Abstract
Catalytic cross-metathesis is a central transformation in chemistry, and yet, corresponding methods for stereoselectively generating acyclic trisubstituted alkenes in either isomeric form do not exist. The key problems are lack of chemoselectivity, namely, the preponderance of side reactions involving only the less hindered starting alkene, ensuing nonproductive processes of homo-metathesis byproducts, and formation of short-lived methylidene complexes. In contrast, in catalytic cross-coupling, another widely used process, substrates are more distinct and homocoupling is less of a problem. Here, we show that through cross-metathesis reactions involving E- or a Z-trisubstituted alkenes, easily prepared from commercially available starting materials by cross-coupling processes, many otherwise desirable and difficult-to-access linear E- or Z-trisubstituted alkenes can be synthesized efficiently and in exceptional stereoisomeric purity (up to >98% E or 95% Z). Utility is highlighted through concise stereoselective syntheses of biologically active compounds such as indiacen B (anti-fungal) and coibacin D (anti-inflammatory).
Linear E- and Z-trisubstituted alkenes occur widely in nature and are used regularly in preparative chemistry1,2 (for example, in catalytic enantioselective hydrogenations3, allylic substitutions4, or conjugate additions5). Several approaches have been developed for generating acyclic trisubstituted alkenes, but these have key shortcomings. Unless an α-alkoxy ketone is involved6, Wittig-type transformations are minimally stereoselective7,8. Protocols for converting alkynes or carbonyl-containing compounds to trisubstituted alkenes entail lengthy sequences9,10,11, strongly acidic or basic conditions10,11,12,13, and/or just one stereoisomer12,14 can be accessed (see the Supplementary Information, Section 1, for extended bibliography). The higher energy Z isomers can be obtained only if there is a suitable directing group15,16. There are no catalytic, high yielding, broadly applicable, and stereoselective methods for formation of trisubstituted alkenes, particularly in either stereoisomeric form. Especially desirable would be strategies that provide access to E- as well as Z-trisubstituted alkenyl chlorides and bromides, which are found in biologically active natural products17, and may be used to access countless other alkenes through cross-coupling.
The main challenges
There are only a small number of reports on synthesis of trisubstituted alkenes by cross-metathesis18,19,20,21. In just two cases stereoisomerism is a concern18,20, and, in each instance, reactions are either minimally selective or afford the E isomer preferentially because stereoselectivity results from substrate control.
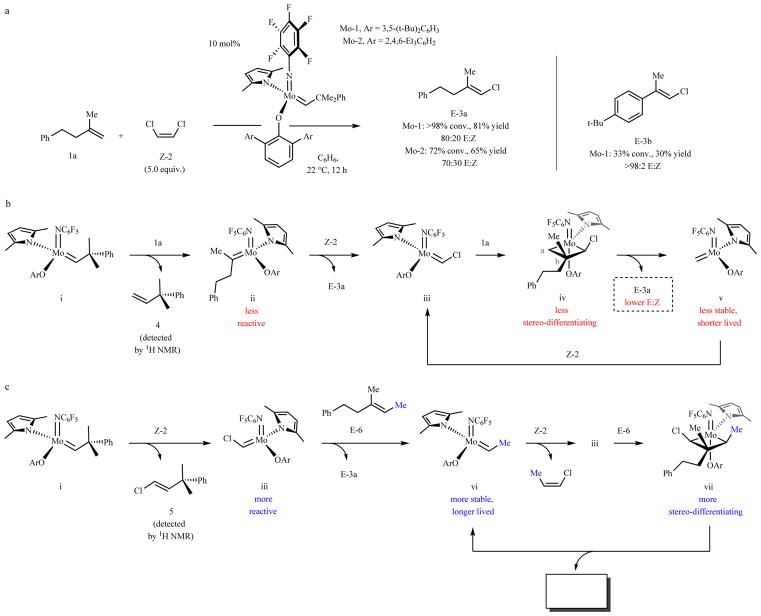
Designing methods for kinetically controlled E- or Z-selective22,23 synthesis of trisubstituted alkenes is difficult24 for several reasons. The metallacyclobutane intermediates are relatively hindered, and there is a smaller energy differences between the E and Z isomers25 (compared to 1,2-disubstituted alkenes). There is also an inherent lack of chemoselectivity: in cross-metathesis, when a trisubstituted alkene is desired, typically one starting material is a monosubstituted and the other a 1,1-disubstituted alkene, both containing an unsubstituted terminal alkenyl methylene unit. Consequently, ethylene can be generated as the byproduct of cross-metathesis or due to homo-metathesis of the less hindered/more reactive reaction partner. Ethylene formation leads to an unstable methylidene complex26, causing low turnover numbers and/or frequencies. It was therefore not surprising that reaction of 1,1-disubstituted alkene 1a with Z-1,2-dichloroethene (Z-2; Fig. 1a) needed 10 mol% Mo-1 or Mo-2 along with 12 hours to furnish 3a in 81% and 65% yield with moderate stereoselectivity (80:20 and 70:30 E:Z, respectively); control experiments indicated minimal post-metathesis isomerization. The transformation involving 4-tert-butyl-α-methyl styrene was less efficient (3b, 30% yield) but more stereoselective, owing to better substrate control.
Fig. 1. The challenge of developing stereoselective trisubstituted alkene cross-metathesis.
a, Reaction between 1,1-disubstituted alkene 1a and Z-2 required 10 mol% loading for ≥72% conversion in 12 hours, affording E-3a in ≤80:20 E:Z ratio. Formation of E-3b was sluggish but more stereoselective due to substrate control. b, Inefficiency and low stereoselectivity is probably low stability of methylidene v and minimal size difference between the substituents in 1a. c, With a trisubstituted alkene (E-6), catalysis is initiated by reaction with Z-2 to generate iii, which is more robust than a methylidene complex. Moreover, the intermediacy of metallacyclobutane vii (vs. iv), should lead to superior stereoselectivity. Conv. and isomeric ratios determined by analysis of 1H NMR spectra of unpurified mixtures; yields are for isolated and purified products. See the Supplementary Information for details.
The above transformations begin with monosubstituted alkene 4 being generated exclusively (Fig. 1b), revealing that initiation entails reaction of Mo complex i with 1a (not Z-2) to give disubstituted alkylidene ii. Reaction of ii with Z-2 may subsequently lead to the putative chloro-substituted alkylidene iii27, which may then react with 1a to give methylidene v and 3a via metallacyclobutane iv, with the quaternary carbon center at the less hindered Cβ28 (for more detailed analysis, see Extended Data Fig. 1). Hence, despite the absence of a terminal alkene, sufficient ethylene is generated so that the short lifetime of methylidene species v translate to the need for high catalyst loadings and extended reaction times. High E selectivity is possible only when one Cβ substituent in iv is much larger.
