Abstract
Efforts to reduce the public health burden of tobacco use have not equally benefited all members of society, leading to disparities in tobacco use as a function of ethnicity/race, socioeconomic position, physical/behavioral comorbidity, and other factors. Although multilevel transdisciplinary models are needed to comprehensively understand sources of tobacco-related health disparities (TRHD), the incorporation of psychopharmacology into TRHD research is rare. Similarly, psychopharmacology researchers have often overlooked the societal context in which tobacco is consumed. In an effort to facilitate transdisciplinary research agendas for studying TRHD and the psychopharmacology of tobacco use, this article introduces a novel paradigm, called “sociopharmacology.” Sociopharmacology is a platform for investigating how contextual factors amplify psychopharmacological determinants of smoking to disproportionately enhance vulnerability to smoking in populations subject to TRHD. The overall goal of sociopharmacology is to identify proximal person-level psychopharmacological mechanisms that channel distal societal-level influences on TRHD. In this article I describe: (1) sociopharmacology’s overarching methodology and theoretical framework; (2) example models that apply sociopharmacology to understand mechanisms underlying TRHD; (3) how sociopharmacological approaches may enhance the public health impact of basic research on the psychopharmacology of tobacco use; and (4) how understanding sociopharmacological mechanisms of TRHD might ultimately translate into interventions that reduce TRHD.
Introduction
Over the past 50 years, tobacco control efforts have dramatically reduced the prevalence of and morbidity and mortality from tobacco use.1–3 Importantly, the decline in smoking prevalence has been disproportionately accounted for by demographic majority groups and the socioeconomically advantaged.1,3,4 One unfortunate consequence of this trend is the emergence of disparities in tobacco use uptake, maintenance, and cessation relative to the general population among the following groups: (1) ethnic/sexual minorities (eg, blacks, American Indian/Alaskan Natives, immigrants, and lesbians), (2) individuals of lower socioeconomic position (eg, lower income, education, and employment), (3) individuals with health problems (eg, physical illness or disability, psychiatric disorder, and substance use disorder), and (4) other disadvantaged populations (eg, people with a criminal justice history). In the United States, for example, 42% of the population smoked 50 years ago and smoking was more common among certain advantaged groups relative to the general population (eg, college educated).1 Presently, the prevalence of smoking in the United States is around 18%. Within the current population of US smokers, 25% have incomes below the poverty line,1 12.3% are under criminal justice supervision,5,6 one-third have a psychiatric condition,7 10.6% are unemployed,8 86.8% do not have a college degree,9 and one-quarter have a non-nicotine substance use disorder.10,11 Accordingly, understanding the mechanisms that disproportionately promote smoking in these “disparity groups” relative to general population will be key to understanding and eliminating tobacco-related health disparities (TRHD) and ultimately impacting the overall tobacco burden.
Given the complexity of multilayered influences on social disadvantage and tobacco addiction, “multilevel” transdisciplinary approaches that acknowledge the intersection of sociocontextual (eg, culture, class) and individual-level (eg, psychology, biology) factors are necessary to address TRHD.12 Despite the need for such work, multilevel empirical studies on TRHD are scant, potentially because focused frameworks to guide such research are lacking. To facilitate multilevel transdisciplinary research of TRHD, this article proposes a novel paradigm, which I call “sociopharmacology.” As a synthesis of theory and methods of social epidemiology and psychopharmacology, sociopharmacology is a platform for studying how the broader social context amplifies individual-level psychopharmacological determinants of smoking to disproportionately enhance vulnerability to smoking in disparity groups relative to the general population. The overall goal is to identify proximal person-level psychopharmacological mechanisms that channel distal sociocontextual influences on TRHD. In addition to its specific relevance for TRHD, sociopharmacology may enhance the public health significance of basic research on the psychopharmacology of tobacco use, as described in greater detail below.
In this article, I define sociopharmacology and provide a unifying framework for applying sociopharmacology to empirical research on TRHD. Then, in exemplary applications of the paradigm, I describe two sociopharmacologally-informed models. Finally, after noting sociopharmacology’s limitations, the article concludes by describing implications for basic research on the psychopharmacology of nicotine and interventions for reducing TRHD.
Theoretical Framework and Methodology of Sociopharmacology
Sociopharmacology is the synthesis of social epidemiology approaches to studying TRHD and psychopharmacology approaches to studying tobacco addiction. Therefore, I briefly outline these two approaches before describing sociopharmacology (Table 1 for a comparison of the three approaches).
Table 1.
Theory and Methods of Social Epidemiology, Psychopharmacology, and Sociopharmacology
| Social epidemiology | Psychopharmacology | Sociopharmacology | |
|---|---|---|---|
| Level of analysis | Focus at the sociocontextual and system level | Focus at the individual level | Focus on interactions across sociocontextual and individual levels |
| Assumes determinants of tobacco use differ across populations | Assumes determinants of tobacco use are common across populations | Assumes a common set of determinants of tobacco use that differ in strength or relevance across populationsIdentifies proximal factors that channel distal influences on tobacco use behavior | |
| Identifies distal influences on tobacco use behavior | Identifies proximal influences on tobacco use behavior | ||
| Example theoretical constructs | Discrimination | Drug reward | Discrimination serving as a conditioned cue triggering tobacco use |
| Cultural acceptance of smoking | Drug-induced reward enhancement | Nicotine-induced distraction away from attending to cues reflecting neighborhood disorder | |
| Targeted marketing of tobacco products to disparity groups | Negative reinforcement | Nicotine-induced enhancement of the reward value of reinforcers in socioeconomically-deprived environmentsGreater value placed on the arousal-enhancing effects of nicotine for blue-collar workers whose jobs require high levels of arousal | |
| Physical environment (eg, density of tobacco retailers in communities with high prevalence of people from TRHD groups) | Withdrawal | ||
| Reduced access to health care | Behavioral economic value | ||
| Biases in the legal system | Drug-induced changes in cognitive performance | ||
| Neighborhood deprivation and crime | Conditioning of drug-related and other cues | ||
| Social class | Alternative reinforcers | ||
| Clustering of biological vulnerability | Pharmacodynamic and pharmacokinetic processes underlying drug effects | ||
| Methodology | Naturalistic, correlational, or descriptive designs | Experimental designs | Quasi- or fully-experimental designs crossing a sociodemographic TRHD variable or social determinant of TRHD with a psychopharmacological manipulation |
| Large community samples | Smaller samples | Correlational designs examining the relation between a social determinant of TRHD and a psychopharmacological cause or consequence of tobacco use | |
| Data often collected in the field | Data often collected in the laboratory | Value on both internal and external validity | |
| High value on generalizability and ecological validity | High value on internal validity and isolating narrow mechanisms | ||
| Evidence for causal effects is modest | Evidence for causal effects is strong | ||
| Statistical control emphasized | Experimental control emphasized | ||
| Basic methodology: studying naturalistic associations of sociodemographic variables or social constructs to tobacco use variables | Basic methodology: studying the effects of experimentally-manipulated tobacco administration or deprivation on variables indicative of addiction liability | ||
| Intervention | Target policy and system-level change | Pharmacotherapy | Combination of pharmacotherapy and/or behavioral interventions that offset sociocontextual and psychopharmacological determinants of tobacco use |
| Individual-level interventions are adapted for use in specific populations | Interventions are not generally adapted for use for specific populations | Targeting psychopharmacological mechanisms linking sociocontextual factors and tobacco use when social factors are immutable | |
| Behavioral interventions incorporate sociological constructs (eg, culturally-adapted counseling) | Behavioral interventions that target psychopharmacological processes (eg, contingency management to reinforce abstinence) | Personalized medicine tailored to population based on biological/social factors (eg, polygenetic risk score, sociodemographic risk assessment) | |
| Risk propensity assessment |
TRHD = tobacco-related health disparities.
