Abstract
Type 2 diabetes mellitus (DM) has reached pandemic proportions and effective prevention strategies are wanted. Its onset is accompanied by cellular distress, the nuclear factor erythroid 2-related factor 2 (Nrf2) is a transcription factor boosting cytoprotective responses, and many phytochemicals activate Nrf2 signaling. Thus, Nrf2 activation by natural products could presumably alleviate DM. We summarize function, regulation and exogenous activation of Nrf2, as well as diabetes-linked and Nrf2-susceptible forms of cellular stress. The reported amelioration of insulin resistance, β-cell dysfunction and diabetic complications by activated Nrf2 as well as the status quo of Nrf2 in precision medicine for DM are reviewed.
Keywords: Nrf2, type 2 diabetes, natural products, hyperglycemia, cellular stress, insulin sensitivity, β-cell function, vascular complications
1. Introduction
Diabetes mellitus (DM) refers to several metabolic disorders with distinct etiology but one common characteristic: hyperglycemia due to defects in insulin secretion, insulin action or both which is associated with a high risk for severe long-term complications. A rough classification discriminates between insulin-dependent or type 1, insulin-independent or type 2 DM and several other specific types such as gestational diabetes. Type 1 mainly affects young patients, is caused by autoimmune destruction of insulin producing pancreatic β-cells and consequent lack of insulin and accounts for 5-10 % of all DM patients (Katsarou et al., 2017). Type 2 or non-insulin dependent DM develops in genetically predisposed individuals, increases with age, obesity and lack of physical activity and makes up 90-95% of all diabetic cases. It is mainly associated with insulin resistance and hyperinsulinemia, followed by insulin deficiency due to β-cell failure (Chatterjee et al., 2017). With 425 million affected people in 2015 and estimated over 590 million diabetic people by 2035 the disease cluster is pandemic and ranked as one of the four main non-communicable diseases by the WHO (2016). Managing hyperglycemia is regarded as key factor for lowering the risk of diabetic complications. There are distinct classes of drugs to lower blood glucose levels ranging from metformin (inhibiting hepatic glucose production) over thiazolinediones (insulin-sensitizing peroxisome proliferator activated receptor (PPAR) γ agonists) to dipepti-dylpeptidase 4 inhibitors (gliptines, prolonging incretin action and boosting insulin secretion). Nonetheless, many patients do not reach a glycated hemoglobin level below 7% (Inzucchi et al., 2012; Inzucchi and Majumdar, 2016). The chronic hyperglycemia leads to a higher risk for micro-and macrovascular complications including retinopathy, nephropathy, neuropathy, coronary heart disease, peripheral vascular and cerebrovascular diseases (Astrup, 2011; Solomon et al., 2017; Zimmermann and Nentwig, 1989). In addition, diabetic individuals carry a higher cancer risk (Gregg et al., 2016). The disease and its complications put a massive strain on ageing sedentary societies, both on patients and carers’ side. Ways to reduce onset and complications of the chronic disease and hereby reduce the physical and psychological distress of affected people are sought for. Nuclear factor erythroid 2-related factor 2 (Nrf2) is a basic leucine zipper transcription factor that belongs to the Cap’n’Collar (CNC) family. Upon activation by various stimuli (including oxidative, electrophilic or proteotoxic stress as well as exogenous small (natural) molecules) it is one of the main players to protect and restore cellular homeostasis. Reduced clearance of oxidative stress or misfolded and aggregated proteins, indicative for an insufficient Nrf2 activity, is often associated with the etiology of diabetes and its consequences. This work reviews the current knowledge on the role of activated Nrf2 for insulin sensitivity, β-cell function and long-term complications of diabetes. Moreover, natural products targeting the Nrf2 signaling pathway are critically assessed for their potential to serve as supplements in preventive or adjuvant therapies.
2. The stress responsive transcription factor Nrf2 and its regulation
2.1. Structure and function of Nrf2
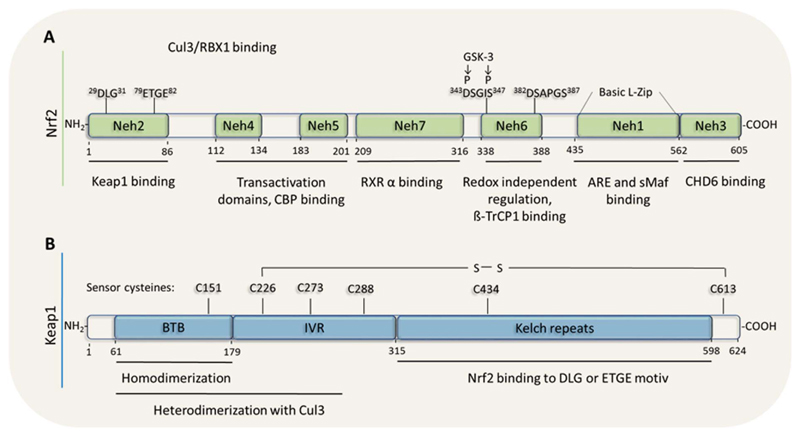
First mainly discussed as transcription factor fighting against oxidative stress, Nrf2 is now recognized for alleviating many faces of stress including xenobiotics, excessive nutrient /metabolite supply, inflammation or accumulation of misfolded proteins. Correspondingly, the activity of Nrf2 is an integral of pleiotropic signals and subject to a fine-tuned regulation on the transcriptional, posttranscriptional and posttranslational level. The Nrf2 protein contains seven functional domains, the DNA binding domain Nrf2-ECH-homology (Neh)1, the (Kelch-Like ECH-Associated Protein) Keap1 binding domain (degron) Neh2, the transactivation domains Neh3-5, the β-transducin repeats-containing protein (β-TrcP) binding domain (redox-independent degron) Neh6 and the retinoid X receptor α (RXRα) binding domain Neh7. The arrangement of the mentioned domains of Nrf2 is shown in Fig. 1A (Baird and Dinkova-Kostova, 2011; Carmona-Aparicio et al., 2015; Dayalan Naidu et al., 2015; Hayes and Dinkova-Kostova, 2014).
Fig. 1.
Domain structure of Nrf2 and Keap1. (A) The different functional domains Neh1 -7 of Nrf2, involved in DNA-, Keap 1-, coactivator-, βTrcP and RXRα-binding, are depicted. (B) The functional domains of Keap 1: The Broad complex, Tramtrack and Bric-a-Brac (BTB) domain and the double-glycine repeat (DGR) domain are responsible for protein – protein interactions. The intervening region (IVR) separates those domains and the N terminal part is, together with the BTB domain, responsible for dimerization to Cul3. In the region of the Kelch domain it can interact with Nrf2 (Neh2 domain).
The abundance of Nrf2 is usually low, mainly due to a short half-life of the protein and continuous degradation. Under stress Nrf2 escapes the proteasomal degradation machinery, accumulates and translocates from the cytosol to the nucleus where it dimerizes with small musculoaponeurotic fibrosarcoma (Maf) proteins at the Neh1 domain. The heterodimer binds to antioxidant response element (ARE) sequences (TGCnnn(G/C)TCA(T/C) in the promoter of target genes and triggers their expression. Nrf2 target genes include antioxidant enzymes such as heme oxygenase 1 (HMOX-1), y-glutamylcysteine synthetase (yGCS), peroxiredoxin (PRDX) 1, glutathione reductase (GR), thioredoxinreductase (TXNRD)1 or sulfiredoxin (SRXN), drug metabolizing and detoxification enzymes (NAD(P)H quinone dehydrogenase 1 (NQO1), glutathione-S-transferase (GST), UDP-glucuronosyltransferase (UGT)) or metabolic enzymes and regulators (glucose-6-phosphate dehydrogenase (G6PDH), transketolase, malic enzyme, RXRα, PPARγ-coactivator 1 β (PGC1-β)) (Chorley et al., 2012; Hu et al., 2006b, 2006a; Jain et al., 2006; Kwak et al., 2003b; Malhotra et al., 2010; Thimmulappa et al., 2002; Wu et al., 2012). Additionally, other gene classes including those involved in protein transport, ubiquitination, phosphorylation, cell cycle, growth and apoptosis have been identified as potentially Nrf2-dependent and are altered in studies with known Nrf2 activators. Notably, Nrf2 also negatively influences the expression of selected genes. It apparently leads to downregulation of fatty acid synthase, acetyl-CoA carboxylase or ATP-citrate lyase, which are key enzymes in fatty acid biosynthesis (Tanaka et al., 2008). So far it is not clear whether this is due to direct repression, miRNA mediated mechanisms or other means. ChIP-Seq (Chromatin Immunoprecipitation Sequencing) analyses, however, showed direct binding of Nrf2 in the vicinity or promoters of the interleukins (IL)-1β and 6 which resulted in impaired recruitment of RNA polymerase 2 and repression of the proinflammatory cytokines (Kobayashi et al., 2016).
2.2. Regulation of Nrf2 activity
2.2.1. Keap1-dependent degradation (canonical pathway)
The canonical activation pathway affects the stability of Nrf2. Under non-stressed conditions, two molecules of Keap1, also referred to as inhibitor of Nrf2 (INrf2), bind to one molecule Nrf2 at the Neh2 domain of Nrf2 occupying the DLG and ETGE motif. Being an adaptor for Cul3-Rbx1 E3 ubiquitin ligases, Keap1 leads to Nrf2 ubiquitination and proteasomal degradation. Like this, basal levels of Nrf2 are usually kept quite low. Upon an oxidative or electrophilic insult multiple sensor cysteines in Keap1 (Kobayashi et al., 2009) are oxidized or covalently modified. The resulting conformational change of Keap1 either interferes with the Nrf2/Keap1 interaction or with the alignment of Keap1 with the E2 ubiquitin conjugating enzyme, putting a break on sequestration, ubiquitination and degradation of Nrf2. This results in Nrf2 accumulation, nuclear translocation and transcriptional activation of respective target genes. After detoxification of the initial insult and restoration of the homeostatic balance Keap1 might translocate into and escort Nrf2 out of the nucleus, as suggested by one study (Sun et al., 2007). The domain structure of Keap1 including redox-sensitive cysteine residues is shown in Fig. 1B (Baird and Dinkova-Kostova, 2011; Bhakkiyalakshmi et al., 2015; Carmona-Aparicio et al., 2015).
2.2.2. Non-canonical regulation of Nrf2 abundance
Besides the canonical pathway several other ways have been identified to influence the stability or degradation of Nrf2. They act upon cues that are partly redox-independent and derive from cellular metabolism, such as glycogen metabolism or autophagy (Hayes and Dinkova-Kostova, 2014). Glycogen synthase kinase (GSK) 3β phosphorylates Nrf2 at the Neh6 degron (after a priming phosphorylation by a so far unknown kinase) and hereby mediates ubiquitin-mediated proteasomal degradation via the ubiquitin E3 ligase βTrcP (Cuadrado, 2015; Rada et al., 2012). Another way of proteasomal depletion of Nrf2 is regulated by the endoplasmic reticulum (ER)-resident E3 ubiquitin ligase Hrd1, a mechanism that so far was only found in liver cirrhosis (T. Wu et al., 2014b). In addition, also autophagy influences Nrf2 abundance. p62, also known as sequestosome-1, is one factor that targets specific cargoes for autophagy. Phosphorylation of p62 at Ser 351 by several kinases (including the inflammatory kinase TGF-β-activated kinase (TAK) or the nutrient/energy sensor kinases mammalian target of rapamycin (mTOR) and AMP-activated kinase (AMPK)) leads to sequestration and lysosomal degradation of Keap1 and thus to accumulation of Nrf2 (Hashimoto et al., 2016; Ichimura et al., 2013; Komatsu et al., 2010; Lee et al., 2016; Rhee and Bae, 2015). P62 can also compete with Nrf2 for Keap1 and thereby increase the number of Nrf2 molecules escaping Keap1-mediated ubiquitination and degradation (Hast et al., 2013). Of note, p62 is a Nrf2 target gene, creating a positive feed-forward activation loop (Jiang et al., 2015). A similar mechanism of competition for Nrf2 or Keap1 binding and hereby reducing the number of Nrf2/Keap1 complexes is reported for other proteins including the cell cycle regulator p21 (Chen et al., 2009), the Ras GTPase-activating-like protein IQGAP1 (Kim et al., 2013), Wilms tumor gene on the X chromosome (WTX), partner and localizer of BRCA2 (PALB2) or dipeptidyl-peptidase 3 (DPP3) (Hast et al., 2013). A general decrease in the number of Keap1 molecules can be achieved by downregulation of Keap1 at the transcriptional and translational level as well as by degradation (Cheng et al., 2017).
Nrf2 abundance can also be regulated via its synthesis. This includes regulation on the transcriptional level, e.g. by AhR-mediated transcription, by modulation of Myc and Jun binding to the Nrf2 promoter (DeNicola et al., 2011) or Notch signaling (Wakabayashi et al., 2014), on the posttranscriptional level via mRNA stability or altered translational efficiency (W. Li et al., 2010b; Perez-Leal et al., 2013) or on a epigenetic level. The latter comprise DNA methylation, histone modification or microRNA (Guo et al., 2015). In this context it has been reported that the Nrf2 promoter is methylated and silenced (Yu et al., 2010) in certain cancer cells. Furthermore, several microRNAs have been identified to control Nrf2- or Keap1 levels including miR144, miR34a, miR28 or miR200a. (Eades et al., 2011; Narasimhan et al., 2012; Sangokoya et al., 2010; Yang et al., 2011).
