Abstract
Utilization of flow diverting devices is accompanied with dual antiplatelet therapy to reduce the risk of thromboembolic events, even though this increases the risk of hemorrhagic complications. The updated Pipeline Flex embolization device with Shield Technology has been created using a phosphorylcholine coating that reduces thrombogenicity and possibly reduces the need for dual antiplatelet therapy. However, because of the potential risk to patients of utilizing a pipeline embolization device without dual antiplatelet therapy, the pipeline embolization device with Shield Technology has not been tested in human subjects without dual antiplatelet therapy, and its contribution to preventing thromboembolic events is therefore unknown. We report a case in which a patient, following complications that limited his absorption of dual antiplatelet therapy, had low levels of dual antiplatelet therapy medications in his bloodstream following treatment for an intracranial aneurysm with a pipeline embolization device with Shield Technology. The patient recovered without signs of luminal stenosis or thromboembolic event.
Keywords: Aneurysm, flow diversion, complication, antiplatelet therapy
Introduction
The pipeline embolization device (PED) was one of the earliest widely utilized flow diverting devices (FDDs).1 Since its invention, it has been applied for a wide range of intracranial aneurysm (IA) types.2 However, the PED and other FDDs present several known risks. Firstly, occlusion following FDDs can take years, and thus present a persistent rupture risk for several years following the intervention. Furthermore, FDDs are also associated with an increased risk of thromboembolic events3 and thus necessitate dual antiplatelet therapy (DAT) for a prolonged period; Such medical regimen, in turn, leads to the increased risk of hemorrhagic complications.4 Thrombogenicity and accompanying complications were addressed by the development of new generation Pipeline Flex embolization device with Shield Technology (PED Shield) in the year 2015.5
The PED Shield is a second-generation PED treated with phosphorylcholine (PC). PC is covalently bound to the mesh of the device, rendering the PED Shield the least thrombogenic of any FDD.6 This feature allows for the possibility that the PED Shield could be used effectively in patients who have a restricted antiplatelet regimen (i.e. unresponsive patients or patients with contraindications to DAT). However, clinical practice has presented few ethically justifiable opportunities to test this hypothesis. We report the use of the PED Shield in a patient who was initially treated effectively with DAT, but shortly after the intervention became unresponsive to medication, offering vague but existing evidence of PED Shield’s function without antiplatelet regimen.
Clinical case
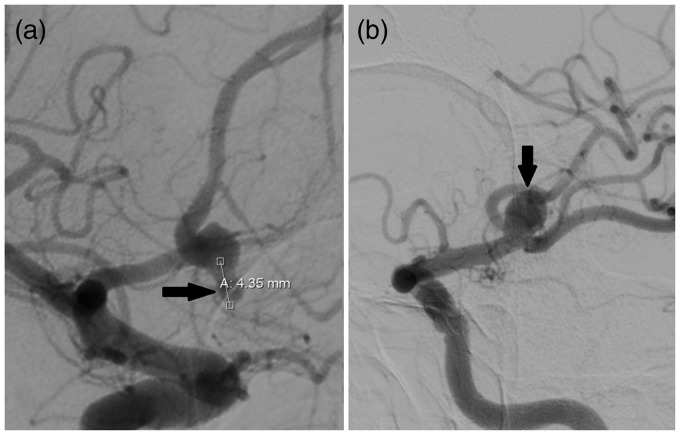
A 65-year-old male presented to our department with two aneurysms: one left middle cerebral artery (MCA) large saccular aneurysm and one partially thrombosed giant anterior communicating artery (aComm) aneurysm. Diagnosis of aneurysms was verified by Digital Subtraction Angiography (DSA) (Figure 1).
Figure 1.
DSA with right carotid injection (a), showing remnant of partially thrombosed giant anterior communicating artery (aComm) aneurysm (arrow) and left carotid injection (b) showing left Middle Cerebral Artery (lMCA) saccular aneurysm (arrow).
In our department, every patient undergoing FDD placement receives an initial loading dose of antiplatelet medications (600 mg of clopidogrel and 300 mg of aspirin) followed by a daily dose of 75 mg of each medication. We assess response to antiplatelet regimen using light transmittance aggregometry (LTA) with the Chrono-Log 490 (Chrono-Log Corporation, USA) which examines platelet aggregation by measuring optical density in response to adenosine diphosphate (ADP) and epinephrine. We target at least a twofold decrease from the patient’s initial aggregation percentages. Intraoperatively, 5000 IU of heparin are administered intravenously. All interventions are performed under conditions of general anesthesia in a Philips Xper Allura FD20 (Koninklijke Philips N.V., The Netherlands) biplane angiosuite.
