Abstract
Prostate Cancer is the forth most common type of cancer. Prostate-specific membrane antigen (PSMA) is anchored in the cell membrane of prostate epithelial cells. PSMA is highly expressed on prostate epithelial cells and strongly up-regulated in prostate cancer. Therefore it is an appropriate target for diagnostic and therapy of prostate cancer and its metastases. This article discusses several articles on radionuclide treatments in prostate cancer and the results on PSMA therapy with either beta or alpha emitters as a salvage therapy.
Keywords: PSMA, Prostate cancer, Radioligand therapy, Metastatic disease
Background
Prostate cancer is the fourth most common type of cancer affecting the European male population (excluding non-melanoma skin cancer) [1]. Currently, one in every six males are at risk of being affected by prostate cancer, and the risk of death because of metastatic prostate cancer is one in every 30 [2]. Castration-resistant prostate cancer (CRPC) is defined by disease progression despite castrated levels of testosterone, and may present as either a continuous rise in serum prostate-specific antigen (PSA) levels, the progression of pre-existing disease, and/or the appearance of new metastases [3].
In patients who fail the initial therapy with curative intent (i.e. radical prostatectomy, external beam radiotherapy [EBRT], brachytherapy) treatment options include androgen-deprivation therapy (ADT) along with chemotherapy in the case of disease progression [4]. A combination of ADT with docetaxel in hormone-sensitive patients improves median overall survival (OS) by 13.6 months compared to ADT alone [5, 6]. In CRPC patients, a more recent approach using abiraterone and enzalutamide prolongs median survival by up to 3.9 and 4.8 months, respectively [7, 8]. Chemotherapy treatment with docetaxel and cabazitaxel is often associated with side effects but prolongs OS for a few months [4, 9, 10]. Furthermore, treatment for diffused or painful bone metastases using radium-223-chloride (223Ra), which targets only the osteoblastic lesions and does not treat the nodal and visceral metastases, improves median OS by 3.6 months [11].
Positron emission tomography/computed tomography (PET/CT) using Gallium-68-labelled (Ga-68) ligands that target the prostate-specific membrane antigen (PSMA) is a sensitive and specific diagnostic method that is dedicated to poorly differentiated prostate cancer. Eiber et al. [12] reported that sensitivity increased to 100% with an increasing PSA velocity of 5 ng/ml/year or greater and with a Gleason score of eight or more. The use of PET/CT to target PSMA with Ga-68 for diagnostic and radioligand therapy (RLT) with Lutetium-177 offers a new theranostics approach using the same ligand for diagnostics and therapy [13]. Since 2013, an increasing number of centres worldwide has begun employing radioligand therapy (RLT) using 177Lu-PSMA [14–17].
The aim of this review is to discuss the current trend of using 177Lu-PSMA therapy, including dosimetry, side effects, treatment efficacy and survival rates, while referring to the literature and examining the prospects for prostate cancer therapy with targeted alpha therapy.
Indications for RLT
Metastatic castration-resistant prostate cancer (mCRPC) patients can undergo treatment with taxane-based therapies (docetaxel and cabazitaxel) and with second line hormonal therapies (including enzalutamide and abiraterone). Both these therapies moderately improve patient survival time, but they are only temporarily effective and patients can develop resistance [18, 19]. Hence, more specific targeted therapies have to be developed for eliminating the prostate cancer visceral and bony lesions. Studies have shown that PSMA is overexpressed in around 90–100% of local prostate cancer lesions, along with many bony lesions and lymph node metastases. Furthermore, many studies have shown that the PSMA expression levels increase in the case of metastatic, high-grade and castration-resistant prostate cancer [20–22] (Fig. 1). The current essential inclusion criteria, as stated in the 2016 consensus recommendations of the German Society of Nuclear Medicine [23], cover:
histologically detected prostate carcinomas;
non-resectable metastases;
tumour progression under guidelines therapy;
detected PSMA expression of the tumour;
reasonable haematological function (leukocyte count > 2.0 × 109/L, thrombocyte > 75 × 109/L);
normal or slightly decreased renal function (creatinine < 2 x the upper standard limit);
sufficient liver function (aspartate aminotransferase [AST] or alanine aminotransferase [ALT] < 5 x the upper standard limit); and
a six-week interval with myelosuppressive therapy.
Fig. 1.
