Abstract
Apolipoprotein E (apoE), a plasma lipoprotein well known for its important role in lipid and cholesterol metabolism, has also been implicated in many neurological diseases. In this study, we examined the effect of apoE on the pathophysiology of traumatic spinal cord injury (SCI). ApoE-deficient mutant (apoE−/−) and wild-type mice received a T9 moderate contusion SCI and were evaluated using histological and behavioral analyses after injury. At 3 days after injury, the permeability of spinal cord-blood-barrier, measured by extravasation of Evans blue dye, was significantly increased in apoE−/− mice compared to wild type. The inflammation and spared white matter was also significantly increased and decreased, respectively, in apoE−/− mice compared to the wild type ones. The apoptosis of both neurons and oligodendrocytes was also significantly increased in apoE−/− mice. At 42 days after injury, the inflammation was still robust in the injured spinal cord in apoE−/− but not wild type mice. CD45+ leukocytes from peripheral blood persisted in the injured spinal cord of apoE−/− mice. The spared white matter was significantly decreased in apoE−/− mice compared to wild type ones. Locomotor function was significantly decreased in apoE−/− mice compared to wild type ones from week 1 to week 8 after contusion. Treatment of exogenous apoE mimetic peptides partially restored the permeability of spinal cord-blood-barrier in apoE−/− mice after SCI. Importantly, the exogenous apoE peptides decreased inflammation, increased spared white matter and promoted locomotor recovery in apoE−/− mice after SCI. Our results indicate that endogenous apoE plays important roles in maintaining the spinal cord-blood-barrier and decreasing inflammation and spinal cord tissue loss after SCI, suggesting its important neuroprotective function after SCI. Our results further suggest that exogenous apoE mimetic peptides could be a novel and promising neuroprotective reagent for SCI.
Keywords: Apolipoprotein E, Spinal cord-blood-barrier, Neuroprotection, Treatment, Spinal cord injury
1. Introduction
Spinal cord injury (SCI) is a devastating traumatic neurological disorder, significantly disabling about 300,000 Americans and millions worldwide (Sekhon and Fehlings, 2001). Currently, there are no effective treatments for this devastating neurological disorder. Pathophysiology of SCI involves the primary and secondary injuries (Hulsebosch, 2002). The secondary injury is a series of progressive pathophysiological responses initiated by the initial mechanical injury, which exacerbates the injury and eventually leads to greater functional loss after SCI. Compromised blood-spinal cord-barrier (BSCB), edema, catecholamines, oxidative damage, excitotoxicity, inflammation, nitric oxide and apoptosis all contribute to the secondary injury following SCI (Hall and Springer, 2004). Identification of reagents, which could block multiple secondary injury pathways to decrease the injury and functional losses, will potentially lead to development of novel therapies for SCI.
Apolipoprotein E (apoE) is one of the most abundant apolipoproteins in the brain and is mainly synthesized by astrocytes, but also in some neurons after injury (Kim et al., 2009; Laskowitz et al., 1998). In addition to its important role in lipid and cholesterol metabolism (Wang and Eckel, 2014), apoE has also been implicated in the physiopathology of many neurological diseases (Giau et al., 2015; Mahley, 2016). For example, the gene dosage of the APOE4 allele is associated with increasing risk and early onset of Alzheimer's disease (Toops et al., 2016; Zhu et al., 2015; Huang et al., 2017). APOE4 is also associated with significantly more severe disability, earlier progression, and poorer recovery following relapse in multiple sclerosis patients (Fazekas et al., 2001; Fazekas et al., 2000; Chapman et al., 2001) and poor neurological outcome following stroke (Sheng et al., 1999a; Laskowitz et al., 1997b), closed head injury (Jordan et al., 1997; Wilson et al., 2002) or cerebral hemorrhage (Alberts et al., 1995; McCarron et al., 1999). Animal experiments show ApoE knockout (apoE−/−) mice are especially vulnerable to neurological injuries. In a mouse experimental autoimmune encephalomyelitis (EAE), a model of human multiple sclerosis, apoE−/− mice show worse disability with poorer recovery and more inflammatory infiltrates in the CNS compared with wild-type (WT) controls (Karussis et al., 2003). ApoE−/− mice also have increased infarct volumes and higher mortality rates than WT after stroke (Sheng et al., 1999a; Sheng et al., 1998; Laskowitz et al., 1997b). In closed head injury paradigms, apoE−/− mice have decreased levels of antioxidant compounds (Lomnitski et al., 1999a; Lomnitski et al., 1997) and impaired functional recovery and neuronal loss (Chen et al., 1997a; Han and Chung, 2000). Notably, treatment with exogenous apoE decrease the anatomical and functional deficits in the animal models of several neurological diseases, such as ischemic stroke (Horsburgh et al., 2000; Tu et al., 2017), of Alzheimer's disease (Sarantseva et al., 2009), traumatic brain injury (Wang et al., 2007; Lynch et al., 2005; Laskowitz et al., 2010; Kaufman et al., 2010; Hoane et al., 2009; Laskowitz et al., 2017; Cao et al., 2016), or EAE (Li et al., 2006). These studies suggest that apoE plays important roles in neuroprotection after neurological diseases and injuries.
Although extensively studied in many neurological diseases, especially in Alzheimer's diseases, the role of apoE in SCI has not been well investigated. Previous study shows that APOE4 is associated with worse neurological recovery and a longer length of stay in rehabilitation in individuals with traumatic cervical spinal cord injury (Jha et al., 2008), suggesting the potential role of apoE in SCI. The expression of apoE is significantly increased after traumatic SCI in both rats and mice (Schmitt et al., 2006; Seitz et al., 2003). However, the role of apoE in SCI remains to be elucidated. In this study, we examined the function of endogenous apoE in the pathophysiology of traumatic SCI using apoE−/− mice. We also evaluated the therapeutic potential of exogenous apoE after SCI. We report here that the anatomical and functional deficits are much greater in apoE−/− mice following traumatic SCI. Importantly, exogenous apoE mimetic peptides decrease the injury size and promote functional recovery after SCI. Our results suggest that endogenous apoE plays an important neuroprotective role after SCI, and exogenous apoE mimetic peptides might be a novel and promising neuroprotective reagent for SCI.
2. Materials and methods
All animal care and surgical interventions were undertaken in strict accordance with the Public Health Service Policy on Humane Care and Use of Laboratory Animals, Guide for the Care and Use of Laboratory Animals (https://grants.nih.gov/grants/olaw/guide-for-the-care-and-use-of-laboratory-animals.pdf), and with the approval of Animal Welfare Committee at the University of Texas Health Science Center at Houston (UTHealth). C57BL/6 (WT) mice and apoE−/− mice in C57BL/6 background were purchased from the Jackson Laboratory (The Jackson Laboratory, Bar Harbor, ME) and maintained in the animal facility at UTHealth. All mice used for these experiments were females between the ages of 10 and 16 weeks. Pairs of female WT and KO mice were matched by ages with differences of 2 weeks. All animals used for this study are shown in Table 1. Female mice were used in this study to avoid bladder complications and urolithiasis that frequently occur in male mice following SCI.
Table 1.
Animals used for experiments.
| Experiments | EB exp at 1 d PI |
EB exp at 3 d PI |
Histology at 3 d PI |
Behavioral and histology at 42 d PI |
Exogenous apoE treatment
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| EB exp at 3 d PI
|
Behavioral and histology at 21 d PI
|
|||||||||
| WT Ctr | ApoE−/− Ctr |
ApoE−/− plus Cog112 |
Ctr | Antp | Cog112 | |||||
| C57BL/6 mice | 4 | 4 | 6 | 10 | 4 | |||||
| ApoE−/− mice | 4 | 4 | 6 | 10 | 4 | 4 | 10 | 10 | 10 | |
2.1. Contusion injury and surgical procedure
The surgical procedure for SCI was described previously (Fan et al., 2013). After anesthetization with a mixed solution of ketamine (80 mg/kg, ip) and xylazine (10 mg/kg, ip), mice received a dorsal laminectomy at the 9th thoracic vertebral (T9) level to expose the spinal cord and then a moderate T9 contusive injury using an Infinite Horizons impactor (Precision Systems and Instrumentation) at 60 kdyn with the spine stabilized using steel stabilizers inserted under the transverse processes one vertebra above and below the injury (Hill et al., 2009). Afterwards, the wound was sutured in layers, bacitracin ointment (Qualitest Pharmaceuticals, Huntsville, AL) was applied to the wound area, 0.1 ml of a 20 mg/ml stock of gentamicin (ButlerSchein, Dublin, OH) was injected subcutaneously, and the animals recovered on a water-circulating heating pad. Then mice received analgesic agent, buprenorphine (0.05 mg/kg, SQ; Reckitt Benckiser, Hull, England) twice a day for three days. Bladders were emptied manually until automatic voiding returned spontaneously. The animals were survived for 3, 21 or 42 days per the experimental design (Table 1).
2.2. Behavioral assessment
Open-field locomotor tests with the Basso-Mouse-Scale (BMS) (Basso et al., 2006) were performed at 1, 3, 7 days after injury and then once every week for 6 weeks. All the animals were coded and behavioral assessments were performed by two investigators blinded with respect to the treatment groups. The mean BMS scores were tallied by injured groups and plotted as a function of time post-injury. Changes in BMS scores over time for the groups were analyzed using a Repeated Measures ANOVA with the between groups factor. Differences among the groups over the survival times were found by using Tukey HSD post hoc t-tests.
2.3. Histological analyses
The spared white matter in the injured epicenter was determined as previously described (Cao et al., 2005). Briefly, at designated time points after SCI (Table 1), mice were briefly anesthetized with a mixed solution of ketamine/xylazine and perfused transcardially with 0.01 M phosphate buffered saline (PBS) (pH 7.4), followed by 4% paraformaldehyde in 0.01 M phosphate buffer (PB). The spinal cord segments with the injury were removed, cryoprotected in 30% sucrose buffer overnight at 4 °C and embedded in OCT compound. Serial 20 µm thick sections through the entire injury site were cut transversely on a cryostat. Two sets of slides were stained with the iron-eriochrome cyanine R (EC) and Cresyl violet, respectively, to identify myelinated white matter and residual spared tissue. The lesion epicenter was defined as the section containing the least amount of spared white matter. White matter sparing was defined as tissue showing normal myelin appearance and density (lacking cysts, degeneration). Septae or fibrous bands of tissue observed within and/or spanning areas of cystic cavitation were not considered to represent spared tissue. The total cross-sectional area of the spinal cord and the lesion boundary were measured with a Zeiss Observer Z1 inverted fluorescence microscope with the stereological stage and Zen Digital Imaging system (Carl Zeiss). An unbiased estimation of the percentage of spared tissue was calculated using the Cavalieri method (Michel and Cruz-Orive, 1988). The percent spared white matter (WM) areas were calculated by dividing the spared white matter area to the total spinal cord volume in the respective cross spinal cord sections. Mean values of spared WM among different injured groups were calculated and statistically compared using Repeated ANOVA followed by Tukey's post hoc testing.
