Abstract
Background: In a European cohort, it was previously reported that 35% of oropharyngeal cancer (OPC) patients were human papillomavirus type-16 (HPV16) seropositive up to 10 years before diagnosis vs 0.6% of cancer-free controls. Here, we describe the kinetics of HPV16-E6 antibodies prior to OPC diagnosis.
Methods: We used annual serial prediagnostic blood samples from the PLCO Cancer Screening Trial. Antibodies to HPV were initially assessed in prediagnostic blood drawn at study enrollment from 198 incident head and neck cancer patients (median years to cancer diagnosis = 6.6) and 924 matched control subjects using multiplex serology, and subsequently in serial samples (median = 5/individual). Available tumor samples were identified and tested for HPV16 RNA to define HPV-driven OPC.
Results: HPV16-E6 antibodies were present at baseline in 42.3% of 52 OPC patients and 0.5% of 924 control subjects. HPV16-E6 antibody levels were highly elevated and stable across serial blood samples for 21 OPC patients who were seropositive at baseline, as well as for one OPC patient who seroconverted closer to diagnosis. All five subjects with HPV16-driven OPC tumors were HPV16-E6-seropositive, and the four subjects with HPV16-negative OPC tumors were seronegative. The estimated 10-year cumulative risk of OPC was 6.2% (95% confidence interval [CI] = 1.8% to 21.5%) for HPV16-E6-seropositive men, 1.3% (95% CI = 0.1% to 15.3%) for HPV16-E6-seropositive women, and 0.04% (95% CI = 0.03% to 0.06%) among HPV16-E6-seronegative individuals.
Conclusions: Forty-two percent of subjects diagnosed with OPC between 1994 and 2009 in a US cohort were HPV16-E6 seropositive, with stable antibody levels during annual follow-up for up to 13 years prior to diagnosis. Tumor analysis indicated that the sensitivity and specificity of HPV16-E6 antibodies were exceptionally high in predicting HPV-driven OPC.
Oropharyngeal cancer (OPC) incidence has increased in parts of the developed world (1–11), likely due to human papillomavirus type-16 (HPV16) (1–3). In the United States, incidence of OPC has risen more than 200% during the past three decades, and HPV16 infection has been estimated to be responsible for more than 70% of cases diagnosed in the 2000s (1).
In a European cohort, we identified HPV16-E6 antibody positivity as a potential biomarker for OPC (12). In this study, 35% of OPC patients were seropositive for HPV16-E6 compared with 0.6% of controls. The proportion of HPV16-E6-seropositive OPC was similar to the attributable fraction of HPV-positive OPC tumors in Europe from this time (13). When the lead time between the collection of the single blood sample and OPC diagnosis was evaluated, the proportion of HPV16-E6-seropositive cases was similar in cases with a blood draw within five and 10 or more years of OPC diagnosis. Intriguingly, this finding suggests that testing HPV16-E6 in a single blood draw might predict a large proportion of future OPC cases, particularly in countries with large HPV-attributable OPC fractions such as the United States (1). However, a number of questions remain unanswered, including the time between HPV infection to malignant transformation and seroconversion, the kinetics of the antibody response approaching OPC diagnosis, and the sensitivity of the HPV16-E6 biomarker in a prospective setting. Few cohort studies have the necessary serial blood samples or tumor specimens available to address such novel questions.
In the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial (PLCO) (14), blood samples were collected annually over six years from screening arm participants, allowing for the investigation of the kinetics of potential biomarkers. Subsequently, a tumor retrieval effort was also conducted across the United States. PLCO enrolled participants (n > 154 000) from 1993 to 2001, with follow-up ongoing, spanning a time when 50% to 70% of OPC cases in the United States were attributed to HPV16 infection (1).
Methods
Study Cohort
PLCO is a randomized trial (clinicaltrials.gov trial registration: NCT00339495) to determine the effects of prostate, lung, colorectal, and ovarian cancer screening on disease-specific mortality (14); 154 935 individuals were recruited between 1993 and 2001 from 10 US screening centers. Eligibility criteria included age 55 to 74 years and no history of prostate, lung, colorectal, or ovarian cancer. Relevant exclusion criteria were current cancer treatment (except nonmelanoma skin cancer) and concurrent participation in another cancer-screening study. Participants provided written informed consent, and the research was approved by the local ethics committees and the National Cancer Institute Institutional Review Board.
Eligible participants were randomly assigned in a 1:1 ratio either to the control arm (who followed their normal health care routine) or to the intervention arm. In the intervention arm, study participants were screened for prostate (men), lung, colorectal, and ovarian (women) cancer over six years, and blood was collected at each of these annual study visits. Participants with positive screening results were referred to their physicians for diagnostic follow-up. Because blood was not collected among participants in the control arm, we nested our study in the intervention arm.
At study entry, participants completed a questionnaire covering demographic information, smoking, and other variables. Incident cancer cases were identified during follow-up using self-report, death certificates, linkage to the National Death Index, and information from next of kin. Certified tumor registrars abstracted pathology reports, and cancer diagnoses were verified by review of medical records.
