogen-free, microisolator cages with free access to laboratory chow and water with a 12 h light-dark cycle. Animal procedures were approved by the Animal Care and Use Committee of the National Institute of Dental and Craniofacial Research, National Institutes of Health (ASP 16-802).
RNA preparation and next-generation sequencing.
Six adult mice (3 male and 3 female) were euthanized by exposure to CO2, followed by cervical dislocation, and the parotid, submandibular, and sublingual glands immediately removed and placed in RNAlater Stabilization Solution (Thermo Fisher Scientific, Waltham, MA, catalog #AM7020) at 4°C. The submandibular and sublingual glands are encapsulated and, thus, were easily separated from the surrounding tissue, while the parotid gland is more diffuse and was removed with the aid of a dissecting microscope to exclude surrounding connective tissue. A single parotid and submandibular gland were used from each animal. The sublingual glands are considerably smaller than the other two major salivary glands; thus the left and right glands were pooled from each animal. The 18 gland samples were shipped overnight to Otogenetics (Atlanta, GA). Total RNA was extracted with the RNeasy Micro Kit (Qiagen, Valencia, CA; catalog #74004). The integrity and purity of total RNA were assessed using Agilent Bioanalyzer or TapeStation and OD260/280. cDNA was generated from high-quality total RNA with the SMARTer PCR cDNA Synthesis Kit with modified oligo(dT) primers (Clontech Laboratories, Mountain View, CA; catalog #634926), and adapters were removed by digestion with RsaI. The resulting 1–2 μg of cDNA were fragmented using Bioruptor (Diagenode, Denville, NJ) and profiled using Agilent Bioanalyzer or TapeStation. Illumina libraries were made from qualified fragmented cDNA using the SPRIworks HT Reagent Kit (Beckman Coulter, Indianapolis, IN; catalog #B06938). The quality, quantity, and size distribution of the Illumina libraries were determined using Agilent Bioanalyzer or Tapestation. The libraries were then submitted for Illumina HiSeq2500 sequencing according to the standard operation with v1 or v2 chemistry, and 100–106 nucleotide (nt) paired end reads were generated and checked for data quality using FastQC (Babraham Institute, Cambridge, UK). Each of the 18 sample libraries were sequenced at ~40 million reads.
Analysis and comparison of RNA-Seq data.
The data generated by Otogenetics Corporation were subjected to analysis using the platform provided by DNAnexus (Mountain View, CA) and then 1) mapped against the mouse mm10 reference genome with STAR 2.4.0j, 2) measured for expression levels of genes and transcripts with Cufflinks 2.2.1, and 3) compared across the three major salivary glands with Cufflinks/Cuffdiff 2.2.1 for significance (q < 0.05). The data from Cufflinks/Cuffdiff 2.2.1 were outputted as fragments per kilobase of transcript per million of mapped reads (FPKM) values, which is a normalized count of a transcript’s abundance, and can be visualized using cummeRbund in RStudio for quality control. The transcriptional output was calculated by adding all FPKMs for a given salivary gland. The differentially expressed genes for pairwise comparisons of the glands were then called using q value <0.05 as cut-off. Genes considered significantly differentially expressed across the glands were called in at least two of the three between-gland comparisons. The principal component analysis (PCA) plot, heat maps, and cluster dendrograms were created in R using the FPKM values for the 18 individual samples. The heat maps were self-organizing for both samples and genes. Expression clusters were identified using Mfuzz package in Bioconductor/R. Gene Ontology (GO) term annotations were derived from the Gene Ontology Consortium database (http://www.geneontology.org), which was further supplemented with InterProScan output (18). The GO enrichment analysis was performed using FUNC packages (43).
RESULTS
Transcriptomes and validation of the three major murine salivary glands.
RNA-Seq allows for simultaneous and unbiased quantitative evaluation of expression for all 23,000+ mouse protein-coding genes with STAR 2.4.0j (mouse mm10 reference genome downloaded from Illumina Igenomes; GEO reviewer link record GSE96747: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?token=mrwlyaoqpjmfpir&acc=GSE96747). A data matrix of FPKM for the parotid (PG), sublingual (SLG) and submandibular (SMG) salivary glands was generated to discover differences in salivary gland gene expression. We set the threshold to FPKM ≥0.1 for a gene to be considered expressed and found that each of the murine adult salivary glands expressed more than 13,000 protein-coding genes (Table 1; genes listed in Supplemental Table S1). (The online version of this article contains supplemental material.) Of the 14,371 protein-coding genes detected, 87% were ubiquitously expressed in all major salivary glands and ~93% were detected in at least two of the three glands, suggesting that these latter genes likely play roles in the basic biology of adult salivary glands, while less than 3% of the 14,371 detected genes were exclusively expressed in a single salivary gland type (Table 1).
Table 1.
Uniquely and commonly expressed protein-coding genes
| Salivary Glands | Expressed Genes, n (FPKM ≥0.1) |
|---|---|
| PG | 13,229 [298; 2.1%]* |
| SLG | 13,411 [345; 2.4%]* |
| SMG | 13,597 [384; 2.7%]* |
| PG, SLG, and SMG | 14,371 |
| Common genes | 13,334 [92.9%]* |
Shown are the numbers of uniquely and commonly expressed protein-coding genes in the murine parotid (PG), sublingual (SLG), submandibular (SMG) glands. Genes were called expressed with fragments per kilobase of transcript per million of mapped reads (FPKM) ≥ 0.1 in the PG, SLG, SMG, and total number of genes expressed in all 3 glands (PG, SLG, and SMG).
Data in brackets represent the number and % of uniquely expressed genes in each gland type relative to total number of genes expressed in all 3 glands. Common genes are those expressed in at least 2 glands; n = 6 for each gland type.
To validate our RNA-Seq results, the gene expression patterns for known salivary gland-specific secretory proteins were evaluated. A comparison between these 20 secretory protein genes with the 18 salivary gland samples revealed three prominent clusters that correlated with the three major glands (Fig. 1), consistent with previous reports of the secretory proteins expressed in the major salivary glands (Supplemental Table S2). Note that several of the 20 secretory protein genes analyzed for data validation purposes are not differentially expressed genes, e.g., Muc1, Muc20, and Cstb. Examples of secretory proteins exhibiting dominant expression in a specific salivary gland include two members of the demilune cell and parotid protein family Dcpp2 and Dccp3, respectively, which were expressed at more than 18-fold greater levels in the SLG compared with the PG and SMG, and parotid secretory protein (PSP, Plunc2)/Bpifa2, which was significantly more abundant (>50-fold) in the PG compared with the SMG and SLG. On the other hand, proline rich, lacrimal 1 (Muc 10)/Prol1 expression was more than 60-fold greater in the SMG relative to the PG and SLG. Submandibular gland protein C/SmgC was >500-fold more abundant in the SLG and SMG than in the PG, while mucin 19/Muc19 was expressed at a 2,000-fold greater level in the SLG than in the PG and SMG.
Fig. 1.
