Abstract
Background
Chemotherapy preference refers to a patient’s interest in receiving chemotherapy. This study examined whether chemotherapy preference was associated with toxicity, efficacy, quality of life (QoL), and functional outcomes during and after completion of adjuvant chemotherapy in older women with breast cancer.
Methods
This study is a secondary analysis of CALGB 49907, a randomized trial that compared standard adjuvant chemotherapy versus capecitabine in patients age 65 years or older with breast cancer. A subset of 145 patients completed a questionnaire to describe chemotherapy preference pre-treatment. The association of this pre-treatment preference with the patient’s perception of self-health, predicted and actual QoL, patient- and professional-reported toxicity, mental health, self-rated function, and survival was studied during and after treatment.
Results
The median age of patients was 71 years and 47% had a high preference for chemotherapy. On baseline demographics, the low preference group had a higher proportion of white patients (95% vs. 78%, p=0.004). Before treatment, low chemotherapy preference was associated with greater nausea/vomiting (p=0.008). Mid-treatment, low preference was associated with lower QoL, worse social, emotional and physical function (all p≤0.02) and worse nausea/vomiting, cancer symptoms and financial worries (all p<0.05). The association noted mid-treatment, resolved after treatment completion except with financial worries which persisted at 24 months. Low preference was associated with higher rates of grade 3–5 adverse events (53% vs. 34%, p=0.02) but was not associated with survival.
Conclusion
Low chemotherapy preference prior to treatment initiation was associated with lower QoL, worse physical symptoms and self-rated function and more adverse events midtreatment. There is no association of chemotherapy preference with survival.
Keywords: Adjuvant Chemotherapy, Breast Cancer, Decision-making, Elderly
Background
There is limited information about the perceptions of older women with breast cancer regarding adjuvant chemotherapy. Older women may have treatment goals that differ from those of younger women with breast cancer. While older adults do not differ from their younger counterparts in terms of acceptance of chemotherapy, it is known that they differ in terms of willingness to trade survival for current quality of life (QoL).1 Common factors implicated in refusal of cancer treatment among older adults include the risk of adverse events (AEs), fear about the discomfort of the treatments, and transportation difficulties.2 These issues are particularly pertinent in older women faced with the decision regarding adjuvant chemotherapy where the therapy has a potential benefit but also carries a significant risk of side effects. Many women find the decision regarding adjuvant chemotherapy to be difficult, with several factors contributing to the decision as to whether receipt of chemotherapy is considered worthwhile.3,4 Older women are often offered fewer opportunities to discuss chemotherapy compared to younger patients and are more likely to harbor negative attitudes and beliefs toward treatment.5
It is not known whether a patient’s “pre-conceived notions” about the impact of adjuvant chemotherapy on QoL might actually have a bearing on QOL during chemotherapy. Such perceptions, especially about toxicity and the impact of adjuvant chemotherapy on QoL may have a major bearing on the decision to accept adjuvant chemotherapy. Further, such perception may also impact the patient’s view and reporting of side effects during chemotherapy. For example, a patient who is very apprehensive of chemotherapy might report more symptoms during chemotherapy. Thus, various factors including perceived risks and benefits of chemotherapy, patients’ perception of their own health and hence, tolerance of chemotherapy may contribute to their willingness to receive chemotherapy when offered. In this manuscript this is referred to as “chemotherapy preference.” To the best of our knowledge, chemotherapy preference prior to treatment has not been studied formally as a factor that may impact cancer outcomes in older adults, especially the frequency of AEs and survival has not been studied.
We hypothesized that patients with low preference for chemotherapy may report more adverse effects during chemotherapy. As a consequence, such patients may report a poorer QoL during treatment and greater declines in physical function compared to patients who have a higher preference for chemotherapy. The latter would be more accepting of the adverse effects since they assign a higher value to the benefit chemotherapy might offer. It is also possible that patients with low preference for chemotherapy may suffer from greater anxiety or depression during chemotherapy treatment and that some of these effects may persist after completion of chemotherapy. Similarly, it is not known whether initial preference for chemotherapy may have an impact on efficacy outcomes, disease-free and overall survival. This unplanned secondary data analysis was undertaken to provide greater clarity on chemotherapy preference and outcomes in older patients with breast cancer who all eventually received postoperative chemotherapy.
Patients and Methods
Patients
In Cancer and Leukemia Group B (CALGB) 49907, older patients (≥65 years of age at study entry) with early stage breast cancer were randomized to standard adjuvant chemotherapy (doxorubicin and cyclophosphamide [AC] or cyclophosphamide, methotrexate and 5-fluorouracil [CMF]) versus capecitabine, which was considered the investigational study arm.6 (CALGB is now part of the Alliance for Clinical Trials in Oncology.) Of the 633 patients enrolled on CALGB 49907 between September 15, 2001 and December 29, 2006, a pre-planned subset of 350 patients participated in a QoL substudy. Of these 350 patients, 145 patients completed the baseline assessment regarding chemotherapy preference. The objective of this secondary analysis is to assess whether patients’ baseline chemotherapy preference (defined as high or low), is associated with the following during and after completion of chemotherapy: self-reported toxicity, professional-reported toxicity, changes in self-reported function, mental health, QoL, recurrence-free survival and overall survival. This group of patients was also asked to rate their health prior to chemotherapy and to predict the QoL of a hypothetical patient receiving chemotherapy. Each participant signed an IRB-approved, protocol-specific informed consent in accordance with federal and institutional guidelines.
