Abstract
Dysregulation of glutamate homeostasis in the interstitial fluid of the brain is strongly implicated in causing synaptic dysfunction in many neurological and psychiatric illnesses. In the case of Alzheimer's disease (AD), amyloid β (Aβ)-mediated disruption of synaptic plasticity and memory can be alleviated by interventions that directly remove glutamate or block certain glutamate receptors. An alternative strategy is to facilitate the removal of excess glutamate from the nervous system by activating peripheral glutamate clearance systems. One such blood-based system, glutamate oxaloacetate transaminase (GOT), is activated by oxaloacetate, which acts as a co-substrate. We report here that synthetic and AD brain-derived Aβ-mediated inhibition of synaptic long-term potentiation in the hippocampus is alleviated by oxaloacetate. Moreover the effect of oxaloacetate was GOT-dependent. The disruptive effects of a general inhibitor of excitatory amino acid transport or TNFα, a pro-inflammatory mediator of Aβ action, were also reversed by oxaloacetate. Furthermore, another intervention that increases peripheral glutamate clearance, peritoneal dialysis, mimicked the beneficial effect of oxaloacetate. These findings lend support to the promotion of the peripheral clearance of glutamate as a means to alleviate synaptic dysfunction that is caused by impaired glutamate homeostasis in the brain.
Keywords: Alzheimer's disease, amyloid beta, glutamate clearance, long-term potentiation, TNFα
Introduction
The extent of synaptic loss between principal glutamatergic neurons in the mesotemporal regions of the brain correlates strongly with cognitive impairment in Alzheimer's disease (AD) patients (Davies et al. 1987; DeKosky and Scheff 1990; Terry et al. 1991). Associated changes in synaptic markers in AD brain include a reduction or mis-localization of the major excitatory amino acid transporter type 2, EAAT2 / GLT-1 (Masliah et al. 1996; Woltjer et al. 2010; Scott et al. 2011; Overk and Masliah 2014; Rudy et al. 2015), which will lead to impaired glutamate homeostasis.
In animal models, amyloid β (Aβ), a pathognomonic peptide of AD, has been reported to disrupt excitatory amino acid transporters and increase glutamate release from neurons and glia (Matos et al. 2008; Talantova et al. 2013). The resultant increase in extracellular glutamate concentration (O'Shea et al. 2008; Lei et al. 2015) may help explain why Aβ interferes with synaptic function and plasticity. We and others have shown that certain soluble aggregates of Aβ are very potent and rapid disrupters of synaptic plasticity, inhibiting long-term potentiation (LTP) in areas such as the hippocampus (Cullen et al. 1997; Lambert et al. 1998; Klyubin et al. 2008; Shankar et al. 2008; Spires-Jones and Hyman 2014; Welzel et al. 2014; Jeronimo-Santos et al. 2015; Willem et al. 2015). Evidence that excessive glutamatergic activity is likely a major cause of such disruption is provided by the ability of antagonists of NMDA and metabotropic glutamate 5 (mGlu5) receptors to abrogate the deficits (Klyubin et al. 2011; Rammes et al. 2011; Ronicke et al. 2011; Hu et al. 2014; Hamilton et al. 2015). Indeed, pretreatment with an enzyme-based scavenger of extracellular glutamate in vitro prevented Aβ-mediated disruption of synaptic plasticity in hippocampal slices (Li et al. 2009; Varga et al. 2015).
Recently, drugs that reduce free extracellular glutamate concentration by increasing the number of functional transporters have been reported to reduce cognitive impairment in transgenic mice overexpressing human disease-causing mutant Aβ precursor protein (Takahashi et al. 2015; Zumkehr et al. 2015). However, agents targeting glutamate receptors or transporters directly in the brain are likely to have dose-limiting side effects. One alternative approach, being developed for other neurological disorders mediated by excessive glutamate, is to promote brain glutamate homeostasis by enhancing its clearance from blood (Gottlieb et al. 2003; Zlotnik et al. 2007; Castillo et al. 2016) across the blood–brain barrier (O'Kane et al. 1999; Teichberg et al. 2009; Cederberg-Helms et al. 2014). Thus, the metabolic intermediate oxaloacetate has been reported to act as a blood-based glutamate “scavenger” or “grabber” by increasing the deamination of glutamate to 2-ketoglutarate via the enzyme glutamate oxaloacetate transaminase (GOT, also known as aspartate aminotransferase). Such an action can rapidly promote glutamate efflux from the brain and thereby alleviate deficits in animal models of stroke, subarachnoid hemorrhage, and traumatic brain injury (Marosi et al. 2009; Campos et al. 2011; Boyko, Melamed et al. 2012; Zlotnik et al. 2012).
Here, we report that systemic treatment with oxaloacetate not only can prevent but also can reverse the disruption of synaptic plasticity by Aβ, both synthetic and soluble AD brain-derived, and TNFα, a pro-inflammatory mediator of Aβ action. The time window for the beneficial effect of oxaloacetate was extended by increasing the blood levels of the converting enzyme GOT. Moreover, peritoneal dialysis, an alternative means of indirectly lowering brain glutamate, also abrogated the inhibition of LTP by Aβ. These findings strongly suggest that enhancing peripheral clearance of glutamate can protect the hippocampus against the synaptic disruptive effects caused by Aβ- and TNFα-mediated impaired glutamate homeostasis.
Materials and Methods
Animals and Surgery
Adult (250–350 g, 8–11 weeks old) male Wistar rats were housed under a 12-h light–dark cycle at room temperature (19–22 °C). The animal care and experimental protocol were approved by the Department of Health, Republic of Ireland, and the Irish Health Products Regulatory Authority in accordance with EU law. The surgical and electrophysiological methods are similar to those used previously (Hu et al. 2014). Anesthesia was induced and maintained with urethane (1.5–1.6 g/kg, i.p.). Monopolar recording electrodes (75 μm inner core diameter, 112 μm external diameter; 3.4 mm posterior to bregma and 2.5 mm lateral to midline) and twisted bipolar stimulating electrodes (50 μm inner core diameter, 75 μm external diameter; 4.2 mm posterior to bregma and 3.8 mm lateral to midline) were constructed from Teflon-coated tungsten wires. Field excitatory postsynaptic potentials (fEPSPs) were recorded from the stratum radiatum in the CA1 area of the dorsal hippocampus in response to stimulation of the ipsilateral Schaffer collateral-commissural pathway. The final placement of electrodes was optimized by using electrophysiological criteria and confirmed via postmortem analysis.
In order to inject drugs or Aβ into the brain, a stainless steel cannula (22 gauge, 0.7 mm outer diameter) was implanted ipsilaterally above the right lateral ventricle (1 mm lateral to the midline and 4 mm below the surface of the dura). Agents were injected intracerebroventricularly (i.c.v.) via an internal cannula (28 gauge, 0.36 mm outer diameter) at a rate of 1 μL/min with a Hamilton syringe. Verification of the placement of the cannula was performed postmortem by checking the spread of ink dye after i.c.v. injection.
Electrophysiology
Test fEPSPs were evoked by square wave pulses (0.2 ms duration) at a frequency of 0.033 Hz and an intensity that triggered a 50% maximum response as determined after constructing an input/output curve. Baseline synaptic transmission was recorded for at least 30 min. LTP was induced using 200 Hz high frequency stimulation (HFS) consisting of 3 sets of 10 trains of 20 stimuli (inter-set interval 5 min). The stimulation intensity was not changed during HFS.
Drugs and Chemicals
Oxaloacetate, TNFα, DL-threo-beta-benzyloxyaspartic acid (TBOA), and chemicals for peritoneal dialysis were purchased from Sigma-Aldrich. The oxaloacetate dose chosen (35 mg/kg in 1 mL, i.v.) was based on previous research (Perez-Mato et al. 2014). The dose of recombinant glutamate oxaloacetate transaminase type 1 (rGOT) (Megazyme) given (0.39 mg/rat i.v.) was previously reported to significantly increase GOT serum concentration for over 20 h (Ruban et al. 2015) .
Synthetic Aβ1-42
Aβ-derived diffusible ligands (ADDLs), an aggregated preparation of synthetic Aβ1-42 (synthesized by Dr James I. Elliott at Yale University, New Haven, CT), used in this study were described previously (Hu et al. 2014). As shown in figure 4 of Hu et al. (2014), these preparations contain predominantly protofibrillar assemblies. Briefly, a peptide solution was prepared in anhydrous dimethylsulfoxide and subsequently diluted with Hams F-12 media. After incubation, the solution was centrifuged at 16 100 × g to remove large preformed aggregates. Aliquots of ADDLs were frozen on dry ice and stored at −80 °C until needed. Depending on the batch of ADDLs, the amount used to strongly block LTP was either 350 or 580 pmol (in 5–8 μl).
AD Brain Extracts
Human tissue was obtained and used in accordance with local IRB guidelines. We studied extracts of temporal cortex from 2 patients’ brains: AD1 from a 92-year-old woman and AD2 from a 95-year-old woman. Both women had histories of dementia and on postmortem examination were confirmed to have AD. AD1 extract was reported previously (An et al. 2013), and similar methods were used for AD2 extract. Briefly, frozen tissue was allowed to thaw on ice, chopped into small pieces, and then homogenized in 5 volumes of ice-cold 20 mM Tris-HCl, pH 7.4, containing 150 mM NaCl Tris-buffered saline (TBS) with 25 strokes of a Dounce homogenizer (Fisher). Water-soluble Aβ was separated from membrane-bound and plaque Aβ by centrifugation at 91 000 × g and 4 °C in a TLA 55 rotor (Beckman Coulter) for 78 min. To eliminate bioactive small molecules and drugs, the supernatant was exchanged into ammonium acetate using a 5 mL Hi-trap desalting column (GE Healthcare Bio-Sciences AB) (AD1 extract) or was dialysed into TBS using Slide-A-Lyzer Dialysis cassettes (Thermo Scientific) (AD2 extract). Thereafter, the extracts were divided into 2 parts: 1 aliquot was immunodepleted (ID) of Aβ by 3 rounds of 12 h incubations with the anti-Aβ polyclonal rabbit antibody AW7, and protein A at 4 °C. The second portion was not manipulated (AD). The amount and form of Aβ was analyzed in samples by immunoprecipitation with AW7 and by western blotting using a combination of the C-terminal monoclonal antibodies, 2G3 and 21F12. The amount of Aβ present was determined by reference to standard quantities of synthetic Aβ1-42. AD1 extract contained approximately 2.2 ng/ml of Aβ monomer and approximately 0.76 ng/ml of SDS-stable dimer (for details, see fig. 6 of An et al. 2013), and the extract AD2 contained approximately 62 ng/ml of Aβ monomer and approximately 27 ng/ml of SDS-stable dimer (to be reported in more detail, including western blot of extract, in another publication, see Supplementary Figure 1).
