Abstract
Atropisomerism is a dynamic type of axial chirality that is ubiquitous in medicinal chemistry. There are several examples of stable atropisomeric US FDA-approved drugs and experimental compounds, and in each case the atropisomers of these compounds possess drastically different biological activities. Rapidly interconverting atropisomerism is even more prevalent, and while such compounds are typically considered achiral, they bind their protein targets in an atroposelective fashion, with the nonrelevant atropisomer contributing little to the desired activities. It has been recently demonstrated that various properties of an interconverting atropisomer can be modulated through the synthesis of atropisomer stable and pure analogs. Herein we discuss examples of atropisomerism in drug discovery as well as challenges and opportunities moving forward.
Keywords: : atropisomer, chirality, drug discovery, kinase inhibitor, target selectivity
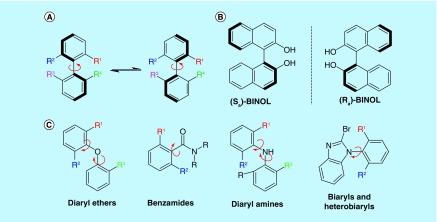
The implications of chirality on the biological activity of a drug have long been appreciated, in part due to examples such as thalidomide where severe complications were caused by one enantiomer of a racemic or rapidly racemizing mixture. Atropisomerism is a type of chirality that arises from differential substitution about a bond, typically between two sp2-hybridized atoms. Atropisomerism arises in many common scaffolds in drug discovery including biaryls, diaryl ethers, diaryl amines, benzamides and anilides (Figure 1). Atropisomerism differs from other instances of chirality in that racemization can occur spontaneously via bond rotation, rather than the process of bond breaking and bond making needed for the racemization of other instances of chirality. Atropisomeric small molecules can thus span the gamut of stereochemical stability with examples that are exceedingly stable when there is a large degree of hindrance to rotation about the chiral axis, as with BINOL (1,1′-Bi-2-naphthol, Figure 1B) which has a half-life of racemization at room temperature (t 1/2(rt)) of approximately 2 million years. Conversely, when there is little hindrance to rotation about the chiral axis, atropisomers can racemize freely, as is the case with myriad pharmaceuticals and chemical probes. Indeed, a cursory analysis of approximately 1900 small molecule drugs in the US FDA Drug Bank reveals that approximately 15% of FDA-approved small molecules contain at least one atropisomeric axis, with the vast majority existing as rapidly interconverting atropisomers [1]. Furthermore, an additional approximately 10% of structures are ‘proatropisomeric’, meaning simple modifications about the axis can break the symmetry and render these drugs atropisomeric. The prevalence of atropisomerism in drug discovery has been increasing over the last decade. Indeed, if you bias the analysis to FDA-approved small-molecule drugs since 2011, approximately 30% of small-molecule drugs contain an axis of chirality, with another 16% being proatropisomeric. Perhaps unsurprisingly, certain classes of inhibitors are made up almost exclusively of scaffolds that display atropisomerism, for example, approximately 80% (24/30 at the time of this writing) of FDA-approved kinase inhibitors possess at least one rapidly interconverting axis of atropisomerism [2].
Figure 1. . Atropisomerism is a type of chirality that is potentially present in many common scaffolds in drug discovery.
Atropisomers can exist as either stable isolable enantiomers or rapidly interconverting racemizing mixtures.
The current ‘industry standard’ approach is to avoid stable atropisomerism when possible, and to treat rapidly interconverting atropisomers as achiral [3,4]. Despite this, rapidly interconverting atropisomers typically interact with their target protein in an atropisomer selective fashion, and it has become increasingly appreciated that each possible atropisomeric conformation of a molecule can possess different drug properties and target profiles [5,6]. In this perspective, we discuss the current state of atropisomerism in drug discovery as well as challenges and opportunities moving forward. We place particular effort to convince medicinal chemists that rapidly interconverting atropisomers should not be readily dismissed as achiral and also to emphasize that in many cases the synthesis of atropisomerically stable analogs can improve different properties of a lead compound and is a worthwhile endeavor to include in structural optimization campaigns.
Classification of atropisomer stereochemical stabilities
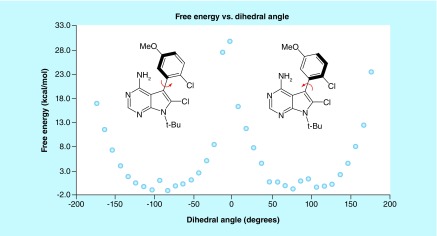
The stereochemical stability of an atropisomeric lead compound is of utmost importance when considering how to further develop it. Figure 2 displays a computational energy map that describes the changes in energy as a chiral axis is rotated [7]. Atropisomers will have two different planar conformations that represent local energy maximums, with the planar conformation that possesses the lower ΔG‡ defining the stereochemical stability. LaPlante et al. have computationally surveyed several industrial screening decks for potentially atropisomeric compounds, leading them to classify atropisomers into three categories based on the amount of energy needed for the chiral axis to racemize via rotation [8,9]. Class 1 atropisomers possess barriers to rotation around the chiral axis of <84 kJ/mol (20 kcal/mol) and racemize on the minute or faster time scale at room temperature; class 2 atropisomers possess a barrier to rotation between 84 and 117 kJ/mol (20–28 kcal/mol) and racemize on the hour to month time scale at room temperature; and class 3 atropisomers possess a barrier to rotation >117 kJ/mol (28 kcal/mol) and racemize on the year or greater timescale at room temperature. Stereochemically stable class 3 atropisomers (which possess a racemization t 1/2[37°C] >6 months) have found their way into the everyday vernacular of chemistry with ligands such as BINAP being widely viewed as privileged chiral scaffolds for catalysis, and natural products such as vancomycin representing important and indispensable therapeutics. As class 3 atropisomers are stable for prolonged periods at physiological conditions they are typically treated in a manner similar to point chiral molecules. Conversely, stereochemically unstable class 1 atropisomers are perhaps the most common manifestation of atropisomerism in medicinal chemistry; however, the ‘latent’ chirality of such molecules is often disregarded and these compounds are typically treated as ‘achiral’. Class 2 atropisomers, represent a conundrum in drug discovery as they racemize on an intermediate time scale that can cause complications when characterizing the activities of each atropisomer in early stage development. In fact, a seminal review has characterized class 2 atropisomerism as ‘a lurking menace’ and suggests avoiding intermediate atropisomerism when at all possible [4].
Figure 2. . Barrier to rotation energy diagram.
M06–2X/6–31+G(d)//RB3LYP/6–31G(d); RB3LYP/6–31G(d) thermal corrections.
