Abstract
The newly described hypothalamic peptide, phoenixin, is produced in the hypothalamus and adenohypophysis, where it acts to control reproductive hormone secretion. Both phoenixin and its receptor GPR173 are expressed in the hypothalamic supraoptic (SON) and paraventricular (PVN) nuclei, suggesting additional, nonreproductive effects of the peptide to control vasopressin (AVP) or oxytocin (OT) secretion. Hypothalamo-neurohypophysial explants released AVP but not OT in response to phoenixin. Intracerebroventricular administration of phoenixin into conscious, unrestrained male and female rats significantly increased circulating AVP, but not OT, levels in plasma, and it increased immediate early gene expression in the supraoptic nuclei of male rats. Bath application of phoenixin in hypothalamic slice preparations resulted in depolarization of PVN neurons, indicating a direct, neural action of phoenixin in the hypothalamus. Our results suggest that the newly described, hypothalamic peptide phoenixin, in addition to its effects on hypothalamic and pituitary mechanisms controlling reproduction, may contribute to the physiological mechanisms regulating fluid and electrolyte homeostasis.
Keywords: electrophysiology, hypothalamus, oxytocin, phoenixin, radioimmunoassay, vasopressin
INTRODUCTION
A bioinformatics approach allowed us to identify a previously unrecognized gene sequence, conserved across species, which encoded the novel peptide hormone phoenixin (11). The hypothalamus contained the most abundant phoenixin immunoreactivity of all tissues examined, and specific binding of labeled phoenixin peptide was highest in the hypothalamus, with lesser amounts in ovary and anterior pituitary gland. We demonstrated that phoenixin acts in both the hypothalamus and pituitary gland to augment luteinizing hormone (LH) release, an effect that could be blocked by compromise of the phoenixin receptor GPR173 in the hypothalamus or pituitary gland (8, 11). During our characterization of the hypothalamic sites that expressed phoenixin and GPR173, we observed strong signal for both peptide and receptor in the supraoptic nucleus (SON) and paraventricular nucleus (PVN) (8). This led to our hypothesis that phoenixin acts in the hypothalamus to stimulate the release of vasopressin and/or oxytocin. Studies presented here demonstrate the action of phoenixin to activate magnocellular neurons in the hypothalamic paraventricular nucleus and to stimulate vasopressin secretion in vitro and in vivo.
METHODS
Animals.
Male and female Sprague-Dawley rats (225–275 g, Envigo, Indianapolis, IN) were housed individually (lights on 0600, lights off 1800, 23–25°C) with free access to laboratory chow and water at the St. Louis University site. At the Queen’s University site, male Sprague-Dawley rats (Charles River, Quebec, Canada) aged postnatal day 21–28 (~50–100 g), housed in groups of three under similar conditions, were used. All procedures were approved by the Saint Louis University or Queen’s University Animal Care and Use Committees.
Peptides.
Rat phoenixin-20 amide, ANG II, and both vasopressin (AVP) and oxytocin (OT), (for iodination and construction of the radioimmunoassay standard curves) were purchased from Phoenix Pharmaceuticals (Burlingame, CA). Solutions were made fresh daily and discarded without reuse following each experiment.
In vitro hormone release.
Rats were killed by rapid decapitation and a hypothalamo-neurohypophysial (HNS) explant containing the SON and projections to the posterior lobe of the pituitary dissected, as previously described (2, 7, 10). Explants were immersed in 2 ml of DMEM containing 0.1% BSA (both Sigma-Aldrich, St. Louis, MO) and 1% penicillin-streptomycin (Invitrogen, Carlsbad, CA) and were incubated at 37°C in 5% CO2. The medium was changed every hour for 2 h, and then four consecutive, 20-min preincubations (1 ml) were conducted. Media from the 20-min preincubations were saved. Then 100 nM of phoenixin (rat phoenixin-20 amide; Phoenix Pharmaceuticals, Burlingame, CA) in 1 ml medium was added to the explant, and this incubation was terminated by media removal after 20 min. Two more incubation periods, with medium alone, preceded incubation in 1 ml of medium containing 60 mM KCl, and then a final control medium incubation was conducted. Media were stored frozen until inclusion in the AVP and OT radioimmunoassays. Data are presented as picograms of hormone released during each 20-min incubation period.
Electrophysiology.