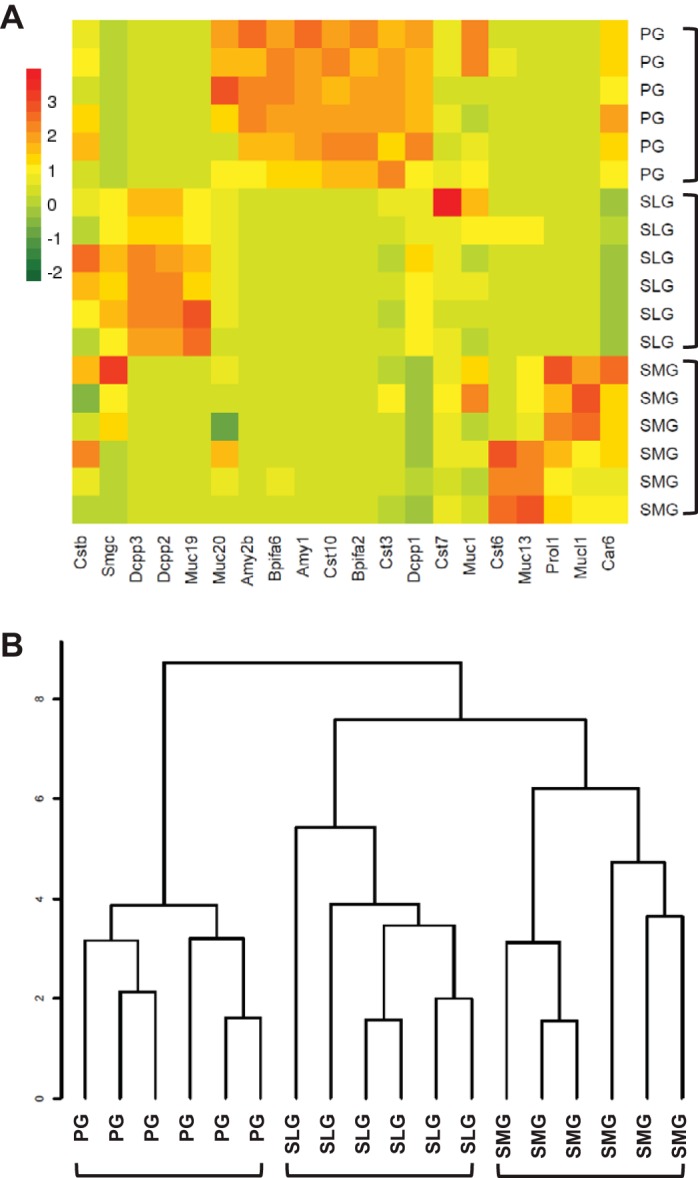
Comparison of secretory protein genes expressed in murine parotid, sublingual, and submandibular glands. Heat map (A) and the resulting cluster dendrogram (B) self-organize for the 18 gland samples and 20 secretory protein genes (listed in Supplemental Table S2). The data segregated into 3 major clusters by gland type: parotid (PG, n = 6), sublingual (SLG, n = 6), and submandibular (SMG, n = 6) glands.
To further validate our RNA-Seq data, we compared the adult male SMG transcriptome generated in the present study with the adult male SMG transcriptome previously reported (12) using the same age and mouse background (12 wk old, C57BL/6). Gluck et al. (12) identified 246 protein-coding genes enriched in the adult male SMG transcriptome, 220 of which were detected in our male SMG transcriptome (Supplemental Table S3). The 90% overlap in detected protein-coding genes in the two male SMG transcriptomes confirms the reproducibility of the RNA-Seq method.
Highly expressed genes in the three major murine salivary glands.
Among the expressed protein-coding genes, we selected the ones most highly expressed for further examination, as these genes generally hold keys to understanding the genetic and biochemical foundation of a given organ system. To identify the highly expressed genes (HEGs) in each gland, the sorted FPKMs were individually graphed on a logarithmic scale and a log FPKM cut-off value of 4 was used, as revealed by the turning points on the graphs (blue lines; Fig. 2). We observed in each of the major salivary glands ~700 HEGs with log FPKM values >4 (Fig. 3). Allowing that the three major salivary glands have similar biological functions, it was therefore not surprising that 685 of the 901 total HEGs (~76%) in the PG, SLG, and SMG presented with high expression levels in at least two of the three salivary glands (Fig. 3; list of HEGs given in Supplemental Table S4). In contrast, 7.8, 11.0, and 5.2% of the HEGs (PG, SLG, and SMG, respectively) displayed enriched expression in a single gland, indicating that the latter group of HEGs contributes to the unique transcriptomic signature of each gland. The 901 different HEGs in the three salivary glands represent only ~6.3% of the total number of expressed genes (14,371), but these highly expressed genes dominated expression, contributing 96.6, 87.8, and 93.4% of the transcriptional outputs in PG, SLG, and SMG, respectively (total HEG FPKMs divided by total FPKMs for each gland).
Fig. 2.
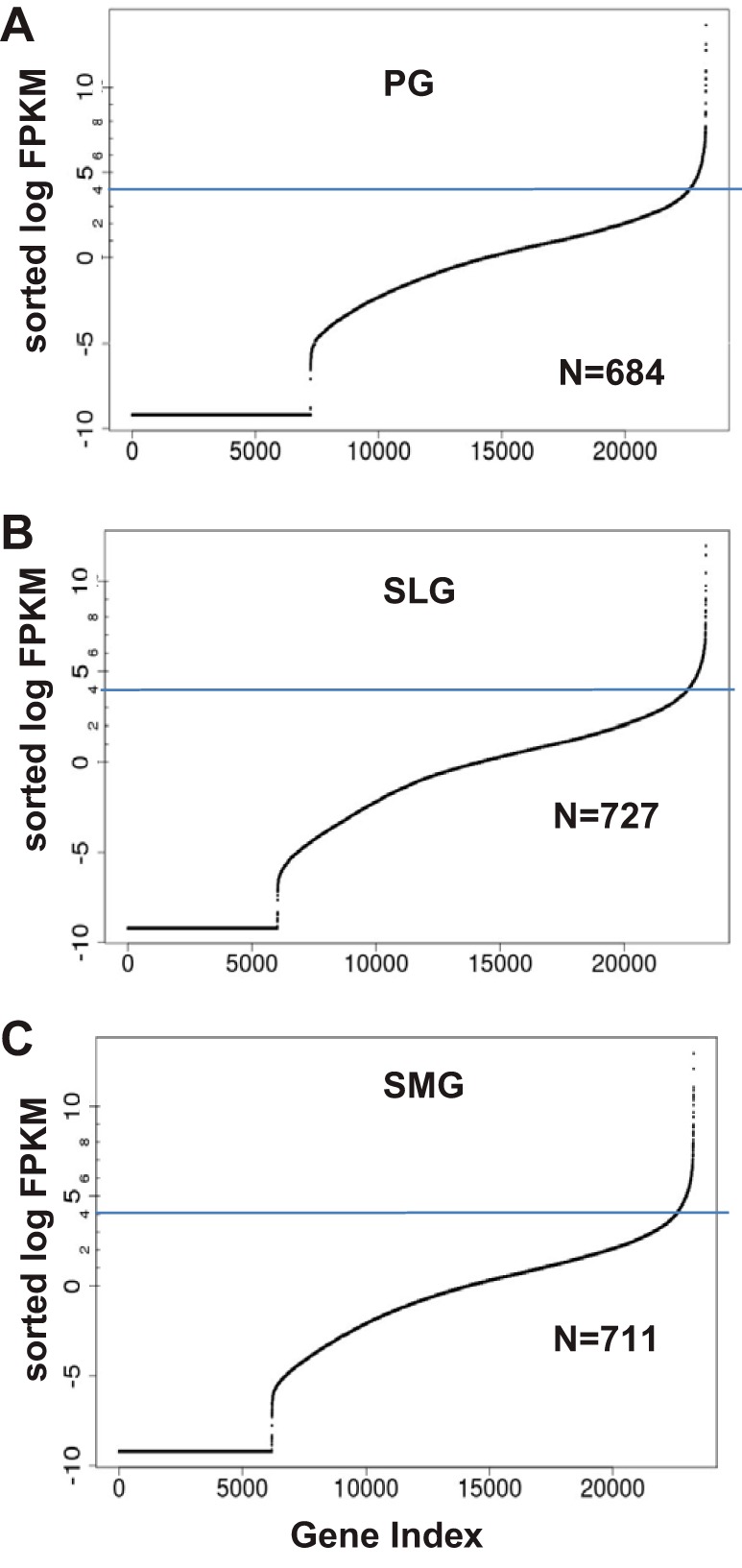
Highly expressed genes (HEGs) in the transcriptomes of the murine parotid (PG), sublingual (SLG), and submandibular (SMG) glands. HEGs in each gland were graphed on a logarithmic scale and identified as the log fragments per kilobase of transcript per million of mapped reads (FPKM) cut-off value of ≥4 for PG, SLG, and (SMG from the data in Supplemental Table S4. We observed 684, 727, and 711 HEGs with FPKMs greater than the turning point in the PG (A), SLG (B), and SMG (C), respectively.