Stable atropisomers (class 3) in drug discovery
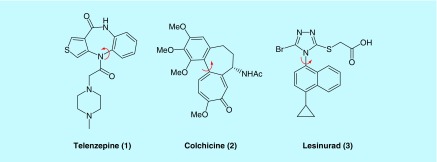
There is a growing body of literature illustrating the impacts of axial chirality on the activities of a compound. A key point that has become apparent from this work is that atropisomers often have markedly divergent activities. For example, it has become clear that in many cases the majority of activity (toward a desired target) belongs to one atropisomer, while the other atropisomer contributes very little [5]. These differences are embodied by three notable small molecule atropisomeric FDA-approved drugs: (1) telenzepine, (2) colchicine, (3) and lesinurad (Figure 3). The most discussed example of an atropisomeric FDA-approved drug in the literature is perhaps the antimuscarinic telenzepine which possesses a t(1/2) to racemization at 20°C of approximately 1000 years, and a 500-fold difference in potency between atropisomers [4,10]. (Ra,7S)-colchicine, which inhibits microtubule polymerization, is an example of an FDA-approved atropisomeric natural product whose enantiomer is notably (∼40-fold) less cytotoxic. Colchicine is also interesting from a fundamental perspective as the atropisomeric axis is constrained in a medium-sized 7-membered ring, leading to a significantly lower than expected barrier to rotation due to a phenomena related to Bringmann's ‘lactone effect’ [11,12]. Despite this, colchicine behaves as a class 3 atropisomer due to an instance of point chirality (the acetamide at the seventh position) that significantly stabilizes one diastereomer over the other, effectively rendering (Ra,7S)-colchicine indefinitely atropisomerically stable [13]. It should be noted that removal of the 7-acetamido group results in an atropisomer with a barrier to rotation of approximately 92 kJ/mol (∼22 kcal/mol) that racemizes on the minute to hour time scale [14]. Finally, lesinurad, a hURAT1 inhibitor approved for the treatment of gout was discovered to exist as a mixture of stable atropisomers after initial approval, with the (Sa)-atropisomer proving to be a 3× more potent hURAT1 inhibitor [15]. It is also worth noting that the atropisomers displayed markedly different pharmacokinetic profiles, underscoring another factor that should be taken into account when dealing with an atropisomer drug candidate.
Figure 3. . US FDA-approved small-molecule drugs that are atropisomerically stable.
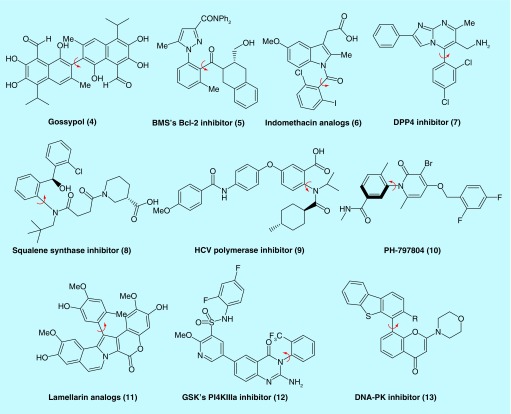
There have also been many recent examples in the medicinal chemistry literature of experimental atropisomeric compounds (Figure 4). The natural product gossypol (4) and its derivatives have been studied extensively as Bcl-2 inhibitors with the (Ra)-atropisomer possessing the bulk of the desired anticancer activities [16,17]. The divergent activities of gossypol's atropisomers were also highlighted in a study where gossypol was studied as a male contraceptive in which the (Ra)-atropisomer sterilized three of five hamsters while the (Sa)-atropisomer displayed no such activity [18]. It is worth noting that gossypol is an example of an atropisomeric natural product that is produced in nature with a moderate degree of enantiopurity that varies from source to source [19]. Another example of an atropisomerically stable Bcl-2 inhibitor (5) was reported by a team at Bristol-Myers Squibb (BMS) in 2009 [20,21]. In these studies, a series of atropisomeric Bcl-2 inhibitors with varying barriers to racemization were synthesized and evaluated. As with colchicine, the atropisomeric axis of the lead BMS compound was not stereochemically stable on its own; however, a point chiral center in the molecule stabilizes the Bcl-2 active atropisomer relative to other diastereomers, resulting in an equilibration at room temperature to a mixture that is primarily the active atropisomer. Nonetheless, the BMS team was able to separate and evaluate each atropisomer (as well as amide rotamers of each atropisomer) and found that the (Ra)-atropisomer inhibited Bcl-2 up to 30-fold more than the (Sa)-atropisomer. The observed activity for the (Sa)-atropisomer was attributed to interconversion to the (Ra)-atropisomer over the course of the experiment.
Figure 4. . Atropisomeric stable compounds from the medicinal chemical literature.
Another seminal demonstration of differential activities among atropisomers was published in 2011 by Takahashi and Natsugari wherein they synthesized atropisomeric analogs (6) of the COX1/2 inhibitor indomethacin finding only the (Ra)-atropisomer possessed any observable COX1 inhibition [22]. While these derivatives were an order of magnitude less potent than indomethacin itself, they displayed no observable activity toward COX2. Another example was disclosed by a BMS team in the context of a suite of serine dipeptidyl peptidase IV (DPP4) inhibitors containing a biaryl axis (7) [23]. During the initial structural optimization they found DPP4 exhibited preferences for ortho substitution on an aryl ring, resulting in their top compounds existing as stable atropisomers. After separation, the (Sa)-atropisomers of the two leading compounds were 74- and 27-fold more active toward DPP4 than the (Ra)-atropisomer. Ichikawa happened upon atropisomerism in a different fashion, as his group's potent squalene synthase inhibitor (8), unintentionally, existed as an interconverting mixture of atropisomers [24]. X-ray co-crystallography displayed only the (S, Ra)-diastereomer bound. Evaluation of subsequent atropisomerically stable analogs in vitro confirmed the x-ray crystallography data, showing squalene synthase inhibitor's preference for the (Ra)-atropisomer was up to 130-fold greater than (Sa)-atropisomer depending on the substrate. In a similar scenario as Ichikawa, LaPlante et al. designed a hepatitis C viral (HCV) polymerase inhibitor (9) with a racemization half-life of 69 min (class 2 atropisomer) [25]. They found that while the (R a) atropisomer inhibited HCV NS5B polymerase the (S a) atropisomer inhibited an HIV matrix protein, representing one of the first precedence that a nonrelevant atropisomer can lead to undesired off-target effects.
Finally, as mentioned earlier, kinase inhibitors are heavily biased toward atropisomeric scaffolds. As such, there have been several instances of atropisomerically stable compounds in the field of kinase inhibition. For example, a team at Pfizer found a lead p38 inhibitor to be a class 3 atropisomer. Upon separating the atropisomers they discovered the (Sa)-atropisomer (PH-797804) (10) to be 100-fold more potent toward p38 than the (Ra)-atropisomer. Yoshida has synthesized analogs of the kinase inhibiting natural product lamellarin (11), and serendipitously found them to be atropisomerically stable, with one atropisomer being consistently less potent, but arguably more selective, across a panel of eight kinases [26]. Yoshida's work represents another early example that suggests atropisomerically pure compounds may possess improved target selectivities. A team at GlaxoSmithKline (GSK) has recently disclosed an inhibitor of type III phosphatidylinositol 4-kinase α (PI4KIIα) (12) as a potential HCV therapeutic [27]. After extensive optimization they arrived to an atropisomerically stable lead molecule of which they found the (Sa)-atropisomer to be one order of magnitude more potent toward PI4KIIIα. Finally, Cano and colleagues saw similar potency trends after restricting rotation about an axis of a known DNA-PK inhibitor (13) through addition of alkyl groups adjacent to a biaryl axis [28]. For each analog, the (-)-atropisomers were 80–100-fold more potent than the (+)-atropisomers. Put together, these examples suggest that leveraging atropisomer conformation via the synthesis of atropisomerically pure compounds can lead to more potent compounds (compared with racemic mixtures), by ensuring that the compound is preorganized into the desired active conformation for a particular target.
