Abstract
A reliable determination of blood pH, PCO2, and [HCO3−] is necessary for assessing the acid-base status of a patient. However, most acid-base disorders are first recognized through abnormalities in serum total CO2 concentration ([TCO2]) in venous blood, a surrogate for [HCO3−]. In screening patients on the basis of serum [TCO2], we have been concerned about the wide limits of normal for serum [TCO2], 10–13 mEq/L, reported by many clinical laboratories. Indeed, we have encountered patients with serum [TCO2] values within the lower or upper end of the normal range of the reporting laboratory, who subsequently were shown to have a cardinal acid-base disorder.
Here, we present a patient who had a serum [TCO2] within the lower end of the normal range of the clinical laboratory, which resulted in delayed diagnosis of a clinically important “hidden” acid-base disorder. To better define the appropriate limits of normal for serum [TCO2], we derived the expected normal range in peripheral venous blood in adults at sea level from carefully conducted acid-base studies. We then compared this range, 23 to 30 mEq/L, to that reported by 64 clinical laboratories, 2 large commercial clinical laboratories, and the major textbook of clinical chemistry. For the most part, the range in the laboratories we queried was substantially different than that we derived and that published in the textbook, with some laboratories reporting values as low as 18–20 mEq/L and as high as 33–35 mEq/L. We conclude that the limits of values of serum [TCO2] reported by clinical laboratories are very often inordinately wide and not consistent with the range of normal expected in healthy individuals at sea level. We suggest that the limits of normal of serum [TCO2] at sea level be tightened to 23–30 mEq/L. Such correction will ensure recognition of the majority of “hidden” acid-base disorders.
Keywords: Reference Values, Chemistry, Clinical, Carbon Dioxide, Delayed Diagnosis, dioxotechnetium, Clinical Laboratory Services, Blood Gas Analysis, Laboratories, Research, Serum Total CO2 Concentration
Introduction
The presence of an acid-base disorder can affect clinical outcome, and it can be a clue to an underlying disease (1). Therefore, it is essential to assess whether the acid-base status of a patient is normal or consistent with a particular acid-base disorder.
Although a reliable determination of blood pH, PCO2, and [HCO3−] is necessary for assessing the acid-base status of a patient, most acid-base disorders are first recognized by clinicians through abnormalities in serum total CO2 concentration ([TCO2]) in venous blood, a surrogate for [HCO3−] (2).
In screening patients on the basis of serum [TCO2], we have been concerned about the wide limits of normal for serum [TCO2] reported by clinical laboratories, often on the order of 10–13 mEq/L. Indeed, we have encountered many patients who had serum [TCO2] values within the lower or upper end of the normal range of the reporting laboratory, in whom assessment of blood gases revealed data consistent with a cardinal acid-base disorder. Recognition of these “hidden” acid-base disorders had important implications for the patients’ health.
In this feature, we present a patient who had an acid-base disorder that defied recognition by her internist for a prolonged period, because her serum [TCO2] was within the normal range (lower end) of the clinical laboratory. This patient’s case underscores the potential limitations of using the prevailing expansive range of normal for serum [TCO2] in screening for acid-base disorders. We examine the basis for the plethora of currently used normal ranges for serum [TCO2] and propose their substitution by a version with a more limited range.
Patient Presentation
A 37-year-old woman was referred for evaluation of recurrent nephrolithiasis. In the past 2 years, she had several episodes of renal colic, passed five stones spontaneously, and undergone two urologic procedures. Stone analysis had shown 90% calcium phosphate and 10% calcium oxalate monohydrate. Five years ago, she was diagnosed with Sjögren syndrome. Other than some fatigue and dryness in her eyes and mouth, she was asymptomatic. Her only medication was artificial tears.
Physical examination was unremarkable except for a dry mouth. Serum creatinine was 0.8 mg/dl, and eGFR was >60 ml/min per 1.73 m2. Serum [Na+] was 138 mEq/L, [K+] was 3.4 mEq/L, [Cl−] was 108 mEq/L, [TCO2] was 20 mEq/L (normal, 19–30 mEq/L), anion gap was 10 mEq/L, albumin was 4.5 g/dl, calcium was 9.8 mg/dl, phosphorus was 3.7 mg/dl, and uric acid was 6.8 mg/dl. Similar levels of serum [TCO2] (20, 22, and 21 mEq/L; normal, 20–31 mEq/L) and serum [K+] (3.5, 3.6, and 3.5 mEq/L) had been obtained by her physician on three previous visits.
