Abstract
Background:
In vitro and in vivo studies have described a number of different antibiotic solutions for irrigation of the pocket in implant-based breast augmentation in an attempt to prevent the formation of capsular contracture (CC). Our objective was to evaluate the evidence that antibiotic irrigation reduced the rate of CC.
Methods:
A systematic search of MEDLINE, EMBASE, and CENTRAL was conducted from inception to January 2016. We included studies which examined the use of intraoperative antibiotic irrigation in women undergoing primary breast augmentation. Our primary outcome was the rate of CC. Included studies were assessed for methodological quality using validated tools.
Results:
Seven studies were included in the final analysis: 1 randomized controlled trial (RCT) and 6 non-randomized studies. The mean follow-up ranged from 14 to 72 months. The rate of CC was less than 2% in 8 studies, between 3% and 6% in 4 studies, and 13.9% in 1 study. Included studies demonstrated significant clinical and methodological heterogeneity. The solitary low-quality RCT concluded that antibiotic irrigation was superior to saline irrigation. Three non-randomized studies demonstrated no significant difference in the rate of CC with the use of antibiotics. One non-randomized controlled study showed that the use of mixture of antibiotic and povidone-iodine significantly lowered the rate of CC.
Conclusions:
The available evidence on the use of antibiotic irrigation to prevent CC is weak and it is based on studies with high risk of bias. Methodologically robust studies are necessary to answer the question whether antibiotic breast pocket irrigation prevents CC.
Keywords: breast, augmentation, implant, antibiotic, capsular contracture
Abstract
Historique :
Des études in vitro et in vivo ont décrit plusieurs solutions antibiotiques pour irriguer la cavité en cas d’augmentation mammaire par implant afin de prévenir la formation de contractures capsulaires (CC). Les chercheurs voulaient évaluer les données selon lesquelles l’irrigation antibiotique réduisait le taux de CC.
Méthodologie :
Les auteurs ont effectué une recherche systématique dans MEDLINE, EMBASE et CENTRAL entre le début de l’étude et janvier 2016. Ils ont inclus des études sur l’examen de l’irrigation antibiotique intraopératoire chez des femmes qui avaient subi une augmentation mammaire primaire. Les résultats primaires étaient le taux de CC. Les chercheurs ont évalué la qualité méthodologique des études à l’aide d’outils validés.
Résultats :
Les auteurs ont inclus sept études dans l’analyse définitive, soit un essai aléatoire et contrôlé (EAC) et six études non aléatoires. Le suivi moyen a duré de 14 à 72 mois. Le taux de CC était inférieur à 2 % dans huit études, se situait entre 3 % et 6 % dans quatre études et correspondait à 13,9 % dans une étude. Ces études présentaient une hétérogénéité clinique et méthodologique marquée. Dans la seule EAC, qui était de mauvaise qualité, l’irrigation antibiotique était considérée comme supérieure à l’irrigation par un soluté physiologique. Trois études non aléatoires n’ont démontré aucune différence significative du taux de CC avec l’utilisation d’antibiotiques. Une étude non aléatoire et contrôlée a révélé que le mélange d’antibiotique et de polyvidone iodée réduisait le taux de CC de manière significative.
Conclusions :
Les données probantes sur l’utilisation de l’irrigation d’antibiotiques pour prévenir la CC sont faibles et fondées sur des études comportant un fort risque de biais. Des études robustes sur le plan méthodologique s’imposent pour déterminer si l’irrigation de la cavité mammaire par un antibiotique prévient la CC.
Background
Biofilm formation and subclinical infection have been implicated in the formation of capsular contractures (CC)1–3 and recently have even been investigated as a potential cause of anaplastic large-cell lymphoma (ALCL).4 The use of antibiotic irrigation solution with or without antiseptic is a common practice among American Society of Plastic Surgery (ASPS) members to reduce CC rates. The classic triple antibiotic solution and mixed antibiotic solution with povidone-iodine are used by 53% and 31% of ASPS members, respectively. Despite that, CC remains the main reason for reoperation in the same group of surgeons surveyed (36%).5
In the year 2000, the Food and Drug Administration (FDA) recommended against contact between povidone-iodine and breast implant, as concerns were raised about povidone-iodine causing higher implant deflation rates.6 As a result, Adams et al6,7 proposed the classic triple antibiotic irrigation solution as an alternative implant irrigant. The triple antibiotic irrigation solution is currently the most utilized irrigant by ASPS members as reported by a recent survey (53.0% for triple antibiotic irrigant vs 13.2% for povidone-iodine only).5 Unlike povidone-iodine irrigation, the evidence for the use antibiotic irrigation for implants has not been critically examined. Our aim is to evaluate the evidence of the use of intraoperative antibiotic irrigation in adult women undergoing primary breast augmentation in reducing the rate of moderate to severe CC.