2.2.3. Posttranslational modifications
Nrf2 is furthermore subject to posttranslational modifications including phosphorylation, acetylation and distinct interaction partners which may fine-tune stability, nuclear translocation, nuclear residence or transactivation capacity. Phosphorylation at Ser40 by protein kinase C (PKC) (Bloom and Jaiswal, 2003), at Ser550 by AMPK (Joo et al., 2016b, 2016a) (both in murine Nrf2) or at Thr395, Ser433 and Thr439 by cyclin dependent kinase 5 (Cdk5) (in human Nrf2; Jimenez-Blasco et al., 2015) are thought to lead to enhanced stability and/or nuclear accumulation. Phosphorylation by GSK3β increases a) nuclear export of Nrf2 (Salazar et al., 2006, p. 3) and, as mentioned above, b) βTrcP-mediated degradation (Rada et al., 2011). The mitogen-activated kinases (MAPK), like extracellular signal regulated kinase (ERK), Jun-N-terminal kinase (JNK) and p38, phosphoinositide-3-kinase (PI3K)/Akt, casein kinase, double stranded RNA activated protein kinase (PKR) like endoplasmic reticulum kinase (PERK) and protein kinase A (PKA) have also been reported to positively regulate Nrf2 activation in several studies (Cullinan et al., 2003; Kulkarni et al., 2014; Yu et al., 2000). Thus, Nrf2 can integrate signals from kinases downstream of membrane receptors, and translate them into cell protective transcriptional responses. It needs to be noted, though, that direct interaction between the respective kinase and endogenous Nrf2 in living cells has not been proven for each kinase, and phosphorylation sites and their relevance for the entire Nrf2 transcriptome also remain elusive in some cases. MAPK have been suggested to be responsible for phosphorylation at Ser215, 408 and 577 of Nrf2. Serine to alanine mutations in Nrf2 at the respective sites did, however, hardly alter the transcriptional activity of Nrf2 (Sun et al., 2009). Therefore, although the kinases may influence Nrf2 signaling, indirect effects on e.g. protein synthesis or phosphorylation of transcriptional cofactors/coactivators need to be considered (Shen et al., 2004).
Acetylation of Nrf2 at several lysines within the Neh1 domain by p300 increases promoter binding of Nrf2 (Sun et al., 2007), but acetylated CnC (the Nrf2 homologue in Drosophila) shows reduced activity due to inhibitory interaction with the bromodomain-containing BET protein Fs(1)h (Chatterjee et al., 2016). The histone deacetylase Sirt2 removes acetyl groups from lysines 506 and 598 of Nrf2, leading to reduced total and nuclear Nrf2 levels (Yang et al., 2017). Apparently, site and context of acetylation are decisive for the impact on Nrf2 activity.
Moreover, the process and extent of transactivation of target genes are open to modulation by distinct Nrf2 interactomes at the respective promoters (Hussong et al., 2014). For instance, RXRα binding to the Neh7 domain of Nrf2 at ARE sites reduces transactivation of Nrf2 target genes (Wang et al., 2013; J. Wu et al., 2014a).
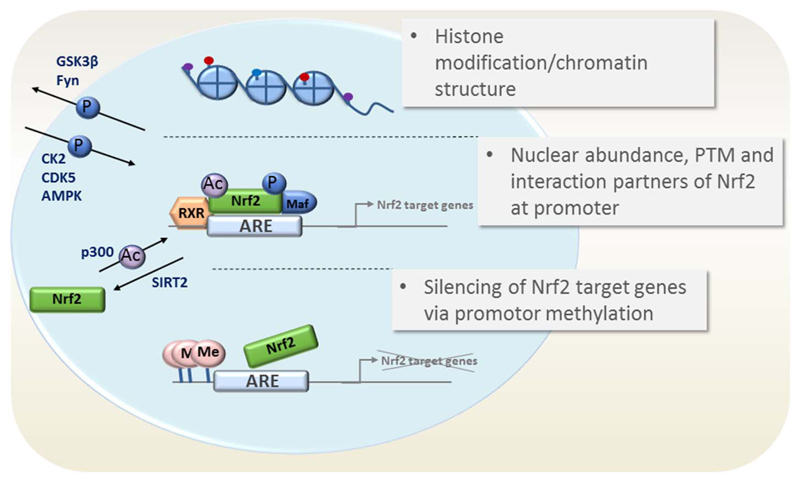
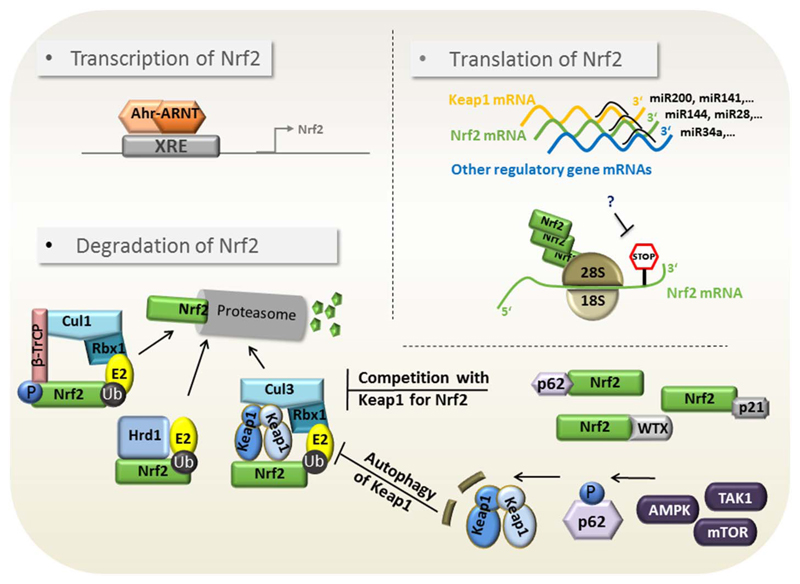
Nrf2-dependent transcription is highly regulated, able to integrate pleiotropic cues from different nodes in the cellular signal transduction network and can –based on approximately 200 target genes- also elicit evenly variable responses to cellular stressors. Figs. 2 and 3 illustrate the main points of Nrf2 regulation on the level of target gene transactivation and Nrf2 abundance, respectively. The depicted endogenous control points are also susceptible to pharmacological modulation, as seen in the next chapter.
Fig. 2.
Control of transcription and transactivation of Nrf2 target genes. Possible mechanisms impinging on Nrf2 target gene expression are depicted. They include (i) altered chromatin structure via histone (de)acetylation, (ii) altered nuclear abundance of Nrf2 (via PTM of Nrf2) (iii) an altered transactivation activity based on interaction partners and PTM of Nrf2 or (iv) promoter silencing of Nrf2 target genes.
Fig. 3.
Control of Nrf2 abundance. Possible mechanisms impinging on cellular Nrf2 levels are depicted. They include (i) altered transcription of Nrf2 mRNA (ii) altered translation of Nrf2 mRNA via miRNA or translational repression and (iii) altered degradation of Nrf2 protein via direct interference with Keap1, βTrcP1, Hrd1-mediated proteasomal degradation as well as reduction of the Nrf2/Keap1 complexes via p62-mediated autophagic degradation of Keap1 or competitive binding of Nrf2.
3. Prominent modes of Nrf2 activation by phytochemicals
Rather than providing an exhaustive list of the steadily increasing number of natural products that are reported to positively influence Nrf2 signaling, we will briefly summarize the different main mechanisms of how Nrf2 activation can be achieved by natural products. This is accompanied by Table 1, depicting selected examples for prominent mechanisms. For a comprehensive collection of natural Nrf2 activators the reader is referred to previous excellent reviews on that topic (Chun et al., 2014; Jadeja et al., 2016; Lu et al., 2016; Magesh et al., 2012; Qin and Hou, 2016; Stefanson and Bakovic, 2014).
Table 1.
Possible modes of Nrf2 activation by natural compounds and selected examples.
| Site of action | Exemplary natural products | Model | References |
|---|---|---|---|
| Abundance of Nrf2 | |||
| Modification of cysteines in Keap1 | Sulforaphane | in vitro, HepG2 and HEK293 cells | (Eggler et al., 2005; Hong et al., 2005a, 2005b; Hu et al., 2011) |
| Curcumin | NRK-52E and LLCPK1 cells | (Balogun et al., 2003) | |
| Quercetin | HepG2 cells | (Tanigawa et al., 2007, p. 2) | |
| Xanthohumol | Murine Hepa1c1c7 cells | (Dietz et al., 2005) | |
| GSK3β/TrcP inhibition | Sulforaphane Salidroside Esculentoside A |
Male Sprague–Dawley Rats “HepG2 cells |
(Cai et al., 2017; Shang et al., 2015; Wang et al., 2016) |
| General proteasome inhibition | Lactacystin | Murine neuroblastomas | (Dick et al., 1996; Fenteany et al., 1995) |
| Clasto-lactacystin β-lactone | Cos7 cells, Human Jurkat T and prostate cancer (LNCaP, PC-3) cells | (Kobayashi et al., 2004; Nam et al., 2001) | |
| EGCG | Human Jurkat T and prostate cancer (LNCaP, PC-3) cells | (Nam et al., 2001) | |
| Resveratrol | mouse macrophages, | (Qureshi et al., 2012) | |
| Upregulation of p62/autophagy | Hydroxytyrosol | human retinal pigment epithelial cells | (Hwa Ko et al., 2017; Qiu et al., 2017; Zou et al., 2012) |
| Didydromyricetin | C57BL/6 mice | ||
| Rapamycin | THP-1 derived macrophages | ||
| Epigenetic regulation | Curcumin | TRAMP-C1 cells and in TRAMP mouse model | (Khor et al., 2011) |
| Sulforaphane | TRAMP-C1 cells | (Zhang et al., 2013) | |
| Z-ligustilide and Radix angelica sinensis) | TRAMP-C1 cells | (Su et al., 2013) | |
| Overcoming translational repression | Apigenin | ARE-HepG2 cells, HEK-293T cells | (Perez-Leal et al., 2017) |
| PPI disruption | EGCG | RAW264.7 cells | (Chiou et al., 2016) |
| Nuclear localization and transactivation potential of Nrf2 (via PTM) | |||
| Phosphorylation by PKC | Curcumin (via PKC delta) | human monocytes, Caco-2-cells | (Lin et al., 2015; Rushworth et al., 2006; Zhai et al., 2013) |
| Phosphorylation by p38 | Quercetin | Human hepatocytes | (Yao et al., 2007) |
| Curcumin | NRK-52E cells, LLC-PKi cells | (Balogun et al., 2003) | |
| VSMC cells | (Kang et al., 2008) | ||
| Red ginseng oil | HepG2 cells | (Bak et al., 2016) | |
| Phosphorylation by ERK | Quercetin | Human hepatocytes | (Yao et al., 2007) |
| Curcumin | NRK-52E and LLCPK1 cells | (Balogun et al., 2003) | |
| Sulforaphane | Caco-2 cells | (Jakubíková et al., 2005) | |
| EGCG | Bovine aortic endothelial cells (BAECs) | (Wu et al., 2006) | |
| Resveratrol | PC12 cells | (Chen et al., 2005) | |
| Cinnamic Aldehyde | HepG2 cells (ERK1/2, Akt, and JNK signaling pathways activated) | (Huang et al., 2011) | |
| Phosphorylation by PI3K/Akt | Lycium barbarum polysaccharide | C57BL/6J mice and HepG2 cells | (Yang et al., 2014) |
| EGCG | Bovine aortic endothelial cells (BAECs) | (Wu et al., 2006) | |
3.1. Covalent modification of Keap1
Keap1 possesses 27 cysteine residues which function as sensors for oxidative or electrophilic insults. Whenever such cysteines are oxidized or alkylated, Keap1 changes conformation so that Nrf2 can escape from the Keap1-mediated ubiquitination and is stabilized. Of note, a so-called cysteine code has been suggested, i.e. (i) specific cysteines of Keap1 are more prone to modification than others and (ii) different Nrf2 activating compounds show a bias for certain cysteines (Kobayashi et al., 2009; Suzuki et al., 2013). Whether observable distinct biological responses to different Nrf2 activators may be caused by modifications of specific Keap1 residues is still to be uncovered. Cysteines playing an essential part in the regulation are cysteines 151, 273, and 288. Keap1-dependent Nrf2 inducers possess the ability to react with sulfhydryl groups by oxido-reduction, alkylation or thiol disulfide interchange. They show remarkable structural diversity and include Michael acceptors (olefins or acetylene conjugated with electron withdrawing groups), oxidizable diphenols and diamines, conjugated polyenes, hydroperoxides, trivalent arsenicals, heavy metals, isothiocyanates, dithiocarbamates, dithiolthiones, or vicinal dimercaptans (Talalay et al., 1988). Examples of natural products covalently modifying Keap1 are sulforaphane or curcumin (see Table 1).
