Abstract
Loss-of-function mutations in PPARG cause familial partial lipodystrophy type 3 (FPLD3) and severe metabolic disease in many cases. Missense mutations in PPARG are present in ~1:500 people. Whilst mutations are often binarily classified as ‘benign’ or ‘deleterious’, prospective functional classification of all missense PPARG variants suggests that their impact is graded. Furthermore, in testing novel mutations with both prototypic ‘endogenous’ (e.g. prostaglandin J2 (PGJ2)) and synthetic ligands (thiazolidinediones, tyrosine agonists), we observed that synthetic agonists selectively rescue function of some PPARγ mutants. Here, we report FPLD3 patients, harbouring two such PPARγ mutations (R308P, A261E). Both PPARγ mutants exhibit negligible constitutive or PGJ2-induced transcriptional activity but respond readily to synthetic agonists in vitro, with structural modelling providing a basis for such differential ligand-dependent responsiveness. Concordant with this, dramatic clinical improvement was seen following pioglitazone treatment of the patient with R308P mutant PPARγ. A patient with A261E mutant PPARγ also responded beneficially to rosiglitazone, though cardiomyopathy precluded prolonged thiazolidinedione use. These observations indicate that detailed structural and functional classification can be used to inform therapeutic decisions in patients with PPARG mutations.
Peroxisome proliferator-activated receptor gamma (PPARγ) is a nuclear receptor originally identified in adipocytes (1). Although widely expressed, cell-based, loss-of-function studies clearly attest to its primary role in regulating adipogenesis and adipocyte function, with rodent knockout studies robustly corroborating these data (2,3). Heterozygous, dominant negative, loss-of-function mutations in human PPARG were first described in 1999 (4) with subsequent identification of many more receptor defects (5–9). Clinical findings in such cases have refined the phenotype, now known as familial partial lipodystrophy type 3 (FPLD3), characterised by a paucity of limb fat, preserved abdominal fat, insulin resistant diabetes, dyslipidaemia with particularly labile, diet-sensitive, hypertriglyceridaemia, polycystic ovarian syndrome and hypertension.
Like many nuclear receptors PPARγ has an amino-terminal activation domain (AF1), a central DNA-binding domain (DBD) and a carboxy-terminal ligand-binding domain (LBD). PPARγ heterodimerises with retinoid X receptor alpha (RXR) and transcriptional activation is triggered by ligand-binding, resulting in the release of a corepressor complex and recruitment of a coactivator complex. Fatty acids and eicosanoids can activate PPARγ, with PGJ2 considered prototypic of such putative endogenous PPARγ ligands (10–11). Indeed, structural studies suggest that the ligand binding pocket of PPARγ is ‘promiscuous’ and can accommodate several different fatty acids (3). Thiazolidinediones (TZDs), a class of synthetic PPARγ agonists, promote adipogenesis and improve insulin sensitivity, underpinning their therapeutic use as insulin sensitizers in patients with T2DM (12).
The fact that as many as 1:500 people may have missense mutations in PPARG recently prompted Majithia et al (7) to generate and functionally characterise all possible missense PPARG mutations, to expedite clinical interpretation of the growing number of missense variants identified in patients. This resource should aid prompt functional classification of novel PPARG variants. For individuals with established loss-of-function mutations and disease phenotype, therapeutic possibilities are limited. Current options include strict dietary fat and calorie restriction, metformin, insulin, GLP1 agonists and leptin has been tried in patients with very low leptin levels (13). Isolated reports of TZD use also exist (9,14–15) but responses were variable (summarised in Supplementary Table 1).
