Abstract
Bortezomib, which is a potent proteasome inhibitor, has been used as a first-line drugs to treat multiple myeloma for a few decades, and radiotherapy has frequently been applied to manage acute bone lesions in the patients. Therefore, it was necessary to investigate what the benefits might be if the two therapies were applied simultaneously in the treatment of multiple myeloma. Since it was known that radiotherapy and proteasome inhibitors could increase the expression of NKG2D ligands through induction of protein synthesis and suppression of protein degradation of NKG2D ligands, respectively, we supposed that the combined treatment might further enhance the expression of NKG2D ligands. In this study, we analyzed the expression level of NKG2D ligands using multiplex PCR and flow cytometry after treatment of IM-9 and RPMI-8226 myeloma cells with bortezomib and ionizing radiation; we then assayed the susceptibility to NK-92 cells. Although the expression of only some kinds of NKG2D ligands were increased by treatment with bortezomib alone, five kinds of NKG2D ligands that we assayed were further induced at the surface protein level after combined treatment with ionizing radiation and bortezomib. Furthermore, combined treatment made myeloma cells more susceptible to NK-92 cells, compared with treatment with bortezomib alone. In conclusion, the combination therapy of ionizing radiation plus the proteasome inhibitor bortezomib is a promising therapeutical strategy for enhancing NK cell–mediated anticancer immune responses.
Keywords: NKG2D ligands, bortezomib, myeloma, radiation
INTRODUCTION
Multiple myeloma is a kind of hematologic malignancy that has increased numbers of immature, abnormal or atypical plasma cells in the bone marrow and typical bone lesions. It accounts for ~10% of hematologic malignancies in the USA [1]. A proteasome inhibitor, bortezomib had been approved to treat myeloma by the US FDA and then became the first-line drugs ~10 years ago [2–4]. As a result, outcomes of the treatment of myeloma were greatly improved. However, multiple myeloma cells have developed resistance to this chemotherapeutic agent, and refractory myeloma remains an incurable disease in patients who cannot receive bone marrow transplantation. Another common modality applied to treat myeloma is radiotherapy, which has the advantage of causing direct and immediate cell death focused in a local area, and also having an abscopal effect, a phenomenon of tumor regression in sites distant from the targeted fields of irradiation. It is well accepted that this abscopal effect might be mediated by activation of the immune system in patients [5]. In the past several decades, it has become increasingly clear that NK cells play an important role in the recognition and killing of myeloma cells, with differences in NK cell numbers and activity depending on the stage of the disease [6–10]. Proteasome inhibitors can regulate the expression of natural killer group 2, member D (NKG2D) ligands, which bind to NK2D receptors and play an important role in the NK, γδ+ and CD8+ T cell–mediated immune response to transformed cells [11, 12], through preventing the degradation of intracellular proteins [13–16]. The role of bortezomib in the NK cell–mediated immune responses is currently being studied. Therefore, it was proposed that both bortezomib and radiotherapy might be used simultaneously to affect the immune system in myeloma patients and thus to modulate disease progression. In this study, we investigated whether a combination of bortezomib and radiotherapy could induce NKG2D ligands in myeloma cells and thus enhance the NK cell–mediated immune response. This could lead to using the activated NK cell–mediated immune system against myeloma cells, through combined treatment with bortezomib and ionizing radiation, to improve outcomes for and reduce recurrence rates in myeloma patients.
MATERIALS AND METHODS
Cell lines and reagents
The two multiple myeloma cell lines, IM-9 and RPMI-8226, used in this study were obtained from the Korean Cell Line Bank (Seoul, Korea). These cell lines were maintained in RPMI-1640 media supplemented with 10% fetal bovine serum (FBS) (Gibco, Grand Island, NY, USA), 2 mM L-glutamine, 100 μg/ml streptomycin, and 100 U/ml penicillin. The NK-92 cell line was obtained from the American Type Culture Collection (Manassas, VA, USA) and maintained in alpha-Minimum Essential Modified medium supplemented with 12.5% (v/v) FBS, 12.5% (v/v) horse serum, 2 mM L-glutamine, 0.1 mM 2-mercaptoethanol, 200 U/ml of recombinant human interleukin-2, 100 mg/ml streptomycin, and 100 U/ml penicillin. All cell lines were cultured at 37°C in a humidified atmosphere containing 5% CO2. Bortezomib was purchased from SELLECK chemicals (Houston, TX, USA) and dissolved in 99.7% dimethyl sulfoxide (DMSO; Sigma-Aldrich, St Louis, MO, USA) at a concentration of 10 μM. Each myeloma cell line was treated with bortezomib at concentration of 2.5 nM to 10 nM.
