Abstract
Although central melanocortin 4 receptor (MC4R) blockade abolishes the central nervous system (CNS)-mediated anorexogenic, antidiabetic, and cardiovascular actions of leptin, chronic MC4R stimulation fails to completely mimic the effects of leptin. Because neuropeptide Y (NPY) and MC4R exert opposite effects on cardiovascular and metabolic functions, we tested the role of NPY in offsetting the long-term actions of MC4R activation. Wild-type (WT) and NPY-deficient (NPY−/−) mice were implanted with telemetry probes for measuring mean arterial pressure (MAP) and heart rate (HR) 24 h/day. After the mice recovered from surgery and stable baseline measurements, the MC3/4R agonist melanotan II (MTII, 120 μg·kg−1·day−1 iv) was infused for 7 days followed by a recovery period. No major differences between groups were observed at baseline except for slightly higher food intake and HR in NPY−/− mice (4.3 ± 0.2 vs. 3.4 ± 0.2 g/day and 567 ± 14 vs. 522 ± 13 beats/min). Chronic MTII infusion reduced food intake in both groups while causing transient increases in MAP and HR only in WT mice (peaks of 11 ± 3 mmHg and 126 ± 13 beats/min). To examine whether NPY deficiency would amplify the antidiabetic effects of MC4R activation, diabetes was induced with streptozotocin (STZ) 1 wk before baseline measurements were taken, and the same experimental protocol was followed. In WT and NPY−/− mice, STZ-induced diabetes led to similar hyperphagia, hyperglycemia, and weight loss, which were not reversed by chronic MTII treatment. Our results demonstrate that chronic MC4R activation, even in NPY-deficient mice, does not mimic chronic antidiabetic, cardiovascular, or metabolic actions of leptin, and that NPY is not essential for hyperphagia or cardiovascular changes associated with diabetes.
Keywords: central nervous system, food intake, glucose regulation, hypertension, melanocortins, obesity
INTRODUCTION
In the past few decades, we have witnessed a marked increase in the prevalence of obesity and its associated metabolic and cardiovascular consequences, including diabetes, high blood pressure, stroke, and other cardiovascular events (3, 5, 15, 18). Although the precise mechanisms responsible for disrupted body weight regulation leading to overfeeding and metabolic and cardiovascular abnormalities are not completely understood, ineffective leptin action appears to play an important role.
Leptin, an adipocyte-derived peptide that circulates in proportion to the amount of body fat, is a major regulator of body weight homeostasis by suppressing appetite and by increasing sympathetic nerve activity (SNA) to various tissues to promote thermogenesis (19). Leptin-induced activation of proopiomelanocortin (POMC) neurons has been shown to stimulate the release of α-melanocyte stimulating hormone (α-MSH), the endogenous agonist of melanocortin receptor type 4 (MC4R), in various hypothalamic and extrahypothalamic regions (6, 14, 31). Leptin, however, not only stimulates POMC neurons to produce α-MSH but also inhibits a group of neurons situated in close proximity to POMC neurons in the arcuate nucleus (ARC) that oppose the actions of POMC activation. These neurons express neuropeptide Y (NPY) and agouti-related peptide (AgRP), which work to counteract the effects of the melanocortin system by either blocking MC4R activity or opposing the action of MC4R on target neurons (21, 22, 24). Acute studies have demonstrated, for example, that NPY suppresses activation of MC4R via inhibition of POMC neurons (24). Also, NPY levels in the central nervous system (CNS) of diabetic mice are markedly elevated and have been postulated to mediate the hyperphagia observed in these mice since diabetic NPY−/− mice have been reported to exhibit attenuated hyperphagia compared with WT diabetic mice (30). Chronic intracerebroventricular NPY infusion has also been shown to reduce heart rate (HR), whereas MC4R activation increases HR (7, 16, 20, 31).
We and others have shown that blockade of the CNS melanocortin system greatly attenuates chronic metabolic and cardiovascular actions of leptin. For example, deletion of leptin receptors in proopiomelanocortin (POMC) neurons markedly attenuated the ability of leptin to increase blood pressure (BP) and to improve glucose regulation, as evidenced by a reduction in fasting insulin and blood glucose levels (10). Despite strong evidence showing that MC4R activation is an important downstream event for leptin to exert its effects, chronic MC4R activation using pharmacological agonists did not mimic the antidiabetic or anorexic effects of leptin. For instance, we found only transient and less profound reductions in food intake and body weight in rats infused with the MC4R agonist MTII compared with the effects of chronic leptin infusion (23). We also showed that chronic MC4R stimulation failed to normalize glucose levels in diabetic rats, whereas blockade of MC4R completely abolished the antidiabetic effects of leptin (8).
