Abstract
Objective
Studies of sex differences in heart rate variability (HRV) typically have not accounted for the influence of family history (FH) of cardiovascular disease (CVD). This study evaluated sex differences in HRV response to speech stress among men and women (age range 30–49 years) with and without a documented FH of CVD.
Methods
Participants were 77 adults (mean age=39.8 + 6.2 years; range: 30–49 years; 52% female) with positive FH (FH+, n=32) and negative FH (FH-, n=45) of CVD, verified with relatives of participants. Cardiac activity for all participants was recorded via electrocardiogram during a standardized speech stress task with three phases: 5-minute rest, 5-minute speech, and 5-minute recovery. Outcomes included time domain and frequency domain indicators of HRV and heart rate (HR) at rest and during stress. Data were analyzed with repeated measures analysis of variance, with sex and FH as between subject variables and time/phase as a within subject variable.
Results
Women exhibited higher HR than did men and greater HR reactivity in response to the speech stress. However, women also exhibited greater HRV in both the time and frequency domains. FH+ women generally exhibited elevated HRV, despite the elevated risk of CVD associated with FH+.
Conclusions
Although women participants exhibited higher HR at rest and during stress, women (both FH+ and FH-) also exhibited elevated HRV reactivity, reflecting greater autonomic control. Thus, enhanced autonomic function observed in prior studies of HRV among women is also evident among FH+ women during a standardized stress task.
Keywords: autonomic function, cardiovascular disease, family history, heart rate variability, parasympathetic nervous system, sex differences
Cardiovascular disease (CVD) remains the leading cause of death in the world [1] despite major reductions in the incidence of CVD during recent decades. Reduced CVD incidence has resulted, in part, from greater awareness of risk factors for CVD and better management of risk. Risk factors for CVD can be categorized into major non-modifiable risk factors (older age, male sex, positive family history), major modifiable risk factors (e.g., hypertension, diabetes, high cholesterol), and modifiable behavioral factors (e.g., smoking, low physical activity, poor diet, excessive alcohol consumption, stress) [2,3]. Excess physiological reactivity to stress also has been posited as a CVD risk factor. Sex is a non-modifiable factor of particular interest because women generally develop CVD approximately 10 years later in life than do men [4]. Studies of sex differences in autonomic function, both at rest and during psychological stress, reveal that premenopausal women generally exhibit higher heart rate (HR) [5] at rest and during stress, relative to men.
One explanation for the paradoxical finding of higher HR and lower CVD risk is that women also may have higher parasympathetic autonomic activity than men, thereby providing protection from at least some forms of CVD (arrhythmia, coronary heart disease). Data from a number of studies indicate greater relative vagal autonomic control among women, as reflected by several parameters in the frequency domain of HRV including lower low frequency (LF) power and lower ratio of LF to high frequency (HF), LF/HF power [6–9]. In addition, a recent meta-analysis of 172 studies evaluating sex differences in HRV confirmed that women generally exhibit higher HR than men, but that women also exhibit greater vagally-mediated autonomic control than men [10].
Whereas past studies of sex differences typically have evaluated HRV at rest or during exercise, postural manipulation, or 24-hour monitoring, fewer studies have addressed psychological/behavioral mechanisms in a controlled laboratory environment. Studies of HRV in the context of a laboratory stressor facilitate evaluation of psychosocial mechanisms that may contribute to any observed sex differences.
Positive family history (FH) of CVD is a significant risk factor for CVD among offspring [11]. Thus, understanding the influence of FH on CVD is critical as an indicator of genetic or family environment influences in sex-related stress responses. Middle-aged individuals with a positive FH of CVD, for example, exhibit exaggerated cardiovascular reactivity to a variety of laboratory stressors, with men having a more consistent and robust response than women [5,12]. Wright and colleagues [13] examined HRV responses to stress among young adults with (n=75) and without (n=16) a FH of CVD. In response to psychosocial stress (combination of Stroop task and public speaking task), positive family history of CVD (FH+) was associated with greater HR and HRV reactivity in response to stress only among women. FH did not have an influence on HRV among men. This study examined only the time domain measures of HRV, specifically the root mean square of successive differences (RMSSD); and FH of CVD was defined very broadly to incorporate parent or grandparent history of diabetes, heart disease, high cholesterol, or high blood pressure. FH+ participants did not necessarily have a family history of heart disease, but could be defined as FH+ with a history of two or more related conditions (hypertension, diabetes, high cholesterol) in either parent or a history of three or more related conditions in any grandparent, or a combination of parent and grandparent risk. The resulting sample of FH+ participants represented individuals with greater risk of cardiovascular-related disease, but this operationalization of FH+ is broader than typically used to define FH+ in identifying individuals with elevated genetic risk of heart disease. In addition, the sample was relatively young (age range 18–25).
