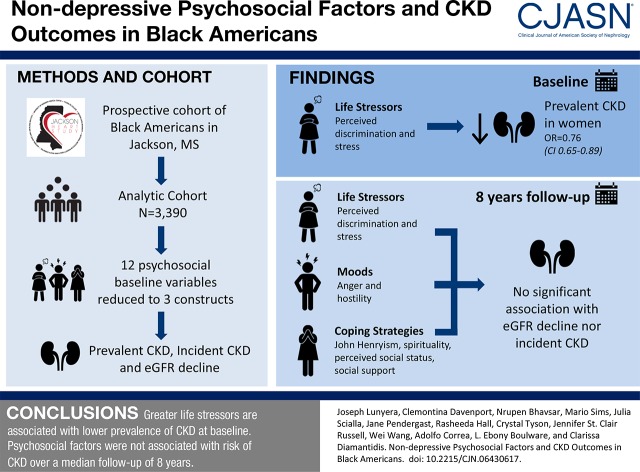
Abstract
Background and objectives
Established risk factors for CKD do not fully account for risk of CKD in black Americans. We studied the association of nondepressive psychosocial factors with risk of CKD in the Jackson Heart Study.
Design, setting, participants, & measurements
We used principal component analysis to identify underlying constructs from 12 psychosocial baseline variables (perceived daily, lifetime, and burden of lifetime discrimination; stress; anger in; anger out; hostility; pessimism; John Henryism; spirituality; perceived social status; and social support). Using multivariable models adjusted for demographics and comorbidity, we examined the association of psychosocial variables with baseline CKD prevalence, eGFR decline, and incident CKD during follow-up.
Results
Of 3390 (64%) Jackson Heart Study participants with the required data, 656 (19%) had prevalent CKD. Those with CKD (versus no CKD) had lower perceived daily (mean [SD] score =7.6 [8.5] versus 9.7 [9.0]) and lifetime discrimination (2.5 [2.0] versus 3.1 [2.2]), lower perceived stress (4.2 [4.0] versus 5.2 [4.4]), higher hostility (12.1 [5.2] versus 11.5 [4.8]), higher John Henryism (30.0 [4.8] versus 29.7 [4.4]), and higher pessimism (2.3 [2.2] versus 2.0 [2.1]; all P<0.05). Principal component analysis identified three factors from the 12 psychosocial variables: factor 1, life stressors (perceived discrimination, stress); factor 2, moods (anger, hostility); and, factor 3, coping strategies (John Henryism, spirituality, social status, social support). After adjustments, factor 1 (life stressors) was negatively associated with prevalent CKD at baseline among women only: odds ratio, 0.76 (95% confidence interval, 0.65 to 0.89). After a median follow-up of 8 years, identified psychosocial factors were not significantly associated with eGFR decline (life stressors: β=0.08; 95% confidence interval, −0.02 to 0.17; moods: β=0.03; 95% confidence interval, −0.06 to 0.13; coping: β=−0.02; 95% confidence interval, −0.12 to 0.08) or incident CKD (life stressors: odds ratio, 1.07; 95% confidence interval, 0.88 to 1.29; moods: odds ratio, 1.02; 95% confidence interval, 0.84 to 1.24; coping: odds ratio, 0.91; 95% confidence interval, 0.75 to 1.11).
Conclusions
Greater life stressors were associated with lower prevalence of CKD at baseline in the Jackson Heart Study. However, psychosocial factors were not associated with risk of CKD over a median follow-up of 8 years.
Podcast
This article contains a podcast at https://www.asn-online.org/media/podcast/CJASN/2018_01_03_CJASNPodcast_18_2_L.mp3
Keywords: chronic kidney disease; Epidemiology and outcomes; ethnicity; risk factors; psychosocial factors; weathering hypothesis; Female; Odds Ratio; glomerular filtration rate; Hostility; African Americans; Prevalence; Spirituality; Confidence Intervals; Comorbidity; Principal Component Analysis; Follow-Up Studies; Pessimism; Renal Insufficiency, Chronic; Social Support; Adaptation, Psychological; Anger; Humans
Introduction
CKD affects approximately 30 million adults in the United States, with millions more at increased risk of developing CKD (1). Black Americans are disproportionately affected by CKD and suffer a twofold higher mortality rate and threefold greater incidence of ESRD compared with whites (2,3). These racial disparities are, in part, due to increased prevalence of established risk factors for CKD, particularly hypertension and diabetes, among black Americans. Genetic predisposition accounts for some, but not all, of the residual disparate risk of CKD among this group (4–6).
Despite improved recognition of established risk factors for CKD among black Americans, little progress has been made to narrow disparities in kidney outcomes among black Americans compared with whites (7). A growing body of research suggests that social determinants of health may also contribute to these unexplained disparate kidney outcomes (8,9). Psychosocial factors, including negative affective moods, psychosocial stressors, and coping mechanisms, are thought to affect health outcomes via several physiologic (e.g., elevations in serum cortisol) and behavioral (e.g., poor diet and physical inactivity) mechanisms (10,11), which may affect kidney outcomes (12,13). However, the effect of these psychosocial factors on kidney health remains less clear.
Using data from the Jackson Heart Study (JHS), an all-black cohort that allows for longitudinal examination of the effects of exposures on human health, we examined the association between several psychosocial factors and CKD outcomes among high-risk black Americans.
Materials and Methods
Study Population
The JHS is a prospective cohort comprising 5301 black Americans ages 21–94 years old at enrollment from the tricounty (Hinds, Madison, and Rankin) area of the Jackson, Mississippi metropolitan area. As previously detailed (14–16), the JHS participants, including prior participants from the Atherosclerosis Risk in Communities Study and their family members, were enrolled from 2000 to 2004 using probability-based sampling (from the Jackson driver’s license registry) and random sampling (from a commercially available list) (15) approaches. Supplementary recruitment approaches included targeted advertisements (radio and newspapers) and first degree relatives of the JHS participants. After providing written informed consent, participants were examined at baseline (examination 1: 2000–2004) and two subsequent follow-up visits (examination 2: 2005–2008 and examination 3: 2009–2013). Institutional review boards at the University of Mississippi Medical Center, Jackson State University, and Tougaloo College approved the JHS protocol.
For this analyses, we included the JHS participants who had complete data for the primary exposures of interest and whose CKD status could be determined. Participants who reported being on dialysis at baseline or follow-up were excluded.