These observations suggest that the chronic cardiovascular and metabolic actions of leptin may require MC4R activity but that the responses to chronic MC4R activation may be offset by compensatory mechanisms that are inhibited by leptin. Activation of MC4R and NPY exerts reciprocal actions, and compensatory stimulation of NPY neurons may be responsible for the blunted effects of chronic MC4R activation. In this study, we examined whether the BP, HR, food intake, and antidiabetic responses to chronic MC4R activation are enhanced in normoglycemic and diabetic NPY−/− mice compared with WT normoglycemic and diabetic mice.
METHODS
All experimental protocols and procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Mississippi Medical Center, Jackson, MS. Mice were placed in a 12-h dark and light cycle and given free access to food (control diet no. CA 170955 or sodium-deficient diet no. CA 170950; Harlan-Invigo) and water throughout the study.
Animals.
Male neuropeptide Y-deficient (NPY−/−; n = 15) and wild-type WT (n = 10) mice were purchased from The Jackson Laboratory and housed in the University of Mississippi Medical Center Laboratory Animal Facility. The characterization of NPY−/− mice showing lack of NPY expression in the arcuate nucleus and thalamic reticular nucleus and their baseline phenotype have been described previously (25, 29).
Surgical procedures.
Under isoflurane anesthesia, male 20- to 24-wk-old NPY−/− and WT mice were implanted with telemetric pressure transmitter probes in the left carotid artery for determination of mean arterial pressure (MAP) and heart rate (HR) 24 h/day using computerized methods for data collection, as previously described (12). Daily MAP and HR were obtained from the average of 24 h of recording using a sampling rate of 1,000 Hz with duration of 10 s for every 10-min period. A venous catheter was also implanted in the jugular vein for infusions of saline vehicle or melanocortin 3/4 (MC3/4R) agonist (MTII), which readily penetrates the blood-brain barrier (4, 13). The venous catheter was tunneled subcutaneously, exteriorized between the scapulae, and passed through a spring connected to a mouse swivel (Instech) mounted on the top of the plastic metabolic cage. The venous catheter was connected through a sterile filter to a syringe pump for continuous saline and MTII infusions. Food and water were offered ad libitum throughout the experiment, and room temperature was maintained at 23 to 24°C. A normal sodium intake of ∼460 μmol/day was maintained via the constant saline infusion combined with sodium-deficient rodent chow.
Chronic infusion of a MC3/4R agonist, MTII.
After an 8- to 10-day postsurgery recovery period and 5 days of stable baseline control measurements, MTII was added to the continuous saline vehicle infusion (3.0 ml/day) at the rate of 200 µg·kg−1·day−1 in separate groups of instrumented WT (n = 5) and NPY−/− mice (n = 7), which was followed by a 5-day posttreatment period, during which only saline vehicle was infused intravenously. MAP, HR, urine volume, and food and water intake were recorded daily. Blood samples (100 µl) were collected via a tail snip after 6 h of fasting (8 AM to 2 PM) during the control period (day 5), on the last day of MTII infusion (day 7 of treatment), and at the end of the posttreatment period (day 5 after stopping MTII infusion) for measurements of blood hormone concentrations.
Measurement of blood pressure and heart rate.
MAP and HR were measured by telemetry 24 h/day for 4 consecutive days during baseline and 7 days of chronic MTII infusion using computerized methods for data collection, as previously described (12). Daily MAP and HR were obtained from the average of 12:12 light-dark recording using a sampling rate of 500 Hz with a duration of 10 s every 10-min period.
Plasma hormones and glucose measurements.
Plasma leptin and insulin concentrations were measured by an ELISA kit (R & D Systems and Crystal Chem), and plasma glucose concentrations were determined using the Beckman glucose analyzer 2 (28).
Induction of diabetes.
After 4–5 days of stable control measurements, insulin-deficient diabetes was induced by a single intraperitoneal injection of streptozotocin (STZ; 150 mg/kg) and dissolved in 0.2 ml of 0.05 M citrate buffer, pH 4.5. Successful induction of diabetes was confirmed by achieving blood glucose levels >300 mg/dl measured 4–5 days after injection.
Statistical analyses.
The results are expressed as means ± SE. Significant differences between two groups were determined by Student’s t-test, one-way ANOVA with repeated measures, and two-way ANOVA, followed by the Dunnett’s post hoc test when appropriate. A P value of <0.05 indicates a significant difference.
RESULTS
Chronic effects of MTII on food and water intake, body weight, and urine volume in NPY−/− mice.
When compared with WT controls, NPY−/− mice at 20 wk of age showed increased food intake and slight but not significant increases in body weight (Table 1 and Fig. 1, A and C). NPY−/− mice had lower plasma glucose levels (177 ± 8 vs. 220 ± 19 mg/dl) and similar water intake and urine volume compared with WT mice (Table 1).
Table 1.
