Abstract
The tongue is an elaborate complex of heterogeneous tissues with taste organs of diverse embryonic origins. The lingual taste organs are papillae, composed of an epithelium that includes specialized taste buds, the basal lamina, and a lamina propria core with matrix molecules, fibroblasts, nerves, and vessels. Because taste organs are dynamic in cell biology and sensory function, homeostasis requires tight regulation in specific compartments or niches. Recently, the Hedgehog (Hh) pathway has emerged as an essential regulator that maintains lingual taste papillae, taste bud and progenitor cell proliferation and differentiation, and neurophysiological function. Activating or suppressing Hh signaling, with genetic models or pharmacological agents used in cancer treatments, disrupts taste papilla and taste bud integrity and can eliminate responses from taste nerves to chemical stimuli but not to touch or temperature. Understanding Hh regulation of taste organ homeostasis contributes knowledge about the basic biology underlying taste disruptions in patients treated with Hh pathway inhibitors.
Keywords: Hh pathway disruption, taste organ niches, taste cell progenitors, fungiform and circumvallate papillae, tongue innervation, taste and cancer treatments
1. The Tongue and Taste Organs
Gustatory papillae on the mammalian tongue are composed of epithelial, connective, neural, and vascular tissues and specialized taste bud cells, assembled in taste organs adapted to detect chemical, tactile, and temperature stimuli. Not only are the taste papillae a composite of multiple tissues, but they reside in the tongue, an elaborate organ also composed of heterogeneous tissues. To perform primary functions in evaluating nutrients and eating, the tongue must be a sensory and motor neural tour de force while maintaining protective barrier functions for lingual tissue integrity. In sum, the tongue and its resident taste organs are highly complex, with varied tissues and cell types that require precise molecular regulation. This review discusses how Hedgehog (Hh) signaling regulates morphological, cellular, and functional homeostasis of the lingual taste organs. Whereas we focus mainly on the tongue, the lingual taste papillae, and the taste buds, we also include reference to other oral taste buds and dispersed chemoreceptors. The emphasis here is on maintenance and homeostasis and a synthesis of current ideas and propositions rather than providing a complete catalog of the literature. A brief summary of taste development is included.
The tongue sits in the mouth at a physical transition between the skin and the gastrointestinal system and is the first organ to confront a myriad of consumables and orchestrate decisions to reject or ingest. Similar to the skin, the tongue has a stratified squamous epithelium, seated on an underlying basal lamina over the lingual connective tissue, or lamina propria, and muscle. As in skin, the tongue includes ectodermal specializations. Similar to the gut, the tongue is a mucosa that is moist and includes specialized cells of simple epithelial type. In both skin and the gastrointestinal system, continuous cell turnover is required for homeostasis and functional integrity, with epithelial tissue turnover times ranging from a few to several days (1, 2). The tongue epithelium also turns over continuously, with a replacement time of 3 to 5 days in mouse (3, 4), and short- and long-lived taste bud cell life spans range from approximately 2 to 21 or more days (5–7).
Within the tongue, the lingual taste organs are exquisite sensory systems of papillae and resident taste buds that further exemplify skin-related and gut-related associations (6–8). The papillae are covered with a stratified squamous epithelium; are attached to an underlying basal lamina; have taste bud(s) resident in the papilla epithelium; encompass stromal cells and fiber elements of the papilla connective tissue core; and include vessels, nerves, and Schwann cells in the papilla core. The taste buds are organized collections of 50–100 specialized, polarized cells in the papilla epithelium, connected by junctions and oriented to the oral environment with apical microvilli that extend into a taste pore, and basal extensions to the basal lamina. Fewer cuboidal cells are seated on the basal lamina. The taste cells have complex specialized interactions with nerve fibers that can include classical synapses, and they share several molecular phenotypes with gut cells, or simple epithelia.
The three gustatory papillae are the fungiform (FGP), circumvallate (CVP), and foliate (FOLP) (8). Nontaste tongue papillae, the filiform (FILIFP), are spinous adaptations that have roles in eating and manipulating the food bolus and have been compared to hairs of the skin (9). Our emphasis in this review is on the rodent FGP and CVP (Figure 1).
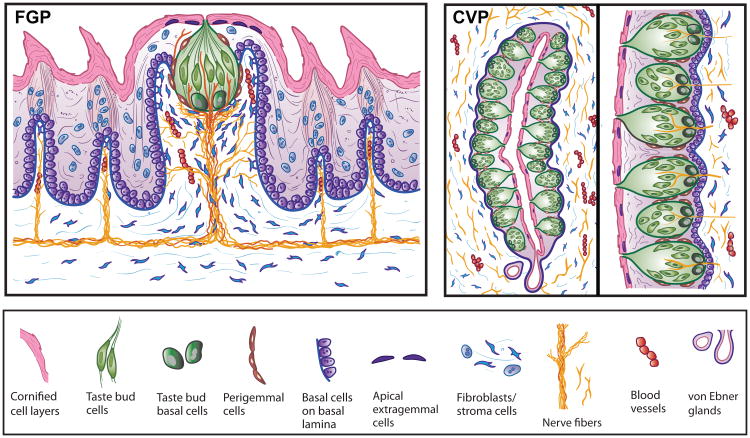
Figure 1.
Diagram of tissue architecture for the (a) fungiform (FGP) and (b) circumvallate (CVP) papillae. The dorsal tongue and luminal surfaces of the taste organs are covered with layers of cornified, desquamating cells. Within the tongue, FGP and CVP are complex organs that are home to specialized collections of taste cells, the taste buds, and are composed of an epithelium covering stromal cell compartments, with fibroblasts, nerves, and blood vessels in the lamina propria. The taste buds and general papilla tissues share several cell characteristics; however, the FGP and CVP embryonic derivations are ectodermal and endodermal, respectively, and the comparative tissue structure is very different. The FGP (illustrated in sagittal section) in mouse and rat accommodates one apical taste bud and is innervated by lingual and taste bud-specific, chorda tympani nerves. Spinous filiform, nontaste papillae surround the FGP, innervated by the lingual nerve. The CVP is illustrated in an orientation horizontal to the dorsal tongue surface. (i) One half or one wall of the CVP is included to the left, with an inset on the right (ii) to emphasize taste bud distribution along the epithelium. The CVP contains a few hundred taste buds in mouse and rat and is innervated by the glossopharyngeal nerve. The luminal aspects of taste bud cells in the CVP open onto a papilla trench, and contents from lingual von Ebner's glands empty into the base of the trenches.
The tongue and taste organs are remarkable in commanding innervation from three cranial ganglia: the trigeminal, geniculate, and petrosal (10). The trigeminal ganglion (Vth) innervates the anterior tongue, FGP, and FILIFP via the lingual nerve; it also innervates the ophthalmic area and face, the maxilla and mandible, and nonlingual structures of the mouth. The geniculate ganglion (VIIth) provides a particular innervation to taste buds in the anterior tongue and soft palate via chorda tympani and greater superficial petrosal nerves, respectively. The petrosal ganglion (IXth) neurons innervate taste buds on the posterior tongue, via the glossopharyngeal nerve, and in the pharynx; they also innervate the posterior lingual and pharyngeal mucosa and chemoreceptor cells in the carotid body (11, 12). These three ganglia have varied embryonic origins, developmental trajectories, and neurotrophic regulation and support (13, 14), but details are beyond the scope of this review. Notably, taste buds on the epiglottis, thought to function principally in upper airway protection, are innervated by yet another ganglion, the nodose (Xth), via the superior laryngeal nerve (15, 16).
Taste bud cells have been termed paraneurons, as well as neuroepithelial, neuroendocrine, neuromodulator, and chemoreceptor cells. These varied terms reflect the nature of the taste bud cells, which have epithelial, neuronal, and secretory properties to differing degrees. The taste field of research generally classifies taste bud cells in four types, but studies cross tongue regions and species (the cell types are reviewed in 17–19): Type I or dark NTPdase2+ cells, the most numerous, with glia-like properties and processes that wrap neighboring cells; Type II or light PLCβ2+ cells, reportedly responding to sweet, bitter, and umami stimuli; Type III, SNAP25+ cells, least frequently observed, that have classical synapses with innervating fibers; and Type IV or basal cells. There is evidence that the taste bud cell types are of differing lineages with some overlap and include similarities to enteroendocrine cells in the gastrointestinal system (20), neuroepithelial cells in the airways (21, 22), and keratinocytes in skin (23). Furthermore, the distinctive cell and molecular characteristics of taste bud cells suggest multiple stem cell types.
How are the taste organs, specialized cell types, and cell turnover regulated in unique tissue contexts, so that sensory function and homeostasis are sustained? Continuing the long-standing attention to the taste papilla as an entire organ (10), we emphasize that an appreciation of complete taste organ biology is necessary to understand the regulation of homeostasis and sensory function. Our focus is on organ complexity and compartments or niches that support and enable signaling. Potential roles for stroma and nerves in concert with epithelium are highlighted in asking, What is Hh signaling doing in various niches? Because deregulated Hh signaling is related to numerous diseases, and disease and disease treatment often are associated with taste disturbance (see Section 10), it is compelling to know how the Hh pathway maintains taste organs and function.
2. Hedgehog Signaling
Hh is not only a powerful regulator of organ development, differentiation, maintenance, and renewal in several tissues, but it also has diverse regulatory roles depending on the organ system (24). For example, Hh signaling in adult lung maintains quiescence in epithelium and connective tissue (25), whereas a robust proliferation in villus crypts in the small intestine and varied cell activity among niches in the hair follicle require different Hh regulatory roles (1, 26).
Hh signaling has been comprehensively reviewed (27–29). To briefly summarize, signaling is initiated when the Hh ligand engages the transmembrane receptor Patched 1 (Ptch1) releasing inhibition of the Smoothened receptor (Smo). In the absence of Hh ligand, the receptor Ptch1 with coreceptor binding proteins Cdon, Boc, and Gas1 inhibits Smo; but when secreted Hh binds to Ptch1, the repression of Smo is released, and signal transduction is initiated. Smo, via cytoplasmic intermediaries, activates a cascade of Gli transcription factors (Gli1, Gli2, and Gli3) that translocate to the nucleus and initiate Hh target gene transcription.
Generally, Gli1 is a target and an activator of the Hh pathway, Gli2 is the major activator, and Gli3 acts principally as a repressor in signaling (30). Another Hh binding protein, Hhip1 (31), plays an essential role for ligand-dependent, feedback antagonism of Hh signaling (32). In vertebrates the primary cilium is critical to Hh signaling (29).
Although several signaling pathways participate in the complex development and maintenance of tongue tissue, nontaste papillae, and gustatory papillae (33, 34), we focus here on the Hh pathway and the Sonic Hedgehog (Shh) ligand. Shh is the most broadly expressed of the three vertebrate Hh ligands (24) and the only ligand with reported expression in taste bud cells (18, 35, 36). Investigations in taste have focused mainly on Shh, Ptch1, Smo, and the Gli transcription factors; however, roles in taste homeostasis for many of the components in the Hh pathway are not known, creating a major knowledge gap. Shh signaling regulates development and patterning of taste papillae (37), but pathway functions in taste bud differentiation and maintenance are less well understood and only more currently emphasized (18, 38). Recent reviews have discussed taste papilla development (33, 39, 40). Therefore, we provide only a brief summary here, including some key topics that are not often referenced.