2.4. Immunohistochemistry
After blocking with 10% donkey serum in Tris buffered saline (TBS) containing 0.3% Triton X-100 (TBST) for 1 h at room temperature (RT), the sections were incubated in TBST containing 5% donkey serum, polyclonal rabbit anti-glial fibrillary acidic protein (GFAP, a marker for astrocytes, 1:200, Dako) and monoclonal mouse anti-CD68 (also called ED1, a marker that labels macrophages and activated microglia, 1:200, Pharmigen) overnight at 4 °C. After three washes of 10 min in TBS, sections were incubated in TBST containing 5% donkey serum, donkey anti-rabbit FITC-conjugated ‘Fab’ fragments (1:100, Jackson-ImmunoRes Lab, Baltimore, MD) and donkey anti-mouse Texas red-conjugated ‘Fab’ fragments (1:200, Jackson-ImmunoRes Lab) for 1 h at RT. The sections were rinsed in TBS and cover-slipped with antifade mounting medium (Molecular Probes, Eugene, OR). To detect the extravasation of circulating IgG in the spinal cord after injury, the cryostat sections were blocked with 10% donkey serum in TBST for 1 h at RT and then TBST containing 5% donkey serum and donkey anti-mouse IgG Texas red-conjugated ‘Fab’ fragments (1:200, Jackson-ImmunoRes Lab) for 1 h at RT. The sections were rinsed in TBS and cover-slipped with antifade mounting medium (Molecular Probes, Eugene, OR). Apoptotic neurons and oligodendrocytes were detected by terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labeling (TUNEL). TUNEL staining was performed on the spinal cord slides according to the manufacturer's manual (R & D systems, Minneapolis, MN). Zeiss Observer Z1 inverted fluorescence microscope with Apotome and stereological stage with mosaic tissue capture capabilities was used to capture representative images. The density of ED1 labeling above background was quantified using threshold-based measures within the whole spinal cord cross sections. The Zen (Carl Zeiss) or ImageJ quantification software programs were used to determine proportional area measurements. The percentages of ED1+ area were calculated by dividing the total ED1 areas by the total spinal cord area in the respective cross sections. Mean values of percent lesion area and spared WM among different injured groups were calculated and statistically compared using one-way ANOVA followed by Tukey's post hoc testing.
2.5. Vascular leakage
Vascular leakage was quantified using the established Evans blue (EB) dye technique (Kakinuma et al., 1998). EB dye was injected intraperitoneally (40 mg/kg) at 3 days after SCI. Distribution of the dye was confirmed by a visible change in the mouse skin color within 1 h after injection. Overnight (around 14 h) after receiving injections, the mice were anesthetized with a mixed solution of ketamine/xylazine and intracardially perfused with saline and thoroughly rinsed 20 min until colorless effluence flew out of the right atrium. Spinal cords (1.5 cm from the injury both caudally and rostrally, total 3 cm length) were removed and incubated in 1 ml formamide (Sigma, St. Louis, MO) for 72 h at RT. Extracts were centrifuged at 13,000 rpm at 4 °C for 10 min. Supernatant was used for spectrophotometric measurement at 620 nm and compared to standard curve of Evans blue. Mean values of the amount of EB in spinal cord in WT and apoE−/− groups were statistically compared using t testing.
2.6. Isolation and culture of spinal cord neurons and glial cells
Thoracic spinal cords at postnatal day 2 or 3 from WT or apoE−/− mice were mechanically but gently dissociated in Hank's Buffered Salt Solution (HBSS) (Invitrogen, Carlsbad, CA) and filtered through 70 µm Hydrophilic Nylon Net Filter (Millipore, Bedford, MA) in order to eliminate tissue debris. The obtained single-cell suspension was spun down (1500 g, 10 min), plated onto poly-lysine/laminin-coated 24-well plates at 100,000 cells per well, and cultured in grown medium consisting of F12/DMEM medium (Invitrogen), N2 and B27 supplements (each at 1×; Invitrogen), 5% fetal bovine serum and penicillin-streptomycin solution (Invitrogen). The growth medium was changed every other day. At 7 days in vitro, spinal cord cultures from WT or apoE−/− mice were randomly divided into two groups, control or N-methyl-d-aspartate (NMDA) treatment group, which was treated with saline or 10 µM NMDA in growth medium for 30 min, respectively. All cultures were rinsed with HBSS for three times and continued to grow in growth medium for 1 more day. The cultures were then immunostained as described previously (Wang et al., 2011; Fan et al., 2014). To detect the surface membrane antigens, O1 (an oligodendrocyte marker), cells cultured on 24-well plates were incubated with mouse-anti-O1 (1:500, Millipore, Billerica, MA) at 4 °C for 45 min. After fixation with 4% paraformaldehyde, cells were incubated in Texas Red-conjugated donkey anti-mouse IgM for 1 h at room temperature (RT). For recognition of other antigens, cells cultured on 24 well plates were fixed with 4% paraformaldehyde. Mouse monoclonal antibodies against β-tubulin III (1: 5000, BioLengend, San Diego, CA) and rabbit polyclonal antibodies against GFAP (1: 2000; DakoCytomation, Denmark) were applied overnight at 4 °C. Then, the appropriate fluorophore conjugated-secondary antibodies 1:200 (Jackson ImmunoResearch, Baltimore, MD) were applied and the nuclei were counterstained with DAPI. Controls were performed with species-specific IgG or sera and with inappropriate secondary antibodies. Both showed negligible background. Total cellular counts for each experimental well were obtained in 10 fields under 20 × objective from three independent culture wells. The result for each experimental condition was verified a minimum of three times. The average numbers of neurons or oligodendrocytes in different groups were calculated and statistically compared using one-way ANOVA followed by Tukey's post hoc testing.
2.7. Treatment of apoE mimetic peptide after SCI
The apoE-mimetic peptide was initially derived from the receptor-binding domain of apoE protein (i.e., apoE133–149) to simulate the bioactivities of the holo-protein (Lynch et al., 2003; Laskowitz et al., 2001). COG112 was designed by fusion of apoE133–149 with a protein transduction domain antennapedia (Antp) to enhance blood-brain barrier (BBB) and cell membrane penetration. COG112 has demonstrated more potent anti-inflammatory activity and therapeutic efficacy in EAE mice (Wei et al., 2013; Li et al., 2006). ApoE-mimetic COG112, and antennapedia (Antp) were synthesized by PolyPeptide Laboratories (San Diego, CA) using standard Fmoc-based chemistry. All peptides were purified by high-performance liquid chromatography (HPLC) to a purity of > 95%. The peptide sequence of COG112 is acetyl-RQI-KIWFQNRRMKWKKCLRVR-LASHLRKLRKRLL-amide. The prefix peptide Antp was found lack of anti-inflammatory activity previously with a sequence of acetyl-RQIKIWFQNRRMKWKK-amide (Li et al., 2006).
ApoE−/− mice received a T9 moderate (60 kdyn IH) contusion injury as described above and then were randomly assigned to one of the following treatment groups: 1) vehicle control, i.e., lactated Ringer's buffer; 2) peptide control Antp (1 mg/kg); and 3) COG112 (1 mg/kg). The mice received the first treatment by tail vein injection at 30 min post-injury and then intraperitoneally twice a day for 3 weeks. At 3 days post-injury, 4 mice from each group were used for testing blood-spinal cord-barrier (BSCB) function by EB technique as described above. The rest mice in each group were tested for locomotion using BMS scores at 1, 3, 7 days after injury and then once every week for 3 weeks. Animals were sacrificed at the end of week 3 for histological and immunohistochemistry examination.
3. Results
3.1. The permeability of BSCB is increased in apoE−/− mice after acute SCI
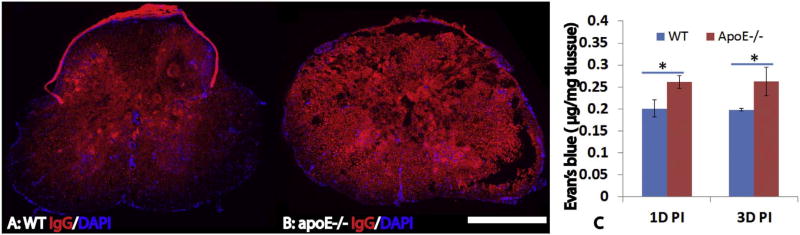
Previous studies show that apoE plays important roles in the function of blood-brain-barrier (BBB) (Bell et al., 2012; Fullerton et al., 2001; Hafezi-Moghadam et al., 2007; Tai et al., 2016). To test whether a lack of endogenous apoE further deteriorates the integrity of blood-spinal cord-barrier (BSCB) after injury, we detected the extravasation of IgG using immunohistochemistry. At 3 days after SCI, the extravasation of circulating IgG into the spinal cord was significantly increased in the injured epicenter as well as the rostral and caudal spinal cords in apoE−/− mice compared to WT mice (Fig. 1A, B). We further quantified the permeability of BSCB following SCI using Evan Blue (EB) technique. Evans blue dye is an azo dye that binds with high affinity to albumin. The Evans blue–albumin (EBA) complex (MW 69 kDa) then penetrates organs lacking blood–tissue barriers but is excluded from the spinal cord by the BSCB. Thus, the presence of EBA in the spinal cord is evidence of increased permeability of the BSCB. At 1 and 3 days after SCI, the amount of Evans blue in the injured spinal cord was significantly increased in apoE−/− mice compared to WT (Fig. 1C, p < 0.05). These results show that permeability of BSCB is further increased in apoE−/− mice following SCI, suggesting that the endogenous apoE plays an important role in the integrity of BSCB.
Fig. 1.
Increased permeability of blood-spinal cord-barrier (BSCB) in apoE deficient mice after SCI. BSCB permeability was evaluated by the extravasation of IgG using immunohistochemistry (A, B). Compared to wild type B6 mice (A), the extravasation of circulating IgG in the injury epicenter was significantly increased in apoE−/− mice at 3 days after SCI (B). Quantification of BSCB permeability using Evan Blue technique also showed that the amount of EB in the injured spinal cord was significantly increased in apoE−/− mice compared to wild type B6 mice at 1 day and 3 days after SCI. Scale bar = 500 µm in A, B. Data in C represent the mean ± SD, N = 4, stars represent p < 0.05.