Unanesthetized rats were decapitated, and their brains were quickly removed and placed in ice-cold (0–4°C) slicing solution consisting of the following (in mM): 87 NaCl, 2.5 KCl, 25 NaHCO3, 0.5 CaCl2, 7 MgCl2, 1.25 NaH2PO4, 25 glucose, and 75 sucrose, bubbled with 95% O2-5% CO2. A tissue block containing the hypothalamus was obtained, and 300-μm coronal slices containing the PVN were cut using a vibratome (VT1000 S; Leica, Nussloch, Germany). Slices were then incubated at 32°C for a minimum of 1 h in a holding chamber containing artificial cerebrospinal fluid (aCSF) consisting of the following (in mM): 124 NaCl, 2.5 KCl, 20 NaHCO3, 2 CaCl2, 1.3 MgSO4, 1.24 KH2PO4, and 3 glucose, bubbled with 95% O2-5% CO2.
Brain slices were transferred and secured to a recording chamber continuously perfused at a rate of 1.5–2 ml/min with carbogenated (95% O2-5% CO2) aCSF heated to 32°C. PVN neurons were visualized at ×40 magnification using infrared differential interference contrast optics on an upright microscope (Scientifica, East Sussex, UK). Borosilicate glass pipettes (World Precision Instruments, Sarasota, FL) were pulled on a micropipette puller (P-97; Sutter Instrument, Novato, CA) to resistances between 2.5 and 5 MΩ when filled with the intracellular solution consisting of the following (in mM): 125 potassium gluconate, 10 KCl, 2 MgCl2, 0.1 CaCl2, 5.5 EGTA, 10 HEPES, 2 NaATP, adjusted to pH 7.2 with KOH. After a high-resistance seal (minimum 1 GΩ) was established, whole cell access was obtained by applying brief suction to rupture the cell membrane. All salts used to prepare the slicing solution, aCSF, and intracellular recording solution were obtained from Sigma Pharmaceuticals (Oakville, Ontario, Canada). Current-clamp recordings were obtained using a MultiClamp 700B amplifier (Molecular Devices, Sunnyvale, CA), filtered at 2.4 kHz and sampled at 10 kHz using a Micro1401 mk II interface and Spike2 software (Cambridge Electronic Design, Cambridge, UK) for offline analysis. Magnocellular (MNC) PVN neurons were characterized on the basis of their electrophysiological fingerprint in response to a standard current pulse protocol (9). Neurons in which a large A-type K+ current was present were classified as MNC. To determine the effect of phoenixin on the excitability of MNC PVN neurons, rat phoenixin-20 amide was bath-applied to cells for 120 s. A neuron was classified as being responsive to phoenixin if the change in membrane potential in any single 100-s period after phoenixin administration was at least twice the amplitude of the SD of the baseline membrane potential obtained during the 100-s period immediately before peptide application. A calculated liquid junction potential of 15 mV has been subtracted from all reported membrane potentials.
In vivo experiments.
Right lateral cerebroventricle cannulas were implanted as previously described (5, 12, 13) under ketamine (70 mg/kg; Ketaset, Fort Dodge Animal Health, Fort Dodge, IA) and xylazine (9 mg/kg; TranquiVed, Vedco, St. Joseph, MO) anesthesia. Buprenorphine (1.0 mg/kg; Reckitt & Coleman, Richmond, VA) analgesia was administered subcutaneously during surgery. Animals were allowed to recover to presurgery body weight before use (minimally 5 days). Patency and placement of the cannula were tested by a dipsogenic dose of ANG II (25 pmol in 2 μl sterile 0.9% NaCl) 2–3 days before experimentation. On the day of the experiment, rats were moved to a quiet room and allowed to habituate for at least 2 h. Phoenixin-20 amide (1.0 or 3.0 nmol in males; 3.0 nmol in females) dissolved in 2.0 μl of sterile, isotonic saline (0.9% NaCl), or saline vehicle alone was injected via the indwelling cannula in conscious, unrestrained rats. Experiments were conducted between 1000 and 1300. Males were killed by decapitation 10, 20, or 30 min later, females at 20 min, and trunk blood was collected into heparinized tubes for plasma collection by centrifugation (3,000 g, 6°C, 30 min).
In a second protocol, a separate cohort of male rats bearing indwelling lateral ventricle cannulas received intracerebroventricular administration of siRNA targeting either Gpr173 or the control, eGFP, once per day for two consecutive days. Details of the siRNA constructs have been previously published by us (8). On the third day, rats were moved to a quiet room, and 2 h later, 2 μl of saline or saline containing 3.0 nmol of phoenixin-20 amide were injected intracerebroventricularly, as described above. Twenty minutes later, rats were killed, and trunk blood was collected, as described above. Hypothalami were collected and extracted for analysis of Gpr173 mRNA levels by qPCR, as described in our earlier work (8).
