Case report: Initial presentation
A 12-year-old boy presented with a two-day history of gross hematuria and vomiting. Further questioning revealed a one-year history of position-induced paroxysmal headaches, polyuria, polydipsia, nocturia, dehydration, and lethargy. He denied any syncopal episodes, palpitations, night sweats, blurry vision, or anxiety attacks. He was noted to have normal pubertal development. An abdominal ultrasound was performed, and showed an 8.1 × 7.5 cm bladder mass on the posterior bladder wall with hypervascular components (Fig. 1). The patient was brought to the operating theater for cystoscopy, which revealed a large, necrotic mass on the postero-lateral bladder wall. Bladder biopsy was performed. There were significant blood pressure fluctuations during the biopsy. Final pathology reported the mass to be a bladder paraganglioma (PGL).
Fig. 1.
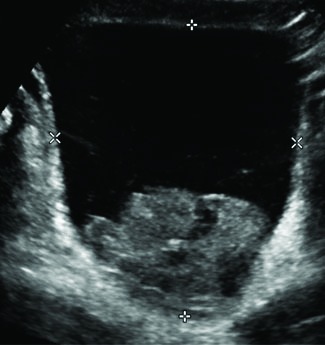
Transverse ultrasound image through the bladder identifies a large lobulated solid mass in the base of the bladder.
The patient received a two-week course of alpha-adrenergic blockade with prazosin and amlodipine for blood pressure control prior to definitive surgery. Echocardiogram did not reveal significant cardiomyopathy. Positron emission/computed tomography (PET/CT) scan showed no evidence of metastatic disease, but avid F18-fludeoxyglucose ([F-18] FDG) uptake was noted at the tumour site (Fig. 2).
Fig. 2.
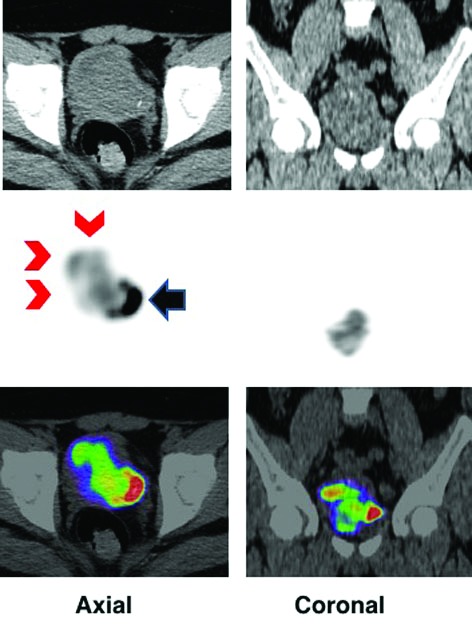
F18-fludeoxyglucose positron emission tomography–computed tomography (FDG PET/CT) image in axial and coronal projections show increased FDG activity in the bladder mass (red chevron) that is indenting the bladder (black arrow). First row is CT, second PET, and third fusion of PET/CT.
Management
The patient was brought back to operating theatre for treatment via partial cystectomy. The tumour was located near the right ureteric opening, and a ureteric stent was placed for six weeks. Pathology reported the resected PGL had negative surgical margins and no invasion into extravesicular fat. The patient was followed with urine metanephrine and blood pressure measurements every three months for surveillance. Subsequent medical genetics consultation revealed an underlying succinate dehydrogenase B (SDHB) gene mutation. First-degree patient relatives were subsequently investigated for the SDHB mutation.
