Abstract
Mitochondrial dynamics contribute to the regulation of mitochondrial shape as well as various mitochondrial functions and quality control. This is of particular interest in the beta-cell because of the key role mitochondria play in the regulation of beta-cell insulin secretion function. Moreover, mitochondrial dysfunction has been suggested to contribute to the development of Type 2 Diabetes. Genetic tools that shift the balance of mitochondrial fusion and fission result in alterations to beta-cell function and viability. Additionally, conditions that induce beta-cell dysfunction, such as exposure to a high nutrient environment, disrupt mitochondrial morphology and dynamics. While it has been shown that mitochondria display a fragmented morphology in islets of diabetic patients and animal models, the mechanism behind this is currently unknown. Here, we review the current literature on mitochondrial morphology and dynamics in the beta-cell as well as some of the unanswered question in this field.
Keywords: Mitochondrial dynamics, mitochondrial morphology, fusion, fission, beta-cells, diabetes
Mitochondrial Dynamics
Mitochondria are dynamic organelles that function as heterogeneous networks. They move from one location to another and undergo continuous fusion and fission events, together termed mitochondrial dynamics. As such, this process regulates mitochondrial morphology, number, location, and function (1,2). Mitochondrial dynamics have been demonstrated to contribute to mitochondrial function in a number of systems including pancreatic beta-cells, muscle, and neurons. It has been established that mitochondrial dynamics can influence various aspects of mitochondrial biology including mitochondrial biogenesis, bioenergetics, heterogeneity and elimination (3).
Proteins that regulate mitochondrial fusion and fission have been identified. In mammals, fusion is regulated by at least three mitochondrially localized GTPases: mitofusin 1 (Mfn1), mitofusin 2 (Mfn2), and optic atrophy protein 1 (Opa1) (4,5). Mfn1 and Mfn2 are localized to the outer mitochondrial membrane, while OPA1 is an inner mitochondrial membrane protein. Mitochondrial fusion is a two-step process where inner and outer membrane fusion occurs as separate events, with fusion of the outer membranes occurring first, which is subsequently followed by inner membrane fusion. Fission is mediated by the transmembrane protein Fis1 and the cytosolic GTPase dynamin related protein 1 (Drp1/DNM1L); with Fis1 as the rate limiting factor in fission in some models (6,7). Drp1 translocates from the cytosol to scission sites (Fis1 sites) on the outer mitochondrial membrane to initiate fission events (8). In addition to Fis1, another outer mitochondrial membrane fission factor has recently been identified, Mff (9). It is a mitochondrial fission factor that also serves in the recruitment of Drp1 to mitochondria. Mff-dependent mitochondrial fission has been shown to be independent of Fis1 (10). Interestingly, it has been recently shown that contact between mitochondria and endoplasmic reticulum (ER) identifies sites of mitochondrial fission (11).
Mitochondrial dynamics facilitate the maintenance of a metabolically efficient mitochondrial population and disruption of either fusion or fission alters mitochondrial morphology and functionality. Manipulation of mitochondrial dynamics proteins and the balance between mitochondrial fusion and fission have been shown to contribute to a number of diseases. There is strong evidence correlating the dysfunctional glucose stimulated insulin secretion (GSIS) in models of type 2 diabetes with mitochondrial dysfunction, including changes in respiratory chain activity (12,13,14,15). Mitochondrial dynamics and morphology have been shown to be regulators of mitochondrial function in a number of cell types (3). These observations gave rise to the hypothesis that an impaired balance between fusion and fission contribute to the deterioration of beta-cell function in the progression of diabetes. The aim of this review is to examine the current literature describing mitochondrial morphology and dynamics in the beta-cell under normal physiological conditions as well as under high nutrient conditions and in type 2 diabetes. Emphasis will be placed on how changes in the fusion/fission balance disrupt both mitochondrial and beta-cell function. We will also address unanswered question and future directions of study in the field of beta-cell mitochondrial dynamics.
Characteristics of Beta-Cell Mitochondria
Mitochondria are essential for beta-cell function. Mitochondrial metabolism serves to integrate nutrients and generate signals necessary for insulin secretion. The mitochondrial generation of ATP in response to glucose is integral to the downstream closure of ATP-sensitive K+ channels, consequently leading to plasma membrane depolarization, opening of voltage-gated Ca2+ channels, and insulin exocytosis (16). GSIS occurs in two distinct phases, first the triggering phase and then a second amplifying phase, and mitochondrial metabolic function have been shown to contribute to both of these phases (16,17). Beta-cell lines depleted of mitochondrial DNA (mtDNA) are unable to secrete insulin in response to stimulatory glucose levels (18). This demonstrated that mitochondria, specifically electron transport chain activity (ETC), are required for normal beta-cell function. The role of mitochondria in the beta-cell can be further appreciated when examining mitochondrial membrane potential (∆ψmt) in response to elevated glucose concentrations. Heart et al. demonstrated that beta-cell mitochondria respond to increasing glucose concentrations with a corresponding increase in ∆ψmt (19). This increase in ∆ψmt is positively correlated with an increase in insulin secretion.
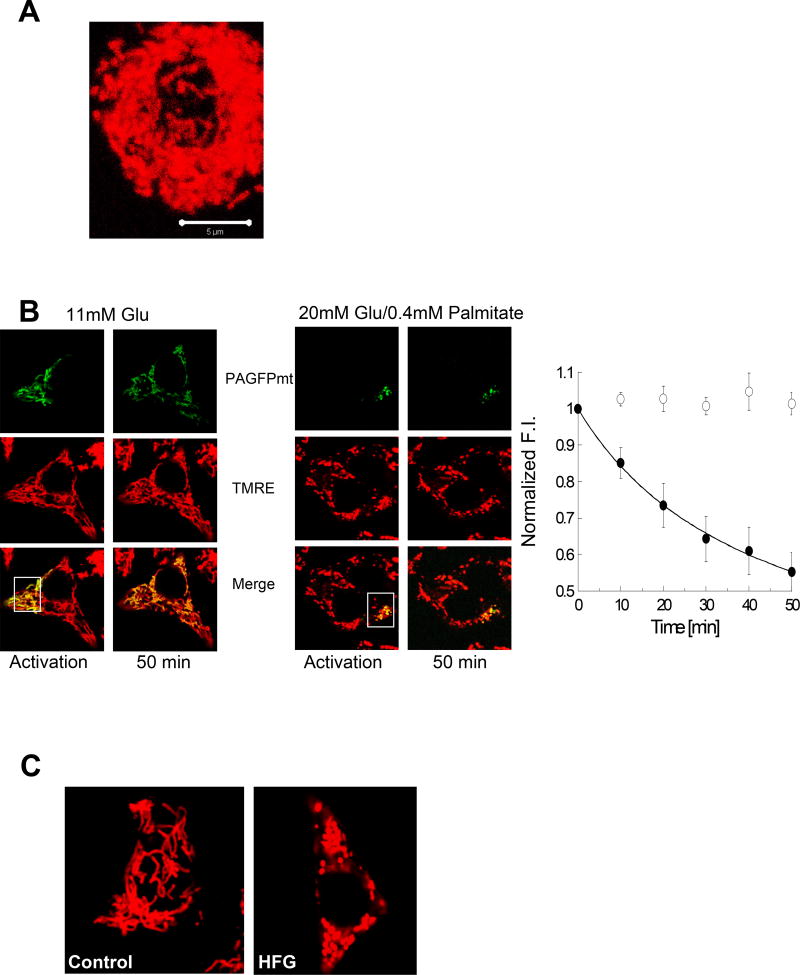
The morphology of beta-cell mitochondria has been characterized in both human and animal islets. Beta-cell mitochondria exhibit tubular networks that vary in size depending on the source of the beta-cells and experimental conditions (i.e. nutrient levels). Park et al. measured mitochondria length and quantified morphology in primary human pancreatic beta-cells and the rat beta-cell line, INS-1 cells. They observed that in both beta-cell types, mitochondria formed tubular networks throughout the cytosol. The mean length of mitochondria in human beta-cells was measured at 3.5µm (20). INS-1 cells were found to have a more elongated mitochondrial network with an average mitochondrial length of approximately 7.5µm (20). Molina et al. also found that mitochondria in INS-1 cells are more tubular than in primary beta-cells. In that study, mitochondrial length and architecture were examined in primary mouse islets with mitochondrial-targeted photo-activatable GFP (PA-GFPmt), which is a matrix targeted GFP with a point mutation that allows it to be photo-converted to an activated state with 2-photon excitation. It was revealed that beta-cell mitochondria from mouse islets were rod-shaped and relatively small in size (Figure 1A), with the majority of mitochondria less than 2 µm in length (21). There are a number of potential explanations for the difference in mitochondrial network length in INS-1 cells, which have an insulinoma cell origin, versus primary beta-cells including differences in nutrient requirements and utilization, disruptions in cell-cell interactions that are crucial to cells within an islet, and active cell proliferation in INS-1 cells. All of these fundamental differences in cell lines compared with primary beta-cells could alter energy requirements and influence mitochondrial morphology. Moreover, in regards to morphology, the short mitochondrial length of primary beta-cells could be attributed to reduced frequency of mitochondrial fusion or alternatively to fusion events that are quickly followed by fission events. PA-GFPmt was utilized to differentiate between these two possibilities.
