Abstract
Background: Current HPV vaccines do not protect against all oncogenic HPV types. Following vaccination, type replacement may occur, especially if different HPV types competitively interact during natural infection. Because of their common route of transmission, it is difficult to assess type interactions in observational studies. Our aim was to evaluate type replacement in the setting of HPV vaccine randomized controlled trials (RCTs).
Methods: Data were pooled from the Costa Rica Vaccine Trial (CVT; NCT00128661) and PATRICIA trial (NCT001226810)—two large-scale, double-blind RCTs of the HPV-16/18 AS04-adjuvanted vaccine—to compare cumulative incidence of nonprotected HPV infections across trial arms after four years. Negative rate difference estimates (rate in control minus vaccine arm) were interpreted as evidence of replacement if the associated 95% confidence interval excluded zero. All statistical tests were two-sided.
Results: After applying relevant exclusion criteria, 21 596 women were included in our analysis (HPV arm = 10 750; control arm = 10 846). Incidence rates (per 1000 infection-years) were lower in the HPV arm than in the control arm for grouped nonprotected oncogenic types (rate difference = 1.6, 95% confidence interval [CI] = 0.9 to 2.3) and oncogenic/nononcogenic types (rate difference = 0.2, 95% CI = −0.3 to 0.7). Focusing on individual HPV types separately, no deleterious effect was observed. In contrast, a statistically significant protective effect (positive rate difference and 95% CI excluded zero) was observed against oncogenic HPV types 35, 52, 58, and 68/73, as well as nononcogenic types 6 and 70.
Conclusion: HPV type replacement does not occur among vaccinated individuals within four years and is unlikely to occur in vaccinated populations.
Despite the availability of effective screening methods, cervical cancer remains a leading cause of cancer death in women worldwide (1). Because of the high cost and infrastructure required to successfully implement screening, cervical cancer has become a sentinel disease of economic inequality. Human papillomavirus (HPV) is a necessary cause of cervical cancer, and prophylactic vaccination now offers the best opportunity to reduce the global burden of cervical and other HPV-related cancers (2,3). However, current vaccines do not offer protection against all HPV types and the theoretical possibility that other types may emerge to fill the vacated ecological niche following successful elimination of vaccine-targeted types has remained a concern for over 10 years (4). This is a concept referred to as “type replacement” (5). Aside from potential issues related to duration of protection, type replacement is the main biological phenomenon that may limit the success of vaccine efforts aimed at reducing morbidity and mortality associated with cervical HPV infection.
While more than 40 different anogenital HPV types exist, only 13 are classified as definite or probable carcinogens (6,7). In descending order, the most common oncogenic types implicated in cervical cancer globally include: 16, 18, 58, 33, 45, 31, 52, 35, 59, 39, 51, 56, and 68 (8). The first two types listed here (HPVs 16 and 18) are responsible for approximately 70% of cervical cancer cases worldwide, whereas the first seven types are responsible for approximately 90% of cases (9–11). Prior to 2015, the HPV-6/11/16/18 vaccine, Gardasil, and the HPV-16/18 AS04-adjuvanted vaccine, Cervarix, were the only commercial HPV vaccines available (12,13). Among HPV-naïve individuals, both of these first-generation vaccines are nearly 100% efficacious against the respective target types and provide variable degrees of cross-protection against other related types, particularly HPVs 31, 33, and 45 (14–17). The seven most common oncogenic HPV types are targeted by the nonavalent Gardasil 9 vaccine (18).
Type replacement previously occurred following pneumococcal vaccination due to creation of an open ecological niche and other biological properties of the virus strains (19). HPV is much more genetically stable than Streptococcus pneumonia, and therefore emergence of escape mutants or entirely new HPV types is not likely to occur. Competition during natural infection between the existing vaccine targeted and nontargeted HPV types is probably a requirement for type replacement to occur (5). Many investigators have used observational (cohort or cross-sectional) data to evaluate HPV type competition among unvaccinated women, and there has been little evidence of competitive interactions (5). Nonetheless, vaccination against only a proportion of HPV types may introduce a competitive advantage for some nonvaccine types that could lead to type replacement.
The National Cancer Institute–sponsored Costa Rica HPV-16/18 vaccine trial (CVT) and the GSK-sponsored multisite PApilloma TRIal against Cancer In young Adults (PATRICIA) are two large-scale, double-blind randomized controlled trials (RCTs) of the HPV-16/18 AS04-adjuvanted vaccine using similar design and methodology (16,20,21). To evaluate type replacement at the individual level, we pooled CVT and PATRICIA data and compared rates of nonvaccine/nonprotected incident HPV infections across arms of these two trials.
Methods
Study Design and Laboratory Procedures
Women randomly assigned to receive either the HPV-16/18 AS04-adjuvanted vaccine or hepatitis A vaccine (HAV) in CVT (NCT00128661, n = 7466) (16,20) and PATRICIA (NCT001226810, n = 18 729) (21) were considered for inclusion in our analysis. In CVT, most participants were 18 to 25 years of age (four were age 17 or 26–27 years), and in PATRICIA, most were age 15 to 25 years (26 were age 14 or 26–33 years). Recruitment for the two trials took place over a similar period (2004 to 2005), and while CVT participants were all from Costa Rica (Guanacaste Province or selected areas of Puntarenas Province), PATRICIA participants came from multiple countries in Europe, Latin America, North America, and the Asia-Pacific region. Because the trials were generally harmonized at the design phase, they share many important features; for example, the same vaccines were administered on the same schedule (enrollment, one month, and six months), the same HPV DNA and serology assays were used with testing done in the same laboratories, and referral algorithms for additional work-up (repeat cytological testing or colposcopy) were also similar. The main difference between protocols is that unless women had abnormal cytology triggering additional work-up and more intensified follow-up, CVT participants were observed annually whereas PATRICIA participants were seen every six months. Women with low-grade squamous intraepithelial neoplasia or who were positive for HR-HPV types with atypical squamous cells of undetermined significance were referred for additional work-up, including excisional treatment of high-grade lesions.