Interconverting atropisomers (class 1) & ‘proatropisomeric’ compounds in drug discovery
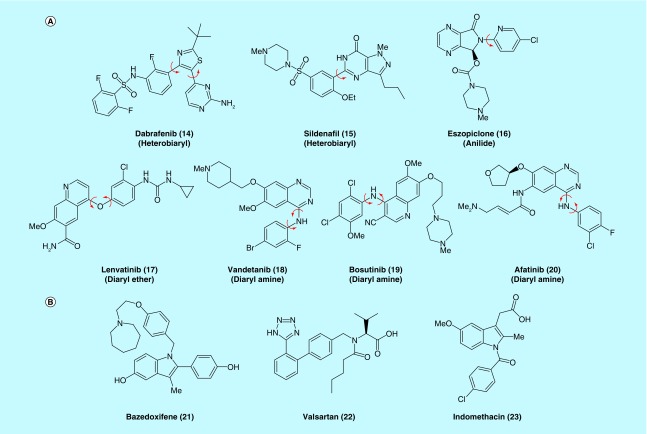
Class 1 atropisomers have become increasingly prevalent in drug discovery (Figure 5). As mentioned in the introduction, approximately 30% of FDA-approved small-molecule drugs since 2011 possess at least one interconverting axis of chirality. As early stage medicinal chemistry is currently biased toward reaction classes (i.e., cross-coupling, amide formation, SNAr) that often yield atropisomeric molecules, pharmaceutical compound collections are becoming increasingly inundated with rapidly interconverting atropisomerism, thus one may expect that the prevalence of atropisomers in drug discovery will only continue to increase over the foreseeable future [29,30].
Figure 5. . Racemizing atropisomeric and proatropisomeric FDA-approved drugs.
(A) US FDA-approved class one atropisomers. (B) FDA-approved proatropisomeric compounds.
Examples of marketed drugs that exist as rapidly interconverting atropisomers include the heterobiaryls dabrafenib (14) and sildenafil (15) and the anilide eszopiclone (16). While these drugs are treated as achiral, examination of co-crystal structures reveals that they bind their respective targets in an atroposelective manner. For example, both atropisomeric axes of dabrafenib are found to interact with its primary target BRAF in the (Sa)-atropisomeric conformation [31]; sildenafil is found to bind phosphodiesterase 5 (PDE5) in the (Sa)-atropisomeric conformation [32]; and eszopiclone is found to interact with the GABA Type A receptor in the (Ra)-atropisomer conformation [33]. Furthermore, an additional 15% of FDA-approved small molecules such as the selective estrogen receptor modulator bazedoxifene (21), the type 1 angiotensin receptor inhibitor valsartan (22), and the COX inhibitor indomethacin (23) are ‘proatropisomeric’ (prochiral), wherein simple modification about the axis (perhaps during a medical chemistry campaign) can break symmetry and render them atropisomeric.
While a large number of rapidly interconverting atropisomers are scaffolds such as biaryls or heterobiaryls that are readily recognized as potentially atropisomeric, there are also numerous instances of scaffolds whose atropisomerism is often overlooked. For example, diaryl ethers are often not considered to be atropisomeric as they possess lower stereochemical stabilities than biaryls; however, many diaryl ethers in the literature are known to have barriers to racemization >117 kJ/mol (28 kcal/mol) [34–37]. Diaryl ethers are also perhaps more complex than more traditional atropisomer classes as they innately have two potential axes of chirality. Lenvatinib (17), is an example of a rapidly interconverting diaryl ether-based kinase inhibitor that is a near pan inhibitor of the VEGFR, EGFR, FGFR, PDGFR and RET families of kinases and also possesses low nM activities toward certain members of the ABL, SRC and Ephrin families [38]. A recent crystal structure of lenvatinib bound to VEGFR2 with both axes of the diaryl ether in the (Sa)-atropisomeric conformation has been recently published, offering evidence that diaryl ethers do bind their target in an atropisomer-specific fashion [39].
Diaryl amines are another nontraditional atropisomeric scaffold that are prevalent throughout drug discovery, particularly among kinase inhibitors, with the FDA-approved vandetanib (18), bosutinib (19) and afatinib (20) representing examples that exist as rapidly interconverting atropisomers. The atropisomerism of diaryl amines has been largely ignored save some seminal work by Kawabata in which his group resolved the atropisomers of a series of diaryl amines and found several to be atropisomeically stable [40,41]. Beyond this, relatively little is known about the factors that render a diaryl amine atropisomerically stable. Nonetheless, co-crystal structures of vandetanib (bound to RET) [42], bosutinib (bound to ABL) [43] and afatinib (bound to EGFR) [44] have each been recently published, with each compound bound to their respective targets in the (Sa, Sa)-atropisomer conformation. Because of these precedences for the atroposelective binding of diaryl amines to their targets, this latent chirality should not be overlooked.
Stable atropisomerism as a design element to increase target selectivity
Rapidly interconverting atropisomers are typically treated as achiral compounds; however, in reality at any given moment these molecules exist as racemic mixture of interconverting enantiomers, not too dissimilar from how the infamous point-chiral drug thalidomide exists [45]. Furthermore, as demonstrated in the previous section, such compounds will likely interact with their respective biological targets in an atropisomer-specific manner, with the nonrelevant atropisomer contributing little to the desired activities [5]. The documented differential biology among atropisomers can result in the nonrelevant atropisomer possessing undesired off-target activities that can lead to side effects in patients or muddle experimental results. Inspired by this, we recently hypothesized that atropisomerism may be leveraged as a design element to increase the target selectivity of promiscuous small molecules that possess a rapidly interconverting axis of atropisomerism [7]. In other words, atropisomerically pure and stable analogs of known promiscuous rapidly interconverting atropisomers would possess increased target selectivities via removal of any off-target activities associated with the nonrelevant atropisomer.
While one may argue that the equilibrium between interconverting atropisomers may be biased by one protein target effecting an ‘in situ dynamic kinetic resolution’ therefore removing the nonrelevant atropisomer, this can be offset if a second target prefers the other atropisomer and biases the equilibrium in the opposite direction. In a biological system the compound will be exposed to perhaps hundreds of proteins that possess affinity for either atropisomer, thus it is likely there will be significant amounts of both atropisomers, and thus effects from the activities of both atropisomers would be expected. When the interconverting atropisomer is rigidified no equilibrium is at play and the situation is perhaps simplified.
