Abstract
This short perspective assesses the present landscape for desktop 3D printing to design and fabricate sensors, in particular, those associated with microfluidics and multiplexing. Lots of advanced devices have already been reported, and this article briefly surveys interesting achievements. Microfluidics can be designed and optimized faster and more easily on low cost desktop 3D printers that with competing methods. Rapid prototyping leads directly to a final (marketable) product fabricated on the same 3D printer. While resolution is not as good as lithographic approaches, very often channel and feature resolution on the order of 100 μm obtainable with SLA 3D printers is perfectly suitable for the desired sensing device. Two examples from our team’s research are used to illustrate how using a 3D printer along with simple automation can reduce a complex microfluidic sensing procedure to a much simpler automated one. Future possibilities for sensor technology are discussed.
Keywords: 3D printing, sensors, microfluidics, biomarker proteins, DNA
Graphical abstract

We humans have long realized the value of materials printing. For example, one-dimensional screen printing, used extensively today for making electrodes as well as T-shirt designs, was first developed in Asia around 500 B.C.1 Additive manufacturing or 3D printing technologies emerged much later, in the mid-1980s, to complement earlier materials deposition techniques including ink jet and screen printing.
The first 3D printers were much too expensive to be widely used in developing routine analytical devices or sensor systems. However, about a decade ago, desktop 3D printers in the cost range $1000–4000 began to appear. These inexpensive printers offer revolutionary opportunities for rapidly designing, optimizing, and fabricating novel, high performance, low cost bioanalytical devices.2-5 Early on, 3D printing was viewed as mainly a rapid prototyping tool, but has now moved well beyond this stage, and advanced fabrications are quickly moving into production.6 Essentially, development of a series of ever improving device prototypes can lead to a final marketable product. Examples of nonsensor applications include precision machine and automotive parts,7 prosthetic implants,8,9 pharmaceutical, electronic, and bioresearch products,6,10-12 scaffolds for tissue engineering,13,14 and batteries.15 Community “fab labs” have emerged where local people can design and 3D print things for themselves using simple fabrication tools.16
Desktop 3D printing of polymers is simple, cheap, and versatile. Printing proceeds in an uninterrupted layer-by-layer fashion with resolution approaching the 50–100 μm range for printing a single layer. In our research team’s experience, 3D printing is well adapted for the fabrication of microfluidic single analyte and multiplexed sensor systems. Bioanalytical devices can be fabricated as complete 3D structures in a single step, enabling rapid prototyping and fabrication for sensors systems with accompanying microfluidic reagent and sample delivery. It is much faster to achieve optimized versions of such microfluidic platforms that with most competing techniques like photo- and soft lithography,17-19 precision cutting, or molding.20 Even though these competing methods have proved very reliable and successful, drawbacks of lithographic approaches include the need to fabricate a master or mask that can often involve significant cost, difficulty in accessing a mask fabrication device, and slow turn-around time.19 While soft lithography was developed by its innovators to be low cost,17-19 it is still a method that fabricates devices a layer at a time and can be somewhat labor intensive. Using a 3D printer, design and fabrication processes are under full control of the operator. The device plan is produced in silico using computer-aided design (CAD) software, processed with “slicing” software, uploaded to the printer. Devices are then ready to be printed.5 The printing is still layer-by-layer, but with no interruption between layers. If unexpected errors in the design arise, the CAD file can be modified, and corrected devices can be printed rapidly, often on the same day. On the other hand, unanticipated design problems using photo- or soft lithography approaches very often required fabricating a new master or mask, with associated cost and time lags. Rapid optimization by 3D-printing, often called rapid prototyping, is part of the reason for its increasing high popularity in addition to its low cost.
