Abstract
Cell mediated immune (CMI) responses are crucial for the clearance of human papillomavirus (HPV) infection and HPV-associated lesions. Activated CD8 T cells are critical effector cells in recognizing and killing HPV-infected or HPV-transformed cells. CD4 T cells provide help for priming the generation and maintenance of CD8 T cells as well as for tumors immunity. An ideal therapeutic HPV peptide-based vaccines should induce both a robust CD8 T-cell response as well as a CD4 T-cell response for ensuring their efficiency. Candida skin test reagent was demonstrated to be able to induce the secretion of IL-12 by Langerhans cells and T-cell proliferation in vitro by our group, which indicated the potential of Candida to enhance CMI response. In this current study, we designed a novel HPV peptide-based vaccine which includes HPV16 E7 peptides and Candida as an adjuvant. The immune response induced by the vaccine was comprehensively evaluated. The results showed that the vaccine induced significant HPV-specific CD8 T-cell and Th1 CD4 T-cell responses as well as humoral immune response. It is interesting that Candida alone induced significant polarization of Th1 response and the production of IFN-ɣ, which indicated Candida alone may be used as a potential immunotherapeutic reagent not only for HPV-associated lesions but also for other viral infection or even cancers.
Keywords: adjuvant, Candida, E7, human papillomavirus, peptide, vaccine
1. Introduction
Persistent infection of high-risk human papillomavirus (HPV) is a necessary cause for the occurrence of high-grade cervical intraepithelial neoplasia (CIN), vulvar intraepithelial neoplasia (VIN) and vaginal intraepithelial neoplasia (VaIN), which may develop to cervical cancer, vulvar cancer and vaginal cancer respectively [1,2,3]. Among high risk HPV causing cancers and their precursors, HPV type 16 (HPV16) is the commonest type [4]. Three licensed prophylactic vaccines (Cervarix, Gardasil 4 and Gardasil 9) have been demonstrated to be able to provide efficient protection against HPV infection [5]. However, they have little therapeutic activity for patients with pre-established HPV infections or HPV-associated lesions. Therefore, it is urgent to develop therapeutic HPV vaccines designed for controlling HPV persistent infection or HPV-associated lesions.
Cell mediated immune (CMI) responses are crucial to kill HPV-infected or HPV-transformed cells. In general, most (90%) of HPV infections may be cleared by the hosts’ immune system within 3 years after the initial infection [6], whereas the remaining 10% may become persistent and further develop to lesion with varying severity, even cancer [6, 7]. This may be due to the failure of inducing CMI responses [8, 9]. In HPV-specific CMI mediated clearance of HPV infection and HPV-associated lesions, activated CD8 T cells are crucial effector cells due to their ability of recognizing and killing viral infected cells. CD4 T-cells play important roles by priming the generation of CD8 T cells as well as maintaining CD8 T cells [10]. Moreover, CD4 T cells induced by vaccines clearly have an important role in successful tumor immunity and maintenance of long-term tumor antigen-specific memory responses in vaccinated mice with established tumors [10, 11]. Therefore, an ideal therapeutic HPV vaccines should induce both a robust CD8 T-cell response as well as a CD4 T-cell response for ensuring their efficiency.
The expression of oncoproteins E6 and E7 of high risk HPV types is essential for malignant progress of HPV-associated cancers and maintenance of malignant phenotype of HPV-transformed cells [12]. Oncoprotein E7 is highly conserved and only constitutively expressed in HPV-infected cells or HPV-transformed cells, but not in normal cells. It would be less expensive to produce E7 peptides compared to E6 peptides as E7 is much shorter. Moreover, the correlation between CMI response to HPV16 E7 peptides and regression of HPV-associated lesions has been demonstrated [13, 14]. Therefore, oncoprotein E7 represents an ideal target of HPV therapeutic vaccines. HPV16 E7 peptides covering E7 protein were incorporated in this current novel vaccine.
Candida skin test reagent (Nielsen BioSciences, San Diego, CA) is a colorless extract of Candida albicans. It has been approved by Food and Drug Administration for human use. Application of Candida to treat HPV-induced common warts resulted in resolution of treated warts and distant untreated warts [15,16]. Specific anti-HPV T-cell responses were demonstrated in the complete responders [16]. In our previous study, Candida was demonstrated to be able to induce the secretion of IL-12 by Langerhans cells (LCs) and T-cell proliferation in vitro, which indicated the potential of Candida to enhance cellular immune response [17]. In this current study, we designed a novel HPV therapeutic vaccine which contains HPV16 E7 peptides and Candida as an adjuvant. While the peptides are designed to mount virus-specific T-cell responses, Candida is expected to further enhance the response by general promotion of Th1 response. The immune response was comprehensively evaluated by examining splenocytes proliferation, intracellular cytokine staining (ICS) of splenocytes, cytometric bead array for Th1 and Th2 cytokines in serum, transcription factors of splenocytes, and specific antibodies. It was demonstrated that the vaccine induced significant HPV-specific CD8 T-cell and Th1 CD4 T-cell responses as well as humoral-mediated immune response. It is interesting that Candida alone induced significant the polarization ofTh1 T-cell response and the production of IFN-ɣ, which indicated Candida alone may be used as a potential immunotherapeutic reagent for HPV-associated lesions, such as CIN, VIN and VaIN, or even cancers.
2. Methods
2.1 Adjuvant and peptides
Candida skin test reagent was purchased from Nielsen BioSciences, Inc.(San Diego, CA). Three peptides (HPV16 E7 1–35, HPV16 E7 36–70, HPV16 E7 71–98) covering the HPV16 E7 protein were produced at the GL Biochem (Shanghai) Ltd. (Shanghai, China). Two of them were 35aa (HPV16 E7 1–35, HPV16 E7 36–70) in length and the third one was 28aa ( HPV16 E7 71–98) in length. The purity (95%) of the synthesized peptides was determined by HPLC and the molecular weight was determined by mass spectrometry. The sequences of the peptides used in this study are described as follows:
E7 1–35: MHGDTPTLHE YMLDLQPETT DLYCYEQLND SSEEE
E7 36–70: DEIDGPAGQA EPDRAHYNIV TFCCKCDSTL RLCVQ
E7 71–98: STHVDIRTLE DLLMGTLGIV CPICSQKP
2.2 Mice and immunization
Six to 8-week-old female C57BL/6 mice were purchased from Beijing Huafukang Bioscience Co. Ltd. (Beijing, China). All the mice were housed in specific pathogen-free condition. Mice were randomly divided into four groups. Three experimental groups were immunized intradermally with (1) 150 μl of Candida, (2) 50 μg each of 3 E7 peptides, (3) the vaccine containing 150 μl of Candida and 50 μg each of three E7 peptides in a total volume of 200 μl, respectively. These reagents were mixed well before injection and were administered to mice at two sites (100 μl at each site). Mice immunized with PBS were used as a control group. All groups were boosted with the same amount twice more with a 3-week interval. After 2 weeks post the 3rd immunization, mice were sacrificed by cervical dislocation. All animal experiments were carried out in accordance with the standard guidelines for the proper use and care of animals, which were approved by the laboratory animal care and use committee of the China Medical University.