Exposure to ionizing radiation and combined treatment
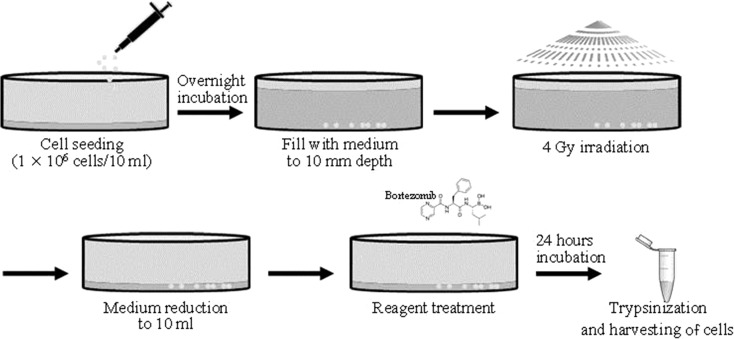
To irradiate the myeloma cells, we used a 6 MeV Clinac iX Linear Accelerator (Varian Medical Systems, Palo Alto, CA, USA) in the Department of Radiation Oncology of Pusan National University Yangsan Hospital (Yangsan, South Korea). The 1 × 106 cells were seeded in 100 mm–diameter culture dishes with 10 ml PRMI-1640 medium. The following day they were irradiated at a rate of 8 Gy/min for 30 s to a 200 × 200 mm area, under a 10 mm depth coverage of medium (created by the addition of 68 ml RPMI-1640 medium). The 10 mm depth of medium mimicked the condition of a tumor mass located in the subcutaneous layer. For the combined treatment, bortezomib treatment was applied just after the ionizing radiation (IR). The irradiated myeloma cells were allowed to recover for 24 h after reduction of the medium to 10 ml (Fig. 1).
Fig. 1.
The protocol for combined treatment with bortezomib and IR. The 1 × 106 cells were seeded in a 100 mm–diameter culture dish with 10 ml PRMI-1640 medium. The next day they were covered with a 10 mm depth-coverage of medium by the addition of 68 ml RPMI-1640 medium and were then irradiated at a rate of 8 Gy/min for 30 s to a 200 × 200 mm area. They were treated with bortezomib just after the ionizing radiation treatment. The irradiated myeloma cells were allowed to recover for 24 h after reduction of the medium to 10 ml.
Total RNA extraction and multiplex reverse transcription PCR
Total RNA extraction and reverse transcription PCR (RT-PCR) were performed as previously described [17]. Briefly, the total RNA was extracted using an RNeasy® Mini Kit (Qiagen GmbH, Hilden, Germany) from the cell lines, following the manufacturer’s protocol. One microgram of extracted total RNA was used to synthesize cDNA using 100 pmol of random primers (Takara Bio Inc., Kyoto, Japan) and 100 U of M-MLV reverse transcriptase (Promega Co., Fitchburg, WI, USA). The resulting cDNA was used in the PCR reaction with the QIAGEN® Multiplex PCR Kit (Qiagen GmbH). Seven pairs of primer sets were purchased from Bioneer Co. Ltd (Daejeon, South Korea) and used to investigate the expression of genes, including ribosomal protein L19 (RPL19), MICA, MICB, ULBP1–3 and β-actin (ACTB). ACTB and RPL19 were used as a loading control and a degradation marker, respectively. The PCR products were analyzed using ethidium bromide (Sigma-Aldrich, St Louis, MO, USA)–stained 2.0% agarose gel electrophoresis and quantitated by image analyzing software, Quantity One (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Flow cytometry
To determine the surface expression of the NKG2D ligands on cancer cells, the cells were incubated with mouse anti-MICA, anti-MICB, anti-ULBP1–3 (R&D systems, Minneapolis, MN, USA), which were NKG2D ligand–specific monoclonal antibodies (mAbs), and the corresponding isotype controls at 10 μg/m, followed by incubation with goat anti-mouse-PE conjugated (BD Phamingen Inc., San Diego, CA., USA). The analysis was performed on a FACS Caliber® (Becton Dickinson, Mountain View, CA., USA) using CellQuest software (Becton Dickinson), and the cell surface expression was quantified by the value of the mean fluorescence intensities (MFIs) obtained with the specific mAbs.
NK cell–mediated cytotoxicity assay
NK cell–mediated cytotoxicity was determined with DELFIA® EuTDA Cytotoxicity Reagents (PerkinElmer Life Sciences, Waltham, MA, USA). The precise procedure has been described previously [18]. Briefly, the target cells (1 × 106 cells/ml) were incubated with freshly prepared 10 μM of BATDA, a fluorescence-enhancing ligand, in 2 ml culture medium for 30 min at 37°C, and washed. Next, 100 μl of BATDA-labeled target cells (5000 cells) were transferred into a round-bottom sterile plate. These target cells were co-cultured with NK-92 cells for 2 h at an effector/target ratio ranging from 2.5:1 to 10:1. After incubation, 20 μl of the supernatant from each well was transferred to the wells of flat-bottom 96-well plates. Next, 180 μl of the europium (Eu) solution was added to form highly fluorescent and stable chelates (EuTDA). The fluorescence of the EuTDA chelates was measured with time-resolved fluorometry (Victor3, PerkinElmer). The percentage specific release was calculated from (experimental release – spontaneous release)/(maximum release – spontaneous release) × 100 (%). In the blocking experiments, the blocking anti-NKG2D mAb (R&D systems) was added to the suspension of the NK-92 cells and incubated for 30 min before they were co-cultured with the target cells. All experiments were done in triplicate.