3. Development and Taste
3.1. Prenatal Taste Function
Early taste buds are observed in the human tongue from 7–9 weeks of gestation (41), indicating a lengthy prenatal opportunity for taste system development and function. Lingual taste buds, papillae, and peripheral and central neural responses to chemical stimuli are well studied in fetal and postnatal sheep, which has a relatively long gestation (147 days). The sheep has long been an important animal model for understanding fetal physiology (42–44). The sheep fetus swallows large quantities of amniotic fluid in patterned bouts, providing occasion to stimulate the taste system in utero (45). Indeed, early taste buds are observed from about 80 days of gestation (46). Neural responses to lingual stimulation from chorda tympani and glossopharyngeal nerves and the brainstem nucleus of solitary tract are present in utero from at least the second half of gestation, so taste function is a prenatal event that continues postnatally, as in humans (47). Whereas roles for Hh signaling in the fetal taste system are not directly demonstrated, it is clear that the Hh pathway participates in prenatal physiological regulation because ewes that have grazed on pasture containing the Hh pathway antagonist cyclopamine produce lambs with radical craniofacial abnormalities (48, 49). Prenatal taste function and amniotic fluid swallowing provide circumstances for maternal dietary components to stimulate and influence peripheral taste circuits (50). Although the mammalian taste system is functional at birth, there is substantial plasticity and shaping by newborn experience as taste buds and circuits mature postnatally (51).
3.2. Rodent Whole Tongue Organ Cultures
Whereas extensive studies established early fetal taste function in sheep, demonstrations of molecular regulation of taste organ development are limited to rodents. In rat and mouse, which have relatively short gestations, taste papilla placodes appear prenatally, and the papillae develop and differentiate in a patterned array (34, 37, 52); however, taste buds differentiate over a long postnatal period (53–55). From an organ culture system of the entire embryonic rodent tongue, in which FGP and CVP form in defined locations and patterns, it was determined that intact sensory innervation is not essential for taste papilla development and patterning (52). Knowing that nerves are not essential for papilla development, attention turned to signaling regulators and focused on the Hh pathway. Embryonically, Shh in central FGP placode cells is bracketed by Shh signaling cells that express Ptch1 and Gli1 (35). In embryonic tongue cultures, blockade of Shh signaling was achieved with the antagonists cyclopamine and jervine and a blocking antibody (37, 56, 57). The evidence supported Shh signaling as a principal, required regulator of tongue development as well as FGP formation, patterning, and differentiation, with concentration dependence in temporal and spatial contexts (58). Furthermore, blocking Shh signaling in organ culture led to the formation of multiple FGP on nonpapilla tongue regions, e.g., the intermolar eminence. Thus, there is a nonpapilla epithelium where FGP gene(s) are suppressed by Shh (see 58, figure 12).
In the whole tongue culture system, regulatory roles in inhibiting or supporting FGP development and patterning were established for several pathway components: Bmp 2,4,7 (56, 59), noggin (59), Egf and PI3K/Akt signaling (60), noncanonical Wnt (61), and canonical Wnt and Wnt/Shh interactions (62, 63). Notably, there were no effects on the CVP number or placement in these studies. However, the CVP in rodents is a single large organ at a midline posterior location and therefore not as tractable for studying patterned distributions. An in-depth study of the CVP structure has not been conducted, nor have FOLP organs been examined. With genetic models, however, regulation of CVP number by the fibroblast growth factor pathway was shown (64), and roles for LGN, an adaptor protein in mitotic spindle orientation, in FILIFP morphogenesis were recently reported (65).
4. Locations of Hedgehog Signaling In Adult Taste Organs
The FGP are numerous and patterned in the adult mammalian tongue, in contrast to the single, centered CVP and clustered, lateral FOLP on mouse and rat posterior tongue (8). In rodents, the FGP contain one taste bud each. On the other hand, CVP and FOLP have a few hundred taste buds in epithelial papilla walls. The accessible taste organs provide a unique circumstance to study signaling pathways that regulate morphological and functional homeostasis in sensory organs in the adult. Hh signaling components are distributed throughout the FGP (Figure 2). Shh is principally restricted to taste bud cells where the ligand is prominent, as demonstrated in reporter mice, in situ hybridization, and immunoreactions (18, 35). In reporter mice, Ptch1lacZ+ and Gli1lacZ+ cells are in perigemmal locations, in the basal cells of the FGP wall, and in nonproliferative stromal cells within the FGP core (35). Furthermore, Hh-responding Gli1lacZ+ cells are identified in a highly proliferative epithelial cell zone at the base of the FGP. Importantly, Gli1lacZ+ cells also are located in nerve bundles of the tongue and in the FGP core, well positioned to respond to proposed expression of Shh in nerve fibers of the geniculate and trigeminal ganglia (38). Shh is observed in cells of the trigeminal ganglion (66), and in recent studies using reporter mice and immunoreactions, we have observed Shh in the geniculate ganglion (B.L. Allen, A.A. Dlugosz, A. Kumari, C.M. Mistretta, unpublished observations). Gli2lacZ+ cells in FGP epithelium overlap expression with that of Ptch1 and Gli1 but are also in a nonoverlapping location in basal cells of the tongue epithelium between FGP.
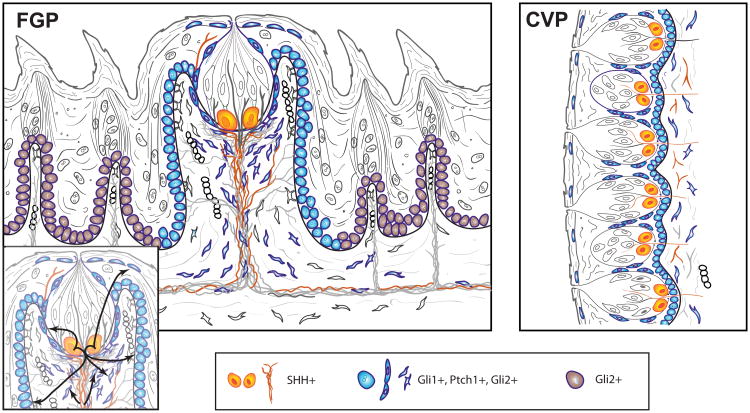
Figure 2.
Hh signaling components in the (a) FGP and (b) CVP. Building on Figure 1, Hh signaling components are located in FGP and CVP. Within the epithelium of taste organs, Shh is principally restricted in taste bud cells, but Shh expression is also in nerve fibers of the tongue and FGP or CVP core, identified with mouse reporter lines (B.L. Allen, A.A. Dlugosz, A. Kumari, C.M. Mistretta, unpublished data). Gli1+, Ptch1+, and Gli2+ cells are within the basal layer of the epithelium of the lateral FGP walls, in perigemmal and apical extragemmal cells, and in stromal cells of the papilla core. Gli2+ cells also extend in basal epithelial cells beyond the FGP. The inset illustrates paracrine signaling flow from Shh+ taste bud cells to Hh-responding cells in the lateral papilla walls, perigemmal and apical extragemmal cells, to stromal cells, and from Shh+ nerves to fibroblasts/stromal cells. Within the CVP, Gli1+, Ptch1+, and Gli2+ cells are within the basal layer of the papilla epithelium, in perigemmal and apical extragemmal cells, and in stromal cells of the papilla core. Paracrine signaling from Shh+ cells within the taste bud to Hh-responding cells is proposed similar to the FGP. Abbreviations: CVP, circumvallate papillae; FGP, fungiform papillae; Hh, Hedgehog; Shh, Sonic Hedgehog.
In the CVP, Shh expression is within taste bud cells; Ptch1+ and Gli1+ Hh-responding cells are perigemmal (38, 67), and Gli1lacZ+ Hh-responding cells are also observed in lamina propria cells of the CVP (38) (Figure 2). Gli2lacZ+ cells are also seen in perigemmal and lamina propria cells of the CVP (B.L. Allen, A.A. Dlugosz, A. Kumari, C.M. Mistretta, unpublished data).
Based on the intense expression of Shh in taste bud cells and positions of responding cells, paracrine signaling from taste buds to Hh-responding cells was proposed (35, 36, 38) (Figure 2). The idea of potential niches or compartments for Hh activity (35) that might use distinctive signaling mechanisms was emphasized. The lack of a complete map for Hh signaling components, including antagonists, in taste organs represents a major gap in our knowledge.
5. The Locations Of Stem and Progenitor Cells In Adult Lingual Tissues
To understand signaling regulation in taste organ homeostasis, it is important to know where the taste bud cell progenitor and stem cells reside. Studies using autoradiography (5), mosaic mice with X chromosome inactivation (68), and lineage tracing (23, 35) conclude that cells from the taste bud perigemmal region migrate into and contribute to taste bud cells. In addition, K14+ and Gli1+ Hh-responding cells from the basal cells of the FGP epithelium contribute to taste bud cells, perigemmal cells, and stratified squamous epithelial cells of the FGP and FILIFP (35). Because Gli1+ cells at the base of FGP walls are progenitors for taste bud cells, these Hh-responding cells are potential progenitors for cells that secrete the ligand.
By tracking cell proliferation at single-cell resolution, rapid cell divisions were observed in intragemmal taste and basal cells as well as perigemmal cells (69). Mitotic cells within the taste bud were also observed in turnover studies tracking specific cell types (7) and by electron microscopy (70). Although potential taste cell progenitors were identified in perigemmal and taste bud cell populations, it also was proposed that slow-cycling, candidate stem cells are within the taste bud in the basal cell population (69). The basal taste bud cells are known to be those that express Shh. In contrast, Shh+ basal cells in the taste bud were recently reported as postmitotic cells that are immediate precursors of all taste bud cell types in the FGP, CVP and soft palate, and not as stem cells (18).
Although Shh+ progenitors were observed in mouse basal taste bud cells (18, 71), others reported that these progenitors, when traced from the embryo, begin to disappear around weaning age and are absent at approximately 4 months postnatal (72). However, Shh+ cells are in the taste bud through at least 12 months of age, and these were proposed as internal, long-lived taste cell progenitors (35). Therefore, questions remain about the origin/derivation of the Shh+ cells within taste buds.
LGR5 (leucine-rich repeat-containing G-protein coupled receptor 5), a Wnt signaling target and receptor for R-spondins, serves as a stem cell marker in the intestine (73) and hair follicles in the skin (74) and was identified as a stem/progenitor cell marker for cells within taste buds of the CVP (75, 76). The Lgr5+ cells give rise to taste cell types, perigemmal cells, and self-renewing cells at the bottom of the CVP trench. However, evidence for Lgr5 as a stem cell marker in FGP is not firm (76), and Lgr5 does not have robust expression in FGP cells. Lgr6, an Lgr5 paralog, marks cells that can give rise to taste cells in the FGP and CVP and is a possible stem cell marker for both (75).
