Abstract
In general, the mammalian whole body mass-specific metabolic rate correlates positively with maximal urine concentration (Umax) irrespective of whether or not the species have adapted to arid or mesic habitat. Accordingly, we hypothesized that the thick ascending limb (TAL) of a rodent with markedly higher whole body mass-specific metabolism than rat exhibits a substantially higher TAL metabolic rate as estimated by Na+-K+-ATPase activity and Na+-K+-ATPase α1-gene and protein expression. The kangaroo rat inner stripe of the outer medulla exhibits significantly higher mean Na+-K+-ATPase activity (~70%) compared with two rat strains (Sprague-Dawley and Munich-Wistar), extending prior studies showing rat activity exceeds rabbit. Furthermore, higher expression of Na+-K+-ATPase α1-protein (~4- to 6-fold) and mRNA (~13-fold) and higher TAL mitochondrial volume density (~20%) occur in the kangaroo rat compared with both rat strains. Rat TAL Na+-K+-ATPase α1-protein expression is relatively unaffected by body hydration status or, shown previously, by dietary Na+, arguing against confounding effects from two unavoidably dissimilar diets: grain-based diet without water (kangaroo rat) or grain-based diet with water (rat). We conclude that higher TAL Na+-K+-ATPase activity contributes to relationships between whole body mass-specific metabolic rate and high Umax. More vigorous TAL Na+-K+-ATPase activity in kangaroo rat than rat may contribute to its steeper Na+ and urea axial concentration gradients, adding support to a revised model of the urine concentrating mechanism, which hypothesizes a leading role for vigorous active transport of NaCl, rather than countercurrent multiplication, in generating the outer medullary axial osmotic gradient.
Keywords: comparative physiology, countercurrent multiplication, loop of Henle, renal outer medulla, sodium transport
INTRODUCTION
In general, mammalian renal medullary thickness relative to kidney size and whole body mass-specific metabolism are positively correlated with maximal urine concentrating ability (Umax) (8, 12). The latter relationship occurs (with exceptions) irrespective of whether or not the species have adapted to arid or mesic habitat (8, 12). In addition, medullary thick ascending limb (TAL) mitochondria density as a percentage of cell volume generally increases with decreasing body mass (1). These interspecies relationships suggest that the TAL active Na+ reabsorption rate may be one determinant of the ability of mammals with relatively high whole body mass-specific metabolism to produce a higher Umax than mammals with relatively low whole body mass-specific metabolism.
The corticomedullary osmotic gradient of the mammalian kidney, which consists chiefly of NaCl and urea (24), plays a fundamental role in maintaining Na+ and water balance by enabling the kidney to produce urine with an osmolality substantially higher than that of plasma (55). The active transport component of TAL Na+ reabsorption is fundamental to this process, playing a direct role in the production of the osmotic gradient in the outer medulla by way of countercurrent multiplication (60) and an indirect role in producing the gradient in the inner medulla, where transepithelial solute transport is predominantly passive, the gradient is steepest, and urea is the dominant solute. The nature of that indirect role remains elusive.
Existence of a high body mass-specific TAL metabolic rate would suggest that these animals might, in some way, use the active Na+ transport step to generate a steeper corticomedullary osmotic gradient and produce a higher Umax compared with animals with a low body mass-specific TAL metabolic rate. However, there is little or no experimental support suggesting that interspecies variations in TAL Na+-K+-ATPase activity significantly impact countercurrent multiplication and Umax (1).
Na+ reabsorption in the TAL involves secondary active transport in the apical membrane [Na+-K+-2Cl cotransporter isofrom 2 (NKCC2)] driven by primary active transport in the basolateral membrane (Na+-K+-ATPase) (50). Voltage-driven, passive, paracellular lumen-to-interstitial Na+ transport maintains electroneutrality and increases the efficiency of Na+ reabsorption compared with that of an entirely transcellular Na+ reabsorptive pathway (25, 26), and Na+/H+ exchanger 3 (NHE3) and renal outer medullary potassium channel (ROMK) also contribute to overall Na+ handling (19, 51).
Within a species, Na+-K+-ATPase activity has been shown to positively correlate with Na+ reabsorption rates measured in individual segments along the length of the nephron (20, 31). In the rat TAL, increased NKCC2 activity, occurring at least in part through increased trafficking, phosphorylation, and protein-protein interactions, is the primary mechanism for hormonal regulation of the Na+ reabsorption rate (2). Increased transcellular Na+ delivery from apical NKCC2 to basolateral Na+-K+-ATPase leads to stoichiometric increases in O2 consumption (41), basolateral Na+ transport, and tubular Na+ reabsorption through increased Na+-K+-ATPase activity; however, there is little or no evidence that regulated increases in Na+-K+-ATPase protein expression and trafficking are associated with increased Na+ reabsorption in the TAL (22, 32, 37, 44, 53).
The TAL accounts for the vast majority of total Na+-K+-ATPase protein and activity in the inner stripe of the outer medulla (ISOM) (20, 32, 49). The overarching role of active Na+ transport by the TAL is underscored by the fact that the rate of Na+ reabsorption is the major determinant of medullary oxygen consumption (11).
To evaluate a potential relationship between body mass-specific TAL active Na+ transport rate and Umax, we sought to determine whether or not a correlation exists among TAL Na+-K+-ATPase activity, protein, mRNA, TAL mitochondrial density and corresponding outer medullary-inner medullary axial Na+, and urea tissue concentration gradients in the kangaroo rat and the Munich-Wistar rat (MW rat), two species in which the former has a nearly 10-fold lower body mass than the latter. For comparison with a second rat strain, Na+-K+-ATPase activity, Na+-K+-ATPase protein expression with water ad libitum and with 72-h water restriction, Na+-K+-ATPase mRNA expression, and mitochondrial density were also measured in the Sprague-Dawley rat (SD rat), a rat strain that is more readily available than the MW rat, but historically less commonly used for medullary micropuncture and microperfusion studies of the urine concentrating mechanism compared with the MW rat. The basal whole body metabolic rate for kangaroo rat is ~4.0 (13) and for rat is ~1.0–1.7 (ml O2·g−1·h−1) (6, 48).
The kangaroo rat, a desert rodent, can produce a substantially higher urine osmolality than the rat, and is capable of reaching more than 6,000 mosmol/kg H2O, compared with a maximum of ~3,000 mosmol/kg H2O, for the SD, MW, and other laboratory rat strains (10). However, mammals tend to exist in a moderately antidiuretic state on a day-to-day basis when provided with adequate food and fluids. Throughout our study, all animals were provided with food and/or fluids ad libitum and produced a urine that was ~50–60% of their individual Umax, confirming their moderately antidiuretic state. An experimental caveat is that the kangaroo rat obtains adequate fluids from a diet of foliage and seeds and is believed to produce sufficient metabolic water but does not drink (free) water.
The results support the idea that an inverse relationship, such as occurs between body mass and whole body mass-specific metabolism, extends to body mass and the active membrane transport step that generates and sustains the corticomedullary Na+ concentration gradient in the renal outer medulla.
METHODS
Animals.
Adult kangaroo rats, Dipodomys merriami, were trapped at Santa Rita Experimental Range (Green Valley, AZ) and were housed in the animal facility at The University of Arizona. Tissue was obtained from adult kangaroo rats at a mean captivity period of ~3 mo. Adult MW rats were reared in The University Animal Care Facility at The University of Arizona and adult SD rats were obtained from Envigo (Indianapolis, IN). Tissue was obtained from adult MW and SD rats at a mean age of ~5–6 mo.
As kangaroo rats survive on dry seeds, grain, and vegetation with no water (28), they were provided with Kaytee birdfeed (Songbird Blend; Kaytee Products, Chilton, WI) ad libitum and one leaf of spinach three times each week; they do not drink water nor do they eat commercial prepared rodent chow (62, 63). MW rats and SD rats were provided with rodent chow (no. 7001; Envigo) and water ad libitum. Both diets are grain based and include predominantly millet, corn, peanuts, and sunflower with added vitamin A and D (Kaytee) or wheat, corn, soybean with added multiple micronutrients, and multiple vitamins (Envigo). Unpublished estimates from Kaytee suggest that the Kaytee dietary Na+ might be reduced by as much as ~50% compared with rodent chow. All animals were male unless otherwise stated. Rats were held on a 14:10-h light-dark cycle at 22–23°C with 40–60% humidity; kangaroo rats were held on a 12:12-h light-dark cycle at 21–22°C with 40–60% humidity. Blood glucose was measured via tail snip (Ascensia Elite XL glucometer; Bayer, Mishawaka, IN). Urine osmolalities were measured with a vapor pressure osmometer (Wescor, Logan, UT). For water restriction, SD rats were provided with ~40% normal water intake for 72 h. Normal daily water consumption by rats is ~10% of body weight (42, 65). Appropriate volumes of water were determined by weighing animals at 12-h intervals. All experiments were conducted in accordance with National Institutes of Health Guide for the Care and Use of Laboratory Animals (1996) and were approved by The University of Arizona Institutional Animal Care and Use Committee.
Tissue preparation for protein and RNA studies.
Animals were euthanized with CO2, and kidneys were rapidly removed and weighed, placed onto a 4°C microscope stage, and immediately bisected with a sagittal incision. The ISOM was dissected, either complete or bisected as described below and placed into a microfuge tube, frozen in liquid nitrogen and stored at −80°C for later analyses.
Measurement of glomerular filtration rate in conscious kangaroo rats.