Statistical analysis
To evaluate the altered level of gene expression, the mean fold of gene expression in proteasomal inhibitor–treated cells was compared with that in the vehicle-treated control cells. The standard error (SE) of the mean was calculated. For comparison of groups, the unpaired Student’s t-test was performed. A P value below 0.05 was considered statistically significant in all experiments.
RESULTS
IR and bortezomib treatment induced the expression of NKG2D ligands
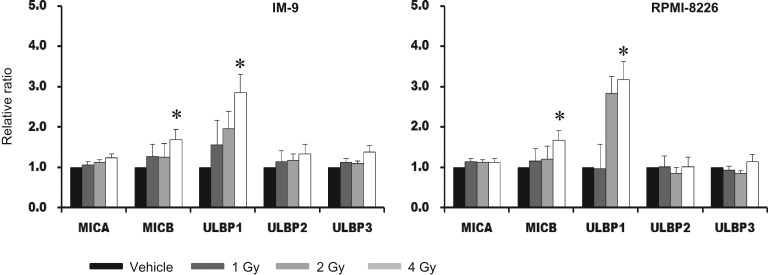
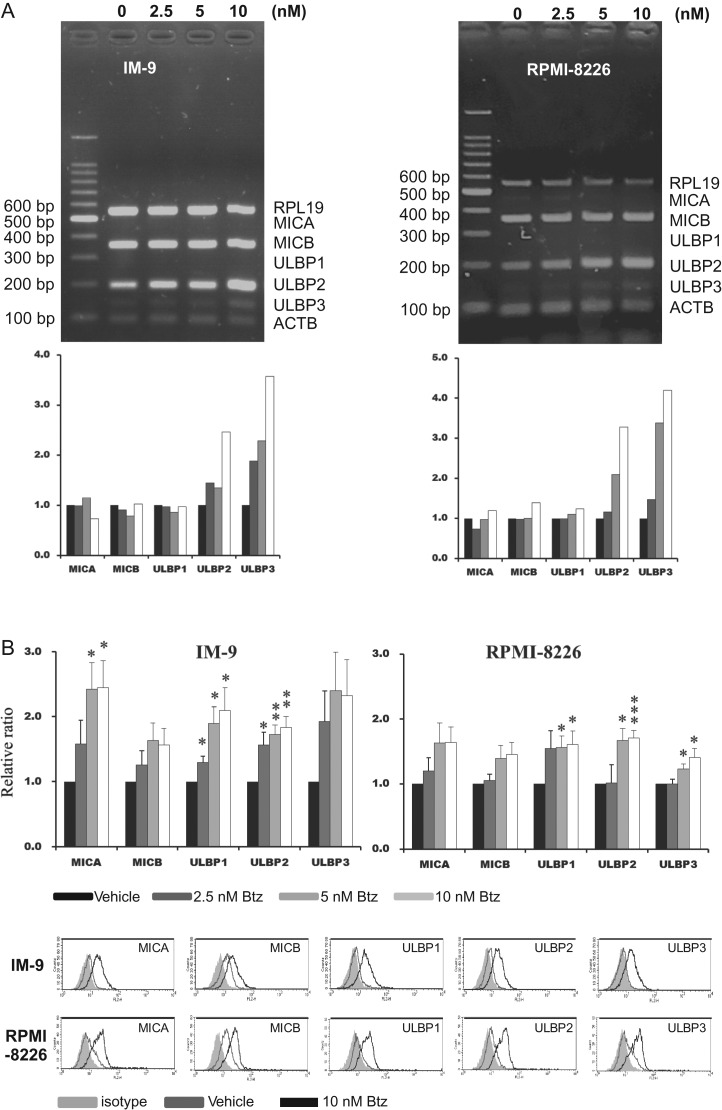
We found that IR could induce surface proteins of MICB and ULBP1 on myeloma cells without increasing mRNA (Fig. 2). The RT-PCR data were not represented—4 Gy of IR was most effective and >6 Gy dose induced a significant amount of cell death. Bortezomib stimulated transcription of ULBP2 and ULBP3 in IM-9 cells and RPMI-8226 cells (Fig. 3A). It was assumed that reduced degradation of factors involved in transcription might increase transcription. Surface proteins of all NKG2D ligands had an induced trend after 24 h treatment with bortezomib. Significant induction of MICA, ULBP1 and ULBP 2 was demonstrated compared with in the vehicle (DMSO) -treated group in IM-9 cells. Expression of ULBP1, ULBP2 and ULBP 3 was increased compared with in the vehicle-treated group in RPMI-8226 cells (Fig. 3B).