More highly substituted alkenes as substrates
Use of a trisubstituted alkene, such as E-6 (Fig. 1c) could improve efficiency and stereoselectivity (Fig. 1c). Complex i would react first with Z-1,2-dichloroethene (Z-2 vs. E-6) to afford chloro-alkylidene iii; indeed, treatment of a mixture of Z-2 and E-6 with Mo-1 or Mo-2 generated chloro-alkene 5 exclusively (based on 1H NMR analysis). Reaction via metallacyclobutane vii would be more stereoselective compared to the less substituted iv, because the competing addition mode would yield a less stable metallacyclobutane with the Cα methyl group oriented towards the larger aryloxide ligand. Another advantage would be the intermediacy of ethylidene vi, as opposed to methylidene (v), leading to longer catalyst lifetime and improved efficiency. If successful, a solution would come to light based on the counterintuitive principle that efficiency and stereoselectivity can be improved by using a more hindered substrate.
The possibility of a trisubstituted alkene substrate poses new challenges. One is the need to promote efficient reactions of more highly substituted alkenes, and if trisubstituted alkenes are difficult to obtain, why consider using them as starting materials? The answer to the latter question is that some trisubstituted alkenes are easy to synthesize from readily available starting materials by catalytic cross-coupling.
E- and Z-trisubstituted alkenyl chlorides
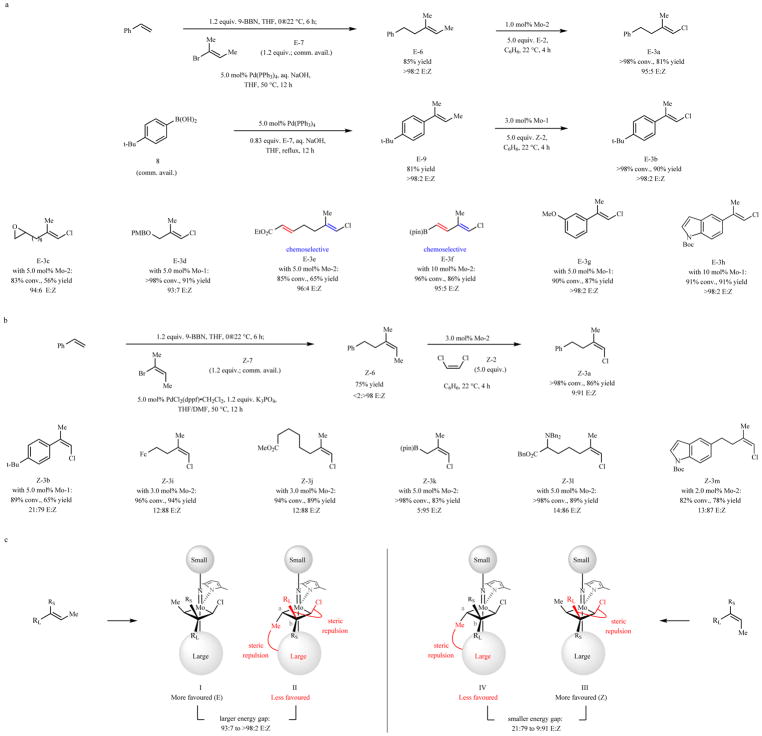
We prepared E-6 (Fig. 2a)29 by hydroboration of styrene and cross-coupling of the resulting alkylborane with commercially available E-2-bromo-2-butene (E-7; 85% yield, >98% E). Subjection of E-6 and E-2 (used without purification) to 1.0 mol% Mo-2 afforded E-3a in 81% yield (>98% conv.) and 95:5 E:Z selectivity after just four hours (compared to 65% yield and 70:30 E:Z, 10 mol% Mo-2, 12 h); reaction with Z-2 led to similar stereoselectivity but yield was lower (50%). Cross-coupling of arylboronic acid 8, which is purchasable, and E-7 delivered E-9 in 81% yield (>98% E); ensuing cross-metathesis with 3.0 mol% Mo-1 and Z-2 afforded E-3b in 90% yield and >98% stereoretention after four hours (compared to 30% yield, 10 mol% Mo-1, 12 h).
Fig. 2. Synthesis of Z- and E-Trisubstituted alkenyl chlorides.
a, E-Trisubstituted alkene substrates can be accessed by hydroboration of a monosubstituted alkene followed by cross-coupling with E-7. Subsequent cross-metathesis with Z- or E-2 was highly efficient, affording products in exceptional isomeric purity. Synthesis of E-3b shows an alternative way of merging cross-coupling and cross-metathesis. The approach is broadly applicable. b, Z-trisubstituted alkenyl chlorides may be accessed efficiently. c, Differences in steric pressure in metallacyclobutanes leading to E- and Z-trisubstituted alkene products provides an explanation for why transformations leading to the latter isomers are less selective: the energy difference between I and II (leading to E isomers) is larger than that separating III and IV (to give Z isomers). Conv. and isomeric ratios determined by analysis of 1H NMR spectra of unpurfied mixtures; yields are for isolated and purified products. See the Supplementary Information for details. Boc, tert-butoxycarbonyl; Fc, ferrocenyl; pin, pinacolato; Bn, benzyl; RL, larger substituent; RS, smaller substituent, PMB, para-methoxybenzyl; dppf, 1,1′-bis(diphenylphosphino)ferrocene.
E-Trisubstituted alkenyl chlorides 3c-h (Fig. 2a) were isolated in 56–91% yield and 93:7 to >98:2 E:Z selectivity. The trialkylaluminum reagents necessary for zirconocene-catalyzed carbometallation approach are not compatible with an epoxide30 (see 3c; lower yield due to difficult purification), a carboxylic ester (see 3e), a B(pin) (pin, pinacolato) group (see 3f) or a Boc-protected (Boc, tert-butoxycarbonyl) indole moiety31 (see 3h). Reactions leading to dienes 3e-f were chemoselective, as cross-metathesis involving the electron deficient but less substituted enoate or alkenyl–B(pin) groups is less favored. Compounds 3b and 3g-h were secured in >85% yield and as a single stereoisomer, although higher catalyst loading was needed for the last product. Cross-metathesis with aryl alkenes is often particularly difficult.