Psychosocial Measurements
We included 12 psychosocial variables assessed at baseline as primary exposure variables (16): daily discrimination, lifetime discrimination, burden of lifetime discrimination, global stress, John Henryism, spirituality, hostility, anger in, anger out, pessimism, social support, and perceived social status.
Perceived discrimination was assessed using instruments validated specifically for use within the JHS cohort (17). To assess daily discrimination, participants rated the frequency of occurrence of nine discriminatory experiences, such as being treated with less courtesy and receiving poor service at a restaurant, on a scale ranging from none (=0) to several (=7) times per day in response to the root question: “How often on a day-to-day basis do you have the following experiences?” The mean score across these nine items composed the daily discrimination score. To assess lifetime discrimination, a nine-item questionnaire was used to capture unfair treatment in social domains, such as school, health care, job market, and housing. Participants self-reported unfair treatments in each domain over their lifetime on a yes =1 or no =0 scale. We summed counts from these domains to generate the lifetime discrimination score ranging from zero to nine. To assess burden of lifetime discrimination, participants who reported at least one instance of lifetime discrimination were asked to quantify the burden by rating (1) how stressful each unfair treatment was on a scale from not stressful =1 to very stressful =3, (2) its interference with living a full and productive life rated from not at all =1 to a lot =4, and (3) whether it increased difficulty in life rated from not at all =1 to a lot =4. We summed the responses to generate the burden of lifetime discrimination score (18).
The Global Perceived Stress Scale, adapted from the Survey of Recent Life Experiences Scale, the Perceived Stress Scale, and the Life Events Scale and validated for use in the JHS, was used to evaluate stress (16). The eight-item questionnaire captures eight potential stress-triggering contexts: employment, relationships, neighborhood, caring for others, legal problems, medical problems, racism and discrimination, and meeting basic needs. Participants rated the severity of stress experienced for each item during the 12-month period preceding the baseline examination on a scale ranging from not stressful =0 to very stressful =3. We derived the Global Perceived Stress score, ranging from 0 to 24, by summing responses to the eight items on the questionnaire.
John Henryism was measured using the 12-item John Henryism Scale for Active Coping, which seeks to evaluate the degree to which an individual engages in effortful active coping (19). Participants were asked to rate their agreement with statements, including “I’ve always felt that I could make of my life pretty much what I wanted to make of it”; “once I make up my mind to do something, I stay with it until the job is completely done”; and “hard work has really helped me get ahead in life.” Participants responded to each statement using four responses: completely true =4, somewhat true =3, somewhat false =2, or completely false =1. Summed responses across these 12 items generated a composite John Henryism score ranging from 12 to 60.
A six-item version of the Daily Spiritual Experience Scale assessed participants’ spirituality. Questionnaire items captured daily spiritual experiences in six domains, including feeling God’s presence, feeling God’s love, and being spiritually touched by creation. Participants rated the frequency of each experience on a scale from never =1 to many times =5 per day. We summed responses across these six domains to generate a composite spirituality score ranging from 5 to 30.
Hostility was assessed using the sum of 27 true/false items derived from the Cook–Medley Hostility Scale (20), which comprised three unequally weighted subscales: cynicism, hostile affect, and aggressive response (15). A positive response to each item on this subscale received a score of one and zero otherwise. We summed scores to generate a summary score, which ranged from 0 to 27.
The Spielberger Trait Anger Scale assessed anger. The 16-item Spielberger scale consists of two separate eight-item subscales for anger in and anger out. Participants rated each item on the questionnaire on a Likert-type scale ranging from almost never =0 to almost always =5 on the basis of how often they expressed that anger item using the response: “I keep things in” (anger in) or “I express my anger” (anger out). Items on the scale included anger reactions, such as “I do things like slam doors” or “I am secretly quite critical of others.” We summed responses across items in each subscale to generate the two anger scores, which ranged from 0 to 22 for anger in and from 0 to 23 for anger out.
The three-item revised Life Orientation Test assessed pessimism via the following statements: “If something can go wrong for me, it will”; “I hardly ever expect things to go my way”; and “I rarely count on good things happening to me.” Participants rated each item on a four-point scale ranging from strongly disagree =0 to strongly agree =4. We summed scores from the three items to yield a summary score ranging from 0 to 12.
Social support was measured using three Lubben-type questions derived from the Interpersonal Support Evaluation List and the Lubben Scale (21), which captured functional and structural components of social support, respectively. We summed scores from Likert-type responses to these questions to get a composite social support score ranging from 0 to 12. We measured subjective socioeconomic status by using one question which asked participants to rate their standing in the community on a reversed scale ranging from high standing =1 to low standing =10.
Demographics and Comorbidities
Participants self-reported demographic characteristics: education, household income, employment, and smoking status. We included data for the following established risk factors for CKD in our analyses: diabetes, hypertension, cardiovascular disease (CVD) history, and body mass index, all of which were measured at baseline. We defined diabetes as fasting glucose ≥126 mg/dl, hemoglobin A1c ≥6.5%, or self-reported use of diabetic medication within 2 weeks before clinic examination. Hypertension was defined as BP≥140/90 mm Hg or self-reported use of BP-lowering medications. CVD history encompassed self-reported history of heart attack, myocardial infarction, stroke, angioplasty in neck arteries, or history of myocardial infarction on the basis of electrocardiographic findings (22).
CKD Outcome Measurements
The primary outcomes were prevalent CKD at baseline, eGFR decline, and incident CKD. We defined prevalent CKD as either eGFR<60 ml/min per 1.73 m2 assessed using the Chronic Kidney Disease Epidemiology Collaboration equation (23) or albuminuria defined as spot urine albumin-to-creatinine ratio (ACR) ≥30 mg/g. If spot ACR was missing, a 24-hour urine was used to determine ACR. eGFR decline was determined as the annual rate of kidney function decline between examinations 1 and 3 using the equation 365.25× (eGFR at examination 1 − eGFR at examination 3)/(number of days between examinations 1 and 3). Incident CKD was defined as new eGFR <60 along with a 25% decline in eGFR at examination 3 compared with examination 1 or new-onset albuminuria at examination 3 among those without CKD at examination 1.
