Abstract
HER2/neu is expressed in the majority of in situ breast cancers, but maintained in 20–30% of invasive breast cancer (IBC). During breast tumorigenesis, there is a progressive loss of anti-HER2 CD4pos Th1 (anti-HER2Th1) from benign to ductal carcinoma in situ, with almost complete loss in IBC. This anti-HER2Th1 response can predict response to neoadjuvant therapy, risk of recurrence and disease-free survival. Vaccines consisting of HER2-pulsed type I polarized dendritic cells (DC1) administered during ductal carcinoma in situ and early IBC can efficiently correct anti-HER2Th1 response and have clinical impact on the disease. In this review, we will discuss the role of anti-HER2Th1 response in the three phases of immunoediting during HER2 breast cancer development and opportunities for reversing these processes using DC1 vaccines alone or in combination with standard therapies. Correcting the anti-HER2Th1 response may represent an opportunity for improving outcomes and providing a path to eliminate escape variants.
Keywords: : adoptive cell therapy, checkpoint inhibitor, chemotherapy, dendritic cell, immunotherapy, multimodality, radiotherapy, targeted therapy
The rationale for targeting HER2
Although HER2 (ERBB2) is transiently expressed during fetal and normal breast development, as well as during breast growth in pregnancy, overexpression of HER2/neu can contribute to breast tumorigenesis. HER2/neu has become the most studied tumor-associated antigen (TAA), as it is a molecular oncodriver overexpressed in 20–30% of invasive breast cancers (IBC) and in about 13–56% of ductal carcinoma in situ (DCIS) lesions [1].
HER2/neu is an immunogenic protein that elicits both humoral and cellular immune responses in patients with HER2pos tumors, but patients with HER2/neu overexpressing tumors often demonstrate diminished existing immunity directed against the protein [2]. In IBC, HER2/neu is associated with a heightened risk for invasion, suggesting a crucial role for HER2/neu in stimulating a tumorigenic microenvironment. Whether by ‘immunoediting’ or other immune evasion mechanisms, a diminished cellular immune response to HER2/neu in the tumor microenvironment of HER2pos breast cancer (HER2posBC) is associated with poorer prognosis [2]. On the contrary, an increased cellular and humoral response against HER2/neu has been associated with decreased tumor development and improved outcomes [3]. Clinical implications of HER2/neu expression in DCIS (HER2posDCIS) are not as clear, but present data suggest that it predicts the presence of invasive foci [4], and increases risk of disease recurrence, although only approximately 20% of HER2posDCIS recurs as HER2posBC [5]. This low rate of HER2posDCIS conversion to HER2posIBC may be due to pathway and phenotype instability in HER2pos cancer cells, with natural or induced immunity possibly shaping the resulting tumor phenotype [6].
With the exception of antibody-based HER2 targeting, HER2-directed immunotherapy in BC has not been as successful as initially expected, there has only been limited success in the setting of advanced disease where tumor cells have already escaped immunosurveillance. The future of HER2-targeted vaccination trials should be geared toward early-stage HER2posBC, potentially halting progression of HER2posDCIS to IBC (Figure 1) or situations to prevent recurrence. HER2 vaccines must be created based on immunologic principles of circumventing tolerance, a primary mechanism of escape, by strengthening the weak pre-existent anti-HER2 CD4pos Th1 (anti-HER2Th1) immune response [6].
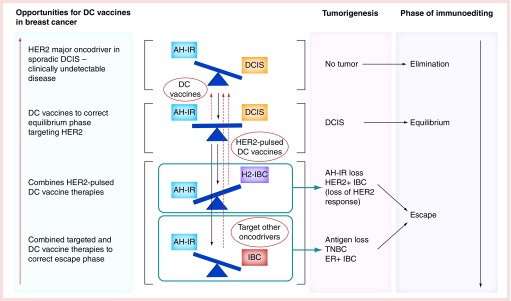
Figure 1. . Immunoediting process in breast cancer with the potential sites of immunotherapy and targeted therapies in HER2-positive, triple-negative and ER-positive breast cancer.
AH-IR: Anti-HER2 immune response; DC: Dendritic cell; DCIS: Ductal carcinoma in situ; ER + BC: Estrogen receptor positive breast cancer; H2IBC: HER2-positive invasive breast cancer; IBC: Invasive breast cancer; IR: Immune response; TNBC: Triple-negative breast cancer.
Loss of anti-HER2 CD4pos Th1 during breast tumorigenesis
Recently, we have identified a progressive loss of anti-HER2Th1 immune response in HER2posBC patients relative to healthy controls, with an early and progressive decrease in immune competence in patients with HER2posBC [7]. Specifically, there is a loss of anti-HER2Th1 response during breast tumorigenesis, where healthy patients have a strong anti-HER2Th1 immune response that is diminished in patients with DCIS and nearly absent in patients with IBC [7]. This supports the hypothesis that the level of circulating anti-HER2Th1 response correlates to immune response in patients with HER2posBC after treatment [7]. It is likely that this immune deficit begins in DCIS and continues to decline with disease progression. This depressed anti-HER2Th1 response is driven predominantly by Th1 phenotypes. In patients who had undergone neoadjuvant therapies, preserved anti-HER2Th1 response was associated with pCR and improved disease-free survival (DFS), while a depressed response was found in patients without pathologic complete response (pCR) and recurrent disease after treatment [8]. These findings may potentially lead to the use of anti-HER2Th1 monitoring for patients receiving HER2-targeted therapies to identify those at risk of clinicopathologic failure.
Role of immune response & immunoediting during breast tumorigenesis
The breast parenchyma normally harbors immune cells such as cytolytic CD8pos T cells, Th1 cells and NK cells that aid in development, lactation, involution and may participate in immunosurveillance of the breast [9]. The presence of high-density CD8pos T cells in tumors and nearby stroma is associated with improved prognosis in BC, suggesting that immune effector cells have identified the malignant cells and are actively mounting an appropriate immune response. Potent immune effectors act by eliminating tumors directly via exocytosis of cytotoxic granules, or indirectly, by producing INF- γ to activate Th1 immune response. It is thought that under immunosurveillance, the host eliminates evolving tumor cells continuously during elimination, until some malignant cells manage to outnumber and stress the immune response, evading immune recognition through a process described as ‘immunoediting’ [9]. Initially malignant clones are effectively eliminated by the immune response but as the tumor grows and expands a state of tumor equilibrium is reached (Figure 1). If this process becomes ineffective or fails, malignant cells may escape from immunological control to proliferate and manifest clinically as invasive and metastatic cancer [10].
The immunoediting phenomenon is evident during breast tumorigenesis mediated by HER2. HER2/neu is present in nonmalignant cells at basal levels, but overexpression leads to malignant transformation. We hypothesize that in the initial stage of malignant transformation, the mounted immune response is robust enough against this HER2/neu overexpression and may eliminate HER2posDCIS from becoming clinically apparent. If elimination is not achieved, HER2/neu overexpressing tumor cells and the immune response develop a state of equilibrium where the anti-HER2Th1 immune response is unable to completely eliminate malignant cells, but is able to prevent them from becoming invasive. This equilibrium state may be clinically manifested as HER2posDCIS presenting with microcalcifications on mammography [11]. These calcifications may be a reflection of necrosis caused by immune effectors cells (anti-HER2Th1 response) attempting to eliminate tumor cells during this period of homeostasis (Figure 1). The anti-HER2Th1 response although diminished remains somewhat active in DCIS [7]. This phase of tumor equilibrium in DCIS may be boosted using DC vaccines, which, by stimulating both CD4pos and CD8pos T cells and IL-12, can maximize INF-γ production and the functionality of Th1 cells thereby tip the equilibrium state toward elimination again (Figure 1).
