THE OLDER MYELOMA PATIENT: EVALUATING AGING AND UNDERSTANDING FITNESS
Overview
Multiple myeloma (MM) is an incurable plasma cell malignancy of older adults. The median age at diagnosis is 69 years, and in the next 15 years the incidence of MM is expected to double in this age demographic [1,2]. Novel therapeutics and the routine use of autologous stem cell transplant have led to substantial improvements in overall response rates and durable remissions [3]. In contrast with younger MM patients, older patients have only modest improvements in overall survival [4–6]. MM deaths overall are highest in patients aged 75 years and greater, and early mortality is most common in those 70 years and older [3,7]. The disparity in overall survival for aging adults is multifactorial and is secondary to comorbidities, treatment strategy, toxicities, physiologic reserve, and therapy discontinuation.
Transplant “eligibility” is an active and important area of MM investigation. Autologous stem cell transplant is established in younger populations to improve survival over nontransplant therapy [8,9] and has progression-free survival advantages over delayed transplant [10]. Autologous stem cell transplant is feasible and an efficacious component of therapy for older patients with MM as well [11]. Older adults mobilize sufficient numbers of stem cells and can tolerate transplant with excellent outcomes, resulting in increased numbers of older adults undergoing autologous transplant [11]. Nearly half of autologous transplants are done in adults 60 years and greater, and this number will only increase as the population ages [12]. Referral bias for transplant still exists, likely because of historic reports depicting conflicting tolerance, response rates, and survival [13].
Transplant Eligibility
Transplant eligibility is matter of estimating a patients’ physiologic reserve for an intensive therapy [14]. Identifying and intervening on factors that contribute to vulnerability in pretransplant MM patients is imperative to balance quality of life with an efficacious therapy. One method to identify and resolve occult health factors is a geriatric assessment (GA), which is a global evaluation of the health of older adults. A GA goes beyond the disease-focused history and aims to identify unrecognized issues to intervene and prevent future complications. GA tools are established metrics to accurately assess risk of morbidity and mortality in cancer populations [15,16]. The GA consists of a multidimensional evaluation of functional status, fall history, social support, cognitive and psychological status, sensory loss, nutritional status, comorbidities, and a polypharmacy evaluation.
Table 1 depicts a set of tools often used in a cancer-specific GA. GAs have been shown to predict mortality and toxicity, independent of performance status and age [17]. Traditional metrics, such as Karnofsky performance status, are often overestimated by clinicians and are a poor indicator of treatment toxicity risk [18].
Table 1.
Geriatric Evaluation for Older AML Candidacy for Allogeneic Transplant
| Domains | Common Tools | Additional Geriatric Questions |
|---|---|---|
| Comorbidity | HCT-CI | Remote cancer, urinary problems, visual or hearing impairment, diastolic dysfunction, prior renal impairment, osteoporosis |
| Function | IADL Timed up and go, grip strength, 4-m walk | Arthritis, falls, maximum ability for physical activity, exercise, balance |
| Social support and function | Illness-specific subscales of social support Center-specific tool | Backup caregiver, alcohol use, stairs at home, person preparing meals, power of attorney |
| Cognition | Mini-Mental State Exam or Montreal cognitive assessment | Prior confusion, memory impairment and duration Assess retention of information without family member interjection |
| Psychological | Geriatric Depression Scale Mental Health Inventory 17 | Sleep problems, motivation for transplant, coping skills, preparation for setbacks, life goals (eg, specific event) |
| Nutritional status | Weight loss, body mass index | Last dental evaluation, dentures, use of supplements, effect of prior therapy on nutrition and weight |
| Polypharmacy | >5 medications | Over-the-counter medications, side effects from prior medication, clarify remote allergies |
The treatment approach for MM is heterogeneous because of concerns for frailty and tolerance in older adults. Primary dose reductions and therapy discontinuations result in worse outcomes in older adults with MM [19,20]. Although it is known that functional assessments can improve on outcomes in cancer patients, feasibility, practicality, and disease specificity are barriers to implementation [21–23]. Nonetheless, a simple GA based on age, comorbidities, cognition, and physical function can predict mortality in the MM population. Frailty assessments in MM patients were predictive of death independent of treatment, cytogenetics, or stage [20].
Biomarkers of Physiologic Age
Novel aging biomarkers are being explored to provide a rapid measure of physiologic fitness [24,25]. Ideally, an aging biomarker would capture vulnerability by estimating physiologic reserve and risk for chemotherapy and/or transplant toxicity. Exploring biomarkers of aging in a cancer population is particularly challenging, because many aging biomarkers are evaluated in a community-dwelling population without a cofounding factor of cancer. Despite these challenges, many potential biomarkers of aging are being explored to evaluate the relationship among chronologic aging, frailty syndromes, and cancer. Candidate biomarkers include molecular markers, inflammatory markers, immunosenescence panels, serum and hematologic parameters, and hormones (Table 2). Each of these biomarkers has associations with aging and frailty and variable relationships with autologous hematopoietic stem cell transplant and myeloma. Inflammatory markers such as IL-6, C-reactive protein, tumor necrosis factor-α, and D-dimer have reported associations with frailty in oncology studies [26]. Inflammatory panels such as the senescence-associated secretory phenotype has a well-established relationship with aging, gerontologic conditions, and frailty [27].
Table 2.
Aging and Frailty Assessments
| Aging | Frailty | Transplant | Myeloma | ||
|---|---|---|---|---|---|
| Molecular markers | p16INK4A | x | x | ||
| Leukocyte telomere length | x | ||||
| DNA methylation | x | ||||
| miRNA | |||||
| Immune dysregulation | Immunosenescence | x | x | x | |
| SASP | x | x | |||
| Heme parameters | Anemia | x | x | ||
| Serum markers | IL-6 | x | x | x | x |
| CRP | x | x | |||
| NT-proBNP | x | x | |||
| Albumin | x | x | x | ||
| D-dimer | x | x | |||
| TNF | |||||
| sICAM-1 | x | ||||
| Clinical tools | GA metrics | x | x | x | x |
SASP indicates senescence-associated secretory phenotype; CRP, C-reactive protein; NT-pro-BNP, N-terminal fragment of B-type natriuretic peptide; TNF, tumor necrosis; sICAM-1, Soluble intercellular adhesion molecule-1.