We used isotope dilution mass spectrometry (IDMS)–calibrated serum creatinine for all GFR estimations. The JHS technique for reconciling serum creatinine measurements between the baseline and follow-up assessments has been previously reported (24,25). Briefly, at examination 1, serum creatinine was measured using a multipoint enzymatic spectrophotometric assay, but at examination 3, it was measured using IDMS. To calibrate examination 1 serum creatinine to that of examination 3, it was remeasured in 2006 in a random sample of 206 JHS participants using the enzymatic method on a Roche Modular P Chemistry Analyzer and traced to IDMS for all JHS participants.
Statistical Analyses
Baseline continuous covariates are described using mean±SD or median (interquartile range), and categorical covariates are summarized as frequency and proportion. Primary exposures were compared across baseline CKD status using Mann–Whitney nonparametric tests for continuous variables and chi-squared tests for categorical variables. Separately for each outcome, the 12 psychosocial variables were reduced into fewer uncorrelated psychosocial constructs using principal component analysis (PCA)—a data reduction technique that converts correlated variables into linearly uncorrelated components (which often represent underlying constructs) by identifying and expressing patterns in data. We retained components (henceforth referred to as factors) with eigenvalues greater than or equal to one, which represent factors accounting for a greater variance compared with that of the original variable. To facilitate interpretation of the retained factors, we used varimax rotation techniques, which redistribute the total variance among the retained factors, and we considered psychosocial variables having a factor load ≥0.4 to compose a given factor. Generalized linear (eGFR decline) or logistic (prevalent and incident CKD outcomes) regression models were then used to examine associations of the retained factors with the respective CKD outcome. We assessed model quality for fit, predictive accuracy, and model assumptions. Multivariable regression models were sequentially adjusted for demographics (age and sex); established CKD risk factors (diabetes, hypertension, and CVD); and sociobehavioral factors: family income (≤1.5 times the poverty level versus >1.5 times the poverty level versus those who did not know their income or declined to answer), employment status (working for pay versus not working for pay), educational attainment (less than or equal to high school diploma versus greater than high school diploma), smoking status (never versus current/former smoker), and body mass index. The eGFR decline model was also adjusted for baseline eGFR and ACR.
Because current conceptual models suggest that age and sex might have a role in moderating the relationship between psychosocial factors and CKD outcomes (12), we conducted two sensitivity analyses to determine the effects of age (dichotomized at the cohort median) and sex on the relation between psychosocial factors and CKD outcomes. An interaction term between the potential modifier (age or sex) and each factor was added to the corresponding regression model. All P values are two sided at a 0.05 significance level. SAS 9.4 (SAS Institute, Cary, NC) and R 3.1.3 (R Core Team, Vienna, Austria) were used for all analyses.
Results
Participant Characteristics
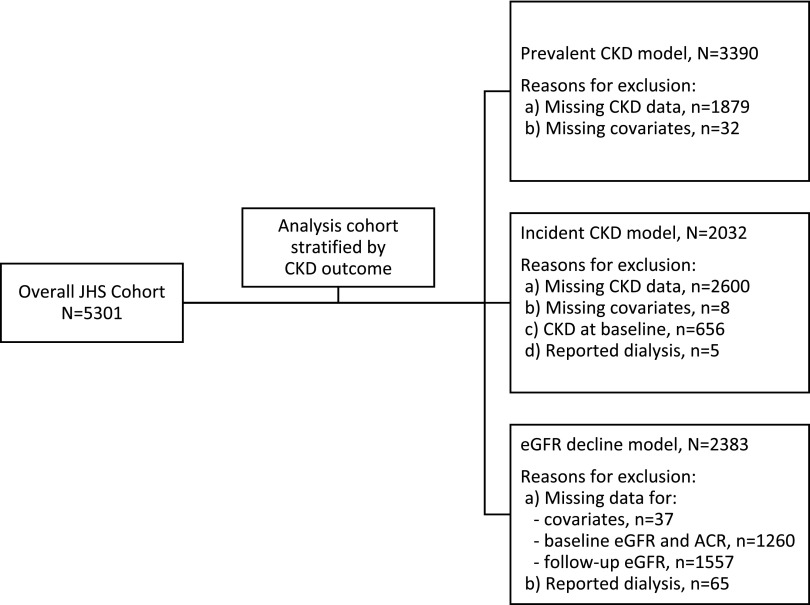
Of the 5301 JHS participants, 3390 (64%) met inclusion criteria for this analysis (Figure 1). Participants in the analysis cohort were similar to those who did not meet inclusion criteria across the majority of psychosocial measures and comorbidities. Nonetheless, those included in this analysis were slightly younger (mean age =55 [SD=13] versus 56 [SD=12] years old), more likely to have greater than high school (versus less than or equal to high school) diploma (64% versus 57%), and more likely to be classified as middle to high income (69% versus 61%) (Supplemental Table 1). Participants in the analysis cohort were predominantly women (63%), and the majority had hypertension (61%); 22% had diabetes, and 11% reported a history of CVD. Mean (SD) eGFR was 94 (24) ml/min per 1.73 m2 (Table 1).
Figure 1.
Flow diagram describing derivation of analysis cohort from the original Jackson Heart Study (JHS) cohort. ACR, albumin-to-creatinine ratio.
Table 1.