Although lineage tracing patterns for K14+ and Gli1+ cells are similar and indicate a common progenitor in the basal epithelial cells of the FGP (23, 35) for tongue keratinocytes and taste bud cells, distinct stem cells for FILIFP maintenance are reported (77). In multicolor lineage tracing, one Bmi1+ stem cell was identified at the base of each interpapilla pit and maintained the keratinized epithelial cells but not the taste buds (77). A separate stem cell for FILIFP is also supported with the identification of another marker, Tcf3, of the Lef/Tcf family (78). Lineage clones of Tcf3 populated the entire interpapillary pit and contributed to neighboring FILIFP, suggesting a mosaic derivation with contributions from at least three distinct individual stem cells (K14+, Bmil+, Tcf3+) (78).
Beyond taste bud progenitor and stem cells in the epithelium per se, it is proposed that neural crest-derived cells in the taste organ contribute to cells of the taste bud in FGP, CVP, FOLP, and soft palate of early postnatal and mature mice (79, 80). Some results are disputed because the Wnt1Cre mouse model does not produce label in taste bud cells even after 4 months of lineage trace from embryo stages (40, 72). However, neural crest-derived cells within the taste organ core, Vimentin+ stromal fibroblasts (see Section 7) and Dermo1+ mesenchymal cells and derivatives, either from embryo or mature FGP, reportedly contribute to all taste bud cell types in the adult (79).
Identifying taste cell stem and progenitors reveals similarities to skin and intestine regarding concepts and markers. We continue to emphasize the complexity of taste organs and the proposition that taste bud cells have more than one stem cell and progenitor type, with contributions from epithelium, lamina propria, and taste buds, and several niches to support stem and progenitor cells (35, 38). Current data propose several stem/progenitor cell types for taste bud cells, FGP versus CVP taste cell types, FGP epithelial cells, FILIFP epithelial cells, and interpapilla epithelial cells.
6. The Role of The Hedgehog Pathway In Taste Organ Homeostasis
Our focus in understanding how Hh regulates taste cell maintenance and integrity is on the taste organ, including epithelial and connective tissue cells and innervation (35, 38). The taste bud cells do not exist and cannot function in integral taste sensation if isolated. Therefore, an understanding of the whole organ is required.
The Hh signaling roles in development were demonstrated more than a decade ago (37) (see Section 3). However, studies in postnatal animals were only conducted more recently, and the Hh pathway has emerged as a principal regulator of taste organ maintenance, renewal, and regeneration. Genetic mouse models to study the Hh pathway in taste organs include reporters, Hh signaling activators, suppressors, and inhibitors, and Shh misexpression (Table 1). The location of Hh signaling elements in FGP and CVP are described and illustrated in Figure 2.
Table 1. Mouse models for Hedgehog (Hh) signaling and taste organ maintenance.
| Mouse models | Function | Expression pattern/Location/Phenotype | Ref. |
|---|---|---|---|
| Reporters | |||
| ShhlacZ | identify Shh-producing cells | in basal cells of taste bud, of FGP and CVP | 35, 67 |
| Gli1lacZ | identify HH-responding cells | in basal epithelial cells, perigemmal cells, and stromal cells, of FGP and CVP | 35, 38 |
| Gli2lacZ | identify Gli2-expressing cells | in basal cells throughout the lingual epithelium; and in perigemmal cells and stromal cells, of FGP and of CVP (unpublished data) | 35 |
| Ptch1lacZ | Identify HH-responding cells | in basal epithelial cells, perigemmal cells, of FGP and CVP; and in stromal cells, of FGP and of CVP (unpublished data) | 35, 67 |
| Lineage trace | |||
| Gli1-CreERT2;R26RlacZ | identify Gli1+ HH-responding cells and descendents | progeny in TB, epithelium and stroma of FGP and nearby FILIFP | 35 |
| Shh-CreERT2;ROSAlacz | identify embryonic SHH+ cells and descendents | progeny in adult TB (Type I and II cells) of FGP, but SHH+ cells lost after 4 months; also in intragemmal basal and perigemmal cells, of FGP | 72 |
| Shh-CreERT2;R26RlacZ/EGFP | identify SHH+ cells and descendents | progeny in all 3 TB cell types, of FGP, CVP and soft palate | 18 |
| HH misexpression | |||
| K14-CreERT2;SHH-YFP | constitutive expression of SHH in K14+ cells | ectopic K8+ clusters induced in FILIFP, molecular similarities to taste cells, not innervated | 81 |
| HH constitutive activation | |||
| K5-rtTA;TRE-GLI2* | GLI2, oncogeneic form, expressed in K5+ cells | loss of FGP and TB, and FILIFP spines; some FGP retain thin TB and innervation; suprabasal cell proliferation | 35 |
| HH/GLI suppression | |||
| K5-rtTA;tetO-Gli2ΔC4 | Gli2 dominant- negative repressor expressed in K5+ cells | TB reduced and/or lost, proliferation reduced, innervation and Gli1lacZ+ cells in stroma retained in FGP and CVP | 38 |
| K5-Cre;R26- LSL-rtTA;tetOGli2ΔC4 | Gli2 dominant- negative repressor expressed in K5+ cells and progeny | FGP, CVP, and FILIFP structure disrupted; TB and cell proliferation reduced; innervation and Gli1lacZ+ cells in stroma retained in FGP and CVP | 38 |
| K5-rtTA;tetO-Cre;Gli2fl/fl | Gli2 deleted in K5+ cells | TB reduced and Gli1lacZ+ cells eliminated except from stroma; cell proliferation reduced and innervation retained in FGP and CVP | 38 |
| K5-rtTA;tetO-Cre; Gli2fl/fl;Gli1lacZ/lacZ | Gli2 in K5+ cells and Gli1 globally deleted | TB reduced, innervation retained; cell proliferation reduced; Gli1lacZ+ cells eliminated except from stroma in FGP and CVP | 38 |
| HH/SMO inhibition | |||
| K5rtTA;tetO-Cre;Smofl/fl | Smo deleted in K5+ cells | TB reduced; innervation and Gli1lacZ+ cells in stroma retained in FGP | 86 |
| R26rtTA M2+/wt;tetO-Cre;Smofl/fl | Smo deleted globally | TB rapidly reduced; innervation and Gli1lacZ+ cells in stroma retained in FGP | 82 |
| LDE225 oral gavage | Pharmacologic SMO blockade | in FGP, all TB cell types and cell proliferation reduced; taste nerve chemical responses reduced and/or lost; innervation and Gli1lacZ+ cells in stroma retained | 83, 85 |
| Vismodegib oral gavage | Pharmacologic SMO blockade | in CVP, TB smaller; cell proliferation reduced | 84 |
| HH/WNT interaction | |||
| SHH-CreERT2;Ctnnb1(Ex3)/fl;R26RYFP | β-catenin activated in SHH+ cells | increased Type I TB type cells in non-cell autonomous manner, in FGP and CVP | 87 |
Abbreviations: CVP, circumvallate papilla; FGP, fungiform papilla; FILIFP, filiform papilla; Shh, Sonic Hedgehog; TB, taste bud.
With misexpression of the Shh ligand there were ectopic, noninnervated collections of taste cell-like structures that formed in the suprabasal lingual epithelium outside of the FGP (81) (Table 1). It was proposed that Shh can drive the entire program of taste bud differentiation without the need for nerves. However, if taste buds are defined as collections of 50 to 100 taste cells, connected by junctions, polarized to access a taste pore, and innervated to transmit taste sensation centrally, the ectopic structures are not taste buds but collections of cells that express taste cell molecular markers. However, this work clearly demonstrates that misexpression of Shh can lead to a new pattern of taste cell-like expression outside of the taste organs.
On the other hand, activating the Hh pathway in a ligand-independent manner in mice with constitutive activation of an oncogenic form of GLI2 in K5+ basal epithelial cells instigated a rapid loss of FGP and taste buds, and spines were eliminated from FILIFP (35) (Table1). Some thin taste buds remained with intact innervation, and there was unusual cell proliferation in suprabasal layers of the epithelium. The data indicated that constitutive Hh signaling stimulates cell proliferation and represses taste bud differentiation. Thus, FGP, taste bud, and epithelial integrity require balanced Hh signaling.
By expressing a Gli2-dominant-negative transgene as a Gli repressor or by conditional deletion of epithelial Gli2, various genetic models of Hh/Gli suppression were used to determine whether Hh regulates taste organ homeostasis (38, 82) (Table 1). After Hh/Gli signaling suppression in K5+ cells or cells throughout the epithelium, there were FGP and CVP taste organ disruption and taste bud loss, with decreased cell proliferation in specific papilla compartments. Cell death by classic apoptosis was not a prominent feature of taste organ disruption. Gli1lacZ+ Hh-responding cells were lost from the epithelium but remained in the stromal core. Nerves were retained, as were stromal cells in the taste organ core. Hh/Gli suppression was relieved by stopping doxycycline treatment and thus the transgene expression. Taste organ recovery with epithelial and taste bud regeneration subsequently occurred, but some FGP did not recover where there was a complete loss of all taste bud cells. Overall, however, taste bud progenitors were retained during epithelial Gli blockade and were readily reactivated during recovery, with innervation and stromal cells that had remained during Hh/Gli suppression already in place. Thus, in a direct test of Hh signaling in regulating taste organs, the data indicate that physiologic Hh signaling targets taste papilla epithelial cells as an essential regulator of homeostasis.
Hedgehog pathway inhibition (HPI) at the Smo receptor has also been studied with a pharmacologic block by the drugs LDE225 or Vismodegib (83, 84) (see Section 10). After oral gavage in mice for 16–28 days, the number of intact FGP and taste buds is essentially eliminated (83). Over half of the few remaining FGP are both misshapen and have no intact taste buds. Notably, this was the first study to demonstrate that neurophysiological taste sensation is dependent on Hh signaling and indeed is eliminated after HPI. Importantly, effects are modality specific because tactile and temperature responses from the chorda tympani nerve are retained after HPI. In recent studies we have noted that there is recovery after cessation of LDE225 treatment, all taste bud cell types are renewed within regenerated taste buds, and chorda tympani nerve responses to taste stimuli return (85). Effects with Vismodegib, investigated in the CVP, are more moderate, although there is a reduction in taste bud cells (84).
To further understand Smo inhibition and taste organ homeostasis, mouse models were developed with Smo deleted from the epithelium or globally. From recent observations in both deletion models, the morphology of FGP and taste buds was substantially altered (82, 86). The data further emphasize the epithelial contribution in taste bud maintenance that is regulated by Hh signaling.
When FGP and taste buds after Hh/Gli suppression are compared to the taste organs after Smo inhibition or Smo deletion (Table 1), comparable FGP effects are evident (Figure 3). The FGP acquires a pointed, heavily cornified apex, and taste bud cells are eliminated or much reduced. However, innervation to the FGP core is retained. In concert with Hh/Gli suppression and Smo inhibition, the Gli1lacZ+, Hh-responding cells are not observed in basal epithelial cells of the papilla or in perigemmal cells but remain within the FGP connective tissue core (A. Kumari, A.N. Ermilov, B.L. Allen, A.A. Dlugosz, C.M. Mistretta, unpublished data). Possible noncanonical Hh signaling within the taste organ stromal core should be investigated.