During the first stage of treatment, the subject underwent uneventful stent-assisted coiling of the MCA aneurysm. Six months later, he was scheduled for FDD implantation for his aComm aneurysm. Since the first intervention, he had been continuously treated with daily doses of clopidogrel and aspirin and, as measured by LTA, his epinephrine-agonized aggregation had reduced from 75% to 32% and his ADP-agonized aggregation had reduced from 68% to 28%.
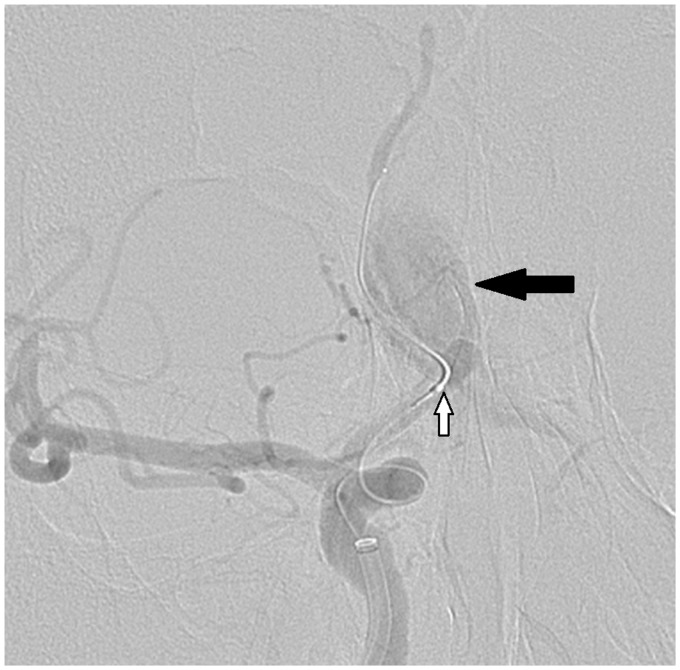
During the scheduled PED Shield implantation, intraoperative perforation of the A2 segment of the anterior communicating artery wall occurred (see Figure 2). Extravasation was halted by temporary balloon occlusion followed by telescopic implantation of a second PED Shield. Immediate postoperative computed tomography (CT) showed signs of subarachnoid hemorrhage with formation of interhemispheric hematoma and ventricular breakthrough (see Figure 3(a)). Immediate external ventricular drainage (EVD) with Codman Bactiseal through Frazier’s point (see Figure 3(b)) was performed.
Figure 2.
DSA shows extravasation (black arrow) due to intraoperative perforation of ACA wall with balloon microcatheter (white arrow).
Figure 3.
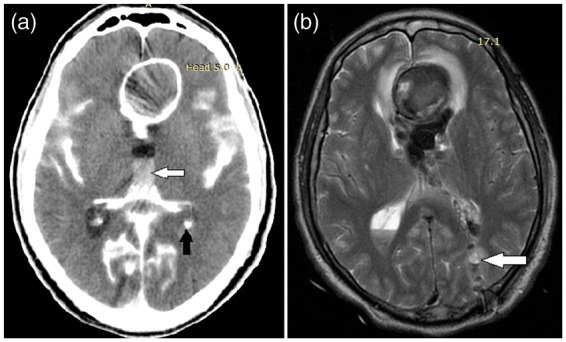
Computed tomography (CT) scan (a) reveals massive subarachnoid hemorrhage (SAH) with interhemispheric hematoma formation (white arrow) and ventricular breakthrough (black arrow). Postoperative magnetic resonance imaging (MRI) (b) shows position of the external ventricular drainage (EVD) (white arrow).