Broad-spectrum polymerase chain reaction (PCR)–based HPV DNA testing (DDL Diagnostic Laboratory) was conducted at each clinic visit, as described previously (22–24). Briefly, the assay used is based on amplification and probe hybridization with the SPF10 HPV DNA enzyme immunoassay (DEIA) system followed by typing with the LiPA25 version 1 method (Labo Biomedical Products, Rijswijk, the Netherlands). All specimens, including those collected from women undergoing more intensified follow-up in CVT, underwent PCR testing. To ensure that HPV16 and HPV18 infections were not missed, all specimens positive for HPV DNA by DEIA but negative for these types by LiPA25 were retested with type-specific primers/probes for the presence of HPV16 and HPV18 DNA. In PATRICA, type-specific PCR results were available for additional HPV types (31, 33, 35, 45, 52, 58, and 59); however, to ensure consistency, these type-specific results were excluded from our primary pooled analyses and considered in study-specific sensitivity analyses. HPV16 and HPV18 serological status was also assessed at baseline, using a virus-like particle-based direct enzyme-linked immunosorbent assay (GlaxoSmithKline Biologicals) and dichotomized according to standard cutoffs (25).
Approval of clinical protocols and other study material was obtained from independent ethics committees or institutional review boards, and all participants in each trial provided written informed consent prior to enrollment.
Statistical Analysis
The current post hoc analysis followed a statistical analysis plan prepared in advance. The primary end point was incident cervical HPV infection with types for which the HPV-16/18 vaccine has not shown evidence of efficacy against, that is, excluding HPVs 6, 11, 16, 18, 31, 33, 45, 51, and 74 (16,17,26). Incident infection was defined as an infection during follow-up that was not present during the vaccination phase (entry to six-month visit) or in instances where the infection was present during the vaccination phase that cleared during follow-up before reappearing.
Incidence rates and associated 95% confidence intervals (CIs) were calculated for groups of oncogenic types (HPVs 35, 39, 52, 56, 58, 59, and 68/73) and oncogenic/nononcogenic types (HPVs 34, 35, 39, 40, 42, 43, 44, 52, 53, 54, 56, 58, 59, 66, 68/73, and 70). Grouped rates were expressed per 1000 infection-years as the ratio of number events to the total combined follow-up time for each HPV type that a woman was at risk of acquiring in the respective groups. Incidence rates were also calculated for the individual types listed above, plus additional types (HPVs 6, 11, 51, and 74) for which evidence of HPV-16/18 vaccine efficacy is less consistent. Because calculation of incidence rates for individual types was based on total follow-up time at risk for each type separately, rates for these analyses were expressed per 1000 person-years. Importantly, for a woman to initially be considered at risk, it was required that she be negative for all nine HPV types that the vaccine is suspected to offer protection against, that is, the types that may potentially be replaced. Although we did not exclude women infected with these HPV types, these infections must have cleared before a woman was considered at risk. While infection- or person-time began one day after enrollment, outcome assessment began at the 12-month study visit, that is, the first visit potentially attended by a woman after receiving her third vaccine dose (≥301 days after enrollment).
Over the four-year follow-up period, type replacement was evaluated by comparing cumulative rates of HPV infection between the HPV vaccine and control arms. In each of our analyses, rate difference and vaccine efficacy estimates were reported, representing the absolute and percentage change (reduction or increase) in the outcome of interest, respectively. Negative estimates would be interpreted as evidence of type replacement if the associated 95% confidence interval excluded zero. This analysis was conducted at the infection level and therefore, to account for lack of independence between infections occurring within the same individual, generalized estimating equation methods were utilized in comparing rates across arms. All statistical tests were two-sided, and a P value of less than .05 was considered statistically significant.
In our primary analyses, single time HPV detection was considered the outcome, and in situations where nonprotected types were present during the vaccination phase, women were considered to be at risk for that particular type immediately following clearance, that is, following a single negative HPV test. Single time clearance of vaccine-protected types present at enrollment was also considered sufficient before a woman could be considered at risk for any nonprotected HPV type. Additional analyses were performed excluding and restricted to women positive at entry for any of the HPV types that the vaccine has shown evidence of efficacy against, stratified by year of participant follow-up, restricted to results from annual study visits, reporting results from each trial separately, focusing on persistent incident infections (two-time positivity at consecutive clinic visits), requiring two consecutive negative tests to confirm clearance in instances where infection was present during the vaccination phase, and restricted to women virginal at study entry but who initiated sexual activity during follow-up. These sensitivity analyses were conducted to ensure that our reported findings were robust to the definition of cohort and outcome that we used. In an effort to ensure that the outcomes evaluated were true new infections, we also performed additional analyses excluding infections that were present during the vaccination phase, cleared, and were reacquired (same HPV type) during follow-up.