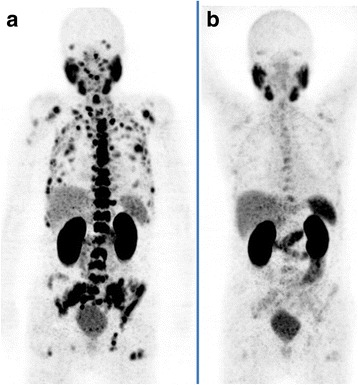
A 83-year-old patient with castration-resistant prostate cancer (Gleason-Score:9) and an increasing prostate-specific antigen (PSA) level. He had a history of prostatectomy and radiation therapy of prostate bed. The 68 Ga-PSMA PET scan showed a diffuse bone and bone marrow involvement (a). The PSA and ALP levels prior to the first cycle of Lu-PSMA therapy were 261 ng/ml and 659 U/l, respectively. The patient received 2 cycles of Lu-PSMA and the PSA level decreased continuously during cycles from 261 to 9.0 ng/ml (8 weeks after the second cycle). The ALP showed also a decreasing value from 659 to 81 U/l (8 weeks after the second cycle). The PSMA-PET (b) 8 weeks after the second cycle showed a significant response with significant regression of PSMA
Activity level
The standard administered activity of 177Lu-PSMA has varied across the literature as institutions undertake safety and toxicity trials [16]. Single injected doses have ranged from 3 to 9.3 GBq, with up to nine injections given to patients, generally at a minimum of six-week intervals [14, 24–31].
Response rate
Up to 80% of patients with mCRPC will have a treatment response to 177Lu-PSMA shown by any PSA decline [14, 24–30, 32–34] (Table 1).
Table 1.
Overview of published trials on treatment efficacy and OS
| First author (n: number of patients) | Compound used | Activity | PSA fall ≥ 50% | CT (RECIST) | PSMA PET | Symptomatic response | Biochemical/radiological PFS | Overall survival |
|---|---|---|---|---|---|---|---|---|
| Vallabhajosula et al. 2005 [46] | 177Lu-J591 | 0.3–2.7 GBq/m2for 177Lu | NA | NA | NA | NA | NA | NA |
| Lu (n: 35) = 177 | 90Y-J591 | 0.18–0.7 GBq/m2for 90Y | ||||||
| n (28) = 90Y | ||||||||
| Zechmann et al. 2014 [37] (n: 28) | 131I-MIP 1095 | 2.0–7.2 GBq | 61% | NA | NA | 23% CR | Median BPFS 126 days | NA |
| PD 14% | 61% PR | |||||||
| Ahmadzadehfar et al. 2015 [14] (n: 10) | 177Lu-PSMA-617 | 4.1–6.1 GBq | 50% | NA | NA | NA | NA | NA |
| PD 30% | ||||||||
| Ahmadzadehfar et al. 2016 [26] (n: 24) | 177Lu-PSMA-617 | 4.1–7.1 GBq | 42% | PR 40% | PR 80% | NA | NA | NA |
| PD 21% | SD 55% | SD 0% | ||||||
| PD 5% | PD 20% | |||||||
| Kratochwil et al. 2016 [32] (n: 30) | 177Lu-PSMA-617 | 4–6 GBq | 43–72% | NA | NA | NA | NA | NA |
| PD 27% | ||||||||
| Baum et al. 2016 [51] (n: 56) | 177Lu-PSMA I&T | 3.6–8.7 GBq | 59% | PR 20% | PR 56% | 33% PR | Medial radiological PFS 13.7 months | |
| PD 11% | SD 52% | SD 8% | ||||||
| PD 28% | PD 36% | |||||||
| Rahbar et al. 2016 [25] (n: 74) | 177Lu-PSMA-617 | 6 MBq | 31% | NA | NA | NA | NA | NA |
| PD 23% | ||||||||
| Rahbar et al. 2016 [39] (n: 28) | 177Lu-PSMA-617 | 7 MBq | 32–50% | NA | NA | NA | NA | 29.4 vs. 19.7 weeks |
| PD 20% | ||||||||
| Heck et al. 2016 [48] (n: 22) | 177Lu-PSMA-I&T | 7–7.8 GBq | 33% | PR 11% | Integrated CR 5% | 14% CR | Median PFS 175 days | NA |
| PD 32% | SD 56% | SD 63% | 42% PR | |||||
| PD 33% | PD 32% | |||||||
| Yadav et al. 2017 [52] (n: 31) | 177Lu-PSMA-617 | 1.1–5.5 MBq | Mean pre and post | CR 33% | Analgesic score reduced from 2.5 to 1.8 | Median PFS 12 months | Median OS 15 weeks | |
| 275/41 | PR 50% | |||||||
| PD 20% | SD 17% (n = 6) | |||||||
| Fendler et al. 2016 [23] (n: 15) | 177Lu-PSMA-617 | 6 MBq | 60% | PR 27% | NA | NA | NA | NA |
| SD 40% | ||||||||
| PD 33% | ||||||||
| Kratochwil et al. 2016 [36] (n: 2) | 225Ac-PSMA-617 | 4–6 GBq | 100% | NA | NA | NA | NA | NA |
| Ahmadzadehfar et al. 2017 [27] (n: 52) | 177Lu-PSMA-617 | 5.6–6 MBq | 44% 1st cycle | NA | NA | NA | NA | Median OS 60 weeks |
| 59.6% | ||||||||
| 2nd cycle | ||||||||
| 59.6% 3rd cycle | ||||||||
| Rahbar et al. 2017 [16] (n: 145) | 177Lu-PSMA-617 | 2–8 MBq | 45% | PR 45% | NA | NA | NA | NA |
| SD 28% |
NA not available, MBq mega becquerel, CR complete response, PD progressive disease, PR partial response, OS overall survival, PFS progressive free survival, BPFS biochemical progressive free survival, PSMA prostate specific membrane antigen, PSA prostate specific antigen, RECIST response evaluation criteria in solid tumors, PET positron emission tomography, CT computed tomography
Studies using 177Lu-PSMA-617 and 177Lu-PSMA-I&T have observed a reduction in PSA levels by 50% or more in 32–60% of patients. Moreover, 47% of patients have experienced a stable disease [14, 24–30, 32–34]. In 2016, a group from Heidelberg, Germany, started the first human treatments with 255Ac-PSMA-617 in two patients with red marrow infiltration and resistance to other therapies, and these patients showed complete responses to the therapy [35, 36]. A study by Zechmann et al., using a 131I-labelled PSMA ligand, showed a 50% or greater decline in PSA in more than 60% of patients [37]. This finding was in line with a recent study by Afshar-Oromieh et al. who studied 36 patients who had received PSMA-RLT with 131I-IMP-1095, and found the best therapeutic effect was achieved by the first therapy, which showed that PSA declined by more than 50% in 70.6% of the patients. The second and third therapies from their study showed reduced effectiveness [38].