Distinct changes in the immune system are reported with aging [28,29] and are being explored with frailty [30,31]. Many of the distinct changes in T cell dysfunction found in MM patients parallels that of aging and frail populations. T cell dysfunction is commonly reported in MM where markers of immunosenescence are associated with relapsed disease [32]. Clonal expansions of T cells are common with aging and are also present with long-term survival in MM [33], although the biology of clonal expansions may differ. Anemia, inherent to MM, has been independently associated with functional disability in older adults [34]. miRNA expression profiles have been shown to correlate with age in healthy populations [35], cardiovascular disease [36,37], but not frailty in solid tumor cancer populations [38]. Other biomarkers, such as N-terminal fragment of B-type natriuretic peptide, have recently been shown as useful predictors of survival, independent of age and performance status in MM patients [39]. Markers of T cell immunosenescence and exhaustion have recently been reported to have an association with relapse after MM transplant [40].
A molecular marker of cellular senescence is peripheral blood T cell p16INK4A (p16). p16 mRNA accumulates in aging tissues and increases with chronologic aging and with internal and external stresses [41–44]. Over the human lifespan, p16 expression levels increase more than 16-fold in peripheral blood T cells [45]. There is considerable evidence that p16INK4A is 1 of the most robust and validated aging biomarkers. p16INK4a is being explored for risk of chemotherapy toxicity and transplant tolerance [46,47]. External stressors can trigger p16 expression such as physical inactivity [48], tobacco use, or chemotherapy [47,49].
Aging biomarkers paired with geriatric metrics may provide a novel method to gauge risk of transplant toxicity in the MM population. The need for objective biomarkers of aging is especially important in the field of MM because of the aged population affected, heterogeneity in older adult fitness, and diverse treatment strategies that are available. Ultimately, an individualized approach to patient fitness based on geriatric metrics and aging biology can aid the clinician in decision-making for myeloma transplant.
THE OLDER ACUTE MYELOID LEUKEMIA PATIENT: CANDIDACY AND OPTIMIZATION FOR ALLOGENEIC TRANSPLANT
Overview
The potent antileukemic effects of allogeneic hematopoietic cell transplantation must be balanced against transplantassociated morbidity and mortality, especially for older patients. Reduced-intensity allogeneic transplant achieves rates of 30% to 50% in 2-year survival for acute myeloid leukemia (AML) in patients 60 years and older. Emerging data highlight the importance of physiologic reserve beyond chronologic age alone. Multidimensional health surveys such as the GA encompassing comorbidity, function, social support, cognition, nutrition, and other domains identify unrecognized and prognostically relevant health impairments. Age-related vulnerabilities may predispose to toxicity because of inherent physiologic stress of transplant. Enrollment on prospective studies is essential to delineate the most effective risk-stratification tools to inform transplant candidacy. Interventions to attenuate transplant risks based on health vulnerabilities in older patients are proposed, although prospective studies are mandatory.
Epidemiology of AML in Older Adults
Advancing age represents the primary risk factor for AML diagnosis and disease fatality. The median age at diagnosis in Western countries is around 67 to 70 years, and older age is strongly associated with worse survival (seer.cancer.gov) [50]. Adverse biologic factors inherent to the disease, competing health conditions, and less treatment together interfere to limit long-term success. The biology of older age as a risk for AML and inferior outcomes remain an area of interest, although age-related clonal myeloid hematopoiesis appears to predispose to leukemia and possibly chemotherapy resistance clones [51].
A large series of AML patients enrolled on Cancer and Leukemia Group B trials illustrates the influence of age on outcomes. Patients 60 years and older had less frequent favorable risk disease using the European LeukemiaNet criteria and more frequent adverse cytogenetics (31% adverse disease for older versus 22% in patients under 60 years). For the favorable risk group, only 24% of older patients achieved 3-year disease-free survival compared with 55% in younger patients. Nontransplant protocol–based treatment rarely accomplished leukemia control for intermediate and adverse risk disease: rates of 3-year disease-free survival were 11% and 6%, respectively [52]. The large United Kingdom National Cancer Research Institute AML16 trial of intensive therapy showed similar results, with rates of 5-year survival of around 14% to 15% [53].
Older Age and Allogeneic Transplant for AML
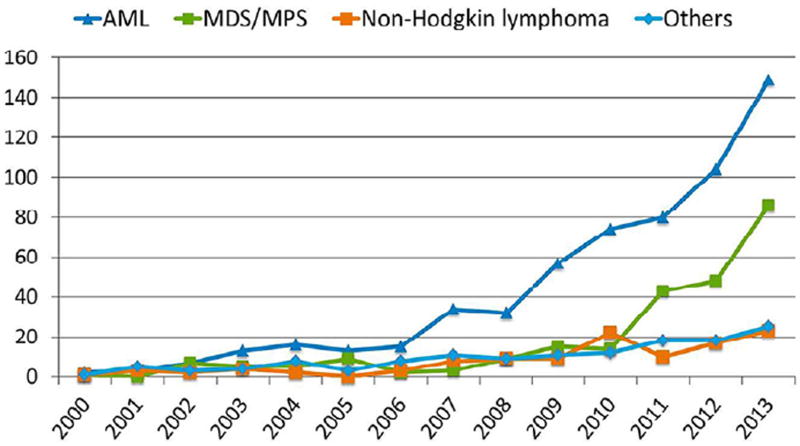
The historical barrier of older age to allogeneic hematopoietic cell transplant has slowly been lifted over the past 2 decades. The Center for International Blood and Marrow Transplant Research (CIBMTR) showed 22% of allogeneic transplant recipients were 60 years or older in 2007 to 2013 among common malignant conditions [54]. Likewise, US allografts for patients 70 years and older have risen 10-fold over the past decade, with AML as the leading indication (Figure 1) [55]. Few older AML patients receive allogeneic transplant; a population study of AML diagnosed between 2000 and 2007 showed only 4% of AML patients 65 to 74 years underwent allografts [56]. Swedish data described a marked uptick in transplant use for those 61 to 70 years in recent years yet no transplants for those 70 years and older for AML [57].
Figure 1.
Allogeneic transplant malignant indications and change over time in the United States reported to the Center for International Blood and Marrow Transplantation Research.
