Abstract
Patient: Female, 14
Final Diagnosis: Myopericarditis
Symptoms: Cardiac tamponade • dyspnea • pericardial effusion • tachycardia
Medication: —
Clinical Procedure: Cardiopulmonary resuscitation
Specialty: Infectious Diseases
Objective:
Unusual clinical course
Background:
Influenza viruses induce uncomplicated infections in most cases in individuals with no known predisposing factors. Acute febrile illness is generally limited to upper respiratory symptoms and several constitutional symptoms, including headache, lethargy, and myalgia. However, influenza A virus is a cause of severe morbidity and mortality worldwide. Some patients are at risk for serious and fatal complications. Cardiac involvement is a well-known condition, but, clinically apparent influenza myocarditis is not common. Few reports exist regarding recurrent fulminant influenza myocarditis.
Case Report:
We report here a fatal case of heart failure following myocarditis in a 14-year-old female who had seasonal flu symptoms but was otherwise healthy. H3N2 influenza virus infection was detected by molecular analyses of throat and nasal swabs, suggesting damage to myocardial cells caused directly by the virus.
Conclusions:
Pericardial effusion myopericarditis may occur during influenza virus infection in young individuals, even those with no known predisposing factors. Physicians need to be aware that acute myopericarditis can be a fatal complication of recent influenza virus infection in all patients with instable hemodynamics. Early diagnosis and treatment could reduce, in some cases, the risk of severe cardiac events. However, this sudden and fatal outcome was difficult to predict in a healthy young female with no known risk factors.
MeSH Keywords: Cardiac Tamponade; Orthomyxoviridae; Phylogeny; Sequence Analysis, RNA
Background
Influenza is an acute febrile illness inducing uncomplicated infections in most cases in healthy individuals. However, every year, the global burden of influenza is believed to be 3 to 5 million cases of severe illness. Up to 650 000 deaths annually are associated with respiratory diseases from seasonal influenza, according to new estimates by the World Health Organization, the United States Center for Disease Control and Prevention (US-CDC) and global health partners. In the developed world, most influenza-associated deaths occur in the elderly, but fatal outcomes occur regularly in young adults and children. Some patients are at risk for serious and fatal complications affecting multiple organ systems [1]. In cases of seasonal influenza infection, cardiac involvement is a well-recognized condition, but myopericarditis and pericardial effusion are rarely present. Myocarditis is an inflammatory disease of the myocardium associated with cardiac dysfunction. Viruses are the most prevalent infectious cause of myocarditis and pericarditis. The etiological agents of viral myocarditis include enteroviruses, adenoviruses, parvoviruses, cytomegalovirus, and influenza viruses [2]. In 1945, Finland et al. reported the first 2 patients with influenza myocarditis [3]. Influenza myocarditis leading to a fatal outcome (fulminant form) does exist, with a reported mortality rate of 28% among myocarditis patients in pandemic and post-pandemic seasons in Japan [2]. A few case reports [4,5] represent the incidental diagnoses of influenza-associated acute myocarditis, but the true prevalence remains unknown [6–8]. This prevalence is not known because of the lack of comprehensive screening, with few clinical cases and autopsy findings reported in the literature [4,5,9].
Here, we report a fatal case of myopericarditis associated with a possible cardiac tamponade in a 14-year-old female patient with the flu but no known risk factors for severe complications. We discuss the role of the influenza virus in myopericarditis.