Selection of Cases and Controls
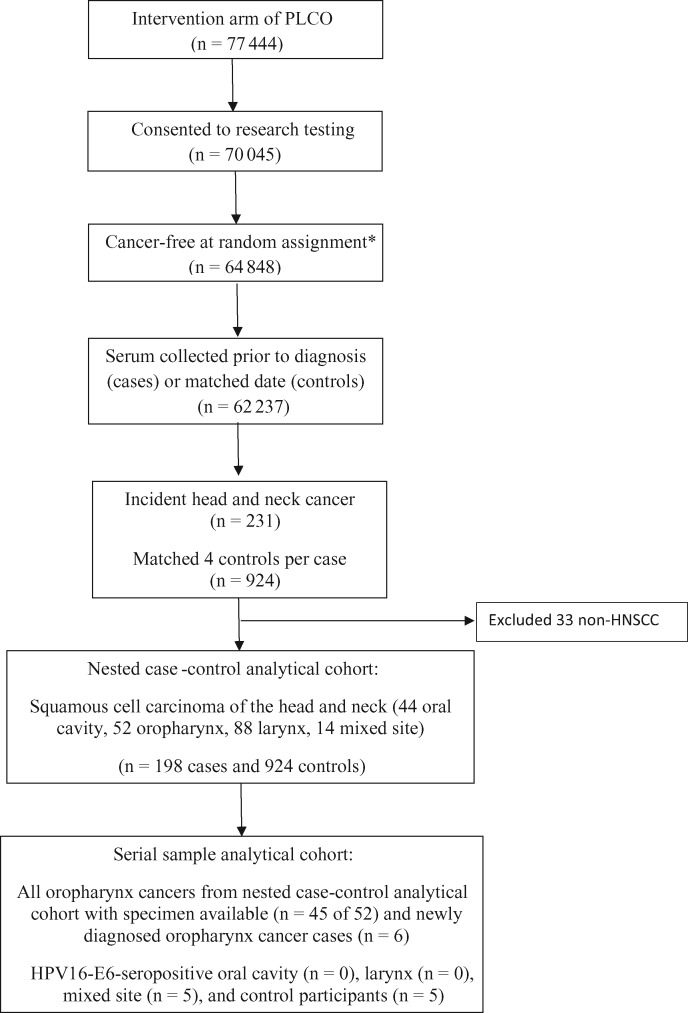
There were 77 444 individuals in the intervention arm of PLCO; 70 045 (90.4%) consented to research testing, 64 848 (83.7%) had no cancer prior to random assignment and at least one day of follow-up, and 62 237 (80.4%) had serum collected prior to cancer diagnosis (cases) or the matched date (controls). Within this subcohort, 231 incident head and neck cancer (HNC) cases were identified during the follow-up, including cancers of the oral cavity (C020-C023, C030-C050, C060-69), oropharynx (C019, C024, C051, C052, C090-C109), and larynx (C320-C329); a “mixed sites” category was included to account for overlapping lesions (C028, C029, C058, C059, C140, C142, C148, C149).
For each case, four controls were randomly chosen from appropriate risk sets consisting of all cohort members alive and free of cancer (except NMSC) at the time of diagnosis of the index case. Matching criteria were year of blood draw (calendar and study year), birth year, sex, race, and smoking status (never, former, current). After excluding 33 cases with nonsquamous cell carcinomas, the final study population included 198 cases and 924 controls. Of these, 52 cases had OPC diagnosed during follow-up.
Serology analyses were run in two phases in order to justify the use of serial samples from PLCO (Figure 1). We initially conducted a nested case-control analysis using the serum samples collected at baseline (at enrollment or close to it; “baseline serum”), mirroring our initial study in the European cohort (12). Results at baseline were similar to our initial findings [12], so we obtained access to serial samples for 1) 45 of 52 OPC cases regardless of baseline HPV16-E6 serostatus (two excluded due to lack of specimen; five HPV16-E6-seronegative cases originally classified as oral cavity and thus did not meet criteria for serial sample testing), 2) five HPV16-E6-seropositive mixed-site cases, 3) five HPV16-E6-seropositive controls, and 4) six new OPC cases that occurred following completion of the nested case-control study (diagnosed between June 2010 and August 2013). In 2013, PLCO undertook collection of tumor tissue from multiple cancer sites, including HPV-driven cancers (HNSCC and anogenital).
Figure 1.
CONSORT flow diagram. HPV = human papillomavirus; HNSCC = head and neck squamous cell carcinoma. *The inclusion of cancer-free at random assignment excludes rare cancers amongst controls.
Serologic Analyses
Briefly, antigens were bacterially expressed, affinity-purified fusion proteins with N-terminal Glutathione S-transferase (15,16). Median fluorescence intensity (MFI) values were dichotomized based on previously established cut-points (12); specifically for HPV16-E6, a prespecified cutoff for seropositivity of MFI values greater than 1000 used as this cutoff resulted in optimal specificity without losing sensitivity in our previous study (12). Quality control (QC) measures for the multiplex serology assay were attained by intermixing four QC samples (A, B, C, and D) with the study samples across the 14 plates tested (based on available volume, between 11 and 14 aliquots were made and tested as blinded replicates). Intraclass correlation coefficients (ICCs) across all antigens for the QC samples were sample A 0.97 (95% CI = 0.95 to 0.99), sample B 1 (95% CI = 1 to 1), sample C 0.98 (95% CI = 0.96 to 0.99), and sample D 0.99 (95% CI = 0.98 to 0.99).
Tumor Analyses
DNA and RNA were extracted from formalin-fixed paraffin-embedded (FFPE) tissue sample sections (17); water samples were included to monitor cross-contamination. HPV DNA analysis was conducted using Multiplex Papillomavirus Genotyping (18,19). Samples positive for HPV and/or beta-globin were considered DNA valid. HPV RNA analysis, that is, detection of viral transcripts, was performed by HPV type-specific reverse transcription polymerase chain reaction (RT-PCR) and hybridization assays (17), which amplify HPV E6*I and ubiquitin C (ubC) cDNA as a cellular mRNA QC. Specimens that were HPV E6*I and/or ubC mRNA-positive were considered RNA valid.