3.2. The inflammation and neural tissue loss are increased in apoE−/− mice after acute SCI
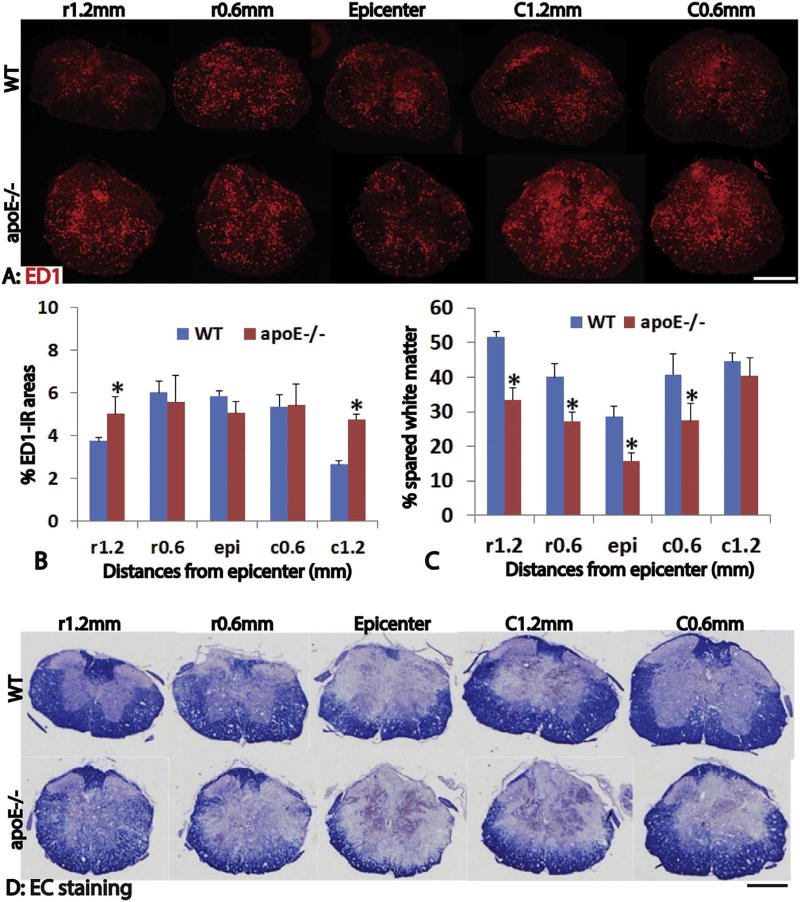
Previous studies suggest that apoE plays important roles in regulating inflammation after neurological diseases (Laskowitz et al., 2000; Laskowitz et al., 2001; Li et al., 2006; Lei et al., 2016). To test the function of endogenous apoE in inflammation after SCI, we quantified the number of ED1+ macrophages and activated microglia in the injured spinal cord using immunohistochemistry. ED1+ macrophages were found throughout the injured spinal cord around the epicenter at day 3 post-injury (Fig. 2A). The percentages of ED1 immunoreactive (IR) areas to the total spinal cord areas were not significantly different between apoE−/− and wild type mice spinal cords at epicenter, 0.6 mm rostral and caudal from epicenter (Fig. 2A, B). However, the percentages of ED1-IR areas were significantly increased in the injured spinal cord at 1.2 mm rostral and caudal from epicenter in apoE−/− mice (Fig. 2B, p < 0.05). These results show that a lack of endogenous apoE in apoE−/− mice increases the inflammation after SCI, especially at the spinal cord around the epicenter.
Fig. 2.
Increased inflammation and neural tissue loss in apoE deficient mice after acute SCI. Inflammation was evaluated by immunohistochemistry of ED1, a marker for macrophage and activated microglia. Three days after SCI, inflammation was significantly increased in apoE deficient mice, especially at 1.2 mm from injury epicenter both rostrally and caudally (A, B). Importantly, the spared white matter shown by EC staining was significantly decreased in apoE deficient mice compared to wild type mice (C, D). Scale bar = 500 µm in A and D. Data in B and C represent the mean ± SD, N = 4, stars represent p < 0.05.
We further quantified the spared white matter areas using EC staining on day 3 post-injury (Fig. 2C, D). The areas of spared white matter were significantly reduced in apoE−/− mice, by as much as 81% at the epicenter in apoE−/− than that in wild type mice (Fig. 2C, D). The percentages of spared white matter area to the total spinal cord area were significantly decreased in apoE−/− mice compared to WT mice at the injured epicenter, 0.6 mm away from epicenter rostrally and caudally, and 1.2 mm rostrally, respectively (Fig. 2C, D). These results indicate that a lack of endogenous apoE decreases the spared white matter following SCI.
3.3. The apoptosis of neurons and oligodendrocytes is increased in apoE−/− mice after acute SCI
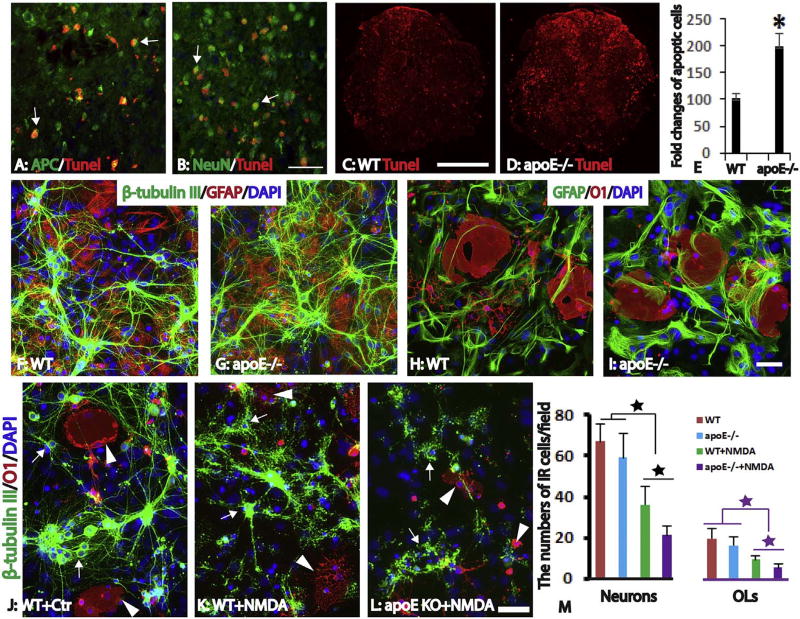
To test effects of endogenous apoE on the survival of neural cells, we examined the apoptosis in the injured spinal cord using TUNEL staining. At 3 days post-injury, many apoptotic cells were found throughout the injured spinal cord at both WT and apoE−/− mice (Fig. 3A – D). Many TUNEL+ cells were CC1+ oligodendrocytes in white matter (Fig. 3A) and NeuN+ neurons in gray matter (Fig. 3B). The number of TUNEL+ cells were greatest at the injury epicenter and gradually decreased both caudally and rostrally. The number of TUNEL + cells in the epicenter was significantly increased in apoE−/− mice compared to WT mice (Fig. 3C, D). It was doubled in apoE−/− compared to WT mice (Fig. 3E, p < 0.05). These results indicate that apoptosis of both neurons and oligodendrocytes is significantly increased in apoE−/− mice, suggesting the neuroprotective roles of endogenous apoE in preventing the cell death of neurons and glial cells after SCI.
Fig. 3.
Increased neuron and oligodendrocyte loss in apoE−/− mice in vivo and in vitro. Robust apoptosis shown by TUNEL staining was observed in the injured spinal cord at 3 days after SCI (A–D). At the injury epicenter, most TUNNEL + apoptotic cells are oligodendrocytes (A, arrows) and neurons (B, arrows). The number of apoptotic cells in the injured epicenter were significantly increased in apoE−/− mice (C–D) and doubled in apoE−/− mice compared to WT mice (E). To further examine the effects of endogenous apoE in the survival of neurons and oligodendrocytes, neurons, astrocytes and oligodendrocytes were isolated from postnatal day 3 spinal cord of WT or apoE−/− mice and co-cultured for 7 days (F–I). In the control culture condition, the morphology and number of neurons (F, G) or oligodendrocytes (H, I) was not significantly different between WT or apoE−/− mice. Treatment with NMDA resulted in the death of neurons and oligodendrocytes derived from WT (K) and apoE−/− (L) mice. The surviving neurons (arrows) and oligodendrocytes (arrowheads) from WT or apoE−/− mice were significantly decreased after NMDA treatment compared to its respective un-treated counterpart (Fig. J–M). Importantly, the numbers of survived neurons or oligodendrocytes from apoE−/− spinal cord were further decreased to only half of those from WT mice spinal cord after NMDA treatment (M). Scale bar = 50 µm in A, B, 500 µm in C, D, 50 µm in F–I and J–L. Data in E and M represent the mean ± SD, N = 4 (E) and 6 (M), stars represent p < 0.05.
We further examined the neuroprotective roles of endogenous apoE in neurons and oligodendrocytes using an in vitro culture of spinal cord. Neurons and glial cells, including astrocytes and oligodendrocytes, were isolated from postnatal spinal cord of WT or apoE−/− mice and continued to grow in vitro for 7 days. To mimic the in situ spinal cord, we used the mixture culture which included neurons, astrocytes and oligodendrocytes (Fig. 3F – I). After being culturing for 7 days, neurons matured with multiple long neurites (Fig. 3F, G). Oligodendrocyte precursor cells or immature oligodendrocytes were also differentiated into mature oligodendrocytes with rich processes which form membrane sheets (Fig. 3H, I). Neurons and oligodendrocytes were integrated well with co-cultured astrocytes (Fig. 3F – I). The morphologies and numbers of neurons and oligodendrocytes from WT and apoE−/− mice were not significantly different (Fig. 3F–I, M), suggesting that apoE is not necessary for the development of neurons and oligodendrocytes. We then tested the effects of excitotoxicity in neurons and oligodendrocytes from WT and apoE−/− mice. Compared to control culture (Fig. 3J), treatment with NMDA resulted in the death of neurons and oligodendrocytes derived from WT (Fig. 3K) and apoE−/− (Fig. 3L) mice. The surviving neurons and oligodendrocytes from WT or apoE−/− mice were significantly decreased after NMDA treatment compared to its respective un-treated counterparts (Fig. 3J – M). Of importance, the numbers of surviving neurons or oligodendrocytes from apoE−/− spinal cord were further decreased to only half of those from WT mice spinal cord after NMDA treatment (Fig. 3M). These data suggest that endogenous apoE in WT mice plays an important role in protecting neurons and oligodendrocytes from excitotoxicity.
3.4. The long-term anatomical deficits are increased in apoE knockout mice
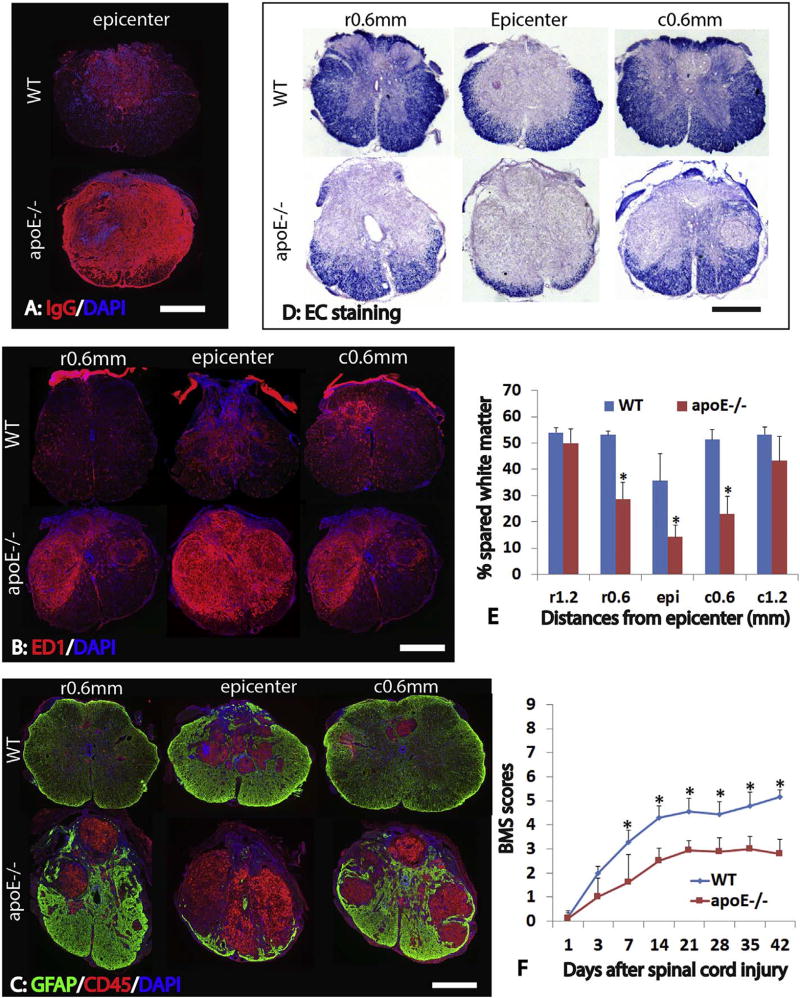
To test whether lack of endogenous apoE causes long term deficits after SCI, we examined the injured spinal cord at 42 days post-injury histologically. The BSCB was still compromised in apoE−/− mice at 42 days after SCI. Compared to WT mice, the extravasation of circulating IgG was significantly increased in the injured spinal cord epicenter in apoE−/− mice (Fig. 4A). The inflammation was significantly increased in the apoE−/− mice compared to WT mice at 42 days after SCI. The numbers of ED1-IR macrophages and active microglia cells were significantly increased not only at the injured epicenter but also at 0.6 mm from epicenter rostrally and caudally in apoE−/− mice (Fig. 4B). In addition, the numbers of CD45+ lymphocytes were also significantly increased at the injured spinal cord in apoE−/− mice (Fig. 4C). These results suggest that inflammation is not only increased but also persists for a longer time in the injured spinal cord of apoE−/− mice following contusion.