At present photo- and soft lithography have the capability to provide much better feature resolution than desktop 3D printing. However, some stereolithographic (SLA) 3D-printers can achieve channel widths approaching 150 μm and solid structural features as small as 95 μm at 0.35 μm roughness.21 These dimensions are well suited for many applications in microfluidics, and <100 μm resolution is not often needed for practical chemical or bioanalytical sensors. Other types of 3D printing also have respectable statistics, but resolution is not as good. Looking to the future, we may anticipate advances that result in improved 3D printer resolution, as well as speed.22
Different types of printers fabricate microfluidic devices with different properties, as well documented in a recent report.21 To investigate laminar flow and mixing, simple Y junctions with 500 μm internal diameters were printed and tested for efficiency in mixing two different input solutions. A fused deposition modeling (FDM)-printed device achieved complete mixing 15 mm past the Y junction for 25–100 μL/min flow rates, attributed to rough features within the printed Y. For the Polyjet printed Y, distance to complete mixing depended on flow rate, with complete mixing at 15 mm for 25 μL/min. SLA printers gave very low mixing at 25–100 μL/min, characteristic of low surface roughness. Thus, FDM seems to be best if you need good mixing, and SLA is the best choice when good adherence to laminar flow is needed.
Due to the practicality of soft polydimethylsiloxane (PDMS) for microfluidic devices, an early report (2002) described printing masks for PDMS soft-lithography microfluidics using a “solid object” printer.23 To illustrate progress in just the few years since then, PDMS itself has been printed recently using a Inkredible 3D printer and specialized PDMS ink to improve mechanical and cell adhesion properties. A diverse range of polymers are now possible to print,24 but most desktop 3D printers are designed for only a few standard choices. Notable achievements related to sensing include 3D printed smart phone adaptors for imaging and sensing,25-28 and metal ion,29 chemical,30 and gas31 sensor systems. Optically transparent devices can be 3D printed to facilitate sensing by light detection.32,33 A comprehensive review of 3D sensor technology has recently appeared.34 Other remarkable achievements related to measurement science include a low cost 3D printed scanning electrochemical microscope35 and 3D printed optics for surface plasmon resonance.36
Our research team is interested in making devices that enable molecular cancer diagnostics and chemical toxicity screening. Our focus involves low cost approaches to microfluidics, and we have naturally gravitated toward 3D printing in recent years. In the remainder of this Sensor Issues, two microfluidic systems developed in our lab using an SLA 3D-printer are briefly described to illustrate the ease of fabricating low cost functional microfluidic sensor devices that convert relatively complex, labor intensive multiplexed assays into procedures that require the operator only to add reagents and sample to the device, start the procedure, and complete a simple measurement. This is achieved with a degree of automation that can be as simple as using a commercial programmable syringe pump or building a micropump system controlled by an inexpensive microprocessor.
The first example is a chemiluminescence immunosensor array for the measurement of proteins in serum. The classic example of this type of measurement is the enzyme-linked immunosorbent assay (ELISA), still widely used for clinical protein-based diagnostics.37 Immunoassays are good examples of labor-intensive procedures that can be automated in multiplexed form by using microfluidics and automatic flow control. In multiplexed chemiluminescent immunoassays, we need a sensor chip spotted with multiple antibodies, one or more for each analyte protein. We then need to add the sample, detection antibodies with associated labels for each analyte protein, and one or more reagents to develop the signal. Between each of these steps, extensive washed with buffers that may contain detergents and blocking proteins such as bovine serum albumin or casein are needed to minimize nonspecific binding (NSB). Detection by chemiluminescence (CL) can be done with a low-light sensitive charge-coupled device (CCD) camera after signal-developing reagents are added.
Figure 1 illustrates a 3D printed microfluidic device designed to hold sample, all necessary reagents, and wash buffer in the exact quantities needed.38 It is bonded to an antibody array in a detection chamber and interfaced with a syringe pump programmed to deliver sample and reagents, and stop for incubations at the correct time to facilitate binding events on the sensor array. The operator simply loads the sample and reagents into their chambers, starts the programmed pump, and makes a simple camera measurement at the end.
Figure 1.
