Abstract
Thrombotic microangiopathy can manifest in a diverse range of diseases and is characterized by thrombocytopenia, microangiopathic hemolytic anemia, and organ injury, including AKI. It can be associated with significant morbidity and mortality, but a systematic approach to investigation and prompt initiation of supportive management and, in some cases, effective specific treatment can result in good outcomes. This review considers the classification, pathology, epidemiology, characteristics, and pathogenesis of the thrombotic microangiopathies, and outlines a pragmatic approach to diagnosis and management.
Keywords: Complement, glomerular disease, atypical hemolytic uremic syndrome, Thrombotic Microangiopathies, Purpura, Thrombotic Thrombocytopenic, Acute Kidney Injury, kidney, Anemia, Hemolytic
Introduction
Thrombotic microangiopathy (TMA), a pathologic description, is characterized by a clinical presentation with thrombocytopenia, microangiopathic hemolytic anemia (MAHA), and organ injury (1,2). It can manifest in a diverse range of conditions and presentations, but AKI is a common prominent feature because of the apparent propensity of the glomerular circulation to endothelial damage and occlusion (3). Early recognition is important: TMAs are associated with significant mortality and morbidity, including ESRD, although prompt initiation of supportive and specific management can transform outcome. In particular, the prognoses of thrombotic thrombocytopenic purpura (TTP) and atypical hemolytic uremic syndrome (aHUS) have been revolutionized following the elucidation of the pathogenic mechanisms and the introduction of effective therapy. Challenges and controversies remain, however. In this review, we will discuss the classification, pathology, epidemiology, characteristics, and pathogenesis of the TMAs, and outline an approach to diagnosis and management.
Classification of Thrombotic Microangiopathies
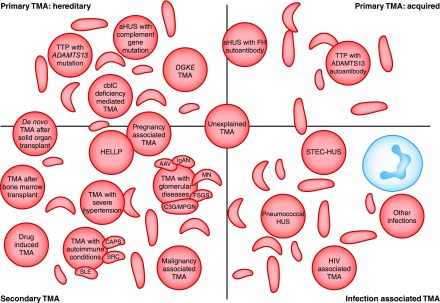
The classification of the TMAs is challenging and constantly evolving. Historical classifications were on the basis of clinical findings: TTP for predominant neurologic involvement and hemolytic uremic syndrome (HUS) for kidney dominant disease. Classifications evolved with greater understanding of the molecular basis of disease: TTP was defined by severe ADAMTS13 deficiency, hemolytic uremic syndrome caused by shiga toxin–producing Escherichia coli (STEC-HUS) was defined by the presence of shiga toxin–producing bacteria, and aHUS was broadly used for all other causes of TMA. The discovery of the role of complement dysregulation in a proportion of patients with aHUS subsequently led to the acceptance of the term complement-mediated TMA to refer to this subgroup (2,4). It should be recognized that differences in the historical and contemporary literature over nomenclature make interpretation difficult: aHUS may refer specifically to complement mediated TMA, or be more loosely applied to any TMA that is not TTP or STEC-HUS. In this review, we adopt the term complement-mediated aHUS when the cause is defined as such, and use aHUS where the cause is ill defined. Current classifications describe primary TMAs, known as either acquired (e.g., factor H (FH) autoantibodies, ADAMTS13 autoantibodies) or inherited (e.g., complement mutations, ADAMTS13 mutations); secondary TMAs; and infection-associated TMAs (2) (Figure 1). Although a useful framework for discussion, these terms do not account for the increasing recognition that patients with an underlying complement risk factor often require a secondary trigger for TMA to manifest. Additionally, with increasing reports of eculizumab nonresponse, a more clinically orientated classification such as eculizumab-responsive and eculizumab-resistant aHUS are likely to be introduced.
Figure 1.
Thrombotic microangiopathies are classified into: Inherited or acquired primary; secondary; or infection associated TMAs. Current classifications define primary TMAs as hereditary (mutations in ADAMTS13, MMACHC (cb1c deficiency), or genes encoding complement proteins) or acquired (autoantibodies to ADAMTS13, or autoantibodies to complement FH, which is associated with homozygous CFHR3/1 deletion). TMA is associated with various infections: in STEC-HUS and pneumococcal HUS, distinct mechanisms result in TMA; in other infections, the processes are not defined and in some cases the infection may trigger manifestation of a primary TMA. Secondary TMAs occur in a spectrum of conditions, and in many cases the pathogenic mechanisms are multifactorial or unknown. The classification presented here is not unequivocal: in some secondary TMAs, for example pregnancy-associated TMA or de novo TMA after transplantation, a significant proportion of individuals will have a genetic predisposition to a primary TMA. AAV, ANCA-associated vasculitis; ADAMTS13, a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13; aHUS, atypical hemolytic uremic syndrome; C3G, C3 glomerulopathy; CAPS, catastrophic antiphospholipid syndrome; cblC, cobalamin C type; DGKE, gene encoding diacylglycerol kinase ε; FH, factor H; HELLP, syndrome of hemolysis, elevated liver enzymes, and low platelets; HUS, hemolytic uremic syndrome; IgAN, IgA nephropathy; MN, membranous nephropathy; MPGN, membranoproliferative GN; SRC, scleroderma renal crisis; STEC, shiga toxin–producing Escherichia coli; TMA, thrombotic microangiopathy; TTP, thrombotic thrombocytopenic purpura.
Pathology
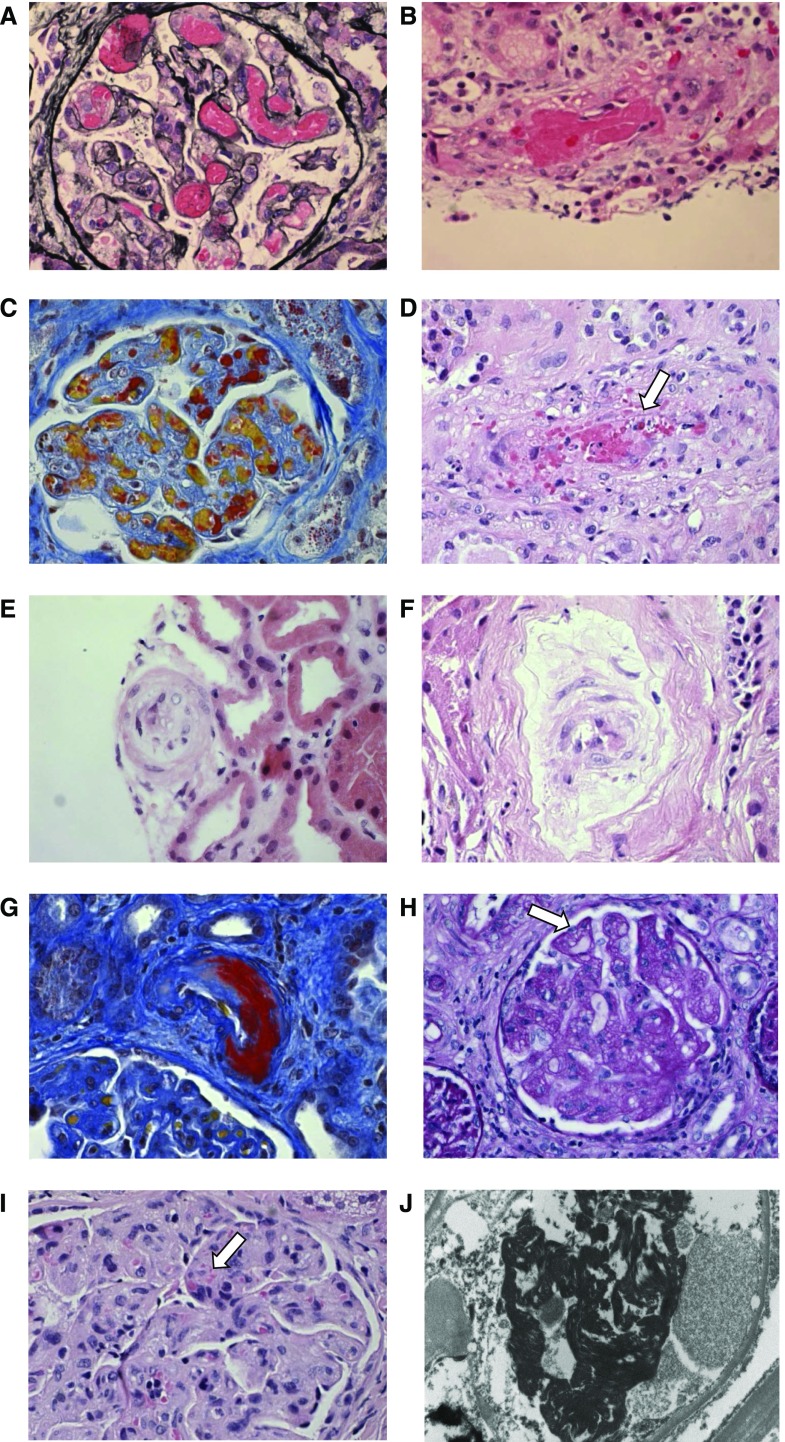
TTP is characterized by unusually large multimers of vWf- and platelet-rich thrombi in capillaries and arterioles (5), although with current practice, pathologic specimens are rarely available. In complement-mediated aHUS and other TMAs with more pronounced AKI, kidney biopsy is more frequently performed, although is not requisite for the diagnosis. The pathologic findings reflect tissue responses to endothelial injury, including endothelial swelling and mesangiolysis in active lesions, and double contours of the basement membrane in chronic lesions (Figure 2) (reviewed by Goodship et al. (6)). Overt fibrin platelet thrombosis may be absent from renal biopsies of TMA, which has recently led to a suggested reclassification to microangiopathy with or without thrombosis (6). Evidence of TMA has also been reported in a number of glomerular diseases (7) and autoimmune diseases; however, in published clinicopathologic studies, only a small proportion of individuals with pathologic evidence of TMA in these contexts had concurrent clinical and laboratory evidence (8,9). It is not possible, on the basis of current knowledge, to establish the cause of TMA from the histopathologic morphology, although this may change with further research (6).
Figure 2.
The pathological features of thrombotic microangiopathies reflect the tissue responses to endothelial injury. (A) Glomerular paralysis with capillary loops containing abundant erythrocytes (silver, ×400). (B) Thrombus in an artery (hematoxylin and eosin, ×400). (C) Glomerular capillary lumina containing fibrin thrombi (red) and erythrocytes (yellow) (Martius Scarlet Blue, ×400). (D) Erythrocyte fragments within the arterial vessel wall (arrow) (hematoxylin and eosin, ×400). (E) Mucoid thickening and obliteration of the lumen of a small artery (hematoxylin and eosin, ×400). (F) Myxoid intimal thickening of small artery (hematoxylin and eosin, ×400). (G) Fibrinoid necrosis of arterial wall (Martius Scarlet Blue, ×400). (H) Reduplication of glomerular basement membrane (arrow) and fibrillary mesangium (periodic acid–Schiff, ×400). (I) Glomerulus with endothelial swelling and erythrocyte fragmentation (arrow) (hematoxylin and eosin, ×400). (J) Electron micrograph demonstrating fibrin tactoids (black) in glomerular capillary (×10,000).
Clinical and Laboratory Features
The clinical presentation of TMA reflects hemolysis and ischemic organ dysfunction, and depends on the underlying disease etiology; AKI is a common manifestation of TMAs, although rarely a severe feature of TTP (10). Extrarenal manifestations are reported in thrombotic microangiopathies, with the published data referring primarily to those observed in complement-mediated aHUS and STEC-HUS (Table 1), although it is not known whether they are a consequence of the TMA, a direct effect of complement activation or shiga toxin, or complications of AKI, such as severe hypertension and uremia (6).
Table 1.
Extrarenal manifestations reported in thrombotic microangiopathies
| * † Neurologic involvement, including seizures and altered consciousness |
| * † Pancreatitis |
| * † Cardiac involvement/myocardial infarction |
| * † Gastrointestinal involvement (including diarrhea, vomiting, abdominal pain) |
| * Cerebral artery thrombosis/stenosis |
| * Extracerebral artery stenosis |
| * Digital gangrene/skin |
| * Ocular involvement |
| * Hepatitis |
| * Pulmonary involvement |
The defining laboratory features comprise thrombocytopenia, resulting from platelet aggregation and consumption, and MAHA, identified by evidence of erythrocyte fragmentation on peripheral blood film microscopy, which occurs in areas of turbulent flow in the microcirculation due to partial occlusion by platelet aggregates (1). Raised lactate dehydrogenase is a result of cell lysis and tissue ischemia (1), haptoglobin is low, and the direct antiglobulin (Coombs) test is negative (except in pneumococcal HUS). Presentation with AKI reflects the consequences of ischemia in the kidney.