If the process of equilibrium continues, the tumor may remain stagnant and suspended as DCIS with no disease progression to invasion. The alternative to equilibrium in DCIS would be tumor progression and developing invasion (IBC). If progression occurs, there are likely two scenarios where the immune response would be ineffective at halting disease progression. First, the anti-HER2Th1 immune response further erodes over time [7] and becomes virtually absent allowing tumor escape. This hyporesponsive state may be due to a chronic exhaustion of the immune system leading to increased inhibitory receptors [11], decreased effector cytokines or impaired cytotoxicity, allowing for tumor escape. The result is continued expression of HER2 on the IBC. The second scenario is one where anti-HER2Th1 response is sustained, but tumor cells manage to evade immunosurveillance via phenotypic shifting. In this scenario, the immune system remains responsive, but tumor cells lose or downregulate HER2/neu expression resulting in a new phenotype, such as triple-negative breast cancer (TNBC) or estrogen receptor positive BC (ERposBC) (Figure 1). There are numerous examples of ERpos non-HER2 primary tumors developing systemic metastasis that are HER2pos and also examples of HER2pos primary tumors that lose expression of HER2 [12]. Using our understanding of immunoediting and immune escape, there are numerous opportunities to use DC vaccines to boost anti-HER2 immunity when needed and boost immunity against oncodrivers involved with escape to prevent recurrence.
Activation of type I polarized DC drive strong anticancer Th1 responses
Dendritic cells (DCs) are a heterogeneous group of specialized APCs [13], found in lymphoid and nonlymphoid tissue. They are classified according to their ability to elicit a specific immune response based on polarization of a T-cell response. DCs are typically found in immature form, and maturation is triggered by proinflammatory cytokines such as IL-1, IL-6, IFN-γ and TNF-α [14]. Additional signals such as bacterial lipopolysaccharide and IFN-γ drive IL-12 production leading to pulsed type I polarized DC1 [15]. Once maturation and activation occurs, migration to nearby lymph nodes is facilitated [16], where activated DCs deliver costimulatory signals essential for T-cell activation such as CD40, CD80, CD86, CD46 ligands and Toll-like receptors [17]. DCs signal activation and polarization of T cells is crucial for the differentiation of CD8pos T cells into cytotoxic T lymphocytes (CTLs) [18] and for the polarization of CD4pos T cells into their different effectors (Th1, Th2 and Th17) [19].
During BC development and progression, CD4pos T cells may be a crucial element in the tumor ‘immunoediting’ process. Therefore, there is increasing interest in activating CD4pos T cells because of their role as helpers in maintaining CD8pos cells as functionally active [20] and indirect effects on other innate cells such as NK, macrophages and DCs [21]. CD4pos are significantly increased during breast cancer and the subsets dynamically change with disease progression. In early-tumor stages, Th1 cells are the dominant population of CD4pos T cells, while in the advanced tumor stages, FoxP3pos Treg and Th17 cells become the dominant populations [22]. Th1 cells secrete high-level cytokines such as IFN-γ and TNF-α, both key components of initiating Th1-polarized response and to the antitumor function of DC1 vaccines [19]. DC1s are very efficient in the presentation of antigens and production of IL-12 [23] that polarizes T cells toward the IFN-γ Th1 phenotype [24]. IL12 has multiple roles with inherent antitumor effect, antiangiogenic capabilities, activation of NK cells as well as enhancing adaptive immunity and improving sensitization to tumor antigens [25].
Despite their ability to prime an immune response, results have been disappointing when testing DC vaccines in late-stage BC. One reason may be because, in advanced disease, DCs are unable to mount a strong enough immune response to overcome the overwhelming immunosuppressive tumor microenvironment present in tumors that have escaped immunosurveillance. This may be one reason why, in early stages of BC, DC vaccines have enjoyed some clinical successes [3]. Another potential reason for the ineffectiveness of DC vaccines in late-stage disease is that inflammatory type DC vaccines have been used in most studies that do not necessarily drive strong Th1 response [15]. Increasing understanding of the dynamics of DCs activation, treatment of early-stage BC and different adjuvant settings will allow for improvements that warrant further investigation.
Effectiveness of DC1 vaccines administered in the equilibrium phase of DCIS
We suggest that HER2posDCIS represents the equilibrium phase of immunoediting, where Th1 immune response is in homeostasis with cancer cells in the ducts. Supporting this argument, data suggest that a strong Th1 proinflammatory signature in tumor microenviroment is associated with improved outcomes [13]. Therefore, if we can stimulate a strong Th1 immune response during the equilibrium phase of disease, it may be possible to prevent recurrence of the preinvasive lesions or halt disease progression to IBC.
A group of 27 patients with HER2posDCIS received HER2-pulsed DC1 vaccines in the neoadjuvant setting [2]. These patients all required surgical resection of their HER2posDCIS. In vaccinated subjects pCR was achieved in 18.5% of all patients (ERneg = 40 vs ERpos 5.9%) suggesting shift from equilibrium to elimination. Among those patients without a pCR in about 50% of HER2 expression was eradicated in residual DCIS (sustained HER-2/neu expression ERneg =10% vs ERpos = 47.1% [p = 0.04]). Postvaccination phenotypes were significantly different between ERpos and ERneg subjects (p =0.01). The conversion of HER2posDCIS to HER2neg phenotypes after vaccination demonstrated the potential process of tumor escape in that a single target may not be sufficient to completely treat or eradicate disease. Interestingly, most of the DCIS lesions that changed were ERpos and subsequent trials have demonstrated this group can have similar complete response rate by combining antiestrogen therapy with DC1 vaccination (Lowenfeld L, Press Oncoimmunology 2016, Manuscript submitted). This suggests that future experimental BC vaccines may be more effective by either targeting multiple oncodrivers or combining with other therapies that block additional pathways to prevent the escape phase of immunoediting [26].
Postimmunization, sensitization of T cells to at least one class II peptide was observed in 22 of 25 evaluable subjects, while 11 of 13 subjects were successfully sensitized to class I peptides. Perhaps most importantly, anti-HER2 peptide responses were observed up to 52 months postimmunization. These data show even in the presence of early-stage BC, such DC1 are potent inducers of durable Th1-polarized immunity, suggesting potential clinical value for development of cancer immunotherapy [27]. There is no significant difference in immune response detected systemically after vaccination in patients with HER2pos or ERneg and ERpos tumors, but complete tumor regression was significantly more common in patients with ERneg compared with ERposDCIS [28]. This proposed the concept of ER signaling as an escape pathway in HERposBC resistance to anti-HER2-targeted therapies. When looking at the effect of antiestrogen therapy in combination with DC1 vaccination, there is an increase rate of pCR and decreases recurrence in patients with ERpos/HERposBC. Interestingly, the addition of antiestrogen therapy increased the anti-HER2Th1 response in regional sentinel nodes (Lowenfeld L, Press Oncoimmunology 2016, Manuscript submitted).
The increased interest in targeted vaccination against HERposBC has shown promising results. This data provide rationale for developing vaccinations to reduce recurrence in patients with ERneg and HER2posDCIS in whom there personalized therapies other than standard surgery and radiation do not exist. There is a large push in the community to develop novel, rationale-targeted therapies for DCIS that also provide protection against new breast events.
The other area where DC1 vaccines may have clinical benefit is in patients with HER2pos IBC where the anti-HER2Th1 response is severely compromised (Figure 1) [7]. These patients have substantial risk of recurrence and include those patients with residual disease following neoadjuvant therapy, and advanced stage III and IV patients that are no evidence of disease (NED). Ongoing research with DC and non-DC vaccines in early-stage trials are currently opened and results are pending. (Table 1). We have demonstrated that the anti-HER2 Th1 response can be restored using DC1 vaccines in these patients [29]. Whether or not restoration of Th1 response translates to diminished recurrence must await larger trials.