Z-Trisubstituted alkenyl halides were synthesized by incorporating a minor procedural change (Fig. 2b). With commercially available Z-7, by an otherwise identical sequence as before, we prepared Z-6 in 75% yield as a single stereoisomer (>98% Z). Cross-metathesis with Z-2 and 3.0 mol% Mo-2 afforded Z-3a in 86% yield and 91:9 Z:E ratio after four hours. Additional examples are provided in Fig. 2b (3i-m). Halogenated allyl–B(pin) compound 3k, amenable to catalytic diastereo- and enantioselective additions to electrophiles, was prepared in 83% yield and 95:5 Z:E ratio. Preparation of 3l (89% yield, 86:14 Z:E) shows that a Lewis basic trialkylamine is tolerated. Reactions with aryl alkenes were efficient but less stereoselective than the related E-selective processes [e.g., Z-3b, 65% yield (pure Z isomer), 79:21 Z:E]. Z-trisubstituted alkenes cannot be accessed by carboalumination without a properly situated directing group13,15.
Transformations affording E-alkenyl chlorides (Fig. 2a) are generally more stereoretentive compared to those furnishing Z isomers (Fig. 2b). This can be accounted for based on repulsive interactions within the metallacycle intermediates. For processes affording E alkenes (Fig. 2c, left panel), the intermediacy of I is likely favored because of the steric pressure in II, caused by the proximity of the methyl group oriented towards the sizeable aryloxide ligand (Cα substituents are nearer to the sizeable ligand compared to those at Cβ28). There is also the propinquity of the larger alkenyl group (RL) and the adjacent chloride substituent (vs. RS and Cl in I). With processes affording Z isomers (Fig. 2c, right panel), the energy gap between III and IV is probably smaller, because now it is within the metallacycle leading to the Z alkene (III) that RL and the chlorine atom are oriented in the same direction. Therefore, Z:E ratios are lower for reactions with a larger group at the fully substituted carbon of the alkene (e.g., aryl group in 3b), as there is more steric pressure in III with a phenyl group as the larger Cβ substituent (RL). Similarly, Z-3k is generated with greater stereoretention (95% compared to ≤91% Z) because the substrate, accessed by a phosphine–Ni-catalyzed diene hydroboration32, bears a larger n-Bu unit cis to the CH2B(pin) moiety (vs. Me); IV is destabilized further by a stronger repulsion between the Cα substituent and the aryloxide ligand (n-Bu instead of Me).
E- and Z-trisubstituted alkenyl bromides
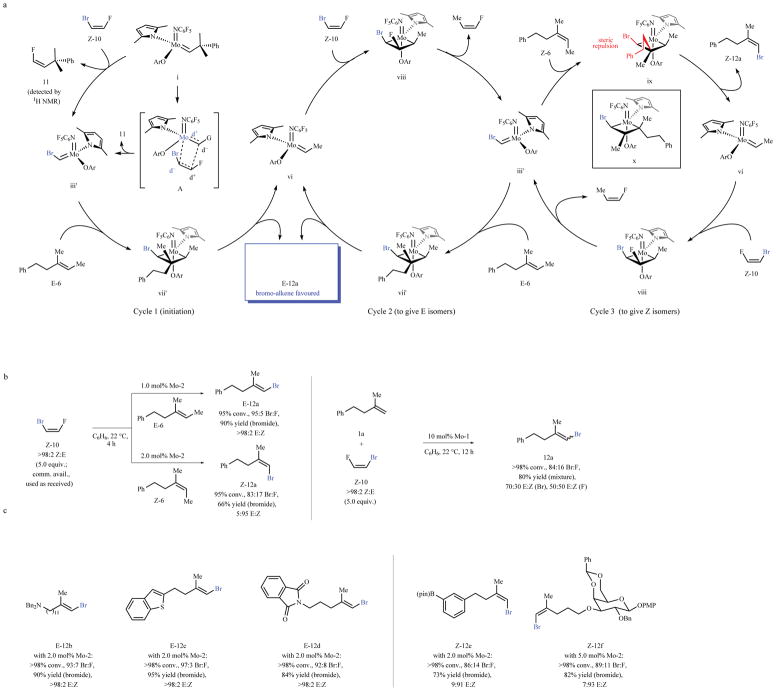
The pathway in Fig. 1c and the formerly established electronic and steric factors,28,33 imply that with a dihaloalkene containing two different halogen atoms [e.g., Z-1-bromo-2-fluoroethene (Z-10), Fig. 3a], the metallacyclobutane (cf. A, Cycle 1, Fig. 3a) generating alkylidene iii′ and an alkenyl fluoride should be favored. Indeed, treatment of Mo-1 or Mo-2 with Z-10 afforded alkenyl fluoride 11 (based on 1H NMR analysis). The ensuing transformation via alkylidene iii′ and metallacyclobutane vii′ (Cycle 1, Fig. 3a) would then give the alkenyl bromide product. This is unlike the reactions with mono- or 1,2-disubstituted alkenes, which involve bromo-substituted alkylidenes and produce fluoro-substituted alkenes33.
Fig. 3. Synthesis of Z- and E-trisubstituted alkenyl bromides.
a, Attributes of a Mo alkylidene dictate that reaction with Z-10 preferentially generates a bromo-substituted alkylidene and an alkenyl fluoride byproduct (e.g., 11) via A. The subsequent steps should afford E- or Z-trisubstituted alkenyl bromides (Cycles 2 and 3, respectively). b, Reaction between Z-10 and E-6 afforded E-12a in 90% yield, 95:5 Br:F ratio, and >98:2 E:Z selectivity; with Z-6, Z-12a was obtained in 66% yield, 83:17 Br:F ratio, and 5:95 E:Z selectivity. The difference in bromo:fluoro selectivity probably originates from the increased steric pressure in metallacyclobutane ix (Cycle 3, Fig. 3a), leading to intermediacy of x and alkenyl fluoride byproduct (via ii, Fig. 1). When 1a was used, 12a was generated with lower efficiency and stereoselectivity. c, The method has considerable scope and may be used with substrates containing acetals. Conv. and isomeric ratios determined by analysis of 1H NMR spectra of unpurified mixtures; yields are for isolated and purified products. See the Supplementary Information for details. Bn = benzyl; pin = pinacolato; PMP = para-methoxyphenyl.
In practice (Fig. 3b, left panel), with 1.0 mol% Mo-2, cross-metathesis between Z-10 and E-6 led to the formation of trisubstituted alkenyl bromide E-12a in 95:5 bromo:fluoro selectivity, 90% yield (pure bromide) and with complete retention of stereochemistry (>98% E). The transformation involving Z-10 and Z-6 generated Z-12a in 66% yield (pure bromide) and 95% stereoisomeric purity. Akin to reactions of alkenyl chlorides (Fig. 2), when 1,1-disubstituted alkene 1a was used (instead of Z- or E-6; Fig. 3b), 12a was formed with much lower stereoselectivity (70:30 E:Z). Additional cases are shown in Fig. 3c (12b-f), including 12f, which contain acetal groups, which are problematic with trialkylaluminum compounds34.
