Abstract
Influenza viral RNA shedding was detected in persons with very few or no symptoms, albeit for a shorter duration and in lower amounts than with symptomatic infections, reflecting a potential for influenza virus transmission from asymptomatic infections to close contacts.
Keywords: influenza virus, viral shedding, asymptomatic, public health, epidemiology.
Abstract
Background.
Influenza virus infections are associated with a wide spectrum of disease. However, few studies have investigated in detail the epidemiological and virological characteristics of asymptomatic and mild illness with influenza virus infections.
Methods.
In a community-based study in Hong Kong from 2008 to 2014, we followed up initially healthy individuals who were household contacts of symptomatic persons with laboratory-confirmed influenza, to identify secondary infections. Information from daily symptom diaries was used to classify infections as symptomatic (≥2 signs/symptoms, including fever ≥37.8°C, headache, myalgia, cough, sore throat, runny nose and sputum), paucisymptomatic (1 symptom only), or asymptomatic (none of these symptoms). We compared the patterns of influenza viral shedding between these groups.
Results.
We identified 235 virologically confirmed secondary cases of influenza virus infection in the household setting, including 31 (13%) paucisymptomatic and 25 (11%) asymptomatic cases. The duration of viral RNA shedding was shorter and declined more rapidly in paucisymptomatic and asymptomatic than in symptomatic cases. The mean levels of influenza viral RNA shedding in asymptomatic and paucisymptomatic cases were approximately 1–2 log10 copies lower than in symptomatic cases.
Conclusions.
The presence of influenza viral shedding in patients with influenza who have very few or no symptoms reflects their potential for transmitting the virus to close contacts. These findings suggest that further research is needed to investigate the contribution of persons with asymptomatic or clinically mild influenza virus infections to influenza virus transmission in household, institutional, and community settings.
Influenza virus infections are associated with a wide range of clinical manifestations, ranging from asymptomatic infection to critical and fatal illness [1]. Although an average of one-third of infections were asymptomatic in a meta-analysis of challenge studies [2], this proportion varied by the viral inoculum dose and the method of administration [3]. Our recent systematic review reported that a pooled mean of 16% (95% confidence interval, 13%–19%) of virologically confirmed influenza virus infections identified in prospective community-based studies were asymptomatic [4]. In that review, we also found evidence for a further proportion of infected patients who were infected but not shedding virus, or shedding at a low level not picked up by virological assessments but indicated by serological testing [4]. Asymptomatic persons and those with mild illness associated with influenza virus infection have rarely been investigated systematically in community studies [5–7]. Therefore, the role of asymptomatic and mildly ill persons with influenza virus infection in the transmission of influenza viruses in the community is largely unknown.
Data are limited on the duration and pattern of influenza viral shedding, and the potential infectivity of asymptomatic and mildly ill persons compared with those with more symptomatic and systemic illness manifestations associated with influenza virus infection. The objective of the current study was to describe the characteristics of asymptomatic and paucisymptomatic (defined below) secondary cases of influenza virus infection identified in a prospective community-based household study conducted over 7 years in Hong Kong, including patterns of clinical illness and quantitative virus shedding, in comparison with symptomatic infections.
METHODS
Recruitment and Follow-up of Participants
A community-based randomized controlled trial investigating the efficacy of nonpharmaceutical interventions against influenza virus transmission in households was conducted in Hong Kong during 2008 [8]. An observational study with similar recruitment and follow-up, but no randomly allocated interventions, was conducted during the influenza A(H1N1) 2009 pandemic [9] and continued through 2014. In these studies, patients were recruited as household index case patients from primary healthcare providers if they presented with acute respiratory illness (ARI), defined as having ≥2 of 7 signs/symptoms (fever ≥37.8°C, headache, myalgia, cough, sore throat, runny nose, and sputum production) and were the first member in their household with a recent ARI.
Index case patients who tested positive for influenza with the QuickVue Influenza A + B rapid diagnostic test (Quidel) were invited to participate in further follow-up, during which the whole household was visited 3 times: on the day of recruitment, and 3 and 6 days later. Nose and throat swab (NTS) specimens were collected from all household members at every home visit, regardless of the presence or absence of respiratory symptoms. Detection of influenza A or B virus and quantification of viral RNA shedding on combined NTS specimens was done with standard reverse-transcription polymerase chain reaction (RT-PCR) testing [10, 11]. Quantitative viral dilutions were also performed to detect median tissue culture infectious dose and determine replicating influenza viral load [12]. Daily symptom diaries including the 7 signs/symptoms listed above were completed by all household members and digital thermometers were distributed to each household for standardized recording of daily tympanic temperature.