Recurrence
Three years post-resection, a routine endocrinological surveillance test detected elevated urine norepinephrine, normetanephrine, and dopamine. An urgent FDG PET/CT scan revealed three avid soft tissue masses: a 5 × 9 mm right anterolateral lesion between the bladder wall and the inguinal canal, an enlarged 9 × 11 mm right external iliac node, and a non-specific density in the left paraspinal muscle at the level of the ninth rib. A two-week preoperative course of alpha-adrenergic blockade was administered in preparation for surgery. An intraoperative gamma probe procedure was used for lesion localization. Right-sided avid common iliac nodes were sampled and PGL cells were histologically confirmed in one lymph node. Thirteen obturator nodes anterior to the bladder were resected and revealed benign histology, with evidence of histiocytosis in five nodes. Paraspinal muscle biopsies were negative. Of the lesions visualized using PET/CT imaging, a single lymph node was positive for PGL cells. Endocrine consultation and bloodwork were negative for signs of disease post-resection. Further cytogenetic studies demonstrated a normal male karyotype 46XY with a balanced translocation t(5;19)(q31;p13). Continual surveillance is comprised of regular ultrasonography and urine catecholamine testing every three months with PET/CT scans only if either of these methods indicates abnormalities. Our centre elected to perform regular cystoscopy for evidence of local recurrence or a new bladder primary. Currently, evidence around this protocol is lacking from the literature.
Discussion
Clinical presentation
Pheochromocytomas (PCC) and PGLs are rare catecholamine-secreting tumours arising from either chromaffin tissue in the adrenal medulla or embryonic neural crest cells, respectively.1,2 PGLs may be found anywhere from the subocciput to the pelvic cavity.1 The incidence of these tumours is 2–8 per million, with most patients being diagnosed in the third to fourth decade.3 PCCs are associated with a lower rate of malignancy at less than 10%, as opposed to PGLs, which have a malignancy rate up to 40%.2 Bladder PGLs are extremely rare, making up less than 0.05% of all bladder tumours, and less than 1% of all PCCs and PGLs. Malignancy rates specific to bladder PGLs are reported to be from 5–15%, and are higher in hereditary conditions.4 It is, therefore, advisable to genetically screen patients presenting with malignant PCCs or PGLs for SDHx mutations.5 Zimmerman et al was the first to report a case of PGL of the bladder in 1953, and since that time there have been fewer than 200 cases described.6,7 Bladder PGLs typically present with painless gross hematuria (50–60%), hypertension (65–80%), headaches, diaphoresis, and palpitations during micturition.8,9 Most of these tumours are hormonally active (83%) and are commonly located in the submucosa, either in the dome or the posterior wall near the trigone (41%).9,10
Familial paraganglioma syndromes
Hereditary forms of PCC and PGL include von Hippel-Lindau disease, multiple endocrine neoplasia type 2, and type 1 neurofibromatosis. Associated predisposing genes include VHL, RET, NF1, SDHB, SDHC, SDHD, and SDHAF2, which may account for 40–45% of PCCs and PGLs.3,11
Following a medical genetics consultation, our patient was found to have SDHB mutation. The succinate dehydrogenase enzyme is also known as Complex II in the mitochondrial electron transport chain, and consists of four subunits (A–D), with subunits B, C, and D associated with PCC and PGL. SDHB-related tumour susceptibility (also known as PGL4) is inherited in an autosomal dominant manner. Compared to mutations in the other subunits, SDHB mutations are associated with a higher frequency of abdominal and pelvic PGLs, but lower frequency of head and neck PGLs.12
Mutations in the B subunit, a tumour suppressor gene, have been associated with more aggressive clinical outcomes, with high malignancy rates (34–71%)13 and frequent occurrence of multifocal disease.12 Rates of penetrance vary between studies. Some reported 80–100% by age 70, whereas other studies and reviews reported 45–52% by ages 35–60.3,12,14,15 However, not only is it important for first-degree relatives of affected individuals to undergo genetic testing, but also patients presenting with apparently sporadic PCCs and PGLs, especially when exhibiting qualities of PGL4, such as having malignant multifocal or extra-adrenal tumours, or early-onset presentation. Regular screening of at-risk patients and family members and early detection of disease reduces morbidity and complications.15
One proposed model relating SDHB mutations to PCC describes a pseudohypoxic state caused by impaired oxidative phosphorylation resulting from partial or complete loss of activity of the SDH enzyme.14 It has been proposed that this induces the HIF-1 (hypoxia-inducible factor 1) pathway, which is known to cause the upregulation of genes involved in cellular metabolism, cell proliferation, apoptosis, glucose transport, and angiogenesis.16
Investigations and imaging
Preoperative diagnostic tests include 24-hour urine or plasma fractionated catecholamines and metanephrines. However, these tests may be normal in certain tumours that only produce small levels of catecholamines or exclusively secrete dopamine. Therefore, measuring plasma dopamine or its metabolite may be useful.17 Chromogranin A is stored and released with catecholamines and can be measured in the plasma for identifying PCCs with a sensitivity of 83–89%. Chromogranin A may be useful for confirmatory testing in the setting of mildly elevated free plasma metanephrine levels, differentiating between benign and malignant disease, and gauging tumour response and relapse.1
There is a scarcity of evidence-based biochemical surveillance protocols for patients with hereditary PGL syndromes. This is due to the broad spectrum of genotypes and associated phenotypic patterns of tumour secretion products, location, and behaviour. SDHB mutations are generally associated with large extra-adrenal tumours and high malignant potential. Furthermore, a predilection for dopamine and dopamine metabolite (methoxytyramine) secretion has been associated with SDHB gene mutations.