Table 1. . Clinical trials of immunotherapy in early-stage breast cancer.
| Phase | Study | Vaccine type | NCI identifier | Status |
|---|---|---|---|---|
| I | Folate receptor binding peptide vaccine | Peptide/protein | NCT02019524 | Open |
| I | GP2-GM-CSF versus AE37 + GM-CSF versus GM-CSF alone | Peptide/protein | NCT00524277 | Open |
| I | NY-EOS-1 vaccine + sirolimus | Peptide/protein | NCT01522820 | Open |
| I | MUC1-peptide for triple-negative breast cancer | Peptide/protein | NCT00986609 | Open |
| II | Trastuzumab + GM-CSF/HER-2 E75 peptide | Peptide/Protein | NCT01570036 | Open |
| III | NeuVax TM | Peptide/protein | NCT01479244 | Open |
| I | Pilot-breast cancer vaccine + Poly-ICLC | Celullar vaccine | NCT01532960 | Open |
| II | GSK2302024A (Wilms tumor 1-specific therapy) + adjuvant therapy | Celullar vaccine | NCT01220128 | Open |
| II | Preoperative cryotherapy +/- ipilimumab (anti-CTLA-4) | Checkpoint inhibitors | NCT01502592 | Open |
| I/II | HER-2/Neu-pulsed DC1 vaccine combined with trastuzumab for patients with DCIS | Pulsed dentritic cells | NCT02336984 | Open |
| I/II | Randomized trial of HER-2/Neu-pulsed DC1 vaccine for patients with DCIS | Pulsed dentritic cells | NCT02061332 | Open |
| I/II | Nelipepimut-S E75 (HER2) NeuVax TM | Peptide/protein | Closed | |
| I/II | GP2 + GM-CSF | Peptide/protein | Closed | |
| AE37 + GM-CSF | Peptide/protein | Closed | ||
| AE37/GP2 + GM-CSF | Peptide/protein | Closed | ||
| I | A HER-2/Neu-pulsed DC1 vaccine for patients with DCIS | Pulsed dentritic cells | NCT00107211 | Closed |
DCIS: Ductal carcinoma in situ; MUC-1: Mucin-1.
Use of DC1 vaccines during tumor escape in combination with anti-HER2 therapies
Loss of anti-HER2Th1 immune response is associated with lack of pCR to adjuvant chemotherapy and HER2-targeted therapies [7] as well as predicts increased of recurrence and diminished DFS [8]. Though DC vaccination has not yet proven to be an effective treatment of BC, other adjuvant treatment modalities have been shown to aid in promoting the immune response to tumor cells when given in combination with DC vaccines. These treatments often work synergistically with vaccination by aiding T-cell recognition of tumor cells. Some therapies, such as chemotherapy or radiation, may induce changes in the tumor microenvironment, allowing a more complete response to DC vaccination. In combination with other therapies, such as HER2-targeted therapies, DC vaccination can aid in overcoming resistance [29,30]. Hence, pCR may be best achieved using combination therapy with DC vaccination (Figure 2).
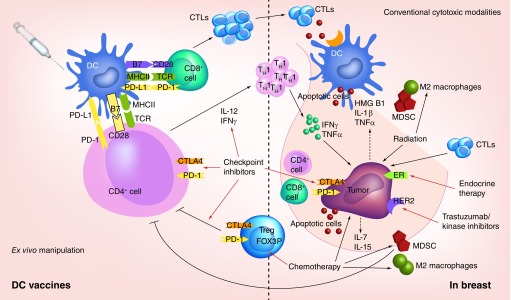
Figure 2. . Multimodality enhancement of dentritic cells-based immunotherapy.
In ex vivo manipulation, monocyte precursorsare sequentially matured and loaded with antigen to be injected. Lymphatics serve as sites of T-cell co-stimulation, DCs present antigen to T cells in the context of MHCClass I/II molecules, activating antigen-specific CD4posTh1 cells or CD8pos CTLs. These effector and helper populations migrate to the tumor bed, where they target tumor cells or elaborate cytokines (e.g., IFN-γ and TNF-α) mediating apoptosis. In conventional cytotoxic modalities radiation of tumor cells induces release of TAAs, pro-inflammatory cytokines (IL-1β, TNF-α), or endogenous TLR agonists (HMGB-1), activating DCs to prime antigen-specific CTL responses; antigens may also bepresented by stromal cells for destruction by CTLs. Chemotherapy generates an immune recovery cytokine environment and inhibits Treg and MDSC function. Endocrine and mAb-based targeted therapies halt downstream nuclear signaling and inhibit proliferation. Checkpoint inhibitors (CTLA-4 and PD-1) are aimed at recovering T-cell cytotoxicity and muting Treg and MDSCs.
CTL: Cytotoxic T lymphocyte; mAb: Monoclonal antibody; MDSC: Myeloid-derived suppressor cell; TLR: Toll-like receptor.
Adjuvant chemotherapy
The tumor microenvironment may play a role in the recognition and response of the immune system to tumor cells. It has been observed that a high level of lymphocytic infiltration in breast tumors predicts a better prognosis and a better response to chemotherapy [31]. Chemotherapy has the potential to augment the tumor microenvironment by increasing CD4pos T-cell infiltration (Figure 2), demonstrating a positive correlation to pCR [32]. Because vaccination, especially with DCs, can stimulate activation of T cells and potentially improve lymphocytic infiltration of breast tumors, concomitant or sequential administration of vaccines with low-dose chemotherapy may improve treatment outcomes. Sequential administration of a low dose of the chemotherapeutic drug, cyclophosphamide, along with vaccination against HER2 has been shown to induce de novo HER2-specific T helper-dependent immunity [33]. Low-dose doxorubicin, paclitaxel and methotrexate have also been shown to enhance HER2-specific immunity with vaccination as well as stimulate DC maturation and differentiation [33–35]. Therefore, chemotherapy may be better utilized in order to maximize the effectiveness of the antitumor immune response.
Combinations of vaccines with HER2-directed therapy
Trastuzumab is a monoclonal antibody that targets the HER2/neu protein by binding to the extracellular domain, triggering HER2 internalization and degradation. The use of trastuzumab induces passive immunity to HER2, although a major limitation of this therapy after continued use is the development of drug resistance. About 70% of patients who initially respond to therapy experience progression of the disease within approximately a year, suggesting the development of an acquired resistance to the antibody [36]. Patients undergoing neoadjuvant trastuzumab therapy plus chemotherapy may not show pCR due to depressed anti-HER2Th1 cell response [29]. Conversely, combination use of trastuzumab with vaccination against HER2 can significantly sensitize CTLs to tumor cells expressing the peptide and result in increased tumor cell lysis specific to HER2 [30,37]. In addition, it has recently been demonstrated that the anti-HER2Th1 response is positively correlated with pCR in patients given neoadjuvant chemotherapy plus trastuzumab. Those patients with a depressed anti-HER2Th1 response did not show pCR, but it was shown that this could be augmented with use of HER2-targeted DC vaccination [29]. Additionally, there is a strong association between immune gene expression and DFS following treatment with adjuvant trastuzumab, suggesting that there is a subset of HER2pos tumors with a high level of immunologic activity [38]. Thus, DC vaccination may prove to be a useful adjunct to trastuzumab therapy by restoring immune response to HER2.
Pertuzumab another anti-HER2 mAb, similar to trastuzumab, targets the extracellular dimerization domain of the HER2 receptor. Developed in attempts to combat resistance to trastuzumab, pertuzumab prevents HER2/HER3 heterodimerization [39,40]. Coadministration of the two therapies has been shown to enhance clinical effectiveness compared with either one administered singly [40,41]. Pertuzumab has not yet been studied in combination with vaccination against cancer, but, considering its similar mechanism of action and synergistic effects with trastuzumab, DC vaccination could prove to be a clinically effective adjunct.
Similarly, lapatinib, a tyrosine kinase inhibitor (TKI) that disrupts the HER2/neu and EGFR, is used to treat HER2posBC. Like trastuzumab, patients may develop resistance fairly early. In attempts to combat this, vaccination against HER2 was given concomitantly with lapatinib in a clinical trial, which demonstrated safety of the combined treatment, but due to small sample size, no additional immunologic benefits were observed [42]. However, another TKI, axitinib, showed improved therapeutic efficacy when combined with DC vaccination in a preclinical model of murine melanoma [43]. Although DC vaccination combined with TKIs has not yet shown improved outcomes in breast cancer, improved anti-HER2 immunity acquired by vaccination may improve pCR and overcome resistance seen with continued use of TKIs [29,37].