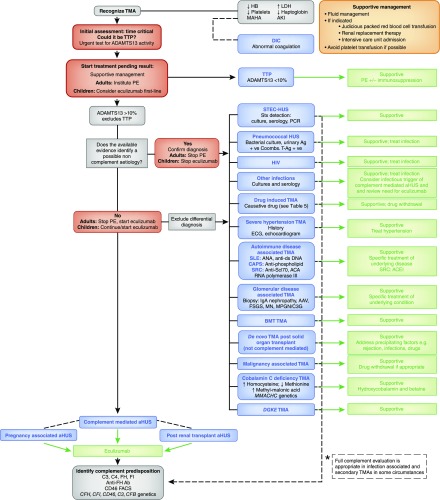
Once routine biochemical and hematologic analysis have demonstrated a TMA, investigations are aimed at determining the underlying disease cause and excluding other differential diagnoses, with the most urgent test being an ADAMTS13 assay (Figure 3). The epidemiology, pathogenesis, and management of TMAs are summarized in Table 2.
Figure 3.
A diagnostic algorithm for the investigation and management of a patient presenting with thrombotic microangiopathy. Supportive treatment is essential. This comprises fluid management and, if indicated, judicious packed red blood cell transfusion, intensive care unit admission, and RRT. Platelet transfusion may worsen the TMA and so should be avoided if possible. The complete evaluation of a patient presenting with TMA comprises complement analysis and investigations for all conditions in which TMA can manifest, and usually enables the disease cause to be established. However, collating the results of the requisite investigations may take considerable time. The clinical evaluation during the acute presentation of a TMA requires some time-critical decision-making, which should focus initially on the consideration of TTP, because immediate management is imperative given the high mortality rate if untreated, and therefore in adults, PE should be instituted on the presumption that it is TTP unless other evidence is available that strongly suggests an alternative cause. In children, in whom TTP is rarer, first-line treatment with eculizumab should be considered if complement-mediated aHUS is suspected and should not be delayed while ADAMTS13 activity is determined. In the absence of a defined cause, complement-mediated aHUS is presumed and treatment with eculizumab is recommended, pending the complete evaluation. Eculizumab is not universally available; in these circumstances, treatment should comprise PE, and there may be a role for liver transplantation. *Full complement evaluation is recommended (Kidney Disease: Improving Global Outcomes consensus [6]) in individuals with pregnancy-associated aHUS and de novo transplantation-associated aHUS because of the high prevalence of rare genetic variants described in these subgroups, and in cases of STEC-HUS that result in ESRD, as rare genetic variants have been described after HUS recurrence in a subsequent kidney transplant. Additionally, it is recognized that infection can trigger manifestation of complement-mediated aHUS. In other secondary cases of aHUS, insufficient evidence exists to recommend a full genetic evaluation, although rare genetic variants have been described in many of these presentations. In cases where the role of complement is as yet unclear, the clinician should decide on the evaluation on the basis of the potential clinical consequences of a positive result (e.g., listing for renal transplantation). +ve, positive; AAV, ANCA-associated vasculitis; Ab, antibody; ACA, anticentromere antibody; ACEI, angiotensin-converting enzyme inhibitor; ADAMTS13, a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13; Ag, antigen; aHUS, atypical hemolytic uremic syndrome; ANA antinuclear antibody; anti-ds DNA, antidouble-stranded DNA; anti–Scl70, anti-topoisomerase I antibody; BMT; bone marrow transplant; C3G, C3 glomerulopathy; CAPS, catastrophic antiphospholipid syndrome; DGKE, gene encoding diacylglycerol kinase ε; DIC, disseminated intravascular coagulation; FH, factor H; FI, factor I; Hb, hemoglobin; HUS, hemolytic uremic syndrome; LDH, lactate dehydrogenase; MAHA, microangiopathic hemolytic anemia; MN, membranous nephropathy; MPGN, membranoproliferative GN; PE, plasma exchange; SRC, scleroderma renal crisis; STEC, shiga toxin–producing Escherichia coli; Stx, shiga toxin; T-Ag, Thomsen–Friedenreich antigen; TMA, thrombotic microangiopathy; TTP, thrombotic thrombocytopenic purpura.
Table 2.
The thrombotic microangiopathies: epidemiology, pathogenesis, and management
| TMA | Epidemiology | Pathogenesis | Management Recommendations |
| Complement-mediated aHUS | Incidence (UK): 0.42 per million per yr (32) | Complement dysregulation caused by: | Eculizumab |
| Hereditary: incomplete penetrance; can present at any age; “trigger” required | Hereditary: heterozygous mutations in CFH, CFI, CD46, C3, and CFB (27,28) | If eculizumab is not available: PE, liver transplantation may be considered. | |
| Acquired: anti-FH Ab aHUS more common in children | Acquired: anti-FH Ab (19,21) | ||
| TTP/ADAMTS13 deficiency mediated TMA | Incidence (US): 0.37 per 100,000 per yr (46); adults 2.9 per million per yr, children 0.1 per million per yr (2) | ADAMTS13 deficiency caused by: Hereditary: recessive (homozygous or compound heterozygous) ADAMTS13 mutations | PE |
| Female>Male (44) | Immunosuppression (steroids/rituximab) indicated if acquired | ||
| Adults > children (2) | Acquired: autoantibody-mediated inhibition | ||
| Cobalamin C deficiency | Incidence of cblC deficiency: 1:37,000–1:100,000 births (50) | Disorder of cblC metabolism results from recessive (homozygous or compound heterozygous) mutations in the MMACHC gene | Metabolic therapy recommended: efficacy of hydroxocobalamin and betaine established (50) |
| Can present in adulthood as well as childhood | Pathogenic mechanisms causing TMA remain undetermined | ||
| DGKE TMA | Rare | Recessive (homozygous or compound heterozygous) mutations in DGKE (36) | Insufficient evidence to determine optimal management |
| Presents aged <1 yr (36) | Loss of DGKE results in prothrombotic state independently of complement activation (51) | Reports of both nonresponse (36) and response (31) to eculizumab | |
| STEC-HUS | E. coli O157 predominant causative pathogen: incidence (UK): 7.1 per million per yr; Latin America, 10–17 per 100,000 children per yr (113) | Enteric infection with Stx-producing pathogens | Supportive care is recommended; high proportion may require dialysis. No intervention evaluated in an RCT was superior to supportive care for any outcome (systematic review [114]) |
| Peak incidence in children <5 yr (115) and approximately 15% of children with enteric infection develop HUS (59) | Stx binds to Gb3, which is highly expressed in the kidney, is internalized, and inhibits protein synthesis (63) | Antibiotics: controversy remains, studies inconclusive, may worsen outcome, therefore currently not recommended; RCT of azithromycin ongoing (NCT02336516) | |
| E. coli O104: European outbreak in 2011 exceptional for high HUS occurrence rate (24%), severity, and high proportion of adults (60) | The consequent endothelial injury results in intravascular fibrin generation (59) | PE: not recommended, no benefit established (no RCTs) (46) | |
| Other Stx producing pathogens e.g., Shigella dysenteriae | Complement activation observed (116) but role is not defined | Eculizumab: not recommended; no benefit demonstrated in retrospective analyses (60,68,69); RCT ongoing (NCT02205541) | |
| Outbreaks and sporadic cases | |||
| Pneumococcal HUS | Rare. Limited data available from published cohorts, and incidence unknown (117,118). 10-yr cumulative incidence rate of 1.2 per 100,000 children reported (NZ) (119). Associated with pneumonia and empyema in children <2 yr (72) | Neuraminidase cleaves sialic acid residues from glycoproteins on erythrocyte, platelet, and endothelial cell membranes, exposing the cryptic T antigen to which IgM in the plasma can then bind, resulting in cell damage and TMA (73). | Supportive management and treatment of infection recommended |
| HIV-associated TMA | Pre-HAART era: incidence of 1.5%–7% (75,120) | Pathogenic mechanisms remain undetermined | Supportive management and treatment of infection recommended |
| HAART era: incidence 0.3% (77) | |||
| Pregnancy-associated TMA | Complement-mediated aHUS: pregnancy triggers complement-mediated TMA in approximately 20% of women with aHUS (81) | Complement gene mutations in up to 86% (81) | Eculizumab; start immediately (do not wait for genetic analysis) if TTP has been excluded and presentation is not suggestive of HELLP |
| A proportion are primary TMAs; differential diagnosis includes complement-mediated aHUS, TTP, and HELLP | TTP: 10%–36% of women with TTP present during pregnancy (44) | vWf increases in normal pregnancy and consumes ADAMTS13; in women with a genetic predisposition, its activity can fall low enough for TTP to manifest (81) | PE |
| HELLP: incidence: 0.5%–0.9% of all pregnancies; complicates 5%–10% of cases of severe preeclampsia (121) | Mechanisms are poorly understood (80) | Definitive treatment is delivery (86). No role for PE (46) | |
| Increased levels of endoglin and sFlt-1 may play a role in endothelial dysfunction (79,86). | |||
| Drug-mediated TMA | True incidence unknown | Immune mediated: drug-dependent antibodies, e.g., quinine (2) | Recommendation is drug discontinuation |
| Reported incidences of TMA include: | Toxicity mediated: multiple mechanisms, e.g., CNIs, IFN, chemotherapy agents, VEGF inhibitors (2) | Ticlopidine-associated TMA: as per TTP (46,92) (PE) | |
| Approximately 1:1000 patient-yr for high-dose IFN-β (90) | Ticlopidine-associated TMA is mediated by acquired ADAMTS13 deficiency (92) | ||
| 2%–10% of patients treated with mitomycin (122) | |||
| 1%–4.7% of patients treated with tacrolimus (122) | |||
| VEGF inhibitors: unknown (91) | |||
| De novo TMA after solid organ transplant | Incidence after kidney transplantation: 0.8% in USRDS data (123); up to 14% in single-center studies (94) | Multifactorial | Treatment of precipitating factors, e.g., AMR, infection, drug withdrawal |
| Incidence: 4% in liver transplant and 2.3% in lung transplant recipients (93) | Complement gene mutations reported in up to 29% (renal transplants) (96) | Eculizumab where complement-mediated aHUS is possible | |
| TMA after bone marrow transplant | Multisystem TMA complicates 10%–40% of allogenic BMTs (97,98) | Multifactorial | No evidence-based effective management strategy |
| No established benefit with PE (46) | |||
| Favorable outcomes with eculizumab compared with historical controls reported in retrospective analysis (101), but prospective trials needed to establish consensus | |||
| Severe hypertension-associated TMA | Incidence: variously reported; case series of severe hypertension have identified TMA in 27%–44% (13,124–127) | Pathogenic mechanisms unclear | No evidence supporting any strategy other than BP management |
| Malignancy-associated TMA | Unknown | Multifactorial | Discontinuation of causative chemotherapy agents |
| TMA with glomerular diseases | TMA can occur in association with IgA nephropathy, ANCA-associated vasculitis, membranous nephropathy, FSGS, and MPGN/C3G (13) | Pathogenic mechanisms unclear | Limited evidence for management of the TMA |
| Hereditary and acquired complement defects in MPGN/C3G | Role of complement inhibition in C3G/aHUS crossover unclear | ||
| TMA with autoimmune conditions | SLE: concurrent TMA reported in up to 8%–15% of biopsies (9,128) | Pathogenic mechanisms unclear | SLE: no evidence for specific TMA management in addition to SLE management as recommended in international guidelines |
| CAPS: TMA in 14% in international registry (112) | Some evidence of complement activation in SLE and CAPS (13) | CAPS: efficacy of eculizumab suggested in case reports and series; prospective trial (not RCT) to enable renal transplantation ongoing (NCT01029587) | |
| SRC: occurs in approximately 10% of people with SSc; TMA in 45%–50% (111,129) | SRC: ACEIs significantly reduce mortality (111) |
aHUS, atypical hemolytic uremic syndrome; UK, United Kingdom; PE, plasma exchange; FH, factor H; Ab, antibody; TTP, thrombotic thrombocytopenic purpura; ADAMTS13, a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13; TMA, thrombotic microangiopathy; US, United States; cblC, cobalamin C type; STEC-HUS, hemolytic uremic syndrome caused by shiga toxin–producing Escherichia coli; Stx, shiga toxin; RCT, randomized controlled trial; HUS, hemolytic uremic syndrome; Gb3, globotriaosylceramide; NZ, New Zealand; T antigen, Thomsen–Friedenreich antigen; HAART, highly active antiretroviral therapy; HELLP, syndrome of hemolysis, elevated liver enzymes, and low platelets; CNI, calcineurin inhibitor; VEGF, vascular endothelial growth factor; USRDS, United States Renal Data System; AMR, antibody-mediated rejection; BMT, bone marrow transplants; MPGN, mesangioproliferative GN; C3G, C3 glomerulopathy; CAPS, catastrophic antiphospholipid syndrome; SRC, scleroderma renal crisis; SSc, systemic sclerosis; ACEI, angiotensin-converting enzyme inhibitor.