Figure 1.
Mitochondrial morphology and dynamics in beta-cells. A. Three-dimensional reconstruction of the mitochondria within an entire primary mouse beta-cell. Mitochondria are densely packed with the beta-cell. B. Mitochondrial fusion assays in INS1 cells demonstrate normal mitochondrial fusion in control cells and inhibition of mitochondrial fusion in cells treated with HFG for 24 hours. Fusion of the photoactivated fraction with unlabeled mitochondria spreads and thereby dilutes the activated PA-GFP throughout the network. The overall rate of mitochondrial fusion activity is quantified by measuring fluorescence intensity (FI) with a faster decrease in FI indicative of a higher rate of fusion (right). C. Mitochondrial fragmentation in INS1 cells exposed to HFG for 24 hours.
Visualizing mitochondrial length and morphology provides a snap-shot of mitochondrial architecture but does not provide information about the ability of mitochondria to participate in fusion and fission events and the frequency at which dynamics occurs. PA-GFPmt can be utilized to monitor fusion and fission events as well as quantify the rate of fusion throughout a cell (21,22). Individual mitochondrial fusion events can be examined by monitoring the transfer of PA-GFPmt from a single mitochondrion that was photoactivated to an unlabeled mitochondrion. Mitochondrial fission events can be monitored on an individual basis in an analogous manner. Using a slightly different experimental protocol, the rate of mitochondrial fusion can be quantified by photoactivation of PA-GFPmt in a subset of the mitochondrial population (approximately 30% of mitochondria) and then measuring the dilution of the PA-GFPmt signal throughout the mitochondrial network over time (Figure 1B). Through the utilization of this tool, Molina et al. determined that beta-cells from islets isolated from C57Bl6 mice do undergo fusion and fission events. This study found that fusion was often followed by fission in mouse beta-cells, which, therefore, maintained pre-fusion size and morphology of mitochondria (21). The rate of fusion was found to be similar between INS-1 cells, dissociated mouse beta-cells and beta-cells in an intact islet (21).
Disruptions in Mitochondrial Fusion and Fission Alter Beta-Cell Function
Manipulating the expression of mitochondrial dynamics proteins can have dramatic effects on mitochondrial morphology in beta-cells. This effect on morphology can be dependent on the extent of overexpression or knockdown of mitochondrial dynamics proteins. This is evident when examining the overexpression of OPA1 in INS-1 cells, which stimulates a pro-fusion state. Mild overexpression of OPA1 increased the density of the mitochondria and resulted in a more elongated mitochondrial morphology. On the other hand, a higher level of OPA1 overexpression led to a fragmented mitochondrial morphology (21). Shifting the mitochondrial dynamics balance towards fusion was also induced by overexpression of wild type (WT) Mfn1, dominant negative Drp1 (Drp1K38A or Drp1-DN), or Fis1 knockdown. Overexpression of WT Mfn1 caused perinuclear aggregation of “super-fused” mitochondria (20). Drp1-DN also leads to a super-fusion state but without perinuclear localization at the concentrations tested (21,23). Interestingly, inhibition of Fis1 expression by approximately 90% using RNAi does not result in changes to mitochondrial morphology (21,23). Pro-fission states have been achieved in INS-1 cells by overexpression of hFis1, Drp1, and Mfn1-DN. All of these conditions resulted in mitochondrial fragmentation (20).
The role of mitochondrial morphology and mitochondrial dynamics proteins in the regulation of beta-cell function has been addressed by using genetic tools to change the balance between fusion and fission proteins in INS-1 and beta-cells, as outlined in Table 1. Disruption of steady state fusion or fission in beta-cells has been shown to disrupt mitochondrial morphology and function thereby influencing beta-cell function under control conditions. Induction of a pro-fission state with overexpression of Drp1 increased ROS production, decreased GSIS by approximately 20%, and increased apoptosis in INS-1 cells (24,25). Furthermore, overexpression of hFis1 in INS-1 cells has been shown to reduce the amount of ATP that is produced at basal and stimulatory glucose concentrations. This was accompanied by increased glucose stimulated lactate production (20). Overexpression of hFis impaired glucose-induced hyperpolarization of mitochondria, which could account for the decrease in ATP production. These data, described by Park et al., represents a shift from mitochondrial oxidative phosphorylation to glycolysis. They also explored how these changes in mitochondrial metabolism affected the insulin secretion pathway. Both cytosolic and mitochondrial calcium release was decreased in response to stimulatory glucose concentrations (20). All of the metabolic effects of hFis1 overexpression culminated with decreased GSIS. However, these effects are not necessarily due solely to mitochondrial fragmentation. When fragmentation was induced in INS-1 cells by the overexpression of the dominant negative form of Mfn1 (Mfn1-DN), no statistically significant changes occurred in any of the parameters that were altered in response to hFis1 overexpression. This is in agreement with data in other cell types demonstrating that some mitochondrial dynamics proteins influence mitochondrial function in a manner that is not directly related to its role in regulating mitochondrial morphology (3).
Table 1.