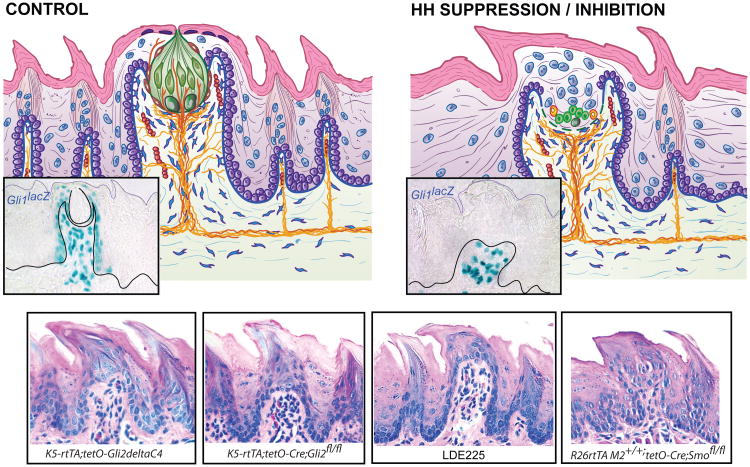
Figure 3.
Effects of Hh signaling suppression or inhibition on FGP morphology and Hh-responding cells. FGP diagrams illustrate Gli1lacZ+, Hh-responding cells (insets), for (a) control mouse tongue and (b) after Hh signaling suppression or inhibition. An H&E stained FGP image is included as an inset in a, the control diagram. This provides a comparison for images in c–f. (c–f) Four examples of papilla morphology after Hh signaling repression with taste bud cell loss are presented in H&E staining. Examples represent four different models for Hh/Gli suppression, or Smo inhibition or Smo deletion: (c) epithelial expression of a dominant negative-repressor form of Gli2 (ΔC4); (d) genetic conditional deletion of Gli2 from epithelium; (e) pharmacological blockade of Smo with the Hh pathway inhibitor LDE225; and (f) global conditional deletion of Smo. After Hh signaling suppression in all models, Hh-responding cells are eliminated in the epithelium but retained in papilla stroma (as shown in the inset in panel b from the Gli1lacz Hh signaling reporter mouse). With Hh signaling disruption, the FGP loses an intact apical taste bud, and across models, the papilla acquires a pointed, heavily keratinized spinous apex. Abbreviations: FGP, fungiform papillae; Hh, Hedgehog; Shh, Sonic Hedgehog; Smo, smoothened receptor.
Interactions between Hh and other major signaling pathways in postnatal taste organ homeostasis are not widely studied. However, the roles of Wnt/β-catenin signaling in mature taste bud cell renewal were recently addressed (71). It was proposed that β-catenin signaling may regulate proliferation of progenitor cells outside of taste buds and differentiation of immature cells within taste buds. Furthermore, differentiation to individual cell types associated with β-catenin signaling levels within lingual epithelial progenitors was proposed (87). Although high signaling levels induce Type I cells, low levels may lead to Type III cells. It was suggested that Wnt/β-catenin acts upstream of Shh signaling in taste cell differentiation. Interestingly, Shh suppresses the Wnt/β-catenin pathway, reflecting an essential interaction for fungiform development (62) and maintenance in adults (88). Although the Wnt and Shh pathways are known to interact, their combined role in tongue maintenance and homeostasis is understudied, but it is undoubtedly important in taste organ regulation.
A distinction between anterior and posterior tongue taste organs is drawn on the basis of embryonic origins (8, 33, 38). However, organ disruption and taste bud loss both in FGP and CVP papilla were demonstrated recently in genetic Hh/Gli signaling suppression (38, 82). After Hh/Gli signaling blockade, taste buds were lost from the CVP, although papilla innervation was retained. The general CVP structure was not altered. These are apparently the first data to demonstrate a direct role for Hh signaling in CVP and taste bud maintenance. The data are particularly important in indicating that Hh maintains homeostasis in taste organs of diverse origin.
7. Hedgehog Signaling In Taste Organ Stroma
In reviewing Hh pathway regulatory effects on taste organ homeostasis, it is apparent that although Hh signaling in the lingual epithelium is essential to taste papilla and taste bud integrity, thinking beyond the epithelium is also critical. The importance of stromal cells in the papilla connective tissue core, adjacent to taste bud cells and their supporting epithelium, is largely ignored in taste research. However, our group has long emphasized the importance of lingual lamina propria stromal cells in understanding signaling regulation in FGP development (10, 34, 61, 89) and homeostasis (35, 38) and made explicit proposals that an FGP niche includes apical connective tissue cells (35).
Fibroblasts, the principal stromal cells, are functionally diverse and respond to signals from epidermal cells and the stromal matrix (90). Within stromal fibroblasts, the intermediate filament vimentin is dynamic in cell adhesion, migration, and signaling (91). Recently, we demonstrated that vimentin-positive fibroblast cells remain within the stromal core of the FGP after Hh repression, in association with Hh-responding cells (38). Many of the vimentin-positive cells near the papilla basal lamina and epithelium have a migratory-like morphology, with numerous, long filopodia-like extensions that might be cytonemes or invadopodia (92).In other systems, both Shh-producing and responding cells extend long specialized filopodia that function in Shh signaling and trafficking Shh packaged in particles (93). The FGP core is replete with vimentin-positive cells that send extensions into the heparan sulfate proteoglycan-positive basal lamina, and in the apical FGP core this region is occupied by a nerve net. Thus, nerves and Hh-responding stromal cells in the FGP are in physical juxtaposition to the basal lamina, a potential Shh signaling domain where Shh is chemically bound and constrained for continued molecular interactions with both epithelial basal progenitors and connective tissue cells.
Important roles for stromal cells in regulating lineage of nearby epidermal cells were shown for the hair follicle (94) and tail skin (95). Moreover, activated fibroblasts accumulate during pancreas tumorogenesis and participate in Hh signaling (96), and in response to epithelial Smo deletion, mesenchymal cells of adult lung increase proliferation (25). Stromal cells in the FGP core are Gli1lacZ+, Hh-responding cells. We propose that in epithelium-specific suppression of the Hh pathway in the tongue, there is potential for activating stromal cells to migrate, associate with basal lamina and molecules, and possibly even cross the basal lamina to regulate epithelial and taste bud cell lineages. Furthermore, activation of the stromal compartment could be a compensatory mechanism for revitalizing a compromised epithelium.
8. Hedgehog Signaling Interacting With Innervation
Nerves, labeled with antibodies to neurofilament or P2×3, remain within the tongue, FGP, and CVP and project into lingual epithelium after Hh/Gli suppression (38, 82). Thus, with Hh suppression, at the light microscopic level the general extent of taste organ innervation is not disrupted, even in the context of taste bud loss, nor is the directed projection of nerve fibers to innervate taste papillae affected. These data indicate that taste bud-specific Shh is not a major target-derived factor in lingual nerve maintenance, nor is innervation alone sufficient to maintain taste bud cells when Hh signaling is suppressed in the epithelium.
When Shh is overexpressed in K14+ basal lingual epithelial cells, taste-like cells form in lingual epithelium in areas between FGP (81). These cells and cell clusters do not meet the definition of complete taste buds and are not innervated, which is essential for taste sensation. Whereas this study indicates that a molecular signature for taste-like cells can be induced with overexpression of Shh ligand, it suggests that there is no direct connection between the ectopic cells and innervation (see Section 6). Indeed, solitary chemoreceptor cells in the gut, lung, and nasal passages can express, for example, K8, gustducin, and TrpM5 (97–99), but these are not taste cells because they do not carry taste sensation to the central nervous system.
In mouse models of Hh/Gli suppression, although taste bud cells are eliminated, nerves are retained in FGP and CVP (38). However, it is long known that after nerve cut the taste buds degenerate and will regenerate after nerves grow back (100–103). Shh and Ptch expressions rapidly disappear in and around CVP taste bud cells after glossopharyngeal nerve cut, whereas K8-expressing taste bud cells are retained for a few days (67, 104). In ongoing studies, at 21 days after unilateral cut of the lingual/chorda tympani nerve innervation to the anterior tongue, FGP, and taste buds, we observed taste bud elimination in approximately 80% of FGP compared to the uncut side of the tongue (105). With elimination of taste buds, Shh expression in the epithelium was also eliminated, as were Hh-responding, Gli1lacZ+ cells in perigemmal and basal papilla epithelial cells. However, in most FGP, Hh-responding cells remained in the stroma in association with neurofilament-positive nerve fibers. We propose that sustained taste bud generation and regeneration are directly associated with the presence of Hh-responding cells in the taste organ.
Although there is no direct evidence for Shh expression in taste organs as a survival or guidance factor for nerves, Shh in nerve fibers could instead function in signaling to the Hh-responding stroma cells for taste organ structural and functional homeostasis. We have proposed that neurofilament-positive nerve fibers transport Hh ligand into the FGP core and thereby maintain Hh signaling in the stromal core, even in the absence of taste bud-derived Shh (38). In reporter mice in studies in progress, Shh-expressing nerve fibers were identified in the FGP stromal core and directed into the taste bud, and Shh+ neurons were in the geniculate ganglion (B.L. Allen, A.A. Dlugosz, A. Kumari, C.M. Mistretta, unpublished data). Dorsal root ganglion sensory neurons are a source of Shh, signaling to Gli1+ cells in the hair follicle upper bulge in a perineural niche necessary for stem cell regulation (106), and Shh-expressing nerve fibers regulate touch dome stem cells in skin (107). Furthermore, via Hh signaling, sensory nerves have proposed roles in hair follicle tumor formation (108). Interactions between Hh signaling and innervation in taste bud maintenance, degeneration, and regeneration are understudied, and further investigation will likely bring new results for understanding homeostasis in the taste system.
9. Hedgehog Signaling and Taste Organ Niches
The taste organ niche is a dynamic tissue microenvironment where stem and/or progenitor cells that reside with neighboring differentiated cells and matrix components are maintained and regulated (109, 110). Signaling compartments or niches where Hh signals in regulating taste organ homeostasis were proposed (35). However, because the stem cells are not formally identified in FGP, niche identifications are not confirmed. Discussion here is restricted to the FGP, but obvious parallels exist with the CVP.