Household members other than the index case patient were referred to as household contacts. A secondary case patient was defined as any household contact who was negative for influenza at the first home visit and was subsequently found to be positive for influenza virus by either RT-PCR or virus culture. For the clinical symptoms of these laboratory-confirmed secondary infections, a case patient with ARI was defined as a contact who had ≥2 respiratory signs or symptoms from the 7 listed above. A subset of these patients also met the criteria for influenza-like illness (ILI), which we defined as fever ≥37.8°C plus cough or sore throat. A paucisymptomatic case patient was defined as a contact who reported only 1 sign/symptom on ≥1 day of follow-up and ≤1 of the 7 signs/symptoms on every day of follow-up. Asymptomatic case patients were defined as those who reported none of the 7 signs/symptoms listed above on any day during follow-up. Among case patients with ARI, a further subcategory of ILI was defined as the presence of fever (≥37.8°C) plus cough and/or sore throat [9].
Statistical Analysis
We calculated the proportion of asymptomatic and paucisymptomatic cases among secondary infections by different influenza viral types/subtypes (prepandemic A(H1N1), A(H1N1)pdm09, A(H3N2), and influenza B), and compared these proportions using Z tests. We analyzed the time from symptom onset to alleviation with Kaplan-Meier estimates and compared the symptomatic duration between paucisymptomatic and ARI case patients with log-rank tests. To study the determinants of clinical presentation among secondary case patients, we modeled the odds of being symptomatic (meeting the ARI case definition), compared with clinically asymptomatic or paucisymptomatic, by a logistic regression model adjusting for relevant demographics (age and sex), medical factors (comorbid conditons and vaccination history), and familial role of index case patients (parent or child).
The geometric mean of log10 viral shedding assessed by quantitative RT-PCR was compared between the asymptomatic, paucisymptomatic, and ARI groups, stratified by viral type/subtype. For undetectable values, half of the lower limit of detection (ie, 450 copies/mL) was imputed when calculating daily geometric means. Patterns of influenza viral RNA shedding over time were analyzed by plotting the viral copies obtained from the quantitative RT-PCR cycle threshold values by the day of first symptom onset (day 0) for ARI and paucisymptomatic case patient groups. We compared the trajectories of the decline in viral RNA shedding between these patient groups using linear random effects model [12]. We also examined the duration of influenza viral RNA shedding by analyzing data on detection of viral RNA by RT-PCR in the NTS specimens collected on days 0, 3, and 6, accounting for the interval censoring. We fitted log-normal regression models to investigate the correlation between the duration of viral RNA shedding and classification of case patients as ARI, paucisymptomatic, and asymptomatic. All analyses were conducted with R software, version 3.2.2 (R Foundation for Statistical Computing).
RESULTS
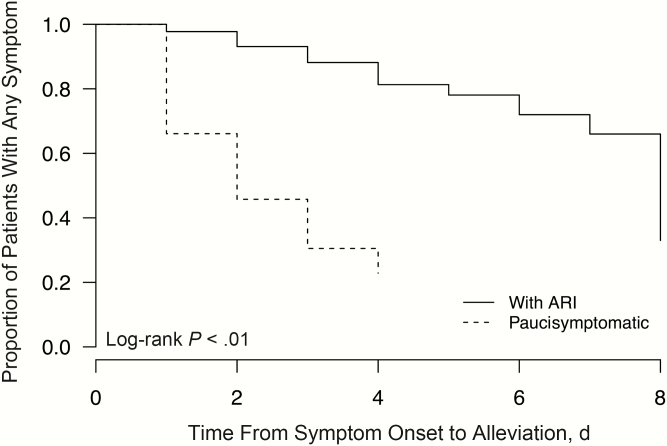
Over the study period of 7 years, we enrolled 852 households each with an index case patient who tested positive for influenza by rapid influenza diagnostic test, and we obtained NTS specimens from a total of 2645 household contacts from these households. Among all household contacts, 235 (8.9%) had laboratory-confirmed secondary influenza virus infection identified by RT-PCR and/or virus culture. Among these secondary case patients, 179 (76%) had ARI, including 83 (35%) with ILI, and 56 (24%) reported ≤1 sign/symptom, including 31 paucisymptomatic patients (13%) reporting only 1 symptom and 25 asymptomatic patients (11%) (Table 1). On stratification by type and subtype of influenza A virus, the proportion of asymptomatic and paucisymptomatic patients varied from 6% to 21% but differences were not statistically significant (P = .29; χ2 test) (Table 1). The asymptomatic fraction varied from 6% for A(H1N1)pdm09 to 20% for influenza B, but these differences were not significant (P = .09; χ2test) (Table 1). When paucisymptomatic patients were compared with those with ARI, the time from symptom onset to alleviation was significantly shorter (P < .01; log-rank test), with a median symptomatic duration of 2 days, versus 8 days for patients with ARI (Figure 1).