Methods
To measure preferences, a previously validated, modified time trade-off approach was utilized to evaluate the benefit, defined as time of life gained, women would require to choose chemotherapy in a hypothetical situation, irrespective of the chemotherapy agent used. Using a ping-pong response pattern, women were asked the following question: “If you were this…patient, would you agree to chemotherapy if it has a 50/50 chance of adding (5 years…down to one week) to your life?” Choosing chemotherapy for the shortest period of gain (i.e., 1 week) indicates the highest preference for chemotherapy, whereas not choosing chemotherapy for even a 5-year gain represents the lowest preference.3,7 Because about half the women indicated that they would choose chemotherapy if it provided 12 months of life extension, the preferences were dichotomized at this threshold. Thus, for the purpose of these current analyses, women who chose chemotherapy for an increase in overall survival of ≤12 months were designated as “high chemotherapy preference” and those who chose >12 months were designated “low chemotherapy preference.” The assessment of patient reported adverse events, changes in function, QoL using validated tools were carried out at mid-treatment and at 1, 12, 18 and 24 months after completion of chemotherapy.
The association of chemotherapy preference (assessed pre-chemotherapy) and the following variables were assessed:
(i) Perception of self-health: The baseline health assessment was obtained by asking patients to rate their health on a linear scale with higher numbers representing better perceived self-health. (ii) Perceived QoL on chemotherapy: Patients were provided a hypothetical scenario of a patient undergoing adjuvant chemotherapy with a narrative regarding common side effects of chemotherapy. Patients were asked to rate the quality of life of this patient during chemotherapy. (iii) Patient-reported adverse events, changes in function and QoL (based on EORTC-QLQ-C30, a validated questionnaire developed to assess the quality of life of patients with cancer).8 (iv) Patient-reported physical and emotional condition, social life and quality of life (based on the Subjective Significance Questionnaire): The Subjective Significance Questionnaire is a 5-item, multi-dimensional self-rated questionnaire that assess patients’ perceived changes in physical, emotional and social functioning and in global quality of life. It provides a method for interpreting the meaningfulness of changes in scores as derived from a general questionnaire, the EORTC QLQ-C30.9 QoL and function (physical, role, social, cognitive and emotional) were also captured as a part of the EORTC QLQ-C30 in this group of patients. (v) Anxiety and depression (based on Hospital Anxiety and Depression Scale [HADS]): The HADS is a validated, self-assessment scale that has been found to be a reliable instrument for detecting states of depression and anxiety in the setting of a hospital medical outpatient clinic.10 (vi) Observed grade 3–5 adverse events (AEs) by NCI common toxicity criteria (CTC v2.0).11 (vii) Overall survival (OS) and recurrence-free survival (RFS).
Statistical Analysis
Data collection and statistical analyses were conducted by the Alliance Statistics and Data Center. Continuous variables were summarized as the mean ± standard deviation or as the median and range. Comparisons of continuous variables between groups were made with a two-sample t-test or Wilcoxon rank sum test, whichever was more appropriate for the distribution. Categorical variables were summarized as frequencies and percentages. Comparisons of categorical variables between groups were made with a chi-square test or Fisher’s exact test if expected cell sizes were too small for the chi-square test. Kaplan-Meier survival curves for OS and RFS were compared by log-rank test. The unadjusted hazard ratios were calculated using a Cox regression analysis. OS is defined as time from registration until death from any cause or date that the patient was last known to be alive. RFS is defined as the time from registration until first recurrence, death from any cause, or the date patient was last known to be alive.
All tests were two-sided, and p-values less than 0.05 were considered to be statistically significant. Since these were hypothesis generating secondary data analyses, an adjustment for multiple comparison was not performed. Analyses were performed using SAS version 9.3 (SAS Institute Inc., Cary, North Carolina) on a database locked on May 28, 2013. Data quality was ensured by review of data by the Alliance Statistics and Data Center and by the study chairperson following Alliance policies.
Results
The baseline demographics and tumor characteristics of patients (median age 71 years) who provided chemotherapy preference at baseline (n=145) were not different from the remainder of the women in the QoL subset who did not provide their chemotherapy preference (n=206) [Supplementary Table S1]. Similarly, they were largely similar to those women who were not included in the QoL substudy (n=282) [Supplementary Table S2]. Of the 145 patients included, 68 (47%) women had a high chemotherapy preference. Chemotherapy preference groups (high versus low) did not differ in age, type of surgery, hormone receptor status, performance status, tumor and nodal stage, number of positive nodes, chemotherapy assignment, education, comorbidity, marital or employment status. However, the low preference group had a higher proportion of white women (95% vs. 78%, p=0.004) [Table 1].
Table 1.