Results
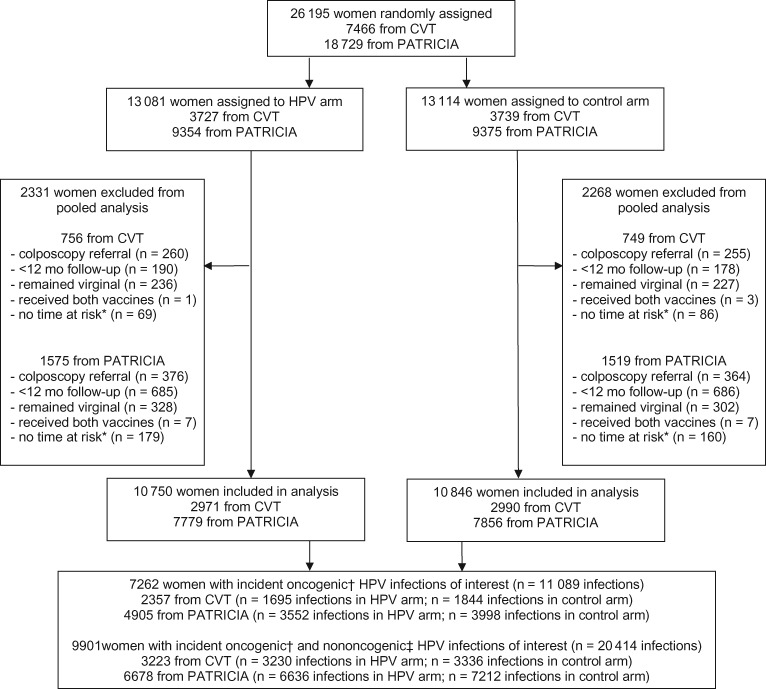
In total, 26 195 women were randomly assigned to the HPV arm (CVT, n = 3727; PATRICIA, n = 9354) and control arm (CVT, n = 3739; PATRICIA, n = 9375) (Figure 1). Women were excluded from our analysis if they were referred for colposcopy prior to their 12-month visit (n = 1255), were under observation for less than 12 months (n = 1739), remained virginal during follow-up (n = 1093), received both HPV and control vaccines (n = 18), or contributed no time at risk because of prevalent infection with a vaccine-protected type that did not clear before the final study visit (n = 494). After applying these restrictions, 2331 women were excluded in the HPV arm and 2268 in the control arm, resulting in a total of 21 596 eligible women (HPV arm, n = 10 750; control arm, n = 10 846). There was nearly perfect balance across arms for baseline characteristics, including age, sexual history, cytology status, HPV 16/18 serology status, and HPV DNA status, as well as total follow-up time and number of clinic visits (Supplementary Table 1, available online).
Figure 1.
Trial profile for Costa Rica Vaccine Trial (CVT) and PATRICIA trial pooled analysis. *Women may have contributed no time at risk because of prevalent infection with a vaccine-protected type that did not clear before their final study visit (excluded from analysis). †Oncogenic infections of interest include HPV types 35, 39, 51, 52, 56, 58, 59, and 68/73. ‡Nononcogenic HPV types of interest include HPV types 6, 11, 34, 40, 42, 43, 44, 53, 54, 66, 70, and 74. HPV = human papillomavirus.
In our primary analyses, the overall incidence rate was lower in the HPV arm compared with the control arm for oncogenic HPV types not protected by the HPV-16/18 vaccine (rate difference = 1.6, 95% confidence interval [CI] = 0.9 to 2.3; efficacy = 10.8%, 95% CI = 6.1% to 15.4%) and for comparable oncogenic/nononcogenic types (rate difference = 0.2, 95% CI = −0.3 to 0.7; efficacy = 7.7%, 95% CI = -10.5% to 22.9%) (Table 1). In our analyses restricted to women who were either negative or positive at enrollment for any of the nine HPV types that the vaccine has shown evidence of efficacy against (nonoverlapping strata), incidence rates (even if much higher in the baseline positive group) remained lower in the HPV arm for both outcomes (Table 2). Results also remained similar when stratified according to year of participant follow-up and when restricting HPV results to annual study visits only (Supplementary Tables 2 and 3, respectively, available online). Similarly, results remained consistent in our study-specific analyses, with no evidence of heterogeneity across study (P = .40 for oncogenic HPV types and P = .23 for nononcogenic HPV types), as well as in our PATRICIA analyses incorporating additional type-specific PCR results (Supplementary Table 4, available online).
Table 1.
Overall efficacy of the HPV16/18 vaccine against oncogenic and oncogenic/nononcogenic HPV infections excluding types that the vaccine has shown evidence of efficacy against HPVs 6, 11, 16, 18, 31, 33, 45, 51, and 74*
| HPV infection | HPV |
Control |
Rate difference (95% CI) | Efficacy (95% CI), % | ||
|---|---|---|---|---|---|---|
| No. of cases | Rate per 1000 infection-years (95% CI) | No. of cases | Rate per 1000 infection-years (95% CI) | |||
| Oncogenic HPV infection (types 35, 39, 52, 56, 58, 59, and 68/73) | 5247 | 13.2 (12.7 to 13.7) | 5842 | 14.8 (14.3 to 15.3) | 1.6 (0.9 to 2.3) | 10.8 (6.1 to 15.4) |
| Oncogenic or nononcogenic HPV infection (types 34, 35, 39, 40, 42, 43, 44, 52, 53, 54, 56, 58, 59, 66, 68/73, and 70) | 9866 | 2.8 (2.4 to 3.1) | 10548 | 3.0 (2.6 to 3.4) | 0.2 (-0.3 to 0.7) | 7.7 (-10.5 to 22.9) |
CI = confidence interval; HPV = human papillomavirus.
Table 2.
Efficacy of the HPV-16/18 vaccine against oncogenic and oncogenic/nononcogenic HPV infections, excluding types that the vaccine has shown evidence of efficacy against: HPVs 6, 11, 16, 18, 31, 33, 45, 51, and 74
| HPV infection | HPV |
Control |
Rate Difference (95% CI) | Efficacy (95% CI), % | ||
|---|---|---|---|---|---|---|
| No. of cases | Rate per 1000 infection-years (95% CI) | No. of cases | Rate per 1000 infection-years (95% CI) | |||
| Baseline negative* | ||||||
| Oncogenic HPV infection (types 35, 39, 52, 56, 58, 59, and 68/73) | 4556 | 12.4 (11.9 to 12.9) | 4997 | 13.8 (13.2 to 14.3) | 1.3 (0.6 to 2.1) | 9.7 (4.4 to 14.7) |
| Oncogenic or nononcogenic HPV infection (types 34, 35, 39, 40, 42, 43, 44, 52, 53, 54, 56, 58, 59, 66, 68/73, and 70) | 8567 | 2.2 (1.9 to 2.6) | 9031 | 2.4 (2.0 to 2.8) | 0.2 (-0.4 to 0.7) | 6.9 (-18.4 to 26.7) |
| Baseline positive† | ||||||
| Oncogenic HPV infection (types 35, 39, 52, 56, 58, 59, and 68/73) | 691 | 21.6 (19.7 to 23.7) | 845 | 26.3 (24.1 to 28.7) | 4.7 (1.7 to 7.7) | 17.8 (6.7 to 27.5) |
| Oncogenic or nononcogenic HPV infection (types 34, 35, 39, 40, 42, 43, 44, 52, 53, 54, 56, 58, 59, 66, 68/73, and 70) | 1299 | 8.2 (7.1 to 9.4) | 1517 | 9.6 (8.4 to 11.0) | 1.4 (-0.3 to 3.1) | 14.7 (-3.7 to 29.7) |
Excluding women positive at entry for any of these HPV types that the vaccine has shown evidence of efficacy against. CI = confidence interval; HPV = human papillomavirus.