Case Report
On January 30, 2015, a 14-year-old female patient developed a flu-like condition with a high fever (39°C). Her general condition was otherwise satisfactory and there were no functional impairments. She was seen by a general practitioner, who prescribed antipyretics. The next day, the patient developed abdominal pain, bilateral leg pain, and became afebrile at 35°C. Her neck remained supple and her skin showed no signs of blotch or purpura. The parents called the emergency medical services at approximately 6:30 p.m. Thirty minutes later, the patient’s clinical condition worsened. At the arrival of the paramedical team, she was having difficulty breathing with tachypnea, she also described referred pain to the abdomen and thereafter lost consciousness. That team observed tachycardia (200 beats/min) and low peripheral oxygen saturation (60%): they were equipped with a simple pulse oximeter whose monitor displays only peripheral oxygen saturation values of hemoglobin and pulse. The waveform was not viewable. The team did not have enough time to take blood pressure before the patient went into cardiorespiratory arrest. The paramedical team initiated a cardiac massage and employed an automated external defibrillator. Thereafter, a team of emergency physicians arrived for prehospital intensive care. They intubated and ventilated the patient, administered epinephrine (15 mg intravenously and 3 mg intratracheally), norepinephrine (1 µg/kg/min) and bicarbonates, and performed cardiopulmonary resuscitation. The medical team did not have a cardiac ultrasound with them. Death was pronounced at 8:30 p.m., after 1 hour of resuscitation efforts.
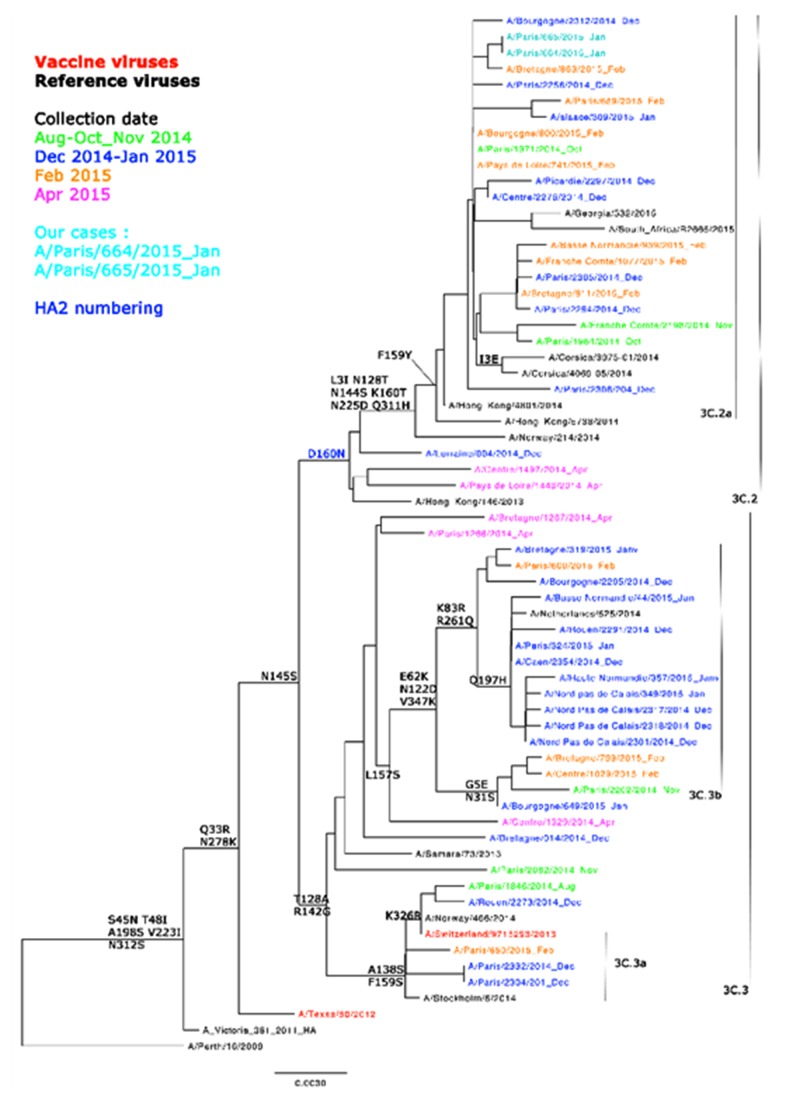
Several samples of fluids and tissues collected during the autopsy were subjected to molecular analyses. Real time (RT)-PCR analyses of both nasopharyngeal and throat specimens were positive for A (H3N2) influenza. The patient’s myocardial tissue, pericardial fluid, and cerebrospinal fluid were negative for influenza virus. An aliquot of the respiratory sample was inoculated onto MDCK-cells to isolate the virus and characterize antigens by hemagglutination inhibition assay. The viral strain had antigenic similarities to A/Switzerland/9715293/2013, which was the most widely circulating influenza virus that season. Genetic characterization was performed by conventional sequencing of the hemagglutinin (HA) and neuraminidase (NA) genes. For phylogenetic analyses, nucleotide sequences were aligned to A (H3N2) reference