Brief Background on Social Epidemiology and Psychopharmacology
Social Epidemiology
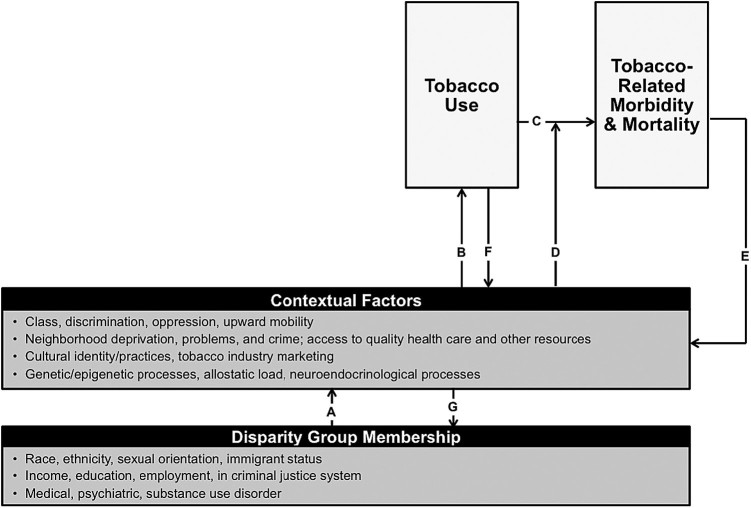
Social epidemiology often focuses on cross-population differences and distal societal-level influences on health. Social epidemiological explanations of TRHD purport that members of disparity groups are disproportionately subject to social, psychological, and biological contexts (Figure 1, path “A”), which in turn increase their vulnerability to tobacco use (Figure 1, path “B”).13,14 For instance, relative to the general population, certain disparity groups may experience more discrimination, live in communities with greater cultural acceptance of smoking, receive less tobacco prevention programming, have greater likelihood of genetic variants associated with tobacco use or tobacco-related health consequences, and experience more biological dysregulation due to chronic social stress, each of which may increase tobacco use vulnerability.13–16 Higher severity and chronicity of tobacco use directly increases tobacco-related morbidity and mortality in disparity groups relative to the general population (Figure 1, “C”).1,17 Furthermore, sociocontextual and system-level factors that are common in disparity populations, such as reduced access to and quality of health care, may increase vulnerability to the health consequences of tobacco use (Figure 1, “D”).18 The tobacco-related health consequences that individuals and families in disparity populations face may further cause social disadvantage (eg, reducing opportunities for upward mobility due to medical and financial consequences of tobacco-related disease; Figure 1, “E”).19 Also, increased tobacco use in disparity groups, relative to the overall population, may feed back into promoting cultural norms for smoking in those communities and further stigmatization and social ostracization (Figure 1, “F”).20,21 The recapitulation of social disadvantage provoked by TRHD (Figure 1, “E” and “F”) may further marginalize individuals to become “members” of multiple disparity groups (eg, an individual with a psychiatric disorder who becomes a chronic smoker and has to deal with the sequelae of tobacco-related health consequences may have even more challenges to gaining employment; Figure 1, “G”).19
Figure 1.
Social epidemiology framework for understanding tobacco-related health disparities.
Social epidemiology methods typically emphasize generalizability and apply descriptive, naturalistic studies in which correlations among social factors and smoking characteristics are investigated in large community samples, with data collection often occurring in the field.22 Interventions based on social epidemiology research findings often target policy and systems change or the incorporation of sociological constructs into psychosocial interventions (eg, culturally-adapted interventions; Table 1, left-hand column).
Psychopharmacology
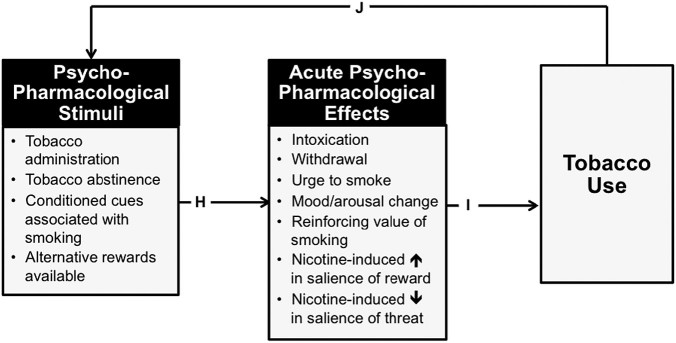
Psychopharmacology focuses on individual-level psychobiological processes that proximally cause or result from acute drug use; these processes are assumed to be applicable to all people within a population irrespective of sociodemographic variation.23 Psychopharmacology purports that tobacco-related psychopharmacological stimuli that acutely alter the brain and behavior provoke acute behavioral changes (eg, nicotine-induced mood enhancement, abstinence-induced mood dysregulation; Figure 2, path “H”), which in turn modify immediately subsequent motivation to smoke (Figure 2, “I”).24 For example, introduction of nicotine into the brain via smoking stimulates nicotinic-acetylcholine receptors, which promotes the release of other neurotransmitters, including dopamine, which may underlie the smoking’s mood-enhancing psychoactive effects, and in turn reinforces smoking behavior and promote motivation to continue smoking.24 Importantly, the resulting tobacco use leads to additional exposures to psychopharmacological stimuli, which recapitulate these processes and provide positive feedback loops that cycle towards addiction (Figure 2, “J”). For instance, chronic tobacco use leads to conditioning processes whereby cues repeatedly associated with smoking (eg, smoking-related stimuli such as ashtrays, locations such as bars, and internal cues such as anxiety) provoke psychopharmacological effects like urge to smoke, which in turn lead to more tobacco use.25 Moreover, chronic nicotine exposure can dysregulate a number of biological systems through pharmacodynamic changes and metabolic adaptations, ultimately affecting nicotine metabolism pathways (eg, CYP2A6)26 and brain dopaminergic, serotoninergic, adrenergic, cholinergic, gabergic, glutamatergic, and other systems,27,28 which can enhance the psychoactive effects of pharmacological stimuli exposure (eg, abstinence-induced reductions in nicotine levels that provoke withdrawal symptoms in chronic smokers).
Figure 2.
Psychopharmacology framework for understanding tobacco addiction.
Psychopharmacology methods typically emphasize internal validity and apply experimental designs under tightly controlled conditions in the laboratory, with the goal of isolating individual casual mechanisms that underlie the behavioral effects of drugs.23 The basic design involves investigating the effects of experimentally-manipulated nicotine administration or deprivation on subjective, physiological, and behavioral responses; additional manipulations (eg, crossing a candidate pharmacotherapy) or factors (eg, participant-level moderators such as level of nicotine dependence) can be super-imposed on this design. Outcome measures typically correspond to the addiction liability of nicotine (eg, mood changes, cognitive performance enhancement, self-administration of tobacco, behavioral choices to consume tobacco or receive money). Pharmacotherapy is the primary treatment approach informed by psychopharmacology (see Table 1; center column).
Sociopharmacology’s Overarching Theoretical Framework
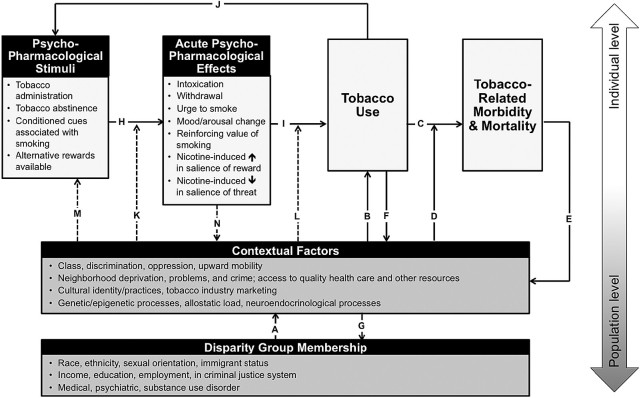
The sociopharmacology framework for understanding TRHD is detailed in Figure 3, with dotted lines representing sociopharmacological mechanisms of TRHD that integrate sociocontextual and psychopharmacological processes. Two key interactive effects that have downstream effects on population-level TRHD are identified in the framework. First, members of disparity groups may be more vulnerable to the acute behavioral effects of tobacco-related psychopharmacological stimuli because of biopsychosocial contexts surrounding disparate populations (Figure 3, “K”). For instance, individuals from certain ethnic/racial minority groups may be more likely to have a genetic variant that increases sensitivity to the psychoactive effects of nicotine. In another example, the experience of discrimination may provoke negative affect states, which may magnify smoking’s acute distress-alleviating effects. Related to this mechanism, chronic experience of discrimination and other forms of social stress may perhaps result in dysregulation of neuroendocrinological, inflammatory, and epigenetic mechanisms, which may alter one’s allostatic load.16 This is important because some of the biological consequences of these social stressors may intersect with pathways that are affected by nicotine (eg, noradrenergic systems, corticotropin releasing factor), which could perhaps have synergistic effects in modulating the psychoactive effects of tobacco-related psychopharmacological stimuli.16,29 Second, social factors moderate the extent to which acute psychopharmacological effects translate into motivation to smoke (Figure 3, “L”). For instance, members of disparity populations may also place a larger value on tobacco’s mood-modulating effects in order to counteract the stress caused by discrimination. As a result, tobacco’s mood-modulating effects may engender stronger motivation to smoke for disparity populations experiencing discrimination. Other specific examples of these pathways are: (1) people from East Asian cultures that discourage the emotional expression of anger30 may report little irritability during nicotine withdrawal, even though irritability is a robust effect of tobacco abstinence in general samples31,32 (eg, “K”); (2) nicotine’s pharmacological effects on pleasure and feelings of friendliness33 and social closeness may benefit the incorporation smoking into certain social practices for some cultures (“N”); and (3) blue-collar workers might be more motivated to use tobacco for its stimulating properties, which may enhance physical labor performance, and ultimately the reinforcing value of tobacco for such individuals (“L”).