Material and Methods
Protocol and Eligibility Criteria
The Preferred Reporting Items for Systematic Reviews and Meta-Analysis guideline was followed in the performance and consequently reporting this review.8 The population of interest in this study consisted of adult female patients undergoing primary breast augmentation surgery with any type of implants (saline or silicone). The intervention being considered was the use of intraoperative irrigation of the pocket and/or the implant with any antibiotic-containing solution. The control was the use of intraoperative irrigation with non-antibiotic solution, saline, or nothing. The primary outcome of interest was post-operative rate of high-grade CC based on Baker’s classification (III and IV) with an at least 6 months of follow-up.9 Only in vivo studies that compared antibiotic-containing solution irrigation with a control to assess the effect of preventing CC were included in this systematic review. We excluded case reports, expert opinions, and in vitro studies from our analysis.
Search Strategy and Study Selection
To identify eligible studies, the following electronic databases were searched with the help of a medical librarian from inception to January 27, 2016: MEDLINE, EMBASE, and Cochrane Central Register of Controlled Trials. The search strategy included the following key words: “Anti-Bacterial Agents” or “Therapeutic Irrigation” or “irrigant” or “Anti-Bacterial Agents” or “antibiotic solution” or “Anti-Infective Agents” and “capsular contracture” or “Implant Capsular Contracture” or “Prosthesis-Related Infections” and “Breast Implants” or “Breast Implants” or “Prostheses and Implants or “Mammaplasty” or “Breast Implantation” or “Plastic Surgery” or “Breast.” Additionally, we used manual cross-referencing to identify further studies for potential inclusion.
The search was limited to papers published in English peer-reviewed journals. In a first step, 2 independent assessors (O.A.S. and N.J.) screened titles and abstracts to assess eligibility for inclusion. When inclusion was uncertain, a third assessor (S.A.Y.) acted as arbitrator. Second, 2 authors then independently reviewed the studies based on the full text paper and eligibility was decided in a consensus process. Any disagreement was resolved through discussion and agreement between the reviewers. Inter-observer reliability of both the screening (selection of studies) and the assessment of methodological quality was calculated.
Data Extraction and Items
The following data were extracted from each article and used for comparisons: author, journal, year of publication, level of evidence, age, sample size, study design, funding, surgical technique, implant utilized, antibiotic solution used for irrigation, CC rate, hematoma, seroma, infection, follow-up period, and study results. Data were extracted by the same 2 independent reviewers using an Excel (Microsoft Corp, Redmond, Washington) data collection spreadsheet designed a priori.
Assessment of Methodological Quality
Two independent assessors (O.A.S. and N.J.) appraised the methodological quality of included randomized controlled trials (RCTs) using the Cochrane risk of bias tool.10 This tool evaluates 6 items: randomization, allocation concealment, blinding, incomplete outcome data, absence of selective reporting, and other issues (eg, power calculations, groups baseline imbalance). Observational non-randomized studies were assessed using the methodological index for non-randomized studies (MINORS) scale, which is a 12-item valid instrument devised to assess the methodological quality of non-randomized surgical studies, whether controlled or non-controlled.11 Each item was given either “low risk” for bias (2 points) if adequately reported and executed or “high risk” for bias (1 point) if it is reported but inadequately performed. In case of no reporting or when it is not clear, the item was given “unclear” (0 point).11 A high-quality study was defined as a study that scored more than 60% of maximum score, which mean 16 or higher in controlled studies and 10 or higher in non-controlled studies.11,12 The Oxford Centre for Evidence-Based Medicine’s Levels of Evidence was utilized to determine and assign the level of evidence in the included studies.13
Analysis of Heterogeneity
All comparative studies included in the analysis were assessed for clinical and methodological heterogeneity to determine the suitability of quantitative analysis. We predetermined broad Population, Intervention, Control and Outcome (PICO) criteria for inclusion, which inevitably include heterogonous data. Heterogeneity across the studies was also taken into account in formulating the final conclusion.
Statistical Analysis
Kappa statistic was performed as a measure of inter-reviewer agreement for screening and assessment of quality of studies. In case comparative studies did not calculate measure of association, the odds ratio was calculated as well as 95% confidence interval for that estimate. Meta-analysis was considered only when there is no significant heterogeneity.