3.2. Interruption of the Keap1/Nrf2 interaction
Next to direct modification of Keap1, compounds can weaken the Nrf2/Keap1 protein-protein interaction (PPI) by directly occupying the sites of mutual Nrf2/Keap1 binding. Such compounds interrupt the protein/protein interface, a feature which is optimally accomplished by peptides mimicking one of the interaction partners. However, also non-peptidic small molecules were identified to act as PPI modulators, including epigallocatechin-3-gallate (EGCG) (Chiou et al., 2016; Wells, 2015; Yasuda et al., 2016). An indirect variation of this theme is the upregulation of alternative binding partners of Nrf2 or Keap1, such as p21 or p62 (e.g. by rapamycin (Hwa Ko et al., 2017)), which may then outcompete the respective protein from the Nrf2/Keap1 complex. Compounds selectively interfering with TrcPβ /Nrf2 interface have not been reported up to now.
3.3. Epigenetic regulation
A way to further boost Nrf2 abundance is to increase de novo synthesis. This is of special interest when the Nrf2 promoter is epigenetically silenced. In this case demethylation of the promoter by inhibitors of DNA methyltransferases (DNMT) or activators of DNA demethylases could lead to increased Nrf2 expression. Histone deacetylase inhibitors or activators of histone acetyl transferases could boost Nrf2-dependent gene expression by loosening up the chromatin or by (de)acetylation of Nrf2 itself. Additionally, compounds affecting microRNAs that control the expression of Nrf2 or Keap1 also fall into this category of epigenetic regulators. Examples of such epigenetic Nrf2 modulators of natural origin include curcumin or sulforaphane (see Table 1).
3.4. Modulation of kinase activities
Phosphorylation of Nrf2 influences its abundance and activity. Therefore, natural compounds capable of modulating certain kinase activities can have a pronounced influence on Nrf2 signaling. Direct or indirect inhibitors of GSK3β can activate Nrf2 signaling (Gameiro et al., 2017; Rojo et al., 2012), as they are likely to blunt βTrcP-mediated degradation and impede nuclear exclusion of Nrf2. Indirect inhibition can be obtained by activators of AMPK or PI3K/AKT kinase. Activation of AMPK or PKC has also been positively associated with direct Nrf2 phosphorylation and activity/abundance. Thus, direct or indirect activators of these two kinases are thought to contribute to elevated Nrf2 signaling as well. The same applies for compounds activating MAPK (ERK, JNK and p38) signaling, although their detailed mode of interaction with Nrf2 is not completely resolved yet. In addition, mTOR inhibition or AMPK activation is able to trigger autophagy and thus presumably p62-mediated Keap1 degradation and Nrf2 activation. Natural modulators of kinase activities with an influence on Nrf2 signaling comprise chlorogenic acid, xanthohumol, β-lapachone, or berberine (see also Table 1) (Han et al., 2017; Lv et al., 2017; Mo et al., 2014; Park et al., 2016; Zimmermann et al., 2015).
3.5. Overcoming translational repression
Due to a specific codon composition in the 3’ portion of Nrf2 mRNA, basal translation of the transcript is normally repressed. This repression can be overcome through the activation of a still unknown factor, which might be a protein or nucleic acid that increases Nrf2 translation and is susceptible to modulation by natural products, as exemplified by apigenin (Perez-Leal et al., 2017, 2013).
3.6. Other mechanisms
General inhibitors of the proteasome activity also lead to Nrf2 accumulation and activation, as exemplified in several studies (Dreger et al., 2009; Fujie et al., 2016; Luo et al., 2011). Natural products fitting in this category may comprise resveratrol or EGCG (see also Table 1). Moreover, as a highly interacting and integrative signaling hub Nrf2 may also be influenced by modulation of cross-talking signaling pathways, such as the arylhydrocarbon receptor, PPAR γ, RXR or the nuclear factor κ B (NF-κB) pathway (Wakabayashi et al., 2010).
3.7. Concluding considerations
It is notable that many natural compounds impinge on more than one point in the Nrf2 signaling network, such as xanthohumol which covalently modifies Keap1 (Liu et al., 2005) and activates AMPK (Lv et al., 2017; Zimmermann et al., 2015) or sulforaphane that alkylates Keap1 (Hong et al., 2005a) and acts as epigenetic regulator (Royston and Tollefsbol, 2015). The same applies for the protein targets affected by the natural products. For instance, activation of AMPK may lead to direct phosphorylation and stabilization of Nrf2, to inhibition of GSK3β and βTrcP-triggered degradation as well as increased autophagy of Keap1. This poly- and network- pharmacology may result in an additive, synergistic but sometimes also antagonistic effect on the finally obtained Nrf2 response. Moreover, the individual mechanisms leading to Nrf2 activation are per se rarely specific for Nrf2 but also affect other signal transducers in a cell. Likewise, molecules carrying a Michael system, often also classified as pan-assay interference compounds, will modify accessible cysteines of proteins other than those of Keap1 (as in IKK β (Kwok et al., 2001), p65 (Lyss et al., 1998), PTEN (Pitha-Rowe et al., 2009), STAT3 (Heiss et al., 2016)), or compounds that inhibit DNMTs will epigenetically also wake promoters of genes besides Nrf2 or those under the control of Nrf2. The inherent polypharmacology of natural products as well as the often rather unspecific mechanisms leading to Nrf2 activation complicate predictions of the expected biological response elicited by a natural product and the unambiguous identification of the underlying mechanisms as Nrf2 activation. The situation gets even more complex when the investigated natural product exerts a concentration-dependent biphasic response on Nrf2 signaling and related regulatory hubs (Calabrese et al., 2008). Bioavailability and metabolism of natural products are further crucial parameters to be considered when Nrf2 activation is targeted orally in an in vivo setting and may vary markedly between different compounds. For instance, whereas the bioavailability of sulforaphane is generally high with a value of up to 82% when orally applied to Wistar albino rats (Hanlon et al., 2008), curcumin and resveratrol only show low bioavailability with values of 1% (Yang et al., 2007) and < 1% (Walle, 2011), respectively. Different formulations have already been shown to improve bioavailability of few selected polyphenolic compounds such as curcumin and to boost delivery to target tissues (Holder et al., 1978; Sharma et al., 2001, Prasad et al., 2014). Further work needs to be invested in this respect in order to increase the value of additional bioactive natural products for potential human use. At this point it should not be forgotten, though, that by activation of Nrf2 exogenous small molecules accelerate metabolization, detoxification and thus termination of their own bioactivity by inducing the Nrf2 target genes GST, UGT or NQO1.
4. Cellular distress in DM and Nrf2
4.1. Oxidative Stress
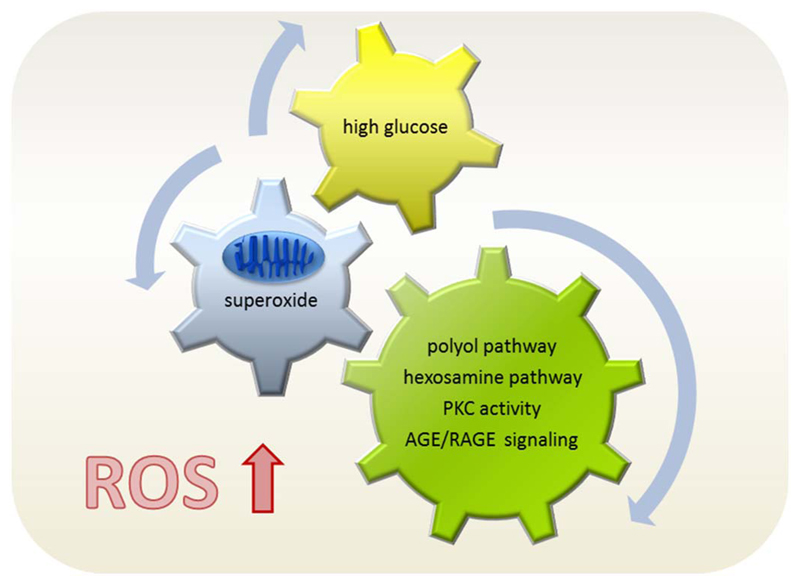
Concomitant hyperglycemia, hyperinsulinemia and inflammation characterize type 2 DM and contribute to a pro-oxidative milieu. DM is a disease of increased redox-stress, e.g. the imbalance between production of reactive oxygen species (ROS) and antioxidant defense mechanisms with a tilt to the oxidants and disturbed redox signaling. Diabetic patients were reported to display both an abnormally high production of ROS and a reduced expression of antioxidant enzymes (Fath El-Bab et al., 2013; Fiorentino et al., 2013; Gorin and Block, 2013; Jha et al., 2016; Newsholme et al., 2016; Rochette et al., 2014; Schaffer et al., 2012). Aberrantly high cellular glucose levels and subsequent redox stress appear as upstream trigger for most of the tissue damage and severe long term complications associated with DM (Brownlee, 2001; Sheetz and King, 2002). Glucose is usually funneled through glycolysis and mitochondrial oxidative phosphorylation when exceeding the capacity of the mitochondrial electron transport chain, electrons leak directly to O2 and form superoxide resulting in damage of DNA, lipids and proteins (Palmeira et al., 2007). Superoxide-triggered DNA damage, poly-ADP-ribose-polymerase activation, and finally inhibition of glycolytic enzyme glycerine-aldehyde-phosphate dehydrogenase lead to an accumulation of glucose and glycolytic metabolites that are diverted into the polyol pathway and PKC activation. Increased enzymatic conversion of glucose to the polyalcohol sorbitol is accompanied by decreases in NADPH and glutathione and hereby aggravated redox stress. Additionally, elevated levels of diacylglycerol boost PKC activity which in turn contributes e.g. to insulin resistance (by serine phosphorylation of insulin receptor substrate (IRS)) and activation of NADPH oxidases (NOX) producing more superoxide (Hoffman et al., 1991; Idris et al., 2001; Rastogi et al., 2017). Interestingly, PKC also activates Nrf2 signaling, likely as a counterbalance for the initiated ROS production. Moreover, glucose-induced redox stress is one major culprit for uncoupling and dysfunction of endothelial NO synthase (eNOS), the onset of endothelial dysfunction and subsequent micro-and macrovascular complications (H. Li et al., 2014a). The biochemical pathways responsible for ROS production under hyperglycemia are summarized in Fig. 4.
Fig. 4.
Biochemical routes of ROS production under hyperglycemia. High cellular glucose levels lead to an overwhelmed electron transport chain in the mitochondrial membrane, resulting in superoxide production and finally in activation of alternative metabolic pathways which further aggravate the cellular redox load.
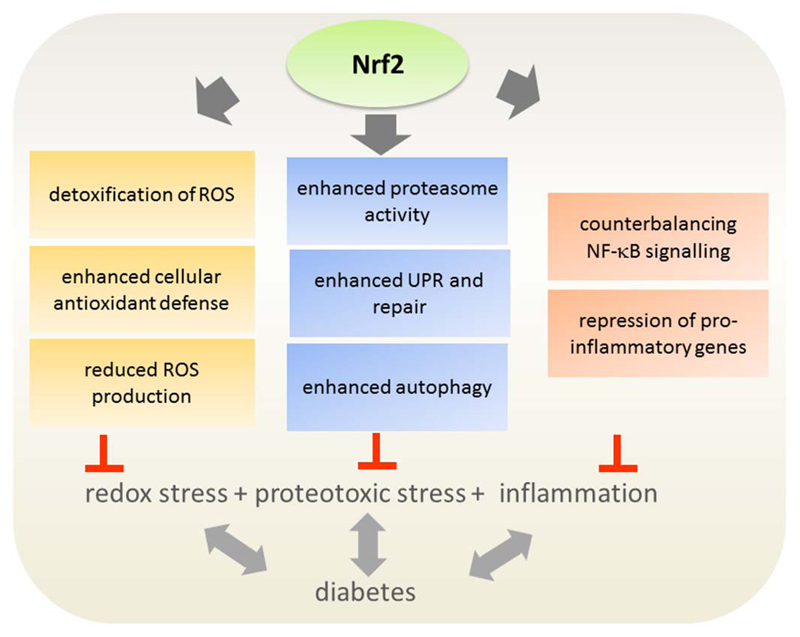
Activation of Nrf2 can counteract the pro-oxidative situation by launching expression of enzymes which (i) directly detoxify ROS, such as superoxide dismutase (SOD) and catalase or (ii) elevate the cellular antioxidant defense by raising glutathione or NADPH levels, such as γGCS, GR, malic enzyme, or G6PDH, (iii) by increasing mitochondrial biogenesis and function and hereby reducing mitochondrial superoxide production, (iv) by inducing the expression of enzymes of the pentose phosphate pathway and hereby drawing glucose off the polyol pathway, (v) by preserving endothelial function and (vi) lowering blood glucose levels (Aleksunes et al., 2010; Bhakkiyalakshmi et al., 2015; Heiss et al., 2009; Uruno et al., 2015). Thus, Nrf2 activation appears as conceivable solution for the oxidative stress associated with hyperglycemia. By increasing several enzymes of antioxidant response Nrf2 may furthermore deliver more promising results than the supplementation with a single antioxidant that only stoichiometrically scavenges ROS and failed to provide significant benefit for diabetic complications so far (Jung and Kwak, 2010).
