Abstract
Heme is an essential cofactor for proteins involved in diverse biological processes such as oxygen transport, electron transport, and microRNA processing. Free heme is hydrophobic and cytotoxic, implying that specific trafficking pathways must exist for the delivery of heme to target hemoproteins which reside in various subcellular locales. Although heme biosynthesis and catabolism have been well characterized, the pathways for trafficking heme within and between cells remain poorly understood. Caenorhabditis elegans serves as a unique animal model for uncovering these pathways because, unlike vertebrates, the worm lacks enzymes to synthesize heme and therefore is crucially dependent on dietary heme for sustenance. Using C. elegans as a genetic animal model, several novel heme trafficking molecules have been identified. Importantly, these proteins have corresponding homologs in vertebrates underscoring the power of using C. elegans, a bloodless worm, in elucidating pathways in heme homeostasis and hematology in humans. Since iron deficiency and anemia are often exacerbated by parasites such as helminths and protozoa which also rely on host heme for survival, C. elegans will be an ideal model to identify anti-parasitic drugs that target heme transport pathways unique to the parasite.
Keywords: Heme, Iron, Porphyrin, Helminths, C. elegans, Micronutrient, Anemia
Introduction
Heme is an iron-containing porphyrin that serves as a cofactor for proteins involved in numerous cellular functions including hemoglobin for oxygen binding, cytochromes for electron transfer, and guanylate cyclase for cell signaling (Ponka 1999; Severance and Hamza 2009). In humans, over sixty percent of iron in the body is utilized as the heme moiety of hemoglobin. Additionally, iron is most efficiently absorbed from the diet as heme-iron (West and Oates 2008). Defects in any step of heme synthesis result in disorders collectively known as the porphyrias, while reduction in heme synthesis due to iron deficiency leads to anemia (Ajioka et al. 2006; Hamza and Dailey 2012). However, despite its necessity, free heme is also cytotoxic due to its inherent peroxidase reactivity and capacity to produce reactive oxygen species within the cell (Balla et al. 1991; Sassa 2002; Vincent 1989). Moreover, heme is hydrophobic due to the porphyrin side chains and, a priori, cannot freely diffuse through the aqueous cytosol. Therefore, cells must be able to carefully regulate, compartmentalize, and transport heme to target hemoproteins whilst preventing toxicity. Although the hemoprotein myoglobin was the first protein to be crystallized over 50 years ago, the mechanisms by which heme is transported within and between cells after synthesis in the mitochondria or how hemoproteins are assembled have remained elusive (Perutz et al. 1960; Severance and Hamza 2009). This is primarily due to the difficulty in uncoupling heme synthesis from downstream processes such as heme trafficking.
The soil-dwelling nematode, Caenorhabditis elegans, is a unique model in which to study heme homeostasis. Worms offer several general advantages as a model organism as they are genetically tractable, optically transparent, amenable for genetic screens and cell biological studies, and have a high percentage of genes that are conserved in humans (The C. elegans Sequencing Consortium 1998). More specifically and crucially for studying heme homeostasis, C. elegans is a heme auxotroph (Rao et al. 2005) (Fig. 1). All heme that is utilized by the worm must be transported across the intestinal brush border where it can be used directly by the intestine, stored for later use, or exported to extraintestinal tissues. Heme destined for extraintestinal tissues must be exported from the basolateral membrane of the intestine to the pseudocoelom, the worm's circulatory system. Once in the pseudocoelom, extraintestinal tissues will have access to intestinal-derived heme. Using C. elegans as an animal model, organismal heme trafficking pathways can be studied in the absence of confounding heme synthesis. Based on transcriptomic analyses, RNAi screens, and phenotypic analyses in C. elegans, several proteins which facilitate heme metabolism through intestinal uptake, intra- and intercellular distribution, and oxidation/reduction of heme have been identified and will be discussed (Chen et al. 2011, 2012; Korolnek et al. 2014; Rajagopal et al. 2008; Severance et al. 2010).
Fig. 1.