Radioimmunoassays.
Vasopressin and oxytocin levels in incubation medium from the HNS explant experiments were determined by radioimmunoassays (RIAs) without extraction. Vasopressin levels in plasma collected from trunk blood were determined, as previously detailed (1, 13) following acidification (0.5 ml 1 N HCl/1.0 ml plasma) and centrifugation (4 min, 6,000 g, 6°C). Supernatants were applied to a C-18 column (500 mg, Sep Pak; Thermo Fisher, Rockville, MD) that had been activated by the elution of 4 ml of methanol and washed with 10 ml of distilled water. Once the supernatant had entered the gel bed, the columns were washed (gravity flow) with 10 ml 0.4% acetic acid, followed by elution of the peptide with 4 ml acetonitrile-4% acetic acid (75/25 ratio), eluates were dried in a rotary evaporator (Speed Vac; Savant Instruments, Hyderabad, India), and then they were reconstituted in RIA buffer (0.1% gelatin in 0.05 M PBS, pH 7.0). Internal purification controls were included by extraction of 1.0 ml plasma from a male donor pool alone or containing 5, 20, or 50 pg synthetic AVP. Recovery efficiency was >90%. Because not all the extracted samples were included in the same assay, the plasma pools and recoveries served as between-assay controls. Interassay and intra-assay coefficients of variability were computed for each assay and were less than 8%. Synthetic AVP was iodinated using the chloramine-T method (1). Details of the generation and characterization of the polyclonal antivasopressin antibody (code: 728-4) have been published previously (1).
Oxytocin levels were determined in plasma samples following precipitation of large plasma proteins by the addition of 1 ml of ice-cold methanol, centrifugation (30 min, 6°C, 3,000 g), evaporation of the supernatants (Speed Vac, as above), and reconstitution in assay buffer (0.1% gelatin in 0.05 M PBS, pH 7.0). OT was iodinated using the chloramine-T method, as previously described (3, 4). Plasma pools alone or spiked with 25, 50, or 100 pg OT served as controls. Details of generation and characterization of our polyclonal anti-OT antibody (code: 326-6) have been published previously (4). As with the AVP RIA, plasma pools were included in all RIAs, and interassays and intra-assays of variability were calculated for each RIA (in all cases <10%).
Immediate early gene detection.
Phoenixin-20 amide (3.0 nmol) or saline vehicle was injected via the indwelling cerebroventricular cannulas, as detailed above in male rats. Two hours later, rats were deeply anesthetized with pentobarbital sodium (Sleepaway, 100 mg/kg ip; Henry Schein, Chicago, IL) and perfused (transcardiac) with 0.9% saline followed by 4% paraformaldehyde, brains were removed and fixed for 4 h in 4% PFA solution in PBS, pH 7.4, and stored at 4°C in 0.1 M PBS containing 20% (wt/vol) sucrose. Four sets of coronal sections (40 µm) of the forebrain were sectioned on a cryostat (Leica, CM1950). One of the sets of the brain sections was preincubated for 15 min in a blocking solution composed of 10% (vol/vol) normal donkey serum (NDS, Jackson Immunoresearch, West Grove, PA) and 0.3% (vol/vol) Triton X-100 (Fisher Scientific, Hampton, NH) in 0.1 M PBS followed by rinses in PBS (3 × 10 min). Subsequently, sections were incubated with rabbit anti-c-Fos (1:2,000; Santa Cruz Biotechnology, Santa Cruz, CA), in PBS containing 1% (vol/vol) NDS and 0.3% Triton-X-100 for 24 h at 4°C, to label c-Fos. Sections were rinsed in PBS between the steps. After this, sections were incubated for 2 h in donkey anti-rabbit Alexa Fluor 594 (1:300, Jackson Immunoresearch) and rinsed with PBS containing DAPI (1:10,000; Thermo Fisher) (3 × 5 min). Then, the sections were mounted onto slides in 0.5% gelatin and allowed to air-dry for 10–15 min before being coverslipped using Prolong Gold antifade reagent (Life Technologies, Carlsbad, CA). The sections were examined in a fluorescence microscope with the appropriate filter.
Statistics.
All radioimmunoassay data were expressed in terms of picograms of peptide per milliliter of plasma or incubation medium and reported as means ± SE. Data were analyzed for significant differences (P < 0.05) by Student’s t-test (HNS explant data, preincubation vs. hormone release during phoenixin exposure; hormone levels in siRNA-pretreated animals following phoenixin administration) or ANOVA (plasma levels, time, and treatment effects) with Scheffé’s post hoc testing. Baseline membrane potential in responsive vs. nonresponsive neurons exposed to phoenixin in slice preparations was analyzed by Student’s t-test.