Peritoneal Dialysis
Similar methods to those described by (Godino Mdel et al. 2013; Pawlaczyk et al. 2015) were used. A precut silicone catheter (inner diameter 0.5 mm, outer diameter 1.5 mm) (Helix Medical) was used for rat peritoneal dialysis. One hour before starting peritoneal dialysis, the catheter was implanted surgically under general anesthesia with the help of a guiding stick. A midline incision was made through the upper abdominal skin, and a hole was pierced close to linea alba using a 2-mm-diameter needle. The catheter (2–3 cm long) was inserted in the incision. A hole for the outlet was made at the level of the lower abdomen. Peritoneal dialysis was initiated 90 min prior to HFS by the infusion by gravity (flow rate 1.5–2.0 mL/min) of dialysate containing glucose 8.3 mM, NaCl 140 mM, CaCl2 1.75 mM, MgCl2 0.5 mM, lactic acid 3.5 mM, and NaH2PO4 10 mM (pH 6.5). The outlet was closed to let the dialysate remain in the peritoneal cavity, and then removed every 20 min. This was followed by a second infusion with fresh dialysate. Animals in the sham peritoneal dialysis group underwent the exact same surgical procedures, but the implanted cannula was not perfused with dialysate.
Serum Glutamate Assay
Blood samples were collected in test tubes, centrifuged at 600 × g for 10 min, serum was removed and immediately frozen, and stored at –80 °C. Serum glutamate concentration was determined by means of a glutamate assay kit (Cat. No. MAK004, Sigma-Aldrich) following the manufacturer's technical instructions.
Data Analysis
The strength of synaptic transmission is expressed as a percentage of the baseline fEPSP amplitude recorded over a 30-min period. The magnitude of LTP was measured at 3 h post-HFS and expressed as the mean ± SEM % baseline. No data were excluded. Control experiments were interleaved randomly throughout experimental sets. For statistical analysis and graphical display, EPSP amplitude was grouped into 10-min epochs. One-way ANOVA followed by Bonferroni's multiple comparison test were used to compare the magnitude of LTP between multiple groups; unpaired and paired Student's t-tests were used to compare between two groups and within groups, respectively. Serum glutamate concentration is expressed as the mean ± SEM percentage of the level in a sample taken before injection or before starting peritoneal dialysis, and analysed statistically in a similar manner. A P value of <0.05 was considered statistically significant.
Results
Systemic Pretreatment with Oxaloacetate Prevents Aβ-Mediated Disruption of Synaptic Plasticity
We studied the effect of oxaloacetate on Aβ-mediated inhibition of LTP using a single bolus injection of a dose (35 mg/kg, i.v.) that previously has been reported to reduce free blood and brain glutamate concentration (Campos et al. 2011; Perez-Mato et al. 2014). This dose of oxaloacetate fully prevented the disruption of hippocampal synaptic plasticity caused by Aβ.
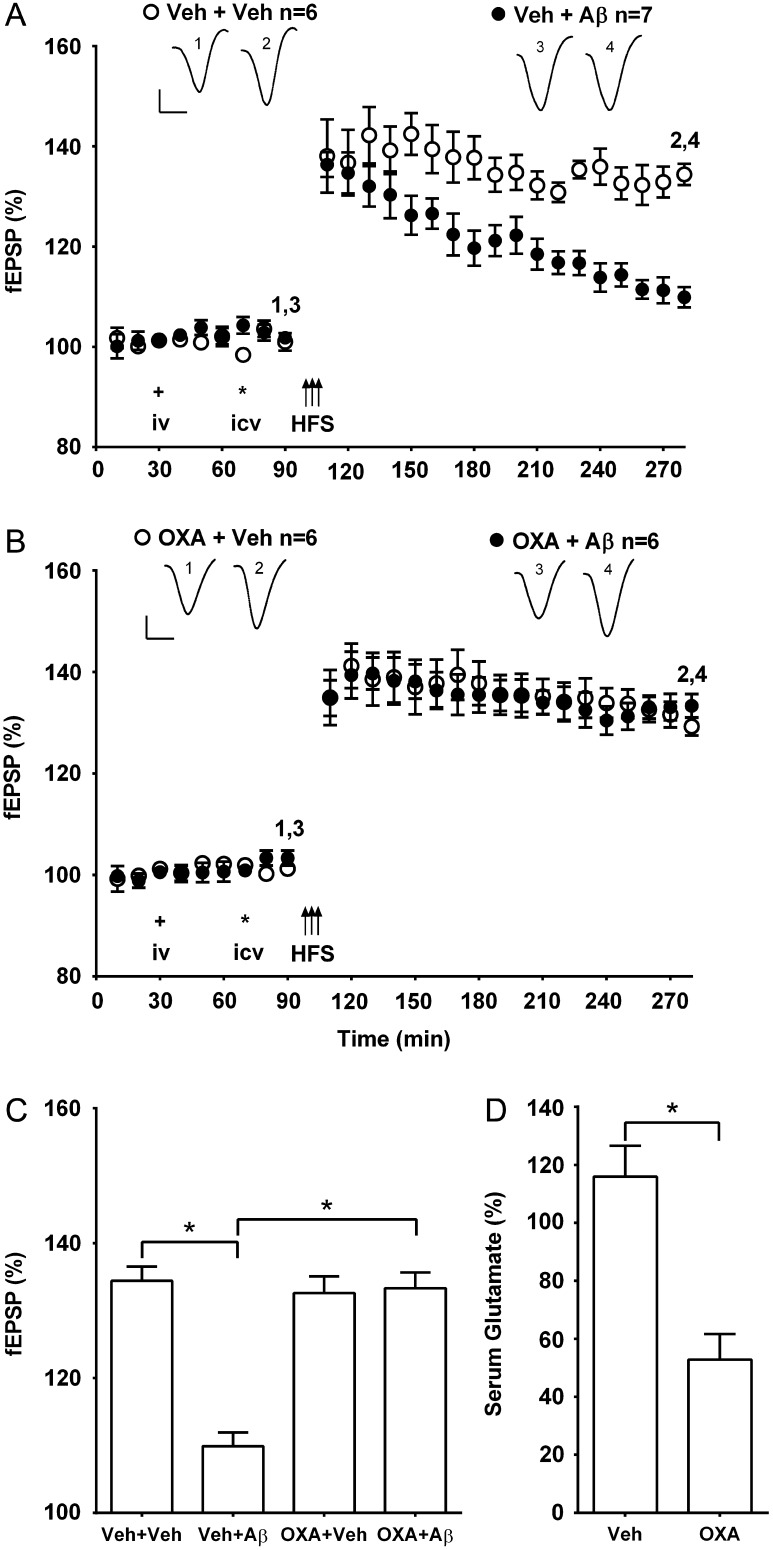
The ADDL preparation of synthetic Aβ1-42 (Aβ, i.c.v.), when applied 20 min prior to HFS, significantly inhibited LTP in vehicle (1 mL, i.v.) pre-injected rats (109.9 ± 2.0%, mean ± SEM% pre-injection baseline fEPSP amplitude at 3 h post-HFS, n = 7; P < 0.05 compared with 134.4 ± 2.1% in controls, n = 6, ANOVA followed by Bonferroni test) (Fig. 1A,C). In contrast, in animals that received oxaloacetate 40 min before the administration of the Aβ, the application of HFS 20 min later triggered robust LTP (133.3 ± 2.3%, n = 6; P < 0.05 compared with Aβ; P > 0.05 compared with vehicle control) (Fig. 1B,C). Injection of the same dose of oxaloacetate in the absence of Aβ, did not significantly affect the magnitude of LTP (129.3 ± 1.8%, n = 6; P > 0.05 compared with vehicle control group) (Fig. 1B,C), baseline synaptic transmission (100.5 ± 0.9% pre-injection baseline fEPSP amplitude at 3 h, n = 4; P > 0.05 compared with 102.6 ± 1.6%, n = 4 in vehicle-injected rats, unpaired t-test, data not shown), or background electroencephalography measures of theta and gamma power (see Supplementary Table 1).
Figure 1.
Systemic pretreatment with the blood-based glutamate scavenger oxaloacetate prevents Aβ-mediated inhibition of LTP. (A,B) Time course graphs showing the effect of oxaloacetate on Aβ-mediated inhibition of LTP. (A) The application of HFS (arrows) in control animals pretreated both i.v. (plus symbol) and i.c.v. (asterisk) with the appropriate vehicle (Veh + Veh) induced robust LTP that was relatively stable for at least 3 h (P < 0.05 compared with pre-HFS baseline, paired t-test). In contrast, in animals injected with the ADDL preparation of synthetic Aβ1-42 (Aβ, i.c.v.) 20 min before HFS and 40 min after systemic vehicle (Veh + Aβ), a decremental LTP was induced (P > 0.05 compared with pre-HFS baseline at 3 h post-HFS). (B) Importantly, in animals pretreated with oxaloacetate (35 mg/kg, i.v.) 40 min before Aβ (OXA + Aβ) the application of HFS 20 min later triggered stable LTP (P < 0.05 compared with pre-HFS baseline). Moreover, this dose of oxaloacetate did not significantly affect control LTP (OXA + Veh) (P < 0.05 compared with pre-HFS baseline). Insets show representative fEPSP traces at the times indicated. Calibration bars: vertical, 1 mV; horizontal, 10 ms. (C) Summary bar chart comparing the magnitude of synaptic potentiation at 3 h post-HFS between treatment groups. Values are the mean ± SEM fEPSP amplitude expressed as a percentage of the pre-HFS baseline. (D) Compared with vehicle-injected controls this dose of oxaloacetate significantly reduced serum concentration of glutamate 1 h after treatment, (n = 4 per group). Values are the mean ± SEM expressed as a percentage of the pre-injection baseline. *P < 0.05, one-way ANOVA followed by Bonferroni test or unpaired t-test.
We confirmed that oxaloacetate reduced serum glutamate levels to 52.8 ± 8.8% (mean ± SEM percentage of the pre-injection concentration) at 1 h post-injection, P < 0.05 compared with 115.9 ± 10.7% in vehicle-treated animals (n = 4 per group) (Fig. 1D).