strains. The virus was found to belong to the 3C.2a clade, the most frequent of the H3N2 strains circulating during the winter of 2014–2015 (Figure 1). Regarding resistance to NA inhibitors, no significant mutations were detected. Whole genome sequencing was performed with next-generation sequencing (NGS) on both primary specimens (nasal and nasopharyngeal) and cultured isolates. NGS was performed by the Mutualized Platform for Microbiology at the Institute Pasteur. No significant mutations were detected. The patient was examined for other viral respiratory agents, such as respiratory syncytial virus, human rhino-virus, and human metapneumovirus, but the results were negative in both nasopharyngeal and throat specimens.
Figure 1.
Phylogenetic analyses. The nomenclature of influenza viruses is defined according to type (e.g., A), place of origin (e.g., Paris), strain number (e.g., 664), and year of isolation (e.g., 2015). The strains in red and black correspond respectively to the vaccine strains and to the reference strains. Nucleotide sequences were aligned to A (H3N2) reference strains. The virus (in light blue) was found to belong to the 3C.2a clade, the most frequent of the H3N2 strains circulating during the winter of 2014–2015.
The autopsy showed a pericardium in tension and an abundant pericardial effusion post mortem (160 mL), which is a high volume considering the reduced size of the heart of a child compared to that of an adult. Moreover, the dramatic and sudden clinical deterioration may have been due to a rapid constitution of this pericardial effusion. Microscopically, there was an important interstitial edema with an abundant mononuclear inflammatory infiltrate. Many foci sources of myocardial necrosis with contraction bands were observed in both ventricles and the septum. These observations indicated an intense myocarditis. Also, a lymphocytic inflammatory infiltrate in the epicardium indicated pericarditis (Figure 2). The lungs presented congestive walls with a lymphoplasmacytic inflammatory infiltrate. In the right lung, this infiltrate was abundant with a hemorrhagic alveolar edema. The autopsy showed nascent influenza pneumonia.
Figure 2.
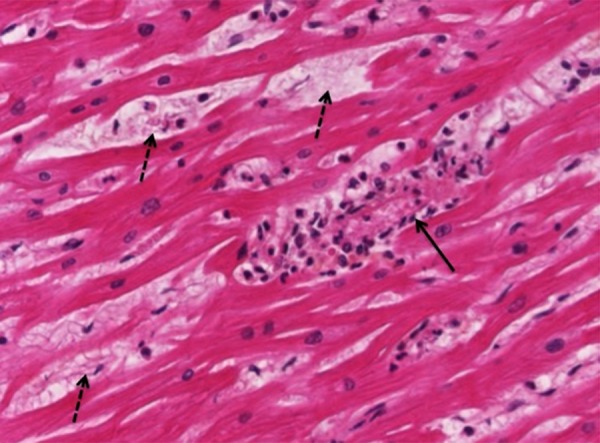
Illustration of the cardiac tissue, post-mortem histopathological examination (Hemalin Phloxin Safran coloration; 400×). Inflammatory infiltrate with myocardic necrosis is shown in one field of cardiac tissue (solid arrow); multifocal lesions with myocardic edema are shown (dashed arrows).