Table 1.
Characteristics and Clinical Symptoms of 235 Naturally Acquired Secondary Influenza Virus Infection With Differential Clinical Presentations
| Characteristics and Symptoms | Case Patients, No. (%) | ||||
|---|---|---|---|---|---|
| ARI Case Patients | Paucisymptomatic | Asymptomatic |
|||
| All Secondary Case Patients, No. | ILI |
ARI Not Meeting Definition of ILI | |||
| Overall | 235 | 83 (35) | 96 (41) | 31 (13) | 25 (11) |
| Influenza type/subtype | |||||
| Pandemic A(H1N1) | 33 | 9 (27) | 15 (45) | 7 (21) | 2 (6) |
| Seasonal A(H1N1) | 73 | 28 (38) | 33 (45) | 7 (10) | 5 (7) |
| Seasonal A(H3N2) | 69 | 24 (35) | 29 (42) | 8 (12) | 8 (12) |
| Seasonal B | 49 | 15 (31) | 16 (33) | 8 (16) | 10 (20) |
| Characteristics | |||||
| Male sex | 91 | 33 (40) | 37 (39) | 10 (32) | 11 (44) |
| Age group | |||||
| 0–15 y | 61 | 35 (43) | 14 (15) | 8 (26) | 4 (16) |
| 16–45 y | 134 | 34 (42) | 65 (68) | 18 (58) | 17 (68) |
| ≥46 y | 37 | 12 (15) | 16 (17) | 5 (16) | 4 (16) |
| Received influenza vaccine in past 1 y | 28 | 12 (15) | 10 (10) | 3 (10) | 3 (12) |
| Ever smoker | 20 | 4 (5) | 13 (14) | 1 (3) | 2 (8) |
| Underlying medical condition | 34 | 13 (16) | 14 (15) | 4 (13) | 3 (12) |
| Symptoms reported | |||||
| Cough | 162 | 81 (98) | 78 (81) | 3 (10) | … |
| Runny nose | 171 | 74 (89) | 87 (91) | 10 (32) | … |
| Sore throat | 132 | 62 (75) | 66 (69) | 4 (13) | … |
| Sputum | 127 | 62 (75) | 62 (65) | 3 (10) | … |
| Fever (≥37.8°C) | 97 | 83 (100) |
6 (6) |
8 (26) |
… |
| Headache | 104 | 49 (59) | 49 (51) | 6 (19) | … |
| Myalgia | 98 | 47 (57) | 49 (51) | 2 (6) | … |
Abbreviations: ARI, acute respiratory illness; ILI, influenzalike illness.
Figure 1.
Proportion of patients with acute respiratory illness (ARI) (solid line) and paucisymptomatic patients (dashed line) remaining symptomatic in naturally acquired influenza virus infections by day since first symptom onset (day 0).
Apart from fewer smokers in the paucisymptomatic group, the sex and age distribution and the proportion receiving influenza vaccination in the past year or having chronic illness were generally similar among the 3 groups of patients with differential clinical presentations (Table 1). On logistic regression, the adjusted odds of being symptomatic were not significantly associated with age, sex, vaccination history, smoking status, or underlying conditions (Table 2).
Table 2.
Determinants of Having a Symptomatic Clinical Presentation (ARI)
| Factor | Adjusted ORa (95% CI) |
|
|---|---|---|
| All Data | Adults Only | |
| Age group | ||
| 0–15 y | 1.00 | … |
| 16–45 y | 0.71 (0.34–1.51) | 1.00 |
| >45 y | 0.78 (0.29–2.08) | 1.10 (0.46–2.64) |
| Sex | ||
| Female | 1.00 | 1.00 |
| Male | 1.01 (0.53–1.89) | 0.80 (0.37–1.77) |
| Received influenza vaccine in past 1 y | ||
| No | 1.00 | 1.00 |
| Yes | 1.17 (0.45–3.06) | 1.35 (0.42–4.34) |
| Ever smoker | ||
| No | … | 1.00 |
| Yes | … | 2.25 (0.56–8.97) |
| Underlying medical condition | ||
| No | … | 1.00 |
| Yes | … | 1.00 (0.38–2.65) |
Abbreviations: ARI, acute respiratory illness; CI, confidence interval; OR, odds ratio.
aOR for meeting ARI criteria among all study participants with polymerase chain reaction–confirmed influenza.