Systemic Co-treatment with the Enzyme Glutamate Oxaloacetate Transaminase Prolongs the Ameliorative Effect of Oxaloacetate on the Disruptive Action of Aβ
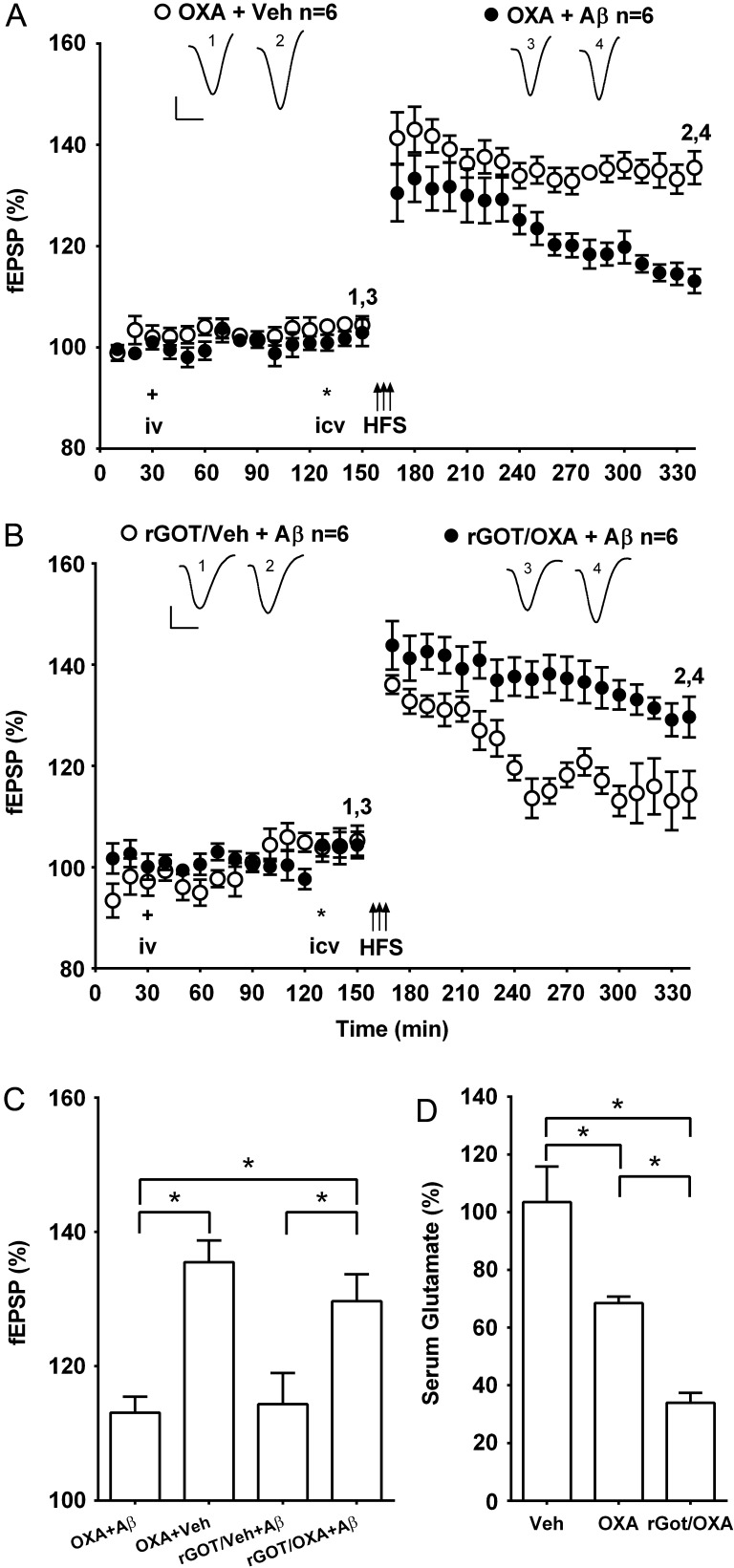
Next we examined if the beneficial effect of oxaloacetate was sustained, by injecting it 2 h, instead of 1 h, prior to HFS. The same dose of oxaloacetate that prevented the inhibition of LTP when administered 40 min before the Aβ now failed to abrogate the inhibition of LTP (113.1 ± 2.4%, n = 6; P < 0.05 compared with 135.5 ± 3.2%, n = 6, in animals that received oxaloacetate in the absence of Aβ) (Fig. 2A,C). We attempted to prolong the effect of oxaloacetate by increasing the blood concentration of GOT using recombinant protein type 1 (rGOT), thereby enhancing oxaloacetate-mediated clearance of glutamate from blood (Zlotnik et al. 2007; Perez-Mato et al. 2014; Ruban et al. 2015). Whereas pretreatment alone with a dose (0.39 mg/rat) of rGOT 2 h prior to HFS failed to abrogate the inhibition of LTP by Aβ (114.4 ± 4.7%, n = 6; P > 0.05 compared with pre-HFS baseline), application of HFS after co-treatment with oxaloacetate now induced robust LTP in the presence of Aβ (129.7 ± 4.0%, n = 6, P < 0.05 compared with baseline or rGOT + vehicle group) (Fig. 2B,C). We compared the effect of oxaloacetate and oxaloacetate plus rGOT on serum glutamate levels at 2 h in order to determine if the glutamate levels correlated with their differential efficacy in preventing the inhibition of LTP by Aβ. Although glutamate concentration was reduced significantly by oxaloacetate alone (63.5 ± 6.6%, n = 4, P < 0.05 compared with 103.5 ± 12.4%, n = 4 in controls), the level was reduced further in the presence of rGOT (36.4 ± 5.8%, n = 4, P < 0.05 compared with oxaloacetate alone) (Fig. 2D).
Figure 2.
The beneficial effect of systemic pretreatment with oxaloacetate against Aβ-mediated inhibition of LTP is prolonged by co-treatment with glutamate-oxaloacetate transaminase. (A,B) Time course graphs showing the effect of oxaloacetate on Aβ-mediated inhibition of LTP. (A) In animals that received oxaloacetate i.v. (plus symbol, 35 mg/kg) 1 h 40 min prior to i.c.v. (asterisk) vehicle (OXA + Veh) the application of HFS (arrows) 20 min later induced robust LTP that was relatively stable for at least 3 h. In contrast, in animals injected with Aβ (i.c.v.) 20 min before HFS and 1 h 40 min after systemic oxaloacetate (OXA + Aβ), a decremental LTP was induced. (B) We tested a dose (0.39 mg/rat) of type 1 recombinant GOT (rGOT) that alone did not prevent the inhibition of LTP by Aβ (rGOT/Veh + Aβ) (P > 0.05 compared with pre-HFS baseline at 3 h post-HFS). However, HFS induced robust LTP when this dose of rGOT was co-injected with oxaloacetate 2 h previously (rGOT/OXA + Aβ) (P < 0.05 compared with pre-HFS baseline). Insets show representative fEPSP traces at the times indicated. Calibration bars: vertical, 1 mV; horizontal, 10 ms. (C) Summary bar chart comparing the magnitude of synaptic potentiation between treatment groups at 3 h post-HFS. Values are the mean ± SEM fEPSP amplitude expressed as a percentage of the pre-HFS baseline. (D) Bar chart comparing serum glutamate concentration at 2 h after the treatment with vehicle (Veh), oxaloacetate alone (OXA) or oxaloacetate with rGOT (rGOT/OXA) (n = 4 per group). *P < 0.05, one-way ANOVA followed by Bonferroni test.
Brief Peritoneal Dialysis Prevents Aβ-Mediated Disruption of Synaptic Plasticity
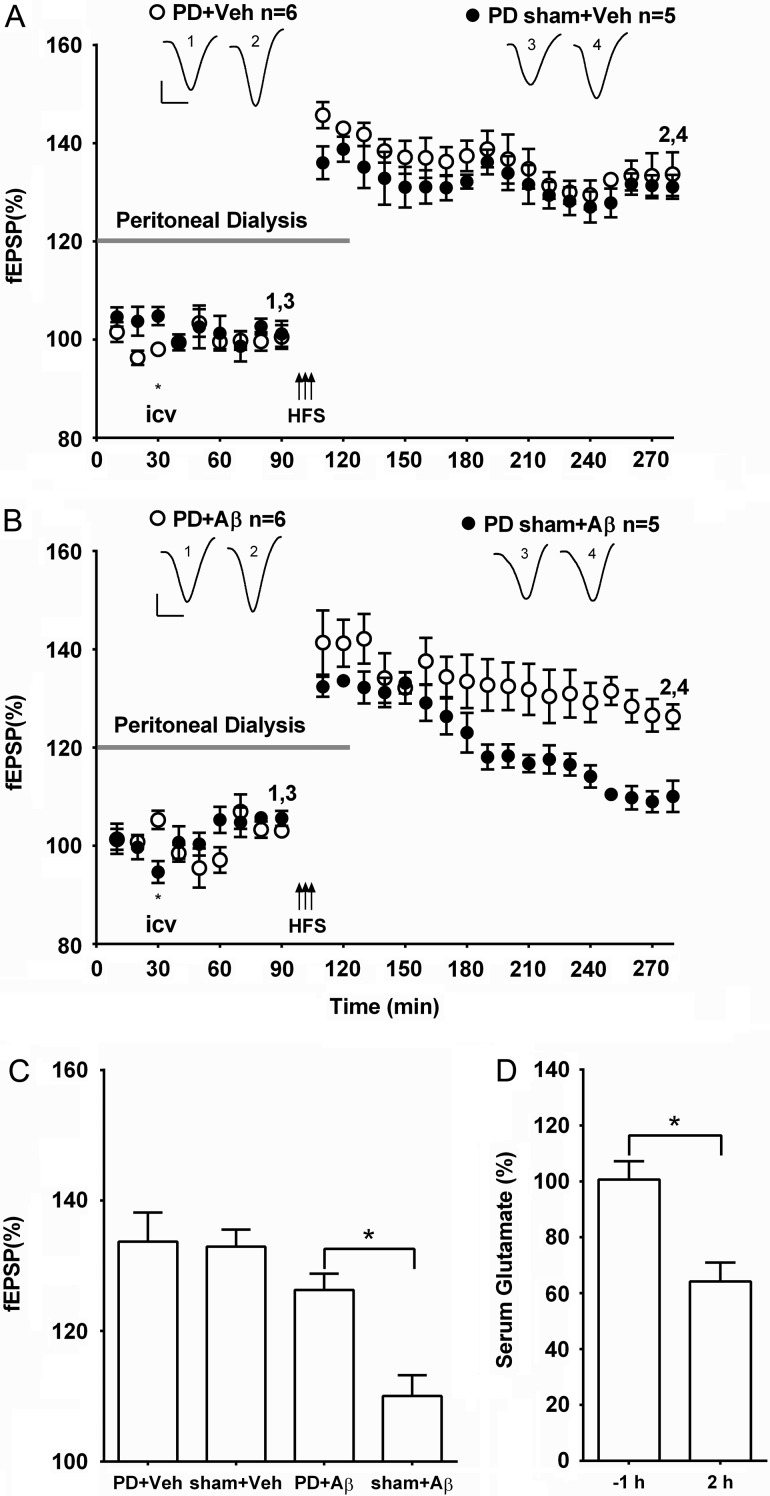
We hypothesized that other treatments that increase peripheral glutamate clearance should mimic the beneficial effect of systemic treatment with oxaloacetate. Peritoneal dialysis is a clinically used procedure whereby fluids and dissolved substances are exchanged between the blood and the dialysate across the peritoneum. Brief peritoneal dialysis of rats with a glutamate-free dialysate has previously been reported to decrease blood glutamate concentration and thereby reduce excitotoxicity in the brain caused by excess glutamate following cerebral ischemia (Godino Mdel et al. 2013). Consistent with the results that we obtained after treatment with oxaloacetate, we found that peritoneal dialysis for 2 h prevented the inhibition of LTP by Aβ.