Imaging studies for bladder PGLs include ultrasonography, CT, magnetic resonance imaging (MRI), and molecular imaging scans using radiotracers. Bladder PGLs appear as hypoechoic, well-defined lesions on ultrasonography; however, these findings are non-specific.9 CT has a much higher sensitivity for extra-adrenal, metastatic, or recurrent tumours. Smaller lesions may appear homogenous on CT, while larger lesions appear heterogeneous due to hemorrhage and low-density necrotic areas. MRI is particularly useful in cases where ionizing radiation should be avoided, such as for children or pregnant women. PCCs are isointense to the liver, kidneys, and muscles on T1 imaging. On T2 imaging, the hypervascularity of the tumour makes it appear characteristically bright. MRI allows for visualization of vessel invasion and may be performed using contrast without risk of catecholamine release. Overall, the sensitivity of detecting extra-adrenal, metastatic, or recurrent PCC is 90% vs. 93–100% for primary adrenal disease.18 The specificity associated with ultrasonography, CT, and MR modalities is lower than that of molecular imaging.
Molecular imaging uses radiotracers to image human tissues; in combination with CT, this approach provides metabolic and functional detail layered onto anatomically accurate CT images. Depending on the nuclear properties of the radiotracers, either gamma radiation or positrons will be emitted, as they accumulate in tissues, corresponding to SPECT/CT and PET/CT respectively. Relevant SPECT/CT scintigraphy methods for PCC and PGL imaging use spectrated radiopharmaceuticals, including meta-123, 131I-benzylguanidine (mIBG) and somatostatin (SS) analogues labelled with 111In.
mIBG is a catecholamine analogue that shares both the uptake and storage mechanism with norepinephrine in the adrenal gland and lacks post-synaptic activity.10,11 Amino acid-based tracers, such as 111In-pentetreotide (an SS analogue) bind to SS receptors expressed by neuroendocrine tumours and are, therefore, relevant to PGL and PCC imaging.19 mIBG and 111In-pentetreotide are indicated for detection, staging, treatment (131I), and monitoring therapy of PGL and PCC, among other neuroendocrine tumours, although mIBG is considered the mainstay of PGL and PCC scintigraphy.20 The sensitivity and specificity of mIBG for PCC and PGL are reported to be 85–90% and 89–95%, respectively. 19,20 m-123I-BG has a higher sensitivity than m-131-BG for imaging these tumours.21 Similar sensitivity and specificity is documented in malignant PCC or PGL using SS receptor analogue scintigraphy, although these values are based on smaller sample sizes.19 In the case of metastatic PCC, the sensitivity of 111In-pentetreotide scintigraphy has been reported to be higher than that achieved using mIBG.20 Importantly, the sensitivity of m-123I-BG for imaging PCC and PGL in familial SDHx mutation patients is <50%.22
PET/CT is the primary investigative molecular modality for PCC and PGL at our institution. There are several relevant radiolabelled isotopes; these include [F-18]FDG, which targets metabolically active cells, as well as 18F-fluoro-DOPA ([F-18]-DOPA), which interacts with catecholamine metabolism. 22 [F-18]-DOPA is associated with a sensitivity and specificity of 91% and 95%, respectively.23 Studies limited by small sample sizes suggest a role in staging and restaging for [F-18]-DOPA PET/CT in familial PGL syndromes with low malignant potential.24 As PGL cells become phenotypically less differentiated, the catecholamine metabolic function is hypothesized to become less regulated, reducing the utility of [F-18]-DOPA, but improving the utility of [F-18] FDG.24 Using [F-18]FDG is reported to be 90% sensitive in metastatic or highly malignant PGL; however, [F-18]FDG accumulates in all rapidly dividing tissues and, therefore, remains non-specific for PCCs and PGLs.25 Other radiolabeled isotopes, such as [C-11]hydroxyephedrine and [C-11]ephedrine, are specific for PGLs, but the complex radiopharmaceutical development infrastructure required to feasibly produce these isotopes limits their utility in many centres, including ours.18
