Abstract
The aim of this paper is to provide the reader with a view of the Endocrine Disruptor Chemical (EDC) research field and its relevance to human health. My perspective is from working on the effects of EDCs that act via the androgen (A) or estrogen (E) signaling pathways in a regulatory agency for the last four decades with the objective of producing data that risk assessors could use to reduce the uncertainty in risk assessment. In vitro and in vivo data from our studies has contributed to regulatory agencies decision-making since the 1990s (https://www3.epa.gov/pesticides/chem_search/cleared_reviews/csr_PC-113201_7-Apr-98_238.pdf). From the start, we were evaluating the utility of in vitro and short-term in vivo effects to predict the adverse effects in developing animals [1; 2]. This approach has expanded greatly to include what is now known as Adverse Outcome Pathways (AOP) and networks (AOPn) [3; 4]. The AOP framework for the effects of chemicals that disrupt androgen signaling during sexual differentiation of the fetal male rat provides biological context for extrapolating mechanistic information from in vitro and in vivo assays in rodents to other species including humans. Such an approach has biological validity because the E and A pathways are highly conserved in vertebrates, including humans and laboratory animals.
Keywords: Adverse Outcome Pathways and Networks, Endocrine disrupters and human health, EDC screening and testing, Dose response
1 Introduction
The development of life-stage and tissue-specific AOPs for EDCs can reduce the uncertainty in extrapolating of the effects of EDCs from in vitro and in vivo studies in laboratory animals to humans. When the key events (KEs) and molecular initiating event (MIEs) in a pathway are highly conserved among species, as is the case for the androgen (A) and estrogen (E) signaling pathways, there is greater certainty that disruption of the pathway will produce adverse developmental effects among the species. For example, (anti)androgenic chemicals like the AR antagonist flutamide or the androgen agonist trenbolone [5] produce species-typical alterations of the reproductive system in vertebrates [6], because the androgen receptor (AR) pathway is highly conserved from fish to humans.
We are developing an AOP network that includes multiple “antiandrogen” AOPs because a single AOP does not capture all molecular initiating events (MIE) and key events (KE) that contribute to the disruption of male rat sexual differentiation (the adverse outcome pathway (AOP)) (Figure 1) [7]. While some of the AOPs share common downstream events (KEs), others do not. Our goal is to develop assays that quantify MIEs and KEs in each AOP and to establish the degree of perturbation needed to reach the next step thereby enabling us to predict with reasonable accuracy the dosage levels of individual chemicals and mixtures that cause adverse reproductive effects in male rat offspring after exposure during sexual differentiation. At present we are able to predict the dose that produces F1 male rat reproductive tract abnormalities from the suppression of fetal testosterone synthesis [7]. We also are attempting to quantify KEs that are predictive for other AOPs in the “antiandrogen” AOPn.
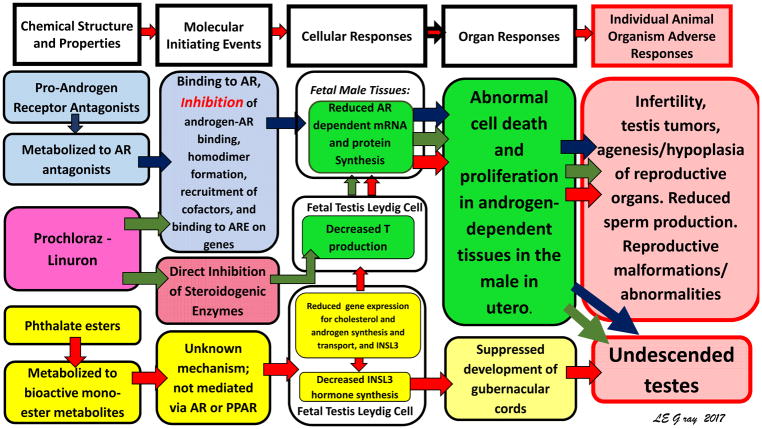
Figure 1. Antiandrogen AOP network (and Graphical Abstract).
Adverse outcome pathway network for disrupted androgen- and insulin-like hormone 3 (INSL3)-dependent reproductive development in male rats. The first column of the AOP network identifies three classes of chemicals known to disrupt the androgen-signaling pathways via three different mechanisms of action. Different colored arrows indicate the pathway through which each set of chemicals exerts its affects: …blue arrows, androgen receptor (AR antagonists); green arrows, dual mechanism of action chemicals (AR antagonists and steroid enzyme inhibitors); and red arrows, phthalates (molecular initiating event unknown, but known to inhibit fetal testosterone (T) production). [7] Abbreviations: AR-androgen receptor; ARE- androgen receptor response element on target gene; mRNA- messenger ribonucleic acid.
The initial identification of chemicals that disrupt the E and A pathways relies on execution and interpretation of valid in vitro and short-term in vivo screening assays. The following provides some background on the EDC field and value and limitations of certain screening approaches.
Furthermore, we have found that downstream KEs like fetal testosterone production or inhibition of androgen-dependent tissue growth are better predictors of adverse outcomes of in utero phthalate exposure or AR antagonist exposure, respectively, than are the in vitro assessment of MIEs. There is a much stronger quantitative relationship between the adverse effects of AR antagonists in utero with the effects in an in vivo AR screening assay than with assays of AR binding and in vitro gene expression.