Transplant outcomes for older AML patients
In a review of studies of adults 60 years and older undergoing allogeneic transplant for AML among 749 patients, 13 studies reported rates of overall survival at 1, 2, and 3 years of 58%, 45%, and 38%, respectively [58]. Devine et al. [59] published a multi-institutional prospective study of a low-intensity fludarabine-busulfan-antithymocyte globulin regimen using matched related and unrelated donors for AML in first remission. Patients 60 to 74 years of age achieved a favorable 2-year survival rate of 48% and a low nonrelapse mortality (NRM) rate of 14%. Registry data for AML and myelodysplastic syndrome patients 70 years and older using all donor sources and conditioning regimens showed 2-year survival rate of 38% and 2-year NRM rate of 30% [55]. Haploidentical and umbilical cord graft studies in older AML patients have been described [60–62].
Advancing age still confers greater risks of transplant-related mortality for AML in first remission [63]. However, limiting the comparison to HLA matched grafts after reduced-intensity conditioning showed no major influence of age alone [64,65]. A report by Versluis et al. [66] derived from prospective AML studies of patients 60 years and older revealed reduced-intensity transplant from matched related or unrelated donors improved 5-year overall survival rates at 35% versus 26% for other forms of consolidation therapy (hazard ratio [HR] = .71, P = .017).
Provisional Recommendations on Candidacy
GA and risk categorization
No well-designed studies have characterized the morbidity and risks of transplant in older adults of hematopoietic cell transplant versus nonhematopoietic cell transplant approaches. Decisions to pursue allogeneic hematopoietic cell transplant demand individualization and attention to disease status, donor type, regimen, center experience, and patient goals. A GA has been the standard tool within geriatric oncology to better characterize health in older patients. The GA in Table 1 was adopted from the Cancer and Aging Research Group and supplemented by additional medical history that may be relevant to transplant [67,68]. The extensive resources committed for transplant and appreciable risks may justify this low-risk low-cost evaluation. Table 3 illustrates a schema used at our institution that divides patients into anticipated transplant-associated mortality risk into fit (ie, consider similar to younger patients), vulnerable (higher risk but transplant risks may be manageable, review optimization strategies), and unfit (avoid transplant because of excessive risks unless on trial or exceptional circumstances). This schema has not been tested and should not be considered a guideline. The available data and/or rationale for such criteria are summarized below.
Table 3.
Provisional Framework for Allogeneic Hematopoietic Cell Transplant Eligibility Criteria for AML Patients 60 Years and Older with Adequate Disease Control
| Factor | Fit (All of the Below) | Vulnerable (Any of the Below) | Usually Exclude or Trial (Any of the Below) |
|---|---|---|---|
| Age, yr | <66 | 66–75 | >75 |
| Comorbidity | HCT-CI 0–2 | HCT-CI 3–5 | HCT-CI 6+ or severe organ dysfunction |
| KPS, % | 90–100 | 70–80 | <70 |
| Function | |||
| IADL* | Normal | Any limitation | Limitations and HCT-CI 4+ |
| Performance† | No impairment | Impaired | Severely limited |
| Cognitive | No impairment | Mild cognitive impairment, prior confusion | Moderate cognitive impairment |
| Other factors | Normal | Weight loss, single or limited caregiver, falls, albumin < 3.5 g/dL, limited motivation | Weight loss, single or limited caregiver, falls, albumin < 3.5 g/dL, limited motivation + others |
KPS indicates Karnofsky performance score.
IADL used here omits the category of ability to do laundry.
Performance was measured by timed up and go, 4-m walk, and grip strength; 6-minute walk used for prehabilitation baseline.
Age
The preponderance of data supporting allografting for older AML patients has been in patients 65 years or less and sparse for 70 years and above [55,69]. Ideally, patients above 75 years of age would undergo transplant on clinical trials specifically addressing age-related issues. Chronologic age alone between 60 and 75 years will not usually dictate transplant candidacy, and complementary evaluation may aide decision-making. Patients younger than 60 years may also benefit from a comprehensive health evaluation.
Comorbidity
The hematopoietic cell transplantation–specific comorbidity index (HCT-CI) summates various comorbid conditions extracted from history and objective testing [70]. In adults 60 years and older undergoing nonablative HLA matched allografts, Sorror et al. [71] showed a borderline significant increase in NRM for intermediate comorbidity of HCT-CI scores of 1 to 2 (HR, 1.69; 95% confidence interval [CI], .91 to 3.14) and 3 or more (HR, 2.0; 95% CI, 1.12 to 3.6) relative to no comorbidity and inferior survival for those with the higher comorbidity. In a registry validation, rates of 1-year allogeneic transplant NRM were 17%, 21%, and 26% for HCT-CI scores of 0, 1 to 2, and 3, respectively (P < .001). NRM did not differ for HCT-CI scores of 1 to 2 relative to 0 after reduced-intensity regimens. High comorbid burden (ie, HCT-CI score of 5 or more) exacted a profound effect on NRM (HR, 1.77; 95% CI, 1.50 to 2.10). Similar effect sizes for NRM and survival have been reported by degree of comorbid burden [72]. Comorbidity and age are additive to the prognostic effects on NRM and survival [71,73,74]. Thus, older patients entering transplant with HCT-CI scores of 3 to 5 are vulnerable, and at our center we simply avoid transplant for patients with HCT-CI scores of 6 unless exceptional circumstances or trial. Following the scoring guidelines by Sorror [75] will maximize accuracy in tabulating comorbidity and likely prognostic discrimination.
GA and Functional Status
Functional status
Low physician rated Karnofsky performance status (eg, <60% to 70%) often excludes patients from allogeneic transplant consideration and among older transplanted patients. A Karnofsky performance status of 80% or less may have worse outcomes relative to 90% to 100% [76].
Patient-reported and/or performance-based function is an essential core element of a multidimensional GA. We reported a high frequency of patient-reported functional impairments for patients 50 years and older before allografting because 40% had at least 1 limitation in a core functionally tool, the Lawton Instrumental Activities of Daily Living (IADL), encompassing the ability to manage medications, finances, meals, grocery shop, telephone, transportation and driving, and housekeeping/chores [77]. Other reports from Houston and San Francisco have likewise demonstrated a high frequency of functional vulnerabilities by GA testing before transplant [78,79]. In a Chicago cohort (N = 203) the presence of any IADL limitation independently imparted high risks of NRM and inferior survival but not relapse rates. Adverse effects were amplified in patients 60 and older (HR, 3.3) relative to patients 50 to 59 (HR, 1.9), although CIs overlapped. Patient function through a self-report quality of life instrument also conveyed worse 1-year survival rates in a multi-institutional study by Wood et al. [80] of all ages supporting the premise of patient-reported function for prognostication.