Radiation
Radiation is an important part of anticancer therapy, with nearly two-thirds of patients with cancer receiving it at some point during the course of their treatment [44]. Radiation is known for its ability to damage DNA of tumor cells, eventually causing enough injury to prohibit cellular proliferation and/or cause tumor cell death. Along with the antitumor properties that radiation has on its own, it may also have the ability to amplify the effects induction of apoptosis and necrosis; so APCs are ultimately attracted to this environment. This radiation-induced tumor microenvironment increases expression of Fas, MHC class I molecules and several other cell surface proteins [45], which provoke a DC-mediated antigen-specific immune response [46], therefore stimulating a CTL response. Correspondingly, the goal of vaccination is to induce an antigen-specific immune response to tumor cells, so addition of radiotherapy to vaccination should have a synergistic effect. This effect has been shown in a preclinical model of subcutaneous murine colorectal carcinoma where, after combination therapy of local radiation and vaccination, there was a massive infiltration of T cells that was not seen in either modality alone [47]. Prostate cancer patients treated with radiotherapy in combination of anti-PSA DC vaccines were able to increase immune response against PSA, but there was no clear synergistic effect of the combination of radiation with vaccination [45]. At this point, there have been no studies examining the effect of radiation therapy in combination with DC vaccines in the treatment of BC, however the ability of radiation to enhance MHC class I expression and consequently CTL infiltration of tumor has been shown in other forms of cancer immunotherapy using monoclonal antibodies against molecules such as cytotoxic T-lymphocyte associated antigen 4 (CTLA-4) and PD-1 [48–50]. Ultimately, radiotherapy may provide a useful adjunct to DC vaccination in the treatment of BC to affect the tumor microenvironment.
Checkpoint inhibitors
Tumors may utilize immune checkpoint pathways by expressing ligands that, under normal circumstances, would prevent aberrantly activated T cells from causing autoimmunity [51,52]. Tumors may effectively hide from the immune system with expression of these ligands. By blocking the interaction of ligands with immune effector cells using monoclonal antibodies (mAb), the tumor cells may now be easily ‘seen’ by the immune system. Hence, two of these checkpoint pathways that are currently areas of major investigation are the PD-1/PD-L1 and CTLA-4/B7 pathways, which have demonstrated significant success in melanoma, lung cancer and colon cancer [53–55] – all tumors with significant production of neoantigens.
PD-1 is expressed on effector immune cells – including T cells, NK cells, activated monocytes and DCs (Figure 2) [51]. Contact of PD-1 with its ligand, PDL-1, results in T-cell inactivation and apoptosis [56] and inhibits the activation of tumor-antigen-specific T cells [57]. Expression of PDL-1 on tumor cells may render them immunologically invisible. Like the PD-1 pathway, the CTLA-4 is a significant immune checkpoint receptor exploited in cancer. CTLA-4 is a CD28 homolog, which is upregulated upon T-cell activation and competes with CD28 for binding to APC ligands [58]. Tumor cells can express the CTLA-4 receptor on their surface [59] and counteract the activity of the T-cell costimulatory receptor, CD28 [60] impairing tumor-reactive T cells.
mAb against PD-1 and CTLA4 may restore the function of disabled CD8pos T cells in cancer as well as preventing the depletion of activated memory B cells, resulting in a more robust immune response [61]. PD-1/PD-L1 blockade can neutralize the inhibition of DC–T-cell interactions, restore APC function, repair T-cell function by increasing IFN-γ production, promoting T-cell proliferation [52] and T-cell targeting of tumors (Figure 2).
The restoration of T-cell function with the use of mAb against these checkpoint inhibitors presents a synergistic opportunity for administration of DC vaccines. Using PD-1 mAb in conjunction with DC vaccination has shown an increase in CD4pos and CD8pos T-cell responses [56]. Along with restoring function of disabled immune cells, blocking the PD-1/PD-L1 checkpoint may further enhance the effectiveness of DC vaccination by preventing suppression of DC maturation [52]. In a murine breast cancer model, administration of a monoclonal antibody against PD-L1 in combination with DC vaccination induced more potent protective immunity than just DC vaccination alone [52]. Anti-HER2 DC vaccination combined with anti-CTLA4 therapy in a murine mammary carcinoma model also exhibited a significant increase in the frequency of tumor infiltrating CD4pos and CD8pos T cells [62]. A probable mechanism for the success in this therapeutic combination is the restoration and promotion of a robust Th1 response, encouraging cytotoxic CD8pos T-cell response and infiltration into the tumor [62]. Checkpoint inhibitors and DC vaccination seem to be an advantageous synergistic combination for activation of the immune system in cancer therapy for which there are ongoing trials [58]. Ultimately, the immune response needs help targeting oncodrivers, and the combination of checkpoint inhibitors with DC vaccines may prove to be the most successful combination.
DC vaccines for breast cancer escape with loss of HER2 oncodriver expression
Genetic profiling has allowed us to identify different oncodrivers associated with IBC as well as the four principal subtypes. HER2pos expressing tumor cells, as previously mentioned, may undergo phenotypic shifting or loss of immune response under anti-HER2 pressure (Figure 1) which may allow for disease progression to HER2negIBC with subtypes such as basal like (TNBC) or Luminal A (ERposBC). With this in mind, modulating the immune responses using DC pulsed with antigens against other oncodrivers may aid in eliminating residual BC cells and prevent recurrence of escape variants that lose HER2/neu expression. We will discuss some of the potential oncodriver targets in the different subtypes of BC.
ER-positive breast cancer oncodrivers
MUC1
Epithelial Mucin-1 (MUC1) is a large transmembrane protein and is the most widely expressed of the mucins, located on the apical surface of human epithelial cells lining glands or ducts. It is an overexpressed antigen found in about 90% of BC and is a cancer-specific class of vaccine target [63]. Elevated levels of MUC1 on the tumor have been associated with invasiveness, tumor growth, metastatic properties leading to poor prognosis in colon, pancreas, breast and bladder cancer [64,65]. MUC1 has been found to stimulate ER-mediated transcription, antagonize tamoxifen and contribute to ER-induced growth and survival of BC cells [66]. These findings support for the idea that MUC1 is crucial to ER function in BC. It contributes to tumorigenesis by reducing degradation of EGFR, increasing cell proliferation and inhibiting cell death by protecting against oxidative and genotoxic stress [67–69]. MUC1 has been studied as a target in cancer immunotherapy due to the ability to induce humoral immune responses in healthy subjects and in cancer patients [70]. Patients vaccinated with MUC1 core peptide-pulsed DC vaccines with MUC1-positive cancer were also found to have a reduction in tumor size or tumor marker levels as well as prolonged survival, suggesting that MUC1 is sufficiently immunogenic to be used as a target for DC vaccination [71]. More recently, MUC1 peptide vaccine study was able to induced INF gamma CD4pos and CD8pos T cells that recognized tumor-associated MUC1. Although only T-cell preactivation outside the tumor bed, either in culture or by repetitive vaccination, showed to overcome escape phase of tumor ‘immunoediting’ [72].
HER2-positive breast cancer – other oncodrivers
hTERT
Human telomerase reverse transcriptase (hTERT) expression has been found to be increased in radioresistant HER2posBC, while HER2 reduction has been shown to cause hTERT/telomerase activity (TA) downregulation. A widely expressed tumor antigen, hTERT, is expressed in more than 85% of all human cancers [73] and absent in most normal human cells [73], making it a favorable target for immunotherapy. hTERT is not a cellular growth receptor or signal transducer, but rather overexpression, prolongs tumor cellular survival by maintaining chromosomal integrity and protecting telomeric DNA [74]. TA has been found in up to 75% of breast carcinoma in situ lesions. Proto-oncogenes and growth factors, such as p53 and HER2, have been associated with the regulation of TA. Studies have shown that HER2 mediates hTERT expression through activation of NF-κB and c-myc [75]. In the setting of immunotherapy, it has been observed that the hTERT peptide, I540, binds with high affinity to HLA-A2, and can be used to generate specific CTLs in vitro that lyse a wide range of hTERT-positive tumor cell lines [73]. Overall, hTERT-specific immunity has been achieved with induction of CD8pos tumor infiltrating lymphocytes [76,77]. Although there was some immunity to the TAA peptide hTERT, trials have not yet proven DC vaccination against hTERT to be an effective therapeutic strategy.