Restricted to women positive at entry for any of these HPV types that the vaccine has shown evidence of efficacy against.
Applying a more conservative outcome definition (ie, two-time positivity to confirm incidence), our rate difference estimates reduced from 1.6 to 0.1 for oncogenic types, and from 0.2 to 0.0 for oncogenic/nononcogenic HPV types (Supplementary Table 5, available online). But regardless of whether clearance of infections present during the vaccination phase was confirmed with two consecutive negative tests or a single test before a specific HPV type could be considered a new incident infection, our results remained similar (Supplementary Table 6, available online). In the truly naïve cohort, which includes women virginal at study entry but who initiated sexual activity during follow-up, similar HPV incidence rates were observed across arms for oncogenic types (rate difference = 1.2, 95% CI = −0.9 to 3.2) and for oncogenic/nononcogenic types (rate difference = −0.6, 95% CI = −1.9 to 0.7) (Supplementary Table 7, available online). Finally, after combining all the restrictions applied in Supplementary Table 5 (available online; two-time positivity to confirm incidence), Supplementary Table 6 (available online; two negative tests to confirm clearance), and Supplementary Table 7 (available online; virginal at study entry), similar incidence rates were observed across arms for oncogenic HPV types (rate difference = 0.7, 95% CI = −0.4 to 1.8) and oncogenic/nononcogenic HPV types (rate difference = 0.1, 95% CI = -0.5 to 0.7) (Supplementary Table 8, available online). Results also remained consistent in our analyses excluding re-acquired infections (Supplementary Table 9, available online).
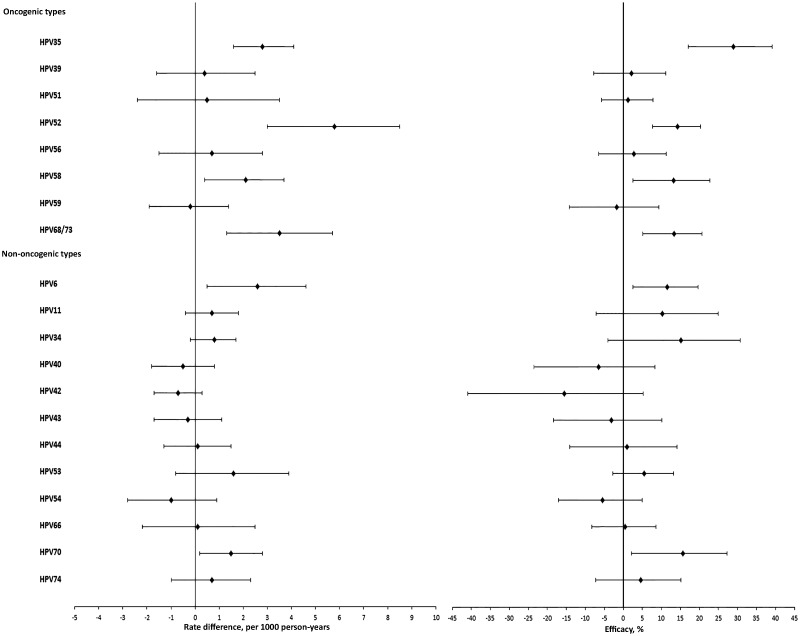
Rate difference and efficacy estimates for the individual nonprotected HPV types are presented in Figure 2. A statistically significant positive rate difference was observed for HPV types 6, 35, 52, 58, 68/73, and 70 (range = 1.5–5.8). For the types where a negative rate difference was observed (HPVs 40, 42, 43, 54, and 59), none of the estimates were statistically significant (95% CI included zero) and HPV59 was the only oncogenic type.
Figure 2.
Rate difference (rate in the control minus the vaccine arm) and efficacy of the HPV-16/18 vaccine against individual oncogenic and nononcogenic HPV infections (excluding types that the vaccine has shown consistent and strong evidence of efficacy against: HPVs 16, 18, 31, 33, and 45). The error bars represent the 95% confidence intervals. HPV = human papillomavirus.
Discussion
Our findings, revealing similar or higher incidence of nonprotected HPV types in the control arm compared with the HPV arm across all our analyses, provide strong evidence that type replacement is unlikely to occur among vaccinated individuals. Although previous observational studies also provide little evidence of competitive interactions between HPV types (suggesting low risk of replacement), because of correlated exposures it has been difficult in these studies to determine if risk of a particular HPV type is lower given infection with another type (Supplementary Table 10, available online). Despite adjustment or other statistical methods that have been utilized, these approaches are unlikely to account for all bias associated with this lack of independence between infections in studies evaluating HPV type competition.
In well-executed RCTs, the confounding of HPV acquisition rates by sexual behavior and other risk factors is greatly minimized. RCTs therefore provide a good setting to evaluate HPV type replacement. Although the number of incident infections (especially for less common HPV types) may be low in individual trials, pooling data across similar trials maximizes power. CVT and PATRICIA are the only completed trials with similar designs evaluating the HPV-16/18 vaccine, which makes our analysis the largest and most powerful pooling effort possible at this time to evaluate type replacement. In addition to providing strong evidence against replacement, we also observed statistically significant albeit low protective efficacy against certain other oncogenic and nononcogenic incident infections. A low level of protection against these additional HPV types suggests that the HPV-16/18 vaccine may be more effective in preventing precancerous/cancerous cervical lesions than originally anticipated.