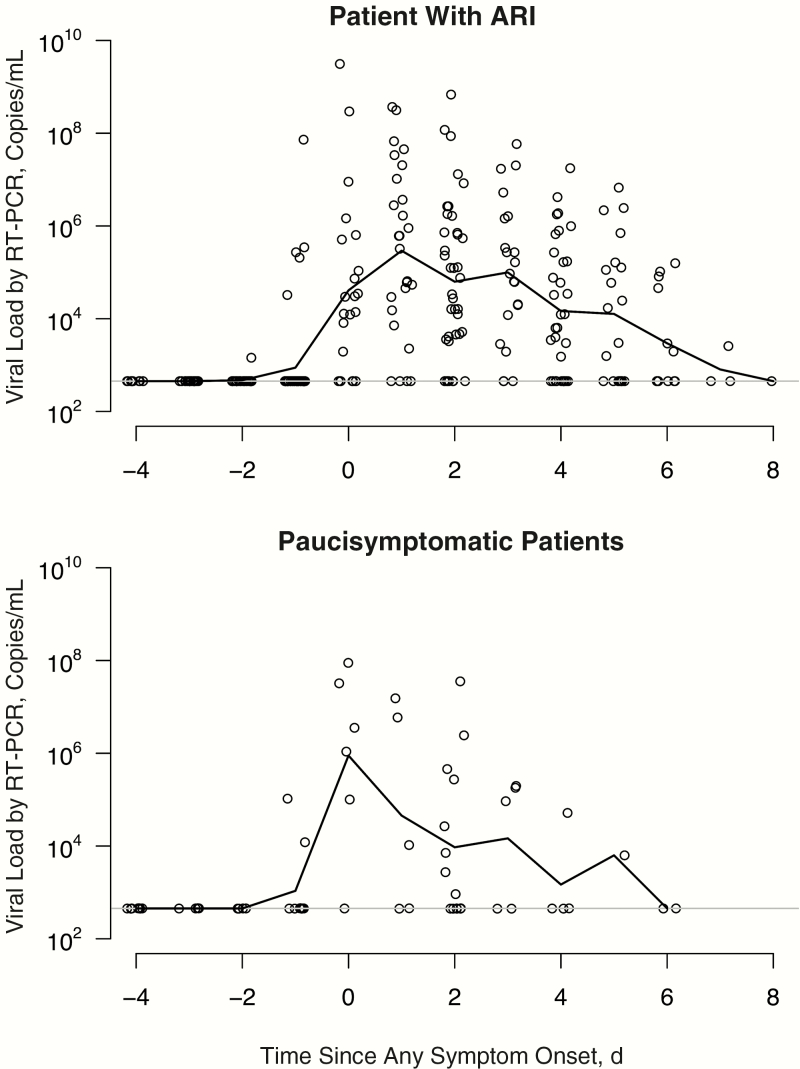
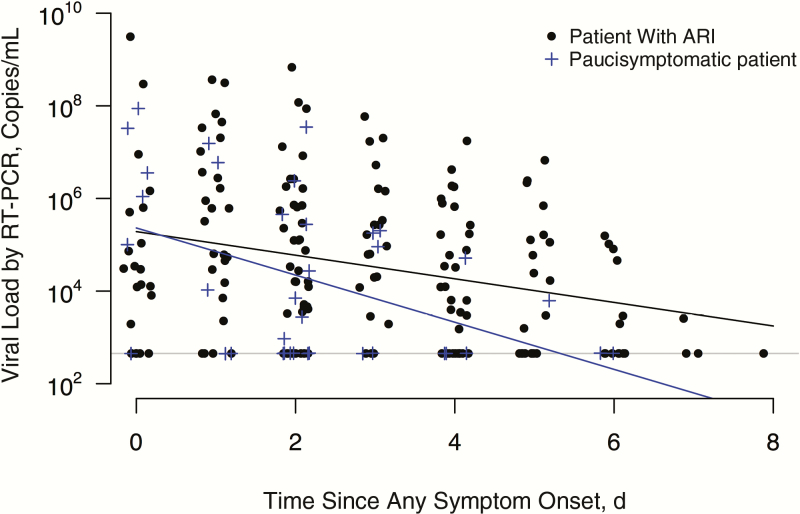
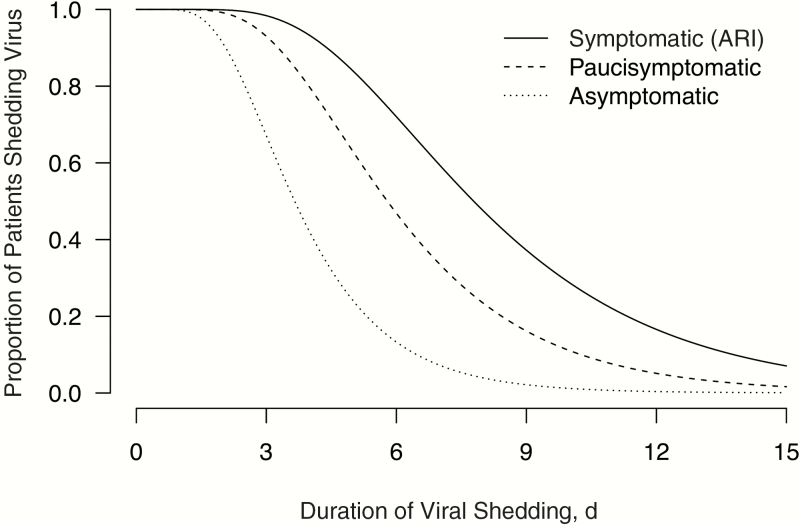
As assessed by quantitative RT-PCR, patients with ARI generally had detectable influenza viral RNA from 2 days before the appearance of clinical symptoms, peaking on day 1 and subsiding gradually through day 8 after initial symptom onset (Figure 2). Paucisymptomatic case patients followed a similar overall pattern of viral RNA detection with a shorter duration, starting up to 2 days before illness onset, peaking on the day of onset, and extending to about day 6 after symptom onset. This shorter observed duration of viral shedding was also consistent with the faster decline in viral RNA load as measured by RT-PCR in paucisymptomatic patients compared with those who had ARI (Figure 3). Because the exact timing of infection could not be precisely determined for asymptomatic patients, we used the interval-censored data on detection of virus by RT-PCR with respect to when these NTS specimens were collected on days 0, 3, and 6 of the follow-up visit, and we found that the duration of viral RNA detection was significantly shorter (P < .01) for paucisymptomatic and asymptomatic patients than for symptomatic patients with ARI (Figure 4).
Figure 2.
Patterns of viral shedding in naturally acquired influenza virus infections by day since first symptom onset (day 0) in patients with acute respiratory illness (ARI) (upper panel) and paucisymptomatic patients (lower panel). RT-PCR, reverse-transcription polymerase chain reaction.
Figure 3.
Rate of viral load decline as measured by reverse-transcription polymerase chain reaction (RT-PCR) in paucisymptomatic patients (blue line) and patients with acute respiratory illness (ARI) (black line).