The preference for the bromo-alkenyl product is higher for the E isomers (92:8–97:3 compared to 83:17–89:11 bromo:fluoro, respectively). This might be because for Z alkene substrates, steric repulsion between the alkyl group and Br in the more favorable ix renders formation of the alternative metallacycle x to be more competitive (Cycle 3, Fig. 3a). Collapse of x would generate disubstituted alkylidene ii (Fig. 1b), which can then react with Z-10 with the expected sense of selectivity (see A, Cycle 1, Fig. 3a) to give more of the alkenyl fluoride byproduct. Reactions with 1,2-dibromoethene were considerably less efficient.
Other types of E- or Z-trisubstituted alkenes
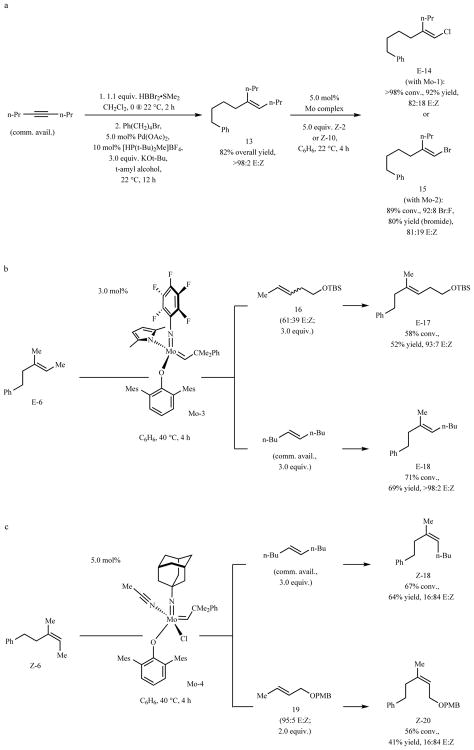
Trisubstituted alkenes with a longer chain alkyl unit (instead of a methyl group) may be prepared (Fig. 4a). Hydroboration35 of 4-octyne followed by catalytic cross-coupling36 afforded 13 in 82% overall yield (>98% E). Subsequent catalytic cross-metathesis generated chloride 14 in 92% yield and 82:18 E:Z selectivity; bromide 15 was obtained in 92:8 bromo:fluoro selectivity, 80% yield (pure bromide) and the same stereoisomeric purity. The diminished stereoretention probably originates from the smaller size difference between the alkyl groups (i.e., n-Pr and (CH2)4Ph) positioned at Cβ of the corresponding metallacyclobutanes. This strategy is attractive especially when the use of higher order not-as-readily-available trialkylaluminum reagents would be a less desirable option (compared to Me3Al)15.
Fig. 4. Synthesis of E- or Z-trisubstituted non-halogenated alkenes.
a, Trisubstituted alkenes with substituents other than a methyl group can be prepared efficiently and stereoselectively. b, The present strategies may be utilized to synthesize non-halogenated alkenes. An isomeric mixture of 1,2-disubstituted alkenes may be used, and sterically less hindered Mo-3 (vs. Mo-2) allowed for higher efficiency to be attained. c, Z-Trisubstituted alkenes may be obtained in a similar manner (e.g., Z-6); as with the alkenyl halides, reactions are less stereoretentive than when E isomers are generated. For higher yield in these instances, involving especially hindered metallacyclobutanes, a Mo chloride complex is needed. Conv. and isomeric ratios determined by analysis of 1H NMR spectra of unpurified mixtures; yields are for isolated and purified products. See the Supplementary Information for details. TBS, tert-butyldimethylsilyl; PMB, para-methoxybenzyl; Mes, 2,4,6-trimethylphenyl.
Non-halogenated trisubstituted alkenes may be prepared efficiently and stereoselectively (Fig. 4b–c). Treatment of E-6 with a 61:39 E:Z mixture of 1,2- disubstituted homoallylic ether 16 and 5.0 mol% Mo-3 led to the formation of E-17 in 52% yield and 93:7 E:Z ratio (Fig. 4b). Similarly, E-18 was obtained in 69% yield as a single stereoisomer (>98% E). Because of the more sizeable reaction partners (vs. a Z-1,2-dihaloethene), use of a less sterically congested complex with mesityl-substituted (mesityl, 2,4,6-trimethylphenyl) aryloxide ligand led to higher efficiency (i.e., Mo-3 instead of Mo-2). Since the catalyst can react with either 1,2-disubstituted alkene isomer to generate the same alkylidene, this starting material need not be stereoisomerically pure; cross-metathesis between a 1,1-disubstituted and an E- or Z-1,2-disubstituted alkene can lead to stereoisomeric mixtures (see Fig. 1b–c).
Z-Trisubstituted alkenes (Z-18 and Z-20, Fig. 4c) were accessed likewise. Because these transformations proceed via more congested metallacycles (see III-IV vs. I-II, Fig. 2c), use of monoaryloxide chloride species Mo-437 led to improved efficiency; for example, Z-18 was isolated in 28% yield when pyrrolide complex Mo-3 was used (compared to 64% yield). Mo chloride complexes are ineffective in promoting reactions that afford alkenyl halides, as decomposition of the purported chloro- or bromo-substituted metallacyclobutanes is probably facile37. The present approach complements a recent study regarding stereoselective synthesis of trisubstituted alkenes starting from carboxylic acids and involving alkenylzinc reagents, which are often derived from alkenyl halide precursors38.