Patient and tumor baseline characteristics: High versus low chemotherapy preference
| High preference N = 68 N (%) |
Low preference N = 77 N (%) |
p-value | |
|---|---|---|---|
|
| |||
| Race | |||
| White | 53 (77.9) | 73 (94.8) | |
| Other | 13 (19.1) | 3 (3.9) | 0.004 |
| Unknown | 2 (2.9) | 1 (1.3) | |
|
| |||
| Age, | |||
| 65–69 years | 29 (42.7) | 29 (37.7) | |
| 70–80 years | 37 (54.4) | 41 (53.3) | 0.31 |
| > 80 years | 2 (2.6) | 7 (9.1) | |
|
| |||
| Primary surgery | |||
| Less than mastectomy | 29 (42.6) | 43 (55.8) | 0.11 |
| Mastectomy | 39 (57.4) | 34 (44.2) | |
|
| |||
| ER status | |||
| Negative | 19 (27.9) | 32 (41.6) | 0.087 |
| Positive | 49 (72.1) | 45 (58.4) | |
|
| |||
| PR status | |||
| Negative | 32 (47.1) | 42 (54.6) | 0.37 |
| Positive | 36 (52.9) | 35 (45.4) | |
|
| |||
| Performance status | |||
| 0–1 | 67 (98.5) | 75 (97.4) | 0.99 |
| 2 | 1 (1.5) | 2 (2.6) | |
|
| |||
| T Stage | |||
| T1 | 27 (40.3) | 34 (46.0) | 0.71 |
| T2 | 38 (56.7) | 37 (50.0) | |
| T3 | 2 (3.0) | 3 (4.0) | |
|
| |||
| N Stage | |||
| N0 | 25 (39.7) | 31 (40.8) | |
| N1 | 31 (49.2) | 39 (51.3) | 0.84 |
| N2 | 6 (9.5) | 6 (7.9) | |
| N3 | 1 (1.6) | 0 | |
|
| |||
| Number of positive nodes | |||
| 0 | 25 (36.8) | 32 (42.1) | |
| 1 | 15 (22.1) | 18 (23.7) | 0.77 |
| 2 | 13 (19.1) | 10 (13.2) | |
| 3 or more | 15 (22.1) | 16 (21.0) | |
|
| |||
| Treatment arm | |||
| CMF or AC | 29 (42.6) | 39 (50.6) | 0.33 |
| Capecitabine | 39 (57.4) | 38 (49.4) | |
|
| |||
| Highest level of education | |||
| Grades 1–11 | 11 (16.4) | 5 (6.8) | |
| High school graduate | 18 (26.9) | 33 (44.6) | 0.09 |
| Some college | 20 (29.8) | 20 (27.0) | |
| College/Advanced degree | 18 (26.9) | 16 (21.6) | |
|
| |||
| Marital status | |||
| Separated/Divorced | 6 (9.0) | 4 (5.3) | |
| Married | 38 (56.7) | 39 (52.0) | 0.70 |
| Single, never married | 7 (10.4) | 10 (13.3) | |
| Widowed | 16 (23.9) | 22 (29.3) | |
|
| |||
| Employment status | |||
| Employed Full/Part Time | 8 (11.9) | 5 (6.7) | |
| Retired | 43 (64.2) | 54 (72.0) | 0.47 |
| Other | 16 (23.9) | 16 (21.3) | |
CMF: Cyclophosphamide/Methotrexate/5-Fluorouracil; AC: Doxorubicin/Cyclophosphamide
Chemotherapy Preference, Self-health and Perceived QoL
Patients with a low preference for chemotherapy perceived their health to be the same as women with a high preference for chemotherapy at pre-, mid-, and post-treatment up to 24 months. [Table 2] Patients with a low preference for chemotherapy perceived the QoL on chemotherapy for a hypothetical patient to be worse than women with a high preference for chemotherapy (p=0.006) [Table 3].
Table 2.
Association of patient reported self-health with chemotherapy preference
| Time Points | Pre-Treatment | Mid-Treatment | 1 month post chemotherapy | 12 months post chemotherapy | 18 months post chemotherapy | 24 months post chemotherapy | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chemotherapy Preference | High | Low | P | High | Low | P | High | Low | P | High | Low | P | High | Low | P | High | Low | P |
| Indicate how good or bad your own health is today | 0.58 | 0.54 | 0.22 | 0.94 | 0.38 | 0.30 | ||||||||||||
| 0–50 | 2 (3.0) |
4 (5.4) |
4 (9.8) |
4 (8.9) |
4 (9.1) |
8 (12.9) |
3 (5.4) |
3 (5.3) |
5 (10.0) |
2 (3.7) |
4 (7.8) |
4 (7.8) |
||||||
| 51–75 | 10 (15.2) |
15 (20.3) |
3 (7.3) |
8 (17.8) |
4 (9.1) |
14 (22.6) |
8 (14.3) |
10 (17.5) |
9 (18.0) |
12 (22.2) |
8 (15.7) |
8 (15.7) |
||||||
| 76–90 | 36 (54.6) |
41 (55.4) |
26 (63.4) |
24 (53.3) |
28 (63.6) |
29 (46.8) |
32 (57.1) |
33 (57.9) |
27 (54.0) |
25 (46.3) |
34 (66.7) |
27 (52.9) |
||||||
| 91–100 | 18 (27.3) |
14 (18.9) |
8 (19.5) |
9 (20.0) |
8 (18.2) |
11 (17.7) |
13 (23.2) |
11 (19.3) |
9 (18.0) |
15 (27.8) |
5 (9.8) |
12 (23.5) |
||||||
Table 3.