Results
Study Selection and Characteristics
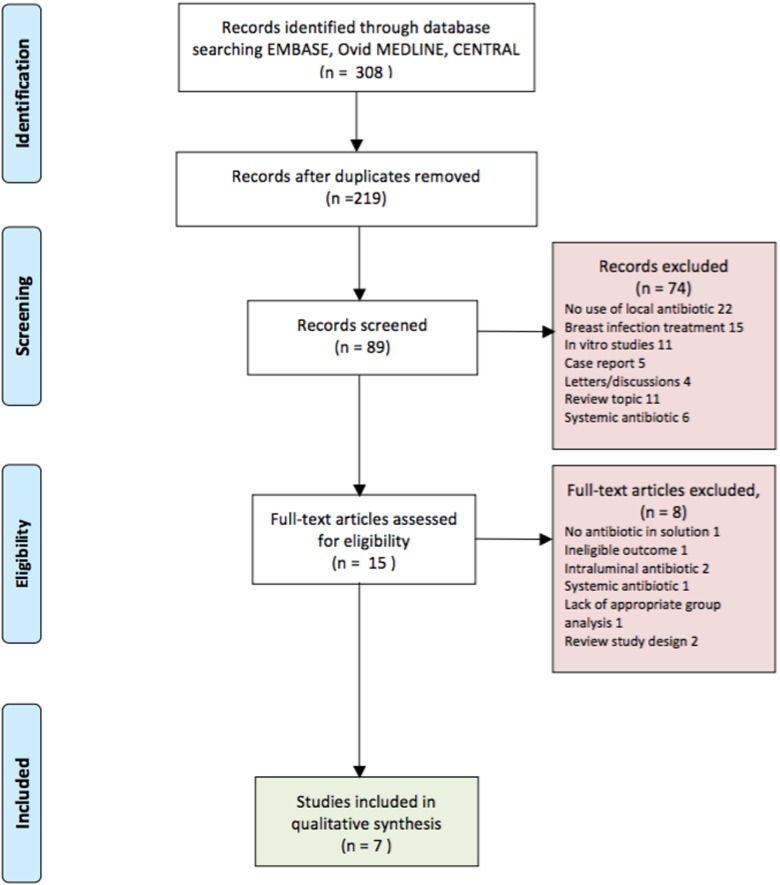
A total of 308 potentially eligible articles were identified in the initial literature search (Figure 1). After screening the title and abstract, 293 articles were excluded. Eight studies were excluded after full-text review.2,14–21 A total of 7 studies were ultimately included in our analysis.7,22–27 These include 1 prospective RCT, 1 prospective cohort study, and 3 retrospective controlled cohort study. Two retrospective non-controlled studies were included. The inter-reviewer agreement was 0.78 for study selections, which corresponds to a substantial agreement. A total of 8892 patients underwent primary breast augmentation across the 7 studies, which were included in this review, with mean follow-up ranging from 14 to 72 months. The age range of those patients was 18 to 86 years. The most common single antibiotic used was bacitracin, which was used in 6 studies, commonly combined with other antibiotics. Gentamicin was used in 5 studies. The well-known triple antibiotic solution was used in 2 studies.7,27 One study received funding from an industry-based institution.26 Table 1 summarizes the characteristics of the included studies in this systematic review.
Figure 1.
Search strategy flow diagram.
Table 1.
Summary of Studies Included in the Review.
| Reference | Study Design | Sample Size | Mean Follow-Up (months) | Mean Patient Age ± SD (years) | Surgical Technique | Type of Implant Used | Type of Antibiotic Irrigation | Funding |
|---|---|---|---|---|---|---|---|---|
| Adams et al7 | Prospective non-controlled trial | 248 | 14 | 35 (range: 18-86) | Dual plane 1 (83%), dual plane 2/3 (15.4%), subglandular (0.6%), retropectoral (0.6%) | Saline or silicone; no further detail | Bacitracin, cefazolin, gentamicin, normal saline | Not stated |
| Blount et al23 | Retrospective controlled cohort | 856 | 14.9 | 33.5 ± 8.5 (range: 17-68) | The submuscular space (99.4%), subglandular (0.6%) | Saline (n = 39), silicon (n = 446), smooth implant (n = 551), textured implant (n = 19) | Bacitracin, cefazolin, gentamicin, Betadine saline | No funding received |
| Burkhardt et al24 | Prospective, randomized control, double blind | 124 | 22 | Not specified | Retroglandular | The Heyer-Schulte saline implants | Bacitracin, oxacillin, triamcinolone | Not stated |
| Giordano et al25 | Retrospective controlled cohort | 330 | 22 | 35 ± 8 (18-58) | Dual plane | Anatomically shaped, texturized silicone gel; Natrelle 410 or 510; Allergan, Irvine, California | Cefuroxime, gentamicin + povidone-iodine solution | No funding received |
| Pfeiffer et al22 | Retrospective controlled cohort | 436 | Group 1: 87, group 2: 21.6 | 34 (18-62) | Submuscular (99.8%), subglandular (0.2%) | Textured Polytech Silimed double-lumen implants—silicone | Cephalothin + saline/epinephrine | No funding received |
| Drinane et al27 | Prospective controlled cohort | 55 | 21.6 | 34.2 (22-50) | Subpectoral plane | Allergan Natrelle silicone breast implants | Cefazolin, gentamicin, bacitracin | No funding received |
| Gutowski et al26 | Retrospective non-controlled case series | 504 | 72 | Not specified | Subpectoral (50.3%) submammary (49.7%) | Saline-filled implants (mentor, 67.4%; Heyer-Schulte, 29.3%; McGhan, 1.6%; Dow Corning, 0.6%; and unknown, 1.0%) | Not specified | Funded by the Plastic Surgery Educational Foundation |
Type of Antibiotic Solutions and Irrigation Route
Table 2 summarizes the type, doses, and route of antibiotics used across the studies. Overall, all studies only irrigated pockets with antibiotics. Three studies bathed the implant and irrigated breast pockets.7,25,27 Only 1 study bathed the implant, irrigated breast pockets, and cleansed skin-surrounding incision with antibiotics.7
Table 2.