Primary TMAs
Complement-Mediated aHUS
Complement-mediated aHUS is prototypical of disease occurring as a consequence of complement dysregulation. The complement system is a regulated cascade network of signaling and amplification that incorporates >30 plasma and cell surface–bound proteins, and was reviewed in detail earlier in this series (11). A key physiologic role involves stimulating the inflammatory response and opsonization and lysis of pathogens to protect the host from infection (encapsulated organisms in particular); it is a fundamental component of the innate immune system, and modulates the adaptive immune system. In addition, it facilitates the disposal of potentially injurious immune complexes and damaged host cells (12).
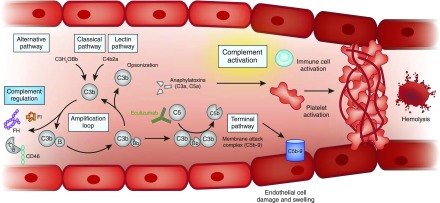
Complement activation is initiated by three pathways: the classical pathway is activated by immune complexes and other molecules (e.g., C-reactive protein), and activation of the lectin pathway results from protein interactions with pathogens (11). The alternative pathway may also be initiated by pattern recognition molecules, but crucially, it is a positive feedback loop that is constitutively active due to spontaneous hydrolysis (tickover) of C3, and is recruited by the classical and lectin pathways; this enables a rapid response against pathogens, but leaves the host vulnerable to bystander damage if the amplification loop is unchecked. The system is therefore tightly regulated by plasma proteins, including FH and factor I (FI), and cell surface proteins, such as membrane cofactor protein (CD46) (13). Defects in these regulators or in the alternative pathway components can lead to complement dysregulation, with activation of the terminal complement pathway and generation of the anaphylatoxin C5a and the membrane attack complex (C5b-9), resulting in a complement-mediated aHUS (Figure 4). The relative roles of these effector molecules in causing disease remains to be established.
Figure 4.
Unfettered complement activation ultimately results in thrombus formation, platelet consumption, vascular occlusion and mechanical hemolysis. Complement is activated by the alternative, classic, and lectin pathways. The alternative pathway of complement is a positive amplification loop. C3b interacts with factor B, which is then cleaved by factor D to form the C3 convertase C3bBb. Unchecked, this leads to activation of the terminal complement pathway with generation of the effector molecules, the anaphylatoxin C5a and the membrane attack complex (C5b-9). To protect host cells from bystander damage, the alternative pathway is downregulated by complement regulators including factor H (FH), factor I (FI), and CD46. In complement-mediated aHUS, activating mutations in C3 and CFB and loss-of-function mutations in CFH, CFI, and CD46, in addition to autoantibodies to FH, result in overactivation of the alternative pathway. This leads to immune cell and platelet activation and endothelial cell damage and swelling, with consequent thrombus formation, platelet consumption, vascular occlusion, and mechanical hemolysis. There is evidence that complement is activated in other TMAs, but whether this is a causative, disease modifier, or bystander phenomenon has not yet been elucidated. Eculizumab is a humanized mAb that binds to C5 and prevents activation of the terminal pathway; it thereby inhibits the generation of the effector molecules that cause TMA in individuals in whom a primary defect in complement underlies the TMA pathogenesis. Its role in the treatment of other TMAs is undefined. aHUS, atypical hemolytic uremic syndrome; TMA, thrombotic microangiopathy.
In complement-mediated aHUS, dysregulated complement activation primarily occurs on endothelial cell surfaces, and although abnormal serum levels of complement components such as C3 may be observed, normal levels do not exclude complement-mediated disease (6,14). There is evidence of complement activation (plasma levels and tissue staining) in many other TMAs, but whether this is a primary event, a disease modifier, or an inconsequential bystander phenomenon has not yet been definitively established (13).
Hereditary Complement-Mediated aHUS
Molecular evidence that CFH mutations are associated with aHUS was first described in genetic research published in 1998 (15), and since then a multitude of studies have determined heterozygous pathogenic activating mutations in the genes encoding the alternative pathway components C3 and factor B, and loss-of-function mutations in the genes encoding the regulators FH (including CFH/CFHR fusions), FI, and CD46 (reviewed in Kavanagh et al. [14]).
These genetic mutations are not causative but are instead predisposing, with penetrance incomplete. Penetrance of disease is age-related and has been reported to be as high as 64% by the age of 70 years for individuals carrying a single genetic mutation (6). This suggests that additional disease risk modifiers are important. Around 3% of patients have more than one mutation, with increased penetrance per additional mutation. Haplotypes (particular combinations of single nucleotide polymorphisms) in CFH and CD46 also modify penetrance (14). Together, these still do not answer the question of why some individuals do not develop disease until later in life. This is best explained by the need for an environmental trigger (e.g., pregnancy or infection) to unmask a latent complement defect. Complement activation is a common factor in many of these triggering events: it occurs in the placenta at the fetomaternal interface in normal pregnancy and is the normal physiologic response to infection. Counseling of family members and offering genetic screening are important considerations.
Historically the outcome of complement mediated aHUS at the initial presentation was very poor, as were outcomes after kidney transplantation: recurrence was 68% and 5-year death-censored graft survival was 51% (16). The outcome of kidney transplantation is predicted largely by the underlying genetic abnormality, with the highest risk associated with CFH, CFB, and C3 mutations and the lowest with CD46 mutations (17). In the pre-eculizumab era, one option for individuals with a defect in a complement protein predominantly synthesized in the liver (FH, FI, factor B, and C3) was combined liver and kidney transplantation (14); however, short-term risks were significant and international experience was limited (18), and some people were therefore considered to be untransplantable.
Acquired Complement-Mediated aHUS
aHUS associated with autoantibodies against FH was first reported in 2005 (19), and functional analyses have demonstrated disruption of complement regulation by multiple mechanisms (20). There is a strong association with homozygous deletion of CFHR3 and CFHR1, which encode the proteins FHR3 and FHR1 (21), although the mechanism is not understood; CFHR3/1 deletion is a common polymorphism, and it is not present in all individuals who develop FH autoantibodies (22). It predominantly presents in childhood, frequently with a gastrointestinal prodrome. Autoantibodies against FI have also been reported, but they are rare and their functional relevance remains to be established (23).
Plasma Exchange
Plasma exchange (PE) still remains the initial treatment of choice until ADAMTS13 activity is available to exclude TTP as a diagnosis. It should be initiated in adults as soon as the diagnosis of TMA is suspected. In addition to removing ADAMTS13 autoantibodies and replacing ADAMTS13 in TTP, PE will also replace faulty complement regulators and remove FH autoantibodies and hyperfunctional complement components in complement-mediated aHUS. In children, PE is associated with a high rate of complications (24) and TTP is rare, therefore PE is not considered as a first-line treatment when eculizumab is available. In many parts of the world, the cost of eculizumab precludes its use, and PE remains the only treatment for complement-mediated aHUS (25).
Complement-Inhibiting Therapy
The evidence for the central role of primary complement defects in pathogenesis summarized above provided the mechanistic rationale for treating complement-mediated aHUS with complement-inhibiting therapy. Eculizumab is a recombinant humanized mAb that functionally blocks C5, and trials published in 2013 demonstrated its efficacy (26). Although these were single-arm studies rather than randomized, controlled trials, the historically poor outcomes (up to 77% of patients with CFH mutations had died or reached ESRD by 3–5 years) justified such study designs (27,28). These findings have been replicated in subsequent extension studies (29), prospective (nonrandomized) studies (30,31), and cohort analysis (32). In the prospective trials, complete TMA response was achieved in approximately 65% after 26 weeks of eculizumab therapy in both adults (26) and children (31).
Eculizumab was approved by the US Food and Drug Administration and the European Medicines Agency for use in aHUS in 2013 (see Table 3 for practical considerations) (33). In those presenting late in the course of disease who do not recover kidney function, the high recurrence rate after kidney transplantation necessitates preemptive eculizumab (Table 4) (6,16). The trials included patients in whom no complement mutation or FH autoantibody had been identified, and the majority responded to complement-inhibiting therapy; therefore, negative genetic and autoantibody investigations do not exclude the diagnosis of complement-mediated aHUS.
Table 3.
Eculizumab: practical considerations
| Eculizumab: Practical Considerations |
| Before administration |
| Meningococcal vaccination mandatory |
| Tetravalent ACWY conjugated vaccine + multicomponent serogroup B vaccine |
| Prophylaxis |
| Long-term antibiotic prophylaxis recommended |
| Administration |
| Intravenous infusion |
| Maintenance therapy is administered every 14 d |
| Monitoring |
| CH50 and AH50 <10% |
| Eculizumab trough level 100 µg/ml |
| Hematologic indicators of TMA |
| Patient education |
| Vigilance regarding meningococcal infection |
| Counseling family members |
| Genetic screening |
| When to stop? |
| Continue during intercurrent illness, unless infection with encapsulated organism, due to high risk of TMA relapse in this context |
| Withdrawal |
| Systematic investigation in clinical trials is being undertaken |
| May be appropriate in some patientsa, with monitoring: liaise with specialist center |
This guidance is based on our practice in the United Kingdom, and the 2015 Kidney Disease: Improving Global Outcomes Controversies Conference consensus recommendations (6). Eculizumab (Soliris; Alexion Pharmaceuticals) is a recombinant humanized mAb that functionally blocks C5, and is approved by the US Food and Drug Administration and the European Medicines Agency for use in atypical hemolytic uremic syndrome. ACWY, meningococcal serotypes; CH50, total complement hemolytic activity; AH50, alternative pathway hemolytic activity; TMA, thrombotic microangiopathy; aHUS, atypical hemolytic uremic syndrome.
For example, if there is no renal recovery after 6 months of treatment.
Table 4.
Approach to kidney transplantation when thrombotic microangiopathy results in established renal disease
| Complete Complement Evaluation before Renal Transplant Listing Is Recommended if Thrombotic Microangiopathy Results in ESRDa | ||
| Risk Stratification | Inclusion Criteria | Management Strategy |
| High | Pathogenic complement mutations | Prophylaxis with eculizumab (KDIGO global panel suggest plasma exchange and liver transplantation may also be considered) |
| Previous early recurrence | ||
| Moderate | No complement mutation, or variant of unknown significance | |
| Isolated CFI mutation | ||
| Detectable anti-factor H antibody | ||
| Low | Isolated CD46 mutationb | No prophylaxis |
| Previously positive but now consistently negative anti-factor H antibody | ||
Delay transplantation until at least 6 months after starting dialysis as late renal recovery with eculizumab treatment has been reported. Living related kidney donation should only be considered if a genetic or acquired cause is identified in the recipient and is not present in the intended donor. Recommendations are on the basis of the 2015 Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference consensus recommendations (6).
Complete complement evaluation should include: serum levels of C3, C4, factor H, factor I; anti-factor H antibody; CD46 FACS; and CFH, CFI, CD46, C3, and CFB genetics.
Allograft cells express functional CD46.
The current license for eculizumab is for lifelong treatment, but this is not evidence based. Withdrawal of eculizumab has been described in a large series of patients with aHUS, with relapse reported in around one third, exclusively in those with complement mutations (34). That rapid reintroduction of eculizumab returned kidney function to baseline suggests that a disease-driven intermittent regime could replace long-term therapy, although prospective trials are required.
With increased clinical use, evidence is emerging of nonresponse to eculizumab. In the recent pediatric trial, Greenbaum et al. (31) highlighted that, for those with a rare genetic variant in the complement system or an autoantibody to FH, all had an improvement in eGFR, whereas 27% of individuals without an identified complement abnormality failed to show an improvement. It is not clear whether this represents late presentation of disease or true nonresponse. A polymorphism in the C5 gene (p.R885H) has been demonstrated to prevent eculizumab binding to C5 (35), and nonresponders to eculizumab should have genetic screening for this single nucleotide polymorphism and alternative treatment strategies considered. More recently, individuals presenting with a TMA with failure to respond to eculizumab have been demonstrated to have genetic variants in the noncomplement genes DGKE (36) and INF2 (37).
The primary concern with terminal complement blockade is increased susceptibility to infection with encapsulated organisms, particularly Neisseria infections (33,38). For this reason, meningococcal vaccination is considered mandatory; however, serologic response is variable (39), and the efficacy of vaccination in the context of complement blockade is uncertain (6). Long-term antibiotic prophylaxis is therefore recommended for the duration of treatment and up to 3 months after withdrawal (6), although meningococcal infection can occur despite these precautions (40,41). Thus, patient education regarding vigilance is essential. Other concerns may become apparent as use of complement-inhibiting therapy in clinical practice expands: hepatotoxicity in association with eculizumab has been reported in children (42), and deposition of eculizumab has been reported in individuals with C3 glomerulopathy (C3G) (but not yet in aHUS), although the clinical significance is unclear (43).