Fig. 3.
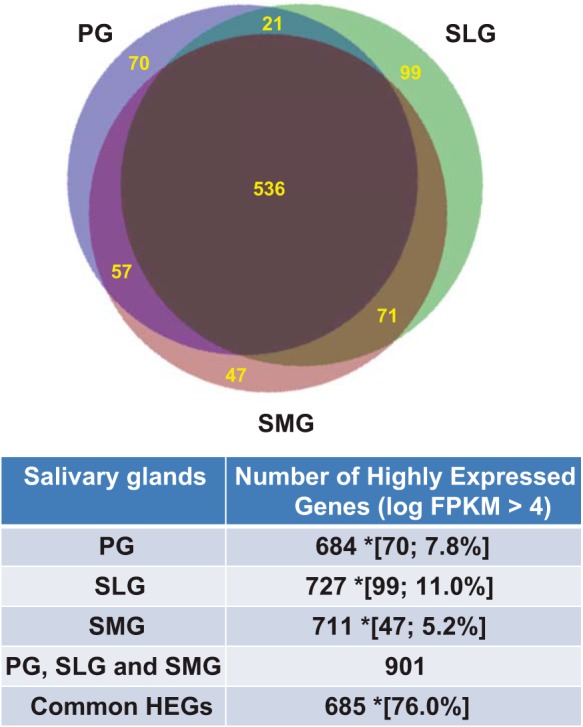
Comparison of highly expressed genes among the murine major salivary glands. The relationship between the highly expressed protein-coding genes (HEGs) in the parotid (PG), sublingual (SLG), submandibular (SMG) glands, and the total number of HEGs expressed in all 3 glands (PG, SLG, and SMG) as listed in Supplemental Table S4, with a log FPKM cut-off value of ≥4. *Data in brackets represent the number and % of unique HEGs in a single gland type relative to the total number of HEGs expressed in all 3 glands. Common HEGs are those expressed in at least 2 glands. n = 6 for each gland type.
GO enrichment analyses (refinement P value <0.005) for the overlapping HEGs revealed many common as well as exclusive molecular functions, cellular components, and biological processes across the three glands. The GO terms for the common HEGs were mainly related to protein synthesis, energy metabolism, membrane traffic/secretion, and intercellular adhesion (Supplemental Table S5; molecular functions, cellular components, and biological processes, respectively), consistent with the common organ maintenance and secretion functions for many of these genes. We also noticed HEGs-associated GO terms that were unevenly distributed across the three glands. For instance, molecular function GO:0005497 (androgen binding) was only associated with the HEGs in SMG, a gland known to be androgen sensitive (7). The biological process GO:0006888 (ER to Golgi vesicle-mediated transport) was only associated with the HEGs in SLG, where these ER-Golgi intermediate compartment proteins are highly expressed, as well as in other mucous cells (45). The enriched Go term “ER to Golgi vesicle-mediated transport GO:0006888” includes four genes that were more highly expressed in SLG compared with PG and SMG, including Copb2, Ergic1, Lman1, and Sec23b. These genes were expressed in all three glands, but at significantly higher levels in SLG. Consequently, this does not dismiss a role for these genes related to “ER to Golgi vesicle-mediated transport” in the PG and SMG but may indicate that elevated expression levels are required in the mucous cells of SLG to facilitate the movement of large mucin cargo proteins. Although such differences reflect gland-specific transcription, there were many more common than unique enriched GO terms (Supplemental Table S5), revealing that the three major salivary glands are very similar in most respects.
Differentially expressed genes in the three major murine salivary glands.
Differentially expressed genes are likely to underlie specific biological differences between the major salivary glands. In keeping with the unique functional properties among these glands, we found that 1,991 genes, ~14% of the 14,371 protein-coding genes expressed in the adult murine PG, SMG, and SLG transcriptomes, were expressed at significantly different levels across the three glands (q < 0.05; Supplemental Table S6; pairwise comparisons: SLG vs. PG, SMG vs. PG and SMG vs. SLG). Note also, that of the 14,371 genes expressed in the three major salivary glands, 66, 232, and 532 genes were differently expressed in male vs. female PG, SLG, and SMG, respectively (genes differently expressed by gender are indicated by an “X” in Supplemental Table S1). It is already known that there are sex differences in mouse SMG gene expression (7) and morphology (41); however, it is beyond the scope of the current paper to describe the details of differences in gene expression related to gender in the three major salivary glands.
Figure 4A shows a gland-pairwise comparison of differentially expressed genes based on a Cuffdiff q < 0.05 value. The number of the pairwise differential genes indicated that the SLG vs. PG difference was larger than the SMG vs. PG or SMG vs. SLG differences (2,296 > 1,524 and 1,817, respectively). Each of the three major salivary glands also exhibited a characteristic transcriptional signature, as revealed by PCA performed on the 1,991 differentially expressed genes (Fig. 4B). When the first two principal components (PC1–2) were plotted, 69% of the data variation was accounted for, and the 18 samples clustered into three distinct groups by gland type (PG, SLG, and SMG = blue, green, and red symbols, respectively; n = 6 for each gland type). The clustering pattern of the PCA plot suggested that many of the differentially expressed genes are unique to a single gland type, i.e., the variation in gene expression is much greater between the three glands than among the six samples from an individual gland type. Together, these comparisons of the differentially expressed genes in the three salivary gland groups (Fig. 4, A and B) reveal that the SLG displays more divergence from the PG than the SMG.
Fig. 4.
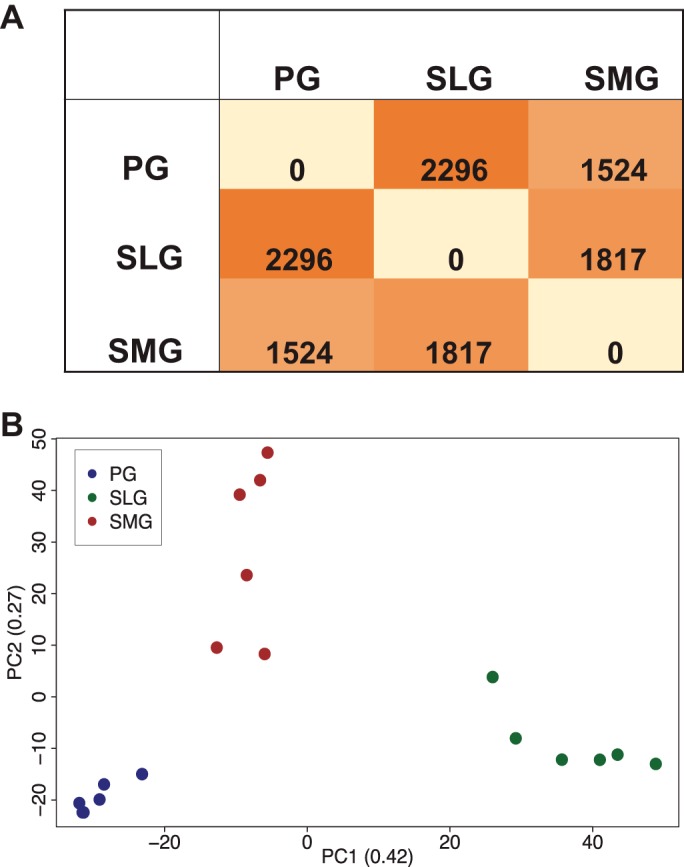
Comparison of differentially expressed genes in the murine major salivary glands. A: number of differentially expressed genes for pairwise comparisons of differentially expressed genes in the major salivary glands based on a Cuffdiff q < 0.05 value. B: principal component analysis (PCA) plot shows a comparison of transcriptional profiles obtained by RNA-Seq of mouse parotid (PG, blue symbols) in comparison to sublingual (SLG, green symbols), and submandibular (SMG, red symbols) glands. Data from Supplementary Table S6 (SMG vs. PG, SMG vs. SLG, and SLG vs. PG) were used to generate A and B; n = 6 for each gland type.