Glomerular filtration rate (GFR) measurements were performed by determining the plasma kinetics of the GFR marker FITC-Sinistrin (Fresenius-Kabi, Linz, Austria) following an intravenous bolus injection as previously described (18, 58). Briefly, FITC-sinistrin (2% in sterile saline, which was also used to establish a standard curve) was injected into the retro-orbital plexus of kangaroo rats (2 µl/g body wt) during brief isoflurane anesthesia. At 3, 5, 7, 10, 15, 35, 56, and 75 min after injection, blood was collected from the tip of the tail into a Na+-heparinized 10-µl microcap (Hirschmann Laborgeräte, Eberstadt, Germany). After centrifugation, plasma was diluted 1:10 in 0.5 M HEPES (pH 7.4) and fluorescence was determined with a Nanodrop ND-3300 fluorospectrometer (Thermo Fisher Scientific, Waltham, MA) by pipetting 2 µl of samples onto the pedestal. GFR was calculated by a two-compartment model of two-phase exponential decay (GraphPad Prism, San Diego, CA).
Measurement of nephron number in kangaroo rat kidney.
Kidneys were perfused via the aorta first with PBS then with a saturated solution of Alcian Blue 8GS (Sigma-Aldrich, St. Louis, MO), excised and decapsulated. Each kidney was immersed in 50% ETOH and incubated for 24 h at 4°C on a rocker (changing ETOH after the first 4 h) and then transferred to a solution of 50% ETOH and 1% NH4OH (1:1 by volume) for 1.5 h at room temperature. Kidneys were placed into a 25-ml Erlenmeyer flask containing 20% HCl, covered with Parafilm M and incubated at 37°C for ~2.5 h. The HCl was suctioned off, and the kidney was rinsed with H2O (3× volume). The kidney, including the final rinse, was poured into a 250-ml volumetric flask, with the Erlenmeyer flask being rinsed several times and the rinses being added to the volumetric flask. The mixture was agitated until homogenous, and the volume was adjusted to 250 ml. Before glomeruli were counted, this mixture was poured into a beaker and mixed with a stir bar so that no visible tissue pieces remained. While being stirred, 1-ml samples were removed and placed into a clear-bottomed counting chamber, coverslipped, and mounted on the stage of a compound microscope for counting of the blue-stained glomeruli.
Immunoblotting.
Protein from the ISOM of one kidney per animal was processed and blotted, and the blot results were quantified as described below. For tissue lysate purification, samples were homogenized in isolation buffer consisting of the following (in mM): 300 mannitol, 12 HEPES (pH 7.6), and protease inhibitors prepared according to the manufacturer’s instructions (1 tablet/10 ml buffer; Complete Mini, cat. no. 11836153001; Roche Diagnostics, Indianapolis, IN) and then centrifuged at 1,100 g for 15 min at 4°C. The supernatant was collected and centrifuged at 147,000 g for 45 min at 4°C. The remaining pellet was resuspended in isolation buffer and stored at −80°C for later blotting. The protein sample was mixed with 5× SDS/dithiothreitol gel-loading buffer and heated for 15 min at 60°C. Samples were separated with 8% or 12% SDS-PAGE. Protein was quantified with a BSA protein standard using the MicroBCA protein assay kit (cat. no. 23235; Thermo Fisher Scientific).
Proteins were transferred to nitrocellulose, blocked in AquaBlock (EastCoast Bio, North Berwick, ME), and incubated with anti-Na+-K+-ATPase α1-mouse antibody (1:1,000, Cat. No. A277; Sigma-Aldrich) and anti-β-actin rabbit antibody (1:7,000, cat. no. SC-47778; Santa Cruz Biotechnology, Dallas, TX) in blocking buffer overnight at 4°C. After being washed, the membrane was incubated for 1 h at 4°C with secondary antibodies (IRDye 800CW, goat anti-mouse, 1:20,000 and IRDye 680RD, goat anti-rabbit, 1:20,000; LI-COR, Lincoln, NE). Proteins were visualized (Odyssey Infrared Imaging System), quantified (Odyssey 2.1), and normalized by comparison to β-actin The mass of protein bands was estimated using Prestained SDS-PAGE Standards (broad range, cat. no. 1610318; Bio-Rad, Hercules, CA). The linearity of the detection system was confirmed with serial dilution of sample protein.
Real-time PCR.
Total RNA was extracted from the ISOM of one kidney from each animal with TRIzol Reagent (Life Technologies, Austin, TX) and quantified on a NanoDrop Lite spectrophotometer (Thermo Fisher Scientific). First-strand cDNA was synthesized from 400 ng of DNase I-treated total RNA with Maxima H Minus reverse transcriptase (Thermo Fisher Scientific). The resulting cDNA was then used to quantify the mRNA expression levels of Na+-K+-ATPase α1 (GenBank M28647) and β-actin (based on GenBank KJ696744) in the MW rat samples using the primers listed in Table 1. Partial cDNA sequences for the same genes were also cloned from the kangaroo rat kidney Na+-K+-ATPase α1 (GenBank KR732616) and β-actin (GenBank KJ696744) to design specific primers for mRNA quantification (Table 1). All primer sets were validated by sequencing of products. Real-time PCR was performed using SYBR Select Master Mix for CFX (Life Technologies) on a Bio-Rad CFX96 Real-Time System (Bio-Rad Laboratories). Melt-curve analysis confirmed production of a single product, and no-template controls were run in parallel. Changes in gene expression were calculated based on the relative standard curve method (52). Standard curves were generated by serial dilution of one random sample.
Table 1.
Primers used for real-time PCR analysis of kangaroo rat, Munich-Wistar rat, and Sprague-Dawley rat
| Gene Name/Species | Forward (5′–3′) | Reverse (5′–3′) |
|---|---|---|
| β-Actin | ||
| Kangaroo rat | gcccatctacgagggctac | agctgtggtggtgaagctg |
| Munich-Wistar rat | agccatgtacgtagccatcc | tctcagctgtggtggtgaag |
| Sprague-Dawley rat | agccatgtacgtagccatcc | tctcagctgtggtggtgaag |
| Na+-K+-ATPase α1 | ||
| Kangaroo rat | ccttcttttccaccaactgc | aaggatacccccaggaacac |
| Munich-Wistar rat | tatctgcaaatggctgcaag | cccagtgtacacaacgatgc |
| Sprague-Dawley rat | tatctgcaaatggctgcaag | cccagtgtacacaacgatgc |
Na+-K+-ATPase activity.
Na+-K+-ATPase activity was determined for the ISOM of the kangaroo rat and the MW rat. The kangaroo rat ISOM was left intact (1 ISOM/assay) while the MW rat ISOM was divided in half along the corticomedullary axis (0.5 ISOM/assay). Tissue was placed into 0.5 to 1.0 ml 2× ATPase assay buffer consisting of the following (mM): 200 NaCl, 10 KCl, 6 MgCl2, 2 EGTA, and Halt protease inhibitor cocktail (1:100, Life Technologies), pH 7.4. The tissue was homogenized on ice using a Misonix 3000 Sonicator, and the homogenate was centrifuged at 13,000 g for 30 min at 4°C. The supernatant was immediately assayed for Na+-K+-ATPase activity with a portion stored at −80°C for protein analysis, which was quantified with a BSA protein standard (MicroBCA; Thermo Fisher Scientific).
Tissue ATP hydrolysis was determined by measuring release of inorganic phosphate. Na+-K+-ATPase activity is the difference between ATP hydrolysis in the presence and absence of ouabain and is expressed as moles of ATP hydrolyzed per gram of protein per minute (64). In brief, hydrolysis assays consisted of tissue supernatant plus 2× ATPase assay buffer (experimental) or assay buffer only (control) in a final volume of 200 μl. Each assay (run in duplicate) included 80 μl l-histidine (200 mM), 5 μl alamethicine (20 mg/ml in ETOH), ouabain (10 mM final concentration) dissolved in DMSO or DMSO alone, and 70 μl H2O. The tubes were incubated in the dark in a shaking water bath for 5 min at 37°C, and then 40 µl of ATP (20 mM) were added to each tube to start the reaction, which continued for 30 min at 37°C. Reactions were stopped by the addition of 150 µl 15% ice-cold trichloroacetic acid.
The tubes were centrifuged at 4°C, and 400 μl of supernatant were removed for assay of inorganic phosphate. A total of 400 μl 4% FeSO4 in 1.25% ammonium molybdate solution (prepared in 2.1 N sulfuric acid) was added to these tubes and to a set of standards containing NaH2PO4 (0–500 nM). Samples were read at 750 nm in a 96-well plate reader (Victor V-3; Perkin Elmer, Waltham, MA).
Electron microscopy.
The ISOM was prepared for electron microscopy by trimming to a size of ~1 mm3 and was immersion-fixed with Karnovsky’s fixative containing 2.5% glutaraldehyde and 2% formaldehyde in PIPES buffer (100 mM), pH 7.4 for 1–3 h at 4°C. Tissue was then immersed in a solution of glycine (100 mM) and PIPES (100 mM) (1:1 by volume) for 15 min, rinsed in PIPES, postfixed with 1% osmium tetroxide in PIPES buffer (100 mM) for 60 min, washed with distilled water, incubated in 2% aqueous uranyl acetate for 20 min, ethanol dehydrated, and embedded in Spurrs epoxy resin. Thin sections were cut on a Leica Ultracut microtome (Leica, Deerfield, IL) and stained with 3% lead citrate. Sections were imaged using a Tecnai Spirit Biotwin electron microscope (FEI, Hillsboro, OR) and photographed with an AMT sidemount 4-megapixel camera (Advanced Microscopy Techniques, Woburn, MA).
TAL tubule density and mitochondria density.