Discussion
Influenza A virus infection can cause a number of complications in the pulmonary (viral or bacterial pneumonia), neurological (encephalopathy, Guillain Barré Syndrome, etc.), renal, cardiac (myocarditis, pericarditis), and muscular systems [10]. Among these complications, myopericarditis is rare but potentially lethal. It comprises a wide spectrum of cardiac involvement, from mild myocarditis to cardiogenic shock. Clinical myocarditis or myocardial damage has also been reported in many studies from Japan [11], Taiwan [10], Canada [12], the USA [13], and Italy [8]. Although enteroviruses, especially group B coxsackieviruses, appear to be the most frequently implicated viral agents in myopericarditis, molecular studies conducted in the United States and in Italy have shown that influenza viruses account for 2% to 10% of all viral agents isolated from myocardial tissues [14,15].
Cardiac involvement usually manifests between 4 and 7 days after the onset of influenza. The most common symptom is worsening dyspnea. ECG abnormalities, elevated cardiac enzymes, and left ventricular dysfunction are also observed. A host immune-mediated inflammatory pathology is the main hypothesis evoked to explain these severe cardiac events. Rarely, myocyte damage may be due to a direct cytolytic effect of the virus, which usually plays a major role in cases with early myocardial involvement [8]. In our case, cardiac involvement occurred early in the course of the infection, less than 12 hours after the onset of fever. The change was so sudden that the patient had not been hospitalized, and resultantly, a search for cardiac enzymes was not performed. The first, and most likely, hypothesis is that a pericardial effusion caused cardiac tamponade and cardiorespiratory arrest, despite the lack of recorded data for cardiac tamponade, compared to that data described in the literature [16,17]. The arguments for this hypothesis are: abdominal referred pain, tachypnea, tachycardia, rapid cardiac arrest, significant pericardial effusion found at autopsy associated with a pericardium in tension and no shock delivered by the semi-automatic defibrillator (no arrhythmia requiring external electrical shock). The second hypothesis for the cause of the death is a hemorrhagic pulmonary edema and a cardiogenic shock due to left ventricular dysfunction, and probably life-threatening VT-like arrhythmia. In addition, the infectious context, especially a viral infection, turn us towards a cardiac tamponade. Indeed, idiopathic pericarditis, usually presumed to be post-viral is the most common cause of an inflammation-related perdicardial effusion in the United States and Western Europe [17,18]. Considering the early cardiovascular involvement in our patient (<48 hours), the cardiac tamponade and heart failure following myopericarditis were probably directly due to the H3N2 influenza virus, suggesting a cardiac tropism. Indeed, a cytokine cascade that enhances or modifies viral receptor exposure on endothelial cells lining the myocardial tissue may increase viral tropism for myocardial tissue [19]. Proinflammatory cytokines, including TNF-α, are considered important in the initiation and development of inflammatory cardiomyopathies [20]. Human studies have demonstrated that infection of the myocardium by the influenza A virus is associated with increased expression of TNF-α and its receptors (TNFRI and TNFRII) in the myocardium. Depressed myocardial function paired with elevated myocardial TNF-α messenger RNA and protein levels have been observed in patients with myocarditis. TNF-α may depress myocardial contractility through a number of processes, including nitric-oxide-mediated mechanisms and direct actions on intracellular calcium handling in the myocardium [21].
Some patients have important risk factors for complications or death. These may include obesity, pregnancy, young age (<5 years), immunosuppression, or chronic diseases (respiratory, renal, and cardiac diseases, etc). In our case, the patient did not exhibit predisposing factors.