The use of complement-inhibiting therapy in TMAs other than complement-mediated aHUS is controversial; case studies have reported success but the inherent publication bias needs to be considered when interpreting these, and given that the role of complement in the pathogenesis of other TMAs has not yet been defined, interventional trials are likely to be needed before a consensus can be achieved (13).
Thrombotic Thrombocytopenic Purpura
TTP is mediated by ADAMTS13 deficiency: in hereditary TTP, this results from recessive mutations (homozygous or compound heterozygous) in the ADAMTS13 gene, and in acquired TTP, ADAMTS13 activity is inhibited by autoantibodies (2). ADAMTS13 is a metalloproteinase enzyme that functions to cleave vWf; deficiency results in unusually large vWf multimers and consequent occlusive microvascular platelet aggregation (2). The incidence of TTP is higher in adults than in children (2), and there is a female predominance (44). Neurologic manifestations are common and laboratory characteristics in comparison to aHUS are reportedly more severe thrombocytopenia (<30×109/L) and less severe AKI (serum creatinine, 1.7–2.3 mg/dl) (45), but these are not absolute and should not be relied upon in clinical practice (10), where the diagnosis is confirmed by ADAMTS13 activity <10% (5). TTP was historically almost universally fatal, but after the introduction of PE treatment, mortality decreased to <10% (46). In acquired TTP, immunosuppressive therapies (e.g., glucocorticoids and rituximab) have reduced the relapse rate (47–49).
Cobalamin C Deficiency
TMA can manifest in methylmalonic aciduria and homocystinuria, cobalamin C type, which is the most common inherited form of functional cobalamin (vitamin B12) deficiency (50). It is caused by recessive (homozygous or compound heterozygous) mutations in the MMACHC gene, can present in adulthood as well as childhood, and the phenotype may comprise developmental, ophthalmologic, neurologic, cardiac, and kidney manifestations, although severity varies. The pathophysiologic mechanisms that cause endothelial damage and subsequent TMA have not yet been determined (50). Mortality is high if untreated or if there is cardiopulmonary involvement, but metabolic therapy (hydroxocobalamin and betaine) is very effective (50).
DGKE TMA
Recessive (homozygous or compound heterozygous) DGKE mutations causing TMA was first reported in 2013 (36), and this defined a cohort of patients with aHUS caused by complement-independent mechanisms (51). Only a small number of cases have been published, but it appears to present in patients aged <1 year and commonly results in progressive CKD and ESRD (36). There is insufficient evidence to determine optimal management; there are reports of both response (31) and nonresponse (36) to eculizumab, as well as response (52,53) and nonresponse (36) to plasma therapy. Concomitant mutations in complement genes have been reported (54). Genetic pleiotropy is seen, whereby DGKE mutations have also been associated with mesangioproliferative GN (MPGN) (55).
Other Genetic Associations
Genetic variants in thrombomodulin (THBD) have been reported in association with aHUS (56,57), although this finding was not replicated by Fremeaux-Bacchi et al. (27). Bu et al. (58) reported rare genetic variants in plasminogen (PLG) in aHUS; however, replication analysis will be required to clarify this disease association. Functionally significant mutations in INF2 have been seen to segregate in families with TMAs, but it remains to be seen whether this is a primary TMA or secondary phenomenon in association with FSGS (37).
Infection-Associated TMAs
STEC-HUS
STEC-HUS is more common than aHUS, with a peak incidence in children aged <5 years (Table 2). The predominant causative pathogen is E. coli O157; enteric infection can occur sporadically or in the context of an outbreak, and around 15% of children with enteric infection develop HUS (59). The E. coli O104 outbreak in Europe in 2011 was exceptional because of the high STEC-HUS occurrence rate (24%) and the high proportion of adults affected (60).
Shiga toxin translocates through the intestinal epithelium and is thought to bind to leukocytes and circulate to the kidneys (61). The shiga toxin is then internalized via the Gb3 receptor and is cleaved to release a protease into the cytoplasm. This protease inhibits ribosomal function and protein synthesis, leading to cell death. It can also activate signaling pathways, inducing an inflammatory response in affected cells (62,63).
The distinction between STEC-HUS and complement-mediated aHUS may not be clinically obvious: approximately 5% of patients with STEC-HUS do not have diarrhea (64), and approximately 30% of patients with complement-mediated aHUS have concurrent diarrhea at presentation (6). Therefore, all individuals with TMA should be investigated for STEC-HUS (Figure 3). Rarely, complement gene mutations are detected, and in these cases the clinical picture is unusually severe (13,65). In STEC-HUS resulting in ESRD, it is recommended to screen for mutations before transplantation. STEC-HUS is considered to be a self-limiting illness, with good outcomes relative to other TMAs: 70% fully recover, the mortality rate is approximately 1%–4%, ESRD occurs in 3% of patients, and CKD occurs in 9%–18% of patients (66). There is some evidence of complement activation (13), but the role of complement-inhibiting therapy has not yet been defined. A case series in 2011 first reported efficacy of eculizumab in three children with severe disease (67), and a significant proportion of those affected in the 2011 E. coli O104 outbreak were treated with eculizumab; no benefit over supportive care or PE was demonstrated in retrospective analyses (60,68,69), although a direct comparison is difficult because of the inherent disadvantages of retrospective analyses, for example there were no controls, patients received multiple therapies administered with inconsistency, and those treated with eculizumab had more severe disease. In this self-limiting condition, only a randomized, controlled trial will resolve this controversy; one is underway in France (Clinicaltrials.gov identifier: NCT02205541) and another is due to start recruiting in the United Kingdom. For now, the recommendation remains supportive management.
Pneumococcal HUS
TMA may occur in adults and children in the context of invasive Streptococcus pneumoniae infection, and the high morbidity and mortality rate usually reflects the severity of the infection (70,71). In a recently published North American cohort, pneumococcal HUS was most commonly observed in children aged <2 years with pneumonia and empyema who were extremely unwell and frequently required prolonged hospitalization and intensive care unit admission; the mortality rate was 3%, and 33% developed ESRD (72). The hypothesized mechanism is that pneumococci produce neuraminidase, which cleaves sialic residues from glycoproteins on erythrocyte, platelet, and endothelial cell membranes, exposing the cryptic Thomsen–Friedenreich antigen (T antigen), to which IgM in the plasma can then bind, resulting in cell damage and TMA (73). The Coombs test is positive. Additionally, it has been suggested that cleavage of sialic acid may reduce FH binding, resulting in impaired endothelial complement regulation, thus contributing to disease pathogenesis (74). Supportive management and treatment of the infection should be the focus.
HIV-Associated TMA
TMA has been reported in association with HIV, more commonly in the pre-highly active antiretroviral therapy era (75–77), and in association with lower CD4+ cell counts and higher viral RNA levels (77). The pathogenic mechanisms are poorly understood, although endothelial damage is thought to be the primary event. Again, there is no evidence for any treatment strategy other than supportive care and antiretroviral treatment.
Other Infections
TMA has been reported in association with a wide range of viral, bacterial, fungal, and parasitic infections, although it is frequently unclear if this is a direct effect of the pathogen, a side effect of treatment, or a trigger that unmasks a latent complement defect (reviewed by Nester et al. [4]). For instance, Caprioli et al. (78) reported undefined infectious triggers in >70% of patients with complement-mediated aHUS and CFH, CFI, or CD46 mutations. Supportive management with appropriate treatment of the infection is recommended, and complement evaluation should be undertaken.
Secondary TMAs
Pregnancy-Associated TMA
The differential diagnosis of TMA in pregnancy includes the primary TMAs complement-mediated aHUS and TTP, as well as the TMA-like presentation occurring in the syndrome of hemolysis, elevated liver enzymes, and low platelets (HELLP) (79), which is part of the clinical spectrum of preeclampsia (80).
In around 20% of women with aHUS, the disease onset appears to be triggered by pregnancy, occurring most often in the postpartum period (81,82). Outcomes were historically poor, with 76% developing ESRD despite PE (81). More recently, it has become clear that a high proportion will have identifiable complement mutations (>50%), and that pregnancy acts as a trigger in those with an underlying genetic predisposition (81). In the era of eculizumab, this high prevalence of complement mutations in pregnancy-associated HUS provides the biologic rationale for complement inhibition. Increasing data in both paroxysmal nocturnal hemoglobinuria (83) and aHUS (84) suggests that eculizumab is safe during pregnancy.
Similarly, a significant proportion of women with TTP present during pregnancy (80), particularly in the second and third trimesters (81). This might be explained by the physiologic increase in vWf during pregnancy, which consumes ADAMTS13, so in women with a genetic predisposition, its activity can fall low enough for TMA to manifest (81). PE is recommended on the basis of observational data and knowledge of pathogenesis (85).
The pathogenesis of preeclampsia and HELLP are poorly understood (80), although there is some evidence to suggest that increased circulating levels of the syncytiotrophoblast- derived antiangiogenic factors, soluble endoglin and the soluble form of the vascular endothelial growth factor receptor (sFlt-1), may contribute to the observed endothelial dysfunction (79,86). Although there is significant overlap in the clinical presentations of HELLP and TMA, in HELLP, the predominant glomerular pathology is endotheliosis (79). In contrast to pregnancy-associated aHUS, only a minority (8%–10%) of patients with preeclampsia and HELLP syndrome harbor complement gene variants, mostly of unknown or nonpathogenic significance (87). There is some evidence that complement is activated in preeclampsia and HELLP, although it is unclear whether it plays a role in pathogenesis; of note, preeclampsia has occurred in women taking eculizumab for paroxysmal nocturnal hemoglobinuria (83) and aHUS (84). Management should be supportive, and the definitive treatment of HELLP is expedited delivery, although expectant management may be considered if the woman is not at or near term (86).
In clinical practice, a pragmatic approach is usually taken in peripartum cases of TMA: if expedited delivery does not result in resolution of the TMA then standard management is instituted (Figure 3).
Drug-Mediated TMA
In published reports TMA has been associated with a large number of drugs, although definitive causality has only been established in relatively few (reviewed by Al-Nouri et al. [88]). Drug-mediated TMA occurs by two main mechanisms: immune-mediated damage and direct toxicity (Table 5). For example, quinine induces the development of autoantibodies reactive with either platelet glycoprotein Ib/IX or IIb/IIIa complexes, or both (89). In contrast, IFN-β (90) and bevacizumab (91) cause TMAs by a dose-dependent toxicity. There are no trials to guide management, and the recommendation is supportive care and discontinuation of the causative drug. However, ticlopidine has been reported to be associated with anti-ADAMTS13 antibodies with resultant TTP, and therefore PE is recommended (92). It is crucial that a full evaluation is undertaken (Figure 3) even if drug-mediated TMA is suspected, including an urgent ADAMTS13 assay.
Table 5.
Drugs with evidence supporting a causal association with thrombotic microangiopathya
| Immune-Mediated TMA | Direct Drug-Induced Toxicity | Other |
| Quinine: Drug-dependent antibodies |
|
Ticlopidine: ADAMTS13 autoantibodyb |
| VEGF inhibitors, e g., bevacizumab, sunitinib | ||
| Chemotherapeutic agents, e.g., gemcitabine, mitomycin | ||
| Recreational drugs, e.g., cocaine |
Even if drug-mediated TMA is suspected, a full evaluation should still be undertaken as shown in Figure 3, including an urgent ADAMTS13 assay. TMA, thrombotic microangiopathy; VEGF, vascular endothelial growth factor; TTP, thrombotic thrombocytopenic purpura; ADAMTS13, a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13.
De Novo TMA after Solid Organ Transplant
De novo TMA has been reported to occur after kidney, liver, pancreas, lung, and heart transplantation (93,94). The pathogenic mechanisms are not well understood, but it is likely to be multifactorial, with ischemia-reperfusion injury, antibody-mediated rejection, viral infections such as cytomegalovirus, and immunosuppressant drugs, especially calcineurin inhibitors (CNIs), contributing to an “endothelial damaging milieu” (95). In one series of de novo TMA after kidney transplantation, complement mutations were identified in 29% (96), providing a rationale for complement-inhibiting therapy especially where the primary diagnosis was not incompatible with complement-mediated aHUS. In many cases, however, supportive treatment and addressing the precipitating factors (CNI discontinuation or dose reduction, treatment of antibody-mediated rejection and viral infections) may be sufficient to stop the TMA (13).