The current study was designed to evaluate sex differences in HRV response to speech stress among men and women (age range 30–49 years) with and without a documented FH of heart disease. Men and women in the fourth and fifth decade of life were selected for study because they are likely to be experiencing age-related changes in physical function and cardiovascular fitness, but not yet likely to have a diagnosis of CVD. The study utilized a more stringent definition of FH+ than the Wright et al. [13] study and incorporated both time domain and frequency domain indicators of HRV, with the goal of both replicating past findings in this area of research and further extending the work to better explicate possible mechanisms underlying observed sex differences in heart disease risk.
Parental history of myocardial infarction (MI) was used as the indicator of CVD risk to identify individuals with heightened genetic/environmental risk of CVD because both paternal and maternal history of MI increases risk of MI in adult children [14], and because parental history of MI is associated with greater magnitude of blood pressure and heart rate response to stress [15]. In addition, previous investigations have indicated that reports of parental MI by adult offspring are accurate due to the salience of the medical event [16,17], and awareness of parental MI does not require broader knowledge about parent health status or health care utilization. Thus, adults were recruited who would be considered higher risk due to parent history of MI. Based on prior studies, it was hypothesized that women would exhibit better autonomic control as reflected by lower LF and LF/HF ratio and higher vagally-mediated HRV than men. In addition, consistent with data from Wright et al. [13], it was hypothesized that HRV reactivity would be greater among women than among men, and that reactivity would be most pronounced among FH+ women.
Methods
Participants
This study included 77 adults (mean age=39.8 + 6.2 years; range: 30–49 years; 52% female) with confirmed FH [both positive (n=32) and negative (n=45)] of CVD. The sample included somewhat more women (56%) than men with positive FH. As shown in Table 1, there were only five self-reported smokers in the study, with most of them (n=4) in the FH+ group.
Table 1.
| Women (n=40) | Men (n=37) | |||
|---|---|---|---|---|
| FH+ (n=18) | FH−(n=22) | FH+ (n=14) | FH−(n=23) | |
| Variable | M (SE) or % | M (SE) or % | M (SE) or % | M (SE) or % |
| Age (years) | 42.9 (1.4) a | 37.5 (1.3)a | 38.9 (1.6) | 40.2 (1.3) |
| Ethnicity (% white) | 78 | 82 | 86 | 70 |
| Body mass index | 29.6 (1.2) a | 25.0 (1.1)a,b | 28.1 (1.4) | 28.8 (1.1)b |
| Cigarette smokers (%) | 11 | 0 | 14 | 4 |
| Baseline heart rate (bpm) Peak HF (hz)1 |
64.9 (2.6) 0.27 (0.01) |
65.7 (2.5) 0.25 (0.01) |
60.4 (2.9) 0.28 (0.02) |
59.8 (2.2) 0.26 (0.01) |
Note: Means in a row sharing superscripts are significantly different:
p<.01,
p<.05;
FH+=positive family history of cardiovascular disease; FH−=negative family history of cardiovascular disease;
Mean (SD) central frequency of the high frequency (HF) spectral peak
Data were collected as part of the Healthy Heart Project, designed to evaluate cardiovascular, lipid, and neuroendocrine responses to stress among community-residing healthy adults with and without a FH of CVD. Prospective participants were recruited via on-line and print advertising for a study requiring three visits to the laboratory, including a screening visit, followed by two experimental visits, separated by at least one week. At the screening visit, participants completed a thorough review of medical history and had a blood draw for measurement of cholesterol, as well as a resting electrocardiogram (ECG). The two subsequent visits included an exercise stress at one visit and a speech stress at the other visit, with the order of visits randomly assigned. HRV data for this study came from the speech stress visit.
Prospective participants were screened by telephone prior to scheduling an initial screening visit. The telephone screen included questions pertaining to sex and family history of CVD, with the goal of recruiting balanced numbers of men and women with and without FH of CVD. In addition, women in the study were premenopausal with normal menstrual cycles (menstrual periods for the previous 3 months occurring every 26–34 days) as assessed by self-report. Women were excluded if they were pregnant, nursing, or using oral contraceptives during the previous 6 months. Prospective participants also were excluded if taking medication for diabetes, hypertension, or CHD; or if diagnosed with renal disease, hyperlipidemia, or any other metabolic disease. Any potential participant with a history of cancer, hepatitis, epilepsy, psychiatric illness, hypertension, or CHD was excluded. A total of 277 prospective participants passed the initial telephone screen and were scheduled for a visit, but 48 of them did not attend the screening visit and could not be contacted or were not interested in re-scheduling. Of the 229 participants who completed the screening visit, 15 were excluded due to still not meeting study criteria (e.g., age limit, use of psychiatric medication, elevated resting blood pressure, difficulty with blood draw, abnormal electrocardiogram). Prospective participants completed a detailed questionnaire and interview concerning FH of CVD, and MI specifically. In addition, participants completed the Beck Depression Inventory to confirm the absence of significant depression. No participants were excluded due to depression scores. Following the screening visit, 53 participants did not complete further study visits, primarily due to time conflicts with work or other life demands, resulting in a sample of 161 participants who completed the screening visit and at least one additional visit. Eleven of those participants did not complete the speech stress visit, resulting in 150 participants with HRV data for this study. Participants received up to $200 for completing all visits. Participants who completed only the screening visit received $10.