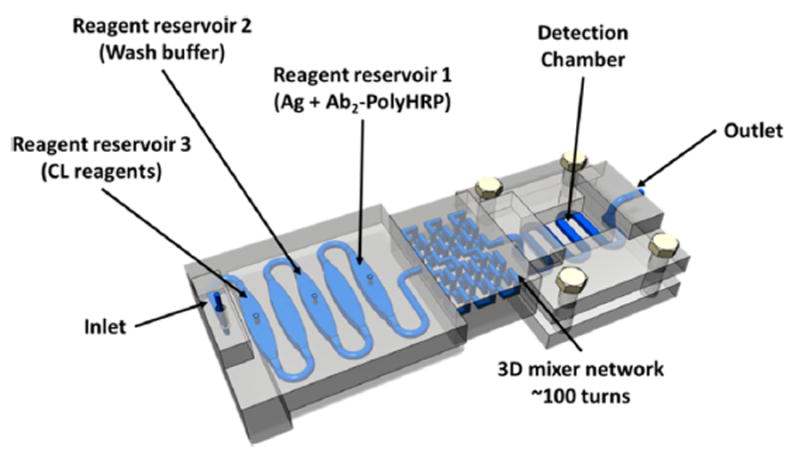
Model of 3D-printed unibody immunoarray for automated detection of proteins (Ag = protein antigen) by chemiluminescence (CL). Reprinted with permission from Tang, C.; Vaze, A.; Rusling, J. Automated 3D-printed unibody immunoarray for chemiluminescence detection of cancer biomarker proteins. Lab on a Chip, 2017, 17, 484–489. Copyright Royal Society of Chemistry, 2017.
Three 125 μL reservoirs that hold sample and reagents on the left end of the device (Figure 1) are separated by equal volume air-filled chambers to prevent unwanted mixing of solutions. A 3D network mixer was located downstream of the reservoirs to ensure complete mixing of solutions that pass through to the 30 μL serpentine-channel detection channel. A replaceable poly(L-lysine) coated glass slide decorated with the appropriate capture antibody (Ab1) spots is housed in a detection chamber and bonded to a serpentine channel under which the capture antibody (Ab1) spots on the chip lie. A transparent quartz window covers the detection chamber.38
Solutions of detection antibodies (Ab2) tagged with biotin and a streptavidin poly(horseradish peroxidase) (polyHRP) label are premixed to make Ab2-polyHPR conjugates, and then loaded into reservoir 1. Wash buffer of 0.5% casein and 0.05% Tween-20 in PBS is loaded into reservoir 2, and 1:1 diluted FemtoWest chemiluminescence (CL) cocktail is loaded into reservoir 3.40 Then, 10 μL of sample or standard (in dilute calf serum) is added into reservoir 1, and the pump system is activated. The sample/label/antibody mixture in reservoir 1 passes through the mixer first, and is delivered to fill the serpentine channel on the sensor chip, and then the pump stops for 15 min to allow incubation. During this period, Ab2-polyHPR-analyte-protein conjugates that have formed in the mixer find their respective cognate Ab1 spots on the sensor chip and bind. Then, the pump resumes flow and washing occurs, followed by filling the detection channel with CL reagents, and immediate CCD camera measurement for 60 s. Typical calibration data output is shown in Figure 2. The entire assay takes 30 min, and detection limits of ~0.5 pg/mL were achieved for prostate specific antigen (PSA) and platelet factor-4 (PF-4) in diluted calf serum.38 Results for human serum samples showed good correlation with ELISA assays.
Figure 2.
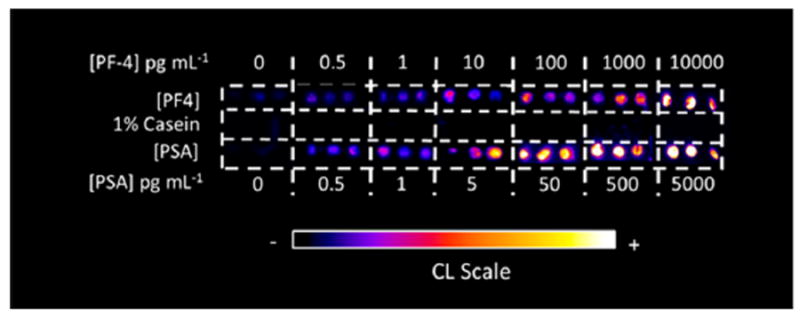
Recolorized, reformatted CL output obtained from the automated 3D printed immunoarray. Each concentration was measured in triplicate for a mixture of standard proteins prostate specific antigen (PSA) and platelet factor-4 (PF-4) in dilute calf serum.