Expression of transcription factors in the three major murine salivary glands.
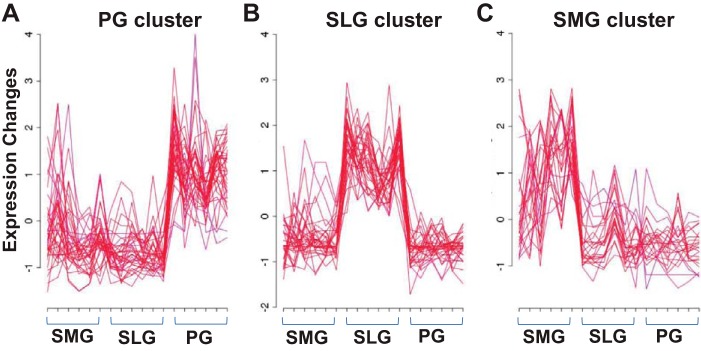
The Riken transcription factor (TF) database (http://genome.gsc.riken.jp/TFdb/; 20) documents 1,675 mouse TF genes. We found that 94.5% (n = 862) of the 912 different TF genes expressed in the three salivary glands were common to at least two of the three glands (Fig. 5). The remaining 50 differentially expressed TF genes (5.5%) that were enriched in only the PG, SLG, or SMG contributed ~4.9% to the total number of 1,027 differentially expressed genes detected exclusively in a single salivary gland (298, 345, and 384 genes, respectively, for the PG, SLG, and SMG; see Table 1). Of these 912 TFs, 106 were differently expressed across the three glands (n = 6 for each gland type). The differently expressed TFs grouped into three distinct clusters by gland type including 40 highly expressed in PG, 37 in SLG, and 29 in SMG according to the fuzzy clustering algorithm with a cut-off of membership ≥0.8 (Fig. 6; TF genes listed in Supplemental Table S7).
Fig. 5.
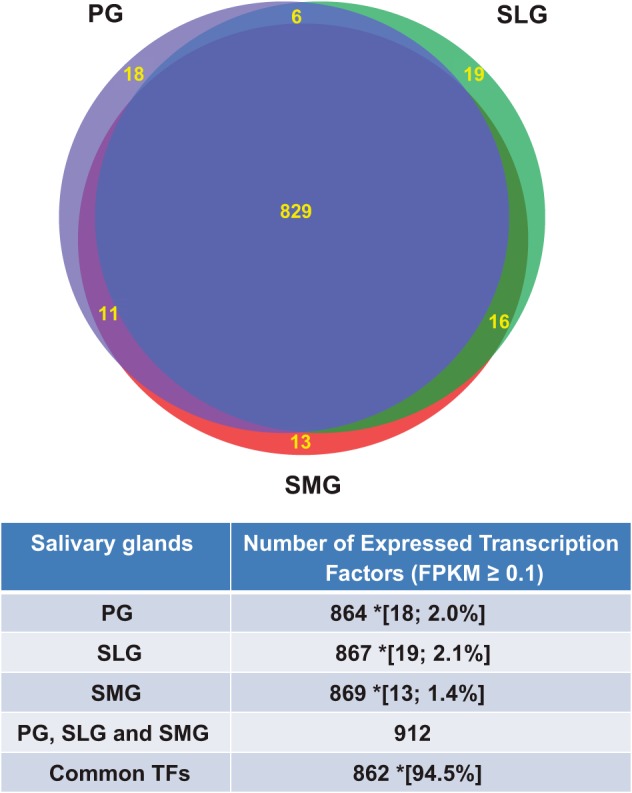
Comparison of transcription factor genes expressed in the murine major salivary glands. Relationship between the differentially expressed (DE) transcription factor genes (TFs) in the parotid (PG), sublingual (SLG), submandibular (SMG), and total number of DE TFs expressed in all 3 glands (PG, SLG, and SMG), as identified in Supplemental Table S1 (TF Genes) with a log FPKM ≥0.1. *Data in brackets represent the number and % of uniquely DE TFs in a single gland type relative to total number of DE TFs expressed in all 3 glands. Common DE TFs are those expressed in at least 2 glands; n = 6 for each gland type.
Fig. 6.
Comparison of differentially expressed (DE) transcription factor (TF) genes in the murine major salivary glands. Fuzzy clustering algorithm was used to visualize the gland-dependent DE TF genes. Of the 106 DE TF genes, 92 met the min.mem ≥0.8 cut-off and were used to generate Fig. 6 (Supplementary Table S7). The data self-organized into 3 clusters according to gland type: parotid, PG cluster (A), sublingual, SLG cluster (B), and submandibular, SMG cluster (C); n = 6 for each gland type.
Gene coexpression networks often reflect the association between individual or a group of genes and their regulatory factors. Thus, we evaluated how the subpopulations of differentially expressed genes in each gland clusters with the differentially expressed TFs (DE TFs) based on coexpression patterns (Fig. 7). Pairwise Pearson correlation coefficients between the 1,991 DE genes and 106 DE TFs based on FPKMs across the 18 samples were calculated, and a cut-off of 0.9 for the Pearson correlation coefficient was used to indicate the coexpression for each pair. We found two prominent clusters where 241 and 99 (340 total) differentially expressed genes coexpressed with 20 and 16 DE TFs highly expressed in the SLG (large and small green boxes, respectively), while another cluster of 197 differentially expressed genes coexpressed with 8 DE TFs highly expressed in the PG (blue box; Supplemental Table S8). Note that the 16 DE TFs associated with the smaller green box were a subset of the 20 DE TFs highly expressed in the SLG.
Fig. 7.
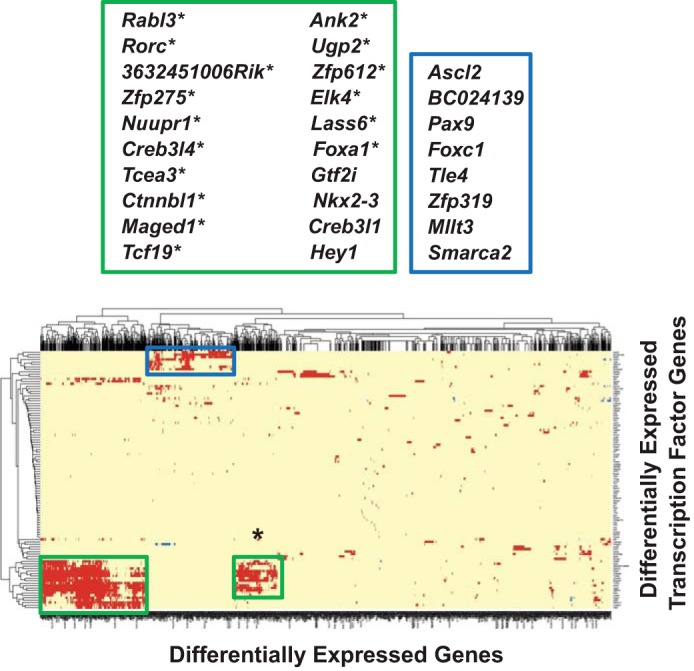
Cluster analysis of differentially expressed protein-coding genes (DE genes) vs. differentially expressed transcription factors (DE TFs) in the murine major salivary glands. Heat map and the resulting cluster dendrogram self-organized for the 106 DE TFs and 1,991 DE genes (listed in Supplementary Table S1), based on 18-sample FPKM values. Pairwise Pearson correlation coefficients were calculated with a cut-off of 0.9. Two clusters were evident where 241 and 99 (340 total) DE genes coexpressed with 20 and 16 DE TFs highly expressed in the SLG, respectively (green boxes). The 16 DE TFs associated with the smaller green box are a subset of the 20 DE TFs highly expressed in the SLG and are indicated by an asterisk. A 3rd cluster (blue box) of 197 DE genes coexpressed with 8 DE TFs highly expressed in the PG (see Supplemental Table S8).