Statistical Analyses
Characteristics of the cancer cases and controls were evaluated. Utilizing the baseline samples, odds ratios (ORs) and 95% confidence intervals (CIs) were calculated (oral cavity, oropharynx, larynx) using unconditional (to allow calculation of OR for rare exposures) logistic regression adjusted for matching variables for seropositivity by HPV16 proteins. Further adjustments for alcohol (never/ever/missing) and education (up to high school [HS] completion/post-HS or some college/college graduate or higher/missing) did not qualitatively alter the odds ratios. Differences in descriptive characteristics by HPV16-E6 serostatus were assessed using chi-square tests.
The kinetics of HPV16-E6 antibody levels were evaluated graphically by plotting the MFI values on a semi-log scale for each serial serum sample for mixed-site cases and controls who were HPV16-E6 seropositive at baseline and all OPC cases. To determine if the slope of the oropharynx cases was statistically significantly different from zero, we fit a linear regression to the log-transformed marker values as a function of time since blood draw and used a generalized estimation equations (GEE) approach to accommodate correlations among observations on the same person in the variance computation (20).
HPV16 mRNA positivity was considered a marker for HPV-driven tumors (17) and applied as gold standard for assessing the sensitivity and specificity of HPV16-E6 serology (21).
The absolute risk of OPC was estimated in the PLCO population by HPV16-E6 serology status. Weights were applied based on the sampling probabilities for each study participant. Because all incident OPC cases in the PLCO cohort were included in the study, they were each assigned a sampling probability of 1. The sampling probability for each control participant being sampled for HPV16-E6 testing was calculated based on age, sex, race, smoking status, calendar year of blood draw, and time in follow-up. Logistic regression with sampling selection as the outcome was used; a model-based approach was necessary to cross-classify the six variables as stratification would have resulted in a large number of strata (likely with some cells having few individuals, resulting in difficultly calculating robust probability estimates). The inverse of the sampling probabilities was then used as weights in a Cox model to calculate 10-year cumulative risk of OPC, overall and by sex, age, and HPV16-E6 serostatus, not accounting for competing risks (22). Confidence limits were calculated by jackknifing (ie, taking repeated n-1 samples of the population) the logit of the cumulative risk (23). To determine whether incidence differed by individual characteristics, covariates were included in weighted Cox models to calculate hazard ratios and P values. Because we calculated cumulative risk over the first 10 years postenrollment, 14 OPC cases that occurred after 10 years were excluded from this analysis.
All P values are two-sided, and statistical analyses were conducted using SAS version 9.2 (Raleigh, NC, USA). A P value of less than .05 was considered statistically significant.
Results
Baseline Characteristics
Eighty two percent of study participants were male, median age at study enrollment (and first blood draw) was 63 years (interquartile range [IQR] = 59–67 years), and 40.8% were smokers at enrollment. Among cases, median age at cancer diagnosis was 69.5 years (IQR = 65.0–75.0 years), median time between blood draw and diagnosis was 6.6 years (IQR = 3.3–10.3 years), and median calendar year of diagnosis was 2005 (IQR = 2002–2007; range = 1994–2009). On average, five serial samples drawn annually during the follow-up were available for the OPC cases (median = 5, IQR = 4 to 6). Age, sex, and smoking status (matching variables) were similar among cases and controls but differed across cancer sites (Supplementary Table 1, available online).
HPV16-E6 Seropositivity and Risk of OPC
Seropositivity to HPV16-E6 was present in the baseline serum of 42.3% (n = 22 of 52) cases with OPC and 0.5% (n = 5 of 924) of controls (OR = 140, 95% CI = 40.2 to 491) (Table 1). By anatomic subsite within the oropharynx, 58.8% of tonsillar cancer (n = 10 of 17), 50.0% of base of tongue cancer (n = 12 of 24), and 0.0% of other oropharynx cancer (n = 0 of 11) were HPV16-E6 seropositive prior to diagnosis.
Table 1.