In characterising the properties of all possible PPARG missense mutations (7) we were struck by two observations. First, the spectrum of functional scores exhibited by the range of all missense PPARG variants suggested that even mutations associated with a monogenic disease are likely to perturb protein function to a variable degree, predisposing to a similarly variable phenotype, rather than fitting an arbitrary designation as disease-causing or benign. Such gradation of PPARγ dysfunction is also likely to translate into differential, graded responses to metabolic stress, and to molecularly-targeted therapeutic interventions. Second, we noted that a few variants, like R308P, manifested a clearly abnormal transcriptional response to prototypic ‘natural’ ligand (e.g. PGJ2), whereas their function when tested with a synthetic agonist was near normal (7). These in vitro observations suggested that patients harbouring such receptor mutants might respond to treatment with synthetic PPARγ agonists. Here, we report the dramatic clinical response of a patient, harbouring the R308P PPARγ variant, following treatment with rosiglitazone. We also describe a further novel A261E PPARγ mutation, present in two apparently unrelated families, with similarly discordant responses to PGJ2 versus synthetic PPARγ agonists.
Research design and methods
Participants provided informed written consent and investigations were approved by local research ethics committees and conducted in accordance with the Declaration of Helsinki.
Assessing transcriptional activity of PPARγ mutants
Characterisation of transcriptional activity of PPARγ variants was undertaken as described previously (16). In brief, 293EBNA cells, cultured in DMEM/10%FCS were transfected with Lipofectamine2000 in 96-well plates and assayed for luciferase and β-galactosidase activity following a 36-hour incubation with or without ligand with results representing the mean +/- SEM of at least three independent experiments in triplicate.
Structural modelling of PPARγ mutants
Crystallographic modelling of PPARγ mutants was undertaken using PPARγ structures (1PRG, 2PRG, 1FM9, 2ZK1, 3DZY, 2XKW) with different ligands, with results illustrated using MacPyMOL (Molecular Graphics System, Schrödinger, LLC).
Further methodological details are available in the supplementary material.
Results
Identification of PPARG mutations
Two different heterozygous missense mutations in the ligand binding pocket of PPARγ were identified in patients presenting with typical features of FPLD3; an Arg308Pro (R308P) mutation was detected in a New Zealand woman, and an Ala261Glu (A261E) mutation was identified in two unrelated women from South Africa (see supplementary material for further clinical details and Table 1 for biochemical results).
Table 1.
Biochemical findings in probands with FPLD3.
| PPARG mutation | Arg308Pro Proband 1 |
Ala261Glu Proband 2 |
Ala261Glu Proband 3 |
Normal range |
|---|---|---|---|---|
| Gender | Female | Female | Female | |
| Age at time of assessment- years | 16 | 22 | 39 | |
| Age at first presentation- years | 16 | 20 | 30 | |
| Height – m | 1.46 | 1.53 | 1.45 | |
| Weight – kg | 48.0 | 61.0 | 55.0 | |
| BMI* - kg/m2 | 23 | 26 | 26 | |
| Total body fat- % | 20 | NA | NA | |
| Predicted body fat - %** | 27 | NA | NA | |
| Truncal fat - % | 22 | NA | NA | |
| Leg fat - % | 18 | NA | NA | |
| Hypertension | No | No | Yes | |
| T2DM or IGT¶ | Yes | Yes | Yes | |
| PCOS§ | Yes | Yes | Yes | |
| NAFLD♯ | Yes | NA | NA | |
| Triglyceride - mmol/L | 13.0 | 16.6 | 11.3 | <1.7 |
| HDL-Cholesterol - mmol/L | 0.4 | 0.5 | 0.5 | >1.0 |
| Total-Cholesterol - mmol/L | 4.7 | 8.2 | 5.1 | <5.1 |
| Insulin - pmol/L | 405 | 1253 | NA | <60 |
| Glucose - mU/L | 22.4 | 6.4 | 12.3 | <6.1 |
| Glycated hemoglobin -mmol/mol | 61 | NA | 78 | 20-40 |
| ALT U/L | 20 | 9 | <30 | |
| GGT U/L | 32 | 18 | <35 | |
| Familial co-segregation | Unaffected mother and sibling are mutation negative | Affected male sibling with the mutation. | Affected male sibling with the mutation. | |
| ***Functional score | −0.932 | −3.798 | −3.798 |
NA denotes not available. Fat mass and distribution was assessed with dual energy X-ray absorptiometry (DXA) performed using a GE-lunar iDXA (software version 15).