All participants in the study reported only one parent with history of MI. Participants provided the name and address of the parent and agreed to allow study staff to contact parents to verify history of CVD. If the parent with history of MI was deceased, the surviving spouse was contacted for confirmation. Mailings included a brief letter explaining the study and indicating that the respondent’s child had given permission for parental contact. The mailing included a one-page ‘medical history questionnaire’ asking the parent to indicate whether or not he/she had had a prior heart attack and, if yes, the date and their age at the time. Parents were provided with a self-addressed, stamped envelope to return the questionnaire and a gift card was enclosed in appreciation of their participation. For participants to be included in the study, information from the parent had to be consistent with the participant’s self-report. Confirmation of reported medical history was received from approximately half (51%, n=77) of the parents of study participants.
Procedure
After completing the preliminary screening session, eligible subjects were invited to continue their participation on a separate testing day. Participants were required to be fasting (12 hours) and were told to refrain from exercise and from consuming alcohol during the 24 hours prior to the appointment. Participants completed informed consent and a packet of questionnaires. Body weight and height were assessed on a calibrated balance scale, and body mass index (BMI) was calculated as a measure of weight relative to height (kg/m2).
Participants then completed the laboratory protocol in a sound-attenuated and temperature-controlled laboratory. Heart rate was measured by placing electrodes in a modified lead II configuration on the participant’s chest. Cardiac activity data was recorded continuously via a 7-lead ECG at a sampling rate of 1000 Hz using a Mindware™ 2000D (MW2000D) Impedance Cardiograph package. Participants were seated in a comfortable recliner and a catheter was inserted by a study nurse for blood draws during the final three minutes of each period of the task. Blood data were not analyzed for this study.
The stress reactivity task was an impromptu videotaped speech with three periods. Following a 5-minute resting baseline, the participant was given a set of videotaped instructions requiring that he/she mentally prepare (2-min) and then deliver (3-min) a videotaped speech about a hypothetical, ambiguous situation in which the participant was unjustly accused of a crime but shares some responsibility for the accusation, and must defend him/herself. Participants were told to speak directly into the video camera, and to continue speaking for the entire 3-min period. They were told that speeches were being video-recorded on a closed-circuit system, and were immediately evaluated on the basis of poise, articulation, and effectiveness. After the 5-minute speech stress, participants remained seated quietly during a 5-minute recovery period.
Cardiac data were evaluated using the following procedures from each 5-minute period: baseline, task, and recovery. Variability between successive r-spikes (or variability within inter-beat intervals (IBIs)) was obtained from ECG recordings to calculate HRV. Successive IBIs, in milliseconds, were extracted using HRV 2.51 Analysis software (Mindware Technologies, LTD), and were written in a text file for analysis using the Kubios HRV analysis package 2.0 [18]. Kubios was used to calculate both time- and frequency-domain indices of HRV in accordance with Task Force [19] guidelines. Artifacts within the R-to-R series were visually detected, and a correction was applied to differentiate and remove artifacts (parsing out abnormal IBIs from the overall mean IBI) using a piecewise cubic spline interpolation method. The mean standard deviation of RR intervals (SDNN) was used as an index of HRV. Additionally, the RMSSD, measured in milliseconds, was used as a stable and valid time-domain measure of vagally-mediated HRV [19,20]. Autoregressive estimates from spectral analyses yielded both low-frequency power HRV (LF, 0.04 – 0.15 Hz) and high-frequency power HRV (HF, 0.15–0.4 Hz). Generally, LF may represent activity of both sympathetic and parasympathetic nervous systems, but is primarily of parasympathetic influence [19]. HF is considered both a stable and valid index of vagally-mediated HRV [19,20]. The LF/HF ratio was calculated to approximate sympathovagal balance [19,21]. RMSSD, HF, and LF values were natural log transformed (ln) to fit the assumptions of linear analyses. The peak of the HF spectral component of HRV was used as an index of respiratory frequency [22].