In the second example, presented in less detail, an automated 3D printed microfluidic array was developed to assess potential genotoxicity from metabolites of chemicals that might be present in environmental samples.39 Here, a 3D printed microfluidic system (Figure 3) simply delivers aqueous samples to a microwell array containing films of metabolic enzymes, a Ru(polyvinylpyridine) (RuPVP) polymer, and DNA where enzyme reactions and detection occur. Different enzymes can be placed in different wells to metabolize chemicals in the sample. If the chemical or its metabolites damage DNA in the wells by adduct reactions or strand breaks, increased electrochemiluminescence (ECL) is obtained from RuPVP, which uses guanine moieties in the DNA as co-reactants. Damaged DNA provides more ECL light from the film than intact ds-DNA because the guanine moieties on the damaged, disordered DNA are more readily available to Ru sites in the polymer to increase the catalytic rate of ECL production.40 Sample or standard solutions flow into the microwells on the array and are metabolized there by enzymes during a flow incubation period. Reactive metabolites will react with DNA in the same wells, and DNA damage is detected by ECL with a CCD camera. The rate of increase of ECL is proportional to the rate of DNA damage.40 This particular array analyzes 3 samples simultaneously in 5 min, but throughput can be easily increased by redesigning the microfluidic delivery system and array. We used this device to compare cigarette and e-cigarette smoke extracts and found that DNA-damage from e-cigarettes was about the same as from unfiltered cigarettes. In another application, wastewater showed a high DNA damage rate that was lowered to acceptable levels by pollution treatment plant processing.39
Figure 3.
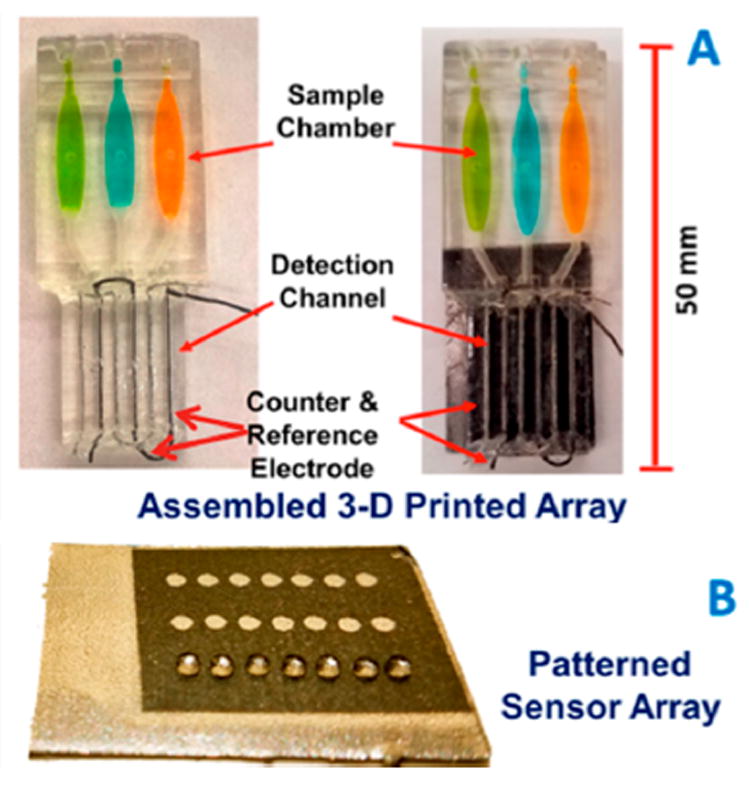
Automated genotoxicity screening array: (A) 3D printed arrays without (left) and with (right) microwell chip with sample chambers containing dye solutions; (B) microwell-patterned pyrolytic graphite array. First row holds 1 μL water droplets retained by hydrophobic microwell boundaries. Microwells hold films of DNA, various metabolic enzymes, and RuPVP. A microprocessor controls 3 micropumps (one per channel) to analyze 3 samples per assay. Adapted from Kadimisetty, K.; Malla, S.; Rusling, J. F. Automated 3-D Printed Arrays to Evaluate Genotoxic Chemistry: E-Cigarettes and Water Samples. ACS Sensors 2017, 2, 670–678. Copyright American Chemical Soc., 2017.