Genetic manipulation of mitochondrial fusion and fission proteins in beta-cells and the resulting effect on mitochondrial morphology, mitochondrial function, and beta-cell function.
| Manipulation | Model | Effect on Morphology |
Effect on Mitochondrial Function |
Effect on Beta- Cell Function |
References | |
|---|---|---|---|---|---|---|
| Pro-Fusion | OPA1 OEx (low) |
|
|
Decreased APs containing mitochondria (mitophagy) in INS1 cells | N/A | (21,23) |
| OPA1 OEx (high) | INS1 cells | Fragmented | N/A | N/A | (21) | |
| Mfn1 OEx | INS1 cells | Perinuclear aggregation of super-fused mitochondria | Decreased basal and glucose stimulated ATP | Impaired GSIS | (20) | |
| Fis1 RNAi | INS1 cells | No Effect | Decreased:
|
Decreased GSIS | (21,23) | |
| Drp1 DN |
|
|
Decreased:
|
No statistically significant change in GSIS | (21,23,25) | |
| Pro-Fission | OPA1 KO | Beta-cell specific mouse model |
|
Decreased:
|
|
(26) |
| Mfn1 DN | INS1 cells | Fragmented | No statistically significant changes | No effect on GSIS | (20) | |
| hFis1 OEx |
|
Fragmented | Decreased:
|
Decreased:
|
(20) | |
| Drp1 OEx | INS1 cells | Fragmented |
|
|
(24,25) |
Abbreviations: ∆ψmt – mitochondrial membrane potential; APs – autophagosomes; DN – dominant negative; GSIS – glucose stimulated insulin secretion; KO – knockout; OEx – overexpression; ROS – reactive oxygen species
Induction of mitochondrial fusion in beta-cells has been shown to effect mitochondrial as well as beta-cell function. Overexpression of WT-Mfn1 in INS-1 cells decreased both basal and glucose stimulated ATP, while increasing glucose stimulated lactate production (20). GSIS was also impaired in these cells. Decreasing mitochondrial fission had similar effects. Fis1 RNAi and Drp1-DN both decreased maximal respiratory capacity in INS-1 cells demonstrating impaired mitochondrial function (23). Fis1 knockdown was also shown to decrease glucose stimulated insulin secretion in INS-1 cells (23).
A knockout mouse of OPA1 in insulin-producing cells has been generated by utilizing the Cre/LoxP system, with Cre-expression driven by the rat insulin promoter 2 (RIP2). Zhang et al. demonstrated that RIP2-OPA1KO mice had impaired mitochondrial and beta-cell function. The mitochondria in islets from RIP2-OPA1KO mice were shorter and fragmented with abnormal cristae structure compared with control islets (26). RIP2-OPA1KO mice displayed hallmarks of diabetes including hyperglycemia in both the fed and fasted state, impaired glucose tolerance, and decreased insulin response to glucose injection in vivo (26). Consistent with the in vivo finding, isolated RIP2-OPA1KO islets had decreased GSIS, which could be attributed to the decrease in glucose-stimulated ATP production. Beta-cell OPA1 knockout was found to compromise glucose stimulated oxygen consumption and ETC activity (26). Currently, this work represents the only published example of a knockout animal of a mitochondrial dynamics protein in beta-cells/insulin producing cells. It provides compelling evidence that changes in the beta-cell fusion/fission balance can directly influence mitochondrial function, which may subsequently disrupt beta-cell function and lead to whole body metabolic adaptation/dysfunction. This demonstrates both the need for a well functioning mitochondrial population in the beta-cell and how changes in beta-cell function alone can affect whole body metabolism. Unpublished data from our lab is in agreement that mitochondrial fusion is necessary for normal beta-cell function. A beta-cell specific knockout of Mfn2 demonstrates mitochondrial fragmentation, bioenergetics dysfunction, changes in insulin secretion, and whole body metabolic adaptations.
There is still much to be discovered about the role these proteins play in the beta-cell both in vitro and in vivo. For example, it is currently unknown how these proteins change with age, in vivo nutrient conditions, or in the development of diabetes. As more data emerges we will be able to discern the contribution of these proteins to mitochondrial and beta-cell function and whether they play a role in the development of the age-related decline in beta-cell function and/or diabetes.
Mitochondrial Dynamics and Autophagy
Mitochondrial dynamics have been shown to influence the general maintenance of the mitochondrial population through the regulation of mitochondrial degradation. Macroautophagy, herein referred to as autophagy, is the process by which macromolecular cytosolic components, including mitochondria, are degraded via sequestration into a double-membrane structure (termed the autophagosome (AP)), which fuses with the lysosome to deliver the enclosed material for degradation. The relevance of autophagy in the beta-cell is an active field of investigation. Multiple studies indicate that autophagy plays an important role in regulating beta-cell mass, viability, and function (27,28). Additionally, there is evidence that alterations in beta-cell autophagy contribute to the development of diabetes (29). A mouse model of beta-cell deficient autophagy via knockout of Atg7, a gene that is essential for autophagosome formation, displayed hallmarks of diabetes including hyperglycemia, glucose intolerance, and decreased beta-cell mass. These mice also have decreased basal and glucose stimulated insulin secretion as well as decreased glucose-induce changes to cytosolic Ca2+ concentrations (30). Much of the data in the beta-cell-specific Atg7KO mouse suggest that mitochondrial dysfunction may be involved in the observed phenotype because of the role autophagy plays in mitochondrial quality control. Indeed, beta-cell mitochondria in these mice were swollen, a finding normally associated with decreased bioenergetic capacity (30). This was in agreement with the finding that inhibition of autophagy in INS-1 cells results in reduced maximal respiratory capacity (23). Additionally, it has been shown that beta-cell autophagy is sensitive to the surrounding nutrient environment. Ebato et al. showed that autophagy was necessary for the increase in beta-cell mass that occurs in mice fed a HFD (31). Studies have also been performed in INS-1 cells addressing the effect high free fatty acids have on autophagy. Las et al. showed that beta-cell autophagy was altered in response to fatty acids in a biphasic manner (32). In the short term, INS-1 cells increased autophagic flux; however, the long term effect of free fatty acids is to decrease autophagic flux. The addition of high glucose concentrations to the free fatty acids (HFG) resulted in a synergistic effect on the inhibition of autophagic flux (32). Interestingly, these same conditions are known to alter mitochondrial function, morphology, and dynamics, which will be expanded upon in the next section.