Predictors of the response
Ferdinandus et al. evaluated the prognostic value of various pre-therapeutic parameters on therapy response, based on changes in PSA after the first cycle of RLT. Their multivariate analysis of these parameters, which considered any decrease in PSA after 2 months, showed that patients with a high platelet count or a regular need for analgesics had a significantly worse response to the first RLT cycle. When a PSA decline of ≥ 50% was considered, patients with a regular need for analgesics showed a worse response in the multivariate analysis; however, other pre-therapeutic parameters had no impact on the response to RLT. In this study, the standard uptake value maximum of 68Ga-PSMA-11 was not a significant predictor of the response to RLT. One explanation for this could be that more aggressive tumours may express higher PSMA levels. However, despite the better uptake of 177Lu-PSMA-617 due to the rapid growth of metastases, the response rate did not correlate with the uptake, which could be due to different washout times of 177Lu-PSMA-617 in the respective metastases [29].
Survival
Rahbar et al. reported a potential survival benefit of 177Lu-PSMA, where they matched the patient population (n = 28) to a historical cohort of 20 patients receiving the best supportive care (BSC) to examine potential survival benefits. Apart from the more heavily pre-treated patients and the more visceral metastases in the 177Lu-PSMA group, the groups were comparable. This finding highlights that the estimated median survival period was 29.4 weeks, being significantly longer than the survival time in the historical control group at 19.7 weeks [39].
In a study by Ahmadzadehfar et al. with 52 patients who underwent a total of 190 cycles of RLT, 80.8% of patients showed a decline in PSA levels 2 months after the first cycle, with 44.2% showing a PSA decline of ≥ 50%. The median OS was 60 weeks in all patients. The median OS was significantly longer for patients who showed a PSA decline after the first cycle compared to patients without a PSA decline (68 versus 33 weeks respectively) [27]. In another study from the same group, 100 patients who received a total of 347 cycles of 177Lu-PSMA (median three cycles) were analysed. All patients had a history of therapy with abiraterone or enzalutamide, or both. In total, 70% of the patients had at least one line of chemotherapy and 36% had a history of radionuclide therapy with 223Ra. Sixty-nine patients showed a decline in PSA 2 months after the first cycle, and 38 of these patients showed a PSA decline of ≥ 50%. The median OS was 60 weeks. In the multivariate analysis, the median OS was significantly longer in those patients without hepatic involvement, with high levels of albumin and haemoglobin (Hb), and with low levels of AST. Moreover, in the univariate analysis, a PSA decline after the first RLT, as well as any decline > 50%, were significant predictors of a longer OS. A decline in PSA levels of more than 14% was the most important response parameter with regard to OS [40]. In a bicentric study, 104 patients were treated with 351 cycles of 177Lu-PSMA-617. All of them had a history of therapy with at least one line of chemotherapy as well as either abiraterone or enzalutamide. Thus, in this study, the patients received all recommended guideline therapies. A PSA decline occurred in 70 (67%) patients, with a PSA decline ≥ 50% in 34 (33%) patients after the first cycle. The median OS was 56.0 weeks (95% CI: 50.5–61.5). Any initial PSA decline, an initial alkaline phosphatase (ALP) < 220 U/L and a cumulative injected activity of ≥18.8 GBq were associated with a longer survival. A step-by-step analysis revealed a PSA decline of ≥ 20.9% as the most noticeable cut-off prognosticating longer survival, which remained an independent prognosticator of improved OS in the multivariate analysis [41]. These studies have shown that responders to PSMA therapies live longer than non-responders, and a PSA response should not necessarily be defined as a PSA decline of > 50%. Interestingly, prior therapies, such as chemotherapy, had no impact on OS.
Dosimetry
The distribution of small molecules of PSMA ligands in tissue is quick and, over time, the uptake in prostate cancer tissue increases, whereas the uptake in healthy tissue declines [42]. In normal healthy tissue, salivary glands have the highest PSMA binding, followed by normal kidney tissue.