It is appreciated that target selectivity is an important factor in drug development. This has proven one of the most difficult issues in the development of small molecule kinase inhibitors [46–48]. Aberrant kinase activity is involved in many different diseases, focusing research efforts toward the development of small molecule kinase inhibitors, resulting in an expanding number of FDA-approved kinase inhibitors-based therapeutics [49,50]. A large degree of active site conservation throughout the kinome has led to most kinase inhibitors to possess promiscuous activities toward many kinases [47,51]. This polypharmacology can lead to unwanted, often severe, side effects thus largely hindering efforts toward kinase inhibitor-based therapeutics [52–54]. Kinase inhibitors are also commonly used as chemical probes to study the underpinnings of cellular processes and diseases; however, these studies are often convoluted due to a lack of kinase inhibitor selectivity [46,47,55]. In recent years, chemists have designed ‘selectivity filters’ that take advantage of uncommon features of a kinase to modulate the selectivity of kinase inhibitors [56–60]. While these approaches have led to selective kinase inhibitors, and FDA-approved therapeutics, a general selectivity filter has remained unobtainable [57,61].
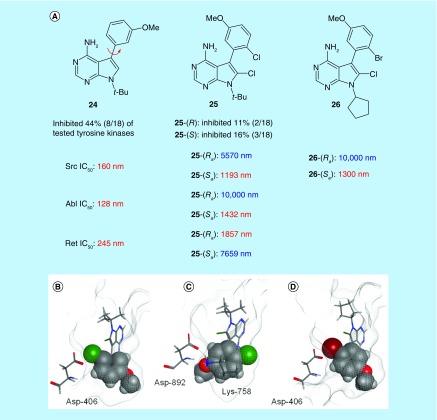
As selectivity is an unsolved problem for kinase inhibitors, and atropisomerism is ubiquitous in kinase inhibitors, this field was an ideal arena to study the effects of atropisomerism on target selectivity. We initially chose to study the pyrrolopyrimidine (PPY) scaffold, a common class of multikinase inhibitors that are related to the better-known pyrazolopyrimidine ligands (i.e., PP1) [62]. We synthesized and separated atropisomerically stable PPY analogs (25 in Figure 6) via HPLC on a chiral stationary phase. After assigning each compound's stereochemical conformation through small molecule x-ray crystallography and circular dichroism, we subjected each atropisomer, and a rapidly interconverting control (24) to a panel of 18 kinases. The atropisomeric analogs displayed increased selectivity compared with 24 across the panel with each atropisomer inhibiting different kinases, albeit at the expense of potency.
Figure 6. . Demonstration that control of atropisomer conformation can modulate the selectivity of a promiscuous small molecule.
These data are exemplified when looking at the IC50 data of these compounds against a series of kinases (SRC, RET and ABL [Figure 6A]). As with the profiling data, the atropisomeric analogs possessed enhanced selectivities compared with 24. For example, 25-(Ra) inhibited RET kinase, but possessed reduced potency toward ABL and SRC. Conversely the 25-(Sa) configuration was less potent toward RET, while maintaining activity toward SRC and ABL. These data represented one of the first intentional demonstrations that different members of highly conserved families of enzymes such as kinases can prefer different atropisomer conformations of the same inhibitor, and that this can be harnessed to modulate inhibitor promiscuity.
To obtain a better understanding of the observed effects we turned to molecular modeling. We examined each docked atropisomer of 25 in RET and SRC. In SRC, the 25-(Sa) atropisomer is predicted to fit in the binding site in a manner consistent with CGP77675 (a PPY-like compound, Protein Data Bank 1YOL) making the same hydrogen bonds with the hinge region and having similar dihedral angles about the chiral axis (Figure 6B). The docked poses suggest that SRC's preference for 25-(Sa) may be caused by steric clashes between the methoxy group on 25-(Ra) with a conserved Lys. In RET, the active atropisomer 25-(Ra) has a binding mode similar to that of PP1 (a structurally similar ligand, PDB 2IVV) making the same hydrogen bonds with the hinge region and having similar dihedral angles between the two aryl ring systems (Figure 6C). The majority of RET's preference for 25-(Ra) appears to be caused by destabilizing steric interactions between the aryl chloride in 25-(Sa) and Asp 892 that causes the docked pose to shift up in the active site (compared with 25-(Ra)), straining the hydrogen bonds between the pyrrolopyrimidine ring and the neighboring Glu 805 and Ala 807 residues. Furthermore, 25-(Ra) is predicted to possess stabilizing interactions between the PPY-Cl and Asp 892 that is not present with 25-(Sa) (Figure 6C). These experiments illustrate that the existence of an inherent atropisomer preference can be predicted in silico, thus representing a tool that allows for rapid assessment of the prospect of using atropisomerism as a selectivity filter to a target kinase a priori.
Conclusion
Over the past decade atropisomerism has become increasingly prevalent in drug discovery. While chemists recognize stable atropisomerism as a form of chirality, we often overlook interconverting atropisomerism as chiral. While such molecules may not rotate light, they have the potential to interact with proteins in a chiral manner, something that had been only recently appreciated in the field. We feel that the presented data above, coupled with many of the discussed examplesillustrate, fundamentally, that in many cases control of atropisomeric conformation can be used as a strategy to modulate different drug properties including the selectivity profile of small molecules. As rapidly interconverting atropisomerism is ubiquitous in drug discovery, controlling it has the potential to represent a general and useful tool to add to the medicinal chemistry tool book.
Future perspective
The conformational preorganization of small molecules is typically achieved via macrocyclization, a classic and indispensable strategy in drug discovery that is applicable to any flexible scaffold [63–65]. While there have been numerous success stories, macrocyclization-based strategies often require significantly more complex syntheses and often result in unintended structural consequences that can lead to a loss of all desired activities. Employing atropisomerism (when possible), on the other hand, represents a more straightforward process where small substitutions adjacent to an axis are replaced by larger ones (i.e., an H replaced with a Cl). This will result in syntheses that are comparable in complexity to that of the parent molecule and a lessened risk of undesired structural perturbations. Furthermore, as a cursory analysis of Figure 2 demonstrates, atropisomerically stable compounds typically retain approximately 80 degrees conformational flexibility (low-energy conformations) about the chiral axis, perhaps lessening the risk of completely ablating the activity toward a target. While this might lessen risk, it also leads to a minimal reduction of any entropic penalty of binding compared with macrocyclization, especially coupled with the fact that only one bond (the chiral axis) is being rigidified. It is enticing to draw a relation between leveraging atropisomerism and the ‘magic methyl’ effect, as in many cases the magic methyl effect has been found to be caused by conformational effects that bias against planar and near planar conformations, while still maintaining access to both possible atropisomeric conformations [66,67]. Atropisomerically stable compounds take this a step further by completely precluding the other atropisomer and further biasing against more planar conformations. Finally, rigidifying an interconverting atropisomeric axis requires fairly minimal structural modifications compared to macrocyclization which should allow for further modifications to optimize other drug properties as needed while still staying within the framework of ‘drug likeness’. This has the potential to aid in the development of new selective small molecules with appropriate (and tunable) properties for drug development. More broadly, as atropisomerism is becoming more prevalent throughout drug discovery there are likely to be increasing opportunities to exploit atropisomer conformation to modulate the potency, selectivity and perhaps even pharmacokinetics of a lead molecules across many classes of targets. While the outlook is certainly bright there are several challenges that need to be overcome when dealing with atropisomerism in drug development. We will discuss these challenges in the upcoming paragraphs.