Baseline characteristics of participants stratified by CKD status at baseline
| Baseline Covariates | All, n=3390 | Baseline CKD Status | |
|---|---|---|---|
| No CKD, n=2734 | CKD, n=656 | ||
| Demographics | |||
| Age, yr, mean±SD | 55±13 | 53±13 | 62±13 |
| Men, n (%) | 1266 (37) | 1040 (38) | 226 (34) |
| Education: less than or equal to high school diploma, n (%) | 1221 (36) | 878 (32) | 343 (52) |
| Lower middle income, n (%) | 1051 (31) | 778 (28) | 273 (42) |
| Not employed, n (%) | 2118 (62) | 1853 (68) | 265 (40) |
| Comorbidities | |||
| BMI, kg/m2, mean±SD | 32±7 | 31±7 | 33±8 |
| Former/current smoker, n (%) | 1015 (30) | 798 (29) | 217 (33) |
| Hypertension, n (%) | 2051 (61) | 1496 (55) | 555 (85) |
| Diabetes, n (%) | 727 (21) | 442 (16) | 285 (43) |
| History of CVD, n (%) | 366 (11) | 215 (8) | 151 (23) |
| CKD awareness,a n (%) | 182 (5) | 86 (3) | 96 (15) |
| eGFR, mean±SD | 93±24 | 99±18 | 71±31 |
| ACR, median (IQR) | 6 (4–12) | 5 (4–9) | 63 (33–194) |
| Psychosocial measures [range],b mean±SD | |||
| Perceived discrimination | |||
| Daily [0–54] | 9.29±8.96 | 9.69±9.03 | 7.61±8.47 |
| Lifetime [0–9] | 2.95±2.16 | 3.06±2.18 | 2.5±2.01 |
| Burden of lifetime [3–10] | 3.68±2.42 | 3.73±2.39 | 3.46±2.54 |
| Anger | |||
| In [0–22] | 5.32±3.48 | 5.32±3.51 | 5.31±3.3 |
| Out [0–23] | 4.41±3.11 | 4.4±3.09 | 4.45±3.18 |
| Hostility [0–27] | 11.59±4.85 | 11.49±4.77 | 12.11±5.19 |
| John Henryism [12–60] | 29.77±4.44 | 29.72±4.36 | 30.02±4.82 |
| Stress [0–24] | 5.0±4.31 | 5.2±4.36 | 4.16±4 |
| Pessimism [0–12] | 2.02±2.14 | 1.97±2.12 | 2.27±2.23 |
| Low spirituality [5–30] | 6.71±4.66 | 6.74±4.7 | 6.62±4.43 |
| Social status [1–10] | 3.37±2.03 | 3.42±2.03 | 3.2±2.06 |
| Perceived social support [0–12] | 6.04±2.69 | 6.1±2.67 | 5.8±2.73 |
BMI, body mass index; CVD, cardiovascular disease; ACR, albumin-to-creatinine ratio; IQR, interquartile range.
Self-reported history of kidney disease.
Range of scores from scales used to assess psychosocial measures. With the exception of social status (which we scored on a reverse scale), higher scores indicate greater rating of each measure.
Psychosocial Variables at Baseline
Distributions of scores for the psychosocial variables are presented in Table 1. The mean (SD) score for the daily discrimination variable was 9.29 (8.96). The mean (SD) score for perceived stress was 5.0 (4.31). The mean (SD) score for hostility was 11.59 (4.85). The mean (SD) score for John Henryism was 29.77 (4.44). The mean (SD) score for perceived social support was 6.04 (2.69), and the mean (SD) score for pessimism was 2.02 (2.14). Participants with CKD (versus no CKD) at baseline had lower perceived daily, lifetime, and burden of discrimination; lower perceived stress; higher hostility; higher John Henryism; and higher pessimism (all P<0.05) (Table 1).
Psychosocial Factors and CKD Outcomes
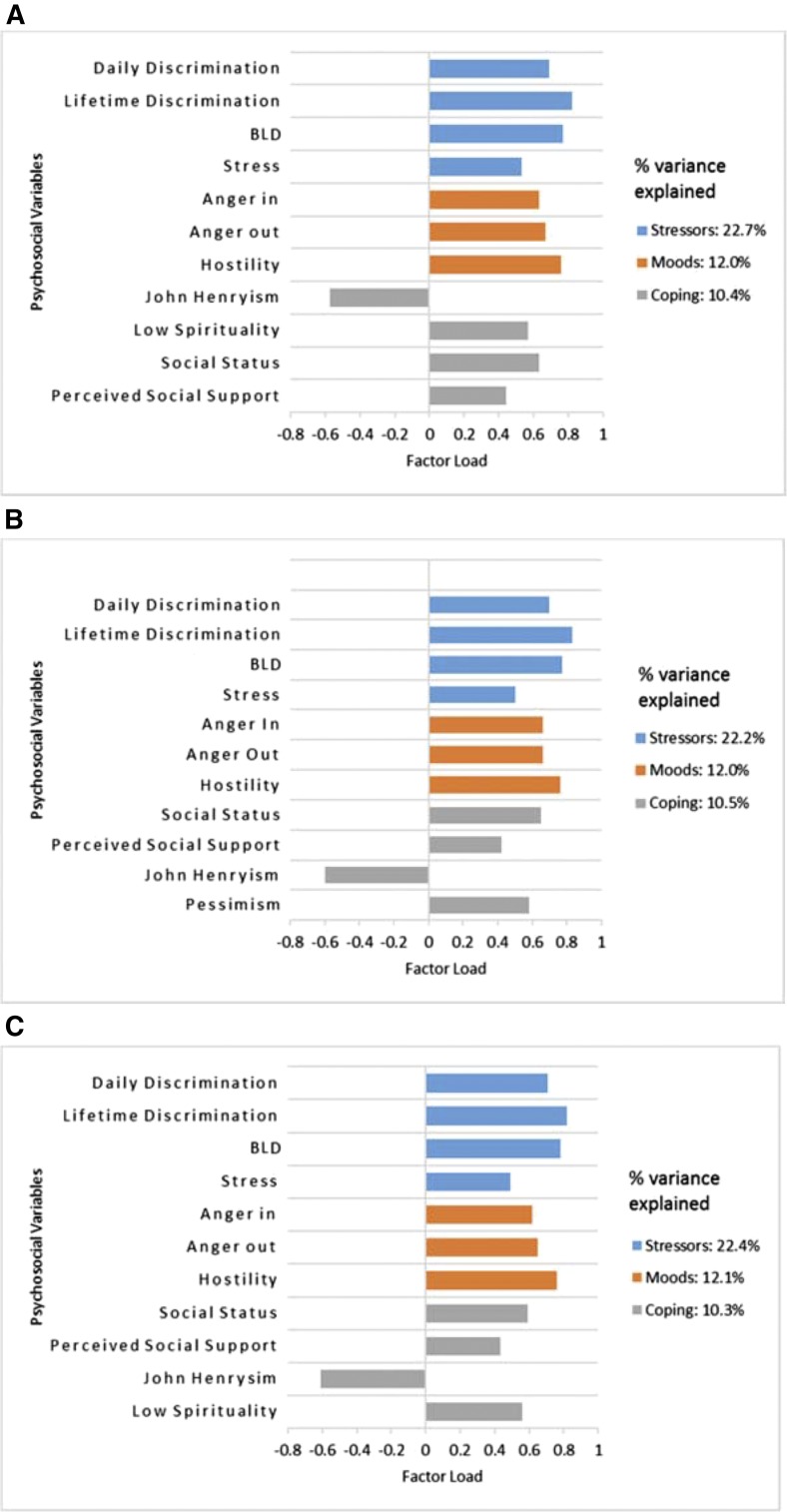
PCA identified three psychosocial factors: life stressors, moods, and coping. Across all CKD outcomes, life stressors encompassed discrimination (daily, lifetime, and burden of lifetime) and stress variables; moods included hostility and anger, and coping included John Henryism, social status, and perceived social support. Coping additionally included low spirituality for the prevalent and incident CKD outcomes and pessimism for eGFR decline. With the exception of John Henryism, all psychosocial variables loaded positively on psychosocial factors (Figure 2).