Risk associated with HPV16 serology status for patients with cancer of the oral cavity, oropharynx, larynx, and mixed sites compared with cancer-free controls*
| HPV16 proteins | Controls (n = 924) | Oral cavity (n = 44) |
Oropharynx (n = 52) |
Larynx (n = 88) |
Mixed sites (n = 14) |
||||
|---|---|---|---|---|---|---|---|---|---|
| No. (%) | No. (%) | OR (95% CI) | No. (%) | OR (95% CI) | No. (%) | OR (95% CI) | No. (%) | OR (95% CI) | |
| HPV16 oncoproteins | |||||||||
| E6 | |||||||||
| Seronegative | 919 (99.5) | 44 (100) | 1.0 | 30 (57.7) | 1.0 | 88 (100) | 1.0 | 9 (64.3) | 1.0 |
| Seropositive | 5 (0.5) | 0 (0) | 0 (NE) | 22 (42.3) | 140.47 (40.21 to 490.77) | 0 (0) | 0 (NE) | 5 (35.7) | >999.99 (106.64 to > 999.99) |
| E7 | |||||||||
| Seronegative | 863 (93.3) | 43 (97.7) | 1.0 | 36 (69.2) | 1.0 | 80 (90.9) | 1.0 | 13 (92.9) | 1.0 |
| Seropositive | 62 (6.7) | 1 (2.3) | 0.35 (0.04 to 3.23) | 16 (30.8) | 6.11 (2.82 to 13.23) | 8 (9.1) | 1.31 (0.50 to 3.39) | 1 (7.1) | 1.85 (0.19 to 18.49) |
| HPV16 early proteins | |||||||||
| E1 | |||||||||
| Seronegative | 901 (97.6) | 44 (100) | 1.0 | 43 (82.7) | 1.0 | 87 (98.9) | 1.0 | 11 (78.6) | 1.0 |
| Seropositive | 22 (2.4) | 0 (0) | 0 (NE) | 9 (17.3) | 6.81 (2.33 to 19.84) | 1 (1.1) | 0.17 (0.02 to 1.87) | 3 (21.4) | 2.82 (0.39 to 20.49) |
| E2 | |||||||||
| Seronegative | 911 (98.6) | 41 (93.2) | 1.0 | 38 (73.1) | 1.0 | 84 (95.5) | 1.0 | 10 (71.4) | 1.0 |
| Seropositive | 13 (1.4) | 3 (6.8) | 2.99 (0.50 to 18.08) | 14 (26.9) | 29.24 (10.30 to 82.99) | 4 (4.5) | 3.84 (0.83 to 17.79) | 4 (28.6) | 63.26 (7.35 to 545.82) |
| HPV16 late protein | |||||||||
| L1 | |||||||||
| Seronegative | 864 (93.6) | 40 (90.1) | 1.0 | 37 (71.2) | 1.0 | 82 (93.2) | 1.0 | 11 (78.6) | 1.0 |
| Seropositive | 60 (6.7) | 4 (9.1) | 1.02 (0.25 to 4.23) | 15 (28.8) | 8.33 (3.80 to 18.28) | 6 (6.8) | 1.75 (0.62 to 4.93) | 3 (21.4) | 3.39 (0.62 to 18.61) |
Odds ratios were adjusted for matching criteria: year of entry into study, year the material was collected, study year of cancer diagnosis (for cases; the same year was used for the matched control), birth year, sex, smoking status (never/former/current), alcohol (never/ever/missing), education (up to high school [HS] completion/post-HS or some college/college graduate or higher/missing). HPV16-E4 was only tested for in the serial sample analysis and not in the analysis of baseline samples. CI = confidence interval; HPV = human papilloma virus; NE = nonestimable; OR = odds ratio.
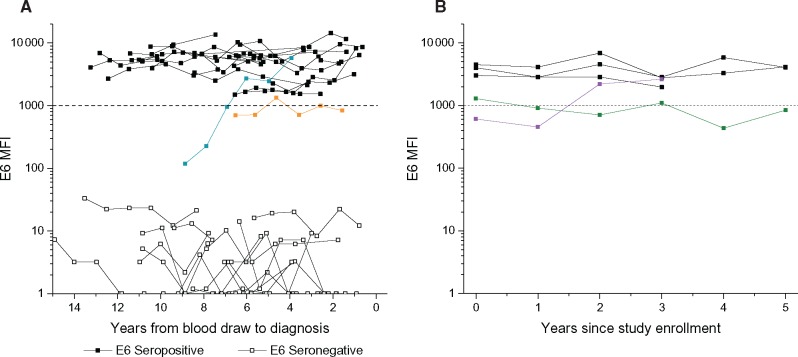
In serial blood samples, individual study participants’ HPV16-E6 levels remained highly elevated and stable for all OPC cases who were HPV16-E6 seropositive at baseline (Figure 2A). The slope of the HPV16-E6-seropositive cases was not different from zero (P = .54), indicating no change over time in average MFI values. For only one OPC case (tonsillar cancer, age 75 years at diagnosis) who was HPV16-E6 seronegative at baseline, nine years prior to diagnosis, HPV16-E6 antibody levels increased during the first three visits and then remained high and stable until diagnosis (highlighted in blue in Figure 2A). This indicates that this case had not fully seroconverted at study enrollment, but then developed strong seropositivity against HPV16-E6 during the follow-up, similar to the OPC cases who were already seropositive. In addition, one OPC case (tonsillar cancer, age 65 years at diagnosis) had low anti-HPV16-E6 levels that fluctuated between positive and negative (Figure 2A, highlighted in orange). Conversely, for the remaining OPC cases that were HPV16-E6 seronegative at baseline, HPV16-E6 antibody levels remained low over the entire follow-up period, well below the threshold for seropositivity. Individual plots of HPV16-L1, -E1, -E2, -E4, -E6, and -E7 serologic markers of all OPC cases (and HPV16-E6-seropositive controls) are shown in Supplementary Figure 1, A to C (available online).
Figure 2.
Kinetics of HPV16-E6 in prediagnostic samples from oropharyngeal cancer cases and selected controls. A) This panel shows HPV16-E6 (MFI) values by years from blood draw to diagnosis among (OPC) cases. HPV16-E6 levels remained clearly positive and stable for all OPC cases who were HPV16-E6 seropositive at baseline. One OPC case who was HPV16-E6 seronegative at baseline displayed increasing levels during the first three visits, after which strong and stable levels were established, similar to the other OPC cases that were seropositive at baseline (highlighted in blue). One OPC case had HPV16-E6 levels that fluctuated between positive and negative (highlighted in orange). B) This panel shows HPV16-E6 MFI values by year from time since study enrollment among controls. Of the five HPV16-E6-seropositive controls, three were consistently positive across serial samples; one seroconverted over time (highlighted in purple), and one was HPV16-E6 positive in two of six serial samples (highlighted in green). HPV = human papillomavirus; MFI = median fluorescence intensity; OPC = oropharyngeal cancer.