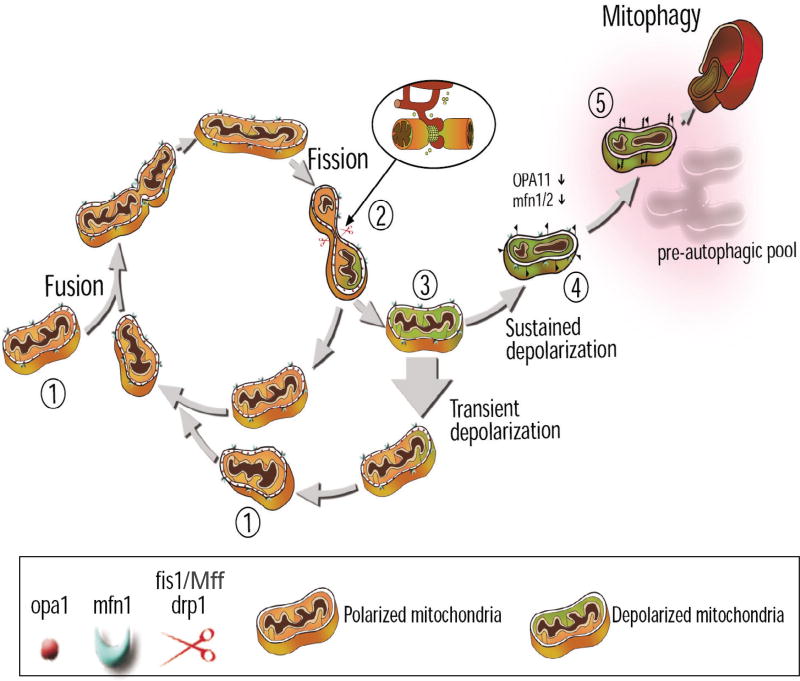
Twig et al. demonstrated that mitochondrial fusion and fission play a prominent role in regulating mitochondrial autophagy, termed mitophagy, which is the process by which dysfunctional mitochondria are degraded by autophagy. This mitochondrial quality control cycle (sometimes referred to as the life cycle of the mitochondrion) was first demonstrated in INS-1 cells (23). This study demonstrated that a mitochondrial fusion event is most often followed by a fission event that generates energetically uneven daughter mitochondria: a hyperpolarized and a depolarized unit (23). While the hyperpolarized daughter mitochondrion was readily available to refuse with the mitochondrial network, the depolarized mitochondrion was unable to undergo subsequent fusion events if the depolarization was sustained (approximately 22% of the time). This demonstrated that mitochondrial fusion can be selective for dysfunctional mitochondria. The study also reported on the observation that the mitochondria with sustained depolarization were targeted for autophagy. However, engulfment by APs could not account for the decreased fusion capacity of the depolarized mitochondria. This was because the induction of autophagy did not occur for at least one hour post depolarization (23). Instead, a decrease in OPA1 expression was found to be responsible for decreased fusion capacity in depolarized mitochondria (23). Consequently, selective mitochondrial fusion, through the regulation of fusion proteins, functions as a quality control mechanism, which contributes to the maintenance of a healthy, well-functioning mitochondrial population. An overview of the mitochondrial quality control cycle is shown in Figure 2.
Figure 2.
The quality control cycle of a mitochondrion. Mitochondrial fusion (1), fission (2), and autophagy (5) contribute to the segregation of dysfunctional mitochondria. The mitochondrion shifts between a networked post-fusion state and a solitary post-fission state. The inlay displays contact sites between ER and mitochondria, which has recently been shown to participate in the fission process (2). Following a fission event, daughter mitochondria may either maintain membrane potential (orange mitochondrion) or depolarize (green mitochondrion). If depolarization occurs, the mitochondrion is unlikely to undergo further fusion events for the entire depolarization interval (3). If mitochondrial depolarization is transient and membrane potential is restored, fusion capacity is also restored (1). However, if mitochondrial membrane potential depolarization is sustained (4), the mitochondrion cannot re-fuse with the network, due to a reduction in OPA1 levels, and elimination by autophagy occurs (5) (Figure adapted from (64,65)).
To further demonstrate the requirement of mitochondrial dynamics in targeting mitochondria for autophagy, Twig et al. examined the consequence of disrupting the balance of fusion or fission on mitophagy in INS-1 cells. They found that in a pro-fusion state, provoked by either enhancing fusion with OPA1 overexpression or inhibiting fission with Drp1-DN or Fis1 RNAi, mitophagy was reduced, which was established as a decrease in the fraction of APs containing mitochondria (23). It should be noted that inhibition of fission did not affect the total number of lysosomes or the autophagic degradation of ER. This demonstrated that fission was necessary to segregate mitochondrial units for autophagic degradation. In addition to the segregation of depolarized mitochondria, they also provided additional evidence that fission plays a functional role in segregating dysfunctional units through quality control. Inhibition of autophagy, achieved through inhibition of mitochondrial fission or the autophagic machinery, resulted in increased oxidized mitochondrial proteins and decreased respiratory capacity, which could be due to accumulation of dysfunctional mitochondria within the network (23). Interestingly, direct addition of H2O2 to INS-1 cells induces mitochondrial fragmentation (12), which demonstrates the cyclic nature of mitochondrial dysfunction and mitochondrial dynamics. Genetic alterations in mitochondrial dynamics proteins often lead to mitochondrial dysfunction, while mitochondrial stressors frequently results in alterations to mitochondrial morphology and dynamics. This demonstrates that the cellular regulation of mitochondrial dynamics and function is highly coupled. Therefore, a functional quality control mechanism is required to remove dysfunctional units through mitophagy in order to prevent further deterioration of the network.
Altogether, these studies indicate that beta-cell mitochondrial dynamics and morphology contribute to beta-cell mitophagy. Disruption in this mitochondrial quality control mechanism is expected to result in the accumulation of depolarized, damaged mitochondria. Moreover, autophagy plays a very important role in maintaining beta-cell mass and function and has been shown to be dysfunctional under high nutrient conditions and in diabetes. The link between mitophagy and beta-cell function in the development of diabetes needs to be further elucidated.
Beta-Cell Mitochondrial Dynamics are Sensitive to High Nutrient Environments
Beta-cells are sensitive to changes in extracellular nutrients, a property used for the generation and integration of nutrient signals and, in response, insulin secretion. Mitochondria play an essential role in this process by metabolizing nutrients and generating signals that are required for both the triggering and amplifying pathways of insulin secretion. It was therefore hypothesized by Molina et al. that mitochondrial morphology may also be sensitive to changes in nutrients and that these morphological changes may play a role in the pathogenesis of diabetes. This hypothesis was tested in INS-1 cells exposed to elevated levels of free fatty acids and glucose (HFG). This kind of high nutrient environment, sometimes referred to as glucolipotoxicity, has been shown to disrupt beta-cell function leading to altered insulin secretion and apoptosis and is often used as an in vitro model of diabetes (33,34). The combined effect of HFG treatment in beta-cells resulted in mitochondrial fragmentation within 4 hours of exposing the cells to these conditions (Figure 1C) (21). The individual effects of high free fatty acids or high glucose on beta-cell mitochondrial morphology were also explored in this study. While an elevated free fatty acid concentration (0.4mM palmitate) induced mitochondrial fragmentation in as little as 4 hours, high glucose levels (20mM glucose) alone did not result in fragmentation at 4 hours and caused only a minor increase in fragmentation at 24 hours (21). This demonstrated that while high glucose in the presence of free fatty acids exacerbated fragmentation, high glucose alone only had a minor effect on morphology.
More studies need to be completed to understand the consequence of a high nutrient environment on mitochondrial morphology in vivo. Fex et al. have shown that beta-cell from mice fed a high fat diet have an increased mitochondrial area compared to mice fed a normal diet (35). An increase in mitochondrial area could be indicative of increased biogenesis or swollen mitochondria. Interestingly, this increase in mitochondrial area was not accompanied by an increase in the mean number of mitochondria per beta-cell suggesting that these mitochondria were swollen. Furthermore, Del Guerra et al. demonstrated altered beta-cell mitochondrial morphology and density by electron microscopy in normal, human islets that were cultured in alternating normal (5mM) and high (16.7mM) glucose concentrations (36). Round mitochondria with poorly-defined cristae structure and increased density volume were observed in the islets exposed to intermittent high glucose exposure (36). Further imaging of mitochondria in beta-cells from animals fed a high fat diet and isolated human islets exposed to high nutrient levels would allow for enhanced quantification of changes in mitochondrial architecture in response to nutrients.