2. Background on Endocrine Disruptors
Beginning in 1991, a series of workshops organized by Theo Colborn defined the term “endocrine disruptor” and issued a consensus statement about the effects of EDCs on human and ecosystem health [8]. Concerns about the potential effects of EDCs led to the passage in 1996 the Food Quality Protection and Safe Drinking Water Acts that mandated that EPA establish a screening program for estrogens and other substances and in 1998, the Endocrine Disrupter Screening and Testing Advisory Committee (EDSTAC) proposed an EDC screening and testing strategy to the EPA. This approach was subjected to evaluation, assay validation and published for review in 2004 and in 2009 EPA announced the availability of the screening battery (detailed time-line (https://www.epa.gov/sites/production/files/2016-04/documents/edsp-timeline-042016.pdf).
3. Comments on the EDSP Tier 1 Screening (T1S) battery
3.1
The EDSP T1S battery contains 11 in vitro and short term in vivo assays to screen chemicals for their ability to display estrogenic, (anti)androgenic, or (anti)thyroid (EAT) activities, or inhibit the hypothalamic-pituitary-gonadal axis as it relates to EAT activity. (https://www.epa.gov/test-guidelines-pesticides-and-toxic-substances/series-890-endocrine-disruptor-screening-program).
3.2
Reports that the existing battery fails to detect the EAT activity for some chemicals have not been substantiated and “better”, validated (robust, reproducible) short-term assays have not been developed.
3.3
The current in vitro assays in the battery can be improved, replaced or enhanced using state-of-the-art recombinant proteins [9]to assess receptor binding and new cell based assays that can discriminate agonists from antagonists [10; 11; 12]. This would reduce animal use and improve the quality of the data.
3.4
In vivo short-term assays are included due to current limitations of in vitro assays.
3.4.1
In vitro assays currently lack absorption, distribution, metabolism and excretion (ADME), they do not account for tissue and species specific effects, MIEs are not known for all adverse outcome pathways (phthalates, for example) and a significant percentage of the “chemical universe” is not amenable to in vitro testing [13].
As a consequence of these limitations, we are unable to predict the in vivo effects of antiandrogenic chemicals in the Hershberger Assay (HA) or in the male offspring exposed in utero. In contrast, the HA results do enable us to predict the doses of AR antagonists that disrupt male reproductive development in utero [14]. When in vitro ER and AR data are from high through-put assays (HTS) one also need to consider the purity of the sample (about 25% of the Tox21 samples evaluated to date are less than 90% pure and about 8% are less than 5% pure), and some assays may not be optimized and the HTS data can display considerable inter- and intra-assay variability [15]. In addition, some unlikely chemicals are listed with “active” ER AUC model scores (defined as AUC>0.1) including trenbolone (AUC=0.475), dihydrotestosterone (AUC=0.4), testosterone propionate (AUC=.392), propyl thiouracil (AUC=.144), triiodothyronine (AUC=.135) for example (https://www.epa.gov/endocrine-disruption/endocrine-disruptor-screening-program-edsp-estrogen-receptor-bioactivity).
3.4.2
In general, it appears that E and A in vitro assays have lower positive predictive rates than negative predictive rates (there are more false positives than false negatives). In addition, when multiple MIEs are affected in vitro it is not possible to predict which of these will drive the toxicity in vivo [14].
4. Results from the EDSP T1S battery [16]
Since the first list of chemicals for screening was proposed in 2007 and publication of the final T1S Test Guidelines in 2009, 52 chemicals have been screened using the T1S Battery. (https://www.epa.gov/endocrine-disruption/endocrine-disruptor-screening-program-timeline).
4.1
None of these displayed estrogenic activity whereas two were antiandrogenic (https://www.epa.gov/endocrine-disruption/endocrine-disruptor-screening-program-tier-1-screening-determinations-and).
4.2
Executing T1S was more resource intensive than expected and competent laboratories had difficulty meeting some of the performance criteria described in the Test Guidelines [17]. Major issues arose from dose-setting for the in vivo assays. Overt-toxicity confounded identification of EDC activity and some of the Test Guidelines contain erroneous performance criteria.