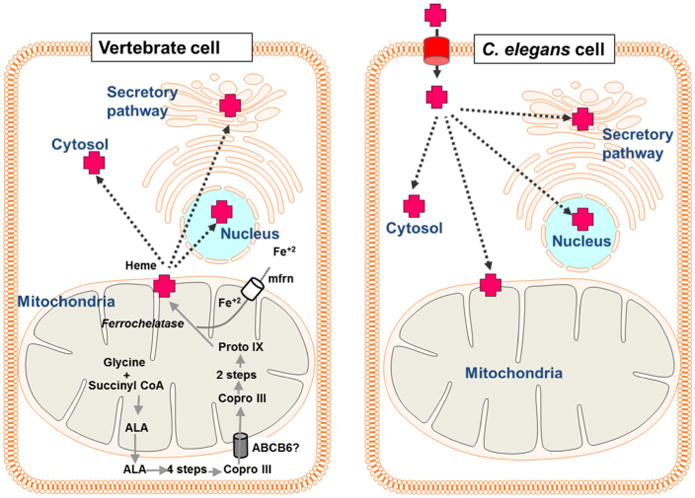
Heme trafficking in vertebrates and C. elegans. In vertebrate cells, heme is synthesized via an eight-step enzymatic pathway that begins with the synthesis of aminolevulinic acid (ALA) from glycine and succinyl CoA in the mitochondria. ALA is then transported from the mitochondria into the cytosol where the subsequent four steps occur. The intermediate coproporphyrinogen III (Copro III) is transported back into the mitochondria for the final three steps of heme synthesis. The terminal step is the insertion of ferrous iron into the protoporphyrin IX (proto IX) ring by ferrochelatase. Heme is then transported from the mitochondria to hemoproteins in various subcellular locations, including the cytosol, the secretory pathway, and the nucleus. Because C. elegans lacks all eight heme biosynthetic enzymes, all heme must be imported into the cell before incorporation into hemoproteins
Heme acquisition
The intestine plays an essential role in systemic heme homeostasis as this organ is the port of entry for heme into the worm's body, and thus expresses several genes necessary for adaptation to environmental fluctuations in heme bioavailability. Although heme stores derived from maternal stores are sufficient for embryogenesis and early larval development, during later larval stages and in adult worms all heme is acquired exclusively through diet. The HEME RESPONSIVE GENE-4 (HRG-4) is a heme importer predicted to be a permease with four transmembrane domains (Rajagopal et al. 2008). HRG-4 is specifically expressed in the C. elegans intestine in low heme and localizes to the apical intestinal membrane where it imports dietary heme across the brush border (Rajagopal et al. 2008; Severance et al. 2010; Yuan et al. 2012). RNAi knockdown of hrg-4 results in an intestinal heme deficiency signal, diminished intestinal accumulation of the fluorescent heme analog zinc mesoporphyrin (ZnMP), and protection from the toxic heme analog gallium protoporphyrin IX (GaPP) (Severance et al. 2010). These results indicate that HRG-4 imports heme from the lumen into the intestine. Interestingly, a genetic deletion of hrg-4 does not result in perturbed growth, suggesting redundant pathways for heme uptake. This redundancy is due to HRG-4 paralogs - HRG-5 and HRG-6. hrg-5 and hrg-6 flank hrg-4 on chromosome IV and likely arose from hrg-4 gene duplication. However, unlike hrg-4 which is upregulated over a 100-fold by heme depletion, hrg-5 and hrg-6 are not transcriptionally regulated by heme and therefore may transport heme under heme sufficient conditions when hrg-4 is downregulated. Within the intestine, at least a portion of heme gets compartmentalized into lysosomal-like vesicles. A fourth HRG-4 paralog, HRG-1, mediates the transport of heme from these vesicles. Unlike the other three paralogs, hrg-1 is located on the X chromosome and is the most divergent with respect to its C. elegans paralogs. Notably, HRG-1 phylogenetic analyses predict orthologs in other organisms suggesting that hrg-1 may be the ancestral gene. HRG-1 is highly upregulated at low heme specifically in the intestine and localizes to ZnMP-containing intracellular vesicles. RNAi depletion of hrg-1 results in vesicular ZnMP accumulation, indicating that under heme limiting conditions, upregulation of HRG-1 allows compartmentalized heme to be mobilized for utilization by the intestine and extraintestinal tissues (Rajagopal et al. 2008). Interestingly, worms harboring mutations in both hrg-1 and hrg-4 exhibit a synthetic growth phenotype in low heme (Yuan et al. 2012). This suggests that after transport into the intestine, heme may be partitioned into distinct compartments. It is also possible that the endolysosomal HRG-1 traffics via the apical plasma membrane and compensates for the lack of HRG-4 (Fig. 2).
Fig. 2.