2.3 Analysis of splenocytes proliferation
Spleens were harvested and single-cell suspensions of splenocytes were prepared as follows. Spleens from individual mice in all groups were harvested and mechanically homogenized in 10 ml of RPMI 1640 supplemented with 10% heat-inactivated FCS, 100 units/ml penicillin, 100 μg/mL streptomycin. Processed cells were filtered using 106 μm filters and red blood cells were depleted using Red Blood Cell Lysis buffer. After washing with PBS, splenocytes were re-suspended in complete culture medium at a concentration of 1× 107 cells/ml.
Splenocytes proliferation was determined using CellTiter 96® AQueous One Solution Cell Proliferation Assay (Promega, Madison, WI, USA). The assay was performed in triplicate wells by culturing splenocytes (2.5×106 cells/ml) in 200 μl of complete culture medium in each well of a 96-well plate. Wells containing cells alone (negative control), cells and 10μM peptides, and cells and 10μg/ml concanavalin (ConA; positive control) from Solarbio Science & Technology Co., Ltd. (Beijing, China) were set up. After 6 days of incubation, 40 μl of CellTiter 96® AQueous One Solution Reagent (Promega) were added to each well and the plate was incubated at 37°C for 3.5 h. The absorbance was measured at 490 nm using BioTek Synergy-2 Multi Plate Reader (Bio-Rad 680, USA). The results were shown as the stimulation index (SI), which is the ratio of the average optical density (OD) value at 490nm of individual wells containing both cells and HPV16 E7 peptides to that of corresponding control wells containing cells alone.
2.4 Analysis of HPV16 E7-specific CD8 and CD4 T-cell responses using intracellular cytokine staining (ICS) followed by flow cytometry analysis
HPV16 E7-specific CD8 and CD4 T-cell responses induced by the vaccine were evaluated using an ICS method. Single-cell suspension of splenocytes were prepared as described above. For intracellular IFN-ɣ staining, splenocytes were plated at 1×106 per well in 96-well plates and re-stimulated with peptides (10 μM) for 4 h at 37 °C. Phorbol 12-myristate 13-acetate (PMA, 1 μg/ml) (Sigma-Aldrich, St. Louis, MO, USA) and ionomycin (50 μg/ml, Sigma-Aldrich) were added and the cells were incubated for 5 h in the presence of Protein Transport Inhibitor Cocktail (500μg/ml, Sigma-Aldrich) for the final 4 h at 37 °C. After stimulation, the cells were first surface stained with FITC-labeled anti-mouse CD4 and PerCP-labeled anti-mouse CD8 for 30 min at 4°C. Subsequently, the cells were washed with buffer, fixed and permeabilized with Cytofix/Cytoperm Solution (Biolegend, San Diego, CA, USA) according to the manufacturer’s instructions. Then, the cells were intracellularly stained with PE-labeled anti-mouse IFN-ɣ (clone XMG1.2) for 30 min at 4°C, washed and fixed with 4% paraformaldehyde (Biolegend). The corresponding isotype control (RTK2071) were used for the staining to minimize the influence of nonspecific binding. All staining procedures were protected from light and performed on ice. All the antibodies were purchased from Biolegend (San Diego, CA, USA). The data was acquired using a FACS Fortessa flow cytometer (BD Biosciences, San Jose, CA, USA) and analyzed using a Flowjo 7.6.1 software (Tree Star, Inc., Ashland, OR, USA).
2.5 Analysis of Th1 and Th2 cytokine in serum using Cytometric Bead Array
To evaluate induced Th1 and Th2 cell responses, cytokines in serum were determined using BD™ Cytometric Bead Array. Blood was harvested from mice in each group at 2 weeks after the 3rd immunization. The serum was collected after centrifugation of blood samples and stored at −80°C immediately. The concentration of Th1-related cytokines (IFN-ɣ, TNF-α and IL-2) and Th2-related cytokines (IL-4 and IL-5) in the serum were measured using mouse Th1/Th2 cytokine kit (BD Biosciences) according to the manufacturer’s protocol. The data was acquired using a FACS Fortessa flow cytometer and analyzed using a Flowjo 7.6.1 software.
2.6 Analysis of Th1, Th2, Treg cells using intranuclear transcript factors staining followed by flow cytometry analysis
For transcript factors staining, the assay was performed using the Foxp3 staining buffer kit, according to the manufacturer’s protocol for True-Nuclear™ Transcription Factor Buffer Set (Biolegend). The PE-labeled anti-mouse FoxP3, APC-labeled anti-mouse GATA3, PE-labeled anti-mouse T-bet antibodies were used in combination with a PerCP-labeled anti-mouse CD25 antibody and the above mentioned FITC-labeled anti-mouse CD4 antibodies. All the antibodies were purchased from Biolegend. The data was acquired using a FACS Fortessa flow cytometer and analyzed using a Flowjo 7.6.1 software.
2.7 Evaluation of humoral-mediated immune responses
Anti-HPV antibody levels were determined using an indirect ELISA. Ninety-six well plates were coated with HPV16 E7 peptides (2 μg/ml) overnight at 4 °C. The plates were washed three times with PBS containing 0.05% (v/v) Tween 20 (PBST) and then incubated with blocking dilute (Dakewe Biotech Co., Ltd., Shenzhen, China) to prevent nonspecific binding for 60 min at 37 °C. After washing the plates with PBST three times, the plates were incubated with serially diluted serum from individual mice in triplicate for 90 min at 37 °C. After washing the plates with PBST, the horseradish peroxidase-conjugated goat anti-mouse IgG (1:5,000) (Jackson ImmunoResearch Laboratories Inc., PA, USA) was added to each well for 2 h. Then the plates were developed using the peroxidase substrate solution as per manufacturer instructions, and the sample OD value was read at 450nm using a microplate reader (Bio-Rad 680, USA). HPV-specific antibody titers were determined as the reciprocal of the highest dilution that provided a reading equal or two-fold greater than the average OD generated in the PBS group.