RESULTS
Phoenixin stimulated AVP but not OT release from HNS explants.
After preincubation for 180 min, AVP and OT releases into the incubation medium were stable and detectable. Exposure to 100 nM phoenixin-20 amide resulted in an ~2.5-fold increase in AVP release (P < 0.05 vs. preincubation levels), but no change in OT release (Fig. 1). At the end of the incubation, hormone release in response to depolarizing concentration of KCl was verified. As demonstrated in Fig. 1, the magnitude of AVP release to KCl (5.6-fold) exceeded that observed in response to phoenixin (2.5-fold). Furthermore, the failure of phoenixin to stimulate OT release was not due to unresponsiveness of the explants, as KCl stimulated robust OT release at the end of the incubation period (56-fold).
Fig. 1.
Vasopressin (AVP) and oxytocin (OT) release from hypothalamo-neurohypophysial explants in response to 100 nM phoenixin-20 amide, expressed as picogram of peptides released per 20-min incubation period (means ± SE; group sizes: n = 9). *P < 0.05 vs. preincubation period, paired Student’s t-test. ***P < 0.001 vs. preincubation.
Phoenixin depolarized magnocellular neurons.
Whole cell current-clamp recordings were obtained from 16 electrophysiologically characterized MNC PVN neurons (9). These cells exhibited a mean resting membrane potential of −66.6 ± 0.9 mV, a mean input resistance of 918 ± 38 MΩ, and action potential amplitudes greater than 60 mV. Bath application of 10 nM phoenixin-20 amide elicited responses in 69% of cells (n = 11/16), all of which were depolarizations (7.1 ± 0.9 mV; n = 11; Fig. 2). These depolarizations were often accompanied by an increase in the firing frequency of action potentials. The phoenixin-mediated changes in membrane potential and action potential discharge were completely reversible in the majority of the cells following washout of peptide. These responses returned to baseline within a 30 min of recording. The remaining 31% (n = 5/16) of tested MNC PVN neurons did not respond to phoenixin application. There was no correlation between the baseline membrane potential of neurons and a response to phoenixin (responding cells, −66.3 ± 1.3 mV vs. nonresponding cells, −67.4 ± 1.0 mV, paired Student's t-test, P = 0.33).
Fig. 2.
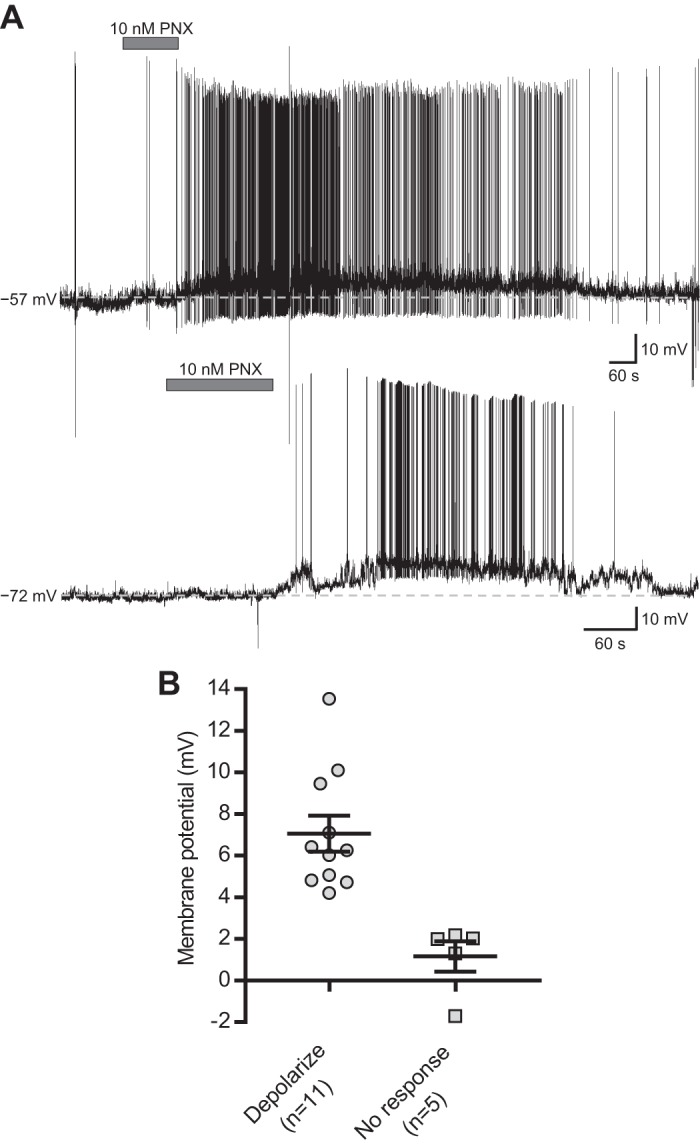
Phoenixin (PNX) depolarizes magnocellular (MNC) paraventricular nucleus (PVN) neurons. A: representative current-clamp recordings from two MNC PVN neurons in slice preparation showing that bath application of 10 nM PNX (horizontal gray bar) caused a depolarization of the membrane potential. Both neurons returned to the baseline membrane potential (dashed line) following washout of PNX. B: range of responses of all MNC PVN neurons (n = 16) elicited by bath application of 10 nM PNX. The horizontal black bars represent the mean change in membrane potential ± SE, while each individual point represents the response of a single MNC neuron. Mean depolarization, 7.1 ± 0.9 mV.