Synthesis of biologically active compounds
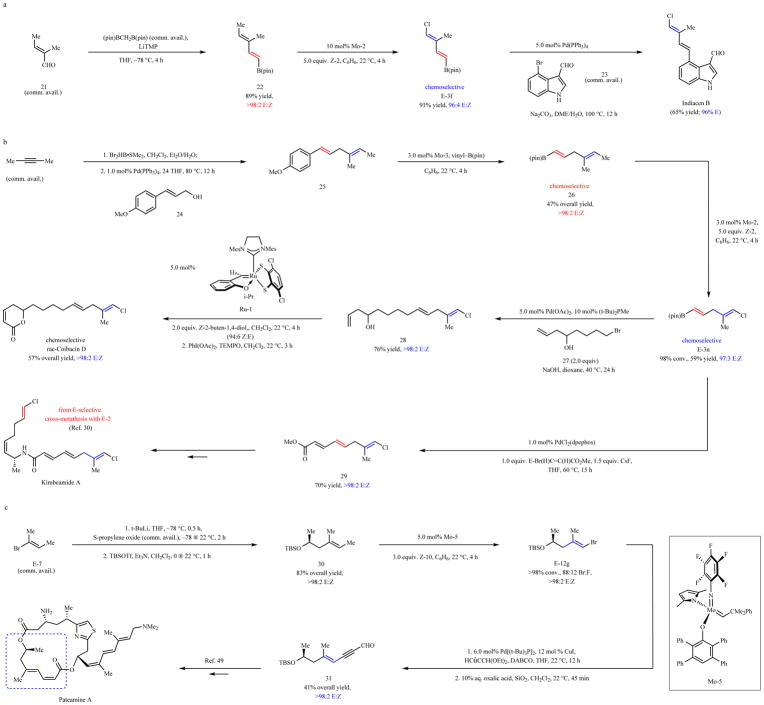
The first application pertains to stereoselective synthesis of naturally occurring anti-fungal agent indiacen B31,39,40 (Fig. 5a). Diene E-3f was prepared from enal 21 and bis[(pinacolato)boryl]methane (both are commercially available) via 22 in two steps41, including a chemoselective and stereoretentive cross-metathesis, in 91% yield and 96% E:Z ratio. Indiacen B was obtained after an additional catalytic step in 65% yield. The three-step route, affording the target molecule in 54% overall yield, compares favorably to a previously reported seven-step synthesis31, which involved zirconocene-catalyzed methyl-aluminum addition to an alkyne, generating the final product in 16% overall yield.
Fig. 5. Synthesis of biologically active compounds.
a, Indiacen B (anti-fungal) was synthesized stereoselectively in 54% overall yield in three steps. b, For synthesis of coibacin D (anti-inflammatory), diene 25, prepared by catalytic cross-coupling, was transformed to the desired target by a sequence of four catalytic processes: two chemo- and stereoselective cross-metathesis to give E-3n via 26, a cross-coupling reaction to afford 28 and a Ru-dithiolate catalyzed cross-metathesis. Dienoate 29, which may be used to prepare kimbeamide A (anti-tumor). c, Cross-metathesis of homoallylic silyl ether 30, synthesized in 83% yield from E-7, afforded E-12g in 88:12 bromo:fluoro ratio. Subsequent cross-coupling afforded 31, a fragment used in a total synthesis of pateamine A (immunosuppressant). Conv. and isomeric ratios determined by analysis of 1H NMR spectra of unpurified mixtures; yields are for isolated and purified products. See the Supplementary Information for details. pin, pinacolato; DME, dimethoxyethane; TEMPO, 3,3,5,5-tetramethyl-1-pyrroline-1-oxide; Mes, 2,4,6-trimethylphenyl; dpephos, bis[(2-diphenylphosphino)phenyl]ether; TBSOTf, tert-butyldimethylsilyl triflate; DABCO, 1,4-diazabicyclo[2.2.2]octane; LiTMP, lithium 2,2,6,6-tetramethylpiperidide.
Preparation of anti-leishmanial and anti-inflammatory compound coibacin D42 highlights a series of five catalytic processes (Fig. 5b). Stereoisomerically pure E,E-diene 25 was accessed in 72% yield by a two-step procedure involving hydroboration of 2-butyne and cross-coupling of the resulting alkenylboronic acid with allylic alcohol 2443. Compound E-3n was then obtained via E-alkenyl–B(pin) intermediate 26 through two chemo-selective and stereoretentive cross-metathesis reactions. The first was the conversion of 25 to 26 by a transformation involving 3.0 mol% Mo-3 and vinyl–B(pin); use of the slightly more hindered complex Mo-3 (instead of Mo-1) allowed for exceptional stereocontrol (see Extended Data Fig. 2 for details), and the less hindered aryl alkene underwent reaction exclusively. A second cross-metathesis was performed with 3.0 mol% Mo-2 and 1,2-dichloroethene Z-2 (5.0 equiv.), delivering E-3n in 59% yield and 97:3 E:Z selectivity; in this case, despite being more hindered, it was the trisubstituted alkene that reacted preferentially. Homoallylic alcohol 28 was accessed in 76% yield after another efficient and chemoselective cross-coupling between E-3n and 27. This was followed by a third kinetically controlled chemoselective cross-metathesis with 28, Z-2-butene-1-4,diol and catechothiolate complex Ru-144,45. The resulting Z-allylic alcohol, generated in 94:6 Z:E selectivity was transformed to racemic coibacin D in 57% overall yield (>98:2 E,E at the acyclic alkene sites). Coibacin D was accordingly obtained in seven steps (longest linear sequence), 12% overall yield, and as a single alkene isomer, comparing favorably with the previously reported 4% overall yield after six steps to give racemic coibacin D as a 75:25 mixture of alkene isomers46. Moreover, alkenyl chloride E-3n was converted to dienoate 29 (70% yield, >98% E), a compound applicable to synthesis of anti-tumor agent kimbeamide A47. The requisite amine fragment has been prepared through kinetically E-selective cross-metathesis28.
Alkenyl chlorides can be ineffective in cross-coupling and, in such cases, the corresponding bromo-alkene is needed. One instance relates to stereoselective preparation of enyne 31 (Fig. 5c), a compound used to prepare immunosuppressant and anti-cancer48 natural product pateamine A49. Conversion of E-7 to homoallylic silyl ether 30 involved enantiomerically pure and commercially available S-propylene oxide, and was accomplished in two straightforward steps. Cross-metathesis between 30 and Z-1-bromo-2-fluoroethene (Z-10; 3.0 equiv.) with 5.0 mol% Mo-5 afforded E-12g in 88:12 bromide:fluoride selectivity and as a single alkenyl bromide isomer. A catalyst with a smaller aryloxide ligand was needed for higher efficiency because a metallacyclobutane with a larger Cβ substituent must be accommodated (for example, 63% conv. to E-12g with Mo-2 under otherwise identical conditions). Cross-metathesis was again followed by cross-coupling, this time between E-12g and 3,3-diethoxy-1-propyne (commercially available). Cross-coupling with the related alkenyl chloride was ineffective (<2%). Unmasking of the diethyl acetal group afforded 31 (41% overall yield for three steps). The fragment was thus synthesized by a shorter route (five compared to eight steps) and in similar yield (34% compared to 33% overall yield reported previously48).