2.8 Statistical analysis
SPSS 19.0 software (SPSS Inc., Chicago, IL, USA) was used for statistical analyses. The GraphPad Prism 5 software (GraphPad Software Inc., La Jolla, CA, USA) was used to perform figures. Data were described as means ± standard deviation (s.d.). Statistical significance was determined with one-way analysis of variance (ANOVA) followed by the Dunnett’s test for group comparisons. Differences were considered to be statistically significant when the p values were < 0.05, and statistically significant comparisons are indicated in the figures.
3. Results
3.1 Splenocytes proliferation in vitro
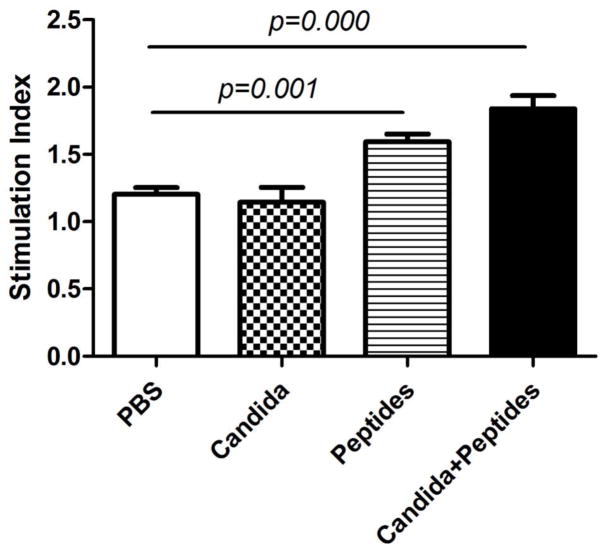
The spleens were obtained from immunized mice at 2 weeks after 3rd immunization. Significantly increased proliferation was shown in groups immunized with the vaccine (p=0.000) and peptides (p=0.001) when compared with the control group immunized with PBS (Fig. 1). No increase was seen with the Candida alone group.
Fig. 1. Splenocytes proliferative response in vitro.
Immunized C57BL mice (n=3) were sacrificed at 2 weeks after the 3rd immunization. The splenocytes were incubated with ConA or HPV peptides for 6 days. The stimulation index are shown. Data are shown as mean ± s.d. Statistically significant differences (p < 0.05) are indicated.
3.2 Induction of cellular immune response
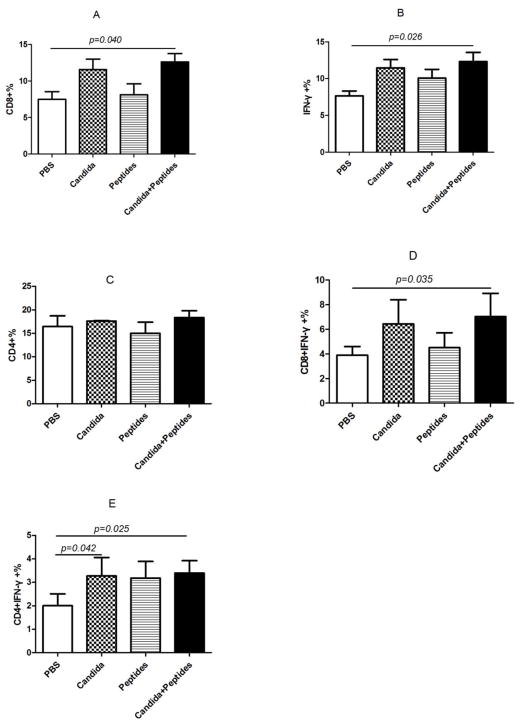
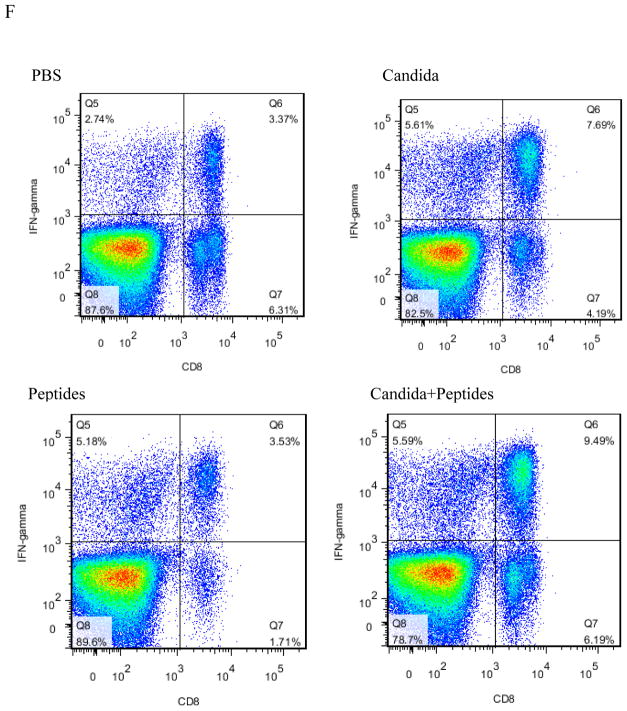
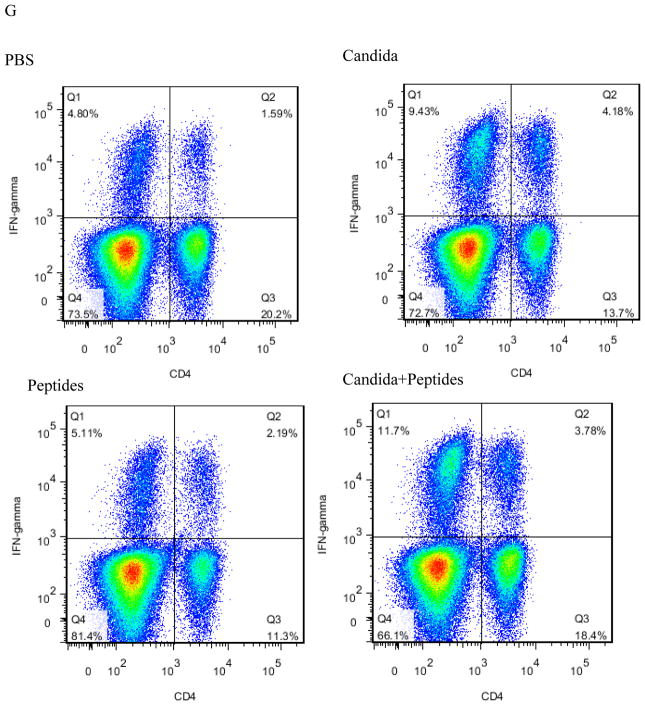
To evaluate the CMI response induced by the vaccine, we determined the percentage of CD8 (Fig. 2A), IFN-ɣ producing (Fig. 2B), CD4 (Fig. 2C), IFN-ɣ producing CD8 (Fig. 2D), IFN-ɣ producing CD4 (Fig. 2E) cells in the splenocytes stimulated with HPV16 E7 peptides and PMA/ionomycin by flow cytometry. The percentage of the cell population expressing CD8 (p=0.040, Fig. 2A) and IFN-ɣ molecule (p=0.026, Fig. 2B) significantly increased only in the vaccine group compared to the PBS control group. Some increases were observed for Candida alone in the percentages of CD8 and CD4 cells, and for E7 peptides for CD4 cells (Fig. 2A and 2C), but they were not statistically significant. The percentage of IFN-ɣ producing CD8 T cells increased significantly in the vaccine group (p=0.035) compared to PBS group (Fig. 2D). The percentage also increased in Candida-treated group but not significantly, and peptides did not have any effect (Fig. 2D). The results showed the percentage of IFN-ɣ producing CD4 T cells in the splenocytes increased significantly in vaccine group (p=0.025) and Candida alone group (p=0.042) compared to the PBS control group (Fig. 2E). The peptide alone group showed an increase over the PBS control group, but the difference was not statistically significant. Representative dot plots from one mouse (out of four mice) for IFN-ɣ producing CD8 cells (Fig. 2F), and IFN-ɣ producing CD4 cells (Fig. 2G) were shown respectively.