Fig. 4.
Increased anatomical deficiency in apoE−/− mice after SCI. At 6 weeks after SCI, the extravasation of circulating IgG was significantly increased in the injured spinal cord epicenter in apoE−/− mice compared to WT ones (A). The numbers of ED1+ macrophages and active microglial cells were significantly increased at the injured epicenter as well as at 0.6 mm from epicenter rostrally and caudally in apoE−/− mice compared to WT ones (B). Similarly, the numbers of CD45+ lymphocytes were significantly increased in these injury levels in apoE−/− mice (C). Conversely, the spared white matter was significantly decreased in apoE−/− mice at the injured epicenter as well as 0.6 mm rostrally and caudally from the epicenter (D, E). Importantly, locomotion function assessed by Basso-Mouse-Score (BMS) was significantly worsen in apoE−/− mice starting at 1 week after SCI (F). Scale bar = 500 µm in A, B, and C. Data in D and E represent the mean ± SD, N = 6 (D) and 10 (E), stars represent p < 0.05.
To examine the long-term effects of endogenous apoE in the injury size, we further evaluated the areas of spared white matter in the injured spinal cord at 42 days post-injury. The spared white matter was significantly decreased in the apoE−/− mice compared to WT mice (Fig. 4D, E). The percentages of spared white matter to the whole spinal cord were significantly decreased at the injured epicenter, 0.6 mm rostrally and caudally in apoE−/− mice (Fig. 4E). These results show that a lack of endogenous apoE leads to more white matter loss, suggesting endogenous apoE is neuroprotective after SCI.
3.5. Locomotor recovery is impaired in apoE−/− mice after SCI
To evaluate the effects of apoE on locomotor recovery after SCI, we assessed locomotor function using Basso-Mouse-Scale (BMS), an open-field locomotor test for mice. Following SCI, all animals displayed immediate hindlimb paralysis (Fig. 4E). BMS was scored 0 and 0.5 on day 1 post-injury. Then gradual recovery in hindlimb locomotion was observed in both apoE−/− and WT groups. However, locomotor recovery was significantly slower in apoE−/− mice, and plateaued at about 21 days. BMS scores were significantly lower in apoE−/− mice compared to WT mice from days 7 to 42 post-injury (Fig. 4F, p < 0.05). These results suggest that endogenous apoE is important for functional recovery after SCI.
3.6. Exogenous apoE improves the functional recovery in apoE−/− mice after SCI
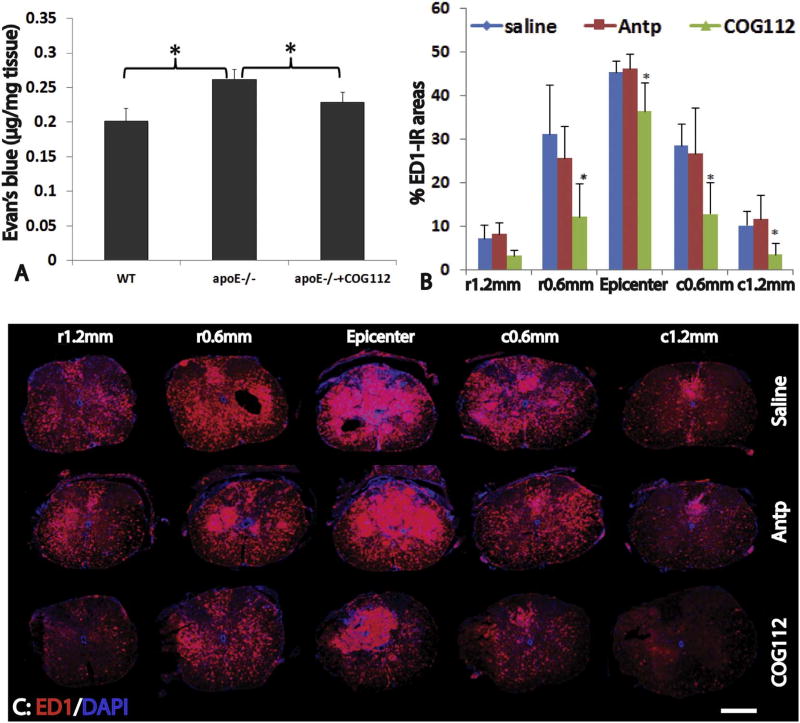
In order to further investigate the effects of exogenous apoE on SCI, apoE−/− mice were treated with apoE mimetic peptide after SCI. Previous studies showed that the apoE-mimetic peptides, which were derived from the receptor-binding domain of apoE protein, (i.e., apoE133–149), could bind apoE receptors to simulate the bioactivities of the holo-protein (Li et al., 2006; Singh et al., 2008; Li et al., 2010). We first tested whether administration of apoE peptides could decrease the permeability of BSCB in apoE−/− mice following SCI. After receiving treatment of apoE peptides starting at 30 min following SCI and then daily for 3 days, the amount of Evans blue in the injured spinal cord was significantly decreased in apoE−/− mice treated with apoE peptides compared to ones receiving control peptides or saline (p < 0.05) (Fig. 5A). Consistent with the EB experiment, the immunostaining also showed that extravasation of circulating IgG in the injured spinal cord was significantly decreased in apoE peptide-treated apoE−/− mice compared the control peptide- or saline-treated apoE−/− mice (not shown). These results show that exogenous apoE could reduce the dysfunction of BSCB in apoE−/− mice following SCI.
Fig. 5.
Treatment of exogenous apoE mimetic peptides decreases the BSCB permeability and inflammation in apoE−/− mice after SCI. At 3 days after SCI, permeability of BSCB shown by the extravasation of Evan Blue was significantly increased in apoE−/− mice compared to WT (A). However, the deteriorated permeability of BSCB in apoE−/− mice was significantly attenuated after treatment with exogenous apoE mimic peptides following SCI (A). Importantly, treatment of exogenous apoE mimic peptides also significantly decreased inflammation in apoE−/− mice at 21 days after SCI (B, C). Scale bar = 500 µm in C. Data in A and B represent the mean ± SD, N = 4 (D) and 6 (E), stars represent p < 0.05.
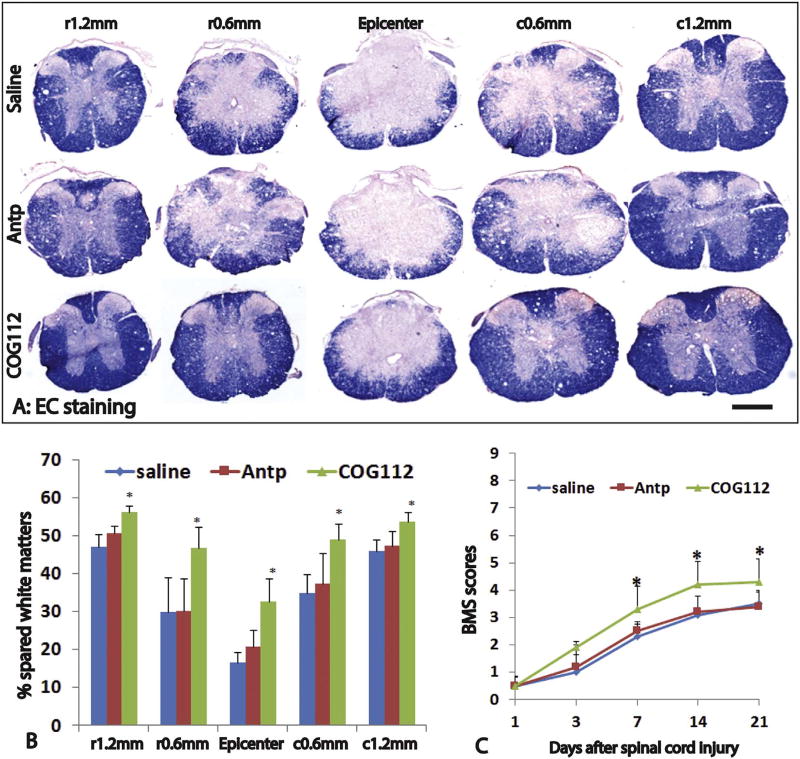
We further examined the effects of exogenous apoE in inflammation and spared white matter after the behavioral test at 3 weeks post-injury. The number of ED1+ cells in the injured spinal cord were significantly decreased in apoE−/− mice treated with exogenous apoE peptide compared to ones treated with control peptide or saline (Fig. 5B, C). The percentages of ED1+ IR areas were significantly lower in apoE peptide treated mice than saline or control peptide treated mice at the lesion epicenter, 0.6 mm caudal, 1.2 mm caudal to the epicenter or 0.6 mm rostral to the epicenter (Fig. 5B, C). There was no significant difference between the saline- or control peptide-treated mice at all examined spinal cords. We also quantified the spared white matters using EC staining (Fig. 6A, B). The apoE mimetic peptide treatment mice contained more white matter in the injured spinal cord than the two control groups did (Fig. 6A, B). For example, at the injury epicenter, the percentage of spared white matter area in apoE treatment group was 1.98 and 1.58 fold to ones in control peptide group and saline group, respectively. In addition to the injury epicenter, the percentages of spared white matter were also significantly increased in apoE peptide treatment mice compared to control peptide or saline treatment mice at 0.6 mm rostral and caudal from epicenter. The percentages of spared white matter were not significantly different between apoE−/− mice treated with saline or control peptide at all examined spinal cord levels. These results show that treatment with exogenous apoE peptides decreases the inflammation and increases the spared white matter, further confirming the neuroprotective roles of apoE after SCI.
Fig. 6.
Treatment of exogenous apoE mimetic peptides improves the anatomical and functional recovery in apoE−/− mice after SCI. The spared white matter was significantly increased in increased in apoE−/− mice treated by exogenous mimic apoE peptides compared to ones treated by saline or control Antp peptides (A, B). Importantly, locomotion function was also significantly increased in apoE−/− mice treated by exogenous mimic apoE peptides compared to ones treated by saline or control Antp peptides from 1 week to 3 weeks post-injury, the longest time for treatment (C). Scale bar = 500 µm in A. Data in B and C represent the mean ± SD, N = 6 (B) and 10 (C), stars represent p < 0.05.
