Abstract
Background:
Clinical trials have shown the positive effects of local insulin therapy in the formation of new vessels and fibrosis in acute and chronic diabetic wounds without major adverse effects.
Objective:
The aim of this study was to investigate the effects of local insulin use on wound healing in non-diabetic patients.
Methods:
A randomized, split-plot, double-blind, placebo-controlled trial was conducted. Ten non-diabetic patients with full-thickness acute wounds were recruited (5 due to trauma, 3 to burns, and 2 to pressure). All wounds received standard bedside treatment. Each wound was divided into 2 zones. One side received a standard care plus insulin, while the other received standard care plus injection of saline solution. A biopsy specimen was taken from both sites on days 0 and 14. The amount of blood vessel growth and the percentage of fibrosis were evaluated.
Results:
A significant difference in the number of new vessels was observed on the insulin-treated site (70.6 [29.21]) compared to saline only (26.5 [34.3]; P < .04). The percentage of fibrosis (insulin 34.7 [28.02] vs saline 27.8 [29.9]) showed no significant difference. No adverse events related to the study occurred. The clinical implications of this study are considerable in terms of the formation of blood vessels but not fibrosis.
Conclusion:
We suggest that local insulin administration is a safe therapeutic option for angiogenesis in wounds of non-diabetic patients.
Keywords: angiogenesis, wound healing, insulin, fibrosis, granulation tissue
Abstract
Historique :
Les essais cliniques démontrent les effets positifs de l’insulinothérapie localisée pour former de nouveaux vaisseaux ou une fibrose en cas de plaies aiguës ou chroniques causées par le diabète, sans entraîner de réactions indésirables majeures.
Objectif :
La présente étude visait à évaluer les effets de l’utilisation localisée d’insuline chez des patients non diabétiques.
Méthodologie :
Les chercheurs ont réalisé un essai aléatoire et contrôlé contre placebo, en parcelles divisées et à double insu. Ils ont recruté dix patients non diabétiques ayant des plaies aiguës de pleine épaisseur (cinq à cause d’un traumatisme, trois à cause de brûlures et deux à cause de pression). Toutes les plaies ont fait l’objet de soins standards au chevet du patient et chacune a été divisée en deux zones. Une zone faisait l’objet de soins standards avec l’ajout d’insuline et l’autre zone, de soins standards avec l’injection de soluté physiologique. Les chercheurs ont prélevé une biopsie dans chaque zone les jours 0 et 14. Ils ont évalué la croissance des vaisseaux sanguins et le pourcentage de fibrose.
Résultats :
Les chercheurs ont observé une différence significative dans le nombre de nouveaux vaisseaux de la zone traitée à l’insuline (70,6 ± 29,21) par rapport à celle traitée à l’aide de soluté physiologique (26,5 ± 34,3; P <0,04). Ils n’ont pas constaté de différence significative dans le pourcentage de fibrose (insuline 34,7 ± 28,02 et soluté physiologique 27,8 ± 29,9) ni de réactions indésirables liées à l’étude. Les conséquences cliniques de la présente étude sont considérables à l’égard de la formation de vaisseaux sanguins, mais pas de la fibrose.
Conclusion :
Selon les chercheurs, l’administration localisée d’insuline serait sécuritaire pour l’angiogenèse des plaies des patients non diabétiques.
Background
Wound healing is the result of a complex series of reactions and interactions among cells and mediators.1 Epithelization, angiogenesis, granulation tissue formation, and collagen deposition are the principal steps in this building process of wound healing. Angiogenesis is a prominent feature of the proliferative phase of healing as the restoration of blood flow to damaged tissues provides oxygen and nutrients required to support the growth and function of reparative cells.2
Insulin has been recognized for its clinical benefits on wound healing for several decades,3 aside from its effect as a regulator of glucose, lipid, and protein metabolism. However, such benefits are archived only on wounds of experimental animals and diabetic patients. Insulin is a peptide hormone and growth factor with several physiological roles and can potentially help restore the integrity of damaged skin; consequently, it is of interest in the investigation of wound healing.4–6
A previous study of diabetic patients demonstrated that local insulin injections for treating wounds increased the formation of blood vessels and fibrosis without any major side effects.7
Compared with previous studies in which insulin was used to treat diabetic wounds, we included nondiabetic patients.5,7 Therefore, the aim of this study was to investigate the effects of local insulin administration to treat acute wounds in nondiabetic patients as well as to evaluate its effect on glucose plasma concentrations, with the objective to prepare the wound to receive a skin graft after 14 days of treatment.