A slow 4-m walk speed also predicted higher mortality in the Chicago series. In studies of adult patients of all ages, cardiopulmonary fitness tools of 6-minute walk distance or cycle ergometry may risk-stratify patients [81,82]. Whether patient-reported function, performance-based function, or both will be optimal is unknown. Other studies in geriatrics and geriatric oncology support the IADL to stratify for outcome using this convenient survey tool [67,83].
Other Health Domains
Emotional health
The Houston and Chicago series showed surveys of emotional health uncovered a higher frequency of emotional health impairments than by the HCT-CI alone [78]. The Mental Health Inventory-5 demonstrated 36% of patients in the Houston study expressed anxiety or depression. Using the Short-Form 36 quality of life instrument mental composite summary subscale, slightly over half of the patients in the Chicago GA indicated mental health at least 1 standard deviation below population normals [74]. Emotional health and motivation are clearly relevant factors, although the attendant risks from these disturbances require additional study.
Cognition
The Houston series on GA described a 16% prevalence of mild cognitive impairment before transplant [78]. In 27 unselected patients 60 years and older before allografting, we found a 10% rate of cognitive impairment by the Mini-Mental Status Exam (A. Artz and M.Q. Lacy, personal communication, 2015). A diagnosis of cognitive impairment should not be based on screening tests alone. One approach to establish cognitive impairment is to perform neuropsychological testing and/or consult geriatrics. The consequences of allografting in cognitive impairment are unknown, although Klepin et al. [84] reported pretreatment cognitive impairment negatively affected early survival after receipt of AML induction therapy in older adults. Therefore, one must exercise caution in pursuing allogeneic transplant if cognitive deficits are uncovered.
Composite models and biomarker
Data-driven models should be developed to maximally guide candidacy. Multidimensional risk scores using comorbidity, function, and age, if not other markers as used in geriatric oncology and noncancer older populations [67,85], will likely emerge. For example, we found a combination of high comorbidity (ie, HCT-CI scores of 3 or more) and functional impairment by IADL produced a simple 3-level scoring system with appreciable discrimination: 2-year survival rates exceeded 60% for no risk factors compared with 29% and 0% for those with 1 or 2 risk factors in patients 60 years and older, respectively. Biomarkers such as elevated C-reactive protein and depressed albumin add prognostication discrimination to comorbidity and age [72,86]. Hypoalbuminemia (<3.5 g/dL) can be applied as a readily available biomarker in light of the established and independent risks of NRM.
Regimen Intensity
The decision of regimen and graft sources is beyond the scope of this review, yet risk stratification must account for the intended regimen and graft source. Unfortunately, a low-intensity regimen may not necessarily improve outcomes for older adults because of heightened risks of relapse. The preliminary data of a benefit in relapse-free survival of myeloablative chemotherapy for AML and myelodysplastic syndrome in patients up to age 65 years with low comorbidity suggest one may consider an ablative regimen for older fit AML patients [87].
Transplant Optimization
Rationale
Multidisciplinary optimization strategies to reduce transplant morbidity are attractive when considering the substantial risks associated with specific vulnerabilities and rapid transplant growth in patients 70 years and older. The promise has not been prospectively tested.
Standard Platform
Team approach
Our center has attempted to leverage vulnerabilities detected by GA by assembling an interdisciplinary team to develop targeted interventions as outlined in Table 4. The team consists of a dietician, social worker, geriatric oncologist, transplant physician (often separate from the primary transplant physician), transplant nurse, transplant advanced practice provider, physical therapist, and a data or clinical coordinator. Most team members exist in transplant programs. Other team members to consider include a pharmacist, psychologist, and patient advocate. Cultivating relationships with medical subspecialists for prevalent comorbid conditions such as cardiac and pulmonary dysfunction promotes a mindset of optimal monitoring and management and away from clearance alone.
Table 4.
Standard Optimization Platform for Older Allogeneic Transplant Recipients Used at University of Chicago
| Health Area | Standard Optimization Recommendations |
|---|---|
| GA | Interventions individualized based on GA-detected limitations in Table 2. Multidisciplinary team meeting and written optimization plan before transplant. |
| Comorbid conditions | Cultivate preferred subspecialists and request post-transplant follow-up. Referral to geriatrics or geriatric oncology for geriatric syndromes (eg, falls, incontinence). |
| Function | Structured prehabilitation based on baseline activity and limitations. Home safety assessment (fall risk, stairs, proximity of bathroom). |
| Social support | Pretransplant family meeting, caregiver present during cytopenia and “on-call” if issues. Request secondary caregivers. Enlist caregivers in optimization plan. |
| Cognition | Delirium awareness and precautions, medication avoidance, encourage presence of caregivers, add written material to verbal instructions, call patient to reinforce education. |
| Emotional health | Offer cognitive therapy and support group to avoid additional medications unless required |
| Nutrition | Develop nutritional plan before transplant included preferred supplements if used. |
| Medications | Reduce unnecessary medications. Re-evaluate days 30 to 100 post-transplant. |
| Other | Infection mitigation plan for grandchildren, evaluate remote drug allergies (eg, penicillin allergy in childhood), more frequent follow-up, smooth survivorship transition from transplant center. |
Specific interventions
Randomized transplant studies of exercise during and after transplant have shown only modest benefits, and data were primarily derived in younger adults at lower risk of functional disability [88]. Structured exercise may be preferred over low intensity education based on the reduction in disability in nontransplant older adults [89]. Starting before transplant (prehabilitation) will likely maximize yield, although extended time for prehabilitation in AML patients may not be feasible. The conditioning regimen of transplant may still be an opportunity to gain strength and endurance and can be combined with prehabilitation. Medication avoidance can follow the updated Beers criteria and requires diligence [90].
Augmented social support can facilitate patient monitoring, compliance, and optimization across domains. Older adults often rely on older spouses or siblings who may have their own health issues. A family meeting of all potential caregivers may combat patient reluctance to enlist children or other social support. We routinely request a backup care-giver in case the primary caregiver becomes ill and inquire about the health of the primary caregiver. During anticipated cytopenia and toxicities from conditioning therapy (when such a regimen is used), a fortified caregiver presence (ie, 24/7 available) is a consideration.