Using Mindware Technologies® Impedance Cardiography Analysis software, mean pre-ejection period (PEP) was also calculated (in milliseconds) in accordance with previously published guidelines [23]. PEP is defined as the duration between initial ventricular depolarization and opening of the aortic valve (beginning of left ventricle ejection). Thus, greater PEP values reflect lower sympathetic nervous system activity.
Data analysis
The Proc Mixed procedure in SAS was utilized to conduct repeated measures analyses of HRV parameters with FH and sex as between subjects factors and time (baseline, stress-task, recovery) as a within subjects factor. Analyses were performed on mean HR as well as both time and frequency measures of HRV. Time domain indicators included SDRR and RMSSD. Frequency domain indicators included HF, LF, and the LF/HF ratio. In addition, PEP was evaluated as an indicator of beta-adrenergic influence on cardiac function. Repeated measures analyses of variance (ANOVAs) were performed on all cardiac outcomes (mean HR, SDNN, RMSSD, LF, HF, LF/HF ratio, and PEP) to evaluate the influence of sex and FH on HRV over the course of the laboratory task, and to evaluate interactions among the three factors (i.e., FH, sex, time). All significant interactions were evaluated with tests of simple effects, and Cohen’s d was calculated as an indicator of effect size.
Results
Preliminary ANOVAs were conducted comparing data from the 77 participants in this study with the 72 participants for whom parental status of CVD could not be confirmed. Results indicated no significant differences in age or BMI, and no differences on any of the resting HRV measures. Similarly, chi-square analyses indicated no differences by sex or PH in the two groups. Thus, the sample available for this study appears to be representative of the full sample recruited.
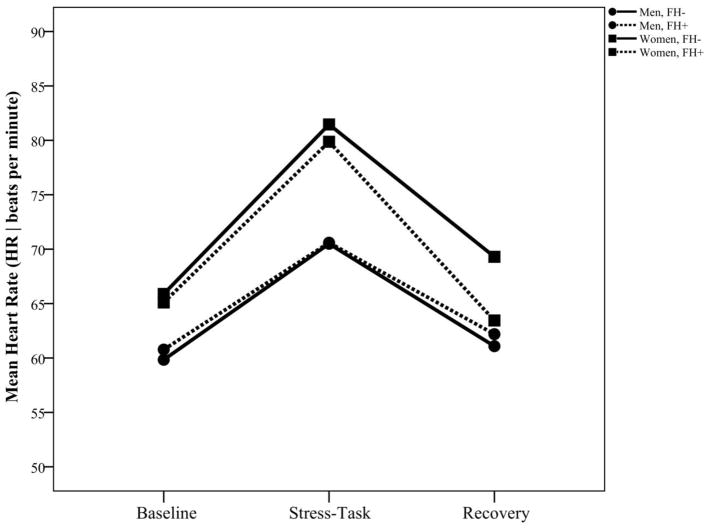
Correlational analyses revealed that age and BMI were not significantly associated with HRV, possibly due to the relatively restricted ranges in both age and BMI in this sample. As shown in Table 1, ANOVAs revealed no sex difference or FH difference in respiration frequency, using central frequency of the HF spectral peak as an index. Repeated measures analysis of HR indicated a sex main effect [F(1, 70)=9.43, p=.003, d=.53], as well as a time by sex interaction [F(2, 119)=3.37, p=.038, d=.48]. Tests of simple effects indicated that both men and women exhibited significant increases in HR from baseline to task (ps<.001) and decreases from task to recovery (ps<.001), but change in HR from baseline to task was greater in women (15.2 bpm) than men (10.2 bpm; [F(1, 61)= 9.21, p=.004, d=.78]. Thus, consistent with data from prior studies [e.g., 10,13], overall HR was higher among women than among men, and HR reactivity was greater among women, as shown in Figure 1. Due to the sex difference in HR, correlational analyses were conducted evaluating the association of HR with other HRV parameters. Analyses revealed that resting HR was associated with baseline RMSSD [r(69)=−.51, p<.001], therefore HR was included as a covariate in analyses of RMSSD.
Figure 1.
Heart rate (HR) at rest, during speech task, and during recovery among women and men with and without a family history of cardiovascular disease.