In our study, we were only able to evaluate HPV type replacement at the individual level, which may be considered a limitation of this investigation. Ongoing surveillance studies (comparing the prevalence of HPV types in vaccinated communities across pre- and postvaccination periods) and/or community-randomized studies monitoring the effectiveness of vaccination will provide important information about the community level effect of vaccination (27). Studies reporting reduced rates of vaccine-targeted HPV types and related precancerous lesions in vaccinated populations have already been published (28); however, some studies have reported higher rates of nontargeted types (29,30). While surveillance studies offer the advantage of monitoring type replacement in settings where exposure to circulating viral types may have changed more dramatically because of high vaccine coverage (31), nonrandomized comparisons across populations and different time periods could lead to bias associated with different HPV risk groups being compared, representativeness of the samples, or changes in other relevant factors across time periods.
The use of consensus PCR assays may lead to an apparent increase in some nonprotected HPV types because of diagnostic artifacts, that is, technical “unmasking” caused by reduced competition for reagents between vaccine-protected and nonprotected HPV types and improved amplification of nonprotected types that were previously present, but missed because of competition within the assay (5,32). It is important to recognize that a reported increase in specific HPV types caused by unmasking would not be representative of the true change in HPV prevalence.
In our analyses requiring two-time positivity to confirm incidence (intended to avoid inclusion of cases caused by viral deposition), effect estimates were slightly lower, but not meaningfully different, compared with our main analyses. To explore if the discrepant cases in this analysis may have been caused by an assay sensitivity issue and differential competition for reagents across arms, we evaluated if the cases that were persistent (+, +) vs those that were not (+, -) differed by infection status with some other HPV type(s) at the second visit and across arms. Among infections that did not persist, prevalence of infection with one or more other HPV type(s) at the second visit was actually lower in the control arm compared with the HPV arm (35.0% vs 44.5%, respectively); and within each arm, prevalence of other type(s) at the second visit was similar among infections that did and did not persist. This suggests that our attenuated estimates were unlikely caused by an assay sensitivity issue.
Women with prevalent HPV infection are known to be at greater risk of acquiring additional HPV infections due to higher HPV exposure and may represent the ideal subpopulation to evaluate type replacement. As expected, based on our previous observations in our analyses restricted to women infected with at least one vaccine-protected type at enrollment (33), we observed the highest incidence rates and also somewhat higher rate difference estimates. These estimates were, however, not meaningfully different compared with our original results. Among the opposite low-risk group of women virginal at study entry and with low incidence rates, we observed a small negative (albeit statistically nonsignificant) rate difference for oncogenic/nononcogenic HPV types, which was the only negative estimate across all of our grouped analyses.
A limitation of pooling results from PATRICIA and CVT is that despite harmonization at the design phase, study protocols and HPV testing methodology were slightly different. For example, in PATRICIA, type-specific PCR results were available for HPV types 31, 33, 35, 45, 52, 58, and 59, whereas only LiPA25 results were available for these types in CVT. As a result, we were forced to exclude type-specific PCR results for these types from our main pooled analyses. Although we do not expect that our conclusions would be different had type-specific PCR results been available in both studies for these types, higher efficacy was observed in our study-specific PATRICIA analyses that included all available PCR results. Additionally, while women in PATRICA had regular six month visits, those in CVT attended only annual visits unless they had abnormal cytology results prompting additional visits. Because women in the HPV arm may be less likely to develop early precancerous lesions resulting in additional follow-up visits, women in the control arm may be more likely to have their transient HPV infections detected. Fortunately, women in the HPV and control arms had the same total follow-up time and number of visits, which supports our findings from analyses restricted to annual visits revealing consistent results. Balance across arms for measured risk factors also assures us that randomization was successful and that any statistically significant difference in observed incidence rates may be attributed to HPV vaccination.
Millions of women worldwide have now been vaccinated with either Cervarix (bivalent) or Gardasil (quadrivalent) HPV vaccines, and despite the arrival of Gardasil 9—a new nonavalent version that targets five additional oncogenic types—all vaccines remain in widespread use. In the current study, type replacement was not observed among HPV-vaccinated women in populations with low vaccine coverage. Although we cannot confirm absence of replacement in populations with high vaccine coverage in this study, as the most comprehensive analysis to date, our results provide important reassurance that HPV vaccination is unlikely to cause type replacement in vaccinated populations.
Funding
The Costa Rica HPV Vaccine Trial is a long-standing collaboration between investigators in Costa Rica and the National Cancer Institute (NCI). The trial is sponsored and funded by the NCI (contract N01-CP-11005), with funding support from the National Institutes of Health Office of Research on Women's Health. GlaxoSmithKline Biologicals SA provided vaccine and support for aspects of the trial associated with regulatory submission needs of the company under a Clinical Trials Agreement (FDA BB-IND 7920) during the four-year, randomized blinded phase of our study. John T. Schiller and Douglas R. Lowy report that they are named inventors on US Government-owned HPV vaccine patents that are licensed to GlaxoSmithKline and Merck and for which the National Cancer Institute receives licensing fees. They are entitled to limited royalties as specified by federal law. The PATRICIA trial was funded by GlaxoSmithKline Biologicals SA.