Figure 2.
With the exception of John Henryism, all psychosocial variables loaded positively on the PCA-identified psychosocial factors in relation to the CKD outcomes. [A] prevalent CKD at baseline, [B] decline in eGFR, and [C] incident CKD after median follow-up of 8 years; derived from separate principal component analyses (PCA). Factor load values signify the direction of the correlation between a variable and the construct to which it loads: positive values signify a positive correlation, and negative values indicate a negative correlation. BLD, Burden of Lifetime Discrimination.
At baseline, life stressors were negatively associated with prevalent CKD (odds ratio [OR], 0.76; 95% confidence interval [95% CI], 0.65 to 0.89) in models adjusted for demographic characteristics, established CKD risk factors, and other psychosocial factors. Other psychosocial factors were not significantly associated with prevalent CKD at baseline (Table 2).
Table 2.
Associations between psychosocial factors and CKD outcomes in the Jackson Heart Study
| Variable | Prevalent CKD, No. of Events/N or OR (95% CI)a | Incident CKD, No. of Events/N or OR (95% CI)a | eGFR Decline, N or β (95% CI)b |
|---|---|---|---|
| CKD outcome definition | |||
| Total | 656/3390 | 250/2032 | 2382 |
| eGFR<60 ml/min per 1.73 m2 | 241/656 | 92/250 | NA |
| ACR≥30 mg/g | 334/656 | 135/250 | NA |
| Both eGFR and ACR | 81/656 | 23/250 | NA |
| Psychosocial factorsc | |||
| Model 1 | |||
| Stressors | 0.80 (0.69 to 0.93)d | 1.09 (0.91 to 1.30) | 0.03 (−0.07 to 0.13) |
| Moods | 1.16 (1.01 to 1.33)d | 1.06 (0.88 to 1.28) | 0.06 (−0.04 to 0.16) |
| Coping | 1.09 (0.94 to 1.27) | 0.92 (0.76 to 1.11) | −0.02 (−0.12 to 0.08) |
| Model 2 | |||
| Stressors | 0.77 (0.66 to 0.90)d | 1.08 (0.89 to 1.30) | 0.06 (−0.03 to 0.16) |
| Moods | 1.14 (0.99 to 1.31)e | 1.05 (0.87 to 1.27) | 0.02 (−0.07 to 0.12) |
| Coping | 1.09 (0.94 to 1.27) | 0.91 (0.75 to 1.10) | <−0.01 (−0.10 to 0.09) |
| Model 3 | |||
| Stressors | 0.76 (0.65 to 0.89)d | 1.07 (0.88 to 1.29) | 0.08 (−0.02 to 0.17) |
| Moods | 1.12 (0.96 to 1.29) | 1.02 (0.84 to 1.24) | 0.03 (−0.06 to 0.13) |
| Coping | 1.09 (0.93 to 1.27) | 0.91 (0.75 to 1.11) | −0.02 (−0.12 to 0.08) |
Model 1 was adjusted for age and sex. Model 2 was model 1 plus established CKD risk factors (diabetes, hypertension, and cardiovascular disease). Model 3 was model 2 plus family income, employment status, educational attainment, smoking status, and body mass index. The eGFR decline model (linear regression) was additionally adjusted for baseline eGFR and ACR. OR, odds ratio; 95% CI, 95% confidence interval; NA, not applicable; ACR, albumin-to-creatinine ratio.
Estimates are from logistic regression (binary outcome).
Estimates are from linear regression (continuous outcome variable; milliliters per minute per 1.73 m2 per year).
Psychosocial factors are underlying constructs derived using principal component analysis separately for each outcome. Each factor features the following psychosocial variables that loaded heavily on it: (1) stressors: discrimination (daily, lifetime, and burden of lifetime) and stress across all CKD outcomes; (2) moods: hostility and anger across all CKD outcomes, and (3) coping: John Henryism, social status, and perceived social support across all CKD outcomes; it additionally included low spirituality for the prevalent and incident CKD outcomes and pessimism for eGFR decline outcome.
P<0.05.
P<0.10.
Median follow-up for the cohort was 8 years (interquartile range, 7–8). Of the 2382 participants included in the analysis for eGFR decline, median eGFR decline was 1.06 ml/min per 1.73 m2 per year (interquartile range, 0.07–2.19 ml/min per 1.73 m2). There were 250 participants who developed incident CKD during follow-up (12% of the 2032 participants whose CKD status could be determined at examination 3). Psychosocial factors were not associated with incident CKD in fully adjusted models: life stressors (OR, 1.07; 95% CI, 0.88 to 1.29), moods (OR, 1.02; 95% CI, 0.84 to 1.24), or coping (OR, 0.91; 95% CI, 0.75 to 1.11). Additionally, psychosocial factors were not associated with eGFR decline (Table 2).
Sensitivity Analyses
Results of age- and sex-stratified sensitivity analyses are displayed in Tables 3 and 4, respectively. The association between life stressors and prevalent CKD at baseline differed by sex; however, the interaction effect was not statistically significant (P value for interaction by sex =0.09). In fully adjusted models, life stressors were negatively associated with prevalent CKD at baseline among women only (OR, 0.78; 95% CI, 0.62 to 0.98). There was no evidence of modification by age or sex of the associations between psychosocial factors and longitudinal CKD outcomes: P>0.05.
Table 3.