4.2. Proteotoxic stress
One manifestation of redox stress in DM is the covalent modification of proteins including oxidation of sulphur containing amino acids, glutathionylation or nitrosylation (Chaudhari et al., 2014). In addition, high glucose levels in DM can lead to unwanted non-enzymatic glycation or enzymatic glycosylation of proteins. Reducing sugars react with free amino groups through a series of reactions resulting in Schiff bases being converted via Amadori rearrangements to advanced glycation end products (AGE) (Nowotny et al., 2015; Singh et al., 2001). Next to altered function of the macromolecule, extracellular AGE can bind to receptors for AGE (RAGE) on the membrane of susceptible cells and lead to formation of ROS, pro-inflammatory and pro-coagulant signaling, entangling hyperglycemia, redox stress, inflammation and thrombosis (Singh et al., 2001). The hexosamine biosynthesis pathway, also fueled by elevated sugar levels, provides UDP-N-acetyl glucosamine and substrate for O-glycosyltransferases (OGT). This enzymatic O-glycosylation is to be discerned from the complex N-or O-linked glycosylation pattern usually conferred to transmembrane or secretory proteins at the ER or Golgi. All these mentioned posttranslational modifications lead to changes in protein conformation, function, solubility and stability and challenge the delicate and dynamic equilibrium among protein synthesis, folding and degradation, also referred to as proteostasis. Not surprisingly, proteotoxic stress has been correlated with DM in several studies (Grattagliano et al., 1998; Su and Dai, 2016; Telci et al., 2000a, 2000b).
Cellular proteostasis is normally brought about by different molecular chaperones, the unfolded protein stress response (UPR), the ubiquitin proteasome system (UPS), or autophagic clearance (Höhn et al., 2017). To prevent an accumulation of oxidatively damaged proteins in phases of (mild) oxidative stress, heat shock proteins are induced that prevent accumulation of damaged proteins, as exemplified by Hsp70, which is also involved in proper folding of nascent proteins. Several chaperones, including Hsp70, have been reported to be under the control of Nrf2 and heat shock factor (HSF)-1. HSF-1 is one of the master regulators of protein homeostasis (Dayalan Naidu et al., 2015; Ji et al., 2016). Of note, Nrf2 and HSF-1 share overlapping target genes, suggesting a fine-tuned team work between the two stress-responsive transcription factors (Dayalan Naidu et al., 2015).
The ER is the major site of protein folding and posttranslational modifications. Disruption of quality control mechanisms results in accumulation of misfolded or unfolded proteins and increased ER stress. To counter such situations the cell launches the UPR, organized by three sensor ER transmembrane proteins: IRE1 (inositol requiring kinase 1), PERK and activating transcription factor 6 (ATF6). The net result of their activation is increased expression of chaperones, a downsized de novo protein synthesis, and removal of aggregated proteins by ER-stress associated proteasomal degradation (ERAD) or autophagy. If stress is too severe and exceeds the capacity of the defense mechanism, cells undergo apoptosis (Sozen and Ozer, 2017). Among the various transcription factors that are induced or repressed by the UPR, Nrf2 plays a major role in regulating the non-antioxidant and antioxidant response triggered by the UPR. Nrf2 activation is interrelated with the UPR sensor PERK, controls genes involved in the ER redox control and disulphide formation and directly activates ubiquitin/proteasome genes involved in ERAD (Cominacini et al., 2015; Glover-Cutter et al., 2013).
There are two main pathways of removing damaged, unfolded, or dysfunctional proteins, namely the UPS and the autophagic machinery. The 26S proteasome is a large proteolytic complex consisting of the core 20S proteasome and a 19S regulator. One of the most important regulators of the proteasomal system is Nrf2 which is able to induce transcription of 19 out of 36 tested subunits of the proteasomal system (Kwak et al., 2003a). Whereas the UPS may be regarded as a major degradation system for short-lived proteins, autophagy is mainly responsible for the degradation of long-lived proteins and other larger cellular contents like organelles. Evolved as a recycling-response to starvation, autophagy is also hampered in DM mainly due to reduced AMPK activation and increased mTOR activity during hyperinsulinemia and hyperglycemia. There is a mutual crosstalk between Nrf2 signaling and autophagy: Activated Nrf2 can positively regulate the expression of p62, also known as sequestosome-1 and targeting specific cargoes for autophagy. p62 in turn, boosts cellular Nrf2 activity, since phosphorylation of p62 brings about autophagic degradation of Keap1 or disruption of the Keap1/Nrf2 complex (Glick et al., 2010; Lamark et al., 2009; Xiao et al., 2017).
In addition, some Nrf2 target genes, including glyoxylase 1 and aldoketoreductase, are involved in reducing carbonyl stress and the repair of modified proteins (Ellis, 2007; Xue et al., 2012).
Taken together, during (pre-)DM several cell types may experience a disturbed proteostasis by aberrant PTM of proteins, an overwhelmed folding capacity of the ER or accumulation of dysfunctional proteins. The role of Nrf2 target genes in relieving proteotoxicity and maintaining a healthy protein turnover comprises damage prevention and repair as well as the removal of damaged proteins by the UPS and autophagy.
4.3. Inflammation
DM is closely linked with an uncontrolled immune response. Whereas in type 1 DM β-cells are attacked by the own immune system, the onset of type 2 DM is accompanied by a chronic inflammation, mainly caused by preceding and coinciding obesity in the affected individual. The ongoing growth of adipose tissue causes hypoxia through inadequate vascularization. This attracts immune cells which release proinflammatory cytokines and cause chronic low-grade inflammation in the fat tissue. The inflammatory signaling causes elevated redox stress and decreased insulin sensitivity in adipocytes (e.g. serine phosphorylation of IRS 1 by proinflammatory kinases) and other peripheral tissue (e.g. by reduced secretion of adiponectin and increased secretion of resistin) (Goldfine et al., 2011; Romeo et al., 2012; Shoelson, 2006; Shoelson et al., 2003; Wellen and Hotamisligil, 2005). Moreover, in obesity the gut microbiome changes which may result in an elevated intestinal permeability and an increased leakage of lipopolysaccharides (LPS) aggravating the inflammatory condition (Dapito et al., 2012; Hua et al., 2016).
Activation of Nrf2 is generally thought to counter inflammation (Ahmed et al., 2017; Cardozo et al., 2013; Kim et al., 2010). Besides a direct repression of cytokine promoters via proximal binding (Kobayashi et al., 2016), Nrf2 also engages in a vivid functional crosstalk with the pro-inflammatory transcription factor NF-κB (Wakabayashi et al., 2010; Wardyn et al., 2015). Although several natural products (e.g. sulforaphane, curcumin or EGCG) activate Nrf2 signaling with a concomitant repression of NF-κB and its target genes, the assumable picture of a completely antagonistic effect of the two transcription factors is too simple, also in light of the fact that both factors share inducing stimuli such as ROS, LPS, flow shear stress, oxidized low-density lipoproteins, or cigarette smoke (Ahn, 2005; Anwar et al., 2005; Carayol et al., 2006; Go et al., 2004; Hosoya et al., 2005; Knorrwittmann et al., 2005; Rushworth et al., 2005). Rather, robust but fine-tuned NF-κB and Nrf2 activities appear essential for a proper inflammatory response and its resolution. In situations of an imbalance between Nrf2 and NF-κB pathways (as in chronic inflammation) exogenous activation of Nrf2 may help to put a break on NF-kB signaling.
Nrf2 is also discussed to be involved in the activation of inflammasomes. Those are protein complexes made up of a sensor and scaffold protein, an adaptor protein as well as caspase 1 with an important role in immunity and pathogenesis of diseases like atherosclerosis and type 2 DM (Strowig et al., 2012). Upon stress inflammasomes assemble, caspase-1 is activated and in turn processes immature (pro-)forms of cytokines (e.g. IL-1β and -18) for secretion (Dinarello, 2009). Recently, Nrf2 expression has been reported to be required for inflammasome activation in murine cells in vitro and in vivo (Freigang et al., 2011; Zhao et al., 2014), but this requirement was controversially discussed since high concentrations of certain Nrf2 activators seem to inhibit inflammasome activation (Greaney et al., 2016; X. Liu et al., 2016c; Maier et al., 2015; Miglio et al., 2015). A recent study may have solved this discrepancy by showing that expression of Nrf2 indeed supports inflammasome activation in murine and human cells. However, Nrf2 activating compounds block activation of caspase-1 in different cell types and hereby dampen inflammasome-dependent inflammation in vivo. Both effects are not caused by changes in Nrf2 target gene expression, but rather by an interaction of components of the Nrf2/Keap1/Cul3/Rbx1 complex with caspase-1, demonstrating a physical link to inflammasomes (Garstkiewicz et al., 2017).
The increased pro-oxidative, proteotoxic and inflammatory conditions in DM (type 2) tend to “stress out” the delicate redox- and protein homeostasis and endanger functionality and viability of cells and tissues. Increased Nrf2 activity and expression of its target genes may alleviate those insults (for summary see Fig. 5). Notably, Nrf2 is upregulated in the early phase of diabetes, suggesting that it acts as the body´s natural defense against hyperglycemia-induced damage. However, the adaptive process seems to fail in later stages in which the Nrf2 system is driven beyond its limits by chronic glucose stimulation. Therefore, exogenous activation of the Nrf2 signaling pathway by natural products looks like a plausible mean to support endogenous anti-stress systems and prevent the development or progression of DM and its complications (Tan and de Haan, 2014). In the following we will challenge this notion with the existing scientific evidence regarding the benefits of Nrf2 activation and natural Nrf2 activators in the context of insulin resistance, β-cell dysfunction, and micro-and macrovascular problems.
Fig. 5.
Potential cellular stress relief by Nrf2 activation in diabetes. Hyperglycemia and obesity as encountered in (type 2) DM disturb the cellular redox balance and proteostasis as well as elevate the inflammatory status. Nrf2 and its various target genes may counteract these insults and successfully increase the cellular detoxification, repair and survival capacities.
5. Insulin sensitivity
The term insulin sensitivity refers to the ability of myocytes, hepatocytes, white adipocytes, endothelial cells and others to respond to insulin and to mediate the metabolic effects of insulin, such as increased glucose and fatty acid uptake and storage as glycogen and triacylglycerol, respectively, as well as decreased gluconeogenesis. Those cells may become insulin-resistant resulting in elevated plasma glucose levels despite the presence of high insulin levels (hyperinsulinemia). This is a typical phenomenon in the pathogenesis of type 2 DM (Martin et al., 1992). Possible mechanisms for the decreased response to insulin could be a reduced number or a defect of insulin receptors as well as impaired signaling from the ligand-activated insulin receptor to the metabolic PI3K/Akt/mTOR signaling pathway. The latter can be caused by factors like high levels of free fatty acids, low grade chronic inflammation, or oxidative stress (Lucidi et al., 2010; Shoelson, 2006; Tangvarasittichai, 2015; Wang, 2010). Since oxidative and cellular distress in general seem to be main factors in the development of insulin resistance (Tangvarasittichai, 2015; Wiernsperger, 2003), keeping or restoring homeostasis by Nrf2 activation appears as a viable approach to prevent or alleviate impaired insulin signaling.
5.1. Insulin sensitivity and Nrf2
Indeed, chemical (de Souza et al., 2016; Nagata et al., 2017; Saha et al., 2010) or genetic (Uruno et al., 2013; J. Xu et al., 2013a) activation of Nrf2 increased insulin sensitivity in mice. In line with these data Nrf2 deficiency resulted in increased ROS levels leading to higher blood glucose levels and impaired insulin signaling in murine models (obese mice compared to obese Nrf2 knockout mice) (Beyer et al., 2008; Xue et al., 2013). Moreover, hepatic insulin resistance occurred as adverse effect in Nrf2 knockout mice upon exposure to high fat diet (HFD). This might be traced down to increased oxidative stress and an elevated pro-inflammatory status. Accordingly, increased activation of NF-κB and its downstream effectors IL-6 and tumor necrosis factor (TNF)-α were detected in Nrf2 knockout mice (Z. Liu et al., 2016b). In the context of hepatic insulin resistance, promising results have also been obtained with bardoxolone-methyl (CDDO-Me), a derivative of the natural oleanolic acid and a well-known potent Nrf2 activator. It was shown to prevent the induction of liver steatosis and insulin resistance in mice on HFD. Alongside the impeded development of insulin resistance, CDDO-Me overcame alterations in the expression of protein tyrosine phosphatase 1B, IRS, or insulin receptor (all key players in the insulin signaling pathway) as well as prevented hepatic macrophage infiltration and inflammation. However, if these beneficial effects are regulated only via Nrf2 activation remains to be elucidated (Camer et al., 2015). The effects of Nrf2 activation have also been investigated in the murine skeletal muscle (SkM), either upon SkM-specific Keap1 knockout or administration of the triterpenoid Nrf2 activator 1-[2-cyano-3,12-dioxooleane-1, 9(11)-dien-28-oyl] imidazole (CDDO-Im). In both cases the blood glucose level was decreased and the expression of the glycogen branching enzyme and a subunit of phosphorylase kinase (PhKα) was upregulated by activated Nrf2. This was accompanied by a reduced muscle glycogen content, increased glucose uptake and improved glucose tolerance. In consistence with this data an insulin tolerance test indicated, that Nrf2 induction, via Keap1 knockout or CDDO-Im administration, enhanced insulin sensitivity in mice on HFD (Uruno et al., 2016, 2013). Another study revealed fibroblast growth factor 21 (FGF21) as Nrf2-dependent gene. Knockdown of Keap1 led to an upregulation of FGF21 protein in the plasma and increased hepatic mRNA levels in a diabetic mouse model (db/db and high-calorie induced obesity model, 8 weeks). FGF21 is known for ameliorating hyperglycemia and insulin resistance (Coskun et al., 2008; Kharitonenkov et al., 2005; Xu et al., 2009). Furthermore, FGF21 was also upregulated when activating Nrf2 with CDDO-Im in db/db, but not in db/db Nrf2 knockout mice (Furusawa et al., 2014). Similar results have been shown in vitro where FGF21 levels were decreased in Nrf2 knockdown 3T3-L1 cells (Kim et al., 2017). A more recent work employed selenocystein-tRNA knockouts (TrspRIPKO mice). These mice suffered from increased oxidative stress and severe insulin resistance due to the absence of any seleno-proteins. When Nrf2 signaling was activated by crossing Keap1-floxed with the TrspRIPKO mice, oxidative stress was diminished and leptin and insulin sensitivity were improved (Yagishita et al., 2017).