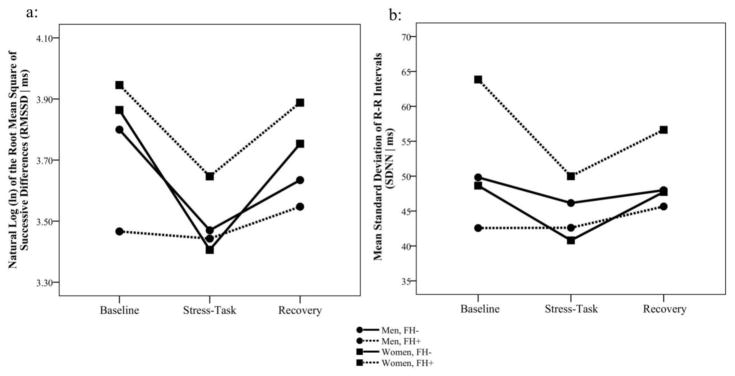
Examination of time domain HRV parameters revealed a sex main effect for RMSSD [F(1, 64)=18.24, p<.001, d=1.07] as well as interactions of time by sex [F(2, 115)=3.28, p=.041, d=.48] and time by FH [F(2, 115)=3.89, p=.023, d=.52] for RMSSD, as shown in Figure 2a. Tests of simple effects indicated significant decreases in RMSSD for men (Δ=0.17 ms, p=.005) and women (Δ=0.38 ms, p<.001) from baseline to task, as well as increases in men (Δ=0.13 ms, p=.043) and women (Δ=0.29 ms, p<.001) from task to recovery. Although change in RMSSD from baseline to task did not differ by sex, the change in RMSSD from task to recovery was greater among women [F(1, 52)= 17.64, p<.001, d=1.16]. Follow up analysis of the time by FH interaction indicated that recovery values among FH- participants were significantly lower than baseline values [Difference=0.14 ms, t(115)=2.44, p=.016, d=.46], but there was no difference between baseline and recovery among FH+ participants. Analyses of SDNN revealed a sex by FH interaction [F(1, 70)=4.91, p=.030, d=.53], evident in Figure 2b. Simple effects testing indicated that among FH+ participants, SDNN was higher for women (51.3 ms, SE=2.40) than for men [45.8 ms, SE=2.54; t(70)=2.51, p=.014, d=.60]; and that there was a FH difference of 11.1 ms among women [t(70)=2.31, p=.024, d=.55], but no FH effect among men. Together, the data indicate that women exhibited greater stress-related changes in time domain HRV parameters than men, especially in the recovery phase, and that FH+ was associated with greater HRV, primarily in FH+ women.
Figure 2.
Time domain variables of heart rate variability (HRV) at rest, during speech task, and during recovery among women and men with and without a family history of cardiovascular disease. a: Root mean square of successive differences (RMSSD); b: Standard deviation of RR intervals (SDNN).
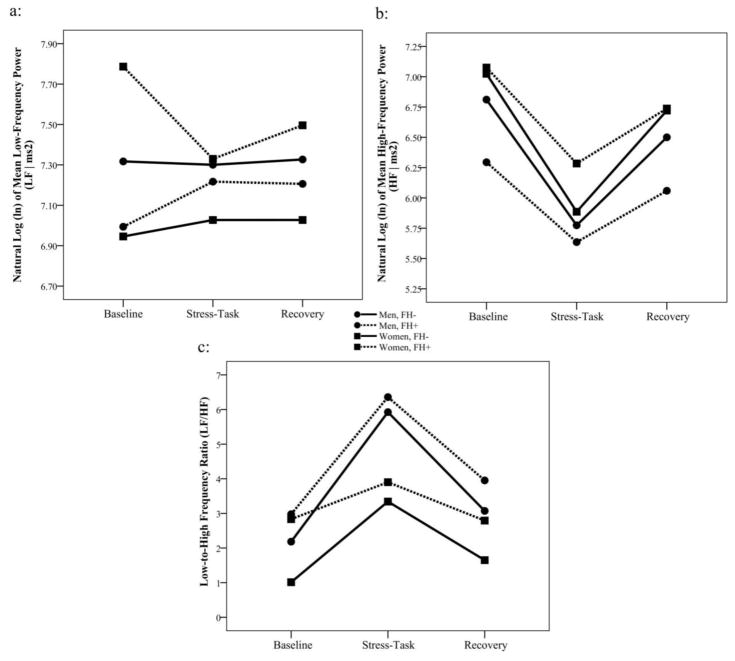
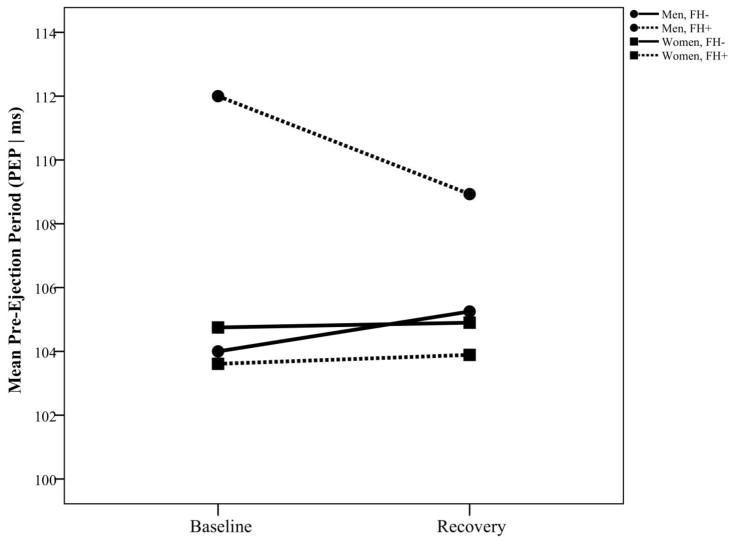
A similar pattern of results was evident in the frequency domain indicators. There was a sex by FH interaction for LF [F(1, 68)=5.50, p=.022, d=.57], with LF being significantly higher among FH+ women (7.54 ms2, SE=0.154) than among FH- women (7.00 ms2, SE=0.143), but no FH differences among men, as shown in Figure 3a. Also, there was an overall sex difference for HF, with women exhibiting higher HF (6.63 ms2, SE=0.136) than men [6.13 ms2, SE=0.143; F(1, 69)=6.29, p=.015, d=.60] throughout the laboratory task, but no interactions with time or with FH (Figure 3b). For the LF/HF ratio, there was an overall sex difference [F(1, 69)=8.22, p=.006, d=.69], with men exhibiting a higher ratio (4.08, SE=0.380) than women (2.58, SE=0.358, Figure 3c). In addition, for PEP (measured only at baseline and recovery) there was a sex main effect [F(1, 70)=4.97, p=.029, d=.53], indicating that PEP values overall were higher for men (107.5 ms, SE=1.064) than for women (104.3 ms, SE=1.002). In addition, there was a sex by FH interaction [F(1, 70)=5.60, p=.021, d=.57], with men having higher values than women (Difference = 6.71 ms) only in FH+ individuals (t(70)=3.08, p=.003, d=.65), as shown in Figure 4.
Figure 3.
Frequency domain variables of heart rate variability (HRV) at rest, during speech task, and during recovery among women and men with and without a family history of cardiovascular disease. a: Low frequency (LF) power HRV; b: High frequency (HF) power HRV; c: LF/HF ratio.
Figure 4.