First and foremost, when designing an atropisomerically stable analog one must take appropriate precautions to ensure that the analog is sufficiently stereochemically stable as to avoid racemization (atropisomer interconversion) under physiological conditions. Some recent guidelines set forth by LaPlante state that a candidate should have a high enough barrier to rotation such that it racemizes <0.5% after 24 h in vivo [8]. Avoiding any racemization caused by metabolism, this would suggest compounds with barriers higher than 114.2 kJ/mol (27.3 kcal/mol) are needed. Even with an adequately high barrier to racemization, further experimental validation that includes detailed studies of the enantiopurity of the molecule in vivo will be required. Furthermore, computational tools that allow for the prediction of barriers to rotation (before synthesis) will also be quite useful. Such tools exist and have been pioneered by LaPlante and coworkers [3]. These tools will especially be useful when dealing with atropisomeric scaffolds that are less well studied such as diaryl ethers [35] and diaryl amines [40,41]. In addition, as more systems are experimentally studied there will be an increased number of datasets of barrier to rotations across sets of pharmaceutically relevant scaffolds which will result in an improved institutional knowledge on what will sufficiently rigidify a specific atropisomeric scaffold. Based on what is known thus far it is expected that halogen, alkyl, amino and thiol substitutions will be most broadly useful as conformational locking substitutions, whereas sp2 (aryl, carbonyl), hydroxyl and fluoro substitutions will likely only be useful in certain systems that already possess a fairly large barrier [68]. Finally, as demonstrated with the natural product colchicine, the addition of a point chiral center also represents a potential strategy to ‘rigidfy’ a rapidly interconverting axis by ‘locking’ the axis in the thermodynamically most stable diastereomer. While enticing, such a strategy will likely require a great deal of serendipity to generate the sought after diastereomer. It should also be noted that the diastereomeric ratio can very well be condition dependent and can change over time, representing an added layer of complexity that must be addressed.
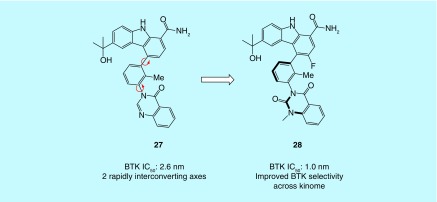
One drawback of our previous work was the loss of potency observed for atropisomeric PPY analogs. While incorporating ‘conformational locking’ substitutions may lead to decreased potency in some cases, any loss of potency can likely be mitigated by the careful choice of ‘conformational locking’ groups and consideration of other factors, for example, potency may be regained elsewhere in the context of a larger structural optimization effort if necessary. Support for this assertion has recently been disclosed in the literature by a team at BMS in the context of BMS-986142, a reversible BTK inhibitor (27) (Figure 7) [69]. In this work they took a lead compounds that possessed two rapidly interconverting axes of atropisomerism and found notable increases in potency (20-fold between atropisomers for one axis, five fold between atropisomers for the other axis) and selectivity (BTK over JAK) (28). This work also demonstrates that when an atropisomeric axis is locked into an active conformation, several different ‘conformational locking’ substitutions should be evaluated as trends in potency and selectivity can change to some degree.
Figure 7. . Example of a selective and potent stable atropisomeric inhibitor.
While most of the examples discussed in the previous sections were largely serendipitous discoveries, moving forward, it is needed to be demonstrated that the control of atropisomerism can be applied to a lead compound de novo. Thus, another potential challenge is how to determine when rigidifying an axis of atropisomerism is a viable strategy to improve a particular drug property. With regards to target selectivity, in an ideal situation one would obtain co-crystal structures of the lead compound toward the desired target as well as its off-targets, and observe differential atropisomeric binding, this is not feasible in many (most) situations. A more realistic approach might be to computationally dock the lead compound across a directed panel of proteins that includes the target and off-targets, as we described with our work on PPYs (Figure 6). If these studies predict that the lead compound will interact with the panel in an atropisomerically differential fashion, then it would be worthwhile to evaluate some atropisomerically stable analogs. This level of inquiry mirrors that of other selectivity filters such as covalently targeting nonconserved cysteines in a kinase active site [56]. Finally, as with colchicine and the BMS Bcl-2 inhibitor, the addition of a chiral center can often bias a rapidly interconverting atropisomer to the active (or inactive) conformation. If a major (perhaps unexpected) change in potency/selectivity is observed when incorporating a point chiral center in the presence of a rapidly interconverting atropisomer, one should consider this potential biasing as a possible explanation, and perhaps incorporate some stable atropisomers in their next series of compounds.
Accessing atropisomerically stable compounds in an enantiopure fashion is another major challenge facing stable atropisomers in drug development. Most of the examples above, including our work, rely on chromatographic separation to obtain atropisomers. While appropriate for early stage medicinal chemistry, this is inefficient from a material-throughput perspective when only one atropisomer is needed and the nonrelevant atropisomer (half of the material) will go to waste or, theoretically, be subjected to additional cycles of racemization and separation. Either way this becomes a hindrance when handed off to process chemistry, or when extensive structural optimization is needed, requiring the synthesis and separation of many atropisomeric analogs. While advances in large-scale analytical separations and resolutions would certainly improve this situation, the ultimate solution will likely also need to include the development of new atroposelective methodologies (both catalytic and auxiliary based) that can be employed on many of the common atropisomeric scaffolds in drug discovery including heterobiaryls, diaryl ethers and diaryl amines. While there has been seminal examples in the literature over the past decade, the field of atroposelective synthesis is much less studied than that of point chirality, with the majority of atroposelective examples being studied on biaryls [11,70–79]. As controlling atropisomer conformation becomes a more prevalent strategy in drug discovery we expect there to be increasing need and opportunities for the development of innovative new atroposelective methodologies and expect that the field will be up for the challenge.
Executive summary.
Atropisomerism is a type of chirality that can exist as either stable enantiomers or a rapidly interconverting racemate.
There is growing body of work that atropisomers can have vastly different biological profiles.
Rapidly interconverting atropisomerism is ubiquitous throughout drug discovery, however, it is often overlooked.
While rapidly interconverting atropisomers may appear achiral, they will bind their target in an enantioselective fashion with the nonrelevant atropisomer contributing little to the desired activities, while possessing the potential to cause off-target effects.
Over the past few years work from our group as well as others have demonstrated that the selectivity of a promiscuous compound can be improved by rigidifying it into the relevant conformation for the desired target.
