Abstract
Brown adipose tissue (BAT) is considered protective against obesity and related cardiometabolic dysfunction. Indeed, activation of BAT improves glucose homeostasis and attenuates cardiovascular disease development. However, whether a reduction in BAT mass perturbs metabolic function and increases risk for cardiovascular disease remains largely unknown. To address this question, C57BL/6J male mice underwent a sham procedure or surgical bilateral excision of interscapular BAT (iBATx) and were fed a normal chow or a Western diet for 18 wk, creating four groups (n = 10/group). Mice were housed at 25°C. As expected, the Western diet increased final body weight and adiposity; however, contrary to our hypothesis, iBATx did not potentiate adiposity independent of diet. Furthermore, iBATx did not affect indexes of glycemic control (HbA1c, fasting glucose and insulin, and glucose area under the curve during a glucose tolerance test) and produced minimal-to-no effects on lipid homeostasis. The absence of metabolic disturbances with iBATx was not attributed to regrowth of iBAT or a “browning” or proliferative compensatory response of other BAT depots. Notably, iBATx caused an increase in aortic stiffness in normal chow-fed mice only, which was associated with an increase in aortic uncoupling protein-1. Collectively, we demonstrated that, at 25°C (i.e., limited thermal stress conditions), a substantial reduction in BAT mass via iBATx does not disrupt systemic glucose metabolism, challenging the current dogma that preservation of BAT is obligatory for optimal metabolic function. However, iBATx caused aortic stiffening in lean mice, hence supporting the existence of an interplay between iBAT and aortic stiffness, independent of alterations in glucose homeostasis.
Keywords: aortic stiffness, brown adipose tissue, glycemic control, metabolic function, UCP-1
INTRODUCTION
Brown adipose tissue (BAT) is characterized by an abundance of mitochondria, capillaries, and uncoupling protein-1 (UCP-1) relative to less metabolically active white adipose tissue depots. BAT is believed to play a protective role against obesity and related cardiometabolic dysfunction by virtue of its role in nonshivering thermogenesis (8). However, in addition to its thermogenic function, accumulating evidence in rodents and humans indicates that activation of BAT improves glucose tolerance, insulin sensitivity (3, 10, 27, 41, 42), lipid homeostasis (13), and protects against the pathogenesis of cardiovascular disease (4). Conversely, we (33, 52) and others (18, 39, 40) have shown that, in the setting of obesity, BAT becomes dysfunctional (i.e., impaired thermogenesis) and lipid-laden (i.e., whitening), which are associated with impaired glucose homeostasis. Similarly, evidence in humans (7, 37, 38, 45) and rodents (9) supports the notion that whitening of BAT and related metabolic dysfunction may contribute to the development of aortic stiffness, which is an established independent predictor for cardiovascular disease and mortality (28).
While studies using genetic models of BAT dysfunction (e.g., UCP-1 knockout mice) support the concept that BAT is vital for optimal metabolic health (52), whether deficiency in BAT perturbs metabolic function and promotes aortic stiffening remains largely unknown. Accordingly, we hypothesized that a substantial loss of BAT mass, via surgical excision of interscapular BAT (iBAT), which constitutes approximately one-third of BAT mass in rodents (30, 35, 44, 49), would impair glycemic control in lean mice, but to a lesser extent in obese mice, such that obesity-related differences in metabolic function would be partially normalized. That is, given that whitening of BAT has been implicated as a contributor to metabolic dysregulation in obesity, we reasoned that loss of whitened and dysfunctional BAT would be mostly inconsequential. Stated differently, we posited that BAT is required for optimal metabolic health in lean mice, whereas it becomes largely dispensable in obese mice. Furthermore, in view of current literature supporting the link between BAT dysfunction, glycemic dysregulation, and increased cardiovascular disease risk, we hypothesized that metabolic derangements associated with removal of BAT would lead to aortic stiffening.
MATERIALS AND METHODS
Animals and surgery.
All procedures received prior approval by the Institutional Animal Care and Use Committee of the University of Missouri, which is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. Forty C57BL/6J male mice (catalog no. 000664, Jackson Laboratory, Bar Harbor, ME) were housed two per cage in an environmentally controlled animal facility maintained at 25°C on a 12:12-h light-dark cycle, with lights on from 0700 to 1900, in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals (8th ed.). Recent evidence suggests that a housing temperature of 25°C, combined with pair-housing (21) and provision of shelter (19, 20), limits thermal stress, creating an environment that more appropriately mimics human physiology. Indeed, standard housing conditions (e.g., 20–22°C) are known to cause significant thermal stress, which has concerning implications for a study that attempts to model human disease (17). At 8 wk of age, mice were randomized (n = 20/group) to undergo sham or bilateral iBAT lipectomy (iBATx) surgery to considerably reduce (by ~30%) BAT mass. Surgeries were performed under constant administration of isoflurane (via inhalation). Briefly, after anesthesia was induced, mice were placed in sternal recumbency. The hair from the neck to just below the scapulae was shaved, and the surgical site was prepared in an aseptic manner. A 2-cm incision was made along the dorsal midline on sham-operated and iBATx mice. In the iBATx group, the iBAT pads (bilateral) were removed (iBAT fat pads, Fig. 1A) and cleaned of white adipose tissue. In the sham-operated group, the iBAT pads were exposed but not removed. The incision was closed using absorbable subcuticular sutures. All mice were given buprenorphine (0.03 mg/kg sc) before recovery. The mice were monitored daily under the supervision of a veterinarian throughout a 1-wk postoperative period. All mice survived the surgery and gained an average of 1.78 ± 0.19 g body wt during the 1st wk. After surgery, the mice were randomized to continue the normal chow diet or switched to a Western diet for 18 wk, thus creating four groups in a 2 × 2 factorial design (n = 10/group): normal chow-sham, normal chow-iBATx, Western diet-sham, and Western diet-iBATx. Mice were given ad libitum access to respective diets and water. The normal chow diet (3.35 kcal/g of food) consisted of 13.4% kcal fat and 56.7% kcal from carbohydrate (Laboratory Rodent Diet 5001*, Laboratory Diet), and the Western diet (4.65 kcal/g of food) consisted of 46% kcal from fat and 36.0% kcal from carbohydrate, with sucrose content of 17.5% and high-fructose corn syrup content of 17.5% (Test Diet modified 58Y1, 5APC) (6). Body weight was recorded weekly, and food intake was assessed over a 10-day period from week 14 to 15.
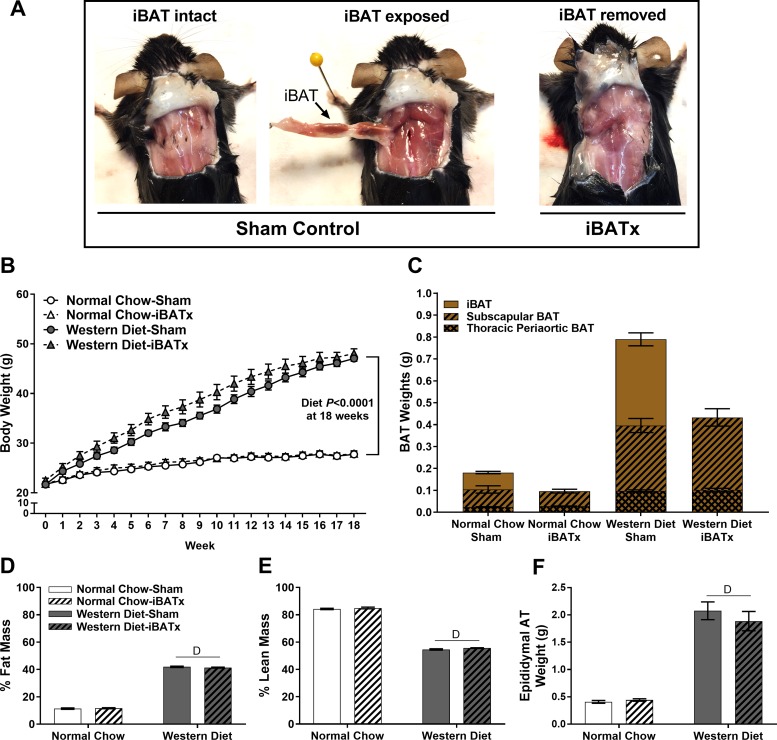
Fig. 1.
Summary of interscapular brown adipose tissue (iBAT) surgery (iBATx), body weight, and tissue morphology. A: intact and surgically excised iBAT in male mice at 26 wk of age. B: body weight growth curve over 18 wk of the study intervention; △ are underneath ○. C: summation of BAT fat pads demonstrating a reduction in cumulative BAT weight following iBATx and no BAT regrowth. D and E: percent fat mass and lean mass as determined by EchoMRI. F: epididymal adipose tissue (AT) weight. Values are means ± SE; n = 10/group. DMain effect of diet (P < 0.05).
After 18 wk of dietary intervention (i.e., at 26 wk of age), mice were euthanized after a 5-h fast via CO2 asphyxiation. Blood was immediately taken via cardiac puncture and placed in EDTA-coated tubes, and whole blood and the plasma fraction were stored at −80°C until analysis. Tissues were quickly excised, weighed, and then flash-frozen in liquid nitrogen and stored at −80°C until analysis. Segments of the thoracic aorta (cleaned of adipose tissue) and adipose tissues were stored in 10% formalin (48 h) and then transferred to 70% ethanol until histological assessment.
Additionally, adipose samples from male C57BLKS/J (wild-type, n = 10; catalog no. 000662, Jackson Laboratories) and leptin receptor-deficient (db−/db−) mice (n = 10; catalog no. 000642, Jackson Laboratories) were retrospectively examined to further interrogate the relationship between the periaortic BAT phenotype and aortic stiffness. Mice were pair-housed (within genotype) at 25°C and given ad libitum access to normal chow and water and euthanized at 21 wk of age.
Glucose tolerance.