Phoenixin administration resulted in early gene activation.
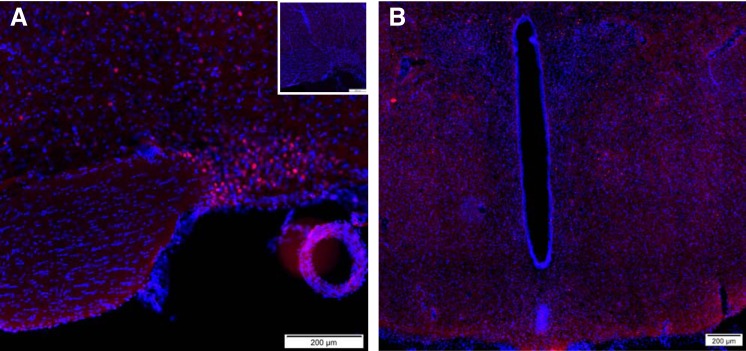
Early gene activation in cells of the supraoptic nucleus was observed in rats injected with 3.0 nmol of phoenixin (Fig. 3A), but not saline vehicle (Fig. 3A, inset). At this time point, only scattered cells positive for c-Fos immunoreactivity were observed in the paraventricular nucleus, and they did not localize to any one subnuclear region (Fig. 3B). Additional c-Fos-positive cells were observed in hypothalamic areas more related to reproductive function, including anterior hypothalamic area, the arcuate nucleus, and the ventromedial hypothalamic nuclei. Scattered immunoreactive cells also were observed in the cortical nucleus of the amygdala and the paraventricular thalamic nucleus (not shown).
Fig. 3.
Lateral ventricle administration of 3.0 nmol of phoenixin-20 amide, but not saline vehicle, resulted in early gene activation, as mirrored by c-Fos immunoreactivity, in cells of the supraoptic nucleus. A: supraoptic nucleus. A, inset: lack of staining in saline-injected control. B: paraventricular nucleus.
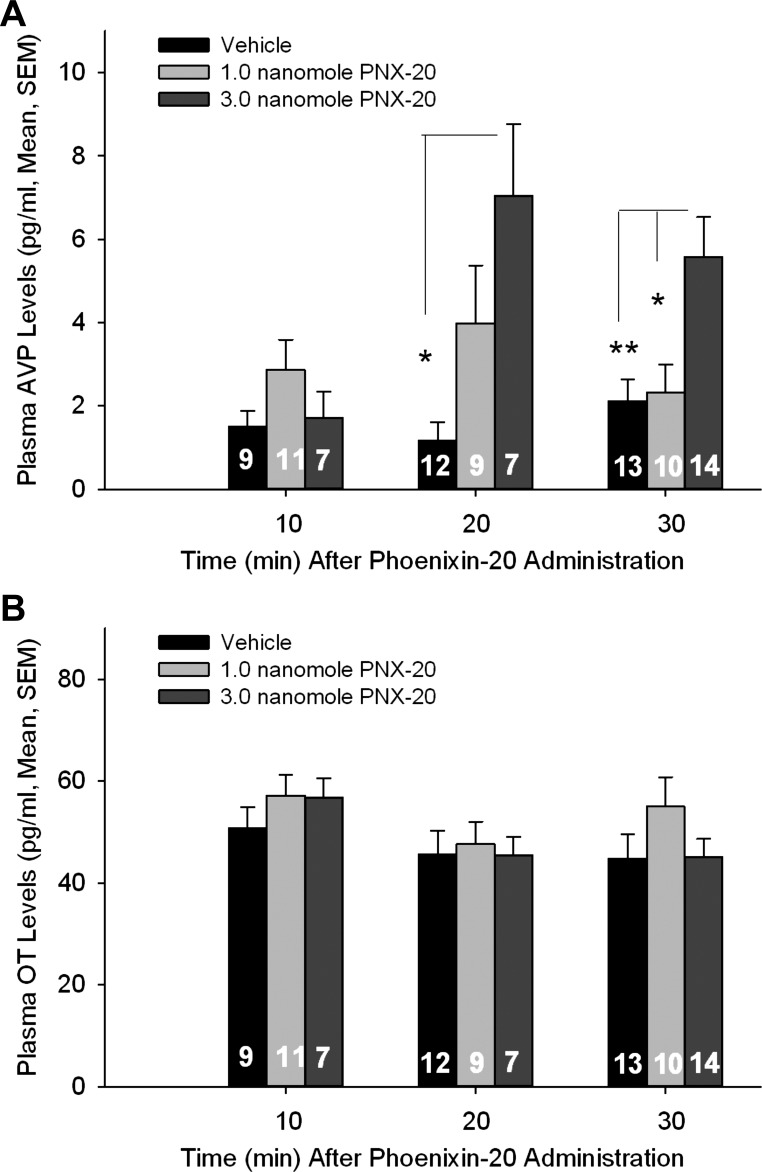
Phoenixin stimulated AVP but not OT release in vivo.
No significant differences in AVP or OT levels were detected as a function of time after vehicle injection. Similarly, no time or dose effect on plasma OT levels was observed following phoenixin administration. On the other hand, both time- and dose-related effects of phoenixin administration on plasma AVP levels were observed (Fig. 4, A and B). In male rats, plasma AVP levels were elevated compared with those present in vehicle-administered control following the 1.0-nmol phoenixin dose at 20 min postinjection; however, this difference did not attain significance. Plasma AVP levels were significantly elevated in