Notes
Authors: Joseph E. Tota, Frank Struyf, Marko Merikukka, Paula Gonzalez, Aimée R. Kreimer, Dan Bi, Xavier Castellsagué, Newton S. de Carvalho, Suzanne M. Garland, Diane M. Harper, Naveen Karkada, Klaus Peters, Willy A. J. Pope, Carolina Porras, Wim Quint, Ana Cecilia Rodriguez, Mark Schiffman, John Schussler, S. Rachel Skinner, Júlio Cesar Teixeira, Cosette M. Wheeler, Rolando Herrero, Allan Hildesheim*, Matti Lehtinen*; for the Costa Rica Vaccine Trial and the PATRICIA study groups
* Authors contributed equally to this work.
Affiliations of authors: Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JET, ARK, MS, AH); GSK Vaccines, Wavre, Belgium (FS, DB); National Institute for Health and Welfare, Oulu, Finland (MM); Proyecto Epidemiológico Guanacaste, Fundación INCIENSA, San José, Costa Rica (PG); Cancer Epidemiology Research Program, Catalan Institute of Oncology (ICO)-IDIBELL, CIBER-ESP, L'Hospitalet de Llobregat, Spain (XC); Department of Gynecology and Obstetrics, Federal University of Paraná, Infectious Diseases in Gynecology and Obstetrics Sector/Clinics Hospital, Curitiba, Brazil (NSdC); Department of Microbiology and Infectious Diseases, Royal Women's Hospital, Parkville, Victoria, Australia (SMG); Murdoch Childrens Research Institute, Parkville, Victoria, Australia (SMG); Department of Obstetrics and Gynaecology, University of Melbourne, Victoria, Parkville, Australia (SMG); Geisel School of Medicine at Dartmouth, Hanover, NH (DMH); GSK Vaccines, Bangalore, India (NK); Berner Heerweg 157, Hamburg, Germany (KP); Department of Gynaecology, University Hospital KU Leuven Gasthuisberg, Leuven, Belgium (WAJP); Proyecto Epidemiológico Guanacaste, Fundación INCIENSA, San José, Costa Rica (CP, ACR, RH); DDL Diagnostic Laboratory, Rijswijk, the Netherlands (WQ); Information Management Systems, Rockville, MD (JS); Vaccines Trials Group, Telethon Institute for Child Health Research, Perth, WA, and Sydney University Discipline of Paediatrics and Child Health, Children’s Hospital Westmead, Sydney, NSW, Australia (SRS); Department of Gynecology, Oncology Division—CAISM, State University of Campinas, Campinas, Brazil (JCT); Department of Pathology and Obstetrics and Gynecology, University of New Mexico Health Sciences Center, Albuquerque, NM (CMW); Section of Early Detection and Prevention, International Agency for Research on Cancer, Lyon, France (RH); University of Tampere, School of Public Health, Tampere, Finland (ML).
JET, FS, MM, DB, AH, and ML formed the manuscript core writing team. All authors, including group coauthors listed below, qualify for authorship in adherence with the International Committee of Medical Journal Editors guidelines. All authors contributed to study design, acquisition of data or statistical analyses, and interpretation of results. All authors commented upon a draft and approved the final version of the report.
The NCI and Costa Rica investigators, not the funders, are responsible for the design and conduct of CVT; collection, management, analysis, and interpretation of the data; and preparation of the manuscript. GlaxoSmithKline Biologicals SA and other investigators coordinated the collection, analysis, and interpretation of the data, as well as the preparation of the manuscript.
We extend a special thanks to the women of Guanacaste and Puntarenas, Costa Rica, who gave of themselves in participating in this effort. In Costa Rica, we acknowledge the tremendous effort and dedication of the staff involved in this project; we would like to specifically acknowledge the meaningful contributions by Loreto Carvajal, Rebeca Ocampo, Cristian Montero, Diego Guillen, Jorge Morales, and Mario Alfaro. In the United States, we extend our appreciation to the team from Information Management Services (IMS), which was responsible for the development and maintenance of the data system used in the trial and serves as the data management center for this effort, especially Jean Cyr, Julie Buckland, John Schussler, and Brian Befano. We thank Dr. Diane Solomon (CVT: medical monitor and QC pathologist) for her invaluable contributions during the randomized blinded phase of the trial and the design of the LTFU and Nora Macklin (CVT) and Kate Torres (LTFU) for their expertise in coordinating the study. We thank the members of the Data and Safety Monitoring Board charged with protecting the safety and interest of participants during the randomized, blinded phase of our study (Steve Self, Chair, Adriana Benavides, Luis Diego Calzada, Ruth Karron, Ritu Nayar, and Nancy Roach) and members of the external Scientific HPV Working Group who have contributed to the success of our efforts over the years (Joanna Cain, Chair, Diane Davey, David DeMets, Francisco Fuster, Ann Gershon, Elizabeth Holly, Silvia Lara, Henriette Raventós, Wasima Rida, Luis Rosero-Bixby, Kristen Suthers, Amber D’Souza, Richard Roden, and Peter Gilbert). The members of the PATRICIA trial would also like to thank all study participants and their families and acknowledge the work of the central and local study coordinators and staff members of the sites that participated in this study.