Type of Antibiotics Solution and Method of Irrigation Used Across the Studies.
| Study | Irrigation Method | Intraoperative Irrigation | Systemic Preoperative Antibiotic | Systemic Post-Operative Antibiotic |
|---|---|---|---|---|
| Adams et al7 | Implants bathed, pockets irrigated, and skin surrounding incision cleansed with antibiotics | Bacitracin (50 000 U), gentamicin (80 mg], cefazolin (1 g], (IV; UD] | Preoperative IV antibiotics cefazolin or vancomycin/gentamicin for allergic patients (UD] | Antibiotics for 5 days (not specified) |
| Blount et al23 | Pocket irrigated | Cefazolin (1 g), bacitracin (50 000 U), gentamicin (80 mg) in normal saline in 11.7% of patients. Bacitracin (50 000 U) in 18.5% of patients. Betadine was used in 0.6% of patients | Cefazolin (1 g) IV | |
| Burkhardt et al24 | Pocket irrigated | Bacitracin (50 000 U), oxacillin (250 mg), cephalothin (1 g per 100 cc) | - | - |
| Giordano et al25 | Group A: IV cephalothin, group B: IV cefuroxime; implant bathed and pocket irrigated. | 10 mL 10% povidone-iodine solution with 750 mg of cefuroxime and 80 mg of gentamicin diluted in 15 mL of 0.9% sodium chloride solution | Administered perioperatively: cephalothin 1.5 g IV (group A) or cefuroxime 750 mg (group B) | Group A: Cefalexin 750 mg BID for 1 week, group B: levofloxacin 500 mg OD for 5 days. |
| Pfeiffer et al22 | Pocket irrigated | Cefuroxime (1.5 g) and epinephrine (1 mg) | - | - |
| Drinane et al27 | Implant bathed and pocket irrigated | Bacitracin 50 000 U, cefazolin 1 g, gentamicin 80 mg, and 500 mL of normal saline | - | - |
| Gutowski et al26 | Pocket irrigated | Not specified | - | - |
Abbreviations. IV, Intravenous; BID, twice a day; OD, once daily; UD, as directed.
Overall Results and Recommendation in Included Individual Studies
Capsular contracture
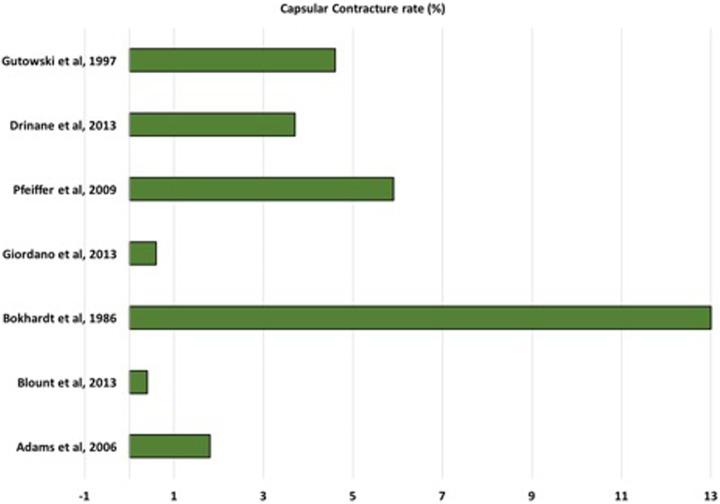
Overall, the rate of grade III/IV CC in all studies that used intraoperative antibiotic irrigation ranged from 0.4% to 13.9% (Figure 2). Of these, the rate of CC was less than 2% in 8 studies, between 3% and 6% in 4 studies,22,26,27 and 13.9% in 1 study.24 The rate of grade III/IV CC in the 5 comparative studies that used saline irrigation ranged from 3.5% to 40%. In an RCT, Burkhardt et al24 found that the use of local antibiotic decreased the incidence of CC by 50% with more than 6-month follow-up. Giordano et al25 found in their retrospective comparative study that the use of the combination of povidone-iodine and antibiotic irrigation in breast augmentation resulted in significant lower rate of CC by a total of 10 cases with approximately 2 years mean follow-up. In another retrospective controlled study, Blount et al23 found that CC was significantly lower with antibiotic irrigation use. However, when a multivariate logistic regression model was used to control for other variables that are potentially associated with CC, no statistical significance and wide confidence interval were found (P = .066; 95% CI: 0.88-55.30). In support of the previous study’s finding, Pfeiffer et al22 suggested that the use of topical antibiotics was not associated with lower rate of the development of CC. Similarly, results from Drinane et al’s27 prospective comparative study revealed no difference between the triple antibiotic breast irrigation and saline irrigation in lowering the incidence or severity of CC. Included comparative studies are summarized in Table 3.
Figure 2.
Rate of capsular contracture (%) in included individual studies.
Table 3.
