Abstract
Purpose
The aim was to evaluate the impact of protocol changes in selective ophthalmic arterial infusion (SOAI) for treatment of retinoblastoma (Rb).
Methods
A retrospective review was completed of 35 patients with Rb who were treated with SOAI between March 2010 and January 2017. Treatment details were tabulated for each SOAI session. SOAI protocol was changed in June 2015, and differences before and after this change were evaluated using two-tail chi-square tests and independent sample t-tests to note any differences in technical complications, need for enucleation, and other outcome variables
Results
125 SOAI sessions occurred. No technical complications occurred during the study. Two complications (1.6%) occurred in the postoperative setting. Both complications occurred prior to the change in protocol. Comparing the complication rates between the two protocols showed no significant difference (2.2% versus 0.0%; p = 0.505); 29 of 43 (67.4%) eyes had their vision preserved overall.
Conclusions
SOAI is an effective treatment for Rb. The refined protocol described herein was associated with fewer complications.
Keywords: Interventional neurooncology, intra-arterial chemotherapy, retinoblastoma
Introduction
Retinoblastoma (Rb) is the most common intraocular cancer in pediatric patients, accounting for 4% of all pediatric cancer.1 It is estimated that there are 200–300 new cases each year in the United States.1 In America, the overall 5-year survival rate for Rb is 97%.2 However, advanced cases—in which eye preservation is desired as opposed to traditional enucleation—often require extensive treatment that is associated with significant side effects. Retinoblastomas are staged by the International Classification of Retinoblastoma based on tumor size and extent, secondary effects (e.g., glaucoma, hemorrhage, uveitis), and the presence and extent of subretinal and vitreous tumor seeds.3 Approximately 40% of cases are bilateral. Germline mutation of the RB1 gene increases chances of developing secondary non-ocular cancers.4 Rarely, “trilateral” cancer occurs, with bilateral ocular lesions accompanied by a pineal-region tumor. It has been reported that about 5% of patients with germline mutation of RB1 develop trilateral Rb.5
Current treatment goals for Rb are to prevent death from intracranial spread or metastasis, preserve vision, avoid enucleation, and minimize the chance of developing secondary cancer from radiation therapy.6 Currently, treatment options include laser photocoagulation, cryotherapy, systemic chemotherapy, enucleation, radiotherapy, intra-vitreal chemotherapy, and selective intra-arterial chemotherapy (IAC). Prior to the advent of IAC, Group A to D tumors would be treated with systemic chemotherapy including vincristine, etoposide, and carboplatin in conjunction with photocoagulation or cyrotherapy.7 Historically, enucleation is the most common treatment for unilateral Group D and E Rb cases.7
The first reported use of IAC was in 1958 by Reese et al. involving injection of triethylene melamine in the carotid artery.8 In the 1990s, IAC was further advanced by the Kaneko group by performing selective ophthalmic arterial infusion (SOAI) of melphalan by super selective catheterization of the ophthalmic artery.9 This group performed SOAI on 187 patients and had a success rate of 97.5% with no major complications.9 With SOAI, the Kaneko group achieved eye preservation rates of 100% in Group A, 88% in Group B, 65% in Group C, 45% in Group D, and 30% in Group E.10 In the past decade, Gobin and colleagues have further advanced these techniques for advanced Rb patients.11 Gobin’s group reported that 70% of all eyes treated with SOAI had an ocular event free survival at two years.12 There are many advantages of SOAI when treating Rb, including control of intraocular tumor, resolution of retinal detachment, globe salvage, and minimal systemic side-effects.13 We previously reported early results of SOAI for Rb in our group.6 This study adds to these data, providing long-term results and updating treatment profiles after modifications to techniques.