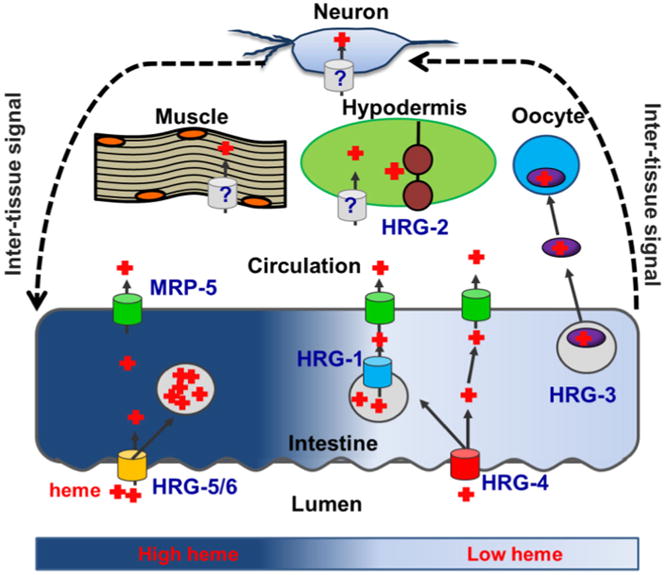
Model of heme homeostasis in C. elegans. In order to adapt to varying environmental heme conditions, C. elegans has adopted several mechanisms to ensure that tissue heme requirements are fulfilled while preventing heme toxicity. In high environmental heme conditions, expression of high affinity heme transporters is repressed, and excess heme is compartmentalized into vesicles. When the worm encounters low environmental heme, high affinity heme importers HRG-4 and HRG-1 are expressed to increase intestinal heme uptake and release heme stored in vesicles, respectively. The heme exporter MRP-5 facilitates heme availability to extraintestinal tissues over a broad heme range. HRG-3 is secreted by the intestine during severe heme deficiency to ensure that oocytes and developing embryos acquire sufficient heme. HRG-2 is upregulated to increase heme utilization in the hypodermis. It is likely that homeostatic adaptation is controlled at the systemic level by bidirectional signaling between the intestine and extraintestinal tissues
Heme transport assays in the budding yeast Sac-charomyces cerevisiae have identified the amino acid residues within HRG-4 and HRG-1 that are important for heme transport (Yuan et al. 2012). HRG-4 transports heme by utilizing a histidine in the exoplasmic (E2) loop and a conserved FARKY motif near the C terminus. Under low heme conditions when maximum transport activity is required, an additional tyrosine in the predicted second transmembrane domain (TMD2) is necessary. HRG-1, meanwhile, requires both a histidine in TMD2 and the FARKY motif. For optimal activity under heme-limiting conditions, HRG-1 requires a histidine in the E2 loop. The presence of tyrosines rather than histidines in HRG-4 may help stabilize oxidized heme imported from the lumen (Goodwin et al. 2000; Liu et al. 1999).
Interestingly, despite both genes being upregulated in the intestine by low heme, hrg-1 and hrg-4 appear to be transcriptionally regulated by different pathways. The heme-dependent regulation of hrg-1 is driven by a heme-responsive element (HERE) in conjunction with GATA sites, elements that bind the GATA transcription factor ELT-2 and are responsible for intestinal transcription in the worm (Hawkins and McGhee 1995; Sinclair and Hamza 2010). Although the hrg-4 5′ flanking region also has GATA sites, it does not have a HERE, indicating that different pathways are responsible for the heme-responsiveness of hrg-1 and hrg-4.
Intertissue heme transport
Before intestinal heme can be utilized by extraintestinal tissues, it must be transported across the intestinal basolateral membrane. Unlike the redundancy observed for heme importers, there appears to be one main intestinal heme exporter in the C. elegans intestine, MRP-5 (Fig. 2). MRP-5 is an ABC-transporter that localizes to the basolateral membrane and basolateral sorting vesicles of the C. elegans intestine (Korolnek et al. 2014). Both a genetic deletion and RNAi depletion for mrp-5 result in embryonic lethality due to heme being trapped in the mother's intestine, showing a lack of functional redundancy in intestinal heme export. This lethality can be overcome by supplementing the worm growth medium with excess heme (>200 μM) which may enter the worm's circulation via low-affinity transporters. Alternatively, heme can intercalate into membrane lipids and may traverse the cell membrane in the absence of transporters. In addition to embryonic lethality, depletion of MRP-5 leads to accumulation of ZnMP in the intestine and protection from GaPP toxicity, presumably by preventing GaPP from being exported from the intestine to other tissues. MRP-5 is also expressed in extraintestinal tissues such as the hypodermis, pharynx and a subset of neurons, although it is unclear whether MRP-5 exports heme from these tissues or provides heme to hemoproteins in the secretory pathway, a function attributed to mammalian MRP5 (Korolnek et al. 2014). Interestingly, mrp-5 is upregulated by low heme and has a putative HERE in its promoter, although the heme-dependent fold change in expression level is less dramatic than hrg-1 (Korolnek et al. 2014; Severance et al. 2010; Sinclair and Hamza 2010). Nevertheless, this suggests that hrg-1 and mrp-5 may be regulated by the same transcriptional pathway.