Summary of Findings of Included Comparative Studies.
| Study | Antibiotic Solution | Design (LOE) | Mean Follow-Up (range) | Rate of CC in Intervention Group | Rate of CC in Control Group | OR (95% Confidence Interval) | Outcome |
|---|---|---|---|---|---|---|---|
| Blount et al23 | Bacitracin, cefazolin, gentamicin, ± Betadine saline | Retrospective controlled cohort (III) | 14.9 (no range) | 1/260 (0.4%) | 23/591 (3.9%) | 0.10 (0.01-0.71) | Significance reduction in CC rate when compared with saline.a No association was found after controlling for confounders (P = .066; 95% CI: 0.88-55.30)b |
| Giordano et al25 | Cefuroxime gentamicin + povidone-iodine solution | Retrospective controlled cohort (III) | 22 (12-72) months | 1/165 (0.60%) | 10/165 (6%) | 0.09 (0.01-0.75) | Significant lower rate of CCa |
| Drinane et al27 | Cefazolin, gentamicin, bacitracin | Prospective controlled cohort (III) | 21.6 (0.9-2.9) months | 1/27 (3.7%) | 1/28 (3.5%) | 1.04 (0.06-17.49) | No decrease in the rate of CC was observeda |
| Pfeiffer et al22 | Cephalothin + saline/epinephrine | Retrospective controlled cohort (III) | Antibiotic group: 87 (85.2-99.3) months, saline group: 21.6 (7.8-33.8) months | 12/203 (5.9%) | 17/211 (8%) | 0.55 (0.29-1.04) | No decrease in the rate of CC was observeda |
| Burkhardt et al24 | Bacitracin, oxacillin, triamcinolone | Prospective, randomized control, double blind (I) | 22 (18-40) months | 5/36 (13.9%) | 15/37 (40%) | 0.24 (0.07-0.75) | Significance reduction in CC rate.a No precise detail on follow-up |
Abbreviations: CC, capsular contracture; LOE, level of evidence; OR, odds ratio.
aResults generated using bivariate analysis.
bResult generated by multivariate regression analysis (odds ratio was not reported).
Analysis of Heterogeneity
From clinical perspective, the type of used implants, their generation, their technique and position, incision type, and type of antibiotic solution used for irrigation were significantly variable both between and within studies. Therefore, pooling data from a non-homogenous population may erroneously produce an association between antibiotic irrigation and developing CC when, in fact, no real association between them exists. From methodological perspective, different study designs have been identified from our search, with significantly variable length of follow-up. All of these observed variation might produce the “mixing apples and oranges” problem of comparing vastly different population.
Risk of Bias in Included Studies and Level of Evidence
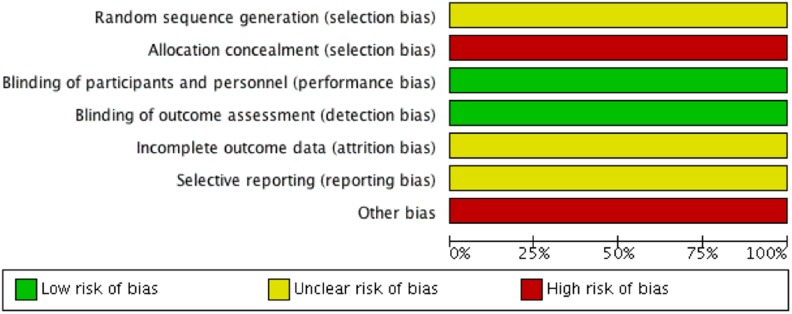
The overall methodological quality of non-randomized studies was low (Table 4). The MINORS scores of the included non-randomized observational studies showed poor methodological and reporting quality. These studies scored less than 60% of the maximum MINORS score, except 1 controlled study which scored 20 of 24 in MINOR score.25 The Cochrane risk of bias figure of the included RCT is shown in Figure 3.24 The authors of this trial did not explicitly describe the method of randomization and allocation to allow readers to determine the appropriateness and validity of the process. Moreover, the fate of all patients was not well described in the article. There was high risk of selective reporting and lack of reporting of the effect size and adjustment of the other potential confounding factors to draw an association between local use of antibiotic irrigation and developing severe CC. Six studies were observational in design; 3 had a comparison group,22,25,27 and 2 were uncontrolled case series.7,23 Two comparative retrospective studies used historical control as they compared CC rates after a change in the surgeon’s practice, after antibiotic irrigation was incorporated into their practice (selection bias). In addition, this longitudinal temporal comparison does not take into account several other factors that may account for the reduced CC rate as the field of breast augmentation evolved over time (eg, incision site, plane selection, different generations of implants, use of talc-free gloves, improved aseptic/no-touch techniques, Keller funnels, meticulous hemostasis, etc).1–3,28 Only Blount et al23 study attempted a multivariate analysis to control for smoking, type of implant, and site of incision.
Table 4.