Robust LTP was induced in control animals (vehicle i.c.v., 5 µl) that underwent peritoneal dialysis starting 90 min prior to HFS and ending 30 min post-HFS (133.7 ± 4.5%, n = 6; P > 0.05 compared with 131.1 ± 2.4%, n = 5, in the sham dialysis + vehicle group) (Fig. 3A,C). Moreover, pre-injection of Aβ 60 min prior to HFS failed to inhibit LTP in animals that underwent the same peritoneal dialysis protocol (126.3 ± 2.5%, n = 6; P > 0.05 compared with the dialysis + vehicle group; P < 0.05 compared with 110.0 ± 3.2%, n = 5, in sham dialysis + Aβ group) (Fig. 3B,C).
Figure 3.
Acute peritoneal dialysis prevents Aβ-mediated inhibition of LTP. (A,B) Time course graphs showing the effect of peripheral peritoneal dialysis on Aβ-mediated inhibition of LTP. (A) HFS induced robust LTP in animals that underwent peritoneal dialysis (gray bar) for 2 h, starting 30 min before i.c.v. injection of vehicle and finishing 30 min post-HFS (PD + Veh) (P < 0.05 compared with pre-HFS baseline). Similarly, in animals that underwent the same surgical protocol, but without initiating peritoneal dialysis (PD sham + Veh), HFS also triggered stable LTP (P < 0.05 compared with pre-HFS baseline). (B) Whereas, the surgical protocol had no effect on Aβ-mediated inhibition of LTP (PD sham + Aβ) (P > 0.05 compared with pre-HFS baseline) HFS induced robust LTP in animals that had 2 h peritoneal dialysis with Aβ (PD + Aβ) (P < 0.05 compared with pre-HFS baseline). Insets show representative fEPSP traces at the times indicated. Calibration bars: vertical, 1 mV; horizontal, 10 ms. (C) Summary bar chart comparing the magnitude of synaptic potentiation at 3 h post-HFS between treatment groups. Values are the mean ± SEM fEPSP amplitude expressed as a percentage of the pre-HFS baseline. (D) Compared with pretreatment values peritoneal dialysis significantly reduced serum concentration of glutamate 2 h after commencing treatment (n = 4, paired t-test). *P < 0.05.
The peritoneal procedure was found to decrease serum glutamate levels to 64.2 ± 6.8% at the end of 2 h dialysis (P < 0.05 compared with 100.7 ± 6.5% in samples taken 1 h before starting dialysis, n = 4) (Fig. 3D), similar to what has been reported previously (Godino Mdel et al. 2013).
Systemic Treatment with Oxaloacetate after Aβ Reverses Disruption of Synaptic Plasticity
We reasoned that if the disruptive effects of Aβ on synaptic plasticity is mediated by the presence of excess glutamate at the time of conditioning stimulation, then application of the glutamate scavenger oxaloacetate after Aβ might also abrogate the inhibition of LTP.
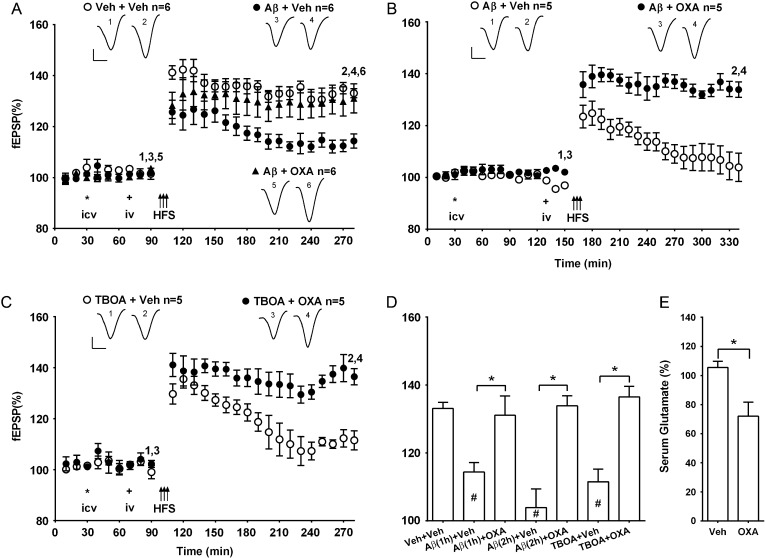
Therefore, we reversed the sequence of the injections. In one set of experiments, oxaloacetate was administered 20 min prior to the start of the 10-min HFS protocol and 40 min after the Aβ injection. LTP was significantly enhanced in these animals (131.1 ± 5.7%, n = 6) compared with rats that received i.v. vehicle injection after the Aβ (114.4 ± 2.8%, n = 6, P < 0.05) and similar in magnitude to that induced in a vehicle control group (133.1 ± 1.8%, n = 6, P > 0.05) (Fig. 4A,D). We also tested the ability of oxaloacetate treatment to rapidly reduce blood glutamate levels and found that they significantly declined 30 min post-injection (72.0 ± 9.7%, P < 0.05 compared with 107.8 ± 5.4% in controls, n = 4 per group) (Fig. 4E).
Figure 4.
The inhibition of LTP by Aβ or the glutamate transporter inhibitor TBOA is abrogated by treatment with oxaloacetate. (A,B) Time course graphs showing the effect of posttreatment with oxaloacetate on Aβ-mediated inhibition of LTP. HFS (arrows) was applied either (A) 1 h or (B) 2 h after Aβ. (A) In animals pretreated first i.c.v. (asterisk) with Aβ and subsequently i.v. (plus symbol) with vehicle (Aβ + Veh) the application of HFS induced a small synaptic potentiation (P < 0.05 at 3 h post-HFS, compared with pre-HFS baseline or veh + veh group). In contrast, in animals injected with Aβ and subsequently treated with oxaloacetate (35 mg/kg, i.v.) (Aβ + OXA) the application of HFS 20 min later triggered robust and stable LTP (P < 0.05 compared with pre-HFS baseline; P > 0.05 compared with veh + veh group). (B) Similar findings were obtained when oxaloacetate was injected 2 h after Aβ. (C) Moreover, oxaloacetate reversed the ability of TBOA to inhibit LTP. Thus, in animals pretreated first with i.c.v. TBOA (asterisk, 6 nmol, i.c.v.) and subsequently with i.v. (plus symbol) vehicle (TBOA + Veh) the application of HFS induced transient synaptic potentiation (P > 0.05 at 3 h, compared with pre-HFS baseline). In contrast, in animals injected with TBOA and subsequently treated with oxaloacetate (35 mg/kg, i.v.) (TBOA + OXA) the application of HFS 20 min later triggered robust and stable LTP (P < 0.05 compared with pre-HFS baseline). Insets show representative fEPSP traces at the times indicated. Calibration bars: vertical, 1 mV; horizontal, 10 ms. (D) Summary bar chart comparing the magnitude of synaptic potentiation at 3 h post-HFS between treatment groups. Values are the mean ± SEM fEPSP amplitude expressed as a percentage of the pre-HFS baseline. (E) Compared with vehicle-injected controls oxaloacetate significantly reduced serum concentration of glutamate 30 min after treatment (n = 4 per group, unpaired t-test). *P < 0.05, # P < 0.05 compared with Veh + Veh, one-way ANOVA followed by Bonferroni test or unpaired t-test..
Similarly, in another set of animals that had been injected with Aβ 2 h prior to HFS, the inhibition of LTP by Aβ was abrogated if they received a subsequent i.v. injection of oxaloacetate (133.9 ± 3.0%, n = 5) rather than vehicle (103. 9 ± 5.5%, n = 5; P < 0.05) (Fig. 4B,D).
Systemic Treatment with Oxaloacetate After the Glutamate Uptake Inhibitor TBOA Reverses the Disruption of Synaptic Plasticity
In order to provide complementary evidence that increasing extracellular glutamate in the brain inhibited LTP in vivo and that systemic administration of oxaloacetate can neutralize such an action, we tested the effect of the general excitatory amino acid transporter inhibitor, TBOA (Montiel et al. 2005) , on LTP in the absence and presence of oxaloacetate. We chose a dose (6 nmol, i.c.v.) of TBOA that did not significantly affect baseline synaptic transmission (96.7 ± 1.8%, n = 4, at 3 h post-injection, P > 0.05 compared with pre-injection baseline, paired t-test, and 103.9 ± 3.3%, n = 5 in vehicle-treated group, unpaired t-test, data not shown). One hour after the i.c.v. injection of TBOA and 20 min after i.v. vehicle, the application of HFS failed to induce LTP (111.5 ± 3.8%, n = 5). In contrast, when oxaloacetate was administered (35 mg/ml, i.v.) 40 min after the TBOA, the HFS induced robust and stable LTP (136.5 ± 3.2%, n = 5, P < 0.05 compared with TBOA + vehicle group) (Fig. 4C,D).