Association of chemotherapy preference with perceived quality of life (QoL) for a hypothetical patient (pre-chemotherapy)
| Perceived QoL on chemotherapy (hypothetical patient) | High chemotherapy preference N (%) |
Low chemotherapy preference N (%) |
P value |
|---|---|---|---|
| 0–50 | 9 (14.1) | 20 (14.1) | 0.006 |
| 51–75 | 24 (37.5) | 35 (50.7) | |
| 76–90 | 23 (35.9) | 11 (15.9) | |
| 91–100 | 8 (12.5) | 3 (4.4) |
Association of Chemotherapy Preference with Patient Reported Symptoms (Based on EORTC QLQ-C30) [Supplementary Table S3]
There were no differences at baseline, mid-treatment or post-treatment in patient reported outcomes (PROs) for dyspnea, pain, fatigue and insomnia based on chemotherapy preference. At baseline (pre-chemotherapy), patients with low preference had higher mean scores for nausea and vomiting than patients with high preference (mean score 3.9 vs. 0.8, p = .0008), but no differences were noted in terms of financial worries. At the mid-treatment assessments, patients with low preference reported higher scores for nausea/vomiting (mean score 15.9 vs. 6.4, p = .002), financial worries (mean score 18.5 vs. 10.1, p=.043), and cancer symptoms (mean score 25.0 vs. 19.1, p=0.022). Post-chemotherapy, patients with low preference reported higher scores for constipation at one month assessment (mean score 28.9 vs. 11.1, p = 0.0004). Patients with low preference were more likely to endorse financial worries at the 24-month assessment post chemotherapy (mean 19.9 vs. 7.0, p = 0.004).
Association with Quality of Life and Function
The QoL was captured based on two separate, validated instruments embedded in the trial. Based on the Subjective Significance Scale, women with low preference reported lower quality of life as well as worse scores for social, emotional and physical function mid-treatment, compared to women with high preference [Table 4]. These differences resolved post-treatment and were not noted at assessments obtained at 1, 12, 18 and 24 months after completion of chemotherapy.
Table 4.
Patient reported function and quality of life (QoL) based on Subjective Significance Questionnaire
| Assessment Time Points | Mid-Treatment | 1 Month Post-chemotherapy | 12 month Post-Chemotherapy | 18 Months Post chemotherapy | 24 Month Post-chemotherapy | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chemotherapy preference | High | Low | P | High | Low | P | High | Low | P | High | Low | P | High | Low | P |
| Has your overall physical condition gotten: | |||||||||||||||
| Any worse | 8 (13.6) |
23 (34.3) |
0.005 | 8 (15.1) |
13 (18.6) |
0.74 | 4 (7.0) |
3 (5.2) |
0.65 | 7 (13.7) |
8 (14.0) |
0.87 | 6 (11.8) |
10 (18.2) |
0.59 |
| About the same | 29 (49.2) |
33 (49.2) |
25 (47.2) |
35 (50.0) |
23 (40.4) |
19 (32.8) |
20 (39.2) |
25 (43.9) |
27 (52.9) |
29 (52.7) |
|||||
| Any better | 22 (37.3) |
11 (16.4) |
20 (37.7) |
22 (31.4) |
30 (52.6) |
36 (62.1) |
24 (47.1) |
24 (42.1) |
18 (35.3) |
16 (29.1) |
|||||
| Has your overall emotional state gotten: | |||||||||||||||
| Any worse | 3 (5.1) |
12 (17.9) |
0.012 | 3 (5.6) |
8 (11.4) |
0.40 | 2 (3.5) |
3 (5.2) |
0.99 | 3 (5.9) |
5 (8.8) |
0.87 | 0 | 5 (9.1) |
0.10 |
| About the same | 33 (55.9) |
42 (62.7) |
33 (61.1) |
44 (62.9) |
23 (40.4) |
24 (41.4) |
26 (51.0) |
29 (50.9) |
35 (68.6) |
33 (60.0) |
|||||
| Any better | 23 (39.0) |
13 (19.4) |
18 (33.3) |
18 (25.7) |
32 (56.1) |
31 (53.4) |
22 (43.1) |
23 (40.4) |
16 (31.4) |
17 (30.9) |
|||||
| Has ability to enjoy your social life gotten: | |||||||||||||||
| Any worse | 4 (6.9) |
17 (25.4) |
0.003 | 6 (11.1) |
10 (14.3) |
0.15 | 1 (1.8) |
2 (3.4) |
0.73 | 4 (7.8) |
4 (7.0) |
0.64 | 2 (3.9) |
5 (9.1) |
0.65 |
| About the same | 31 (53.4) |
38 (56.7) |
27 (50.0) |
44 (62.9) |
27 (47.4) |
24 (41.4) |
24 (47.1) |
32 (56.1) |
34 (66.7) |
35 (63.6) |
|||||
| Any better | 23 (39.7) |
12 (17.9) |
21 (38.9) |
16 (22.9) |
29 (50.9) |
32 (55.2) |
23 (45.1) |
21 (36.8) |
15 (29.4) |
15 (27.3) |
|||||
| Has your overall quality of life gotten: | |||||||||||||||
| Any worse | 5 (8.5) |
18 (27.3) |
0.004 | 8 (14.8) |
11 (15.9) |
0.14 | 1 (1.8) |
4 (6.9) |
0.19 | 4 (7.8) |
5 (8.8) |
0.96 | 3 (5.9) |
6 (10.9) |
0.63 |
| About the same | 31 (52.5) |
36 (54.6) |
23 (42.6) |
40 (58.0) |
24 (42.1) |
17 (29.3) |
23 (45.1) |
27 (47.4) |
31 (60.8) |
34 (61.8) |
|||||
| Any better | 23 (40.0) |
12 (18.2) |
23 (42.6) |
18 (26.1) |
32 (56.1) |
37 (63.8) |
24 (47.1) |
25 (43.9) |
17 (33.3) |
15 (27.3) |
|||||
Based on the EORTC questionnaire, there were no significant differences in the overall QoL at any time point between patients with low versus high chemotherapy preference. Patients with low chemotherapy preference had lower mean scores for cognitive function at baseline compared to those with high preference (84.3 vs. 90.0, p = 0.02). They also had lower mean scores for role functioning (72.5 vs. 85.1, p = 0.01) and social functioning (76.8 vs. 87.9, p= 0.02) during treatment. The mean scores for role functioning improved for both groups 24 months post-therapy but remained worse for patients with low preference (81.0 vs. 90.1, p = 0.05). Using the Hospital Anxiety and Depression Scale assessments, there were no significant differences in mean scores of anxiety and depression at baseline, mid- treatment, or posttreatment, between the preference groups. [Supplementary Table S4].