A kidney ultrasound disclosed several nonobstructing stones bilaterally and bilateral medullary calcifications. Venous blood gases revealed pH 7.32, PvCO2 38 mm Hg, [HCO3−] 19 mEq/L, and PvO2 42 mm Hg. Urinalysis showed specific gravity 1.010, pH 6.5, 1+ protein, and 1+ leukocyte esterase. Microscopic examination of the urine sediment showed two to four wbc per hpf, no casts, and occasional crystals. A urine culture revealed no growth. Spot urine albumin-to-creatinine ratio was 120 mg/g, and protein-to-creatinine ratio was 350 mg/g. The urine anion gap was 22 mEq/L ([Na+] 55 + [K+] 32 − [Cl−] 65). A 24-hour urine collection had a volume of 2050 ml and contained calcium 230 mg (normal, <250 mg/d), oxalate 15 mg (normal, <45 mg/d), uric acid 425 mg (normal, <700 mg/d), and citrate 92 mg (normal, >320 mg/d). A diagnosis of type 1 renal tubular acidosis caused by Sjögren syndrome was made. Potassium citrate, 10 mEq three times daily, was prescribed. Over the subsequent 3 years, serum [TCO2] has been on the order of 25–28 mEq/L, and urine citrate has been >600 mg/d; no new stones have developed, and nephrocalcinosis has diminished.
Discussion
The patient presented underscores the serious limitations of the existing screening method for recognizing the presence of acid-base disorders. Three routine measurements of serum [TCO2] obtained over several years were within the lower end of the normal range of the clinical laboratory; therefore, the internist did not probe the acid-base status of the patient further, thereby missing a highly consequential acid-base disorder. This patient highlights our view that the range of normal for serum [TCO2] requires reassessment.
Screening for Acid-Base Disorders
The physiologic approach to assessing acid-base status views blood pH as being determined by the prevailing levels of carbonic acid (that is, PCO2, the respiratory component) and [HCO3−] (the metabolic component) (1,2). However, [HCO3−] is not measured directly. The standard blood gas analyzer measures pH and PCO2, from which [HCO3−] is calculated using the Henderson–Hasselbalch equation.
Because of convenience and wide availability, directly measured serum [TCO2] in venous blood, a surrogate for [HCO3−], is routinely used in screening for acid-base disorders (2). The underlying rationale is that both metabolic and respiratory disorders (the latter by virtue of the secondary responses of [HCO3−] to changes in PCO2) are associated with abnormalities in [HCO3−]. Serum [TCO2] is largely composed of HCO3− but also includes dissolved CO2 and carbonic acid as well as negligible amounts of carbonate and carbamino compounds (CO2 bound reversibly to amino groups of proteins) (3). The amount of dissolved CO2 (including carbonic acid) in serum can be estimated by multiplying PCO2 by the solubility coefficient of CO2, which is 0.0301 mEq/L per millimeter of mercury at body temperature (at a PCO2 of 40 mm Hg, this amounts to 1.2 mEq/L). Thus, serum [TCO2] normally exceeds [HCO3−] by 1–1.5 mEq/L, depending primarily on the prevailing PCO2. As an example, an arterial blood sample of a subject with a pH of 7.40, PaCO2 of 40 mm Hg, and a calculated [HCO3−] of 24 mEq/L would have a serum [TCO2] of approximately 25.2 mEq/L (24+40×0.0301). On average, this arterial sample corresponds to a peripheral venous sample with a pH of 7.38, PvCO2 of 46 mm Hg, and a calculated [HCO3−] of 26 mEq/L (the higher [HCO3−] in venous blood reflects the fact that the bulk of CO2 transported from tissues to the lungs is carried as HCO3−). Therefore, serum [TCO2] in this venous blood is expected to equal approximately 27.4 mEq/L (26+46×0.0301). Indeed, serum [TCO2] in peripheral venous blood is normally approximately 2–3 mEq/L higher than in arterial blood. Note that, in each case (arterial or venous blood), calculated [HCO3−] corresponds to approximately 95% of measured serum [TCO2]. It is this virtual quantitative equivalency that has led many to use the terms [HCO3−] and [TCO2] (or [CO2]) in serum interchangeably. The quantitative difference between [HCO3−] and [TCO2] is further minimized by loss of dissolved CO2 during processing of the uncapped sample for measurement (4). The old manometric technique for measuring serum [TCO2] has been replaced by more efficient techniques adapted for the autoanalyzer (3). An electrometric method is on the basis of the release of all CO2 in the sample after addition of strong acid and monitoring its diffusion through a membrane by a pH electrode, the pH change being proportional to the serum [TCO2]. In an enzymatic assay (5), CO2 reacts with phosphoenolpyruvate under the influence of the enzyme phosphoenolpyruvate carboxylase, leading to the oxidation of NADH to NAD+ and a decrease in absorbance, which is proportional to serum [TCO2].