Body weight, food intake, urine volume, and water intake in normoglycemic WT and NPY−/− mice
| Body Weight, g | Food Intake, g/day | Urine Volume, ml/day | Water Intake, ml/day | |
|---|---|---|---|---|
| WT mice (n = 5) | ||||
| Control | 26.3 ± 0.4 | 3.4 ± 0.2 | 2.2 ± 0.3 | 3.9 ± 0.3 |
| MITII infusion | 25.4 ± 0.7 | 2.8 ± 0.1 | 3.2 ± 0.6 | 4.6 ± 0.8 |
| Recovery | 27.1 ± 0.5 | 3.3 ± 0.4 | 3.7 ± 0.6 | 6.6 ± 1.0 |
| NPY−/− (n = 7) | ||||
| Control | 27.3 ± 1.9 | 4.3 ± 0.2# | 2.7 ± 0.5 | 4.3 ± 0.5 |
| MTII infusion | 27.1 ± 1.4 | 3.5 ± 0.3 | 1.4 ± 0.4 | 3.4 ± 0.3 |
| Recovery | 29.8 ± 1.3 | 3.6 ± 0.3 | 1.1 ± 0.4* | 2.8 ± 0.3* |
Values are means ± SE. WT, wild type; NPY, neuropeptide Y; MTII, melanotan II. Paired and unpaired Student’s t-test.
P < 0.05 compared with control period;
P < 0.05 compared with WT mice.
Fig. 1.
Food intake (A), net cumulative food intake (B), body weight (C), plasma leptin (D), and insulin concentrations (E) in wild-type (WT; n = 5) and neuropeptide Y (NPY)−/− (n = 7) mice in response to intravenous infusion of the melanocortin 3/4 receptor (MC3/4R) agonist melanotan II (MTII) for 7 days in WT and NPY−/− mice. *P < 0.05 compared with control period, 2-way ANOVA with Tukey’s post hoc multiple comparison test; #P < 0.05 NPY−/− mice compared with WT mice, 1-way ANOVA with repeated measures.
Chronic MC4R activation with MTII infusion caused only a transient reduction in food intake in WT and NPY−/− mice (Fig. 1A). The similar effect of MC4R agonist on food intake in WT and NPY−/− mice is more evident when analyzing the net cumulative decrease in food intake during the 7 days of MC4R agonist infusion (Fig. 1B).
Chronic effects of MTII on plasma leptin and insulin levels in WT and NPY−/− mice.
NYP−/− mice had slightly higher plasma leptin levels (Fig. 1D) but lower plasma insulin levels (Fig. 1E) compared with WT controls. Chronic MTII infusion caused a significant reduction in plasma leptin levels in both groups but significantly increasing plasma insulin levels in WT mice.
MAP and HR responses to chronic MC4R agonist.
When compared with WT mice, the NPY−/− mice had higher MAP and HR (Fig. 2, A and C), and chronic MC4R activation caused only a transient increase in MAP (Fig. 2, A and B) as well as in HR (Fig. 2, C and D) in WT but not in NPY−/− mice.
Fig. 2.
Mean arterial pressure (MAP; A), change (Δ) in MAP (B), heart rate (HR; C), and change (Δ) in HR responses to intravenous infusion of the MC3/4R agonist MTII for 7 days in WT (n = 5) and NPY−/− (n = 7) mice. *P < 0.05 compared with control period, 2-way ANOVA with post hoc Tukey’s multiple comparison; #P < 0.05 NPY−/− mice compared with WT mice, 1-way ANOVA with repeated measures.
Chronic effects of MTII on food and water intake, body weight, and urine volume in diabetic NPY−/− mice.
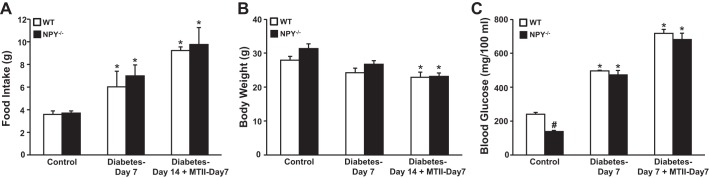
Induction of type 1 diabetes with STZ was associated with significant increases in food intake and plasma glucose (Fig. 3, A and C) and a slight decrease in body weight (Fig. 3B). Chronic MTII infusion did not reverse the hyperphagia associated with STZ-induced diabetes in WT or NPY−/− mice (Fig. 3A). MC4R agonism with MTII caused significant weight loss in both groups (Fig. 3B) but failed to reduce glucose levels in WT and NPY−/− mice (Fig. 3C). We also observed significant increases in water intake and urine volume in both groups (Table 2). Thus, NPY−/− mice exhibited reduced plasma glucose levels and similar increases in food intake, blood glucose, and weight loss compared with WT mice.
Fig. 3.
Food intake (A), body weight (B), and blood glucose (C) responses to diabetes and diabetes plus intravenous infusion of the MC3/4R agonist MTII for 7 days in WT (n = 5) and NPY−/− (n = 8) mice. Blood samples for glucose were obtained on day 5 of the control period, day 7 of diabetes, and on day 14 of diabetes plus day 7 of MC3/4R agonist infusion. *P < 0.05 compared with control period; #P < 0.05 NPY−/− mice compared with WT mice, 1-way ANOVA with repeated measures.