4.3
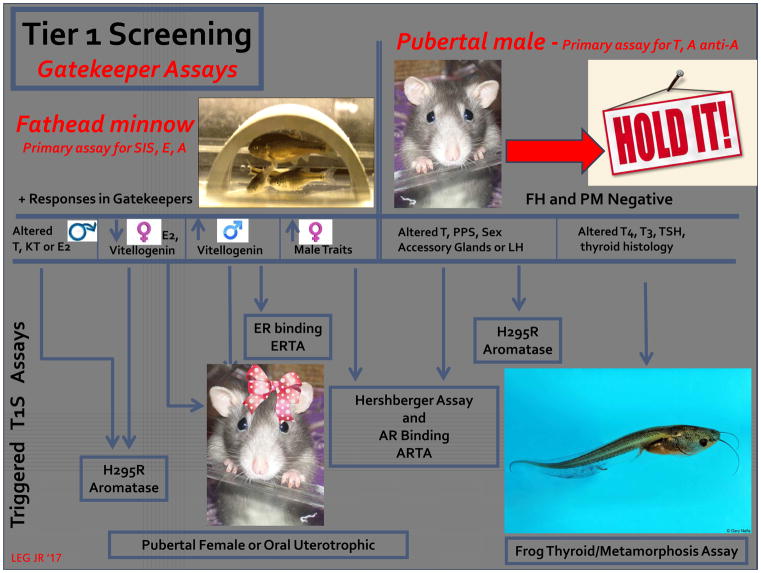
Based upon an evaluation of the published literature we ([4; 18; 19]) proposed tiering the 11 T1S assays with two “gatekeeper” assays in the first tier. If these two assays were negative then no other screening would take place. This concept, however, has not been rigorously evaluated experimentally (Figure 2).
Figure 2.
A potential approach to streamlining the eleven EPA, EDSP Tier 1 Screening assays based upon a “weight of evidence” review of the literature on the effects of chemicals in the test guideline protocols [4; 18]. In this approach, initially two “gatekeeper” assays, the fathead minnow short-term reproductive test and the pubertal male rat assay, are conducted. If these are negative, then no other screening is necessary. This relies upon the fish assay for the detection of estrogens, and chemicals that inhibit steroidogenesis. Androgens and antiandrogens and chemicals that alter thyroid function are detected in the pubertal male assay. If either gatekeeper assay is positive (in the absence of overt toxicity) then additional screening is triggered on a mode-of-action basis [4; 16; 18]. (abbreviations: T1S- EPA, EDSP tier 1 screening assay battery; AR – androgen receptor; ARTA- androgen receptor transcriptional antagonist assay; T – testosterone; PPS – preputial separation; LH – luteinizing hormone; T4 – thyroxine; T3 – triiodothyronine; TSH – thyroid stimulating hormone; FH – fathead minnow reproduction test; PM – pubertal male assay; E2 – 17 beta estradiol; KT – ketotestosterone; H295R – cell line used to assess steroidogenesis in vitro.
4.4
In summary, given the rate of progress since 2009 and the resources required to execute T1S, it is uncertain how much useful data will be available for use in hazard identification and risk assessment in the near term.
5. Unresolved issues in the field of EDC research
At present, the study of EDCs and other scientific fields are mired in debate in many areas and it is unclear how science and regulation can move forward in a constructive fashion without different groups beginning to discuss these differences of opinion in a constructive manner.
5.1 Reproducibility of many EDC studies, or lack thereof
5.1.1
The scientific community has recently focused on the issue of reproducibility of important research observations including studies on EDCs. There is increasing awareness that the results of many high-profile studies cannot be reproduced. As a result, a Reproducibility Initiative was initiated as collaboration between Science Exchange, PLOS, figshare and Mendeley to encourage journals to establish guidance for authors and peer reviewers that assures the proper execution of studies and adequate peer review.
5.1.2
There should be mandatory reporting requirements in the methods of publications that assure: 1) proper experimental design for multigenerational and transgenerational studies, 2) proper statistical analyses of litter-based effects and lineage effects over generations, and 3) proper documentation and reporting of chemical source, lot, purity, and CAS numbers.
5.1.3
Finally, scientists should be objectively testing hypotheses, not attempting to prove them, and there needs to be objectivity in interpretation of results that does not exaggerate the significance of the findings.
6. Low dose exposures
The term “low dose” has several different definitions. In some cases, scientists are referring to “environmentally relevant” levels of EDCs. However, not all “environmental” levels of EDCs are low [20; 21; 22] [23; 24]. In other cases, scientists are referring to lowest dose levels used in their experiment or dose levels below the no observed adverse effect level (NOAEL), the reference dose (RfD), or acceptable daily intake (ADI). “Low dose” is a useful descriptive term but authors need to define how they are using the term in their publication or presentation.
7. Shape of the dose response curve in the “low” dose region (linear, threshold or non-monotonic (NMDR))
The shape of the dose response curve in the low dose region has been debated since the late 1940s [25]. The debate originally focused on linear-no-threshold (LNT) vs threshold responses in the low dose range for cancer and noncancer related effects. While it is generally assumed that EDCs and other noncancer effects display thresholds for adverse effects, the shape of the dose response curve in the low dose region (linear versus threshold) cannot be addressed empirically because very low dosage levels of EDCs would require infinitely large sample sizes to determine statistically significant differences in effect from a zero dose control (OECD, 2012; ENV/JM/MONO(2011)47). At a 2009 EPA funded workshop [25], most “participants concluded that for population-level risk analyses, in the absence of mode of action (MOA)-based dose–response models, the most appropriate low-dose extrapolation approach for both cancer and noncancer end points is linear, no-threshold extrapolation from the range of observed responses, recognizing the effects of population variability as well as additivity to background disease and exposures on the dose–response function”.