HER3
HER3 is another member of the EGFR family. Some studies have suggested that combination of HER2 and HER3 are crucial in cell growth, tumorigenesis and directly contribute in acquired resistance to therapies in HER2posBC and ERposBC. Although HER3 overexpression and activation is seen in invasive BC, a few studies have looked at it in the setting of DCIS, in which HER2pos status is frequently seen [78]. HER3 can channel ErbB signaling to PI3K pathway leading to its adeptness to favor tumor growth. Murine models have shown that overexpression of HER3 is seen in conjunction with high levels of HER2 [79]. This is thought to occur via gene amplification, transcription or translation. In cells with high HER2 and HER3, downregulation of HER3 affects both receptors, implying association of the two for signal maintenance [79]. This may have impact in HER2-targeted therapies and provides resistance against pertuzumab, mTOR inhibitors and tamoxifen. In the case of tamoxifen, downregulation of ERBB3 in MCF-7 cells rendered resistant by HER2 overexpression promotes Tamoxifen-induced apoptosis. This relationship allows for a chance at improving efficacy of HER2-targeted therapies, by hindering linking of HER2 to HER3 potentially using DC vaccines. HER-3 expression is also a poor prognostic factor in TNBC [80] as it can heterodimerize with EGFR as well as transduce signals with c-MET.
Triple-negative breast cancer oncodrivers
c-MET & EGFR
c-MET and EGFR amplification and overexpression have been associated with TNBC and basal-like subgroups. EGFR is found in 50% of BC and c-MET is overexpressed in 20–30% of cases. c-MET is a tyrosine kinase receptor that binds to ligand EGFR or HGF for organ development, but when an anomalous activation occurs it can lead to tumor development [81]. Overexpression has been associated with increasing tumor size, increased nodal involvement [82], decreased DFS, decreased overall survival and poor response to chemotherapy [83].
c-MET amplification is associated with treatment failure to trastuzumab and shorter time to disease progression [84] in HER2posBC, and resistance to anti-EGFR therapies in TNBC [85]. Currently, Phase II trials of anti-c-MET therapies are underway in the setting of advanced BC using targeted therapies such as tyrosine kinase inhibitors and monoclonal antibodies.
Combined c-MET/EGFR therapies have been studied in head and neck, lung and colon cancer and more recently for TNBC. EGFR/MET inhibition is synergic and therefore targeting both may provide for insight to establishing an effective therapeutic strategy in the setting on TNBC were limited therapies are available [85]. This role of c-MET and EGFR in BC merits further investigation and potentially using them as a DC vaccine target to block both pathways.
P53
p53 is a tumor suppressor gene, regulator of the cell cycle, cellular proliferation and apoptosis, which is found to be mutated in 25% of BC. It is most commonly seen in TNBC in about 50–80% [86]. Overexpression is associated with poor response to chemotherapy [87], DFS and overall survival [88]. Tumors cells are able to bypass the G1 checkpoint and complete the cell cycle, even in the presence of DNA damage in the setting of a p53 mutation [86]. Th1 response is significantly more prevalent in patients with tumors exhibiting high expression of p53. With this in mind, future immunotherapeutic approaches may potentially be geared toward Th1 polarization to allow a more effective immunotherapy in patients with advanced disease with p53 mutation [89].
Conclusion
Cancer immunotherapy is an important feature in the rapidly changing landscape of cancer treatment. Because breast cancer may represent a classic example of immunoediting, DC-based cancer vaccines can harness the potential to reinvigorate the immune system, moving toward elimination of tumor cells and shifting away from the equilibrium and escape stages. However, a one-size-fits-all theory will not likely be possible with DC vaccines. The use of vaccines in early stages of breast cancer, such as in DCIS, has claimed more success than in late-stage use, likely because, in DCIS, the tumor cells and the immune response have achieved a state of equilibrium. Vaccines may be able to more readily tip the scales in favor of the immune system, thereby eliminating equilibrium and moving toward elimination of the tumor. Although DC vaccines have enjoyed some success in early stages of breast cancer, they still have been limited success when used in later stages. This limited success has shifted focus toward using DC vaccines in the adjuvant setting to prevent recurrence. Vaccination in combination with targeted molecular and immune therapies that provide an additive or synergistic response, as well as using DC with cytotoxic therapies such as chemotherapy and radiation remain underexplored. Combinations with checkpoint inhibitors are rapidly moving forward. DC vaccination therapy may also be used to target numerous TAAs, giving it the potential to be a personalized tumor treatment of tumor immune escape for essentially any phenotypic subtype of breast cancers. The versatility of DC vaccines lends itself to being a powerful tool in the future of cancer therapy.
Future perspective
In the past, monotherapy with DC vaccines did not meet the expectations as a standalone therapy in advanced breast cancer. As we move forward, the focus of DC vaccines is being shifted to early disease and use in adjuvant settings. In addition, DC vaccines are being incorporated in combination with conventional therapies such as radiation, chemotherapy and antiestrogen therapy as well as in combination with other immune adjuvants in the treatment of early-stage breast cancer. It is presumed that such uses of DC vaccines will reduce recurrence and could potentially be developed for primary prevention. The synergistic effects will yield improved outcomes as the era of personalized medicine continues.
Executive summary.
Targeting HER2 & loss of anti-HER2
HER2/neu is associated with a heightened risk for invasion and metastasis, suggesting a crucial role for HER2/neu during breast tumorigenesis. We have observed a progressive loss of anti-HER2 CD4pos Th1 (anti-HER2Th1) immune response during tumorigenesis in patients with HER-2 expressing breast cancer. HER2 vaccines can restore anti-HER2Th1 immune responses.
Role of immune response & immunoediting during breast tumorigenesis
Since healthy women have robust anti-HER-2 CD4 Th1 responses, we hypothesize that in the initial stage of malignant transformation, the mounted immune response is robust enough against this HER2/neu overexpression and may eliminate HER2posDCIS or invasive breast cancer (IBC) from becoming clinically apparent. If elimination is not achieved, HER2/neu overexpressing tumor cells and the immune response develop into a stalemate of equilibrium where the anti-HER2Th1 immune response is unable to completely eliminate malignant cells, but is able to prevent them from becoming invasive. This equilibrium state may be clinically manifested as HER2posDCIS and may remain in this state of equilibrium and the anti-HER-2 Th1 response further erodes allowing tumor escape as HER-2posIBC or anti-HER2Th1 response is sustained but ineffectual and tumor cells manage to evade immunosurveillance via phenotypic shifting.
Activation of type I polarized DC drive strong anticancer Th1 responses
CD4pos T cells may be a crucial element in the antitumor immune response because of their role as helpers in keeping CD8pos cells functionally active. Th1 cells secrete high-level cytokines such as IFN-γ and TNF-α, both key components in antitumor function. These cytokines can induce apoptosis of HER-2 expressing breast cancer cells and upregulate MHC class I expression making the cells more susceptible to CD8-mediated cytolysis. Dendritic cell (DC) polarized toward high levels of IL-12 production specifically drive anti-HER-2 CD4 Th1.
Effectiveness of DC1 vaccines administered in the equilibrium phase of DCIS
We suggest that HER2posDCIS represents the equilibrium phase of immunoediting where Th1 immune response is in homeostasis with cancer cells in the ducts. Stimulating a strong Th1 immune response may lead to eradication or return to the elimination phase. Results from ductal carcinoma in situ patients treated with HER-2-pulsed DC1 demonstrated that some patients who received HER2-pulsed DC1 vaccines alone achieved pCR. In addition, others have remaining tumor with loss of HER-2 suggesting tumors can escape. Combining vaccines targeting multiple oncodrivers or combination therapies may prevent immunoediting.