Of the five HPV16-E6-seropositive controls, three were consistently positive across serial samples; one seroconverted over time, and one was HPV16-E6 positive in two of six serial samples (Figure 2B;Supplementary Figure 1D, available online). None developed an HPV-related cancer (ie, anogenital or oropharynx) during the documented follow-up period.
Sensitivity and Specificity of HPV16-E6 Antibodies
Tumor specimens were retrieved from nine OPC cases (six tonsil and three base of tongue). Of these tumor samples, five were identified as HPV-driven based on evidence of HPV16 transcriptional activity (presence of RNA), all of which were HPV16-E6 positive in corresponding enrolment serum samples (sensitivity = 100%, 95% CI = 47.8% to 100%). Similarly, the four HPV RNA-negative (not HPV-driven) tumors were all HPV16-E6-seronegative (specificity = 100%, 95% CI = 39.8% to 100%; data not shown).
HPV16 Seropositivity for Non-E6 Proteins and Risk of OPC
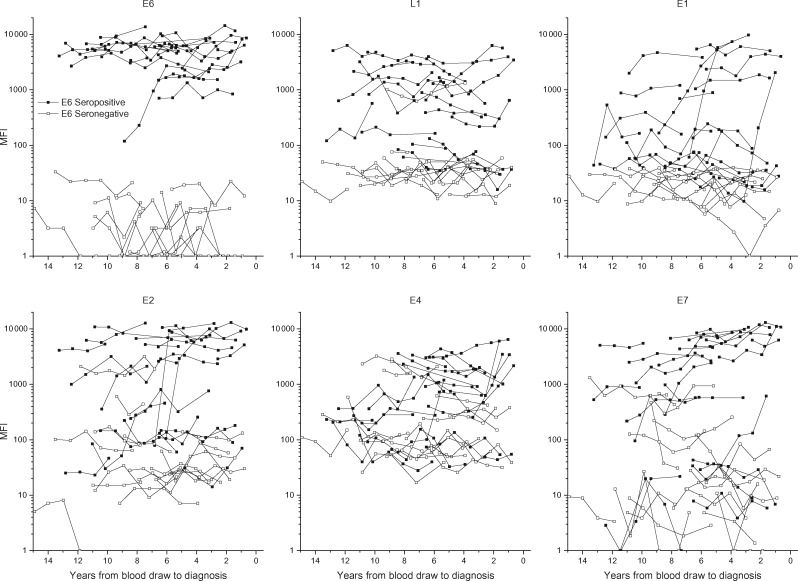
Increases in OPC risk were observed in the baseline serum for all tested HPV16 proteins, including E7 (OR = 6.1, 95% CI = 2.8 to 13.2), E1 (OR = 6.8, 95% CI = 2.8 to 19.8), E2 (OR = 29.2, 95% CI = 10.3 to 83.0), and L1 (OR = 8.3, 95% CI = 3.8 to 18.3) (Table 1). After stratifying OPC cases on E6 seropositivity, antibody levels for the other serologic markers were higher in cases who were already E6 seropositive (Figure 3).
Figure 3.
HPV16 antibody levels of multiple HPV proteins in serial samples leading up to diagnosis of oropharyngeal cancer (OPC), by HPV16-E6 serostatus in the baseline sample. HPV = human papillomavirus; MFI = median fluorescence intensity; OPC = oropharyngeal cancer.
Cumulative Risk of OPC by HPV16-E6 Seropositivity Status
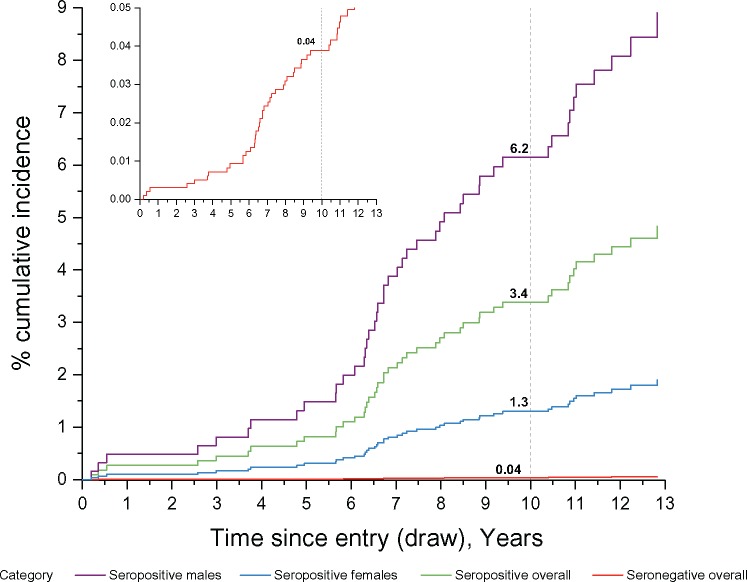
Because HPV16-E6-seropositive OPC cases were more likely to be male (94.5%, n = 21 of 22) compared with HPV16-E6-seronegative OPC (73.3%, n = 22 of 30, P = .04) and to be never smokers (45.5% among HPV16-E6 seropositive cases [n = 10 of 22] compared with 16.7% of HPV16-E6 seronegative cases [n = 5 of 30], P = 0.02), we estimated cumulative risks of OPC both overall and stratified by sex and smoking status. Incidence of OPC did not vary by age group (Ptrend = .71) (Table 2) and thus was not included as a covariate. The overall 10-year cumulative incidence per 100 000 person-years within PLCO was 6.5 (95% CI = 4.6 to 9.2) overall, and incidence rates were higher among males (10.6, 95% CI = 7.4 to 15.4) than among females (2.6, 95% CI = 1.2 to 5.4), as well as in ever smokers than in never smokers irrespective of sex (Table 2). Using the weighted Cox model, we estimated the 10-year cumulative risk for OPC at 6.2% (95% CI = 1.8% to 21.5%) for HPV16-E6 seropositive males and 1.3% (95% CI = 0.1% to 15.3%) for HPV16-E6 seropositive females, while the 10-year cumulative risk of OPC among HPV16-E6 seronegative subjects was extremely low (0.04%, 95% CI = 0.03% to 0.06%) (Figure 4).