TMA after Bone Marrow Transplant
A multisystem TMA complicates 10%–40% of allogenic bone marrow transplants (97,98) and is associated with significant mortality, variously reported as 21%–75% (13). Again, this is likely to be multifactorial, with risk factors including CNIs, graft versus host disease, HLA mismatch, chemotherapy, radiation therapy, and infections (99). Rare genetic variants in aHUS associated genes (98) and FH autoantibodies (100) have been infrequently reported in post bone marrow transplant TMA.
The optimal treatment strategy remains controversial; favorable outcomes with eculizumab have been described in uncontrolled retrospective analyses (101) and there is some evidence to suggest that complement is activated (13), but prospective trials are likely needed in order to establish a consensus.
Severe Hypertension-Associated TMA
TMA can occur in association with severe hypertension, and should be managed with antihypertensive and supportive treatment. The pathogenic mechanisms that result in endothelial damage and TMA are unclear (102,103). Conversely, any patient with a TMA can have severe hypertension, thus a clinical conundrum can arise in the initial evaluation of a patient presenting acutely with TMA. In practice, failure of BP control and supportive management to control the TMA will often result in the pragmatic initiating of PE or eculizumab until complement evaluation is available. In the majority of patients with TMA associated with severe hypertension, renal function and MAHA usually recover with management of BP (102,104). In a retrospective case series, genetic analysis identified rare variants in complement genes in patients for whom TMA was initially attributed to severe hypertension; eight out of nine patients progressed to ESRD despite management of hypertension (103). This may be particularly relevant when considering kidney transplant assessment in an individual with ESRD attributed to severe hypertension with TMA, or if TMA occurs again after transplantation.
Malignancy-Associated TMA
When TMA occurs in association with malignancy it can be difficult to distinguish between TMA caused by chemotherapy and TMA caused by malignancy (105). It is possible that the causative mechanism in disseminated malignancy involves erythrocyte shearing after direct contact with microvascular embolic tumor cells (106). Prognosis is poor because of malignancy-related mortality (107,108), and there is no evidence to support any treatment strategy other than withdrawal of causative chemotherapy agents.
TMA Associated with Glomerular Diseases
TMA can occur in association with IgA nephropathy, ANCA-associated vasculitis, membranous nephropathy, FSGS, and MPGN/C3G, although it may be a histopathologic finding without biochemical or clinical manifestation (13).
In MPGN/C3G, the hereditary and acquired complement defects are similar, although subtly different to those seen in complement-mediated aHUS, and it is perhaps unsurprising that concurrent (7) and sequential (109) manifestation of C3G and TMA has been reported. Mutations in CFH are observed in both complement-mediated aHUS and C3G; the reason for this genetic pleiotropy is not fully understood, but the location of the mutation within the gene may be important. In aHUS, the majority of mutations are located at the C-terminal of FH, which binds to C3b and glycosaminoglycans on host cells to mediate cell surface protection (14), whereas in C3G, mutations are more often located at the N-terminal of FH, which mediates complement regulation in the fluid phase (110).
Genetic pleiotropy has also been reported in INF2- and DGKE-mediated disease. In addition to the FSGS normally seen in INF2-mediated disease (37) and the MPGN seen in DGKE-mediated disease (55), TMAs have been reported in both cases that seemed nonresponsive to eculizumab.
TMA Associated with Autoimmune Diseases
TMA can occur in association with autoimmune diseases including SLE, catastrophic antiphospholipid syndrome (CAPS), and scleroderma renal crisis (SRC); again, the mechanisms remain unclear, although there is some evidence of complement activation in SLE and CAPS (13). For SLE, CAPS, and SRC with TMA, treatment should be of the underlying condition. The influence of TMA on outcome of SLE is uncertain, as case series have reported both no difference and worse outcome (13); treatment should be immunosuppression according to international guidelines, and there is no evidence to suggest that any additional treatment specifically directed at the TMA is beneficial. The high mortality of SRC is significantly reduced with angiotensin-converting enzyme inhibitors (111). In CAPS, the international registry (522 episodes) reported the incidence of TMA to be 14%, and overall mortality was 37%, with no subgroup analysis according to treatment. The most frequent treatment regimens used were anticoagulation plus glucocorticoids (19%) or anticoagulation, glucocorticoids and PE with or without intravenous Ig (18%), with only 0.2% receiving eculizumab (112). There is some evidence from mouse models and observational clinical data that complement may be involved in the pathophysiology of CAPS; successful use of eculizumab has been reported in case reports and series, and a prospective trial of preemptive eculizumab to enable renal transplantation is ongoing (Clinicaltrials.gov identifier: NCT01029587) (13).
Diagnosis and Management
Establishing the cause of a TMA is usually possible after a complete evaluation. However, the results of the requisite investigations are not available immediately, and in clinical practice, the scenario is often one of a severely ill patient with TMA for whom immediate management decisions are required. The acute decision-making is time-critical: the initial priority should be the consideration of TTP, because urgent management is imperative given the high mortality if untreated, and therefore in adults, PE should be instituted (after obtaining a sample for ADAMTS13 activity testing) on the presumption that it is TTP unless other evidence is available that strongly suggests an alternative cause. If the ADAMTS13 result excludes TTP, then complement-mediated aHUS is presumed and treatment with eculizumab is recommended, pending the complete evaluation. In children, in whom PE may not be appropriate and is high risk, treatment with eculizumab has been recommended by Kidney Disease: Improving Global Outcomes before the availability of the ADAMTS13 result, with the caveat that clinical deterioration on eculizumab should necessitate immediate plasma therapy (6). An algorithm for the real-time investigation and management of a patient presenting with TMA is illustrated in Figure 3. Once the full evaluation has been completed, the indication for eculizumab can be reviewed.
Summary
In summary, TMA can manifest in a diverse range of diseases and can be associated with significant morbidity and mortality. For some forms of TMA, such as TTP and complement-mediated aHUS, research has now defined the molecular mechanisms of disease leading to targeted therapy and improved patient outcomes. However, the optimal eculizumab treatment duration and the specific patient populations who will benefit remain undefined.
Disclosures
D.K. has received honoraria for consultancy work from Alexion Pharmaceuticals, and is a director of and scientific advisor to Gyroscope Therapeutics.
Acknowledgment
V.B. has received funding from the Northern Counties Kidney Research Fund and is a Medical Research Council/Kidney Research UK Clinical Research Training Fellow. D.K. has received funding from the Wellcome Trust, The Medical Research Council and Kidney Research UK.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Moake JL: Thrombotic microangiopathies. N Engl J Med 347: 589–600, 2002 [DOI] [PubMed] [Google Scholar]
- 2.George JN, Nester CM: Syndromes of thrombotic microangiopathy. N Engl J Med 371: 654–666, 2014 [DOI] [PubMed] [Google Scholar]
- 3.Kerr H, Richards A: Complement-mediated injury and protection of endothelium: Lessons from atypical haemolytic uraemic syndrome. Immunobiology 217: 195–203, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nester CM, Barbour T, de Cordoba SR, Dragon-Durey MA, Fremeaux-Bacchi V, Goodship TH, Kavanagh D, Noris M, Pickering M, Sanchez-Corral P, Skerka C, Zipfel P, Smith RJ: Atypical aHUS: State of the art. Mol Immunol 67: 31–42, 2015 [DOI] [PubMed] [Google Scholar]
- 5. Scully M, Cataland S, Coppo P, de la Rubia J, Friedman KD, Kremer Hovinga J, Lammle B, Matsumoto M, Pavenski K, Sadler E, Sarode R, Wu H; International Working Group for Thrombotic Thrombocytopenic Purpura: Consensus on the standardization of terminology in thrombotic thrombocytopenic purpura and related thrombotic microangiopathies. J Thromb Haemost 15: 312–322, 2017. [DOI] [PubMed]
- 6.Goodship TH, Cook HT, Fakhouri F, Fervenza FC, Fremeaux-Bacchi V, Kavanagh D, Nester CM, Noris M, Pickering MC, Rodriguez de Cordoba S, Roumenina LT, Sethi S, Smith RJ; Conference Participants: Atypical hemolytic uremic syndrome and C3 glomerulopathy: Conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) controversies conference. Kidney Int 91: 539–551, 2017 [DOI] [PubMed] [Google Scholar]
- 7.Manenti L, Gnappi E, Vaglio A, Allegri L, Noris M, Bresin E, Pilato FP, Valoti E, Pasquali S, Buzio C: Atypical haemolytic uraemic syndrome with underlying glomerulopathies. A case series and a review of the literature. Nephrol Dial Transplant 28: 2246–2259, 2013 [DOI] [PubMed] [Google Scholar]
- 8.El Karoui K, Hill GS, Karras A, Jacquot C, Moulonguet L, Kourilsky O, Frémeaux-Bacchi V, Delahousse M, Duong Van Huyen JP, Loupy A, Bruneval P, Nochy D: A clinicopathologic study of thrombotic microangiopathy in IgA nephropathy. J Am Soc Nephrol 23: 137–148, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barrera-Vargas A, Rosado-Canto R, Merayo-Chalico J, Arreola-Guerra JM, Mejía-Vilet JM, Correa-Rotter R, Gómez-Martín D, Alcocer-Varela J: Renal thrombotic microangiopathy in proliferative lupus nephritis: Risk factors and clinical outcomes: A Case-Control study. J Clin Rheumatol 22: 235–240, 2016 [DOI] [PubMed] [Google Scholar]
- 10.Phillips EH, Westwood JP, Brocklebank V, Wong EK, Tellez JO, Marchbank KJ, McGuckin S, Gale DP, Connolly J, Goodship TH, Kavanagh D, Scully MA: The role of ADAMTS-13 activity and complement mutational analysis in differentiating acute thrombotic microangiopathies. J Thromb Haemost 14: 175–185, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thurman JM, Nester CM: All things complement. Clin J Am Soc Nephrol 11: 1856–1866, 2016. [DOI] [PMC free article] [PubMed]