Pre-ejection period (PEP) at rest and during recovery (following speech stress) among women and men with and without a family history of cardiovascular disease.
Discussion
These data extend findings of prior studies documenting higher HR among women [10] and the apparent paradox of lower risk of CVD among women than among men. The present data indicate that vagally-mediated HRV in response to a controlled stressor remains generally higher among women than among men, as demonstrated in a number of past studies [10], thereby helping to explain the paradox. In addition, data from this study expand on the only prior study evaluating sex differences in HRV response to a laboratory stressor [13] among FH+ individuals, reflecting greater time domain HRV among FH+ women. These data indicate that both time and frequency domain indicators of HRV response to a laboratory stressor were generally higher among women than among men, thereby reflecting better autonomic tone in the context of acute stress, and possibly conferring lower CVD risk among women. The observed effect sizes generally ranged from medium to large, and are comparable to prior studies of sex differences in HRV (10).
Consistent with study hypotheses, the higher LF/HF ratio among FH+ individuals is suggestive of greater sympathetic response to stress in the FH+ participants, generally reflecting increased cardiovascular risk. However, among FH+ women both time and frequency dimensions of HRV were relatively elevated (e.g., SDNN, RMSSD, HF, LF). Thus, among FH+ women there was evidence of greater parasympathetic activation (including lower LF/HF ratio). These data are consistent with data from past studies among healthy males and females ranging in age from teenagers to octogenarians [9], and the LF/HF ratio data are consistent with resting HRV data from the Atherosclerosis Risk in Communities (ARIC) study [8], as well as 24-hour HRV data [7] and resting HRV data among young and middle-aged adults [6]. Overall, these data suggest that higher parasympathetic activity among women relative to men at rest is sustained during the psychological stress of a speech task. In particular, FH+ women who would typically be identified as having higher risk for CVD, generally exhibited relatively higher parasympathetic response to stress, consistent with lower risk of CVD. Women had lower PEP than men, reflecting greater sympathetic activity. Because PEP was measured only during the two resting states (baseline and recovery), these data suggest greater beta-adrenergic drive to the heart at rest in women than in men. In combination with higher vagally-mediated HRV at rest among women, the PEP data suggest that women had greater autonomic drive (both sympathetic and parasympathetic) at rest than men.
The physiological basis for the sex effects may originate in the central nervous system. It has been found that oxytocin-type neurons from the paraventricular nucleus synapse on cardiovagal neurons in the nucleus tractus solitarius, dorsal motor nucleus of the vagus, and the nucleus ambiguus [24,25]. Excitation of these neurons increases vagal outflow while having no effect on sympathetic outflow [25]. Of particular relevance to the present study, these neurons seem to be involved in maintaining greater vagal tone, especially during stressful events [25,26]. Oxytocin-related effects may be particularly evident in women and may therefore help explicate the current results among FH+ women.