Mitochondrial fragmentation can be a result of increased fission or inhibition of fusion. Molina et al. addressed this by using the mitochondrial fusion assay, discussed previously, to determine the cause of fragmentation in INS-1 cells and beta-cells exposed to high fat and glucose. They found that beta-cells exposed to HFG displayed a decreased mitochondrial fusion capacity (Figure 1B). Compensation of decreased fusion with inhibition of mitochondrial fission using Fis1 RNAi was able to correct a number of the cellular dysfunctions that occur in response to HFG. Fis1 RNAi restored mitochondrial morphology and protected INS-1 cells from apoptosis in response to the toxic nutrient environment. Interestingly, restoring mitochondrial morphology with Fis1 RNAi did not restore GSIS. This implies that while morphology may play the predominant role in regulating apoptosis under these conditions in INS-1 cells, it is not responsible for the decrease in GSIS in response to HFG.
The role of mitochondrial dynamics in apoptosis is a relevant area of study in the beta-cell. Increased beta-cell apoptosis is a significant contributor to the eventual loss of beta-cell mass that occurs in response to high nutrient levels and in diabetes (37,38,39). HFG activation of beta-cell death pathways includes cleaved caspase-3, demonstrating involvement of a mitochondrial apoptosis pathway. The importance of mitochondrial dynamics in apoptosis in beta-cells is revealed by data demonstrating that preservation of mitochondrial morphology protects from nutrient-induced apoptosis. As well as evidence demonstrating that Drp1 mediates beta-cell apoptosis in response to high glucose and ER stress (24,25). Prolonged periods of high glucose in INS-1 cells induce both Drp1 and cytochrome c expression (25). Overexpression of WT Drp1 was shown to induce mitochondrial pathways of apoptosis and this apoptosis was further enhanced under high glucose conditions. However, Drp1-DN was able to prevent high glucose-induced apoptosis. In these experiments, Drp1-DN exerted a protective effect on GSIS, which is in contrast to what was observed in Fis1 RNAi cell under HFG (21,25). However, Drp1-DN was not able to completely restore GSIS (25).
More research needs to be conducted to understand whether changes in mitochondrial dynamics play a role in regulating insulin secretion and apoptosis in beta-cells in response to high nutrients in vivo. We already know that under control culturing conditions Fis1 RNAi results in decreased GSIS in INS-1 cells (morphology is unchanged in these cells), so it is possible that this contributed to the inability of Fis1 RNAi to restore GSIS under HFG. Additionally, the role of Drp1 in HFG-mediated beta-cell death should also be studied. The cause of decreased fusion capacity under HFG should be further examined both in vitro and in vivo. It is possible that something intrinsic to a decrease in fusion capacity besides morphology, for instance a change in the expression of a fusion or fission protein, contributes to the decreased GSIS.
Evidence for Changes in Beta-Cell Mitochondrial Dynamics in Diabetes
Alterations to mitochondrial morphology and function have been found in beta-cells of patients with diabetes and diabetic animal models. Bindokas et al. demonstrated that there is an increase in mitochondrial superoxide in response to glucose in normal islets. In Zucker Diabetic Fatty (ZDF) rats, there was a significantly higher level of mitochondrial superoxide compared with lean controls (40). Moreover, they found that islets isolated from ZDF rats exhibit fragmented mitochondrial morphology. Higa et al. have also demonstrated altered mitochondrial morphology in beta-cells of ZDF rats. They observed large, swollen mitochondria with apparent mitochondrial remodeling and altered cristae structure (41). ZDF rats are not the only diabetic animal model to display impaired mitochondrial morphology. Mitochondrial fragmentation was also observed in diabetic Goto Kakizaki (GK) rats (42,43). Interestingly, Drp1 mRNA and gene expression was increased over time in islets from GK rats (25). However, the primary role of mitochondrial dysfunction in the development of diabetes in GK rats is not firmly established and needs to be elucidated under control conditions where mitochondrial dysfunction is not exacerbated by high nutrient levels. Diabetic MKR mice have beta-cells with swollen mitochondria with altered cristae structure and displayed a reduced degree of hyperpolarization in response to glucose (15). Mitochondrial swelling was also reported in beta-cells of young cattle that developed spontaneous diabetes mellitus (44,43).
Diabetic islets from patients have also been investigated. Anello et al. have shown that diabetic beta-cells have reduced ATP levels, a lower ATP/ADP ratio and impaired hyperpolarization of the mitochondrial membrane. Increased protein expression of UCP-2, complex I and the ATP synthase of the respiratory chain, and a higher level of reactive nitrogen species were also found in type 2 diabetic islets (45). Additionally, diabetic β-cells displayed an increase in mitochondrial volume albeit a similar number of total mitochondria compared with control beta-cells, suggesting the presence of swollen mitochondria (45). These beta-cells also have a vastly different architecture than the control beta-cells and appear to be fragmented with disrupted cristae morphology.
There are still a number of questions regarding the changes that occur to mitochondrial architecture in diabetic patients and animal models of diabetes. While it is known that mitochondrial morphology is changed to a more fragmented state, we do not know the mechanism. We can hypothesize that this fragmentation is due to decreased fusion activity because of available in vitro data, but this is still unknown. Indeed, unpublished data from our lab indicates that inhibition of mitochondrial fusion through the downregulation of Mfn2 may contribute to this mitochondrial fragmentation. Alterations in the balance of fusion and fission need to be investigated in models of diabetes as well as how changes in the expression of mitochondrial dynamics proteins could be contributing to the fragmented mitochondrial morphology and mitochondrial dysfunction. If differences in mitochondrial dynamics are found in beta-cells of diabetic models, the functional significance of these in vivo changes will need to be determined. Additionally, whether the alterations to mitochondrial architecture are a cause or consequence of diabetes will need to be considered.
Mitochondrial Dynamics May Influence Other Regulators of Beta-Cell Function
While not yet studied in the beta-cell, it has been demonstrated in other systems and cell types that changes in mitochondrial dynamics can influence ROS production, mitochondrial calcium homeostasis, and bioenergetic efficiency. Alterations in any of these parameters could alter beta-cell insulin secretion function or viability. All of these are areas that need to be further explored in the beta-cell as they may provide a link between mitochondrial dynamics and the regulation of beta-cell nutrient handling, insulin secretion, and viability.
There is growing evidence demonstrating a relationship between mitochondrial dynamics and oxidative stress. One of the main sources of ROS is the mitochondrial ETC and because of the close proximity of mitochondrial proteins and mtDNA to the source as well as less efficient mitochondrial DNA proofreading enzymes, mitochondria are also the main target of ROS. Decreased ∆ψmt and an increase in ROS production have been demonstrated in HeLa cells with decreased Fis1 expression (46). As was already discussed, inhibition of mitochondrial fission with Fis1 RNAi in INS-1 cells reduces mitophagy and leads to the accumulation of oxidized proteins (23). Additionally, Yu et al. demonstrated that mitochondria undergo rapid fragmentation after high glucose exposure with a concomitant increase in ROS in rat liver and myoblast cell lines. They found that mitochondrial fragmentation was necessary for the high glucose-induced respiration responsible for the increased ROS production and that inhibition of fission with Drp1-DN prevented this ROS production (47). In INS-1 cells, overexpression of WT Drp1 has been shown to enhance ROS production in response to high glucose concentrations while expression of Drp1-DN inhibits this ROS production (25).
The connections between mitochondrial dynamics, autophagy, and ROS have a number of implications that should be addressed in beta-cells. ROS has an important dose-dependent regulatory role in the beta-cell. Low concentrations ROS with acute exposure has been shown to induce insulin secretion while high, chronic concentrations are detrimental to the beta-cell and induce apoptosis (48). Therefore alterations in mitochondrial dynamics could alter ROS levels and influence insulin secretion or susceptibility to apoptosis. This is especially important in the context of mitochondrial dynamics functioning as a quality control mechanism.