Kabasakal et al. [15] reported their dosimetry results with 177Lu-PSMA-617 and showed the highest radiation estimated doses in parotid glands and kidneys. Calculated radiation-absorbed doses per megabecquerel were 1.17 ± 0.31 mGy for parotid glands and 0.88 ± 0.40 mGy for kidneys. The radiation dose given to bone marrow was significantly lower than those of kidney and parotid glands (p < 0.05). The calculated radiation dose to bone marrow was 0.03 ± 0.01 mGy/MBq.
These results were reproduced by Delker et al. [43] who reported their dosimetry results with 177Lu-PSMA-617 and calculated a mean absorbed dose to bone marrow, kidneys, liver, spleen, and salivary glands of 0.012 Gy/GBq, 0.6 Gy/GBq, 0.1 Gy/GBq, 0.1 Gy/GBq and 1.4 Gy/GBq respectively.
There are several profound fears with regards to the damage caused to salivary glands. Based on external beam radiotherapy (EBRT) data, irreversible damage to salivary glands occurs after the administration of 30–40 Gy. With a mean absorbed dose of 1.4 Gy/GBq of 177Lu-PSMA-617, and the absence of permanent xerostomia or hypogeusia in the initial treatment studies, the salivary glands do not appear to be a dose-limiting organ [43].
The second fear concerns the absorbed dose to kidney tissues [15, 43], where, based on the EBRT data, a dose of 23 Gy may result in permanent damage. The mean absorbed kidney dose of 177Lu-PSMA is 0.53–0.8 Gy/GBq, quite like the absorbed kidney dose mentioned in the published data on 177Lu-DOTATATE (0.64 ± 0.16 Gy/GBq) [44].
In a study with 135 patients undergoing diagnostics using 68Ga-PSMA PET-CT, Gaertner et al. [45] compared three groups of patients with low, moderate, and high tumour loads according to their tumour volume. Their results indicate that patients with high tumour loads may receive less toxicity in their non-target organs.
As a result of previous findings, the safety and efficacy of targeted radionuclide therapies can be improved using patient-specific dosimetry, which may help to guide successful tumour dosing and act as an early indicator of organ toxicity.
Toxicity
Myelosuppression
Myelotoxicity is a classic non-stochastic (deterministic) effect. This effect is characterised by a sigmoidal, dose-response relationship [46].
A report from a German multicentre study [16] showed that grade 3–4 hematologic adverse events occurred in 18 of 145 patients (12%). Furthermore, one (0.7%) patient experienced severe leukopenia, 11 (8%) patients experienced anaemia, two (2%) patients experienced thrombocytopenia, and four (3%) patients had a combination of these conditions.
Ahmadzadehfar et al. [28] showed in a retrospective analysis of 49 patients, who had undergone three cycles of RLT with at least 2 months of follow up after the last cycle, that there was no CTC 4° haematotoxicity in the entire study population. Relevant anaemia, thrombocytopenia and leukopenia (CTC 3°) occurred during the observation period after the third cycle in four (8.2%), three (6.1%) and zero patients, respectively. The patients were divided into two groups with regard to their history of therapy with 223Ra. Group 1 included 20 patients who had received therapy with 223Ra (median six cycles) prior to 177Lu-PSMA-617 therapy. Group 2, which was the control group regarding haematotoxicity, was comprised of 29 patients without any history of a bone-targeted radionuclide therapy. There was no significant difference between the groups regarding relevant haematotoxicity. Thus, according to the results of this study, performing repeated cycles of 177Lu-PSMA-617 after 223Ra seems to be safe, with a very small probability of haematotoxicity [28].
Renal toxicity
Due to the physiological expression of PSMA in kidneys, many researchers have been concerned about probable radiation toxicity to the kidneys. Along with glomerular filtration rate (GFR) and creatinine levels, renal scintigraphy should be performed with Tc-MAG3 before therapy for overruling any significant obstructive disease. Any relevant obstructive disease must be treated in order to decrease the radiation dose to the diseased kidneys. Recently, Yordanova et al. [47] reported on 55 patients treated with 177Lu-PSMA-617, where 14 (25%) showed a (sub-)acute toxicity of CTC 1° and only one patient had a CTC 2° according to the creatinine value. No grade 3–4 acute loss of renal function was detected, and this was in line with the German multicentre study [16]. A decreased GFR was observed in 16 patients (29%) where four had CTC 1° and 12 had CTC 2° toxicity [47]. It has been suggested that conditions that may affect renal function and increase the radiation absorbed dose to the kidneys occur in patients who are older-age men, who have had prior chemotherapy and who have accompanying diseases, such as hypertension [47] (Table 2).
Table 2.