Glucose tolerance tests (GTT) were performed at 17 wk postsurgery (i.e., at 25 wk of age). Briefly, after a 5-h fast, glucose in blood from the tail vein was measured: the tail was nicked, and blood was sampled by a hand-held glucometer (Alpha Trak, Abbott Laboratories, Abbott Park, IL). A baseline measurement of blood glucose was determined before administration of a sterile solution of 50% dextrose (2 g/kg body wt ip), as previously performed (52). Glucose was measured 15, 30, 45, 60, and 120 min after the glucose injection, and glucose area under curve (AUC) was calculated from baseline.
Body composition.
Body fat and lean mass were measured by a nuclear magnetic resonance imaging whole body composition analyzer (EchoMRI, 4-in-1/1100, Echo Medical Systems, Houston, TX) within 1 wk of euthanasia.
Aortic pulse wave velocity.
Doppler ultrasound (Indus Mouse Doppler System, Webster, TX) was used as previously described (14) to assess pulse wave velocity for in vivo determination of arterial stiffness. At ∼3 h before they were euthanized, the mice were anesthetized by isoflurane inhalation (1.75% in 100% oxygen stream) and positioned supine on a heating board with their legs secured to ECG electrodes. Determination of pulse wave velocity was based on the transit time method, calculated as the difference in arrival times of pulse waves at the descending aorta proximal to the aortic arch followed immediately by measurement at the descending aorta proximal to the iliac bifurcation. Each pulse wave arrival time was measured as the time from the peak of the ECG R wave to the leading foot of the pulse wave at which time velocity begins to rise at the start of systole. The distance between the thoracic and abdominal aorta regions was measured with a ruler to the nearest 0.1 cm and then divided by transit time. At least ∼35 pulse waves per aortic site were captured and averaged for each animal. Data are expressed in centimeters per second and as fold difference. The investigator analyzing pulse waveforms was blinded to the treatment interventions.
Fasting plasma parameters.
Plasma glucose, insulin, cholesterol, HDL, LDL, triglycerides, and nonesterified fatty acid (NEFA) were assayed by a commercial laboratory (Comparative Clinical Pathology Services, Columbia, MO) on an Olympus AU680 automated chemistry analyzer (Beckman-Coulter, Brea, CA) as recommended in the manufacturer’s guidelines. The homeostatic model assessment of insulin resistance (HOMA-IR) was determined as a measure of insulin resistance {[fasting glucose (mg/dl) × insulin (mU/l)]/405.1} (29), while adipose tissue insulin resistance (AT-IR) was calculated as the product of fasting insulin (mU/l) and fasting NEFA (mmol/l) (26). In addition, whole blood samples were analyzed for hemoglobin A1c (HbA1c) at the University of Missouri Diabetes Diagnostic Laboratory using a boronate affinity HPLC method (ultra2, Trinity Biotech, Kansas City, MO), a secondary reference method for the National Glycohemoglobin Standardization Program.
Liver triglycerides.
Hepatic triglyceride concentration was determined using a commercially available kit (L-type TG M, Wako Pure Chemical Industries, Osaka, Japan), as previously described (48).
Histological assessment.
Dissected iBAT, subscapular BAT (sBAT), thoracic periaortic BAT, subcutaneous (inguinal region) and epididymal white adipose tissue, and thoracic aortic rings were placed in 10% formalin and embedded in paraffin, and 5-µm sections were stained for tissue morphology: adipose depots with hematoxylin and eosin and aortic rings with Verhoeff-Van Gieson. Tissue sections were imaged under a photomicroscope (model BX34, Olympus, Melville, NY) equipped with an Olympus SC30 optical microscope accessory CMOS color camera. Mouse aortas were imaged at ×10 magnification with a ×10 objective and analyzed using ImageJ software (NIH public domain, NIH, Bethesda, MD). Circumferences of the aorta lumen and tunica media (excluding the external elastic lamina) were traced using the free tracing tool. The lumen area was subtracted from the area of the tunica media, and the radius of the vessel was determined using the following equation: A = πr2, where A is area and r is radius. An average of three circumference traces were averaged for each vessel.
Adipocyte size (area, µm2) was assessed in subcutaneous adipose tissue. Adipocyte size was calculated from the average of ~100 adipocytes per animal from ≥3 different captured images (i.e., fields of view). Cross-sectional areas of the adipocyte perimeter traces were obtained and analyzed using ImageJ software. Additionally, the distribution of adipocytes per discrete cell size was examined as previously described (24). All procedures were performed by an investigator who was blinded to the groups.
Gene expression.
mRNA expression was determined in interscapular, subscapular, thoracic periaortic, subcutaneous, and epididymal adipose tissues. Tissues were homogenized in TRIzol solution (TissueLyser LT, Qiagen, Valencia, CA), and total RNA was isolated using the RNeasy lipid tissue homogenization kit according to the manufacturer’s instructions (Qiagen). RNA was assessed for purity and concentration using a spectrophotometer (NanoDrop, Thermo Scientific, Wilmington, DE). First-strand cDNA was synthesized from total RNA using a high-capacity cDNA reverse-transcription kit (Applied Biosystems, Carlsbad, CA). Quantitative real-time PCR was performed using the StepOne Plus sequence detection system (Applied Biosystems). Primers were purchased from Integrated DNA Technologies (Coralville, IA) or Sigma Aldrich (St. Louis, MO). A 20-µl reaction mixture containing iTaq UniverSYBR Green SMX (10 µl; Bio-Rad, Hercules, CA), appropriate concentrations of gene-specific primers (0.8 µl, 1,000 nmol stock), cDNA template (4.0 µl, 750 ng/µl), and RNase-free water (5.2 µl) was loaded in a single well of a 96-well plate. Gene primer sequences are listed in Table 1. PCRs were performed in duplicate under thermal conditions as follows: 95°C for 10 min, followed by 40 cycles of 95°C for 15 s, and 60°C for 60 s. A dissociation melt curve analysis was performed to verify the specificity of the PCR products. GAPDH and 18S rRNA were used as housekeeping control genes. mRNA expression values were calculated by the cycle threshold (∆∆CT) method (25) and are presented as fold difference compared with a control group, which was set at 1.
Table 1.
PCR primer sequences
| Sequence (5′ → 3′) | ||
|---|---|---|
| Gene | Forward | Reverse |
| TNFα | CTATGTCTCAGCCTCTTCTC | CATTTGGGAACTTCTCTCATC |
| IL-1β | AAATGGAGACCTGGAATCAG | CCTTCTTACTTCACTGTCTTTG |
| IL-6 | TCCAGTTGCCTTCTTGGGAC | AGTCTCCTCTCCGGACTTGT |
| MCP-1 | GGCTGGAGAGCTACAAGAGG | GGTCAGCACAGACCTCTCTC |
| CD68 | TGTTCAGCTCCAAGCCCAAA | GTACCGTCACAACCTCCCTG |
| UCP-1 | CACGGGGACCTACAATGCTT | ACAGTAAATGGCAGGGGACG |
| PGC1α | CCCTGCCATTGTTAAGACC | TGCTGCTGTTCCTGTTTTC |
| PRDM16 | CAGCACGGTGAAGCCATTC | GCGTGCATCCGCTTGTG |
| Cidea | TGCTCTTCTGTATCGCCCAGT | GCCGTGTTAAGGAATCTGCTG |
| FGF-21 | GTACCTTCTACACAGATGACGAA | CGCCTACCACTGTTCCATCCT |
| 18S | TCAAGAACGAAAGTCGGAGG | GGACATCTAAGGGCATCAC |
| GAPDH | CCAGCTACTCGCGGCTTTA | GAGGGCTGCAGTCCGTATTT |
TNFα, tumor necrosis factor-α; IL-1β, interleukin (IL)-1β, MCP-1, monocyte chemoattractant protein-1; UCP-1, uncoupling protein-1; PGC1α, peroxisome proliferator-activated receptor-γ coactivator-1α; PRDM16, PR domain zinc finger protein 16; Cidea, cell death-inducing DNA fragmentation factor-α (DFFA)-like effector A; FGF-21, fibroblast growth factor 21; 18S, 18S rRNA; GAPDH, glyceraldehyde phosphate dehydrogenase.
The browning-to-inflammatory gene expression ratio across groups was calculated as an average composite score (average of fold differences) of classical BAT-associated genes [UCP-1, peroxisome proliferator-activated receptor-γ coactivator-1α (PGC1α), PR domain zinc finger protein 16 (PRDM16), cell death-inducing DNA fragmentation factor-α (DFFA)-like effector A (Cidea), and fibroblast growth factor-21 (FGF-21)] vs. inflammatory genes [interleukin (IL)-1β, tumor necrosis factor (TNF)-α, IL-6, monocyte chemoattractant protein-1 (MCP-1), and CD68]. BAT-associated (i.e., browning) genes were selected following careful examination of previous literature (12, 15, 23, 34). The fold difference of classical browning genes and inflammatory genes and the browning-to-inflammatory gene expression ratio were then regressed on the variable of aortic stiffness (represented as fold difference), and a polynomial correlation was assessed.
Western blotting.
Triton X-100 tissue lysates of the aorta (cleaned of periaortic BAT), iBAT, sBAT, thoracic periaortic BAT, subcutaneous and epididymal adipose tissue, and the quadriceps muscle group were prepared in 1:1 Laemmli buffer. Prepared protein samples (8 µg/lane for periaortic BAT and UCP-1 between-tissue comparison blot; 9 µg/lane for aorta; and 10 µg/lane for iBAT, sBAT, subcutaneous and epididymal adipose tissue, and quadriceps muscle) were separated via Criterion Tris-glycine eXtended-PAGE precast gels (Bio-Rad). Proteins were transferred onto polyvinylidene difluoride membranes and blocked overnight in 5% nonfat dry milk. Membranes were probed for UCP-1 (1:1,000 dilution; catalog no. U6382, Sigma-Aldrich), PGC1α (1:1,000 dilution; catalog no. 516557, Sigma-Aldrich), oxidative phosphorylation (Oxphos) cocktail (1:2,000 dilution; catalog no. 110413, Abcam), adipose triglyceride lipase (ATGL, 1:1,000 dilution; catalog no. 2138, Cell Signaling Technology), sarcolipin (1:500 dilution; catalog no. ABT13, Millipore Sigma), and β-tubulin (1:1,000 dilution; catalog no. 2146, Cell Signaling Technology). Intensity of individual protein bands was quantified via densitometry using FluoroChem HD2 (version 3.4.0.0, AlphaView). Adipose tissue blots were corrected to total protein stain on the membrane (1% amido black; Sigma-Aldrich) or blot standard. Aortic and skeletal muscle blots were normalized to β-tubulin. Values are expressed as fold difference.
