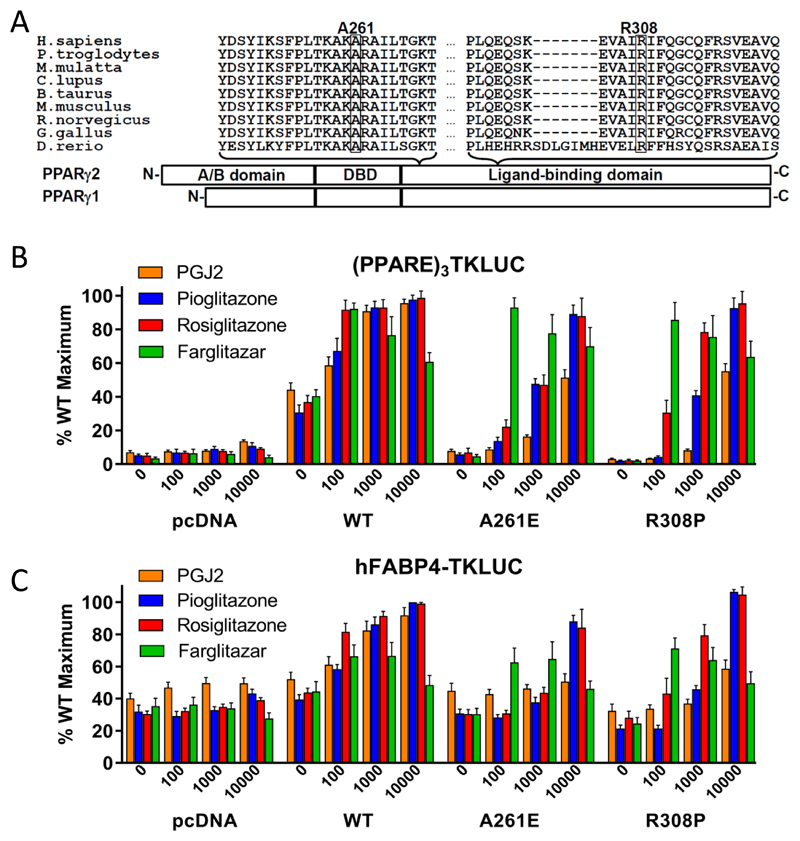
1A. Schematic representation of the three major domains of PPARγ, showing the locations of the two mutations and the conservation of the mutated residues between species (A261, R308 – PPARγ2 nomenclature).
1B. Transcriptional responses of empty vector (pcDNA), R308P or A261E mutant PPARγ2 to PGJ2 and Rosiglitazone, Pioglitazone and Farglitazar (doses in nM on x-axis) when tested with a (PPARE)3TKLUC reporter construct and Bos-β-gal internal control plasmid. Results are expressed as a percentage of the maximum activation achieved with wild type (WT) PPARγ2 and represent the mean ± SEM of at least three independent experiments in triplicate.
1C. Transcriptional responses of empty vector (pcDNA), R308P or A261E mutant PPARγ2 to PGJ2 and Rosiglitazone, Pioglitazone and Farglitazar (doses in nM on x-axis) when tested with a hFABP4-Luc reporter construct and Bos-β-gal internal control plasmid. Results are expressed as a percentage of the maximum activation achieved with wild type (WT) PPARγ2 and represent the mean ± SEM of at least three independent experiments in triplicate.