Besides these mainly “pro-Nrf2” findings, there are also several reports suggesting a negative role of Nrf2 for insulin sensitivity: In a study with HFD-induced obese mice Nrf2 activation, via knockdown of Keap1, elicited an impaired glucose metabolism and an increased insulin resistance in 6-week-old mice. However, this observation was transient as 8-week-old mice displayed no significant differences in glucose tolerance and insulin sensitivity between the two groups (Xu et al., 2012). Also another study observed attenuated insulin resistance, improved glucose tolerance and partial protection from HFD induced obesity in Nrf2 knockout mice compared to their wild type fellows (180 days observation period). This might due to increased mRNA expression and protein levels of FGF21 in the knockout group. The negative influence of Nrf2 on FGF21 was confirmed by lowered levels of FGF21 in ST-2 cells, which overexpressed Nrf2 (Chartoumpekis et al., 2011). An ameliorated insulin sensitivity in case of Nrf2 deficiency was corroborated by other investigators as well (Meakin et al., 2014; Meher et al., 2012): Meher et al. observed a decreased insulin resistance and reduced expression of inflammatory genes in the stromal vascular fraction in a myeloid Nrf2 knockout. Moreover, those knockout mice were protected from HFD-induced inflammation of adipose tissue. Meakin et al. reported that Nrf2 knockout mice on HFD showed increased insulin sensitivity compared to wild type mice (168 days observation period). However, the knockout group showed a higher incidence rate of nonalcoholic steatohepatitis and was not protected from obesity. This is of special interest, since increased lipid accumulation is usually positively correlated with insulin resistance. The role of Nrf2 for insulin sensitivity seems to depend on a substantial and context-dependent (transcriptional) influence on metabolic hubs and pathways, including FGF21 or lipid ana-and catabolism. The influence of Nrf2 on metabolism is also reflected by a significantly increased frequency of congenital intrahepatic shunts in Nrf2 knockout mice (shunts were formed in 2/3 of Nrf2 knockout mice). These shunts bypass the portal blood flow from the liver, which results in increased hepatic oxygen and changes in protein expression and function in Nrf2 knockout mice (Skoko et al., 2014).
Besides that metabolic facet, Nrf2 is mainly believed to boost on insulin sensitivity via upregulation of antioxidant genes and reduction of redox stress. In contrast to this notion, enhanced levels of antioxidants were found in obese and type 2 diabetic animals and patients suffering from insulin resistance and type 2 DM (Costa et al., 2002; Jiménez-Osorio et al., 2014). Another key finding was that insulin resistance, induced by hyperinsulinemia, is connected to permanent Nrf2 activation (Wang et al., 2012; Xu et al., 2012). However, it is not absolutely clear whether elevated antioxidant signaling is causally contributing to insulin resistance, or a consequence of hyperinsulinemia, indicative for the cells’ attempt to restore homeostasis and prevent deterioration. In contrast to increased Nrf2 expression in type 2 DM associated hyperinsulinemia, insulin negatively regulates Nrf2 expression in a type 1 DM mouse model (Ghosh et al., 2017).
Table 2 gives an overview on the partly controversial results on Nrf2 activation and insulin sensitivity.
Table 2.
Influence of Nrf2 on insulin sensitivity.
| Nrf2 | Model system | Insulin sensitivity | Suggested mechanism | Reference |
|---|---|---|---|---|
| ↓ | ob/ob Nrf2-KO mice | ↓ | Increased ROS levels | (Beyer et al., 2008; Xue et al., 2013) |
| ↑ | db/db mice: Keap1-KO or CDDO-Im | ↑ | FGF21 induction | (Furusawa et al., 2014) |
| ↓ | 3T3-L1 Nrf2-KO cells | ↓ | FGF21 downregulation | (Kim et al., 2017) |
| ↓ | HFD induced ob Nrf2-KO mice, long term (180 d) | ↑ | FGF21 induction | (Chartoumpekis et al., 2011) |
| ↓ | HFD induced ob Nrf2-KO mice | ↓ | Increased oxidative stress, NF-κB, IL-6 and TNF-α | (Z. Liu et al., 2016c) |
| ↑ | HFD induced ob Keap1-KO mice or CDDO-Im | ↑ | Increased PhKα expression | (Uruno et al., 2016, 2013) |
| ↑ | TrspRIPKO crossed with Keap1-floxed mice | ↑ | Oxidative stress of TrspRIPKO is diminished | (Yagishita et al., 2017) |
| ↑ | HFD induced ob mice, CDDO-Me | ↑ | Inhibition of protein tyrosine phosphatase 1B | (Camer et al., 2015) |
| ↑ | HFD induced ob Keap1-KO mice | ↓ | higher blood glucose, triglyceride, fatty acid and insulin levels | (Xu et al., 2012) |
| ↓ | Nrf2 KO myeloid cells | ↑ | reduced IL-1ß induction | (Meher et al., 2012) |
| ↓ | HFD Nrf2 KO mice | ↑ | increased H2O2 levels inhibit protein tyrosine phosphatase 1B | (Meakin et al., 2014) |
5.2. Natural Nrf2 activators and insulin sensitivity
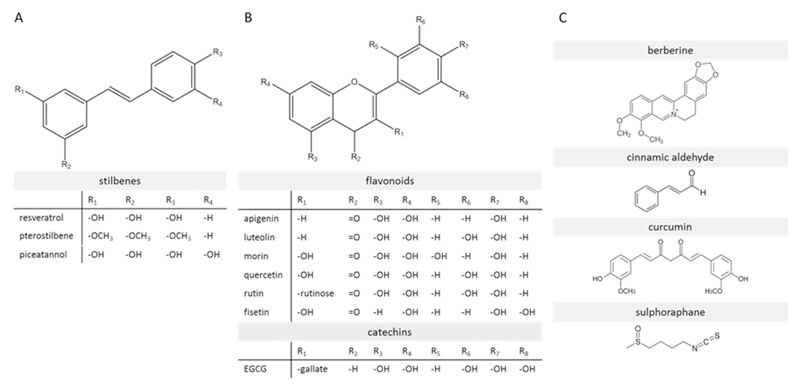
Several natural compounds, shown to activate Nrf2 signaling, are under investigation for their ability to ameliorate insulin resistance, including stilbenes. As shown in Fig. 6A stilbenes have an aromatic character and show complete conjugation of their double bonds. Here we focus on three prominent examples of that group with the ability to induce Nrf2 activity. Despite their similar structure, different mechanisms of Nrf2 activation have been proposed. Resveratrol is supposed to act via upstream kinases, mainly via the ERK pathway (Cheng et al., 2012) and piceatannol was suggested as direct binding partner of the Nrf2 repressor Keap1 (Lee et al., 2010). The mechanistic details for pterostilbene are not well understood, but Nrf2-Keap1 dissociation and phosphorylation of Nrf2 were already linked to that compound (Bhakkiyalakshmi et al., 2016a). Resveratrol is presumably the most prominent representative of this group of Nrf2 activating substances. Its ability to activate Nrf2 signaling and subsequently reduce oxidative stress has been shown in several studies (Baur et al., 2006; Cheng et al., 2015, 2012; Fischer et al., 2017; Palsamy and Subramanian, 2011). Cheng et al. reported that resveratrol protects from insulin resistance, induced by methylglyoxal (MG, a reactive dicarbonyl) in HepG2 cells and in murine models and connected this at least partially to Nrf2 activation (Cheng et al., 2015, 2012). Furthermore, increased insulin sensitivity and activation of AMPK by that stilbene has been reported in vivo (Baur et al., 2006). AMPK appears to be connected to the Nrf2 pathway (Joo et al., 2016a; Zimmermann et al., 2015). Based on these promising results, resveratrol underwent also clinical trials. In a recent one, including 43 patients with diabetes, orally administered resveratrol managed to improve insulin sensitivity significantly within a time period of 1 month (Zare Javid et al., 2017). In this trial, no investigation regarding any causal relationship with Nrf2 or any other potential mechanisms was performed. Pterostilbene showed anti-diabetic properties, which are at least partially Nrf2 driven, in several studies. Activation of Nrf2 by pterostilbene has been shown in vitro and in vivo in pancreatic and liver tissue leading to induction of downstream target genes, increased insulin secretion, decreased blood glucose and ROS levels (Bhakkiyalakshmi et al., 2016a, 2016b; Chiou et al., 2011; Fischer et al., 2017). Therefore, also an improvement of the insulin sensitivity by pterostilbene is likely. This was already shown in vivo more than a decade ago, although no investigations into the correlation with Nrf2 were performed at that time (Grover et al., 2005). The structural derivative piceatannol binds to Keap1 and induces the Nrf2/HMOX-1 signaling axis (Lee et al., 2010). In the presence of piceatannol, impaired insulin signaling was restored in vitro via Nrf2 activation (Jeong et al., 2015).
Fig. 6.
Structure of prominent natural activators of Nrf2 signaling (with particular relevance for this review). (A) Stilbenes (B) flavonoids (e.g. apigenin, luteolin, rutin,) and catechins (EGCG) (C) other relevant natural compounds (e.g. cinnamic aldehyde, curcumin, sulforaphane).
The isothiocyanate sulforaphane (Fig. 6C) is another well-known natural Nrf2 activator (Wu et al., 2013). In contrast to the previously discussed stilbenes it is a weak pro-oxidant interacting with the critical cysteine thiols at position 151,489 and 583 of Keap1 (Hong et al., 2005a). Sulforaphane and also its precursor glucoraphanin have been identified to alleviate insulin resistance and to increase glucose tolerance in vivo (de Souza et al., 2016; Nagata et al., 2017). This positive effect was abolished in Nrf2 knockout mice, indicating that Nrf2 is a responsible target for the improvement of insulin sensitivity by sulforaphane (Nagata et al., 2017). Compared to resveratrol or curcumin, sulforaphane shows a rather high bioavailability due its lipophilic character, which enables passive diffusion through the cell membrane (Hanlon et al., 2008). Moreover, sulforaphane obtained from broccoli sprouts, has already been tested in clinical trials involving patients suffering from DM type 2. In addition to a favorable impact on the lipid profile (Bahadoran et al., 2012a) and inflammatory markers (Mirmiran et al., 2012), sulforaphane led to a significant improvement of insulin sensitivity and lower fasting glucose levels (Bahadoran et al., 2012b) The authors suggested that the ameliorated insulin resistance resulted from the antioxidant enzymes induced by activated Nrf2. The flavonoids rutin and quercetin (Fig. 6B) also showed the ability to lower plasma glucose levels and decrease oxidative stress by activating Nrf2 (Sun et al., 2015; Tian et al., 2016). In addition, both compounds also acted as insulin sensitizer based on in vivo investigations in an obese mice model using an extract of Chrysobalanus icaco L. containing the two flavonoids (White et al., 2016). However, no detailed and reliable mechanistic studies were performed to prove a connection between these flavonoids, Nrf2 activation and increased insulin sensitivity. Curcumin (Fig. 6C), from Curcuma longa, was identified as an activator of Nrf2 (Wu et al., 2013) and its downstream targets like HMOX-1 (Balogun et al., 2003). Different mechanisms were suggested for its positive effects on insulin sensitivity in several studies. In fructose-fed rats curcumin activated Akt and ERK1/2, which are upstream kinases of Nrf2, in the liver (J.-M. Li et al., 2010a). Additionally, inhibition of inflammation and oxidative stress and/or a direct interaction with the insulin receptor are possible mechanisms. Furthermore, AMPK was suggested as another target of curcumin, influencing insulin signaling (Na et al., 2011). Despite uncertain mechanistic details and inconclusive clinical trials so far, several studies reported increased Nrf2 levels after curcumin administration, mostly in murine models, and linked it to an improved insulin sensitivity (He, 2012; Weisberg et al., 2008; S.-G. Zhao et al., 2011a). The French lilac contains galegine and is traditionally used to lower the blood glucose level (Perla and Jayanty, 2013). A derivative of galegine, metformin, is successfully used as blockbuster antidiabetic drug for more than two decades. Metformin increases insulin sensitivity and decreases glucose levels especially in the liver (Stumvoll et al., 1995). Although the responsible mode of action of the drug is not fully understood and current models range from inhibition of mitochondrial complex I and AMPK activation (Andrzejewski et al., 2014) to inhibition of glycerol phosphate dehydrogenase and an altered hepatic redox state (Madiraju et al., 2014), the insulin sensitization is thought to be driven mainly by AMPK activation. However, AMPK and Nrf2 seem to engage in a crosstalk (Habib et al., 2016; Joo et al., 2016b, Onken and Driscoll, 2010), and metformin was also shown to lead to Nrf2 activation (Ashabi et al., 2015; Prasad et al., 2017). Therefore, a participating role of Nrf2 for the insulin sensitizing properties of metformin cannot be excluded and is even likely.