Use of DC1 vaccines during tumor escape in combination with anti-HER2 therapies
Persistent diminished anti-HER2Th1 immune response is associated with lack of pCR to neoadjuvant chemotherapy and HER2-targeted therapies. Ongoing clinical trials as being performed to assess whether restoring anti-HER-2 CD4 Th1 can prevent recurrence or combined with initial therapies to cause more complete responses to standard therapy. Combining the anti-HER-2 therapies and other immune modulating agents such as checkpoint inhibitors are subjects of future trials to augment effective anti-HER-2 CD4 Th1 responses.
DC vaccines for breast cancer escape with loss of HER2 oncodriver expression
Ineffectual anti-HER-2 Th1 responses may lead HER2pos expressing tumor cells to undergo phenotypic shifting or loss of immune response under anti-HER2 pressure which may allow for disease progression to HER2negIBC with subtypes such as basal like (triple-negative breast cancer) or Luminal A (ERposBC). Modulating immune responses using DC pulsed with antigens against other oncodrivers such as Mucin-1, c-MET, human telomerase reverse transcriptase, HER3 may aid in eliminating residual BC cells and prevent recurrence of escape variants that lose HER2/neu expression.
Footnotes
Financial & competing interests disclosure
Work from our laboratory cited in this review was funded by NIH R01 CA096997, Henle Fund Research Fellowship (*) and Pennies in Action® Foundation. No external funding was secured for this study. The authors have no financial relationships or conflicts of interest relevant to this article to disclose. Work from our laboratory cited in this review was funded by National Institutes of Health R01 CA096997, Henle Fund Research Fellowship (*) and Pennies in Action® Foundation. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: •• of considerable interest
- 1.Witton CJ, Reeves JR, Going JJ, Cooke TG, Bartlett JM. Expression of the HER1–4 family of receptor tyrosine kinases in breast cancer. 2003;200(3):290–297. doi: 10.1002/path.1370. [DOI] [PubMed] [Google Scholar]
- 2.Czerniecki BJ, Koski GK, Koldovsky U, et al. Targeting HER-2/neu in early breast cancer development using dendritic cells with staged interleukin-12 burst secretion. 2007;67(4):1842–1852. doi: 10.1158/0008-5472.CAN-06-4038. [DOI] [PubMed] [Google Scholar]
- 3.Disis ML, Calenoff E, Mclaughlin G, et al. Existent T-cell and antibody immunity to HER-2/neu protein in patients with breast cancer. 1994;54(1):16–20. [PubMed] [Google Scholar]
- 4.Roses RE, Paulson EC, Sharma A, et al. HER-2/neu overexpression as a predictor for the transition from in situ to invasive breast cancer. 2009;18(5):1386–1389. doi: 10.1158/1055-9965.EPI-08-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hassett MJ, Jiang W, Habel LA, et al. Characteristics of second breast events among women treated with breast-conserving surgery for DCIS in the community. 2016;155(3):541–549. doi: 10.1007/s10549-016-3692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harada S, Mick R, Roses RE, et al. The significance of HER-2/neu receptor positivity and immunophenotype in ductal carcinoma in situ with early invasive disease. 2011;104(5):458–465. doi: 10.1002/jso.21973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Datta J, Rosemblit C, Berk E, et al. Progressive loss of anti-HER2 CD4+ T-helper type 1 response in breast tumorigenesis and the potential for immune restoration. 2015;4(10):e1022301. doi: 10.1080/2162402X.2015.1022301. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• First description of the progressive and specific loss of CD4+ Th1 immunity to a molecular oncodriver during breast tumorigenesis. Points into the negative clinical and pathologic outcomes associated with depressed anti-HER2 Th1 immunity and implies that immune restoration with vaccination or other immune modulating strategies may be an option to diminish tumor progression or prevent recurrence.
- 8.Datta J, Fracol M, Mcmillan MT, et al. Association of depressed anti-HER2 T-helper type 1 response with recurrence in patients with completely treated HER2-positive breast cancer: role for immune monitoring. 2015;2(2):242–246. doi: 10.1001/jamaoncol.2015.5482. [DOI] [PubMed] [Google Scholar]
- 9.Hennighausen L, Robinson GW. Signaling pathways in mammary gland development. 2001;1:467–475. doi: 10.1016/s1534-5807(01)00064-8. [DOI] [PubMed] [Google Scholar]
- 10.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. 2011;331(6024):1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 11.Wherry E. T cell exhaustion. 2011;12(6):492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 12.Zhu YY SW, Ji TF, Guo XQ, Hu Y, Yang Jl. The variation and clinical significance of hormone receptors and HER-2 status from primary to metastatic lesions in breast cancer patients. 2015;37(6):675–684. doi: 10.1007/s13277-015-4649-7. [DOI] [PubMed] [Google Scholar]
- 13.Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. 2013;31:563–604. doi: 10.1146/annurev-immunol-020711-074950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bancherau J, Briere F, Caux C, et al. Immunobiology of dendritic cells. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 15.Kono M, Nakamura Y, Suda T, et al. Enhancement of protective immunity against intracellular bacteria using type-1 polarized dendritic cell (DC) vaccine. 2012;30(16):2633–2639. doi: 10.1016/j.vaccine.2012.02.026. [DOI] [PubMed] [Google Scholar]
- 16.Sallusto F, Schaerli P, Loetscher P, et al. Rapid and coordinated switch in chemokine receptor expression during dendritic cell maturation. 1998;28(9):2760–2769. doi: 10.1002/(SICI)1521-4141(199809)28:09<2760::AID-IMMU2760>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 17.Austyn J. Death, destruction, danger and dendritic cells. 1999;5(11):1232–1233. doi: 10.1038/15182. [DOI] [PubMed] [Google Scholar]
- 18.Curtsinger JM, Johnson CM, Mescher MF. CD8 T cell clonal expansion and development of effector function require prolonged exposure to antigen, costimulation, and signal 3 cytokine. 2003;171(10):5165–5171. doi: 10.4049/jimmunol.171.10.5165. [DOI] [PubMed] [Google Scholar]
- 19.Kaiko Ge HJ, Beagley KW, Hansbro PM. Immunological decision-making: how does the immune system decide to mount a helper T-cell response? 2008;123(3):326–338. doi: 10.1111/j.1365-2567.2007.02719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Antony PA, Piccirillo CA, Akpinarli A, et al. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. 2005;174(5):2591–2601. doi: 10.4049/jimmunol.174.5.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao FG, Khammanivong V, Liu WJ, Leggatt GR, Frazer IH, Fernando GJ. Antigen-specific CD4+ T-cell help is required to activate a memory CD8+ T cell to a fully functional tumor killer cell. 2002;62(22):6438–6441. [PubMed] [Google Scholar]
- 22.Huang Y, Ma C, Zhang Q, et al. CD4+ and CD8+ T cells have opposing roles in breast cancer progression and outcome. 2015;6(19):17462–17478. doi: 10.18632/oncotarget.3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scott P. IL-12: initiation cytokine for cell-mediated immunity. 1993;260(5107):496–497. doi: 10.1126/science.8097337. [DOI] [PubMed] [Google Scholar]
- 24.Pulendran B. Pulendran modulating TH1/TH2 responses with microbes, dendritic cells, and pathogen recognition receptors. 2004;29(1):187–196. doi: 10.1385/IR:29:1-3:187. [DOI] [PubMed] [Google Scholar]
- 25.Cintolo JA, Datta J, Mathew SJ, Czerniecki BJ. Dendritic cell-based vaccines: barriers and opportunities. 2012;8(10):1273–1299. doi: 10.2217/fon.12.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharma A, Koldovsky U, Xu S, et al. HER-2 pulsed dendritic cell vaccine can eliminate HER-2 expression and impact ductal carcinoma in situ . 2012;118(17):4354–4362. doi: 10.1002/cncr.26734. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Results from this study suggest that targeting the HER-2/neu pathway in early breast cancer and ductal carcinoma in situ using DC1 pulsed with HER-2/neu peptides boosted anti-HER-2 CD4 Th1 responses and caused regression in some of these lesions, especially those with estrogen-independent HER-2/neupos ductal carcinoma in situ.