TAL tubule density was calculated as the total TAL cross-sectional area divided by the total ISOM cross-sectional area in paraffin-embedded tissue sections obtained from ~500 μm above the outer medullary-inner medullary boundary (images are not shown). TALs and collecting ducts were labeled using fluorescence immunohistochemistry for the chloride channel ClC-K2 and the water channel aquaporin 2, respectively, as described previously (33, 55) (1 section per animal).
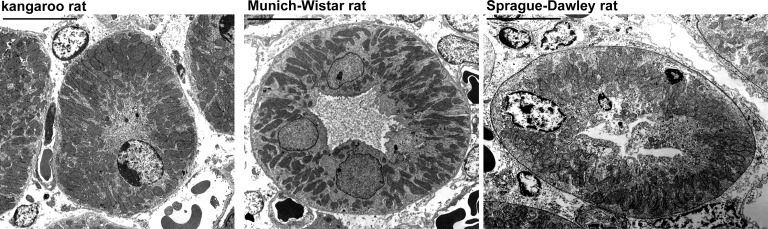
TAL mitochondria density was calculated as the total mitochondria area divided by the total TAL intracellular area including nuclei in electron micrographs of individual TAL transverse sections (×2,550; ~500–700 pixel radius at a resolution of ~256 pixels/in.) (Fig. 1). Intracellular area was taken as the total area encompassed by the basal and apical plasma membranes. Luminal area was excluded. The cellular area, mitochondria density, and nuclear number for each species were calculated as the mean of three TAL measurements from each of five kangaroo rats, five MW rats, and three SD rats.
Fig. 1.
Electron micrographs of the thick ascending limb (TAL) from the inner stripe of the outer medulla (ISOM) of the kangaroo rat, Munich-Wistar rat and Sprague-Dawley rat. The mitochondria density in the kangaroo rat is significantly higher compared with the Munich-Wistar and Sprague-Dawley rats. See results for details. Scale bars = 10 μm.
Each TAL, mitochondrion (see TAL mitochondria density), and cross-sectional area was outlined using drawing tools in Photoshop CS5 (Adobe, San Jose, CA), and area was calculated using the Image Processing Toolkit (Reindeer Graphics, Asheville, NC).
Medullary Na+, K+, urea and water composition.
Medullary Na+, K+, urea, and water composition was determined using a method described by Schmidt-Nielsen and colleagues (61). Briefly, two kidneys from each kangaroo rat and one kidney from each MW rat were removed and the ISOM, inner medullary base (IM1), and inner medullary tip (IM2) were isolated on a microscope stage at 4°C. Tissue wet weight was immediately measured, and dry weight was determined after overnight drying. Water composition was calculated as the difference between wet and dry weights. Na+, K+ and urea were extracted from the dry tissue into aqueous solution. Na+ and K+ were assayed with atomic absorption spectrophotometry (45), and urea was assayed with reagents from BioAssay Systems (Hayward, CA). Results for each kangaroo rat were combined as the mean of two kidneys.
Statistical analysis.
Data combined from three or more samples are reported as means ± SE (n = number of replicates). The statistical significance of differences between means was determined with Student’s unpaired t-test, and multiple comparisons were evaluated, as indicated, with one-way ANOVA with Tukey’s post hoc test or with a two-way, repeated-measures, mixed-model ANOVA with post hoc Bonferroni adjustments calculated for each tested effect (zone, species, or zone × species) using GraphPad Prism 6 (Graphpad) and SPSS Statistics for Windows, Version 25 (IBM, Armonk, NY). Differences in the means were considered significant with P < 0.05; the critical value of P was two tailed.
RESULTS
Rodent fluid homeostasis and renal structure.
Kangaroo rat urine osmolality was approximately threefold higher than that of the MW and SD rats, whereas urine osmolality of MW and SD rats was not significantly different from each other (kangaroo rat: 4,066 ± 248, n = 3; MW rat: 1,378 ± 67, n = 6; SD rat: 1,382 ± 44, n = 3; mosmol/kg H2O; one-way ANOVA with Tukey’s post hoc test), with all three species producing nearly 50–60% of their Umax (8). The total kidney mass-to-body mass ratio for kangaroo rats was ~40–80% greater compared with MW or SD rats (Table 2). The two-kidney GFR of awake kangaroo rats is ~320 μl/min (Table 3) and, based on ~20,000 nephrons per kidney (nephron number is reported below), has a calculated mean single nephron GFR of ~8 nl/min, which is about equivalent to that of the mouse (71, 72) and ~25% that of the MW rat (68, 69). The kangaroo rat two-kidney GFR normalized to body mass (40 g) or kidney mass (0.46 g) is ~8 or ~700 µl·min−1·g−1, respectively. The rat two kidney GFR normalized to body mass (400 g) or kidney mass (2.23 g) is ~6.4 or ~1,090 µl·min−1·g−1, respectively. The hematocrit in kangaroo rat was ~49%, indicating that kangaroo rats are in fluid balance presenting with a hematocrit that is in a comparable range as described for other rodents (27, 46). Along these lines, plasma glucose levels (Table 3) also fall into the range found in other rodents (23).
Table 2.
Body and kidney mass of kangaroo rat, Munich-Wistar rat, and Sprague-Dawley rat
| Species | n | Body Mass, g | Left Kidney Mass, g | Right Kidney Mass, g | Total Kidney Mass, g | Total Kidney Mass/Body Mass |
|---|---|---|---|---|---|---|
| Kangaroo rat | 8 | 40.7 ± 1.6a | 0.23 ± 0.0a | 0.23 ± 0.01a | 0.46 ± 0.02a | 0.011 ± 0.001a |
| Munich-Wistar rat | 6 | 401.0 ± 55.3b | 1.11 ± 0.08b | 1.12 ± 0.08b | 2.23 ± 0.16b | 0.006 ± 0.001b |
| Sprague-Dawley rat | 3 | 326.9 ± 10.3b | 1.22 ± 0.03b | 1.26 ± 0.06b | 2.48 ± 0.09b | 0.008 ± 0.000b |
Values are means ± SE. The statistical significance between sample means for each column was determined with a one-way ANOVA and Tukey’s post hoc test.
Values in each column that share the same letter are not significantly different from each other.
Table 3.
Glomerular filtration rate and plasma characteristics of kangaroo rat
| Body Weight, g | n | Glucose, mg/dl | Hematocrit, % | Sinistrin Clearance, μl/min |
|---|---|---|---|---|
| 43.0 ± 2.2 | 6 | 161.2 ± 2.2 | 49 ± 1 | 322 ± 21 |
Values are means ± SE. Glomerular filtration rate was determined by FITC-sinistrin clearance in awake animals. See text for details on other measurement techniques. Genders were not recorded for these animals.
The total nephron number in the kangaroo rat kidney was determined by counting glomeruli in six kidneys from three animals (1 female, 2 males). The body weight for these three animals was 41.7 ± 0.8 g. The mean number of nephrons per kidney for these three animals was 20,317 ± 543 (19,600 female; 20,600 first male, 20,750 second male), comparable to the nephron number of 19,000 reported previously for Dipodomys sp (59). For comparison, the MW and SD rats have between 30,000 and 38,000 nephrons per kidney (3, 5, 34, 59).
TAL mitochondria density.
We hypothesized that the TAL mitochondria volume per cell volume would be greater in the kangaroo rat than in the MW rat, in part because of greater body mass-specific metabolism (1, 9). TAL mitochondria density was calculated as the ratio of cross-sectional mitochondria area (μm2) to cross-sectional cellular area (μm2) measured in electron micrographs (Fig. 1). Cross-sectional cellular areas were 472 ± 71, 741 ± 67, and 824 ± 84 μm2 for kangaroo rat, MW rat and SD rat, respectively, significantly smaller in the kangaroo rat compared with the MW and SD rats. Mitochondria density was significantly higher in the kangaroo rat (0.55 ± 0.05) compared with the MW rat (0.37 ± 0.05) and the SD rat (0.35 ± 0.02). For both cellular area and mitochondria density, there was no significant difference between MW and SD rats. The number of nuclei per cross-sectional cellular area was not significantly different among the three species; kangaroo rat (0.003 ± 0.001), MW rat (0.004 ± 0.000), and SD rat (0.004 ± 0.001). All morphological comparisons were conducted with five kangaroo rats, five MW rats, and three SD rats as described in methods and analyzed with one-way ANOVA with Tukey’s post hoc test.
Na+-K+-ATPase activity of the ISOM.
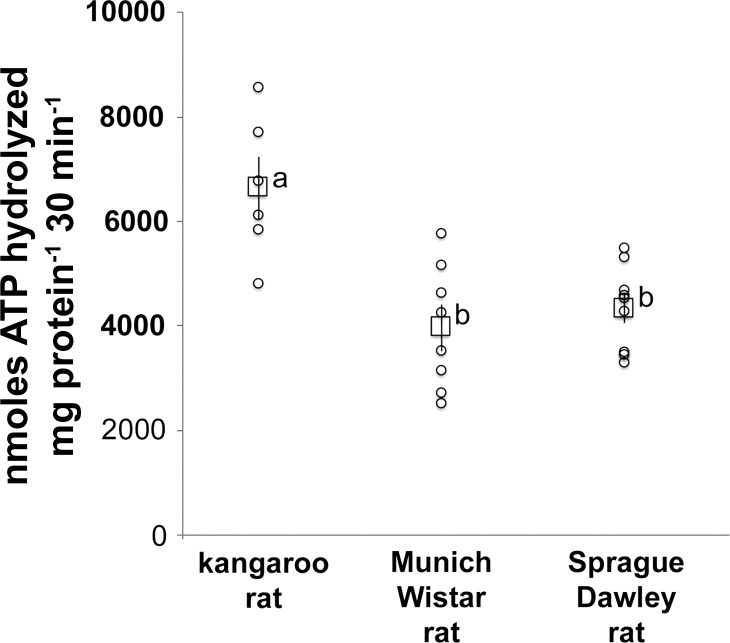
Na+-K+-ATPase activity (nmol·ATP hydrolyzed mg protein−1·30 min−1) measured as the difference in ATP hydrolyzed in the presence and absence of ouabain was ~70% higher in kangaroo rat compared with MW rat (Fig. 2). The Na+-K+-ATPase activity in the SD rat was not significantly different from that of the MW rat (Fig. 2). The TAL tubule density in the ISOM of the kangaroo rat (0.31 ± 0.03, n = 4) was not significantly different from that of the MW rat (0.32 ± 0.06, n = 5). Tubule density of the SD rat was not determined.