The study had several limitations, including a relatively smaller sample size. In recruiting and retaining participants, the age range of the sample may have posed an unexpected challenge due to the demands of work and home life in this age group limiting the time available for study participation. In addition, the procedures employed to confirm parental history of CVD required verification from parents who never had in-person contact with study personnel and may have had less motivation for complying with study requests. This study also is limited by the cross-sectional design, precluding causal interpretation. Although participants were ostensibly healthy and capable of strenuous exercise, it is possible that sub-clinical changes in cardiovascular function may have influenced the observed sex differences. Furthermore, all women in the study were pre-menopausal, thus cardioprotective effects of female hormones may partially explain the observed effects. It is also important to consider that the physiological outcomes of this study (i.e., HRV and PEP) are not direct measures of sympathetic or parasympathetic activity, and are reliant on end-organ sensitivity. Strengths of the study include the use of a controlled laboratory stressor, the use of both time and frequency domain measures of HRV, and recruitment of adults with parental confirmation of CVD family history.
The study results reflect greater HR reactivity and greater HRV among women than among men in the context of a laboratory stressor. Because parasympathetic activation in response to stress among FH+ women appeared to be greater than that of men, sex differences in the reactivity of the cardiovascular system may be a mediator in the relationship between the genetic or environmental influences of FH and risk for differential development of CVD later in life. Future research with longitudinal follow up among FH+ adults would help to clarify the mechanisms by which genetic and environmental risk for CVD may lead to disease in both men and women.
Highlights.
Women exhibit higher heart rate than men, but also higher heart rate variability
Men and women with family history of heart disease had sympathetic stress response
Women with family history of heart disease also had parasympathetic stress response
Better autonomic tone in women during stress reflects lower risk of heart disease
Acknowledgments
This work was supported by the National Institutes of Health (Grant HL068956 from the National Heart Lung and Blood Institute; and Grant UL1TR001070 from the National Center for Advancing Translational Sciences).
Footnotes
Disclosures
The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the U.S. Government.
Conflicts of interest and source of funding
The authors have no competing interests to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367:1747–57. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- 2.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després J-P, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB on behalf of the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2016 update. Circulation. 2016;133:e38–e360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 3.Thayer JF, Yamamoto SS, Brosschot JF. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int J Cardiol. 2010;141:122–131. doi: 10.1016/j.ijcard.2009.09.543. [DOI] [PubMed] [Google Scholar]
- 4.Anand SS, Islam S, Rosengren A, Franzosi MG, Steyn K, Yusufali AH, Keltai M, Diaz R, Rangarajan S, Yusuf S on behalf of the INTERHEART Investigators. Risk factors for myocardial infarction in women and men: insights from the INTERHEART study. Eur Heart J. 2008;29:932–940. doi: 10.1093/eurheartj/ehn018. [DOI] [PubMed] [Google Scholar]
- 5.Stoney CM, Matthews KA. Sex differences in physiological responses to stress and in coronary heart disease: A causal link? Psychophysiology. 1987;24:127–131. doi: 10.1111/j.1469-8986.1987.tb00264.x. [DOI] [PubMed] [Google Scholar]
- 6.Agelink MW, Malessa R, Baumann B, Majewski T, Akila F, Zeit T, Ziegler D. Standardized tests of heart rate variability: normal ranges obtained from 309 healthy humans, and effects of age, gender, and heart rate. Clin Auton Res. 2001;11:99–108. doi: 10.1007/BF02322053. [DOI] [PubMed] [Google Scholar]
- 7.Jensen-Urstad K, Storck N, Bouvier F, Ericson M, Lindbland LE, Jensen-Urstad M. Heart rate variability in healthy subjects is related to age and gender. Acta Physiologica. 1997;160:235–241. doi: 10.1046/j.1365-201X.1997.00142.x. [DOI] [PubMed] [Google Scholar]