Published data on myelosuppression and complaints after therapy
| Anemia | Thromcytopenia | Leucopenia | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First Author year | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
| Deb et al. 1996 | X | X | - | - | X | X | X | - | X | X | X | - |
| Vallabhajousula et al. 2005 | - | - | - | - | X | X | X | X | - | - | - | - |
| Bander et al. 2005 | - | - | - | - | X | X | X | X | - | - | - | - |
| Tagawa et al. 2013 | X | X | X | - | X | X | X | X | X | X | X | X |
| Zechmann et al. 2014 | - | - | - | - | X | X | X | - | X | X | X | _ |
| Ahmadzadehfar et al. 2016 | X | X | X | - | X | - | - | - | X | X | - | - |
| Kratochwil et al. 2016 | X | X | X | - | X | X | X | - | X | X | - | - |
| Baum et al. 2016 | X | X | - | - | - | - | - | - | X | X | - | - |
| Rahbar et al. 2016 | X | X | - | - | X | - | - | X | - | - | - | |
| Rahbar et al. 2016 | - | - | X | - | X | - | - | - | - | - | - | - |
| Heck et al. 2016 | X | X | - | X | X | - | - | X | X | - | - | |
| Yadav et al. 2016 | X | X | X | - | - | - | - | - | - | - | - | - |
| Fendler et al. 2016 | X | X | - | - | X | X | - | - | X | X | X | - |
| Kratochwil et al. 2016 | X | X | - | - | X | - | - | - | X | - | - | - |
| Ahmadzadehfar et al. 2017 | X | X | X | - | X | X | X | - | X | X | - | - |
Salivary glands
Although the salivary glands contain highly differentiated cells and their proliferation rate is slow, they are exceptionally radiosensitive organs. Due to the high binding of PSMA ligands, damage to the salivary glands and the development of xerostomia is a frequent side effect of radiation therapy which decreases the patient’s quality of life. In the studies by Ahmadzadehfar et al. [26–28, 40], Heck et al. [48], and Rahbar et al. [14, 25, 41], patients received an ice pack collar for 30 min before and for up to 4 h after the administration of 177Lu-PSMA-617 to induce vasoconstriction and reduce PSMA binding to the salivary glands. Transient xerostomia or hypogeusia occurred in 4–37% of patients with or without an ice pack collar [16, 26, 48].
Toxicity of 225Ac-PSMA
As stated earlier, 225Ac-PSMA therapy can be used in the patients who are unresponsive to 177Lu-PSMA therapy or show pronounced bone marrow infiltration. 225Ac-PSMA radiation consists of short-ranged alpha particles which kill tumour cells but spare the bone marrow cells. In a recent study, many patients reported the success of their 225Ac-PSMA therapy [36]. Also, in the case of the preclinical models of neuroendocrine tumours, 225Ac-DOTATOC was very effective in controlling the tumours, thus indicating that 225Ac-PSMA therapy was very effective [49]. In one recent study, researchers treated 40 patients using 225Ac-PSMA therapy and noted that four patients had to discontinue their treatment because of xerostomia. Twenty four patients (63%) showed more than a 50% decrease in their PSA levels while 33 (87%) patients showed some PSA response. The median duration for tumour control after the last-line of 225Ac-PSMA-617 therapy was 9 months, while five patients endured a response for ≥ 2 years [36, 50]. None of the patients showed any hematologic toxicity, and xerostomia was the only clinical side effect observed [36].
Conclusions
177Lu- and 225Ac-based PSMA-targeted therapies appear to be promising and effective treatments for advanced prostate cancer. The new types of diagnostic tracers show a high sensitivity and specificity in the imaging of prostate cancers, even in patients with very low PSA levels, which has helped in the diagnostics, especially for staging and follow up during RLT. Therefore, prospective randomised trials are required to determine the impact of 177Lu-PSMA on survival, toxicities, dosimetry, and to rigorously assess the clinical benefits compared to other treatments for prostate cancer, including chemotherapy, EBRT, and androgen blockade. We suggest that a new type of nuclear medicine specialist should be established to perform and further develop radionuclide therapy: theranostics specialists should be trained in the fields of radionuclide treatment, radionuclide imaging, oncology, and radiation therapy to cover all aspects of these complex therapies and to ensure that this new treatment option is accepted worldwide.
Acknowledgments
We are grateful to the nursing staff of the treatment ward in our department. We give special thanks to our study nurse, Mrs. Ulrike Kuhn-Seifer (Department of Nuclear Medicine Bonn) and to the patients for their willingness to undergo an investigational treatment and consent to the publications of the results.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Abbreviations
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- CRPC
Castration-resistant prostate cancer
- CTC
Common toxicity criteria
- EBRT
External beam radiotherapy
- Hb
Haemoglobin
- MBq
Megabecquerel
- OS
Overall survival
- PET/CT
Positron emission tomography/computed tomography
- PSA
Prostate-specific antigen
- PSMA
Prostate-specific membrane antigen
- RLT
Radioligand therapy
- SUV
Standard uptake value
Authors’ contributions
Conception and design: HA, ME. Research: HA, ZHA. Manuscript writing: ZHA, HA, ME. All authors read and approved the final manuscript.