Fig. 2.
The expression of NKG2D ligands after ionizing radiation (IR) at the surface protein level. After IR treatment with doses of 1, 2 and 4 Gy, and a 24 h recovery phase, using IM-9 and RPMI-8226 cells, we obtained the mean fluorescence intensities (MFIs) using flow cytometry. Average relative folds of MFI were calculated in comparison with those of untreated cells. The surface expression of NKG2D ligands, including MICA, MICB and ULBP 1–3, were evaluated. All experiments were repeated three times, and P values of <0.05 are indicated by an asterisk.
Fig. 3.
The expression of NKG2D ligands after treatment with bortezomib and ionizing radiation at both mRNA and surface protein levels. (A) The mRNAs of NKG2D ligands were amplified by the multiplex RT-PCR method in IM-9 and RPMI-8226 cells after treatment with 2.5, 5 and 10 nM bortezomib for 24 h. This was analyzed by 2% agarose DNA electrophoresis and quantitated by image analyzing software. (B) After treatment with bortezomib or vehicle for 24 h, we compared the mean fluorescence intensities (MFIs) obtained from flow cytometry with those of vehicle-treated cells (upper panel). All experiments were repeated four times and a representative histogram was created (lower panel). P values were presented as single asterisks (<0.05), double asterisks (<0.01) and triple asterisks (<0.001).
Combination of both bortezomib treatment and IR increased the expression of NKG2D ligands
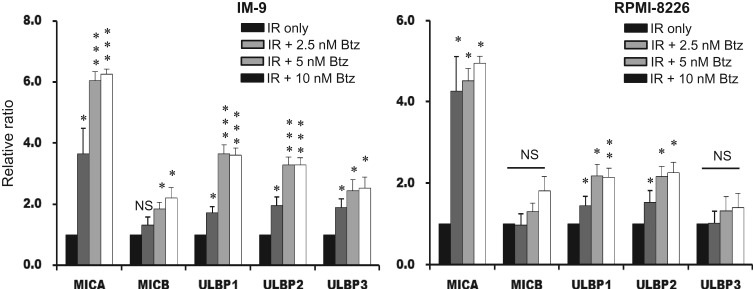
To determine the effect of the combined treatment of proteasome inhibition and IR on the expression of NKG2D ligands, myeloma cells were treated with 2.5, 5 or 10 nM bortezomib immediately after irradiation and incubated for 24 h. Induction of all of the assayed NKG2D ligands was increased in the combined treatment myeloma cells compared with in the vehicle-treated group. In IM-9 cells treated with the combination of IR and bortezomib, expression of MIA, MICB and ULBP 1–3 was increased compared with in the 4 Gy IR group. In RPMI-8226 cells, expression of MICA and ULBP 1,2 was increased compared with in the 4 Gy IR group (Fig. 4). There was no further increase in NKG2D ligands after treatment with >10 nM concentration of bortezomib in either group of cells.
Fig. 4.
The significant induction of NKG2D ligands after combined treatment with bortezomib and ionizing radiation (IR). The expression of NKG2D ligands was evaluated using flow cytometry in IM-9 and RPMI-8226 myeloma cells. The bars represent the MFI ratios of NKG2D ligands for the various groups: IR plus 2.5, 5 and 10 nM bortezomib, and IR only. P values are indicated by a single asterisk (<0.05), double asterisks (<0.01) or triple asterisks (<0.001).