The body-mass index (BMI) is the weight in kilograms divided by the square of the height in meters.
Predicted body fat = (1.48*BMI)-7
Type 2 diabetes mellitus (T2DM) or impaired glucose tolerance (IGT) – yes or no indicates the presence or absence of either of these conditions.
Polycystic ovary syndrome (PCOS) - yes or no indicates the presence or absence of this syndrome.
Non-alcoholic fatty liver disease (NAFLD) – yes indicates NAFLD as confirmed by ultrasound and magnetic resonance spectroscopy.
Functional score as derived from http://miter.broadinstitute.org/
Functional studies of PPARγ mutants
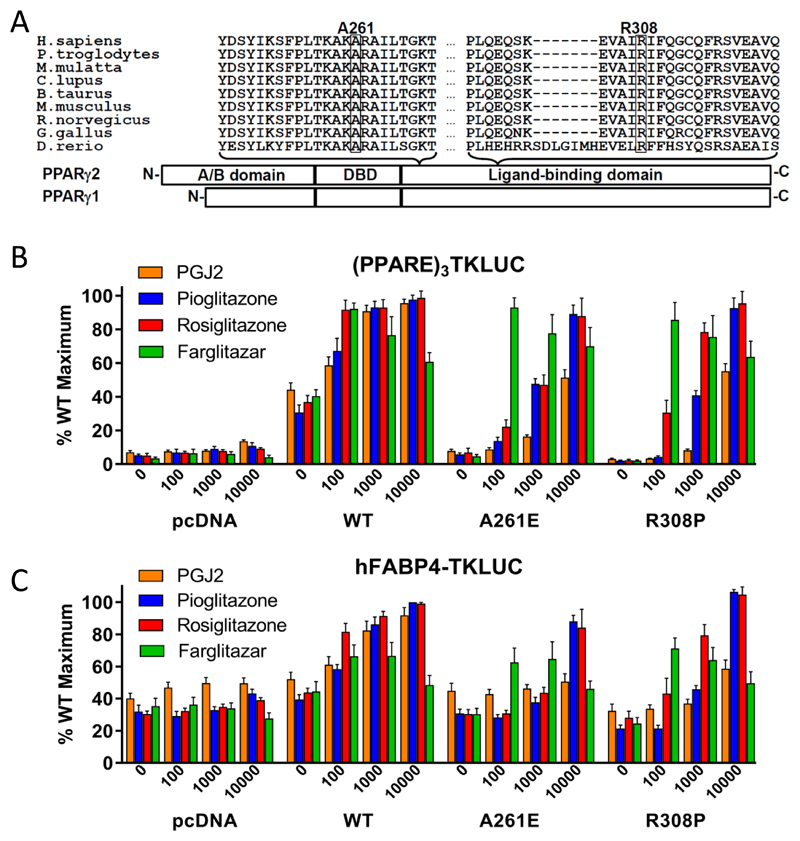
Both Arginine 308 and Alanine 261 in PPARγ are highly conserved (Figure 1A). In transfection assays using reporter constructs containing either synthetic {(PPARE)3TK-LUC} or natural (human FABP4-LUC) enhancer/promoter elements, both R308P and A261E mutants exhibited negligible basal transcriptional activity and minimal responsiveness with PGJ2 (Figure 1B, C). However, moderate (100nM farglitazar) or higher concentrations (1µM rosiglitazone; 10µM pioglitazone) of synthetic agonists restored transcriptional activity comparable to wild type receptor (Figure 1B, C). Interestingly, the R308P variant returned an intermediate, non-diagnostic, functional score when tested in a high-throughput cellular assay (Majithia et al (7); Table 1), reflecting a similar discordance between failure to respond to PGJ2 and activation with rosiglitazone. These results suggest that the R308P mutant is transcriptionally resistant to both natural ligands present endogenously within transfected cells and PGJ2, with such loss-of-function likely contributing to the patient’s lipodystrophic phenotype.