Associations between psychosocial factors and CKD outcomes in the Jackson Heart Study stratified at median age (55 years old)
| Psychosocial Factorsa Stratified by Age | Prevalent CKD | Incident CKD | eGFR Decline | |||
|---|---|---|---|---|---|---|
| OR (95% CI)b | P Value for Interaction | OR (95% CI)b | P Value for Interaction | β (95% CI)c | P Value for Interaction | |
| Stressors | 0.70 | 0.85 | 0.79 | |||
| Age <55 yr | 0.78 (0.62 to 0.98) | 1.07 (0.77 to 1.49) | 0.08 (−0.05 to 0.20) | |||
| Age ≥55 yr | 0.74 (0.59 to 0.91) | 1.03 (0.82 to 1.30) | 0.10 (−0.04 to 0.25) | |||
| Moods | 0.61 | 0.27 | 0.43 | |||
| Age <55 yr | 1.05 (0.84 to 1.31) | 0.84 (0.59 to 1.19) | −0.01 (−0.14 to 0.11) | |||
| Age ≥55 yr | 1.13 (0.93 to 1.36) | 1.06 (0.84 to 1.33) | 0.06 (−0.08 to 0.21) | |||
| Coping | 0.28 | 0.47 | 0.36 | |||
| Age <55 yr | 1.15 (0.92 to 1.44) | 0.81 (0.57 to 1.16) | −0.08 (−0.20 to 0.05) | |||
| Age ≥55 yr | 0.97 (0.78 to 1.20) | 0.95 (0.75 to 1.20) | 0.02 (−0.14 to 0.17) | |||
OR, odds ratio; 95% CI, 95% confidence interval.
Psychosocial factors are underlying constructs derived using principal component analysis separately for each outcome. Each factor features the following psychosocial variables that loaded heavily on it: (1) stressors: discrimination (daily, lifetime, and burden of lifetime) and stress across all CKD outcomes; (2) moods: hostility and anger across all CKD outcomes; and (3) coping: John Henryism, social status, and perceived social support across all CKD outcomes; it additionally included low spirituality for the prevalent and incident CKD outcomes and pessimism for eGFR decline outcome.
Estimates are from logistic regression (binary outcome).
Estimates are from linear regression (continuous outcome variable; milliliters per minute per 1.73 m2 per year); all models were adjusted for age, sex, body mass index, family income, employment status, educational attainment, smoking status, diabetes, hypertension, and self-reported history of cardiovascular disease; eGFR decline model (linear regression) was additionally adjusted for baseline eGFR and albumin-to-creatinine ratio.
Table 4.
Associations between psychosocial factors and CKD outcomes in the Jackson Heart Study stratified by sex
| Psychosocial Factorsa Stratified by Sex | Prevalent CKD | Incident CKD | eGFR Decline | |||
|---|---|---|---|---|---|---|
| OR (95% CI)b | P Value for Interaction | OR (95% CI)b | P Value for Interaction | β (95% CI)c | P Value for Interaction | |
| Stressors | 0.09 | 0.77 | 0.21 | |||
| Men | 0.90 (0.68 to 1.18) | 0.91 (0.64 to 1.30) | −0.01 (−0.17 to 0.14) | |||
| Women | 0.69 (0.57 to 0.84) | 1.09 (0.86 to 1.38) | 0.12 (−0.01 to 0.24) | |||
| Moods | 0.39 | 0.07 | 0.45 | |||
| Men | 1.22 (0.93 to 1.60) | 0.72 (0.48 to 1.08) | <−0.01 (−0.16 to 0.15) | |||
| Women | 1.07 (0.89 to 1.28) | 1.16 (0.91 to 1.48) | 0.03 (−0.09 to 0.16) | |||
| Coping | 0.55 | 0.93 | 0.68 | |||
| Men | 1.03 (0.78 to 1.36) | 0.87 (0.61 to 1.26) | −0.04 (−0.19 to 0.12) | |||
| Women | 1.10 (0.91 to 1.33) | 0.91 (0.72 to 1.17) | <0.01 (−0.12 to 0.13) | |||
OR, odds ratio; 95% CI, 95% confidence interval.
Psychosocial factors are underlying constructs derived using principal component analysis separately for each outcome. Each factor features the following psychosocial variables that loaded heavily on it: (1) stressors: discrimination (daily, lifetime, and burden of lifetime) and stress across all CKD outcomes; (2) moods: hostility and anger across all CKD outcomes; and (3) coping: John Henryism, social status, and perceived social support across all CKD outcomes; it additionally included low spirituality for the prevalent and incident CKD outcomes and pessimism for eGFR decline outcome.
Estimates are from logistic regression (binary outcome).
Linear regression (continuous outcome variable; milliliters per minute per 1.73 m2 per year). All models were adjusted for age, sex, body mass index, family income, employment status, educational attainment, smoking status, diabetes, hypertension, and self-reported history of cardiovascular disease; the eGFR decline model (linear regression) was additionally adjusted for baseline eGFR and albumin-to-creatinine ratio.
Results from fitting regression models for the associations between psychosocial variables and CKD outcomes before performing PCA were generally consistent with the findings from PCAs (Supplemental Table 2). Pairwise correlations between psychosocial variables are presented in Supplemental Table 3. Replacing hypertension with systolic BP in the models also yielded similar results (Supplemental Table 4).
Discussion
In this large prospective study of nearly 3400 black Americans, life stressors were negatively associated with prevalent CKD at baseline after adjusting for demographics, established CKD risk factors, and other psychosocial factors (moods and coping). This relationship was only evident among participants who were women. After a median follow-up of 8 years, psychosocial factors identified via PCA (stressors, moods, or coping) were not significantly associated with eGFR decline or incident CKD, and these findings were consistent across sex and age categories in the JHS cohort. Thus, we found limited evidence to support an independent association of psychosocial factors with kidney health among black Americans.
To our knowledge, this study is the first to examine the relation between nondepressive psychosocial factors and CKD outcomes in black Americans. Prior studies have predominantly focused on and have shown a positive association between depression and kidney disease outcomes. However, few studies have evaluated the potential effect of other less commonly characterized psychosocial determinants on kidney health (26–30). The unique availability of a comprehensive array of psychosocial variables within the JHS cohort and the use of a principal component analytic approach permitted the characterization of a psychosocial phenotype. These phenotypic constructs were largely consistent across all CKD outcomes that we examined, suggesting that these multidimensional constructs accurately captured inter-relations among psychosocial variables as they pertain to their influence on kidney outcomes—which may be important in understanding the relation between exposure to psychosocial stressors and kidney outcomes (12,13). By contributing the first empirical evidence, our findings highlight the complexity of the relationship between negative psychosocial exposure and health outcomes among black Americans.
Although our findings do not universally substantiate a role of psychosocial factors as CKD risk factors among black Americans, they suggest a possible role among black women. It has been suggested that black women experience earlier deterioration in health than black men, because in addition to racism, they also experience sexism (31,32). Despite its disproportionate adverse health effects, this double jeopardy is thought to improve resilience in black women—termed the weathering hypothesis. In light of this hypothesis, the negative association between life stressors and prevalent CKD among women only may reflect the unique ability of black women to manage psychosocial stressors more effectively than black men. Further studies should, therefore, delineate specific behavioral responses (33) that might offset the health consequences of negative social influences in black environments.