Although MRP-5 is required for embryonic survival over a broad range of heme concentrations, C. elegans has adapted an “emergency” mechanism at critically low heme conditions to ensure that oocytes developing within the germline receive the heme required for embryogenesis and larval development (Chen et al. 2011). This is accomplished by upregulation of HRG-3, an ∼8 kDa protein that is expressed in the intestine and binds to heme in a 2:1 (protein:heme) stoichiometric ratio (Fig. 2) (Chen et al. 2011). Although hrg-3 expression is upregulated more than 900 fold during heme deficiency, it is barely detectable when worms are grown in the presence of 6 μM heme. HRG-3 is secreted from the intestine and is internalized by developing oocytes, consistent with the observation that hrg-3 null worms lay dead eggs or larvae that arrest at the first larval (L1) stage. Taken together, these data indicate that HRG-3 may function as a heme chaperone to deliver maternal heme to developing oocytes and other tissues under severe heme limitation. HRG-3 trafficking is reminiscent of the C. elegans vitellogenins, or yolk precursor proteins, which are also internalized by developing oocytes after secretion from the mother's intestine (Grant and Hirsh 1999; Spieth and Blumenthal 1985). However, RNAi knockdown of the vitellogenins or the oocyte yolk receptor RME-2 does not affect HRG-3 secretion or trafficking, indicating that HRG-3 is not part of the vitellogenin complex. Consequently, the receptor for HRG-3 or the mechanism for its internalization into oocytes have yet to be elucidated although we speculate, due to the presence of maternally derived HRG-3 within oocytes and its persistence to late larval stages, that heme-bound HRG-3 is internalized into oocytes. It is unclear whether HRG-3 acquires heme in the secretory pathway or in the pseudocoelom post secretion, or if heme loading is dependent upon MRP-5. Further analyses will be required to establish the relationship between different mechanisms of intestinal heme export. Interestingly, HRG-3 is expressed in zygotes, all larval stages, and in males, suggesting HRG-3-dependent heme delivery functions beyond the germline.
Systemic heme homeostasis
In addition to HRGs in the intestine, HRG-2 is a single pass type I transmembrane protein that localizes to the ER and apical plasma membrane of the hypodermis (Chen et al. 2012) (Fig. 2). Along with the TMD, HRG-2 has a thioredoxin -like fold and a glutathione S-transferase-like domain on the carboxyl terminus. Under heme-limiting conditions, hrg-2 is upregulated over 200-fold in the hypodermis. A genetic deletion of hrg-2 results in aberrant cytochrome heme profiles, and ectopic expression of HRG-2 in a heme deficient strain of yeast facilitates growth in submicromolar concentrations of exogenous heme. Together these data suggest that HRG-2 is involved in heme utilization in the hypodermis. Although HRG-2 can bind heme, as a single -pass membrane protein it is unlikely that HRG-2 itself is a heme transporter. However, it may function as on oxidoreductase, which may facilitate heme uptake, similar to how DcytB and Steap3 facilitate iron uptake by reducing circulating iron which is in the Fe(III) oxidation state (McKie et al. 2000; Ohgami et al. 2005).
Presently, there is a gap in our knowledge for how heme availability in extraintestinal tissues such as the hypodermis, muscle, neurons, and germline is correlated with intestinal heme status. A clue comes from depletion of mrp-5, which is expressed at heme concentrations where hrg-1, hrg-4, hrg-2, and hrg-3 are not expressed. Unexpectedly, knockdown of mrp-5 results in a heme deficiency signal in the intestine, i.e. upregulation of hrg-1, despite also resulting in intestinal heme accumulation (Korolnek et al. 2014; Severance et al. 2010). This result suggests that when heme is trapped in the intestine, heme-depleted extraintestinal tissues signal to the intestine to release stored heme. Thus, it appears that though the intestine is the source of all heme in C. elegans, extraintestinal tissue have regulatory input over intestinal heme transport by regulating HRG-1 and possibly other transporters, indicating that heme homeostasis is maintained at an organismal level through an intertissue communication network (Fig. 2). This hypothesis is supported by the results of a genome-wide screen for regulators of heme homeostasis, in which ∼30 % of the candidate genes affected intestinal heme transport despite being expressed exclusively in extraintestinal tissues (manuscript under preparation). Along these lines, it is interesting that the worm has adopted several transcriptional pathways for the upregulation of intestinal genes in low heme. We speculate that individual regulatory circuit responds to specific inputs or simuli. For example, while mrp-5 depletion results in upregulation of hrg-1 through the HERE, hrg-3 is downregulated under the same conditions, suggesting that the HERE responds primarily to extraintestinal signals while the regulation of hrg-3 is dominated by intestinal heme status. In this model, as hrg-1 and mrp-5 both have a HERE, heme mobilization from storage vesicles in the intestine would be released to the extraintestinal tissues for general usage as an initial heme deficiency response, but the directed delivery of heme to embryos would not occur until intestinal heme stores were sufficiently low. These different mechanisms would enable tight control over systemic heme homeostasis and possibly establish a hierarchy of tissue heme distribution under heme limiting conditions. In support of this concept, RNAi knockdown of mrp-5 or hrg-4 results in upregulation of hrg-1, but only knockdown of mrp-5 results in upregulation of hrg-2(Korolnek et al. 2014). This suggests that the intestinal heme stores are mobilized prior to the upregulation of heme-responsive genes in extraintestinal tissues, which are only turned on once systemic heme availability is sufficiently low. The “sensors”, transcription factors or otherwise, that detect heme levels and initiate a response to varying heme conditions in each tissue have yet to be elucidated. Uncovering how each tissue senses and communicates heme levels will be crucial to understanding organismal heme homeostasis.