1A. Schematic representation of the three major domains of PPARγ, showing the locations of the two mutations and the conservation of the mutated residues between species (A261, R308 – PPARγ2 nomenclature).
1B. Transcriptional responses of empty vector (pcDNA), R308P or A261E mutant PPARγ2 to PGJ2 and Rosiglitazone, Pioglitazone and Farglitazar (doses in nM on x-axis) when tested with a (PPARE)3TKLUC reporter construct and Bos-β-gal internal control plasmid. Results are expressed as a percentage of the maximum activation achieved with wild type (WT) PPARγ2 and represent the mean ± SEM of at least three independent experiments in triplicate.
1C. Transcriptional responses of empty vector (pcDNA), R308P or A261E mutant PPARγ2 to PGJ2 and Rosiglitazone, Pioglitazone and Farglitazar (doses in nM on x-axis) when tested with a hFABP4-Luc reporter construct and Bos-β-gal internal control plasmid. Results are expressed as a percentage of the maximum activation achieved with wild type (WT) PPARγ2 and represent the mean ± SEM of at least three independent experiments in triplicate.
Structural modelling
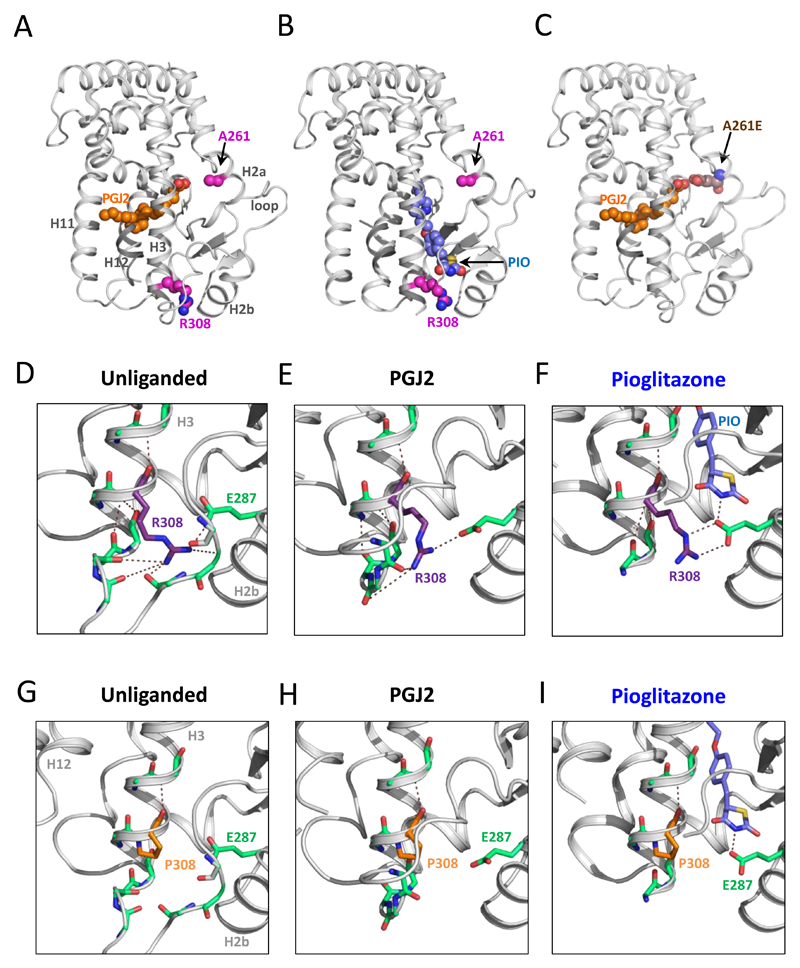
In the crystal structure of the PPARγ LBD, Ala261 and Arg308 are situated on different sides of the ligand binding pocket (Figure 2A). Arg308, located close to the amino terminus of helix 3 (Figure 2A, B), participates in an extensive hydrogen-bond network (Figure 2D-F) involving E287 in helix 2 and residues in the loop between helix 2-3. In the unliganded receptor, R308 also makes hydrogen bonds within helix 3 (Figure 2D). Upon ligand binding the loop between helix 2-3 adopts varying conformations, depending on the nature of the ligand (Supplementary Figure 1). Mutation R308P would completely disrupt both the intra and inter-helical hydrogen bond networks (Figure 2G). While PGJ2 does not alter the structural architecture of this region (Figure 2H); binding of farglitazar, rosiglitazone and pioglitazone can potentially alter the conformation of the loop between helix 2-3, thereby providing a mechanism that counteracts the destabilising effect of the R308P mutation, preserving transcriptional responsiveness to these synthetic ligands (Figure 2F,I; Supplementary figure 1B,C,D). In keeping with this prediction, proton nuclear magnetic resonance (NMR) spectral analysis confirmed that pioglitazone can bind effectively to the R308P mutant (Supplementary figure 2).