Figure 4.
Estimated duration of viral shedding from the start of virus shedding (0 on the x-axis), accounting for interval censoring in the data on detection of virus by reverse-transcription polymerase chain reaction at 3-day intervals, for patients with acute respiratory illness (ARI) (solid line), paucisymptomatic patients (dashed line), and asymptomatic patients (dotted line).
Stratifying by different influenza A viral subtypes, the overall quantity of viral shedding as reflected by the mean viral load varied across secondary case patients with different clinical manifestations. Among patients with seasonal A(H3N2) influenza, mean viral RNA loads were generally similar between asymptomatic, paucisymptomatic, and ARI patient groups. For A(H1N1)pdm09, seasonal A(H1N1) and B virus infections, the mean levels of viral RNA shedding in the asymptomatic and/or paucisymptomatic case patients were approximately 1–2 log10 copies lower than in symptomatic patients (Table 3).
Table 3.
Overall Quantity of Viral Shedding (Mean Viral Load) in Cases Patients With Different Clinical Symptoms, Stratified by Influenza Viral Subtypes
| Influenza Type/Subtype | Case Patients with ARIa | Paucisymptomatic Case Patients | Asymptomatic Case Patients | |||
|---|---|---|---|---|---|---|
| No. | Mean Viral Load, Log10 Copies/mL | No. | Mean Viral Load, Log10 Copies/mL | No. | Mean Viral Load, Log10 Copies/mL | |
| Pandemic A(H1N1) | 24 | 4.7 | 7 | 4.3 | 2 | 2.7 |
| Seasonal A(H1N1) | 61 | 5.6 | 7 | 6.6 | 5 | 4.0 |
| Seasonal A(H3N2) | 53 | 5.5 | 8 | 4.9 | 8 | 5.5 |
| Seasonal B | 31 | 5.5 | 8 | 3.6 | 10 | 4.4 |
| Overall | 179 | 5.4 | 31 | 4.8 | 25 | 4.5 |
Abbreviation: ARI, acute respiratory illness.
aIncluding patients with influenzalike illness.