Statistical analysis.
GraphPad Prism (version 7.0, GraphPad Prism Software, La Jolla, CA) was used for statistical analysis. A two-way analysis of variance was utilized to test diet × surgical treatment interactions. Student’s t-tests were performed when appropriate (i.e., normal chow vs. Western diet or wild-type vs. db−/db−). Values are means ± SE. Significance was determined when P < 0.05.
RESULTS
iBATx does not alter body composition.
Figure 1A depicts the iBAT fat pad in sham-operated mice and the absence of iBAT in iBATx mice at 26 wk of age. At 18 wk following iBATx, no evidence of iBAT regrowth was noted in any of the iBATx mice (Fig. 1A). Western diet-fed mice were significantly heavier than normal chow-fed mice after 18 wk (Fig. 1B). Body weight from week 4 to 14 was greater for Western diet-iBATx than Western diet-sham mice (P < 0.05). However, this effect was lost after 14 wk with no influence of iBATx on body weight at the end point. Furthermore, iBATx did not affect energy intake in normal chow-fed (13.86 ± 0.59 and 13.91 ± 0.59 kcal/day for iBATx and sham, respectively, P = 0.91) or Western diet-fed (13.58 ± 0.29 and 13.28 ± 0.17 kcal/day for iBATx and sham, respectively, P = 0.70) mice over a 10-day period from week 14 to 15. In addition, because neither iBAT regrew nor sBAT or periaortic BAT compensated in growth, total BAT mass in iBATx mice remained markedly reduced throughout the study (Fig. 1C). Accordingly, no differences in sBAT or periaortic BAT weight were observed between sham-operated and iBATx mice (all P > 0.05). At the end point, Western diet-fed mice exhibited increased body fat mass (P < 0.05) and reduced percent lean body mass (P < 0.05), while no effects of iBATx were noted (Fig. 1, D and E). Epididymal adipose tissue mass was also increased in Western diet-fed mice (P < 0.05) with no effects of iBATx (Fig. 1F).
iBATx does not disrupt glycemic control and has minimal-to-no effects on lipid homeostasis.
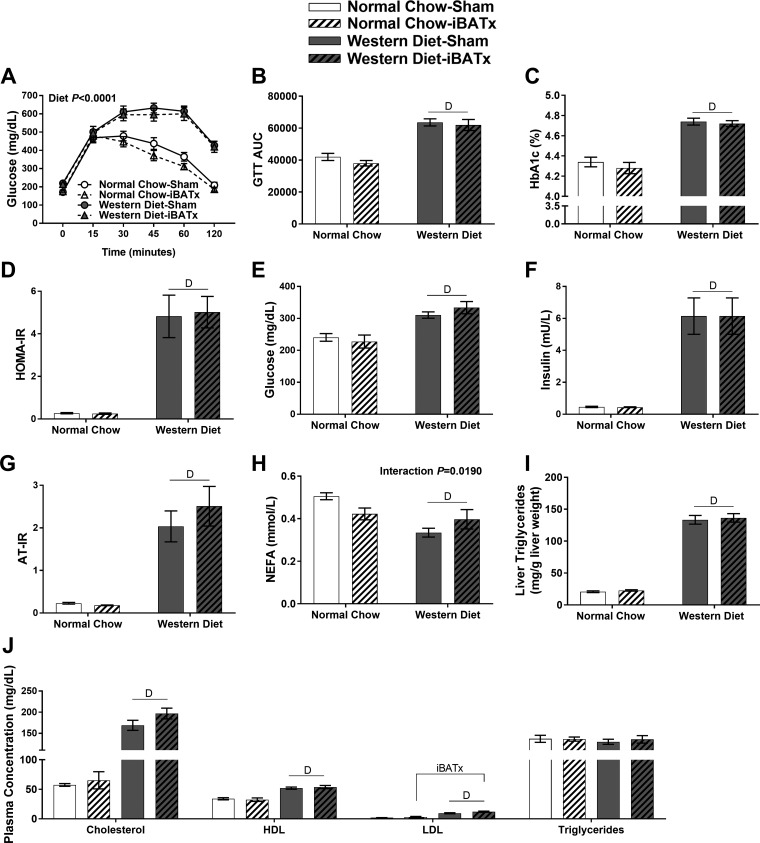
As expected, Western diet-fed mice displayed glucose intolerance and elevated HbA1c, HOMA-IR, fasting glucose, fasting insulin, and AT-IR compared with normal chow-fed mice (P < 0.05; Fig. 2, A–G); however, none of these variables was influenced by loss of iBAT. An interaction (diet × iBATx) was found with fasting NEFA, whereby NEFA were reduced in normal chow-iBATx mice and increased in Western diet-iBATx mice relative to sham-operated controls (P < 0.05; Fig. 2H). The Western diet increased liver triglycerides (Fig. 2I) with no effect of iBATx. Plasma cholesterol, LDL, HDL, and triglycerides were elevated in Western diet-fed mice (Fig. 2J), while iBATx produced a small, but significant, main effect (i.e., increased) on LDL levels only.
Fig. 2.
Interscapular brown adipose tissue surgery (iBATx) does not alter glycemic control and minimally affects lipid homeostasis. A: glucose tolerance test (GTT) conducted at 25 wk of age shows blood glucose levels following intraperitoneal injection of glucose (2 g/kg body wt). B: glucose area under the curve (AUC) during GTT. C: fasting hemoglobin A1c (HbA1c). D: homeostatic model assessment of insulin resistance (HOMA-IR). E and F: fasting plasma glucose and plasma insulin at 26 wk of age. G–J: fasting adipose tissue insulin resistance (AT-IR), nonesterified fatty acids (NEFA), liver triglycerides, and plasma cholesterol (total, HDL, and LDL) and triglycerides. Values are means ± SE; n = 10/group. DMain effect of diet (P < 0.05). iBATxMain effect of iBATx (P < 0.05).
iBATx increases aortic stiffness in normal chow-fed mice.
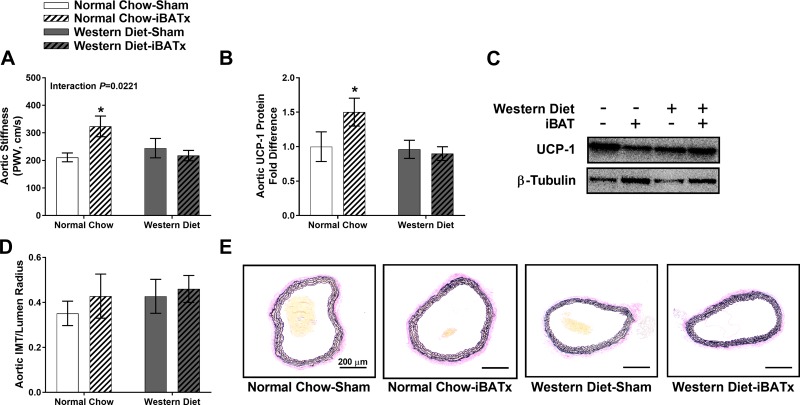
iBATx increased aortic stiffness in normal chow-fed, but not Western diet-fed, mice (P < 0.05 relative to normal chow-sham; interaction P < 0.05; Fig. 3A). The increase in aortic stiffness in normal chow-iBATx mice was associated with increased UCP-1 protein expression in the thoracic aorta (P < 0.05; Fig. 3, B and C). This iBATx-associated induction of aortic UCP-1 was absent in Western diet-fed mice. No differences were found in the intima-media thickness of the aorta among groups (Fig. 3, D and E).
Fig. 3.
Interscapular brown adipose tissue (iBAT) surgery (iBATx) increases aortic stiffness in normal chow-fed mice. A: aortic stiffness as determined by pulse wave velocity (PWV) in normal chow- and Western diet-fed mice following sham operation or iBATx. B and C: aortic uncoupling protein-1 (UCP-1) content and Western blot images. D: ratio of intima-media thickness (IMT) to lumen radius of the thoracic aorta. E: histological images of thoracic aorta stained for Verhoeff-Van Gieson. Magnification ×10 with ×10 objective. Values are means ± SE; n = 9–10/group. *Effect of iBATx (P < 0.05).
iBATx does not enhance browning in other BAT depots.
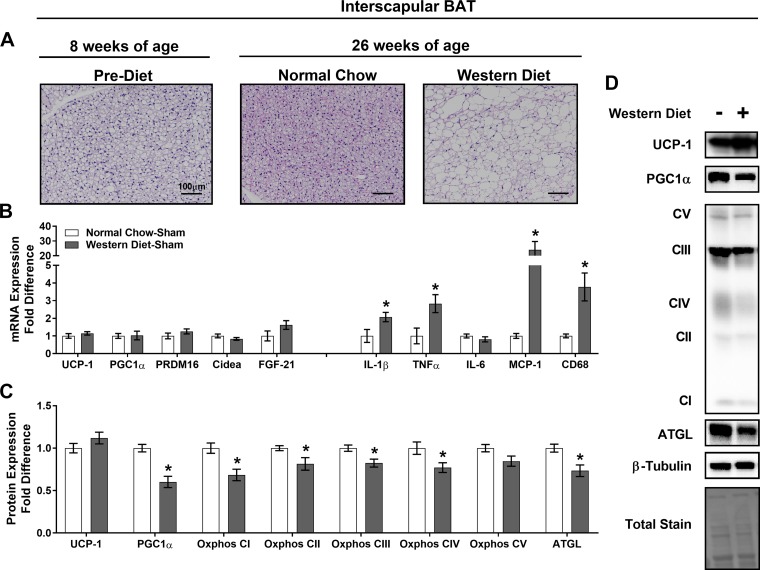
In iBAT, the Western diet increased lipid deposition in sham-operated mice, characterized histologically by a loss of multilocular cells (Fig. 4A). Western diet-sham mice exhibited increased gene expression of select inflammatory markers, while browning genes were not altered in iBAT (Fig. 4B). The Western diet decreased iBAT PGC1α, Oxphos complexes I–IV, and ATGL proteins (Fig. 4, C and D), indicative of BAT dysfunction.