Normal Range of Serum [TCO2]
On the basis of several sources (6), the normal range of [HCO3−] in arterial blood in adults at sea level extends from 21 to 27 mEq/L, whereas the corresponding range in peripheral venous blood is from 23 to 29 mEq/L. These ranges incorporate the fact that [HCO3−] in women is approximately 1 mEq/L lower than that in men (6). Importantly, [HCO3−] values in the normal range are highly and directly dependent on the prevailing PCO2; as much as 50% of the variability in [HCO3−] in blood is attributable simply to variations in PCO2 (7). Low-normal [HCO3−] values are associated with low-normal PCO2 values and high-normal pH values, whereas high-normal [HCO3−] values are associated with high-normal PCO2 values and low-normal pH values, indicating that PCO2 is the independent variable in these relationships (7). Considering that the normal range of PCO2 in arterial blood is from 36 to 44 mm Hg and that this range in peripheral venous blood is from 42 to 50 mm Hg (6), we can add the contribution of dissolved CO2 to the above ranges of calculated [HCO3−] to arrive at the expected normal ranges of serum [TCO2]. Thus, in arterial blood, the expected normal range of [TCO2] is from approximately 22.1 mEq/L (21+36×0.0301) to approximately 28.3 mEq/L (27+44×0.0301), and in venous blood, it is from approximately 24.3 mEq/L (23+42×0.0301) to approximately 30.5 mEq/L (29+50×0.0301). However, if a variable amount of the dissolved CO2 is lost during processing of the sample (4), the normal range of [TCO2] will tend to approximate that of [HCO3−] (23–29 mEq/L in venous blood). To accommodate this variable loss of dissolved CO2, we recast the expected normal range of serum [TCO2] in venous blood as 23–30 mEq/L.
Importantly, in its latest review, Lab Tests Online, the result of a collaborative led by the American Association of Clinical Chemistry and including 18 other laboratory and medical organizations in the United States and Canada, endorses a normal range of serum [TCO2] in venous blood of 23–29 mEq/L for adults up to 60 years of age (8). This endorsement is on the basis of the normal range provided in the authoritative Tietz Textbook of Clinical Chemistry and Molecular Diagnostics (4) and a review of the literature.
If the expected limit of normal of serum [TCO2] in venous blood is 23–30 mEq/L as derived from our own analysis and approximated by Lab Tests Online, it seems appropriate that the normal range reported by clinical laboratories would be similar. To examine this issue, we compiled the normal ranges of serum [TCO2] in venous blood reported by the clinical laboratories of our own institutions, 20 additional institutions from our regions selected at random (ten from California and ten from Massachusetts), and 42 academic medical centers (of 65 queried) throughout the United States (total of 64).