Our study had several limitations. First, we did not find the influenza virus in the myocardial biopsy or pericardial liquid of the patient. Therefore, we cannot confirm the virological etiology of the myocarditis. However, a recently-published report described a similar situation wherein viral RNA was detected neither in the serum nor in the pericardial effusion of a 9-year-old female patient with influenza-associated myocarditis [22]. Mice models have shown that virus concentrations are much lower in the serum and myocardium than they are in the respiratory tract, and that the severity of cardiomyocyte injury does not seem to correlate with viral titer in the heart [23]. Thus, the influenza virus appears to be rarely detected in myocardium biopsies from patients with myocarditis, even when it directly invades the myocardium [2]. In our case, although we could not confirm that the myocarditis was caused directly by influenza virus infection, it is nonetheless highly likely that the virus had infected the myocardium. A second weakness of our study is that due to the rapid worsening of the cardiac involvement, we were not able to assess biochemical parameters, measure cytokines, or obtain an echocardiogram to explore the possibility of systolic dysfunction with an impaired left ventricular function.
Conclusions
To conclude, our study suggests that pericardial effusion myopericarditis may occur during influenza virus infection in young patients, even those with no known predisposing factors. The occurrence of complications, such as tamponade or hemorrhagic pulmonary edema, is unpredictable. Instable hemodynamics and respiratory distress are signs that must alert and lead to rescue intervention. Early diagnosis and treatment could reduce, in some cases, the risk of severe cardiac events. Vaccination is also important in the prevention of influenza infections. Nevertheless, a risk-factor based influenza vaccination program for children would not prevent these fatal cases as the reasons underlying susceptibility to severe disease remains occult.
Footnotes
Conflicts of interest
None.
References:
- 1.Jain S, Kamimoto L, Bramley AM, et al. Hospitalized patients with 2009 H1N1 influenza in the United States, April–June 2009. N Engl J Med. 2009;361:1935–44. doi: 10.1056/NEJMoa0906695. [DOI] [PubMed] [Google Scholar]
- 2.Ukimura A, Ooi Y, Kanzaki Y, et al. A national survey on myocarditis associated with influenza H1N1pdm2009 in the pandemic and postpandemic season in Japan. J Infect Chemother. 2013;19:426–31. doi: 10.1007/s10156-012-0499-z. [DOI] [PubMed] [Google Scholar]
- 3.Finland M, Parker J, Jr, Barnes MW, Joliffe LS. Acute myocarditis in inflenza A infections: 2 cases of non-baxterial myocarditis, with isolation of virus from the lung. Am J Med Sci. 1945;209:455–68. [Google Scholar]
- 4.Adams CW. Postviral myopericarditis associated with the influenza virus; Report of eight cases. Am J Cardiol. 1959;4:56–67. doi: 10.1016/0002-9149(59)90193-6. [DOI] [PubMed] [Google Scholar]
- 5.Onitsuka H, Imamura T, Miyamoto N, et al. Clinical manifestations of influenza a myocarditis during the influenza epidemic of winter 1998–1999. J Cardiol. 2001;37:315–23. [PubMed] [Google Scholar]
- 6.Davoudi AR, Maleki AR, Beykmohammadi AR, Tayebi A. Fulminant myopericarditis in an immunocompetent adult due to pandemic 2009 (H1N1) influenza A virus infection. Scand J Infect Dis. 2012;44:470–72. doi: 10.3109/00365548.2011.631575. [DOI] [PubMed] [Google Scholar]