GA-guided interventions
Based on the GA, specific vulnerabilities often emerge for which individualized therapy can be directed often by nonphysician team members (eg, dietician, physical therapist, social worker). For example, dentures may prompt regular dietician input and modified nutritional recommendations. Subtle or mild cognitive impairment justifies greater attention to medication avoidance, focus on sleep hygiene, and consideration of serial monitoring for delirium (including caregiver education) when hospitalized and/or geriatrics consultation. Weakness in a specific arm may guide placement of central venous catheters, modified exercises, initiation of an assist device (eg, cane, walker), and special instructions on rising from a bed or chair.
Timing
Time to transplant may be 1 of the most modifiable factors and a key reason to characterize fitness for transplant. The high rates of relapse and short disease-free survival in older adults justify moving quickly to allogeneic transplant for appropriate candidates. A common clinical dilemma for the vulnerable older AML patient is the tendency toward a sluggish pursuit of transplant because of uncertainty about the harms and benefits of transplant. Here, the decision-making process for transplant may be as important as the criteria used [91,92].
Future Directions
Transplant studies must ensure adequate representation of older adults and compare results with nontransplanted treatment strategies. Large prospective studies incorporating multidimensional tools such as GAs are required to better inform transplant candidacy. Finally, optimization strategies should be tested to reduce transplant-associated morbidity and perhaps extend transplant to older and more vulnerable AML patients.
Footnotes
Financial disclosure: The authors have nothing to disclose.
Conflict of interest statement: There are no conflicts of interest to report.
References
- 1.Surveillance E End Results (SEER) Program. [Accessed September 2016];SEER*Stat Database. Available at: www.seer.cancer.gov.
- 2.Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA. Future of cancer incidence in the united states: burdens upon an aging, changing nation. J Clin Oncol. 2009;27:2758–2765. doi: 10.1200/JCO.2008.20.8983. [DOI] [PubMed] [Google Scholar]
- 3.Kumar SK, Dispenzieri A, Lacy MQ, et al. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia. 2014;28:1122–1128. doi: 10.1038/leu.2013.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pulte D, Gondos A, Brenner H. Improvement in survival of older adults with multiple myeloma: results of an updated period analysis of SEER data. Oncologist. 2011;16:1600–1603. doi: 10.1634/theoncologist.2011-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brenner H, Gondos A, Pulte D. Recent major improvement in long-term survival of younger patients with multiple myeloma. Blood. 2008;111:2521–2526. doi: 10.1182/blood-2007-08-104984. [DOI] [PubMed] [Google Scholar]
- 6.Turesson I, Velez R, Kristinsson SY, Landgren O. Patterns of multiple myeloma during the past 5 decades: stable incidence rates for all age groups in the population but rapidly changing age distribution in the clinic. Mayo Clin Proc. 2010;85:225–230. doi: 10.4065/mcp.2009.0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Warren JL, Harlan LC, Stevens J, Little RF, Abel GA. Multiple myeloma treatment transformed: a population-based study of changes in initial management approaches in the United States. J Clin Oncol. 2013;31:1984–1989. doi: 10.1200/JCO.2012.46.3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Attal M, Blaise D, Marit G, et al. Consolidation treatment of adult acute lymphoblastic-leukemia—a prospective, randomized trial comparing allogeneic versus autologous bone-marrow transplantation and testing the impact of recombinant interleukin-2 after autologous bone-marrow transplantation. Blood. 1995;86:1619–1628. [PubMed] [Google Scholar]
- 9.Cavo M, Palumbo A, Zweegman S, et al. Upfront autologous stem cell transplantation (ASCT) versus novel agent-based therapy for multiple myeloma (MM): a randomized phase 3 study of the European Myeloma Network (EMN02/HO95 MM trial). Abstract no. 8000. Presented at the 2016 American Society of Clinical Oncology Annual Meeting; Chicago, IL. June 3, 2016. [Google Scholar]
- 10.Attal M, Lauwers-Cances V, Hulin C, et al. Autologous transplantation for multiple myeloma in the era of new drugs: a phase III study of the Intergroupe Francophone du Myelome (IFM/DFCI 2009 trial) Blood. 2015:126. [Google Scholar]
- 11.Wildes TM, Finney JD, Fiala M, et al. High-dose therapy and autologous stem cell transplant in older adults with multiple myeloma. Bone Marrow Transplant. 2015;50:1075–1082. doi: 10.1038/bmt.2015.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pasquini MC. [Accessed September 2016];ZXCuaoohsctCSS. 2015 Available at: http://www.cibmtr.org.