The examples above are meant to illustrate the ease and low cost of fabrication of 3D printed microfluidic systems that can convert relatively complicated experimental assays into trouble free automated processes. Multiplexing is easy to achieve, and both system can easily be redesigned to analyze a larger collection of analytes or samples. Cost of both devices is less than $1.00, with all the optimization advantages mentioned in earlier paragraphs of this Sensor Issues. Automation of these microfluidic approaches is also easier and cheaper than ever, and can utilize a commercial programmable pump or home-built microprocessor-pump system. In many cases, low cost batteries39 or even a supercapacitor (for ECL detection)41 can power detection. One drawback at present is illustrated in both systems. That is, the detection array needs to be fabricated separately and bonded with adhesive onto the 3D-printed microfluidic device.
Both examples above utilize detection systems and external pumps interfaced to a 3D-printed microfluidic unit. In Figure 1, detection chips containing antibodies need to be secured leak-free to the microfluidic device. It would be excellent to have a 3D printer with the capability of making detection and microfluidics parts of the device in a single unit, e.g., a combined 3D–1D printer. However, how can we attach the antibodies to sensor spots or microwells if the system is closed? In some sensor devices, we may also want to assemble nanomaterials into sensor microwells to enhance sensitivity, e.g., for determination of low abundance proteins in serum.42 Multiple-material 3D printers are starting to appear on the market,43 but full assembly of a high sensitivity CL or ECL microfluidic immunoarray would need to print 3 very different types of features and integrate them together. These include printing polymer for the microfluidics, printing a conductive material like some form of graphite with microwells for sensing, then printing nanomaterial films in the sensor microwells and attaching antibodies. The ability to do these types of things routinely for device fabrication would be a major advance that we may perhaps anticipate in the next 5–10 years.
So, what about incorporating pumps or even 3D-printed pumps into a microfluidic sensor device? This is certainly an option, and 3D-printed pump designs have been published.44,45 Pumps should be cheap, reliable, and easy to print, and not add cost to the analytical system. In microfluidic systems designed for bioanalysis of human fluids, e.g., blood, there is an advantage to using external pumps and a very low cost disposable microfluidic sensor unit that can be destroyed after use to avoid pathogen exposure issues. A preprogrammed external pumping system, used in Figure 3, has the advantage of readily accepting plug-in microfluidic units for multiple repetitive uses. On-board pumps also raise the issue of device-to-device flow reproducibility. As in conventional microfluidics, the designer must weigh performance, cost, and ease of fabrication, and simplicity of use to design the appropriate system that fits the sensor problem at hand. As illustrated above, this is where 3D printing shines due to the ease of simple and rapid design optimization!
In closing, our research team has great future plans for 3D printing of sensors with microfluidics, particularly in low cost, multiplexed tools for clinical and point-of-care protein-based disease diagnostics and chemical and drug toxicity screening. A driving force for this work is to bring potentially life-saving multiplexed molecular bioanalysis into hospital and point-of-care environments at a cost that our already expensive medical system can easily bear.
We hope that this article stimulates researchers including beginning graduate and undergraduate students to explore 3D-printing capabilities. From a practical viewpoint, you no longer need sophisticated and expensive machines to do microfluidics. Relatively complex systems can be designed in an afternoon, and printed the next day! Design ideas can be tested by printing them, and error corrections are fast. Desktop 3D printers provide an easy to use platform to interface your creativity with advanced fabrication abilities, and really, the sky (or your imagination) is the limit.