In addition, while statistically determined no observed adverse effect levels (NOAELs) are useful in chemical hazard assessments, there can be molecular interactions occurring below these values such that the NOAEL is not a “true” NOAEL. For example, we find that when we combine 15 to 20 chemicals below the NOAEL of each chemical, all of the male offspring display reproductive tract malformations [26].
7.1
It has been proposed that EDCs represent a special category of toxicants that frequently display “low dose” NMDR relationships [27] confounding the identification of NOAELs and lowest observed adverse effect levels (LOAELs). Although the robust, multigenerational dose response studies that address this question are generally negative, the dogma is widely held in some EDC circles [28]. What is evident about E and A active chemicals is that they can simultaneously induce both adverse and beneficial effects due to the widespread expression of hormone receptors throughout the body and that the consequences of exposure vary greatly among different life stages, sexes, and tissues. Clearly, all the effects of E and A active chemicals are not adverse, as some are very beneficial.
NMDRs are frequently seen in vitro, but often they typically occur at high, not low, concentrations and often result from cytotoxicity [29]. Although uncommon, there are robust examples of NMDRs for adverse effects in vertebrates (e.g. [30] [31]) [32] [33]) (Figure 3). However, there is little compelling evidence that when biologically plausible NMDRs are found that these effects are adverse, low-dose effects that would alter the risk assessment of an EDC [34]. The issue of NMDRs has been reviewed by the USEPA and currently being reviewed by the NAS (http://dels.nas.edu/Study-In-Progress/Unraveling-Dose-Toxicity-Case-Studies/DELS-BEST-14-07).
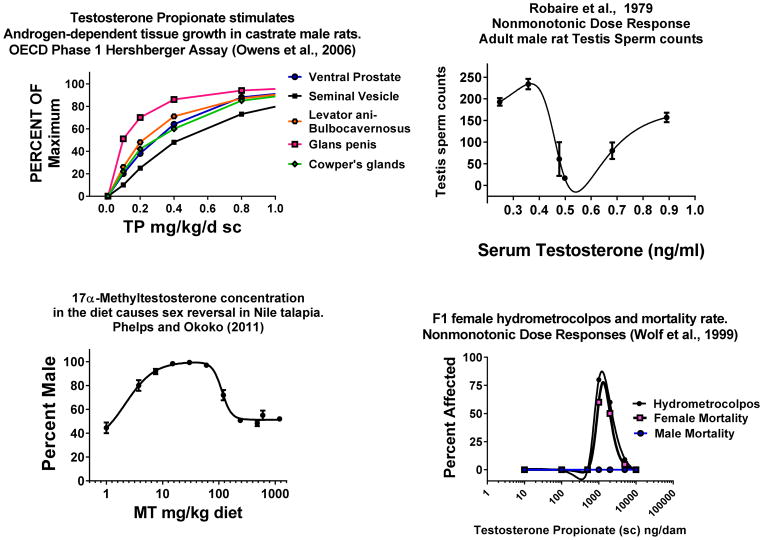
Figure 3.
Robust in vivo NMDRs and an apparent LNT dose response in vertebrate reproductive tissues from Owens et al., 2006 [47], Robaire et al., 1979 [30], Phelps and Okoko et al., 2011 [33] and Wolf et al., 2002 [32].
Upper left panel displays the dose-related responses of five androgen-dependent tissues weighed after ten days of treatment with testosterone propionate (sc). These responses all appear to be linear in the low dose range. The data are from the OECD interlaboratory Hershberger Assay validation study conducted in 15 laboratories (n=90, with 6 rats per dose per laboratory)
Upper right panel displays a nonmonotonic dose response (NMDR) in testis sperm counts following long-term treatment with testosterone (sc implants). Testis weight also shows a similar NMDR, whereas serum testosterone and LH did not.
Lower left panel displays a NMDR for the effects of methyl testosterone in the diet on the sex ratio of Nile tilapia.
Lower right panel. In utero administration of testosterone propionate (sc) induces NMDRs for reproductive tract malformations and mortality in female rat offspring. Mortality was only seen in female offspring and only after puberty.
While there are a reasonable number of robust multigenerational studies with estrogens, steroid synthesis inhibitors and antiandrogens that have included a broad range of dosage levels including low to high dose levels, other EDC modes of action have not been adequately studied. In addition, there is less information on the shape of the dose response curve in in vivo EDC screening studies. It is noteworthy that in the 21 day fathead minnow reproduction assay there are significant temporal changes in the biological response over time [35].
We reviewed the literature on LNT, threshold and NMDR responses from case studies of chemicals that disrupt reproductive development and function via the androgen (A) and estrogen (E) signaling pathways [36] and reached the following conclusions (https://www.epa.gov/sites/production/files/2016-01/documents/nmdr.pdf) [37; 38].
7.2 Conclusions about dose response for EDCs
NMDR curves are biologically plausible and occur frequently in vitro, but these often occur at high concentrations that are not relevant in vivo [39].
EDCs induce some responses that appear to be LNT in vivo (Fig 3).
NMDR curves are more common in short- versus long-term exposures, with upstream, mechanistic events versus downstream adverse effects [35].
Different tissues can display different dose-related responses (LNT versus threshold) to the same EDC (e.g. vinclozolin [40]).