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Consent for publication
The consent form was signed by the patient presented in Fig. 1.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zool Hilmi Awang, Email: zoolhilmi_75@hotmail.com.
Markus Essler, Email: markus.essler@ukbonn.de.
Hojjat Ahmadzadehfar, Phone: +49 228 287 19858, Email: hojjat.ahmadzadehfar@ukbonn.de.
References
- 1.Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374–1403. doi: 10.1016/j.ejca.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 2.Nelson WG, De Marzo AM, Isaacs WB. Prostate cancer. N Engl J Med. 2003;349:366–381. doi: 10.1056/NEJMra021562. [DOI] [PubMed] [Google Scholar]
- 3.Saad F, Chi KN, Finelli A, et al. The 2015 CUA-CUOG guidelines for the management of castration-resistant prostate cancer (CRPC) Can Urol Assoc J. 2015;9:90–96. doi: 10.5489/cuaj.2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cornford P, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG guidelines on prostate Cancer. Part II: treatment of relapsing, metastatic, and castration-resistant prostate Cancer. Eur Urol. 2017;71:630–642. doi: 10.1016/j.eururo.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373:737–746. doi: 10.1056/NEJMoa1503747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.James ND, Sydes MR, Clarke NW, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387:1163–1177. doi: 10.1016/S0140-6736(15)01037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chi KN, Kheoh T, Ryan CJ, et al. A prognostic index model for predicting overall survival in patients with metastatic castration-resistant prostate cancer treated with abiraterone acetate after docetaxel. Ann Oncol. 2016;27:454–460. doi: 10.1093/annonc/mdv594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–1197. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 9.de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147–1154. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 10.Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 11.Parker C, Sartor O. Radium-223 in prostate cancer. N Engl J Med. 2013;369:1659–1660. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 12.Eiber M, Maurer T, Souvatzoglou M, et al. Evaluation of hybrid (6)(8)Ga-PSMA ligand PET/CT in 248 patients with biochemical recurrence after radical prostatectomy. J Nucl Med. 2015;56:668–674. doi: 10.2967/jnumed.115.154153. [DOI] [PubMed] [Google Scholar]
- 13.Braat A, Ahmadzadehfar H. Lutetium-177 labelled PSMA ligands for the treatment of metastatic castrate-resistant prostate cancer. Tijdschr Nucl Geneesk. 2016;38:1627–1634. [Google Scholar]
- 14.Ahmadzadehfar H, Rahbar K, Kurpig S, et al. Early side effects and first results of radioligand therapy with (177)Lu-DKFZ-617 PSMA of castrate-resistant metastatic prostate cancer: a two-centre study. EJNMMI Res. 2015;5:114. doi: 10.1186/s13550-015-0114-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kabasakal L, AbuQbeitah M, Aygun A, et al. Pre-therapeutic dosimetry of normal organs and tissues of (177)Lu-PSMA-617 prostate-specific membrane antigen (PSMA) inhibitor in patients with castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging. 2015;42:1976–1983. doi: 10.1007/s00259-015-3125-3. [DOI] [PubMed] [Google Scholar]
- 16.Rahbar K, Ahmadzadehfar H, Kratochwil C, et al. German multicenter study investigating 177Lu-PSMA-617 Radioligand therapy in advanced prostate cancer patients. J Nucl Med. 2017;58:85–90. doi: 10.2967/jnumed.116.183194. [DOI] [PubMed] [Google Scholar]
- 17.Ahmadzadehfar H, Aryana K, Pirayesh E, et al. The Iranian Society of Nuclear Medicine practical guideline on radioligand therapy in metastatic castration-resistant prostate cancer using 177Lu-PSMA. Iran J Nucl Med. 2018;26:2–8. [Google Scholar]
- 18.Heidenreich A, Bastian PJ, Bellmunt J, et al. EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2014;65:467–479. doi: 10.1016/j.eururo.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 19.Heidenreich A, Porres D. Prostate cancer: treatment sequencing for CRPC--what do we know? Nat Rev Urol. 2014;11:189–190. doi: 10.1038/nrurol.2014.36. [DOI] [PubMed] [Google Scholar]