Methods
A randomized, double-blind, controlled trial with a split-plot design was performed in 10 patients (6 men and 4 women, mean age 33 ± 11.04 years) who were recruited from the emergency department of the Hospital Central “Dr Ignacio Morones Prieto”, in San Luis Potosí, México. Inclusion criteria of the study included participants between the ages 18 and 65 years with full-thickness acute wounds of more than 40 cm2, with the first wound debridement having been completed less than 24 hours prior. The exclusion criteria included the following: history of diabetes mellitus, infection, peripheral arterial disease, malnutrition, collagen diseases, renal and hepatic failure.
Wounds were located on the upper extremities (n = 3, avulsion trauma), the lower extremity (n = 4, fire burn), and sacrum (n = 3, pressure; Table 1). The wounds were divided into 2 zones of equal size and a randomized treatment allocation sequence, derived using the statistical package R v.3.1.1. One side received insulin injection, while the other received saline solution injection.
Table 1.
Demographic Information.
| Patients | Sex | Age | Area of the Body Affected | Cause of the Wound | Size of the Wound (cm2) |
|---|---|---|---|---|---|
| 1 | Female | 32 | Lower limb | Trauma | 80 |
| 2 | Female | 23 | Sacrum | Pressure | 100 |
| 3 | Male | 24 | Lower limb | Trauma | 40 |
| 4 | Female | 60 | Abdomen | Burn | 225 |
| 5 | Male | 32 | Lower limb | Burn | 136 |
| 6 | Female | 30 | Upper limb | Burn | 84 |
| 7 | Male | 45 | Sacrum | Pressure | 81 |
| 8 | Male | 31 | Lower limb | Trauma | 64 |
| 9 | Male | 28 | Upper limb | Trauma | 60 |
| 10 | Male | 25 | Upper limb | Trauma | 60 |
The treatment protocol was as follows: first, we divided the wound in 2 halves. Next, we applied 0.1 mL of either neutral protamine Hagedon insulin or sterile saline solution at a depth of 1 to 2 mm at the centre of each half. This volume corresponds to 10 units of a tuberculin syringe, and no further dilution was used. No further doses were applied on the wound borders. In all cases, we ensured that the point of administration of the insulin was at least 2 cm away from the point of administration of the saline solution and 2 cm away from the wound border. All doses on consecutive days were applied at the same sites. Insulin injection was applied after breakfast. Capillary blood glucose was measured before and 3 hours after the insulin was administered. After 14 days of treatment, the wound received an autologous skin graft regardless of the trial result, keeping in line with the International Society for Burn Injuries Practice Guidelines for Burncare guidelines.8
This study was approved by the hospital’s ethics committee (Reg. 71-13). All patients gave signed informed consent prior to participating in the study. Good clinical practice, privacy regulations, and the ethical principles that have their origin in the Declaration of Helsinki in its current review were followed.
Tissue Biopsy
Biopsy specimens were obtained on day 0 and day 14, just prior to grafting. Hematoxylin and eosin staining were used to evaluate blood vessel growth, and the percentage of fibrosis was determined via Masson’s trichrome stain. The images were photographed with a digital camera (Olympus SP-320; Olympus America Inc, Allentown, Pennsylvania) mounted on a microscope (Olympus CX31; Olympus) connected to a personal computer, and the number of blood vessels was counted in 1 randomly selected field. Images were processed using the public domain software ImageJ v1.44 (National Institutes of Health, Bethesda, Maryland). Fibrosis was evaluated by quantifying the percentage area of staining. Image analysis was done with a 10× magnification of the 4-μm processed sections.
Biopsies were analyzed by 2-blinded independent pathologists. Intraobserver and interobserver correlation was 0.987 (95% confidence interval [CI]: 0.945-1) and 0.9805 (95% CI: 0.949-1), respectively, for number of vessels; and 0.979 (95% CI: 0.936-1) and 0.993 (95% CI: 0.969-1), respectively, for the percentage of fibrosis area.
Statistical Analysis
The results were expressed as mean (standard deviation). Statistical analysis was performed using a paired t test. Differences were considered statistically significant at P < .05. R version 3.1.1, R commander version 2.0-95, and 95% confidence intervals were used for statistical analysis.