Fig. 4.
Western diet increases markers of interscapular brown adipose tissue (iBAT) inflammation and reduces iBAT mitochondrial content. A: iBAT morphology in hematoxylin-eosin-stained sections at 8 and 26 wk of age. Magnification ×20 with ×10 objective. B: expression of browning and inflammatory genes [uncoupling protein-1 (UCP-1), peroxisome proliferator-activated receptor-γ coactivator-1α (PGC1α), PR domain zinc finger protein 16 (PRDM16), cell death-inducing DNA fragmentation factor-α (DFFA)-like effector A (Cidea), fibroblast growth factor 12 (FGF-12), interleukin (IL)-1β (IL-1β), tumor necrosis factor-α (TNF-α), IL-6, monocyte chemoattractant protein-1 (MCP-1), and CD68] in iBAT, represented as fold difference from normal chow-sham. C: protein level determination of mitochondrial markers [UCP-1, PGC1α, and oxidative phosphorylation (Oxphos) complexes I–V (CI–C5)] and adipose triglyceride lipase (ATGL) in iBAT as determined by Western blot analysis, represented as fold difference from normal chow-sham. D: representative Western blots, for which experimental conditions were optimized for within-tissue comparisons. Values are means ± SE; n = 8–10/group. *Effect of iBATx (P < 0.05).
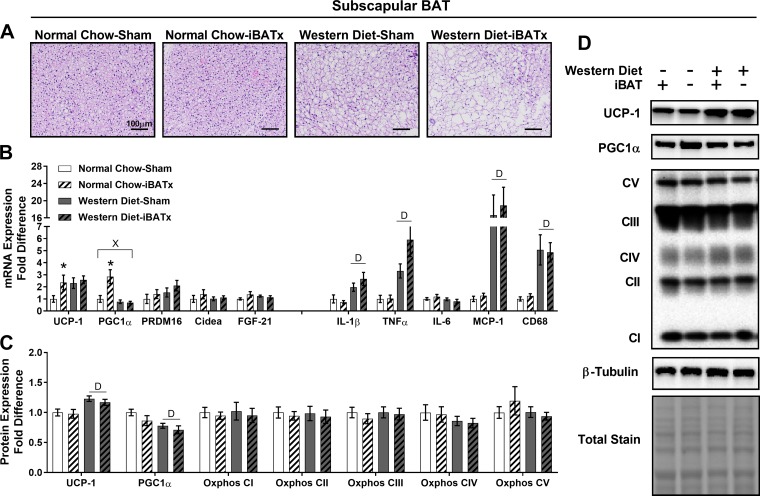
iBATx did not alter sBAT morphology; however, Western-diet fed mice exhibited an increase in adipocyte size relative to normal chow-fed mice (Fig. 5A). In sBAT, normal chow-iBATx mice exhibited upregulation of select browning genes (UCP-1 and PGC1α) with no alterations in inflammatory gene expression (P < 0.05; Fig. 5B). A diet × iBATx interaction was found in PGC1α, where the induction with iBATx was lost with Western diet feeding (P < 0.05). Independent of diet, iBATx did not alter sBAT protein levels of UCP-1, PGC1α, or Oxphos (Fig. 5, C and D). However, the Western diet increased sBAT UCP-1 and PGC1α protein expression in sham-operated and iBATx mice (main effect of diet P < 0.05).
Fig. 5.
Interscapular brown adipose tissue surgery (iBATx) does not alter characteristic BAT protein markers in subscapular BAT. A: subscapular BAT morphology at 26 wk of age in hematoxylin-eosin-stained sections. Magnification ×20 with ×10 objective. B: expression of browning and inflammatory genes in subscapular BAT, represented as fold difference from normal chow-sham. C: protein level determination of browning and mitochondrial markers in subscapular BAT as determined by Western blot analysis, represented as fold difference from normal chow-sham. D: representative Western blots, for which experimental conditions were optimized for within-tissue comparisons. Values are means ± SE; n = 8–10/group. *Effect of iBATx (P < 0.05). DMain effect of diet (P < 0.05). ×Diet × surgical treatment interaction effect (P < 0.05).
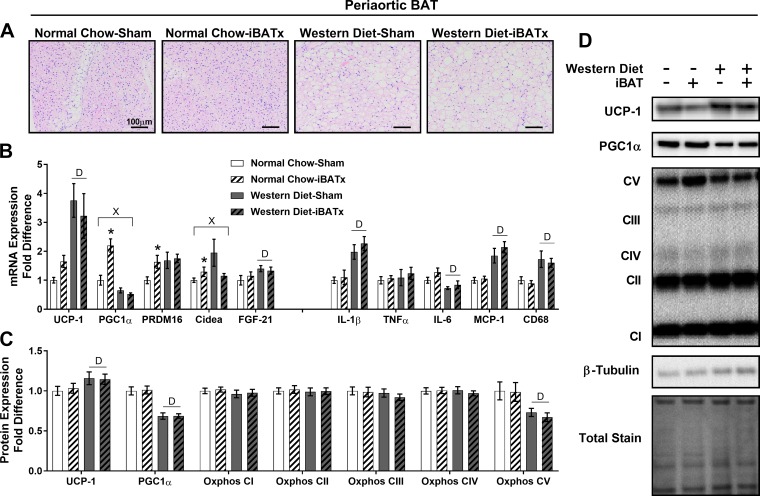
In periaortic BAT, iBATx in normal chow-fed mice did not alter tissue morphology relative to sham-operated controls, whereas thoracic periaortic adipocytes were larger in Western diet- than normal chow-fed controls with no apparent effect of iBATx (Fig. 6A). In periaortic BAT, normal chow-iBATx mice exhibited upregulation of select browning gene (PGC1α, PRDM16, and Cidea) expression and no alterations in inflammatory gene expression (Fig. 6B). A diet × iBATx interaction was found for the browning genes PGC1α and Cidea, where the induction with iBATx was suppressed by the Western diet (P < 0.05). Independent of diet, iBATx did not affect periaortic BAT UCP-1, PGC1α, or Oxphos protein expression (Fig. 6, C and D). In iBATx and sham-operated groups, the Western diet increased periaortic BAT UCP-1 content while decreasing PGC1α and Oxphos complex V protein expression relative to normal chow-fed mice.
Fig. 6.
Interscapular brown adipose tissue surgery (iBATx) does not alter characteristic BAT protein markers in periaortic BAT. A: periaortic BAT morphology at 26 wk of age in hematoxylin-eosin-stained sections. Magnification ×20 with ×10 objective. B: expression of browning and inflammatory genes in periaortic BAT, represented as fold difference from normal chow-sham. C: protein level determination of browning and mitochondrial markers in periaortic BAT as determined by Western blot, represented as fold difference from normal chow-sham. D: representative Western blots, for which experimental conditions were optimized for within-tissue comparisons. Values are means ± SE; n = 8–10/group. *Effect of iBATx (P < 0.05). DMain effect of diet (P < 0.05). ×Diet × surgical treatment interaction effect (P < 0.05).
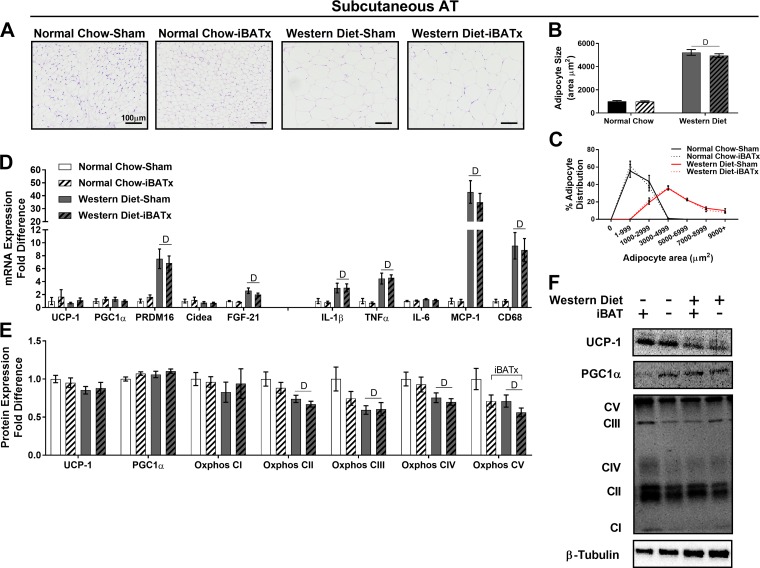
Morphology of subcutaneous adipose tissue was not altered in normal chow-iBATx relative to normal chow-sham mice, whereas adipocyte size was increased in Western diet-sham compared with normal chow-sham mice (Fig. 7, A and B). The percentage of large adipocytes was greater in Western diet- than chow-fed mice, and this distribution was not affected by iBATx (Fig. 7C). In subcutaneous adipose tissue, normal chow-iBATx mice exhibited no alterations in browning or inflammatory gene expression (Fig. 7D). Independent of diet, iBATx did not affect protein expression of UCP-1, PGC1α, or Oxphos complexes I–IV in subcutaneous adipose tissue, whereas Oxphos complex V protein expression was decreased (Fig. 7, E and F). In iBATx and sham-operated groups, the Western diet decreased subcutaneous adipose tissue Oxphos complexes II–V protein expression relative to normal chow-fed mice.