- 20.Bostwick DG, Pacelli A, Blute M, Roche P, Murphy GP. Prostate specific membrane antigen expression in prostatic intraepithelial neoplasia and adenocarcinoma: a study of 184 cases. Cancer. 1998;82:2256–2261. doi: 10.1002/(SICI)1097-0142(19980601)82:11<2256::AID-CNCR22>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 21.Silver DA, Pellicer I, Fair WR, Heston WD, Cordon-Cardo C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res. 1997;3:81–85. [PubMed] [Google Scholar]
- 22.Wright GL, Jr, Haley C, Beckett ML, Schellhammer PF. Expression of prostate-specific membrane antigen in normal, benign, and malignant prostate tissues. Urol Oncol. 1995;1:18–28. doi: 10.1016/1078-1439(95)00002-Y. [DOI] [PubMed] [Google Scholar]
- 23.Fendler WP, Kratochwil C, Ahmadzadehfar H, et al. 177Lu-PSMA-617 therapy, dosimetry and follow-up in patients with metastatic castration-resistant prostate cancer. Nuklearmedizin. 2016;55:123–128. [PubMed] [Google Scholar]
- 24.Rahbar K, Bogemann M, Ahmadzadehfar H. 177Lu-PSMA-617 radioligand therapy of mCRPC: evaluation criteria of response. Eur J Nucl Med Mol Imaging. 2017;44:166–167. doi: 10.1007/s00259-016-3530-2. [DOI] [PubMed] [Google Scholar]
- 25.Rahbar K, Schmidt M, Heinzel A, et al. Response and tolerability of a single dose of 177Lu-PSMA-617 in patients with metastatic castration-resistant prostate Cancer: a multicenter retrospective analysis. J Nucl Med. 2016;57:1334–1338. doi: 10.2967/jnumed.116.173757. [DOI] [PubMed] [Google Scholar]
- 26.Ahmadzadehfar H, Eppard E, Kurpig S, et al. Therapeutic response and side effects of repeated radioligand therapy with 177Lu-PSMA-DKFZ-617 of castrate-resistant metastatic prostate cancer. Oncotarget. 2016;7:12477–12488. doi: 10.18632/oncotarget.7245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahmadzadehfar H, Wegen S, Yordanova A, et al. Overall survival and response pattern of castration-resistant metastatic prostate cancer to multiple cycles of radioligand therapy using [177Lu]Lu-PSMA-617. Eur J Nucl Med Mol Imaging. 2017;44:1448–1454. doi: 10.1007/s00259-017-3716-2. [DOI] [PubMed] [Google Scholar]
- 28.Ahmadzadehfar H, Zimbelmann S, Yordanova A, et al. Radioligand therapy of metastatic prostate cancer using 177Lu-PSMA-617 after radiation exposure to 223Ra-dichloride. Oncotarget. 2017;8:55567–55574. doi: 10.18632/oncotarget.15698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferdinandus J, Eppard E, Gaertner FC, et al. Predictors of response to Radioligand therapy of metastatic castrate-resistant prostate Cancer with 177Lu-PSMA-617. J Nucl Med. 2017;58:312–319. doi: 10.2967/jnumed.116.178228. [DOI] [PubMed] [Google Scholar]
- 30.Kulkarni HR, Singh A, Schuchardt C, et al. PSMA-based Radioligand therapy for metastatic castration-resistant prostate Cancer: the Bad Berka experience since 2013. J Nucl Med. 2016;57:97S–104S. doi: 10.2967/jnumed.115.170167. [DOI] [PubMed] [Google Scholar]
- 31.Rathke H, Giesel FL, Flechsig P, et al. Repeated (177)Lu-labeled PSMA-617 Radioligand therapy using treatment activities of up to 9.3 GBq. J Nucl Med. 2018;59:459–465. doi: 10.2967/jnumed.117.194209. [DOI] [PubMed] [Google Scholar]
- 32.Kratochwil C, Giesel FL, Stefanova M, et al. PSMA-targeted radionuclide therapy of metastatic castration-resistant prostate Cancer with 177Lu-labeled PSMA-617. J Nucl Med. 2016;57:1170–1176. doi: 10.2967/jnumed.115.171397. [DOI] [PubMed] [Google Scholar]
- 33.Fendler WP, Reinhardt S, Ilhan H, et al. Preliminary experience with dosimetry, response and patient reported outcome after 177Lu-PSMA-617 therapy for metastatic castration-resistant prostate cancer. Oncotarget. 2017;8:3581–3590. doi: 10.18632/oncotarget.12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heck MM, Retz M, D'Alessandria C, et al. Systemic Radioligand therapy with (177)Lu labeled prostate specific membrane antigen ligand for imaging and therapy in patients with metastatic castration resistant prostate Cancer. J Urol. 2016;196:382–391. doi: 10.1016/j.juro.2016.02.2969. [DOI] [PubMed] [Google Scholar]
- 35.Kratochwil C, Bruchertseifer F, Rathke H, et al. Targeted alpha-therapy of metastatic castration-resistant prostate Cancer with 225Ac-PSMA-617: dosimetry estimate and empiric dose finding. J Nucl Med. 2017;58:1624–1631. doi: 10.2967/jnumed.117.191395. [DOI] [PubMed] [Google Scholar]
- 36.Kratochwil C, Bruchertseifer F, Giesel FL, et al. 225Ac-PSMA-617 for PSMA-targeted alpha-radiation therapy of metastatic castration-resistant prostate Cancer. J Nucl Med. 2016;57:1941–1944. doi: 10.2967/jnumed.116.178673. [DOI] [PubMed] [Google Scholar]