Adverse Events and Survival
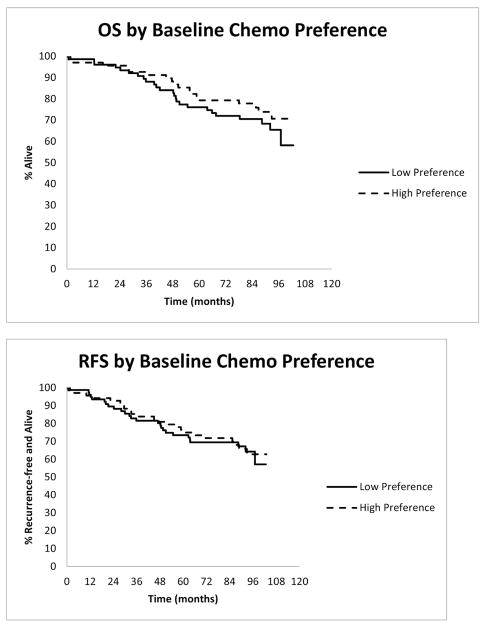
Professionally reported grade 3–5 AEs on all patients were evaluated as collected on CALGB 49907. The median follow-up duration is 7.53 years. Patients with low preference for chemotherapy had statistically significantly higher rates of grade 3–5 AEs compared to those with high chemotherapy preference (53% vs. 34%, p=0.02) during treatment but these did not persist post-therapy [Supplementary Table S5]. Chemotherapy preference was not significantly associated with OS [HR=0.75 (95% CI: 0.41, 1.38), p=0.36] or RFS [HR=0.94, (95% CI: 0.54, 1.66), p=0.84] [Figure 1].
Figure 1.
Discussion
Data from the Early Breast Cancer Trialists’ Collaborative Group suggests a decreasing benefit from adjuvant chemotherapy with increasing age; however, the authors acknowledged that too few women over the age of 70 were included in randomized clinical trials to reliably inform these data.12 In contrast, a pooled analysis of patients enrolled in randomized clinical trials for node-positive disease demonstrated that older women appear to derive similar benefit from the experimental chemotherapy arm as younger individuals.13 These data underscore the challenge of discussing adjuvant chemotherapy in older patients with breast cancer and emphasize the importance of the research presented here. There is limited information of how a patient’s perception of adjuvant chemotherapy might impact outcomes, especially among older women with breast cancer, and such perceptions likely weigh heavily into these patients’ decision making, particularly given the lack of decisive guidance from prior prospective clinical trials.
In the present study, we found that in older women with early stage breast cancer, chemotherapy preference is associated with certain outcomes. Low preference for chemotherapy is associated with lower QoL, poorer function in some domains, worse patient-reported physical symptoms, as well as higher incidence of professionally assessed adverse events during chemotherapy. However, notably, these mid-therapy declines are largely resolved after therapy. The significance of the findings is two-fold: First, these findings can help inform older patients with low chemotherapy preference that they are more likely to have a decline in function, worse physical symptoms and toxicity during adjuvant chemotherapy but that these typically reverse after completion of chemotherapy. Such information, if provided at the time of adjuvant chemotherapy discussion, may allow these patients greater ease with the decision regarding chemotherapy. Second, these findings may be useful for medical oncologists who may dissuade older women from adjuvant chemotherapy due to concerns of chemotherapy-associated toxicity and an irreversible decline in function. As evidenced in the current cohort, the majority of decline observed in function, as well as symptoms during treatment in patients with low preference, largely normalized after completion of therapy. The reversible nature of the decline in QoL was previously reported as it pertains to the entire cohort of patients, irrespective of chemotherapy preference.14 As a counter-point, these data may be considered in older patients with borderline indication for adjuvant chemotherapy who are reluctant to accept chemotherapy, noting that such a patient may be at a higher risk for AEs and/or decrement in QoL and function during treatment. Such a consideration may then “tip the decision balance” away from a chemotherapy recommendation. Thus, a detailed conversation with patients with low preference could temper expectations and prepare the patient better for chemotherapy. Additionally, proactive strategies to address reported symptoms may be targeted to patients with low preference (such as more frequent phone calls or visits) to manage symptoms and provide reassurance.