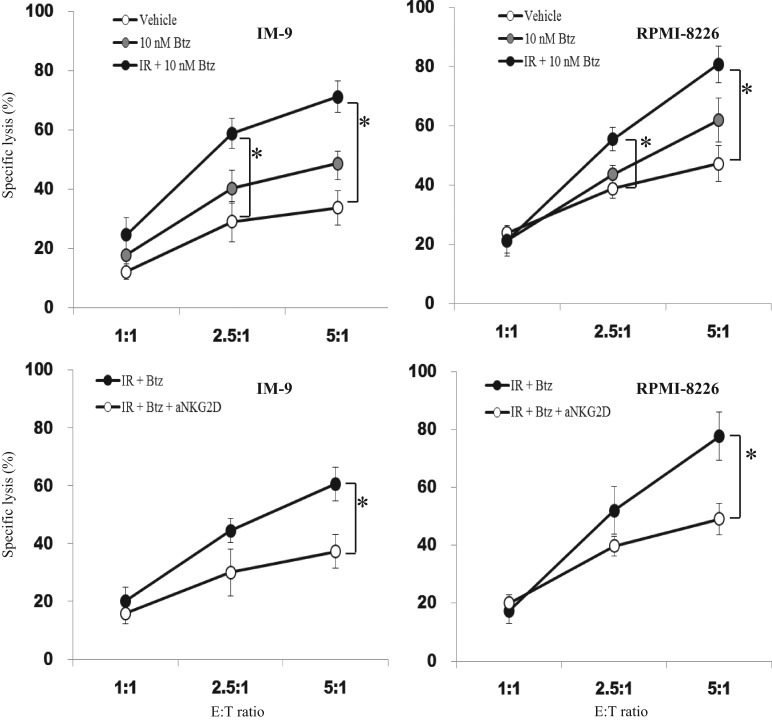
Bortezomib treatment and ionizing radiation enhanced NK cell–mediated cancer cell lysis
Cytotoxicity against two kinds of myeloma cells was further enhanced after combined treatment with bortezomib and IR (Fig. 5). Although 10 nM bortezomib treatment tended to enhance the cytotoxicity of the NK-92 cells by increasing the expression of several kinds of NKG2D ligands, the increase was not significant. When we performed an NKG2D receptor–blocking assay, the magnitude of the cytotoxicity was found to be significantly reduced. Adding IR to bortezomib treatment would be beneficial for killing the target cells through stimulation of NK cell–mediated immune responses by induction of NKG2D ligands.
Fig. 5.
Enhanced NK cell–mediated cancer cell lysis by combined treatment with bortezomib and ionizing radiation (IR). After treatment with 10 nM bortezomib with or without IR in IM-9 and RPMI-8226 cells, the myeloma cells (target cells) and NK-92 cells (effector cells) were cocultured for 4 h. The cytotoxicity of NK-92 cells compared with that of two kinds of myeloma cells was assayed (upper panel). Blank circles represents vehicle (DMSO)-treated cells. Gray-filled circles and black-filled circles represent bortezomib and combined treatment with IR and bortezomib, respectively. The blocking assay was performed after treating the myeloma cells with anti-NKG2D blocking antibody for half an hour. Black-filled circles represent combined treatment with IR and bortezomib, and blank circles represent NKG2D blocked cells, respectively. A single asterisk indicates P < 0.05.
DISCUSSION
Although multiple myeloma was previously an incurable type of hematologic malignancy, the clinical outcome for patients with multiple myeloma has been greatly improved over the last few decades. A significant survival advantage was achieved after using bortezomib, which was first approved for the treatment of relapsed/refractory multiple myeloma in 2003 and which became the first-line drug treatment in 2008 [19, 20]. Bortezomib reversibly inhibits the threonine residue of the 20S unit and inhibits proteasome activity. At first, it was thought that bortezomib prevented IκBα from degradation and inhibited the nuclear factor kappa B (NF-κB) pathway in myeloma [21]. However, a different report claimed that canonical NF-κB activity was activated after treatment with bortezomib through enhanced activation of IκB kinase in myeloma cells [22]. Although it is unclear which molecule really exhibited the anti-myeloma effect after treatment with bortezomib, bortezomib showed several anti-cancer effects through arrest of the cell cycle, induction of apoptosis and prevention of the cytokine loop [23–29]. Recent reports suggest that bortezomib has apparent immunomodulatory effects through decrease in anti-apoptotic proteins, induction of apoptosis, and inhibition of the transcription factor NF-κB in immune cells [30–33]. With respect to bone marrow transplantation, it was known that bortezomib could prevent GVHD and maintain GVT through depletion of alloreactive T cells and preservation of regulatory T cells [34, 35]. It was likely that bortezomib might have a role as an immune stimulator in myeloma patients and thus contribute to better clinical outcomes. Since the expression of NKG2D ligands is dependent on the balance between gene expression and protein degradation, it was reasonable to assume that a proteasome inhibitor could regulate the expression of some NKG2D ligands through modulating the rate of protein turnover. Currently, eight kinds of NKG2D ligands have been identified in humans, and these are grouped into the MIC family (MICA and MICB) and the ULBP family (ULBP 1–6); their expression is generally restricted in normal cells. The affinities of MICA/MICB and ULBP1 are 0.5 and 1.1 μM, respectively [36, 37]. However, till now it has not been known which ligands are more potent in activating NKG2D+ cells. It is known that the expression of NKG2D ligands is significantly increased in infected cells and transformed cells [38, 39]. Although the reason for the presence of the various NKG2D ligands is not clear, it has been assumed that they make cells responsive to various external stimuli. In this study, we found that bortezomib could increase the mRNA of ULBP2 and ULBP3, through unknown mechanisms. Butler et al. found increased ULBP1 transcription after inhibition of proteasomes, and Geng et al. suggested that reduced degradation of factors involved in transcription could be responsible for increasing the transcription of genes [13, 40]. However, it was not known which NKG2D ligands were the main ones transcribed after treatment with bortezomib, how the type of cell might affect transcription, and what stimuli might be required. In this study, it was demonstrated that bortezomib significantly increased the surface proteins of several NKG2D ligands in myeloma cells. Although there are previous reports that bortezomib treatment results in enhancement of NK cell–mediated immune responses against many kind of cancer cells (including myeloma) through induction of NKG2D ligands, suppression of MHC class I molecules, or facilitated apoptosis of cancer cells [13–16, 41–43], it appears that the anti-cancer immune responses are not sufficient to eradicate whole tumor cells and need enhancement by other modalities in myeloma patients under treatment of bortezomib.