When an adverse effects of an EDC is non-monotonic, other effects often display monotonic responses at lower dosage levels.
The shape of the dose response curve can be affected by several factors, including life stage, route of exposure, target tissue, and species differences in EDC pathways or ADME.
A number of robust multigenerational studies of estrogens and antiandrogens have been executed and NMDR curves are uncommon at low dosage levels.
Multigenerational test guidelines can be enhanced on a case-by-case basis to improve the sensitivity to low dose effects of some EDCs.
8. EDC testing
8.1 Reproductive test guidelines
Comprehensive testing for EDCs requires a full life-cycle evaluation. Studies that examine male rat fertility require exposures that cover a minimum of a full cycle of spermatogenesis and one-generation studies that do not include mating of the F1 to produce an F2 generation fail to evaluate several potential developmental alterations in the F1 on mating behavior, ovulation, implantation, pregnancy maintenance, parturition, lactation and maternal behavior. In this regard, the National Toxicology Program has developed a new study design, termed the modified one generation (MOG) reproduction study that includes measurements of developmental and reproductive toxicity. Exposure starts during gestation, with a focus on F1 animals, and the MOG is adequately powered to detect lower dose effects in the F1 [41].
8.2 Statistical power to detect low-dose effects
A number of publications have demonstrated that the current sample sizes used in multigenerational and in vivo EDC screening tests lack sufficient statistical power to statistically detect low incidence adverse effects (e.g. p< 0.05) ([42; 43; 44]). For example, Blystone et al., (2010) [44] reported that examining all the males rather than one male per litter in a multigenerational study of diethyl hexyl phthalate (DEHP) lowered the NOAEL an order of magnitude.
8.3 Adequacy of endpoints in standard test guidelines to detect adverse low dose effects
While recent changes to multigenerational and one-generation protocol have improved the ability of these test guidelines to detect some of the low dose effects for AR antagonists, studies of EDCs with other modes of action could include additional endpoints to enhance the ability to detect low dose effects. For example, proliferation of mammary tissue in the male rat occurs with some estrogenic chemicals at dose levels below the endpoints currently included in the protocols [45]. In addition, the more sensitive effects of androgens in utero on the female reproductive tract including retention of male sex accessory tissues [46] and the developmental effects of anti-thyroid chemicals are not typically evaluated in multigenerational protocols.
10. Mixture effects of diverse chemicals and incorporation of the best available science into risk assessments
While there have been extensive discussions on the effects of mixtures of EDCs and there is considerable agreement that EDCs that affect common target organs act generally in a dose-additive manner regardless of the mechanism of action, there has not been a great deal of regulatory action on this issue. Clearly, each individual has a unique exposure profile of chemicals that act via a variety of modes of action but it is unclear if these exposures are problematic.
11. Conclusions
The successful development of quantitative AOPns for EDCs requires the investigator or teams of researchers to integrate 21st century in vitro and molecular tools with 20th century biology including toxicology, endocrinology, experimental design, statistics, histology, anatomy, physiology, and biochemistry. In the absence of a comprehensive biological perspective, computational toxicology of in vitro data sets can result in some rather unrealistic MIE, KE and AO linkages in an AOP and biologically implausible predictions.
This article presents my views on the EDC field. The concerns and controversies expressed above are not in any way unique to this field of research and do not in any way take away from the great potential of this research to be on the forefront of toxicological risk assessment. The study of AOPs and development of AOPns provides a framework for incorporating cellular and molecular MIEs and key events into pathways that can be linked to specific adverse reproductive outcomes in a quantitative manner and facilitates the extrapolation of effects from EDC screening and testing studies to humans.
COT Highlights.
Extrapolation of EDC-induced cellular and molecular alterations into Adverse Outcome Pathways and Networks can facilitate the extrapolation of “upstream” effects to downstream adverse outcomes and can reduce the uncertainty in extrapolation of effects in vitro and animals studies to humans and other species.
Understanding the strengths and limitations of EDC screening and testing approaches
Near-term steps to improve EDC screeging and testing
Controversies in the EDC field of research
Reproducibility of many studies on the developmental effects of EDCs
Study design, statistical analysis, objective data interpretation
Low dose effects of EDCs: Linear not threshold, threshold or nonmonotonic?