Systemic Pretreatment or Posttreatment with Oxaloacetate Abrogates the Disruption of Synaptic Plasticity by AD Brain-Derived Aβ
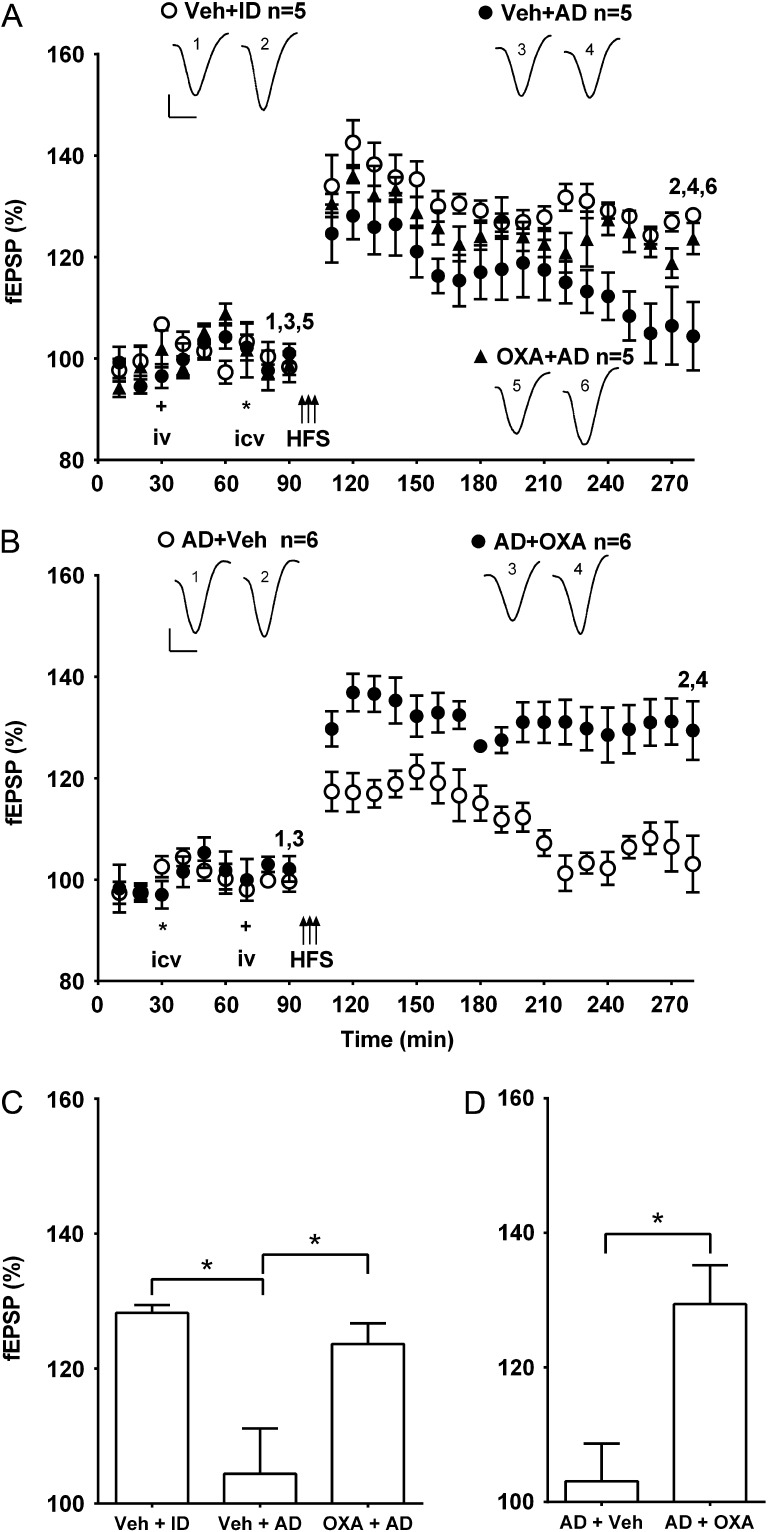
In order to help determine if the potential beneficial effect of systemic treatment with oxaloacetate extends to the assemblies and concentration of Aβ species found in human brain, we also examined its efficacy against the disruptive action of Aβ-containing soluble extracts of AD brain. Similar to our previous reports (Barry et al. 2011; Freir et al. 2011; An et al. 2013), injection of small volumes of soluble extract inhibited LTP in an Aβ-dependent manner. Here, 6 µl of AD1 extract strongly inhibited LTP (Fig. 5). The inhibitory effect was mediated by Aβ since injection of the same extract that had been immunodepleted with the polyclonal anti-Aβ antibody AW7 (see also fig. 6 of An et al., 2013, for the western blot of this extract before and after immunodepletion) failed to inhibit LTP.
Figure 5.
Systemic pretreatment or posttreatment with oxaloacetate abrogates the inhibition of LTP by Aβ-containing AD brain extracts. (A,B) Time course graphs showing the effect of AD brain extracts on LTP in the presence of either (A) pretreatment or (B) posttreatment with oxaloacetate. (A) The magnitude of LTP was significantly reduced in animals injected with brain extract AD1 (AD, 6 µl i.c.v.) 20 min before HFS and 40 min after systemic vehicle (Veh + AD) (P > 0.05 compared with pre-HFS baseline at 3 h post-HFS). In contrast, the same extract that had been immunodepleted of Aβ (Veh + ID) had no effect on LTP (P < 0.05 compared with pre-HFS baseline). Importantly, in animals pretreated with oxaloacetate (35 mg/kg, i.v.) 40 min before AD (OXA + AD) the application of HFS 20 min later triggered stable LTP (P < 0.05 compared with pre-HFS baseline). (B) In animals pre-treated first i.c.v. (asterisk) with brain extract AD2 from a different patient (8 µl), and subsequently i.v. (plus symbol) with vehicle (AD + Veh), the application of HFS induced a decremental synaptic potentiation (P > 0.05 compared with pre-HFS baseline at 3 h post-HFS). In contrast, in animals injected with AD and subsequently treated with oxaloacetate (35 mg/kg, i.v.) (AD + OXA) the application of HFS 20 min later triggered stable LTP (P < 0.05 compared with pre-HFS baseline). Insets show representative fEPSP traces at the times indicated. Calibration bars: vertical, 1 mV; horizontal, 10 ms. (C,D) Summary bar charts comparing the magnitude of synaptic potentiation at 3 h post-HFS between groups (C) pre- and (D) post-treated with oxaloacetate. Values are the mean ± SEM fEPSP amplitude expressed as a percentage of the pre-HFS baseline. *P < 0.05.
Oxaloacetate both prevented and reversed the inhibition of LTP by the Aβ-containing extract. Thus, injection of AD1 extract 40 min after pretreatment with oxaloacetate no longer inhibited LTP (123.6 ± 3.1%, n = 5, P < 0.05 compared with 104.4 ± 6.7%, n = 5 in the extract treated with vehicle group, P > 0.05 compared with 128.3 ± 1.2%, n = 5 in immunodepleted samples). Similarly, i.v. administration of oxaloacetate 40 min after a soluble extract from a different AD patient (8 µl of AD2 extract, i.c.v.) reversed the inhibition of LTP (129.4 ± 5.8%, n = 6, P < 0.05 compared with 103.1 ± 5.6% in the vehicle-pretreated animals, n = 6).
Systemic Pretreatment with Oxaloacetate Attenuates the Disruption of Synaptic Plasticity by the Cytokine TNFα
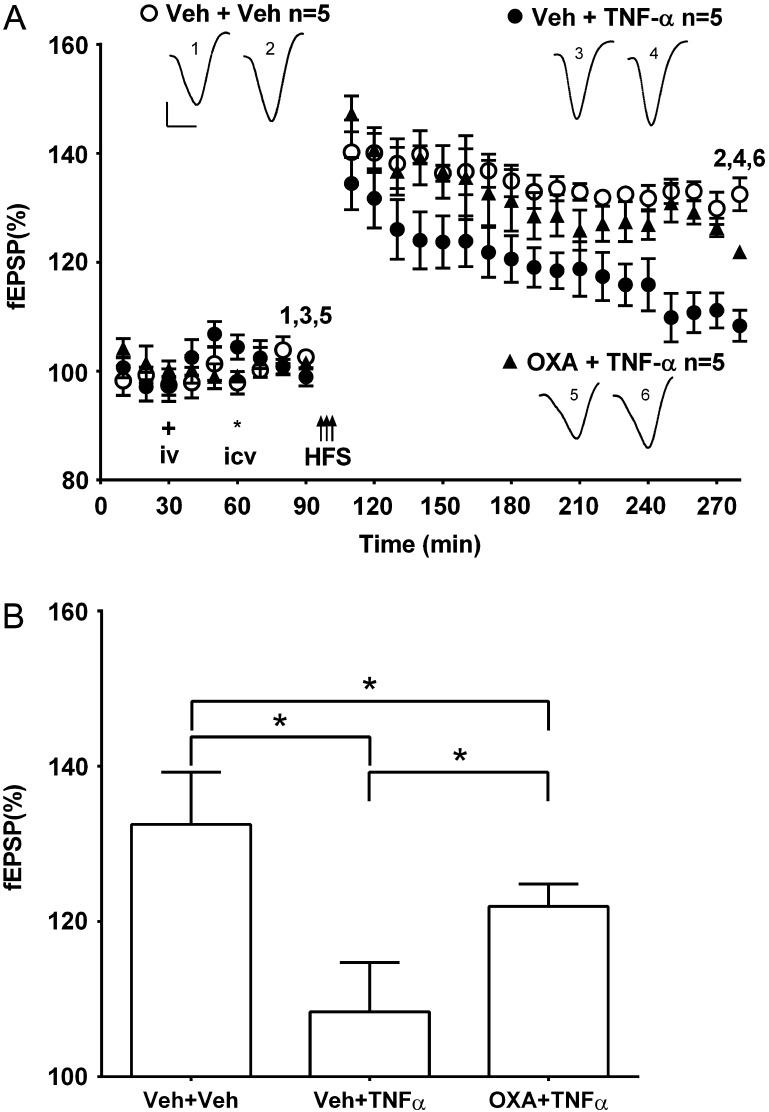
Since the inhibition of LTP by Aβ is prevented by agents that decrease TNFα (Wang et al. 2005; Hu et al. 2009) and TNFα can increase extracellular glutamate concentration via a variety of different mechanisms (Zou and Crews 2005; Takeuchi et al. 2006; Olmos and Lladó 2014; Jing et al. 2015; Shi et al. 2016), we wondered if oxaloacetate might prevent the inhibition of LTP by TNFα.
Consistent with previous findings (Curran and O'Connor 2003; Hu et al. 2009), a dose (1.2 pmol, i.c.v.) of TNFα that did not significantly affect baseline synaptic transmission (103.5 ± 2.0%, n = 4 at 3 h post-injection, P > 0.05 compared with pre-injection baseline, paired t-test, data not shown), strongly inhibited LTP. Thus in animals given TNFα 40 min after systemic vehicle treatment and 20 min prior to HFS, LTP was significantly impaired (108.4 ± 2.9%, n = 5; P < 0.05 compared with 132.5 ± 3.0%, n = 5, in the vehicle + vehicle control group) (Fig. 6). However, in animals pretreated with oxaloacetate (35 mg/kg, i.v.) 40 min prior to TNFα and 60 min prior to HFS, the magnitude of potentiation at 3 h was intermediate between these two treatment groups (121.9 ± 1.3%, n = 5, P < 0.05 compared with either the vehicle + vehicle control group or the vehicle + TNFα group) (Fig. 6).
Figure 6.
Systemic pretreatment with oxaloacetate reduces TNFα-mediated inhibition of LTP. (A) Time course graphs showing the effect of oxaloacetate on TNFα-mediated inhibition of LTP. The application of HFS (arrows) in animals pretreated i.v. (plus symbol) with vehicle and i.c.v. (asterisk) with TNFα (1.2 pmol) (Veh + TNFα) induced decremental LTP (P > 0.05 compared with pre-HFS baseline). In animals pretreated with oxaloacetate (35 mg/kg in 1 mL, i.v.) 40 min before TNFα (OXA + TNFα) the application of HFS 20 min later triggered a small but stable LTP (P < 0.05 compared with pre-HFS baseline). Insets show representative fEPSP traces at the times indicated. Calibration bars: vertical, 1 mV; horizontal, 10 ms. (B) Summary bar chart comparing the magnitude of synaptic potentiation at 3 h post-HFS between treatment groups. Values are the mean ± SEM fEPSP amplitude expressed as a percentage of the pre-HFS baseline. *P < 0.05.