C. elegans as a model for vertebrate heme homeostasis
Although C. elegans is unique in that it is a free-living heme auxotroph, data suggest that heme homeostatic mechanisms discovered in C. elegans are conserved in metazoans. As noted previously, HRG-1 is involved in the mobilization of heme stored in lysosome-like compartments in the intestine (Rajagopal et al. 2008; Yuan et al. 2012). In mammals, the majority of heme serves as a cofactor for hemoglobin in red blood cells. Remarkably, >90 % of heme–iron is recycled daily by macrophages that reside within the reticuloendothelial system through erythrophagocytosis (EP) of senescent red blood cells. After EP and subsequent red blood cell lysis, heme must be transported across the phagolysosomal membrane before degradation by heme oxygenase-1 (HMOX1) for iron reutilization. This process is mediated by the mammalian HRG1 homolog (White et al. 2013). During EP, Hrg1 localizes to the phagolysosome in mouse bone marrow-derived macrophages and depletion of hrg1 by siRNA following EP results in lower mRNA expression of Hmox1, the iron exporter Fpn1, and reduction in the accumulation of iron storage protein ferritin, all markers of cellular heme and iron levels. In addition to macrophages, HRG1 is expressed in brain, heart, kidney, muscle, placenta, and intestine, suggesting a role for HRG1 beyond heme transport out of phagolysosomes (Rajagopal et al. 2008). Accordingly, in zebrafish, morpholino knockdown of hrg1 results in anemia, hydrocephalus, and a curved body with shortened yolk tube. Remarkably these phenotypes can be rescued with C. elegans HRG1 despite the two proteins sharing only 20 % identity. This demonstrates that mechanisms of heme homeostasis are conserved between the heme auxotroph C. elegans and vertebrates that synthesize heme.
In addition to heme import, mechanisms of heme export are also conserved from C. elegans to mammals. In C. elegans, MRP-5 is required for heme export from the intestine, and is therefore crucial for systemic heme homeostasis. Interestingly, depletion of mrp5 in zebrafish embryos by morpholino knockdowns result in severe anemia phenocopying hrg1 morphants, suggesting a similar function of Mrp5 in vertebrates by which Mrp5 mediated heme export is critical for organismal heme homeostasis. In mammalian cells, MRP5 localizes to both the plasma membrane and endosomal compartments where it appears to facilitate incorporation of heme into hemoproteins in the secretory pathway. Therefore, in addition to its role in cellular heme export, MRP5 may transport heme from the cytosol to the secretory pathway.
In C. elegans, heme homeostasis is maintained at a systemic level through intertissue heme trafficking and cell-non-autonomous signaling. Although it was presumed that in heme prototrophs, such as vertebrates, heme levels are regulated exclusively by a cell's capacity to synthesize and regulate its own heme i.e. cell-autonomous mechanisms, recent evidence suggests that an intercellular and intertissue heme transport system may also exist (Cao and O'Brien 2013; Haldar et al. 2014; Keel et al. 2008; Yang et al. 2010). Considering depletion of heme transporters in vertebrates appears to disrupt systemic heme homeostasis, we postulate that the roundworm C. elegans may prove to be a powerful genetic model to decipher how organs and tissues communicate and integrate systemic heme homeostasis (Korolnek and Hamza 2014; Korolnek et al. 2014; Rajagopal et al. 2008).
Relevance to parasitology
Parasitic worms, termed helminths, are the most prevalent infectious agents of humans in developing countries (Hotez et al. 2008). Moreover, iron deficiency is exacerbated by helminthic infections (Stephenson et al. 2000). In addition to compromising human health, helminths cause enormous economic losses each year through both animal and plant parasitism (de Almeida Engler et al. 2005; Waller 2006). Like C. elegans, helminths including Strongyloides, Ancylostoma, Haemonchus, Trichuris and Ascaris are unable to synthesize heme de novo but must scavenge host heme for survival (Rao et al. 2005). In Ancylostoma and Haemonchus, an ordered proteolytic cascade is involved in hemoglobin digestion and utilization, which presumably serves as a heme source for these parasites (Knox 2011; Williamson et al. 2004). It has also been postulated that heme-regulated, heme-binding members of the glutathione-S-transferase (GST) family in these same genera are involved in transport and detoxification of heme (Atamna and Ginsburg 1995; Perally et al. 2008). In fact, much of the recent attention on vaccination against hookworms has focused on hemoglobin degrading proteases and GSTs (Hotez et al. 2013). Similarly, C. elegans has several heme-regulated proteases and GSTs (Severance et al. 2010). Moreover, the presence of HRG-1 in helminths suggests that mechanisms of heme transport are conserved in parasitic worms (Fig. 3). Taken together, these data indicate that despite the differences in heme sources, mechanisms of heme acquisition are similar between blood-feeders and free -living worms (Severance et al. 2010). Plausibly, C. elegans could be an excellent model for discovery of anthelminthics that target heme acquisition pathways. Drug resistance is already rampant and there is an urgent need for safe and efficacious anthelminthics (Gilleard 2006). Drugs disrupting heme acquisition would deprive parasites of a specific yet crucial micronutrient, thus inhibiting their ability to grow and reproduce.