Acknowledgments
Funding. The support for the work on this review was provided by the USEPA
Footnotes
Disclaimer
This Manuscript has been reviewed in accordance with the policy of the National Health and Environmental Effects Research Laboratory, U.S. Environmental Protection Agency, and approved for publication. Approval does not signify that the contents necessarily reflect the views or policy of the Agency nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kelce WR, Monosson E, Gamcsik MP, Laws SC, Gray LE., Jr Environmental hormone disruptors: evidence that vinclozolin developmental toxicity is mediated by antiandrogenic metabolites. Toxicology and applied pharmacology. 1994;126:276–85. doi: 10.1006/taap.1994.1117. [DOI] [PubMed] [Google Scholar]
- 2.Kelce WR, Monosson E, Gray LE. Biology of reproduction. SOC STUDY REPRODUCTION; 1603 MONROE ST MADISON, WI 53711–2021: 1994. IN-VITRO IN-VIVOEVIDENCE THAT VINCLOZOLIN (V) IS AN ENVIRONMENTAL ANTIANDROGEN; pp. 102–102. [Google Scholar]
- 3.Ankley GT, Bennett RS, Erickson RJ, Hoff DJ, Hornung MW, Johnson RD, Mount DR, Nichols JW, Russom CL, Schmieder PK, Serrrano JA, Tietge JE, Villeneuve DL. Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment. Environmental toxicology and chemistry/SETAC. 2010;29:730–41. doi: 10.1002/etc.34. [DOI] [PubMed] [Google Scholar]
- 4.Ankley GT, Gray LE. Cross-species conservation of endocrine pathways: a critical analysis of tier 1 fish and rat screening assays with 12 model chemicals. Environmental toxicology and chemistry/SETAC. 2013;32:1084–7. doi: 10.1002/etc.2151. [DOI] [PubMed] [Google Scholar]
- 5.JECFA. Trenbolone acetate, Toxicological evaluation of certain veterinary drug residues in food. International Programme on Chemical Safety; Geneva, Switzerland: 1988. [Google Scholar]
- 6.Hotchkiss AK, Ankley GT, Wilson VS, Hartig PC, Durhan EJ, Jensen KM, Martinovi D, Gray LE. Of mice and men (and mosquitofish): antiandrogens and androgens in the environment. BioScience. 2008;58:1037–1050. [Google Scholar]
- 7.Howdeshell KL, Hotchkiss AK, Gray LE., Jr Cumulative effects of antiandrogenic chemical mixtures and their relevance to human health risk assessment. International journal of hygiene and environmental health. 2016 doi: 10.1016/j.ijheh.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colborn T, Clement C. Chemically-Induced Alterations in Sexual and Functional Development: the Wildlife/Human Connection. Princeton Scientific Publishing Co., Inc; Princeton, NJ: 1992. [Google Scholar]
- 9.Hartig PC, Cardon MC, Blystone CR, Gray LE, Jr, Wilson VS. High throughput adjustable 96-well plate assay for androgen receptor binding: a practical approach for EDC screening using the chimpanzee AR. Toxicology letters. 2008;181:126–131. doi: 10.1016/j.toxlet.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 10.Hartig PC, Bobseine KL, Britt BH, Cardon MC, Lambright CR, Wilson VS, Gray LE., Jr Development of two androgen receptor assays using adenoviral transduction of MMTV-luc reporter and/or hAR for endocrine screening. Toxicol Sci. 2002;66:82–90. doi: 10.1093/toxsci/66.1.82. [DOI] [PubMed] [Google Scholar]
- 11.Wilson VS, Bobseine K, Lambright CR, Gray LE., Jr A novel cell line, MDA-kb2, that stably expresses an androgen- and glucocorticoid-responsive reporter for the detection of hormone receptor agonists and antagonists. Toxicol Sci. 2002;66:69–81. doi: 10.1093/toxsci/66.1.69. [DOI] [PubMed] [Google Scholar]
- 12.Wilson VS, Bobseine K, Gray LE., Jr Development and characterization of a cell line that stably expresses an estrogen-responsive luciferase reporter for the detection of estrogen receptor agonist and antagonists. Toxicol Sci. 2004;81:69–77. doi: 10.1093/toxsci/kfh180. [DOI] [PubMed] [Google Scholar]
- 13.Richard AM, Truong H, Wolf M, Thillainadarajah I. ToxCast Chemical Inventory: Data Management & Data Quality Considerations. 2014. [Google Scholar]
- 14.Gray LEJ. Quantification of the uncertainties in extrapolating from in vitro androgen receptor (AR) antagonism to key events in in vivo screening assays and adverse reproductive outcomes in F1 male rats. The Toxicologist. 2017:139. [Google Scholar]
- 15.Gray LE, Evans N, Wilson VS. Anti- and Androgenic Activities in MDA-kb2 Cells: A Comparison of Performance in 96 Well versus HTS Assays. The Toxicologist. 2015;144(1):174. [Google Scholar]