Table 2.
Annual incidence of oropharyngeal cancer overall and by HPV16-E6 serostatus, stratified by covariates of interest*
| Characteristic | Overall |
HPV16-E6 seronegative |
HPV16-E6 seropositive |
|||
|---|---|---|---|---|---|---|
| No. of cancers | Annual incidence per 100 000, rate (95% CI) | No. of cancers | Annual incidence per 100 000, rate (95% CI) | No. of cancers | Annual incidence per 100 000, rate (95% CI) | |
| Overall | 36 | 6.5 (4.6 to 9.2) | 22 | 3.9 (2.5 to 6.0) | 14 | 339 (55 to 2083) |
| Male never smokers | 8 | 7.5 (3.9 to 14.2) | 3 | 5.5 (2.4 to 12.2) | 5 | 497 (66 to 3721) |
| Male ever smokers | 20 | 12.6 (8.3 to 19.0) | 12 | 6.9 (2.5 to 18.7) | 8 | 632 (181 to 2212) |
| Female never smokers | 1 | 2.0 (0.9 to 4.3) | 1 | 1.3 (0.2 to 8.1) | 0 | 120 (61 to 2342) |
| Female ever smokers | 7 | 3.4 (1.4 to 7.9) | 6 | 1.6 (032 to 8.3) | 1 | 153 (15 to 1517) |
CI = confidence interval; HPV = human papilloma virus.
Figure 4.
Cumulative 10-year risk of oropharyngeal cancer by sex and HPV16-E6 serostatus. Using the weighted Cox model, we estimated the 10-year cumulative risk for oropharyngeal cancer (OPC) overall at 3.4% (95% CI = 0.6% to 20.8%); by sex, the 10-year cumulative risk was 6.2% (95% CI = 1.8% to 21.5%) for HPV16-E6-seropositive males and 1.3% (95% CI = 0.1% to 15.3%) for HPV16-E6-seropositive females. The 10-year cumulative risk of OPC among HPV16-E6-seronegative subjects was extremely low (0.04%, 95% CI = 0.03% to 0.06%). A separate graph of the HPV16-E6-seronegative individuals is nested in the figure in order to better visualize the curve. Please note that the y-axis scale differs in the two graphs. HPV = human papillomavirus.
HPV16-E6 Seropositivity and Non-OPC/Mixed Site Cancer Risk
Mixed-site tumors had increased odds of HPV16-E6 seropositivity (Table 1; Supplementary Material, available online), and the results from the baseline sample appeared representative of the positives throughout the serial samples (Supplementary Figure 1C, available online). No associations with HPV16 antibodies were observed for oral cavity or laryngeal cancer (Table 1). HPV serology and tumor results generally corresponded among non-OPC cases (Supplementary Material, available online).
Discussion
This is the first study to evaluate the kinetics of HPV16-E6 antibodies in serial samples of OPC cases leading up to cancer diagnosis. In a US-based cohort and during a calendar period where approximately half of OPCs were caused by HPV16 (1), 42.3% of OPC cases were HPV16-E6 seropositive up to 13 years prior to cancer diagnosis, with antibody patterns that were high and stable throughout follow-up. This is also the first study to determine the sensitivity and specificity of the biomarker using serostatus in prediagnostic samples and viral transcriptional activity as the gold standard (21). While the tumor analysis was based on small numbers, it did confirm that the sensitivity and specificity of HPV16-E6 antibodies were exceptionally high (estimated at 100%) in predicting HPV-driven OPC cases.
This study informs our understanding of the etiology of HPV-driven OPC. In particular, the data strongly suggest that precursor states manifest many years prior to clinical presentation of OPC and will help establish the important role of HPV infection throughout the carcinogenic process. These results may also have translational implications, in particular for secondary prevention by using HPV16-E6 serology for OPC screening and early detection. Whether this is a realistic scenario will primarily depend on population incidence rates of OPC and the proportion of cases caused by HPV16 infection. In the PLCO cohort, a population of relatively low OPC incidence relative to the general population (24), we estimated the 10-year absolute risk to be 6.2% for HPV16-E6-seropositive men. This implies that one in 17 seropositive PLCO men age 55 to 74 years would have underlying HPV-driven OPC over the course of 10 years, although this positive predictive value would improve in higher-incidence settings. Our analysis suggested a four-fold absolute risk ratio between HPV16-E6-seropositive male and female PLCO participants, suggesting screening females would be inefficient. Identification of a population at high risk of HPV-driven OPC, such as white men born since the mid-1940s (25), would further improve efficiency (nb, smoking did not achieve additional risk stratification).