Fig. 2. Induction of T cell responses.
Immunized C57BL mice (n=4) were sacrificed at 2 weeks after the 3rd immunization. The splenocytes were incubated with PMA and ionomycin for 5 h after initial stimulation with peptides for 4 h. The frequency of CD8 cells (A), CD4 cells (B), IFN-ɣ producing cells (C), IFN-ɣ producing CD8 cells (D), and IFN-ɣ producing CD4 cells (E) are shown. Representative dot plots from one mouse (out of four mice) for IFN-ɣ producing CD8 cells (F), and IFN-ɣ producing CD4 cells (G) are shown respectively. Data are shown as mean ± s.d. Statistically significant differences (p < 0.05) are indicated.
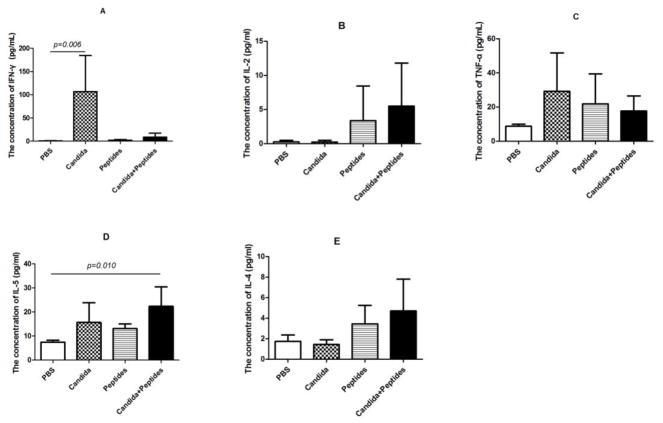
3.3 Induction of Th1 and Th2 cytokines in serum
The priming of CD4 T cell response is an essential step in vaccination due to the key role of Th cells in developing both effector and memory immune responses. In order to evaluate the induction of Th differentiation by the vaccine, Th1-associated cytokines (IFN-ɣ, IL-2 and TNF-α) and Th2-associated cytokines (IL-4 and IL-5) in serum were determined using a Cytometric Bead Array. Remarkably, mice immunized with Candida alone generated statistically significant increased IFN-ɣ compared to control group but not with peptide alone. Unexpectedly, the vaccine didn’t induced significant increased IFN-ɣ production compared to the control group (Fig. 3A). While the increases in IL-2 (Fig. 3B) and TNF-α (Fig. 3C) in the vaccine group were more noticeably, they were not statistically significant. As for Th2 cytokines, immunization of mice with the vaccine elicited statistically significant increased IL-5 but the increases were not significant for Candida alone or peptide alone (Fig. 3D). While increased IL-4 levels were observed with the peptide and vaccine groups, the increases were not statistically significant (Fig. 3E).
Fig. 3. Induction of Th1 and Th2 cytokines in serum.
The serum from immunized C57BL mice (n=4) were collected at 2 weeks after the 3rd immunization. Th1 cytokines (IFN-ɣ, IL-2 and TNF-α) and Th2 cytokines (IL-4 and IL-5) in serum were determined by CBA. The levels of IFN-ɣ (A), IL-2 (B), IL-5 (C), TNF-α (D) and IL-4 (E) in immunized mice are shown. Data are shown as mean ± s.d. Statistically significant differences (p < 0.05) are indicated.