- 12.Ricklin D, Hajishengallis G, Yang K, Lambris JD: Complement: A key system for immune surveillance and homeostasis. Nat Immunol 11: 785–797, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brocklebank VKD: Complement C5 inhibiting therapy for the thrombotic microangiopathies: Accumulating evidence, but not a panacea. Clin Kidney J, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kavanagh D, Goodship TH, Richards A: Atypical hemolytic uremic syndrome. Semin Nephrol 33: 508–530, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Warwicker P, Goodship TH, Donne RL, Pirson Y, Nicholls A, Ward RM, Turnpenny P, Goodship JA: Genetic studies into inherited and sporadic hemolytic uremic syndrome. Kidney Int 53: 836–844, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Le Quintrec M, Zuber J, Moulin B, Kamar N, Jablonski M, Lionet A, Chatelet V, Mousson C, Mourad G, Bridoux F, Cassuto E, Loirat C, Rondeau E, Delahousse M, Frémeaux-Bacchi V: Complement genes strongly predict recurrence and graft outcome in adult renal transplant recipients with atypical hemolytic and uremic syndrome. Am J Transplant 13: 663–675, 2013 [DOI] [PubMed] [Google Scholar]
- 17.Kavanagh D, Richards A, Goodship T, Jalanko H: Transplantation in atypical hemolytic uremic syndrome. Semin Thromb Hemost 36: 653–659, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Saland J: Liver-kidney transplantation to cure atypical HUS: Still an option post-eculizumab? Pediatr Nephrol 29: 329–332, 2014 [DOI] [PubMed] [Google Scholar]
- 19.Dragon-Durey MA, Loirat C, Cloarec S, Macher MA, Blouin J, Nivet H, Weiss L, Fridman WH, Frémeaux-Bacchi V: Anti-Factor H autoantibodies associated with atypical hemolytic uremic syndrome. J Am Soc Nephrol 16: 555–563, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Blanc C, Roumenina LT, Ashraf Y, Hyvärinen S, Sethi SK, Ranchin B, Niaudet P, Loirat C, Gulati A, Bagga A, Fridman WH, Sautès-Fridman C, Jokiranta TS, Frémeaux-Bacchi V, Dragon-Durey MA: Overall neutralization of complement factor H by autoantibodies in the acute phase of the autoimmune form of atypical hemolytic uremic syndrome. J Immunol 189: 3528–3537, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Moore I, Strain L, Pappworth I, Kavanagh D, Barlow PN, Herbert AP, Schmidt CQ, Staniforth SJ, Holmes LV, Ward R, Morgan L, Goodship TH, Marchbank KJ: Association of factor H autoantibodies with deletions of CFHR1, CFHR3, CFHR4, and with mutations in CFH, CFI, CD46, and C3 in patients with atypical hemolytic uremic syndrome. Blood 115: 379–387, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brocklebank V, Johnson S, Sheerin TP, Marks SD, Gilbert RD, Tyerman K, Kinoshita M, Awan A, Kaur A, Webb N, Hegde S, Finlay E, Fitzpatrick M, Walsh PR, Wong EKS, Booth C, Kerecuk L, Salama AD, Almond M, Inward C, Goodship TH, Sheerin NS, Marchbank KJ, Kavanagh D: Factor H: Autoantibody associated atypical haemolytic uraemic syndrome in children in the United Kingdom and Ireland [published online ahead of print July 24, 2017]. Kidney Int doi:10.1016/j.kint.2017.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kavanagh D, Pappworth IY, Anderson H, Hayes CM, Moore I, Hunze EM, Bennaceur K, Roversi P, Lea S, Strain L, Ward R, Plant N, Nailescu C, Goodship TH, Marchbank KJ: Factor I autoantibodies in patients with atypical hemolytic uremic syndrome: Disease-associated or an epiphenomenon? Clin J Am Soc Nephrol 7: 417–426, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loirat C, Fakhouri F, Ariceta G, Besbas N, Bitzan M, Bjerre A, Coppo R, Emma F, Johnson S, Karpman D, Landau D, Langman CB, Lapeyraque AL, Licht C, Nester C, Pecoraro C, Riedl M, van de Kar NC, Van de Walle J, Vivarelli M, Frémeaux-Bacchi V; HUS International: An international consensus approach to the management of atypical hemolytic uremic syndrome in children. Pediatr Nephrol 31: 15–39, 2016 [DOI] [PubMed] [Google Scholar]
- 25.Hofer J, Giner T, Safouh H: Diagnosis and treatment of the hemolytic uremic syndrome disease spectrum in developing regions. Semin Thromb Hemost 40: 478–486, 2014 [DOI] [PubMed] [Google Scholar]
- 26.Legendre CM, Licht C, Muus P, Greenbaum LA, Babu S, Bedrosian C, Bingham C, Cohen DJ, Delmas Y, Douglas K, Eitner F, Feldkamp T, Fouque D, Furman RR, Gaber O, Herthelius M, Hourmant M, Karpman D, Lebranchu Y, Mariat C, Menne J, Moulin B, Nürnberger J, Ogawa M, Remuzzi G, Richard T, Sberro-Soussan R, Severino B, Sheerin NS, Trivelli A, Zimmerhackl LB, Goodship T, Loirat C: Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med 368: 2169–2181, 2013 [DOI] [PubMed] [Google Scholar]
- 27.Fremeaux-Bacchi V, Fakhouri F, Garnier A, Bienaimé F, Dragon-Durey MA, Ngo S, Moulin B, Servais A, Provot F, Rostaing L, Burtey S, Niaudet P, Deschênes G, Lebranchu Y, Zuber J, Loirat C: Genetics and outcome of atypical hemolytic uremic syndrome: A nationwide French series comparing children and adults. Clin J Am Soc Nephrol 8: 554–562, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noris M, Caprioli J, Bresin E, Mossali C, Pianetti G, Gamba S, Daina E, Fenili C, Castelletti F, Sorosina A, Piras R, Donadelli R, Maranta R, van der Meer I, Conway EM, Zipfel PF, Goodship TH, Remuzzi G: Relative role of genetic complement abnormalities in sporadic and familial aHUS and their impact on clinical phenotype. Clin J Am Soc Nephrol 5: 1844–1859, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Licht C, Greenbaum LA, Muus P, Babu S, Bedrosian CL, Cohen DJ, Delmas Y, Douglas K, Furman RR, Gaber OA, Goodship T, Herthelius M, Hourmant M, Legendre CM, Remuzzi G, Sheerin N, Trivelli A, Loirat C: Efficacy and safety of eculizumab in atypical hemolytic uremic syndrome from 2-year extensions of phase 2 studies. Kidney Int 87: 1061–1073, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fakhouri F, Hourmant M, Campistol JM, Cataland SR, Espinosa M, Gaber AO, Menne J, Minetti EE, Provôt F, Rondeau E, Ruggenenti P, Weekers LE, Ogawa M, Bedrosian CL, Legendre CM: Terminal complement inhibitor eculizumab in adult patients with atypical hemolytic uremic syndrome: A single-arm, open-label trial. Am J Kidney Dis 68: 84–93, 2016 [DOI] [PubMed] [Google Scholar]
- 31.Greenbaum LA, Fila M, Ardissino G, Al-Akash SI, Evans J, Henning P, Lieberman KV, Maringhini S, Pape L, Rees L, van de Kar NC, Vande Walle J, Ogawa M, Bedrosian CL, Licht C: Eculizumab is a safe and effective treatment in pediatric patients with atypical hemolytic uremic syndrome. Kidney Int 89: 701–711, 2016 [DOI] [PubMed] [Google Scholar]
- 32. Sheerin NS, Kavanagh D, Goodship TH, Johnson S: A national specialized service in England for atypical haemolytic uraemic syndrome-the first year's experience. QJM 109: 27–33, 2016. [DOI] [PubMed]
- 33.Wong EK, Kavanagh D: Anticomplement C5 therapy with eculizumab for the treatment of paroxysmal nocturnal hemoglobinuria and atypical hemolytic uremic syndrome. Transl Res 165: 306–320, 2015 [DOI] [PubMed] [Google Scholar]
- 34.Fakhouri F, Fila M, Provôt F, Delmas Y, Barbet C, Châtelet V, Rafat C, Cailliez M, Hogan J, Servais A, Karras A, Makdassi R, Louillet F, Coindre JP, Rondeau E, Loirat C, Frémeaux-Bacchi V: Pathogenic variants in complement genes and risk of atypical hemolytic uremic syndrome relapse after eculizumab discontinuation. Clin J Am Soc Nephrol 12: 50–59, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nishijima Y, Hirata H, Himeno A, Kida H, Matsumoto M, Takahashi R, Otani Y, Inoue K, Nagatomo I, Takeda Y, Kijima T, Tachibana I, Fujimura Y, Kumanogoh A: Drug-induced thrombotic thrombocytopenic purpura successfully treated with recombinant human soluble thrombomodulin. Intern Med 52: 1111–1114, 2013 [DOI] [PubMed] [Google Scholar]
- 36.Lemaire M, Frémeaux-Bacchi V, Schaefer F, Choi M, Tang WH, Le Quintrec M, Fakhouri F, Taque S, Nobili F, Martinez F, Ji W, Overton JD, Mane SM, Nürnberg G, Altmüller J, Thiele H, Morin D, Deschenes G, Baudouin V, Llanas B, Collard L, Majid MA, Simkova E, Nürnberg P, Rioux-Leclerc N, Moeckel GW, Gubler MC, Hwa J, Loirat C, Lifton RP: Recessive mutations in DGKE cause atypical hemolytic-uremic syndrome. Nat Genet 45: 531–536, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Challis RC, Ring T, Xu Y, Wong EK, Flossmann O, Roberts IS, Ahmed S, Wetherall M, Salkus G, Brocklebank V, Fester J, Strain L, Wilson V, Wood KM, Marchbank KJ, Santibanez-Koref M, Goodship TH, Kavanagh D: Thrombotic microangiopathy in inverted formin 2-mediated renal disease. J Am Soc Nephrol 28: 1084–1091, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Benamu E, Montoya JG: Infections associated with the use of eculizumab: Recommendations for prevention and prophylaxis. Curr Opin Infect Dis 29: 319–329, 2016 [DOI] [PubMed] [Google Scholar]
- 39.Alashkar F, Vance C, Herich-Terhürne D, Preising N, Dührsen U, Röth A: Serologic response to meningococcal vaccination in patients with paroxysmal nocturnal hemoglobinuria (PNH) chronically treated with the terminal complement inhibitor eculizumab. Ann Hematol 96: 589–596, 2017 [DOI] [PubMed] [Google Scholar]
- 40.Cullinan N, Gorman KM, Riordan M, Waldron M, Goodship TH, Awan A: Case report: Benefits and challenges of long-term eculizumab in atypical hemolytic uremic syndrome. Pediatrics 135: e1506–e1509, 2015 [DOI] [PubMed] [Google Scholar]
- 41.Struijk GH, Bouts AH, Rijkers GT, Kuin EA, ten Berge IJ, Bemelman FJ: Meningococcal sepsis complicating eculizumab treatment despite prior vaccination. Am J Transplant 13: 819–820, 2013 [DOI] [PubMed] [Google Scholar]
- 42.Hayes W, Tschumi S, Ling SC, Feber J, Kirschfink M, Licht C: Eculizumab hepatotoxicity in pediatric aHUS. Pediatr Nephrol 30: 775–781, 2015 [DOI] [PubMed] [Google Scholar]
- 43.Herlitz LC, Bomback AS, Markowitz GS, Stokes MB, Smith RN, Colvin RB, Appel GB, D’Agati VD: Pathology after eculizumab in dense deposit disease and C3 GN. J Am Soc Nephrol 23: 1229–1237, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.George JN: The association of pregnancy with thrombotic thrombocytopenic purpura-hemolytic uremic syndrome. Curr Opin Hematol 10: 339–344, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Zuber J, Fakhouri F, Roumenina LT, Loirat C, Frémeaux-Bacchi V; French Study Group for aHUS/C3G: Use of eculizumab for atypical haemolytic uraemic syndrome and C3 glomerulopathies. Nat Rev Nephrol 8: 643–657, 2012 [DOI] [PubMed] [Google Scholar]
- 46.Schwartz J, Padmanabhan A, Aqui N, Balogun RA, Connelly-Smith L, Delaney M, Dunbar NM, Witt V, Wu Y, Shaz BH: Guidelines on the use of therapeutic apheresis in clinical practice-evidence-based approach from the writing committee of the american society for apheresis: The seventh special issue. J Clin Apher 31: 149–162, 2016 [DOI] [PubMed] [Google Scholar]
- 47.Scully M, McDonald V, Cavenagh J, Hunt BJ, Longair I, Cohen H, Machin SJ: A phase 2 study of the safety and efficacy of rituximab with plasma exchange in acute acquired thrombotic thrombocytopenic purpura. Blood 118: 1746–1753, 2011 [DOI] [PubMed] [Google Scholar]
- 48.Clark WF, Rock G, Barth D, Arnold DM, Webert KE, Yenson PR, Kelton JG, Li L, Foley SR; members of Canadian Apheresis Group: A phase-II sequential case-series study of all patients presenting to four plasma exchange centres with presumed relapsed/refractory thrombotic thrombocytopenic purpura treated with rituximab. Br J Haematol 170: 208–217, 2015 [DOI] [PubMed] [Google Scholar]
- 49.Benhamou Y, Paintaud G, Azoulay E, Poullin P, Galicier L, Desvignes C, Baudel JL, Peltier J, Mira JP, Pene F, Presne C, Saheb S, Deligny C, Rousseau A, Feger F, Veyradier A, Coppo P; French Reference Center for Thrombotic M: Efficacy of a rituximab regimen based on B cell depletion in thrombotic thrombocytopenic purpura with suboptimal response to standard treatment: Results of a phase II, multicenter non-comparative study. Am J Hematol 91: 1246–1251, 2016 [DOI] [PubMed] [Google Scholar]