Mitochondrial dynamics and calcium signaling may also be linked (49). The relevance of this in the beta-cell is that increased intracellular calcium concentrations is the primary trigger for insulin granule exocytosis. It has been reported in MEFs that Mfn2 located on mitochondria and ER allows for fusion of these organelles and mitochondrial calcium buffering (50). Elevated cytosolic [Ca2+] and K+ (via a Ca2+-dependent manner) have been shown to induce mitochondria fragmentation and depolarization of ∆ψmt in hippocampal neurons (51,52). Drp1-DN was able to rescue these effects of high Ca2+ levels (52). Moreover, induction of mitochondrial fragmentation alone has also been shown to affect Ca2+ signaling. Fragmentation of mitochondria with overexpression of Drp1 has been shown to block the propagation of mitochondrial Ca2+ signaling (53). Mitochondrial membrane potential is a driving force for Ca2+ uptake into the mitochondrial matrix where it regulates the activity of Kreb Cycle enzymes, which can influence oxidative phosphorylation. This would be significant in beta-cells because nutrient signaling induces ETC activity, ∆ψmt hyperpolarization, and increased ATP synthesis, all of which are necessary for insulin secretion. Additionally, Ca2+ overload can induce the mitochondrial permeability transition pore, ∆ψmt depolarization, and apoptosis (54). Further investigation into this could provide an interesting link between high nutrients levels, mitochondrial metabolism and fragmentation, and apoptosis in beta-cells.
The contribution of mitochondrial dynamics to bioenergetic efficiency could be a particularly interesting area of research in the beta-cell. Changes in proton leak and ∆ψmt are often associated with mitochondrial fragmentation in cells such as skeletal muscle and adipocytes (55,56). This may be relevant in the beta-cell because it is known that mitochondrial fragmentation occurs in response to a high nutrient environment and any associated bioenergetic changes could affect insulin secretion and beta-cell viability. It has been previously shown that INS-1 cells exhibit a high level of ETC proton leak, or oxygen consumption that is not coupled to ATP synthesis. Seventy five percent of the respiration measured from INS1 cells was reported to be uncoupled in comparison to 20% in myoblasts (57,58). Currently, the reason behind this apparent inefficiency of the beta-cell ETC is unknown. However, considering the importance of mitochondrial ATP-production for insulin secretion this finding is certainly intriguing. Mitochondrial proton leak in the beta-cell may play an important role in nutrient regulation and insulin secretion function. It has been suggested that the role of beta-cell proton leak may be to allow higher flux through the TCA cycle, thereby increasing levels of TCA cycle-derived signals for insulin secretion (59). Additionally, it is possible that the changes in mitochondrial morphology and dynamics that occur in response to chronic high nutrient levels contribute to decreased GSIS insulin secretion in part through changes in bioenergetic efficiency. Fragmentation combined with the induction of proton leak could present a mechanism to protect beta-cell from nutrient-induced ROS production because increased proton leak would decrease ∆ψmt and subsequently decrease ROS production.
There is some evidence supporting a relationship between mitochondrial dynamics and bioenergetics. As was already reviewed, there is a decrease in mitochondrial fusion capacity with depolarization of ∆ψmt (23). This is in part due to proteolytic cleavage of OPA1 that inhibits fusion ability (60). Additionally, it has been shown that alterations in the expression of some mitochondrial dynamics proteins results in changes to oxygen consumption and energy production. Mfn2 deficiency in myotubes leads to reduced ∆ψmt and oxygen consumption (61). Furthermore, downregulation of Drp1 expression impaired oxygen consumption and reduced the rate of ATP synthesis in HeLa cells (62). It has also been shown that the use of rotenone to inhibit ETC activity at complex I results in decreased ∆ψmt, increased ROS production, and fragmented mitochondrial morphology (63). These data suggest that both mitochondrial fusion and fission can influence bioenergetics and that this could be a bidirectional relationship.
Summary
Mitochondrial morphology and dynamics have been shown to contribute to multiple aspects of beta-cell function including insulin secretion, quality control, and viability. High nutrient environments and diabetes lead to mitochondrial fragmentation both in vitro and in vivo. While the cause of this fragmentation has been shown to be due to decreased mitochondrial fusion activity in vitro, this has not yet been confirmed in vivo. Future investigations into the role of mitochondrial dynamics in the beta-cell will be needed to provide necessary information regarding the role of mitochondrial dynamics in the preservation of beta-cell function. Additionally, the contribution of mitochondrial dynamics to the age-related decline in beta-cell function and the development of diabetes need to be elucidated. In the future, approaches to maintain the balance between mitochondrial fusion and fission or mitochondrial function in the face of changes in dynamics could be of valuable in the treatment of diabetes.
Clinical Practice Points
- It would be of interest to study the effect different macronutrient levels have on mitochondrial quality control in diabetic patients.
-
◦mtDNA may serve as a useful biomarker
-
◦
Mitochondrial fusion and fission may prove to be a desired therapeutic target in metabolic diseases.
Research Agenda
- Identify changes in beta-cell mitochondrial fusion and fission proteins in vivo that account for the decreased fusion capacity in response to high nutrient levels.
-
◦Do expression levels of these proteins change when beta-cell function is compromised?
-
◦Mitochondrial fragmentation has been established in diabetic islets; however, it is currently unknown whether mitochondrial dynamics are altered.
-
◦
- Determine corresponding changes in mitochondrial function that occur in response to altered mitochondrial dynamics in the beta-cell.
-
◦Do changes in beta-cell mitochondrial dynamics and morphology affect pathways that influence insulin secretion such as calcium, ROS, and/or ATP levels?