Fig. 7.
Interscapular brown adipose tissue surgery (iBATx) does not promote browning of white subcutaneous adipose tissue (inguinal region) in normal chow- and Western diet-fed mice. A: subcutaneous adipose tissue (AT) morphology at 26 wk of age in hematoxylin-eosin-stained sections. Magnification ×20 with ×10 objective. B and C: quantification of adipocyte cell size and adipocyte cell size distribution. D: expression of browning and inflammatory genes in epididymal adipose tissue, represented as fold difference from normal chow-sham. E: protein level of browning and mitochondrial markers in subcutaneous adipose tissue as determined by Western blot analysis, represented as fold difference from normal chow-sham. F: representative Western blots, for which experimental conditions were optimized for within-tissue comparisons. Values are means ± SE; n = 9–10/group. DMain effect of diet (P < 0.05). iBATxMain effect of iBATx (P < 0.05).
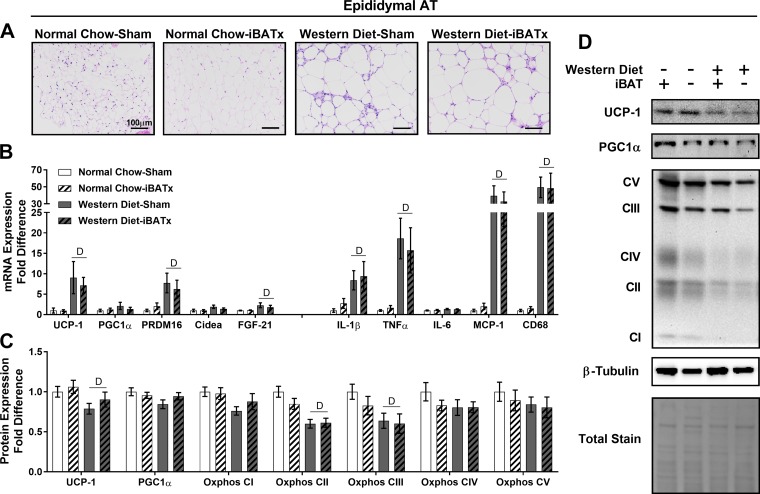
In epididymal adipose tissue, Western diet-fed mice exhibited increased adipocyte size relative to normal chow-fed mice with no apparent effect of iBATx (Fig. 8A). Independent of diet, iBATx did not alter browning or inflammatory gene expression, while expression of select browning and inflammatory genes was increased in Western diet-fed relative to chow-fed mice (Fig. 8B). iBATx did not alter UCP-1, PGC1α, or Oxphos protein levels (Fig. 8, C and D). Western diet-fed mice exhibited reduced UCP-1 protein levels in this white adipose tissue depot relative to normal chow-fed mice.
Fig. 8.
Interscapular brown adipose tissue surgery (iBATx) does not promote browning of white epididymal adipose tissue in normal chow- and Western diet-fed mice. A: epididymal adipose tissue (AT) morphology at 26 wk of age in hematoxylin-eosin-stained sections. Magnification ×20 with ×10 objective. B: expression of browning and inflammatory genes in epididymal adipose tissue, represented as fold difference from normal chow-sham. C: protein level of browning and mitochondrial markers in epididymal adipose tissue as determined by Western blot analysis, represented as fold difference from normal chow-sham. D: representative Western blots, for which experimental conditions were optimized for within-tissue comparisons. Values are means ± SE; n = 8–10/group. DMain effect of diet (P < 0.05).
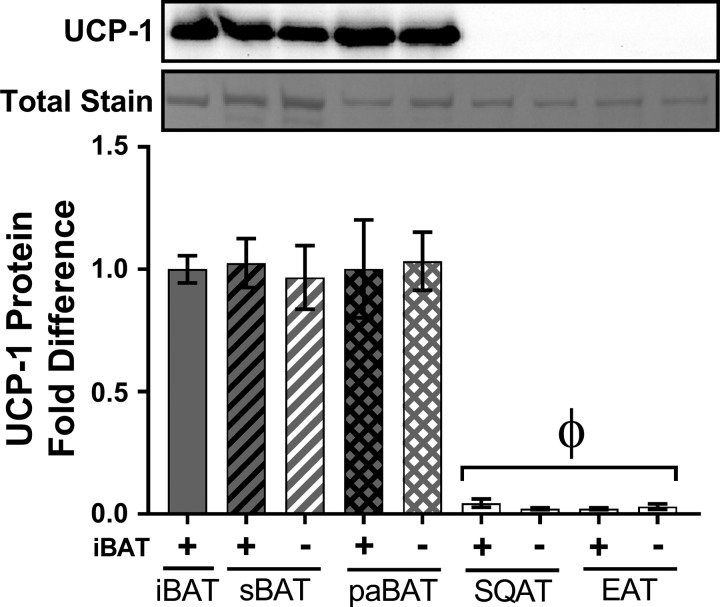
To appreciate the differences in UCP-1 protein content across adipose tissue depots and to further verify that iBATx did not lead to a compensatory induction of UCP-1 beyond iBAT, protein expression of UCP-1 was directly compared among fat depots by loading samples within the same Western blot. As displayed in Fig. 9, all BAT depots exhibited similar expression levels of UCP-1, independent of iBAT removal. Relative to BAT, UCP-1 protein content was virtually undetectable in white adipose tissue (Fig. 9).
Fig. 9.
Interscapular brown adipose tissue surgery (iBATx) does not cause a compensatory induction of UCP-1 protein across adipose tissue depots. UCP-1 protein content in iBAT, subscapular BAT (sBAT), periaortic BAT (paBAT), subcutaneous white adipose tissue (SQAT), and epididymal white adipose tissue (EAT) from chow-fed mice is shown as representative blot of a pooled sample of 3 animals from the same treatment group and densitometric analysis of 3 pooled samples (i.e., total of 9 animals/group). Pooled sample was loaded at 8 µg/lane and optimized for within- and between-tissue comparisons. Values are means ± SE. ΦEffect of all white adipose tissue depots (P < 0.05).
In quadriceps muscle, sarcolipin protein levels were determined as a marker of BAT-independent thermogenesis (2). Neither iBATx nor the Western diet affected skeletal muscle sarcolipin protein content (expressed as fold difference): 1.0 ± 0.04 for normal chow-sham, 0.94 ± 0.06 for normal chow-iBATx, 0.92 ± 0.05 for Western diet-sham, and 1.01 ± 0.05 for Western diet-iBATx (interaction P = 0.18, diet P = 0.89, iBATx P = 0.81).
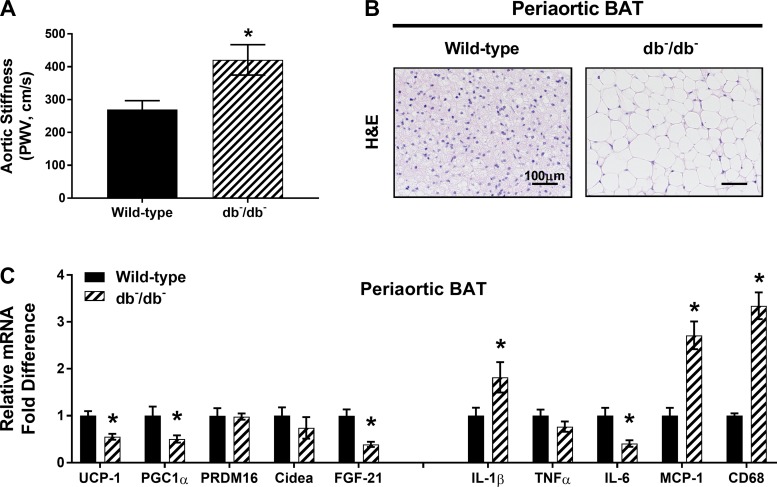
db−/db− Mice exhibit aortic stiffness and inflamed periaortic BAT.
To further elucidate the relationship between periaortic BAT gene expression and aortic stiffness, pulse wave velocity and thoracic periaortic BAT from db−/db− mice were examined. Compared with wild-type controls, db−/db− mice exhibited significant increases in aortic stiffness (Fig. 10A) and periaortic BAT whitening (Fig. 10B). Additionally, inflammatory gene expression in periaortic BAT was enhanced in db−/db− mice relative to wild-type controls (Fig. 10C).
Fig. 10.
Increased aortic stiffness and increased markers of inflammation in periaortic BAT from leptin receptor-deficient (db−/db−) mice. A: aortic stiffness as determined by pulse wave velocity (PWV) in wild-type and db−/db− mice. B: periaortic BAT morphology in hematoxylin-eosin (H&E)-stained sections. Magnification ×20 with ×10 objective. C: expression of browning and inflammatory genes in periaortic BAT, represented as fold difference from wild-type controls. Values are means ± SE; n = 9–10/group. *Effect of db−/db− (P < 0.05).
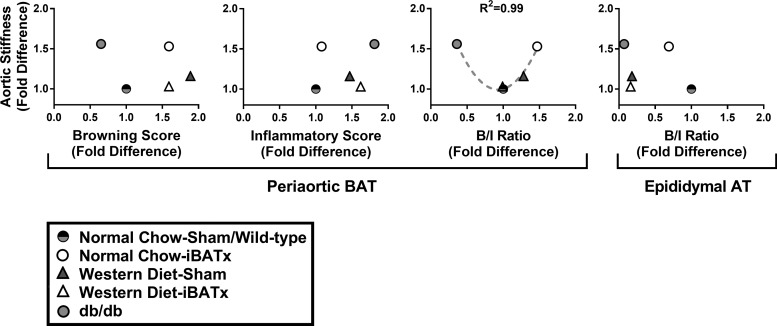
Periaortic BAT browning-to-inflammatory gene expression ratio is associated with aortic stiffness.