Figure 3.
Sociopharmacology framework to understanding tobacco-related health disparities. Note. Arrows with broken lines reflect sociopharmacological mechanisms contributing to tobacco-related health disparities.
Additionally, sociocontextual factors may disproportionately promote exposure to psychopharmacological stimuli in disparity populations (“M”), which may have downstream effects on TRHD. For example, targeted tobacco industry marketing coupled with cultural norms accepting smoking for disadvantaged communities may bombard members of disparity groups with greater exposure to smoking-related cues (“M”) that may provoke urges to smoke. As described in greater detail below, diminished access to resources for individuals in disparity populations can reduce alterative rewards available that may acutely increase the urge and reinforcing value of smoking (“M,” “H”). Finally, some of the acute behavioral effects of psychopharmacological stimuli may have reciprocal effects back on sociocultural processes, such as nicotine-induced social enhancement of certain culturally-normative social practices in which smoking is common (“N”). Collectively, each of these novel sociopharmacological mechanisms (Figure 3, pathways “K” to “N”) innervate with psychopharmacological (“H” to “J”) and sociocontextual (“A” to “G”) mechanisms to jointly underlie TRHD.
Sociopharmacology’s Methodology
Sociopharmacology synthesizes the strengths of social epidemiology (eg, generalizability, incorporation of sociological constructs key to TRHD, relevance to population-specific interventions) and psychopharmacology (eg, internal validity for making causal inferences, incorporation of psychopharmacological theories key to tobacco addiction etiology, relevance to pharmacotherapy). Sociopharmacology’s basic research design is quasi-experimental and involves studying natural variation in a sociocontextual-level variable as a moderator of the effects of experimentally-manipulated tobacco-related psychopharmacological stimuli on behavioral outcomes relevant to tobacco use motivation (eg, mood, smoking urge). An initial sociopharmacological investigation may utilize a standard demographic/clinical variable that explicitly marks “disparity group status” (eg, level of education, psychiatric diagnosis) as the moderator of the psychopharmacological manipulation. Such studies, which are not entirely absent in the literature,34 may provide critical clues to the biopsychosocial contextual factors that mediate such effects (eg, genetics, neighborhood community influences, and culture). Following such initial inquiries, research incorporating a sociocontextual biomarker (eg, c-reactive protein which may indicate inflammatory process due to altered allostatic load provoked by chronic social stress)16 or construct (eg, target of discrimination) as a moderator would further elucidate sociopharmacological mechanisms underlying TRHD. There have been some examples of this approach in the literature that are focused on some biological contextual moderators (eg, genetically-determined racial differences in nicotine effects);35 however, exploration of sociocultural moderators of psychopharmacological effects is scant.
The basic design proposed above is a prototype for many other design iterations, including: (1) manipulating the sociocontextual variable rather than solely measuring its natural variation (eg, experimentally priming the activation of cultural identities in participants through vignettes); (2) manipulating a psychopharmacological variable other than nicotine/tobacco exposure (eg, non-nicotine drug administration, exposure to smoking-related cues); (3) nonexperimental designs (eg, measuring retrospective reports of subjective drug effects); and (4) naturalistic designs that incorporate a sociocontextual construct and a psychopharmacological process (eg, studying correlations between socioeconomic position and responses to behavioral economic questionnaires assessing the relative reward value of smoking). Many other designs not described here that meaningfully address intersections between sociocontextual and psychopharmacological processes to explain TRHD would also fit into the sociopharmacology framework.
Potential Applications of Sociopharmacology
To illustrate the utility of the approach, I offer two theoretical models that can fall under the sociopharmacology framework described above.
Socially-Determined Stress and Tobacco’s Psychopharmacological Effects on Threat Processing
In comparison to the general population, disparity groups are subjected to higher socially-determined stress, including neighborhood crime, area disorder (eg, vandalism, litter), and discrimination.14 Each of these social determinants of stress has been linked with tobacco use, often over and above other confounding factors; such linkages have often been interpreted as evidence that disparity populations use tobacco to cope with socially-determined stress.36–38 I propose the following additional sociopharmacological explanation for the link between socially-determined stress and smoking.
Stimuli that signal potential threat tend to automatically capture and hold one’s attention.39 This process is believed to be evolutionarily-hardwired to allow individuals to be prepared to quickly react to threatening stimuli to prevent potential harm.40 Importantly, nicotine has attentional filtering properties that enhance one’s ability to maintain attention on targets and prevent distraction from peripheral cues, an effect believed to be mediated by nicotinic-acetylcholine receptor stimulation.41,42 Basic psychopharmacology research has shown that nicotine administration reduces attentional distraction by threatening stimuli.41,43 This psychopharmacological effect of nicotine may be particularly valued by disparity populations that regularly encounter aversive stimuli, such as discriminatory actions by others, dilapidated environments, and criminal acts going on in the neighborhood. Nicotine may help disadvantaged individuals avoid focusing their attention on aversive stimuli saturated in the environment and potentially buffer against the effects of socially-determined stressors on subjective negative affect. As a result, nicotine’s effects on threat processing may be more reinforcing and thus have a stronger effect on motivation to smoke in disparity populations facing socially-determined stress.
Individuals who repeatedly smoke in response to socially-determined stress may develop a specific conditioned association between social stress triggers and smoking. As a result, situations in which socially-determined stress arises (eg, experiencing discrimination, witnessing crime) may become triggers for smoking urges, which may in turn provoke smoking. Psychopharmacology research suggests that stressors in general can become powerful conditioned cues that produce reinstatement of drug-taking behavior, even following extended periods of abstinence.44,45 Hence, cue-induced reinstatement of smoking behavior by socially-determined stress may be a specific, proximal mechanism to account for the disproportionately high relapse rates in some disparity populations; this hypothesis is consistent with some empirical research.46
Sociopharmacology studies could address the abovementioned predictions. For example, regular smokers from neighborhoods with high and low rates of crime could be administered nicotine (vs. placebo) following a period of acute smoking abstinence and then perform a modified Stroop task measuring distraction by stimuli associated with crime. The task would instruct participants to name the ink color of crime-related and neutral words presented in various colors (eg, blue, green, and red) as quickly as possible while ignoring the meaning. Attentional interference towards crime related words would be reflected by longer latency to name the color of crime-related (vs. neutral) words due to greater distraction by the meaning of these cues. Based on the model described above, I would predict that: (1) nicotine would diminish attentional interference from crime related words, particularly for the smokers from high-crime neighborhoods (neighborhood crime status × drug interaction); and (2) individual differences in the degree of nicotine-induced suppression of attentional interference by crime-related stimuli in smokers from high crime neighborhoods would correlate with smoking level, chronicity, and dependence. If these predictions were supported, smokers from high-crime neighborhoods may particularly benefit from nicotine replacement formulations administered on an acute as needed basis (eg, nicotine lozenge). Such forms of nicotine replacement could be taken in response to acute episodes of socially-determined stress to prevent a ruminative response that prolongs and intensifies the stress state, and in turn, possibly prevents a stress-induced lapse.