We then tested the effects of exogenous apoE peptides in the locomotion recovery after SCI. All injured animals showed paralysis with no or slight hindlimb movement on day 1 after injury and then gradual recovery in hindlimb locomotion (Fig. 6C). BMS scores in animals treated with the apoE peptide COG112 were higher than those treated with saline, or control peptide Antp at days 7, 14 and 21 but not on day 1 or day 3 post-injury. There was no statistically significant difference in the BMS scores between saline group and control peptide Antp group at all tested time points. These results show that delivery of exogenous apoE peptides promotes locomotion recovery in apoE−/− mice after SCI.
4. Discussion
In this study, we showed the worsening dysfunction of BSCB, the increased inflammation, the greater neural tissue loss, and importantly, the worse locomotion functional deficits in apoE−/− mice after SCI. Previous studies show that expression of apoE is significantly increased after traumatic SCI in both rats and mice (Schmitt et al., 2006; Seitz et al., 2003). However, the role of endogenous apoE in SCI remains unknown. The results in our present study suggest that endogenous apoE plays important protective roles following traumatic SCI. These results are consistent with previous studies regarding pleiotropic roles of apoE in other neurological disorders. For example, apoE−/− mice have increased infarct volumes, worse functional outcomes and higher mortality rates than WT control mice following focal (Laskowitz et al., 1997c) and global (Sheng et al., 1999b; Horsburgh et al., 2000) ischemia. And the increased anatomical and functional deficits in apoE−/− mice are independent of vascular changes (Bart et al., 1998). Similarly, apoE−/− mice have decreased levels of antioxidant compounds and impaired functional recovery and neuronal loss after the closed head injury paradigms (Chen et al., 1997b; Lomnitski et al., 1999b) or the controlled cortical impact brain injury model (Zhong et al., 2017). Furthermore, apoE−/− mice are cognitively impaired (Gordon et al., 1996; Oitzl et al., 1997) and show signs of neurodegeneration in the central nervous system without injuries (Masliah et al., 1997). Importantly, polymorphisms in apoE may also contribute to the risk of developing Alzheimer's disease (AD) and other neurological diseases in humans. Multiple genome-wide association studies indicate that APOE4 is by far the strongest genetic risk factor for AD, increasing the risk of developing disease by 400% to 1500% for apoE4 carriers compared with apoE3/3 carriers (Kim et al., 2009; Mahley and Huang, 2006; Zlokovic, 2013; Huang et al., 2017; Zhu et al., 2015). APOE may also increases the risk of developing MS and cognitive dysfunction in patients with MS (Shi et al., 2011; Yin et al., 2012), although this remains controversial (Lill et al., 2012). Furthermore, APOE4 is associated with worse neurological recovery and a longer length of stay in rehabilitation in individuals with traumatic cervical spinal cord injury (Jha et al., 2008). Taken together, these studies show that endogenous apoE plays important roles in neurological functions in the normal central nervous system or after injuries.
Although the exact mechanisms by which a lack of endogenous apoE leads to greater functional loss after SCI remain to be studied, the results in present studies suggest that the increased dysfunction of BSCB could be one of these important mechanisms. We show that the permeability of BSCB is significantly increased in apoE deficient mice after acute SCI, and the dysfunction of BSCB persists even at 42 days after injury. The increased permeability of BSCB is associated with the exaggerated inflammation, greater neural tissue loss and worse locomotor outcomes in apoE deficient mice. These results suggest that endogenous apoE plays a vital role in the integrity of the BSCB after SCI. Our results are consistent with previous studies showing that apoE deficiency increases the permeability of blood-brain-barrier (BBB) in vivo and in vitro (Bell et al., 2012; Nishitsuji et al., 2011; Fullerton et al., 2001; Hafezi-Moghadam et al., 2007; Teng et al., 2017). For example, apoE−/− mice have been shown to increase BBB integrity damage in many animal models, such as brain injury (Methia et al., 2001; Teng et al., 2017), hyperlipidemia, and/or atherosclerosis, and aged mice (Grinberg and Thal, 2010; Badaut et al., 2012). Since BSCB plays an important role in maintaining the microenvironment and normal functions of the spinal cord, disruption of BSCB caused by SCI leads to the leakage of blood constituents, such as inflammation cells, immune proteins and other large molecules, which collectively initiate and/or contribute to a “vicious cycle” of pathophysiological processes, resulting in progressive tissue loss and neurological deficits after injury (Popovich et al., 1996; Whetstone et al., 2003). BSCB permeability is inversely correlated with locomotion function after SCI (Cohen et al., 2009; Patel et al., 2009). The worsening anatomic and functional deficits in apoE deficient mice shown in the present study could be at least in part caused by the exaggerated BSCB dysfunction due to a lack of endogenous apoE. We further show that treatment with exogenous apoE mimetic peptides can partially replace endogenous apoE to decrease the BSCB permeability, thus reducing the anatomic and functional deficits in apoE deficient mice after SCI. Previous study show that delivery of exogenous apoE mimetic peptides is able to significantly decrease brain-blood-barrier (BBB) disruption and reduce the neuronal loss and functional deficits after subarachnoid hemorrhage (Pang et al., 2017). These studies suggest that exogenous apoE mimetic peptides could be a novel therapeutic agent to decrease the dysfunction of BSCB or BBB after SCI or other neurological diseases.
Another mechanism by which apoE deficient mice have greater anatomical and functional deficits following SCI could be exaggerated inflammation due to lack of endogenous apoE. Our results show that the number of ED1+ microphages in the injured spinal cord are significantly increased in apoE deficient mice at acute (3 days post-injury) and chronic (42 days post-injury) stages. The number of T cells are also increased in apoE deficient mice after SCI. The increased permeability of BSCB may contribute to the exaggerated inflammation in apoE deficient mice after SCI by enhancing the influx of inflammation cells in peripheral blood, such as macrophages, T lymphocytes, etc. In addition, apoE can directly modulate the immune responses. For example, apoE suppresses microglial activation and its release of the inflammatory cytokines TNFα and IL-6 both in vitro and in vivo (Lynch et al., 2003; Laskowitz et al., 1997a; Laskowitz et al., 2001). ApoE inhibits mitogen induced lymphocyte proliferation (Zhang et al., 2010). ApoE has also shown to inhibit T-cell activation by reducing the density of immune stimulatory proteins on antigen-presenting cells (Tenger and Zhou, 2003). In apoE−/− mice, macrophages stimulate T-cell activation more effectively as antigen-presenting cells than in WT mice (Tenger and Zhou, 2003). Thus, lack of endogenous apoE could lead to increased inflammation responses after SCI as observed in the present study. Inflammatory and immune responses play important roles not only in neuronal cell death but also in posttraumatic white matter degeneration after SCI (Schwab and Bartholdi, 1996; Donnelly and Popovich, 2008). Our results show that increased inflammation correlates with a dramatic loss of white matter in apoE−/− mice, suggesting that apoE is essential for restricting inflammation and lesion size. In addition, endogenous apoE may directly protect neurons and oligodendrocytes from cell death after SCI. Our results show that the number of apoptotic neurons and oligodendrocytes is significantly increased in apoE−/− mice at 3 days after SCI. Our in vitro data also show that loss of neurons and oligodendrocytes induced by NMDA treatment is significantly increased in the spinal cord culture of apoE−/− mice compared to WT, suggesting the endogenous apoE from astrocytes in WT spinal cord culture may protect the co-cultured neurons and oligodendrocytes from excitotoxicity (Qiu et al., 2003; Aono et al., 2003). Lack of endogenous apoE may contribute to exaggerated cell death of neurons and oligodendrocytes in apoE−/− mice after acute SCI (Fig. 3). Although inflammation is not significantly different at the injury epicenter or at spinal cord areas around the epicenter at 3 days after SCI between apoE−/− mice and WT (Fig. 2), the increased loss of neurons oligodendrocytes may at least partially result in the greater tissue loss observed in these spinal cord areas in apoE−/− mice after acute SCI (Fig. 2). Previous studies show that decrease of astrogliosis increase the injury and lead to more severe functional deficits after SCI, suggesting astroglial scar plays an important neuroprotective role following SCI (Faulkner et al., 2004; Herrmann et al., 2008). The reactive astrocytes may restrict the inflammation cells to spread to the spared spinal cord and prevent the further damage after traumatic SCI (Sahni et al., 2010). Our present study show that endogenous apoE may directly protect neurons and oligodendrocytes from apoptosis. The endogenous apoE in the central nervous system is mainly synthesized by astrocytes and significantly increase after injury (Kim et al., 2009; Laskowitz et al., 1998). Secretion of apoE to protect neurons and oligodendrocytes from cell death could be another potential mechanism by which the reactive astrocytes provide neuroprotection after SCI. Robust apoptosis has been detected in the injured epicenter after acute SCI and majority of apoptotic cells are neurons and oligodendrocytes (Fig. 3). However, significant apoptotic astrocytes have not been observed in the injured spinal cord in either WT or apoE−/− mice. The astrocytes are activated and form astroglial scar after SCI in both WT and apoE−/− mice (Fig. 4). Astrogliosis is not significantly different between WT and apoE−/− mice following SCI.
We further tested whether delivery of exogenous apoE would provide neuroprotection in apoE deficient mice after SCI. Due to its large size, apoE does not readily cross the blood–brain barrier (Linton et al., 1991). Given the impracticality of intrathecal delivery, it is unlikely that intact apoE could serve as a viable therapeutic strategy in acute neurological disease. To address this issue, a series of small apoE mimetic peptides derived from its receptor-binding region have been created (Laskowitz and Vitek, 2007; Laskowitz et al., 2006; Guptill et al., 2017). These peptides retain the anti-inflammatory and neuroprotective effects of the native apoE protein yet cross blood-brain-barrier (Lynch et al., 2003; Laskowitz et al., 2001; Laskowitz et al., 2007; Laskowitz et al., 2017). In the present study, we used apoE peptide, COG112, which is designed by fusion of apoE133–149 with a protein transduction domain antennapedia (Antp) to enhance blood-brain barrier (BBB) and cell membrane penetration. COG112 has demonstrated more potent anti-inflammatory activity and therapeutic efficacy in EAE mice than peptides without Antp (Li et al., 2006; Wei et al., 2013; Li et al., 2010). Our current study extends the therapeutic potentials of COG112 in SCI. Our results show that COG112 decreases inflammation after SCI. In addition, COG112 protects BSCB and reduces its permeability after SCI. As discussed above, these two mechanisms are not exclusive but synergistic. Of importance, we show that these synergistic mechanisms by COG112 decrease the injury and promote locomotor recovery, suggesting that exogenous apoE peptide, COG112, could at least partially replace the endogenous apoE to provide neuroprotection after SCI in apoE−/− mice. Consistent with the results in our present study, previous study show that delivery of COG112 decreases inflammation and demyelination and promote remyelination and functional recovery after a local chemical demyelination in rat (Gu et al., 2013). Similarly, treatment with another apoE mimetic peptide, COG1410, immediately after injury for 14 days reduces the inflammation and the neural tissue damage and ameliorates locomotion deficits in a rat thoracic SCI model (Wang et al., 2014). These studies indicate that exogenous apoE mimetic peptides can provide additional neuroprotection after SCI even after the expression of endogenous apoE is increased following injury. Neuroprotection of exogenous apoE mimetic peptides has also been demonstrated in several other neurological disease models, such as ischemia stroke (Tu et al., 2017; Wang et al., 2013), intracerebral hemorrhage (Lei et al., 2016; Laskowitz et al., 2012), traumatic brain injury (Laskowitz et al., 2017; Laskowitz et al., 2007), EAE, a model of human multiple sclerosis (Li et al., 2006), or the peripheral nerve injury (Li et al., 2010). These studies suggest that apoE mimetic peptides could be an important neuroprotective reagent after SCI and other neurological diseases.