- 27.Koski GK, Koldovsky U, Xu S, et al. A novel dendritic cell-based immunization approach for the induction of durable Th1-polarized anti-HER-2/neu responses in women with early breast cancer. 2012;35(1):54–65. doi: 10.1097/CJI.0b013e318235f512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fracol M, Xu S, Mick R, et al. Response to HER-2 pulsed DC1 vaccines is predicted by both HER-2 and estrogen receptor expression in DCIS. 2013;20(10):3233–3239. doi: 10.1245/s10434-013-3119-y. [DOI] [PubMed] [Google Scholar]
- 29.Datta J, Berk E, Xu S, et al. Anti-HER2 CD4 (+) T-helper type 1 response is a novel immune correlate to pathologic response following neoadjuvant therapy in HER2-positive breast cancer. 2015;17:71. doi: 10.1186/s13058-015-0584-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mittendorf EA, Storrer CE, Shriver CD, Ponniah S, Peoples GE. Investigating the combination of trastuzumab and HER2/neu peptide vaccines for the treatment of breast cancer. 2006;13(8):1085–1098. doi: 10.1245/ASO.2006.03.069. [DOI] [PubMed] [Google Scholar]
- 31.Denkert C, Loibl S, Noske A, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. 2010;28(1):105–113. doi: 10.1200/JCO.2009.23.7370. [DOI] [PubMed] [Google Scholar]
- 32.García-Martínez E, Gil GL, Benito AC, et al. Tumor-infiltrating immune cell profiles and their change after neoadjuvant chemotherapy predict response and prognosis of breast cancer. 2014;16:1–17. doi: 10.1186/s13058-014-0488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Emens LA, Asquith JM, Leatherman JM, et al. Timed sequential treatment with cyclophosphamide, doxorubicin, and an allogeneic granulocyte-macrophage colony-stimulating factor-secreting breast tumor vaccine: a chemotherapy dose-ranging factorial study of safety and immune activation. 2009;27(35):5911–5918. doi: 10.1200/JCO.2009.23.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaneno R, Shurin GV, Tourkova IL, Shurin MR. Chemomodulation of human dendritic cell function by antineoplastic agents in low noncytotoxic concentrations. 2009;7:58. doi: 10.1186/1479-5876-7-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pfannenstiel LW, Lam SS, Emens LA, Jaffee EM, Armstrong TD. Paclitaxel enhances early dendritic cell maturation and function through TLR4 signaling in mice. 2010;263(1):79–87. doi: 10.1016/j.cellimm.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gajria D, Chandarlapaty S. HER2-amplified breast cancer: mechanisms of trastuzumab resistance and novel targeted therapies. 2011;11(2):263–275. doi: 10.1586/era.10.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Disis ML, Wallace DR, Gooley TA, et al. Concurrent trastuzumab and HER2/neu-specific vaccination in patients with metastatic breast cancer. 2009;27(28):4685–4692. doi: 10.1200/JCO.2008.20.6789. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Reported safety and tolerability of combination therapy of trastuzumab and a HER2/neu-specific vaccine, as well as demonstrated the potential use of vaccines to boost and maintain pre-existing immunity to HER2/neu with immunization. Moreover, they showed epitope spread, which could be involved in modulating systemic mediators of tumor-induced immune suppression.
- 38.Perez EA, Thompson EA, Ballman KV, et al. Genomic analysis reveals that immune function genes are strongly linked to clinical outcome in the North Central Cancer Treatment Group n9831 Adjuvant Trastuzumab Trial. 2015;33(7):701–708. doi: 10.1200/JCO.2014.57.6298. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Discussed the importance of identifying patients who are likely to benefit from trastuzumab on the basis of evaluation of an immune response gene signature. Patients with low immune response signature did not obtain as significant benefit from trastuzumab as those with high immune response signature. This opens the possibility for future clinical trials designed to evaluate therapeutic approaches that might enhance the immune signature within HER2-positive tumors and thereby sensitize the tumors to biologic therapies. HER-2-pulsed DC1 vaccines may be one way to boost the tumor immune signature.
- 39.Zanardi E, Bregni G, De Braud F, Di Cosimo S. Better together: targeted combination therapies in breast cancer. 2015;42(6):887–895. doi: 10.1053/j.seminoncol.2015.09.029. [DOI] [PubMed] [Google Scholar]
- 40.Gollamudi J, Parvani JG, Schiemann WP, Vinayak S. Neoadjuvant therapy for early-stage breast cancer: the clinical utility of pertuzumab. 2016;8:21–31. doi: 10.2147/CMAR.S55279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, Phase II trial. 2012;13(1):25–32. doi: 10.1016/S1470-2045(11)70336-9. [DOI] [PubMed] [Google Scholar]
- 42.Hamilton E, Blackwell K, Hobeika AC, et al. Phase 1 clinical trial of HER2-specific immunotherapy with concomitant HER2 kinase inhibition [corrected] 2012;10:28. doi: 10.1186/1479-5876-10-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bose A, Lowe DB, Rao A, Storkus WJ. Combined vaccine+axitinib therapy yields superior antitumor efficacy in a murine melanoma model. 2012;22(3):236–243. doi: 10.1097/CMR.0b013e3283538293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Verbrugge I, Hagekyriakou J, Sharp LL, et al. Radiotherapy increases the permissiveness of established mammary tumors to rejection by immunomodulatory antibodies. 2012;72(13):3163–3174. doi: 10.1158/0008-5472.CAN-12-0210. [DOI] [PubMed] [Google Scholar]
- 45.Gulley JL, Arlen PM, Bastian A, et al. Combining a recombinant cancer vaccine with standard definitive radiotherapy in patients with localized prostate cancer. 2005;11(9):3353–3362. doi: 10.1158/1078-0432.CCR-04-2062. [DOI] [PubMed] [Google Scholar]
- 46.Friedman E. Immune modulation by ionizing radiation and its implications for cancer immunotherapy. 2002;8(19):1765–1780. doi: 10.2174/1381612023394089. [DOI] [PubMed] [Google Scholar]
- 47.Chakraborty MA, Scott I, Coleman N, et al. External beam radiation of tumors alters phenotype of tumor cells to render them susceptible to vaccine-mediated T-cell killing. 2004;64:4328–4337. doi: 10.1158/0008-5472.CAN-04-0073. [DOI] [PubMed] [Google Scholar]
- 48.Demaria S, Kawashima N, Yang AM, et al. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. 2005;11:728–734. [PubMed] [Google Scholar]
- 49.Vanpouille-Box C, Diamond JM, Pilones KA, et al. TGFbeta Is a master regulator of radiation therapy-induced antitumor immunity. 2015;75(11):2232–2242. doi: 10.1158/0008-5472.CAN-14-3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reits EA, Hodge JW, Herberts CA, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. 2006;203(5):1259–1271. doi: 10.1084/jem.20052494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ge Y, Xi H, Ju S, Zhang X. Blockade of PD-1/PD-L1 immune checkpoint during DC vaccination induces potent protective immunity against breast cancer in hu-SCID mice. 2013;336(2):253–259. doi: 10.1016/j.canlet.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 53.Ribasa C, Comin-Anduix B, Chmielowski B, et al. Dendritic cell vaccination combined with CTLA4 blockade in patients with metastatic melanoma. 2009;15(19):6267–6276. doi: 10.1158/1078-0432.CCR-09-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. 2013;369(2):134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rosenblatt J, Glotzbecker B, Mills H, et al. PD-1 blockade by CT-011, anti-PD-1 antibody, enhances ex vivo T-cell responses to autologous dendritic cell/myeloma fusion vaccine. 2011;34(5):409–418. doi: 10.1097/CJI.0b013e31821ca6ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ashley C-M, Jeremy BF, Leisha AE. Immune targeting in breast cancer. 2015;29(5):375. [PubMed] [Google Scholar]