Fig. 3.
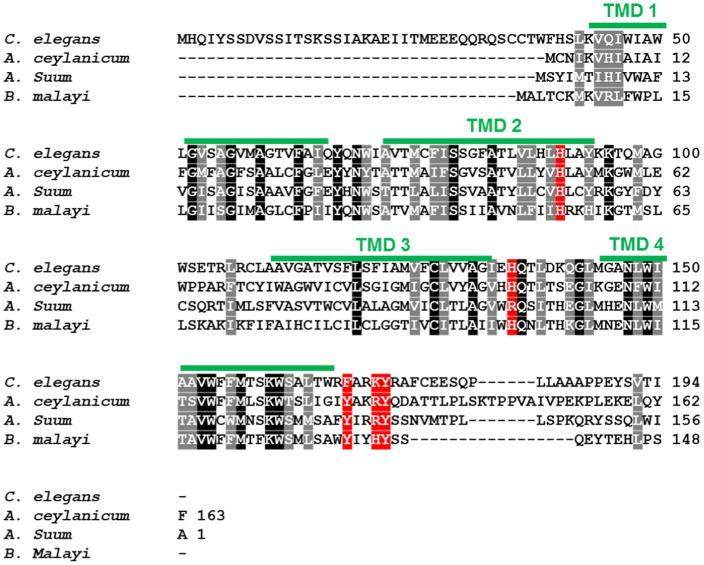
HRG-1 is conserved in helminthic species. ClustalW alignment of HRG-1 in C. elegans, Ancylostoma ceylanicum, Ascaris suum, and Brugia malayi. Identical residues are indicated by white lettering with black background. Similar residues are indicated by white lettering with grey background. Potential conserved (identical or similar) heme interacting residues are indicated by white lettering with red background. Transmembrane domains of C. elegans HRG-1 are indicated by green lines
Exploiting heme acquisition pathways for drug discovery extends beyond just helminths. Parasitic protozoa such as trypanosomatids and plasmodium, which cause diseases such as Chaga's disease and malaria, respectively, also scavenge host heme to meet nutritional requirements (Chang and Chang 1985; Chang et al. 1975; Dutta et al. 2008; Ke et al. 2014). As in multicellular organisms, the ortholog of HRG-1 in Leishmania amazonensis, LHR1, mediates heme uptake and it is likely that other parasitic protozoa utilize similar uptake mechanisms (Huynh et al. 2012). Consequently, uptake pathways discovered in C. elegans can be utilized for therapies against protozoa.
Conclusions
Heme is an essential yet cytotoxic porphyrin. Therefore, heme transport must be a highly regulated process. Although C. elegans are bloodless worms, i.e. they lack a dedicated RBC-dependent circulatory system, it has already proven to be an ideal animal model to study blood formation, anemia, and iron metabolism. Importantly, this microscopic animal straddles both sides of the fence: it has led to the discovery of intracellular and intercellular heme trafficking pathways in humans as well as pathways for heme acquisition by parasites that exacerbate human iron deficiency.
Contributor Information
Jason Sinclair, Department of Animal & Avian Sciences, University of, Maryland, 2413 Animal Sciences Center, College Park, MD 20742, USA, ; Department of Cell Biology & Molecular Genetics, University of Maryland, 2413 Animal Sciences Center, College Park, MD 20742, USA.
Iqbal Hamza, Department of Animal & Avian Sciences, University of, Maryland, 2413 Animal Sciences Center, College Park, MD 20742, USA; Department of Cell Biology & Molecular Genetics, University of Maryland, 2413 Animal Sciences Center, College Park, MD 20742, USA.