Fig. 2.
Na+-K+-ATPase activity in homogenates of the ISOM of the kangaroo rat (n = 6), Munich-Wistar rat (n = 8) and Sprague-Dawley rat (n = 9). Individual values (○) and means ± SE (☐) are shown. The statistical significance between sample means was determined with a one-way ANOVA and Tukey’s post hoc test. Mean values that share the same letter are not significantly different from each other.
Expression of Na+-K+-ATPase α1-protein in the ISOM.
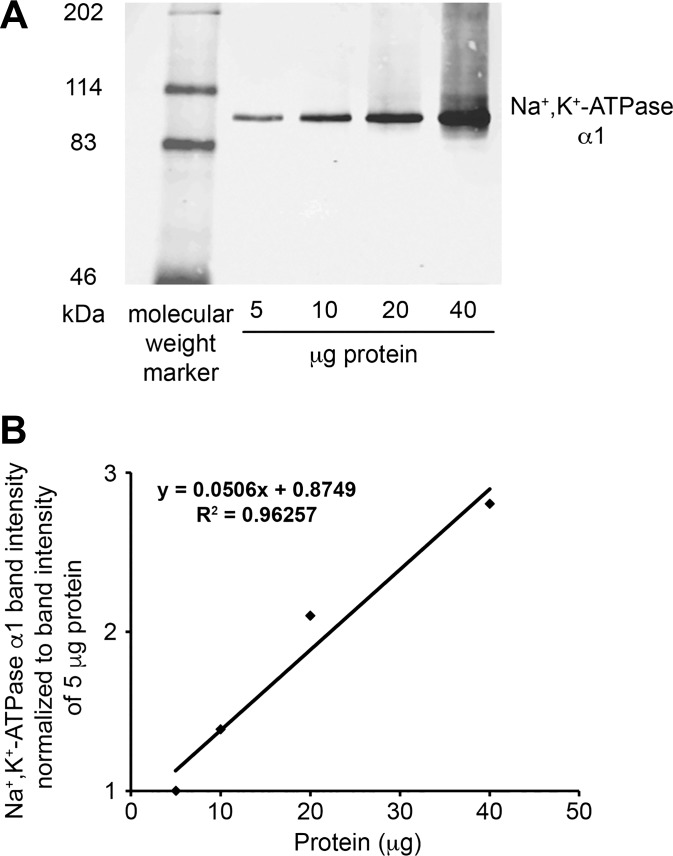
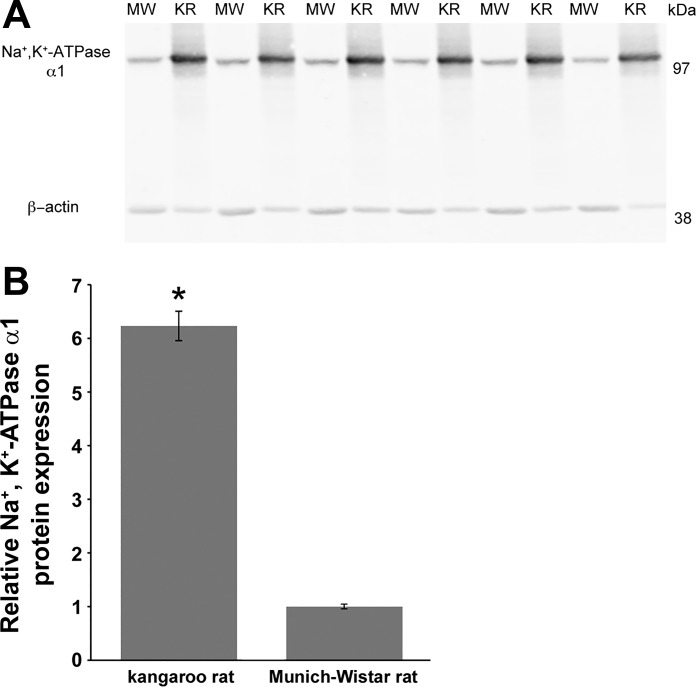
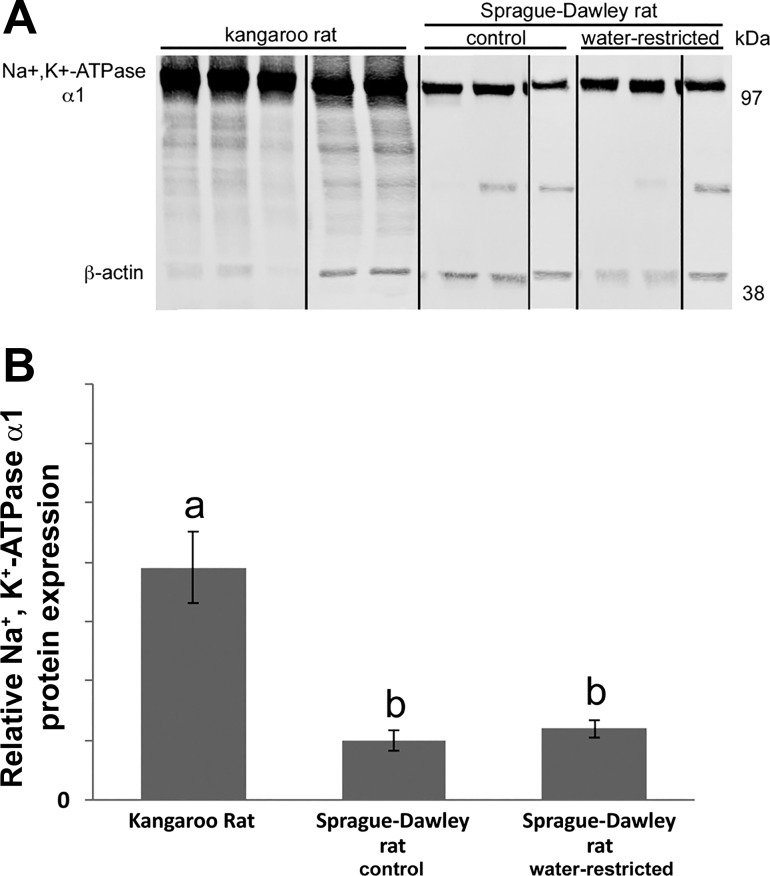
The detection of Na+-K+-ATPase α1-protein by immunoblot was linear for a range of 5 to 40 µg total protein (Fig. 3). Figures 4 and 5 show representative Na+-K+-ATPase α1-immunoblots from gels loaded with tissue protein from kangaroo rat, MW rat and SD rat ISOM. Na+-K+-ATPase α1-protein abundance in the kangaroo rat was approximately sixfold higher compared with the MW rat. Similarly, Na+-K+-ATPase α1-protein abundance in the kangaroo rat was approximately fourfold higher compared with the SD rat. In an effort to ensure strong β-actin intensity, the kangaroo rat Na+-K+-ATPase α1-protein band is overexposed. Consequently, kangaroo rat Na+-K+-ATPase α1-protein expression is likely underestimated, which accounts for its apparently lower expression relative to the SD rat (~4-fold) compared with the MW rat (~6-fold). Na+-K+-ATPase α1-protein expression in the 72-h water-restricted SD rat is not significantly different from the control SD rat (water ad libitum). Urine osmolality (mosmol/kg H2O) was significantly higher in water-restricted SD rats (1,950 ± 161; n = 3) compared with control SD rats (1,234 ± 65; n = 3; Student’s unpaired t-test).
Fig. 3.
The linearity of the Na+-K+-ATPase α1-protein immunoblot detection system. The detection of Na+-K+-ATPase α1-protein by immunoblot was linear for a range of 5–40 µg total protein. A: immunoblot with 5–40 μg of protein from Munich-Wistar rat ISOM applied to each lane. B: quantification of Na+-K+-ATPase α1 normalized to band intensity measured for 5 µg total protein.
Fig. 4.
Na+-K+-ATPase α1-protein expression in the ISOM of the kangaroo rat (KR) and the Munich-Wistar rat (MW). A: immunoblots with 20 μg of protein applied to each lane; each lane represents a different animal. B: quantification of Na+-K+-ATPase α1 immunoblot normalized to β-actin; n = 6 for each species. Kangaroo rat data are expressed relative to the mean of the Munich-Wistar rat, which was set to 1. *Significant difference in protein expression between species by Student’s unpaired t-test.
Fig. 5.