The Methodological Quality of the Included Non-Randomized Studies.
| MINORS Criteria | Gutowski et al26 | Adams et al7 | Pfeiffer et al22 | Giordano et al25 | Drinane et al27 | Blount et al23 |
|---|---|---|---|---|---|---|
| 1. A clearly stated aim | Unclear | Low risk | Low risk | Low risk | Low risk | High risk |
| 2. Inclusion of consecutive patients | Low risk | Unclear | Unclear | Low risk | Low risk | Low risk |
| 3. Prospective collection of data | High risk | Low risk | High risk | High risk | Low risk | High risk |
| 4. End points appropriate to the aim of the study | Unclear | Low risk | Low risk | Low risk | Low risk | Unclear |
| 5. Unbiased assessment of the study end point | Unclear | Unclear | High risk | Unclear | High risk | Unclear |
| 6. Follow-up period appropriate to the aim of the study | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| 7. Loss to follow-up less than 5% | Unclear | Unclear | Unclear | Unclear | Low risk | Unclear |
| 8. Prospective calculation of the study size | High risk | High risk | High risk | High risk | High risk | High risk |
| 9. An adequate control groupa | NA | NA | High risk | Low risk | Low risk | Unclear |
| 10. Contemporary groupsa (historical comparison) | NA | NA | High risk | High risk | Low risk | Low risk |
| 11. Baseline equivalence of groupsa | NA | NA | High risk | Unclear | Unclear | Unclear |
| 12. Adequate statistical analysesa | NA | NA | High risk | High risk | High risk | Low risk |
| MINOR score | 6/16 (38%) | 9/16 (56%) | 13/24 (42%) | 14/24 (58%) | 20/24 (83%) | 11/24 (46%) |
Abbreviations. MINOR, Methodological index for non-randomized studies; NA, not applicable.
Figure 3.
The methodological quality of the single included randomized controlled study.
Discussion
The breast implant/pocket irrigation with various antibiotic solutions in breast augmentation is a common clinical practice in North America. The popularity of this practice stemmed from Adams et al’s29 in vitro study, where they reinforced the validity of the association of subclinical colonization/infection and the development of CC. They recommended the use of both antibiotic irrigation (1 g cefazolin and 80 mg gentamicin) solution and povidone-iodine (50 mL) in 500 mL of sterile saline. The rationale to use these agents was to target certain pathogens that are commonly cultured around breast implants, which include Staphylococcus epidermidis, Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli, and Propionibacterium acnes. Following the restriction of the use of povidone-iodine in breast implant augmentation in the year 2000, Adams et al6,7 proposed an alternative solution, which is the classic triple antibiotic irrigation solution, in which bacitracin was added to cefazolin and gentamicin to effectively cover pseudomonas.
After more than a decade and a paradigm shift toward more evidence-based practice, there have been no systematic reviews that critically evaluate the evidence of the practice of using antibiotic irrigation solution in primary breast augmentation. Thus, to our knowledge, this is the first analytical systematic review of the current literature focusing on the use of antibiotic irrigation.
Quality of the Evidence
A major limitation of the current evidence is that there is a paucity of high-level evidence in this area. The articles we reviewed include 1 level II study, 5 level III studies, and 7 level IV studies. There were several methodological flaws not only in retrospective studies but also in higher level of evidence comparative studies (Tables 3 and 4). Overall, the MINORS and the Cochrane risk of bias tool of the studies reviewed are indicative of poor methodological and reporting quality. In addition, the heterogeneity of the population and the intervention between and within studies were significant. Hence, an erroneous association between antibiotic irrigation and the development of CC is likely to be observed.
Capsular Contracture
The highest level of evidence (II) study24 revealed no statistically significant difference between antibiotic irrigation and povidone-iodine irrigation, but antibiotic irrigation decreased the incidence of CC when compared to saline-only solution. However, in addition to the poor methodologic quality and inadequate follow-up, this study was conducted in the year 1986, which is prior to many major advancements in the field of implant-based breast surgery.
Interestingly, we found that the only comparative study that attempted multivariate analysis23 did not actually demonstrate a difference between saline and antibiotic irrigation in the rates of CC formation. This indicates that the initial observed association was confounded by other factors. We believe that this observed discrepancy warrants further investigation.
Moreover, given the fact that CC is oftentimes a delayed event, it is noteworthy to mention that there is a wide range of heterogeneity within and between studies in terms of length of follow-up, which may inevitably affect CC development rates (Table 1). This information is important as the longer the surgeon follows their patients, the higher the likelihood of detecting CC.
Another important observation is that Giordano et al25 who found a significant reduction in the rate of CC was the only author who combined topical antibiotic solution with povidone-iodine. We think that the use of this mixture, which has been recommended by Adams et al29 in 2000 prior to that FDA statement, also deserves further investigation. Hidalgo and Spector30 suggested that the utilization of gentamicin and cefalexin is unnecessary. The former may be redundant since it’s commonly given preoperatively in a systemic fashion, while the latter is perceived as excessive as gram-negative bacteria are rare and not implicated as a common pathogen for CC. They recommended irrigating the breast with a combination of dilute Betadine and antibiotics as an alternative to irrigation with triple antibiotic solution. Nevertheless, this recommendation was not based on robust evidence.
The efficacy of povidone-iodine irrigation in reducing CC in aesthetic breast augmentation was recently examined in a systematic review and meta-analysis by Yalanis et al.12 They concluded that povidone-iodine breast pocket irrigation is effective in reducing CC in implant-based breast augmentation. Although their conclusion was based on studies that have weak methodological quality, their systematic review included slightly higher level of evidence studies than our study. This might indicate that there is relatively better evidence in using povidone-iodine as breast implant irrigation solution as opposed to antibiotic solution. However, further high-quality research is required to compare the 2 solutions.