Acknowledgments
The author thanks NIH for Grant No. ES03154 from the National institute of Environmental Health Sciences (NIEHS), and No. EB016707 from the National Institute of Biomedical Imaging and Bioengineering (NIBIB), and The University of Connecticut for an Academic Plan Project Grant for financial support in preparing this article.
Footnotes
Notes
The author declares no competing financial interest.
References
- 1. [01/01/2018];Silk screen printing. http://www.scholastic.com/browse/article.jsp?id=3754174.
- 2.Gross BC, Erkal JL, Lockwood SY, Chen C, Spence DM. Evaluation of 3D printing and its potential impact on biotechnology and the chemical sciences. Anal Chem. 2014;86:3240–3253. doi: 10.1021/ac403397r. [DOI] [PubMed] [Google Scholar]
- 3.Meng C, Ho B, Ng SH, Ho K, Li H, Yoon Y-J. 3D printed microfluidics for biological applications. Lab Chip. 2015;15:3627–3637. doi: 10.1039/c5lc00685f. [DOI] [PubMed] [Google Scholar]
- 4.O’Neill PF, Ben Azouz A, Vázquez M, Liu J, Marczak S, Slouka Z, Chang HC, Diamond D, Brabazon D. Advances in three-dimensional rapid prototyping of microfluidic devices for biological applications. Biomicrofluidics. 2014;8:052112. doi: 10.1063/1.4898632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bishop GW, Satterwhite-Warden JE, Kadimisetty K, Rusling JF. 3D-printed Bioanalytical Devices. Nanotechnology. 2016;27:284002. doi: 10.1088/0957-4484/27/28/284002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MacDonald E, Wicker R. Multiprocess 3D printing for increasing component functionality. Science. 2016;353:aaf2093. doi: 10.1126/science.aaf2093. [DOI] [PubMed] [Google Scholar]
- 7.(a) [01/23/18];3D printed aircraft parts. http://www.stratasys.com/resources/case-studies/aerospace/piper-aircraft.; (b) [01/23/18];3D printed automotive parts. http://www.stratasys.com/industries/automotive.
- 8.Mannoor MS, Jiang Z, James T, Kong YL, Malatesta KA, Soboyejo WO, Verma N, Gracias DH, McAlpine MC. 3D printed bionic ears. Nano Lett. 2013;13:2634–2639. doi: 10.1021/nl4007744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol. 2014;32:773–785. doi: 10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- 10.Liu C, Liao S, Song J, Mauk MG, Li X, Wu G, Ge D, Greenberg RM, Yang S, Bau HH. A high-efficiency superhydrophobic plasma separator. Lab Chip. 2016;16:553–560. doi: 10.1039/c5lc01235j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. [01/23/18];3D Printing Orthotic and Prosthetic Devices. http://www.sme.org/MEMagazine/Article.aspx?id=8589935763&taxid=1426.