-
◦
Acknowledgments
We are grateful to Drs. Marc Liesa, Guy Las, and Dani Dagan as well as Sam Sereda for insightful comments during the writing of this review.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Chen H, Chan DC. Emerging functions of mammalian mitochondrial fusion and fission. Hum.Mol.Genet. 2005;14(Spec No. 2):R283–R289. doi: 10.1093/hmg/ddi270. [DOI] [PubMed] [Google Scholar]
- 2.Bereiter-Hahn J, Voth M. Dynamics of mitochondria in living cells: shape changes, dislocations, fusion, and fission of mitochondria. Microsc.Res.Tech. 1994;27:198–219. doi: 10.1002/jemt.1070270303. [DOI] [PubMed] [Google Scholar]
- 3.Liesa M, Palacin M, Zorzano A. Mitochondrial dynamics in mammalian health and disease. Physiol Rev. 2009;89:799–845. doi: 10.1152/physrev.00030.2008. [DOI] [PubMed] [Google Scholar]
- 4.Santel A, Fuller MT. Control of mitochondrial morphology by a human mitofusin. J.Cell Sci. 2001;114:867–874. doi: 10.1242/jcs.114.5.867. [DOI] [PubMed] [Google Scholar]
- 5.Misaka T, Miyashita T, Kubo Y. Primary structure of a dynamin-related mouse mitochondrial GTPase and its distribution in brain, subcellular localization, and effect on mitochondrial morphology. J.Biol.Chem. 2002;277:15834–15842. doi: 10.1074/jbc.M109260200. [DOI] [PubMed] [Google Scholar]
- 6.James DI, Parone PA, Mattenberger Y, et al. hFis1, a novel component of the mammalian mitochondrial fission machinery. J.Biol.Chem. 2003;278:36373–36379. doi: 10.1074/jbc.M303758200. [DOI] [PubMed] [Google Scholar]
- 7.Praefcke GJ, McMahon HT. The dynamin superfamily: universal membrane tubulation and fission molecules? Nat.Rev.Mol.Cell Biol. 2004;5:133–147. doi: 10.1038/nrm1313. [DOI] [PubMed] [Google Scholar]
- 8.Yoon Y, Krueger EW, Oswald BJ, et al. The mitochondrial protein hFis1 regulates mitochondrial fission in mammalian cells through an interaction with the dynamin-like protein DLP1. Mol.Cell Biol. 2003;23:5409–5420. doi: 10.1128/MCB.23.15.5409-5420.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gandre-Babbe S, van der Bliek AM. The novel tail-anchored membrane protein Mff controls mitochondrial and peroxisomal fission in mammalian cells. Mol.Biol.Cell. 2008;19:2402–2412. doi: 10.1091/mbc.E07-12-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Otera H, Wang C, Cleland MM, et al. Mff is an essential factor for mitochondrial recruitment of Drp1 during mitochondrial fission in mammalian cells. J.Cell Biol. 2010;191:1141–1158. doi: 10.1083/jcb.201007152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedman JR, Lackner LL, West M, et al. ER tubules mark sites of mitochondrial division. Science. 2011;334:358–362. doi: 10.1126/science.1207385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maechler P, Jornot L, Wollheim CB. Hydrogen peroxide alters mitochondrial activation and insulin secretion in pancreatic beta cells. J.Biol.Chem. 1999;274:27905–27913. doi: 10.1074/jbc.274.39.27905. [DOI] [PubMed] [Google Scholar]
- 13.Mulder H, Ling C. Mitochondrial dysfunction in pancreatic beta-cells in Type 2 diabetes. Mol.Cell Endocrinol. 2009;297:34–40. doi: 10.1016/j.mce.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 14.Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science. 2005;307:384–387. doi: 10.1126/science.1104343. [DOI] [PubMed] [Google Scholar]
- 15.Lu H, Koshkin V, Allister EM, et al. Molecular and metabolic evidence for mitochondrial defects associated with beta-cell dysfunction in a mouse model of type 2 diabetes. Diabetes. 2010;59:448–459. doi: 10.2337/db09-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maechler P. Mitochondria as the conductor of metabolic signals for insulin exocytosis in pancreatic beta-cells. Cell Mol.Life Sci. 2002;59:1803–1818. doi: 10.1007/PL00012507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wiederkehr A, Wollheim CB. Mitochondrial signals drive insulin secretion in the pancreatic beta-cell. Mol.Cell Endocrinol. 2011;353:128–137. doi: 10.1016/j.mce.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 18.Kennedy ED, Maechler P, Wollheim CB. Effects of depletion of mitochondrial DNA in metabolism secretion coupling in INS-1 cells. Diabetes. 1998;47:374–380. doi: 10.2337/diabetes.47.3.374. [DOI] [PubMed] [Google Scholar]
- 19.Heart E, Yaney GC, Corkey RF, et al. Ca2+, NAD(P)H and membrane potential changes in pancreatic beta-cells by methyl succinate: comparison with glucose. Biochem.J. 2007;403:197–205. doi: 10.1042/BJ20061209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park KS, Wiederkehr A, Kirkpatrick C, et al. Selective actions of mitochondrial fission/fusion genes on metabolism-secretion coupling in insulin-releasing cells. J.Biol.Chem. 2008;283:33347–33356. doi: 10.1074/jbc.M806251200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Molina AJ, Wikstrom JD, Stiles L, et al. Mitochondrial networking protects beta-cells from nutrient-induced apoptosis. Diabetes. 2009;58:2303–2315. doi: 10.2337/db07-1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Molina AJ, Shirihai OS. Monitoring mitochondrial dynamics with photoactivatable [corrected] green fluorescent protein. Methods Enzymol. 2009;457:289–304. doi: 10.1016/S0076-6879(09)05016-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Twig G, Elorza A, Molina AJ, et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008;27:433–446. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peng L, Men X, Zhang W, et al. Dynamin-related protein 1 is implicated in endoplasmic reticulum stress-induced pancreatic beta-cell apoptosis. Int.J.Mol.Med. 2011;28:161–169. doi: 10.3892/ijmm.2011.684. [DOI] [PubMed] [Google Scholar]
- 25.Men X, Wang H, Li M, et al. Dynamin-related protein 1 mediates high glucose induced pancreatic beta cell apoptosis. Int.J.Biochem.Cell Biol. 2009;41:879– 890. doi: 10.1016/j.biocel.2008.08.031. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Z, Wakabayashi N, Wakabayashi J, et al. The dynamin-related GTPase Opa1 is required for glucose-stimulated ATP production in pancreatic beta cells. Mol.Biol.Cell. 2011;22:2235–2245. doi: 10.1091/mbc.E10-12-0933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jung HS, Lee MS. Role of autophagy in diabetes and mitochondria. Ann.N.Y.Acad.Sci. 2010;1201:79–83. doi: 10.1111/j.1749-6632.2010.05614.x. [DOI] [PubMed] [Google Scholar]
- 28.Jung HS, Lee MS. Macroautophagy in homeostasis of pancreatic beta-cell. Autophagy. 2009;5:241–243. doi: 10.4161/auto.5.2.7518. [DOI] [PubMed] [Google Scholar]
- 29.Fujitani Y, Ebato C, Uchida T, et al. beta-cell autophagy: A novel mechanism regulating beta-cell function and mass: Lessons from beta-cell-specific Atg7-deficient mice. Islets. 2009;1:151–153. doi: 10.4161/isl.1.2.9057. [DOI] [PubMed] [Google Scholar]
- 30.Jung HS, Chung KW, Won KJ, et al. Loss of autophagy diminishes pancreatic beta cell mass and function with resultant hyperglycemia. Cell Metab. 2008;8:318–324. doi: 10.1016/j.cmet.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 31.Ebato C, Uchida T, Arakawa M, et al. Autophagy is important in islet homeostasis and compensatory increase of beta cell mass in response to high-fat diet. Cell Metab. 2008;8:325–332. doi: 10.1016/j.cmet.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 32.Las G, Serada SB, Wikstrom JD, et al. Fatty acids suppress autophagic turnover in beta-cells. J.Biol.Chem. 2011;286:42534–42544. doi: 10.1074/jbc.M111.242412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poitout V, Robertson RP. Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocr.Rev. 2008;29:351–366. doi: 10.1210/er.2007-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poitout V, Amyot J, Semache M, et al. Glucolipotoxicity of the pancreatic beta cell. Biochim.Biophys.Acta. 2010;1801:289–298. doi: 10.1016/j.bbalip.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fex M, Nitert MD, Wierup N, et al. Enhanced mitochondrial metabolism may account for the adaptation to insulin resistance in islets from C57BL/6J mice fed a high-fat diet. Diabetologia. 2007;50:74–83. doi: 10.1007/s00125-006-0464-4. [DOI] [PubMed] [Google Scholar]