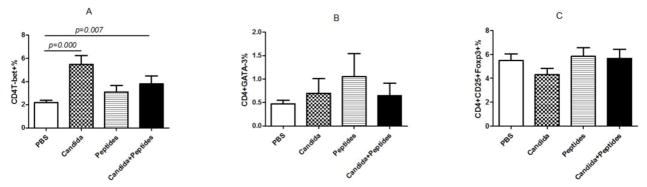
3.4 Induction of Th differentiation
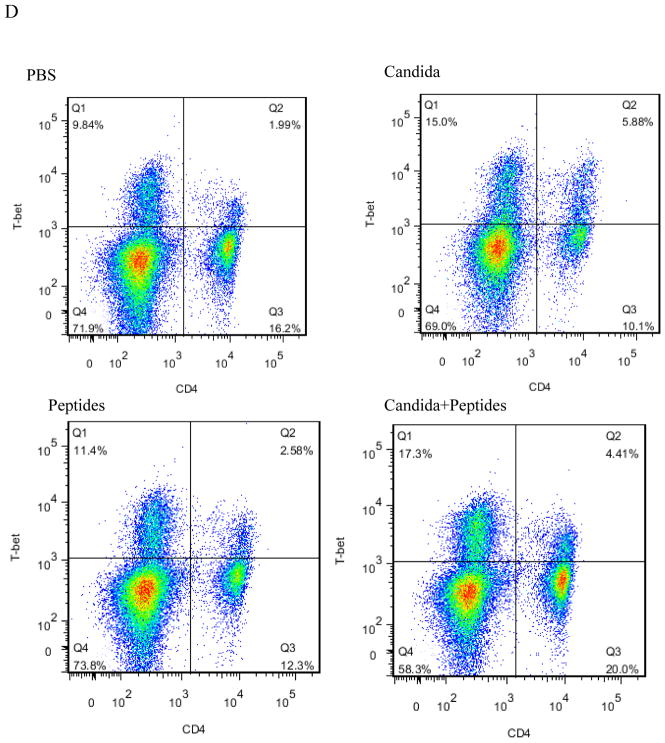
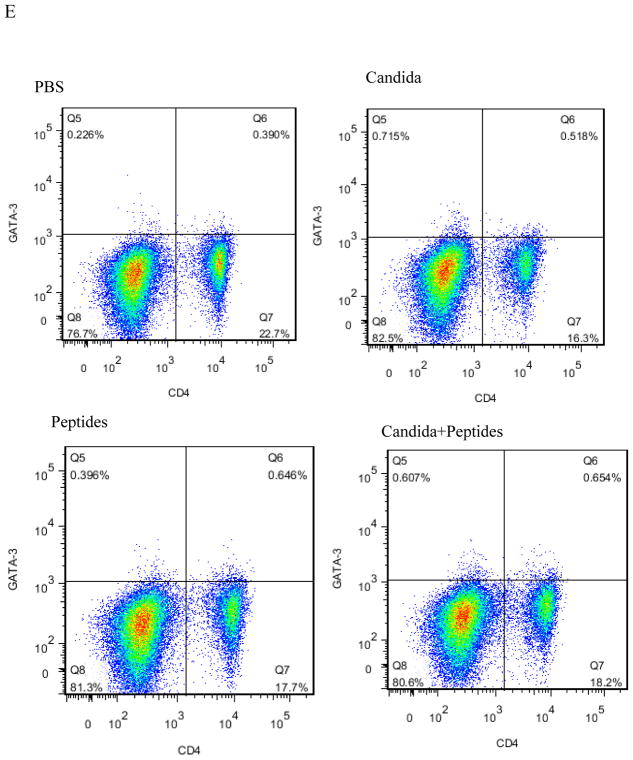
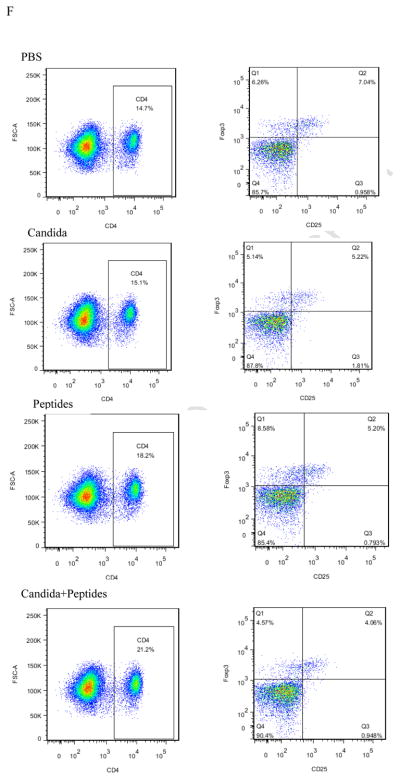
Having observed the Th1-skewed response in Candida group based on the result of significant secretion of IFN-ɣ, we re-examined the Th1 response by determining the incidences of Th1, Th2 and T regulatory cells in the splenocytes. Splenocytes of immunized mice with the vaccine (p=0.007) and Candida alone (p=0.000) showed statistically significant increases in the percentages of CD4+T-bet+ cells compared with the control (Fig. 4A). No significant differences in the level of CD4+GATA-3+ and CD4+CD25+FoxP3+ were shown in the immunized groups compared to the PBS control group (Fig. 4B and 4C). Representative dot plots from one mouse (out of four mice) for CD4+T-bet+ cell (Fig. 4D), CD4+GATA-3+ cells (Fig. 4E), and CD4+CD25+FoxP3+ cells (Fig. 4F) are shown respectively.
Fig. 4. Induction of Th differentiation.
The intracellular cytokine staining of splenocytes in immunized C57BL mice (n=4) was performed at 2 weeks after the 3rd immunization. The frequency of CD4T-bet+ cells (A), CD4+GATA-3+ cells (B) and CD4+CD25+FoxP3+ cells (C) are shown. Representative dot plots from one mouse (out of four mice) for CD4+T-bet+ cell (D), CD4+GATA-3+ cells (E), and CD4+CD25+FoxP3+ cells (F) are shown respectively. Data are shown as mean ± s.d. Statistically significant differences (p < 0.05) are indicated.
3.5 Induction of HPV-specific humoral immune response
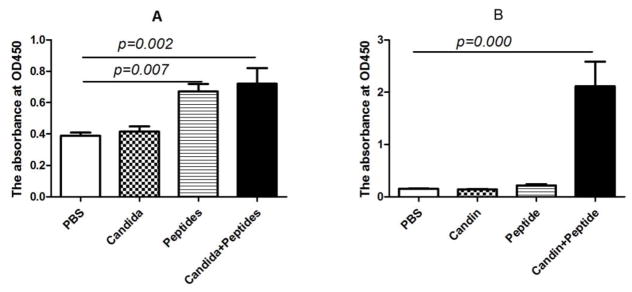
In order to assess the humoral immune response induced by the vaccine, we determined the titer of anti-HPV E7 IgG antibody in serum collected from mice at weeks 3, 6, and 8 by ELISA. The HPV-specific antibodies were first detectable at week 6 in the group immunized with the E7 peptides (p=0.007) and the vaccine (p=0.002, Fig. 5A). Successive immunization induced intensely increased antibody as the endpoint titer was 1:400 at week 6 (Fig. 5A) and was over 1:12 800 at week 8 (Fig. 5B) in group immunized with the vaccine. While the vaccine induced significantly increased HPV-specific antibody at week 8, Candida alone and peptides alone did not (Fib. 5B).
Figure 5. Induction of HPV-specific antibody responses.
Serum samples (n = 4) were collected from immunized mice at weeks 6 and 8. Data are shown as mean ± s.d at 1:400 dilution of serum collected at week 6 (A) and at 1:12 800 dilution (B) of serum collected at week 8. Data are shown as mean ± s.d. Statistically significant differences (p < 0.05) are indicated.