- 13.Offidani M, Leoni P, Corvatta L, et al. ThaDD plus high dose therapy and autologous stem cell transplantation does not appear superior to ThaDD plus maintenance in elderly patients with de novo multiple myeloma. Eur J Haematol. 2010;84:474–483. doi: 10.1111/j.1600-0609.2010.01418.x. [DOI] [PubMed] [Google Scholar]
- 14.Palumbo A, Rajkumar SV, San Miguel JF, et al. International Myeloma Working Group consensus statement for the management, treatment, and supportive care of patients with myeloma not eligible for standard autologous stem-cell transplantation. J Clin Oncol. 2014;32:587–600. doi: 10.1200/JCO.2013.48.7934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pal SK, Katheria V, Hurria A. Evaluating the older patient with cancer: understanding frailty and the geriatric assessment. CA Cancer J Clin. 2010;60:120–132. doi: 10.3322/caac.20059. [DOI] [PubMed] [Google Scholar]
- 16.Rodin MB, Mohile SG. A practical approach to geriatric assessment in oncology. J Clin Oncol. 2007;25:1936–1944. doi: 10.1200/JCO.2006.10.2954. [DOI] [PubMed] [Google Scholar]
- 17.Hamaker ME, Prins MC, Stauder R. The relevance of a geriatric assessment for elderly patients with a haematological malignancy—a systematic review. Leuk Res. 2014;38:275–283. doi: 10.1016/j.leukres.2013.12.018. [DOI] [PubMed] [Google Scholar]
- 18.Hurria A, Togawa K, Mohile S, et al. Predicting chemotherapy toxicity in older adults and the importance of geriatric assessment reply. J Clin Oncol. 2012;30:561–562. doi: 10.1200/JCO.2011.39.4858. [DOI] [PubMed] [Google Scholar]
- 19.Bringhen S, Mateos MV, Zweegman S, et al. Age and organ damage correlate with poor survival in myeloma patients: meta-analysis of 1435 individual patient data from 4 randomized trials. Haematologica. 2013;98:980–987. doi: 10.3324/haematol.2012.075051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palumbo A, Bringhen S, Mateos MV, et al. Geriatric assessment predicts survival and toxicities in elderly myeloma patients: an International Myeloma Working Group report. Blood. 2015;125:2068–2074. doi: 10.1182/blood-2014-12-615187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karnakis T, Gattas-Vernaglia IF, Saraiva MD, Gil-Junior LA, Kanaji AL, Jacob-Filho W. The geriatrician’s perspective on practical aspects of the multidisciplinary care of older adults with cancer. J Geriatr Oncol. 2016;7:341–345. doi: 10.1016/j.jgo.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 22.Caillet P, Paillaud E, Dupuis J. Malignant hematological diseases in the elderly. Soins Gerontol. 2011:45–46. [PubMed] [Google Scholar]
- 23.Hurria A, Cirrincione CT, Muss HB, et al. Implementing a geriatric assessment in cooperative group clinical cancer trials: CALGB 360401. J Clin Oncol. 2011;29:1290–1296. doi: 10.1200/JCO.2010.30.6985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hubbard JM, Cohen HJ, Muss HB. Incorporating biomarkers into cancer and aging research. J Clin Oncol. 2014;32:2611–2616. doi: 10.1200/JCO.2014.55.4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walston JD. Connecting age-related biological decline to frailty and late-life vulnerability. Nestle Nutr Inst Workshop Ser. 2015;83:1–10. doi: 10.1159/000382052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hubbard JM, Jatoi A. Incorporating biomarkers of frailty and senescence in cancer therapeutic trials. J Gerontol A Biol Sci Med Sci. 2015;70:722–728. doi: 10.1093/gerona/glu046. [DOI] [PubMed] [Google Scholar]
- 27.LeBrasseur NK, Tchkonia T, Kirkland JL. Cellular senescence and the biology of aging, disease, and frailty. Nestle Nutr Inst Workshop Ser. 2015;83:11–18. doi: 10.1159/000382054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Czesnikiewicz-Guzik M, Lee WW, Cui D, et al. T cell subset-specific susceptibility to aging. Clin Immunol. 2008;127:107–118. doi: 10.1016/j.clim.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Effros RB. The role of CD8 T cell replicative senescence in human aging. Discov Med. 2005;5:293–297. [PubMed] [Google Scholar]
- 30.Lu Y, Tan CT, Nyunt MS, et al. Inflammatory and immune markers associated with physical frailty syndrome: findings from Singapore longitudinal aging studies. Oncotarget. 2016;7:28783–28795. doi: 10.18632/oncotarget.8939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fahey JL, Schnelle JF, Boscardin J, et al. Distinct categories of immunologic changes in frail elderly. Mech Ageing Dev. 2000;115:1–20. doi: 10.1016/s0047-6374(00)00094-4. [DOI] [PubMed] [Google Scholar]
- 32.Chung DJ, Pronschinske KB, Shyer JA, et al. T cell exhaustion/senescence in relapsed multiple myeloma after autologous stem cell transplantation. Blood. 2015:126. [Google Scholar]
- 33.Bryant C, Suen H, Brown R, et al. Long-term survival in multiple myeloma is associated with a distinct immunological profile, which includes proliferative cytotoxic T-cell clones and a favourable Treg/Th17 balance. Blood Cancer J. 2013:3. doi: 10.1038/bcj.2013.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Owusu C, Cohen HJ, Feng T, et al. Anemia and functional disability in older adults with cancer. J Natl Compr Canc Netw. 2015;13:1233–1239. doi: 10.6004/jnccn.2015.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang HY, Yang H, Zhang CN, et al. Investigation of MicroRNA expression in human serum during the aging process. J Gerontol A Biol Sci Med Sci. 2015;70:102–109. doi: 10.1093/gerona/glu145. [DOI] [PubMed] [Google Scholar]
- 36.Jazbutyte V, Fiedler J, Kneitz S, et al. MicroRNA-22 increases senescence and activates cardiac fibroblasts in the aging heart. Age (Dordr) 2013;35:747–762. doi: 10.1007/s11357-012-9407-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boon RA, Iekushi K, Lechner S, et al. MicroRNA-34a regulates cardiac ageing and function. Nature. 2013;495:107–110. doi: 10.1038/nature11919. [DOI] [PubMed] [Google Scholar]
- 38.Hatse S, Brouwers B, Dalmasso B, et al. Circulating micrornas as easy-to-measure aging biomarkers in older breast cancer patients: correlation with chronological age but not with fitness/frailty status. PLoS One. 2014;9:e110644. doi: 10.1371/journal.pone.0110644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Milani P, Rajkumar SV, Merlini G, et al. N-terminal fragment of the type-B natriuretic peptide (NT-proBNP) contributes to a simple new frailty score in patients with newly diagnosed multiple myeloma. Am J Hematol. 2016;91:1129–1134. doi: 10.1002/ajh.24532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chung DJ, Pronschinske KB, Shyer JA, et al. T-cell exhaustion in multiple myeloma relapse after autotransplant: optimal timing of immuno-therapy. Cancer Immunol Res. 2016;4:61–71. doi: 10.1158/2326-6066.CIR-15-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu Y, Johnson SM, Fedoriw Y, et al. Expression of p16(INK4a) prevents cancer and promotes aging in lymphocytes. Blood. 2011;117:3257–3267. doi: 10.1182/blood-2010-09-304402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Melzer D, Frayling TM, Murray A, et al. A common variant of the p16(INK4a) genetic region is associated with physical function in older people. Mech Ageing Dev. 2007;128:370–377. doi: 10.1016/j.mad.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Helgadottir A, Thorleifsson G, Manolescu A, et al. A common variant on chromosome 9p21 affects the risk of myocardial infarction. Science. 2007;316:1491–1493. doi: 10.1126/science.1142842. [DOI] [PubMed] [Google Scholar]