- 16.Coady KK, Biever RC, Denslow ND, Gross M, Guiney PD, Holbech H, Karouna-Renier NK, Katsiadaki I, Krueger H, Levine SL, Maack G, Williams M, Wolf JC, Ankley GT. Current limitations and recommendations to improve testing for the environmental assessment of endocrine active substances. Integr Environ Assess Manag. 2016 doi: 10.1002/ieam.1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stump DG, O’Connor JC, Lewis JM, Marty MS. Key lessons from performance of the U.S. EPA Endocrine Disruptor Screening Program (EDSP) Tier 1 male and female pubertal assays. Birth defects research. 2014;101:43–62. doi: 10.1002/bdrb.21097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gray LE, Ankley GT. A Two-Tiered-Testing Decision Tree for Assays in the US EPA-EDSP Screening Battery: Using 15 Years of Experience to Improve Screening and Testing for Endocrine Active Chemicals. The Toxicologist. 2015;144(1):183. [Google Scholar]
- 19.Juberg DR, Borghoff SJ, Becker RA, Casey W, Hartung T, Holsapple MP, Marty MS, Mihaich EM, Van Der Kraak G, Wade MG, Willett CE, Andersen ME, Borgert CJ, Coady KK, Dourson ML, Fowle JR, 3rd, Gray LE, Lamb JC, Ortego LS, Schug TT, Toole CM, Zorrilla LM, Kroner OL, Patterson J, Rinckel LA, Jones BR. t4 workshop report--lessons learned, challenges, and opportunities: the U.S. Endocrine Disruptor Screening Program. Altex. 2014;31:63–78. doi: 10.14573/altex.1309171. [DOI] [PubMed] [Google Scholar]
- 20.Kang JJ, Chu SF, Wu ZZ, Chou SW, Tsai SJ, Chiu WT. Crisis management turns Taiwan’s plasticizer nightmare into progressive policy. J Formos Med Assoc. 2012;111:409–11. doi: 10.1016/j.jfma.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 21.Chen PH, Chang KT, Lu YD. Polychlorinated biphenyls and polychlorinated dibenzofurans in the toxic rice-bran oil that caused PCB poisoning in Taichung. Bull Environ Contam Toxicol. 1981;26:489–95. doi: 10.1007/BF01622125. [DOI] [PubMed] [Google Scholar]
- 22.Whorton D, Milby TH, Krauss RM, Stubbs HA. Testicular function in DBCP exposed pesticide workers. J Occup Med. 1979;21:161–6. [PubMed] [Google Scholar]
- 23.Heaton SN, Bursian SJ, Giesy JP, Tillitt DE, Render JA, Jones PD, Verbrugge DA, Kubiak TJ, Aulerich RJ. Dietary exposure of mink to carp from Saginaw Bay, Michigan. 1. Effects on reproduction and survival, and the potential risks to wild mink populations. Arch Environ Contam Toxicol. 1995;28:334–43. doi: 10.1007/BF00213111. [DOI] [PubMed] [Google Scholar]
- 24.McKenzie RA, Freudigmann CL, Mawhinney H, Eaves LE, Green PE, Rees GJ. Dieldrin poisoning and botulism in Australian pelicans (Pelecanus conspicillatus) Australian veterinary journal. 1982;58:148–52. doi: 10.1111/j.1751-0813.1982.tb00627.x. [DOI] [PubMed] [Google Scholar]
- 25.White RH, Cote I, Zeise L, Fox M, Dominici F, Burke TA, White PD, Hattis DB, Samet JM. State-of-the-science workshop report: issues and approaches in low-dose-response extrapolation for environmental health risk assessment. Environmental health perspectives. 2009;117:283–7. doi: 10.1289/ehp.11502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Conley JM, Lambright CS, Evans N, Cardon MC, Wilson VS, Gray LE., Jr Oral exposure to a mixture of 18 anti-androgens with varying mechanisms of action at concentrations below individual chemical effect levels produces reproductive tract malformations in the male rat. The Toxicologist. 2017 [Google Scholar]
- 27.Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR, Jr, Lee DH, Shioda T, Soto AM, Vom Saal FS, Welshons WV, Zoeller RT, Myers JP. Hormones and Endocrine-Disrupting Chemicals: Low-Dose Effects and Nonmonotonic Dose Responses. Endocr Rev. 2012 doi: 10.1210/er.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zoeller RT, Brown TR, Doan LL, Gore AC, Skakkebaek NE, Soto AM, Woodruff TJ, Vom Saal FS. Endocrine-disrupting chemicals and public health protection: a statement of principles from the endocrine society. Endocrinology. 2012;153:4097–4110. doi: 10.1210/en.2012-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Welshons WV, Nagel SC, Thayer KA, Judy BM, Vom Saal FS. Low-dose bioactivity of xenoestrogens in animals: fetal exposure to low doses of methoxychlor and other xenoestrogens increases adult prostate size in mice. Toxicology and industrial health. 1999;15:12–25. doi: 10.1177/074823379901500103. [DOI] [PubMed] [Google Scholar]
- 30.Robaire B, Ewing LL, Irby DC, Desjardins C. Interactions of testosterone and estradiol-17 beta on the reproductive tract of the male rat. Biology of reproduction. 1979;21:455–63. doi: 10.1095/biolreprod21.2.455. [DOI] [PubMed] [Google Scholar]
- 31.Ewing LL, Desjardins C, Irby DC, Robaire B. Synergistic interaction of testosterone and oestradiol inhibits spermatogenesis in rats. Nature. 1977;269:409–11. doi: 10.1038/269409a0. [DOI] [PubMed] [Google Scholar]
- 32.Wolf CJ, Hotchkiss A, Ostby JS, LeBlanc GA, Gray LE., Jr Effects of prenatal testosterone propionate on the sexual development of male and female rats: a dose-response study. Toxicol Sci. 2002;65:71–86. doi: 10.1093/toxsci/65.1.71. [DOI] [PubMed] [Google Scholar]