In the present cohort differences in chemotherapy preference were noted based on race, with Caucasian women more likely to have low preference. While no clear reason exists, a possible explanation may be that “chemotherapy fatigue” among Caucasian women, who form the majority demographic, may result from exposure to other women who have been treated with chemotherapy causing them to be less inclined to try chemotherapy. Alternatively, this finding may be a consequence of cultural beliefs among non-Caucasian women who may be more likely to perceive chemotherapy as an essential part of the treatment regimen. A recent retrospective study of 868 patients with non-metastatic breast cancer across all ages found that African-American women were more likely to receive chemotherapy (48.3% vs. 36.0%, p=0.001), and black race was favorably associated with completing planned chemotherapy compared to white women [Odds Ratio (OR) 2.36, p=0.052, CI (0.99–5.62)].15
Patients who predicted poor QoL for a hypothetical patient during chemotherapy were found to be more likely to have low preference for chemotherapy. While the explanation is intuitive, in that low preference is a surrogate for perceived poor QoL during treatment, the information that the transient decline in QoL reverses after completion of chemotherapy may help such patients have a more favorable outlook towards chemotherapy. Interestingly, the rating of self-health at baseline does not impact chemotherapy preference. Hence, the above finding suggests that the perceptions of chemotherapy preference are independent of the patients’ perception of their own health.
Some of the patient-reported symptoms as captured by EORTC questionnaire were worse during chemotherapy but did not persist long-term. Notably, nausea/vomiting scores were worse in patients with low preference even prior to initiating chemotherapy. Possible explanation for this finding may include either that such tendency to experience nausea/vomiting was the reason for low preference for chemotherapy, or that the thought of chemotherapy evoked a sensation of nausea/vomiting in patients with low preference. In a prior study, patients with breast cancer were asked to choose between potential chemotherapy agents based on the side effect profile of agents. Patient preferences were captured based on the trade-offs that patients are willing to make between treatment side effects. While not limited to older patients nor to early stage disease, the findings were telling nonetheless. Among the mild (grade I/II) side effects, a 5% reduction in the risk of sensory neuropathy, nausea, and motor neuropathy had the greatest impact on the choice of chemotherapy agent. Among severe (grade III/IV) side effects, motor neuropathy, nausea/vomiting, and myalgia made the most difference.16 Thus, the potential and type of anticipated side effects of chemotherapy can play a role in choice of chemotherapy with patients choosing one regimen over another based on toxicity profile. Other studies have evaluated the concept of what potential duration of life added makes the choice of adjuvant chemotherapy seem worthwhile. A recent prospective study comparing the adjuvant chemotherapy preferences of 29 older (≥ 65 years) and 52 younger women with early stage breast cancer, found a similar minimally required benefit in terms of additional 10-year disease-free survival, to accept adjuvant chemotherapy (median, 5% vs. 4% respectively; p=0.13). Factors associated with requiring larger benefits from chemotherapy included single/divorced/widowed status (OR, 0.16; p=0.005), presence of geriatric condition (OR, 0.27; p=0.047), and having a preference to make the treatment decision alone rather than after considering the clinician’s opinion (OR, 0.15; p=0.012).4 A systematic review of 15 studies regarding treatment preferences noted a wide range of individual preferences across different studies. To make adjuvant systemic therapy worthwhile, the median required absolute increase in survival rate was 0.1–10% and the median required additional life expectancy was 1 day to 5 years. Participants in the adjuvant hormonal studies required larger median benefits than those in the adjuvant chemotherapy studies. Factors associated with judging smaller benefits sufficient most often (44%) related to QoL (e.g., less treatment toxicity).17 As found in the present study, others have reported that QoL is not dramatically impacted in older women after completion of breast cancer treatment (surgery, radiation, chemotherapy and hormonal therapy). In a population-based study of a cohort of over 3000 older women treated for breast cancer with curative intent, Neuner et al. followed general and breast cancer related QoL longitudinally for 5 years after diagnosis.18 There were no declines in the general health scores. However, chemotherapy treatment was associated with worse scores in the breast cancer-specific QoL.
Investigators have developed decision quality measuring instruments in patients with breast cancer using psychometric evaluation.19,20 One such instrument, evaluated in women with early stage breast cancer, found that increasing age was negatively associated with the decision to accept chemotherapy while negative hormone receptor status had a positive association.19 However, patient goals did not demonstrate an association with chemotherapy decision–making.. Most patients reported that they were not asked their preference, which suggests that patients’ goals were likely not taken into consideration in the treatment decision-making about chemotherapy. While not limited to older women, this report highlights the need to incorporate patient’s goal in the adjuvant chemotherapy decision-making process, especially given the lower preference for chemotherapy among older patients. Our study suggests that incorporating patient preference for chemotherapy may lead to better acceptance of chemotherapy as well as greater satisfaction with the decision itself.