References
- Ajioka RS, Phillips JD, Kushner JP. Biosynthesis of heme in mammals. Biochim Biophys Acta. 2006;1763:723–736. doi: 10.1016/j.bbamcr.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Atamna H, Ginsburg H. Heme degradation in the presence of glutathione. A proposed mechanism to account for the high levels of non-heme iron found in the membranes of hemoglobinopathic red blood cells. J Biol Chem. 1995;270:24876–24883. doi: 10.1074/jbc.270.42.24876. [DOI] [PubMed] [Google Scholar]
- Balla G, Vercellotti GM, Muller-Eberhard U, Eaton J, Jacob HS. Exposure of endothelial cells to free heme potentiates damage mediated by granulocytes and toxic oxygen species. Lab Invest. 1991;64:648–655. doi: 10.1016/0006-291X(90)92056-6. [DOI] [PubMed] [Google Scholar]
- Cao C, O'Brien KO. Pregnancy and iron homeostasis: an update. Nutr Rev. 2013;71:35–51. doi: 10.1111/j.1753-4887.2012.00550.x. [DOI] [PubMed] [Google Scholar]
- Chang CS, Chang KP. Heme requirement and acquisition by extracellular and intracellular stages of Leishmania mexicana amazonensis. Mol Biochem Parasitol. 1985;16:267–276. doi: 10.1016/0166-6851(85)90069-6. [DOI] [PubMed] [Google Scholar]
- Chang KP, Chang CS, Sassa S. Heme biosynthesis in bacterium-protozoon symbioses: enzymic defects in host hemoflagellates and complemental role of their intracellular symbiotes. Proc Natl Acad Sci USA. 1975;72:2979–2983. doi: 10.1073/pnas.72.8.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Samuel TK, Sinclair J, Dailey HA, Hamza I. An intercellular heme-trafficking protein delivers maternal heme to the embryo during development in C. elegans. Cell. 2011;145:720–731. doi: 10.1016/j.cell.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Samuel TK, Krause M, Dailey HA, Hamza I. Heme utilization in the Caenorhabditis elegans hypodermal cells is facilitated by heme-responsive gene-2. J Biol Chem. 2012;287:9601–9612. doi: 10.1074/jbc.M111.307694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida Engler J, Favery B, Engler G, Abad P. Loss of susceptibility as an alternative for nematode resistance. Curr Opin Biotechnol. 2005;16:112–117. doi: 10.1016/j.copbio.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Dutta S, Furuyama K, Sassa S, Chang KP. Leishmania spp.: delta-aminolevulinate-inducible neogenesis of porphyria by genetic complementation of incomplete heme biosynthesis pathway. Exp Parasitol. 2008;118:629–636. doi: 10.1016/j.exppara.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilleard JS. Understanding anthelmintic resistance: the need for genomics and genetics. Int J Parasitol. 2006;36:1227–1239. doi: 10.1016/j.ijpara.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Goodwin DC, Rowlinson SW, Marnett LJ. Substitution of tyrosine for the proximal histidine ligand to the heme of prostaglandin endoperoxide synthase 2: implications for the mechanism of cyclooxygenase activation and catalysis. Biochemistry. 2000;39:5422–5432. doi: 10.1021/bi992752f. [DOI] [PubMed] [Google Scholar]
- Grant B, Hirsh D. Receptor-mediated endocytosis in the Caenorhabditis elegans oocyte. Mol Biol Cell. 1999;10:4311–4326. doi: 10.1091/mbc.10.12.4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldar M, et al. Heme-mediated SPI-C induction promotes monocyte differentiation into iron-recycling macrophages. Cell. 2014;156:1223–1234. doi: 10.1016/j.cell.2014.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamza I, Dailey HA. One ring to rule them all: trafficking of heme and heme synthesis intermediates in the metazoans. Biochim Biophys Acta. 2012;1823:1617–1632. doi: 10.1016/j.bbamcr.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins MG, McGhee JD. elt-2, a second GATA factor from the nematode Caenorhabditis elegans. J Biol Chem. 1995;270:14666–14671. doi: 10.1074/jbc.270.24.14666. [DOI] [PubMed] [Google Scholar]
- Hotez PJ, Brindley PJ, Bethony JM, King CH, Pearce EJ, Jacobson J. Helminth infections: the great neglected tropical diseases. J Clin Invest. 2008;118:1311–1321. doi: 10.1172/JCI34261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotez PJ, et al. The human hookworm vaccine. Vaccine. 2013;31(Suppl 2):B227–232. doi: 10.1016/j.vaccine.2012.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh C, et al. Heme uptake by Leishmania amazonensis is mediated by the transmembrane protein LHR1. PLoS Pathog. 2012;8:e1002795. doi: 10.1371/journal.ppat.1002795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke H, et al. The heme biosynthesis pathway is essential for plasmodium falciparum development in mosquito stage but not in blood stages. J Biol Chem. 2014 doi: 10.1074/jbc.M114.615831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keel SB, et al. A heme export protein is required for red blood cell differentiation and iron homeostasis. Science. 2008;319:825–828. doi: 10.1126/science.1151133. [DOI] [PubMed] [Google Scholar]
- Knox D. Proteases in blood-feeding nematodes and their potential as vaccine candidates. Adv Exp Med Biol. 2011;712:155–176. doi: 10.1007/978-1-4419-8414-2_10. [DOI] [PubMed] [Google Scholar]