- 7.Bratincsák A, El-Said HG, Bradley JS, et al. Fulminant myocarditis associated with pandemic H1N1 influenza A virus in children. J Am Coll Cardiol. 2010;55:928–29. doi: 10.1016/j.jacc.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Puzelli S, Buonaguro FM, Facchini M, et al. Cardiac tamponade and heart failure due to myopericarditis as a presentation of infection with the pandemic H1N1 2009 influenza A virus. J Clin Microbiol. 2010;48:2298–300. doi: 10.1128/JCM.00418-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guarner J, Paddock CD, Shieh W-J, et al. Histopathologic and immunohistochemical features of fatal influenza virus infection in children during the 2003–2004 season. Clin Infect Dis. 2006;43:132–40. doi: 10.1086/505122. [DOI] [PubMed] [Google Scholar]
- 10.Tseng G-S, Hsieh C-Y, Hsu C-T, et al. Myopericarditis and exertional rhabdomyolysis following an influenza A (H3N2) infection. BMC Infect Dis. 2013;13:283. doi: 10.1186/1471-2334-13-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaji M, Kuno H, Turu T, et al. Elevated serum myosin light chain I in influenza patients. Intern Med. 2001;40:594–97. doi: 10.2169/internalmedicine.40.594. [DOI] [PubMed] [Google Scholar]
- 12.Moore DL, Vaudry W, Scheifele DW, et al. Surveillance for influenza admissions among children hospitalized in Canadian immunization monitoring program active centers, 2003–2004. Pediatrics. 2006;118:e610–19. doi: 10.1542/peds.2005-2744. [DOI] [PubMed] [Google Scholar]
- 13.Brown SM, Pittman J, Miller RR, III, et al. Right and left heart failure in severe H1N1 influenza A infection. Eur Respir J. 2011;37:112–18. doi: 10.1183/09031936.00008210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bowles NE, Ni J, Kearney DL, et al. Detection of viruses in myocardial tissues by polymerase chain reaction: Evidence of adenovirus as a common cause of myocarditis in children and adults. J Am Coll Cardiol. 2003;42:466–72. doi: 10.1016/s0735-1097(03)00648-x. [DOI] [PubMed] [Google Scholar]
- 15.Calabrese F, Carturan E, Chimenti C, et al. Overexpression of tumor necrosis factor (TNF)alpha and TNFalpha receptor I in human viral myocarditis: Clinicopathologic correlations. Mod Pathol. 2004;17:1108–18. doi: 10.1038/modpathol.3800158. [DOI] [PubMed] [Google Scholar]
- 16.Roy CL, Minor MA, Brookhart MA, Choudhry NK. Does this patient with a pericardial effusion have cardiac tamponade? JAMA. 2007;297(16):1810–18. doi: 10.1001/jama.297.16.1810. [DOI] [PubMed] [Google Scholar]
- 17.Vakamudi S, Ho N, Cremer PC. Pericardial effusions: Causes, diagnosis, and management. Prog Cardiovasc Dis. 2017;59(4):380–88. doi: 10.1016/j.pcad.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 18.Imazio M, Gaita F, LeWinter M. Evaluation and treatment of pericarditis: A systematic review [erratum in JAMA 2015; 314: 1978] JAMA. 2015;314:1498–506. doi: 10.1001/jama.2015.12763. [DOI] [PubMed] [Google Scholar]
- 19.Warren-Gash C, Smeeth L, Hayward AC. Influenza as a trigger for acute myocardial infarction or death from cardiovascular disease: A systematic review. Lancet Infect Dis. 2009;9:601–10. doi: 10.1016/S1473-3099(09)70233-6. [DOI] [PubMed] [Google Scholar]
- 20.Mamas MA, Fraser D, Neyses L. Cardiovascular manifestations associated with influenza virus infection. Int J Cardiol. 2008;130:304–9. doi: 10.1016/j.ijcard.2008.04.044. [DOI] [PubMed] [Google Scholar]
- 21.Mamas MA, Nair S, Fraser D. Cardiac tamponade and heart failure as a presentation of influenza. Exp Clin Cardiol. 2007;12:214–16. [PMC free article] [PubMed] [Google Scholar]
- 22.Ito N, Sato M, Momoi N, et al. Influenza A H1N1 pdm09-associated myocarditis during zanamivir therapy. Pediatr Int. 2015;57:1172–74. doi: 10.1111/ped.12712. [DOI] [PubMed] [Google Scholar]
- 23.Kotaka M, Kitaura Y, Deguchi H, Kawamura K. Experimental influenza A virus myocarditis in mice. Light and electron microscopic, virologic, and hemodynamic study. Am J Pathol. 1990;136:409–19. [PMC free article] [PubMed] [Google Scholar]