Even if HPV16-E6-based screening was warranted, additional research on subsequent steps following a positive screen is needed. It is questionable whether current diagnostic imaging technologies would be able to detect asymptomatic early oropharyngeal tumors. PET scans can identify tumors larger than 5 mm, but the relevant oropharynx lesions may be smaller. Diagnostic techniques with higher sensitivity may be needed, and novel imaging techniques, such as transcervical ultrasound, have shown promise in detecting primary tumors when traditional imagining techniques have failed (26). Further, given the survival advantage of HPV16-driven oropharyngeal cancer, the clinical community is investigating dose de-intensification in the treatment of these cancers (27). Given that this marker may identify early cancers, or even precancers, cure may be possible with relatively mild treatment regimes, such as tonsillectomy (28), robotic surgery (29), or therapeutic HPV vaccines (30). Yet, as in all secondary prevention efforts, the pitfalls of screening need to be weighed against the potential gains.
The PLCO tumor retrieval represented a major effort across the United States, but resulted in a relatively low number of OPC tumors available for analysis. Thus, the main limitation to this work is the small number of tumor samples available for HPV RNA analysis, and while the results were encouraging in that the concordance between tumor and serology HPV status was perfect (100%), ongoing parallel efforts by our group will expand this analysis to confirm the high marker sensitivity (21). We were additionally limited by small numbers of OPC overall as OPC is currently a rare cancer. Finally, we did not test all serial samples among oral cavity cases and controls as a check for specificity; yet only one OPC (2%) seroconverted in the serial sample analysis, and it would thus seem unlikely that many seroconverting oral cavity cases and/or controls were missed.
The incidence of HPV-positive OPC may continue to increase in the coming decades (1). Highly effective HPV vaccines, which were first introduced to 11- to 12-year-old girls in the mid-2000s, will not curtail this male-dominated trend until at least 2060, when the vaccinated birth cohorts reach the median age of OPC diagnosis (∼60 years). Thus, research in secondary prevention of OPC is important. This study demonstrates high sensitivity and specificity of the HPV16-E6 biomarker. Furthermore, the antibody response is remarkably stable and strong up to 13 years prior to cancer diagnosis, suggesting that testing HPV16-E6 in a single blood draw might predict the vast majority of future HPV-driven OPCs. Yet, while these data are promising, the incidence of OPC even among white men in the United States is much lower than the incidence of other cancers for which screening is currently recommended, and our data indicate that a large number of people testing positive for HPV16-E6 would not get cancer (0.5% of controls were HPV16-E6 seropositive). High-risk subgroups would likely need to be identified in order to attain a reasonable screening efficacy using the HVP16-E6 serology test.
To consider the translational potential of HPV16-E6 serology in the early detection of OPC, necessary subsequent steps include identifying high-risk groups where screening is warranted, identifying a histologic precursor lesion, improvements in diagnostics, and establishment of low-harm treatment regimens. Screening for oropharyngeal cancer using HPV16-E6 will not be possible until these challenges are satisfactorily solved.
Funding
Costs for the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial (PLCO) were covered by the Divisions of Cancer Prevention and Cancer Epidemiology and Genetics, National Cancer Institute (NCI). Costs for this project nested within PLCO were covered by the NCI Intramural Research Program (ARK).
Notes
Though the funder approved the final version of this manuscript, the funder had no role in the design of the study; the collection, analysis, or interpretation of the data; or the writing of the manuscript.
The authors have no financial disclosures to declare.
We thank John Schiller, PhD, and Anil Chaturvedi, PhD (NCI), for their comments and insights on this manuscript, Dave Castenson (Information Management Systems [IMS]) for programming support, Matt Moore (IMS) for support in the sample selection, Mike Furr (IMS) for assistance in computation of the absolute risk estimates, and Sandra Brown (NCI) for her help with references and formatting the manuscript. No compensation was received for these contributions.
Authors supported the nested study as follows: design, conduct, collection (ARK, MJ, AH, CW, WYH, MPP, MP, PB, TW), management (ARK, MJ, CW, MWF, AM, MPP, MP, PB, TW), analysis (ARK, MJ, EY, HAK, DPC, KALK, DH, RP, NDF, MP, PB, TW), and interpretation of the data (all authors); and preparation (ARK, MP, TW), review (all authors), or approval of the manuscript (all authors). Aimée R. Kreimer and Tim Waterboer had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Supplementary Material
References
- 1. Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;2932:4294–4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hong AM, Grulich AE, Jones D, et al. Squamous cell carcinoma of the oropharynx in Australian males induced by human papillomavirus vaccine targets. Vaccine. 2010;2819:3269–3272. [DOI] [PubMed] [Google Scholar]
- 3. Hammarstedt L, Lindquist D, Dahlstrand H, et al. Human papillomavirus as a risk factor for the increase in incidence of tonsillar cancer. Int J Cancer. 2006;11911:2620–2623. [DOI] [PubMed] [Google Scholar]