Radiotherapy has for a long time frequently been used to manage the acute bone lesions of multiple myeloma. Because it was known that IR can disrupt proteasome complexes [44] and increase the expression of NKG2D ligands on a post-transcriptional level through the cellular response to DNA damage [45, 46], it was suggested that IR might have a role as an immune booster through activation of immune cells, including NK cells [47–50]. Since IR can activate the NF-κB pathway through ATM signaling, and NF-κB is a common target of bortezomib and IR [51, 52], it appeared that activated NF-κB might contribute to expression of MICA [53, 54].
NK cell–based anti-cancer immunotherapy is already well advanced, and destruction of residual cancer cells by specific immune cells during the remission period is ideal for preventing recurrence or resistance. Immunotherapy might be a promising strategy for improving the outcome for myeloma patients. Although it was known that single treatment with bortezomib or radiotherapy could induce NKG2D ligands and modulate NK cell–mediated immune responses independently, the magnitude and duration of the immune responses were not sufficient for eliminating residual cancer cells in vivo. In this study, we demonstrated that radiotherapy could enhance bortezomib-induced immune responses against myeloma cells through further induction of NKG2D ligands. Since bortezomib and radiotherapy have different mechanisms for inducing NKG2D ligands, they will be good partners for combatting myeloma. In the near future, if NK cell–based immunotherapy can be established well and can be applied to treat myeloma, combined treatment with bortezomib and radiotherapy promises to provide increased benefits to myeloma patients.
CONFLICT OF INTEREST
The authors state that there is no conflict of interest regarding the publication of this article.
FUNDING
This work was supported by a Pusan National University Research Grant for 2 years.
REFERENCES
- 1. National Center for Health Statistics Vital Statistics of the United States, 2016. https://www.cdc.gov/nchs/nvss/index.htm (17 May 2017, date last accessed).
- 2. Kouroukis TC, Baldassarre FG, Haynes AE et al. . Bortezomib in multiple myeloma: systematic review and clinical considerations. Curr Oncol 2014;21:e573–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lonial S, Boise LH. Current advances in novel proteasome inhibitor–based approaches to the treatment of relapsed/refractory multiple myeloma. Oncology 2011;2:25–31. [PubMed] [Google Scholar]
- 4. Crawford LJ, Walker B, Irvine AE. Proteasome inhibitors in cancer therapy. J Cell Commun Signal 2011;5:101–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Grass GD, Krishna N, Kim S. The immune mechanisms of abscopal effect in radiation therapy. Curr Probl Cancer 2016;40:10–24. [DOI] [PubMed] [Google Scholar]
- 6. Osterborg A, Nilsson B, Bjorkholm M et al. . Natural killer cell activity in monoclonal gammopathies: relation to disease activity. Eur J Haematol 1990;45:153–7. [DOI] [PubMed] [Google Scholar]
- 7. Frohn C, Hoppner M, Schlenke P et al. . Anti-myeloma activity of natural killer lymphocytes. Br J Haematol 2002;119:660–64. [DOI] [PubMed] [Google Scholar]
- 8. Carbone E, Neri P, Mesuraca M et al. . HLA class I, NKG2D, and natural cytotoxicity receptors regulate multiple myeloma cell recognition by natural killer cells. Blood 2005;105:251–8. [DOI] [PubMed] [Google Scholar]
- 9. El-Sherbiny YM, Meade JL, Holmes TD et al. . The requirement for DNAM-1, NKG2D, and NKp46 in the natural killer cell–mediated killing of myeloma cells. Cancer Res 2007;67:8444–9. [DOI] [PubMed] [Google Scholar]
- 10. Jinushi M, Vanneman M, Munshi NC et al. . MHC class I chain–related protein A antibodies and shedding are associated with the progression of multiple myeloma. Proc Natl Acad Sci U S A 2008;105:1285–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Marcus A, Gowen BG, Thompson TW et al. . Recognition of tumors by the innate immune system and natural killer cells. Adv Immunol 2014;122:91–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Champsaur M, Lanier LL. Effect of NKG2D ligand expression on host immune responses. Immunol Rev 2010;235:267–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Butler JE, Moore MB, Presnell SR et al. . Proteasome regulation of ULBP1 transcription. J Immunol 2009;182:6600–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Valés-Gómez M, Chisholm SE, Cassady-Cain RL et al. . Selective induction of expression of a ligand for the NKG2D receptor by proteasome inhibitors. Cancer Res 2008;68:1546–54. [DOI] [PubMed] [Google Scholar]