Table 2.
Urine volume and water intake in diabetic WT and NPY−/− mice
| Urine Volume, ml/day | Water Intake, ml/day | |
|---|---|---|
| WT mice (n = 5) | ||
| Control | 3.0 ± 0.4 | 5.7 ± 0.7 |
| STZ (day 7) | 26.1 ± 7.4* | 27.2 ± 7.7* |
| MTII (day 7) | 33.6 ± 6.4* | 35.2 ± 6.2* |
| NPY−/− (n = 8) | ||
| Control | 1.4 ± 0.3# | 3.1 ± 0.3# |
| STZ (day 7) | 31.3 ± 6.6* | 34.0 ± 6.5* |
| MTII (day 7) | 41.7 ± 8.3* | 42.7 ± 8.0* |
Values are means ± SE. STZ, streptozotocin; MTII, melanotan II. Paired and unpaired Student’s t-test.
P < 0.05 compared with control period;
P < 0.05 compared with WT mice.
Chronic effects of MTII on BP and HR in diabetic NPY−/− mice.
Chronic MC4R activation or STZ-induced diabetes did not significantly alter MAP in WT or in NPY−/− mice (Fig. 4A). We also did not observe major effects of STZ-induced diabetes or MC4R activation on HR in WT or in NPY−/− mice (Fig. 4B).
Fig. 4.

Mean arterial pressure (MAP; A) and heart rate (HR; B) responses to diabetes (day 7) and diabetes (day 14) plus intravenous infusion of the MC3/4R agonist MTII (day 7) in WT and NPY−/− mice. #P < 0.05 NPY−/− mice compared with WT mice, 1-way ANOVA with repeated measures.
Chronic effects of intracerebroventricular MTII infusion on food intake, body weight, blood glucose, BP, and HR in WT mice.
To test whether the transient responses to chronic MTII infusion observed in WT and NPY−/− mice were due to the fact that we administered MTII peripherally and not directly into the (CNS, we performed experiments on an additional group of WT (n = 5) instrumented with telemetry transmitters and fed a control diet. These mice were implanted with a stainless-steel cannula (33-gauge, 5 mm long) into the brain left lateral ventricle, as previously described (11, 27). After 8 days to recover from surgery and a 5-day control period, mice received an intracerebroventricular infusion of MTII (10 ng/h) using osmotic minipump (1007D, 0.5 μl/h; Durect, Cupertino, CA), and food intake, body weight, blood glucose, BP, and HR were measured. On day 7 of treatment the tubing connecting the osmotic pump with the intracerebroventricular cannula was severed, and the mice were followed for an additional 5-day posttreatment period.
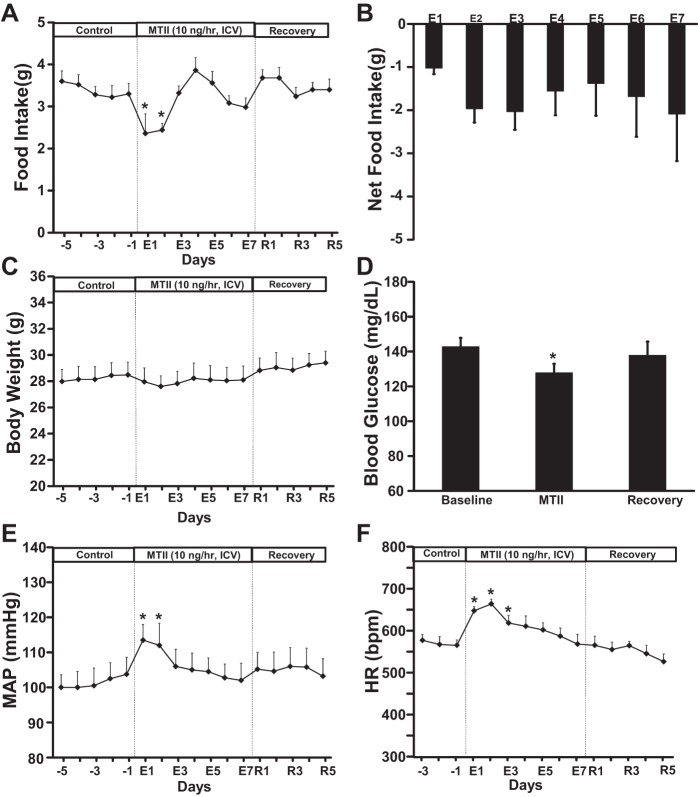
Chronic intracerebroventricular MTII administration evoked similar transient responses of food intake (Fig. 5, A and B), body weight (Fig. 5C), BP, and HR (Fig. 5, E and F) compared with peripheral administration of MTII. Intracerebroventricular MTII infusion also reduced blood glucose levels (Fig. 5D) in a similar fashion as when MTII was administered peripherally. These observations suggest that the route of chronic MTII administration does not markedly influence its metabolic and cardiovascular effects, consistent with previous studies indicating that MTII crosses the blood-brain barrier (17).