Discussion
The present findings clearly show that systemic treatment with the blood-based glutamate scavenger oxaloacetate not only prevents but also reverses the inhibition of LTP by both synthetic Aβ and Aβ-containing AD brain extracts, albeit in a narrow time window. Supporting a peripheral mechanism of action, increasing blood levels of the enzyme GOT prolonged the effect of oxaloacetate. Moreover, peritoneal dialysis mimicked the ability of systemic oxaloacetate to prevent the inhibition of LTP. Consistent with oxaloacetate mediating its protective effect by decreasing excess brain glutamate, systemic treatment with oxaloacetate also prevented the impairment of LTP caused by the general excitatory amino acid transporter inhibitor TBOA. Moreover, oxaloacetate partially reversed the inhibition of LTP by TNFα, a cytokine known to increase extracellular glutamate in the brain and implicated in mediating deleterious synaptic changes in AD and other neurological and psychiatric conditions. Overall, this research indicates that interventions that promote peripheral glutamate clearance can effectively alleviate synaptic plasticity disruption caused by Aβ- and TNFα-mediated excessive extracellular glutamate in the hippocampus.
Aβ can increase brain extracellular glutamate concentration (O'Shea et al. 2008; Lei et al. 2015) apparently through several different mechanisms, including inhibition of excitatory amino acid transporters (Keller et al. 1997; Masliah et al. 2000; Li et al. 2009; Scott et al. 2011), and enhancing release from astrocytes and microglia (Orellana et al. 2011; Talantova et al. 2013). Some of these actions of Aβ are likely to be at least partly mediated by promoting oxidative stress and pro-inflammatory mechanisms (Keller et al. 1997; Bezzi et al. 2001; Vezzani and Viviani 2015). For example, Aβ promotes the release of cytokines such as TNFα that also can potently inhibit glutamate uptake and trigger glutamate release (Vezzani and Viviani 2015) (also see below).
Intravenous administration of oxaloacetate prior to intracerebral injection of Aβ fully prevented the inhibition of LTP, albeit in a narrow time window. The present in vivo findings, taken together with previous reports that in vitro pretreatment of hippocampal slices with a pyruvate-based glutamate scavenger system prevented the inhibition of LTP by Aβ (Li et al. 2009; Varga et al. 2015), strongly implicate a critical role for excess glutamate in mediating synaptic plasticity disruption by Aβ. Oxaloacetate is known to transiently promote glutamate clearance from the brain by reducing blood glutamate concentration. It achieves this by promoting the conversion of glutamate to 2-ketoglutarate by the blood-resident enzyme GOT (Ruban et al. 2015; Silva-Candal et al. 2015; Castillo et al. 2016). Whereas glutamate is readily cleared from the brain, dicarboxylates like oxaloacetate do not measurably cross into the brain from the periphery (Hassel et al. 2002). Significantly, we found that systemic treatment with oxaloacetate alone did not affect baseline hippocampal glutamatergic transmission or control HFS-induced LTP. Under basal conditions or after control conditioning stimulation in the absence of Aβ, normal physiological mechanisms of glutamate clearance and metabolism should be engaged continually and rapidly, thereby ensuring levels remain low without resorting to clearance of glutamate from the brain (O'Kane et al. 1999; Cederberg-Helms et al. 2014). Active glutamate and glutamine uptake via transporters on neurons but especially astrocytes promote glutamate recycling via the glutamate–glutamine cycle in the tripartite synapse. In contrast, in the presence of Aβ, glutamate uptake inhibition, combined with a likely increase in glutamate release from glia and possibly disinhibited neurons (Matos et al. 2008; Talantova et al. 2013; Ulrich 2015), will cause glutamate levels to rise excessively, necessitating the clearance of glutamate from the brain. Oxaloacetate promotes this clearance by increasing the concentration gradient of glutamate between the endothelial cells in the brain vasculature and the blood. It achieves this by promoting glutamate metabolism in blood by GOT. Indeed it is likely that the effects of oxaloacetate on brain glutamate are self-limiting since its effects should be minimal when the brain interstitial levels are below the threshold for activation of glutamate transporters on the abluminal side of endothelial cells in the cerebral microvessels (Gottlieb et al. 2003; Zlotnik et al. 2007; Castillo et al. 2016).
The present experiments were carried out on urethane anaesthetized rats and relatively strong HFS was applied to induce LTP. In animals awake, background EEG would be different and milder conditioning stimulation would be expected to induce robust LTP. It will be important to determine if peripheral glutamate scavenging systems, like oxaloacetate, can disrupt LTP induced by other protocols or background EEG in the absence of anesthesia. In principle, as discussed above, any increase in synaptic glutamate during physiological conditioning stimulation is unlikely to lead to excessive accumulation of glutamate extrasynaptically and hence would not be expected to be influenced by a selective blood-based glutamate scavenger.
In support of an indirect, blood-based, action of oxaloacetate, co-administration of the enzyme GOT, at a time/dose that neither agent alone was effective, prevented the impairment of LTP by Aβ. The beneficial synergism between oxaloacetate and GOT (molecular weight 92 kDa), neither of which penetrate into the brain to any significant extent (Hassel et al. 2002; Boyko, Stepensky et al. 2012), is best explained by their ability to work together to reduce glutamate concentration in blood and thereby indirectly decrease brain glutamate concentration (Boyko, Stepensky et al. 2012). The finding that brief peritoneal dialysis also fully prevented the disruption of synaptic plasticity by Aβ lends additional support to the proposal that interventions that reduce excess glutamate in the brain by increasing the peripheral clearance of glutamate (Godino Mdel et al. 2013; Rogachev et al. 2013) is an efficient strategy in vivo. However, the relationship between the magnitude of the reduction in serum glutamate levels and ability to prevent Aβ’s deleterious effect was not linear. Thus, an approximately 30–50% reduction in serum glutamate was effective against Aβ when tested 30 min/1 h after oxaloacetate alone or 2 h after peritoneal dialysis, but not at 2 h after treatment with oxaloacetate. The use of peripheral serum samples to indicate glutamate concentration gradient across cerebral blood vessels, and hence brain efflux of glutamate, is indirect. Serum samples taken at any given time will also be strongly influenced by other blood:tissue gradients throughout the body. Most other tissues lack a blood–brain barrier-like mechanism and continuous infusion of oxaloacetate has been reported to mobilize glutamate from peripheral tissue, which was associated with the development of rapid tolerance (Gottlieb et al. 2003). Consistent with the development of tolerance to the effect of oxaloacetate on the efflux of glutamate from the brain, a significantly greater reduction in blood glutamate level at 2 h, caused by the addition of rGOT to oxaloacetate, was associated with the simultaneous prevention of Aβ-mediated inhibition. In the case of peritoneal dialysis, glutamate will be irreversibly cleared from the body, and may consequently reduce the likelihood of the development of any rapid tolerance (Zhumadilov et al. 2015). Future studies using more direct measures of glutamate gradient and efflux from the brain will be required to determine how well the peripheral scavenger hypothesis explains these apparent discrepancies.
Importantly, injection of oxaloacetate even after the administration of Aβ was also fully effective in ameliorating the inhibition of LTP. This finding points to the potential beneficial role of this approach to reverse, as well as to prevent, the synaptic plasticity disruption. The time window for this beneficial action extended for over an hour, but in preliminary studies the same acute systemic treatment with oxaloacetate 7 days after Aβ injection was unable to reverse a delayed inhibition of LTP (Zhang et al., unpublished). Corroborating the ability of oxaloacetate to rapidly promote excess glutamate clearance from the brain, we also found that similar posttreatment with oxaloacetate reversed the inhibition of LTP caused by prior acute treatment with the glutamate transporter inhibitor TBOA.
The finding that pretreatment or posttreatment with oxaloacetate also abrogated the inhibition of LTP by Aβ from AD brain provides support for the possible disease relevance of the current findings. Our initial experiments employed ADDLs, a widely used bioactive preparation of synthetic Aβ1-42. However, since it is uncertain if ADDLs accurately reflect the Aβ species present in human brain, we also used the most disease-relevant preparation of Aβ, Aβ-containing extracts of AD brains. The relatively high potency of the Aβ in AD brain extracts indicates that it contains aggregates of Aβ that are particularly effective at disrupting synaptic plasticity and/or that other factors present in the diseased brain potentiate the effects of Aβ (Teich et al. 2016). We have previously reported that Aβ in soluble AD brain extracts not only mediates deleterious effects on synaptic plasticity but also causes synaptic pruning and impairs learning in rodents (Shankar et al. 2008; Borlikova et al. 2013).
Finally, the partial effectiveness of systemic treatment with oxaloacetate in ameliorating the inhibition of LTP by TNFα both substantiates its beneficial effect against Aβ and attests to the potential relevance of this approach to targeting other neurological and psychiatric illnesses that engage pro-inflammatory mechanisms (Raison et al. 2013; Chung et al. 2015). Like Aβ, TNFα can increase extracellular glutamate levels in the brain by increasing the release of glutamate from astrocytes (Bezzi et al. 2001) or microglia (Takeuchi et al. 2006). Moreover, it also can decrease glutamate uptake by inhibiting excitatory amino acid transporters (Kim et al. 2003; Zou and Crews 2005; Chen et al. 2012). Unlike the inhibitory effect of Aβ on LTP, the similar disruptive effect of TNFα was only partly prevented by oxaloacetate. It is possible that the dose of TNFα that was used caused a large increase in glutamate in the interstitial fluid which was only partly cleared by oxaloacetate. Alternatively, other mechanisms of action of TNFα independent of changes in extracellular glutamate concentration may also mediate the inhibition of LTP, such as direct effects on AMPA receptor trafficking (Beattie et al. 2002).
The general approach of promoting the peripheral clearance of glutamate offers a potentially advantageous means of reducing excessive glutamatergic activity in the brain without directly targeting brain receptors or transporters (Matos-Ocasio et al. 2014). Indeed the dynamic regulation of glutamate transporters and receptors that underlies physiological synaptic plasticity may be undermined by such agents (Pita-Almenar et al. 2012; Qi et al. 2013; Park et al. 2014; Jones 2015). Because intravenous oxaloacetate seemed to be associated with the development of tolerance and has not been extensively assessed in toxicity studies, in the long run alternative approaches, including other routes of administering rGOT and different forms of dialysis, may offer more clinically attractive ways of persistently reducing blood glutamate.
In conclusion, interventions that indirectly enhance glutamate homeostasis in the brain provide compelling evidence for the critical role of excess glutamate in mediating synaptic plasticity disruption by Aβ and TNFα. Furthermore, this approach offers certain advantages in the development of novel therapies that target excess glutamate in neurological and psychiatric illnesses.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/.
Funding
Science Foundation Ireland (10/IN.1/B3001 and 14/IA/2571); the Health Research Board of Ireland (HRA-POR-2015-1102); the National Institutes of Health (R01 AG046275).