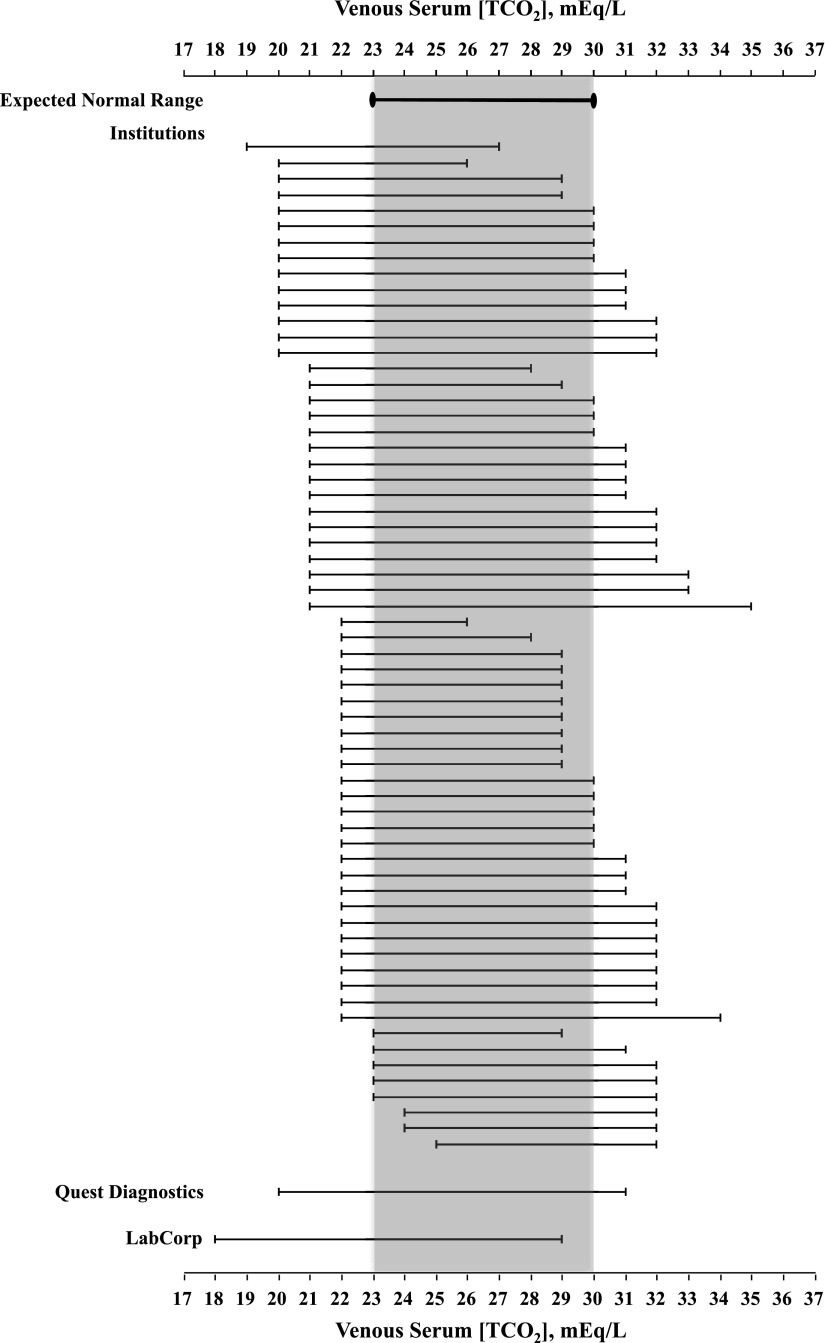
Figure 1 depicts the normal ranges of serum [TCO2] in venous blood reported by each of the 64 clinical laboratories and juxtaposes them against the expected normal range of 23–30 mEq/L. Although we do not claim that this is a completely representative sample of clinical laboratories in the United States, it allows us to make a number of observations. Most striking is the marked variation in the normal range of serum [TCO2] across the sample. Indeed, the lower limit of normal ranges from 19 to 25 mEq/L, whereas the upper limit of normal extends from 26 to 35 mEq/L! Moreover, compared with a span of 8 mEq/L of the expected normal range cited above, 78% of the clinical laboratories have a span ≥9 mEq/L, and 63% have a span ≥10 mEq/L; in 22%, the span is ≥12 mEq/L. The deviations of the limiting values from those of the expected normal range are more common at the lower limit than the higher limit; 88% of the clinical laboratories have a lower limit of normal below that of the expected normal range (23 mEq/L): in 41%, values are ≤21 mEq/L, and in 17%, values are as low as ≤20 mEq/L. By contrast, 52% of the laboratories have an upper limit of normal above that of the expected normal range (30 mEq/L), and 38% have values ≥32 mEq/L.