- 15. Niu C, Jin H, Li M et al. . Low-dose bortezomib increases the expression of NKG2D and DNAM-1 ligands and enhances induced NK and γδ T cell–mediated lysis in multiple myeloma. Oncotarget 2017;8:5954–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shi J, Tricot GJ, Garg TK et al. . Bortezomib down-regulates the cell-surface expression of HLA class I and enhances natural killer cell–mediated lysis of myeloma. Blood 2008;111:1309–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Park SW, Bae JH, Kim SD et al. . Comparison of level of NKG2D ligands between normal and tumor tissue using multiplex RT-PCR. Cancer Invest 2007;25:299–307. [DOI] [PubMed] [Google Scholar]
- 18. Bae JH, Kim JY, Kim MJ et al. . Quercetin enhances susceptibility to NK cell–mediated lysis of tumor cells through induction of NKG2D ligands and suppression of HSP70. J Immunother 2010;33:391–401. [DOI] [PubMed] [Google Scholar]
- 19. Richardson PG, Sonneveld P, Schuster MW et al. . Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Eng J Med 2005;352:2487–98. [DOI] [PubMed] [Google Scholar]
- 20. Richardson PG, Barlogie B, Berenson J et al. . A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med 2003. ;348:2609–17. [DOI] [PubMed] [Google Scholar]
- 21. Pham LV, Tamayo AT, Yoshimura LC et al. . Inhibition of constitutive NF-kappa B activation in mantle cell lymphoma B cells leads to induction of cell cycle arrest and apoptosis. J Immunol 2003;171:88–95. [DOI] [PubMed] [Google Scholar]
- 22. Hideshima T, Ikeda H, Chauhan D et al. . Bortezomib induces canonical nuclear factor-κB activation in multiple myeloma cells. Blood 2009;114:1046–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hideshima T, Mitsiades C, Akiyama M et al. . Molecular mechanisms mediating antimyeloma activity of proteasome inhibitor PS-341. Blood 2003;101:1530–4. [DOI] [PubMed] [Google Scholar]
- 24. Mitsiades N, Mitsiades CS, Richardson PG et al. . The proteasome inhibitor PS-341 potentiates sensitivity of multiple myeloma cells to conventional chemotherapeutic agents: therapeutic applications. Blood 2003;101:2377–80. [DOI] [PubMed] [Google Scholar]
- 25. Hideshima T, Richardson P, Chauhan D et al. . The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer Res 2001;61:3071–6. [PubMed] [Google Scholar]
- 26. Fennell DA, Chacko A, Mutti L. BCL-2 family regulation by the 20S proteasome inhibitor bortezomib. Oncogene 2008;27:1189–97. [DOI] [PubMed] [Google Scholar]
- 27. Nikrad M, Johnson T, Puthalalath H et al. . The proteasome inhibitor bortezomib sensitizes cells to killing by death receptor ligand TRAIL via BH3-only proteins Bik and Bim. Mol Cancer Ther 2005;4:443–9. [DOI] [PubMed] [Google Scholar]
- 28. Voortman J, Checinska A, Giaccone G et al. . Bortezomib, but not cisplatin, induces mitochondria-dependent apoptosis accompanied by up-regulation of noxa in the non–small cell lung cancer cell line NCI-H460. Mol Cancer Ther 2007;6:1046–53. [DOI] [PubMed] [Google Scholar]
- 29. Fahy BN, Schlieman MG, Mortenson MM et al. . Targeting BCL-2 overexpression in various human malignancies through NF-κB inhibition by the proteasome inhibitor bortezomib. Cancer Chemother Pharmacol 2005;56:46–54. [DOI] [PubMed] [Google Scholar]
- 30. Wang X, Ottosson A, Ji C et al. . Proteasome inhibition induces apoptosis in primary human natural killer cells and suppresses NKp46-mediated cytotoxicity. Haematologica 2009;94:470–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Feng X, Yan J, Wang Y et al. . The proteasome inhibitor bortezomib disrupts tumor necrosis factor–related apoptosis-inducing ligand (TRAIL) expression and natural killer (NK) cell killing of TRAIL receptor–positive multiple myeloma cells. Mol Immunol 2010;47:2388–96. [DOI] [PubMed] [Google Scholar]
- 32. Straube C, Wehner R, Wendisch M et al. . Bortezomib significantly impairs the immunostimulatory capacity of human myeloid blood dendritic cells. Leukemia 2007;21:1464–71. [DOI] [PubMed] [Google Scholar]