Materials and methods
According to a protocol approved by the relevant institutional review board, prospectively maintained procedure logs were retrospectively reviewed to identify all patients with Rb treated with SOAI. Patients were reviewed from the initiation of the program at our center in March 2010 through January 2017 to allow for at least three months of follow up. Patient demographics, lesion laterality, and tumor grade were noted. Adjunctive therapy was noted, including external beam radiation therapy or IV chemotherapy performed prior to, concurrently with, or after SOAI. Treatment details were noted for each SOAI session, including site of arterial access, sheath size, use of a guide catheter, use of flow catheter versus over-the-wire technique, and site of microcatheter placement to perform chemotherapy infusion into the ophthalmic artery (Figure 1). Chemotherapy agent and dose were noted, as was duration of each infusion. The technical protocol for our group was changed in June 2015, switching to smaller sheaths, no guide catheter, and faster drug infusion. Guide catheters used in the initial time period were 4 French Non-taper Angle Glidecaths (Terumo Medical Corporation, Tokyo, Japan). Both 1.2 French and 1.5 French Magic microcatheters (Balt Extrusion, Montmorency, France) were used when flow catheters were employed. In cases requiring over-the-wire technique, an SL-10 microcatheter was used with a Synchro 2 soft microwire (Stryker Neurovascular, Fremont, CA). All cases were categorized as pre- or post-change with respect to technique. Any technical complications were noted, as were any delayed complications, response to treatment, preservation of vision, and need for enucleation. All procedures were performed under general endotracheal anesthesia. Infusion was performed. Descriptive statistics were performed to summarize these variables. Two-tail chi-square tests were performed to compare outcome variable means between groups for nominal variables. Independent sample t-tests not assuming equal variances were performed for continuous variables. Statistical analysis was performed in SPSS version 22.0.0.0 (IBM, Armonk, NY).
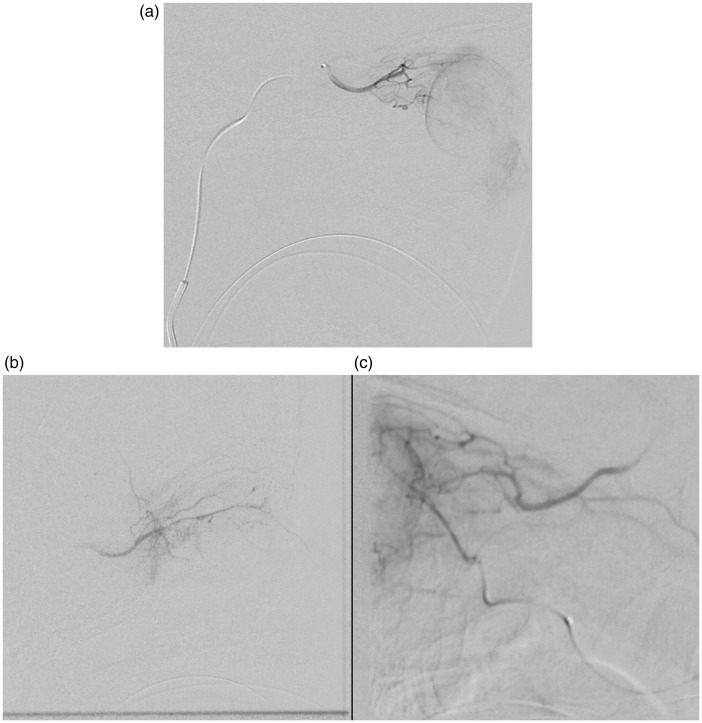
Figure 1.
Angiograms of chemotherapy infusion through three different arteries. (a) Infusion through the ophthalmic artery as it arises from the junction of the cavernous and supraclinoid internal carotid artery. (b) Infusion through the meningo-lacrimal branch of the middle meningeal artery. (c) Infusion through an anterior deep temporal branch of the internal maxillary artery.
Results
A total of 125 treatment sessions were identified, treating 43 eyes in 35 patients. Mean follow up was 954 days (IQR, 397–1741). Demographic, lesion, and treatment variables are listed in Table 1, including comparison of sessions performed according to the older and updated technical protocols.
Table 1.
Changes in SOAI Protocol.
| Protocol time period | Before June 2015 | June 2015 and later |
|---|---|---|
| Femoral sheath | 4 Fr 11 cm Brite Tip (Cordis) | 3.3 Fr 7 cm Super Sheath (PediaVascular) |
| Guide catheter | 4 Fr Glidecatheter (Terumo) | None |
| Wire for Guide Catheter | 0.035” Bentson (Boston Scientific) | None |
| Microcatheter | Magic 1.5 Fr or 1.2 Fr (Balt)* | Magic 1.5 Fr or 1.2 Fr (Balt)* |
| Marathon (Medtronic) | Marathon (Medtronic) | |
| Excelsior SL-10 (Stryker) | Excelsior SL-10 (Stryker) | |
| Wire for microcatheter | Chikai (Asahi) | Chikai (Asahi) |
| Mirage (Medtronic) | Mirage (Medtronic) | |
| Synchro-2 Soft** | Synchro-2 Soft** | |
| Drugs | Melphalan | Melphalan |
| ± carboplatin | ||
| ± topotecan | ||
| Drug infusion rate | 0.5 mL/minute | 1 mL/minute |
1.5 Fr Magic microcatheter was the default first choice. Only in cases of very small meningolacrimal or superficial temporal arterial catheterizations was a 1.2 Fr catheter used.