Supplementary Material
References
- An K, Klyubin I, Kim Y, Jung JH, Mably AJ, O'Dowd ST, Lynch T, Kanmert D, Lemere CA, Finan GM, et al. 2013. Exosomes neutralize synaptic-plasticity-disrupting activity of Abeta assemblies in vivo. Mol Brain. 6:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry AE, Klyubin I, Mc Donald JM, Mably AJ, Farrell MA, Scott M, Walsh DM, Rowan MJ.. 2011. Alzheimer's disease brain-derived amyloid-β-mediated inhibition of LTP in vivo is prevented by immunotargeting cellular prion protein. J Neurosci. 31:7259–7263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Von Zastrow M, Beattie MS, Malenka RC.. 2002. Control of synaptic strength by glial TNFalpha. Science. 295:2282–2285. [DOI] [PubMed] [Google Scholar]
- Bezzi P, Domercq M, Brambilla L, Galli R, Schols D, De Clercq E, Vescovi A, Bagetta G, Kollias G, Meldolesi J, et al. 2001. CXCR4-activated astrocyte glutamate release via TNF[alpha]: amplification by microglia triggers neurotoxicity. Nat Neurosci. 4:702–710. [DOI] [PubMed] [Google Scholar]
- Borlikova GG, Trejo M, Mably AJ, Mc Donald JM, Sala Frigerio C, Regan CM, Murphy KJ, Masliah E, Walsh DM.. 2013. Alzheimer brain-derived amyloid beta-protein impairs synaptic remodeling and memory consolidation. Neurobiol Aging. 34:1315–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyko M, Melamed I, Gruenbaum BF, Gruenbaum SE, Ohayon S, Leibowitz A, Brotfain E, Shapira Y, Zlotnik A.. 2012. The effect of blood glutamate scavengers oxaloacetate and pyruvate on neurological outcome in a rat model of subarachnoid hemorrhage. Neurotherapeutics. 9:649–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyko M, Stepensky D, Gruenbaum BF, Gruenbaum SE, Melamed I, Ohayon S, Glazer M, Shapira Y, Zlotnik A.. 2012. Pharmacokinetics of glutamate-oxaloacetate transaminase and glutamate-pyruvate transaminase and their blood glutamate-lowering activity in naive rats. Neurochem Res. 37:2198–2205. [DOI] [PubMed] [Google Scholar]
- Campos F, Sobrino T, Ramos-Cabrer P, Argibay B, Agulla J, Perez-Mato M, Rodriguez-Gonzalez R, Brea D, Castillo J.. 2011. Neuroprotection by glutamate oxaloacetate transaminase in ischemic stroke: an experimental study. J Cereb Blood Flow Metab. 31:1378–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo J, Loza MI, Mirelman D, Brea J, Blanco M, Sobrino T, Campos F.. 2016. A novel mechanism of neuroprotection: blood glutamate grabber. J Cereb Blood Flow Metab. 36:292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cederberg-Helms HC, Uhd-Nielsen C, Brodin B.. 2014. Glutamate efflux at the blood-brain barrier: cellular mechanisms and potential clinical relevance. Arch Med Res. 45:639–645. [DOI] [PubMed] [Google Scholar]
- Chen CJ, Ou YC, Chang CY, Pan HC, Liao SL, Chen SY, Raung SL, Lai CY.. 2012. Glutamate released by Japanese encephalitis virus-infected microglia involves TNF-alpha signaling and contributes to neuronal death. Glia. 60:487–501. [DOI] [PubMed] [Google Scholar]
- Chung WS, Welsh CA, Barres BA, Stevens B.. 2015. Do glia drive synaptic and cognitive impairment in disease. Nat Neurosci. 18:1539–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen WK, Suh YH, Anwyl R, Rowan MJ.. 1997. Block of LTP in rat hippocampus in vivo by beta-amyloid precursor protein fragments. Neuroreport. 8:3213–3217. [DOI] [PubMed] [Google Scholar]
- Curran BP, O'Connor JJ.. 2003. The inhibition of long-term potentiation in the rat dentate gyrus by pro-inflammatory cytokines is attenuated in the presence of nicotine. Neurosci Lett. 344:103–106. [DOI] [PubMed] [Google Scholar]
- Davies CA, Mann DM, Sumpter PQ, Yates PO.. 1987. A quantitative morphometric analysis of the neuronal and synaptic content of the frontal and temporal cortex in patients with Alzheimer's disease. J Neurol Sci. 78:151–164. [DOI] [PubMed] [Google Scholar]
- DeKosky ST, Scheff SW.. 1990. Synapse loss in frontal cortex biopsies in Alzheimer's disease: correlation with cognitive severity. Ann Neurol. 27:457–464. [DOI] [PubMed] [Google Scholar]
- Freir DB, Fedriani R, Scully D, Smith IM, Selkoe DJ, Walsh DM, Regan CM.. 2011. Abeta oligomers inhibit synapse remodelling necessary for memory consolidation. Neurobiol Aging. 32:2211–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godino Mdel C, Romera VG, Sanchez-Tomero JA, Pacheco J, Canals S, Lerma J, Vivancos J, Moro MA, Torres M, Lizasoain I, et al. 2013. Amelioration of ischemic brain damage by peritoneal dialysis. J Clin Invest. 123:4359–4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb M, Wang Y, Teichberg VI.. 2003. Blood-mediated scavenging of cerebrospinal fluid glutamate. J Neurochem. 87:119–126. [DOI] [PubMed] [Google Scholar]
- Hamilton A, Zamponi GW, Ferguson SS.. 2015. Glutamate receptors function as scaffolds for the regulation of beta-amyloid and cellular prion protein signaling complexes. Mol Brain. 8:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassel B, Brathe A, Petersen D.. 2002. Cerebral dicarboxylate transport and metabolism studied with isotopically labelled fumarate, malate and malonate. J Neurochem. 82:410–419. [DOI] [PubMed] [Google Scholar]
- Hu N-W, Klyubin I, Anwyl R, Rowan MJ.. 2009. GluN2B subunit-containing NMDA receptor antagonists prevent Aβ-mediated synaptic plasticity disruption in vivo. Proc Natl Acad Sci USA. 106:20504–20509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu N-W, Nicoll AJ, Zhang D, Mably AJ, O'Malley T, Purro SA, Terry C, Collinge J, Walsh DM, Rowan MJ.. 2014. mGlu5 receptors and cellular prion protein mediate amyloid-β-facilitated synaptic long-term depression in vivo. Nat Commun. 5:3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeronimo-Santos A, Vaz SH, Parreira S, Rapaz-Lerias S, Caetano AP, Buee-Scherrer V, Castren E, Valente CA, Blum D, Sebastiao AM, et al. 2015. Dysregulation of TrkB receptors and BDNF function by amyloid-beta peptide is mediated by calpain. Cereb Cortex. 25:3107–3121. [DOI] [PubMed] [Google Scholar]
- Jing H, Hao Y, Bi Q, Zhang J, Yang P.. 2015. Intra-amygdala microinjection of TNF-α impairs the auditory fear conditioning of rats via glutamate toxicity. Neurosci Res. 91:34–40. [DOI] [PubMed] [Google Scholar]
- Jones OD. 2015. Astrocyte-mediated metaplasticity in the hippocampus: help or hindrance. Neuroscience. 309:113–124. [DOI] [PubMed] [Google Scholar]
- Keller JN, Pang Z, Geddes JW, Begley JG, Germeyer A, Waeg G, Mattson MP.. 1997. Impairment of glucose and glutamate transport and induction of mitochondrial oxidative stress and dysfunction in synaptosomes by amyloid beta-peptide: role of the lipid peroxidation product 4-hydroxynonenal. J Neurochem. 69:273–284. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Chae SC, Lee DK, Chromy B, Lee SC, Park YC, Klein WL, Krafft GA, Hong ST.. 2003. Selective neuronal degeneration induced by soluble oligomeric amyloid beta protein. FASEB J. 17:118–120. [DOI] [PubMed] [Google Scholar]
- Klyubin I, Betts V, Welzel AT, Blennow K, Zetterberg H, Wallin A, Lemere CA, Cullen WK, Peng Y, Wisniewski T, et al. 2008. Amyloid beta protein dimer-containing human CSF disrupts synaptic plasticity: prevention by systemic passive immunization. J Neurosci. 28:4231–4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klyubin I, Wang Q, Reed MN, Irving EA, Upton N, Hofmeister J, Cleary JP, Anwyl R, Rowan MJ.. 2011. Protection against Abeta-mediated rapid disruption of synaptic plasticity and memory by memantine. Neurobiol Aging. 32:614–623. [DOI] [PubMed] [Google Scholar]
- Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, Morgan TE, Rozovsky I, Trommer B, Viola KL, et al. 1998. Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc Natl Acad Sci USA. 95:6448–6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei M, Xu H, Li Z, Wang Z, O'Malley TT, Zhang D, Walsh DM, Xu P, Selkoe DJ, Li S.. 2015. Soluble Abeta oligomers impair hippocampal LTP by disrupting glutamatergic/GABAergic balance. Neurobiol Dis. 85:111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Hong S, Shepardson NE, Walsh DM, Shankar GM, Selkoe D.. 2009. Soluble oligomers of amyloid beta protein facilitate hippocampal long-term depression by disrupting neuronal glutamate uptake. Neuron. 62:788–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marosi M, Fuzik J, Nagy D, Rakos G, Kis Z, Vecsei L, Toldi J, Ruban-Matuzani A, Teichberg VI, Farkas T.. 2009. Oxaloacetate restores the long-term potentiation impaired in rat hippocampus CA1 region by 2-vessel occlusion. Eur J Pharmacol. 604:51–57. [DOI] [PubMed] [Google Scholar]
- Masliah E, Alford M, DeTeresa R, Mallory M, Hansen L.. 1996. Deficient glutamate transport is associated with neurodegeneration in Alzheimer's disease. Ann Neurol. 40:759–766. [DOI] [PubMed] [Google Scholar]
- Masliah E, Alford M, Mallory M, Rockenstein E, Moechars D, Van Leuven F.. 2000. Abnormal glutamate transport function in mutant amyloid precursor protein transgenic mice. Exp Neurol. 163:381–387. [DOI] [PubMed] [Google Scholar]
- Matos M, Augusto E, Oliveira CR, Agostinho P.. 2008. Amyloid-beta peptide decreases glutamate uptake in cultured astrocytes: involvement of oxidative stress and mitogen-activated protein kinase cascades. Neuroscience. 156:898–910. [DOI] [PubMed] [Google Scholar]
- Matos-Ocasio F, Hernandez-Lopez A, Thompson KJ.. 2014. Ceftriaxone, a GLT-1 transporter activator, disrupts hippocampal learning in rats. Pharmacol Biochem Behav. 122:118–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montiel T, Camacho A, Estrada-Sanchez AM, Massieu L.. 2005. Differential effects of the substrate inhibitor l-trans-pyrrolidine-2,4-dicarboxylate (PDC) and the non-substrate inhibitor DL-threo-beta-benzyloxyaspartate (DL-TBOA) of glutamate transporters on neuronal damage and extracellular amino acid levels in rat brain in vivo. Neuroscience. 133:667–678. [DOI] [PubMed] [Google Scholar]