Given the importance of patients’ concerns regarding chemotherapy side effects and their impact on QoL, it is important that available geriatric assessment-based tools be utilized to predict the risk of chemotherapy-associated toxicity in any given patient.21,22 The identification of those at highest risk can lead to intervention to mitigate such risk (e.g., primary prophylaxis with growth factors), modification in the choice, dose or schedule of chemotherapy regimen in addition to appropriate counseling and education of the patient and caregivers. One finding in the current study that does not lend itself to a simple explanation is the association of low chemotherapy preference with higher incidence of AEs during chemotherapy as graded by the professional team. One recent study reports a rise in inflammatory cytokines during breast cancer treatment that can persist as long as 18 months after completion of all therapy.23 There also appears to be an accumulating evidence of an organic explanation for what was considered a mind-body connection.24,25 Thus, it is plausible that there may be neurocognitive factors that manifest as preference that may trigger a cytokine response, leading to subjective symptoms and objective findings during and after completion of chemotherapy.
There are limitations to this study. While the quality of life substudy was pre-planned and adequately powered, the current study is a post hoc subset analysis. Thus, these data can be considered “hypothesis generating” and ideally should be confirmed in future prospective studies. A detailed assessment regarding the impact of comorbidity and polypharmacy was not assessed in this cohort. A prior subset analysis of the 367 patients who participated on the QoL portion of CALGB 49907 has been reported: Self-reported comorbidity was associated with shorter overall survival (HR = 1.18, 95% CI= 1.06 to 1.33) but not with chemotherapy associated toxicity or time to relapse.26 In the current cohort, there was no association with patient reported comorbidity and chemotherapy preference (p = 0.51). Further, these results are limited to a cohort of patients that agreed to enroll on a clinical trial and the preference was assessed after a decision regarding chemotherapy had been already made. As is common for longitudinal questionnaire based studies, there is inevitable attrition in the number of patients submitting questionnaires with time, raising the question regarding validity and application to the entire population.
Despite these limitations, this study provides several interesting findings. Low chemotherapy preference was associated with white race but not with self-rated health. Patients with low preference have more self-reported symptoms as well as higher rates of professionally assessed adverse events, and a decline in self-reported QoL and some aspects of function during treatment. However, these differences largely resolve after completion of chemotherapy. This information may be useful for oncology professionals in working with older patients with early stage breast cancer faced with decision-making regarding adjuvant chemotherapy.
Supplementary Material
Acknowledgments
Support: Research reported in this publication was supported by grants National Cancer Institute of the National Institutes of Health under Award Number U10CA189823 (Alliance for Clinical Trials in Oncology NCORP Research Grant), U10CA180821 and U10CA180882 (to the Alliance for Clinical Trials in Oncology), U10CA003927, U10CA007968, U10CA011789, U10CA180790, U10CA180838, U10CA180857, UG1CA189858 and U10CA180867. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Presented at Thirty-Seventh Annual CTRC-AACR San Antonio Breast Cancer Symposium; December 9–13, 2014; San Antonio, TX
ClinicalTrials.gov Identifier: NCT00024102
Author Contributions
Study Concept: A Gajra, L McCall, HB Muss, HJ Cohen, A Jatoi, KV Ballman, AH Partridge, L Sutton, BA Parker, G Magrinat, HD Klepin, JM Lafky, A Hurria
Study Design: A Gajra, L McCall, HB Muss, HJ Cohen, A Jatoi, K V Ballman, HD Klepin, JM Lafky, A Hurria
Data Acquisition: A Gajra, L McCall, KV Ballman, A Hurria
Quality Control of Data and Algorithms: A Gajra, L McCall, KV Ballman, A Hurria
Data Analysis and Interpretation: A Gajra, L McCall, KV Ballman, A Hurria
Statistical Analysis: A Gajra, L McCall, K Ballman, A Hurria
Manuscript Preparation: A Gajra, L McCall, A Hurria
Manuscript Editing: A Gajra, L McCall, HB Muss, HJ Cohen, A Jatoi, KV Ballman, AH Partridge, L Sutton, BA Parker, G Magrinat, HD Klepin, JM Lafky, A Hurria
Manuscript Review: A Gajra, L McCall, HB Muss, HJ Cohen, A Jatoi, KV Ballman, AH Partridge, L Sutton, BA Parker, G Magrinat, HD Klepin, JM Lafky, A Hurria
Disclosures and Conflict of Interest Statements
Dr. Gajra serves as a consultant for Bayer and BMS. Dr. Hurria has received research funding from Celgene, Novartis, and GSK as well as served as a consultant for Boehringer Ingelheim Pharmaceuticals, Carevive, Sanofi, GTx, Inc., Pierian Biosciences, and MJH Healthcare Holdings, LLC outside the submitted work. The authors have no other disclosures to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yellen SB, Cella DF, Leslie WT. Age and clinical decision making in oncology patients. J Natl Cancer Inst. 1994;86:1766–70. doi: 10.1093/jnci/86.23.1766. [DOI] [PubMed] [Google Scholar]
- 2.Puts MT, Tapscott B, Fitch M, et al. A systematic review of factors influencing older adults’ decision to accept or decline cancer treatment. Cancer Treat Rev. 2015;41:197–215. doi: 10.1016/j.ctrv.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 3.Mandelblatt JS, Sheppard VB, Hurria A, et al. Breast cancer adjuvant chemotherapy decisions in older women: the role of patient preference and interactions with physicians. J Clin Oncol. 2010;28:3146–53. doi: 10.1200/JCO.2009.24.3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamelinck VC, Bastiaannet E, Pieterse AH, et al. A Prospective Comparison of Younger and Older Patients’ Preferences for Adjuvant Chemotherapy and Hormonal Therapy in Early Breast Cancer. Clinical Breast Cancer. 2016;16:379–88. doi: 10.1016/j.clbc.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Shelton RC, Clarke Hillyer G, Hershman DL, et al. Interpersonal influences and attitudes about adjuvant therapy treatment decisions among non-metastatic breast cancer patients: an examination of differences by age and race/ethnicity in the BQUAL study. Breast Cancer Res Treat. 2013;137:817–28. doi: 10.1007/s10549-012-2370-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muss HB, Berry DA, Cirrincione CT, et al. Adjuvant chemotherapy in older women with early-stage breast cancer. N Engl J Med. 2009;360:2055–65. doi: 10.1056/NEJMoa0810266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Froberg DG, Kane RL. Methodology for measuring health-state preferences--II: Scaling methods. Journal of Clinical Epidemiology. 1989;42:459–71. doi: 10.1016/0895-4356(89)90136-4. [DOI] [PubMed] [Google Scholar]
- 8.EORTC Quality of Life Group. EORTC QOL-C30 (v3) EORTC Quality of Life. 2017 Feb 8; Available from: groups.eortc.be/qol/eortc-qlq-c30. 1995.