DISCUSSION
This study provides a detailed description of the epidemiological mechanism and patterns of influenza viral shedding as assessed by quantitative viral RNA detection in 56 asymptomatic and paucisymptomatic cases of naturally acquired influenza virus infections, including seasonal and pandemic influenza, representing 24% of all 235 secondary household cases we investigated spanning 7 years. The overall asymptomatic fraction among the cases identified by influenza virus type was estimated to be 11% (25 of 235), and varied from 6% to 20% in infections with different influenza A virus subtypes (Table 1). This is comparable with published estimates of the asymptomatic fraction among virologically confirmed cases, and the pooled estimate of 16% in a recent systematic review [4], but much lower than the 30%–50% used in some modeling studies [1]. Accurate estimation of the asymptomatic fraction of influenza virus infections is important, because the potential transmissibility of asymptomatic infections could have implications for transmission dynamics and control of influenza.
We found that the mean levels of influenza viral RNA shedding in the asymptomatic and/or paucisymptomatic case patents were approximately 1–2 log10 copies lower than in symptomatic cases for A(H1N1)pdm09, seasonal A(H1N1) and B virus infections, but similar between the asymptomatic patients and those with typical ARI symptoms associated with A(H3N2) virus infections (Table 3). We and others have previously shown a strong correlation between influenza viral RNA loads detected by RT-PCR and virus infectivity, as measured by quantitative viral culture assays [12–14]. Assuming that the quantity of viral RNA detection is a reasonable proxy for viral shedding and infectiousness [15], this suggests the potential for influenza virus transmission from infected persons to their close contacts even in the absence of clinical symptoms, although asymptomatically infected persons could play a more substantial role in the transmission of influenza A(H3N2) virus. The potential for disease transmission by asymptomatic persons highlights the inadequacy of preventive measures targeting only clinically recognizable cases, such as isolating or excluding patients with symptomatic ARI/ILI from work or school, and further supports the importance of general preventive measures, including good general hygiene practices and influenza vaccination for effective control of epidemics.
For case patients with influenza A(H1N1)pdm09, A(H1N1), or B, the observation of lower quantities of virus shedding by asymptomatic than by symptomatic individuals may imply lower infectiousness in the former. However, it should be noted that we only studied the pattern of viral RNA shedding in the current study and not the potential role of other factors related to symptom variability that may affect disease transmission in real-life settings These included behavioral variation, such as work or school absenteeism that may affect close contact with others in daily activities [16]; differential adoption of preventive measures, such as face masks and hand hygiene, or treatment options in relation to symptom occurrence and disease awareness [8, 17]; and the potential contribution of certain symptoms (eg, coughing and sneezing) in symptomatic patients to enhance virus dispersal [18–20].
Although the dynamics of viral shedding patterns over time has been studied in symptomatic individuals [2], very few studies have systematically examined viral shedding in persons with asymptomatic influenza virus infection [7]. The prospective household transmission study represents an ideal setting to identify and characterize asymptomatic infections because this study design allowed both symptoms and influenza viral shedding to be measured prospectively regardless of symptoms. We were therefore able to ascertain asymptomatic infections in exposed household contacts of all ages, underlying medical conditions, and wide variety of demographic characteristics [7, 19, 21]. We identified viral shedding, though lasting for a shorter duration, in patients with very few or no symptoms. Although secondary cases in a household setting might not be representative of all influenza virus infections occurring in other community settings because of the particular mechanisms of transmission in households [22], it has been estimated that a large proportion of influenza virus transmission in the community occurs in the household setting [23]. Consistently, Loeb et al also reported that the duration of influenza virus shedding was shorter in asymptomatic cases [7]. The correlation between cross-protective T-cell responses with lower virus shedding and milder disease reported in a viral challenge study [24] may be a plausible mechanism for explaining our observation and warrants further investigation.