5.3. Bottom line
Despite some controversy on the role of Nrf2 for insulin signaling, the majority of in vitro and in vivo studies promote Nrf2 as promising potential target and natural Nrf2 activators as promising adjuvant for the improvement of insulin sensitivity. However, studies relying on in vitro tests in human cancer cell lines (such as HepG2) may be considered with some caution as those cells may have a decreased or altered dependency on growth factors and insulin (Lazar and Birnbaum, 2012). Most of the in vivo studies were performed in murine models and extrapolation to the human situation remains to be assessed. Several clinical trials with antioxidant supplements or vitamins (Fu et al., 2016; Pi et al., 2010; Wiernsperger, 2003), as well as Nrf2 activators (de Zeeuw et al., 2013b) so far failed to show beneficial effects on insulin sensitivity or prevention of type 2 DM, presumably due to some off-target and even harmful effects (Bjelakovic et al., 2007; Jain, 2012). Moreover, a study from 2014 revealed HMOX-1, a prominent Nrf2 target gene, as strong positive predictor of metabolic disease in obese subjects. Conditional depletion of HMOX-1 in liver cells and macrophages in mice protected from diet-triggered insulin resistance and inflammation, indicating a causative role of HMOX-1 for “metaflammation” (Jais et al., 2014). Unfavorable effects of chronically high antioxidant levels, e.g. brought about by activation of Nrf2, may be explained by impaired signaling pathways that rely on ROS for signal relay. This applies also for insulin that depends among others on an initial redox burst after receptor binding for transient inhibition of redox sensitive protein tyrosine phosphatases (Pi et al., 2007; Rhee, 2006). Therefore, further systematic investigations using several different models (high fat diet vs genetic induction of diabetes, transient vs constitutive activation of Nrf2, systemic vs tissue specific Nrf2 activation, etc.) and the hyperinsulinemic-euglycemic clamp method as a gold standard for insulin sensitivity (Kim, 2009), are still needed to fully understand the detailed role of Nrf2 for insulin signaling and to develop potential context-dependent Nrf2-based treatment strategies for insulin resistance. Another obstacle encountered with the reported natural Nrf2 activators is often the low bioavailability, the intensive metabolization and the incompletely understood polypharmacology, complicating their application in humans and reliable assignment of an observed effect to Nrf2 activation (see also chapter 3.8).
6. Pancreatic β-cell function
6.1. β-cell function and Nrf2
β-cells are one of five main cell types located in the glucose-sensing mammalian pancreas assembled in the so-called Islands of Langerhans (Lacy, 1967; Pipeleers et al., 1994). They are the only cells within the mammalian organism that can produce the hormone insulin, which is secreted in response to elevated glucose levels in the body. In patients with type 2 DM the suggested biphasic secretion of insulin, most likely initiated by different β -cell subsets, is either abolished or strongly reduced (Ashcroft and Rorsman, 2012; Hosker et al., 1989). In 2016, a study could identify four different subsets of human β -cells that respond differently to glucose and show differences in insulin release kinetics, suggesting different susceptibilities to metabolic stress (Dorrell et al., 2016). Under normal circumstances β -cells regulate plasma glucose levels very tightly, as they directly respond with increased insulin gene transcription and according hormone/protein secretion (Henquin et al., 2006), mainly in response to increased blood glucose levels, but also in reaction to specific hormones or neurotransmitters (Ashcroft and Rorsman, 2012). However, an unhealthy lifestyle, characterized by the intake of high caloric, sugar-rich food and insufficient exercise and energy expenditure and consequent insulin resistance, exposes β -cells to aberrant gluco-lipotoxic stress and can cause their exhaustion, dysfunction, and finally their death (Brownlee, 2001; Ceriello, 2003; Hunter and Stein, 2017; Nishikawa et al., 2000; Sheetz and King, 2002). Although hyperplasia of β-cells will compensate the loss for a while, this coverage cannot be sustained on a permanent basis also because β-cells are inherently prone to stress in conditions of metabolic derangements. They are among the metabolically most active tissues within the human body and highly dependent on oxidative catabolism for ATP synthesis (Pullen and Rutter, 2013). In fact, elevated oxygen consumption at high glucose concentrations appears pivotal to the stimulation of insulin secretion (Rutter et al., 2015). However, despite this high metabolic activity and the fact that ROS are an unavoidable by-product of mitochondrial respiration during glucose stimulation (and may even be required for normal glucose sensing) (Leloup et al., 2009), enzymes involved in antioxidant defense are present at unusually low levels (Lenzen et al., 1996) or encoded by disallowed genes in β-cells (Pullen and Rutter, 2013), which makes them hypersensitive to oxidative harm. Molecular targets for oxidative stress in the β-cells are hereby likely to include duodenal homeobox factor 1 (PDX1), playing an important role in pancreas development and differentiation as well as in maintaining normal β-cell function (Ohlsson et al., 1993). Due to low protein levels of antioxidant enzymes (Lenzen et al., 1996) and therefore little anti-oxidant potential in β-cells/the pancreas (Grankvist et al., 1981), boosting antioxidant signaling by Nrf2 activation seems to be a promising strategy to preserve their function in the context of type 2 diabetes. Accordingly, diabetic Keap1-conditional knockout mice showed improved insulin secretion and suppressed onset of diabetes, which corroborates a key role of the Nrf2/Keap1-axis in disease development (Uruno et al., 2013).
Closely entwined with oxidative stress, proteotoxic stress endangers proper β-cell function (Hasnain et al., 2016). As endocrine cells, they are particularly predisposed to ER stress. In order to meet the insulin demand (especially in the insulin-resistant hyperinsulinemic state) they sustain a high protein synthesis levels and need high folding capacities in the ER. Under these conditions, ER stress and UPR often exceed their repair capacities resulting in β-cell dysfunction. (Ariyasu et al., 2017). Also for the autoimmune type 1 diabetes, reduction of ER stress in pancreatic β-cells showed recently promising results (Morita et al., 2017). The UPR in β-cells engages in a vivid crosstalk with pro-inflammatory signaling cascades, e.g. by potentiating activation of NF-κB or JNK (Eizirik et al., 2013; Liu et al., 2015; Meyerovich et al., 2016; Urano et al., 2000). Recently, induction of misfolded aggregates of the islet amyloid polypeptide (IAPP) appeared to be sufficient for induction of clinical abnormalities typical of type 2 diabetes (Mukherjee et al., 2017).
Chronic inflammation has been identified as another critical factor associated with diabetic β-cells (Donath et al., 2008; Novials et al., 2014). In different diabetic (e.g. db/db mice, GK rats) and non-diabetic (C57BL/6J) rodent models, inflammatory factors like IL-6 or IL-8 were elevated in the pancreas of subjects being fed with HFD, which was associated with an increased infiltration of immune cells like monocytes or neutrophils (Ehses et al., 2007). Also, the histology of islets from patients with type 2 DM shows characteristics of inflammation such as the presence of cytokines, apoptotic cells, infiltrated immune cells and eventually fibrosis. This inflammatory process is likely to be the result of dyslipidemia, hyperglycemia, and increased circulating adipokines (Donath et al., 2008). In studies with LPS-treated rodents Choudhury et al. could demonstrate that inflammatory conditions in the pancreas lead to an accumulation of ROS, which activates NF-κB signaling and thereby leads to an induction of inflammatory cytokines. Conversely, nuclear levels of Nrf2 were decreased together with the activity of its targets. If those conditions are sustained, this might lead to β-cell death via the SAPK/Jun-N-terminal kinase (JNK) apoptotic pathway (Choudhury et al., 2015).
In addition, the pancreatic islet is furthermore highly vascularized. Besides the supply of nutrients and oxygen and the off-transport of hormones, islet capillaries and primarily endothelial cells provide pivotal signals that can enhance β-cell survival and function. In diabetes, the islet endothelial cells express markers of activation and inflammation, and in vitro data suggest that a dysfunctional inflamed islet endothelium contributes to impaired insulin release (Hogan and Hull, 2017).
Taken together, Nrf2 emerges as good target for a potential treatment/prevention of β-cell dysfunction triggered by the viciously entangled triad of oxidative stress, ER stress and inflammation. Accordingly, treatment of pancreatic β-cells with pharmacological Nrf2 activators, such as dh404, CDDO-Im or dimethylfumarate significantly increased expression of the key anti-oxidants enzymes, decreased inflammatory mediators in islets and conferred protection against oxidative stress in β-cells (Fu et al., 2015; W. Li et al., 2014b; Masuda et al., 2015). Nrf2-deficient mice were also markedly more vulnerable to arsenic-induced β-cell damage (Yang et al., 2012), and based on β-cell-specific conditional Nrf2- and Keap1- knockout mice Nrf2 could be proven to have a protective effect against reactive species in β- cells (Yagishita et al., 2014). Moreover, Nrf2 is a major regulator of enzymes involved in mitochondrial metabolism, hence allowing improved handling of elevated glucose and fatty acid levels (Hirotsu et al., 2012; Mitsuishi et al., 2012; Wu et al., 2011). Of particular note, changes in the expression or activity of the Nrf2 heterodimerization partner Maf1, which is involved in the insulin gene expression (Matsuoka et al., 2004), were also implicated in the deleterious effects of oxidative stress on β-cells (Harmon et al., 2009). Increasing MafA abundance by overexpression or impeded degradation ameliorated β-cell function under oxidative stress. However, it should not be forgotten that similar to insulin signaling, β- cells also depend on ROS for proper insulin synthesis and secretion which may be hampered by a persistent and inappropriately timed activation of the antioxidant defense (Pi et al., 2010, 2007).
6.2. Natural Nrf2 activators and β-cell function
In several different in vivo and in vitro studies, phytochemicals that have been shown to activate Nrf2-dependent (antioxidant) signaling positively influenced β-cell mass and function, including the “herbal blockbusters” sulforaphane, pterostilbene and curcumin.
Sulforaphane led to nuclear translocation of Nrf2 and expression of phase 2 enzymes in RINm5F insulinoma cells. Furthermore, it prevented the cytokine-mediated (IL-1β and IFN-γ) decrease in proliferative potential by lowering NO and prostaglandin E2 (PGE2) production and subsequent cytotoxicity (also seen in Nrf2 overexpression experiments). This effect could be pinned down to suppression of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) gene expression and of IL-1β and IFN-γ-induced NF-κB activation. Overexpression experiments of Nrf2 in RIN cells resulted in decreased DNA binding activity of NF-κB, which indicates a role of Nrf2 in inhibition of NF-κB signaling. In experiments with pancreatic islets from Sprague–Dawley rats, pretreatment with sulforaphane restored islet cell insulin secretion and corroborated the data generated in RIN cells. Treatment of mice with sulforaphane prior to streptozotocin (STZ) also led to decreased islet destruction as well as restored islet cell insulin secretion. In those experiments, also NF-κB activation was inhibited upon sulforaphane administration (Song et al., 2009). In a recent study, it was shown that sulforaphane prevented cholesterol-induced β-cell dysfunction, improved mitochondrial function, attenuated the pro-inflammatory NF-κB pathway and increased the expression of antioxidant enzymes downstream of the Nrf2 pathway in Min6-cells (Carrasco-Pozo et al., 2017).
Pterostilbene has also been shown to positively influence β-cells. Pretreatment with this compound led to a concentration-dependently increased viability and decreased apoptosis of cytokine-treated insulin-secreting MIN6 cells. Moreover, pterostilbene led to increased phospho-Nrf2 levels in nuclear fractions as well as decreased levels of NO and iNOS. In STZ-induced mice, pancreatic iNOS mRNA levels were significantly increased in comparison to the controls. However, those levels were significantly reduced upon pterostilbene administration. Detailed analysis of Nrf2 and its downstream targets (e.g. NQO1 or HMOX-1) revealed decreased mRNA expression levels in diabetic mice, which were again elevated upon treatment with pterostilbene. Both, in vitro as well as in vivo data indicate a major protective role of the polyphenol against pancreatic β-cell destruction (Sireesh et al., 2017). In the study of Bhakkiyalakshmi et al (2014) pterostilbene consistently activated Nrf2 and its cytoprotective and antiapoptotic gene expression profile in STZ-treated INSE1-1 cells. However, a stringent proof that the beneficial effects of the polyphenol are Nrf2-dependent is missing.
Rashid and Sil treated Wistar rats with STZ, which led to increased oxidative and inflammatory markers, increased apoptosis and reduced levels of nuclear Nrf2 and Nrf2-dependent defense genes in β-cells. Interestingly, all those deregulated markers could be normalized to standard levels upon treatment with curcumin (Rashid and Sil, 2015). However, to what extent Nrf2 activation, next to many other bioactivities of curcumin, is exactly involved remains elusive. Additionally, Kanitka et al. demonstrated that curcumin protects pancreatic islets against cytokine-induced redox stress, dysfunction and death in vitro and prevents STZ-induced DM in vivo. However, the impact of Nrf2 remained also unassessed (Kanitkar et al., 2009).