male animals receiving the 3.0 nmol phoenixin dose at both 20 and 30 min postinjection, and those levels differed significantly from levels present in the 1.0-nmol phoenixin-administered group at 30 min. In male rats receiving the 3.0-nmol dose of phoenixin, levels of AVP rose across time, such that levels present at 20 and 30 min postinjection differed significantly from those present at 10 min postinjection. In female animals, as in males, AVP but not OT levels, were elevated significantly 20 min following administration of intracerebroventricular 3.0-nmol phoenixin (AVP pg/ml: control 0.8 ± 0.3, n = 6 and phoenixin 3.4 ± 1.0, n = 5; P < 0.025; OT pg/ml: control 42.0 ± 4.3 and phoenixin 40.6 ± 4.5; P = 0.34).
Fig. 4.
Time- and dose-dependent effect of phoenixin-20 amide on plasma vasopressin (AVP; A) and oxytocin (OT; B) levels in conscious male rats. Hormone levels are expressed as picograms of peptide per milliliter plasma (means ± SE; group sizes are indicated within the bars). *P < 0.05, **P < 0.01 vs. 3.0 nmol of phoenixin-20 dose, ANOVA (time and treatment) with Scheffé’s multiple-comparison testing.
When compared with eGFP, mRNA levels in rats pretreated with siRNA against Gpr173, hypothalamic Gpr173 mRNA levels in animals targeted for compromise of the receptor message displayed on average a 53% decrease in Gpr173 mRNA. Phoenixin administration resulted in elevated plasma AVP levels in eGFP siRNA pretreated, but not Gpr173 siRNA pretreated, animals (Gpr173 siRNA-pretreated rats: 0.9 ± 0.2 pg/ml, n = 7; eGFP siRNA pretreated rats: 3.8 ± 1.9 pg/ml, n = 8; P < 0.05). Plasma oxytocin levels were similar to those in control in the previous protocol and did not differ between pretreatment groups (Gpr173 siRNA-pretreated rats: 23.1 ± 2.8 pg/ml, n = 7; eGFP siRNA-pretreated rats: 22.3 ± 2.1 pg/ml, n = 8).