- Korolnek T, Hamza I. Like iron in the blood of the people: the requirement for heme trafficking in iron metabolism. Front Pharmacol. 2014;5:126. doi: 10.3389/fphar.2014.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korolnek T, Zhang J, Beardsley S, Scheffer GL, Hamza I. Control of metazoan heme homeostasis by a conserved multidrug resistance protein. Cell Metab. 2014;19:1008–1019. doi: 10.1016/j.cmet.2014.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Moenne-Loccoz P, Hildebrand DP, Wilks A, Loehr TM, Mauk AG, Ortiz de Montellano PR. Replacement of the proximal histidine iron ligand by a cysteine or tyrosine converts heme oxygenase to an oxidase. Biochemistry. 1999;38:3733–3743. doi: 10.1021/bi982707s. [DOI] [PubMed] [Google Scholar]
- McKie AT, et al. A novel duodenal iron-regulated transporter, IREG1, implicated in the basolateral transfer of iron to the circulation. Mol Cell. 2000;5:299–309. doi: 10.1016/S1097-2765(00)80425-6. [DOI] [PubMed] [Google Scholar]
- Ohgami RS, et al. Identification of a ferrireductase required for efficient transferrin-dependent iron uptake in erythroid cells. Nat Genet. 2005;37:1264–1269. doi: 10.1038/ng1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perally S, Lacourse EJ, Campbell AM, Brophy PM. Heme transport and detoxification in nematodes: subproteomics evidence of differential role of glutathione transferases. J Proteome Res. 2008 doi: 10.1021/pr800395x. [DOI] [PubMed] [Google Scholar]
- Perutz MF, Rossmann MG, Cullis AF, Muirhead H, Will G, North AC. Structure of haemoglobin: a three-dimensional Fourier synthesis at 5.5-A. resolution, obtained by X-ray analysis. Nature. 1960;185:416–422. doi: 10.1038/185416a0. [DOI] [PubMed] [Google Scholar]
- Ponka P. Cell biology of heme. Am J Med Sci. 1999;318:241–256. doi: 10.1097/00000441-199910000-00004. [DOI] [PubMed] [Google Scholar]
- Rajagopal A, et al. Haem homeostasis is regulated by the conserved and concerted functions of HRG-1 proteins. Nature. 2008;453:1127–1131. doi: 10.1038/nature06934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao AU, Carta LK, Lesuisse E, Hamza I. Lack of heme synthesis in a free-living eukaryote. Proc Natl Acad Sci USA. 2005;102:4270–4275. doi: 10.1073/pnas.0500877102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassa S. The porphyrias. Photodermatol Photoimmunol Photomed. 2002;18:56–67. doi: 10.1034/j.1600-0781.2002.180202.x. [DOI] [PubMed] [Google Scholar]
- Severance S, Hamza I. Trafficking of heme and porphyrins in metazoa. Chem Rev. 2009;109:4596–4616. doi: 10.1021/cr9001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severance S, et al. Genome-wide analysis reveals novel genes essential for heme homeostasis in Caenorhabditis elegans. PLoS Genet. 2010;6:e1001044. doi: 10.1371/journal.pgen.1001044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair J, Hamza I. A novel heme response element mediates transcriptional regulation in Caenorhabditis elegans. J Biol Chem. 2010 doi: 10.1074/jbc.M110.167619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spieth J, Blumenthal T. The Caenorhabditis elegans vitellogenin gene family includes a gene encoding a distantly related protein. Mol Cell Biol. 1985;5:2495–2501. doi: 10.1128/MCB.5.10.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson LS, Latham MC, Ottesen EA. Malnutrition and parasitic helminth infections. Parasitology. 2000;121(S1):S23–38. doi: 10.1017/S0031182000006491. [DOI] [PubMed] [Google Scholar]
- The C. elegans Sequencing Consortium. Genome sequence of the nematode C. elegans: a platform for investigating biology. Science. 1998;282:2012–2018. doi: 10.1126/science.282.5396.2012. [DOI] [PubMed] [Google Scholar]
- Vincent SH. Oxidative effects of heme and porphyrins on proteins and lipids. Semin Hematol. 1989;26:105–113. [PubMed] [Google Scholar]
- Waller PJ. From discovery to development: current industry perspectives for the development of novel methods of helminth control in livestock. Vet Parasitol. 2006;139:1–14. doi: 10.1016/j.vetpar.2006.02.036. [DOI] [PubMed] [Google Scholar]
- West AR, Oates PS. Mechanisms of heme iron absorption: current questions and controversies. World J Gastroenterol. 2008;14:4101–4110. doi: 10.3748/wjg.14.4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White C, et al. HRG1 is essential for heme transport from the phagolysosome of macrophages during erythrophagocytosis. Cell Metab. 2013;17:261–270. doi: 10.1016/j.cmet.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson AL, et al. A multi-enzyme cascade of hemoglobin proteolysis in the intestine of blood-feeding hookworms. J Biol Chem. 2004;279:35950–35957. doi: 10.1074/jbc.M405842200. [DOI] [PubMed] [Google Scholar]
- Yang Z, et al. Kinetics and specificity of feline leukemia virus subgroup C receptor (FLVCR) export function and its dependence on hemopexin. J Biol Chem. 2010;285:28874–28882. doi: 10.1074/jbc.M110.119131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X, Protchenko O, Philpott CC, Hamza I. Topologically conserved residues direct heme transport in HRG-1-related proteins. J Biol Chem. 2012;287:4914–4924. doi: 10.1074/jbc.M111.326785. [DOI] [PMC free article] [PubMed] [Google Scholar]