5. Conclusions
Our study shows that endogenous apoE plays important roles in the integrity of BSCB and the regulation of inflammation after SCI. Importantly, endogenous apoE is critical in neuroprotection by preserving the neural tissues and reducing locomotion deficits after SCI. Furthermore, our data indicate that exogenous apoE peptides are able to at least partially replace the endogenous apoE to decrease the neural injuries and locomotion deficits in apoE deficient mice after traumatic SCI. Our data suggest that apoE plays important neuroprotective roles and apoE mimetic peptides could be an important therapeutic reagent to decrease the injury and promote functional recovery after SCI.
Acknowledgments
We thank Ms. Chrystine Gallegos for her proof reading of the manuscript.
Funding
This work was supported by the National Institutes of Health (R01 NS099635), the Staman Ogilvie Fund for Spinal Cord Injury Recovery, Craig H. Neilsen Foundation (385446), and Mission Connect-a project of The TIRR Foundation.
References
- Alberts MJ, Graffagnino C, McClenny C, DeLong D, Strittmatter W, Saunders AM, Roses AD. ApoE genotype and survival from intracerebral haemorrhage. Lancet. 1995;346:575. doi: 10.1016/s0140-6736(95)91411-0. [DOI] [PubMed] [Google Scholar]
- Aono M, Bennett ER, Kim KS, Lynch JR, Myers J, Pearlstein RD, Warner DS, Laskowitz DT. Protective effect of apolipoprotein E-mimetic peptides on N-methyl-d-aspartate excitotoxicity in primary rat neuronal-glial cell cultures. Neuroscience. 2003;116:437–445. doi: 10.1016/s0306-4522(02)00709-1. [DOI] [PubMed] [Google Scholar]
- Badaut J, Copin JC, Fukuda AM, Gasche Y, Schaller K, da Silva RF. Increase of arginase activity in old apolipoprotein-E deficient mice under Western diet associated with changes in neurovascular unit. J. Neuroinflammation. 2012;9:132. doi: 10.1186/1742-2094-9-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bart RD, Sheng H, Laskowitz DT, Pearlstein RD, Warner DS. Regional CBF in apolipoprotein E-deficient and wild type mice during focal cerebral ischemia. Neuroreport. 1998;9:2615–2620. doi: 10.1097/00001756-199808030-00035. [DOI] [PubMed] [Google Scholar]
- Basso DM, Fisher LC, Anderson AJ, Jakeman LB, McTigue DM, Popovich PG. Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J. Neurotrauma. 2006;23:635–659. doi: 10.1089/neu.2006.23.635. [DOI] [PubMed] [Google Scholar]
- Bell RD, Winkler EA, Singh I, Sagare AP, Deane R, Wu Z, Holtzman DM, Betsholtz C, Armulik A, Sallstrom J, Berk BC, Zlokovic BV. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature. 2012;485:512–516. doi: 10.1038/nature11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Q, Zhang YP, Iannotti C, DeVries WH, XM Xu, Shields CB, Whittemore SR. Functional and electrophysiological changes after graded traumatic spinal cord injury in adult rat. Exp. Neurol. 2005;191(Suppl. 1):S3–S16. doi: 10.1016/j.expneurol.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Cao F, Jiang Y, Wu Y, Zhong J, Liu J, Qin X, Chen L, Vitek MP, Li F, Xu L, Sun X. Apolipoprotein E-mimetic COG1410 reduces acute vasogenic edema following traumatic brain injury. J. Neurotrauma. 2016;33:175–182. doi: 10.1089/neu.2015.3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman J, Vinokurov S, Achiron A, Karussis DM, Mitosek-Szewczyk K, Birnbaum M, Michaelson DM, Korczyn AD. APOE genotype is a major predictor of long-term progression of disability in MS. Neurology. 2001;56:312–316. doi: 10.1212/wnl.56.3.312. [DOI] [PubMed] [Google Scholar]
- Chen Y, Lomnitski L, Michaelson DM, Shohami E. Motor and cognitive deficits in apolipoprotein E-deficient mice after closed head injury. Neuroscience. 1997a;80:1255–1262. doi: 10.1016/s0306-4522(97)00007-9. [DOI] [PubMed] [Google Scholar]
- Chen Y, Lomnitski L, Michaelson DM, Shohami E. Motor and cognitive deficits in apolipoprotein E-deficient mice after closed head injury. Neuroscience. 1997b;80:1255–1262. doi: 10.1016/s0306-4522(97)00007-9. [DOI] [PubMed] [Google Scholar]
- Cohen DM, Patel CB, Ahobila-Vajjula P, Sundberg LM, Chacko T, Liu SJ, Narayana PA. Blood-spinal cord barrier permeability in experimental spinal cord injury: dynamic contrast-enhanced MRI. NMR Biomed. 2009;22:332–341. doi: 10.1002/nbm.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly DJ, Popovich PG. Inflammation and its role in neuroprotection, ax-onal regeneration and functional recovery after spinal cord injury. Exp. Neurol. 2008;209:378–388. doi: 10.1016/j.expneurol.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan C, Zheng Y, Cheng X, Qi X, Bu P, Luo X, Kim DH, Cao Q. Transplantation of D15A–expressing glial-restricted-precursor-derived astrocytes improves anatomical and locomotor recovery after spinal cord injury. Int. J. Biol. Sci. 2013;9:78–93. doi: 10.7150/ijbs.5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan C, Wang H, Chen D, Cheng X, Xiong K, Luo X, Cao Q. Effect of type-2 astrocytes on the viability of dorsal root ganglion neurons and length of neuronal processes. Neural Regen. Res. 2014;9:119–128. doi: 10.4103/1673-5374.125339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J. Neurosci. 2004;24:2143–2155. doi: 10.1523/JNEUROSCI.3547-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazekas F, Strasser-Fuchs S, Schmidt H, Enzinger C, Ropele S, Lechner A, Flooh E, Schmidt R, Hartung HP. Apolipoprotein E genotype related differences in brain lesions of multiple sclerosis. J. Neurol. Neurosurg. Psychiatry. 2000;69:25–28. doi: 10.1136/jnnp.69.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazekas F, Strasser-Fuchs S, Kollegger H, Berger T, Kristoferitsch W, Schmidt H, Enzinger C, Schiefermeier M, Schwarz C, Kornek B, Reindl M, Huber K, Grass R, Wimmer G, Vass K, Pfeiffer KH, Hartung HP, Schmidt R. Apolipoprotein E epsilon 4 is associated with rapid progression of multiple sclerosis. Neurology. 2001;57:853–857. doi: 10.1212/wnl.57.5.853. [DOI] [PubMed] [Google Scholar]
- Fullerton SM, Shirman GA, Strittmatter WJ, Matthew WD. Impairment of the blood-nerve and blood-brain barriers in apolipoprotein e knockout mice. Exp. Neurol. 2001;169:13–22. doi: 10.1006/exnr.2001.7631. [DOI] [PubMed] [Google Scholar]
- Giau VV, Bagyinszky E, An SS, Kim SY. Role of apolipoprotein E in neurodegenerative diseases. Neuropsychiatr. Dis. Treat. 2015;11:1723–1737. doi: 10.2147/NDT.S84266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon I, Genis I, Grauer E, Sehayek E, Michaelson DM. Biochemical and cognitive studies of apolipoprotein-E-deficient mice. Mol. Chem. Neuropathol. 1996;28:97–103. doi: 10.1007/BF02815210. [DOI] [PubMed] [Google Scholar]
- Grinberg LT, Thal DR. Vascular pathology in the aged human brain. Acta Neuropathol. 2010;119:277–290. doi: 10.1007/s00401-010-0652-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Z, Li F, Zhang YP, Shields LB, Hu X, Zheng Y, Yu P, Zhang Y, Cai J, Vitek MP, Shields CB. Apolipoprotein E mimetic promotes functional and histological recovery in lysolecithin-induced spinal cord demyelination in mice. J. Neurol. Neurophysiol. 2013;2014:10. doi: 10.4172/2155-9562.S12-010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guptill JT, Raja SM, Boakye-Agyeman F, Noveck R, Ramey S, TM Tu, Laskowitz DT. Phase 1 randomized, double-blind, placebo-controlled study to determine the safety, tolerability, and pharmacokinetics of a single escalating dose and repeated doses of CN-105 in healthy adult subjects. J. Clin. Pharmacol. 2017;57:770–776. doi: 10.1002/jcph.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafezi-Moghadam A, Thomas KL, Wagner DD. ApoE deficiency leads to a progressive age-dependent blood-brain barrier leakage. Am. J. Physiol. Cell Physiol. 2007;292:C1256–C1262. doi: 10.1152/ajpcell.00563.2005. [DOI] [PubMed] [Google Scholar]
- Hall ED, Springer JE. Neuroprotection and acute spinal cord injury: areappraisal. NeuroRx. 2004;1:80–100. doi: 10.1602/neurorx.1.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SH, Chung SY. Marked hippocampal neuronal damage without motor deficits after mild concussive-like brain injury in apolipoprotein E-deficient mice. Ann. N. Y. Acad. Sci. 2000;903:357–365. doi: 10.1111/j.1749-6632.2000.tb06387.x. [DOI] [PubMed] [Google Scholar]
- Herrmann JE, Imura T, Song B, Qi J, Ao Y, Nguyen TK, Korsak RA, Takeda K, Akira S, Sofroniew MV. STAT3 is a critical regulator of astrogliosis and scar formation after spinal cord injury. J. Neurosci. 2008;28:7231–7243. doi: 10.1523/JNEUROSCI.1709-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill RL, Zhang YP, Burke DA, DeVries WH, Zhang Y, Magnuson DS, Whittemore SR, Shields CB. Anatomical and functional outcomes following a precise, graded, dorsal laceration spinal cord injury in C57BL/6 mice. J. Neurotrauma. 2009;26:1–15. doi: 10.1089/neu.2008.0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoane MR, Kaufman N, Vitek MP, McKenna SE. COG1410 improves cognitive performance and reduces cortical neuronal loss in the traumatically injured brain. J. Neurotrauma. 2009;26:121–129. doi: 10.1089/neu.2008.0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsburgh K, McCulloch J, Nilsen M, McCracken E, Large C, Roses AD, Nicoll JA. Intraventricular infusion of apolipoprotein E ameliorates acute neuronal damage after global cerebral ischemia in mice. J. Cereb. Blood Flow Metab. 2000;20:458–462. doi: 10.1097/00004647-200003000-00003. [DOI] [PubMed] [Google Scholar]