DISCUSSION
Phoenixin immunoreactivity was identified in numerous hypothalamic sites (11), in particular, those related to reproductive function, including the medial preoptic area, the anterior hypothalamic area, and the arcuate nucleus, as well as those areas related to the control of fluid and electrolyte homeostasis (e.g., the SON and PVN). At first, our focus was on the potential reproductive actions of phoenixin because of the high levels present in the neuroendocrine hypothalamus and the peptide’s ability to augment gonadotropin-releasing hormone (GnRH)-stimulated luteinizing hormone (LH) release (11). Phoenixin treatment also augmented GnRH upregulation of its own receptor, as well as levels of LH β-chain mRNA in the pituitary gland, further suggesting a physiologically relevant action on reproductive hormone secretion. Indeed, compromise of phoenixin production in hypothalamus interrupted the rat estrous cycle, suggesting a physiologically relevant action of the peptide to control reproductive function. It was not until we identified a potential receptor for phoenixin to be the previously orphaned, G protein-coupled receptor Gpr173 that we were able to determine that phoenixin’s actions related to gonadotropin secretion were due to both a hypothalamic and an anterior pituitary site of action (8).
While characterizing the importance of the hypothalamic action of Gpr173, we observed strong hybridization signal for Gpr173 mRNA in the PVN and SON (8), leading us to hypothesize that endogenous phoenixin, produced in the same nuclei, might control vasopressin and/or oxytocin release. Indeed, we now have demonstrated that phoenixin activated magnocellular neurons in the rat PVN in vitro and stimulated AVP release from HNS explants. Most importantly, we present evidence that phoenixin administered into the lateral cerebroventricle selectively stimulated AVP but not OT release in vivo and activated early gene expression in neurons of the SON.
The concentrations of phoenixin used in the in vitro studies were similar to those employed by us to demonstrate the peptide’s ability to increase gene transcription and augment GnRH-stimulated LH secretion from dispersed pituitary cell cultures, actions of the peptide that could be blocked by knockdown of Gpr173 mRNA levels in those cells (8). Similarly, the doses of phoenixin used in the in vivo study were similar to those we demonstrated to activate gonadotropin secretion in vivo (8). They are similar also to the doses of the truncated form of phoenixin, phoenixin-14, required to demonstrate the orexigenic action of the peptide (6). While the most abundant levels of phoenixin immunoreactivity were detected in hypothalamic extracts (11) compared with other tissues, we do not at this time know the exact concentration of the peptide in cerebrospinal fluid, and certainly cannot estimate levels present in the synaptic cleft. We cannot comment on the physiological relevance of those doses except to say that when hypothalamic Gpr173 levels were lowered by siRNA pretreatment, the in vivo LH response to intracerebroventricular phoenixin administration was absent (8). Similarly, when Gpr173 mRNA levels were lowered by siRNA pretreatment in the current studies, the 3.0 nmol dose of phoenixin-20 amide did not result in increased plasma AVP levels. Both the current results and our previous findings (8) support the hypothesis that the Gpr173 is required for the actions of phoenixin-20 amide on reproductive hormone and vasopressin secretion.
Perspectives and Significance
These in vitro and in vivo results support our original hypothesis and point to a potentially significant role for phoenixin in the control of fluid and electrolyte homeostasis. Many questions remain. Do phoenixin and/or Gpr173 colocalize with either AVP or OT, and could endogenous peptide act in a paracrine fashion on those cells, as has been demonstrated, or other colocalized peptides (e.g., endothelin, dynorphin)? Does expression of either phoenixin or Gpr173 change depending on volume or osmotic stimuli that normally drive or suppress AVP production and/or release? Does hemorrhage-induced or osmotically driven AVP secretion in vivo rely upon endogenous phoenixin production? In other words, would compromise of phoenixin production blunt the AVP response to hemorrhage or hyperosmotic challenge? Since we have now observed that Gpr173 signaling is apparently necessary for phoenixin’s pharmacologic action to stimulate AVP release, would compromise of Gpr173 expression similarly blunt the AVP response to physiologically relevant stimuli? Is there any connection between the actions of phoenixin on reproductive hormone secretion and AVP release (e.g., ovarian cycle-dependent changes or pregnancy-related changes in volume status)? These questions and many others remain, but for now, it is important for researchers in the field of fluid and electrolyte homeostasis to be aware of this initial evidence, suggesting a novel action of phoenixin, just as it is also important for those studying phoenixin’s reproductive effects to recognize this additional hypothalamic action of the peptide.