It may also be that self-reported measurements of psychosocial factors do not accurately capture the adverse effects of psychosocial exposures on health outcomes and that self-report is a suboptimal exposure measurement strategy compared with more downstream measurements, such as allostatic load (34,35). For instance, it has been proposed that allostatic load—a composite biologic measure of the degree of dysregulation in the mediators of homeostasis in the body—might mediate the relation between social determinants and adverse health outcomes, such as CKD (36,37). With this plausible causal chain between exposure to psychosocial stressors and CKD outcomes, the use of psychosocial factors to measure exposure in this observational study may have masked exposure-outcome associations, because these psychosocial factors are farther upstream in the causal pathway. Thus, future investigation should aim at explaining the role of allostatic load in the relation between psychosocial factors and kidney outcomes.
Our study has noteworthy limitations. The instruments used for measuring the psychosocial variables in the JHS have not been crossvalidated in other regions in the United States, which limits the generalizability of our findings to the broader black population. In addition, although designed to measure relatively stable individual psychosocial attributes, it remains unknown whether these instruments are stable over time among the JHS participants. Therefore, our use of a time-invariant measure (at baseline) to characterize a potentially time-variant exposure in a long-term exposure-outcome relationship may have biased our findings toward the null. Future work should explore the longitudinal variability of these psychosocial variables. Furthermore, although participants who had complete data for our analysis were generally similar to those with missing data on the key psychosocial and CKD outcome variables, selection bias remains a concern. Moreover, because we excluded the JHS participants from our cohort who self-reported dialysis, our incident CKD outcome may have overlooked individuals who initiated dialysis during follow-up but lacked CKD at baseline. Last, given the observational design of the study, residual confounding, including depression and quality of life (38), might have additionally contributed to the null associations that we observed. Despite these limitations, the JHS has important strengths that are particularly relevant to examining the associations between psychosocial factors and adverse health outcomes. The JHS is the largest study of CVD in black Americans, and it examined a multitude of psychosocial variables representing a broad dimension of psychosocial domains known to affect human health—the majority of which were included in these analyses. Additionally, the scales used to measure these psychosocial variables have been validated for use within the JHS cohort. Finally, the prospective design of the JHS, with a median follow-up of 8 years, allowed for the assessment of longitudinally derived CKD outcomes.
In conclusion, life stressors were negatively associated with prevalent CKD at baseline in the JHS. No other association between psychosocial factors and CKD outcomes was evident after a median follow-up of 8 years. To understand the underlying factors driving the residual unexplained disparities in CKD outcomes among black Americans, future studies should delineate the potential mediating role of biologic markers in the relation between psychosocial factors and kidney health.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors thank the staffs and participants of the Jackson Heart Study (JHS).
The JHS is supported and conducted in collaboration with Jackson State University contracts HHSN268201300049C and HHSN268201300050C, Tougaloo College contract HHSN268201300048C, and University of Mississippi Medical Center contracts HHSN268201300046C and HHSN268201300047C from the National Heart, Lung, and Blood Institute (NHLBI) and the National Institute for Minority Health and Health Disparities.
The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the NHLBI, the National Institutes of Health, or the US Department of Health and Human Services.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.06430617/-/DCSupplemental.
References
- 1.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Hsu CY, Lin F, Vittinghoff E, Shlipak MG: Racial differences in the progression from chronic renal insufficiency to end-stage renal disease in the United States. J Am Soc Nephrol 14: 2902–2907, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Crews DC, Pfaff T, Powe NR: Socioeconomic factors and racial disparities in kidney disease outcomes. Semin Nephrol 33: 468–475, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Parsa A, Kao WH, Xie D, Astor BC, Li M, Hsu CY, Feldman HI, Parekh RS, Kusek JW, Greene TH, Fink JC, Anderson AH, Choi MJ, Wright JT Jr, Lash JP, Freedman BI, Ojo A, Winkler CA, Raj DS, Kopp JB, He J, Jensvold NG, Tao K, Lipkowitz MS, Appel LJ; AASK Study Investigators; CRIC Study Investigators : APOL1 risk variants, race, and progression of chronic kidney disease. N Engl J Med 369: 2183–2196, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bodonyi-Kovacs G, Ma JZ, Chang J, Lipkowitz MS, Kopp JB, Winkler CA, Le TH: Combined effects of GSTM1 null allele and APOL1 renal risk alleles in CKD progression in the African American study of kidney disease and hypertension trial. J Am Soc Nephrol 27: 3140–3152, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murphy D, McCulloch CE, Lin F, Banerjee T, Bragg-Gresham JL, Eberhardt MS, Morgenstern H, Pavkov ME, Saran R, Powe NR, Hsu CY; Centers for Disease Control and Prevention Chronic Kidney Disease Surveillance Team : Trends in prevalence of chronic kidney disease in the United States. Ann Intern Med 165: 473–481, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Norton JM, Moxey-Mims MM, Eggers PW, Narva AS, Star RA, Kimmel PL, Rodgers GP: Social determinants of racial disparities in CKD. J Am Soc Nephrol 27: 2576–2595, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beydoun MA, Poggi-Burke A, Zonderman AB, Rostant OS, Evans MK, Crews DC: Perceived discrimination and longitudinal change in kidney function among urban adults. Psychosom Med 79: 824–834, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walker RJ, Gebregziabher M, Martin-Harris B, Egede LE: Relationship between social determinants of health and processes and outcomes in adults with type 2 diabetes: Validation of a conceptual framework. BMC Endocr Disord 14: 82, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gebreab SY, Diez-Roux AV, Hickson DA, Boykin S, Sims M, Sarpong DF, Taylor HA, Wyatt SB: The contribution of stress to the social patterning of clinical and subclinical CVD risk factors in African Americans: The Jackson Heart Study. Soc Sci Med 75: 1697–1707, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruce MA, Griffith DM, Thorpe RJ Jr: Stress and the kidney. Adv Chronic Kidney Dis 22: 46–53, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruce MA, Beech BM, Sims M, Brown TN, Wyatt SB, Taylor HA, Williams DR, Crook E: Social environmental stressors, psychological factors, and kidney disease. J Investig Med 57: 583–589, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor HA, Jr: The Jackson Heart Study of the future. Ethn Dis 22[3 Suppl 1]: S1-49–S1-54, 2012 [PubMed] [Google Scholar]