Na+-K+-ATPase α1-protein expression in the ISOM of the kangaroo rat and the Sprague-Dawley rat, the latter with water ad libitum (control) or water restricted for 72 h (see methods). A: immunoblots with 10 μg of protein applied to each lane; each lane represents a different animal. Black lines denote where blot was cut for rearrangement of lanes. B: quantification of Na+-K+-ATPase α1-immunoblot normalized to β-actin; n = 5 (kangaroo rat) and n = 3 (Sprague-Dawley rats). Kangaroo rat and water-restricted Sprague-Dawley rat data are expressed relative to the mean of the control Sprague-Dawley rat, which was set to 1. Kangaroo rats were mixed gender. The statistical significance between sample means was determined with a one-way ANOVA and Tukey’s post hoc test. Mean values that share the same letter are not significantly different from each other.
Na+-K+-ATPase α1-mRNA expression in the ISOM.
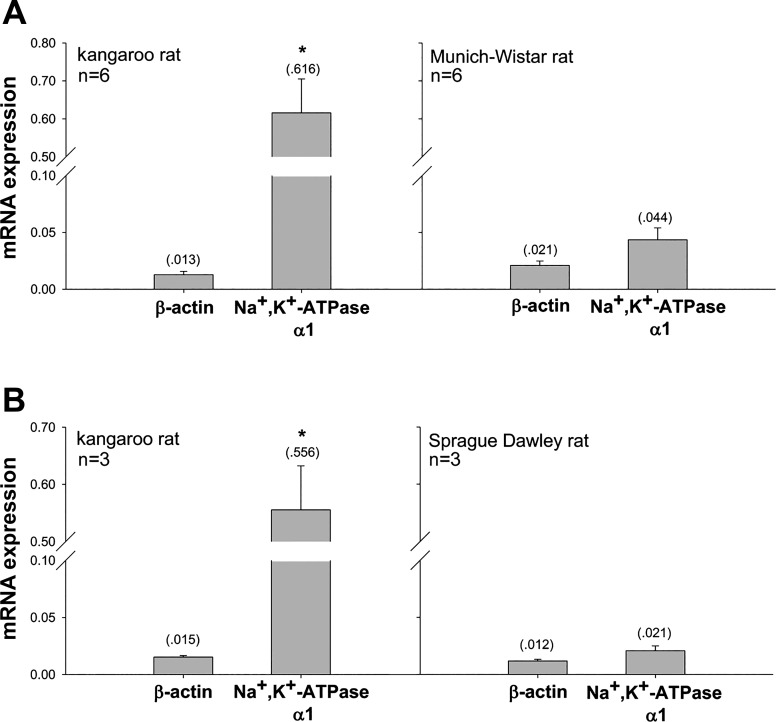
Na+-K+-ATPase α1-mRNA expression in the kangaroo rat ISOM was significantly higher compared with the MW rat (~13-fold) and compared with the SD rat (~25-fold higher) (Fig. 6).
Fig. 6.
Na+-K+-ATPase α1-mRNA expression in the ISOM of the kangaroo rat compared with the Munich-Wistar rat (A) and kangaroo rat compared with the Sprague-Dawley rat (B), as determined by real-time PCR. β-Actin expression was not significantly different between the two species in each panel. Arbitrary units; means shown in parentheses. *Significant difference in gene expression between species by Student’s unpaired t-test.
Medullary Na+, K and urea composition.
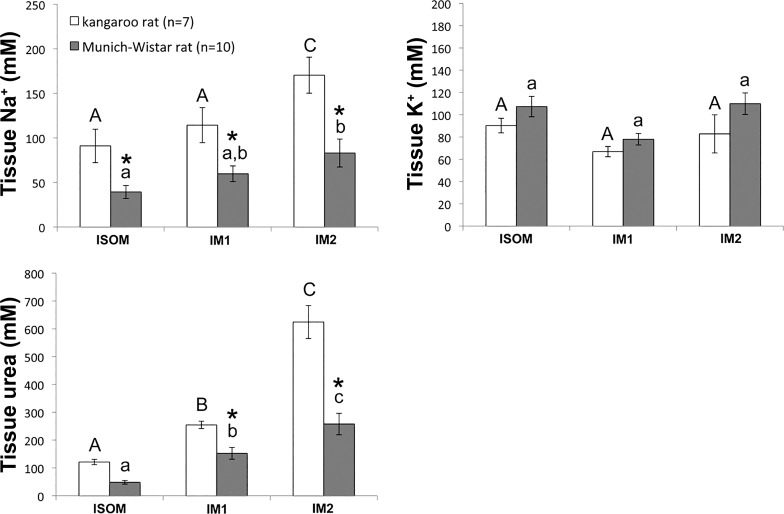
For the kangaroo rat and the MW rat, Na+, K+ and urea concentrations (in mM) as a proportion of tissue water were determined for the ISOM and for the outer and inner 50% of the inner medulla (IM1 and IM2, respectively; Fig. 7). The tissue Na+ concentrations of the kangaroo rat were significantly higher than those of the MW rat in all three zones, whereas the tissue urea concentrations of the kangaroo rat were significantly higher than those of the MW rat only in the IM1 and IM2 zones. For each species, Na+ and urea concentrations increased significantly from the ISOM to the IM2. In contrast, K+ concentrations were not significantly different between species in any of the three zones or within a species in successively deeper zones.
Fig. 7.
Na+, K+ and urea concentrations in the inner and outer medulla of the kangaroo rat and the Munich-Wistar rat. Na+, K+ and urea concentrations were determined in three medullary zones: the ISOM, outer 50% of the inner medulla (IM1), and inner 50% of the inner medulla (IM2). The statistical significance of differences between sample means was determined with a two-way, repeated-measures, mixed-model ANOVA, and post hoc Bonferroni adjustments were calculated for each tested effect (zone, species or zone*species). *Significant differences between species in each zone; bars sharing the same letter of the same case are not significantly different from one another.
DISCUSSION
In this study, we showed that Na+-K+-ATPase activity in the ISOM (in moderately dehydrated animals) is markedly higher in the kangaroo rat than in two strains of rat. Previous studies have shown that Na+-K+-ATPase activity in the isolated TAL from the ISOM is at least twofold higher in the SD or MW rat than in the larger sized New Zealand rabbit (21, 31). Each pairwise comparison has a differential of ~10-fold body mass and an approximately twofold Umax. The combined data support the hypothesis that the level of active Na+ transport in the medullary TAL is inversely proportional to body mass and, if this correlates with the level of Na+ reabsorption rate, would conceivably play a significant role in determining species-dependent Umax. Variations along body mass range likely exist, both smaller mammals producing relatively low Umax (e.g., CD1 mice; Refs. 31, 43) and larger mammals producing high Umax. Higher Na+-K+-ATPase activity in the kangaroo rat than in the rat, and, we speculate, in the rat relative to rabbit, is likely a consequence of higher Na+-K+-ATPase α1-mRNA and protein expression and would be supported by a capacity for higher cellular respiration rates, judging from mitochondrial density. A higher Na+-K+-ATPase activity and higher TAL NaCl reabsorption rate would directly account for Na+ concentrations and indirectly account for urea concentrations of the outer medulla and inner medulla of the kangaroo rat exceeding those of the rat. The ensuing steeper corticomedullary osmotic gradient would contribute to higher Umax in the kangaroo rat compared with rat.
While the kangaroo rat and rat TALs clearly exhibit distinctly different Na+-K+-ATPase activity, some fraction of that difference may relate to adaptations that enable the kangaroo rat to survive in an arid habitat. Correlations between renal Na+-K+-ATPase activity and hydration status in desert-adapted mammals have been shown for the camel and rodent jerboa. In the camel, water restriction leads to increased renal Na+-K+-ATPase gene expression (73), whereas in jerboa, hydration leads to a decline in medullary TAL Na+-K+-ATPase activity (16).
Hydration status in the rat has previously been shown to have a relatively minor impact on TAL Na+-K+-ATPase α1-protein expression levels, in support of our studies with the water-restricted SD rat. Kim et al. (32) found that 7-day water restriction in the SD rat (with consequent urine osmolality of ~2,900 mosmol/kg H2O) results in an ~60% increase in Na+-K+-ATPase α1-protein expression in ISOM homogenates compared with control hydrated SD rat (with urine of ~550 mosmol/kg H2O). This increase of 60% is relatively minor in comparison to the 400–600% higher value that we report in our study for kangaroo rat (with urine of ~4,000 mosmol/kg H2O) compared with hydrated MW and SD rats (with urine of ~1,400 mosmol/kg H2O). While the urine osmolalities of water-restricted rat do not reach the high concentrations that occur in kangaroo rat, a case can be made that kangaroo rat Na+-K+-ATPase expression would be substantially higher than that of rat at equal urine osmolalities. How variations in Na+-K+-ATPase gene or protein expression or activity relate to quantitative differences in interspecies variation of Umax remains poorly understood.
The impact of dietary Na+ on ISOM Na+-K+-ATPase protein expression was not tested; however, little or no change in Na+-K+-ATPase α1-protein expression in SD rat kidney cortex or ISOM occurs following 10- to 100-fold reduction in dietary NaCl composition for 10 days (35, 47). If dietary Na intake were to have an impact on kangaroo rat Na+-K+-ATPase α1-protein expression, then the kangaroo rat kidney is regulated in a markedly different manner from rat.