GRANTS
This article was supported by National Institutes of Health Grant HL-121456 (to W. K. Samson and G. L. C. Yosten) and Canadian Institutes of Health Research Grant MOP 12192 (to A. V. Ferguson).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.G., L.M.S., S.P.L., C.J.H., J.S., G.R.K., G.L.C.Y., and W.K.S. performed experiments; S.G., L.M.S., S.P.L., C.J.H., A.V.F., G.R.K., G.L.C.Y., and W.K.S. analyzed data; S.G., L.M.S., C.J.H., J.S., A.V.F., G.R.K., G.L.C.Y., and W.K.S. edited and revised manuscript; S.G., L.M.S., S.P.L., C.J.H., J.S., A.V.F., G.R.K., G.L.C.Y., and W.K.S. approved final version of manuscript; L.M.S., S.P.L., C.J.H., A.V.F., G.R.K., G.L.C.Y., and W.K.S. interpreted results of experiments; S.P.L., A.V.F., G.L.C.Y., and W.K.S. drafted manuscript; A.V.F., G.R.K., G.L.C.Y., and W.K.S. conceived and designed research; G.R.K. and W.K.S. prepared figures.
REFERENCES
- 1.Samson WK. Atrial natriuretic factor inhibits dehydration and hemorrhage-induced vasopressin release. Neuroendocrinology 40: 277–279, 1985. doi: 10.1159/000124085. [DOI] [PubMed] [Google Scholar]
- 2.Samson WK, Aguila MC, Martinovic J, Antunes-Rodrigues J, Norris M. Hypothalamic action of atrial natriuretic factor to inhibit vasopressin secretion. Peptides 8: 449–454, 1987. doi: 10.1016/0196-9781(87)90008-8. [DOI] [PubMed] [Google Scholar]
- 3.Samson WK, Lumpkin MD, McCann SM. Evidence for a physiological role for oxytocin in the control of prolactin secretion. Endocrinology 119: 554–560, 1986. doi: 10.1210/endo-119-2-554. [DOI] [PubMed] [Google Scholar]
- 4.Samson WK, McDonald JK, Lumpkin MD. Naloxone-induced dissociation of oxytocin and prolactin releases. Neuroendocrinology 40: 68–79, 1985. doi: 10.1159/000124053. [DOI] [PubMed] [Google Scholar]
- 5.Samson WK, Murphy TC. Adrenomedullin inhibits salt appetite. Endocrinology 138: 613–616, 1997. doi: 10.1210/endo.138.2.4943. [DOI] [PubMed] [Google Scholar]
- 6.Schalla M, Prinz P, Friedrich T, Scharner S, Kobelt P, Goebel-Stengel M, Rose M, Stengel A. Phoenixin-14 injected intracerebroventricularly but not intraperitoneally stimulates food intake in rats. Peptides 96: 53–60, 2017. doi: 10.1016/j.peptides.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 7.Sladek CD, Joynt RJ. Characterization of cholinergic control of vasopressin release by the organ-cultured rat hypothalamo-neurohypophyseal system. Endocrinology 104: 659–663, 1979. doi: 10.1210/endo-104-3-659. [DOI] [PubMed] [Google Scholar]
- 8.Stein LM, Tullock CW, Mathews SK, Garcia-Galiano D, Elias CF, Samson WK, Yosten GLC. Hypothalamic action of phoenixin to control reproductive hormone secretion in females: importance of the orphan G protein-coupled receptor Gpr173. Am J Physiol Regul Integr Comp Physiol 311: R489–R496, 2016. doi: 10.1152/ajpregu.00191.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tasker JG, Dudek FE. Electrophysiological properties of neurones in the region of the paraventricular nucleus in slices of rat hypothalamus. J Physiol 434: 271–293, 1991. doi: 10.1113/jphysiol.1991.sp018469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor MM, Baker JR, Samson WK. Brain-derived adrenomedullin controls blood volume through the regulation of arginine vasopressin production and release. Am J Physiol Regul Integr Comp Physiol 288: R1203–R1210, 2005. doi: 10.1152/ajpregu.00781.2004. [DOI] [PubMed] [Google Scholar]
- 11.Yosten GLC, Lyu RM, Hsueh AJ, Avsian-Kretchmer O, Chang JK, Tullock CW, Dun SL, Dun N, Samson WK. A novel reproductive peptide, phoenixin. J Neuroendocrinol 25: 206–215, 2013. doi: 10.1111/j.1365-2826.2012.02381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yosten GLC, Pate AT, Samson WK. Neuronostatin acts in brain to biphasically increase mean arterial pressure through sympatho-activation followed by vasopressin secretion: the role of melanocortin receptors. Am J Physiol Regul Integr Comp Physiol 300: R1194–R1199, 2011. doi: 10.1152/ajpregu.00849.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yosten GLC, Samson WK. Pressor doses of vasopressin result in only transient elevations in plasma peptide levels. Peptides 33: 342–345, 2012. doi: 10.1016/j.peptides.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]