Functional enrichment analysis (refinement P value <0.005) showed that the positively correlated, 340-gene SLG clusters were mainly associated with glycosylation-related membrane trafficking, as five out of 10 molecular function GO terms (GO:0004653, GO:0005004, GO:0004360, GO:0005351, and GO:0030246) were implicated in carbohydrate transfer, and all the seven cellular component GO terms were relevant to intra-/extracellular membrane dynamics (Supplemental Table S9A; for PG GO enrichment analyses, see Supplemental Table S9B). The enriched biological process GO terms contained ones relevant to protein folding/unfolding beyond glycosylation/Golgi dynamics. Together, these GO terms were consistent with the mucous cell phenotype of the SLG.
Figure 8 shows the calculated pairwise Pearson correlation coefficients between the 901 HEGs and the 106 DE TFs in the three glands (Supplemental Tables S4 and S7, respectively). Like the gene coexpression analysis for DE vs. DE TF genes (Fig. 7), prominent clusters of HEGs correlated with DE TFs enriched in the PG and SLG (Supplemental Table S8). Two clusters of 65 and 43 HEGs were highly correlated with 18 and eight DE TFs enriched in the SLG and PG, respectively. Moreover, these 18 DE TFs comprise a subgroup of the 20 DE TFs that correlated with DE protein-coding genes in the SLG cluster, while the eight DE TFs in the PG cluster are the same ones that correlated with DE protein-coding genes. Greater than 70% of the 65 (56 out of 65) and 43 (31 out of 43) HEGs overlapped with the 340 and 197 differentially expressed genes that coexpressed with the SLG- and PG-dominant transcription factors, respectively (overlapping DE TFs and HEGs are indicated by asterisks in Supplemental Table S8).
Fig. 8.
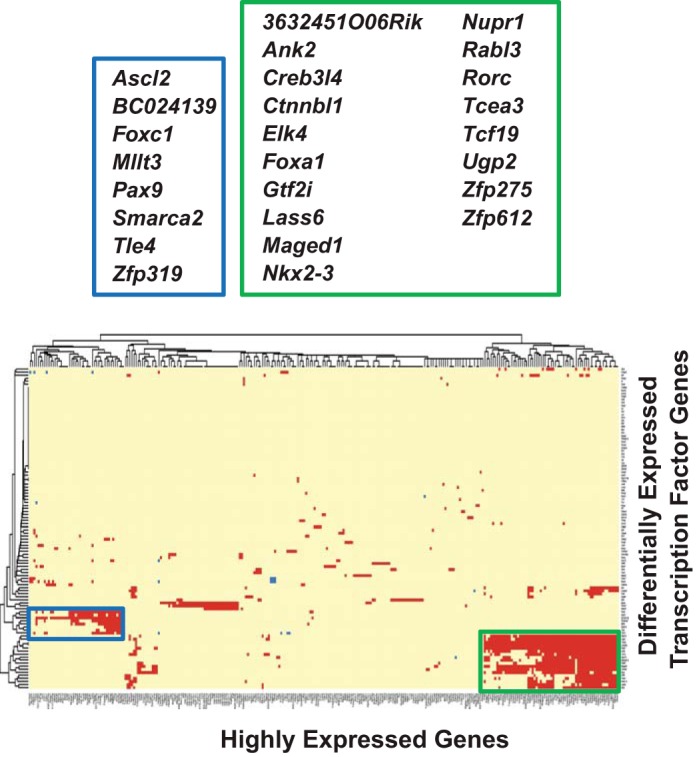
Cluster analysis of highly expressed protein-coding genes (HEGs) vs. differentially expressed transcription factors (DE TFs) in the murine major salivary glands. Heat map and the resulting cluster dendrogram self-organized for the 106 DE TFs vs. 901 HEGs (listed in Supplemental Table S4), based on 18-sample FPKM values. Pairwise Pearson correlation coefficients were calculated with a cut-off of 0.9. Two clusters were evident where 65 and 43 HEGs coexpressed with 18 and 8 DE TFs highly expressed in the SLG (green box) and the PG (blue box), respectively (see Supplemental Table S8).
DISCUSSION
The molecular and genetic mechanisms responsible for differential regulation of the adult PG, SLG, and SMG phenotypes are unknown. Thus, genome-wide expression profiling (RNA-Seq) was used in the present study to provide a rapid and sensitive assay to simultaneously screen for differences in the expression of all protein-coding transcripts in the three major salivary glands of adult mice. Salivary glands are relatively simple organ systems, primarily comprising acinar and duct cells. Accordingly, the total number of protein-coding genes in mouse salivary glands was comparable to less complex organs like the liver, vesicular gland, and heart, but lower than reported for more complex mouse tissues such as the testis, ovary, and lung (28). Moreover, the number of genes detected in the major salivary glands was essentially equivalent to that from distinct cell types in the brain (55). Thus, the number of protein-coding transcripts detected is not unexpected given that salivary glands are relatively simple organs, with few major cell types.
Salivary glands secrete thousands of proteins (10, 54), many of which have defined biological activities. Thus, we compared the list of secretory proteins previously reported in the major salivary glands with the catalog of secretory protein genes detected by RNA-Seq (Supplemental Table S2: secretory protein list with references). The secretory protein genes expressed in the transcriptome of each salivary gland was distinct and consistent with the literature, confirming the robustness of this methodology. As a further validation, we compared our results to those of Gluck et al. (12). There was nearly 90% overlap of our data with the enriched protein-coding transcripts in the male adult murine SMG. The overlapping protein-coding genes included, for example, Muc13, numerous members of the kallikrein gene family (Klk1b), and growth factors (Ngf and Egf), all genes known to be highly enriched in mouse submandibular salivary glands (12, 21, 26, 44). Together, these data suggest that the overlapping genes likely contribute to an adult male SMG transcriptome signature (Supplemental Table S2).
Transcriptional profiling revealed significant differences in the expression of nearly 2,000 protein-coding genes. These differentially expressed genes included a limited number of gland-enriched transcription factor genes in the murine PG, SMG, and SLG. Transcription factors regulate gene expression and, in so doing, are the primary determinants of the sustained programs that control the adult phenotype. Consequently, DE TF genes would be expected to be key components of transcriptome signatures. To date, the transcription factors responsible for regulating the unique adult phenotypes of the PG, SLG, and SMG are unknown, but their roles in gland development and tumorigenesis have been proposed. However, the regulation of salivary gland development by transcription factors has focused on the mouse SMG model, with little attention paid to other mammalian species or salivary glands. Examples of the transcription factors thought to be important in the development of the mouse SMG include Runx1, Six1, and Sox9 (5, 27, 38). Other transcription factors, like SMARCA2, FOXA1, and SOX10 (15, 17, 19, 37), have been proposed as biomarkers for different types of human salivary gland tumors, while Ascl2 and RORc (13, 22) have been implicated in Sjögren’s syndrome. It is not clear if aberrant expression of these latter transcription factors is causative or the consequence of the salivary gland pathogenesis. However, caution is suggested for the use of these transcription factor genes as disease biomarkers since their expression levels vary significantly between the three major salivary glands (see Supplemental Table S1).
It is expected that expression of the adult salivary gland phenotype involves a common set of transcription factors that regulate general biological processes in all three glands, and indeed, there was a greater than 90% overlap in the expression of transcription factor genes in the three major salivary glands. However, a subpopulation of transcription factors was uniquely expressed in each type of salivary gland, and these would likely be responsible for regulating the serous (parotid) vs. the mucous (sublingual) cell phenotype. PCA clustering analyses revealed that the sublingual gland transcriptome was most different compared with the parotid gland, with the PG and SMG transcriptomes displaying the least divergence. SLG specificity appears be driven by the concerted expression of at least some of the 20 DE TF genes that associated with this gland. Of these, the transcription factor Nkx2.3, which is only expressed in the SLG, and Irf6 have been previously linked to the regulation of the mucous cell phenotype in salivary glands (4, 34). The expression of these 20 DE TFs correlated positively with 340 differentially expressed protein-coding genes, suggesting that these genes might be considered part of the adult mouse SLG transcriptome signature (Supplemental Table S8).