Ecological momentary assessment studies that would fall under the sociopharmacology framework could also address pathways linking socially-determined stress and smoking. Participants can be given mobile devices to be taken with them into their natural environment. In addition to repeated assessment of smoking, urges, and mood (which is often standard in ecological momentary assessment), instances in which crime is witnessed, neighborhood disorganization is observed, or discrimination occurs in one’s naturalistic environment could be recorded. I predict that covariation between instances of socially-determined stress, negative affect, smoking would occur within individuals, such that negative affect and smoking is higher during episodes of socially-determined stress exposure.
Dearth of Opportunity for Reward in Disadvantaged Populations and Tobacco’s Reward-Enhancing Effects
Because of social inequities, the opportunity to engage in pleasurable, rewarding experiences is lower, on average, for disparity populations (relative to the general population), and diminished resources and opportunity for reward has been linked with greater likelihood of smoking.37 This finding can be interpreted from a behavioral economic perspective, which purports that vulnerability to tobacco use is higher among individuals with: (1) lower access to experiences that provide pleasure and meaning; and (2) less to lose from social, occupational, and health consequences of tobacco use that may further limit opportunities for alternative nondrug reinforcement.47 Indeed, many TRHD groups arguably meet both of these criteria due to social inequities.21,48 An additional sociopharmacological mechanism may underlie the link between diminished opportunity for reward and TRHD as described below.49
The drive to pursue and experience rewards that promote pleasure, meaning, and a sense of wellbeing is evolutionarily hardwired.50 Basic psychopharmacology research indicates that nicotine is a “reward enhancer” that amplifies the pleasurable and reinforcing effects of other rewards experienced concurrently with nicotine.51 That is, in addition to nicotine acting as a primary reward that causes direct psychoactive effects irrespective of environmental context, the drug also modulates the mood-enhancing effects of rewarding stimuli that are present in the environmental context in which nicotine is consumed.51–53 Correspondingly, regular tobacco users who are acutely abstinent appear to experience withdrawal-related deficits in their ability to emotionally respond to reward-related stimuli.54–56
Disparity populations who have less opportunity to experience reward may be more motivated to smoke because the pharmacological activity of nicotine may magnify the potency of the limited rewards at hand. Accordingly, tobacco use may be a means for enhancing the well-being one derives from their environment when altering one’s environment is difficult or impossible. For instance, if attending a sporting event at the stadium is financially unfeasible, watching the game on television while smoking might magnify the level of pleasure derived from the experience. If one is able to derive greater reward from their environment when smoking, and their environment is otherwise reward deficient, the net gain in reward experience may heighten motivation to continue and escalate tobacco use. Furthermore, given that nicotine withdrawal diminishes reward sensitivity,56 the loss of reinforcement produced by quitting smoking may be particularly profound for disparate populations who may be left with impotent environmental reward structures that are no longer pharmacologically-enhanced by nicotine. Such deficits may produce a strong motivation to resume smoking and reattain tobacco-induced reward enhancement for disparity populations. Elements of this overarching model have been proposed before by Perkins with regards to socioeconomic position.49 Below, I flesh out this hypothesis for socioeconomically disadvantaged individuals and extend it to several other populations subject to TRHD.
Applying the Reward-Enhancement Model to Specific Disparity Populations
Socioeconomic Position
Different indicators of socioeconomic position may have unique influences on opportunity for reward. For example, individuals with lower income have fewer resources to obtain material goods and other commodities that may provide a source of reward and pleasure.57 Those unemployed may lack reward derived from the satisfaction obtained from working,58 and “blue-collar” workers (eg, crafts and kindred occupations, operatives, transportation operatives, and laborers) may find less stimulation and meaning from their jobs and hence experience a deficiency of reward obtained from employment.59 Indeed, smoking is more prevalent among workers whose abilities are underused, have monotonous and repetitive jobs, and report less work satisfaction.60,61 Qualitative research in socioeconomically disadvantaged samples supports the notion that disadvantaged individuals use tobacco as a source of reward and to enhance the limited rewards at their disposal (eg, reporting that smoking while drinking a cup of tea is one of the few “treats” one has).62,63 We found that smokers with lower (vs. higher) education were more likely to choose smoking over an alternative reward following overnight tobacco deprivation,64 suggesting enhanced value of smoking relative to alternative rewards in less educated individuals.
Criminal Justice Populations
Imprisoned individuals have substantial restriction of reward opportunities, and evidence suggests that inmates smoke to manage the lack of stimulation experienced in prison.65 In the reward deficient environment of prison, cigarettes have disproportionate reward value relative to other commodities,66 which might be explained by sociopharmacological processes. Reward restrictions persist for individuals in the criminal justice system post-incarceration, as these individuals often have fewer opportunities of reward post-release because many people are hesitant to socialize with individuals who have been incarcerated and many employers are unwilling to hire individuals with a criminal history.67
Ethnic and Sexual Minorities and Immigrants
Populations in the statistical minority within a community are less likely, on average, than those in the statistical majority to have culturally-relevant rewards available in their immediate environments. That is, community resources for minorities to engage in their own cultural practices, such as religious institutions, will be scarcer for such individuals. In addition, discriminatory practices may prevent access to certain rewards (eg, employment, education, and social status) on the basis of ethnicity or sexual orientation.68,69 Accordingly, nicotine may have greater value as a reward enhancer for minority populations. We found that African American (vs. white and Hispanic) smokers reported greater reductions in acute positive affect but not other affective states or nicotine withdrawal symptoms following acute tobacco abstinence.70 Given the specificity of results to diminished positive affect outcomes, one sociopharmacological explanation for this finding is that the loss of affect-enhancing effects of nicotine may have been more salient for African American smokers who otherwise may have had less alternative rewards available in their environment.
Physical Illness or Disability
Individuals with a severe or chronic physical illness or disability often have restrictions in the types of activities they can engage in, leading to constraints in opportunity for reward.71 Beyond the impact of illness severity and psychosocial variables (age, income, social support, and personality), activity restriction explains significant portions of the variance in well-being among individuals with physical illnesses and disabilities.72 Furthermore, recent evidence in physically-disabled smokers suggests that restriction of valued life activities (social, professional, pleasurable or otherwise meaningful activities) due to mobility impairment is associated with lower quit motivation and success.73,74 Hence, restrictions in opportunities for reward imposed by an illness or disability may motivate tobacco use as a means for enhancing the potency of the limited rewards at one’s disposal.
Behavioral Health Comorbidities
Individuals with behavioral health comorbidities (ie, psychiatric and/or non-nicotine substance use disorder) have higher levels of smoking, lower cessation likelihood, and greater risk of tobacco-related disease than nearly all other groups subject to TRHD.75,76 Sociopharmacology is particularly well-suited for addressing TRHD among individuals with behavioral health comorbidities. Like other disadvantaged groups who face stigma, discrimination, and functional limitations, individuals with behavioral health comorbidities have restricted opportunities and access to rewards that engender happiness and meaning.77–79 On top of social inequities, many psychiatric and substance use disorders are associated with psychobiological disturbances in the brain’s reward system,80–82 which directly blunt the reinforcing and mood-enhancing effects of nondrug rewards. Consequently, individuals with behavioral health comorbidities are likely to derive less reinforcement, happiness, and meaning in their lives than those without such comorbidities even if available environmental rewards were identical across the two groups (which they are not). The combination of social inequities that diminish access to reward with inherent limitations in reward responsivity may produce profound increases in the motivation to smoke to obtain reinforcement for individuals with behavioral health comorbidities.
Individuals with depression, schizophrenia, and other psychiatric conditions are more likely to prefer smoking over nondrug rewards,83,84 experience greater mood enhancement in response to nondrug rewards when concurrently smoking,85 show greater declines in positive mood when abstinent,31 and escalate their smoking behavior when they experience reductions in pleasure obtained from nondrug rewards.86 Our laboratory has addressed this phenomenon by focusing on anhedonia—a cross-cutting psychopathological trait common to several behavioral health conditions that reflects the key process described above (ie, diminished happiness, enjoyment, and ability to experience pleasure in response to rewards).87 We have shown that anhedonia is associated with greater preference for smoking versus alternative rewards88,89 and larger abstinence-induced declines in positive mood,31 reductions in cognitive processing of rewards,90 and increases in urge to smoke for pleasure.89 Similarly, Cook, Spring, and McChargue91 found that anhedonic smokers were able to overcome inherently deficient hedonic responses to a rewarding stimulus (ie, happy music) when administered nicotine. An important next step in research on TRHD among individuals with behavioral health comorbidities will be to study joint influences of diminished reward responsiveness in combination with social inequities in access to healthy and meaningful rewards.