Group coauthors: Costa Rica Vaccine Trial Study Group authors: Bernal Cortés (Proyecto Epidemiologico Guanacaste Fundación INCIENSA, San José, Costa Rica), Teresa M. Darragh (University of California, San Francisco, CA), Silvia Jiménez (Proyecto Epidemiologico Guanacaste Fundación INCIENSA, San José, Costa Rica), Troy J. Kemp (Leidos Biomedical Research, Inc., Frederick National Laboratory for Cancer Research, Frederick, MD), Douglas R. Lowy (United States National Cancer Institute, Bethesda, MD), Joel M. Palefsky (University of California, San Francisco, CA), Ligia A. Pinto (Leidos Biomedical Research, Inc., Frederick National Laboratory for Cancer Research, Frederick, MD), John T. Schiller, PhD (United States National Cancer Institute, Bethesda, MD), Mark E. Sherman (United States National Cancer Institute, Bethesda, MD), Mary K. Sidawy (Georgetown University, Washington, DC), Mark H. Stoler (University of Virginia, Charlottesville, VA), Linda Struijk (DDL Diagnostic Laboratory, Netherlands), Leen-Jan van Doorn (DDL Diagnostic Laboratory, the Netherlands), Sholom Wacholder (United States National Cancer Institute, Bethesda, MD); PATRICIA Study Group authors: F. Xavier Bosch (Network on Cooperative Cancer Research [RTICC] and Unit of Infections and Cancer, Cancer Epidemiology Research Program, Institut Català d’Oncologia, L’Hospitalet de Llobregat, IDIBELL, Barcelona, Spain), Song-Nan Chow (Department of Obstetrics and Gynecology, College of Medicine and the Hospital, National Taiwan University, Taipei, Taiwan), Dominique Descamps (GSK Vaccines, Wavre, Belgium), Francisco Diaz-Mitoma (Advanced Medical Research Institute of Canada, Sudbury, ON, Canada), Gary Dubin (GSK Vaccines, King of Prussia, PA), Maria Julieta Germar, MD (University of the Philippines College of Medicine, Philippine General Hospital, Manila, Philippines), David J. M. Lewis (Clinical Research Centre, University of Surrey, Guildford, UK), Genara Limson (University of the Philippines, College of Medicine, Philippine General Hospital, Makati Medical Centre, Makati City, Philippines), Paulo Naud (University Federal of Rio Grande do Sul, Hospital de Clínica de Porto Alegre, Porto Alegre, Brazil), Brian Ramjattan (First Line Medical Services, St John’s, NL, Canada), Barbara Romanowski (University of Alberta, Edmonton, AB, Canada), Jorge Salmeron (Unidad de Investigación Epidemiológica y en Servicios de Salud, Instituto Mexicano del Seguro Social, Morelos, Mexico), Tino F. Schwarz (Central Laboratory and Vaccination Centre, Stiftung Juliusspital, Academic Teaching Hospital of the University of Wuerzburg, Wuerzburg, Germany), Wiebren A. A. Tjalma (Multidisciplinary Breast Clinic–Gynecological Oncology Unit, Department of Obstetrics and Gynecology, Antwerp University Hospital and University of Antwerp, Antwerpen, Belgium).
Other collaborators: HPV PATRICIA principal investigators/coprincipal investigator collaborators: Australia: S. M. Garland, A. Mindel, S. R. Skinner; Belgium: P. De Sutter, W. A. J. Poppe, W. A. A. Tjalma; Brazil: N. S. De Carvalho, P. Naud, J. C. Teixeira; Canada: F. Y. Aoki, F. Diaz-Mitoma, L. Ferguson, M. Miller, K. Papp, B. Ramjattan, B. Romanowski, P. H. Orr; Finland: D. Apter, T. Karppa, N. Kudjoi, M. Lehtinen, K. Lönnberg, J. Palmroth, J. Paavonen, T. Petaja, L. Tuomivaara; Germany: T. Gent, T. Grubert, W. D. Höpker, K. Peters, K. Schulze, T. F. Schwarz, R. Waddell; Mexico: J. Salmerón; Philippines: C. Crisostomo, M. R. Del Rosario-Raymundo, J. E. Raymundo, M. J. Germar, G. Limson, G. Villanueva, S. Villanueva, J. D. Zamora; Spain: J. Bajo-Arenas, J. Bayas, M. Campins, X. Castellsagué, M. Castro, C. Centeno, M. L. Rodríguez de la Pinta, A. Torné, J. A. Vidart; Taiwan: S. N. Chow, M. H. Yu; Thailand: S. Angsuwathana, U. Jaisamrarn; UK: M. Cruickshank, E. A. Hakim, D. Lewis, A. Szarewski (deceased); USA: R. Ackerman, M. Caldwell, C. Chambers, A. Chatterjee, L. DeMars, P. Fine, W. Huh, T. Klein, J. Lalezari, L. Leeman, S. Luber, M. Martens, C. Peterson, J. Rosen, L. Seidman, R. Sperling, M. Stager, J. Stapleton, A. Waldbaum, C. M. Wheeler.
Other contributors: GSK Vaccines clinical study support: B. Colau, S. Genevrois, N. Martens, N. Houard, S. Poncelet, C. Provenzano, A. Tonglet, C. Van Hoof (Xpe Pharma), D. Descamps, K. Hardt, V. Xhenseval, T. Zahaf.
Laboratory contribution: E. Alt, B. Iskaros, A. Limaye, R. D. Luff, M. McNeeley, B. Winkler (Quest Diagnostics Clinical Trials, Teterboro, NJ); A. Molijn, W. Quint, L. Struijk, M. Van de Sandt, L. J. Van Doorn (DDL Diagnostic Laboratory, Voorburg, the Netherlands).
Endpoint committee: K. P. Klugman, P. Nieminen. Independent Data Monitoring Committee: C. Bergeron, E. Eisenstein, R. Karron, R. Marks, T. Nolan, S. K. Tay.