An increasing kidney mass-to-nephron number ratio with body size is a classic example of allometric scaling (12). Connective and vascular tissue and interstitial cells likely make up an increasing proportion of kidney mass in larger animals, possibly reflecting requirements for enhanced vascular flow and proportionately more support for larger organs and larger animals (12, 57). Mitochondria density relative to cell volume reported here for MW and SD rat TAL is comparable to that reported for MW rat TAL (0.37) (14) and markedly higher than nephron segments generally exhibiting lower Na+ reabsorption rates, such as SD rat distal convoluted tubule (0.26) and connecting tubule (0.21) (30). On the other hand, the TAL mitochondria density relative to cell volume in the kangaroo rat, while higher than that in rat, is lower than in the mouse, Mus musculus (0.65) (1), which has ~25% lower body mass. The higher mitochondria density in the TAL of kangaroo rat than in rat suggests, albeit indirectly, that greater amounts of ATP per cell exist for energizing kangaroo rat Na+-K+-ATPase activity than in the rat. In a mathematical model of the laboratory rat outer medullary urine concentrating mechanism, Layton and Layton (39) found that an elevated TAL active Na+ transport rate may substantially increase the concentrating effect through greater Na+ reabsorption but at a greater energy cost. In the kangaroo rat, an elevated Na+-K+-ATPase activity conceivably plays a role in producing a higher urine osmolality compared with the rat; greater cellular respiration could overcome the higher energy costs.
With the combination of 70% greater Na+-K+-ATPase activity and 50% greater total kidney mass-to-body mass ratio for the kangaroo rat compared with the MW or SD rat, and assuming all other factors remain equal, the contribution of TAL Na+-K+-ATPase activity to overall whole body metabolism would be ~2.5-fold greater in kangaroo rat compared with MW or SD rat. These and other structural and functional adaptations that might underlie the ability of desert mammals to withstand demands of heat and limited water supply in arid habitat have been proposed (4, 15, 54, 67). To the list of renal structural and functional features that correlate with the high Umax in desert rodents (4, 29, 70), we add the involvement of higher ISOM Na+-K+-ATPase activity. However, as already suggested, higher ISOM Na+-K+-ATPase activity may closely reflect adaptations to phenomena associated with lower body mass (e.g., evaporative and respiratory water loss) in addition to adaptations associated with arid habitat.
The kangaroo rat more effectively distributes reabsorbed Na+ along the entire corticomedullary axial length than rat. A role for urea transport by the inner medullary collecting duct in facilitating that Na+ distribution has long been hypothesized (36, 66); however, a mechanism has never been established and that role remains uncertain (17, 55, 56). A more complete understanding of NaCl compartment-to-compartment flows and characterization of tissue composition at higher resolution, both along the corticomedullary axis and perpendicular to the axis within the multiple lateral regions, should lead to a more complete understanding of the linkages between TAL NaCl reabsorption and the corticomedullary osmotic gradient at the level of the inner medulla, the latter arising primarily from passive processes and indirectly from active transport in the ISOM. An alternative hypothesis to the long-held passive mechanism hypothesis of the urine concentrating mechanism and widely accepted countercurrent multiplication theory proposes that the outer medullary corticomedullary osmotic gradient arises principally from vigorous active NaCl transport, with minimal osmotic equilibration by water reabsorption in descending thin limbs; this results in an increasing ratio of Na+ reabsorption in the TAL to water reabsorption with increasing depth along the corticomedullary axis (7, 38, 40). A steeper Na+ gradient in the ISOM alongside higher Na+-K+-ATPase activity in the kangaroo rat compared with rat is consistent with this hypothesis. The absence of aquaporin 1 in short loop descending thin limbs in rodents and humans (74) and presumably little or no water permeability, in addition to the anatomical separation of short loop descending thin limbs from TALs (which lie in the vascular bundle and interbundle regions, respectively), argue against the effectiveness of countercurrent multiplication. TAL active Na+ transport leads to a hyperosmotic solution that is carried by ascending peritubular capillaries to higher medullary levels, where NaCl is reabsorbed from capillaries at these higher levels; water is retained within capillaries due to oncotic and hydrostatic pressure and is returned to general circulation.
Perspectives and Significance
In this study, we have shown that Na+-K+-ATPase activity and protein expression levels in the ISOM of animals provided with food and water sufficient to maintain a urine concentration that is 50–60% of Umax are significantly higher in the kangaroo rat compared with the rat, consistent with the idea that a high body mass-specific metabolic rate may reflect, in part, the active step of Na+ reabsorption in the TAL. From an evolutionary viewpoint, a high Umax in small mammals with a high metabolic rate would counter: 1) the relatively greater diffusive water loss that occurs with larger surface area-to-body mass ratio, and 2) the relatively higher rate of water turnover that occurs in smaller mammals compared with larger mammals.
Given the tight control of the urine concentrating mechanism, the patterns of Na+-K+-ATPase activity and protein expression levels likely correlate to a degree along a range of urine concentrations; however, while water restriction leads to an increase in urine osmolality in rat, it does not lead to a substantial increase in Na+-K+-ATPase protein (Na+-K+-ATPase activity changes are not known). On the other hand, it is difficult to water load a kangaroo rat and the outcome of hydration on Na+-K+-ATPase protein and activity in the kangaroo rat are not known. However, if in a hydrated kangaroo rat, the urine osmolality and Na+-K+-ATPase protein were to approach that of rat, then it would indicate that the kangaroo rat has substantially higher ability to modulate Na+-K+-ATPase compared with rat. Given the complexity of the urine concentrating mechanism, other physiological factors, including glucagon, vasopressin, and other hormones as well as medullary blood flow and epithelial Na+ and urea permeabilities, variably influence concentrating capacity along that range (between and within species).
Tubulovascular functional architecture varies both within and between species that have complex type (rat, mice, and kangaroo rat) and simple type (human, pig, and rabbit) medullas. These structural and functional differences may lead to species-dependent countercurrent and solute recycling mechanisms that differentially influence the role of active Na+ reabsorption in production of the corticomedullary osmotic gradient and concentrated urine (4).
GRANTS
This study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-083338 (to T. L. Pannabecker) and DK-110621 (T. Rieg), National Science Foundation (NSF) Grant IOS-0952885 (to T. L. Pannabecker), Joint Division of Mathematical Sciences/National Institute of General Medical Sciences Initiative under NSF Grant DMS-1263943 (to T. L. Pannabecker), and American Physiological Society/NSF Integrative Organismal Systems Physiology Undergraduate Summer Research Fellowships (to M. Aw).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.M.N., M.S., T.R., and T.L.P. conceived and designed research; M.A., T.M.A., C.M.N., S.N.B., J.J.O., G.W., K.K.E., and T.R. performed experiments; M.A., T.M.A., C.M.N., S.N.B., J.J.O., G.W., K.K.E., M.S., T.R., and T.L.P. analyzed data; M.A., T.M.A., C.M.N., S.N.B., J.J.O., G.W., K.K.E., M.S., T.R., and T.L.P. interpreted results of experiments; M.A., C.M.N., G.W., and T.L.P. prepared figures; C.M.N., K.K.E., T.R., and T.L.P. drafted manuscript; C.M.N., M.S., T.R., and T.L.P. edited and revised manuscript; C.M.N., M.S., T.R., and T.L.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank our University of Arizona colleagues Erica Eggers, Ralph Fregosi, and Mark Borgstrom for advice with statistical analyses, Amrit Mandal for assistance with immunoblotting and Eldon Braun for many discussions.