Fig. 5.
Food intake (A), net cumulative food intake (B), body weight (C), blood glucose concentration (D), MAP (E), and HR (F) responses to intracerebroventricular infusion of the MC3/4R agonist MTII for 7 days. in WT (n = 5). *P < 0.05 compared with control period, 1-way ANOVA with repeated measures.
DISCUSSION
In the present study, we showed that chronic MC4R activation reduced appetite and elicited transient increases in BP and HR in WT mice. NPY deficiency did not amplify these responses. In fact, the BP and HR responses to chronic MC4R activation were less pronounced in NPY-deficient mice. In addition, MTII infusion also failed to lower blood glucose levels in STZ-diabetic WT or NPY−/− mice.
We and others have previously demonstrated that long-term MC4R inhibition has powerful effects on appetite, body weight regulation, glucose homeostasis, and cardiovascular function. For example, chronic pharmacological antagonism of MC4R with SHU-9119 caused marked and sustained increases in food intake and body weight that were associated with hyperinsulinemia, hyperleptinemia, and insulin resistance, whereas HR and blood pressure were significantly reduced (23). These results can also be recapitulated when MC4Rs are deleted using genetic approaches, indicating that lack of normal MC4R function, and not off-target effects of pharmacological antagonists, is the cause of these cardiovascular and metabolic effects of MC4R blockade (9, 32). These previous studies show the importance of the brain melanocortin system, and especially of MC4R, in modulating energy balance and metabolic and cardiovascular functions and highlight the melanocortin system as a potential target for treatment of obesity and metabolic disorders such as diabetes.
Despite the strong impact of MC4R deficiency or pharmacological inhibition on several cardiovascular and metabolic parameters, the responses to chronic MC4R activation have been disappointing, with most studies showing only mild changes that are usually transient (8, 23). In this study we found, for instance, that chronic MTI infusion caused only acute reductions in food intake and no significant weight loss. We also observed acute increases in BP and HR in WT mice that rapidly returned toward baseline values. Taken together, these observations suggest that the long-term effects of MC4R activation may be at least partially offset by compensatory mechanisms.
One potential mechanism that may participate in the waning effect of chronic MC4R activation is the NPY system. NPY neurons are located in close proximity with POMC neurons (22); they induce orexigenic behavior (21, 24) and reduce HR (16) when activated. In addition, activation of Y1 receptors suppresses POMC neuronal activity (1, 24), and NPY opposes the effects of α-MSH when released by axons directed at neurons in other hypothalamic regions that also receive innervation by POMC axons (2). Thus, we hypothesized that preventing compensatory release of NPY would amplify the long-term actions of MC4R activation. However, we found no evidence that the effects of MTII on appetite, body weight, glucose regulation, or cardiovascular function were amplified in NPY−/− mice.
NPY−/− mice were slightly bigger and ate slightly more than control mice, in agreement with previous studies showing that lack of NPY does not have major effects on food intake, except when challenged by prolonged fasting (30). Despite having higher BP and HR, NPY−/− mice did not exhibit the usual increase in BP and HR in response to MC4R activation that was observed in WT controls. One explanation for this lack of transient elevations in BP and HR with chronic MC4R activation may be that they are less prone to stress-induced sympathetic stimulation. We showed that most of the pressor effects of MC4R activation are dependent on sympathetic nervous system (SNS) activation (23), and NPY deficiency may be associated with blunted activity of brain areas responsible for stress-induced modulation of SNS activity. However, additional studies are needed to test this possibility.
We also tested whether lack of compensatory increase in NPY would amplify the effects of MC4R activation to reduce blood glucose levels. We previously showed that leptin has remarkable CNS-mediated antidiabetic effects that are completely abolished by MC4R inhibition (8), whereas chronic MC4R activation causes only modest and transient reductions in glucose levels in STZ-diabetic animals (8). Thus, we induced STZ-diabetes in a subgroup of WT and NPY−/− mice to examine whether chronic MC4R activation would exert long-lasting glucose lowering effects in NPY−/− mice. However, we found that both groups behaved almost exactly the same and that chronic MC4R activation failed to improve glucose levels in diabetic mice. These results suggest that NPY neurons may not be as important in modulating the responses to POMC neurons-MC4R axis stimulation, as previously thought, and that other mechanisms may come into play to offset the long-term effects of MC4R activation. However, these mechanisms remain elusive, and additional studies are needed to unravel them.
One limitation of this study is potential activation of compensatory factors related to the lifelong loss of NPY in the knockout mice. The NPY−/− mice did not exhibit a leaner body weight or anorectic phenotype that might be predicted based on the potent effect of NPY to stimulate feeding and promote adiposity. Although the identity of these potential compensatory factors remains elusive, agouti-related protein (AgRP), which is an endogenous antagonist of MC3/4R, is increased in the hypothalamus of NPY−/− mice (25), but its importance in offsetting the effects of lifelong loss of NPY or chronic MC4R activation is still unclear. Other orexigenic factors, such as orexins, may also be activated by loss of NPY or chronic MTII infusion. However, the role of these orexigenic factors in counteracting the effects of chronic MC4R activation remains to be tested. Our results indicate that activation NPY is not required for restoration of normal food intake or energy balance during chronic activation of MC4R.