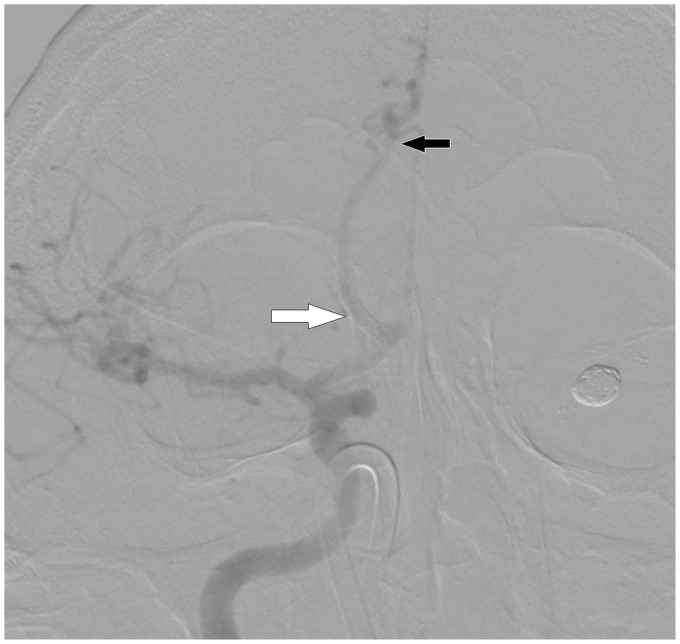
The patient remained stable for the following 10 days at a Glasgow Coma Scale (GCS) value of six. On day 11, he was switched from nasogastric tube (NGT) feeding to partial extra-parenteral feeding because of the appearance of malabsorption signs, possibly due to delayed gastric emptying. Aggregometry on day 12 revealed recovery of aggregometry parameters up to baseline levels (epinephrine-agonized aggregation: 79%; ADP-agonized aggregation: 65%). Because of his existing interhemispheric hematoma and EVD, we rejected the possibility of switching to glycoprotein (GP) IIb/IIIa inhibitors due to the risk of hemorrhagic complications.7 Thus, we decided to risk Anterior Communicating Artery (ACA) occlusion over severe hemorrhage, and left the patient on the same DAT (clopidogrel and aspirin through NGT), rendering him effectively off therapy. For the following 15 days, aggregometry consistently showed baseline measures. During the same period, he gradually improved from GCS value of six to 12. On day 28, enteral feeding was undertaken. The next day, LTA demonstrated a return to a hypoaggregative state. On day 30, we performed DSA, which revealed the patency of the parent artery containing the telescopically implanted PEDs with no signs of luminal stenosis (see Figure 4) despite the lack of antiaggregation for at least 16 days.
Figure 4.
Control DSA showing patency of the right anterior cerebral artery (black arrow) with the implanted pipeline embolization devices (PEDs) (white arrow).
The patient’s representative gave informed consent for publication of the clinical case, and our institutional ethics board authorized manuscript submission.
Discussion
A dual antiplatelet regimen must be balanced in accordance with the risks of thromboembolic and hemorrhagic complications.8 However, while it is difficult to properly extrapolate, cardiointerventional practice still suggests that regimen adjustment does not make a significant impact on the outcome.9 The possibility10 of delayed hemorrhages from procedural complications, silent or treated IA ruptures, or postoperative interventions means that discovery of ways to reduce dependence of FDD patients on DAT in the postoperative period could reduce long-term complication rates. This clinical need inspired the creation of the PED Shield.11
While the safety of the PED Shield has been shown in a clinical trial5 and ex vivo testing has shown significantly lower platelet deposition on the PED Shield without DAT,12 ethical concerns have precluded human testing of FDD treatment without proper antiaggregation. There is an existing experience of compelled PED Shield use with solitary aspirin.13 Our case differs because the patient initially received proper DAT, and hypoaggregation was verified by standard means. However, his complication, which presented a challenge to administering medications,14 meant that DAT was virtually discontinued for several days. This presented the opportunity to demonstrate the patient outcome of using the PED Shield without any antiplatelet regimen. However, we acknowledge that 16 days is quite a brief period and, though the data is lacking, we cannot state that thrombogenicity of a PED without Shield Technology is 100% in every individual. This is a sole case, and we therefore cannot determine how well the PED Shield effectively prevents thrombotic complications or whether it could reduce the need for DAT in FDD patients, but the outcome matches the theory behind the technology and its performance in animal and ex vivo models.
We theorize that the possibility of FDD use without DAT regimen could lead to a breakthrough in aneurysm treatment, with its potential to eliminate a major risk associated with their use. IAs retain a rupture risk for several years after FDD implantation,4 and antiplatelet regimen worsens outcomes in these cases. Moreover, the procedure of FDD implantation itself possesses a certain risk of hemorrhagic complications.10 Refraining from DAT use would reduce the risks associated with these events, leading to the expansion of FDD use.