In summary, the striking differences in the expression of a limited number of transcription factor genes among the three major salivary glands are consistent with the different patterns of gene expression by these glands. Our results suggest that differential expression of protein-coding genes, including transcription factors, likely plays a major role in the regulation of the gene expression programs that oversee the sustained differentiation of each gland type in the adult mouse. Our results underscore the value of monitoring the transcriptomes of the three major murine salivary glands to better understand gland-specific transcriptional regulation of the adult phenotype and pathogenesis.
GRANTS
This study was supported in part by National Institute of Dental and Craniofacial Research Grants R01 DE-018896 and R01 DE-022949 (C. E. Ovitt) and by the Intramural Research Program of the National Institute of Dental and Craniofacial Research, National Institutes of Health (ZIA DE-000738, J. E. Melvin).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
X.G., C.E.O., M.S., and J.E.M. conceived and designed research; X.G. and M.S.O. performed experiments; X.G., M.S.O., M.S., and J.E.M. analyzed data; X.G., M.S.O., C.E.O., M.S., and J.E.M. interpreted results of experiments; X.G., M.S.O., and J.E.M. prepared figures; X.G., M.S.O., C.E.O., and J.E.M. drafted manuscript; X.G., C.E.O., M.S., and J.E.M. edited and revised manuscript; X.G., M.S.O., C.E.O., M.S., and J.E.M. approved final version of manuscript.
Supplemental Data
Supplemental Table 1. Expressed Protein-coding Genes - .xlsx (2.2 MB)
Supplemental Table 2. Secretory Protein Genes - .docx (18 KB)
Supplemental Table 3. Adult Male SMG (Gluck et al., 2016) - .xlsx (16 KB)
Supplemental Table 4. Highly expressed protein-coding genes (HEG) in murine parotid, sublingual, and submandibular salivary glands. - .xlsx (86 KB)
Supplemental Table 5. Enriched GO terms associated with highly expressed genes (HEGs). - .xlsx (84 KB)
Supplemental Table 6. Pairwise comparisons of significant differences in gene expression between salivary glands. - .xlsx (282 KB)
Supplemental Table 7. Expression clustering of differentially expressed transcription factor (DE TF) genes in the murine major salivary glands using Mfuzz/Bioconductor (default cluster membership=0.8) - .xlsx (14 KB)
Supplemental Table 8. Genes involved in co-expression clusters - .xlsx (20 KB)
Supplemental Table 9A. Enriched GO terms associated with the DE genes that were co-expressed with the SLG dominant DE TFs; Supplemental Table 9B. Enriched GO terms associated with the DE genes that were co-expressed with the PG dominant DE TFs - .xlsx (18 KB)
ACKNOWLEDGMENTS
J. E. Melvin is the guarantor of this work, had full access to all the data, and takes full responsibility for the integrity of data and the accuracy of data analysis. The authors wish to thank Yasna Jaramillo for technical assistance.
Present addresses: M. Sincan, Sanford Health, University of South Dakota, Sioux Falls, SD; M. S. Oei, School of Dentistry, University of Maryland, Baltimore, MD.
REFERENCES
- 1.Anil S, Vellappally S, Hashem M, Preethanath RS, Patil S, Samaranayake LP. Xerostomia in geriatric patients: a burgeoning global concern. J Investig Clin Dent 7: 5–12, 2016. doi: 10.1111/jicd.12120. [DOI] [PubMed] [Google Scholar]
- 2.Amerongen AV, Veerman EC. Saliva–the defender of the oral cavity. Oral Dis 8: 12–22, 2002. doi: 10.1034/j.1601-0825.2002.1o816.x. [DOI] [PubMed] [Google Scholar]
- 3.Bekhor I, Wen Y, Shi S, Hsieh CH, Denny PA, Denny PC. cDNA cloning, sequencing and in situ localization of a transcript specific to both sublingual demilune cells and parotid intercalated duct cells in mouse salivary glands. Arch Oral Biol 39: 1011–1022, 1994. doi: 10.1016/0003-9969(94)90052-3. [DOI] [PubMed] [Google Scholar]
- 4.Biben C, Wang CC, Harvey RP. NK-2 class homeobox genes and pharyngeal/oral patterning: Nkx2-3 is required for salivary gland and tooth morphogenesis. Int J Dev Biol 46: 415–422, 2002. [PubMed] [Google Scholar]
- 5.Chatzeli L, Gaete M, Tucker AS. Fgf10 and Sox9 are essential for the establishment of distal progenitor cells during mouse salivary gland development. Development 144: 2294–2305, 2017. doi: 10.1242/dev.146019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen KS, Lim JWC, Richards LJ, Bunt J. The convergent roles of the nuclear factor I transcription factors in development and cancer. Cancer Lett 410: 124–138, 2017. doi: 10.1016/j.canlet.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 7.Chung AG, Belone PM, Bímová BV, Karn RC, Laukaitis CM. Studies of an androgen-binding protein knockout corroborate a role for salivary ABP in mouse communication. Genetics 205: 1517–1527, 2017. doi: 10.1534/genetics.116.194571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Culp DJ, Latchney LR, Fallon MA, Denny PA, Denny PC, Couwenhoven RI, Chuang S. The gene encoding mouse Muc19: cDNA, genomic organization and relationship to Smgc. Physiol Genomics 19: 303–318, 2004. doi: 10.1152/physiolgenomics.00161.2004. [DOI] [PubMed] [Google Scholar]
- 9.Culp DJ, Robinson B, Parkkila S, Pan PW, Cash MN, Truong HN, Hussey TW, Gullett SL. Oral colonization by Streptococcus mutans and caries development is reduced upon deletion of carbonic anhydrase VI expression in saliva. Biochim Biophys Acta 1812: 1567–1576, 2011. doi: 10.1016/j.bbadis.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denny P, Hagen FK, Hardt M, Liao L, Yan W, Arellanno M, Bassilian S, Bedi GS, Boontheung P, Cociorva D, Delahunty CM, Denny T, Dunsmore J, Faull KF, Gilligan J, Gonzalez-Begne M, Halgand F, Hall SC, Han X, Henson B, Hewel J, Hu S, Jeffrey S, Jiang J, Loo JA, Ogorzalek Loo RR, Malamud D, Melvin JE, Miroshnychenko O, Navazesh M, Niles R, Park SK, Prakobphol A, Ramachandran P, Richert M, Robinson S, Sondej M, Souda P, Sullivan MA, Takashima J, Than S, Wang J, Whitelegge JP, Witkowska HE, Wolinsky L, Xie Y, Xu T, Yu W, Ytterberg J, Wong DT, Yates JR III, Fisher SJ. The proteomes of human parotid and submandibular/sublingual gland salivas collected as the ductal secretions. J Proteome Res 7: 1994–2006, 2008. doi: 10.1021/pr700764j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furusawa M, Taira T, Iguchi-Ariga SM, Ariga H. Molecular cloning of the mouse AMY-1 gene and identification of the synergistic activation of the AMY-1 promoter by GATA-1 and Sp1. Genomics 81: 221–233, 2003. doi: 10.1016/S0888-7543(03)00006-5. [DOI] [PubMed] [Google Scholar]
- 12.Gluck C, Min S, Oyelakin A, Smalley K, Sinha S, Romano RA. RNA-seq based transcriptomic map reveals new insights into mouse salivary gland development and maturation. BMC Genomics 17: 923, 2016. doi: 10.1186/s12864-016-3228-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guggino G, Liberto DD, Pizzo ML, Saieva L, Alessandro R, Dieli F, Triolo G, Ciccia F. IL-17 polarization of MAIT cells is derived from the activation of two different pathways. Eur J Immunol 47: 2002–2003, 2017. doi: 10.1002/eji.201747140. [DOI] [PubMed] [Google Scholar]