Figure 1.
The normal range of serum total CO2 concentration ([TCO2]) in venous blood reported by the clinical laboratory of each of 64 institutions sampled and two large commercial laboratories is shown. The expected normal range of 23–30 mEq/L that is on the basis of carefully performed studies on the blood acid-base composition of healthy adults is shown for comparison.
Figure 1 also depicts the normal range of serum [TCO2] in venous blood reported by two large commercial laboratory companies: Quest Diagnostics (with a range of 20–31 mEq/L) and LabCorp (with a range of 18–29 mEq/L).
Taken against the expected limits of normal, the analysis of the normal range of serum [TCO2] of the compiled clinical laboratories underscores the high potential for failing to recognize existing acid-base disorders. Subjects with serum [TCO2] values in venous blood of 18–22 mEq/L, values within the normal range in many clinical laboratories (Figure 1), are expected to have [HCO3−] values in arterial blood of approximately 16–20 mEq/L. Similarly, venous serum [TCO2] values of 31–35 mEq/L (Figure 1) correspond to [HCO3−] values in arterial blood of approximately 29–33 mEq/L. We argue that these are not [HCO3−] values encountered in 95% of healthy subjects, and only a limited number of healthy subjects will have values just outside the expected normal range.
What factors might have conspired to produce the wide variability and substantial deviation from the expected normal range that are observed in the normal limits of serum [TCO2] reported by clinical laboratories? The underlying factors are somewhat unclear. Most of the compiled clinical laboratories had adopted the limits of normal of serum [TCO2] suggested by the manufacturer of the analytic instrument used. These limits are usually on the basis of selected published literature and rarely derived from studies of small samples of apparently normal subjects (cohorts of 50 to a few hundred). Approximately 20% of the clinical laboratories had conducted their own small study of apparently healthy subjects, but details of these studies are unknown. Compared with the expected normal range that is extrapolated from rigorous studies on acid-base composition in normal subjects, the serum [TCO2] studies conducted by manufacturers or clinical laboratories might have been less stringent in subject selection, timing of blood sampling relative to meals (alkaline tide), and sample processing. Extremes of dietary habits (quantity and type of protein and quantity of fruits and vegetables), unbalanced sex representation, mild abnormalities in alveolar ventilation, and early kidney dysfunction might have also contributed (9). Whatever the explanation, the wide range of normal values used by many clinical laboratories can result in a failure to recognize the presence of acid-base disorders, with potentially important implications for the patients’ health.
There are several important caveats to these observations: To be sure, a level of serum [TCO2] falling within the normal limits is not synonymous with normal acid-base status. The limits of normal are sufficiently wide to allow development of mild grades of each of the four cardinal acid-base disorders while serum [TCO2] remains within the normal range. However, the substantially restricted span of the expected normal range highlighted above diminishes the likelihood of such occurrences. Additionally, coexistence of two or more acid-base disorders can maintain serum [TCO2] within the normal range, thereby decreasing the sensitivity of serum [TCO2] in screening for the presence of acid-base disorders. In general, evaluating the serum [TCO2] in the context of the clinical information and other electrolytes will increase its value as a screening tool.
The expected range of normal described above is applicable to sea level. For altitudes above 1500 m (4921 ft), the limits of normal have to incorporate the low serum [TCO2] values reflective of chronic hypocapnia and hypobicarbonatemia consequent to the prevailing hypoxemia (10,11).
Finally, we cannot justify the recommendation of an expanded normal range of serum [TCO2], 23–31 mEq/L, for subjects >60 years of age (8). In view of the direct relationship between blood [HCO3−] and PCO2 within the normal domain (7), [HCO3−] values ≥29 mEq/L, if taken as an extension of the normal domain, must be associated with PCO2 values in the frank hypercapnic range and pH values exceeding the lower range of normal.