Results
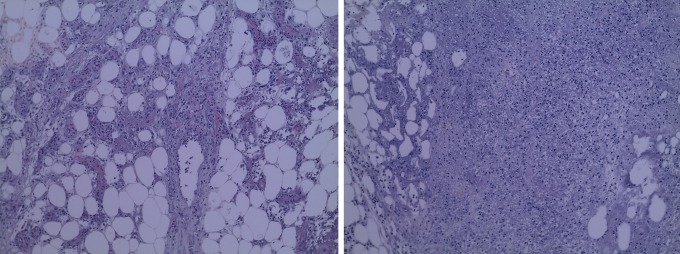
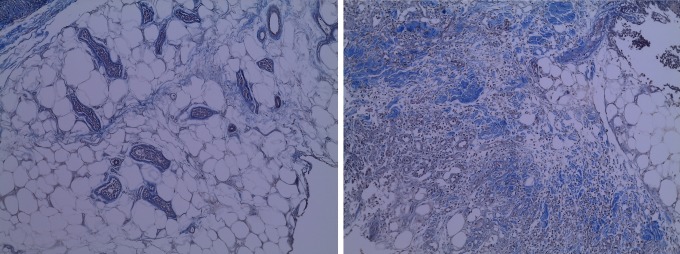
The average wound size in the patients included in the study was 93 (53) cm2. The result of the studied variables can be observed in Table 2. We did not find significant differences in the number of blood vessels (37.9 [43.56] vs 36.4 [28.26]) nor in the percentage of fibrosis (29.3% [33.23%] vs 31.6% [27.11%]) at the beginning of the study (Figures 1–3).
Table 2.
Results of Statistical Analysis.a
| Variable | Day 0 | Day 14 | ||||
|---|---|---|---|---|---|---|
| Insulin | Saline Solution | P | Insulin | Saline Solution | P | |
| No. of vessels | 36.4 (28.26) | 37.9 (43.55) | .9 | 107 (27.11) | 64.4 (33.23) | .04 |
| Fibrosis % | 31.6 (27.1) | 29.3 (33.22) | .8 | 66.3 (26.72) | 57.1 (28.9) | .4 |
aData are presented as mean (standard deviation). t test was used for between-group comparisons.
Figure 1.
Comparing the initial (left) and the final day (right) blood vessel counts.
Figure 2.
Comparing the initial (left) and the final day (right) degree of fibrosis.
Figure 3.
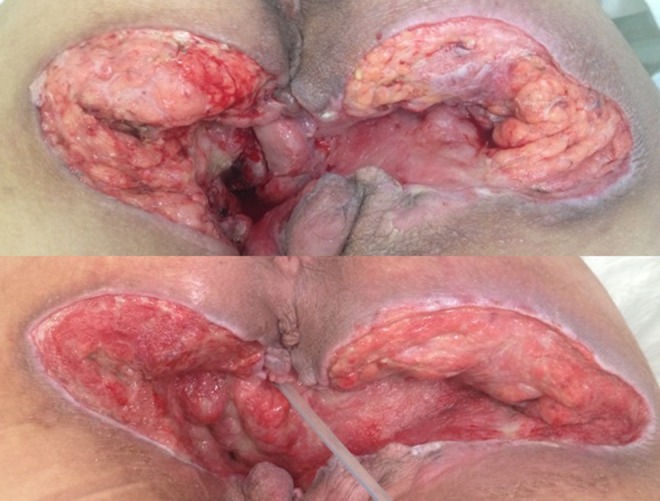
Comparing the insulin-treated site (left) compared to placebo (right) clinical aspect.
After 14 days, significant differences were observed in the number of vessels within the insulin-treated zones (70.6 [29.21]) when compared to saline-only sites (26.5 [34.3]; P < .04). Conversely, the percentage of fibrotic areas did not show significant differences between insulin-treated versus saline-only sites (34.7% [28.02%] vs 27.8.1% [29.9%]; P = .6). Effect size (Cohen’s δ) for the number of blood vessels was 0.805 and 0.334 for the percentage area of fibrosis. No adverse events of hypoglycemia were recorded. Capillary blood glucose levels before treatment (99.59 [11.2] mg/dL) did not significantly differ from those 3 hours after treatment (97.83 [10.3] mg/dL).
Discussion
Compared with previous studies in which local insulin was used in wounds of diabetic patients, we included nondiabetic patients with acute wounds induced by trauma, burns, and pressure. Our previous study7 suggests that insulin improves wound healing in diabetic patients by increasing granulation tissue formation.