- 36.Del GS, Grupillo M, Masini M, et al. Gliclazide protects human islet beta-cells from apoptosis induced by intermittent high glucose. Diabetes Metab Res.Rev. 2007;23:234–238. doi: 10.1002/dmrr.680. [DOI] [PubMed] [Google Scholar]
- 37.El-Assaad W, Buteau J, Peyot ML, et al. Saturated fatty acids synergize with elevated glucose to cause pancreatic beta-cell death. Endocrinology. 2003;144:4154–4163. doi: 10.1210/en.2003-0410. [DOI] [PubMed] [Google Scholar]
- 38.Szabadkai G, Duchen MR. Mitochondria mediated cell death in diabetes. Apoptosis. 2009;14:1405–1423. doi: 10.1007/s10495-009-0363-5. [DOI] [PubMed] [Google Scholar]
- 39.Prentki M, Nolan CJ. Islet beta cell failure in type 2 diabetes. J.Clin.Invest. 2006;116:1802–1812. doi: 10.1172/JCI29103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bindokas VP, Kuznetsov A, Sreenan S, et al. Visualizing superoxide production in normal and diabetic rat islets of Langerhans. J.Biol.Chem. 2003;278:9796–9801. doi: 10.1074/jbc.M206913200. [DOI] [PubMed] [Google Scholar]
- 41.Higa M, Zhou YT, Ravazzola M, et al. Troglitazone prevents mitochondrial alterations, beta cell destruction, and diabetes in obese prediabetic rats. Proc.Natl.Acad.Sci.U.S.A. 1999;96:11513–11518. doi: 10.1073/pnas.96.20.11513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dlaskova A, Spacek T, Santorova J, et al. 4Pi microscopy reveals an impaired three-dimensional mitochondrial network of pancreatic islet beta-cells, an experimental model of type-2 diabetes. Biochim.Biophys.Acta. 2010;1797:1327–1341. doi: 10.1016/j.bbabio.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 43.Mizukami H, Wada R, Koyama M, et al. Augmented beta cell loss and mitochondrial abnormalities in sucrose-fed GK rats. Virchows Arch. 2008;452:383–392. doi: 10.1007/s00428-007-0508-2. [DOI] [PubMed] [Google Scholar]
- 44.Taniyama H, Shirakawa T, Furuoka H, et al. Spontaneous diabetes mellitus in young cattle: histologic, immunohistochemical, and electron microscopic studies of the islets of Langerhans. Vet.Pathol. 1993;30:46–54. doi: 10.1177/030098589303000106. [DOI] [PubMed] [Google Scholar]
- 45.Anello M, Lupi R, Spampinato D, et al. Functional and morphological alterations of mitochondria in pancreatic beta cells from type 2 diabetic patients. Diabetologia. 2005;48:282–289. doi: 10.1007/s00125-004-1627-9. [DOI] [PubMed] [Google Scholar]
- 46.Lee S, Jeong SY, Lim WC, et al. Mitochondrial fission and fusion mediators, hFis1 and OPA1, modulate cellular senescence. J.Biol.Chem. 2007;282:22977–22983. doi: 10.1074/jbc.M700679200. [DOI] [PubMed] [Google Scholar]
- 47.Yu T, Robotham JL, Yoon Y. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc.Natl.Acad.Sci.U.S.A. 2006;103:2653–2658. doi: 10.1073/pnas.0511154103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pi J, Bai Y, Zhang Q, et al. Reactive oxygen species as a signal in glucose-stimulated insulin secretion. Diabetes. 2007;56:1783–1791. doi: 10.2337/db06-1601. [DOI] [PubMed] [Google Scholar]
- 49.Szabadkai G, Simoni AM, Bianchi K, et al. Mitochondrial dynamics and Ca2+ signaling. Biochim.Biophys.Acta. 2006;1763:442–449. doi: 10.1016/j.bbamcr.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 50.de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456:605–610. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- 51.Rintoul GL, Filiano AJ, Brocard JB, et al. Glutamate decreases mitochondrial size and movement in primary forebrain neurons. J.Neurosci. 2003;23:7881–7888. doi: 10.1523/JNEUROSCI.23-21-07881.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li Z, Okamoto K, Hayashi Y, et al. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell. 2004;119:873–887. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 53.Szabadkai G, Simoni AM, Chami M, et al. Drp-1-dependent division of the mitochondrial network blocks intraorganellar Ca2+ waves and protects against Ca2+-mediated apoptosis. Mol.Cell. 2004;16:59–68. doi: 10.1016/j.molcel.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 54.Lemasters JJ, Theruvath TP, Zhong Z, et al. Mitochondrial calcium and the permeability transition in cell death. Biochim.Biophys.Acta. 2009;1787:1395–1401. doi: 10.1016/j.bbabio.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bach D, Pich S, Soriano FX, et al. Mitofusin-2 determines mitochondrial network architecture and mitochondrial metabolism. A novel regulatory mechanism altered in obesity. J.Biol.Chem. 2003;278:17190–17197. doi: 10.1074/jbc.M212754200. [DOI] [PubMed] [Google Scholar]
- 56.Ducluzeau PH, Priou M, Weitheimer M, et al. Dynamic regulation of mitochondrial network and oxidative functions during 3T3-L1 fat cell differentiation. J.Physiol Biochem. 2011;67:285–296. doi: 10.1007/s13105-011-0074-6. [DOI] [PubMed] [Google Scholar]
- 57.Affourtit C, Brand MD. Uncoupling protein-2 contributes significantly to high mitochondrial proton leak in INS-1E insulinoma cells and attenuates glucose-stimulated insulin secretion. Biochem.J. 2008;409:199–204. doi: 10.1042/BJ20070954. [DOI] [PubMed] [Google Scholar]
- 58.Affourtit C, Brand MD. Stronger control of ATP/ADP by proton leak in pancreatic beta-cells than skeletal muscle mitochondria. Biochem.J. 2006;393:151–159. doi: 10.1042/BJ20051280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Affourtit C, Brand MD. On the role of uncoupling protein-2 in pancreatic beta cells. Biochim.Biophys.Acta. 2008;1777:973–979. doi: 10.1016/j.bbabio.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 60.Duvezin-Caubet S, Jagasia R, Wagener J, et al. Proteolytic processing of OPA1 links mitochondrial dysfunction to alterations in mitochondrial morphology. J.Biol.Chem. 2006;281:37972–37979. doi: 10.1074/jbc.M606059200. [DOI] [PubMed] [Google Scholar]
- 61.Pich S, Bach D, Briones P, et al. The Charcot-Marie-Tooth type 2A gene product, Mfn2, up-regulates fuel oxidation through expression of OXPHOS system. Hum.Mol.Genet. 2005;14:1405–1415. doi: 10.1093/hmg/ddi149. [DOI] [PubMed] [Google Scholar]
- 62.Benard G, Rossignol R. Ultrastructure of the mitochondrion and its bearing on function and bioenergetics. Antioxid.Redox.Signal. 2008;10:1313–1342. doi: 10.1089/ars.2007.2000. [DOI] [PubMed] [Google Scholar]
- 63.Benard G, Bellance N, James D, et al. Mitochondrial bioenergetics and structural network organization. J.Cell Sci. 2007;120:838–848. doi: 10.1242/jcs.03381. [DOI] [PubMed] [Google Scholar]
- 64.Twig G, Shirihai OS. The interplay between mitochondrial dynamics and mitophagy. Antioxid.Redox.Signal. 2011;14:1939–1951. doi: 10.1089/ars.2010.3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Westermann B. Organelle dynamics: ER embraces mitochondria for fission. Curr.Biol. 2011;21:R922–R924. doi: 10.1016/j.cub.2011.10.010. [DOI] [PubMed] [Google Scholar]