Conclusions
The current limits of normal of serum [TCO2] reported by clinical laboratories and used by clinicians to screen for acid-base abnormalities are very often inordinately wide and substantially disparate from what should be expected in normal individuals. Therefore, we propose that the more limited range of serum [TCO2] described above, 23–30 mEq/L, be adopted by clinical laboratories. We posit that subjects with a verified serum [TCO2] <23 mEq/L are likely to harbor an acid-base disorder (metabolic acidosis or respiratory alkalosis) and recommend that their acid-base status be assessed with a venous blood gas. Similarly, subjects with a repeated serum [TCO2] >30 mEq/L should be assessed with a venous blood gas for the presence of metabolic alkalosis or respiratory acidosis. This approach will improve the care of patients by ensuring the recognition of the majority of “hidden” acid-base disorders.
Disclosures
None.
Acknowledgments
The authors thank Dr. Ioannis Koulouridis for designing the figure.
JAK received support through unrestricted funds from the University of California at Los Angeles.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Kraut JA, Madias NE: Approach to patients with acid-base disorders. Respir Care 46: 392–403, 2001 [PubMed] [Google Scholar]
- 2.Adrogué HJ, Gennari FJ, Galla JH, Madias NE: Assessing acid-base disorders. Kidney Int 76: 1239–1247, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Adrogué H, Madias NE: Measurement of acid-base status. In: Acid-Base Disorders and Their Treatment, 1st Ed., edited by Gennari FJ, Adrogue HJ, Galla JH, Madias NE, Boca Raton, FL, Taylor and Francis, 2005, pp 775–788 [Google Scholar]
- 4.Schindler E, Brown S, Scott M: Electrolytes and blood gases. In: Tietz Textbook of Clinical Chemistry and Molecular Diagnostics, 6th Ed., edited by Rifai N, Horvath AR, Wittwer CT, St. Louis, MO, Elsevier, 2017, pp 604–625 [Google Scholar]
- 5.Forrester RL, Wataji LJ, Silverman DA, Pierre KJ: Enzymatic method for determination of CO2 in serum. Clin Chem 22: 243–245, 1976 [PubMed] [Google Scholar]
- 6.Adrogué HJ, Madias NE: Normal acid-base values. In: Acid-Base Disorders and Their Treatment, 1st Ed., edited by Gennari FJ, Adrogue HJ, Galla JH, Madias NE, Boca Raton, FL, Taylor and Francis, 2005, pp 789–799 [Google Scholar]
- 7.Madias NE, Adrogué HJ, Horowitz GL, Cohen JJ, Schwartz WB: A redefinition of normal acid-base equilibrium in man: Carbon dioxide tension as a key determinant of normal plasma bicarbonate concentration. Kidney Int 16: 612–618, 1979 [DOI] [PubMed] [Google Scholar]
- 8.American Association of Clinical Chemistry: Total CO2 Reference Range, 2017. Available at: https://labtestsonline.org/understanding/analytes/co2/tab/test. Accessed May 10, 2017
- 9.Goraya N, Simoni J, Jo CH, Wesson DE: A comparison of treating metabolic acidosis in CKD stage 4 hypertensive kidney disease with fruits and vegetables or sodium bicarbonate. Clin J Am Soc Nephrol 8: 371–381, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramirez-Sandoval JC, Castilla-Peón MF, Gotés-Palazuelos J, Vázquez-García JC, Wagner MP, Merelo-Arias CA, Vega-Vega O, Rincón-Pedrero R, Correa-Rotter R: Bicarbonate values for healthy residents living in cities above 1500 meters of altitude: A theoretical model and systematic review. High Alt Med Biol 17: 85–92, 2016 [DOI] [PubMed] [Google Scholar]
- 11.Krapf R, Beeler I, Hertner D, Hulter HN: Chronic respiratory alkalosis. The effect of sustained hyperventilation on renal regulation of acid-base equilibrium. N Engl J Med 324: 1394–1401, 1991 [DOI] [PubMed] [Google Scholar]