Conclusions
We demonstrate that there are two crucial factors for successful development of kinetically controlled stereoretentive cross-metathesis reactions that afford trisubstituted alkenes. A variety of trisubstituted alkene substrates must be readily accessible in stereoisomerically pure form, and a set of catalysts that can catalyze reactions between tri- and 1,2-disubstituted alkenes efficiently and stereoselectively must be available. Accordingly, we show that a sequence beginning with cross-coupling between E- or Z-trisubstituted 2-bromo-2-butene and an organoboron compound and then a stereoretentive cross-metathesis with an appropriate Mo-based complex furnishes E- or Z-trisubstituted alkenes efficiently and in high stereoisomeric purity. The approach, which merges cross-coupling with cross-metathesis, underlines a key difference between two major classes of catalytic processes. Substrates in cross-coupling are more distinct and chemoselectivity is less of a problem, offering facile access to the necessary trisubstituted alkene substrates. Cross-metathesis can then be used to access a wider range of alkenes readily and in high stereoisomeric purity. The relationship between cross-coupling and cross-metathesis has another dimension: the E- or Z-trisubstituted alkenyl halides may be converted to other trisubstituted alkenes with little or no loss of stereochemical purity through another cross-coupling.
By adopting the proper combination of these two important catalytic C–C bond forming transformations, we have been able to address a critical unresolved problem in chemical synthesis. The present study further highlights the attributes of stereogenic-at-Mo complexes as effective alkene metathesis catalysts, which further benefit from the possibility of using them as commercially available paraffin tablets50.
Extended Data
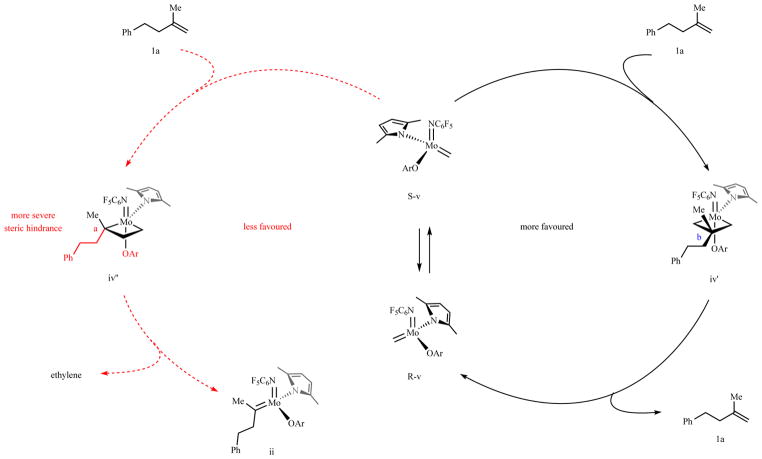
Extended Data Fig. 1. Nonproductive olefin metathesis pathways.
Cross-metathesis between v and 1a via symmetrical metallacyclobutane iv′ (pathway on the right, in black) is more likely than one entailing the intermediacy of complex iv″ (pathway on the left, in red). This is as a result of greater steric pressure between the Ca substituent and the sizeable aryloxide ligand [see: Nguyen, T. T., Koh, M. J., Shen, X., Romiti, F., Schrock, R. R. & Hoveyda, A. H. Science 352, 569–575 (2016)]. Cycloreversion of iv′ would then re-generate v and afford 1a (nonproductive process).
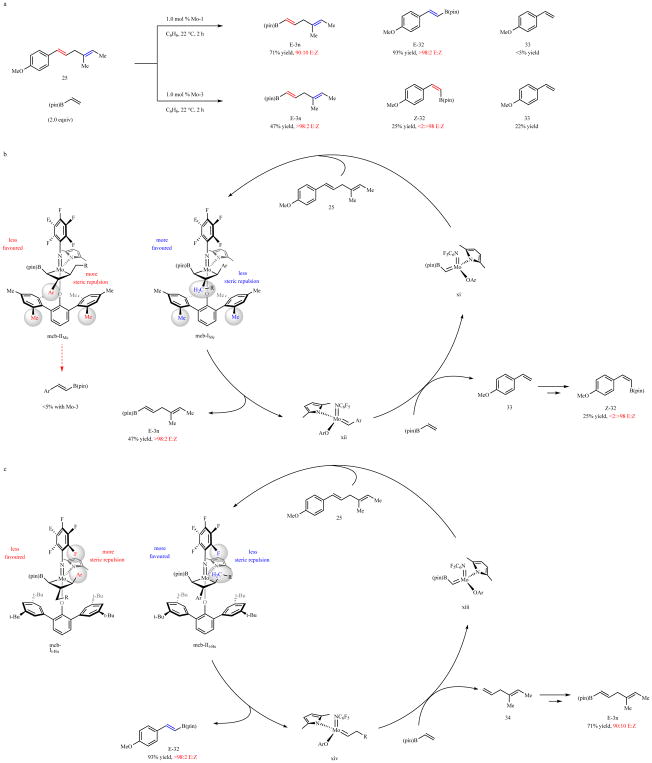
Extended Data Fig. 2. Distinctive pathways for cross-metathesis of 22 and vinyl–B(pin) with Mo-1,2.
a. Cross-metathesis between 25 and vinyl–B(pin) in the presence of Mo1 and Mo-2 results in different product distribution and stereoselectivity profiles. b, The reactions proceed via mcbIMe because of severe steric repulsion between the larger Cb aryl group in mcbIIMe and the Me units of the aryloxide ligand in Mo-3. Byproduct 33 may react with vinyl-B(pin) to furnish Z-32. c, There is less steric pressure at Cβ in mcbIt-Bu and mcbIIt-Bu; consequently, steric repulsion between the Cα metallacyclobutane substituent and an ortho fluorine substituent of the arylimido becomes more of a factor. Thus, cross-metathesis probably proceeds via mcbIIt-Bu to afford the corresponding alkenyl–B(pin) compound (E-32). Ensuing reaction of xiv with vinyl–B(pin) likely affords 34, which may then react with vinyl–B(pin) to furnish E-3n.
Supplementary Material
Acknowledgments
This research was supported by the United States National Institutes of Health, Institute of General Medical Sciences (GM-59426 and, in part, CHE-1362763). M. J. K. and T. J. M. are grateful for support in the form of a Bristol Myers-Squibb Fellowship in Organic Chemistry and a John LaMattina Graduate Fellowship, respectively.
Footnotes
Online Content Methods, along with any additional Extended Data display items and Source Data, are available in the online version of the paper; references unique to these sections appear only in the online paper.
Data Availability The authors declare that findings of this study are available within the paper [and its supplementary files].
The authors declare no competing financial interests.
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
Author Contributions T. T. N., M. J. K. were involved in the discovery, design and development of the cross-metathesis strategies and their applications. T. J. M. carried out the initial exploratory studies with 1,1-disubstituted alkenes. A. H. H. designed and directed the investigations. A.H.H. and R. R. S. conceived the studies that led to the development of Mo complexes used in this study. A. H. H. wrote the manuscript with revisions provided by T. T. N., M. J. K., and T. J. M.