- 37.Zechmann CM, Afshar-Oromieh A, Armor T, et al. Radiation dosimetry and first therapy results with a (124)I/ (131)I-labeled small molecule (MIP-1095) targeting PSMA for prostate cancer therapy. Eur J Nucl Med Mol Imaging. 2014;41:1280–1292. doi: 10.1007/s00259-014-2713-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Afshar-Oromieh A, Haberkorn U, Zechmann C, et al. Repeated PSMA-targeting radioligand therapy of metastatic prostate cancer with 131I-MIP-1095. Eur J Nucl Med Mol Imaging. 2017;44:950–959. doi: 10.1007/s00259-017-3665-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rahbar K, Bode A, Weckesser M, et al. Radioligand therapy with 177Lu-PSMA-617 as a novel therapeutic option in patients with metastatic castration resistant prostate Cancer. Clin Nucl Med. 2016;41:522–528. doi: 10.1097/RLU.0000000000001240. [DOI] [PubMed] [Google Scholar]
- 40.Ahmadzadehfar H, Schlolaut S, Fimmers R, et al. Predictors of overall survival in metastatic castration-resistant prostate cancer patients receiving [177Lu]Lu-PSMA-617 radioligand therapy. Oncotarget. 2017;8(61):103108–16. [DOI] [PMC free article] [PubMed]
- 41.Rahbar K, Boegemann M, Yordanova A, et al. PSMA targeted radioligandtherapy in metastatic castration resistant prostate cancer after chemotherapy, abiraterone and/or enzalutamide. A retrospective analysis of overall survival. Eur J Nucl Med Mol Imaging. 2018;45(1):12–19. [DOI] [PubMed]
- 42.Gourni E, Henriksen G. Metal-based PSMA Radioligands. Molecules. 2017;22(4). [DOI] [PMC free article] [PubMed]
- 43.Delker A, Fendler WP, Kratochwil C, et al. Dosimetry for (177)Lu-DKFZ-PSMA-617: a new radiopharmaceutical for the treatment of metastatic prostate cancer. Eur J Nucl Med Mol Imaging. 2016;43:42–51. doi: 10.1007/s00259-015-3174-7. [DOI] [PubMed] [Google Scholar]
- 44.Guerriero F, Ferrari ME, Botta F, et al. Kidney dosimetry in (1)(7)(7)Lu and (9)(0)Y peptide receptor radionuclide therapy: influence of image timing, time-activity integration method, and risk factors. Biomed Res Int. 2013;2013:935351. doi: 10.1155/2013/935351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gaertner FC, Halabi K, Ahmadzadehfar H, et al. Uptake of PSMA-ligands in normal tissues is dependent on tumor load in patients with prostate cancer. Oncotarget. 2017;8:55094–55103. doi: 10.18632/oncotarget.19049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vallabhajosula S, Goldsmith SJ, Hamacher KA, et al. Prediction of myelotoxicity based on bone marrow radiation-absorbed dose: radioimmunotherapy studies using 90Y- and 177Lu-labeled J591 antibodies specific for prostate-specific membrane antigen. J Nucl Med. 2005;46:850–858. [PubMed] [Google Scholar]
- 47.Yordanova A, Becker A, Eppard E, et al. The impact of repeated cycles of radioligand therapy using [177Lu]Lu-PSMA-617 on renal function in patients with hormone refractory metastatic prostate cancer. Eur J Nucl Med Mol Imaging. 2017;44:1473–1479. doi: 10.1007/s00259-017-3681-9. [DOI] [PubMed] [Google Scholar]
- 48.Heck MM, Retz M, D'Alessandria C, et al. Systemic radioligand therapy with Lu-PSMA-I&T in patients with metastatic castration-resistant prostate cancer. J Urol. 2016;196(2):382–91 [DOI] [PubMed]
- 49.Miederer M, Henriksen G, Alke A, et al. Preclinical evaluation of the alpha-particle generator nuclide 225Ac for somatostatin receptor radiotherapy of neuroendocrine tumors. Clin Cancer Res. 2008;14:3555–3561. doi: 10.1158/1078-0432.CCR-07-4647. [DOI] [PubMed] [Google Scholar]
- 50.Kratochwil C, Bruchertseifer F, Rathke H, et al. Targeted alpha therapy of mCRPC with (225)actinium-PSMA-617: swimmer-plot analysis suggests efficacy regarding duration of tumor-control. J Nucl Med. 2018;59(5):795–802. [DOI] [PubMed]
- 51.Baum RP, Kulkarni HR, Schuchardt C, Singh A, Wirtz M, Wiessalla S, Schottelius M, Mueller D, Klette I, Wester HJ. Lu-Labe led Prostate-Specific Membrane Antigen Radioligand Therapy of Metastatic Castration-Resistant Prostate Cancer: Safety and Efficacy. J Nucl Med. 2016;57(7):1006–13. doi: 10.2967/jnumed.115.168443. [DOI] [PubMed] [Google Scholar]
- 52.Yadav MP, Ballal S, Tripathi M, Damle NA, Sahoo RK, Seth A, Bal C. Lu-DKFZ-PSMA-617 therapy in metastatic castration resistant prostate cancer: safety, efficacy, and quality of life assessment. Eur J Nucl Med Mol Imaging. 2017;44(1):81–91. doi: 10.1007/s00259-016-3481-7. [DOI] [PubMed] [Google Scholar]