There is growing evidence to suggest the utility of gadolinium-68 (GA-68) labelled somatostatin analogue tracers using the PET/CT modality. One such example is GA-68 1, 4, 7, 10-tetraazacyclododecane-1, 4, 7, 10-tetraacetic acid(0)-Tyr(3)-octreotate (GA-68-DOTATATE). Several somatostatin receptor isozymes have been demonstrated in PCC and PGL tissue and, therefore, these receptors make useful imaging targets.26,27 In a prospective study comprised of 17 SDHB mutation-positive patients with metastatic PCCs or PGLs, GA-68-DOTATATE-based tracers demonstrated lesion-based detection rates of 98.6%. This was higher than all other imaging modalities compared, including FDG-18.28 GA-68 tracers are not currently used at our institution.
We used an [F-18]FDG tracer to visualize the presenting and recurrent pathology. Due to the renal excretory pathway of [F-18]FDG, visualization of the bladder lesion was challenging. This issue has previously been reported in renal and bladder PGL cases.18 There was a discrepancy between tumour recurrence visualized on functional [F-18] FDG imaging and positive PGL biopsies taken from the three avid regions following dissection. Current literature has not yet elucidated a clear mechanism for these false positives on imaging. It was previously believed that since SDH-related PGLs, like VHL-related tumours, exhibited the Warburg effect; this explains the effectiveness of [F-18]FDG imaging for these tumours. However, evidence has since suggested that the increased glucose uptake in SDH PGLs is instead associated with increased expression of glucose and hexose transporters through the HIF-1 pathway rather than through metabolic changes.29 If this cellular phenotypic change takes place in the premalignant phase of cellular transformation, we suggest that this could provide a molecular basis for the false positive molecular imaging.
Operative management
Preoperative goals include establishing adequate control of blood pressure and achieving euvolemia. Alpha-blockade for 7–14 days should be used to normalize blood pressure. For patients with cardiac arrhythmias or tachycardias, beta blockade can be used after alpha-blockade has been established. Volume expansion immediately prior to surgery is also recommended. 29
Treatment options for bladder PCC include transurethral resection (TUR), radical cystectomy, and partial cystectomy. Das et al performed a meta-analysis of 100 patients diagnosed with bladder PCCs and their treatment; 7% of patients had TUR, 7% had radical cystectomy, and 86% had partial cystectomy. The authors concluded that in the setting of localized disease, partial cystectomy may be satisfactory. However, extensive disease, positive nodal involvement, or pelvic lymphadenopathy may indicate a radical procedure.7 The equivocal distinction between benign and malignant PCC on pathological examination may not always be definitive. Malignancy is typically proven with demonstration of chromaffin cells in non-chromaffin tissues.30