In the present study, we also limited our observations to male mice at 20–24 wk old. Thus, it is possible that female mice or mice of different ages may respond differently to MTII infusion. These possibilities, however, are still uncertain and remain to be tested. To examine whether the mild transient responses to chronic MC4R activation observed in WT and NPY were due to peripheral administration of MTII, which could result in reduced delivery of the agonist into the CNS, we also administered MTII centrally and found almost identical responses compared with peripheral MTII administration. These results are consistent with the findings of Pierroz et al. (26), who observed similar effects of MTII given peripherally when compared with central infusion.
In summary, NPY deficiency does not cause major alterations in appetite and body weight but is associated with slightly higher BP and HR. As previously demonstrated, chronic MC4R activation evoked only modest and transient reductions in food intake and elevations in BP and HR that were not exacerbated by NPY deficiency. MTII treatment also failed to improve glucose regulation in STZ-diabetes, which was not affected by NPY deficiency. These results demonstrate that the chronic cardiovascular and metabolic effects of MC4R activation are not substantially modified by NPY deficiency and suggest involvement of other CNS pathways in offsetting the long-term actions of MC4R activation.
GRANTS
The authors were supported by grants from the National Heart, Lung, and Blood Institute (PO1-HL-51971) and the National Institute of General Medical Sciences (P20-GM-104357 and U54-GM-115428).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.A.d.S. and J.M.d.C. conceived and designed research; A.A.d.S., J.N.F., and J.M.d.C. performed experiments; A.A.d.S., J.N.F., J.E.H., and J.M.d.C. analyzed data; A.A.d.S., J.E.H., and J.M.d.C. interpreted results of experiments; A.A.d.S. and J.M.d.C. prepared figures; A.A.d.S. and J.M.d.C. drafted manuscript; A.A.d.S., J.N.F., J.E.H., and J.M.d.C. approved final version of manuscript; J.E.H. and J.M.d.C. edited and revised manuscript.
REFERENCES
- 1.Acuna-Goycolea C, Tamamaki N, Yanagawa Y, Obata K, van den Pol AN. Mechanisms of neuropeptide Y, peptide YY, and pancreatic polypeptide inhibition of identified green fluorescent protein-expressing GABA neurons in the hypothalamic neuroendocrine arcuate nucleus. J Neurosci 25: 7406–7419, 2005. doi: 10.1523/JNEUROSCI.1008-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atasoy D, Betley JN, Su HH, Sternson SM. Deconstruction of a neural circuit for hunger. Nature 488: 172–177, 2012. doi: 10.1038/nature11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhupathiraju SN, Hu FB. Epidemiology of obesity and diabetes and their cardiovascular complications. Circ Res 118: 1723–1735, 2016. doi: 10.1161/CIRCRESAHA.115.306825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cai M, Mayorov AV, Ying J, Stankova M, Trivedi D, Cabello C, Hruby VJ. Design of novel melanotropin agonists and antagonists with high potency and selectivity for human melanocortin receptors. Peptides 26: 1481–1485, 2005. doi: 10.1016/j.peptides.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 5.Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A, Marczak L, Mokdad AH, Moradi-Lakeh M, Naghavi M, Salama JS, Vos T, Abate KH, Abbafati C, Ahmed MB, Al-Aly Z, Alkerwi A, Al-Raddadi R, Amare AT, Amberbir A, Amegah AK, Amini E, Amrock SM, Anjana RM, Ärnlöv J, Asayesh H, Banerjee A, Barac A, Baye E, Bennett DA, Beyene AS, Biadgilign S, Biryukov S, Bjertness E, Boneya DJ, Campos-Nonato I, Carrero JJ, Cecilio P, Cercy K, Ciobanu LG, Cornaby L, Damtew SA, Dandona L, Dandona R, Dharmaratne SD, Duncan BB, Eshrati B, Esteghamati A, Feigin VL, Fernandes JC, Fürst T, Gebrehiwot TT, Gold A, Gona PN, Goto A, Habtewold TD, Hadush KT, Hafezi-Nejad N, Hay SI, Horino M, Islami F, Kamal R, Kasaeian A, Katikireddi SV, Kengne AP, Kesavachandran CN, Khader YS, Khang YH, Khubchandani J, Kim D, Kim YJ, Kinfu Y, Kosen S, Ku T, Defo BK, Kumar GA, Larson HJ, Leinsalu M, Liang X, Lim SS, Liu P, Lopez