REFERENCES
- 1.Abrahams S, Greenwald L, Stetson DL. Contribution of renal medullary mitochondrial density to urinary concentrating ability in mammals. Am J Physiol Regul Integr Comp Physiol 261: R719–R726, 1991. [DOI] [PubMed] [Google Scholar]
- 2.Ares GR, Caceres PS, Ortiz PA. Molecular regulation of NKCC2 in the thick ascending limb. Am J Physiol Renal Physiol 301: F1143–F1159, 2011. doi: 10.1152/ajprenal.00396.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baines AD, de Rouffignac C, Deiss S. Functional heterogeneity of nephrons. II. Filtration rates, intraluminal flow velocities and fractional water reabsorption. Pflugers Arch 308: 260–276, 1969. doi: 10.1007/BF00586558. [DOI] [PubMed] [Google Scholar]
- 4.Bankir L, de Rouffignac C. Urinary concentrating ability: insights from comparative anatomy. Am J Physiol Regul Integr Comp Physiol 249: R643–R666, 1985. [DOI] [PubMed] [Google Scholar]
- 5.Barratt LJ, Wallin JD, Rector FC Jr, Seldin DW. Influence of volume expansion on single-nephron filtration rate and plasma flow in the rat. Am J Physiol 224: 643–650, 1973. doi: 10.1152/ajplegacy.1973.224.3.643. [DOI] [PubMed] [Google Scholar]
- 6.Bedford TG, Tipton CM, Wilson NC, Oppliger RA, Gisolfi CV. Maximum oxygen consumption of rats and its changes with various experimental procedures. J Appl Physiol Respir Environ Exerc Physiol 47: 1278–1283, 1979. doi: 10.1152/jappl.1979.47.6.1278. [DOI] [PubMed] [Google Scholar]
- 7.Berliner RW, Levinsky NG, Davidson DG, Eden M. Dilution and concentration of the urine and the action of antidiuretic hormone. Am J Med 24: 730–744, 1958. doi: 10.1016/0002-9343(58)90377-2. [DOI] [PubMed] [Google Scholar]
- 8.Beuchat CA. Body size, medullary thickness, and urine concentrating ability in mammals. Am J Physiol Regul Integr Comp Physiol 258: R298–R308, 1990. doi: 10.1152/ajpregu.1990.258.2.R298. [DOI] [PubMed] [Google Scholar]
- 9.Beuchat CA. Metabolism and the scaling of urine concentrating ability in mammals: resolution of a paradox? J Theor Biol 143: 113–122, 1990. doi: 10.1016/S0022-5193(05)80291-7. [DOI] [PubMed] [Google Scholar]
- 10.Beuchat CA. Structure and concentrating ability of the mammalian kidney: correlations with habitat. Am J Physiol Regul Integr Comp Physiol 271: R157–R179, 1996. doi: 10.1152/ajpregu.1996.271.1.R157. [DOI] [PubMed] [Google Scholar]
- 11.Brezis M, Rosen S. Hypoxia of the renal medulla–its implications for disease. N Engl J Med 332: 647–655, 1995. doi: 10.1056/NEJM199503093321006. [DOI] [PubMed] [Google Scholar]
- 12.Calder WA 3rd, Braun EJ. Scaling of osmotic regulation in mammals and birds. Am J Physiol Regul Integr Comp Physiol 244: R601–R606, 1983. doi: 10.1152/ajpregu.1983.244.5.R601. [DOI] [PubMed] [Google Scholar]
- 13.Dawson WR. The relation of oxygen consumption to temperature in desert rodents. J Mamm 36: 544–553, 1955. doi: 10.2307/1375808. [DOI] [Google Scholar]
- 14.Djouadi F, Bastin J, Gilbert T, Rötig A, Rustin P, Merlet-Benichou C. Mitochondrial biogenesis and development of respiratory chain enzymes in kidney cells: role of glucocorticoids. Am J Physiol Cell Physiol 267: C245–C254, 1994. doi: 10.1152/ajpcell.1994.267.1.C245. [DOI] [PubMed] [Google Scholar]
- 15.Donald J, Pannabecker TL. Osmoregulation in desert-adapted mammals. In: Comparative, Evolutionary and Genetic Models of Sodium and Water Homeostasis, edited by Hyndman KA, Pannabecker TL. New York: Springer, 2015. doi: 10.1007/978-1-4939-3213-9_10. [DOI] [Google Scholar]
- 16.Doucet A, Barlet C, Baddouri K. Effect of water intake on Na-K-ATPase in nephron segments of the desert rodent, Jaculus orientalis. Pflugers Arch 408: 129–132, 1987. doi: 10.1007/BF00581341. [DOI] [PubMed] [Google Scholar]
- 17.Fenton RA, Chou CL, Stewart GS, Smith CP, Knepper MA. Urinary concentrating defect in mice with selective deletion of phloretin-sensitive urea transporters in the renal collecting duct. Proc Natl Acad Sci USA 101: 7469–7474, 2004. doi: 10.1073/pnas.0401704101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fenton RA, Poulsen SB, de la Mora Chavez S, Soleimani M, Busslinger M, Dominguez Rieg JA, Rieg T. Caffeine-induced diuresis and natriuresis is independent of renal tubular NHE3. Am J Physiol Renal Physiol 308: F1409–F1420, 2015. doi: 10.1152/ajprenal.00129.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fenton RA, Poulsen SB, de la Mora Chavez S, Soleimani M, Dominguez Rieg JA, Rieg T. Renal tubular NHE3 is required in the maintenance of water and sodium chloride homeostasis. Kidney Int 92: 397–414, 2017. doi: 10.1016/j.kint.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garg LC, Knepper MA, Burg MB. Mineralocorticoid effects on Na-K-ATPase in individual nephron segments. Am J Physiol Renal Fluid Electrolyte Physiol 240: F536–F544, 1981. doi: 10.1152/ajprenal.1981.240.6.F536. [DOI] [PubMed] [Google Scholar]
- 21.Garg LC, Mackie S, Tisher CC. Effect of low potassium-diet on Na-K-ATPase in rat nephron segments. Pflugers Arch 394: 113–117, 1982. doi: 10.1007/BF00582911. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez-Vicente A, Garvin JL. Angiotensin II-induced hypertension increases plasma membrane Na pump activity by enhancing Na entry in rat thick ascending limbs. Am J Physiol Renal Physiol 305: F1306–F1314, 2013. doi: 10.1152/ajprenal.00064.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hagberg CE, Mehlem A, Falkevall A, Muhl L, Fam BC, Ortsäter H, Scotney P, Nyqvist D, Samén E, Lu L, Stone-Elander S, Proietto J, Andrikopoulos S, Sjöholm A, Nash A, Eriksson U. Targeting VEGF-B as a novel treatment for insulin resistance and type 2 diabetes. Nature 490: 426–430, 2012. doi: 10.1038/nature11464. [DOI] [PubMed] [Google Scholar]
- 24.Hai MA, Thomas S. The time-course of changes in renal tissue composition during lysine vasopressin infusion in the rat. Pflugers Arch 310: 297–317, 1969. doi: 10.1007/BF00587241. [DOI] [PubMed] [Google Scholar]
- 25.Hebert SC. Nephron heterogeneity. Renal physiology. In: Handbook of Physiology, edited by Windhager EE. Bethsda, MD: Am. Physiol. Soc, 1992, p. 875–925. [Google Scholar]
- 26.Hebert SC, Andreoli TE. Ionic conductance pathways in the mouse medullary thick ascending limb of Henle. The paracellular pathway and electrogenic Cl−1 absorption. J Gen Physiol 87: 567–590, 1986. doi: 10.1085/jgp.87.4.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hedrich HJ, Bullock G. The Laboratory Mouse. Amsterdam, The Netherlands: Elsevier, 2004. [Google Scholar]
- 28.Huang Y, Tracy R, Walsberg GE, Makkinje A, Fang P, Brown D, Van Hoek AN. Absence of aquaporin-4 water channels from kidneys of the desert rodent Dipodomys merriami merriami. Am J Physiol Renal Physiol 280: F794–F802, 2001. doi: 10.1152/ajprenal.2001.280.5.F794. [DOI] [PubMed] [Google Scholar]
- 29.Issaian T, Urity VB, Dantzler WH, Pannabecker TL. Architecture of vasa recta in the renal inner medulla of the desert rodent Dipodomys merriami: potential impact on the urine concentrating mechanism. Am J Physiol Regul Integr Comp Physiol 303: R748–R756, 2012. doi: 10.1152/ajpregu.00300.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaissling B, Stanton BA. Adaptation of distal tubule and collecting duct to increased sodium delivery. I. Ultrastructure. Am J Physiol Renal Fluid Electrolyte Physiol 255: F1256–F1268, 1988. doi: 10.1152/ajprenal.1988.255.6.F1256. [DOI] [PubMed] [Google Scholar]
- 31.Katz AI, Doucet A, Morel F. Na-K-ATPase activity along the rabbit, rat, and mouse nephron. Am J Physiol Renal Fluid Electrolyte Physiol 237: F114–F120, 1979. doi: 10.1152/ajprenal.1979.237.2.F114. [DOI] [PubMed] [Google Scholar]
- 32.Kim GH, Ecelbarger CA, Mitchell C, Packer RK, Wade JB, Knepper MA. Vasopressin increases Na-K-2Cl cotransporter expression in thick ascending limb of Henle’s loop. Am J Physiol Renal Physiol 276: F96–F103, 1999. [DOI] [PubMed] [Google Scholar]
- 33.Kim J, Pannabecker TL. Two-compartment model of inner medullary vasculature supports dual modes of vasopressin-regulated inner medullary blood flow. Am J Physiol Renal Physiol 299: F273–F279, 2010. doi: 10.1152/ajprenal.00072.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knepper MA, Danielson RA, Saidel GM, Post RS. Quantitative analysis of renal medullary anatomy in rats and rabbits. Kidney Int 12: 313–323, 1977. doi: 10.1038/ki.1977.118. [DOI] [PubMed] [Google Scholar]
- 35.Knepper MA, Kim GH, Masilamani S. Renal tubule sodium transporter abundance profiling in rat kidney: response to aldosterone and variations in NaCl intake. Ann NY Acad Sci 986: 562–569, 2003. doi: 10.1111/j.1749-6632.2003.tb07254.x. [DOI] [PubMed] [Google Scholar]
- 36.Kokko JP, Rector FC Jr. Countercurrent multiplication system without active transport in inner medulla. Kidney Int 2: 214–223, 1972. doi: 10.1038/ki.1972.97. [DOI] [PubMed] [Google Scholar]
- 37.Kwon TH, Nielsen J, Kim YH, Knepper MA, Frøkiaer J, Nielsen S. Regulation of sodium transporters in the thick ascending limb of rat kidney: response to angiotensin II. Am J Physiol Renal Physiol 285: F152–F165, 2003. doi: 10.1152/ajprenal.00307.2002. [DOI] [PubMed] [Google Scholar]