Postoperatively, the patient should be monitored to prevent possible cardiac and metabolic complications, such as hypoglycemia and hypotension.29 PGL and PCC recurrence is rare (4.6–6.5%); however, followup is recommended. Harari et al suggest following SDHB gene-positive patients or those with greater than three recurrence risk factors (elevated 24-hour urine dopamine, poor pathology characteristics, PASS>6) with plasma metanephrines every six months for two years, then annually for 10 years, and finally every five years for life. Otherwise, for all other patients, yearly plasma metanephrines for five years, then every five years for life is recommended.31
Patients with metastases can receive adjuvant chemotherapy, although reported outcomes are poor.7 Since these tumours arise from neural crest cell origin, a similar chemotherapy regimen to neuroblastoma is used, consisting of cyclophosphamide, vincristine, and dacarbazine (CVD). Huang et al evaluated 18 patients with metastatic PGL treated with CVD regimen. Patients included had good bone marrow, renal, and hepatic function, with Karnofsky score >30%. Patients were followed with serial CT and MIBG imaging every 6–16 weeks. Partial or complete responders had a median survival of 3.8 years post-chemotherapy; all others had a median survival of 1.8 years.31,32
Conclusion
Bladder PGL is a rare catecholamine-secreting neoplasm arising from chromaffin cells of the sympathetic nervous system within the bladder wall. Our patient presented with gross hematuria. Following appropriate diagnostic workup, he was treated with partial cystectomy. He has continued to have surveillance investigations and experienced one episode of biochemical recurrence during which PGL cells were detected in an inguinal lymph node. After undergoing cytogenetic analysis, our patient was found to have a hereditary mutation in the succinate dehydrogenase B gene. The long-term prognosis of this variant is associated with a high malignant potential and stringent surveillance is, therefore, warranted indefinitely. Due to the complex physiological alterations associated with the disease, a multidisciplinary approach to management and treatment is important.
Footnotes
Competing interests: The author reports no competing personal or financial interests related to this work.
This paper has been peer-reviewed.
References
- 1.Andersen KF, Altaf R, Krarup-Hansen A, et al. Malignant pheochromocytomas and paragangliomas — the importance of a multidisciplinary approach. Cancer Treat Rev. 2011;37:111–9. doi: 10.1016/j.ctrv.2010.07.002. https://doi.org/10.1016/j.ctrv.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Vanderveen KA, Thompson SM, Callstrom MR, et al. Biopsy of pheochromocytomas and paragangliomas: Potential for disaster. Surgery. 2009;146:1158–66. doi: 10.1016/j.surg.2009.09.013. https://doi.org/10.1016/j.surg.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 3.Fishbein L, Nathanson KL. Pheochromocytoma and paraganglioma: Understanding the complexities of the genetic background. Cancer Genet. 2012;205:1–11. doi: 10.1016/j.cancergen.2012.01.009. https://doi.org/10.1016/j.cancer-gen.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhalani SM, Casalino DD, Manvar AM. Paraganglioma of the bladder. J Urol. 2011;186:279–80. doi: 10.1016/j.juro.2011.04.032. https://doi.org/10.1016/j.juro.2011.04.032. [DOI] [PubMed] [Google Scholar]
- 5.Lenders JWM, Duh Q-Y, Eisenhofer G, et al. Pheochromocytoma and paraganglioma: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2014;99:1915–42. doi: 10.1210/jc.2014-1498. https://doi.org/10.1210/jc.2014-1498. [DOI] [PubMed] [Google Scholar]