Future Consideration
Future research is suggested to better determine the actual role of topical antibiotic irrigation in breast implant augmentation. Although RCT could be challenging, a longitudinal observational comparative study with adequate follow-up that incorporates all potential risk factors for CC may provide superior evidence than what presently exists. In a recent study, Hu et al detected a number of bacteria when they analyzed specimens of microbiome of the breast implant–associated ALCL.31 Unlike non-neoplastic capsules, the ALCL specimens showed high proportion of Ralstonia spp., which is a gram-negative bacilli found in soil and water.31 This finding generates controversy whether the use of topical antibiotic has a role in preventing ALCL by eliminating the risk of subclinical infection or acts as a precursor to developing ALCL by inducing the development of highly resistant bacteria. We believe that this question warrants further investigations.
Limitation
Although every attempt was made to produce an ideal study, our study has some limitations. There was a high degree of heterogeneity between the analyzed studies; however, as in most systematic reviews, studies brought together will inevitably vary. Another limitation of our systematic review is that we excluded articles that were not in English. Also, there may be ongoing studies, or unpublished data with negative results that we are not aware of, that may impact our review’s findings.
Conclusion
There is inconsistent evidence regarding the effect of antibiotic irrigation on reducing the rate of CC. The available evidence that supports the use of antibiotic is weak and based on significantly heterogeneous data; thus, the truth is presently unknown. Our analysis also suggests that povidone-iodine breast pocket irrigation might have a role in preventing CC and needs to be investigated further. Although most plastic surgeons would be reluctant to change their preferred type of irrigant in implant-based breast surgery, we believe that more methodologically sound studies with an adequate follow-up are warranted, especially in the era of improved aseptic technique and antibiotic resistance.
Acknowledgments
The authors thank Jill E. Hatchette, PhD, from Interdisciplinary Research at IWK Health Centre and Haron Hasan and Omar Al-Sheikh for their feedback on the methodology. The authors also thank our medical librarian, Robin Parker from Department of Community Health and Epidemiology at Dalhousie University for her guidance and feedback in search strategy. Special thanks to Dr Mark W. Clemens from MD Anderson Cancer Center for his input in the discussion regarding the role of antibiotic irrigation and the development of ALCL.
Level of Evidence: Level 3, Therapeutic
Authors’ Note: This work was presented at (1) the 70th Annual Meeting of the Canadian Society of Plastic Surgeons; June 16, 2016; Ottawa, Ontario and (2) Plastic Surgery the Meeting of the American Society of Plastic Surgeons; September 23-37, 2016; Los Angles, California.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Ajdic D, Zoghbi Y, Gerth D, Panthaki ZJ, Thaller S. The relationship of bacterial biofilms and capsular contracture in breast implants. Aesthet Surg J. 2016;36(3):297–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Araco A, Caruso R, Araco F, Overton J, Gravante G. Capsular contractures: a systematic review. Plast Reconstr Surg. 2009;124(6):1808–1819. [DOI] [PubMed] [Google Scholar]
- 3. Chong SJ, Deva AK. Understanding the etiology and prevention of capsular contracture: translating science into practice. Clin Plast Surg. 2015;42(4):427–436. [DOI] [PubMed] [Google Scholar]
- 4. Hu H, Johani K, Almatroudi A, et al. Bacterial biofilm infection detected in breast implant–associated anaplastic large-cell lymphoma. Plast Reconstr Surg. 2016;137(6):1659–69. [DOI] [PubMed] [Google Scholar]
- 5. Hidalgo DA, Sinno S. Current trends and controversies in breast augmentation. Plast Reconstr Surg. 2016;137(4):1142–1150. [DOI] [PubMed] [Google Scholar]
- 6. Adams WP, Jr, Conner WCH, Barton FE, Jr, Rohrich RJ. Optimizing breast-pocket irrigation: the post-Betadine era. Plast Reconstr Surg. 2001;107(6):1596–1601. [DOI] [PubMed] [Google Scholar]
- 7. Adams WP, Jr, Rios JL, Smith SJ. Enhancing patient outcomes in aesthetic and reconstructive breast surgery using triple antibiotic breast irrigation: six-year prospective clinical study. Plast Reconstr Surg. 2006;117(1):30–36. [PubMed] [Google Scholar]
- 8. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W65–W94. [DOI] [PubMed] [Google Scholar]
- 9. Spear SL, Baker JL., Jr Classification of capsular contracture after prosthetic breast reconstruction. Plast Reconstr Surg. 1995;96(5):1119–1123. [PubMed] [Google Scholar]
- 10. Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0. The Cochrane Collaboration; 2011. wwwcochrane-handbookorg. Updated March 2011. Accessed December 23, 2016. [Google Scholar]
- 11. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (MINORS): development and validation of a new instrument. ANZ J Surg. 2003;73(9):712–716. [DOI] [PubMed] [Google Scholar]
- 12. Yalanis GC, Liu E-W, Cheng H-T. Efficacy and safety of povidone-iodine irrigation in reducing the risk of capsular contracture in aesthetic breast augmentation: a systematic review and meta-analysis. Plast Reconstr Surg. 2015;136(4):687–698. [DOI] [PubMed] [Google Scholar]
- 13. Center for Evidence Based Medicine, Oxford. Level of evidence. OCEBM Levels of Evidence Working Group*. “The Oxford Levels of Evidence 2”. http://www.cebm.net/ocebm-levels-of-evidence/. Accessed December 23, 2016.