In addition, morin was found to activate Nrf2 and protect β-cells from genotoxic stress in vitro (Vanitha et al., 2017). 4-Dihydroxyphenylacetic acid, a microbiota-derived metabolite of quercetin, activated Nrf2, launched an antioxidant stress response and prevented β- cell dysfunction induced by high cholesterol (Carrasco-Pozo et al., 2015). Lithospermic acid B protected β- cells from cytokine- induced dysfunction, and a plant extract obtained from Magnolia officinalis, Pueraria lobata, Glycyrrhiza uralensis, and Euphorbia pekinensis (KIOM-79), protected them from STZ-induced apoptosis. Both observations went along with activation of Nrf2 signaling (Ah Kang et al., 2008; Lee et al., 2011). Phycocyanin prevented INS-1 cells from MG-induced apoptosis, an effect which was blunted by knockdown of Nrf2 (Gao et al., 2016).
6.3. Bottom line
In theory, the cytoprotective transcription factor Nrf2 appears as promising potential target for the preservation of β-cell function, in particular under the stress experienced in type 2 DM by hyperglycemia, hyperlipidemia and chronic inflammation. In actual experiments, natural activators of Nrf2 indeed afforded protection of β-cells from various insults, both in vitro and in vivo. However, although most studies did clearly show concomitant activation of Nrf2 signaling and β-cell protection by the studied phytochemical, only few did prove stringent causality. In light of the polypharmacology, the enthusiasm over Nrf2 activation by natural products as mean to protect β-cells should therefore still remain somewhat cautious. Moreover, it must be kept in mind, that a constant or untimely boosting of Nrf2 signaling can also be detrimental, as a) proper insulin synthesis and secretion are dependent on ROS and b) activation of endogenous Nrf2 was correlated with pancreatic cancer and increased drug resistance (Hong et al., 2010) (see also Chapter 7.5).
7. Diabetes-associated complications
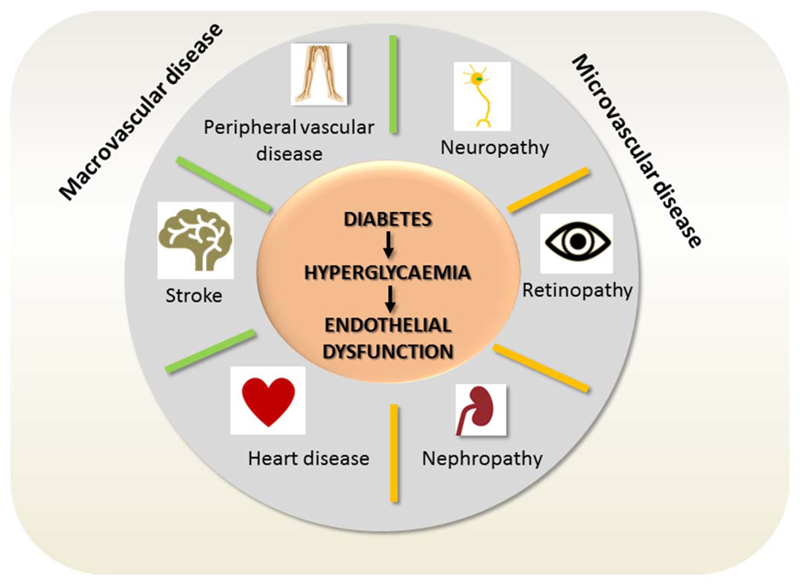
Glucotoxicity is the main culprit of the long term micro-and macrovascular pathologies associated with diabetes as summarized in Fig. 7. Although every cell in the body of diabetic patients is exposed to abnormally high glucose concentrations, vascular and nerve cells are particularly prone to damage. That is because endothelial cells in the vasculature and retina, mesangial cells in the renal glomerulus, and neurons and Schwann cells in peripheral nerves cannot sufficiently reduce the transport of glucose into the cytoplasm under hyperglycemia. They succumb the triggered cascade of oxidative and proteotoxic stress, inflammation and dysfunction, leading to the predominance of macro-and microvascular complications and nephro- and neuropathies in diabetic patients.
Fig. 7.
Diabetes-associated vascular complications. Chronic hyperglycemia is especially harmful for endothelial and nerve cells. Their dysfunction mainly accounts for the increased risk for the depicted micro-and macrovascular complications experienced by diabetic patients.
7.1. Neuropathy
7.1.1. Diabetic Neuropathy and Nrf2
Neuropathy is a common devastating complication of DM and affects about 8% of newly diagnosed patients and more than 50% of patients with longstanding disease (Boulton et al., 2005; Edwards et al., 2008). It is characterized by chronic pain or loss of sensation, recurrent foot ulcerations, and is the leading cause for non-traumatic limb amputation, accounting for significant morbidity and mortality (Gordois et al., 2003; Thomas, 1999). Diabetic neuropathy is thought to occur both from hyperglycemia-induced damage to nerve cells per se and from neuronal ischemia caused by hyperglycemia-induced decreases in neurovascular flow. The hyperglycemic damage can be assigned to the already mentioned altered metabolic pathways, the uncontrolled redox state and ER stress (Albers and Pop-Busui, 2014; Lupachyk et al., 2013a, 2013b; O’Brien et al., 2014; Obrosova et al., 2004; Shakeel, 2015; Stribling et al., 1989; Sytze Van Dam et al., 2013; Zochodne, 2014). Besides hyperglycemia, concomitant hyperlipidemia, obesity and hypertension often contribute to neuronal dysfunction in type 2 diabetics (Grisold et al., 2017).
As a transcription factor increasing the cellular resistance against redox- and ER stress, Nrf2 has been brought forward as promising target to prevent onset and progression of diabetic neuropathy (Kumar and Mittal, 2017; Negi et al., 2011a; Sandireddy et al., 2014; X. Xu et al., 2013b). Consistently, Nrf2 and its target gene HMOX-1 were activated concomitantly with the protective effect of melatonin against STZ- induced DM and diabetic neuropathy (Negi et al., 2011b), and epalrestat, currently used in the treatment of diabetic neuropathy, led to upregulated HMOX-1, SOD, catalase or glutathione levels in a Nrf2-dependent manner in rat Schwann, human SH-SY5Y or bovine aortic endothelial cells (Sato et al., 2013; Yama et al., 2016, 2015). Moreover, Nrf2-mediated HMOX-1 expression reduced formalin-induced inflammatory pain indicating a putative role in preventing sensorimotor alteration at peripheral sites (Rosa et al., 2008). Activated Nrf2 also protected retinal ganglions from redox stress by upregulation of HMOX-1 and the thioredoxin system (Koriyama et al., 2010; Tanito et al., 2007) and dorsal root ganglions from hyperglycemic damage (Vincent et al., 2009). Despite the promising premises and strong indications for successful neuroprotection by Nrf2, unambiguous validation and exploitation of the transcription factor as relevant drug target in diabetic neuropathy in several independent studies is still awaited.
7.1.2. Natural Nrf2 activators and diabetic neuropathy
Prompted by the expected stress relief and potential neuroprotection also phytochemical Nrf2 activators were subjected to animal or cell-based models of diabetic neuropathy. In in vivo models, using STZ-treated diabetic rats, rutin (Fig. 6B) produced a significant inhibition of mechanical and thermal hyperalgesia, cold allodynia, as well as partial restoration of nerve conduction velocities in diabetic rats. Furthermore, it attenuated oxidative stress and neuroinflammation with concomitant upregulated expression of Nrf2 and HMOX-1 in dorsal root ganglions (Tian et al., 2016). Luteolin (Fig. 6B) also dose-dependently alleviated abnormal sensation, improved nerve conduction velocities and nerve blood flow in diabetic rats. In further analyses, the compound successfully lowered ROS and malondialdehyde levels and significantly upregulated the protein levels of Nrf2 and HMOX-1 in diabetic nerves (Li et al., 2015). Fisetin (Fig. 6B) alleviated thermal and mechanical hyperalgesia and deficits in motor nerve conduction velocity in diabetic rats. Expression analyses indicated activation of Nrf2 and inhibition of NF-kB signaling in the treated animals (Sandireddy et al., 2016). Tang Luo Ning recipe (TLN), a traditional Chinese herbal medicine based on Huangqi Guizhi Wuwu decoction, which is used clinically to treat diabetic peripheral neuropathy in China, markedly improved the neurological function including thermal perception threshold and nerve conduction velocity in diabetic rats. Concomitantly, axon atrophy and demyelination were attenuated, ROS levels and apoptosis markers were decreased and protein levels of Nrf2 or γGCS were elevated (X. Yang et al., 2015a). Taurine also partially alleviated neuroinflammation in brains of diabetic rats and enhanced expression of Nrf2 and HMOX-1 (Agca et al., 2014). Sulforaphane (Fig. 6C) improved motor nerve conduction velocity, nerve blood flow, pain behavior and malondialdehyde levels in STZ- induced diabetic rats and elicited increased expression of Nrf2 and downstream targets HMOX-1 and NQO-1 in sciatic nerves (Negi et al., 2011c). The observed promising improvements in diabetic neuronal dysfunction upon administration of the mentioned natural products correlated well with an activation of the Nrf2- stress response signaling, however, causality remained elusive.
From cell-based studies the causality between Nrf2 activation and neuroprotection appears more evident. Likewise, the alcoholic extract of kiwi peel (Actinidia callosa, ACE) or allylisothiocyanate (AITC) inhibited the generation of ROS and the elevation of caspase-3 and capase-9 levels induced by the dicarbonyl MG in Neuro-2A cells (C.-C. Lee et al., 2014b). The transcriptional activity and nuclear translocation of Nrf2, as well as HMOX-1 and γGCS expression, were elevated by ACE or AITC treatment in MG-stressed Neuro-2A cells. The protective effects were attenuated when Nrf2 levels were reduced by knockdown showing a pivotal role of Nrf2. Similarly, siRNA mediated knockdown of Nrf2 overcame the berberine-induced HMOX-1 expression, neurite outgrowth and ROS decrease in H2O2- or high glucose-stressed SH-SY5Y cells (Hsu et al., 2013). The green tea constituent EGCG (Fig. 6B) protected immortalized rat neurons (H 19-7) from glucose-oxidase induced redox stress and cell death. The EGCG-mediated upregulation of the Nrf2 target gene HMOX-1 appeared hereby responsible for this protection as an inhibitor of HMOX-1 activity overcame the protection (Romeo et al., 2009).
7.2. Retinopathy
7.2.1. Diabetic retinopathy and Nrf2
Diabetic patients suffer from many ophthalmic complications including retinopathy (Frank, 2004) which is the leading cause of new blindness in the industrialized nations in persons aged 25–74 years. Approximately 10 million people worldwide have diabetic retinopathy, and these numbers are expected to reach up to 14–15 million in 2050 (Saaddine et al., 2008). Diabetic retinopathy is characterized by abnormal vessel development resulting in hemorrhages, ischemia and infarctions. Being very rich in polyunsaturated fatty acids and directly exposed to damaging UV radiations as well as having a high glucose oxidation rate, the retina is a ‘prime’ target for oxidative damage (Anderson et al., 1984; Catala, 2011; Nabavi et al., 2015; SanGiovanni and Chew, 2005; van Kuijk and Buck, 1992).
Nrf2 has become an attractive subject of investigation on the basic mechanisms of retinal disease (Nabavi et al., 2016). Nrf2 knockout mice develop age-dependent degeneration in the retinal pigment epithelium, indicating that Nrf2- deficiency favors retinal disease (Z. Zhao et al., 2011b), and further emphasized the negative impact of oxidative stress on retinal ganglion cells. Diabetic animals gradually lose these neurons (Barber, 2003; Barber et al., 1998; Gastinger et al., 2006; Kern and Barber, 2008), which is aggravated in Nrf2- knockout subjects (Shanab et al., 2012). Loss of the protection by Nrf2 resulted in exacerbation of the reduced vessel integrity and neural function in the retina during DM (Akimov and Rentería, 2012; Aung et al., 2014, 2013; Barber et al., 2005; C. A. Lee et al., 2014a). A study examining STZ- diabetic rats and human post mortem retina samples further corroborated the involvement of Nrf2 dysregulation (high Nrf2 levels, but low target gene expression) as a likely component of diabetic retinopathy in humans and animals (Zhong et al., 2013). Furthermore, an altered epigenetic regulation of Nrf2, Keap1 or Nrf2 target gene expression due to diabetic metabolic derangements was also suggested for the diabetic retina (Kowluru and Mishra, 2017; Mishra et al., 2014a, 2014b). Overexpression of the Nrf2 target gene HMOX-1 protected retinal ganglion cells in diabetic retinopathy through anti-inflammatory, anti-apoptotic, and anti-proliferative effects (Fan et al., 2012).
Overall, current scientific evidence is in line with the notion that redox stress is causally involved in retinopathy, and that activated Nrf2 may provide relief for the retina. Deletion or reduced effectiveness of Nrf2 tends to exacerbate the functional retinal deficits associated with diabetes. The exploitability of Nrf2 as potential target in the treatment of diabetic retinopathy is underlined by a patent application from 2010: Landers and Bingaman invented an Nrf2- stimulating compound that detoxifies and eliminates cytotoxic metabolites and is to be used for treatment of diabetic retinopathy as well as drusen formation in age-related macular degeneration (Landers and Bingaman, 2010).