The association between the browning-to-inflammatory gene expression ratio and aortic stiffness is presented in Fig. 11. Relative to sham-operated controls, normal chow-iBATx mice exhibited an elevated browning-to-inflammatory ratio in periaortic BAT that was associated with increased aortic stiffness. Despite Western diet-induced metabolic dysfunction, the periaortic BAT browning-to-inflammatory ratio remained unaltered in Western diet-sham and Western diet-iBATx groups, which paralleled no changes in aortic stiffening. To expand on this relationship, data from db−/db− mice and respective controls are also included in the scatter plot of group means in Fig. 11. A U-shaped polynomial relationship of group means was found between the periaortic BAT browning-to-inflammatory gene expression ratio and aortic stiffness (R2 = 0.99). This relationship between the adipose tissue browning-to-inflammatory ratio and aortic stiffness was specific to periaortic BAT (i.e., not apparent in distal adipose tissue depots such as white epididymal adipose tissue; Fig. 11). In addition, this relationship was not present when periaortic BAT browning scores (average of browning genes only) and inflammatory scores (average of inflammatory genes only) were considered independent of each other.
Fig. 11.
Browning-to-inflammatory gene expression ratio (B/I ratio) in periaortic BAT is associated with aortic stiffness. Note U-shaped polynomial relationship between periaortic BAT B/I ratio and aortic stiffness (R2 = 0.99); i.e., high and low periaortic BAT B/I ratios were associated with increased aortic stiffness. Interestingly, the B/I ratio was a stronger determinant of aortic stiffness than the browning or inflammatory phenotype of periaortic BAT alone. This polynomial relationship was much less apparent between epididymal (distal) adipose tissue (AT) and aortic stiffness. Thus the B/I ratio in periaortic BAT may be an important determinant of aortic stiffness in mice (n = 9–10/group).
DISCUSSION
The most salient finding from the current investigation is that, in discordance with our hypothesis, a significant reduction of BAT mass following iBATx did not impair systemic glucose metabolism in lean mice housed at 25°C. Similarly, in obese mice, iBATx was inconsequential to metabolic function. However, despite the lack of metabolic impairments, iBATx caused aortic stiffening in lean (but not obese) mice, supporting the notion that iBAT may be implicated in the regulation of aortic function.
On the basis of previous work indicating that functional BAT contributes to weight maintenance and metabolic function (3, 4, 41, 52), it was logical to hypothesize that bilateral iBATx would impair glycemic control in lean mice. Furthermore, because obesity is associated with whitened and dysfunctional BAT, it was also reasoned that, following consumption of a Western diet, whitened and dysfunctional BAT would be largely unnecessary to the regulation of metabolic function. Consistent with this hypothesis, we found that iBATx did not exacerbate Western diet-induced metabolic dysfunction. However, the finding that removal of a substantial portion of functional BAT in lean mice did not alter glycemic control was in stark contrast with our hypothesis. Along these lines, iBATx did not overtly disrupt lipid homeostasis, as evidenced by minimal-to-no changes in circulating lipid levels and no alterations to hepatic triglycerides (Fig. 2, H–J). Together, these findings indicate that healthy or whitened BAT mass is dispensable for maintenance of glucose and lipid homeostasis at 25°C.
The existing view that BAT exerts metabolic protection is founded on reports that BAT was sympathetically activated via cold exposure or pharmacological intervention (4). Indeed, cold-induced BAT activation enhances glucose uptake, insulin sensitivity, and lipid metabolism in rodents and humans (3, 10, 32, 36). Yet there appears to be less support for the role of BAT in modulation of metabolic function closer to thermoneutrality. Previous work indicated that mice fed a high-fat diet and housed at 30°C were more hyperinsulinemic and hypertriglyceridemic than those housed at 22°C, whereas these responses were normalized via β-adrenergic activation (53). Furthermore, a recent study in humans demonstrated that, under thermoneutral conditions, individuals with nondetectable BAT displayed levels of resting energy expenditure, whole body glucose disposal, and insulin sensitivity similar to those of individuals with detectable BAT (10). Notably, cold exposure increased resting energy expenditure, whole body glucose uptake, and insulin sensitivity solely in subjects with detectable BAT, thus supporting the premise that BAT may function as an antidiabetic tissue, but only when activated. In agreement with this report (10), data from the present study conducted at 25°C reveal that when BAT is not environmentally stimulated by cold exposure, ~30% deficiency of BAT does not disturb metabolic function. That is, long-term loss of iBAT did not affect indexes of glycemic control (HbA1c, HOMA-IR, and glucose AUC during GTT) and produced minimal-to-no effects on lipid homeostasis (Fig. 2). Importantly, the absence of metabolic disturbances evoked by iBATx was not attributed to regrowth of iBAT or to a compensatory expansion of other BAT depots (Fig. 1, A and C).
Although we noted that iBATx caused a mild, but significant, upregulation of browning genes in periaortic BAT and sBAT of normal chow-fed mice, there were no compensatory increases in UCP-1, PGC1α, or Oxphos protein expression in these BAT depots (Figs. 5C and 6C) or in white adipose tissues (Figs. 7E and 8C). Changes in browning genes, most notably UCP-1, reflect the responsiveness or the activation state of the tissue; however, UCP-1 mRNA level may not be a valid marker of BAT recruitment (31). Specifically, BAT recruitment refers to the thermogenic capacity of the tissue when maximally stimulated, which is most accurately reflected at the molecular level by UCP-1 protein content and proteins involved in mitochondrial biogenesis (31). Thus it is unlikely that iBATx caused compensatory BAT recruitment in distal BAT depots, given that integral proteins involved in this process were unaltered in iBATx mice.
In contrast to our findings, previous studies have demonstrated that surgical excision of BAT depots in rodents induces body weight gain (43) and provokes distal BAT compensation in unresected depots (30, 35, 44), whereby tissue weight, markers of lipolysis, and mitochondrial activity were increased. This inconsistency in findings may be attributed to species-specific differences (i.e., rats vs. mice), housing temperature (cold exposure vs. 25°C), study duration, or relative amount of BAT excised (multiple BAT depots vs. iBAT only). Furthermore, our data suggest that the compensatory increase in body weight and adiposity reported by others (35, 43) may be time course-dependent (Fig. 1B), such that BAT functional capacity regresses with prolonged obesity.
Interestingly, despite the lack of metabolic disturbances caused by iBATx, we observed that iBATx mice developed an increase in aortic stiffness, as assessed in vivo by pulse wave velocity, when fed normal chow but not the Western diet. On closer examination of the data, we noted that aortic stiffening occurred only in the setting where periaortic BAT displayed an imbalance between browning and inflammatory gene expression (i.e., induction of browning genes without concomitant inflammation). To better understand this relationship between the phenotype of periaortic BAT and aortic stiffness, we incorporated existing (previously unpublished) data from a cohort of db−/db− male mice that exhibited whitened and inflamed periaortic BAT, along with increased aortic stiffness, relative to wild-type controls. The observation that an imbalance between browning and inflammation in periaortic BAT appears to be a determinant of aortic stiffness held true when data from db−/db− mice were integrated. That is, as illustrated in Fig. 11, the composite browning-to-inflammatory ratio in periaortic BAT related to aortic stiffness in a U-shape-dependent manner, where both lower and higher browning-to-inflammatory ratios in periaortic BAT were associated with increased aortic stiffness. The finding that aortic stiffness did not associate with the browning-to-inflammatory ratio in other remote adipose tissue depots (e.g., epididymal adipose tissue; Fig. 11) further supports the idea that periaortic BAT, likely because of its privileged anatomic location and unique phenotypic makeup, may be a specific modulator of aortic stiffness. However, further research is necessary to corroborate this new hypothesis. Our findings in the aorta support the recently advocated notion that enhanced BAT capacity is not always favorable (1).
After iBAT lipectomy, the loss of iBAT-derived factors secreted into the systemic circulation, termed “batokines” [see extensive reviews (46, 47)], such as FGF-21 and neuregulin 4, may also contribute to alterations in aortic stiffness. Indeed, it has been reported that iBAT-derived factors (i.e., hydrogen peroxide) exert anticontractile effects on mesenteric arteries, blunting the vasoconstrictor actions of norepinephrine and phenylephrine (16). Thus, in the present study, it is possible that iBATx and associated loss of a significant source of batokines may have played a role in promoting aortic stiffness in normal chow-fed mice; however, more work is needed to identify the BAT-derived factors that exert vascular effects.
Additionally, while previous research implicates UCP-1 as protective against metabolic dysfunction (52), UCP-1 has paradoxically been shown to adversely affect cardiovascular tissues. For example, recent work from our group demonstrates that UCP-1-deficient mice have enhanced vasomotor function, despite impaired metabolic health (51). Reciprocally, vascular overexpression of UCP-1 causes atherosclerosis and hypertension (5). Together, our current finding that UCP-1 protein content was selectively increased in the aorta of iBATx mice that exhibited increased aortic stiffening is congruent with the aforementioned studies indicating that respiratory uncoupling may contribute to a dysfunctional vascular phenotype. Our finding that male mice appeared to be protected against Western diet-induced aortic stiffness is supported by previous reports indicating that men are less susceptible than women to metabolic dysfunction-induced aortic stiffening (11, 22, 50). Studies are needed to establish the mechanisms of this sexual dimorphism.
Some considerations should be acknowledged regarding the study design and data interpretation. First, we recognize that, compared with colder ambient temperatures, sympathetic outflow to BAT is relatively low under our experimental housing conditions at 25°C. The present study was intentionally designed to evaluate the role of BAT in conditions that are more human-relevant (i.e., minimal thermal stress) and, thus, where BAT is not largely activated (17). Implementing housing conditions identical to those used here, we previously reported that UCP-1 knockout mice displayed glycemic dysregulation (51) that was exacerbated with a Western diet (52). On the basis of this previous work (51, 52), we posited that removal of a major source of UCP-1-expressing tissue (i.e., iBAT) would evoke metabolic dysfunction, even when mice were kept in nonthermal stress conditions. However, iBATx under such conditions did not cause metabolic dysfunction. These results might have been positive if more BAT had been resected to more closely simulate global UCP-1 knockout, but technically this was not feasible. Nevertheless, we did observe that ~30% BAT removal was associated with increased intramural aortic UCP-1 and aortic stiffness in lean mice. Second, while we reasoned that loss of whitened and dysfunctional BAT would be mostly inconsequential compared with removal of healthy BAT in lean mice, it is possible that functional quantities of BAT persist in Western diet-induced whitened BAT depots, limiting metabolic dysfunction with obesity. That is, we acknowledge that even a little bit of a “good thing” may be better than none at all.