2. A-I. Crystallographic modelling based on structures of unliganded PPARγ (1PRG) or bound to PGJ2 (2ZK1) or pioglitazone (2XKW). One mutated residue (A261) is in the proximity of PGJ2 (A) whereas the other amino acid (R308) is in the vicinity of pioglitazone (B). Substitution of glutamic acid for alanine at residue 261 (A261E) can interfere with PGJ2 binding via steric hindrance (C). The side-chain of arginine 308 (R308) participates in a network of intrahelical (H3) and interhelical (e.g. E287 in H2) hydrogen bonds in unliganded (D) and liganded (E,F) PPARγ. Mutation of this residue to Proline likely disrupts this hydrogen bond network (G). PGJ2, which binds elsewhere in the ligand binding cavity, is unable to prevent loss of such interactions (H), whereas pioglitazone, which binds in the vicinity, hydrogen bonds with E287 and could preserve receptor conformation (I). H, helix.
Ala261 is located in helix 2a (Figure 2A-C) and the size and charge difference of the A261E mutation will cause displacement of helix 2a and the loop to helix 2b, thereby destabilizing the ligand binding pocket. This was confirmed using circular dichroism (CD) studies showing a lower thermal denaturation temperature compared to the wild type receptor (Supplementary Table 1). As PGJ2 docks in this part of the ligand binding cavity, its binding to receptor is expected to be impaired (Figure 2C). In contrast, receptor occupancy by rosiglitazone, farglitazar and pioglitazone is not structurally dependent on this region, correlating with preservation of transcriptional activation of the A261E mutant (Figure 2B, Supplementary figure 1).
Responses to thiazolidinedione therapy
The R308P proband had previously been treated with dietary advice and metformin, but her metabolic control remained suboptimal, so pioglitazone 30mg/day was commenced, resulting in dramatic improvements in glycaemic control and dyslipidaemia (Table 2). Her hirsutism, hyperandrogenism and acanthosis nigricans also improved. These changes were largely sustained over a 3-year period without a substantial change in BMI (23.7 kg/m2 to 22.0 kg/m2 at 12 months and 23.0 kg/m2 at 24 months).
Table 2.