References
- 1.Negishi E, Huang Z, Wang G, Mohan S, Wang C, Hattori H. Recent advances in efficient and selective synthesis of di-, tri-, and tetrasubstituted alkenes via Pd-catalyzed alkenylation–carbonyl olefination strategy. Acct Chem Res. 2008;41:1474–1485. doi: 10.1021/ar800038e. [DOI] [PubMed] [Google Scholar]
- 2.Siau WY, Zhang Y, Zhao Y. Stereoselective synthesis of Z-alkenes. Top Curr Chem. 2012;327:33–58. doi: 10.1007/128_2012_315. [DOI] [PubMed] [Google Scholar]
- 3.Shang G, Li W, Zhang X. In: Catalytic Asymmetric Synthesis. Ojima I, editor. Wiley; 2010. pp. 344–436. [Google Scholar]
- 4.Baslé O, Denicourt-Nowicki A, Crévisy C, Mauduit M. In: Copper-Catalyzed Asymmetric Synthesis. Alexakis A, Krause N, Woodward S, editors. VCH–Wiley; 2014. pp. 85–119. [Google Scholar]
- 5.Alexakis A, Krause N, Woodward S. In: Copper-Catalyzed Asymmetric Synthesis. Alexakis A, Krause N, Woodward S, editors. VCH–Wiley; 2014. pp. 33–68. [Google Scholar]
- 6.Sreekumar C, Darst KP, Still WC. A direct synthesis of Z-trisubstituted allylic alcohols via the Wittig reaction. J Org Chem. 1980;45:4260–4262. [Google Scholar]
- 7.Maryanoff BE, Reitz AB. The Wittig olefination reaction and modifications involving phosphoryl-stabilized carbanions. Stereochemistry, mechanism, and selected synthetic aspects. Chem Rev. 1989;89:863–927. [Google Scholar]
- 8.Taber DF, Meagley RP, Doren DJ. Cyclohexenone construction by intramolecular alkylidene C–H insertion: Synthesis of (+)-cassiol. J Org Chem. 1996;61:5723–5728. [Google Scholar]
- 9.Trost BM, Balls ZT. Addition of metalloid hydrides to akynes: Hydrometallation with boron, silicon, and tin. Synthesis. 2005;2005:853–887. [Google Scholar]
- 10.Wang C, Tobrman T, Xu Z, Negishi E. Highly regio- and stereoselective synthesis of Z-trisubstituted alkenes via propyne bromoboration and tandem Pd-catalysed cross-coupling. Org Lett. 2009;11:4092–4095. doi: 10.1021/ol901566e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Minato A, Suzuki K. A remarkable steric effect in palladium-catalyzed Grignard coupling: regio- and stereoselective monoalkylation and -arylation of 1,1-dichloro-1-alkenes. J Am Chem Soc. 1987;109:1257–1258. [Google Scholar]
- 12.Mun B, Kim S, Yoon H, Kim KH, Lee Y. Total synthesis of isohericerin, isohericerinone, and erinacerin A: development of a copper-catalyzed methylboronation of terminal alkynes. J Org Chem. 2017;82:6349–6357. doi: 10.1021/acs.joc.7b00920. [DOI] [PubMed] [Google Scholar]
- 13.Negishi E, Van Horn DE, Yoshida T. Controlled carbometallation. 20 Carbometalation reaction of alkynes with organoalane-zirconocene derivatives as a route to stereo- and regiodefined trisubstituted alkenes. J Am Chem Soc. 1985;107:6639–6647. [Google Scholar]
- 14.Fleming I, Newton TW, Roessler F. The silylcupration of acetylenes: A synthesis of vinylsilanes. J Chem Soc Perkin Trans 1. 1981:2527–2532. [Google Scholar]
- 15.Ma S, Negishi E. Anti-carbometalation of homopropargyl alcohols and their higher homologues via non-chelation-controlled syn-carbometallation and chelation-controlled isomerization. J Org Chem. 1997;62:784–785. [Google Scholar]
- 16.Lu Z, Ma S. Studies on the Cu(II)-catalyzed regioselective anti-carbometallation of secondary terminal propargyl alcohols. J Org Chem. 2006;71:2655–2660. doi: 10.1021/jo0524021. [DOI] [PubMed] [Google Scholar]
- 17.Gribble GW. Biological activity of recently discovered halogenated marine natural products. Mar Drugs. 2015;13:4044–4136. doi: 10.3390/md13074044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chatterjee AK, Grubbs RH. Synthesis of trisubstituted alkenes via olefin cross-metathesis. Org Lett. 1999;1:1751–1753. doi: 10.1021/ol991023p. [DOI] [PubMed] [Google Scholar]
- 19.Chatterjee AK, Sanders DP, Grubbs RH. Synthesis of symmetrical trisubstituted olefins by cross metathesis. Org Lett. 2002;4:1939–1942. doi: 10.1021/ol0259793. [DOI] [PubMed] [Google Scholar]
- 20.Morrill CM, Funk TW, Grubbs RH. Synthesis of tri-substituted vinyl boronates via ruthenium-catalyzed olefin cross-metathesis. Tetrahedron Lett. 2004;45:7733–7736. [Google Scholar]
- 21.Wang ZJ, Jackson WR, Robinson AJ. An efficient protocol for the cross-metathesis of sterically demanding olefins. Org Lett. 2013;15:3006–3009. doi: 10.1021/ol401194h. [DOI] [PubMed] [Google Scholar]
- 22.Hoveyda AH, Khan RKM, Torker S, Malcolmson SJ. In: Catalyst-controlled stereoselective olefin metathesis in Handbook of Metathesis. Grubbs RH, Wenzel AG, O’Leary DJ, Khosravi E, editors. Wiley–VCH; Weinheim: 2014. pp. 503–562. [Google Scholar]
- 23.Hoveyda AH. Evolution of catalytic enantioselective olefin metathesis: From ancillary transformation to purveyor of stereochemical identity. J Org Chem. 2014;79:4763–4792. doi: 10.1021/jo500467z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoveyda AH, Zhugralin AR. The remarkable metal-catalysed olefin metathesis reaction. Nature. 2007;450:243–251. doi: 10.1038/nature06351. [DOI] [PubMed] [Google Scholar]
- 25.Cuvigny T, du Penhoat H, Julia M. Isomérisation cis trans régiosélective de doubles liaison trisubstitutées. Tetrahedron Lett. 1980;21:1331–1334. [Google Scholar]
- 26.Schrock RR, Hoveyda AH. Molybdenum and tungsten imido alkylidene complexes as efficient olefin-metathesis catalysts. Angew Chem Int Ed. 2003;42:4592–4633. doi: 10.1002/anie.200300576. [DOI] [PubMed] [Google Scholar]