- 44.Liu Y, Sanoff HK, Cho H, et al. Expression of p16(INK4a) in peripheral blood T-cells is a biomarker of human aging. Aging Cell. 2009;8:439–448. doi: 10.1111/j.1474-9726.2009.00489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.LaPak KM, Burd CE. The molecular balancing act of p16(INK4a) in cancer and aging. Mol Cancer Res. 2014;12:167–183. doi: 10.1158/1541-7786.MCR-13-0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosko AE, Hofmeister CC, Benson D, Efebera YA, Huang Y, Burd C. T-cell p16(INK4A) expression increases post-transplant in patients with multiple myeloma. Blood. 2014:124. [Google Scholar]
- 47.Sanoff HK, Deal AM, Krishnamurthy J, et al. Effect of cytotoxic chemotherapy on markers of molecular age in patients with breast cancer. J Natl Cancer Inst. 2014:106. doi: 10.1093/jnci/dju057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Song ZF, von Figura G, Liu Y, et al. Lifestyle impacts on the aging-associated expression of biomarkers of DNA damage and telomere dysfunction in human blood. Aging Cell. 2010;9:607–615. doi: 10.1111/j.1474-9726.2010.00583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rosko A, Hofmeister C, Benson D, et al. Autologous hematopoietic stem cell transplant induces the molecular aging of T-cells in multiple myeloma. Bone Marrow Transplant. 2015;50:1379–1381. doi: 10.1038/bmt.2015.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Juliusson G, Lazarevic V, Horstedt AS, Hagberg O, Hoglund M Swedish Acute Leukemia Registry G. Acute myeloid leukemia in the real world: why population-based registries are needed. Blood. 2012;119:3890–3899. doi: 10.1182/blood-2011-12-379008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jaiswal S, Fontanillas P, Flannick J, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371:2488–2498. doi: 10.1056/NEJMoa1408617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mrozek K, Marcucci G, Nicolet D, et al. Prognostic significance of the European LeukemiaNet standardized system for reporting cytogenetic and molecular alterations in adults with acute myeloid leukemia. J Clin Oncol. 2012;30:4515–4523. doi: 10.1200/JCO.2012.43.4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burnett AK, Russell NH, Hills RK, et al. A comparison of clofarabine with ara-C, each in combination with daunorubicin as induction treatment in older patients with acute myeloid leukaemia. Leukemia. 2016 doi: 10.1038/leu.2016.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pasquini MC, Zhu X. [Accessed September 2016];Current uses and outcomes of hematopoietic stem cell transplantation: CIBMTR Summary Slides. 2015 Available at: http://www.cibmtr.org.
- 55.Muffly L, Pasquini MC, Martens M, et al. Increasing use of allogeneic hematopoietic cell transplantation (HCT) in patients age 70 years and older: a CIBMTR study of trends and outcomes. Bone Marrow Transplant. 2016;22:S68–S69. [Google Scholar]
- 56.Oran B, Weisdorf DJ. Survival for older patients with acute myeloid leukemia: a population-based study. Haematologica. 2012;97:1916–1924. doi: 10.3324/haematol.2012.066100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bower H, Andersson TM, Bjorkholm M, Dickman PW, Lambert PC, Derolf AR. Continued improvement in survival of acute myeloid leukemia patients: an application of the loss in expectation of life. Blood Cancer J. 2016;6:e390. doi: 10.1038/bcj.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rashidi A, Ebadi M, Colditz GA, DiPersio JF. Outcomes of allogeneic stem cell transplantation in elderly patients with acute myeloid leukemia: a systematic review and meta-analysis. Biol Blood Marrow Transplant. 2016;22:651–657. doi: 10.1016/j.bbmt.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Devine SM, Owzar K, Blum W, et al. Phase II study of allogeneic transplantation for older patients with acute myeloid leukemia in first complete remission using a reduced-intensity conditioning regimen: results from Cancer and Leukemia Group B 100103 (Alliance for Clinical Trials in Oncology)/Blood and Marrow Transplant Clinical Trial Network 0502. J Clin Oncol. 2015;33:4167–4175. doi: 10.1200/JCO.2015.62.7273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Blaise D, Furst S, Crocchiolo R, et al. Haploidentical T cell-replete transplantation with post-transplantation cyclophosphamide for patients in or above the sixth decade of age compared with allogeneic hematopoietic stem cell transplantation from an human leukocyte antigen-matched related or unrelated donor. Biol Blood Marrow Transplant. 2016;22:119–124. doi: 10.1016/j.bbmt.2015.08.029. [DOI] [PubMed] [Google Scholar]
- 61.Kasamon YL, Bolanos-Meade J, Prince GT, et al. Outcomes of nonmyeloablative HLA-haploidentical blood or marrow transplantation with high-dose post-transplantation cyclophosphamide in older adults. J Clin Oncol. 2015;33:3152–3161. doi: 10.1200/JCO.2014.60.4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sandhu KS, Brunstein C, DeFor T, et al. Umbilical cord blood transplantation outcomes in acute myelogenous leukemia/myelodysplastic syndrome patients aged >/= 70 years. Biol Blood Marrow Transplant. 2016;22:390–393. doi: 10.1016/j.bbmt.2015.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Horan JT, Logan BR, Agovi-Johnson MA, et al. Reducing the risk for transplantation-related mortality after allogeneic hematopoietic cell transplantation: how much progress has been made? J Clin Oncol. 2011;29:805–813. doi: 10.1200/JCO.2010.32.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McClune BL, Weisdorf DJ, Pedersen TL, et al. Effect of age on outcome of reduced-intensity hematopoietic cell transplantation for older patients with acute myeloid leukemia in first complete remission or with myelodysplastic syndrome. J Clin Oncol. 2010;28:1878–1887. doi: 10.1200/JCO.2009.25.4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sorror ML, Sandmaier BM, Storer BE, et al. Long-term outcomes among older patients following nonmyeloablative conditioning and allogeneic hematopoietic cell transplantation for advanced hematologic malignancies. JAMA. 2011;306:1874–1883. doi: 10.1001/jama.2011.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Versluis J, Hazenberg CL, Passweg JR, et al. Post-remission treatment with allogeneic stem cell transplantation in patients aged 60 years and older with acute myeloid leukaemia: a time-dependent analysis. Lancet Haematol. 2015;2:e427–e436. doi: 10.1016/S2352-3026(15)00148-9. [DOI] [PubMed] [Google Scholar]