Comparison of investigations before and following Pioglitazone treatment.
| Investigations | Before treatment | At 12 month treatment | At 24 month treatment | Reference range |
|---|---|---|---|---|
| Weight (kg) | 49.0 | 48.6 | 50.5 | |
| Diabetes profile | ||||
| HBA1c (mmol/mol) | 61 | 42 | 31 | 20 - 40 |
| Glucose (mmol/L) | 12.0 | 4.0 | 3.7 | < 6.1 |
| Insulin (pmol/L) | 405 | ND* | ND* | 10 -60 |
| Liver enzymes | ||||
| ALT(IU/L) | 64 | 35 | 33 | < 30 |
| GGT(IU/L) | 34 | 16 | 15 | < 35 |
| Hormonal profile | ||||
| Free Testosterone (pmol/L) | 198 | 84 | ND* | < 50 |
| SHBG(nmol/L) | 16 | 14 | ND* | 20 - 90 |
| Free Androgen Index | 450 | 214 | ND* | < 80 |
| FSH (IU/L)# | 8.8 | 4.5 | 7.1 | 3 - 25 |
| LH (IU/L)# | 14.8 | 4.3 | 5.1 | 2.0 - 25 |
| Lipid profile | ||||
| TG (mmol/L) | 13.2 | 2.4 | 1.6 | < 1.7 |
| HDL (mmol/L) | 0.4 | 0.6 | 0.7 | > 1.0 |
| Total Cholesterol (mmol/L) | 4.7 | 3 | 3.8 | < 5.1 |
| LDL(mmol/L) | 2 | 1.3 | 2.4 | < 3.4 |
ND*: not done; # FSH: Follicle stimulating hormone; LH: Luteinizing hormone; LDL: Low density lipids
HDL: High density Lipids; TG: Triglyceride
One of the A261E patients was twice treated with rosiglitazone (4 mg twice daily) when her glycaemic and triglyceride control deteriorated significantly. On each occasion, this intervention was accompanied by substantial falls in her HBA1c as well as improvements in fasting triglyceride levels, though these remained labile (Supplementary figure 3). However, therapy was discontinued because it exacerbated her severe congestive cardiac failure which ultimately caused her death at age 26 years.
Discussion
The remarkable increase in access to and use of next generation sequencing has accelerated discovery of novel Mendelian disorders and detection of mutations in genes known to cause monogenic disorders like FPLD3, where ~1:500 people harbour missense mutations (7). Whilst most are ‘benign’ or mild in their impact others are pathogenic, but likely in a graded rather than binary categorical fashion. As synthetic PPARγ ligands are licensed treatments and given the severity of the metabolic complications seen in FPLD3 patients, TZDs are obvious therapeutic options. Theoretically, FPLD3 patients could be (a) resistant to TZDs due to the extreme deleteriousness of the underlying PPARγ defect, or (b) responsive to therapy with mutations that are unresponsive to low-affinity, endogenous ligands yet activated by higher-affinity synthetic agonists, or (c) potentially ‘hyper-responsive’ to specific “designer” ligands that can overcome the molecular defect that is particular to a specific receptor mutation.
Here, we report two FPLD3-associated PPARγ mutations (A261E, R308P), whose properties fall into the latter categories (b,c above). Despite its transcriptional efficacy with wild type PPARγ, PGJ2, a ligand which is prototypic of the various, endogenous fatty acid and eicosanoid PPARγ activators, was unable to fully activate transcription mediated by A261E or R308P mutants (Figure 1B), whereas exposure to high affinity, synthetic ligands like rosiglitazone, pioglitazone and farglitazar achieved full transcriptional activity. Crystal structures of PPARγ bound to either farglitazar, rosiglitazone, pioglitazone or PGJ2 show differences between these ligands in the nature of their occupancy of the binding cavity (Supplementary Figure 1) and structural modelling provides a plausible basis for differential mutant PPARγ responses to prototypic endogenous versus synthetic ligands. Whilst synthetic agonists do not occupy the region of the pocket where A261 is situated, this residue is in close proximity to the location of PGJ2, and other fatty acid ligands (16). Modelling of the A261E mutation suggests that the alanine to glutamic acid change is likely to perturb PGJ2 binding directly via steric hindrance, whereas receptor interaction with rosiglitazone, pioglitazone and farglitazar would be preserved; correlating with the observed transcriptional responses (Figure 1B-C).