AD, Lozano R, Majeed A, Malekzadeh R, Malta DC, Mazidi M, McAlinden C, McGarvey ST, Mengistu DT, Mensah GA, Mensink GBM, Mezgebe HB, Mirrakhimov EM, Mueller UO, Noubiap JJ, Obermeyer CM, Ogbo FA, Owolabi MO, Patton GC, Pourmalek F, Qorbani M, Rafay A, Rai RK, Ranabhat CL, Reinig N, Safiri S, Salomon JA, Sanabria JR, Santos IS, Sartorius B, Sawhney M, Schmidhuber J, Schutte AE, Schmidt MI, Sepanlou SG, Shamsizadeh M, Sheikhbahaei S, Shin MJ, Shiri R, Shiue I, Roba HS, Silva DAS, Silverberg JI, Singh JA, Stranges S, Swaminathan S, Tabarés-Seisdedos R, Tadese F, Tedla BA, Tegegne BS, Terkawi AS, Thakur JS, Tonelli M, Topor-Madry R, Tyrovolas S, Ukwaja KN, Uthman OA, Vaezghasemi M, Vasankari T, Vlassov VV, Vollset SE, Weiderpass E, Werdecker A, Wesana J, Westerman R, Yano Y, Yonemoto N, Yonga G, Zaidi Z, Zenebe ZM, Zipkin B, Murray CJL. Health effects of overweight and obesity in 195 countries over 25 years: The GBD 2015 Obesity Collaborators. N Engl J Med 377: 13–27, 2017. doi: 10.1056/NEJMoa1614362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cone RD, Cowley MA, Butler AA, Fan W, Marks DL, Low MJ. The arcuate nucleus as a conduit for diverse signals relevant to energy homeostasis. Int J Obes Relat Metab Disord 25, Suppl 5: S63–S67, 2001. doi: 10.1038/sj.ijo.0801913. [DOI] [PubMed] [Google Scholar]
- 7.Correia ML, Morgan DA, Sivitz WI, Mark AL, Haynes WG. Hemodynamic consequences of neuropeptide Y-induced obesity. Am J Hypertens 15: 137–142, 2002. doi: 10.1016/S0895-7061(01)02270-1. [DOI] [PubMed] [Google Scholar]
- 8.da Silva AA, do Carmo JM, Freeman JN, Tallam LS, Hall JE. A functional melanocortin system may be required for chronic CNS-mediated antidiabetic and cardiovascular actions of leptin. Diabetes 58: 1749–1756, 2009. doi: 10.2337/db08-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.da Silva AA, Spradley FT, Granger JP, Hall JE, do Carmo JM. Brain-mediated antidiabetic, anorexic, and cardiovascular actions of leptin require melanocortin-4 receptor signaling. J Neurophysiol 113: 2786–2791, 2015. doi: 10.1152/jn.00911.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.do Carmo JM, da Silva AA, Cai Z, Lin S, Dubinion JH, Hall JE. Control of blood pressure, appetite, and glucose by leptin in mice lacking leptin receptors in proopiomelanocortin neurons. Hypertension 57: 918–926, 2011. doi: 10.1161/HYPERTENSIONAHA.110.161349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.do Carmo JM, da Silva AA, Rushing JS, Pace B, Hall JE. Differential control of metabolic and cardiovascular functions by melanocortin-4 receptors in proopiomelanocortin neurons. Am J Physiol Regul Integr Comp Physiol 305: R359–R368, 2013. doi: 10.1152/ajpregu.00518.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.do Carmo JM, da Silva AA, Wang Z, Freeman NJ, Alsheik AJ, Adi A, Hall JE. Regulation of blood pressure, appetite, and glucose by leptin after inactivation of insulin receptor substrate 2 signaling in the entire brain or in proopiomelanocortin neurons. Hypertension 67: 378–386, 2016. doi: 10.1161/HYPERTENSIONAHA.115.06153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dorr RT, Lines R, Levine N, Brooks C, Xiang L, Hruby VJ, Hadley ME. Evaluation of melanotan-II, a superpotent cyclic melanotropic peptide in a pilot phase-I clinical study. Life Sci 58: 1777–1784, 1996. doi: 10.1016/0024-3205(96)00160-9. [DOI] [PubMed] [Google Scholar]
- 14.Dunbar JC, Lu H. Proopiomelanocortin (POMC) products in the central regulation of sympathetic and cardiovascular dynamics: studies on melanocortin and opioid interactions. Peptides 21: 211–217, 2000. doi: 10.1016/S0196-9781(99)00192-8. [DOI] [PubMed] [Google Scholar]
- 15.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the United States, 2005 to 2014. JAMA 315: 2284–2291, 2016. doi: 10.1001/jama.2016.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gray AL, Johnson TA, Lauenstein JM, Newton SS, Ardell JL, Massari VJ. Parasympathetic control of the heart. III. Neuropeptide Y-immunoreactive nerve terminals synapse on three populations of negative chronotropic vagal preganglionic neurons. J Appl Physiol (1985) 96: 2279–2287, 2004. doi: 10.1152/japplphysiol.00621.2003. [DOI] [PubMed] [Google Scholar]