- 12.Macdonald E, Salas R, Espalin D, Perez M, Aguilera E, Muse D, Wicker RB. 3D printing for the rapid prototyping of structural electronics. IEEE Access. 2014;2:234–242. [Google Scholar]
- 13.Wang MO, Vorwald CE, Dreher ML, Mott EJ, Cheng M, Cinar A, Mehdizadeh H, Somo S, Dean D, Brey EM. Evaluating 3D-Printed biomaterials as scaffolds for vascularized bone tissue engineering. Adv Mater. 2015;27:138–144. doi: 10.1002/adma.201403943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bose S, Vahabzadeh S, Bandyopadhyay A. Bone tissue engineering using 3D printing. Mater Today. 2013;16:496–504. [Google Scholar]
- 15.Sun K, Wei T, Ahn BY, Seo JY, Dillon SJ, Lewis JA. 3D printing of interdigitated Li-Ion microbattery architectures. Adv Mater. 2013;25:4539–4543. doi: 10.1002/adma.201301036. [DOI] [PubMed] [Google Scholar]
- 16.Shapira P. Making the Future. Science. 2017;358:1007. [Google Scholar]
- 17.Whitesides GM, Ostuni E, Takayama S, Jiang X, Ingber DE. Soft lithography in biology and biochemistry. Annu Rev Biomed Eng. 2001;3:335–373. doi: 10.1146/annurev.bioeng.3.1.335. [DOI] [PubMed] [Google Scholar]
- 18.Duffy DC, McDonald JC, Schueller OJ, Whitesides GM. Rapid prototyping of microfluidic systems in poly (dimethylsiloxane) Anal Chem. 1998;70:4974–4984. doi: 10.1021/ac980656z. [DOI] [PubMed] [Google Scholar]
- 19.Qin D, Xia Y, Whitesides GM. Soft lithography for micro-and nanoscale patterning. Nat Protoc. 2010;5:491–502. doi: 10.1038/nprot.2009.234. [DOI] [PubMed] [Google Scholar]
- 20.Chikkaveeraiah BV, Mani V, Patel V, Gutkind JS, Rusling JF. Microfluidic electrochemical immunoarray for ultra-sensitive detection of two cancer biomarker proteins in serum. Biosens Bioelectron. 2011;26:4477–4483. doi: 10.1016/j.bios.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Macdonald NP, Cabot JM, Smejkal P, Guijt RM, Paull B, Breadmore MC. Comparing Microfluidic Performance of Three-Dimensional (3D) Printing Platforms. Anal Chem. 2017;89:3858–3866. doi: 10.1021/acs.analchem.7b00136. [DOI] [PubMed] [Google Scholar]
- 22.Tumbleston JR, Shirvanyants D, Ermoshkin N, Janusziewicz R, Johnson AR, Kelly D, Chen K, Pinschmidt R, Rolland JP, Ermoshkin A, Samulski ET, DeSimone JM. Continuous liquid interface production of 3D objects. Science. 2015;347:1349–1352. doi: 10.1126/science.aaa2397. [DOI] [PubMed] [Google Scholar]
- 23.McDonald JC, Chabinyc ML, Metallo SJ, Anderson JR, Stroock AD, Whitesides GM. Prototyping of microfluidic devices in poly (dimethylsiloxane) using solid-object printing. Anal Chem. 2002;74:1537–1545. doi: 10.1021/ac010938q. [DOI] [PubMed] [Google Scholar]
- 24.Ligon SC, Liska R, Stampfl J, Gurr M, Mülhaupt R. Polymers for 3D Printing and Customized Additive Manufacturing. Chem Rev. 2017;117:10212–10290. doi: 10.1021/acs.chemrev.7b00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liao S, Peng J, Mauk MG, Awasthi S, Song J, Friedman H, Bau HH, Liu C. Smart cup: a minimally-instrumented, smartphone-based point-of-care molecular diagnostic device. Sens Actuators, B. 2016;229:232–238. doi: 10.1016/j.snb.2016.01.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coskun AF, Wong J, Khodadadi D, Nagi R, Tey A, Ozcan A. A personalized food allergen testing platform on a cellphone. Lab Chip. 2013;13:636–640. doi: 10.1039/c2lc41152k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berg B, Cortazar B, Tseng D, Ozkan H, Feng S, Wei Q, Chan RY, Burbano J, Farooqui Q, Lewinski M. Cellphone-based hand-held microplate reader for point-of-care testing of enzyme-linked immunosorbent assays. ACS Nano. 2015;9:7857–7866. doi: 10.1021/acsnano.5b03203. [DOI] [PubMed] [Google Scholar]
- 28.Contreras-Naranjo JC, Wei Q, Ozcan A. Mobile phone-based microscopy, sensing, and diagnostics. IEEE J Sel Top Quantum Electron. 2016;22:392–405. [Google Scholar]