- 9.Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16:139–44. doi: 10.1200/JCO.1998.16.1.139. [DOI] [PubMed] [Google Scholar]
- 10.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica. 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 11.Cancer Therapy Evaluation Program. Common toxicity criteria (CTC) (v.2.0) 2016 Apr 2; Available from https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcv20_4-30-992.pdf. April 20, 1999.
- 12.Early Breast Cancer Trialists’ Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 13.Muss HB, Woolf S, Berry D, et al. Adjuvant chemotherapy in older and younger women with lymph node-positive breast cancer. JAMA. 2005;293:1073–81. doi: 10.1001/jama.293.9.1073. [DOI] [PubMed] [Google Scholar]
- 14.Kornblith AB, Lan L, Archer L, et al. Quality of life of older patients with early-stage breast cancer receiving adjuvant chemotherapy: a companion study to cancer and leukemia group B 49907. J Clin Oncol. 2011;29:1022–8. doi: 10.1200/JCO.2010.29.9859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lipscomb J, Gillespie TW, Goodman M, et al. Black-white differences in receipt and completion of adjuvant chemotherapy among breast cancer patients in a rural region of the US. Breast Cancer Res Treat. 2012;133:285–96. doi: 10.1007/s10549-011-1916-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beusterien K, Grinspan J, Kuchuk I, et al. Use of conjoint analysis to assess breast cancer patient preferences for chemotherapy side effects. The Oncologist. 2014;19:127–34. doi: 10.1634/theoncologist.2013-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamelinck VC, Bastiaannet E, Pieterse AH, et al. Patients’ preferences for surgical and adjuvant systemic treatment in early breast cancer: a systematic review. Cancer Treat Rev. 2014;40:1005–18. doi: 10.1016/j.ctrv.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 18.Neuner JM, Zokoe N, McGinley EL, et al. Quality of life among a population-based cohort of older patients with breast cancer. Breast (Edinburgh, Scotland) 2014;23:609–16. doi: 10.1016/j.breast.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee CN, Wetschler MH, Chang Y, et al. Measuring decision quality: psychometric evaluation of a new instrument for breast cancer chemotherapy. BMC Medical Informatics and Decision Making. 2014;14:73. doi: 10.1186/1472-6947-14-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Resnicow K, Abrahamse P, Tocco RS, et al. Development and psychometric properties of a brief measure of subjective decision quality for breast cancer treatment. BMC Medical Informatics and Decision Making. 2014;14:110. doi: 10.1186/s12911-014-0110-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol. 2011;29:3457–65. doi: 10.1200/JCO.2011.34.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Extermann M, Boler I, Reich RR, et al. Predicting the risk of chemotherapy toxicity in older patients: the Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score. Cancer. 2012;118:3377–86. doi: 10.1002/cncr.26646. [DOI] [PubMed] [Google Scholar]
- 23.Alfano CM, Peng J, Andridge RR, et al. Inflammatory Cytokines and Comorbidity Development in Breast Cancer Survivors Versus Noncancer Controls: Evidence for Accelerated Aging? J Clin Oncol. 2017;35:149–56. doi: 10.1200/JCO.2016.67.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ganz PA, Dougherty PM. Painful Hands and Feet After Cancer Treatment: Inflammation Affecting the Mind-Body Connection. J Clin Oncol. 2016;34:649–52. doi: 10.1200/JCO.2015.64.7479. [DOI] [PubMed] [Google Scholar]
- 25.Ganz PA, Petersen L, Bower JE, Crespi CM. Impact of Adjuvant Endocrine Therapy on Quality of Life and Symptoms: Observational Data Over 12 Months From the Mind-Body Study. J Clin Oncol. 2016;34:816–24. doi: 10.1200/JCO.2015.64.3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klepin HD, Pitcher BN, Ballman KV, et al. Comorbidity, chemotherapy toxicity, and outcomes among older women receiving adjuvant chemotherapy for breast cancer on a clinical trial: CALGB 49907 and CALGB 361004 (alliance) Journal of Oncology Practice/American Society of Clinical Oncology. 2014;10:e285–92. doi: 10.1200/JOP.2014.001388. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.