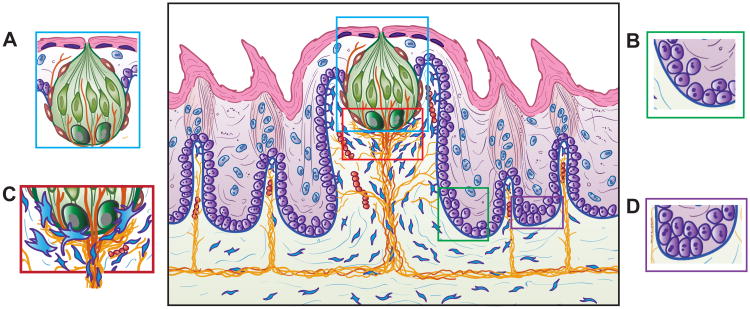
In the FGP and neighboring FILIFP, at least four niches are proposed (Figure 4). Niche 1 covers areas within and around the taste bud, including taste bud and perigemmal cells. Cells include Shh-expressing cells, Hh-responding Ptch1+ and Gli1+ cells, Gli2+ cells, K5+/K14+ cells, p63+ and Ki67+ cells, and intra- and extragemmal nerve fibers (Figure 4a). Shh signaling in a paracrine manner from basal taste bud cells was suggested (35, 38) (Figure 2). Niche 2 encompasses highly proliferative, basal epithelial cells at the base or bottom of the FGP walls, including Ki67+ and p63+ cells, K5/K14+ cells, and Gli1+ Hh-responding cells (Figure 4b). These Hh-responding cells are progenitors for taste bud and perigemmal cells, and keratinocytes of the FGP and neighboring FILIFP (23, 35). Niche 3 is in the apical FGP core and base of the taste bud and includes basal Shh+ taste bud cells and the basal lamina; Hh-responding cells; nerve fibers extending in a net under the taste bud; S100+ Schwann cells; fascicles that include nerve fibers, glia cells, Hh-responding cells and, we propose, vessels; fibroblasts with filopodia, associated with the basal lamina; and capillaries (Figure 4c). This is an especially complex and comprehensive signaling center, with taste cell bipolar extensions to the basal lamina, taste bud basal cells, basal lamina molecules, stromal fibroblast cells, including those that are Hh–responding, and bundles of nerve fibers and Schwann or satellite cells, all in close association. Multiple Shh signaling exchanges are likely, based on taste bud-derived Shh secretion and sequestration of Shh ligand by basal lamina components, for example, heparan sulfate proteoglycans that could modulate Hh gradients and interact with stromal fibroblasts via filipodia extensions. This provides a domain for stromal cells to regulate epithelial progenitors, and importantly, a particular perineural environment around lingual and taste nerve fibers to regulate FGP and taste bud progenitor and stem cells, similar to that proposed in touch dome stem cell maintenance for neural Hh signaling (107). In this signaling hub, nerve fibers not only enter the taste bud but also can directly interact with all other niche components. Furthermore, we have proposed that these nerve fibers express Shh (38). In fact, another niche within Niche 3, could be the bundle of nerve fibers and stromal cells entering the taste bud, echoing the neurovascular bundle (NVB) niche in Hh signaling to mesenchymal stem cells in the mouse incisor (66). Similarly, an NVB in the FGP apical core might signal to the Hh-responding cells, associated with nerve bundles and in the stroma, in organ homeostasis. Niche 4 involves basal epithelial cells at the base or near bottom of the FILIFP, including one Bmi1+ cell and Tcf3+, Gli2+, p63+, Ki67+, and K5/K14+ cells (Figure 4d). The cells are seated on a basal lamina positioned over vimentin+ stromal cells and nerve fibers. These cells contribute to keratinocyte epithelial cells of the interpapilla epithelium and neighboring FILIFP (77, 78).
Figure 4.
FGP with signaling niches or compartments. Building on Figures 1 and 2, four niches are proposed for Hh signaling in specific FGP cell compartments. (a) Niche 1 is within and around the taste bud, including taste bud and perigemmal cells. Signaling from Shh+ taste bud cells to HH-responding, perigemmal proliferative cells, extragemmal apical cells, and extragemmal apical nerves is proposed. (b) Niche 2 represents the highly proliferative, basal epithelial cells at the base or bottom of the FGP walls, including Gli1+ Hh-responding cells that are the progenitors of taste bud cells, perigemmal cells, and stratified keratinocytes of the FGP. These cells are positioned over stromal cells and lingual innervation. (c) In the apical FGP core and base of the taste bud, Niche 3 includes Shh+ basal taste bud cells and the basal lamina, taste cell bipolar extensions to the basal lamina, Hh-responding cells of the stroma, nerve fibers and Shh+ nerve fibers, and fibroblasts with filopodia in close association with the basal lamina. Multiple Shh signaling exchanges are likely, including those to the stromal compartment, based on taste bud-derived Shh secretion and sequestration of Shh ligand by basal lamina components. This also provides a signaling domain for stromal cells to regulate epithelial progenitors, and importantly, a particular perineural environment around lingual and taste nerve fibers to regulate FGP and taste bud progenitor and stem cells. (d) Niche 4 is in basal epithelial cells at the base or near bottom of the FILIFP, including proliferative cells seated on the basal lamina positioned over vimentin+ stromal cells and nerve fibers. Bmi1+, Tcf3+, and Gli2+ cells include progenitors for keratinocytes of the interpapilla epithelium and FILIFP. Abbreviations: FGP, fungiform papillae; FILIFP, spinous filiform papillae; Hh, Hedgehog; Shh, Sonic Hedgehog
10. Hedgehog Signaling and Taste Sensation And Cancer Treatment
Cancer caused by aberrant Hh–pathway function or other mechanisms, as well as treatments with chemotherapy and/or radiotherapy, can cause dysfunctional taste sensation and perception in patients (111–114). However, our focus here is specifically on the effects of recent Hh-targeted cancer therapies on taste homeostasis. Deregulated Hh signaling is associated with many cancers, including basal cell carcinoma (BCC), caused by sporadic mutations with the loss of PTCH1 or activation of SMO (115, 116). Hence, an attractive approach for the treatment of BCC is to develop Hh pathway inhibitors. Hh pathway drug discovery efforts are predominantly focused on targeting SMO (117), and small-molecule inhibitors of SMO, including Vismodegib and LDE225/Sonidegib/Odomzo, are approved by the US Food and Drug Administration (118).
Although Hh pathway inhibitors are effective for advanced BCC treatment, patients using them experience severe taste disturbances (119–122). More than 55% of patients taking Vismodegib for locally advanced and metastatic BCC and 29% of patients using Sonidegib reported dysguesia (118). Adverse taste effects are a significant limiting factor to sustained treatment and consequently can lead patients to discontinue treatment.
We administered LDE225 to mice by oral gavage and demonstrated that Hh signaling is critical for renewal of taste organs and maintaining taste sensation (83) (see Section 6). LDE225 is a specific, small-molecule SMO inhibitor, with mean residence times in the body from approximately 3 to 7 h (123). With increasing LDE225 treatment duration, FGP in mice lose taste buds and become atypical in form, with or without taste buds. The atypical FGP acquire thick, apical keratinized layers and a pointed, spinous protrusion (Figure 3). Not only are FGP and taste buds altered or eliminated, but notably, neurophysiological taste function is disrupted. In chorda tympani nerve recordings, responses to all taste stimuli representing salt, sweet, sour, bitter, and umami qualities were lost (83). Importantly, responses to cold and tactile stimuli were retained, indicating modality-specific effects of Hh pathway inhibition associated with LDE225 treatment.
In current work, we also have noted the reduction and eventual elimination of all three types of taste bud receptor cells with increasing exposure to LDE225 (85), explaining the observed complete, cross-stimulus neurophysiological taste loss and indicating that the progenitors of all three cell types are disrupted. Cell alterations with LDE225 treatment were principally epithelial, whereas innervation and Gli1lacZ+ Hh-responding cells were retained in the FGP stroma (83). Overall, our data indicate that pharmacological disruption of HH signaling by LDE225 is responsible for chemosensory disturbances in patients treated with HH pathway inhibitors. Furthermore, the data reflect a direct requirement for Hh signaling in maintaining adult taste organ and taste sensation.
In comparison, after administering Vismodegib via oral gavage for 15 weeks in mice, there were reported reductions in CVP taste bud size, taste bud cell numbers, and perigemmal cell proliferation (84). However, CVP taste buds were not eliminated after the almost 4-month treatment, nor was Gli1 expression fully inhibited, and there was no demonstrated alteration in taste preference for sucrose or denatonium benzoate. In contrast, after 28 days of LDE225 gavage we observed complete elimination of normal FGP taste buds and neurophysiological taste sensation (83). Notably these studies address either FGP or CVP, however, it is not clear why oral gavage in mice with LDE225 should have such profound effects after approximately one month, whereas effects are moderate after Vismodegib for approximately 4 months. Biologic activity of the two drugs in rodents might be different.
Although Vismodegib and LDE225 are currently used for the treatment of BCC, resistance to the drugs is emerging (124). Thus, other SMO inhibitors or inhibitors of downstream components, such as GLI transcription factors, are being pursued as alternative therapeutics (125–127). Studies of Hh suppression in mice with a Gli2 transgene and Smo and Gli2 deletion predict that drugs targeting these pathway components will have adverse effects on taste function (38, 82, 86).
Disruption in taste sensation can negatively affect quality of life and nutritional status and requires management for taste disturbances (128). Recently in ongoing studies, we determined that the tongue and taste alterations caused by LDE225 treatment in mice are reversible after the drug is discontinued, and taste loss and altered taste organs can recover after a few weeks (85). These findings provide evidence of the rapid reversal of taste loss after cessation of the drug. Translating this study to patient experiences could be very useful for the management of Hh pathway treatment-related taste loss. Furthermore, knowledge of modality-specific effects means that patients should retain tongue touch and temperature sensations. This could be leveraged in designing diets that would still afford lingual sensory stimulation via tactile, cold, and spicy stimuli, for example.
11. Summary and Future Issues
Major effects of the HH pathway on taste organ homeostasis can be summarized as follows.
11.1. Signaling Misexpression or Activation
With Shh misexpression in lingual epithelium, progenitors can overcome some aspects of organ patterning because collections of noninnervated, taste-like cells form in anterior tongue tissue outside of the FGP. In mice with Gli2 constitutive activation, the FGP, taste buds, and FILIP are altered or eliminated, whereas proliferation is observed in suprabasal epithelial layers.
11.2. Signaling Suppression
Cell proliferation is altered in a compartment-specific manner, and differentiation to taste bud cell types is eliminated. Apoptotic cell death apparently is not a pivotal event in taste organ effects. Innervation to the taste organ core and epithelium is not substantially altered at the light microscopic level, and stromal cells, such as Gli1lacZ+ and vimentin+, are not substantially altered in density. Remaining nerve fibers and stromal cells are not sufficient to maintain taste organs in the face of epithelial disruption, indicating that interactions are required between nerves and other components for taste bud integrity. Taste organs recover after release from Hh pathway suppression, and effects are observed in FGP, CVP, and taste buds.
11.3. Signaling Inhibition
FGP and taste bud integrity are lost, with taste bud elimination after HPI. Chorda tympani nerve responses to taste stimuli are eliminated after pharmacologic HPI. The effects on lingual sensory systems are modality specific because HPI does not disrupt chorda tympani nerve responses to tactile and temperature stimuli. In comparing the effects of HPI by pharmacologic agents and genetic models, similar effects are obtained in FGP and taste buds. However, the effects of HPI by pharmacologic agents on the CVP and taste buds are apparently not as severe.