- Huang YA, Zhou B, Wernig M, Sudhof TC. ApoE2, ApoE3, and ApoE4 differentially stimulate APP transcription and Abeta secretion. Cell. 2017;168:427–441. doi: 10.1016/j.cell.2016.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulsebosch CE. Recent advances in pathophysiology and treatment of spinal cord injury. Adv. Physiol. Educ. 2002;26:238–255. doi: 10.1152/advan.00039.2002. [DOI] [PubMed] [Google Scholar]
- Jha A, Lammertse DP, Coll JR, Charlifue S, Coughlin CT, Whiteneck GG, Worley G. Apolipoprotein E epsilon4 allele and outcomes of traumatic spinal cord injury. J. Spinal. Cord Med. 2008;31:171–176. doi: 10.1080/10790268.2008.11760708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan BD, Relkin NR, Ravdin LD, Jacobs AR, Bennett A, Gandy S. Apolipoprotein E epsilon4 associated with chronic traumatic brain injury in boxing. JAMA. 1997;278:136–140. [PubMed] [Google Scholar]
- Kakinuma Y, Hama H, Sugiyama F, Yagami K, Goto K, Murakami K, Fukamizu A. Impaired blood-brain barrier function in angiotensinogen-deficient mice. Nat. Med. 1998;4:1078–1080. doi: 10.1038/2070. [DOI] [PubMed] [Google Scholar]
- Karussis D, Michaelson DM, Grigoriadis N, Korezyn AD, Mizrachi-Koll R, Chapman S, Abramsky O, Chapman J. Lack of apolipoprotein-E exacerbates experimental allergic encephalomyelitis. Mult. Scler. 2003;9:476–480. doi: 10.1191/1352458503ms950oa. [DOI] [PubMed] [Google Scholar]
- Kaufman NA, Beare JE, Tan AA, Vitek MP, McKenna SE, Hoane MR. COG1410, an apolipoprotein E-based peptide, improves cognitive performance and reduces cortical loss following moderate fluid percussion injury in the rat. Behav. Brain Res. 2010;214:395–401. doi: 10.1016/j.bbr.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Basak JM, Holtzman DM. The role of apolipoprotein E in Alzheimer's disease. Neuron. 2009;63:287–303. doi: 10.1016/j.neuron.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskowitz DT, Vitek MP. Apolipoprotein E and neurological disease: therapeutic potential and pharmacogenomic interactions. Pharmacogenomics. 2007;8:959–969. doi: 10.2217/14622416.8.8.959. [DOI] [PubMed] [Google Scholar]
- Laskowitz DT, Goel S, Bennett ER, Matthew WD. Apolipoprotein E suppresses glial cell secretion of TNF alpha. J. Neuroimmunol. 1997a;76:70–74. doi: 10.1016/s0165-5728(97)00021-0. [DOI] [PubMed] [Google Scholar]
- Laskowitz DT, Sheng H, Bart RD, Joyner KA, Roses AD, Warner DS. Apolipoprotein E-deficient mice have increased susceptibility to focal cerebral ischemia. J. Cereb. Blood Flow Metab. 1997b;17:753–758. doi: 10.1097/00004647-199707000-00005. [DOI] [PubMed] [Google Scholar]
- Laskowitz DT, Sheng H, Bart RD, Joyner KA, Roses AD, Warner DS. Apolipoprotein E-deficient mice have increased susceptibility to focal cerebral ischemia. J. Cereb. Blood Flow Metab. 1997c;17:753–758. doi: 10.1097/00004647-199707000-00005. [DOI] [PubMed] [Google Scholar]
- Laskowitz DT, Horsburgh K, Roses AD. Apolipoprotein E and the CNS response to injury. J. Cereb. Blood Flow Metab. 1998;18:465–471. doi: 10.1097/00004647-199805000-00001. [DOI] [PubMed] [Google Scholar]
- Laskowitz DT, Lee DM, Schmechel D, Staats HF. Altered immune responses in apolipoprotein E-deficient mice. J. Lipid Res. 2000;41:613–620. [PubMed] [Google Scholar]
- Laskowitz DT, Thekdi AD, Thekdi SD, Han SK, Myers JK, Pizzo SV, Bennett ER. Downregulation of microglial activation by apolipoprotein E and apoE-mimetic peptides. Exp. Neurol. 2001;167:74–85. doi: 10.1006/exnr.2001.7541. [DOI] [PubMed] [Google Scholar]
- Laskowitz DT, Fillit H, Yeung N, Toku K, Vitek MP. Apolipoprotein E-de-rived peptides reduce CNS inflammation: implications for therapy of neurological disease. Acta Neurol. Scand. Suppl. 2006;185:15–20. doi: 10.1111/j.1600-0404.2006.00680.x. [DOI] [PubMed] [Google Scholar]
- Laskowitz DT, McKenna SE, Song P, Wang H, Durham L, Yeung N, Christensen D, Vitek MP. COG1410, a novel apolipoprotein E-based peptide, improves functional recovery in a murine model of traumatic brain injury. J. Neurotrauma. 2007;24:1093–1107. doi: 10.1089/neu.2006.0192. [DOI] [PubMed] [Google Scholar]
- Laskowitz DT, Song P, Wang H, Mace B, Sullivan PM, Vitek MP, Dawson HN. Traumatic brain injury exacerbates neurodegenerative pathology: improvement with an apolipoprotein E-based therapeutic. J. Neurotrauma. 2010;27:1983–1995. doi: 10.1089/neu.2010.1396. [DOI] [PubMed] [Google Scholar]
- Laskowitz DT, Lei B, Dawson HN, Wang H, Bellows ST, Christensen DJ, Vitek MP, James ML. The apoE-mimetic peptide, COG1410, improves functional recovery in a murine model of intracerebral hemorrhage. Neurocrit. Care. 2012;16:316–326. doi: 10.1007/s12028-011-9641-5. [DOI] [PubMed] [Google Scholar]
- Laskowitz DT, Wang H, Chen T, Lubkin DT, Cantillana V, TM Tu, Kernagis D, Zhou G, Macy G, Kolls BJ, Dawson HN. Neuroprotective pentapeptide CN-105 is associated with reduced sterile inflammation and improved functional outcomes in a traumatic brain injury murine model. Sci Rep. 2017;7:46461. doi: 10.1038/srep46461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei B, James ML, Liu J, Zhou G, Venkatraman TN, Lascola CD, Acheson SK, Dubois LG, Laskowitz DT, Wang H. Neuroprotective pentapeptide CN-105 improves functional and histological outcomes in a murine model of intracerebral hemorrhage. Sci Rep. 2016;6:34834. doi: 10.1038/srep34834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li FQ, Sempowski GD, McKenna SE, Laskowitz DT, Colton CA, Vitek MP. Apolipoprotein E-derived peptides ameliorate clinical disability and inflammatory infiltrates into the spinal cord in a murine model of multiple sclerosis. J. Pharmacol. Exp. Ther. 2006;318:956–965. doi: 10.1124/jpet.106.103671. [DOI] [PubMed] [Google Scholar]
- Li FQ, Fowler KA, Neil JE, Colton CA, Vitek MP. An apolipoprotein E-mimetic stimulates axonal regeneration and remyelination after peripheral nerve injury. J. Pharmacol. Exp. Ther. 2010;334:106–115. doi: 10.1124/jpet.110.167882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lill CM, et al. Closing the case of APOE in multiple sclerosis: no association with disease risk in over 29,000 subjects. J. Med. Genet. 2012;49:558–562. doi: 10.1136/jmedgenet-2012-101175. [DOI] [PubMed] [Google Scholar]
- Linton MF, Gish R, Hubl ST, Butler E, Esquivel C, Bry WI, Boyles JK, Wardell MR, Young SG. Phenotypes of apolipoprotein B and apolipoprotein E after liver transplantation. J. Clin. Invest. 1991;88:270–281. doi: 10.1172/JCI115288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomnitski L, Kohen R, Chen Y, Shohami E, Trembovler V, Vogel T, Michaelson DM. Reduced levels of antioxidants in brains of apolipoprotein E-deficient mice following closed head injury. Pharmacol. Biochem. Behav. 1997;56:669–673. doi: 10.1016/s0091-3057(96)00412-1. [DOI] [PubMed] [Google Scholar]
- Lomnitski L, Chapman S, Hochman A, Kohen R, Shohami E, Chen Y, Trembovler V, Michaelson DM. Antioxidant mechanisms in apolipoprotein E deficient mice prior to and following closed head injury. Biochim. Biophys. Acta. 1999a;1453:359–368. doi: 10.1016/s0925-4439(99)00010-1. [DOI] [PubMed] [Google Scholar]
- Lomnitski L, Chapman S, Hochman A, Kohen R, Shohami E, Chen Y, Trembovler V, Michaelson DM. Antioxidant mechanisms in apolipoprotein E deficient mice prior to and following closed head injury. Biochim. Biophys. Acta. 1999b;1453:359–368. doi: 10.1016/s0925-4439(99)00010-1. [DOI] [PubMed] [Google Scholar]
- Lynch JR, Tang W, Wang H, Vitek MP, Bennett ER, Sullivan PM, Warner DS, Laskowitz DT. APOE genotype and an ApoE-mimetic peptide modify the systemic and central nervous system inflammatory response. J. Biol. Chem. 2003;278:48529–48533. doi: 10.1074/jbc.M306923200. [DOI] [PubMed] [Google Scholar]
- Lynch JR, Wang H, Mace B, Leinenweber S, Warner DS, Bennett ER, Vitek MP, McKenna S, Laskowitz DT. A novel therapeutic derived from apolipoprotein E reduces brain inflammation and improves outcome after closed head injury. Exp. Neurol. 2005;192:109–116. doi: 10.1016/j.expneurol.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Mahley RW. Apolipoprotein E: from cardiovascular disease to neurodegenerative disorders. J. Mol. Med. (Berl.) 2016;94:739–746. doi: 10.1007/s00109-016-1427-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley RW, Huang Y. Apolipoprotein (apo) E4 and Alzheimer's disease: unique conformational and biophysical properties of apoE4 can modulate neuropathology. Acta Neurol. Scand. Suppl. 2006;185:8–14. doi: 10.1111/j.1600-0404.2006.00679.x. [DOI] [PubMed] [Google Scholar]
- Masliah E, Samuel W, Veinbergs I, Mallory M, Mante M, Saitoh T. Neurodegeneration and cognitive impairment in apoE-deficient mice is ameliorated by infusion of recombinant apoE. Brain Res. 1997;751:307–314. doi: 10.1016/s0006-8993(96)01420-5. [DOI] [PubMed] [Google Scholar]
- McCarron MO, Hoffmann KL, DeLong DM, Gray L, Saunders AM, Alberts MJ. Intracerebral hemorrhage outcome: apolipoprotein E genotype, hematoma, and edema volumes. Neurology. 1999;53:2176–2179. doi: 10.1212/wnl.53.9.2176. [DOI] [PubMed] [Google Scholar]
- Methia N, Andre P, Hafezi-Moghadam A, Economopoulos M, Thomas KL, Wagner DD. ApoE deficiency compromises the blood brain barrier especially after injury. Mol. Med. 2001;7:810–815. [PMC free article] [PubMed] [Google Scholar]
- Michel RP, Cruz-Orive LM. Application of the Cavalieri principle and vertical sections method to lung: estimation of volume and pleural surface area. J. Microsc. 1988;150:117–136. doi: 10.1111/j.1365-2818.1988.tb04603.x. [DOI] [PubMed] [Google Scholar]