As atropisomerism becomes more prevalent, new and improved ways to obtain enantiopure atropisomers will be needed.
Footnotes
Financial & competing interests disclosure
JL Gustafson had an NIH grant funded for the atropisomer medicinal chemistry projects. ST Toenjes was bought out from teaching responsibilities with some of these funds and received salary support as well. The Grant ID is R35GM124637. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest
- 1.Law V, Knox C, Djoumbou Y, et al. DrugBank 4.0: shedding new light on drug metabolism. 2014;42(D1):D1091–D1097. doi: 10.1093/nar/gkt1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu P, Nielsen TE, Clausen MH. FDA-approved small-molecule kinase inhibitors. 2015;36(7):422–439. doi: 10.1016/j.tips.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 3.Laplante SR, Edwards PJ, Fader LD, Jakalian A, Hucke O. Revealing atropisomer axial chirality in drug discovery. 2011;6(3):505–513. doi: 10.1002/cmdc.201000485. [DOI] [PubMed] [Google Scholar]
- 4.Clayden J, Moran WJ, Edwards PJ, LaPlante SR. The challenge of atropisomerism in drug discovery. 2009;48(35):6398–6401. doi: 10.1002/anie.200901719. [DOI] [PubMed] [Google Scholar]; • Seminal review addressing atropisomerism in drug discovery.
- 5.Zask A, Murphy J, Ellestad GA. Biological stereoselectivity of atropisomeric natural products and drugs. 2013;25:265–274. doi: 10.1002/chir.22145. [DOI] [PubMed] [Google Scholar]; • Seminal review on differential activities of atropisomers.
- 6.Glunz PE. Recentencounters with atropisomerism in drug discovery. 2018;28(2):53–60. doi: 10.1016/j.bmcl.2017.11.050. [DOI] [PubMed] [Google Scholar]
- 7.Smith DE, Marquez I, Lokensgard ME, Rheingold AL, Hecht DA, Gustafson JL. Exploiting atropisomerism to increase the target selectivity of kinase inhibitors. 2015;54(40):11754–11759. doi: 10.1002/anie.201506085. [DOI] [PubMed] [Google Scholar]; • Seminal work showing control of atropisomeric conformation can be used to modulate the selectivity profile of kinase inhibitors.
- 8.LaPlante SR, Fader LD, Fandrick KR, et al. Assessing atropisomer axial chirality in drug discovery and development. 2011;54(20):7005–7022. doi: 10.1021/jm200584g. [DOI] [PubMed] [Google Scholar]
- 9.LaPlante SR, Edwards PJ, Fader LD, Jakalian A, Hucke O. Revealing atropisomer axial chirality in drug discovery. 2011;6(3):505–513. doi: 10.1002/cmdc.201000485. [DOI] [PubMed] [Google Scholar]
- 10.Eveleigh P, Hulme EC, Schudt C, Birdsall NJ. The existence of stable enantiomers of telenzepine and their stereoselective interaction with muscarinic receptor subtypes. 1989;35(4):477–483. [PubMed] [Google Scholar]
- 11.Bringmann G, Mortimer AJP, Keller Paul A, Gresser Mary J, Garner J, Matthias B. Atroposelective synthesis of axially chiral biaryl compounds. 2005;44:5384–5427. doi: 10.1002/anie.200462661. [DOI] [PubMed] [Google Scholar]
- 12.Bringmann G, Gulder T, Gulder TAM, Breuning M. Atroposelective total synthesis of axially chiral biaryl natural products. 2011;111(2):563–639. doi: 10.1021/cr100155e. [DOI] [PubMed] [Google Scholar]
- 13.Pietra F. Why colchicine does not show mutarotation. With M05–2X density functional in the realm of tricky natural products. 2007;20:1102–1107. [Google Scholar]
- 14.Berg U, Deinum J, Lincoln P, Kvassman J. Stereochemistry of colchicinoids. Enantiomeric stability and binding to tubulin of desacetamidocolchicine and desacetamidoisocolchicine. 1991;19(1):53–65. [Google Scholar]
- 15.Wang J, Zeng W, Li S, et al. Discovery and assessment of atropisomers of (±)-lesinurad. 2017;8(3):299–303. doi: 10.1021/acsmedchemlett.6b00465. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Discovery that lesinurad exists as a racemic mixture of atropisomers where in the atropisomers possess differential activities.
- 16.Dodou K, Anderson RJ, Lough WJ, Small DAP, Shelley MD, Groundwater PW. Synthesis of gossypol atropisomers and derivatives and evaluation of their anti-proliferative and anti-oxidant activity. 2005;13(13):4228–4237. doi: 10.1016/j.bmc.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 17.Kang MH, Reynolds CP. Bcl-2 inhibitors: targeting mitochondrial apoptotic pathways in cancer therapy. 2009;15(4):1126–1132. doi: 10.1158/1078-0432.CCR-08-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matlin SA, Zhou R. (-)-Gossypol: an active male antifertility agent. 1985;31(2):141–149. doi: 10.1016/0010-7824(85)90027-7. [DOI] [PubMed] [Google Scholar]
- 19.Tipanovic RODS, Uckhaber LOSP, Iu JIL, Ell ALAB. Total and percent atropisomers of gossypol and gossypol-6-methyl ether in seeds from pima cottons and accessions of Gossypium barbadense L. 2009;57(2):566–571. doi: 10.1021/jf802756e. [DOI] [PubMed] [Google Scholar]
- 20.Porter J, Payne A, Whitcombe I, et al. Atropisomeric small molecule Bcl-2 ligands: determination of bioactive conformation. 2009;19(6):1767–1772. doi: 10.1016/j.bmcl.2009.01.071. [DOI] [PubMed] [Google Scholar]
- 21.Porter J, Payne A, de Candole B, et al. Tetrahydroisoquinoline amide substituted phenyl pyrazoles as selective Bcl-2 inhibitors. 2009;19(1):230–233. doi: 10.1016/j.bmcl.2008.10.113. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi H, Wakamatsu S, Tabata H, et al. Atropisomerism observed in indometacin derivatives. 2011;13(4):760–763. doi: 10.1021/ol103008d. [DOI] [PubMed] [Google Scholar]
- 23.Connor SPO, Wang Y, Simpkins LM, et al. Synthesis, SAR, and atropisomerism of imidazolopyrimidine DPP4 inhibitors. 2010;20:6273–6276. doi: 10.1016/j.bmcl.2010.08.090. [DOI] [PubMed] [Google Scholar]
- 24.Ichikawa M, Yokomizo A, Itoh M, et al. Discovery of atrop fixed alkoxy-aminobenzhydrol derivatives: novel, highly potent and orally efficacious squalene synthase inhibitors. 2011;19(17):5207–5224. doi: 10.1016/j.bmc.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 25.LaPlante SR, Forgione P, Boucher C, et al. Enantiomeric atropisomers inhibit HCV polymerase and/or HIV matrix: characterizing hindered bond rotations and target selectivity. 2014;57(5):1944–1951. doi: 10.1021/jm401202a. [DOI] [PubMed] [Google Scholar]; • One of the first examples that a nonrelevant atropisomer can inhibit off-target proteins.