12. Summary
The collected data demonstrate an unequivocal place for the Hh pathway as a principal regulator to maintain taste organ homeostasis. Nonetheless, many issues should be addressed to attain a full grasp of the Hh pathway components and the potential interactions between Hh and other signaling pathways. It is apparent that Hh signaling acts to sculpt a fine balance between proliferation and differentiation. Hh signaling is required for homeostasis in taste organ epithelium and in specialized taste bud cells. Specific effects on FGP, CVP, and/or nonlingual taste structures, as well as neurophysiological function and behavioral taste sensation effects, will require additional studies. Other areas for future study include the interactions between taste organ innervation and Hh regulation, distinctive signaling mechanisms and taste organ maintenance roles in various niches, possible niche-specific stem/progenitor cells, potential noncanonical Hh signaling in particular taste organ niches, and a full identification of Hh signaling elements in taste organs.
Acknowledgments
We thank University of Michigan colleagues, collaborators, and Co-Principal Investigators Benjamin L. Allen (Medical School), Robert M. Bradley (School of Dentistry), and Andrzej A. Dlugosz (Medical School) for their continued collaborative work and discussions about signaling pathways that regulate homeostasis in taste organs and taste function, both cited in this review and in progress. We acknowledge funding from the NIH National Institute on Deafness and Other Communication Disorders Multi PI Grant R01 DC014428 (to B.L. Allen, R.M. Bradley, A.A. Dlugosz, C.M. Mistretta) and from the University of Michigan Center for Organogenesis Postdoctoral Fellowship to A.K.
Footnotes
Disclosure Statement: The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Literature Cited
- 1.Barker N, Bartfeld S, Clevers H. Tissue-resident adult stem cell populations of rapidly self-renewing organs. Cell Stem Cell. 2010;7:656–70. doi: 10.1016/j.stem.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 2.Potten CS, Saffhill R, Maibach HI. Measurement of the transit time for cells through the epidermis and stratum corneum of the mouse and guinea-pig. Cell Tissue Kinet. 1987;20:461–72. doi: 10.1111/j.1365-2184.1987.tb01355.x. [DOI] [PubMed] [Google Scholar]
- 3.Cameron IL. Cell proliferation migration and specialization in epithelium of mouse tongue. J Exp Zool. 1966;163:271–83. [Google Scholar]
- 4.Toto PD, Ojha G. Generation cycle of oral epithelium in mice. J Dent Res. 1962;41:388–91. doi: 10.1177/00220345660450037401. [DOI] [PubMed] [Google Scholar]
- 5.Beidler LM, Smallman RL. Renewal of cells within taste buds. J Cell Biol. 1965;27:263–72. doi: 10.1083/jcb.27.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamamichi R, Asano-Miyoshi M, Emori Y. Taste bud contains both short-lived and long-lived cell populations. Neuroscience. 2006;141:2129–38. doi: 10.1016/j.neuroscience.2006.05.061. [DOI] [PubMed] [Google Scholar]
- 7.Perea-Martinez I, Nagai T, Chaudhari N. Functional cell types in taste buds have distinct longevities. PLOS ONE. 2013;8:e53399. doi: 10.1371/journal.pone.0053399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mistretta CM. Developmental neurobiology of taste. In: Getchell T, Doty RL, Bartoshuk L, Snow J, editors. Smell and Taste in Health and Disease. New York: Raven; 1991. pp. 35–64. [Google Scholar]
- 9.Manabe M, Lim HW, Winzer M, Loomis CA. Architectural organization of filiform papillae in normal and black hairy tongue epithelium: dissection of differentiation pathways in a complex human epithelium according to their patterns of keratin expression. Arch Dermatol. 1999;135:177–81. doi: 10.1001/archderm.135.2.177. [DOI] [PubMed] [Google Scholar]
- 10.Mistretta CM, Hill DL. Development of the taste system: basic cell and neurobiology. In: Doty RL, editor. Handbook of Clinical Olfaction and Gustation. New York: Marcel Dekker; 1995. pp. 635–68. [Google Scholar]
- 11.Iturriaga R, Varas R, Alcayaga J. Electrical and pharmacological properties of petrosal ganglion neurons that innervate the carotid body. Respir Physiol Neurobiol. 2007;157:130–39. doi: 10.1016/j.resp.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 12.Eyzaguirre C, Zapata P. Perspectives in carotid body research. J Appl Physiol Respir Environ Exerc Physiol. 1984;57:931–57. doi: 10.1152/jappl.1984.57.4.931. [DOI] [PubMed] [Google Scholar]
- 13.Krimm RF. Factors that regulate embryonic gustatory development. BMC Neurosci. 2007;8(Suppl. 3):S4. doi: 10.1186/1471-2202-8-S3-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schlosser G. Induction and specification of cranial placodes. Dev Biol. 2006;294:303–51. doi: 10.1016/j.ydbio.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 15.Hayakawa T, Kuwahara-Otani S, Maeda S, Tanaka K, Seki M. Calcitonin gene-related peptide immunoreactive sensory neurons in the vagal and glossopharyngeal ganglia innervating the larynx of the rat. J Chem Neuroanat. 2014;55:18–23. doi: 10.1016/j.jchemneu.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Bradley RM, Stedman HM, Mistretta CM. Superior laryngeal nerve response patterns to chemical stimulation of sheep epiglottis. Brain Res. 1983;276:81–93. doi: 10.1016/0006-8993(83)90550-4. [DOI] [PubMed] [Google Scholar]
- 17.Chaudhari N, Roper SD. The cell biology of taste. J Cell Biol. 2010;190:285–96. doi: 10.1083/jcb.201003144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miura H, Scott JK, Harada S, Barlow LA. Sonic hedgehog-expressing basal cells are general post-mitotic precursors of functional taste receptor cells. Dev Dyn. 2014;243:1286–97. doi: 10.1002/dvdy.24121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murray RG. The ultrastructure of taste buds. In: Friedman I, editor. Ultrastructure of Sensory Organs. Amsterdam: North Holland; 1973. pp. 1–81. [Google Scholar]
- 20.Gribble FM, Reimann F. Enteroendocrine cells: chemosensors in the intestinal epithelium. Annu Rev Physiol. 2016;78:277–99. doi: 10.1146/annurev-physiol-021115-105439. [DOI] [PubMed] [Google Scholar]
- 21.Branchfield K, Nantie L, Verheyden JM, Sui P, Wienhold MD, Sun X. Pulmonary neuroendocrine cells function as airway sensors to control lung immune response. Science. 2016;351:707–10. doi: 10.1126/science.aad7969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee RJ, Cohen NA. Bitter and sweet taste receptors in the respiratory epithelium in health and disease. J Mol Med. 2014;92:1235–44. doi: 10.1007/s00109-014-1222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okubo T, Clark C, Hogan BL. Cell lineage mapping of taste bud cells and keratinocytes in the mouse tongue and soft palate. Stem Cells. 2009;27:442–50. doi: 10.1634/stemcells.2008-0611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petrova R, Joyner AL. Roles for Hedgehog signaling in adult organ homeostasis and repair. Development. 2014;141:3445–57. doi: 10.1242/dev.083691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peng T, Frank DB, Kadzik RS, Morley MP, Rathi KS, et al. Hedgehog actively maintains adult lung quiescence and regulates repair and regeneration. Nature. 2015;526:578–82. doi: 10.1038/nature14984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rompolas P, Greco V. Stem cell dynamics in the hair follicle niche. Semin Cell Dev Biol. 2014:25–26. 34–42. doi: 10.1016/j.semcdb.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allen BL, Song JY, Izzi L, Althaus IW, Kang JS, et al. Overlapping roles and collective requirement for the coreceptors GAS1, CDO, and BOC in SHH pathway function. Dev Cell. 2011;20:775–87. doi: 10.1016/j.devcel.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Briscoe J, Therond PP. The mechanisms of Hedgehog signalling and its roles in development and disease. Nat Rev Mol Cell Biol. 2013;14:416–29. doi: 10.1038/nrm3598. [DOI] [PubMed] [Google Scholar]
- 29.Gorojankina T. Hedgehog signaling pathway: a novel model and molecular mechanisms of signal transduction. Cell Mol Life Sci. 2016;73:1317–32. doi: 10.1007/s00018-015-2127-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pan Y, Bai CB, Joyner AL, Wang B. Sonic hedgehog signaling regulates Gli2 transcriptional activity by suppressing its processing and degradation. Mol Cell Biol. 2006;26:3365–77. doi: 10.1128/MCB.26.9.3365-3377.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chuang PT, McMahon AP. Vertebrate Hedgehog signalling modulated by induction of a Hedgehog-binding protein. Nature. 1999;397:617–21. doi: 10.1038/17611. [DOI] [PubMed] [Google Scholar]
- 32.Holtz AM, Peterson KA, Nishi Y, Morin S, Song JY, et al. Essential role for ligand-dependent feedback antagonism of vertebrate hedgehog signaling by PTCH1, PTCH2 and HHIP1 during neural patterning. Development. 2013;140:3423–34. doi: 10.1242/dev.095083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barlow LA, Klein OD. Developing and regenerating a sense of taste. Curr Top Dev Biol. 2015;111:401–19. doi: 10.1016/bs.ctdb.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mistretta CM, Liu HX. Development of fungiform papillae: patterned lingual gustatory organs. Arch Histol Cytol. 2006;69:199–208. doi: 10.1679/aohc.69.199. [DOI] [PubMed] [Google Scholar]
- 35.Liu HX, Ermilov A, Grachtchouk M, Li L, Gumucio DL, et al. Dev Biol. 2013;382:82–97. doi: 10.1016/j.ydbio.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miura H, Kusakabe Y, Harada S. Cell lineage and differentiation in taste buds. Arch Histol Cytol. 2006;69:209–25. doi: 10.1679/aohc.69.209. [DOI] [PubMed] [Google Scholar]
- 37.Mistretta CM, Liu HX, Gaffield W, MacCallum DK. Cyclopamine and jervine in embryonic rat tongue cultures demonstrate a role for Shh signaling in taste papilla development and patterning: fungiform papillae double in number and form in novel locations in dorsal lingual epithelium. Dev Biol. 2003;254:1–18. doi: 10.1016/s0012-1606(02)00014-3. [DOI] [PubMed] [Google Scholar]