Perspectives and Significance
In summary, our findings demonstrate that substantial loss (i.e., ~30% reduction) of BAT mass via iBATx does not disrupt metabolic function in mice under more human-relevant, minimal-thermal-stressed conditions (25°C), indicating that activation of BAT may be required for its participation in metabolic protection. Notwithstanding the lack of metabolic impairments, aortic stiffness was increased with iBATx in lean mice that exhibited a “healthy” BAT profile; on the contrary, Western diet-fed mice that exhibited an “unhealthy” BAT profile were not adversely affected by iBATx. Thus, because aortic stiffening was only manifested with loss of functional iBAT, findings from this investigation support the existence of an interplay between iBAT and aortic stiffness; however, the mechanisms by which aortic function is regulated by iBAT require further investigation.
GRANTS
This work was supported in part by grants from the Cardiometabolic Disease Research Foundation (to J. Padilla), National Heart, Lung, and Blood Institute Grant K01 HL-125503 (to J. Padilla), and American Egg Board Grant 00050021 (to N. C. Winn).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Z.I.G., M.L.G., S.A.H., H.S.S., V.J.V.-P., and J.P. conceived and designed research; Z.I.G., N.C.W., M.L.G., M.L.W., J.R.B., and S.A.H. performed experiments; Z.I.G., N.C.W., and M.L.W. analyzed data; Z.I.G., N.C.W., V.J.V.-P., and J.P. interpreted results of experiments; Z.I.G. prepared figures; Z.I.G. and N.C.W. drafted manuscript; Z.I.G., N.C.W., M.L.G., S.A.H., H.S.S., V.J.V.-P., and J.P. edited and revised manuscript; Z.I.G., N.C.W., M.L.G., M.L.W., J.R.B., S.A.H., H.S.S., V.J.V.-P., and J.P. approved final version of manuscript.
REFERENCES
- 1.Abdullahi A, Jeschke MG. White adipose tissue browning: a double-edged sword. Trends Endocrinol Metab 27: 542–552, 2016. doi: 10.1016/j.tem.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bal NC, Maurya SK, Sopariwala DH, Sahoo SK, Gupta SC, Shaikh SA, Pant M, Rowland LA, Bombardier E, Goonasekera SA, Tupling AR, Molkentin JD, Periasamy M. Sarcolipin is a newly identified regulator of muscle-based thermogenesis in mammals. Nat Med 18: 1575–1579, 2012. doi: 10.1038/nm.2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartelt A, Bruns OT, Reimer R, Hohenberg H, Ittrich H, Peldschus K, Kaul MG, Tromsdorf UI, Weller H, Waurisch C, Eychmüller A, Gordts PL, Rinninger F, Bruegelmann K, Freund B, Nielsen P, Merkel M, Heeren J. Brown adipose tissue activity controls triglyceride clearance. Nat Med 17: 200–205, 2011. doi: 10.1038/nm.2297. [DOI] [PubMed] [Google Scholar]
- 4.Berbée JF, Boon MR, Khedoe PP, Bartelt A, Schlein C, Worthmann A, Kooijman S, Hoeke G, Mol IM, John C, Jung C, Vazirpanah N, Brouwers LP, Gordts PL, Esko JD, Hiemstra PS, Havekes LM, Scheja L, Heeren J, Rensen PC. Brown fat activation reduces hypercholesterolaemia and protects from atherosclerosis development. Nat Commun 6: 6356, 2015. doi: 10.1038/ncomms7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernal-Mizrachi C, Gates AC, Weng S, Imamura T, Knutsen RH, DeSantis P, Coleman T, Townsend RR, Muglia LJ, Semenkovich CF. Vascular respiratory uncoupling increases blood pressure and atherosclerosis. Nature 435: 502–506, 2005. doi: 10.1038/nature03527. [DOI] [PubMed] [Google Scholar]
- 6.Bostick B, Aroor AR, Habibi J, Durante W, Ma L, DeMarco VG, Garro M, Hayden MR, Booth FW, Sowers JR. Daily exercise prevents diastolic dysfunction and oxidative stress in a female mouse model of Western diet induced obesity by maintaining cardiac heme oxygenase-1 levels. Metabolism 66: 14–22, 2017. doi: 10.1016/j.metabol.2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Britton KA, Wang N, Palmisano J, Corsini E, Schlett CL, Hoffmann U, Larson MG, Vasan RS, Vita JA, Mitchell GF, Benjamin EJ, Hamburg NM, Fox CS. Thoracic periaortic and visceral adipose tissue and their cross-sectional associations with measures of vascular function. Obesity (Silver Spring) 21: 1496–1503, 2013. doi: 10.1002/oby.20166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev 84: 277–359, 2004. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 9.Chen JY, Tsai PJ, Tai HC, Tsai RL, Chang YT, Wang MC, Chiou YW, Yeh ML, Tang MJ, Lam CF, Shiesh SC, Li YH, Tsai WC, Chou CH, Lin LJ, Wu HL, Tsai YS. Increased aortic stiffness and attenuated lysyl oxidase activity in obesity. Arterioscler Thromb Vasc Biol 33: 839–846, 2013. doi: 10.1161/ATVBAHA.112.300036. [DOI] [PubMed] [Google Scholar]
- 10.Chondronikola M, Volpi E, Børsheim E, Porter C, Annamalai P, Enerbäck S, Lidell ME, Saraf MK, Labbe SM, Hurren NM, Yfanti C, Chao T, Andersen CR, Cesani F, Hawkins H, Sidossis LS. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes 63: 4089–4099, 2014. doi: 10.2337/db14-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Angelis L, Millasseau SC, Smith A, Viberti G, Jones RH, Ritter JM, Chowienczyk PJ. Sex differences in age-related stiffening of the aorta in subjects with type 2 diabetes. Hypertension 44: 67–71, 2004. doi: 10.1161/01.HYP.0000130482.81883.fd. [DOI] [PubMed] [Google Scholar]
- 12.de Jong JM, Larsson O, Cannon B, Nedergaard J. A stringent validation of mouse adipose tissue identity markers. Am J Physiol Endocrinol Metab 308: E1085–E1105, 2015. doi: 10.1152/ajpendo.00023.2015. [DOI] [PubMed] [Google Scholar]
- 13.De Lorenzo F, Mukherjee M, Kadziola Z, Sherwood R, Kakkar VV. Central cooling effects in patients with hypercholesterolaemia. Clin Sci (Lond) 95: 213–217, 1998. doi: 10.1042/cs0950213. [DOI] [PubMed] [Google Scholar]
- 14.DeMarco VG, Habibi J, Jia G, Aroor AR, Ramirez-Perez FI, Martinez-Lemus LA, Bender SB, Garro M, Hayden MR, Sun Z, Meininger GA, Manrique C, Whaley-Connell A, Sowers JR. Low-dose mineralocorticoid receptor blockade prevents Western diet-induced arterial stiffening in female mice. Hypertension 66: 99–107, 2015. doi: 10.1161/HYPERTENSIONAHA.115.05674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fitzgibbons TP, Kogan S, Aouadi M, Hendricks GM, Straubhaar J, Czech MP. Similarity of mouse perivascular and brown adipose tissues and their resistance to diet-induced inflammation. Am J Physiol Heart Circ Physiol 301: H1425–H1437, 2011. doi: 10.1152/ajpheart.00376.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friederich-Persson M, Nguyen Dinh Cat A, Persson P, Montezano AC, Touyz RM. Brown adipose tissue regulates small artery function through NADPH oxidase 4-derived hydrogen peroxide and redox-sensitive protein kinase G-1α. Arterioscler Thromb Vasc Biol 37: 455–465, 2017. doi: 10.1161/ATVBAHA.116.308659. [DOI] [PubMed] [Google Scholar]
- 17.Ganeshan K, Chawla A. Warming the mouse to model human diseases. Nat Rev Endocrinol 13: 458–465, 2017. doi: 10.1038/nrendo.2017.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao M, Ma Y, Liu D. High-fat diet-induced adiposity, adipose inflammation, hepatic steatosis and hyperinsulinemia in outbred CD-1 mice. PLoS One 10: e0119784, 2015. doi: 10.1371/journal.pone.0119784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaskill BN, Gordon CJ, Pajor EA, Lucas JR, Davis JK, Garner JP. Heat or insulation: behavioral titration of mouse preference for warmth or access to a nest. PLoS One 7: e32799, 2012. doi: 10.1371/journal.pone.0032799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaskill BN, Gordon CJ, Pajor EA, Lucas JR, Davis JK, Garner JP. Impact of nesting material on mouse body temperature and physiology. Physiol Behav 110-111: 87–95, 2013. doi: 10.1016/j.physbeh.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 21.Gordon CJ. Thermal physiology of laboratory mice: defining thermoneutrality. J Therm Biol 37: 654–685, 2012. doi: 10.1016/j.jtherbio.2012.08.004. [DOI] [Google Scholar]
- 22.Gottsäter M, Länne T, Nilsson PM. Predictive markers of abdominal aortic stiffness measured by echo-tracking in subjects with varying insulin sensitivity. J Hum Hypertens 28: 456–460, 2014. doi: 10.1038/jhh.2013.126. [DOI] [PubMed] [Google Scholar]
- 23.Jia R, Luo XQ, Wang G, Lin CX, Qiao H, Wang N, Yao T, Barclay JL, Whitehead JP, Luo X, Yan JQ. Characterization of cold-induced remodelling reveals depot-specific differences across and within brown and white adipose tissues in mice. Acta Physiol (Oxf) 217: 311–324, 2016. doi: 10.1111/apha.12688. [DOI] [PubMed] [Google Scholar]