Proposed Studies to Test the Reward-Enhancement Model
A common method of assessing reinforcement and pleasure derived from activities involves questionnaires measuring the frequency of engagement in and pleasure obtained from various rewarding activities.92 This tool has been further leveraged for substance use by instructing participants to indicate whether each activity is experienced in concert with substance use, yielding two primary outcomes: (1) drug-complementary reinforcement, which is the sum of the product of engagement × frequency ratings for each activity identified as being performed while using the substance; (2) alternative reinforcement, which is the corresponding cross-product for activities identified not be associated with substance use.86 In addition to self-report methods, geocoding of rewards available in the immediate environment (eg, theaters, parks) might also be used to measure reward access and experience. To test if the hypothesized sociopharmacological mechanism may be a source of disparities in tobacco use, smoking-complementary and alternative reinforcement could be explored as statistical mediators of relations between disparity group membership and tobacco use. Consistent with some work in this area,86 I hypothesize that members of disparity groups relative to the general population: (1) derive less alternative reinforcement, which in turn may motivate smoking uptake and maintenance; and (2) derive greater smoking-complementary reinforcement (ie, pleasure from nondrug activities while smoking), which in turn may motivate more pervasive and severe levels of tobacco use.
Researchers might utilize some of the assessments described above to determine the “typical” reward available and then expose participants to their typical reward under varying levels of nicotine administration in a laboratory experiment. I hypothesize that administration of one’s typical reward in the laboratory may generate markedly lower levels of pleasure and greater motivation to smoke in nicotine-deprived versus nicotine-exposed smokers in disparity populations. By contrast, the pleasure and motivation to smoke caused by typical reward exposure would be less robustly modulated by nicotine administration in populations not subject to TRHD (eg, high socioeconomic position).
Limitations
The sociopharmacological approach to addressing TRHD proposed should be considered with several caveats. First and foremost, extant empirical data that applies this approach is virtually absent. Hence, it is possible that some of the key research questions posed by sociopharmacology may not yield definitive insights. That being said, given the novelty of sociopharmacological applications to tobacco research, data that helps to rule out a wide range of potential sociopharmacologically-mediated sources of TRHD may help to sharpen focus on other approaches. Further, some interventions to eliminate sociopharmacological sources of TRHD will be subject to the same challenges facing social epidemiology. For instance, if the abovementioned reward-enhancement model of tobacco disparities ultimately garners empirical support, enhancing access to healthy, nondrug rewards in disparity populations would presumably close the gap in tobacco use between these groups and the overall population. Yet, altering the environmental reward structure of disparity groups at the population level would require a great effort that runs counter to powerful, chronically-entrenched social inequities; though, altering the reward structure for individual persons facing TRHD may be more feasible.
Implications for Efforts to Reduce and Eliminate TRHD
If sociopharmacological mechanisms that disproportionately promote tobacco use in disparity populations can be identified, interventions and policy that counteracts these mechanisms would presumably help to reduce TRHD. Extension of ongoing work on the genomics and pharmacogenomics of smoking cessation treatment and outcome could be straightforwardly adapted to TRHD.93 One example is evidence that there is racial stratification of genes involved in the metabolism of nicotine (eg, CYP2A6); variation in these genes and their biological phenotypes also predict cessation outcome and clinical response to nicotine replacement therapy.35,93 Hence, prediction models using race (or biomarkers that stratify by race and underlie variation in clinical response to smoking cessation medication, such as the nicotine metabolite ratio)93,94 may lead to “personalized” interventions that can be tailored to disparity population membership. Such efforts can ultimately enhance chances for quit success across populations and help to increase equity in cessation outcome.
Combination treatments that disrupt the sociocontextual process via behavioral intervention and disrupt the psychopharmacological process via pharmacotherapy would presumably have synergistic effects in reducing tobacco use among individuals in disparity populations. If the reward-enhancement explanation of TRHD described above was validated, pharmacotherapies that increase responsiveness to reward (eg, nicotine replacement therapy, varenicline)54,95 and behavioral interventions that increase access to meaningful and pleasurable nondrug rewards (eg, behavioral activation treatment) 96 may synergistically reduce smoking in disparity populations.
Sociopharmacology also offers avenues for reducing TRHD caused by sociocontextual processes that are difficult to modify. Indeed, individual level psychopharmacological pathways that channel the sociocontextual influences on TRHD are modifiable and could be disrupted to offset immutable population level sociocontextual influences. For instance, if the threat processing pathway linking socially-determined stress and smoking identified above was supported, interventions that inhibit automatic attentional interference by threatening cues may help smokers affected by discrimination. Attentional retraining interventions in which computer-based platforms that teach individuals to disengage their attention away threatening cues through cognitive exercises have been used successfully in anxiety disorder treatment.97 Applying such interventions to modify attentional interference caused by cues associated with socially-determined stress (eg, racial slurs) could perhaps prove useful for reducing TRHD.
In addition to specific interventional strategies, sociopharmacology may inform the development of more precise risk assessment approaches at the population level. Specifically, prediction models that include environmental, sociodemographic, genetic, and other factors to mark accumulative risk indices98 for likelihood of tobacco use and could be expanded to include psychopharmacological factors (eg, initial psychopharmacological sensitivity to nicotine via retrospective report of early smoking experience).99 One could envision novel prediction models that incorporate “top down” socioenvironmental factors (eg, number of tobacco retailers in close proximity to one residence), “bottom up” biological factors (eg, genetic susceptibility score), and their interaction (eg, inflammatory biomarkers caused by social stress, reporting that nicotine’s stimulating performance-enhancing effects is a reason for smoking for blue-collar workers). The combination of these factors might improve the accuracy of risk models for tobacco use, addiction, and health consequences. Such models could inform policy makers for identifying high risk populations likely to be subject to TRHD as well as the potential biopsychosocical mechanisms that may channel risk to be targeted in intervention.
Implications for Basic Research on the Psychopharmacology of Nicotine and Tobacco
Sociopharmacology may also advance basic science by stimulating psychopharmacology research that considers the social context in which tobacco is consumed. Some evidence suggests that eastern cultures encourage socially-engaging emotional experience (eg, friendliness, guilt), whereas western cultures encourage disengaging emotions (eg, anger, pride).100 Hence, psychopharmacology research in culturally-heterogeneous samples that fails to separate engaging versus disengaging emotions may be at risk for making faulty conclusions. For instance, an experiment that finds no evidence of moderation of the subjective mood-altering effects of nicotine by a candidate pharmacotherapy may conclude that the pharmacotherapy has limited clinical value. In this circumstance, it is possible the pharmacological agent modulated certain domains of mood for some participants and other domains of mood for others; however, the overall effect in the combined sample was masked because mood assessment focused on higher-order dimensions (eg, positive and negative affect) and failed isolate culturally-relevant discrete lower-order mood dimensions. Furthermore, considering the greater context in which tobacco is consumed can only serve to enhance the public health impact of tobacco psychopharmacology research. Sociopharmacology offers one means of achieving this.
Conclusion
Health disparities are among of the most critical challenges facing tobacco control. The origins of TRHD are highly complex and poorly understood. Perhaps sociopharmacology may benefit efforts to understand and eventually reduce TRHD. Irrespective of possible downstream effects on population-level health disparities, it is hoped that, at the very least, this paradigm may stimulate exciting new tobacco research that brings together fields that are typically unintegrated.
Funding
I am grateful to the US National Institute on Drug Abuse (K08-DA025041; R01-DA033296; R21-DA034768; R01-DA026831; T32-DA016184), National Cancer Institute (R25-CA57730), American Cancer Society (RSG-13-163-01), and California Tobacco-Related Disease Research Program (22FT-0062) for their support of my research and training.
Declaration of Interests
None declared.