- 4. Forte T, Niu J, Lockwood GA, et al. Incidence trends in head and neck cancers and human papillomavirus (HPV)-associated oropharyngeal cancer in Canada, 1992–2009. Cancer Causes Control. 2012;238:1343–1348. [DOI] [PubMed] [Google Scholar]
- 5. Blomberg M, Nielsen A, Munk C, et al. Trends in head and neck cancer incidence in Denmark, 1978–2007: Focus on human papillomavirus associated sites. Int J Cancer. 2011;1293:733–741. [DOI] [PubMed] [Google Scholar]
- 6. Reddy VM, Cundall-Curry D, Bridger MW.. Trends in the incidence rates of tonsil and base of tongue cancer in England, 1985–2006. Ann R Coll Surg Engl. 2010;928:655–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Braakhuis BJ, Visser O, Leemans CR.. Oral and oropharyngeal cancer in The Netherlands between 1989 and 2006: Increasing incidence, but not in young adults. Oral Oncol. 2009;459:e85–e89. [DOI] [PubMed] [Google Scholar]
- 8. Mork J, Moller B, Dahl T, et al. Time trends in pharyngeal cancer incidence in Norway 1981–2005: A subsite analysis based on a reabstraction and recoding of registered cases. Cancer Causes Control. 2010;219:1397–1405. [DOI] [PubMed] [Google Scholar]
- 9. Hwang TZ, Hsiao JR, Tsai CR, et al. Incidence trends of human papillomavirus-related head and neck cancer in Taiwan, 1995–2009. Int J Cancer. 2015;1372:395–408. [DOI] [PubMed] [Google Scholar]
- 10. Chaturvedi AK, Anderson WF, Lortet-Tieulent J, et al. Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J Clin Oncol. 2013;3136:4550–4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jemal A, Simard EP, Dorell C, et al. Annual Report to the Nation on the Status of Cancer, 1975–2009, featuring the burden and trends in human papillomavirus(HPV)-associated cancers and HPV vaccination coverage levels. J Natl Cancer Inst. 2013;1053:175–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kreimer AR, Johansson M, Waterboer T, et al. Evaluation of human papillomavirus antibodies and risk of subsequent head and neck cancer. J Clin Oncol. 2013;3121:2708–2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. de Martel C, Ferlay J, Franceschi S, et al. Global burden of cancers attributable to infections in 2008: A review and synthetic analysis. Lancet Oncol. 2012;136:607–615. [DOI] [PubMed] [Google Scholar]
- 14. Prorok PC, Andriole GL, Bresalier RS, et al. Design of the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Control Clin Trials. 2000;21(6 suppl):273S–309S. [DOI] [PubMed] [Google Scholar]
- 15. Waterboer T, Sehr P, Pawlita M.. Suppression of non-specific binding in serological Luminex assays. J Immunol Methods. 2006;309(1–2):200–204. [DOI] [PubMed] [Google Scholar]
- 16. Waterboer T, Sehr P, Michael KM, et al. Multiplex human papillomavirus serology based on in situ-purified glutathione s-transferase fusion proteins. Clin Chem. 2005;5110:1845–1853. [DOI] [PubMed] [Google Scholar]
- 17. Halec G, Schmitt M, Dondog B, et al. Biological activity of probable/possible high-risk human papillomavirus types in cervical cancer. Int J Cancer. 2013;1321:63–71. [DOI] [PubMed] [Google Scholar]
- 18. Schmitt M, Bravo IG, Snijders PJ, et al. Bead-based multiplex genotyping of human papillomaviruses. J Clin Microbiol. 2006;442:504–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schmitt M, Dondog B, Waterboer T, et al. Homogeneous amplification of genital human alpha papillomaviruses by PCR using novel broad-spectrum GP5+ and GP6+ primers. J Clin Microbiol. 2008;463:1050–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liang KY, Zeger SL.. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 21. Waterboer T, Lang Kuhs K, Kreimer A, et al. Sensitivity and specificity of HPV16 serology for HPV-driven oropharyngeal cancer. IPV 2015 abstract book HPV15-0548 2015. 110. http://www.hpv2015.org/Documents/HPV15%20Abstracts%20for%20after%20conference%20unlocked.pdf. Accessed February 10, 2017.
- 22. Korn E, Graubard B.. Analysis of Health Surveys. New York: Wiley-Interscience Publication; 1999:8–50. [Google Scholar]
- 23. Shao J, Tu D.. The Jacknife and Bootstrap. New York: Springer-Verlag; 1995. [Google Scholar]
- 24. Pinsky PF, Miller A, Kramer BS, et al. Evidence of a healthy volunteer effect in the prostate, lung, colorectal, and ovarian cancer screening trial. Am J Epidemiol. 2007;1658:874–881. [DOI] [PubMed] [Google Scholar]
- 25. Brown LM, Check DP, Devesa SS.. Oral cavity and pharynx cancer incidence trends by subsite in the United States: Changing gender patterns. J Oncol. 2012;2012:649498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fakhry C, Agrawal N, Califano J, et al. The use of ultrasound in the search for the primary site of unknown primary head and neck squamous cell cancers. Oral Oncol. 2014;507:640–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yom SS, Gillison ML, Trotti AM.. Dose de-escalation in human papillomavirus-associated oropharyngeal cancer: First tracks on powder. Int J Radiat Oncol Biol Phys. 2015;935:986–988. [DOI] [PubMed] [Google Scholar]
- 28. Fakhry C, Andersen KK, Christensen J, et al. The impact of tonsillectomy upon the risk of oropharyngeal carcinoma diagnosis and prognosis in the Danish Cancer Registry. Cancer Prev Res (Phila). 2015;87:583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mehta V, Johnson P, Tassler A, et al. A new paradigm for the diagnosis and management of unknown primary tumors of the head and neck: A role for transoral robotic surgery. Laryngoscope. 2013;1231:146–151. [DOI] [PubMed] [Google Scholar]
- 30. Trimble CL, Morrow MP, Kraynyak KA, et al. Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: A randomised, double-blind, placebo-controlled phase 2b trial. Lancet. 2015;S0140–S6736. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.