- 24.Laye MJ, Rector RS, Warner SO, Naples SP, Perretta AL, Uptergrove GM, Laughlin MH, Thyfault JP, Booth FW, Ibdah JA. Changes in visceral adipose tissue mitochondrial content with type 2 diabetes and daily voluntary wheel running in OLETF rats. J Physiol 587: 3729–3739, 2009. doi: 10.1113/jphysiol.2009.172601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔCT) method. Methods 25: 402–408, 2001. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 26.Lomonaco R, Ortiz-Lopez C, Orsak B, Webb A, Hardies J, Darland C, Finch J, Gastaldelli A, Harrison S, Tio F, Cusi K. Effect of adipose tissue insulin resistance on metabolic parameters and liver histology in obese patients with nonalcoholic fatty liver disease. Hepatology 55: 1389–1397, 2012. doi: 10.1002/hep.25539. [DOI] [PubMed] [Google Scholar]
- 27.Matsushita M, Yoneshiro T, Aita S, Kameya T, Sugie H, Saito M. Impact of brown adipose tissue on body fatness and glucose metabolism in healthy humans. Int J Obes 38: 812–817, 2014. doi: 10.1038/ijo.2013.206. [DOI] [PubMed] [Google Scholar]
- 28.Mattace-Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA, Asmar R, Reneman RS, Hoeks AP, Breteler MM, Witteman JC. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation 113: 657–663, 2006. doi: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- 29.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412–419, 1985. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 30.Moore BJ, Inokuchi T, Stern JS, Horwitz BA. Brown adipose tissue lipectomy leads to increased fat deposition in Osborne-Mendel rats. Am J Physiol Regul Integr Comp Physiol 248: R231–R235, 1985. doi: 10.1152/ajpregu.1985.248.2.R231. [DOI] [PubMed] [Google Scholar]
- 31.Nedergaard J, Cannon B. UCP1 mRNA does not produce heat. Biochim Biophys Acta 1831: 943–949, 2013. doi: 10.1016/j.bbalip.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 32.Orava J, Nuutila P, Lidell ME, Oikonen V, Noponen T, Viljanen T, Scheinin M, Taittonen M, Niemi T, Enerbäck S, Virtanen KA. Different metabolic responses of human brown adipose tissue to activation by cold and insulin. Cell Metab 14: 272–279, 2011. doi: 10.1016/j.cmet.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 33.Padilla J, Jenkins NT, Vieira-Potter VJ, Laughlin MH. Divergent phenotype of rat thoracic and abdominal perivascular adipose tissues. Am J Physiol Regul Integr Comp Physiol 304: R543–R552, 2013. doi: 10.1152/ajpregu.00567.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosell M, Kaforou M, Frontini A, Okolo A, Chan YW, Nikolopoulou E, Millership S, Fenech ME, MacIntyre D, Turner JO, Moore JD, Blackburn E, Gullick WJ, Cinti S, Montana G, Parker MG, Christian M. Brown and white adipose tissues: intrinsic differences in gene expression and response to cold exposure in mice. Am J Physiol Endocrinol Metab 306: E945–E964, 2014. doi: 10.1152/ajpendo.00473.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rothwell NJ, Stock MJ. Surgical removal of brown fat results in rapid and complete compensation by other depots. Am J Physiol Regul Integr Comp Physiol 257: R253–R258, 1989. doi: 10.1152/ajpregu.1989.257.2.R253. [DOI] [PubMed] [Google Scholar]
- 36.Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J, Iwanaga T, Miyagawa M, Kameya T, Nakada K, Kawai Y, Tsujisaki M. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes 58: 1526–1531, 2009. doi: 10.2337/db09-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seifalian AM, Filippatos TD, Joshi J, Mikhailidis DP. Obesity and arterial compliance alterations. Curr Vasc Pharmacol 8: 155–168, 2010. doi: 10.2174/157016110790886956. [DOI] [PubMed] [Google Scholar]
- 38.Selcuk A, Bulucu F, Kalafat F, Cakar M, Demirbas S, Karaman M, Ay SA, Saglam K, Balta S, Demirkol S, Arslan E. Skinfold thickness as a predictor of arterial stiffness: obesity and fatness linked to higher stiffness measurements in hypertensive patients. Clin Exp Hypertens 35: 459–464, 2013. doi: 10.3109/10641963.2012.746357. [DOI] [PubMed] [Google Scholar]
- 39.Shimizu I, Aprahamian T, Kikuchi R, Shimizu A, Papanicolaou KN, MacLauchlan S, Maruyama S, Walsh K. Vascular rarefaction mediates whitening of brown fat in obesity. J Clin Invest 124: 2099–2112, 2014. doi: 10.1172/JCI71643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shimizu I, Walsh K. The whitening of brown fat and its implications for weight management in obesity. Curr Obes Rep 4: 224–229, 2015. doi: 10.1007/s13679-015-0157-8. [DOI] [PubMed] [Google Scholar]
- 41.Stanford KI, Middelbeek RJ, Townsend KL, An D, Nygaard EB, Hitchcox KM, Markan KR, Nakano K, Hirshman MF, Tseng YH, Goodyear LJ. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Invest 123: 215–223, 2013. doi: 10.1172/JCI62308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stanford KI, Middelbeek RJ, Townsend KL, Lee MY, Takahashi H, So K, Hitchcox KM, Markan KR, Hellbach K, Hirshman MF, Tseng YH, Goodyear LJ. A novel role for subcutaneous adipose tissue in exercise-induced improvements in glucose homeostasis. Diabetes 64: 2002–2014, 2015. doi: 10.2337/db14-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stephens DN, Nash SC, Proffitt C. Dietary obesity in adult and weanling rats following removal of interscapular brown adipose tissue. Pflugers Arch 392: 7–12, 1981. doi: 10.1007/BF00584574. [DOI] [PubMed] [Google Scholar]
- 44.Stern JS, Inokuchi T, Castonguay TW, Wickler SJ, Horwitz BA. Scapular brown fat removal enhances development of adiposity in cold-exposed obese Zucker rats. Am J Physiol Regul Integr Comp Physiol 247: R918–R926, 1984. doi: 10.1152/ajpregu.1984.247.5.R918. [DOI] [PubMed] [Google Scholar]
- 45.Thanassoulis G, Massaro JM, Corsini E, Rogers I, Schlett CL, Meigs JB, Hoffmann U, O’Donnell CJ, Fox CS. Periaortic adipose tissue and aortic dimensions in the Framingham Heart Study. J Am Heart Assoc 1: e000885, 2012. doi: 10.1161/JAHA.112.000885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Villarroya F, Cereijo R, Villarroya J, Giralt M. Brown adipose tissue as a secretory organ. Nat Rev Endocrinol 13: 26–35, 2017. doi: 10.1038/nrendo.2016.136. [DOI] [PubMed] [Google Scholar]
- 47.Villarroya J, Cereijo R, Villarroya F. An endocrine role for brown adipose tissue? Am J Physiol Endocrinol Metab 305: E567–E572, 2013. doi: 10.1152/ajpendo.00250.2013. [DOI] [PubMed] [Google Scholar]
- 48.Wainright KS, Fleming NJ, Rowles JL, Welly RJ, Zidon TM, Park YM, Gaines TL, Scroggins RJ, Anderson-Baucum EK, Hasty AH, Vieira-Potter VJ, Padilla J. Retention of sedentary obese visceral white adipose tissue phenotype with intermittent physical activity despite reduced adiposity. Am J Physiol Regul Integr Comp Physiol 309: R594–R602, 2015. doi: 10.1152/ajpregu.00042.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Waldén TB, Hansen IR, Timmons JA, Cannon B, Nedergaard J. Recruited vs. nonrecruited molecular signatures of brown, “brite,” and white adipose tissues. Am J Physiol Endocrinol Metab 302: E19–E31, 2012. doi: 10.1152/ajpendo.00249.2011. [DOI] [PubMed] [Google Scholar]
- 50.Weng C, Yuan H, Yang K, Tang X, Huang Z, Huang L, Chen W, Chen F, Chen Z, Yang P. Gender-specific association between the metabolic syndrome and arterial stiffness in 8,300 subjects. Am J Med Sci 346: 289–294, 2013. doi: 10.1097/MAJ.0b013e3182732e97. [DOI] [PubMed] [Google Scholar]
- 51.Winn NC, Grunewald ZI, Gastecki ML, Woodford ML, Welly RJ, Clookey SL, Ball JR, Gaines TL, Karasseva NG, Kanaley JA, Sacks HS, Vieira-Potter VJ, Padilla J. Deletion of UCP1 enhances ex vivo aortic vasomotor function in female but not male mice despite similar susceptibility to metabolic dysfunction. Am J Physiol Endocrinol Metab 313: E402–E412, 2017. doi: 10.1152/ajpendo.00096.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Winn NC, Vieira-Potter VJ, Gastecki ML, Welly RJ, Scroggins RJ, Zidon TM, Gaines TL, Woodford ML, Karasseva NG, Kanaley JA, Sacks HS, Padilla J. Loss of UCP1 exacerbates Western diet-induced glycemic dysregulation independent of changes in body weight in female mice. Am J Physiol Regul Integr Comp Physiol 312: R74–R84, 2017. doi: 10.1152/ajpregu.00425.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xiao C, Goldgof M, Gavrilova O, Reitman ML. Anti-obesity and metabolic efficacy of the β3-adrenergic agonist, CL316243, in mice at thermoneutrality compared to 22°C. Obesity (Silver Spring) 23: 1450–1459, 2015. doi: 10.1002/oby.21124. [DOI] [PMC free article] [PubMed] [Google Scholar]