- 50.Beck BB, van Spronsen F, Diepstra A, Berger RM, Komhoff M: Renal thrombotic microangiopathy in patients with cblC defect: Review of an under-recognized entity. Pediatr Nephrol 32: 733–741, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bruneau S, Néel M, Roumenina LT, Frimat M, Laurent L, Frémeaux-Bacchi V, Fakhouri F: Loss of DGKε induces endothelial cell activation and death independently of complement activation. Blood 125: 1038–1046, 2015 [DOI] [PubMed] [Google Scholar]
- 52.Mele C, Lemaire M, Iatropoulos P, Piras R, Bresin E, Bettoni S, Bick D, Helbling D, Veith R, Valoti E, Donadelli R, Murer L, Neunhäuserer M, Breno M, Frémeaux-Bacchi V, Lifton R, Remuzzi G, Noris M: Characterization of a new DGKE intronic mutation in genetically unsolved cases of familial atypical hemolytic uremic syndrome. Clin J Am Soc Nephrol 10: 1011–1019, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Westland R, Bodria M, Carrea A, Lata S, Scolari F, Fremeaux-Bacchi V, D’Agati VD, Lifton RP, Gharavi AG, Ghiggeri GM, Sanna-Cherchi S: Phenotypic expansion of DGKE-associated diseases. J Am Soc Nephrol 25: 1408–1414, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sánchez Chinchilla D, Pinto S, Hoppe B, Adragna M, Lopez L, Justa Roldan ML, Peña A, Lopez Trascasa M, Sánchez-Corral P, Rodríguez de Córdoba S: Complement mutations in diacylglycerol kinase-ε-associated atypical hemolytic uremic syndrome. Clin J Am Soc Nephrol 9: 1611–1619, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ozaltin F, Li B, Rauhauser A, An SW, Soylemezoglu O, Gonul II, Taskiran EZ, Ibsirlioglu T, Korkmaz E, Bilginer Y, Duzova A, Ozen S, Topaloglu R, Besbas N, Ashraf S, Du Y, Liang C, Chen P, Lu D, Vadnagara K, Arbuckle S, Lewis D, Wakeland B, Quigg RJ, Ransom RF, Wakeland EK, Topham MK, Bazan NG, Mohan C, Hildebrandt F, Bakkaloglu A, Huang CL, Attanasio M: DGKE variants cause a glomerular microangiopathy that mimics membranoproliferative GN. J Am Soc Nephrol 24: 377–384, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maga TK, Nishimura CJ, Weaver AE, Frees KL, Smith RJ: Mutations in alternative pathway complement proteins in American patients with atypical hemolytic uremic syndrome. Hum Mutat 31: E1445–E1460, 2010 [DOI] [PubMed] [Google Scholar]
- 57.Delvaeye M, Noris M, De Vriese A, Esmon CT, Esmon NL, Ferrell G, Del-Favero J, Plaisance S, Claes B, Lambrechts D, Zoja C, Remuzzi G, Conway EM: Thrombomodulin mutations in atypical hemolytic-uremic syndrome. N Engl J Med 361: 345–357, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bu F, Maga T, Meyer NC, Wang K, Thomas CP, Nester CM, Smith RJ: Comprehensive genetic analysis of complement and coagulation genes in atypical hemolytic uremic syndrome. J Am Soc Nephrol 25: 55–64, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tarr PI, Gordon CA, Chandler WL: Shiga-toxin-producing Escherichia coli and haemolytic uraemic syndrome. Lancet 365: 1073–1086, 2005 [DOI] [PubMed] [Google Scholar]
- 60.Kielstein JT, Beutel G, Fleig S, Steinhoff J, Meyer TN, Hafer C, Kuhlmann U, Bramstedt J, Panzer U, Vischedyk M, Busch V, Ries W, Mitzner S, Mees S, Stracke S, Nürnberger J, Gerke P, Wiesner M, Sucke B, Abu-Tair M, Kribben A, Klause N, Schindler R, Merkel F, Schnatter S, Dorresteijn EM, Samuelsson O, Brunkhorst R; Collaborators of the DGfN STEC-HUS registry: Best supportive care and therapeutic plasma exchange with or without eculizumab in Shiga-toxin-producing E. coli O104:H4 induced haemolytic-uraemic syndrome: An analysis of the German STEC-HUS registry. Nephrol Dial Transplant 27: 3807–3815, 2012 [DOI] [PubMed] [Google Scholar]
- 61.Brigotti M, Caprioli A, Tozzi AE, Tazzari PL, Ricci F, Conte R, Carnicelli D, Procaccino MA, Minelli F, Ferretti AV, Paglialonga F, Edefonti A, Rizzoni G: Shiga toxins present in the gut and in the polymorphonuclear leukocytes circulating in the blood of children with hemolytic-uremic syndrome. J Clin Microbiol 44: 313–317, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Obrig TG: Escherichia coli Shiga toxin mechanisms of action in renal disease. Toxins (Basel) 2: 2769–2794, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hughes AK, Ergonul Z, Stricklett PK, Kohan DE: Molecular basis for high renal cell sensitivity to the cytotoxic effects of shigatoxin-1: Upregulation of globotriaosylceramide expression. J Am Soc Nephrol 13: 2239–2245, 2002 [DOI] [PubMed] [Google Scholar]
- 64.Brandt JR, Fouser LS, Watkins SL, Zelikovic I, Tarr PI, Nazar-Stewart V, Avner ED: Escherichia coli O 157:H7-associated hemolytic-uremic syndrome after ingestion of contaminated hamburgers. J Pediatr 125: 519–526, 1994 [DOI] [PubMed] [Google Scholar]
- 65.Dowen FWK, Brown AL, Palfrey J, Kavanagh D, Brocklebank V: Rare genetic variants in Shiga toxin–associated haemolytic uraemic syndrome: Genetic analysis prior to transplantation is essential. Clin Kidney J 10: 490–493, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Spinale JM, Ruebner RL, Copelovitch L, Kaplan BS: Long-term outcomes of Shiga toxin hemolytic uremic syndrome. Pediatr Nephrol 28: 2097–2105, 2013 [DOI] [PubMed] [Google Scholar]
- 67.Lapeyraque AL, Malina M, Fremeaux-Bacchi V, Boppel T, Kirschfink M, Oualha M, Proulx F, Clermont MJ, Le Deist F, Niaudet P, Schaefer F: Eculizumab in severe Shiga-toxin-associated HUS. N Engl J Med 364: 2561–2563, 2011 [DOI] [PubMed] [Google Scholar]
- 68.Menne J, Nitschke M, Stingele R, Abu-Tair M, Beneke J, Bramstedt J, Bremer JP, Brunkhorst R, Busch V, Dengler R, Deuschl G, Fellermann K, Fickenscher H, Gerigk C, Goettsche A, Greeve J, Hafer C, Hagenmüller F, Haller H, Herget-Rosenthal S, Hertenstein B, Hofmann C, Lang M, Kielstein JT, Klostermeier UC, Knobloch J, Kuehbacher M, Kunzendorf U, Lehnert H, Manns MP, Menne TF, Meyer TN, Michael C, Münte T, Neumann-Grutzeck C, Nuernberger J, Pavenstaedt H, Ramazan L, Renders L, Repenthin J, Ries W, Rohr A, Rump LC, Samuelsson O, Sayk F, Schmidt BM, Schnatter S, Schöcklmann H, Schreiber S, von Seydewitz CU, Steinhoff J, Stracke S, Suerbaum S, van de Loo A, Vischedyk M, Weissenborn K, Wellhöner P, Wiesner M, Zeissig S, Büning J, Schiffer M, Kuehbacher T; EHEC-HUS consortium: Validation of treatment strategies for enterohaemorrhagic Escherichia coli O104:H4 induced haemolytic uraemic syndrome: Case-control study. BMJ 345: e4565, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Loos S, Ahlenstiel T, Kranz B, Staude H, Pape L, Härtel C, Vester U, Buchtala L, Benz K, Hoppe B, Beringer O, Krause M, Müller D, Pohl M, Lemke J, Hillebrand G, Kreuzer M, König J, Wigger M, Konrad M, Haffner D, Oh J, Kemper MJ: An outbreak of Shiga toxin-producing Escherichia coli O104:H4 hemolytic uremic syndrome in Germany: Presentation and short-term outcome in children. Clin Infect Dis 55: 753–759, 2012 [DOI] [PubMed] [Google Scholar]
- 70.Johnson S, Waters A: Is complement a culprit in infection-induced forms of haemolytic uraemic syndrome? Immunobiology 217: 235–243, 2012 [DOI] [PubMed] [Google Scholar]
- 71.Spinale JM, Ruebner RL, Kaplan BS, Copelovitch L: Update on Streptococcus pneumoniae associated hemolytic uremic syndrome. Curr Opin Pediatr 25: 203–208, 2013 [DOI] [PubMed] [Google Scholar]
- 72.Banerjee R, Hersh AL, Newland J, Beekmann SE, Polgreen PM, Bender J, Shaw J, Copelovitch L, Kaplan BS, Shah SS; Emerging Infections Network Hemolytic-Uremic Syndrome Study Group: Streptococcus pneumoniae-associated hemolytic uremic syndrome among children in North America. Pediatr Infect Dis J 30: 736–739, 2011 [DOI] [PubMed] [Google Scholar]
- 73.Kavanagh D, Goodship TH, Richards A: Atypical haemolytic uraemic syndrome. Br Med Bull 77-78: 5–22, 2006 [DOI] [PubMed] [Google Scholar]
- 74.Blaum BS, Hannan JP, Herbert AP, Kavanagh D, Uhrín D, Stehle T: Structural basis for sialic acid-mediated self-recognition by complement factor H. Nat Chem Biol 11: 77–82, 2015 [DOI] [PubMed] [Google Scholar]
- 75.Alpers CE: Light at the end of the TUNEL: HIV-associated thrombotic microangiopathy. Kidney Int 63: 385–396, 2003 [DOI] [PubMed] [Google Scholar]
- 76.Peraldi MN, Maslo C, Akposso K, Mougenot B, Rondeau E, Sraer JD: Acute renal failure in the course of HIV infection: A single-institution retrospective study of ninety-two patients and sixty renal biopsies. Nephrol Dial Transplant 14: 1578–1585, 1999 [DOI] [PubMed] [Google Scholar]
- 77.Becker S, Fusco G, Fusco J, Balu R, Gangjee S, Brennan C, Feinberg J; Collaborations in HIVORUSC: HIV-associated thrombotic microangiopathy in the era of highly active antiretroviral therapy: An observational study. Clin Infect Dis 39[Suppl 5]: S267–S275, 2004 [DOI] [PubMed] [Google Scholar]
- 78.Caprioli J, Noris M, Brioschi S, Pianetti G, Castelletti F, Bettinaglio P, Mele C, Bresin E, Cassis L, Gamba S, Porrati F, Bucchioni S, Monteferrante G, Fang CJ, Liszewski MK, Kavanagh D, Atkinson JP, Remuzzi G; International Registry of Recurrent and Familial HUS/TTP: Genetics of HUS: The impact of MCP, CFH, and IF mutations on clinical presentation, response to treatment, and outcome. Blood 108: 1267–1279, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fakhouri F, Vercel C, Frémeaux-Bacchi V: Obstetric nephrology: AKI and thrombotic microangiopathies in pregnancy. Clin J Am Soc Nephrol 7: 2100–2106, 2012 [DOI] [PubMed] [Google Scholar]
- 80.George JN, Nester CM, McIntosh JJ: Syndromes of thrombotic microangiopathy associated with pregnancy. Hematology (Am Soc Hematol Educ Program) 2015: 644–648, 2015 [DOI] [PubMed] [Google Scholar]
- 81. Fakhouri F: Pregnancy-related thrombotic microangiopathies: Clues from complement biology. Transfus Apher Sci 54: 199–202, 2016. [DOI] [PubMed]
- 82.Bruel A, Kavanagh D, Noris M, Delmas Y, Wong EKS, Bresin E, Provôt F, Brocklebank V, Mele C, Remuzzi G, Loirat C, Frémeaux-Bacchi V, Fakhouri F: Hemolytic uremic syndrome in pregnancy and postpartum. Clin J Am Soc Nephrol 12: 1237–1247, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kelly RJ, Höchsmann B, Szer J, Kulasekararaj A, de Guibert S, Röth A, Weitz IC, Armstrong E, Risitano AM, Patriquin CJ, Terriou L, Muus P, Hill A, Turner MP, Schrezenmeier H, Peffault de Latour R: Eculizumab in pregnant patients with paroxysmal nocturnal hemoglobinuria. N Engl J Med 373: 1032–1039, 2015 [DOI] [PubMed] [Google Scholar]
- 84. Servais A, Devillard N, Fremeaux-Bacchi V, Hummel A, Salomon L, Contin-Bordes C, Gomer H, Legendre C, Delmas Y: Atypical haemolytic uraemic syndrome and pregnancy: Outcome with ongoing eculizumab. Nephrol Dialysis Transplant 31: 2122–2130, 2016. [DOI] [PubMed]
- 85.Scully M, Thomas M, Underwood M, Watson H, Langley K, Camilleri RS, Clark A, Creagh D, Rayment R, Mcdonald V, Roy A, Evans G, McGuckin S, Ni Ainle F, Maclean R, Lester W, Nash M, Scott R, O Brien P; collaborators of the UK TTP Registry: Thrombotic thrombocytopenic purpura and pregnancy: Presentation, management, and subsequent pregnancy outcomes. Blood 124: 211–219, 2014 [DOI] [PubMed] [Google Scholar]
- 86.Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R: Pre-eclampsia. Lancet 376: 631–644, 2010 [DOI] [PubMed] [Google Scholar]