- 15.Fuqua SR, Wyatt SB, Andrew ME, Sarpong DF, Henderson FR, Cunningham MF, Taylor HA Jr: Recruiting African-American research participation in the Jackson Heart Study: Methods, response rates, and sample description. Ethn Dis 15[4 Suppl 6]: S6-18–S6-29, 2005 [PubMed] [Google Scholar]
- 16.Payne TJ, Wyatt SB, Mosley TH, Dubbert PM, Guiterrez-Mohammed ML, Calvin RL, Taylor HA Jr, Williams DR: Sociocultural methods in the Jackson Heart Study: Conceptual and descriptive overview. Ethn Dis 15[4 Suppl 6]: S6-38–S6-48, 2005 [PubMed] [Google Scholar]
- 17.Sims M, Wyatt SB, Gutierrez ML, Taylor HA, Williams DR: Development and psychometric testing of a multidimensional instrument of perceived discrimination among African Americans in the Jackson Heart Study. Ethn Dis 19: 56–64, 2009 [PMC free article] [PubMed] [Google Scholar]
- 18.Sims M, Diez-Roux AV, Dudley A, Gebreab S, Wyatt SB, Bruce MA, James SA, Robinson JC, Williams DR, Taylor HA: Perceived discrimination and hypertension among African Americans in the Jackson Heart Study. Am J Public Health 102[Suppl 2]: S258–S265, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.James SA: The John Henryism scale for active coping. In: Handbook of Tests and Measurements for Black Populations, Vol. 2, edited by Jones RL, Hampton, VA, Cobb & Henry Publishers, 1996, pp 415–425 [Google Scholar]
- 20.Barefoot JC, Dodge KA, Peterson BL, Dahlstrom WG, Williams RB Jr: The Cook-Medley hostility scale: Item content and ability to predict survival. Psychosom Med 51: 46–57, 1989 [DOI] [PubMed] [Google Scholar]
- 21.Lubben JE: Assessing social networks among elderly populations. Fam Community Health (Bristol) 11: 42–52, 1988 [Google Scholar]
- 22.Keku E, Rosamond W, Taylor HA Jr, Garrison R, Wyatt SB, Richard M, Jenkins B, Reeves L, Sarpong D: Cardiovascular disease event classification in the Jackson Heart Study: Methods and procedures. Ethn Dis 15[4 Suppl 6]: S6-62–S6-70, 2005 [PubMed] [Google Scholar]
- 23.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang W, Young BA, Fülöp T, de Boer IH, Boulware LE, Katz R, Correa A, Griswold ME: Effects of serum creatinine calibration on estimated renal function in African Americans: The Jackson Heart Study. Am J Med Sci 349: 379–384, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Young BA, Katz R, Boulware LE, Kestenbaum B, de Boer IH, Wang W, Fülöp T, Bansal N, Robinson-Cohen C, Griswold M, Powe NR, Himmelfarb J, Correa A: Risk factors for rapid kidney function decline among African Americans: The Jackson Heart Study (JHS). Am J Kidney Dis 68: 229–239, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hedayati SS, Minhajuddin AT, Afshar M, Toto RD, Trivedi MH, Rush AJ: Association between major depressive episodes in patients with chronic kidney disease and initiation of dialysis, hospitalization, or death. JAMA 303: 1946–1953, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loosman WL, Rottier MA, Honig A, Siegert CE: Association of depressive and anxiety symptoms with adverse events in Dutch chronic kidney disease patients: A prospective cohort study. BMC Nephrol 16: 155, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsai YC, Chiu YW, Hung CC, Hwang SJ, Tsai JC, Wang SL, Lin MY, Chen HC: Association of symptoms of depression with progression of CKD. Am J Kidney Dis 60: 54–61, 2012 [DOI] [PubMed] [Google Scholar]
- 29.Chiang HH, Guo HR, Livneh H, Lu MC, Yen ML, Tsai TY: Increased risk of progression to dialysis or death in CKD patients with depressive symptoms: A prospective 3-year follow-up cohort study. J Psychosom Res 79: 228–232, 2015 [DOI] [PubMed] [Google Scholar]
- 30.McKercher C, Sanderson K, Jose MD: Psychosocial factors in people with chronic kidney disease prior to renal replacement therapy. Nephrology (Carlton) 18: 585–591, 2013 [DOI] [PubMed] [Google Scholar]
- 31.Geronimus AT: The weathering hypothesis and the health of African-American women and infants: Evidence and speculations. Ethn Dis 2: 207–221, 1992 [PubMed] [Google Scholar]
- 32.Geronimus AT, Hicken M, Keene D, Bound J: “Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. Am J Public Health 96: 826–833, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fernandez CA, Loucks EB, Arheart KL, Hickson DA, Kohn R, Buka SL, Gjelsvik A: Evaluating the effects of coping style on allostatic load, by sex: The Jackson Heart Study, 2000-2004. Prev Chronic Dis 12: E165, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Diez Roux AV: Conceptual approaches to the study of health disparities. Annu Rev Public Health 33: 41–58, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gibbons MC, Brock M, Alberg AJ, Glass T, LaVeist TA, Baylin S, Levine D, Fox CE: The sociobiologic integrative model (SBIM): Enhancing the integration of sociobehavioral, environmental, and biomolecular knowledge in urban health and disparities research. J Urban Health 84: 198–211, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hickson DA, Diez Roux AV, Gebreab SY, Wyatt SB, Dubbert PM, Sarpong DF, Sims M, Taylor HA: Social patterning of cumulative biological risk by education and income among African Americans. Am J Public Health 102: 1362–1369, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McEwen BS, Gianaros PJ: Central role of the brain in stress and adaptation: Links to socioeconomic status, health, and disease. Ann N Y Acad Sci 1186: 190–222, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Porter A, Fischer MJ, Brooks D, Bruce M, Charleston J, Cleveland WH, Dowie D, Faulkner M, Gassman J, Greene T, Hiremath L, Kendrick C, Kusek JW, Thornley-Brown D, Wang X, Norris K, Unruh M, Lash J: Quality of life and psychosocial factors in African Americans with hypertensive chronic kidney disease. Transl Res 159: 4–11, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.