- 14.Higuchi T, Orita T, Nakanishi S, Katsuya K, Watanabe H, Yamasaki Y, Waga I, Nanayama T, Yamamoto Y, Munger W, Sun HW, Falk RJ, Jennette JC, Alcorta DA, Li H, Yamamoto T, Saito Y, Nakamura M. Molecular cloning, genomic structure, and expression analysis of MUC20, a novel mucin protein, up-regulated in injured kidney. J Biol Chem 279: 1968–1979, 2004. doi: 10.1074/jbc.M304558200. [DOI] [PubMed] [Google Scholar]
- 15.Ho AS, Kannan K, Roy DM, Morris LG, Ganly I, Katabi N, Ramaswami D, Walsh LA, Eng S, Huse JT, Zhang J, Dolgalev I, Huberman K, Heguy A, Viale A, Drobnjak M, Leversha MA, Rice CE, Singh B, Iyer NG, Leemans CR, Bloemena E, Ferris RL, Seethala RR, Gross BE, Liang Y, Sinha R, Peng L, Raphael BJ, Turcan S, Gong Y, Schultz N, Kim S, Chiosea S, Shah JP, Sander C, Lee W, Chan TA. The mutational landscape of adenoid cystic carcinoma. Nat Genet 45: 791–798, 2013. doi: 10.1038/ng.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hong JH, Park S, Shcheynikov N, Muallem S. Mechanism and synergism in epithelial fluid and electrolyte secretion. Pflugers Arch 466: 1487–1499, 2014. doi: 10.1007/s00424-013-1390-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsieh MS, Lee YH, Chang YL. SOX10-positive salivary gland tumors: a growing list, including mammary analogue secretory carcinoma of the salivary gland, sialoblastoma, low-grade salivary duct carcinoma, basal cell adenoma/adenocarcinoma, and a subgroup of mucoepidermoid carcinoma. Hum Pathol 56: 134–142, 2016. doi: 10.1016/j.humpath.2016.05.021. [DOI] [PubMed] [Google Scholar]
- 18.Jones P, Binns D, Chang HY, Fraser M, Li W, McAnulla C, McWilliam H, Maslen J, Mitchell A, Nuka G, Pesseat S, Quinn AF, Sangrador-Vegas A, Scheremetjew M, Yong SY, Lopez R, Hunter S. InterProScan 5: genome-scale protein function classification. Bioinformatics 30: 1236–1240, 2014. doi: 10.1093/bioinformatics/btu031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karpathiou G, Da Cruz V, Casteillo F, Mobarki M, Dumollard JM, Chauleur C, Forest F, Prades JM, Peoc’h M. FOXA1 in HPV associated carcinomas: Its expression in carcinomas of the head and neck and of the uterine cervix. Exp Mol Pathol 102: 230–236, 2017. doi: 10.1016/j.yexmp.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 20.Kanamori M, Konno H, Osato N, Kawai J, Hayashizaki Y, Suzuki H. A genome-wide and nonredundant mouse transcription factor database. Biochem Biophys Res Commun 322: 787–793, 2004. doi: 10.1016/j.bbrc.2004.07.179. [DOI] [PubMed] [Google Scholar]
- 21.Kikkawa Y, Yamanaka N, Tada J, Kanamori N, Tsumura K, Hosoi K. Prorenin processing and restricted endoproteolysis by mouse tissue kallikrein family enzymes (mK1, mK9, mK13, and mK22). Biochim Biophys Acta 1382: 55–64, 1998. doi: 10.1016/S0167-4838(97)00144-1. [DOI] [PubMed] [Google Scholar]
- 22.Kim SM, Kwon JE, Park JS, Seo HB, Jung KA, Moon YM, Lee J, Kwok SK, Cho ML, Park SH. Achaete-scute complex homologue 2 accelerates the development of Sjögren’s syndrome-like disease in the NOD/ShiLtJ mouse. Immunol Lett 190: 26–33, 2017. doi: 10.1016/j.imlet.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 23.Kondo Y, Nakamoto T, Jaramillo Y, Choi S, Catalan MA, Melvin JE. Functional differences in the acinar cells of the murine major salivary glands. J Dent Res 94: 715–721, 2015. doi: 10.1177/0022034515570943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koshizuka Y, Yamada T, Hoshi K, Ogasawara T, Chung UI, Kawano H, Nakamura Y, Nakamura K, Ikegawa S, Kawaguchi H. Cystatin 10, a novel chondrocyte-specific protein, may promote the last steps of the chondrocyte differentiation pathway. J Biol Chem 278: 48259–48266, 2003. doi: 10.1074/jbc.M211639200. [DOI] [PubMed] [Google Scholar]
- 25.Kropp PA, Gannon M. Onecut transcription factors in development and disease. Trends Dev Biol 9: 43–57, 2016. [PMC free article] [PubMed] [Google Scholar]
- 26.Kwon SM, Kim SA, Yoon JH, Yook JI, Ahn SG. Global analysis of gene expression profiles in the submandibular salivary gland of klotho knockout mice. J Cell Physiol 233: 3282–3294, 2018. doi: 10.1002/jcp.26172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laclef C, Souil E, Demignon J, Maire P. Thymus, kidney and craniofacial abnormalities in Six 1 deficient mice. Mech Dev 120: 669–679, 2003. doi: 10.1016/S0925-4773(03)00065-0. [DOI] [PubMed] [Google Scholar]
- 28.Li B, Qing T, Zhu J, Wen Z, Yu Y, Fukumura R, Zheng Y, Gondo Y, Shi L. A Comprehensive mouse transcriptomic bodymap across 17 tissues by RNA-seq. Sci Rep 7: 4200, 2017. doi: 10.1038/s41598-017-04520-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li J, Parada C, Chai Y. Cellular and molecular mechanisms of tooth root development. Development 144: 374–384, 2017. doi: 10.1242/dev.137216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu B, Lague JR, Nunes DP, Toselli P, Oppenheim FG, Soares RV, Troxler RF, Offner GD. Expression of membrane-associated mucins MUC1 and MUC4 in major human salivary glands. J Histochem Cytochem 50: 811–820, 2002. doi: 10.1177/002215540205000607. [DOI] [PubMed] [Google Scholar]
- 31.Lourenço AR, Coffer PJ. SOX4: joining the master regulators of epithelial-to-mesenchymal transition? Trends Cancer 3: 571–582, 2017. doi: 10.1016/j.trecan.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 32.Melnick M, Chen H, Zhou Y, Jaskoll T. An alternatively spliced Muc10 glycoprotein ligand for putative L-selectin binding during mouse embryonic submandibular gland morphogenesis. Arch Oral Biol 46: 745–757, 2001. doi: 10.1016/S0003-9969(01)00027-9. [DOI] [PubMed] [Google Scholar]
- 33.Melvin JE, Yule D, Shuttleworth T, Begenisich T. Regulation of fluid and electrolyte secretion in salivary gland acinar cells. Annu Rev Physiol 67: 445–469, 2005. doi: 10.1146/annurev.physiol.67.041703.084745. [DOI] [PubMed] [Google Scholar]
- 34.Metwalli KA, Do MA, Nguyen K, Mallick S, Kin K, Farokhnia N, Jun G, Fakhouri WD. Interferon regulatory factor 6 is necessary for salivary glands and pancreas development. J Dent Res 97: 226–236, 2017. doi: 10.1177/0022034517729803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nashida T, Yoshimura K, Yoshie S, Mizuhashi F, Shimomura-Kuroki J. Upregulation of Bpifb1 expression in the parotid glands of non-obese diabetic mice. Oral Dis 22: 46–52, 2016. doi: 10.1111/odi.12377. [DOI] [PubMed] [Google Scholar]
- 36.Ni J, Abrahamson M, Zhang M, Fernandez MA, Grubb A, Su J, Yu GL, Li Y, Parmelee D, Xing L, Coleman TA, Gentz S, Thotakura R, Nguyen N, Hesselberg M, Gentz R. Cystatin E is a novel human cysteine proteinase inhibitor with structural resemblance to family 2 cystatins. J Biol Chem 272: 10853–10858, 1997. doi: 10.1074/jbc.272.16.10853. [DOI] [PubMed] [Google Scholar]