- 87.Salmon JE, Heuser C, Triebwasser M, Liszewski MK, Kavanagh D, Roumenina L, Branch DW, Goodship T, Fremeaux-Bacchi V, Atkinson JP: Mutations in complement regulatory proteins predispose to preeclampsia: A genetic analysis of the PROMISSE cohort. PLoS Med 8: e1001013, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Al-Nouri ZL, Reese JA, Terrell DR, Vesely SK, George JN: Drug-induced thrombotic microangiopathy: A systematic review of published reports. Blood 125: 616–618, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gottschall JL, Elliot W, Lianos E, McFarland JG, Wolfmeyer K, Aster RH: Quinine-induced immune thrombocytopenia associated with hemolytic uremic syndrome: A new clinical entity. Blood 77: 306–310, 1991 [PubMed] [Google Scholar]
- 90.Kavanagh D, McGlasson S, Jury A, Williams J, Scolding N, Bellamy C, Gunther C, Ritchie D, Gale DP, Kanwar YS, Challis R, Buist H, Overell J, Weller B, Flossmann O, Blunden M, Meyer EP, Krucker T, Evans SJ, Campbell IL, Jackson AP, Chandran S, Hunt DP: Type I interferon causes thrombotic microangiopathy by a dose-dependent toxic effect on the microvasculature. Blood 128: 2824–2833, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Eremina V, Jefferson JA, Kowalewska J, Hochster H, Haas M, Weisstuch J, Richardson C, Kopp JB, Kabir MG, Backx PH, Gerber HP, Ferrara N, Barisoni L, Alpers CE, Quaggin SE: VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med 358: 1129–1136, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bennett CL, Jacob S, Dunn BL, Georgantopoulos P, Zheng XL, Kwaan HC, McKoy JM, Magwood JS, Qureshi ZP, Bandarenko N, Winters JL, Raife TJ, Carey PM, Sarode R, Kiss JE, Danielson C, Ortel TL, Clark WF, Ablin RJ, Rock G, Matsumoto M, Fujimura Y: Ticlopidine-associated ADAMTS13 activity deficient thrombotic thrombocytopenic purpura in 22 persons in Japan: A report from the Southern Network on Adverse Reactions (SONAR). Br J Haematol 161: 896–898, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Verbiest A, Pirenne J, Dierickx D: De novo thrombotic microangiopathy after non-renal solid organ transplantation. Blood Rev 28: 269–279, 2014 [DOI] [PubMed] [Google Scholar]
- 94.Ponticelli C, Banfi G: Thrombotic microangiopathy after kidney transplantation. Transpl Int 19: 789–794, 2006 [DOI] [PubMed] [Google Scholar]
- 95.Zuber J, Le Quintrec M, Morris H, Frémeaux-Bacchi V, Loirat C, Legendre C: Targeted strategies in the prevention and management of atypical HUS recurrence after kidney transplantation. Transplant Rev (Orlando) 27: 117–125, 2013 [DOI] [PubMed] [Google Scholar]
- 96.Le Quintrec M, Lionet A, Kamar N, Karras A, Barbier S, Buchler M, Fakhouri F, Provost F, Fridman WH, Thervet E, Legendre C, Zuber J, Frémeaux-Bacchi V: Complement mutation-associated de novo thrombotic microangiopathy following kidney transplantation. Am J Transplant 8: 1694–1701, 2008 [DOI] [PubMed] [Google Scholar]
- 97. Chapin J, Shore T, Forsberg P, Desman G, Van Besien K, Laurence J: Hematopoietic transplant-associated thrombotic microangiopathy: Case report and review of diagnosis and treatments. Clin Adv Hematol Oncol 12: 565–573, 2014. [PubMed]
- 98.Jodele S, Zhang K, Zou F, Laskin B, Dandoy CE, Myers KC, Lane A, Meller J, Medvedovic M, Chen J, Davies SM: The genetic fingerprint of susceptibility for transplant-associated thrombotic microangiopathy. Blood 127: 989–996, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dhakal P, Bhatt VR: Is complement blockade an acceptable therapeutic strategy for hematopoietic cell transplant-associated thrombotic microangiopathy? Bone Marrow Transplant 52: 352–356, 2017 [DOI] [PubMed] [Google Scholar]
- 100.Jodele S, Licht C, Goebel J, Dixon BP, Zhang K, Sivakumaran TA, Davies SM, Pluthero FG, Lu L, Laskin BL: Abnormalities in the alternative pathway of complement in children with hematopoietic stem cell transplant-associated thrombotic microangiopathy. Blood 122: 2003–2007, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Jodele S, Dandoy CE, Myers KC, El-Bietar J, Nelson A, Wallace G, Laskin BL: New approaches in the diagnosis, pathophysiology, and treatment of pediatric hematopoietic stem cell transplantation-associated thrombotic microangiopathy. Transfus Apher Sci 54: 181–190, 2016. [DOI] [PMC free article] [PubMed]
- 102.Van Laecke S, Van Biesen W: Severe hypertension with renal thrombotic microangiopathy: What happened to the usual suspect? Kidney Int 91: 1271–1274, 2017 [DOI] [PubMed] [Google Scholar]
- 103.Timmermans SAMEG, Abdul-Hamid MA, Vanderlocht J, Damoiseaux JGMC, Reutelingsperger CP, van Paassen P; Limburg Renal Registry: Patients with hypertension-associated thrombotic microangiopathy may present with complement abnormalities. Kidney Int 91: 1420–1425, 2017 [DOI] [PubMed] [Google Scholar]
- 104.Shavit L, Reinus C, Slotki I: Severe renal failure and microangiopathic hemolysis induced by malignant hypertension--case series and review of literature. Clin Nephrol 73: 147–152, 2010 [DOI] [PubMed] [Google Scholar]
- 105.Izzedine H, Perazella MA: Thrombotic microangiopathy, cancer, and cancer drugs. Am J Kidney Dis 66: 857–868, 2015 [DOI] [PubMed] [Google Scholar]
- 106.George JN: Systemic malignancies as a cause of unexpected microangiopathic hemolytic anemia and thrombocytopenia. Oncology (Williston Park) 25: 908–914, 2011 [PubMed] [Google Scholar]
- 107.Lesesne JB, Rothschild N, Erickson B, Korec S, Sisk R, Keller J, Arbus M, Woolley PV, Chiazze L, Schein PS: Cancer-associated hemolytic-uremic syndrome: Analysis of 85 cases from a national registry. J Clin Oncol 7: 781–789, 1989 [DOI] [PubMed] [Google Scholar]
- 108.Oberic L, Buffet M, Schwarzinger M, Veyradier A, Clabault K, Malot S, Schleinitz N, Valla D, Galicier L, Bengrine-Lefèvre L, Gorin NC, Coppo P; Reference Center for the Management of Thrombotic Microangiopathies: Cancer awareness in atypical thrombotic microangiopathies. Oncologist 14: 769–779, 2009 [DOI] [PubMed] [Google Scholar]
- 109.Cooper M, McGraw ME, Unsworth DJ, Mathieson P: Familial mesangio-capillary glomerulonephritis with initial presentation as haemolytic uraemic syndrome. Nephrol Dial Transplant 19: 230–233, 2004 [DOI] [PubMed] [Google Scholar]
- 110.Zipfel PF, Skerka C, Chen Q, Wiech T, Goodship T, Johnson S, Fremeaux-Bacchi V, Nester C, de Córdoba SR, Noris M, Pickering M, Smith R: The role of complement in C3 glomerulopathy. Mol Immunol 67: 21–30, 2015 [DOI] [PubMed] [Google Scholar]
- 111.Woodworth TG, Suliman YA, Furst DE, Clements P: Scleroderma renal crisis and renal involvement in systemic sclerosis. Nat Rev Nephrol 12: 678–691, 2016 [DOI] [PubMed] [Google Scholar]
- 112.Rodríguez-Pintó I, Moitinho M, Santacreu I, Shoenfeld Y, Erkan D, Espinosa G, Cervera R; CAPS Registry Project Group (European Forum on Antiphospholipid Antibodies): Catastrophic antiphospholipid syndrome (CAPS): Descriptive analysis of 500 patients from the international CAPS registry. Autoimmun Rev 15: 1120–1124, 2016 [DOI] [PubMed] [Google Scholar]
- 113.Fakhouri F, Zuber J, Frémeaux-Bacchi V, Loirat C: Haemolytic uraemic syndrome [published online ahead of print February 25, 2017]. Lancet doi:10.1016/S0140-6736(17)30062-4 [DOI] [PubMed] [Google Scholar]
- 114.Michael M, Elliott EJ, Craig JC, Ridley G, Hodson EM: Interventions for hemolytic uremic syndrome and thrombotic thrombocytopenic purpura: A systematic review of randomized controlled trials. Am J Kidney Dis 53: 259–272, 2009 [DOI] [PubMed] [Google Scholar]
- 115.Lynn RM, O’Brien SJ, Taylor CM, Adak GK, Chart H, Cheasty T, Coia JE, Gillespie IA, Locking ME, Reilly WJ, Smith HR, Waters A, Willshaw GA: Childhood hemolytic uremic syndrome, United Kingdom and Ireland. Emerg Infect Dis 11: 590–596, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Noris M, Mescia F, Remuzzi G: STEC-HUS, atypical HUS and TTP are all diseases of complement activation. Nat Rev Nephrol 8: 622–633, 2012 [DOI] [PubMed] [Google Scholar]
- 117.Waters AM, Kerecuk L, Luk D, Haq MR, Fitzpatrick MM, Gilbert RD, Inward C, Jones C, Pichon B, Reid C, Slack MP, Van’t Hoff W, Dillon MJ, Taylor CM, Tullus K: Hemolytic uremic syndrome associated with invasive pneumococcal disease: The United kingdom experience. J Pediatr 151: 140–144, 2007 [DOI] [PubMed] [Google Scholar]
- 118.Cochran JB, Panzarino VM, Maes LY, Tecklenburg FW: Pneumococcus-induced T-antigen activation in hemolytic uremic syndrome and anemia. Pediatr Nephrol 19: 317–321, 2004 [DOI] [PubMed] [Google Scholar]
- 119.Prestidge C, Wong W: Ten years of pneumococcal-associated haemolytic uraemic syndrome in New Zealand children. J Paediatr Child Health 45: 731–735, 2009 [DOI] [PubMed] [Google Scholar]
- 120.Feinberg JE, Hurwitz S, Cooper D, Sattler FR, MacGregor RR, Powderly W, Holland GN, Griffiths PD, Pollard RB, Youle M, Gill MJ, Holland FJ, Power ME, Owens S, Coakley D, Fry J, Jacobson MA: A randomized, double-blind trial of valaciclovir prophylaxis for cytomegalovirus disease in patients with advanced human immunodeficiency virus infection. AIDS Clinical Trials Group Protocol 204/Glaxo Wellcome 123-014 International CMV Prophylaxis study group. J Infect Dis 177: 48–56, 1998 [DOI] [PubMed] [Google Scholar]
- 121. Crovetto F, Borsa N, Acaia B, Nishimura C, Frees K, Smith RJ, Peyvandi F, Palla R, Cugno M, Tedeschi S, Castorina P, Somigliana E, Ardissino G, Fedele L: The genetics of the alternative pathway of complement in the pathogenesis of HELLP syndrome. J Matern Fetal Neonatal Med 25: 2322–2325, 2012. [DOI] [PubMed]
- 122.Pisoni R, Ruggenenti P, Remuzzi G: Drug-induced thrombotic microangiopathy: Incidence, prevention and management. Drug Saf 24: 491–501, 2001 [DOI] [PubMed] [Google Scholar]
- 123.Reynolds JC, Agodoa LY, Yuan CM, Abbott KC: Thrombotic microangiopathy after renal transplantation in the United States. Am J Kidney Dis 42: 1058–1068, 2003 [DOI] [PubMed] [Google Scholar]
- 124.van den Born BJ, Honnebier UP, Koopmans RP, van Montfrans GA: Microangiopathic hemolysis and renal failure in malignant hypertension. Hypertension 45: 246–251, 2005 [DOI] [PubMed] [Google Scholar]
- 125.van den Born BJ, Koopmans RP, van Montfrans GA: The renin-angiotensin system in malignant hypertension revisited: plasma renin activity, microangiopathic hemolysis, and renal failure in malignant hypertension. Am J Hypertens 20: 900–906, 2007 [DOI] [PubMed] [Google Scholar]
- 126.Akimoto T, Muto S, Ito C, Takahashi H, Takeda S, Ando Y, Kusano E: Clinical features of malignant hypertension with thrombotic microangiopathy. Clin Exp Hypertens 33: 77–83, 2011 [DOI] [PubMed] [Google Scholar]
- 127.Amraoui F, Bos S, Vogt L, van den Born BJ: Long-term renal outcome in patients with malignant hypertension: A retrospective cohort study. BMC Nephrol 13: 71, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Barber C, Herzenberg A, Aghdassi E, Su J, Lou W, Qian G, Yip J, Nasr SH, Thomas D, Scholey JW, Wither J, Urowitz M, Gladman D, Reich H, Fortin PR: Evaluation of clinical outcomes and renal vascular pathology among patients with lupus. Clin J Am Soc Nephrol 7: 757–764, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ghossein C, Varga J, Fenves AZ: Recent developments in the classification, evaluation, pathophysiology, and management of scleroderma renal crisis. Curr Rheumatol Rep 18: 5, 2016 [DOI] [PubMed] [Google Scholar]