- 38.Layton AT, Layton HE. Countercurrent multiplication may not explain the axial osmolality gradient in the outer medulla of the rat kidney. Am J Physiol Renal Physiol 301: F1047–F1056, 2011. doi: 10.1152/ajprenal.00620.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Layton AT, Layton HE. A region-based mathematical model of the urine concentrating mechanism in the rat outer medulla. II. Parameter sensitivity and tubular inhomogeneity. Am J Physiol Renal Physiol 289: F1367–F1381, 2005. doi: 10.1152/ajprenal.00347.2003. [DOI] [PubMed] [Google Scholar]
- 40.Layton AT, Layton HE, Dantzler WH, Pannabecker TL. The mammalian urine concentrating mechanism: hypotheses and uncertainties. Physiology (Bethesda) 24: 250–256, 2009. [DOI] [PubMed] [Google Scholar]
- 41.Layton AT, Vallon V, Edwards A. A computational model for simulating solute transport and oxygen consumption along the nephrons. Am J Physiol Renal Physiol 311: F1378–F1390, 2016. doi: 10.1152/ajprenal.00293.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lim SW, Han KH, Jung JY, Kim WY, Yang CW, Sands JM, Knepper MA, Madsen KM, Kim J. Ultrastructural localization of UT-A and UT-B in rat kidneys with different hydration status. Am J Physiol Regul Integr Comp Physiol 290: R479–R492, 2006. doi: 10.1152/ajpregu.00512.2005. [DOI] [PubMed] [Google Scholar]
- 43.Ma T, Yang B, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS. Generation and phenotype of a transgenic knockout mouse lacking the mercurial-insensitive water channel aquaporin-4. J Clin Invest 100: 957–962, 1997. doi: 10.1172/JCI231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Magyar CE, Zhang Y, Holstein-Rathlou NH, McDonough AA. Downstream shift in sodium pump activity along the nephron during acute hypertension. J Am Soc Nephrol 12: 2231–2240, 2001. [DOI] [PubMed] [Google Scholar]
- 45.Mandal A, Delamere NA, Shahidullah M. Ouabain-induced stimulation of sodium-hydrogen exchange in rat optic nerve astrocytes. Am J Physiol Cell Physiol 295: C100–C110, 2008. doi: 10.1152/ajpcell.90636.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marumo R, Kaizuma S, Nogae S, Kanazawa M, Kimura T, Saito T, Ito S, Matsubara M. Differential upregulation of rat Na-K-Cl cotransporter, rBSC1, mRNA in the thick ascending limb of Henle in different pathological conditions. Kidney Int 54: 877–888, 1998. doi: 10.1046/j.1523-1755.1998.00051.x. [DOI] [PubMed] [Google Scholar]
- 47.Masilamani S, Kim GH, Mitchell C, Wade JB, Knepper MA. Aldosterone-mediated regulation of ENaC alpha, beta, and gamma subunit proteins in rat kidney. J Clin Invest 104: R19–R23, 1999. doi: 10.1172/JCI7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McCarter R, Masoro EJ, Yu BP. Does food restriction retard aging by reducing the metabolic rate? Am J Physiol Endocrinol Metab 248: E488–E490, 1985. doi: 10.1152/ajpendo.1985.248.4.E488. [DOI] [PubMed] [Google Scholar]
- 49.McDonough AA, Magyar CE, Komatsu Y. Expression of Na+-K+-ATPase α- and β-subunits along rat nephron: isoform specificity and response to hypokalemia. Am J Physiol Cell Physiol 267: C901–C908, 1994. doi: 10.1152/ajpcell.1994.267.4.C901. [DOI] [PubMed] [Google Scholar]
- 50.Mount DB. Thick ascending limb of the loop of Henle. Clin J Am Soc Nephrol 9: 1974–1986, 2014. doi: 10.2215/CJN.04480413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mount DB. Transport of sodium, chloride, and potassium. In: Brenner and Rector’s The Kidney (9th ed.). Philadelphia, PA: Elsevier, 2012, p. 158–201. doi: 10.1016/B978-1-4160-6193-9.10005-3. [DOI] [Google Scholar]
- 52.Nawata CM, Hung CC, Tsui TK, Wilson JM, Wright PA, Wood CM. Ammonia excretion in rainbow trout (Oncorhynchus mykiss): evidence for Rh glycoprotein and H+-ATPase involvement. Physiol Genomics 31: 463–474, 2007. doi: 10.1152/physiolgenomics.00061.2007. [DOI] [PubMed] [Google Scholar]
- 53.Nguyen MT, Lee DH, Delpire E, McDonough AA. Differential regulation of Na+ transporters along nephron during ANG II-dependent hypertension: distal stimulation counteracted by proximal inhibition. Am J Physiol Renal Physiol 305: F510–F519, 2013. doi: 10.1152/ajprenal.00183.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pannabecker TL. Aquaporins in desert rodent physiology. Biol Bull 229: 120–128, 2015. doi: 10.1086/BBLv229n1p120. [DOI] [PubMed] [Google Scholar]
- 55.Pannabecker TL. Comparative physiology and architecture associated with the mammalian urine concentrating mechanism: role of inner medullary water and urea transport pathways in the rodent medulla. Am J Physiol Regul Integr Comp Physiol 304: R488–R503, 2013. doi: 10.1152/ajpregu.00456.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pannabecker TL, Dantzler WH, Layton HE, Layton AT. Role of three-dimensional architecture in the urine concentrating mechanism of the rat renal inner medulla. Am J Physiol Renal Physiol 295: F1271–F1285, 2008. doi: 10.1152/ajprenal.90252.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Prange HD, Anderson JF, Rahn H. Scaling of skeletal mass to body-mass in birds and mammals. Am Nat 113: 103–122, 1979. doi: 10.1086/283367. [DOI] [Google Scholar]
- 58.Rieg T. A high-throughput method for measurement of glomerular filtration rate in conscious mice. J Vis Exp 75: e50330, 2013. doi: 10.3791/50330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rytand DA. The number and size of mammalian glomeruli as related to kidney and to body weight, with methods for their enumeration and measurement. Am J Anat 62: 507–520, 1938. doi: 10.1002/aja.1000620406. [DOI] [Google Scholar]
- 60.Sands JM, Layton HE. Advances in understanding the urine-concentrating mechanism. Annu Rev Physiol 76: 387–409, 2014. doi: 10.1146/annurev-physiol-021113-170350. [DOI] [PubMed] [Google Scholar]
- 61.Schmidt-Nielsen B, Graves B, Roth J. Water removal and solute additions determining increases in renal medullary osmolality. Am J Physiol Regul Integr Comp Physiol 244: F472–F482, 1983. doi: 10.1152/ajprenal.1983.244.5.F472. [DOI] [PubMed] [Google Scholar]
- 62.Schmidt-Nielsen K. Desert Animals. London: Oxford, 1964. [Google Scholar]
- 63.Schmidt-Nielsen K, Schmidt-Nielsen B. Water metabolism of desert mammals 1. Physiol Rev 32: 135–166, 1952. doi: 10.1152/physrev.1952.32.2.135. [DOI] [PubMed] [Google Scholar]
- 64.Shahidullah M, Wei G, Delamere NA. DIDS inhibits Na-K-ATPase activity in porcine nonpigmented ciliary epithelial cells by a Src family kinase-dependent mechanism. Am J Physiol Cell Physiol 305: C492–C501, 2013. doi: 10.1152/ajpcell.00057.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Smith CP, Lee WS, Martial S, Knepper MA, You G, Sands JM, Hediger MA. Cloning and regulation of expression of the rat kidney urea transporter (rUT2). J Clin Invest 96: 1556–1563, 1995. doi: 10.1172/JCI118194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stephenson JL. Concentration of urine in a central core model of the renal counterflow system. Kidney Int 2: 85–94, 1972. doi: 10.1038/ki.1972.75. [DOI] [PubMed] [Google Scholar]
- 67.Takei Y, Bartolo RC, Fujihara H, Ueta Y, Donald JA. Water deprivation induces appetite and alters metabolic strategy in Notomys alexis: unique mechanisms for water production in the desert. Proc Biol Sci 279: 2599–2608, 2012. doi: 10.1098/rspb.2011.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thomson SC, Blantz RC. Biophysics of glomerular filtration. Compr Physiol 2: 1671–1699, 2012. doi: 10.1002/cphy.c100089. [DOI] [PubMed] [Google Scholar]
- 69.Thomson SC, Kashkouli A, Singh P. Glucagon-like peptide-1 receptor stimulation increases GFR and suppresses proximal reabsorption in the rat. Am J Physiol Renal Physiol 304: F137–F144, 2013. doi: 10.1152/ajprenal.00064.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Urity VB, Issaian T, Braun EJ, Dantzler WH, Pannabecker TL. Architecture of kangaroo rat inner medulla: segmentation of descending thin limb of Henle’s loop. Am J Physiol Regul Integr Comp Physiol 302: R720–R726, 2012. doi: 10.1152/ajpregu.00549.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vallon V, Platt KA, Cunard R, Schroth J, Whaley J, Thomson SC, Koepsell H, Rieg T. SGLT2 mediates glucose reabsorption in the early proximal tubule. J Am Soc Nephrol 22: 104–112, 2011. doi: 10.1681/ASN.2010030246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vallon V, Richter K, Huang DY, Rieg T, Schnermann J. Functional consequences at the single-nephron level of the lack of adenosine A1 receptors and tubuloglomerular feedback in mice. Pflugers Arch 448: 214–221, 2004. doi: 10.1007/s00424-004-1239-8. [DOI] [PubMed] [Google Scholar]
- 73.Wu H, Guang X, Al-Fageeh MB, Cao J, Pan S, Zhou H, Zhang L, Abutarboush MH, Xing Y, Xie Z, Alshanqeeti AS, Zhang Y, Yao Q, Al-Shomrani BM, Zhang D, Li J, Manee MM, Yang Z, Yang L, Liu Y, Zhang J, Altammami MA, Wang S, Yu L, Zhang W, Liu S, Ba L, Liu C, Yang X, Meng F, Wang S, Li L, Li E, Li X, Wu K, Zhang S, Wang J, Yin Y, Yang H, Al-Swailem AM, Wang J. Camelid genomes reveal evolution and adaptation to desert environments. Nat Commun 5: 5188, 2014. doi: 10.1038/ncomms6188. [DOI] [PubMed] [Google Scholar]
- 74.Zhai XY, Fenton RA, Andreasen A, Thomsen JS, Christensen EI. Aquaporin-1 is not expressed in descending thin limbs of short-loop nephrons. J Am Soc Nephrol 18: 2937–2944, 2007. doi: 10.1681/ASN.2007010056. [DOI] [PubMed] [Google Scholar]