- 26.Yoshida K, Itoyama R, Yamahira M, et al. Synthesis, resolution, and biological evaluation of atropisomeric (a R)-and (a S)-16-methyllamellarins n: unique effects of the axial chirality on the selectivity of protein kinases inhibition. 2013;56(18):7289–7301. doi: 10.1021/jm400719y. [DOI] [PubMed] [Google Scholar]
- 27.Leivers AL, Tallant M, Shotwell JB, et al. Discovery of selective small molecule type III phosphatidylinositol 4-kinase alpha (PI4KIII α) inhibitors as anti hepatitis C (HCV) agents. 2014;57(5):2091–2106. doi: 10.1021/jm400781h. [DOI] [PubMed] [Google Scholar]
- 28.Clapham KM, Rennison T, Jones G, et al. Potent enantioselective inhibition of DNA-dependent protein kinase (DNA-PK) by atropisomeric chromenone derivatives. 2012;10(33):6747–6757. doi: 10.1039/c2ob26035b. [DOI] [PubMed] [Google Scholar]
- 29.Brown DG, Boström J. Analysis of past and present synthetic methodologies on medicinal chemistry: where have all the new reactions gone? 2016;59(10):4443–4458. doi: 10.1021/acs.jmedchem.5b01409. [DOI] [PubMed] [Google Scholar]
- 30.Roughley SD, Jordan AM. The medicinal chemist's toolbox: an analysis of reactions used in the pursuit of drug candidates. 2011;54(10):3451–3479. doi: 10.1021/jm200187y. [DOI] [PubMed] [Google Scholar]
- 31.Foster SA, Whalen DM, Ozen A, et al. Activation mechanism of oncogenic deletion mutations in BRAF, EGFR, and HER2. 2016;29(4):477–493. doi: 10.1016/j.ccell.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 32.Wang H, Liu Y, Huai Q, et al. Multiple conformations of phosphodiesterase-5: implications for enzyme function and drug development. 2006;281(30):21469–21479. doi: 10.1074/jbc.M512527200. [DOI] [PubMed] [Google Scholar]
- 33.Hanson SM, Morlock EV, Satyshur KA, Czajkowski C. Structural requirements for eszopiclone and zolpidem binding to the γ-aminobutyric acid type-A (GABAA) receptor are different. 2008;51(22):7243–7252. doi: 10.1021/jm800889m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bracegirdle A, Clayden J, Lai LW. Asymmetric synthesis of biaryl atropisomers by dynamic resolution on condensation of biaryl aldehydes with (-)-ephedrine or a proline-derived diamine. 2008;4:47. doi: 10.3762/bjoc.4.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Betson MS, Clayden J, Worrall CP, Peace S. Three groups good, four groups bad? Atropisomerism in ortho-substituted diaryl ethers. 2006;45:5803–5807. doi: 10.1002/anie.200601866. [DOI] [PubMed] [Google Scholar]
- 36.Clayden J, Worrall CP, Moran WJ, Helliwell M. Enantioselective synthesis of an atropisomeric diaryl ether. 2008;47(17):3234–3237. doi: 10.1002/anie.200705660. [DOI] [PubMed] [Google Scholar]
- 37.Fuji K, Oka T, Kawabata T, Kinoshita T. The first synthesis of an optically active molecular bevel gear with only two cogs on each wheel. 1998;39(97):1373–1376. [Google Scholar]
- 38.Stjepanovic N, Capdevila J. Multikinase inhibitors in the treatment of thyroid cancer: specific role of lenvatinib. 2014;8:129–139. doi: 10.2147/BTT.S39381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okamoto K, Ikemori-Kawada M, Jestel A, et al. Distinct binding mode of multikinase inhibitor lenvatinib revealed by biochemical characterization. 2015;6(1):89–94. doi: 10.1021/ml500394m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kawabata T, Jiang C, Hayashi K, et al. Axially chiral binaphthyl surrogates with an inner N-H-N hydrogen bond. 2009;131(1):54–55. doi: 10.1021/ja808213r. [DOI] [PubMed] [Google Scholar]
- 41.Hayashi K, Matubayasi N, Jiang C, et al. Insights into the origins of configurational stability of axially chiral biaryl amines with an intramolecular N-H-N hydrogen bond. 2010;75(15):5031–5036. doi: 10.1021/jo100586b. [DOI] [PubMed] [Google Scholar]
- 42.Knowles PP, Murray-Rust J, Kjaer S, et al. Structure and chemical inhibition of the RET tyrosine kinase domain. 2006;281(44):33577–33587. doi: 10.1074/jbc.M605604200. [DOI] [PubMed] [Google Scholar]
- 43.Levinson NM, Boxer SG. Structural and spectroscopic analysis of the kinase inhibitor bosutinib and an isomer of bosutinib binding to the Abl tyrosine kinase domain. 2012;7(4):e29828. doi: 10.1371/journal.pone.0029828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Solca F, Dahl G, Zoephel A, et al. Target binding properties and cellular activity of afatinib (BIBW 2992), an irreversible ErbB family blocker. 2012;343(2):342–350. doi: 10.1124/jpet.112.197756. [DOI] [PubMed] [Google Scholar]
- 45.Jacques V, Czarnik AW, Judge TM, et al. Differentiation of antiinflammatory and antitumorigenic properties of stabilized enantiomers of thalidomide analogs. 2015;112(19):e1471–e1479. doi: 10.1073/pnas.1417832112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arrowsmith CH, Audia JE, Austin C, et al. The promise and peril of chemical probes. 2015;11(8):536–541. doi: 10.1038/nchembio.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bain J, Plater L, Elliott M, et al. The selectivity of protein kinase inhibitors: a further update. 2007;408(3):297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. 2002;298(5600):1912–1934. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- 49.Cohen P, Alessi DR. Kinase drug discovery – what's next in the field? 2012;8(1):96–104. doi: 10.1021/cb300610s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cohen P. Protein kinases – the major drug targets of the twenty-first century? 2002;1(4):309–315. doi: 10.1038/nrd773. [DOI] [PubMed] [Google Scholar]
- 51.Davis MI, Hunt JP, Herrgard S, et al. Comprehensive analysis of kinase inhibitor selectivity. 2011;29(11):1046–1051. doi: 10.1038/nbt.1990. [DOI] [PubMed] [Google Scholar]
- 52.Cornelison M, Jabbour EJ, Welch MA. Managing side effects of tyrosine kinase inhibitor therapy to optimize adherence in patients with chronic myeloid leukemia: the role of the midlevel practitioner. 2012;10(1):14–23. doi: 10.1016/j.suponc.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 53.Mellor HR, Bell AR, Valentin J-P, Roberts RRA. Cardiotoxicity associated with targeting kinase pathways in cancer. 2011;120(1):14–32. doi: 10.1093/toxsci/kfq378. [DOI] [PubMed] [Google Scholar]