- Nishitsuji K, Hosono T, Nakamura T, Bu G, Michikawa M. Apolipoprotein E regulates the integrity of tight junctions in an isoform-dependent manner in an in vitro blood-brain barrier model. J. Biol. Chem. 2011;286:17536–17542. doi: 10.1074/jbc.M111.225532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oitzl MS, Mulder M, Lucassen PJ, Havekes LM, Grootendorst J, de Kloet ER. Severe learning deficits in apolipoprotein E-knockout mice in a water maze task. Brain Res. 1997;752:189–196. doi: 10.1016/s0006-8993(96)01448-5. [DOI] [PubMed] [Google Scholar]
- Pang J, Chen Y, Kuai L, Yang P, Peng J, Wu Y, Chen Y, Vitek MP, Chen L, Sun X, Jiang Y. Inhibition of blood-brain barrier disruption by an apolipoprotein E-mimetic peptide ameliorates early brain injury in experimental subarachnoid hemorrhage. Transl Stroke Res. 2017;8:257–272. doi: 10.1007/s12975-016-0507-1. [DOI] [PubMed] [Google Scholar]
- Patel CB, Cohen DM, Ahobila-Vajjula P, Sundberg LM, Chacko T, Narayana PA. Effect of VEGF treatment on the blood-spinal cord barrier permeability in experimental spinal cord injury: dynamic contrast-enhanced magnetic resonance imaging. J. Neurotrauma. 2009;26:1005–1016. doi: 10.1089/neu.2008.0860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovich PG, Horner PJ, Mullin BB, Stokes BT. A quantitative spatial analysis of the blood-spinal cord barrier. I. Permeability changes after experimental spinal contusion injury. Exp. Neurol. 1996;142:258–275. doi: 10.1006/exnr.1996.0196. [DOI] [PubMed] [Google Scholar]
- Qiu Z, Crutcher KA, Hyman BT, Rebeck GW. ApoE isoforms affect neuronal N-methyl-d-aspartate calcium responses and toxicity via receptor-mediated processes. Neuroscience. 2003;122:291–303. doi: 10.1016/j.neuroscience.2003.08.017. [DOI] [PubMed] [Google Scholar]
- Sahni V, Mukhopadhyay A, Tysseling V, Hebert A, Birch D, McGuire TL, Stupp SI, Kessler JA. BMPR1a and BMPR1b signaling exert opposing effects on gliosis after spinal cord injury. J. Neurosci. 2010;30:1839–1855. doi: 10.1523/JNEUROSCI.4459-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarantseva S, Timoshenko S, Bolshakova O, Karaseva E, Rodin D, Schwarzman AL, Vitek MP. Apolipoprotein E-mimetics inhibit neurodegeneration and restore cognitive functions in a transgenic Drosophila model of Alzheimer's disease. PLoS One. 2009;4:e8191. doi: 10.1371/journal.pone.0008191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt C, Miranpuri GS, Dhodda VK, Isaacson J, Vemuganti R, Resnick DK. Changes in spinal cord injury-induced gene expression in rat are strain-dependent. Spine J. 2006;6:113–119. doi: 10.1016/j.spinee.2005.05.379. [DOI] [PubMed] [Google Scholar]
- Schwab ME, Bartholdi D. Degeneration and regeneration of axons in the le-sioned spinal cord. Physiol. Rev. 1996;76:319–370. doi: 10.1152/physrev.1996.76.2.319. [DOI] [PubMed] [Google Scholar]
- Seitz A, Kragol M, Aglow E, Showe L, Heber-Katz E. Apolipoprotein E expression after spinal cord injury in the mouse. J. Neurosci. Res. 2003;71:417–426. doi: 10.1002/jnr.10482. [DOI] [PubMed] [Google Scholar]
- Sekhon LH, Fehlings MG. Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine. 2001;26:S2–12. doi: 10.1097/00007632-200112151-00002. [DOI] [PubMed] [Google Scholar]
- Sheng H, Laskowitz DT, Bennett E, Schmechel DE, Bart RD, Saunders AM, Pearlstein RD, Roses AD, Warner DS. Apolipoprotein E isoform-specific differences in outcome from focal ischemia in transgenic mice. J. Cereb. Blood Flow Metab. 1998;18:361–366. doi: 10.1097/00004647-199804000-00003. [DOI] [PubMed] [Google Scholar]
- Sheng H, Laskowitz DT, Mackensen GB, Kudo M, Pearlstein RD, Warner DS. Apolipoprotein E deficiency worsens outcome from global cerebral ischemia in the mouse. Stroke. 1999a;30:1118–1124. doi: 10.1161/01.str.30.5.1118. [DOI] [PubMed] [Google Scholar]
- Sheng H, Laskowitz DT, Mackensen GB, Kudo M, Pearlstein RD, Warner DS. Apolipoprotein E deficiency worsens outcome from global cerebral ischemia in the mouse. Stroke. 1999b;30:1118–1124. doi: 10.1161/01.str.30.5.1118. [DOI] [PubMed] [Google Scholar]
- Shi J, JL Tu, Gale SD, Baxter L, Vollmer TL, Campagnolo DI, Tyry TM, Zhuang Y, Kuniyoshi SM. APOE epsilon4 is associated with exacerbation of cognitive decline in patients with multiple sclerosis. Cogn. Behav. Neurol. 2011;24:128–133. doi: 10.1097/WNN.0b013e31823380b5. [DOI] [PubMed] [Google Scholar]
- Singh K, Chaturvedi R, Asim M, Barry DP, Lewis ND, Vitek MP, Wilson KT. The apolipoprotein E-mimetic peptide COG112 inhibits the inflammatory response to Citrobacter rodentium in colonic epithelial cells by preventing NF-kappaB activation. J. Biol. Chem. 2008;283:16752–16761. doi: 10.1074/jbc.M710530200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai LM, Thomas R, Marottoli FM, Koster KP, Kanekiyo T, Morris AW, Bu G. The role of APOE in cerebrovascular dysfunction. Acta Neuropathol. 2016;131:709–723. doi: 10.1007/s00401-016-1547-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng Z, Guo Z, Zhong J, Cheng C, Huang Z, Wu Y, Tang S, Luo C, Peng X, Wu H, Sun X, Jiang L. ApoE influences the blood-brain barrier through the NF-kappaB/MMP-9 pathway after traumatic brain injury. Sci Rep. 2017;7:6649. doi: 10.1038/s41598-017-06932-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenger C, Zhou X. Apolipoprotein E modulates immune activation by acting on the antigen-presenting cell. Immunology. 2003;109:392–397. doi: 10.1046/j.1365-2567.2003.01665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toops KA, Tan LX, Lakkaraju A. Apolipoprotein E isoforms and AMD. Adv. Exp. Med. Biol. 2016;854:3–9. doi: 10.1007/978-3-319-17121-0_1. [DOI] [PubMed] [Google Scholar]
- Tu TM, Kolls BJ, Soderblom EJ, Cantillana V, Ferrell PD, Moseley MA, Wang H, Dawson HN, Laskowitz DT. Apolipoprotein E mimetic peptide, CN-105, improves outcomes in ischemic stroke. Ann. Clin. Transl. Neurol. 2017;4:246–265. doi: 10.1002/acn3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Eckel RH. What are lipoproteins doing in the brain? Trends Endocrinol. Metab. 2014;25:8–14. doi: 10.1016/j.tem.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Durham L, Dawson H, Song P, Warner DS, Sullivan PM, Vitek MP, Laskowitz DT. An apolipoprotein E-based therapeutic improves outcome and reduces Alzheimer's disease pathology following closed head injury: evidence of pharmacogenomic interaction. Neuroscience. 2007;144:1324–1333. doi: 10.1016/j.neuroscience.2006.11.017. [DOI] [PubMed] [Google Scholar]
- Wang Y, Cheng X, He Q, Zheng Y, Kim DH, Whittemore SR, Cao QL. Astrocytes from the contused spinal cord inhibit oligodendrocyte differentiation of adult oligodendrocyte precursor cells by increasing the expression of bone morphogenetic proteins. J. Neurosci. 2011;31:6053–6058. doi: 10.1523/JNEUROSCI.5524-09.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Anderson LG, Lascola CD, James ML, Venkatraman TN, Bennett ER, Acheson SK, Vitek MP, Laskowitz DT. Apolipoprotein E mimetic peptides improve outcome after focal ischemia. Exp. Neurol. 2013;241:67–74. doi: 10.1016/j.expneurol.2012.11.027. [DOI] [PubMed] [Google Scholar]
- Wang R, Hong J, Lu M, Neil JE, Vitek MP, Liu X, Warner DS, Li F, Sheng H. ApoE mimetic ameliorates motor deficit and tissue damage in rat spinal cord injury. J. Neurosci. Res. 2014;92:884–892. doi: 10.1002/jnr.23371. [DOI] [PubMed] [Google Scholar]
- Wei J, Zheng M, Liang P, Wei Y, Yin X, Tang Y, Xue Y. Apolipoprotein E and its mimetic peptide suppress Th1 and Th17 responses in experimental autoimmune encephalomyelitis. Neurobiol. Dis. 2013;56:59–65. doi: 10.1016/j.nbd.2013.04.009. [DOI] [PubMed] [Google Scholar]
- Whetstone WD, Hsu JY, Eisenberg M, Werb Z, Noble-Haeusslein LJ. Blood-spinal cord barrier after spinal cord injury: relation to revascularization and wound healing. J. Neurosci. Res. 2003;74:227–239. doi: 10.1002/jnr.10759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JT, Edwards P, Fiddes H, Stewart E, Teasdale GM. Reliability of postal questionnaires for the Glasgow Outcome Scale. J. Neurotrauma. 2002;19:999–1005. doi: 10.1089/089771502760341910. [DOI] [PubMed] [Google Scholar]
- Yin YW, Zhang YD, Wang JZ, Li BH, Yang QW, Fang CQ, Gao CY, Li JC, Zhang LL. Association between apolipoprotein E gene polymorphism and the risk of multiple sclerosis: a meta-analysis of 6977 subjects. Gene. 2012;511:12–17. doi: 10.1016/j.gene.2012.09.010. [DOI] [PubMed] [Google Scholar]
- Zhang HL, Wu J, Zhu J. The immune-modulatory role of apolipoprotein E with emphasis on multiple sclerosis and experimental autoimmune encephalomyelitis. Clin. Dev. Immunol. 2010;2010:186813. doi: 10.1155/2010/186813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J, Cheng C, Liu H, Huang Z, Wu Y, Teng Z, He J, Zhang H, Wu J, Cao F, Jiang L, Sun X. Bexarotene protects against traumatic brain injury in mice partially through apolipoprotein E. Neuroscience. 2017;343:434–448. doi: 10.1016/j.neuroscience.2016.05.033. [DOI] [PubMed] [Google Scholar]
- Zhu L, Zhong M, Elder GA, Sano M, Holtzman DM, Gandy S, Cardozo C, Haroutunian V, Robakis NK, Cai D. Phospholipid dysregulation contributes to ApoE4-associated cognitive deficits in Alzheimer's disease pathogenesis. Proc. Natl. Acad. Sci. U. S. A. 2015;112:11965–11970. doi: 10.1073/pnas.1510011112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic BV. Cerebrovascular effects of apolipoprotein E: implications for Alzheimer disease. JAMA Neurol. 2013;70:440–444. doi: 10.1001/jamaneurol.2013.2152. [DOI] [PMC free article] [PubMed] [Google Scholar]