It is possible that we may have underestimated the asymptomatic fraction if some of the symptoms reported by participants during follow-up (eg, cough, or runny nose) had a cause other than influenza (eg, poor air quality, cold weather, allergies, or coinfection with other respiratory viruses). Furthermore, we have not considered some other less common symptoms, such as vomiting and diarrhea. On the other hand, examining virologically confirmed infections may bias against the inclusion of very mild infections associated with low levels of viral shedding for a short time that nevertheless stimulate an immune response and provide immunity against reinfection. In an earlier review, Leung et al [4] did find that serological studies tended to estimate a greater fraction of influenza virus infections that were reported as asymptomatic, suggesting the presence of a proportion of patients who are infected but not shedding virus, or shedding at a low level that escaped being picked up by virological testing.
The potential contribution of asymptomatically infected persons to influenza virus transmission depends on the proportion of infected persons who are asymptomatic, the infectiousness of these asymptomatic cases, and likely host factors as well as immune factors in their close contacts. The greatest gap in our knowledge is regarding the relative infectiousness of asymptomatic cases. The substantial viral RNA detection in asymptomatic cases in our study supports the need for and importance of further research in this area. A knowledge of the minimal infectious dose required for influenza virus infection would also be important in determining the relative contribution of asymptomatic cases to the overall transmission of influenza viruses in households and in the community.
Our study did have some potential limitations. Short and mild infections could be missed during the gaps between NTS specimen collections. Recruitment of index case patients required participants to be present at a healthcare provider, possibly resulting in a bias towards index patients with more severe illness and health-seeking behavior. Some primary infections due to out-of-household transmission may have been classified as secondary household cases, although our analysis of genetic sequence data indicated that >95% of subsequent cases in households in our study were likely to be secondary cases rather than coincidental infections from the community [25]. Finally, although Tsang et al [15] previously noted that quantitative detection of influenza viral RNA is a reasonable proxy for infectiousness, detection of viral RNA is not the same as isolation of infectious virus and may not correlate perfectly with an infected person’s infectivity.
In conclusion, our study demonstrated the presence of viral shedding in patients with influenza who have very few or no signs or symptoms, reflecting their potential for transmitting the virus and infecting others. The similar amount of viral RNA detection in asymptomatic case patients and patients with typical ARI symptoms associated with A(H3N2) virus infections in particular highlights the potential for influenza virus transmission from infected persons, even in the absence of clinical symptoms. These findings suggest the need for further studies to investigate and quantify the contribution of persons with asymptomatic or clinically mild influenza virus infections to influenza virus transmission.
Notes
Acknowledgments. We thank all the physicians, nurses, and staff members at the participating centers for facilitating recruitment and the dedicated team of healthcare workers who conducted the home visits.
Disclaimer. This work represents the views of the authors and not necessarily the official policy of the Centers for Disease Control and Prevention.
Financial support. This project was supported by the National Institute of Allergy and Infectious Diseases (NIAID; contract HHSN266200700005C; ADB N01-AI-70005 [NIAID Centers for Excellence in Influenza Research and Surveillance]), the government of the Hong Kong Special Administrative Region (commissioned grant from the Health and Medical Research Fund), the Harvard Center for Communicable Disease Dynamics (grant U54 GM088558 from the National Institute of General Medical Sciences), and the Research Grants Council of the Hong Kong Special Administrative Region, China (project T11-705/14N).