Our results show that local insulin stimulates the development of new microvessels but are not associated with the generation of new connective tissue during wound healing. Therefore, we hypothesize that insulin stimulates healing primarily by increasing blood flow.
It is well-known that insulin application to excision wounds in mice has led to accelerated reepithelization by stimulating angiogenesis and promoting a higher level of maturation of the healing tissue. Apikoglu-Rabus et al reported that insulin administration to cutaneous wounds accelerates wound healing in rats with or without acute diabetes. It has been suggested that alterations in the wound healing process begin to occur even at the onset of diabetes and can be associated with deficiencies in the defence cells involved in normal wound healing in addition to a marked decrease in the collagen production.9
Insulin induces the expression of growth factors, such as vascular endothelial growth factor (VEGF) and insulin like growth factor 1 (IGF-1).9–11 The VEGF is the most potent angiogenic factor, and dermal endothelial cells respond to VEGF by proliferating and forming capillary tubes.1,12 Platelets release IGF-1, a potent chemotactic agent of endothelial cells, during degranulation and similarly in fibroblasts, when stimulated. This results in neovascularization,13 which is critical for successful wound healing. It increases blood flow to damaged tissue, thus providing the oxygen and nutrients required by newly synthesized granulation tissue for collagen deposition and wound epithelization. Efforts have been made to induce new blood vessel formation in order to enhance tissue repair.14
The formation of new blood vessels is necessary to sustain the newly formed granulation tissue. Nevertheless once an abundant collagen matrix has been deposited in the wound, the fibroblast will stop producing collagen. This may be particularly useful in the treatment of chronic or impaired wounds in whose tissue insulin and its receptor might be deficient or dysfunctional and the wound healing impairment is attributed to inadequate blood supply. The wound healing impairment in diabetes can be attributed to several factors including inadequate blood supply, decreased proliferative potential of fibroblasts, and decreased inflammatory changes.
Finally, the local administration of insulin improved the blood supply, and increased cellular traffic, improving the overall quality of healing. Therefore, it is possible to take advantage of these differences to treat various types of wounds in diabetic and non-diabetic patients. The dose of local insulin was chosen because it significantly stimulated healing. Although results from this study suggest that local insulin treatment for wound healing in non-diabetic patients is a promising potential therapy.
In addition, we found that 10 units of local insulin is safe without affecting blood glucose levels, thus demonstrating its potential usefulness in wounds of non-diabetic patients. We suggest that local insulin is suitable for routine clinical use, in wound healing. Better understanding of the physiologic pathways of how insulin contributes to angiogenesis in normal tissue repair will help identify future targets for successful therapies.
The present study did not investigate the changes in signaling on cytokine and growth factor expression after insulin delivery. Our study suggests further investigation of insulin as a potential of treatment in conditions in which blood vessel development is impaired with special consideration for inflammatory mediators.6
Although there are many studies that involve local administration of insulin to wounds, it has been difficult to standardize the treatment for practical application.15–22 The mechanism of action, appropriate dosing, and optimal route of delivery of insulin in this application needs to be characterized, as the current study only examined a single dose of 10 units.
Conclusion
The clinical implications of this study are considerable in showing that local insulin increases the formation of blood vessels but not fibrosis. We propose that local insulin administration is a potentially safe therapeutic option for the stimulation of angiogenesis in wounds of non-diabetic patients.
Acknowledgments
The authors thank Peter M. Mandeville, MA, Amado Nieto-Caraveo, MD, and Antonio Gordillo-Moscoso MD for their contribution to this article.