- 38.Ermilov AN, Kumari A, Li L, Joiner AM, Grachtchouk MA, et al. Maintenance of taste organs is strictly dependent on epithelial Hedgehog/GLI signaling. PLOS Genet. 2016;12(11):e1006442. doi: 10.1371/journal.pgen.1006442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barlow LA. Progress and renewal in gustation: new insights into taste bud development. Development. 2015;142:3620–29. doi: 10.1242/dev.120394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kapsimali M, Barlow LA. Developing a sense of taste. Semin Cell Dev Biol. 2013;24:200–9. doi: 10.1016/j.semcdb.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bradley RM, Stern IB. The development of the human taste bud during the foetal period. J Anat. 1967;101:743–52. [PMC free article] [PubMed] [Google Scholar]
- 42.Beard RW, Nathanielsz PW. Fetal Physiology and Medicine: The Basis of Perinatology. 2nd Vol. 6. New York: Marcel Dekker; 1984. [Google Scholar]
- 43.Dawes GS. Foetal and Neonatal Physiology. Chicago: Year Book Med Publ; 1968. [Google Scholar]
- 44.Longo LD. Rise of Fetal and Neonatal Physiology: Basic Science to Clinical Care. New York: Springer-Verlag; 2013. [Google Scholar]
- 45.Bradley RM, Mistretta CM. Swallowing in fetal sheep. Science. 1973;179:1016–17. doi: 10.1126/science.179.4077.1016. [DOI] [PubMed] [Google Scholar]
- 46.Mistretta CM, Bradley RM. Neural basis of developing salt taste sensation-response changes in fetal, postnatal, and adult sheep. J Comp Neurol. 1983;215:199–210. doi: 10.1002/cne.902150207. [DOI] [PubMed] [Google Scholar]
- 47.Mistretta CM, Bradley RM. The development of taste. In: Blass E, editor. Handbook of Behavioral Neurobiology Vol 8 : Developmental Processes in Psychobiology and Neurobiology. New York: Plenum; 1985. pp. 205–36. [Google Scholar]
- 48.Chen JK, Taipale J, Cooper MK, Beachy PA. Inhibition of Hedgehog signaling by direct binding of cyclopamine to Smoothened. Genes Dev. 2002;16:2743–48. doi: 10.1101/gad.1025302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gaffield W, Keeler RF. Steroidal alkaloid teratogens: molecular probes for investigation of craniofacial malformations. J Toxicol Toxin Rev. 1996;15:303–26. [Google Scholar]
- 50.Mistretta CM, Bradley RM. Taste and swallowing in utero: discussion of fetal sensory function. Br Med Bull. 1975;31:80–84. doi: 10.1093/oxfordjournals.bmb.a071247. [DOI] [PubMed] [Google Scholar]
- 51.Mistretta CM. Taste development. In: Coleman JR, editor. Development of Sensory Systems in Mammals. New York: Wiley-Interscience; 1990. pp. 567–613. [Google Scholar]
- 52.Mbiene JP, Maccallum DK, Mistretta CM. Organ cultures of embryonic rat tongue support tongue and gustatory papilla morphogenesis in vitro without intact sensory ganglia. J Comp Neurol. 1997;377:324–40. doi: 10.1002/(sici)1096-9861(19970120)377:3<324::aid-cne2>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 53.Harada S, Yamaguchi K, Kanemaru N, Kasahara Y. Maturation of taste buds on the soft palate of the postnatal rat. Physiol Behav. 2000;68:333–39. doi: 10.1016/s0031-9384(99)00184-5. [DOI] [PubMed] [Google Scholar]
- 54.Hendricks SJ, Brunjes PC, Hill DL. Taste bud cell dynamics during normal and sodium-restricted development. J Comp Neurol. 2004;472:173–82. doi: 10.1002/cne.20064. [DOI] [PubMed] [Google Scholar]
- 55.Krimm RF, Hill DL. Neuron/target matching between chorda tympani neurons and taste buds during postnatal rat development. J Neurobiol. 2000;43:98–106. [PubMed] [Google Scholar]
- 56.Hall JM, Bell ML, Finger TE. Disruption of sonic hedgehog signaling alters growth and patterning of lingual taste papillae. Dev Biol. 2003;255:263–77. doi: 10.1016/s0012-1606(02)00048-9. [DOI] [PubMed] [Google Scholar]
- 57.Torii D, Soeno Y, Fujita K, Sato K, Aoba T, Taya Y. Embryonic tongue morphogenesis in an organ culture model of mouse mandibular arches: blocking Sonic hedgehog signaling leads to microglossia. In Vitro Cell Dev Biol Anim. 2016;52:89–99. doi: 10.1007/s11626-015-9951-6. [DOI] [PubMed] [Google Scholar]
- 58.Liu HX, MacCallum DK, Edwards C, Gaffield W, Mistretta CM. Sonic hedgehog exerts distinct, stage-specific effects on tongue and taste papilla development. Dev Biol. 2004;276:280–300. doi: 10.1016/j.ydbio.2004.07.042. [DOI] [PubMed] [Google Scholar]
- 59.Zhou Y, Liu HX, Mistretta CM. Bone morphogenetic proteins and noggin: inhibiting and inducing fungiform taste papilla development. Dev Biol. 2006;297:198–213. doi: 10.1016/j.ydbio.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 60.Liu HX, Henson BS, Zhou Y, D'Silva NJ, Mistretta CM. Fungiform papilla pattern: EGF regulates inter-papilla lingual epithelium and decreases papilla number by means of PI3K/Akt, MEK/ERK, and p38 MAPK signaling. Dev Dyn. 2008;237:2378–93. doi: 10.1002/dvdy.21657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu HX, Grosse AS, Iwatsuki K, Mishina Y, Gumucio DL, Mistretta CM. Separate and distinctive roles for Wnt5a in tongue, lingual tissue and taste papilla development. Dev Biol. 2012;361:39–56. doi: 10.1016/j.ydbio.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Iwatsuki K, Liu HX, Gronder A, Singer MA, Lane TF, et al. Wnt signaling interacts with Shh to regulate taste papilla development. PNAS. 2007;104:2253–58. doi: 10.1073/pnas.0607399104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu F, Thirumangalathu S, Gallant NM, Yang SH, Stoick-Cooper CL, et al. Wnt-β-catenin signaling initiates taste papilla development. Nat Genet. 2007;39:106–12. doi: 10.1038/ng1932. [DOI] [PubMed] [Google Scholar]
- 64.Petersen CI, Jheon AH, Mostowfi P, Charles C, Ching S, et al. FGF signaling regulates the number of posterior taste papillae by controlling progenitor field size. PLOS Genet. 2011;7:e1002098. doi: 10.1371/journal.pgen.1002098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Byrd KM, Lough KJ, Patel JH, Descovich CP, Curtis TA, Williams SE. LGN plays distinct roles in oral epithelial stratification, filiform papilla morphogenesis and hair follicle development. Development. 2016;143:2803–17. doi: 10.1242/dev.136010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhao H, Feng J, Seidel K, Shi S, Klein O, et al. Secretion of Shh by a neurovascular bundle niche supports mesenchymal stem cell homeostasis in the adult mouse incisor. Cell Stem Cell. 2014;14:160–73. doi: 10.1016/j.stem.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miura H, Kusakabe Y, Sugiyama C, Kawamatsu M, Ninomiya Y, et al. Shh and Ptc are associated with taste bud maintenance in the adult mouse. Mech Dev. 2001;106:143–45. doi: 10.1016/s0925-4773(01)00414-2. [DOI] [PubMed] [Google Scholar]
- 68.Stone LM, Finger TE, Tam PP, Tan SS. Taste receptor cells arise from local epithelium, not neurogenic ectoderm. PNAS. 1995;92:1916–20. doi: 10.1073/pnas.92.6.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sullivan JM, Borecki AA, Oleskevich S. Stem and progenitor cell compartments within adult mouse taste buds. Eur J Neurosci. 2010;31:1549–60. doi: 10.1111/j.1460-9568.2010.07184.x. [DOI] [PubMed] [Google Scholar]
- 70.Delay RJ, Kinnamon JC, Roper SD. Ultrastructure of mouse vallate taste buds: II. Cell types and cell lineage. J Comp Neurol. 1986;253:242–52. doi: 10.1002/cne.902530210. [DOI] [PubMed] [Google Scholar]
- 71.Gaillard D, Barlow LA. Taste bud cells of adult mice are responsive to Wnt/β-catenin signaling: Implications for the renewal of mature taste cells. Genesis. 2011;49:295–306. doi: 10.1002/dvg.20731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thirumangalathu S, Harlow DE, Driskell AL, Krimm RF, Barlow LA. Fate mapping of mammalian embryonic taste bud progenitors. Development. 2009;136:1519–28. doi: 10.1242/dev.029090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–7. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 74.Jaks V, Barker N, Kasper M, van Es JH, Snippert HJ, et al. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat Genet. 2008;40:1291–99. doi: 10.1038/ng.239. [DOI] [PubMed] [Google Scholar]
- 75.Ren W, Lewandowski BC, Watson J, Aihara E, Iwatsuki K, et al. Single Lgr5- or Lgr6-expressing taste stem/progenitor cells generate taste bud cells ex vivo. PNAS. 2014;111:16401–6. doi: 10.1073/pnas.1409064111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yee KK, Li Y, Redding KM, Iwatsuki K, Margolskee RF, Jiang P. Lgr5-EGFP marks taste bud stem/progenitor cells in posterior tongue. Stem Cells. 2013;31:992–1000. doi: 10.1002/stem.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tanaka T, Komai Y, Tokuyama Y, Yanai H, Ohe S, et al. Identification of stem cells that maintain and regenerate lingual keratinized epithelial cells. Nat Cell Biol. 2013;15:511–18. doi: 10.1038/ncb2719. [DOI] [PubMed] [Google Scholar]
- 78.Howard JM, Nuguid JM, Ngole D, Nguyen H. Tcf3 expression marks both stem and progenitor cells in multiple epithelia. Development. 2014;141:3143–52. doi: 10.1242/dev.106989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Boggs K, Venkatesan N, Mederacke I, Komatsu Y, Stice S, et al. Contribution of underlying connective tissue cells to taste buds in mouse tongue and soft palate. PLOS ONE. 2016;11:e0146475. doi: 10.1371/journal.pone.0146475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu HX, Komatsu Y, Mishina Y, Mistretta CM. Neural crest contribution to lingual mesenchyme, epithelium and developing taste papillae and taste buds. Dev Biol. 2012;368:294–303. doi: 10.1016/j.ydbio.2012.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Castillo D, Seidel K, Salcedo E, Ahn C, de Sauvage FJ, et al. Induction of ectopic taste buds by SHH reveals the competency and plasticity of adult lingual epithelium. Development. 2014;141:2993–3002. doi: 10.1242/dev.107631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mistretta CM. Taste papilla and taste bud maintenance, function and renewal are dependent on epithelial hedgehog signaling; Presented at Int. Symp. Olfaction Taste; 17th, Yokohama, Japan. 2016. [Google Scholar]
- 83.Kumari A, Ermilov AN, Allen BL, Bradley RM, Dlugosz AA, Mistretta CM. Hedgehog pathway blockade with the cancer drug LDE225 disrupts taste organs and taste sensation. J Neurophysiol. 2015;113:1034–40. doi: 10.1152/jn.00822.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang H, Cong WN, Yoon JS, Egan JM. Vismodegib, an antagonist of hedgehog signaling, directly alters taste molecular signaling in taste buds. Cancer Med. 2015;4:245–52. doi: 10.1002/cam4.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kumari A, Ermilov AN, Li L, Allen BL, Bradley RM, et al. Cessation of hedgehog pathway blockade leads to restoration of taste responses despite incomplete taste organ recovery. Assoc Chemorecept Sci. 2016:199. Abstr. [Google Scholar]