- 14. Carlesimo B, Cigna E, Fino P, Rusciani A, Tariciotti F, Staccioli S. Antibiotic therapy of transaxillary augmentation mammoplasty. In Vivo. 2009;23(2):357–362. [PubMed] [Google Scholar]
- 15. Wiener TC. The role of Betadine irrigation in breast augmentation. Plast Reconstr Surg. 2007;119(1):12–15. [DOI] [PubMed] [Google Scholar]
- 16. Vistnes LM. Copsular contracture around silicone implants: the role of intraluminal antibiotics. Plast Reconstr Surg. 1982;69(5):813–814. [DOI] [PubMed] [Google Scholar]
- 17. Huang N, Liu M, Yu P, Wu J. Antibiotic prophylaxis in prosthesis-based mammoplasty: a systematic review. Int J Surg. 2015;15:31–37. [DOI] [PubMed] [Google Scholar]
- 18. Burkhardt BR, Fried M, Schnur PL, Tofield JJ. Capsules, infection, and intraluminal antibiotics. Plast Reconstr Surg. 1981;68(1):43–47. [DOI] [PubMed] [Google Scholar]
- 19. Gylbert L, Asplund O, Berggren A, Jurell G, Ransjö U, Östrup L. Preoperative antibiotics and capsular contracture in augmentation mammaplasty. Plast Reconstr Surg. 1990;86(2):260–267. [PubMed] [Google Scholar]
- 20. Stevens WG, Nahabedian MY, Calobrace MB, et al. Risk factor analysis for capsular contracture: a 5-year Sientra study analysis using round, smooth, and textured implants for breast augmentation. Plast Reconstr Surg. 2013;132(5):1115–1123. [DOI] [PubMed] [Google Scholar]
- 21. Araco A, Gravante G, Araco F, Delogu D, Cervelli V, Walgenbach K. Infections of breast implants in aesthetic breast augmentations: a single-center review of 3,002 patients. Aesthetic Plast Surg. 2007;31(4):325–329. [DOI] [PubMed] [Google Scholar]
- 22. Pfeiffer P, Jørgensen S, Kristiansen TB, Jørgensen A, Hölmich LR. Protective effect of topical antibiotics in breast augmentation. Plast Reconstr Surg. 2009;124(2):629–634. [DOI] [PubMed] [Google Scholar]
- 23. Blount AL, Martin MD, Lineberry KD, Kettaneh N, Alfonso DR. Capsular contracture rate in a low-risk population after primary augmentation mammaplasty. Aesthet Surg J. 2013;33(4):516–521. [DOI] [PubMed] [Google Scholar]
- 24. Burkhardt B, Dempsey P, Schnur P, Tofield J. Capsular contracture: a prospective study of the effect of local antibacterial agents. Plast Reconstr Surg. 1986;77(6):919–930. [PubMed] [Google Scholar]
- 25. Giordano S, Peltoniemi H, Lilius P, Salmi A. Povidone-iodine combined with antibiotic topical irrigation to reduce capsular contracture in cosmetic breast augmentation. Aesthet Surg J. 2013;33(5):675–680. [DOI] [PubMed] [Google Scholar]
- 26. Gutowski KA, Mesna GT, Cunningham BL. Saline-filled breast implants: a plastic surgery educational foundation multicenter outcomes study. Plast Reconstr Surg. 1997;100(4):1019–1027. [DOI] [PubMed] [Google Scholar]
- 27. Drinane JJ, Bergman RS, Folkers BL, Kortes MJ. Revisiting triple antibiotic irrigation of breast implant pockets: a placebo-controlled single practice cohort study. Plast Reconstr Surg Glob Open. 2013;1(7):e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rohrich RJ, Kenkel JM, Adams WP. Preventing capsular contracture in breast augmentation: in search of the Holy Grail. Plast Reconstr Surg. 1999;103(6):1759–1760. [DOI] [PubMed] [Google Scholar]
- 29. Adams WP, Jr, Conner WCH, Barton FE, Jr, Rohrich RJ. Optimizing breast pocket irrigation: an in vitro study and clinical implications. Plast Reconstr Surg. 2000;105(1):334–338. [DOI] [PubMed] [Google Scholar]
- 30. Hidalgo DA, Spector JA. Breast augmentation. Plast Reconstr Surg. 2014;133(4):567e–583e. [DOI] [PubMed] [Google Scholar]
- 31. Johani K, Vickery K, Van Natta B, et al. Bacterial biofilm infection detected in breast implant associated anaplastic large cell lymphoma. Plast Reconstr Surg. 2016;137(6):1659–1569. [DOI] [PubMed] [Google Scholar]