- 6.Zimmerman IJ, Biron RE, MacMahon HE. Pheochromocytoma of the urinary bladder. N Engl J Med. 1953;249:25–6. doi: 10.1056/NEJM195307022490106. https://doi.org/10.1056/NEJM195307022490106. [DOI] [PubMed] [Google Scholar]
- 7.Das S, Bulusu NV, Lowe P. Primary vesical pheochromocytoma. Urology. 1983;21:20–5. doi: 10.1016/0090-4295(83)90116-4. https://doi.org/10.1016/0090-4295(83)90116-4. [DOI] [PubMed] [Google Scholar]
- 8.Piédrola G, López E, Rueda MD, et al. Malignant pheochromocytoma of the bladder: Current controversies. Eur Urol. 1997;31:122–5. doi: 10.1159/000474432. https://doi.org/10.1159/000474432. [DOI] [PubMed] [Google Scholar]
- 9.Thrasher JB, Rajan RR, Perez LM, et al. Pheochromocytoma of urinary bladder: Contemporary methods of diagnosis and treatment options. Urology. 1993;41:435–9. doi: 10.1016/0090-4295(93)90503-3. https://doi.org/10.1016/0090-4295(93)90503-3. [DOI] [PubMed] [Google Scholar]
- 10.Baima C, Casetta G, Vella R, et al. Bladder pheochromocytoma: A 3-year followup after transurethral resection (TURB) Urol Int. 2000;65:176–8. doi: 10.1159/000064868. https://doi.org/10.1159/000064868. [DOI] [PubMed] [Google Scholar]
- 11.Opocher G, Schiavi F. Genetics of pheochromocytomas and paragangliomas. Best Pract Res Clin Endocrinol Metab. 2010;24:943–56. doi: 10.1016/j.beem.2010.05.001. https://doi.org/10.1016/j.beem.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Kantorovich V, King KS, Pacak K. SDH-related pheochromocytoma and paraganglioma. Best Pract Res Clin Endocrinol Metab. 2010;24:415–24. doi: 10.1016/j.beem.2010.04.001. https://doi.org/10.1016/j.beem.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Timmers HJLM, Kozupa A, Eisenhofer G, et al. Clinical presentations, biochemical phenotypes, and genotype-phenotype correlations in patients with succinate dehydrogenase subunit B-associated pheochromocytomas and paragangliomas. J Clin Endocrinol Metab. 2007;92:779–86. doi: 10.1210/jc.2006-2315. https://doi.org/10.1210/jc.2006-2315. [DOI] [PubMed] [Google Scholar]
- 14.Gottlieb E, Tomlinson IPM. Mitochondrial tumour suppressors: A genetic and biochemical update. Nat Rev Cancer. 2005;5:857–66. doi: 10.1038/nrc1737. https://doi.org/10.1038/nrc1737. [DOI] [PubMed] [Google Scholar]
- 15.Favier J, Brière J-J, Strompf L, et al. Hereditary paraganglioma/pheochromocytoma and inherited succinate dehydrogenase deficiency. Horm Res. 2005;63:171–9. doi: 10.1159/000084685. https://doi.org/10.1159/000084685. [DOI] [PubMed] [Google Scholar]
- 16.Semenza GL. HIF-1 and human disease: One highly involved factor. Genes Dev. 2000;14:1983–91. [PubMed] [Google Scholar]
- 17.Eisenhofer G, Siegert G, Kotzerke J, et al. Current progress and future challenges in the biochemical diagnosis and treatment of pheochromocytomas and paragangliomas. Horm Metab Res. 2008;40:329–37. doi: 10.1055/s-2008-1073156. https://doi.org/10.1055/s-2008-1073156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ilias I, Pacak K. Current approaches and recommended algorithm for the diagnostic localization of pheochromocytoma. J Clin Endocrinol Metab. 2004;89:479–91. doi: 10.1210/jc.2003-031091. https://doi.org/10.1210/jc.2003-031091. [DOI] [PubMed] [Google Scholar]
- 19.Pepe G, Bombardieri E, Lorenzoni A, et al. Single-photon emission computed tomography tracers in the diagnostics of neuroendocrine tumours. PET Clin. 2014;9:11–26. doi: 10.1016/j.cpet.2013.08.011. https://doi.org/10.1016/j.cpet.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 20.Ilias I, Chen CC, Carrasquillo JA, et al. Comparison of 6–18F-fluorodopamine PET with 123I-metaiodobenzylguanidine and 111in-pentetreotide scintigraphy in localization of nonmetastatic and metastatic pheochromocytoma. J Nucl Med. 2008;49:1613–9. doi: 10.2967/jnumed.108.052373. https://doi.org/10.2967/jnumed.108.052373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lenders JWM, Eisenhofer G, Mannelli M, et al. Phaeochromocytoma. Lancet. 2005;366:665–75. doi: 10.1016/S0140-6736(05)67139-5. https://doi.org/10.1016/S0140-6736(05)67139-5. [DOI] [PubMed] [Google Scholar]