Synchro 0.014” wires were only used when an Excelsior SL-10 catheter was also used.
No technical complications were noted. Two (1.6%) complications occurred in the postoperative period. One patient seized on postoperative day two, and magnetic resonance imaging revealed a small cerebral infarct referable on the internal carotid artery on the side of treatment; this patient made a full neurological recovery. The second patient experienced systemic toxicity from chemotherapy, which was treated with supportive measures and which resulted in no permanent morbidity. Both complications occurred after changes in the treatment protocol. Comparing complication rates before and after these protocol changes showed no significant difference (2.2% versus 0.0%, respectively; p = 0.505).
Long-term results are summarized according to tumor classification in Table 2. Overall, vision was preserved in 29 (67.4%) eyes, similar to long term results published by Gobin’s group.12 There were no patients in which enucleation was avoided but vision was not preserved. Comparing lesions treated before and after protocol changes reflects changes in referral patterns in our practice. Our group has been referred patients with lower stage disease, and adjunctive intravenous chemotherapy is performed less commonly. Additionally, triple-agent SOAI is more commonly performed, and external beam radiation therapy is no longer utilized.
Table 2.
Comparison of Two SOAI Protocols.
| Protocol |
||||
|---|---|---|---|---|
| Original | Updated | Total | p-value | |
| n = 89 | n = 36 | n = 125 | ||
| Age at diagnosis (mos) | 9.60 | 9.46 | 9.60 | 0.351 |
| Age at first SOAI (mos) | 15.2 | 15.7 | 15.1 | 0.127 |
| Male | 52 (58.4%) | 11 (30.6%) | 63 (50.4%) | 0.004 |
| Weight at treatment (kg) | 10.4 | 11.6 | 11.5 | 0.004 |
| Subretinal seeding | 9 (10.1%) | 13 (36.1%) | 22 (17.6%) | 0.001 |
| Vitreous seeding | 23 (25.8%) | 9 (25.0%) | 32 (25.6%) | 0.557 |
| Retinal detachment | 46 (51.7%) | 27 (75.0%) | 73 (58.4%) | 0.013 |
| Classification | – | – | – | – |
| A | 4 (4.5%) | 0 (0.0%) | 4 (3.2%) | 0.003 |
| B | 11 (12.4%) | 11 (30.6%) | 22 (17.6%) | |
| C | 25 (28.1%) | 9 (25.0%) | 34 (27.2%) | |
| D | 28 (31.5%) | 16 (44.4%) | 44 (35.2%) | |
| E | 21 (23.6%) | 0 (0.0%) | 21 (16.8%) | |
| Right side | 47 (52.8%) | 24 (66.7%) | 71 (56.8%) | 0.111 |
| Bilateral disease | 73 (82.0%) | 21 (58.3%) | 94 (75.2%) | 0.006 |
| Familial | 31 (34.8%) | 4 (11.1%) | 35 (28.0%) | 0.005 |
| Germline mutation | 77 (86.5%) | 24 (66.7%) | 101 (80.8%) | 0.013 |
| IV Chemo | 59 (66.3%) | 30 (83.3%) | 89 (71.2%) | 0.043 |
| Before SOAI | 49 (55.1%) | 23 (63.9%) | 72 (57.6%) | 0.241 |
| Concurrent | 10 (11.2%) | 18 (50.0%) | 28 (22.4%) | <0.001 |
| After SOAI | 24 (27.0%) | 22 (61.1%) | 46 (36.8%) | <0.001 |
| XRT | 17 (19.1%) | 0 (0.0%) | 17 (13.6%) | 0.002 |
| Bilateral SOAI | 40 (44.9%) | 16 (44.4%) | 56 (44.8%) | 0.560 |
| 3.3 French sheath | 0 (0.0%) | 30 (83.3%) | 30 (24.0%) | <0.001 |
| Guide catheter | 89 (100%) | 5 (13.9%) | 94 (75.2%) | <0.001 |
| Flow catheter | 89 (100%) | 31 (86.1%) | 120 (96.0%) | 0.002 |
| OA directly accessed | 82 (92.1%) | 32 (88.9%) | 114 (91.2%) | 0.394 |
| Meningo-lacrimal branch | 5 (5.6%) | 4 (11.1%) | 9 (7.2%) | 0.237 |
| Anterior deep temporal | 3 (3.4%) | 0 (0.0%) | 3 (2.4%) | 0.357 |
| Triple agent SOAI | 0 (0.0%) | 12 (13.5%) | 12 (13.5%) | <0.001 |
| Mean melphalan dose | 4.28 | 3.76 | 4.28 | – |
| Mean carboplatin dose | – | 30 | 30 | – |
| Mean topotecan dose | – | 0.49 | 0.49 | – |