- 67.Hurria A, Mohile S, Gajra A, et al. Validation of a prediction tool for chemotherapy toxicity in older adults with cancer. J Clin Oncol. 2016;34:2366–2371. doi: 10.1200/JCO.2015.65.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol. 2011;29:3457–3465. doi: 10.1200/JCO.2011.34.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brunner AM, Kim HT, Coughlin E, et al. Outcomes in patients age 70 or older undergoing allogeneic hematopoietic stem cell transplantation for hematologic malignancies. Biol Blood Marrow Transplant. 2013;19:1374–1380. doi: 10.1016/j.bbmt.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 70.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sorror ML, Logan BR, Zhu X, et al. Prospective validation of the predictive power of the hematopoietic cell transplantation comorbidity index: a center for international blood and marrow transplant research study. Biol Blood Marrow Transplant. 2015;21:1479–1487. doi: 10.1016/j.bbmt.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vaughn JE, Storer BE, Armand P, et al. Design and validation of an augmented hematopoietic cell transplantation-comorbidity index comprising pretransplant ferritin, albumin, and platelet count for prediction of outcomes after allogeneic transplantation. Biol Blood Marrow Transplant. 2015;21:1418–1424. doi: 10.1016/j.bbmt.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sorror ML, Storb RF, Sandmaier BM, et al. Comorbidity-age index: a clinical measure of biologic age before allogeneic hematopoietic cell transplantation. J Clin Oncol. 2014;32:3249–3256. doi: 10.1200/JCO.2013.53.8157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Muffly LS, Kocherginsky M, Stock W, et al. Geriatric assessment to predict survival in older allogeneic hematopoietic cell transplantation recipients. Haematologica. 2014;99:1373–1379. doi: 10.3324/haematol.2014.103655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sorror ML. How I assess comorbidities before hematopoietic cell transplantation. Blood. 2013;121:2854–2863. doi: 10.1182/blood-2012-09-455063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Alousi AM, Le-Rademacher J, Saliba RM, et al. Who is the better donor for older hematopoietic transplant recipients: an older-aged sibling or a young, matched unrelated volunteer? Blood. 2013;121:2567–2573. doi: 10.1182/blood-2012-08-453860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Muffly LS, Boulukos M, Swanson K, et al. Pilot study of comprehensive geriatric assessment (CGA) in allogeneic transplant: CGA captures a high prevalence of vulnerabilities in older transplant recipients. Biol Blood Marrow Transplant. 2013;19:429–434. doi: 10.1016/j.bbmt.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 78.Holmes HM, Des Bordes JK, Kebriaei P, et al. Optimal screening for geriatric assessment in older allogeneic hematopoietic cell transplantation candidates. J Geriatr Oncol. 2014;5:422–430. doi: 10.1016/j.jgo.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Olin RL, Andreadis C, Martin TG, et al. Comprehensive geriatric assessment identifies significant functional impairments in older hematopoietic cell transplant recipients. Bone Marrow Transplant. 2014;20:S65–S66. [Google Scholar]
- 80.Wood WA, Le-Rademacher J, Syrjala KL, et al. Patient-reported physical functioning predicts the success of hematopoietic cell transplantation (BMT CTN 0902) Cancer. 2016;122:91–98. doi: 10.1002/cncr.29717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wood WA, Deal AM, Reeve BB, et al. Cardiopulmonary fitness in patients undergoing hematopoietic SCT: a pilot study. Bone Marrow Transplant. 2013;48:1342–1349. doi: 10.1038/bmt.2013.58. [DOI] [PubMed] [Google Scholar]
- 82.Jones LW, Devlin SM, Maloy MA, et al. Prognostic importance of pretransplant functional capacity after allogeneic hematopoietic cell transplantation. Oncologist. 2015;20:1290–1297. doi: 10.1634/theoncologist.2015-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Repetto L, Fratino L, Audisio RA, et al. Comprehensive geriatric assessment adds information to Eastern Cooperative Oncology Group performance status in elderly cancer patients: an Italian Group for Geriatric Oncology Study. J Clin Oncol. 2002;20:494–502. doi: 10.1200/JCO.2002.20.2.494. [DOI] [PubMed] [Google Scholar]
- 84.Klepin HD, Geiger AM, Tooze JA, et al. Geriatric assessment predicts survival for older adults receiving induction chemotherapy for acute myelogenous leukemia. Blood. 2013;121:4287–4294. doi: 10.1182/blood-2012-12-471680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cruz M, Covinsky K, Widera EW, Stijacic-Cenzer I, Lee SJ. Predicting 10-year mortality for older adults. JAMA. 2013;309:874–876. doi: 10.1001/jama.2013.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Artz AS, Logan BR, Zhu X, et al. Pre-transplant C-reactive protein (CRP), ferritin and albumin as biomarkers to predict transplant related mortality (TRM) after allogeneic hematopoietic cell transplant (HCT) Blood. 2014;124:422. [Google Scholar]
- 87.Pasquini MC, Logan B, Wu J, et al. Results of a phase III randomized, multi-center study of allogeneic stem cell transplantation after high versus reduced intensity conditioning in patients with myelodysplastic syndrome (MDS) or acute myeloid leukemia (AML): Blood and Marrow Transplant Clinical Trials Network (BMT CTN) 0901. Blood. 2015;126 LBA-8. [Google Scholar]
- 88.Persoon S, Kersten MJ, van der Weiden K, et al. Effects of exercise in patients treated with stem cell transplantation for a hematologic malignancy: a systematic review and meta-analysis. Cancer Treat Rev. 2013;39:682–690. doi: 10.1016/j.ctrv.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 89.Pahor M, Guralnik JM, Ambrosius WT, et al. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA. 2014;311:2387–2396. doi: 10.1001/jama.2014.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63:2227–2246. doi: 10.1111/jgs.13702. [DOI] [PubMed] [Google Scholar]
- 91.Randall J, Keven K, Atli T, Ustun C. Process of allogeneic hematopoietic cell transplantation decision making for older adults. Bone Marrow Transplant. 2016;51:623–628. doi: 10.1038/bmt.2015.241. [DOI] [PubMed] [Google Scholar]
- 92.Artz AS, Chow S. Hematopoietic cell transplantation in older adults: deciding or decision-making? Bone Marrow Transplant. 2016;51:643–644. doi: 10.1038/bmt.2016.86. [DOI] [PubMed] [Google Scholar]