- 29.Su C, Hsia S, Sun Y. Three-dimensional printed sample load/inject valves enabling online monitoring of extracellular calcium and zinc ions in living rat brains. Anal Chim Acta. 2014;838:58–63. doi: 10.1016/j.aca.2014.06.037. [DOI] [PubMed] [Google Scholar]
- 30.Erkal JL, Selimovic A, Gross BC, Lockwood SY, Walton EL, McNamara S, Martin RS, Spence DM. 3D printed microfluidic devices with integrated versatile and reusable electrodes. Lab Chip. 2014;14:2023–2032. doi: 10.1039/c4lc00171k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stach R, Haas J, Tütüncü E, Daboss S, Kranz C, Mizaikoff B. PolyHWG: 3D Printed Substrate-Integrated Hollow Waveguides for Mid-Infrared Gas Sensing. ACS Sens. 2017;2:1700–1705. doi: 10.1021/acssensors.7b00649. [DOI] [PubMed] [Google Scholar]
- 32.Shallan AI, Smejkal P, Corban M, Guijt RM, Breadmore MC. Cost-Effective Three-Dimensional Printing of Visibly Transparent Microchips within Minutes. Anal Chem. 2014;86:3124–3130. doi: 10.1021/ac4041857. [DOI] [PubMed] [Google Scholar]
- 33.Bishop GW, Satterwhite-Warden JE, Bist I, Chen E, Rusling JF. Electrochemiluminescence at bare and DNA-coated graphite electrodes in 3D-printed fluidic devices. ACS Sens. 2016;1:197–202. doi: 10.1021/acssensors.5b00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu Y, Wu X, Guo X, Kong B, Zhang M, Qian X, Mi S, Sun W. The Boom in 3D-Printed Sensor Technology. Sensors. 2017;17:37. doi: 10.3390/s17051166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meloni GN. 3D Printed and Microcontrolled: The One Hundred Dollars Scanning Electrochemical Microscope. Anal Chem. 2017;89:8643–8649. doi: 10.1021/acs.analchem.7b01764. [DOI] [PubMed] [Google Scholar]
- 36.Hinman SS, McKeating KS, Cheng Q. Plasmonic Sensing with 3D Printed Optics. Anal Chem. 2017;89:12626–12630. doi: 10.1021/acs.analchem.7b03967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barry MJ. Prostate-specific–antigen testing for early diagnosis of prostate cancer. N Engl J Med. 2001;344:1373–1377. doi: 10.1056/NEJM200105033441806. [DOI] [PubMed] [Google Scholar]
- 38.Tang C, Vaze A, Rusling J. Automated 3D-printed unibody immunoarray for chemiluminescence detection of cancer biomarker proteins. Lab Chip. 2017;17:484–489. doi: 10.1039/c6lc01238h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kadimisetty K, Malla S, Rusling JF. Automated 3-D Printed Arrays to Evaluate Genotoxic Chemistry: E-Cigarettes and Water Samples. ACS Sensors. 2017;2:670–678. doi: 10.1021/acssensors.7b00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hvastkovs EG, Rusling JF. State-of-the-Art Metabolic Toxicity Screening and Pathway Evaluation. Anal Chem. 2016;88:4584–4599. doi: 10.1021/acs.analchem.5b04772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kadimisetty K, Mosa IM, Malla S, Satterwhite-Warden JE, Kuhns TM, Faria RC, Lee NH, Rusling JF. 3D-printed supercapacitor-powered electrochemiluminescent protein immunoarray. Biosens Bioelectron. 2016;77:188–193. doi: 10.1016/j.bios.2015.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rusling JF, Kadimisetty K, Malla S, Bishop GW, Satterwhite-Warden JE. Low cost 3D-Printed Biosensor Arrays for Protein-based Cancer Diagnostics based on Electrochemiluminescence. Biodevices; Proceedings of the 9th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2016); pp. 17–22. [DOI] [Google Scholar]
- 43. [02/26/18]; https://3dprint.com/tag/multi-material-3d-printer/
- 44. [02/26/18]; https://pdfs.semanticscholar.org/a0e8/8c193d398d43f1cc58706fac42d5717d3427.pdf.
- 45.Alam MN, Hossain F, Vale A, Kouzani A. Design and Fabrication of a 3D Printed Miniature Pump for Integrated Microfluidic Applications. Int J Precis Eng & Manuf. 2017;18:1287–1296. [Google Scholar]