The R308P mutation involves a different part of the ligand binding cavity and this residue does not make direct contact with ligands. Structural modelling suggests that the Arginine to Proline change would disrupt local hydrogen bond networks, with deleterious conformational consequences affecting transcriptional function of the receptor. Rosiglitazone, pioglitazone and farglitazar (but not PGJ2), bind in proximity to R308, possibly stabilising receptor structure. In particular, pioglitazone, which we have shown binds effectively to the R308P mutant receptor, makes a hydrogen bond with E287 in helix 2, as does R308, counteracting the effect of the R308P mutation which is predicted to disrupt this interaction (Figure 2F-I).
In vitro studies with R308P mutant PPARγ mirrored her dramatic and sustained response to pioglitazone therapy. Thus, her case highlights the importance of recognizing and then establishing the genetic basis for severe, early-onset, metabolic disease. Identification of a PPARG mutation enabled early treatment with pioglitazone in preference to other standard glucose-lowering therapies, resulting in substantial clinical improvements in all metabolic abnormalities paralleled by specific redistribution of body fat, away from visceral and with expansion of subcutaneous depots. Moreover, structural modelling and verification with studies of mutant receptor function in vitro, provide a plausible explanation for in vivo observations. Specifically, impaired receptor activation by endogenous ligands presumably mediates diminished adipogenesis and the FPLD3 phenotype; the subsequent profound therapeutic response to pioglitazone likely reflects the ability of this synthetic agonist to bypass or overcome the molecular consequences of this mutation. In patients harbouring A261E mutant PPARγ, we have documented similar, discordant, transcriptional responses to prototypic endogenous ligand versus synthetic agonists. We have shown that this translates into a beneficial therapeutic response to rosiglitazone in one patient with this receptor defect.
Although our observations are based on prismatic case studies, they are supported by structural analyses of the other isolated reports of TZD use in FPLD3 patients (Supplementary table 1); such concordance between structural modelling of PPARγ mutations, transcriptional responses of mutant PPARγ to ligands in vitro, and clinical responses to treatment with synthetic agonists in vivo, highlight the potential for this approach to inform individualised therapeutic choices.
Supplementary Material
Acknowledgements
We are very grateful to the patients for their willingness to participate in these studies. D.B.S. and K.C and J.S are supported by the Wellcome Trust (grants WT107064, WT095564 and WT100237), the MRC Metabolic Disease Unit, the National Institute for Health Research (NIHR) Cambridge Biomedical Research Centre and the NIHR Rare Disease Translational Research Collaboration. Dr. David Savage is the guarantor of this work and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Duality of Interest.
The authors report no potential conflicts of interest relevant to this article.
Author contributions.
M.A., E.S., undertook functional studies of the mutants, analysed data and wrote parts of the manuscript. J.B. and R.M., characterised and treated proband 1, and wrote parts of the manuscript. O.R. performed sequencing studies, identified the PPARG mutations and reviewed the manuscript. C.A., co-ordinated studies and reviewed the manuscript. I.S., S.O’R., R.K.S., L.N., A.R.M. analysed data and reviewed the manuscript. L.F., F.M., J.S., undertook structural modelling of mutations, circular dichroism and NMR studies and reviewed the manuscript. A.D.M. and D.J.B. characterised probands 2 and 3, analysed data and wrote parts of the manuscript. R.M., K.C. and D.B.S. planned studies, analysed data and wrote the manuscript.