4. Discussion
CMI response was demonstrated to be correlated with a favorable clinical trend [18, 19, 20, 21]. In a clinical trial involving patients with HPV-associated vulvar intraepithelial neoplasia, the correlation between the clinical efficacy and T-cell responses induced by the HPV peptides-based vaccine with an incomplete Freund’s adjuvant was assessed. Patients with a complete response had a significantly stronger IFN-ɣ-associated proliferative CD4 T-cell response and a broad response of IFN-ɣ producing CD8 T cells than did patients without a complete response [20]. In this current study, we comprehensively evaluated CD8 T-cell and CD4 T-cell responses induced by a novel HPV therapeutic vaccine in mice. When splenocytes proliferation was examined, statistically significant increases were seen in HPV 16 E7 peptide and the vaccine groups compared to the PBS negative control (Fig. 1). These results corroborate our earlier work using human cells that HPV 16 E6 peptides plus Candida induces T-cell proliferation in vitro[17]. However, in the human system, Candida alone also demonstrated significantly increased T-cell proliferation while the HPV 16 E6 peptides did not [17]. While the different results with the peptides may be due to the differences in their inherent properties, it is not clear why Candida could induce proliferation in the human system but not in mice system with respect to proliferation.
In the current work, the percentages of IFN-ɣ producing CD8 T cells and CD4 T cells in splenocytes were increased in the vaccine group, which indicated that the vaccine induced the generation and activation of HPV-specific CD8 T cells and CD4 T cells. Significant increased CD8 T-cell response as well as CD4 T-cell response induced by vaccine may promise a favorable clinical outcome [20], which would need to be investigated in the future clinical trials. It was interesting that Candida alone induced significant increase in IFN-ɣ producing CD4 cells (Fig. 2E), in IFN-ɣ level in serum (Fig 3A), and in induction of Th1 cells (Fig 4A). Candida alone also induced an increased CD8 T-cell response although this increase did not reach a statistical significance. It is conceivable that Candida may be useful as potential inclusion in designing immunotherapeutic and immunoprophylactic reagents not only for HPV-associated lesions but also for other viral infection or even cancers.
The immune response induced by vaccines is often characterized by a dominance of either CMI or humoral mediated response, which has been attributed to a dichotomy in the cytokine production profiles of Th cells. Th1 cells producing IFN-ɣ and IL-2 favor cellular immune response, and Th2 cells producing IL-4 and IL-5 favor humoral immune responses. IFN-ɣ produced by Th1 cells in turn results in more Th1 cells differentiation from naïve CD4+ T (Th0) cells, representing a positive feedback, while suppressing Th2 cell differentiation. Moreover, IFN-ɣ has been considered to be the most crucial cytokine for immunity against intracellular pathogens and for tumors control [22]. In immunity against viral infection, the importance of IFN-ɣ arises in part from its ability to inhibit viral replication, and more importantly from its immunostimulatory and immunomodulatory effects [23]. In patients with CIN, IFN-ɣ may be a prognostic marker for clearance of high-risk HPV [24]. In this study, Candida alone induced statistically significantly increased IFN-ɣ in serum which demonstrated the potential of Candida alone to enhance CMI response and indicated the promising prognosis in patients with HPV-associated lesions when treated with Candida alone. IL-5 mainly produced by Th2 cells stimulates B cell growth and enhance antibody secretion [25]. The vaccine significantly induced IL-5 production but not IL-4, which was not consistent with the classical paradigm of Th2 cytokines profile. The reason may be that each Thl (IFN-ɣ and IL-2) and Th2 (IL-4 and IL-5) cytokine gene is independently regulated in T cells and that the dichotomy in the cytokine profiles of Thl and Th2 cells is not due to a direct differentiation-inducing effect of regulatory cytokines, but rather to the following selective mechanisms [26]. Candida alone only induced a significant secretion of IFN-ɣ, but not TNF-α. This can be advantageous considering that TNF-α is a prototypical proinflammatory cytokine [27] whose overproduction may result in the occurrence of host tissue injury [28]. Though significant Th1 response was shown in the vaccine group, no significant production of Th1 cytokines (IFN-ɣ, IL-2 and TNF-α) in serum was demonstrated. A closer examination shows that the vaccine did increase IL-2 level multiple fold over the PBS negative control. It may be that there are underlying differences as to how the Candida and vaccine group may induce Th1 differentiation, i.e., Candida may relay on IFN-ɣ production while the vaccine may rely on IL-2 production.
The differentiation of precusor Th0 cells into Th1 or Th2 cells are influenced by local cytokines profiles as the most crucial factors. It is also influenced by the types of antigen presenting cells (APCs), and the costimulatory molecules on APCs [29]. IL-12 is the dominant factor for the differentiation of Th0 into Th1. CD80 is also a factor promoting the differentiation of Th0 into Th1 [30, 31]. In our previous study, Candida has been demonstrated to induce the secretion of IL-12 by LCs and enhance the expression of CD80 on LCs [17]. Therefore, it is inferred that Candida has the capacity of promoting the Th1 polarization. T-bet is a key transcription factor for Th1 differentiation and GATA3 is specific for alternative Th2 differentiation [32]. T-bet directly induces IFN-ɣ production [33] which in turn enhances the expression of T-bet and the development of Th1 cells. The vaccine significantly induced the expression of T-bet which indicated the increased Th1 response that otherwise elicit minimal cellular immune responses with a Th2 bias. The results were consistent with a significantly increased systemic level of Th1 cells induced by an HPV therapeutic vaccine with E6 peptides in the phase I clinical trial by our group [34]. In this vaccine, we incorporated E7 peptides as a more economical and efficacious alternative. Also we evaluated CD8 T-cell responses induced by the vaccine and the immune responses induced by Candida alone which were not included in that clinical trial. It was interesting that Candida alone induced a significant Th1 polarization which confirmed the ability of Candida alone to enhance cellular immune response. Considering the application of Candida alone resulted in resolution of common warts, we believed that Candida alone may be used as a potential immunotherapeutic reagent for other HPV-associated lesions such as cervical intraepithelial neoplasia and vulvar intraepithelial neoplasia. Candida alone group has been included in the ongoing phase II clinical trial by our group.
It has been demonstrated that seropositivity for anti-HPV16 E6/E7 was associated with a better HPV-associated head and neck squamous cell carcinoma prognosis. Patients with oropharyngeal cancer who test positive for anti-HPV16 E6/E7 survived longer than those who tested negative [35]. Though vaccine-induced anti-HPV16 E7 may be not neutralizing antibodies, the ability of Candida to induce humoral mediated immune response was demonstrated which indicated wide applicable prospect of Candida in vaccines designed to boost not only CMI but also humoral mediated responses. If additional peptides from viral L1 or L2 protein are incorporated into the vaccine, the protective antibodies may be induced by the vaccine which will be used as a prophylactic vaccine aside from as a therapeutic vaccine. Though the capacity of the vaccine to induce CMI and humoral mediated responses in healthy animals was demonstrated in this study, it is not clear in the case of preexisting tumors and it needs to be investigated using tumor-bearing animal models in the future.