- 86.Kumari A, Ermilov AN, Li L, Allen BL, Bradley RM, et al. Pharmacologic and genetic disruption of Smoothened reveals dependence of taste organs on Hedgehog signaling. Assoc Chemorecept Sci. 2015:113. Abstr. [Google Scholar]
- 87.Gaillard D, Xu M, Liu F, Millar SE, Barlow LA. β-Catenin signaling biases multipotent lingual epithelial progenitors to differentiate and acquire specific taste cell fates. PLOS Genet. 2015;11:e1005208. doi: 10.1371/journal.pgen.1005208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schneider FT, Schanzer A, Czupalla CJ, Thom S, Engels K, et al. Sonic hedgehog actsas a negative regulator of β-catenin signaling in the adult tongue epithelium. Am J Pathol. 2010;177:404–14. doi: 10.2353/ajpath.2010.091079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mistretta CM. The role of innervation in induction and differentiation of taste organs: introduction and background. Ann NY Acad Sci. 1998;855:1–13. doi: 10.1111/j.1749-6632.1998.tb10542.x. [DOI] [PubMed] [Google Scholar]
- 90.Driskell RR, Watt FM. Understanding fibroblast heterogeneity in the skin. Trends Cell Biol. 2015;25:92–99. doi: 10.1016/j.tcb.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 91.Ivaska J, Pallari HM, Nevo J, Eriksson JE. Novel functions of vimentin in cell adhesion, migration, and signaling. Exp Cell Res. 2007;313:2050–62. doi: 10.1016/j.yexcr.2007.03.040. [DOI] [PubMed] [Google Scholar]
- 92.Snyder JC, Rochelle LK, Marion S, Lyerly HK, Barak LS, Caron MG. Lgr4 and Lgr5 drive the formation of long actin-rich cytoneme-like membrane protrusions. J Cell Sci. 2015;128:1230–40. doi: 10.1242/jcs.166322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sanders TA, Llagostera E, Barna M. Specialized filopodia direct long-range transport of SHH during vertebrate tissue patterning. Nature. 2013;497:628–32. doi: 10.1038/nature12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Morgan BA. The dermal papilla: an instructive niche for epithelial stem and progenitor cells in development and regeneration of the hair follicle. Cold Spring Harb Perspect Med. 2014;4:a015180. doi: 10.1101/cshperspect.a015180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gomez C, Chua W, Miremadi A, Quist S, Headon DJ, Watt FM. The interfollicular epidermis of adult mouse tail comprises two distinct cell lineages that are differentially regulated by Wnt, Edaradd, and Lrig1. Stem Cell Rep. 2013;1:19–27. doi: 10.1016/j.stemcr.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mathew E, Zhang Y, Holtz AM, Kane KT, Song JY, et al. Dosage-dependent regulation of pancreatic cancer growth and angiogenesis by hedgehog signaling. Cell Rep. 2014;9:484–94. doi: 10.1016/j.celrep.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Green BG. Chemesthesis and the chemical senses as components of a “chemofensor complex. Chem Senses. 2012;37:201–6. doi: 10.1093/chemse/bjr119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schutz B, Jurastow I, Bader S, Ringer C, von Engelhardt J, et al. Chemical coding and chemosensory properties of cholinergic brush cells in the mouse gastrointestinal and biliary tract. Front Physiol. 2015;6:87. doi: 10.3389/fphys.2015.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tizzano M, Finger TE. Chemosensors in the nose: guardians of the airways. Physiology. 2013;28:51–60. doi: 10.1152/physiol.00035.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Torrey TW. The relation of taste buds to their nerve fibers. J Comp Neurol. 1934;59:203–20. [Google Scholar]
- 101.Smith DV, Klevitsky R, Akeson RA, Shipley MT. Expression of the neural cell adhesion molecule (NCAM) and polysialic acid during taste bud degeneration and regeneration. J Comp Neurol. 1994;347:187–96. doi: 10.1002/cne.903470204. [DOI] [PubMed] [Google Scholar]
- 102.Zalewski AA. Regeneration of taste buds after reinnervation by peripheral or central sensory fibers of vagal ganglia. Exp Neurol. 1969;25:429–37. doi: 10.1016/0014-4886(69)90137-x. [DOI] [PubMed] [Google Scholar]
- 103.Guth L. The effects of glossopharyngeal nerve transection on the circumvallate papilla of the rat. Anat Rec. 1957;128:715–31. doi: 10.1002/ar.1091280406. [DOI] [PubMed] [Google Scholar]
- 104.Miura H, Kato H, Kusakabe Y, Tagami M, Miura-Ohnuma J, et al. A strong nerve dependence of sonic hedgehog expression in basal cells in mouse taste bud and an autonomous transcriptional control of genes in differentiated taste cells. Chem Senses. 2004;29:823–31. doi: 10.1093/chemse/bjh248. [DOI] [PubMed] [Google Scholar]
- 105.Kumari A, Allen BL, Bradley RM, Dlugosz AA, Mistretta CM. Role of innervation in HH signaling in the adult mouse fungiform taste papilla; Presented at Soc. Neurosci. Meet; 46th, San Diego, CA. 2016. [Google Scholar]
- 106.Brownell I, Guevara E, Bai CB, Loomis CA, Joyner AL. Nerve-derived Sonic hedgehog defines a niche for hair follicle stem cells capable of becoming epidermal stem cells. Cell Stem Cell. 2011;8:552–65. doi: 10.1016/j.stem.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Xiao Y, Thoresen DT, Williams JS, Wang C, Perna J, et al. Neural Hedgehog signaling maintains stem cell renewal in the sensory touch dome epithelium. PNAS. 2015;112:7195–200. doi: 10.1073/pnas.1504177112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Peterson SC, Eberl M, Vagnozzi AN, Belkadi A, Veniaminova NA, et al. Basal cell carcinoma preferentially arises from stem cells within hair follicle and mechanosensory niches. Cell Stem Cell. 2015;16:400–12. doi: 10.1016/j.stem.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hsu YC, Li L, Fuchs E. Emerging interactions between skin stem cells and their niches. Nat Med. 2014;20:847–56. doi: 10.1038/nm.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lander AD, Kimble J, Clevers H, Fuchs E, Montarras D, et al. What does the concept of the stem cell niche really mean today? BMC Biol. 2012;10:19. doi: 10.1186/1741-7007-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Epstein JB, Barasch A. Taste disorders in cancer patients: pathogenesis, and approach to assessment and management. Oral Oncol. 2010;46:77–81. doi: 10.1016/j.oraloncology.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 112.Hong JH, Omur-Ozbek P, Stanek BT, Dietrich AM, Duncan SE, et al. Taste and odor abnormalities in cancer patients. J Support Oncol. 2009;7:58–65. [PubMed] [Google Scholar]
- 113.Nguyen HM, Reyland ME, Barlow LA. Mechanisms of taste bud cell loss after head and neck irradiation. J Neurosci. 2012;32:3474–84. doi: 10.1523/JNEUROSCI.4167-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ruo Redda MG, Allis S. Radiotherapy-induced taste impairment. Cancer Treat Rev. 2006;32:541–47. doi: 10.1016/j.ctrv.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 115.Reifenberger J, Wolter M, Knobbe CB, Köhler B, Schonicke A, et al. Somatic mutations in the PTCH, SMOH, SUFUH and TP53 genes in sporadic basal cell carcinomas. Br J Dermatol. 2005;152:43–51. doi: 10.1111/j.1365-2133.2005.06353.x. [DOI] [PubMed] [Google Scholar]
- 116.Gailani MR, Ståhle-Bäckdahl M, Leffell DJ, Glynn M, Zaphiropoulos PG, et al. The role of the human homologue of Drosophila patched in sporadic basal cell carcinomas. Nat Genet. 1996;14:78–81. doi: 10.1038/ng0996-78. [DOI] [PubMed] [Google Scholar]
- 117.Amakye D, Jagani Z, Dorsch M. Unraveling the therapeutic potential of the Hedgehog pathway in cancer. Nat Med. 2013;19:1410–22. doi: 10.1038/nm.3389. [DOI] [PubMed] [Google Scholar]
- 118.Jacobsen AA, Aldahan AS, Hughes OB, Shah VV, Strasswimmer J. Hedgehog pathway inhibitor therapy for locally advanced and metastatic basal cell carcinoma: a systematic review and pooled analysis of interventional studies. JAMA Dermatol. 2016;152:816–24. doi: 10.1001/jamadermatol.2016.0780. [DOI] [PubMed] [Google Scholar]
- 119.Migden MR, Guminski A, Gutzmer R, Dirix L, Lewis KD, et al. Treatment with two different doses of sonidegib in patients with locally advanced or metastatic basal cell carcinoma (BOLT): a multicentre, randomised, double-blind phase 2 trial. Lancet Oncol. 2015;16:716–28. doi: 10.1016/S1470-2045(15)70100-2. [DOI] [PubMed] [Google Scholar]
- 120.Rodon J, Tawbi HA, Thomas AL, Stoller RG, Turtschi CP, et al. A phase I, multicenter, open-label, first-in-human, dose-escalation study of the oral smoothened inhibitor Sonidegib (LDE225) in patients with advanced solid tumors. Clin Cancer Res. 2014;20:1900–9. doi: 10.1158/1078-0432.CCR-13-1710. [DOI] [PubMed] [Google Scholar]
- 121.Sekulic A, Migden MR, Oro AE, Dirix L, Lewis KD, et al. Efficacy and safety of Vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171–79. doi: 10.1056/NEJMoa1113713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tang JY, Mackay-Wiggan JM, Aszterbaum M, Yauch RL, Lindgren J, et al. Inhibiting the hedgehog pathway in patients with the basal-cell nevus syndrome. N Engl J Med. 2012;366:2180–88. doi: 10.1056/NEJMoa1113538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pan S, Wu X, Jiang J, Gao W, Wan Y, et al. Discovery of NVP-LDE225, a potent and selective smoothened antagonist. ACS Med Chem Lett. 2010;1:130–34. doi: 10.1021/ml1000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Basset-Seguin N, Sharpe HJ, de Sauvage FJ. Efficacy of Hedgehog pathway inhibitors in basal cell carcinoma. Mol Cancer Ther. 2015;14:633–41. doi: 10.1158/1535-7163.MCT-14-0703. [DOI] [PubMed] [Google Scholar]
- 125.Miyazaki Y, Matsubara S, Ding Q, Tsukasa K, Yoshimitsu M, et al. Efficient elimination of pancreatic cancer stem cells by hedgehog/GLI inhibitor GANT61 in combination with mTOR inhibition. Mol Cancer. 2016;15:49. doi: 10.1186/s12943-016-0534-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rimkus TK, Carpenter RL, Qasem S, Chan M, Lo HW. Targeting the sonic hedgehog signaling pathway: review of smoothened and GLI inhibitors. Cancers. 2016;8:22. doi: 10.3390/cancers8020022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tang Y, Gholamin S, Schubert S, Willardson MI, Lee A, et al. Epigenetic targeting of Hedgehog pathway transcriptional output through BET bromodomain inhibition. Nat Med. 2014;20:732–40. doi: 10.1038/nm.3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nagraj SK, Naresh S, Srinivas K, Renjith George P, Shrestha A, et al. Interventions for the management of taste disturbances. Cochrane Database Syst Rev. 2014;11:CD010470. doi: 10.1002/14651858.CD010470.pub2. [DOI] [PubMed] [Google Scholar]