References
- 1.Spiegelman BM. PPAR-gamma: Adipogenic regulator and thiazolidinedione receptor. Diabetes. 1998;47:507–514. doi: 10.2337/diabetes.47.4.507. [DOI] [PubMed] [Google Scholar]
- 2.Lazar M. PPAR gamma, 10 years later. Biochimie. 2005;87(1):9–13. doi: 10.1016/j.biochi.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 3.Hallenborg P, Petersen RK, Kouskoumvekaki I, Newman JW, Madsen L, Kristiansen K. The elusive endogenous adipogenic PPARG agonists: Lining up the suspects. Progress in Lipid Research. 2016;61:149–162. doi: 10.1016/j.plipres.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Barroso I, Gurnell M, Crowley VEF, Agostini M, Schwabe JW, Soos MA, et al. Dominant negative mutations in human PPAR gamma associated with severe insulin resistance, diabetes mellitus and hypertension. Nature. 1999;402:880–883. doi: 10.1038/47254. [DOI] [PubMed] [Google Scholar]
- 5.Semple RK, Chatterjee VKK, O’Rahilly S. PPAR gamma and human metabolic disease. J Clin Invest. 2006;116(3):581–589. doi: 10.1172/JCI28003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agarwal AK, Garg A. Genetic basis of lipodystrophies and management of metabolic complications. Annu Rev Med. 2006;57:297–311. doi: 10.1146/annurev.med.57.022605.114424. [DOI] [PubMed] [Google Scholar]
- 7.Majithia AR, Tsuda B, Agostini M, Gnanapradeepan K, Rice R, Peloso G, et al. Prospective functional classification of all possible missense variants in PPARG. Nat Genet. 2016;48(12):1570–1575. doi: 10.1038/ng.3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robbins AL, Savage DB. The genetics of lipid storage and human lipodystrophies. Trends Mol Med. 2015;21(7):433–438. doi: 10.1016/j.molmed.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Savage DB, Tan GD, Acerini CL, Jebb SA, Agostini M, Gurnell M, et al. Human metabolic syndrome resulting from dominant-negative mutations in the nuclear receptor peroxisome proliferator-activated receptor-γ. Diabetes. 2003;52(4):910–917. doi: 10.2337/diabetes.52.4.910. [DOI] [PubMed] [Google Scholar]
- 10.Forman BM, Tontonoz P, Chen J, Brun RP, Spiegelman BM, Evans RM. 15-Deoxy-Δ12,14-Prostaglandin J2 is a ligand for the adipocyte determination factor PPARγ. Cell. 1995;83(5):803–812. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- 11.Kliewer SA, Lenhard JM, Willson TM, Patel I, Morris DC, Lehmann JM. A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor γ and promotes adipocyte differentiation. Cell. 1995;83(5):813–819. doi: 10.1016/0092-8674(95)90194-9. [DOI] [PubMed] [Google Scholar]
- 12.Soccio RE, Chen ER, Lazar MA. Thiazolidinediones and the promise of insulin sensitization in type 2 diabetes. Cell Metabolism. 2014;20:573–591. doi: 10.1016/j.cmet.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guettier JM, Park JY, Cochran EK, Poitou C, Basdevant A, Meier M, et al. Leptin therapy for partial lipodystrophy linked to a PPAR-γ mutation. Clin Endocrinol (Oxf) 2008;68(4):547–554. doi: 10.1111/j.1365-2265.2007.03095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Francis GA, Li G, Casey R, Wang J, Cao H, Leff T, et al. Peroxisomal proliferator activated receptor-gamma deficiency in a Canadian kindred with familial partial lipodystrophy type 3 (FPLD3) BMC Med Genet. 2006;7:3. doi: 10.1186/1471-2350-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Demir T, Onay H, Savage DB, Temeloglu E, Uzum AK, Kadioglu P, Altay C, Ozen S, Demir L, Cavdar U, Akiinci B. Familial partial lipodystrophy linked to a novel peroxisome proliferator activator receptor -γ (PPARG) mutation, H449L: a comparison of people with this mutation and those with classic codon 482 Lamin A/C (LMNA) mutations. Diabet Med. 2016;33(10):1445–50. doi: 10.1111/dme.13061. [DOI] [PubMed] [Google Scholar]
- 16.Agostini M, Schoenmakers E, Mitchell C, Szatmari I, Savage D, Smith A, et al. Non-DNA binding, dominant-negative, human PPARγ mutations cause lipodystrophic insulin resistance. Cell Metab. 2006;4(4):303–311. doi: 10.1016/j.cmet.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.