| Mean infusion time (min) | 32 | 18 | 29 | |
| Technical complication | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | – |
| Groin hematoma | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | – |
| Stroke | 1 (1.1%) | 0 (0.0%) | 0 (0.0%) | 0.712 |
| Ocular toxicity | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | – |
| Systemic toxicity | 1 (1.1%) | 0 (0.0%) | 1 (0.8%) | 0.712 |
| All complications | 2 (2.2%) | 0 (0.0%) | 2 (1.6%) | 0.505 |
Discussion
Treatment for Rb is continuously improving in terms of effectiveness and safety. SOAI has gained favor due to advances by the Kaneko and Gobin groups. SOAI has been able to help patients avoid enucleation and the side effects of systemic chemotherapy. However, with SOAI come new complications, namely vitreous hemorrhage and retinal ischemia.13 SOAI techniques continue to be optimized to avoid these complications and maintain effectiveness.
The current study expands on previously reported results from our practice.6 This series confirms the effectiveness and safety profile of SOAI treatment of Rb. Overall, 58% of tumors decreased in size, and 67% of eyes were spared from enucleation (Table 3). Furthermore, this study reflects technical advantages of the modified protocol. Changing to a smaller sheath size, eliminating use of guide catheters, and increasing the rate of chemotherapy infusion, SOAI remained effective and had a better safety profile. These changes have reduced opportunities for thromboembolic complications, which are of particular concern in patients with cancer.10,14 While eliminating the use of guide catheters has decreased occurrences of catheter-induced vasospasm in the internal carotid artery, this phenomenon can still occur in the ophthalmic artery or other small branch arteries. Additionally, variable anatomy of the ophthalmic artery renders some vessels exceedingly difficult to catheterize. In such cases, SOAI may require catheterization of the meniningo-lacimal branch of the middle meningeal artery or accessory meningeal or anterior deep temporal branches of the internal maxillary artery.15 Furthermore, catheterization of such vessels may not yield satisfactory flow to the tumor. In such cases, infusion directly into the internal carotid artery with a balloon inflated just distal to the ophthalmic origin may be necessary.15 As of the time of data lock for this series, this technique had not been considered necessary for any cases in our series, but has subsequently been used successfully by our group.
Table 3.
Intermediate Term Clinical Outcomes.
| Mean sessions | Tumor response | Enucleation | Vision recovered | Vision preserved | |
|---|---|---|---|---|---|
| A (n = 1) | 4 | 0 (0.0%) | 1 (100%) | 0 (0.0%) | 0 (0.0%) |
| B (n = 10) | 2.2 | 9 (90.0%) | 1 (10.0%) | 1 (10.0%) | 9 (90.0%) |
| C (n = 11) | 3.1 | 7 (63.6%) | 4 (36.4%) | 0 (0.0%) | 7 (63.6%) |
| D (n = 13) | 3.2 | 5 (38.5%) | 4 (30.8%) | 3 (23.1%) | 9 (69.2%) |
| E (n = 8) | 2.4 | 4 (50.0%) | 4 (50.0%) | 1 (12.5%) | 4 (50.0%) |
| Total (n = 43) | 2.81 | 25 (58.1%) | 14 (32.6%) | 5 (11.6%) | 29 (67.4%) |
Exposure to ionizing radiation during catheterization is another factor warranting attention, particularly considering the radiosensitive biology of patients with germline mutations of the RB1 gene. Protocol changes have dramatically reduced radiation exposure with no change in response to treatment.16 Analysis of potential delayed radiation effects from this procedure is beyond the scope of this investigation.