Level of Evidence: Level 2, Therapeutic
Authors’ Note: Trial registration.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Reference
- 1. Broughton G, Janis JE, Attinger CE. The basic science of wound healing. Plast Recontr Surg. 2006;117(suppl 7): 12S–34S. [DOI] [PubMed] [Google Scholar]
- 2. Broughton G, Janis JE, Attinger CE. Wound healing: an overview. Plast Recontr Surg. 2006;117(suppl 7):1–32. [DOI] [PubMed] [Google Scholar]
- 3. Rosenthal SP. Acceleration of primary wound healing by insulin. Arch Surg. 1968;96(1):53–55. [DOI] [PubMed] [Google Scholar]
- 4. Magkos F, Wang X, Mittendorfer B. Metabolic actions of insulin in men and women. Nutrition. 2010;26(7-8):686–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pierre EJ, Barrow RE, Hawkins HK, et al. Effects of insulin on wound healing. J Trauma 1998;44(2):342–345. [DOI] [PubMed] [Google Scholar]
- 6. Hrynyk M, Neufeld RJ. Insulin and wound healing. Burns. 2014. 40(8):1433–1446. [DOI] [PubMed] [Google Scholar]
- 7. Martínez-Jiménez MA, Aguilar-García J, Valdés-Rodríguez R, et al. Local use of insulin in wounds of diabetic patients higher temperature, fibrosis and angiogenesis. Plast Reconstr Surg. 2013;132(6):1015e–1019e. [DOI] [PubMed] [Google Scholar]
- 8. Isbi Practice Guidelines Committee; Steering Subcommittee; Advisory Subcommittee. ISBI practice guidelines for burn care. Burns. 2016;42(5):953–1021. [DOI] [PubMed] [Google Scholar]
- 9. Apikoglu-Rabus S, Izzettin FV, Turan P, Ercan F. Effect of topical insulin on cutaneous wound healing in rats with or without acute diabetes. Clin Exp Dermatol. 2009;35(2):180–185. [DOI] [PubMed] [Google Scholar]
- 10. Liu Y, Petreaca M, Martins-Green M. Cell and molecular mechanism of insulin-indiced angiogenesis. J Cell Mol Med. 2009;13(11-12):4492–4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grant M, Jerdan J, Merimee T. Insulin-like growth factor-1 modulates endothelial cell chemotaxis. J Clin Endocrinol Metab. 1987;65(2):370. [DOI] [PubMed] [Google Scholar]
- 12. Johnson KE, Wilgus TA. Vascular endothelial growth factor and angiogenesis in the regulation of cutaneous wound repair. Adv Wound Care. 2014;3(10):647–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Aghdam SY, Eming SA, Willenborg S, et al. Vascular endothelial insulin/IGF-1 signaling controls skin wound vascularization. Biochem Biophys Res Commun. 2012;421(2):197–202 [DOI] [PubMed] [Google Scholar]
- 14. Paglia DN, Wey A, Breitbart EA, et al. Effects of local insulin delivery on subperiosteal angiogenesis and mineralized tissue formation during fracture healing. J Orthop Res. 2013. 31(5):783–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Greenway SE, Filler LE, Greenway FL. Topical insulin in wound healing: a randomized, doble-blind, placebo-controlled trial. J Wound Care. 1999; 8(10): 526–528.15. [DOI] [PubMed] [Google Scholar]
- 16. Lui Y, Zhang X, Zhang Z, Fang PY, Xu WS. Effects of topical application of insulin on the wound healing in scalded rats. Zhonghua Shao Shang Za Zhi. 2004;20(2):98–101. [PubMed] [Google Scholar]
- 17. Zhang XJ, Wu X, Wolf SE, Hawkins HK, Chinkes DL, Wolfe RR. Local insulin–zinc injection accelerates skin donor site wound healing. J Surg Res. 2007:142(1):90–96. [DOI] [PubMed] [Google Scholar]
- 18. Rezvani O, Shabbak E, Aslani A, Bidar R, Jafari M, Safarnezhad S. A randomized, double-blind, placebo-controlled trial to determine the effects of topical insulin on wound healing. Ostomy Wound Manage. 2009;55(8):22–28 [PubMed] [Google Scholar]
- 19. Hrynyk M, Martins-Green M, Barron AE, Neufeld RJ. Sustained prolonged topical delivery of bioactive human insulin for potential treatment of cutaneous wounds. Inter J Pharmaceutics. 2010;398(1-2):147–154. [DOI] [PubMed] [Google Scholar]
- 20. Scimeca CL, Bharara M, Fischer TK, Kimbriel H, Mills JL, Armstrong DG. Novel use of insulin in continuous-instillation negative pressure wound therapy as wound chemotherapy. J Diabetes Sci Tech. 2010;4(4):820–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang ZX, Liu XL, Lü L, Zhang L, Ji DL, Liu LH. Effect of insulin by local injection on the level of systemic blood glucose and granulation tissue formation of wound in patients with diabetic foot ulcer. Zhonghua Shao Shang Za Zhi. 2011;27(6):451–455. [PubMed] [Google Scholar]
- 22. Hrynyk M, Martins-Green M, Barron AE, Neufeld R. Alginate-PEG sponge architecture and role in the design of insulin release dressings. Biomacromolecules. 2012;13(5):1478–1485. [DOI] [PubMed] [Google Scholar]