Acknowledgments
The concepts outlined in this article mirrors my own development as a scientist. I am clinical psychologist who initially trained with exceptional researchers applying psychopharmacology and experimental psychopathology to tobacco addiction. Later in my career, I have been fortunate to learn from and collaborate with outstanding public health scientists on the forefront of health disparities research. I am indebted to all those who have provided critical mentoring for me (in chronological order), including Andrew Waters, Christopher Kahler, Suzanne Colby, Jennifer Tidey, Anthony Spirito, Steve Sussman, and Jonathan Samet, as well as the numerous colleagues who, without SRNT, I wouldn’t otherwise had the wonderful opportunity to know and collaborate with. I also thank Matthew Kirkpatrick, Christopher Kahler, and Jonathan Samet for providing valuable feedback on this manuscript before it was submitted as well as Marcus Munafò, the journal’s action editor for this manuscript for obtaining outstanding reviews. This article is an invited paper submitted to Nicotine and Tobacco Research for having received the 2015 Jarvik-Russell Young Investigator Award for Outstanding Early Career Scientific Contributions by the Society for Research on Nicotine and Toabcco.
References
- 1. USDHHS. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [Google Scholar]
- 2. Hiscock R, Bauld L, Amos A, Platt S. Smoking and socioeconomic status in England: the rise of the never smoker and the disadvantaged smoker. J Public Health (Oxf). 2012;34(3):390–396. doi:10.1093/pubmed/fds012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Scollo M, Winstanley M. Tobacco in Australia: Facts and Issues. 4th ed. Melbourne, Austrailia: Cancel Council Victoria; 2012. www.tobaccoinaustralia.org.au. Accessed February 4, 2015. [Google Scholar]
- 4. SAMSHA. Smoking rate among adults with serious psychological distress remains high. Data Spotlight. 2013. [Google Scholar]
- 5. Cropsey KL, Jones-Whaley S, Jackson DO, Hale GJ. Smoking characteristics of community corrections clients. Nicotine Tob Res. 2010;12(1):53–58. doi:10.1093/ntr/ntp172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Glaze LE, Parks E. Correctional Populations in the United States, 2011. Department of Justice, Bureau of Justice Statistics; United States Department of Justice; 2012. www.bjs.gov/content/pub/pdf/cpus11.pdf. Accessed February 4, 2015. [Google Scholar]
- 7.CDC. Vital Signs. Adult Smoking Focusing on People with Mental Illness February 2013. http://www.cdc.gov/media/dpk/2013/docs/dpk-vs-adult-smoking-mental-illnessFACT-SHEET.pdf. [Google Scholar]
- 8. Garrett BE, Dube SR, Trosclair A, Caraballo RS, Pechacek TF. Cigarette smoking—United States, 1965–2008. MMWR Surveill Summ. 2011;60(1):109–113. www.cdc.gov/mmwr/preview/mmwrhtml/su6001a24.htm. Accessed February 4, 2015. [PubMed] [Google Scholar]
- 9. Garrett BE, Dube SR, Winder C, Caraballo RS. Cigarette smoking—United States, 2006–2008 and 2009–2010. CDC Health Disparities and Inequalities Report—United States. 2013;62(3):81 www.cdc.gov/mmwr/preview/mmwrhtml/su6203a14.htm. Accessed February 4, 2015. [PubMed] [Google Scholar]
- 10. Grant BF, Hasin DS, Chou SP, Stinson FS, Dawson DA. Nicotine dependence and psychiatric disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. 2004;61(11):1107–1115. doi:10.1001/archpsyc.61.11.1107. [DOI] [PubMed] [Google Scholar]
- 11. Falk DE, Yi HY, Hiller-Sturmhofel S. An epidemiologic analysis of co-occurring alcohol and tobacco use and disorders: findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Alcohol Res Health. 2006;29(3):162–171. [PMC free article] [PubMed] [Google Scholar]
- 12. Moolchan ET, Fagan P, Fernander AF, et al. Addressing tobacco-related health disparities. Addiction. 2007;102(suppl 2):30–42. doi:10.1111/j.1360-0443.2007.01953.x. [DOI] [PubMed] [Google Scholar]
- 13. Sorensen G, Barbeau E, Hunt MK, Emmons K. Reducing social disparities in tobacco use: a social-contextual model for reducing tobacco use among blue-collar workers. Am J Public Health. 2004;94(2):230–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Krieger N. A glossary for social epidemiology. J Epidemiol Community Health. 2001;55(10):693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Adler NE, Rehkopf DH. U.S. disparities in health: descriptions, causes, and mechanisms. Ann Rev Public Health. 2008;29:235–252. doi:10.1146/annurev.publhealth.29.020907.090852. [DOI] [PubMed] [Google Scholar]
- 16. Djuric Z, Bird CE, Furumoto-Dawson A, et al. Biomarkers of psychological stress in health disparities research. Open Biomark J. 2008;1(1):7–19. doi:10.2174/1875318300801010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Meara ER, Richards S, Cutler DM. The gap gets bigger: changes in mortality and life expectancy, by education, 1981–2000. Health Aff (Millwood). 2008;27(2):350–360. doi:10.1377/hlthaff.27.2.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Birch S, Jerrett M, Eyles J. Heterogeneity in the determinants of health and illness: the example of socioeconomic status and smoking. Soc Sci Med. 2000;51(2):307–317. [PubMed] [Google Scholar]
- 19. Valtorta NK, Hanratty B. Socioeconomic variation in the financial consequences of ill health for older people with chronic diseases: a systematic review. Maturitas. 2013;74(4):313–333. doi:10.1016/j.maturitas.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 20. Karasek D, Ahern J, Galea S. Social norms, collective efficacy, and smoking cessation in urban neighborhoods. Am J Public Health. 2012;102(2):343–351. doi:10.2105/AJPH.2011.300364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ahern J, Galea S, Hubbard A, Syme SL. Neighborhood smoking norms modify the relation between collective efficacy and smoking behavior. Drug Alcohol Depend. 2009;100(1–2):138–145. doi:10.1016/j.drugalcdep.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Honjo K. Social epidemiology: definition, history, and research examples. Environ Health Prev Med. 2004;9(5):193–199. doi:10.1007/BF02898100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. MacKillop J, de Wit H, eds. The Wiley-Blackwell Handbook of Addiction Psychopharmacology. New York, NY: Wiley-Blackwell; 2013. [Google Scholar]
- 24. Benowitz NL. Nicotine addiction. N Engl J Med. 2010;362(24):2295–2303. doi:10.1056/NEJMra0809890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Conklin CA, Tiffany ST. Applying extinction research and theory to cue-exposure addiction treatments. Addiction. 2002;97(2):155–167. [DOI] [PubMed] [Google Scholar]
- 26. Ferguson CS, Miksys S, Palmour RM, Tyndale RF. Differential effects of nicotine treatment and ethanol self-administration on CYP2A6, CYP2B6 and nicotine pharmacokinetics in African green monkeys. J Pharmacol Exp Ther. 2012;343(3):628–637. doi:10.1124/jpet.112.198564. [DOI] [PubMed] [Google Scholar]
- 27. Benowitz NL. Neurobiology of nicotine addiction: implications for smoking cessation treatment. Am J Med. 2008;121(4)(suppl 1):S3–10. doi:10.1016/j.amjmed.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 28. Watkins SS, Koob GF, Markou A. Neural mechanisms underlying nicotine addiction: acute positive reinforcement and withdrawal. Nicotine Tob Res. 2000;2(1):19–37. [DOI] [PubMed] [Google Scholar]
- 29. Yu G, Chen H, Zhao W, Matta SG, Sharp BM. Nicotine self-administration differentially regulates hypothalamic corticotropin-releasing factor and arginine vasopressin mRNAs and facilitates stress-induced neuronal activation. J Neurosci. 2008;28(11):2773–2782. doi:10.1523/JNEUROSCI.3837-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Adam H, Shirako A, Maddux WW. Cultural variance in the interpersonal effects of anger in negotiations. Psychol Sci. 2010;21(6):882–889. doi:10.1177/0956797610370755. [DOI] [PubMed] [Google Scholar]