- Olmos G, Lladó J.. 2014. Tumor necrosis factor alpha: a Link between neuroinflammation and excitotoxicity. Mediators Inflamm. 2014:861231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Kane RL, Martinez-Lopez I, DeJoseph MR, Vina JR, Hawkins RA.. 1999. Na(+)-dependent glutamate transporters (EAAT1, EAAT2, and EAAT3) of the blood-brain barrier. A mechanism for glutamate removal. J Biol Chem. 274:31891–31895. [DOI] [PubMed] [Google Scholar]
- Orellana JA, Froger N, Ezan P, Jiang JX, Bennett MV, Naus CC, Giaume C, Saez JC.. 2011. ATP and glutamate released via astroglial connexin 43 hemichannels mediate neuronal death through activation of pannexin 1 hemichannels. J Neurochem. 118:826–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shea SD, Smith IM, McCabe OM, Cronin MM, Walsh DM, O'Connor WT.. 2008. Intracerebroventricular administration of amyloid β-protein oligomers selectively increases dorsal hippocampal dialysate glutamate levels in the awake rat Sensors (Basel, Switzerland). 8:7428–7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overk CR, Masliah E.. 2014. Pathogenesis of synaptic degeneration in Alzheimer's disease and Lewy body disease. Biochem Pharmacol. 88:508–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park P, Volianskis A, Sanderson TM, Bortolotto ZA, Jane DE, Zhuo M, Kaang BK, Collingridge GL.. 2014. NMDA receptor-dependent long-term potentiation comprises a family of temporally overlapping forms of synaptic plasticity that are induced by different patterns of stimulation. Philos Trans R Soc Lond B Biol Sci. 369:20130131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlaczyk K, Baum E, Schwermer K, Hoppe K, Lindholm B, Breborowicz A.. 2015. Animal models of peritoneal dialysis: thirty years of our own experience. Biomed Res Int. 2015:261813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Mato M, Ramos-Cabrer P, Sobrino T, Blanco M, Ruban A, Mirelman D, Menendez P, Castillo J, Campos F.. 2014. Human recombinant glutamate oxaloacetate transaminase 1 (GOT1) supplemented with oxaloacetate induces a protective effect after cerebral ischemia. Cell Death Dis. 5:e992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pita-Almenar JD, Zou S, Colbert CM, Eskin A.. 2012. Relationship between increase in astrocytic GLT-1 glutamate transport and late-LTP. Learn Mem. 19:615–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Y, Hu NW, Rowan MJ.. 2013. Switching off LTP: mGlu and NMDA receptor-dependent novelty exploration-induced depotentiation in the rat hippocampus. Cereb Cortex. 23:932–939. [DOI] [PubMed] [Google Scholar]
- Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, Haroon E, Miller AH.. 2013. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry. 70:31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rammes G, Hasenjager A, Sroka-Saidi K, Deussing JM, Parsons CG.. 2011. Therapeutic significance of NR2B-containing NMDA receptors and mGluR5 metabotropic glutamate receptors in mediating the synaptotoxic effects of beta-amyloid oligomers on long-term potentiation (LTP) in murine hippocampal slices. Neuropharmacology. 60:982–990. [DOI] [PubMed] [Google Scholar]
- Rogachev B, Tsesis S, Gruenbaum BF, Gruenbaum SE, Boyko M, Klein M, Shapira Y, Vorobiev M, Zlotnik A.. 2013. The effects of peritoneal dialysis on blood glutamate levels: implementation for neuroprotection. J Neurosurg Anesthesiol. 25:262–266. [DOI] [PubMed] [Google Scholar]
- Ronicke R, Mikhaylova M, Ronicke S, Meinhardt J, Schroder UH, Fandrich M, Reiser G, Kreutz MR, Reymann KG.. 2011. Early neuronal dysfunction by amyloid beta oligomers depends on activation of NR2B-containing NMDA receptors. Neurobiol Aging. 32:2219–2228. [DOI] [PubMed] [Google Scholar]
- Ruban A, Cohen-Kashi Malina K, Cooper I, Graubardt N, Babakin L, Jona G, Teichberg VI.. 2015. Combined treatment of an amyotrophic lateral sclerosis rat model with recombinant GOT1 and oxaloacetic acid: a novel neuroprotective treatment. Neurodegener Dis. 15:233–242. [DOI] [PubMed] [Google Scholar]
- Rudy CC, Hunsberger HC, Weitzner DS, Reed MN.. 2015. The role of the tripartite glutamatergic synapse in the pathophysiology of Alzheimer's disease. Aging Dis. 6:131–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott HA, Gebhardt FM, Mitrovic AD, Vandenberg RJ, Dodd PR.. 2011. Glutamate transporter variants reduce glutamate uptake in Alzheimer's disease. Neurobiol Aging. 32:553 e551–511. [DOI] [PubMed] [Google Scholar]
- Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, et al. 2008. Amyloid-beta protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat Med. 14:837–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S, Liang D, Chen Y, Xie Y, Wang Y, Wang L, Wang Z, Qiao Z.. 2016. Gx-50 reduces beta-amyloid-induced TNF-alpha, IL-1beta, NO and PGE expression and inhibits NF- kappaB signaling in a mouse model of Alzheimer's disease. Eur J Immunol. 46:665–676. [DOI] [PubMed] [Google Scholar]
- Silva-Candal Ad, Vieites-Prado A, Gutierrez-Fernandez M, Rey RI, Argibay B, Mirelman D, Sobrino T, Rodriguez-Frutos B, Castillo J, Campos F.. 2015. Blood glutamate grabbing does not reduce the hematoma in an intracerebral hemorrhage model but it is a safe excitotoxic treatment modality. J Cereb Blood Flow Metab. 35:1206–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spires-Jones TL, Hyman BT.. 2014. The intersection of amyloid beta and tau at synapses in Alzheimer's disease. Neuron. 82:756–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Jin S, Wang J, Zhang G, Kawanokuchi J, Kuno R, Sonobe Y, Mizuno T, Suzumura A.. 2006. Tumor necrosis factor-alpha induces neurotoxicity via glutamate release from hemichannels of activated microglia in an autocrine manner. J Biol Chem. 281:21362–21368. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Kong Q, Lin Y, Stouffer N, Schulte DA, Lai L, Liu Q, Chang LC, Dominguez S, Xing X, et al. 2015. Restored glial glutamate transporter EAAT2 function as a potential therapeutic approach for Alzheimer's disease. J Exp Med. 212:319–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talantova M, Sanz-Blasco S, Zhang X, Xia P, Akhtar MW, Okamoto S, Dziewczapolski G, Nakamura T, Cao G, Pratt AE, et al. 2013. Abeta induces astrocytic glutamate release, extrasynaptic NMDA receptor activation, and synaptic loss. Proc Natl Acad Sci USA. 110:E2518–E2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teich AF, Sakurai M, Patel M, Holman C, Saeed F, Fiorito J, Arancio O.. 2016. PDE5 exists in human neurons and is a viable therapeutic target for neurologic disease. J Alzheimers Dis. 52:295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teichberg VI, Cohen-Kashi-Malina K, Cooper I, Zlotnik A.. 2009. Homeostasis of glutamate in brain fluids: an accelerated brain-to-blood efflux of excess glutamate is produced by blood glutamate scavenging and offers protection from neuropathologies. Neuroscience. 158:301–308. [DOI] [PubMed] [Google Scholar]
- Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R.. 1991. Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 30:572–580. [DOI] [PubMed] [Google Scholar]
- Ulrich D. 2015. Amyloid-beta impairs synaptic inhibition via GABA(A) receptor endocytosis. J Neurosci. 35:9205–9210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga E, Juhasz G, Bozso Z, Penke B, Fulop L, Szegedi V.. 2015. Amyloid-beta1-42 disrupts synaptic plasticity by altering glutamate recycling at the synapse. J Alzheimers Dis. 45:449–456. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Viviani B.. 2015. Neuromodulatory properties of inflammatory cytokines and their impact on neuronal excitability. Neuropharmacology. 96:70–82. [DOI] [PubMed] [Google Scholar]
- Wang Q, Wu J, Rowan MJ, Anwyl R.. 2005. β-amyloid inhibition of long-term potentiation is mediated via tumor necrosis factor. Eur J Neurosci. 22:2827–2832. [DOI] [PubMed] [Google Scholar]
- Welzel AT, Maggio JE, Shankar GM, Walker DE, Ostaszewski BL, Li S, Klyubin I, Rowan MJ, Seubert P, Walsh DM, et al. 2014. Secreted amyloid beta-proteins in a cell culture model include N-terminally extended peptides that impair synaptic plasticity. Biochemistry. 53:3908–3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willem M, Tahirovic S, Busche MA, Ovsepian SV, Chafai M, Kootar S, Hornburg D, Evans LD, Moore S, Daria A, et al. 2015. eta-Secretase processing of APP inhibits neuronal activity in the hippocampus. Nature. 526:443–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woltjer RL, Duerson K, Fullmer JM, Mookherjee P, Ryan AM, Montine TJ, Kaye JA, Quinn JF, Silbert L, Erten-Lyons D, et al. 2010. Aberrant detergent-insoluble excitatory amino acid transporter 2 accumulates in Alzheimer disease. J Neuropathol Exp Neurol. 69:667–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhumadilov A, Boyko M, Gruenbaum SE, Brotfain E, Bilotta F, Zlotnik A.. 2015. Extracorporeal methods of blood glutamate scavenging: a novel therapeutic modality. Expert Rev Neurother. 15:501–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotnik A, Gurevich B, Tkachov S, Maoz I, Shapira Y, Teichberg VI.. 2007. Brain neuroprotection by scavenging blood glutamate. Exp Neurol. 203:213–220. [DOI] [PubMed] [Google Scholar]
- Zlotnik A, Tsesis S, Gruenbaum BF, Ohayon S, Gruenbaum SE, Boyko M, Sheiner E, Brotfain E, Shapira Y, Teichberg VI.. 2012. Relationship between glutamate, GOT and GPT levels in maternal and fetal blood: a potential mechanism for fetal neuroprotection. Early Hum Dev. 88:773–778. [DOI] [PubMed] [Google Scholar]
- Zou JY, Crews FT.. 2005. TNF alpha potentiates glutamate neurotoxicity by inhibiting glutamate uptake in organotypic brain slice cultures: neuroprotection by NF kappa B inhibition. Brain Res. 1034:11–24. [DOI] [PubMed] [Google Scholar]
- Zumkehr J, Rodriguez-Ortiz CJ, Cheng D, Kieu Z, Wai T, Hawkins C, Kilian J, Lim SL, Medeiros R, Kitazawa M.. 2015. Ceftriaxone ameliorates tau pathology and cognitive decline via restoration of glial glutamate transporter in a mouse model of Alzheimer's disease. Neurobiol Aging. 36:2260–2271. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.