Potential conflicts of interest. D. K. M. I. has received research funding from Hoffmann–La Roche. J. S. M. P. receives research funding from Crucell and serves as an ad hoc consultant for GlaxoSmithKline and Sanofi. B. J. C. has received research funding from Sanofi Pasteur. All others authors report potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Patrozou E, Mermel LA. Does influenza transmission occur from asymptomatic infection or prior to symptom onset? Public Health Rep 2009; 124:193–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Carrat F, Vergu E, Ferguson NM, et al. Time lines of infection and disease in human influenza: a review of volunteer challenge studies. Am J Epidemiol 2008; 167:775–85. [DOI] [PubMed] [Google Scholar]
- 3. Alford RH, Kasel JA, Gerone PJ, Knight V. Human influenza resulting from aerosol inhalation. Proc Soc Exp Biol Med 1966; 122: 800–4. [DOI] [PubMed] [Google Scholar]
- 4. Leung NH, Xu C, Ip DK, Cowling BJ. Review article: the fraction of influenza virus infections that are asymptomatic: a systematic review and meta-analysis. Epidemiology 2015; 26:862–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hayward AC, Fragaszy EB, Bermingham A, et al. ; Flu Watch Group Comparative community burden and severity of seasonal and pandemic influenza: results of the Flu Watch cohort study. Lancet Respir Med 2014; 2:445–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang TE, Lin CY, King CC, Lee WC. Estimating pathogen-specific asymptomatic ratios. Epidemiology 2010; 21:726–8. [DOI] [PubMed] [Google Scholar]
- 7. Loeb M, Singh PK, Fox J, et al. Longitudinal study of influenza molecular viral shedding in Hutterite communities. J Infect Dis 2012; 206:1078–84. [DOI] [PubMed] [Google Scholar]
- 8. Cowling BJ, Chan KH, Fang VJ, et al. Facemasks and hand hygiene to prevent influenza transmission in households: a cluster randomized trial. Ann Intern Med 2009; 151:437–46. [DOI] [PubMed] [Google Scholar]
- 9. Cowling BJ, Chan KH, Fang VJ, et al. Comparative epidemiology of pandemic and seasonal influenza A in households. N Engl J Med 2010; 362:2175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Peiris JS, Tang WH, Chan KH, et al. Children with respiratory disease associated with metapneumovirus in Hong Kong. Emerg Infect Dis 2003; 9:628–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chan KH, Peiris JS, Lim W, Nicholls JM, Chiu SS. Comparison of nasopharyngeal flocked swabs and aspirates for rapid diagnosis of respiratory viruses in children. J Clin Virol 2008; 42:65–9. [DOI] [PubMed] [Google Scholar]
- 12. Lau LL, Cowling BJ, Fang VJ, et al. Viral shedding and clinical illness in naturally acquired influenza virus infections. J Infect Dis 2010; 201:1509–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tsou TP, Shao PL, Lu CY, et al. Viral load and clinical features in children infected with seasonal influenza B in 2006/2007. J Formos Med Assoc 2012; 111: 83–7. [DOI] [PubMed] [Google Scholar]
- 14. Cheng SM, Vainionpaa R, Zhao P, et al. Detection of influenza B in clinical specimens: comparison of high throughput RT-PCR and culture confirmation. Virus Res 2004; 103:85–90. [DOI] [PubMed] [Google Scholar]
- 15. Tsang TK, Cowling BJ, Fang VJ, et al. Influenza a virus shedding and infectivity in households. J Infect Dis 2015; 212:1420–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ip DK, Lau EH, Tam YH, So HC, Cowling BJ, Kwok HK. Increases in absenteeism among health care workers in Hong Kong during influenza epidemics, 2004–2009. BMC Infect Dis 2015; 15: 586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cheung DH, Tsang TK, Fang VJ, et al. Association of oseltamivir treatment with virus shedding, illness, and household transmission of influenza viruses. J Infect Dis 2015; 212:391–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bischoff WE, Swett K, Leng I, Peters TR. Exposure to influenza virus aerosols during routine patient care. J Infect Dis 2013; 207:1037–46. [DOI] [PubMed] [Google Scholar]
- 19. Thai PQ, Mai le Q, Welkers MR, et al. Pandemic H1N1 virus transmission and shedding dynamics in index case households of a prospective Vietnamese cohort. J Infect 2014; 68:581–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ip DK, Lau LL, Chan KH, et al. The dynamic relationship between clinical symptomatology and viral shedding in naturally acquired seasonal and pandemic influenza virus infections. Clin Infect Dis 2016; 62:431–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Suess T, Remschmidt C, Schink SB, et al. Comparison of shedding characteristics of seasonal influenza virus (sub)types and influenza A(H1N1)pdm09; Germany, 2007–2011. PLoS One 2012; 7: e51653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cowling BJ, Ip DK, Fang VJ, et al. Aerosol transmission is an important mode of influenza A virus spread. Nat Commun 2013; 4:1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hayden FG, Belshe R, Villanueva C, et al. Management of influenza in households: a prospective, randomized comparison of oseltamivir treatment with or without postexposure prophylaxis. J Infect Dis 2004; 189:440–9. [DOI] [PubMed] [Google Scholar]
- 24. Wilkinson TM, Li CK, Chui CS, et al. Preexisting influenza-specific CD4+ T cells correlate with disease protection against influenza challenge in humans. Nat Med 2012; 18:274–80. [DOI] [PubMed] [Google Scholar]
- 25. Poon LL, Chan KH, Chu DK, et al. Viral genetic sequence variations in pandemic H1N1/2009 and seasonal H3N2 influenza viruses within an individual, a household and a community. J Clin Virol 2011; 52: 146–50. [DOI] [PMC free article] [PubMed] [Google Scholar]