- 58.Datta J, Berk E, Cintolo JA, Xu S, Roses RE, Czerniecki BJ. Rationale for a multimodality strategy to enhance the efficacy of dendritic cell-based cancer immunotherapy. 2015;6:271. doi: 10.3389/fimmu.2015.00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Katz T, Avivi I, Benyamini N, Rosenblatt J, Avigan D. Dendritic cell cancer vaccines: from the bench to the bedside. 2014;5(4):e0024. doi: 10.5041/RMMJ.10158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. 2012;12(4):252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zitvogel L, Kroemer G. Targeting PD-1/PD-L1 interactions for cancer immunotherapy. 2012;1(8):1223–1225. doi: 10.4161/onci.21335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Linch S, Kasiewicz MJ, Mcnamara MJ, Hilgart-Martiszus IF, Farhad M, Redmond WL. Combination OX40 agonism/CTLA-4 blockade with HER2 vaccination reverses T-cell anergy and promotes survival in tumor-bearing mice. 2016;113(3):E319–E327. doi: 10.1073/pnas.1510518113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bafna S, Kaur S, Batra SK. Membrane-bound mucins: the mechanistic basis for alterations in the growth and survival of cancer cells. 2010;29(20):2893–2904. doi: 10.1038/onc.2010.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Suzuki H, Shoda J, Kawamoto T, et al. Expression of MUC1 recognized by monoclonal antibody MY.1E12 is a useful biomarker for tumor aggressiveness of advanced colon carcinoma. 2004;21(4):321–329. doi: 10.1023/b:clin.0000046133.35133.cc. [DOI] [PubMed] [Google Scholar]
- 65.Ghosh SK, Pantazopoulos P, Medarova Z, Moore A. Expression of underglycosylated MUC1 antigen in cancerous and adjacent normal breast tissues. 2013;13(2):109–118. doi: 10.1016/j.clbc.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kufe DW. MUC1-C oncoprotein as a target in breast cancer: activation of signaling pathways and therapeutic approaches. 2013;32(9):1073–1081. doi: 10.1038/onc.2012.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rivalland G, Loveland B, Mitchell P. Update on Mucin-1 immunotherapy in cancer: a clincal perspective. 2015;15(12):1773–1787. doi: 10.1517/14712598.2015.1088519. [DOI] [PubMed] [Google Scholar]
- 68.Burchell JM, Mungul A, Taylor-Papadimitriou J. O-linked glycosylation in the mammary gland: changes that occur during malignancy. 2001;6(3):355–364. doi: 10.1023/a:1011331809881. [DOI] [PubMed] [Google Scholar]
- 69.Blixt O, Bueti D, Burford B, et al. Autoantibodies to aberrantly glycosylated MUC1 in early stage breast cancer are associated with a better prognosis. 2011;13(2):R25. doi: 10.1186/bcr2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hanisch FG. Design of a MUC1-based cancer vaccine. 2005;33(4):705–708. doi: 10.1042/BST0330705. [DOI] [PubMed] [Google Scholar]
- 71.Keiichi K, Taguchi O, Ozaki Y, et al. Dendritic cell vaccine immunotherapy of cancer targeting MUC1 mucin. 2003;12:493–502. [PubMed] [Google Scholar]
- 72.Lakshmiarayanan V, Supekar NT, Wei J, et al. MUC1 vaccines, comprised of glycosylated or non-glycosylated peptides or tumor- derived MUC1, can circumvent immunoediting to control tumor growth in MUC1 transgenic mice. 2016;11(1):e0145920. doi: 10.1371/journal.pone.0145920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Domchek SM, Recio A, Mick R, et al. Telomerase-specific T-cell immunity in breast cancer: effect of vaccination on tumor immunosurveillance. 2007;67(21):10546–10555. doi: 10.1158/0008-5472.CAN-07-2765. [DOI] [PubMed] [Google Scholar]
- 74.William CH, Matthew M. Telomerase activation, cellular imortalization and cancer. 2001;33:123–129. doi: 10.3109/07853890109002067. [DOI] [PubMed] [Google Scholar]
- 75.Papanikolaou V, Athanassiou E, Dubos S, et al. hTERT regulation by NF-jB and c-myc in irradiated HER2-positive breast cancer cells. 2011;87(6):609–621. doi: 10.3109/09553002.2011.572112. [DOI] [PubMed] [Google Scholar]
- 76.Märten A, Sievers E, Albers P, et al. Telomerase-pulsed dendritic cells: preclinical results and outcome of a clinical Phase I/II trial in patients with metastatic renal cell carcinoma. 2006;4:Doc02. [PMC free article] [PubMed] [Google Scholar]
- 77.Vonderheide RH, Domchek SM, Schultze JL, et al. Vaccination of cancer patients against telomerase induces functional antitumor CD8+ T lymphocytes. 2004;10:828–839. doi: 10.1158/1078-0432.ccr-0620-3. [DOI] [PubMed] [Google Scholar]
- 78.Holbro T, Beerli RR, Maurer F, Koziczak M, Barbas CF, 3rd, Hynes NE. The ErbB2/ErbB3 heterodimer functions as an oncogenic unit: ErbB2 requires ErbB3 to drive breast tumor cell proliferation. 2003;100(15):8933–8938. doi: 10.1073/pnas.1537685100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Siegel P, Ryan ED, Cardiff RD, Muller WJ. Elevated expression of activated forms of Neu/ErbB-2 and ErbB-3 are involved in the induction of mammary tumors in transgenic mice: implications for human breast cancer. 1999;18:2149–2164. doi: 10.1093/emboj/18.8.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Green AR, Barros FF, Abdel-Fatah TM, et al. HER2/HER3 heterodimers and p21 expression are capable of predicting adjuvant trastuzumab response in HER2+ breast cancer. 2014;145(1):33–44. doi: 10.1007/s10549-014-2925-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Siziopikou KP, Cobleigh M. The basal subtype of breast carcinomas may represent the group of breast tumors that could benefit from EGFR-targeted therapies. 2007;16(1):104–107. doi: 10.1016/j.breast.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 82.Kim YJ, Choi JS, Seo J, et al. MET is a potential target for use in combination therapy with EGFR inhibition in TNBC. 2014;134(10):2424–2436. doi: 10.1002/ijc.28566. [DOI] [PubMed] [Google Scholar]
- 83.Lengyel E, Prechtel D, Resau JH, et al. c-met expression in node positive breast cancer identifies patients with poor clinical outcomes independent of HER2/neu. 2005;113(4):678–682. doi: 10.1002/ijc.20598. [DOI] [PubMed] [Google Scholar]
- 84.Minuti G, Cappuzzo F, Duchnowska R, et al. Increased MET and HGF gene copy numbers are associated with trastuzumab failure in HER2 positive metastatic cancer. 2012;107(5):793–799. doi: 10.1038/bjc.2012.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sohn J, Liu S, Parinyanitikul N, et al. cMET activation and EGFR-directed therapy resistance in triple-negative breast cancer. 2014;5(9):745–753. doi: 10.7150/jca.9696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Turner N, Moretti E, Siclari O, et al. Targeting triple negative breast cancer: is p53 the answer? 2013;39(5):541–550. doi: 10.1016/j.ctrv.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 87.Kandioler-Eckersberger D, Ludwig C, Rudas M, et al. TP53 mutation and p53 overexpression for prediction of response to neoadjuvant treatment in breast cancer patients. 2000;6(1):50–56. [PubMed] [Google Scholar]
- 88.Miller LD, Smeds J, George J, et al. An expression signature for p53 status in human breast cancer predicts mutation status, transcriptional effects, and patient survival. 2005;102(38):13550–13555. doi: 10.1073/pnas.0506230102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Met O, Balslev E, Flyger H, Svane IM. High immunogenic potential of p53 mRNA-transfected dendritic cells in patients with primary breast cancer. 2011;125(2):395–406. doi: 10.1007/s10549-010-0844-9. [DOI] [PubMed] [Google Scholar]