Conclusion
The PED Shield may offer the benefit of reduced use of DAT in patients for whom use or continuation of antiplatelet therapy presents a risk of hemorrhagic complications. Our case demonstrates that a reduced DAT regimen for a substantial period resulted in no thrombotic complications for a patient with the PED Shield. However, as this represents one of the first cases regarding this issue, further investigations are necessary. To be more precise, the only way to get unbiased clear-cut evidence is a prospective randomized trial. At the current state, it's impossible from the ethical standpoint to compare the PED Shield with and without DAT outright, there is nothing to prevent the prospective comparison of the thrombotic event rate of two devices with proper DAT. And in the case of the PED Shield showing significantly lower thrombogenicity in such trial, there would be a possibility to conduct a prospective study with incomplete or absent DAT for the PED Shield device.
Declaration of conflicting interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Lylyk P, Miranda C, Ceratto R, et al. Curative endovascular reconstruction of cerebral aneurysms with the pipeline embolization device: The Buenos Aires Experience. Neurosurgery 2009; 64: 632–343. [DOI] [PubMed] [Google Scholar]
- 2.Rajah G, Narayanan S, Rangel-Castilla L. Update on flow diverters for the endovascular management of cerebral aneurysms. Neurosurg Focus 2017; 42: E2. [DOI] [PubMed] [Google Scholar]
- 3.Kallmes DF, Hanel R, Lopes D, et al. International retrospective study of the pipeline embolization device: A multicenter aneurysm treatment study. AJNR Am J Neuroradiol 2015; 36: 108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park MS, Kilburg C, Taussky P, et al. Pipeline embolization device with or without adjunctive coil embolization: Analysis of complications from the IntrePED Registry. AJNR Am J Neuroradiol 2016; 37: 1127–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martínez-Galdámez M, Lamin SM, Lagios KG, et al. Periprocedural outcomes and early safety with the use of the Pipeline Flex embolization device with Shield Technology for unruptured intracranial aneurysms: Preliminary results from a prospective clinical study. J Neurointerv Surg 2017; 9: 772–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Girdhar G, Li J, Kostousov L, et al. In-vitro thrombogenicity assessment of flow diversion and aneurysm bridging devices. J Thromb Thrombolysis 2015; 40: 437–443. [DOI] [PubMed] [Google Scholar]
- 7.Iskandar SB, Kasasbeh ES, Mechleb BK, et al. Alveolar hemorrhage: An underdiagnosed complication of treatment with glycoprotein IIb/IIIa inhibitors. J Interv Cardiol 2006; 19: 356–363. [DOI] [PubMed] [Google Scholar]
- 8.Ozao-Choy J, Tammaro Y, Fradis M, et al. Clopidogrel and bleeding after general surgery procedures. Am Surg 2008; 74: 721–725. [PubMed] [Google Scholar]
- 9.Cayla G, Cuisset T, Silvain J, et al. Platelet function monitoring to adjust antiplatelet therapy in elderly patients stented for an acute coronary syndrome (ANTARCTIC): An open-label, blinded-endpoint, randomised controlled superiority trial. Lancet 2016; 388: 2015–2022. [DOI] [PubMed] [Google Scholar]
- 10.Leung GKK, Tsang ACO, Lui WM. Pipeline embolization device for intracranial aneurysm: A systematic review. Clin Neuroradiol 2012; 22: 295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marosfoi M, Clarencon F, Langan ET, et al. Acute thrombus formation on phosphorilcholine surface modified flow diverters. J Neurointerv Surg. Epub before print 8 July 2017. DOI: 10.1136/neurintsurg-2017-013175. [DOI] [PMC free article] [PubMed]
- 12.Hagen MW, Girdhar G, Wainwright J, et al. Thrombogenicity of flow diverters in an ex vivo shunt model: Effect of phosphorylcholine surface modification. J Neurointerv Surg 2017; 9: 1006–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiu AHY, Ramesh R, Wenderoth J, et al. Use of aspirin as sole oral antiplatelet therapy in acute flow diversion for ruptured dissecting aneurysms. J Neurointerv Surg 2017; 9: e18. [DOI] [PubMed] [Google Scholar]
- 14.Reintam Blaser A, Malbrain MLNG, Starkopf J, et al. Gastrointestinal function in intensive care patients: Terminology, definitions and management. Recommendations of the ESICM Working Group on Abdominal Problems. Intensive Care Med 2012; 38: 384–394. [DOI] [PMC free article] [PubMed] [Google Scholar]