- 37.Ohtomo R, Mori T, Shibata S, Tsuta K, Maeshima AM, Akazawa C, Watabe Y, Honda K, Yamada T, Yoshimoto S, Asai M, Okano H, Kanai Y, Tsuda H. SOX10 is a novel marker of acinus and intercalated duct differentiation in salivary gland tumors: a clue to the histogenesis for tumor diagnosis. Mod Pathol 26: 1041–1050, 2013. doi: 10.1038/modpathol.2013.54. [DOI] [PubMed] [Google Scholar]
- 38.Ono Minagi H, Sarper SE, Kurosaka H, Kuremoto KI, Taniuchi I, Sakai T, Yamashiro T. Runx1 mediates the development of the granular convoluted tubules in the submandibular glands. PLoS One 12: e0184395, 2017. doi: 10.1371/journal.pone.0184395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pan PW, Käyrä K, Leinonen J, Nissinen M, Parkkila S, Rajaniemi H. Gene expression profiling in the submandibular gland, stomach, and duodenum of CAVI-deficient mice. Transgenic Res 20: 675–698, 2011. doi: 10.1007/s11248-010-9441-2. [DOI] [PubMed] [Google Scholar]
- 40.Pennacchio LA, Bouley DM, Higgins KM, Scott MP, Noebels JL, Myers RM. Progressive ataxia, myoclonic epilepsy and cerebellar apoptosis in cystatin B-deficient mice. Nat Genet 20: 251–258, 1998. doi: 10.1038/3059. [DOI] [PubMed] [Google Scholar]
- 41.Pinkstaff CA. The cytology of salivary glands. Int Rev Cytol 63: 141–261, 1980. doi: 10.1016/S0074-7696(08)61759-3. [DOI] [PubMed] [Google Scholar]
- 42.Prokopovic V, Popovic M, Andjelkovic U, Marsavelski A, Raskovic B, Gavrovic-Jankulovic M, Polovic N. Isolation, biochemical characterization and anti-bacterial activity of BPIFA2 protein. Arch Oral Biol 59: 302–309, 2014. doi: 10.1016/j.archoralbio.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 43.Prüfer K, Muetzel B, Do HH, Weiss G, Khaitovich P, Rahm E, Pääbo S, Lachmann M, Enard W. FUNC: a package for detecting significant associations between gene sets and ontological annotations. BMC Bioinformatics 8: 41, 2007. doi: 10.1186/1471-2105-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saito Y, Yamada A, Suzuki D, Tanaka J, Nagahama R, Kurosawa T, Maki K, Mishima K, Shirota T, Kamijo R. Association of aging with gene expression profiling in mouse submandibular glands. Genom Data 5: 115–119, 2015. doi: 10.1016/j.gdata.2015.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sakulsak N, Wakayama T, Hipkaeo W, Yamamoto M, Iseki S. Cloning and characterization of a novel animal lectin expressed in the rat sublingual gland. J Histochem Cytochem 53: 1335–1343, 2005. doi: 10.1369/jhc.5A6618.2005. [DOI] [PubMed] [Google Scholar]
- 46.Sanz M, Beighton D, Curtis MA, Cury JA, Dige I, Dommisch H, Ellwood R, Giacaman RA, Herrera D, Herzberg MC, Könönen E, Marsh PD, Meyle J, Mira A, Molina A, Mombelli A, Quirynen M, Reynolds EC, Shapira L, Zaura E. Role of microbial biofilms in the maintenance of oral health and in the development of dental caries and periodontal diseases. Consensus report of group 1 of the Joint EFP/ORCA workshop on the boundaries between caries and periodontal disease. J Clin Periodontol 44, Suppl 18: S5–S11, 2017. doi: 10.1111/jcpe.12682. [DOI] [PubMed] [Google Scholar]
- 47.Sheeba CJ, Logan MP. The roles of T-box genes in vertebrate limb development. Curr Top Dev Biol 122: 355–381, 2017. doi: 10.1016/bs.ctdb.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 48.Skliris GP, Hubé F, Gheorghiu I, Mutawe MM, Penner C, Watson PH, Murphy LC, Leygue E, Myal Y. Expression of small breast epithelial mucin (SBEM) protein in tissue microarrays (TMAs) of primary invasive breast cancers. Histopathology 52: 355–369, 2008. doi: 10.1111/j.1365-2559.2007.02955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sotiropoulou G, Anisowicz A, Sager R. Identification, cloning, and characterization of cystatin M, a novel cysteine proteinase inhibitor, down-regulated in breast cancer. J Biol Chem 272: 903–910, 1997. doi: 10.1074/jbc.272.2.903. [DOI] [PubMed] [Google Scholar]
- 50.Sugino H. Comparative genomic analysis of the mouse and rat amylase multigene family. FEBS Lett 581: 355–360, 2007. doi: 10.1016/j.febslet.2006.12.039. [DOI] [PubMed] [Google Scholar]
- 51.Tabak LA. In defense of the oral cavity: structure, biosynthesis, and function of salivary mucins. Annu Rev Physiol 57: 547–564, 1995. doi: 10.1146/annurev.ph.57.030195.002555. [DOI] [PubMed] [Google Scholar]
- 52.Utsunomiya T, Hara Y, Kataoka A, Morita M, Arakawa H, Mori M, Nishimura S. Cystatin-like metastasis-associated protein mRNA expression in human colorectal cancer is associated with both liver metastasis and patient survival. Clin Cancer Res 8: 2591–2594, 2002. [PubMed] [Google Scholar]
- 53.Williams SJ, Wreschner DH, Tran M, Eyre HJ, Sutherland GR, McGuckin MA. Muc13, a novel human cell surface mucin expressed by epithelial and hemopoietic cells. J Biol Chem 276: 18327–18336, 2001. doi: 10.1074/jbc.M008850200. [DOI] [PubMed] [Google Scholar]
- 54.Yan W, Apweiler R, Balgley BM, Boontheung P, Bundy JL, Cargile BJ, Cole S, Fang X, Gonzalez-Begne M, Griffin TJ, Hagen F, Hu S, Wolinsky LE, Lee CS, Malamud D, Melvin JE, Menon R, Mueller M, Qiao R, Rhodus NL, Sevinsky JR, States D, Stephenson JL Jr, Than S, Yates JR III, Yu W, Xie H, Xie Y, Omenn GS, Loo JA, Wong DT. Systematic comparison of the human saliva and plasma proteomes. Proteomics Clin Appl 3: 116–134, 2009. doi: 10.1002/prca.200800140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N, Deng S, Liddelow SA, Zhang C, Daneman R, Maniatis T, Barres BA, Wu JQ. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci 34: 11929–11947, 2014. doi: 10.1523/JNEUROSCI.1860-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Expressed Protein-coding Genes - .xlsx (2.2 MB)
Supplemental Table 2. Secretory Protein Genes - .docx (18 KB)
Supplemental Table 3. Adult Male SMG (Gluck et al., 2016) - .xlsx (16 KB)
Supplemental Table 4. Highly expressed protein-coding genes (HEG) in murine parotid, sublingual, and submandibular salivary glands. - .xlsx (86 KB)
Supplemental Table 5. Enriched GO terms associated with highly expressed genes (HEGs). - .xlsx (84 KB)
Supplemental Table 6. Pairwise comparisons of significant differences in gene expression between salivary glands. - .xlsx (282 KB)
Supplemental Table 7. Expression clustering of differentially expressed transcription factor (DE TF) genes in the murine major salivary glands using Mfuzz/Bioconductor (default cluster membership=0.8) - .xlsx (14 KB)
Supplemental Table 8. Genes involved in co-expression clusters - .xlsx (20 KB)
Supplemental Table 9A. Enriched GO terms associated with the DE genes that were co-expressed with the SLG dominant DE TFs; Supplemental Table 9B. Enriched GO terms associated with the DE genes that were co-expressed with the PG dominant DE TFs - .xlsx (18 KB)