- 33. Wang X, Feng X, Wang J et al. . Bortezomib and IL-12 produce synergetic anti-multiple myeloma effects with reduced toxicity to natural killer cells. Anti-Cancer Drugs 2014;25:282–8. [DOI] [PubMed] [Google Scholar]
- 34. Blanco B, Perez-Simon JA, Sanchez-Abarca LI et al. . Bortezomib induces selective depletion of alloreactive T lymphocytes and decreases the production of Th1 cytokines. Blood 2006;107:3575–83. [DOI] [PubMed] [Google Scholar]
- 35. Blanco B, Perez-Simon JA, Sanchez-Abarca LI et al. . Treatment with bortezomib of human CD4+ T cells preserves natural regulatory T cells and allows the emergence of a distinct suppressor T cell population. Haematologica 2009;94:975–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Paul S, Ming-Ru W, Marie-Louise S et al. . NKG2D ligands as therapeutic targets. Cancer Immun 2013;13:8–21. [PMC free article] [PubMed] [Google Scholar]
- 37. Lanier LL. NKG2D receptor and its ligands in host defense. Cancer Immunol Res 2015;3:575–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Trinchieri G. Biology of natural killer cells. Adv Immunol 1989;47:187–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Biron CA. Activation and function of natural killer cell responses during viral infections. Curr Opin Immunol 1997;9:24–34. [DOI] [PubMed] [Google Scholar]
- 40. Geng F, Wenzel S, Tansey WP. Ubiquitin and proteasomes in transcription. Annu Rev Biochem 2012;81:177–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hallett WH, Ames E, Motarjemi M et al. . Sensitization of tumor cells to NK cell–mediated killing by proteasome inhibition. J Immunol 2008;180:163–70. [DOI] [PubMed] [Google Scholar]
- 42. Ames E, Hallett WH, Murphy WJ. Sensitization of human breast cancer cells to natural killer cell–mediated cytotoxicity by proteasome inhibition. Clin Exp Immunol 2009;155:504–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lundqvist A, Yokoyama H, Smith A et al. . Bortezomib treatment and regulatory T-cell depletion enhance the anti-tumor effects of adoptively infused NK cells. Blood 2009;113:6120–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pervan M, Iwamoto KS, McBride WH. Proteasome structures affected by ionizing radiation. Mol Cancer Res 2005; 3:381–90. [DOI] [PubMed] [Google Scholar]
- 45. Gasser S, Orsulic S, Brown EJ et al. . The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature 2005;436:1186–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Son CH, Keum JH, Yang K et al. . Synergistic enhancement of NK cell–mediated cytotoxicity by combination of histone deacetylase inhibitor and ionizing radiation. Radiat Oncol 2014;9:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Formenti SC. Immunological aspects of local radiotherapy: clinical relevance. Discov Med 2010;9:119–24. [PubMed] [Google Scholar]
- 48. Santin AD, Hermonat PL, Ravaggi A et al. . The effects of irradiation on the expression of a tumour rejection antigen (heat shock protein gp96) in human cervical cancer. Int J Radiat Biol 1998;73:699–704. [DOI] [PubMed] [Google Scholar]
- 49. Schmid TE, Multhoff G. Radiation-induced stress proteins—the role of heat shock proteins (HSP) in anti-tumor responses. Curr Med Chem 2012;19:1765–70. [DOI] [PubMed] [Google Scholar]
- 50. Frey B, Rubner Y, Wunderlich R et al. . Induction of abscopal anti-tumor immunity and immunogenic tumor cell death by ionizing irradiation—implications for cancer therapies. Curr Med Chem 2012;19:1751–64. [DOI] [PubMed] [Google Scholar]
- 51. Kiang JG, Jiao W, Cary LH et al. . Wound trauma increases radiation-induced mortality by activation of iNOS pathway and elevation of cytokine concentrations and bacterial infection. Radiat Res 2010;173:319–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Magné N, Toillon RA, Bottero V et al. . NF-kB modulation and ionizing radiation: mechanisms and future directions for cancer treatment. Cancer Lett 2006;231:158–68. [DOI] [PubMed] [Google Scholar]
- 53. Molinero LL, Fuertes MB, Girart MV et al. . NF-kappa B regulates expression of the MHC class I–related chain A gene in activated T lymphocytes. J Immunol 2004;173:5583–90. [DOI] [PubMed] [Google Scholar]
- 54. Chen Z, Li Z, Chang Y et al. . Relationship between NF-κB, MMP-9, and MICA expression in pituitary adenomas reveals a new mechanism of pituitary adenomas immune escape. Neurosci Lett 2015597:77–83. [DOI] [PubMed] [Google Scholar]