- 22.Angelousi A, Kassi E, Zografos G, et al. Metastatic pheochromocytoma and paraganglioma. Eur J Clin Investig. 2015;45:986–97. doi: 10.1111/eci.12495. https://doi.org/10.1111/eci.12495. [DOI] [PubMed] [Google Scholar]
- 23.Timmers HJLM, Kozupa A, Chen CC, et al. Superiority of fluorodeoxyglucose positron emission tomography to other functional imaging techniques in the evaluation of metastatic SDHB-associated pheochromocytoma and paraganglioma. J Clin Oncol. 2007;25:2262–9. doi: 10.1200/JCO.2006.09.6297. https://doi.org/10.1200/JCO.2006.09.6297. [DOI] [PubMed] [Google Scholar]
- 24.Marzola MC, Chondrogiannis S, Colletti PM, et al. Succinate dehydrogenase mutation-related paragangliomas: Conventional vs. PET/CT diagnostic workup. Nucl Med Commun. 2015;36:657–65. doi: 10.1097/MNM.0000000000000297. https://doi.org/10.1097/MNM.0000000000000297. [DOI] [PubMed] [Google Scholar]
- 25.Timmers HJLM, Chen CC, Carrasquillo JA, et al. Comparison of 18F-fluoro-L-DOPA, 18F-fluoro-deoxyglucose, and 18F-fluorodopamine PET and 123I-MIBG scintigraphy in the localization of pheochromocytoma and paraganglioma. J Clin Endocrinol Metab. 2009;94:4757–67. doi: 10.1210/jc.2009-1248. https://doi.org/10.1210/jc.2009-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharma P, Singh H, Bal C, et al. PET/CT imaging of neuroendocrine tumours with (68)Gallium-labeled somatostatin analogues: An overview and single institutional experience from India. Indian J Nucl Med. 2014;29:2–12. doi: 10.4103/0972-3919.125760. https://doi.org/10.4103/0972-3919.125760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ueberberg B, Tourne H, Redman A, et al. Differential expression of the human somatostatin receptor subtypes SST1 to SST5 in various adrenal tumours and normal adrenal gland. Horm Metab Res. 2005;37:722–8. doi: 10.1055/s-2005-921092. https://doi.org/10.1055/s-2005-921092. [DOI] [PubMed] [Google Scholar]
- 28.Janssen I, Blanchet EM, Adams K, et al. Superiority of [Ga]-DOTATATE PET/CT to other functional imaging modalities in the localization of SDHB-associated metastatic pheochromocytoma and paraganglioma. Clin Cancer Res. 2015;21:3888–95. doi: 10.1158/1078-0432.CCR-14-2751. https://doi.org/10.1158/1078-0432.CCR-14-2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mannelli M. Management and treatment of pheochromocytomas and paragangliomas. Ann N Y Acad Sci. 2006;1073:405–16. doi: 10.1196/annals.1353.044. https://doi.org/10.1196/annals.1353.044. [DOI] [PubMed] [Google Scholar]
- 30.Jain T, Basher R, Gupta N, et al. Unusual presentation of bladder paraganglioma: Comparison of 131I MIBG SPECT/CT and 68Ga DOTANOC PET/CT. World J Nucl Med. 2015;15:65–7. doi: 10.4103/1450-1147.167591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harari A, Inabnet WB. Malignant pheochromocytoma: A review. Am J Surg. 2011;201:693–701. doi: 10.1016/j.amjsurg.2010.04.012. https://doi.org/10.1016/j.amjsurg.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 32.Huang H, Abraham J, Hung E, et al. Treatment of malignant pheochromocytoma/paraganglioma with cyclophosphamide, vincristine, and dacarbazine. Cancer. 2008;113:2020–8. doi: 10.1002/cncr.23812. https://doi.org/10.1002/cncr.23812. [DOI] [PMC free article] [PubMed] [Google Scholar]