- 17.Hadley ME. Discovery that a melanocortin regulates sexual functions in male and female humans. Peptides 26: 1687–1689, 2005. doi: 10.1016/j.peptides.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 18.Hall JE, do Carmo JM, da Silva AA, Wang Z, Hall ME. Obesity-induced hypertension: interaction of neurohumoral and renal mechanisms. Circ Res 116: 991–1006, 2015. doi: 10.1161/CIRCRESAHA.116.305697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haynes WG, Morgan DA, Djalali A, Sivitz WI, Mark AL. Interactions between the melanocortin system and leptin in control of sympathetic nerve traffic. Hypertension 33: 542–547, 1999. doi: 10.1161/01.HYP.33.1.542. [DOI] [PubMed] [Google Scholar]
- 20.Humphreys MH, Ni XP, Pearce D. Cardiovascular effects of melanocortins. Eur J Pharmacol 660: 43–52, 2011. doi: 10.1016/j.ejphar.2010.10.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim ER, Wu Z, Sun H, Xu Y, Mangieri LR, Xu Y, Tong Q. Hypothalamic non-AgRP, -POMC GABAergic neurons are required for postweaning feeding and NPY hyperphagia. J Neurosci 35: 10440–10450, 2015. doi: 10.1523/JNEUROSCI.1110-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim JD, Leyva S, Diano S. Hormonal regulation of the hypothalamic melanocortin system. Front Physiol 5: 480, 2014. doi: 10.3389/fphys.2014.00480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuo JJ, Silva AA, Hall JE. Hypothalamic melanocortin receptors and chronic regulation of arterial pressure and renal function. Hypertension 41: 768–774, 2003. doi: 10.1161/01.HYP.0000048194.97428.1A. [DOI] [PubMed] [Google Scholar]
- 24.Loh K, Herzog H, Shi YC. Regulation of energy homeostasis by the NPY system. Trends Endocrinol Metab 26: 125–135, 2015. doi: 10.1016/j.tem.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 25.Marsh DJ, Miura GI, Yagaloff KA, Schwartz MW, Barsh GS, Palmiter RD. Effects of neuropeptide Y deficiency on hypothalamic agouti-related protein expression and responsiveness to melanocortin analogues. Brain Res 848: 66–77, 1999. doi: 10.1016/S0006-8993(99)01962-9. [DOI] [PubMed] [Google Scholar]
- 26.Pierroz DD, Ziotopoulou M, Ungsunan L, Moschos S, Flier JS, Mantzoros CS. Effects of acute and chronic administration of the melanocortin agonist MTII in mice with diet-induced obesity. Diabetes 51: 1337–1345, 2002. doi: 10.2337/diabetes.51.5.1337. [DOI] [PubMed] [Google Scholar]
- 27.Plum L, Ma X, Hampel B, Balthasar N, Coppari R, Münzberg H, Shanabrough M, Burdakov D, Rother E, Janoschek R, Alber J, Belgardt BF, Koch L, Seibler J, Schwenk F, Fekete C, Suzuki A, Mak TW, Krone W, Horvath TL, Ashcroft FM, Brüning JC. Enhanced PIP3 signaling in POMC neurons causes KATP channel activation and leads to diet-sensitive obesity. J Clin Invest 116: 1886–1901, 2006. doi: 10.1172/JCI27123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Putz GR, Barrett DA II, Witte DL. Evaluation of a glucose oxidase procedure. Am J Med Technol 42: 351–356, 1976. [PubMed] [Google Scholar]
- 29.Schwartz MW, Erickson JC, Baskin DG, Palmiter RD. Effect of fasting and leptin deficiency on hypothalamic neuropeptide Y gene transcription in vivo revealed by expression of a lacZ reporter gene. Endocrinology 139: 2629–2635, 1998. doi: 10.1210/endo.139.5.6000. [DOI] [PubMed] [Google Scholar]
- 30.Sindelar DK, Mystkowski P, Marsh DJ, Palmiter RD, Schwartz MW. Attenuation of diabetic hyperphagia in neuropeptide Y-deficient mice. Diabetes 51: 778–783, 2002. doi: 10.2337/diabetes.51.3.778. [DOI] [PubMed] [Google Scholar]
- 31.Sohn JW, Harris LE, Berglund ED, Liu T, Vong L, Lowell BB, Balthasar N, Williams KW, Elmquist JK. Melanocortin 4 receptors reciprocally regulate sympathetic and parasympathetic preganglionic neurons. Cell 152: 612–619, 2013. doi: 10.1016/j.cell.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tallam LS, da Silva AA, Hall JE. Melanocortin-4 receptor mediates chronic cardiovascular and metabolic actions of leptin. Hypertension 48: 58–64, 2006. doi: 10.1161/01.HYP.0000227966.36744.d9. [DOI] [PubMed] [Google Scholar]