- 54.Laufer S, Bajorath J. New frontiers in kinases: second generation inhibitors. 2014;57(6):2167–2168. doi: 10.1021/jm500195x. [DOI] [PubMed] [Google Scholar]
- 55.Murray AJ. Pharmacological PKA inhibition: all may not be what it seems. 2008;1(22):re4. doi: 10.1126/scisignal.122re4. [DOI] [PubMed] [Google Scholar]
- 56.Barf T, Kaptein A. Irreversible protein kinase inhibitors: balancing the benefits and risks. 2012;55(14):6243–6262. doi: 10.1021/jm3003203. [DOI] [PubMed] [Google Scholar]
- 57.Liu Q, Sabnis Y, Zhao Z, et al. Developing irreversible inhibitors of the protein kinase cysteinome. 2013;20(2):146–159. doi: 10.1016/j.chembiol.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cohen MS, Zhang C, Shokat KM, Taunton J. Structural bioinformatics-based design of selective, irreversible kinase inhibitors. 2005;308(5726):1318–1321. doi: 10.1126/science1108367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu Y, Gray NS. Rational design of inhibitors that bind to inactive kinase conformations. 2006;2(7):358–364. doi: 10.1038/nchembio799. [DOI] [PubMed] [Google Scholar]
- 60.Knight ZA, Shokat KM. Chemical genetics: where genetics and pharmacology meet. 2007;128(3):425–430. doi: 10.1016/j.cell.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 61.Singh J, Petter RC, Kluge AF. Targeted covalent drugs of the kinase family. 2010;14(4):475–480. doi: 10.1016/j.cbpa.2010.06.168. [DOI] [PubMed] [Google Scholar]
- 62.Liu Y, Bishop A, Witucki L, et al. Structural basis for selective inhibition of Src family kinases by PP1. 1999;6(9):671–678. doi: 10.1016/s1074-5521(99)80118-5. [DOI] [PubMed] [Google Scholar]
- 63.Driggers EM, Hale SP, Lee J, Terrett NK. The exploration of macrocycles for drug discovery – an underexploited structural class. 2008;7(7):608–624. doi: 10.1038/nrd2590. [DOI] [PubMed] [Google Scholar]
- 64.Cisar JS, Ferreras JA, Soni RK, Quadri LEN, Tan DS. Exploiting ligand conformation in selective inhibition of non-ribosomal peptide synthetase amino acid adenylation with designed macrocyclic small molecules. 2007;129(25):7752–7753. doi: 10.1021/ja0721521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dinsmore CJ, Bogusky MJ, Culberson JC, et al. Conformational restriction of flexible ligands guided by the transferred NOE experiment: potent macrocyclic inhibitors of farnesyltransferase. 2001;123(9):2107–2108. doi: 10.1021/ja003673q. [DOI] [PubMed] [Google Scholar]
- 66.Schönherr H, Cernak T. Profound methyl effects in drug discovery and a call for new C-H methylation reactions. 2013;52(47):12256–12267. doi: 10.1002/anie.201303207. [DOI] [PubMed] [Google Scholar]
- 67.Leung CS, Leung SSF, Tirado-Rives J, Jorgensen WL. Methyl effects on protein–ligand binding. 2012;55(9):4489–4500. doi: 10.1021/jm3003697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bott G, Field LD, Sternhell S. Steric effects. A study of a rationally designed system. 1980;102(18):5618–5626. [Google Scholar]
- 69.Watterson SH, De Lucca GV, Shi Q, et al. Discovery of 6-Fluoro-5-(R)-(3-(S)-(8-fluoro-1-methyl-2,4-dioxo-1,2-dihydroquinazolin-3(4H)-yl)-2-methylphenyl)-2-(S)-(2-hydroxypropan-2-yl)-2,3,4,9-tetrahydro-1H-carbazole-8-carboxamide (BMS-986142): a reversible inhibitor of Bruton's tyrosine kinase (BTK) conformationally constrained by two locked atropisomers. 2016;59(19):9173–9200. doi: 10.1021/acs.jmedchem.6b01088. [DOI] [PubMed] [Google Scholar]; • An example of increasing potency and selectivity by installing correct conformational locking groups for a kinase inhibitor.
- 70.Bhat V, Wang S, Stoltz BM, Virgil SC. Asymmetric synthesis of QUINAP via dynamic kinetic resolution. 2013;135(45):16829–16832. doi: 10.1021/ja409383f. [DOI] [PubMed] [Google Scholar]
- 71.Gustafson JL, Lim D, Miller SJ. Dynamic kinetic resolution of biaryl atropisomers via peptide-catalyzed asymmetric bromination. 2010;328(5983):1251–1255. doi: 10.1126/science.1188403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tanaka K, Takeishi K, Noguchi K. Enantioselective synthesis of axially chiral anilides through rhodium-catalyzed [2 + 2 + 2] cycloaddition of 1,6-diynes with trimethylsilylynamides. 2006;128(14):4586–4587. doi: 10.1021/ja060348f. [DOI] [PubMed] [Google Scholar]
- 73.Chen Y-H, Cheng D-J, Zhang J, Wang Y, Liu X-Y, Tan B. Atroposelective synthesis of axially chiral biaryldiols via organocatalytic arylation of 2-naphthols. 2015;137(48):15062–15065. doi: 10.1021/jacs.5b10152. [DOI] [PubMed] [Google Scholar]
- 74.Fandrick KR, Li W, Zhang Y, et al. Concise and practical asymmetric synthesis of a challenging atropisomeric HIV integrase inhibitor. 2015;54(24):7144–7148. doi: 10.1002/anie.201501575. [DOI] [PubMed] [Google Scholar]
- 75.Diener ME, Metrano AJ, Kusano S, Miller SJ. Enantioselective synthesis of 3-arylquinazolin-4(3H)-ones via peptide-catalyzed atroposelective bromination. 2015;137(38):12369–12377. doi: 10.1021/jacs.5b07726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Barrett KT, Miller SJ. Enantioselective synthesis of atropisomeric benzamides through peptide-catalyzed bromination. 2013;135(8):2963–2966. doi: 10.1021/ja400082x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yuan B, Page A, Worrall CP, et al. Biocatalytic desymmetrization of an atropisomer with both an enantioselective oxidase and ketoreductases. 2010;49(39):7010–7013. doi: 10.1002/anie.201002580. [DOI] [PubMed] [Google Scholar]
- 78.Armstrong RJ, Smith MD. Catalytic enantioselective synthesis of atropisomeric biaryls: a cation-directed nucleophilic aromatic substitution reaction. 2014;53(47):12822–12826. doi: 10.1002/anie.201408205. [DOI] [PubMed] [Google Scholar]
- 79.Kumarasamy E, Raghunathan R, Sibi MP, Sivaguru J. Nonbiaryl and heterobiaryl atropisomers: molecular templates with promise for atropselective chemical transformations. 2015;115(20):11239–11300. doi: 10.1021/acs.chemrev.5b00136. [DOI] [PubMed] [Google Scholar]