- 8.Liao D, Barnes RW, Chambless LE, Simpson RJ, Sorlie P, Heiss G ARIC investigators. Age, race, and sex differences in autonomic cardiac function measured by spectral analysis of heart rate variability—the ARIC study. Am J Cardiol. 1995;76:906–912. doi: 10.1016/s0002-9149(99)80260-4. [DOI] [PubMed] [Google Scholar]
- 9.Zhang J. Effect of age and sex on heart rate variability in healthy subjects. J Manipulative Physiol Ther. 2007;30:374–379. doi: 10.1016/j.jmpt.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Koenig J, Thayer JF. Sex differences in healthy human heart rate variability: A meta-analysis. Neurosci Biobehav Rev. 2016;64:288–310. doi: 10.1016/j.neubiorev.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 11.Myers RH, Kiely DK, Cupples LA, Kannel WB. Parental history is an independent risk factor for coronary artery disease: the Framingham Study. Am Heart J. 1990;120:963–969. doi: 10.1016/0002-8703(90)90216-k. [DOI] [PubMed] [Google Scholar]
- 12.Light KC, Girdler SS, Sherwood A, Bragdon EE, Brownley KA, West SG, Hinderliter AL. High stress responsivity predicts later blood pressure only in combination with positive family history and high life stress. Hypertension. 1999;33:1458–1464. doi: 10.1161/01.hyp.33.6.1458. [DOI] [PubMed] [Google Scholar]
- 13.Wright CE, O’Donnell K, Brydon L, Wardle J, Steptoe A. Family history of cardiovascular disease is associated with cardiovascular responses to stress in healthy young men and women. Int J Psychophysiol. 2007;63:275–282. doi: 10.1016/j.ijpsycho.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Sesso HD, Lee IM, Gaziano JM, Rexrode KM, Glynn RJ, Buring JE. Maternal and paternal history of myocardial infarction and risk of cardiovascular disease in men and women. Circulation. 2001;104:393–398. doi: 10.1161/hc2901.093115. [DOI] [PubMed] [Google Scholar]
- 15.Stoney CM, Hughes JW. Lipid reactivity among men with a parental history of myocardial infarction. Psychophysiology. 1999;36:1–7. doi: 10.1017/s0048577299981283. [DOI] [PubMed] [Google Scholar]
- 16.Hastrup JL, Hotchkiss AP, Johnson CA. Accuracy of knowledge of family history of cardiovascular disorders. Health Psychol. 1985;4:291–306. doi: 10.1037//0278-6133.4.4.291. [DOI] [PubMed] [Google Scholar]
- 17.Kee F, Tiret L, Robo JY, Nicaud V, McCrum E, Evans A, Cambienet F. Reliability of reported family history of myocardial infarction. BMJ. 1993;307:1528–1530. doi: 10.1136/bmj.307.6918.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tarvainen MP, Niskanen JP, Lipponen JA, Ranta-Aho PO, Karjalainen PA. Kubios HRV–heart rate variability analysis software. Comput Methods Programs Biomed. 2014;113:210–220. doi: 10.1016/j.cmpb.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 19.Task Force of the European Society of Cardiology. Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- 20.Thayer JF, Hansen AL, Johnsen BH. The non-invasive assessment of autonomic influences on the heart using impedance cardiography and heart rate variability. In: Steptoe A, editor. Handbook of behavioral medicine. New York: Springer; 2010. pp. 723–40. [Google Scholar]
- 21.Williams DP, Wiley C, Rahman T, Barton A, Gerardo GM, Hill LK, Koenig J, Spangler D, Thayer JF. Re-examining the association between the low-to-high-frequency ratio and cardiac autonomic balance and regulation: a focus on systolic time intervals. Biomed Sci Instrum. 2017 in press. [Google Scholar]
- 22.Thayer JF, Sollers JJ, Ruiz-Padial E, Vila J. Estimating respiratory frequency from autoregressive spectral analysis of heart period. IEEE Eng Med Biol Mag. 2002;21:41–45. doi: 10.1109/memb.2002.1032638. [DOI] [PubMed] [Google Scholar]
- 23.Allen MT, Fahrenberg J, Kelsey RM, Lovallo WR, Doornen LJ. Methodological guidelines for impedance cardiography. Psychophysiology. 1990;27:1–23. doi: 10.1111/j.1469-8986.1990.tb02171.x. [DOI] [PubMed] [Google Scholar]
- 24.Luiten P, ter Horst G, Karst H, Steffens A. The course of paraventricular hypothalamic efferents to autonomic structures in medulla and spinal cord. Brain Res. 1985;329:374–378. doi: 10.1016/0006-8993(85)90554-2. [DOI] [PubMed] [Google Scholar]
- 25.Higa KT, Mori E, Viana FF, Morris M, Michelini LC. Baroreflex control of heart rate by oxytocin in the solitary-vagal complex. Am J Physiol. 2002;282:R537–45. doi: 10.1152/ajpregu.00806.2000. [DOI] [PubMed] [Google Scholar]
- 26.Higa-Taniguchi KT, Felix JV, Michelini LC. Brainstem oxytocinergic modulation of heart rate control in rats: effects of hypertension and exercise training. Exp Physiol. 2009;94:1103–1113. doi: 10.1113/expphysiol.2009.049262. [DOI] [PubMed] [Google Scholar]