- 33.Phelps RP, Okoko M. A non-paradoxical dose response to 17α-methyltestosterone by Nile tilapia Oreochromis niloticus (L.): Effects on the sex ratio, growth and gonadal development. Aquaculture Research. 2011;42:549–558. [Google Scholar]
- 34.Parrott JL, Bjerregaard P, Brugger KE, Gray LE, Jr, Iguchi T, Kadlec SM, Weltje L, Wheeler JR. Uncertainties in biological responses that influence hazard and risk approaches to the regulation of endocrine active substances. Integr Environ Assess Manag. 2016 doi: 10.1002/ieam.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ankley GT, Villeneuve DL. Temporal changes in biological responses and uncertainty in assessing risks of endocrine-disrupting chemicals: insights from intensive time-course studies with fish. Toxicol Sci. 2015;144:259–75. doi: 10.1093/toxsci/kfu320. [DOI] [PubMed] [Google Scholar]
- 36.Matthiessen P, Ankley GT, Biever RC, Bjerregaard P, Borgert C, Brugger K, Blankinship A, Chambers J, Coady KK, Constantine L, Dang Z, Denslow ND, Dreier DA, Dungey S, Gray LE, Gross M, Guiney PD, Hecker M, Holbech H, Iguchi T, Kadlec S, Karouna-Renier NK, Katsiadaki I, Kawashima Y, Kloas W, Krueger H, Kumar A, Lagadic L, Leopold A, Levine SL, Maack G, Marty S, Meador J, Mihaich E, Odum J, Ortego L, Parrott J, Pickford D, Roberts M, Schaefers C, Schwarz T, Solomon K, Verslycke T, Weltje L, Wheeler JR, Williams M, Wolf JC, Yamazaki K. Recommended approaches to the scientific evaluation of ecotoxicological hazards and risks of endocrine-active substances. Integr Environ Assess Manag. 2017 doi: 10.1002/ieam.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gray LE, Foster PM. Nonmonotonic Dose-Response Curves and Endocrine-Disrupting Chemicals: Fact or Falderal? The Toxicologist. 2013;132(1):341. [Google Scholar]
- 38.Gray LE. Nonmonotonic Dose-Response Curves (NMDRCs) Are Common after Estrogen or Androgen Signaling Pathway Disruption—Fact or Falderal? The Toxicologist. 2013;132(1):341. [Google Scholar]
- 39.Welshons WV, Thayer KA, Judy BM, Taylor JA, Curran EM, vom Saal FS. Large effects from small exposures. I. Mechanisms for endocrine-disrupting chemicals with estrogenic activity. Environmental health perspectives. 2003;111:994–1006. doi: 10.1289/ehp.5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gray LE, Jr, Ostby J, Monosson E, Kelce WR. Environmental antiandrogens: low doses of the fungicide vinclozolin alter sexual differentiation of the male rat. Toxicol Ind Health. 1999;15:48–64. doi: 10.1177/074823379901500106. Find this article online. [DOI] [PubMed] [Google Scholar]
- 41.Foster PM. Regulatory Forum opinion piece: New testing paradigms for reproductive and developmental toxicity--the NTP modified one generation study and OECD 443. Toxicologic pathology. 2014;42:1165–7. doi: 10.1177/0192623314534920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gray L, Barlow N, Howdeshell K, Ostby J, Furr J, Gray C. Transgenerational effects of Di (2-ethylhexyl) phthalate in the male CRL:CD(SD) rat: added value of assessing multiple offspring per litter. Toxicological Sciences. 2009;110:411–425. doi: 10.1093/toxsci/kfp109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hotchkiss AK, Rider CV, Blystone CR, Wilson VS, Hartig PC, Ankley GT, Foster PM, Gray CL, Gray LE. Fifteen years after “Wingspread”--environmental endocrine disrupters and human and wildlife health: where we are today and where we need to go. Toxicol Sci. 2008;105:235–59. doi: 10.1093/toxsci/kfn030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blystone CR, Kissling GE, Bishop JB, Chapin RE, Wolfe GW, Foster PM. Determination of the di-(2-ethylhexyl) phthalate NOAEL for reproductive development in the rat: importance of the retention of extra animals to adulthood. Toxicol Sci. 2010;116:640–6. doi: 10.1093/toxsci/kfq147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Latendresse JR, Bucci TJ, Olson G, Mellick P, Weis CC, Thorn B, Newbold RR, Delclos KB. Genistein and ethinyl estradiol dietary exposure in multigenerational and chronic studies induce similar proliferative lesions in mammary gland of male Sprague-Dawley rats. Reproductive toxicology (Elmsford, NY. 2009 doi: 10.1016/j.reprotox.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 46.Greene RR, Burrill M, Ivy AC. Experimental intersexuality. The effect of antenatal androgens on sexual development of female rats. Am J Anat. 1939;65:415–469. [Google Scholar]
- 47.Owens W, Zeiger E, Walker M, Ashby J, Onyon L, Gray LE., Jr The OECD program to validate the rat Hershberger bioassay to screen compounds for in vivo androgen and antiandrogen responses. Phase 1: use of a potent agonist and a potent antagonist to test the standardized protocol. Environmental health perspectives. 2006:1259–1265. doi: 10.1289/ehp.8751. [DOI] [PMC free article] [PubMed] [Google Scholar]