- 27.Lam JK, Zhu C, Bukhryakov KV, Müller P, Hoveyda AH, Schrock RR. Synthesis and evaluation of molybdenum and tungsten monoaryloxide halide alkylidene complexes for Z-selective cross-metathesis of cyclooctene and Z-1,2-dichloroethylene. J Am Chem Soc. 2016;138:15774–15783. doi: 10.1021/jacs.6b10499. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen TT, Koh MJ, Shen X, Romiti F, Schrock RR, Hoveyda AH. Kinetically controlled E-selective catalytic olefin metathesis. Science. 2016;352:569–575. doi: 10.1126/science.aaf4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chemler SR, Trauner D, Danishefsky SJ. The B-alkyl Suzuki-Miyaura cross-coupling reaction: Development, mechanistic study, and applications in natural product synthesis. Angew Chem Int Ed. 2001;40:4544–4568. doi: 10.1002/1521-3773(20011217)40:24<4544::aid-anie4544>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 30.Miyazawa M, Ishibashi N, Ohnuma S, Miyashita M. Stereospecific internal alkylation of terminal g,d-epoxy acrylates. Tetrahedron Lett. 1997;38:3419–3422. [Google Scholar]
- 31.Anantoju KK, Mohd BS, Maringanti TC. An efficient and concise synthesis of indiacen A and indiacen B. Tetrahedron Lett. 2017;58:1499–1500. [Google Scholar]
- 32.Ely RJ, Morken JP. Stereoselective nickel-catalyzed 1,4-hydroboration of 1,3-dienes. Org Synth. 2011;88:342–352. [Google Scholar]
- 33.Koh MJ, Nguyen TT, Zhang H, Schrock RR, Hoveyda AH. Direct synthesis of Z-alkenyl halides through catalytic cross-metathesis. Nature. 2016;531:459–465. doi: 10.1038/nature17396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mori A, Fujiwara J, Maruoka K, Yamamoto H. Nucleophilic cleavage of acetals using organometallic reagents. J Organomet Chem. 1985;285:83–94. [Google Scholar]
- 35.Brown HC, Bhat NG, Rajagopalan S. Stereoselective synthesis of (E)- and (Z)-disubstituted vinyl bromides via organoboranes. Synthesis. 1986:480–482. [Google Scholar]
- 36.Kirchhoff JH, Matthew R, Netherton MR, Hills ID, Fu GC. Boronic acids: New coupling partners in room-temperature Suzuki reactions of alkyl bromides. Crystallographic characterization of an oxidative-addition adduct generated under remarkably mild conditions. J Am Chem Soc. 2002;124:13662–13663. doi: 10.1021/ja0283899. [DOI] [PubMed] [Google Scholar]
- 37.Koh MJ, Nguyen TT, Lam JK, Torker S, Hyvl J, Schrock RR, Hoveyda AH. Molybdenum chloride catalysts for Z-selective olefin metathesis reactions. Nature. 2017;542:80–85. doi: 10.1038/nature21043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Edwards JT, et al. Decarboxylative alkenylation. Nature. 2017;545:213–218. doi: 10.1038/nature22307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steinmetz H, Mohr KI, Zander W, Jansen R, Gerth K, Müller R. Indiacen A and B: Prenyl indoles from the myxobacterium Sandaracinus amylolyticus. J Nat Prod. 2012;75:1803–1805. doi: 10.1021/np300288b. [DOI] [PubMed] [Google Scholar]
- 40.Marsch N, Jones PG, Lindel T. SmI2-mediated dimerization of indolylbutenones and synthesis of the myxobacterial natural product indiacen B. Beilstein J Org Chem. 2015;11:1700–1706. doi: 10.3762/bjoc.11.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coombs JR, Zhang L, Morken JP. Synthesis of vinyl boronates from aldehydes by a practical boron–Wittig reaction. Org Lett. 2015;17:1708–1711. doi: 10.1021/acs.orglett.5b00480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Balunas MJ, et al. Coibacins A–D, Antileishmanial marine cyanobacterial polyketides with intriguing biosynthetic origins. Org Lett. 2012;14:3878–3881. doi: 10.1021/ol301607q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsukamoto H, Uchiyama T, Suzuki T, Kondo Y. Palladium(0)-catalyzed direct cross-coupling reaction of allylic alcohols with aryl- and alkenylboronic acids. Org Biomol Chem. 2008;6:3005–3013. doi: 10.1039/b804991b. [DOI] [PubMed] [Google Scholar]
- 44.Koh MJ, Khan RKM, Torker S, Yu M, Mikus MS, Hoveyda AH. High-value alcohols and higher-oxidatio-state compounds by catalytic Z-selective cross-metathesis. Nature. 2015;517:181–186. doi: 10.1038/nature14061. [DOI] [PubMed] [Google Scholar]
- 45.Xu C, Shen X, Hoveyda AH. In situ methylene capping: A general strategy for efficient stereoretentive catalytic olefin metathesis. The concept, methodological implications, and applications to synthesis of biologically active compounds. J Am Chem Soc. 2017;139:10919–10928. doi: 10.1021/jacs.7b06552. [DOI] [PubMed] [Google Scholar]
- 46.Kolská K, Ghavre M, Pour M, Hybelbauerová S, Kotora M. Total synthesis of coibacin D by using enantioselective allylation and metathesis reactions. Asian J Org Chem. 2016;5:646–651. [Google Scholar]
- 47.Nunnery JK, et al. Biosynthetically intriguing chlorinated lipophilic metabolites from distant tropical marine cyanobacteria. J Org Chem. 2012;77:4198–4208. doi: 10.1021/jo300160e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Northcote PT, Blunt JW, Munro MHG. Pateamine: A potent cytotoxin from the New Zealand marine sponge, Mycale sp. Tetrahedron Lett. 1991;32:6411–6414. [Google Scholar]
- 49.Romo D, et al. Total synthesis and immunosuppressive activity of (−)-pateamine A and related compounds: Implementation of a β-lactam-based macrocyclization. J Am Chem Soc. 1998;120:12237–12254. [Google Scholar]
- 50.Ondi L, Nagy GM, Czirok JB, Bucsai A, Frater GE. From Box to bench: Air-stable molybdenum catalyst tablets for everyday use in olefin metathesis. Org Proc Res Dev. 2016;20:709–1716. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.