- 31. Leventhal AM, Ameringer KJ, Osborn E, Zvolensky MJ, Langdon KJ. Anxiety and depressive symptoms and affective patterns of tobacco withdrawal. Drug Alcohol Depend. 2013;133(2):324–329. doi:10.1016/J.Drugalcdep.2013.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Leventhal AM, Waters AJ, Moolchan ET, Heishman SJ, Pickworth WB. A quantitative analysis of subjective, cognitive, and physiological manifestations of the acute tobacco abstinence syndrome. Addict Behav. 2010;35(12):1120–1130. doi:10.1016/j.addbeh.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nesic J, Rusted J, Duka T, Jackson A. Degree of dependence influences the effect of smoking on cognitive flexibility. Pharmacol Biochem Behav. 2011;98(3):376–384. doi:10.1016/j.pbb.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Robinson CD, Pickworth WB, Heishman SJ, Waters AJ. The acute tobacco withdrawal syndrome among black smokers. Psychol Addict Behav. 2014;28(1):173–181. doi:10.1037/a0031950. [DOI] [PubMed] [Google Scholar]
- 35. Benowitz NL, Hukkanen J, Jacob P., III Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol. 2009;192:29–60. doi:10.1007/978-3-540-69248-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shareck M, Ellaway A. Neighbourhood crime and smoking: the role of objective and perceived crime measures. BMC Public Health. 2011;11:930. doi:10.1186/1471-2458-11-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ellaway A, Macintyre S. Are perceived neighbourhood problems associated with the likelihood of smoking? J Epidemiol Community Health. 2009;63(1):78–80. doi:10.1136/jech.2007.068767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Borrell LN, Diez Roux AV, Jacobs DR, Jr, et al. Perceived racial/ethnic discrimination, smoking and alcohol consumption in the Multi-Ethnic Study of Atherosclerosis (MESA). Prev Med. 2010;51(3–4):307–312. doi:10.1016/j.ypmed.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vuilleumier P. How brains beware: neural mechanisms of emotional attention. Trends Cogn Sci. 2005;9(12):585–594. doi:10.1016/j.tics.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 40. Ohman A, Mineka S. Fears, phobias, and preparedness: toward an evolved module of fear and fear learning. Psychol Rev. 2001;108(3):483–522. [DOI] [PubMed] [Google Scholar]
- 41. Kassel JD, Shiffman S. Attentional mediation of cigarette smoking’s effect on anxiety. Health Psychol. 1997;16(4):359–368. [DOI] [PubMed] [Google Scholar]
- 42. Evans DE, Drobes DJ. Nicotine self-medication of cognitive-attentional processing. Addict Biol. 2009;14(1):32–42. doi:10.1111/j.1369-1600.2008.00130.x. [DOI] [PubMed] [Google Scholar]
- 43. Asgaard GL, Gilbert DG, Malpass D, Sugai C, Dillon A. Nicotine primes attention to competing affective stimuli in the context of salient alternatives. Exp Clin Psychopharmacol. 2010;18(1):51–60. doi:10.1037/a0018516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liu X, Weiss F. Additive effect of stress and drug cues on reinstatement of ethanol seeking: exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms. J Neurosci. 2002;22(18):7856–7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lu L, Shepard JD, Hall FS, Shaham Y. Effect of environmental stressors on opiate and psychostimulant reinforcement, reinstatement and discrimination in rats: a review. Neurosci Biobehav Rev. 2003;27(5):457–491. [DOI] [PubMed] [Google Scholar]
- 46. Businelle MS, Kendzor DE, Reitzel LR, et al. Mechanisms linking socioeconomic status to smoking cessation: a structural equation modeling approach. Health Psychol. 2010;29(3):262–273. doi:10.1037/a0019285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pampel FC, Krueger PM, Denney JT. Socioeconomic disparities in health behaviors. Annu Rev Sociol. 2010;36:349–370. doi:10.1146/annurev.soc.012809.102529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Spillane NS, Smith GT, Kahler CW. Perceived access to reinforcers as a function of alcohol consumption among one First Nation group. Alcohol Clin Exp Res. 2013;37(suppl 1):E314–321. doi:10.1111/j.1530-0277.2012.01864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Perkins KA. Acute responses to nicotine and smoking: implications for prevention and treatment of smoking in lower SES women. Drug Alcohol Depend. 2009;104(suppl 1):S79–86. doi:10.1016/j.drugalcdep.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 50. Bozarth M. Pleasure systems in the brain. In: Warburton DM, ed. Pleasure: The politics and the Reality. New York, NY: John Wiley & Sons; 1994:5–14. [Google Scholar]
- 51. Caggiula AR, Donny EC, Palmatier MI, Liu X, Chaudhri N, Sved AF. The role of nicotine in smoking: a dual-reinforcement model. Nebr Symp Motiv. 2009;55:91–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dawkins L, Powell J. Effects of nicotine and alcohol on affective responses to emotionally toned film clips. Psychopharmacology (Berl). 2011;216(2):197–205. doi:10.1007/s00213-011-2197-4. [DOI] [PubMed] [Google Scholar]
- 53. Dawkins L, Acaster S, Powell JH. The effects of smoking and abstinence on experience of happiness and sadness in response to positively valenced, negatively valenced, and neutral film clips. Addict Behav. 2007;32(2):425–431. doi:10.1016/j.addbeh.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 54. Dawkins L, Powell JH, West R, Powell J, Pickering A. A double-blind placebo controlled experimental study of nicotine: I--effects on incentive motivation. Psychopharmacology (Berl). 2006;189(3):355–367. doi:10.1007/s00213-006-0588-8. [DOI] [PubMed] [Google Scholar]
- 55. Powell J, Dawkins L, Davis RE. Smoking, reward responsiveness, and response inhibition: tests of an incentive motivational model. Biol Psychiatry. 2002;51(2):151–163. [DOI] [PubMed] [Google Scholar]
- 56. Cook JW, Piper ME, Leventhal AM, Schlam TR, Fiore MC, Baker TB. Anhedonia as a component of the tobacco withdrawal syndrome. J Abnorm Psychol. 2015 Feb;124(1):215–225. doi:10.1037/abn0000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Stevenson B, Wolfers J. Subjective well-being and income: is there any evidence of satiation? Am Econ Rev. 2013;103(3):598–604. doi:10.1257/Aer.103.3.598. [Google Scholar]
- 58. Bowling NA, Eschleman KJ, Wang QA. A meta-analytic examination of the relationship between job satisfaction and subjective well-being. J Occup Organ Psych. 2010;83(4):915–934. doi:10.1348/096317909x478557. [Google Scholar]
- 59. Melamed S, Benavi I, Luz J, Green MS. Objective and subjective work monotony—effects on job-satisfaction, psychological distress, and absenteeism in blue-collar workers. J Appl Psychol. 1995;80(1):29–42. doi:10.1037/0021-9010.80.1.29. [DOI] [PubMed] [Google Scholar]
- 60. Barbeau EM, Krieger N, Soobader MJ. Working class matters: socioeconomic disadvantage, race/ethnicity, gender, and smoking in NHIS 2000. Am J Public Health. 2004;94(2):269–278. doi:10.2105/Ajph.94.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Green KL, Johnson JV. The Effects of Psychosocial Work Organization on Patterns of Cigarette-Smoking among Male Chemical-Plant Employees. Am J Public Health. 1990;80(11):1368–1371. doi:10.2105/Ajph.80.11.1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bancroft A, Wiltshire S, Parry O, Amos A. “It’s like an addiction first thing…afterwards it’s like a habit”: daily smoking behaviour among people living in areas of deprivation. Soc Sci Med. 2003;56(6):1261–1267. [DOI] [PubMed] [Google Scholar]
- 63. Stead M, MacAskill S, MacKintosh AM, Reece J, Eadie D. “It’s as if you’re locked in”: qualitative explanations for area effects on smoking in disadvantaged communities. Health Place. 2001;7(4):333–343. [DOI] [PubMed] [Google Scholar]
- 64. Reitzel LR, Leventhal AM. Socioeconomic status and the reward value of smoking following tobacco abstinence: a laboratory study. Nicotine Tob Res. 2014;16(11):1455–1462. doi:10.1093/ntr/ntu100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Richmond R, Butler T, Wilhelm K, Wodak A, Cunningham M, Anderson I. Tobacco in prisons: a focus group study. Tob Control. 2009;18(3):176–182. doi:10.1136/Tc.2008.026393. [DOI] [PubMed] [Google Scholar]