Taken together, the results in this study showed that the vaccine with HPV 16 E7 peptides and Candida induced significant CMI response and humoral mediated response; Candida alone enhanced CMI response. The results suggested that 1) inoculation of Candida alone or the vaccine with HPV16 E7 peptides and Candida may represent a novel therapeutic reagent to treat patients with HPV-associated lesions including CIN, VIN, VaIN and even cancers, and 2) the potential of Candida as an immunotherapeutic reagent for viral infection whose elimination mainly depends on the CMI.
Highlights.
The vaccine induced HPV-specific CD8 and IFN-ɣ secreting CD4 T-cell responses.
The vaccine induced HPV-specific humoral immune response.
Candida alone induced IFN-ɣ secreting CD4 response and IFN-ɣ production.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No.81472439), and the National Institutes of Health (R01CA143130).
Abbreviations
- ADCC
antibody-dependent cellular cytotoxicity
- APCs
antigen presenting cells
- C. albicans
Candida albicans
- CIN
cervical intraepithelial neoplasia
- CMI
cell mediated immune
- ICS
intracellular cytokine staining
- LCs
Langerhans cells
- HPV
human papillomavirus
- s.d
standard deviation
- Th
T helper
- VaIN
vaginal intraepithelial neoplasia
- VIN
vulvar intraepithelial neoplasia
Footnotes
Author Contributions Statement
Study concept and design: MN and XW. Acquisition of data: XW, YC, BC, and YZ. Analysis and interpretation of data: XW, YC, BC, YZ, MN, XW. All authors were involved in drafting and critical revision of the manuscript. All authors approved the final version.
Conflicts of Interest Statement
Mayumi Nakagawa is one of the inventors named in a patent and patent applications describing PepCan, a therapeutic HPV vaccine containing HPV 16 E6 peptides and Candida. The other authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002;2:342–350. doi: 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]
- 2.Insinga RP, Liaw KL, Johnson LG, Madeleine MM. A systematic review of the prevalence and attribution of human papillomavirus types among cervical, vaginal, and vulvar precancers and cancers in the United States. Cancer Epidemiol Biomarkers Prev. 2008;17:1611–1622. doi: 10.1158/1055-9965.EPI-07-2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Denny L. Epidemiology and Burden of Disease Associated with HPV Infection. Current Obstetrics & Gynecology Reports. 2016;5(3):189–195. [Google Scholar]
- 4.Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ, Munoz N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 5.Mariani L, Venuti A. HPV vaccine: an overview of immune response, clinical protection, and new approaches for the future. J Transl Med. 2010;8:105. doi: 10.1186/1479-5876-8-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deligeoroglou E, Giannouli A, Athanasopoulos N, Karountzos V, Vatopoulou A, Dimopoulos K, Creatsas G. HPV infection: immunological aspects and their utility in future therapy. Infect Dis Obstet Gynecol. 2013;2013:540850. doi: 10.1155/2013/540850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghittoni R, Accardi R, Chiocca S, Tommasino M. Role of human papillomaviruses in carcinogenesis. Ecancermedicalscience. 2015;9:526. doi: 10.3332/ecancer.2015.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Welters MJP, de Jong A, van den Eeden SJF, et al. Frequent display of human papillomavirus type 16 E6-specific memory T-helper cells in the healthy population as witness of previous viral encounter. Cancer Res. 2003;63(3):636–641. [PubMed] [Google Scholar]
- 9.Nakagawa M, Stites DP, Patel S, Farhat S, Scott M, Hills NK, Palefsky JM, Moscicki AB. Persistence of human papillomavirus type 16 infection is associated with lack of cytotoxic T lymphocyte response to the E6 antigens. J Infect Dis. 2000;182(2):595–598. doi: 10.1086/315706. [DOI] [PubMed] [Google Scholar]
- 10.Lin CT, Chang TC, Shaw SW, Cheng PJ, Huang CT, Chao A, Soong YK, Lai CH. Maintenance of CD8 effector T cells by CD4 helper T cells eradicates growing tumors and promotes long-term tumor immunity. Vaccine. 2006;24(37–39):6199–6207. doi: 10.1016/j.vaccine.2006.05.108. [DOI] [PubMed] [Google Scholar]
- 11.Wu CY, Monie A, Pang X, Hung CF, Wu TC. Improving therapeutic HPV peptide-based vaccine potency by enhancing CD4+ T help and dendritic cell activation. J Biomed Sci. 2010;17:88. doi: 10.1186/1423-0127-17-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feller L, Wood NH, Khammissa RA, Lemmer J. Human papillomavirus-mediated carcinogenesis and HPV-associated oral andoropharyngeal squamous cell carcinoma. Part 1: human papillomavirus-mediatedcarcinogenesis. Head Face Med. 2010;6:14. doi: 10.1186/1746-160X-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kadish AS, Timmins P, Wang Y, Ho GY, Burk RD, Ketz J, He W, Romney SL, Johnson A, Angeletti R, Abadi M Albert Einstein Cervix Dysplasia Clinical Consortium. Regression of cervical intraepithelial neoplasia and loss of human papillomavirus (HPV) infection is associated with cell-mediated immune responses to an HPV type 16 E7 peptide. Cancer Epidemiol Biomarkers Prev. 2002;11(5):483–488. [PubMed] [Google Scholar]
- 14.Sarkar AK, Tortolero-Luna G, Follen M, Sastry KJ. Inverse correlation of cellular immune responses specific to synthetic peptides from the E6 and E7 oncoproteins of HPV-16 with recurrence of cervical intraepithelial neoplasia in a cross-sectional study. Gynecol Oncol. 2005;99(3 Suppl 1):S251–261. doi: 10.1016/j.ygyno.2005.07.099. [DOI] [PubMed] [Google Scholar]
- 15.Johnson SM1, Roberson PK, Horn TD. Intralesional injection of mumps or Candida skin test antigens: a novel immunotherapy for warts. Arch Dermatol. 2001 Apr;137(4):451–455. [PubMed] [Google Scholar]