Supplementary Material
References
- 1. Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;652:87–108. [DOI] [PubMed] [Google Scholar]
- 2. Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;1891:12–19. [DOI] [PubMed] [Google Scholar]
- 3. Tota JE, Chevarie-Davis M, Richardson LA, et al. Epidemiology and burden of HPV infection and related diseases: Implications for prevention strategies. Prev Med. 2011;53(suppl 1):S12–S21. [DOI] [PubMed] [Google Scholar]
- 4. Lehtinen M, Paavonen J.. Vaccination against human papillomaviruses shows great promise. Lancet. 2004;3649447:1731–1732. [DOI] [PubMed] [Google Scholar]
- 5. Tota JE, Ramanakumar AV, Jiang M, et al. Epidemiologic approaches to evaluating the potential for human papillomavirus type replacement postvaccination. Am J Epidemiol. 2013;1784:625–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schiffman M, Clifford G, Buonaguro FM.. Classification of weakly carcinogenic human papillomavirus types: Addressing the limits of epidemiology at the borderline. Infect Agent Cancer. 2009;4:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. IARC. Human Papillomaviruses. IARC monographs on the evaluation of carcinogenic risks to humans. Lyon, France: IARC; 2011. [Google Scholar]
- 8. Li N, Franceschi S, Howell-Jones R, et al. Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: Variation by geographical region, histological type and year of publication. Int J Cancer. 2011;1284:927–935. [DOI] [PubMed] [Google Scholar]
- 9. Munoz N, Bosch FX, Castellsague X, et al. Against which human papillomavirus types shall we vaccinate and screen? The international perspective. Int J Cancer. 2004;1112:278–85. [DOI] [PubMed] [Google Scholar]
- 10. de Sanjose S, Quint WG, Alemany L, et al. Human papillomavirus genotype attribution in invasive cervical cancer: A retrospective cross-sectional worldwide study. Lancet Oncol. 2010;1111:1048–1056. [DOI] [PubMed] [Google Scholar]
- 11. Serrano B, Alemany L, Tous S, et al. Potential impact of a nine-valent vaccine in human papillomavirus related cervical disease. Infect Agent Cancer. 2012;71:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Harper DM, Franco EL, Wheeler CM, et al. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet. 2006;3679518:1247–1255. [DOI] [PubMed] [Google Scholar]
- 13. Garland SM, Hernandez-Avila M, Wheeler CM, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;35619:1928–1943. [DOI] [PubMed] [Google Scholar]
- 14. Brown DR, Kjaer SK, Sigurdsson K, et al. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in generally HPV-naive women aged 16-26 years. J Infect Dis. 2009;1997:926–935. [DOI] [PubMed] [Google Scholar]
- 15. Wheeler CM, Kjaer SK, Sigurdsson K, et al. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in sexually active women aged 16-26 years. J Infect Dis. 2009;1997:936–944. [DOI] [PubMed] [Google Scholar]
- 16. Herrero R, Wacholder S, Rodriguez AC, et al. Prevention of persistent human papillomavirus infection by an HPV16/18 vaccine: A community-based randomized clinical trial in Guanacaste, Costa Rica. Cancer Discov. 2011;15:408–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wheeler CM, Castellsague X, Garland SM, et al. Cross-protective efficacy of HPV-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by non-vaccine oncogenic HPV types: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol. 2012;131:100–110. [DOI] [PubMed] [Google Scholar]
- 18. Joura EA, Giuliano AR, Iversen OE, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;3728:711–723. [DOI] [PubMed] [Google Scholar]
- 19. Weinberger DM, Malley R, Lipsitch M.. Serotype replacement in disease after pneumococcal vaccination. Lancet. 2011;3789807:1962–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Herrero R, Hildesheim A, Rodriguez AC, et al. Rationale and design of a community-based double-blind randomized clinical trial of an HPV 16 and 18 vaccine in Guanacaste, Costa Rica. Vaccine. 2008;2637:4795–4808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Paavonen J, Jenkins D, Bosch FX, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: An interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007;3699580:2161–2170. [DOI] [PubMed] [Google Scholar]
- 22. Kleter B, van Doorn LJ, ter Schegget J, et al. Novel short-fragment PCR assay for highly sensitive broad-spectrum detection of anogenital human papillomaviruses. Am J Pathol. 1998;1536:1731–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kleter B, van Doorn LJ, Schrauwen L, et al. Development and clinical evaluation of a highly sensitive PCR-reverse hybridization line probe assay for detection and identification of anogenital human papillomavirus. J Clin Microbiol. 1999;378:2508–2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. van Doorn LJ, Molijn A, Kleter B, et al. Highly effective detection of human papillomavirus 16 and 18 DNA by a testing algorithm combining broad-spectrum and type-specific PCR. J Clin Microbiol. 2006;449:3292–3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dessy FJ, Giannini SL, Bougelet CA, et al. Correlation between direct ELISA, single epitope-based inhibition ELISA and pseudovirion-based neutralization assay for measuring anti-HPV-16 and anti-HPV-18 antibody response after vaccination with the AS04-adjuvanted HPV-16/18 cervical cancer vaccine. Hum Vaccin. 2008;46:425–434. [DOI] [PubMed] [Google Scholar]
- 26. Szarewski A, Skinner SR, Garland SM, et al. Efficacy of the HPV-16/18 AS04-adjuvanted vaccine against low-risk HPV types (PATRICIA randomized trial): An unexpected observation. J Infect Dis. 2013;2089:1391–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lehtinen M, Apter D, Baussano I, et al. Characteristics of a cluster-randomized phase IV human papillomavirus vaccination effectiveness trial. Vaccine. 2015;3310:1284–1290. [DOI] [PubMed] [Google Scholar]
- 28. Drolet M, Benard E, Boily MC, et al. Population-level impact and herd effects following human papillomavirus vaccination programmes: A systematic review and meta-analysis. Lancet Infect Dis. 2015;155:565–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kahn JA, Brown DR, Ding L, et al. Vaccine-type human papillomavirus and evidence of herd protection after vaccine introduction. Pediatrics. 2012;1302:e249–e256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mesher D, Soldan K, Howell-Jones R, et al. Reduction in HPV 16/18 prevalence in sexually active young women following the introduction of HPV immunisation in England. Vaccine. 2013;321:26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lipsitch M. Vaccination against colonizing bacteria with multiple serotypes. Proc Natl Acad Sci U S A. 1997;9412:6571–6576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tota JE, Ramanakumar AV, Villa LL, et al. Evaluation of human papillomavirus type replacement postvaccination must account for diagnostic artifacts: Masking of HPV52 by HPV16 in anogenital specimens. Cancer Epidemiol Biomarkers Prev. 2015;241:286–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Palmroth J, Merikukka M, Paavonen J, et al. Occurrence of vaccine and non-vaccine human papillomavirus types in adolescent Finnish females 4 years post-vaccination. Int J Cancer. 2012;13112:2832–2838. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.