- 66. Lankenau S. Smoke ‘Em if You Got ‘Em: Cigarette Black Markets in U.S. Prisons and Jails. Prison J. 2001;8(2):142–161. doi:10.1177/0032885501081002002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Visher CA, Travis J. Transitions from prison to community: understanding individual pathways. Annu Rev Sociol. 2003;29:89–113. doi:10.1146/Annurev.Soc.29.010202.095931. [Google Scholar]
- 68. Pager D, Shepherd H. The sociology of discrimination: Racial discrimination in employment, housing, credit, and consumer markets. Annu Rev Sociol. 2008;34:181–209. doi:10.1146/Annurev.Soc.33.040406.131740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Drydakis N. Sexual orientation discrimination in the labour market. Labour Econ. 2009;16(4):364–372. doi:10.1016/J.Labeco.2008.12.003. [Google Scholar]
- 70. Bello MS, Pang RD, Cropsey KL, et al. Tobacco Withdrawal Amongst Black, Hispanic, and White Smokers. Annual Scientific Meeting for the Society for Research on Nicotine and Tobacco; 2014; Philadelphia, PA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bisno B, Richardson JL. The relationship between depression and reinforcing events in cancer patients. J Psychosoc Oncol. 1987;5(2):63–71. doi:10.1300/J077v05n02_06. [Google Scholar]
- 72. Williamson GM. The central role of restricted normal activities in adjustment to illness and disability: a model of depressed affect. Rehabil Psychol. 1998;43(4):327–347. [Google Scholar]
- 73. Busch AM, Borrelli B. Valued life activities and readiness to quit smoking among mobility-impaired smokers. Health Psychol. 2012;31(1):122–125. doi:10.1037/A0025218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Busch AM, Fani Srour J, Arrighi JA, Kahler CW, Borrelli B. Valued life activities, smoking cessation, and mood in post-acute coronary syndrome patients. Int J Behav Med. 2014. doi:10.1007/s12529-014-9456-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Williams JM, Ziedonis D. Addressing tobacco among individuals with a mental illness or an addiction. Addict Behav. 2004;29(6):1067–1083. doi:10.1016/J.Addbeh.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 76. Williams JM, Steinberg ML, Griffiths KG, Cooperman N. Smokers with behavioral health comorbidity should be designated a tobacco use disparity group. Am J Public Health. 2013;103(9):1549–1555. doi:10.2105/Ajph.2013.301232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Van Etten ML, Higgins ST, Budney AJ, Badger GJ. Comparison of the frequency and enjoyability of pleasant events in cocaine abusers vs. non-abusers using a standardized behavioral inventory. Addiction. 1998;93(11):1669–1680. [DOI] [PubMed] [Google Scholar]
- 78. Stuart H. Mental illness and employment discrimination. Curr Opin Psychiatr. 2006;19(5):522–526. doi:10.1097/01.Yco.0000238482.27270.5d. [DOI] [PubMed] [Google Scholar]
- 79. Bouman TK, Luteijn F. Relations between the pleasant events schedule, depression, and other aspects of psychopathology. J Abnorm Psychol. 1986;95(4):373–377. doi:10.1037/0021-843x.95.4.373. [DOI] [PubMed] [Google Scholar]
- 80. Dichter GS, Damiano CA, Allen JA. Reward circuitry dysfunction in psychiatric and neurodevelopmental disorders and genetic syndromes: animal models and clinical findings. J Neurodev Disord. 2012;4:1–43. doi:10.1186/1866-1955-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacol. 2001;24(2):97–129. doi:10.1016/S0893-133x(00)00195-0. [DOI] [PubMed] [Google Scholar]
- 82. Hatzigiakoumis DS, Martinotti G, Giannantonio MD, Janiri L. Anhedonia and substance dependence: clinical correlates and treatment options. Front Psychiatry. 2011;2:10. doi:10.3389/fpsyt.2011.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Spring B, Pingitore R, McChargue DE. Reward value of cigarette smoking for comparably heavy smoking schizophrenic, depressed, and nonpatient smokers. Am J Psychiat. 2003;160(2):316–322. doi:10.1176/Appi.Ajp.160.2.316. [DOI] [PubMed] [Google Scholar]
- 84. MacKillop J, Tidey JW. Cigarette demand and delayed reward discounting in nicotine-dependent individuals with schizophrenia and controls: an initial study. Psychopharmacology (Berl). 2011;216(1):91–99. doi:10.1007/S00213-011-2185-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Spring B, Cook JW, Appelhans B, et al. Nicotine effects on affective response in depression-prone smokers. Psychopharmacology (Berl). 2008;196(3):461–471. doi:10.1007/S00213-007-0977-7. [DOI] [PubMed] [Google Scholar]
- 86. Audrain-McGovern J, Rodriguez D, Rodgers K, Cuevas J. Declining alternative reinforcers link depression to young adult smoking. Addiction. 2011;106(1):178–187. doi:10.1111/J.1360-0443.2010.03113.X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Leventhal AM, Zvolensky MJ. Anxiety, depression, and cigarette smoking: a transdiagnostic framework for understanding emotion-smoking comorbidity. Psychol Bull. 2015 Jan;141(1):176–212. doi:10.1037/bul0000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Leventhal AM, Trujillo M, Ameringer KJ, Tidey JW, Sussman S, Kahler CW. Anhedonia and the relative reward value of drug and nondrug reinforcers in cigarette smokers. J Abnorm Psychol. 2014;123(2):375–386. doi:10.1037/a0036384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Leventhal AM, Waters AJ, Kahler CW, Ray LA, Sussman S. Relations between anhedonia and smoking motivation. Nicotine Tob Res. 2009;11(9):1047–1054. doi:10.1093/Ntr/Ntp098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Leventhal AM, Munafo M, Tidey JW, et al. Anhedonia predicts altered processing of happy faces in abstinent cigarette smokers. Psychopharmacology (Berl). 2012;222(2):343–351. doi:10.1007/S00213-012-2649-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Cook JW, Spring B, McChargue D. Influence of nicotine on positive affect in anhedonic smokers. Psychopharmacology (Berl). 2007;192(1):87–95. doi:10.1007/s00213-006-0688-5. [DOI] [PubMed] [Google Scholar]
- 92. Macphillamy DJ, Lewinsohn PM. The pleasant events schedule—studies on reliability, validity, and scale intercorrelation. J Consult Clin Psych. 1982;50(3):363–380. doi:10.1037/0022-006x.50.3.363. [Google Scholar]
- 93. Gold AB, Lerman C. Pharmacogenetics of smoking cessation: role of nicotine target and metabolism genes. Human Genetics. 2012. doi:10.1007/s00439-012-1143-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Derby KS, Cuthrell K, Caberto C, et al. Nicotine metabolism in three ethnic/racial groups with different risks of lung cancer. Cancer Epidemiol Biomarkers Prev. 2008;17(12):3526–3535. doi:10.1158/1055-9965.EPI-08-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Dutra SJ, Stoeckel LE, Carlini SV, Pizzagalli DA, Evins AE. Varenicline as a smoking cessation aid in schizophrenia: effects on smoking behavior and reward sensitivity. Psychopharmacology (Berl). 2012;219(1):25–34. doi:10.1007/s00213-011-2373-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. MacPherson L, Tull MT, Matusiewicz AK, et al. Randomized controlled trial of behavioral activation smoking cessation treatment for smokers with elevated depressive symptoms. J Consult Clin Psych. 2010;78(1):55–61. doi:10.1037/a0017939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Hazen RA, Vasey MW, Schmidt NB. Attentional retraining: a randomized clinical trial for pathological worry. J Psychiatr Res. 2009;43(6):627–633. doi:10.1016/j.jpsychires.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 98. Schwartz J, Bellinger D, Glass T. Expanding the scope of environmental risk assessment to better include differential vulnerability and susceptibility. Am J Public Health. 2011;101(suppl 1):S88–93. doi:10.2105/AJPH.2011.300268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Urban R, Sutfin E. Do early smoking experiences count in development of smoking?: temporal stability and predictive validity of an early smoking experience questionnaire in adolescents. Nicotine Tob Res. 2010;12(12):1265–1269. doi:10.1093/ntr/ntq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Kitayama S, Mesquita B, Karasawa M. Cultural affordances and emotional experience: socially engaging and disengaging emotions in Japan and the United States. J Pers Soc Psychol. 2006;91(5):890–903. doi:10.1037/0022-3514.91.5.890. [DOI] [PubMed] [Google Scholar]