- 16.Kim KH, Horn TD, Pharis J, Kincannon J, Jones R, O’Bryan K, Myers J, Nakagawa M. Phase 1 clinical trial of intralesional injection of Candida antigen for the treatment of warts. Arch Dermatol. 2010;146(12):1431–1433. doi: 10.1001/archdermatol.2010.350. [DOI] [PubMed] [Google Scholar]
- 17.Wang X, Coleman HN, Nagarajan U, Spencer HJ, Nakagawa M. Candida skin test reagent as a novel adjuvant for a human papillomavirus peptide-based therapeutic vaccine. Vaccine. 2013;31(49):5806–5813. doi: 10.1016/j.vaccine.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farhat S, Nakagawa M, Moscicki AB. Cell-mediated immune responses to human papillomavirus 16 E6 and E7 antigens as measured by interferon gammaenzyme-linked immunospot in women with cleared or persistent human papil-lomavirus infection. Int J Gynecol Cancer. 2009;19(4):508–512. doi: 10.1111/IGC.0b013e3181a388c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakagawa M, Gupta SK, Coleman HN, Sellers MA, Banken JA, Greenfield WW. A favorable clinical trend is associated with CD8 T-cell immune responses to the human papillomavirus type 16 e6 antigens in women being studied for abnormal pap smear results. J Low Genit Tract Dis. 2010;14(2):124–129. doi: 10.1097/LGT.0b013e3181c6f01e. [DOI] [PubMed] [Google Scholar]
- 20.Kenter GG, Welters MJ, Valentijn AR, Lowik MJ, Berends-van der Meer DM, Vloon AP, Essahsah F, Fathers LM, Offringa R, Drijfhout JW, Wafelman AR, Oostendorp J, Fleuren GJ, van der Burg SH, Melief CJ. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N Engl J Med. 2009;361(19):1838–1847. doi: 10.1056/NEJMoa0810097. [DOI] [PubMed] [Google Scholar]
- 21.Coleman HN, Greenfield WW, Stratton SL, Vaughn R, Kieber A, Moerman-Herzog AM, Spencer HJ, Hitt WC, Quick CM, Hutchins LF, Mackintosh SG, Edmondson RD, Erickson SW, Nakagawa M. Human papillomavirus type 16 viral load is decreased following a therapeutic vaccination. Cancer Immunol Immunother. 2016;65(5):563–573. doi: 10.1007/s00262-016-1821-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schoenborn JR, Wilson CB. Regulation of interferon-gamma during innate and adaptive immune responses. Adv Immunol. 2007;96:41–101. doi: 10.1016/S0065-2776(07)96002-2. [DOI] [PubMed] [Google Scholar]
- 23.Gattoni A, Parlato A, Vangieri B, Bresciani M, Derna R. Interferon-gamma: biologic functions and HCV therapy (type I/II) (1 of 2 parts) Clin Ter. 2006;157(4):377–386. [PubMed] [Google Scholar]
- 24.Song SH, Lee JK, Lee NW, Saw HS, Kang JS, Lee KW. Interferon-gamma (IFN-gamma): a possible prognostic marker for clearance of high-risk human papillomavirus (HPV) Gynecol Oncol. 2008;108(3):543–548. doi: 10.1016/j.ygyno.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 25.Wetzel GD. Interleukin 5 regulation of peritoneal B-cell proliferation and antibody secretion. Scand J Immunol. 1990 Jan;31(1):91–101. doi: 10.1111/j.1365-3083.1990.tb02747.x. [DOI] [PubMed] [Google Scholar]
- 26.Gerosa F, Paganin C, Peritt D, Paiola F, Scupoli MT, Aste-Amezaga M, Frank I, Trinchieri G. Interleukin-12 primes human CD4 and CD8 T cell clones for high production of both interferon-gamma and interleukin-10. J Exp Med. 1996;183(6):2559–2569. doi: 10.1084/jem.183.6.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Criscione LG, St Clair EW. Tumor necrosis factor-α antagonists for the treatment of rheumatic diseases. Curr Opin Rheumatol. 2002;14:204–211. doi: 10.1097/00002281-200205000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Abuzakouk M, Feighery C, Jackson J. Tumor necrosis factor blocking agents: a new therapeutic modality for inflammatory disorders. Br J Biomed Sci. 2002;59:173–179. doi: 10.1080/09674845.2002.11783656. [DOI] [PubMed] [Google Scholar]
- 29.Zygmunt B, Veldhoen M. T helper cell differentiation more than just cytokines. Adv Immunol. 2011;109:159–196. doi: 10.1016/B978-0-12-387664-5.00005-4. [DOI] [PubMed] [Google Scholar]
- 30.Hilkens CM, Kalinski P, de Boer M, Kapsenberg ML. Human dendritic cells require exogenous interleukin-12-inducing factors to direct the development of naive T-helper cells toward the Th1 phenotype. Blood. 1997 Sep 1;90(5):1920–1926. [PubMed] [Google Scholar]
- 31.Palmer EM, van Seventer GA. Human T helper cell differentiation is regulated by the combined action of cytokines and accessory cell-dependent costimulatory signals. J Immunol. 1997 Mar 15;158(6):2654–2662. [PubMed] [Google Scholar]
- 32.Gagliani N, Huber S. Basic Aspects of T Helper Cell Differentiation. Methods Mol Biol. 2017;1514:19–30. doi: 10.1007/978-1-4939-6548-9_2. [DOI] [PubMed] [Google Scholar]
- 33.Oh S, Hwang ES. The role of protein modifications of T-bet in cytokine production and differentiation of T helper cells. J Immunol Res. 2014;2014:589672. doi: 10.1155/2014/589672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greenfield William W, Stratton Shawna L, Myrick Rebecca S, Vaughn Rita, Donnalley Lisa M, Coleman Hannah N, Mercado Maria, Moerman-Herzog Andrea M, Spencer Horace J, Andrews-Collins Nancy R, Hitt Wilbur C, Low Gordon M, Manning Nirvana A, McKelvey Samantha S, Smith Dora, Smith Michael V, Phillips Amy M, Matthew Quick C, Jeffus Susanne K, Hutchins Laura F, Nakagawa Mayumi. A phase I dose-escalation clinical trial of a peptide-based human papillomavirus therapeuticvaccine with Candida skin test reagent as a novel vaccine adjuvant for treating women with biopsy-proven cervical intraepithelial neoplasia 2/3. Oncoimmunology. 2015;4(10):e1031439. doi: 10.1080/2162402X.2015.1031439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.López RV1, Levi JE, Eluf-Neto J, Koifman RJ, Koifman S, Curado MP, Michaluart-Junior P, Figueiredo DL, Saggioro FP, de Carvalho MB, Kowalski LP, Abrahão M, de Góis-Filho F, Tajara EH, Waterboer T, Boffetta P, Brennan P, Wünsch-Filho V. Human papillomavirus (HPV) 16 and the prognosis of head and neck cancer in a geographical region with a low prevalence of HPV infection. Cancer Causes Control. 2014;25(4):461–471. doi: 10.1007/s10552-014-0348-8. [DOI] [PubMed] [Google Scholar]