In addition to effects of technical changes, this series also reflects changes in the referral pattern of Rb patients for SOAI. The role of adjuvant intravenous chemotherapy and ablative intraocular procedures remain to be determined definitively. In the current study, no differences in outcomes were noted between those with and without adjuvant chemotherapy. However, interpretation of this result is limited by multiple confounding variables.
Our study is limited by a small number of patients due to the rarity of Rb. While data were prospectively logged to reduce opportunities for bias, our study’s retrospective and non-standardized design limits this analysis. Patient and lesion heterogeneity is the result of such nonstandard design. Further long-term follow studies will be warranted from this patient series and the ongoing clinical trials within which they have received treatment.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Grossniklaus HE. Retinoblastoma. Fifty years of progress. The LXXI Edward Jackson Memorial Lecture. Am J Ophthalmol 2014; 158: 875–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Broaddus E, Topham A, Singh AD. Survival with retinoblastoma in the USA: 1975–2004. Br J Ophthalmol 2009; 93: 24–27. [DOI] [PubMed] [Google Scholar]
- 3.Shields CL, Fulco EM, Arias JD, et al. Retinoblastoma frontiers with intravenous, intra-arterial, periocular, and intravitreal chemotherapy. Eye 2013; 27: 253–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lohmann DR, Gallie BL. Retinoblastoma. In: Pagon RA, Bird TD, Dolan CR, et al.(eds). GeneReviews, Seattle, WA: University of Washington, 1993. [Google Scholar]
- 5.De Jong MC, Kors WA, De Graaf P, et al. The incidence of trilateral retinoblastoma: a systematic review and meta-analysis. Am J Ophthalmol 2015; 160: 1116–1126. [DOI] [PubMed] [Google Scholar]
- 6.Thampi S, Hetts SW, Cooke DL, et al. Superselective intra-arterial melphalan therapy for newly diagnosed and refractory retinoblastoma: results from a single institution. Clin Ophthalmol 2013; 7: 981–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meel R, Radhakrishnan V, Bakhshi S. Current therapy and recent advances in the management of retinoblastoma. Indian J Med Paediatr Oncol 2012; 33: 80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reese AB, Hyman GA, Tapley ND, et al. The treatment of retinoblastoma by x-ray and triethylene melamine. AMA Arch Ophthalmol 1958; 60: 897–906. [DOI] [PubMed] [Google Scholar]
- 9.Yamane T, Kaneko A, Mohri M. The technique of ophthalmic arterial infusion therapy for patients with intraocular retinoblastoma. Int J Clin Oncol 2004; 9: 69–73. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki S, Yamane T, Mohri M, et al. Selective ophthalmic arterial injection therapy for intraocular retinoblastoma: the long-term prognosis. Ophthalmology 2011; 118: 2081–2087. [DOI] [PubMed] [Google Scholar]
- 11.Abramson DH, Dunkel IJ, Brodie SE, et al. A phase I/II study of direct intra-arterial (ophthalmic artery) chemotherapy with melphalan for intraocular retinoblastoma initial results. Ophthalmology 2008; 115: 1398–1404. [DOI] [PubMed] [Google Scholar]
- 12.Gobin YP, Dunkel IJ, Marr BP, et al. Intra-arterial chemotherapy for the management of retinoblastoma: four-year experience. Arch Ophthalmol 2011; 129: 732–737. [DOI] [PubMed] [Google Scholar]
- 13.Kaliki S, Shields CL. Retinoblastoma: achieving new standards with methods of chemotherapy. Indian J Ophthalmol 2015; 63: 103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khorana AA, Francis CW, Culakova E, et al. Risk factors for chemotherapy-associated venous thromboembolism in a prospective observational study. Cancer 2005; 104: 2822–2829. [DOI] [PubMed] [Google Scholar]
- 15.Boddu SR, Abramson DH, Marr BP, et al. Selective ophthalmic artery chemosurgery (SOAC) for retinoblastoma: fluoroscopic time and radiation dose parameters. A baseline study. J NeuroInterv Surg. Epub ahead of print 9 November 2016. DOI: 10.1136/neurintsurg-2016-012758. [DOI] [PubMed]
- 16.Cooke DL, Stout CE, Kim WT, et al. Radiation dose reduction in intra-arterial chemotherapy infusion for intraocular retinoblastoma. J NeuroInterv Surg 2014; 6: 785–789. [DOI] [PubMed] [Google Scholar]