7.2.2. Natural Nrf2 activators and diabetic retinopathy
Alleviation of diabetic retinopathy by natural Nrf2 activators has been investigated both in cell models and in vivo. Curcumin was found to reduce retinal oxidative stress, inflammatory markers, vascular endothelial growth factor expression, as well as endothelial and retinal Müller cell dysfunction in in vivo rodent models for diabetes. (Gupta et al., 2011; Kowluru and Kanwar, 2007; Mrudula et al., 2007; Steigerwalt et al., 2012; Zuo et al., 2013). However, none of the studies examined Nrf2 activation in their system or aimed at causally linking the observed bioactivity of curcumin with its potential to activate Nrf2 signaling. The same applies to green tea extracts or its main bioactive ingredient EGCG, which knowingly activate Nrf2 (Na and Surh, 2008). They were able to reduce ocular angiogenesis and vascular permeability as well oxidative stress marker in diabetic retinal cells (H. S. Lee et al., 2014c; Silva et al., 2013). Similarly, lutein and zeaxanthin activate Nrf2 signaling (Wu et al., 2015; Zou et al., 2014) and appear protective in diabetic retinopathy (Neelam et al., 2017). However, their bioactivity was not linked to Nrf2 activation so far either. Grape seed proanthocyanidin extract was also tested for its protective effect on diabetic retinal function in Wistar rats (Sun et al., 2016). Compared to the control diabetic rats, the extract-treated group showed improved retinal structure, increased activity of antioxidant enzymes, reduced malondialdehyde levels, and reduced apoptosis. Song et al (2016) investigated the effect of blueberry anthocyanins on diabetes- induced oxidative stress and inflammation in rat retinas. The anthocyanins increased the antioxidant capacity of the retina and protected from diabetes-induced inflammation. The employed polyphenol mixtures successfully activated Nrf2 signaling in both studies as evident by increased levels of (nuclear) Nrf2 and HMOX-1 expression. However, although the observed beneficial antioxidant effects may be likely regulated through Nrf2/HMOX-1 signaling, the final proof of causality (e.g. by a blunted effect in Nrf2- knockout animals or upon Nrf2/HMOX-1 inhibition) is missing.
In cell-based studies flavonoids have been shown to protect retinal ganglion cells from oxidative stress-induced death (Maher and Hanneken, 2005), however, without taking into account their potential to activate Nrf2. Vitamin E was found to activate Nrf2 signaling (nuclear translocation of Nrf2, induction of HMOX-1, NQO1 and γ-GCS) and prevented stress- induced ROS levels and protein carbonylation in retinal epithelial cells (Feng et al., 2010). EGCG from green tea, phloretin from apple, and [6]-shogaol and [6]-gingerol from ginger prevented MG- induced cytotoxicity in retinal epithelial cells. The investigated compounds restored MG-diminished nuclear translocation of Nrf2 and HMOX-1 expression (Sampath et al., 2016). Furthermore, lignans of Eucommia ulmoides protected retinal endothelial cell against AGE-induced injury in vitro and diabetes- induced vascular dysfunction in vivo (B. Liu et al., 2016a). The Nrf2 activator carnosol increased wound healing capacity in retinal epithelial cells (Foresti et al., 2015). The two latter studies linked the beneficial effects of the investigated compounds to the Nrf2/HMOX-1 axis, as they were overcome by inhibition of HMOX-1.
7.3. Nephropathy
7.3.1. Diabetic Nephropathy and Nrf2
Diabetic nephropathy is the prime cause of chronic kidney disease accounting for nearly half of all end-stage renal diseases and affects 20-30% of DM patients (Dronavalli et al., 2008; Kanwar et al., 2011). It is characterized by renal hypertrophy and basement membrane thickening in the early stage, extracellular matrix (ECM) accumulation, glomerulosclerosis, and interstitial fibrosis in the late stage, which eventually results in the loss of renal function (Cooper, 1998). The pathogenesis of diabetic nephropathy is far from being completely resolved, but once more, hyperglycemia and redox stress appear as primary underlying factors (Debnam and Unwin, 1996). Several in vitro studies showed that high glucose- induced renal damage goes along with excessive production of ROS. In line, many renal cell types including mesangial cells, endothelial cells, and tubular epithelial cells produce high levels of ROS under hyperglycemic conditions (Fridlyand and Philipson, 2005; Kiritoshi et al., 2003; Koya et al., 2003; Lee et al., 2005; Thallas-Bonke et al., 2008). The pro-oxidative facet of diabetic nephropathy advocates Nrf2 activation as a promising strategy for prevention. During the later stages of diabetic nephropathy, transforming growth factor-β1 (TGF-β1) is pivotal in the progression of diabetic nephropathy and glomerulosclerosis by controlling production of many ECM proteins (Border and Ruoslahti, 1990; Yamamoto et al., 1993). Interestingly, there is also interplay between the TGF-β1 and Nrf2 pathways. On the one hand, activation of the TGF-β1 pathway inhibits Nrf2-dependent expression of the γGCS, resulting in elevated ROS levels, which, in turn, further activated the TGF-β1 pathway and excessive production of ECM (Bakin et al., 2005). On the other hand, activation of Nrf2 inhibits the function of TGF-β1 in a liver fibrosis mouse model (Choi et al., 2009). Thus, activation of Nrf2 signaling may be beneficial for preventing or slowing down the progression of diabetic nephropathy by reducing ROS and TGF-β1–mediated ECM production. Indeed, Nrf2- knockout mice suffered greater STZ-induced renal damage, and Nrf2 negatively controlled the promoter activity of TGF-β1 and ECM in human mesangial cells (Jiang et al., 2010). By influencing the UPS, Nrf2 may further boost renoprotection (Goru et al., 2017). Clinical trials with bardoxolone-methyl (CDDO-Me), a potent triterpenoid Nrf2 activator, strongly underlined the potential benefits of Nrf2 for diabetic chronic kidney disease. An exploratory multi-center study included 20 patients with moderate to severe chronic kidney disease and type 2 DM and assessed clinical activity and safety of CDDO-Me (Pergola et al., 2011a). The obtained results showed an improved estimated glomerular filtration rate in 90% of the patients with a paralleled reduction in serum creatinine and blood urea nitrogen, along with an increase in creatinine clearance. Markers of vascular injury and inflammation were also improved by treatment with CDDO-Me without any life-threatening or other drug related adverse events. The following BEAM study (BEAM ClinicalTrials.gov number, NCT00811889) was a phase 2, double-blind, randomized, placebo- controlled trial with > 200 adults suffering from chronic kidney disease. Patients receiving CDDO-Me had significantly increased glomerular filtration rates, as compared with placebo (Pergola et al., 2011b). The subsequent phase 3 BEACON study with a larger cohort of patients with stage 4 chronic kidney disease and type 2 DM (de Zeeuw et al., 2013a) had to be prematurely terminated as adverse cardiovascular events and mortality were noticed in the CDDO-Me arm. A recent study has meanwhile shown that these events were linked to fluid overload in individuals at higher risk for cardiovascular events (de Zeeuw et al., 2013b). Whether the side effects are linked to Nrf2 activation or other off- targets of the highly electrophilic CDDO-Me remains to be elucidated.
7.3.2. Diabetic nephropathy and natural Nrf2 activators
In line with the presumable beneficial impact of Nrf2 activation on diabetic nephropathy, natural Nrf2 activators were heavily investigated in this respect (Al-Waili et al., 2017). Sulforaphane, cinnamic aldehyde or digitoflavone were administered to STZ-induced diabetic wild-type (WT) and Nrf2 knockout mice. The authors could show that Nrf2 activators both improved renal performance and minimized pathological alterations in the glomerulus of STZ-treated wild-type mice, but not in Nrf2 knockout mice (Y. Yang et al., 2015b; Zheng et al., 2011). Nrf2 activation reduced oxidative damage and suppressed the expression of TGF-β1, ECM proteins and p21 both in vivo and in human renal mesangial cells. In addition, Nrf2 activation reverted p21- mediated growth inhibition and hypertrophy of human renal mesangial cells under hyperglycemic conditions (Zheng et al., 2011). A role for the Nrf2 target gene metallothioneine for kidney protection by sulforaphane was also suggested. The antibiotic minocycline stabilized endogenous Nrf2 and protected against inflammasome-mediated diabetic nephrotoxicity in WT, but not in Nrf2 knockout mice (Shahzad et al., 2016). Curcumin was also brought forward as promising renoprotective agent by several studies, which has already been reviewed elsewhere (Soetikno et al., 2013; Trujillo et al., 2013). In patients with overt type 2 diabetic nephropathy, curcumin supplementation attenuated proteinuria, TGF-β and IL-8 levels (Khajehdehi et al., 2011). Although Nrf2 activation and subsequent reduction of oxidative stress and inflammation may contribute to the good outcomes, additional bioactivities of curcumin are likely to be involved, such as inhibition of activator protein 1 and NF-kB signaling (Huang et al., 2013; Pan et al., 2012; Soetikno et al., 2011). Zerumbone from ginger alleviated diabetic nephropathy in STZ-induced rats, markedly improved histological architecture in the diabetic kidney and reversed hyperglycemia- induced p38 MAPK activation and inflammatory cytokines. It further decreased the expression of intercellular adhesion molecule-1, monocyte chemoattractant protein-1 (MCP-1), TGF-β1 and fibronectin in the diabetic kidneys. However, these properties were not clearly assigned to Nrf2 activation. Coenzyme Q10 (ubiquinone) is the lipophilic soluble electron carrier of the mitochondrial electron transport chain. It led to nuclear translocation of Nrf2 (Li et al., 2016) and alleviated redox stress, leukocyte infiltration and glomerulosclerosis in diabetic rats (Ahmadvand et al., 2012; Maheshwari et al., 2014). In another study, it prevented altered mitochondrial function and morphology, glomerular hyperfiltration, and proteinuria in db/db diabetic mice (Persson et al., 2012). Green tea extracts, as well as the main constituent EGCG, also activated Nrf2 signaling and protected kidney function in in vivo DM models (Chennasamudram et al., 2012; Kang et al., 2012; Renno et al., 2008; Yokozawa et al., 2005; Yoon et al., 2014). The same duality of Nrf2 activation and renoprotection under diabetic conditions applies for extracts or individual constituents of garlic (allylcystein, allicin), Nigella sativa (thymoquinone), grape seed (proanthocyanidines), milk thistle (silibinin, silimarin), Sanziguben Granule, or Panax Ginseng (ginsenoside Rg3, panaxytryol) (Al-Trad et al., 2016; Bao et al., 2015; Fallahzadeh et al., 2012; Kang et al., 2013; Liu et al., 2006; Thomson et al., 2016; Chenxue Zhang et al., 2017b). The extent to which Nrf2 activation really contributes to the renoprotective activity of those natural products is not unambiguously clear, though.
7.4. Atherosclerosis
7.4.1. Atherosclerosis and Nrf2
Atherosclerosis is a chronic disease of the arterial wall and refers to the condition in which an arterial plaque mainly made up of fat, cholesterol and calcium hardens and narrows the vessel lumen, limiting the flow of oxygen-rich blood. It is the prime cause for myocardial infarction, stroke and perivascular artery disease and is accelerated by diabetes. Indeed, DM increases risk for cardiovascular disease three- to eightfold. The first critical event and trigger of the development of atherosclerosis is the chronic injury and activation of endothelial cells through different insults including hyperglycemia, hypertension, hyperlipidemia as well as redox-/ER-stress and inflammation. All those features are characteristic for (type 2) DM and may be counteracted by Nrf2 activation (Dong et al., 2017; Giacco and Brownlee, 2010; Gupte et al., 2013; Shah and Brownlee, 2016; Cheng Zhang et al., 2017a). In particular, the Nrf2 target genes HMOX-1, NQO-1 or metallothioneine have gained interest with regard to potential protection from atherosclerosis or restenosis (Ayer et al., 2016; Calay and Mason, 2014; Chapple et al., 2012; Cominacini et al., 2015; He et al., 2015; Mason, 2016; Miao et al., 2013; Mylroie et al., 2015; Oh et al., 2014).
Nonetheless, the picture of Nrf2 activity in cardiovascular function and atherosclerosis appears complex and is dependent on the used model system, the cell type in which Nrf2 is activated and the nature (genetic vs pharmacological; transient vs chronic) of Nrf2 activation (Gupte et al., 2013; Jakobs et al., 2017; Li and Fukagawa, 2010). Nrf2 seems to confer endothelial protection under diabetic conditions as hyperglycemia- or AGE-mediated oxidative stress induces Nrf2 and its target genes in coronary arterial endothelial cells. HFD-induced vascular ROS and endothelial dysfunction are furthermore more severe in Nrf2 knockout mice (Cheng et al., 2011; He et al., 2011; Ungvari et al., 2011), and Nrf2 activation overcomes endothelial activation and dysfunction under hyperglycemia (Heiss et al., 2009; Zakkar et al., 2009). Aged low density lipoprotein receptor (Ldlr) knockout mice on a high-fat and high- cholesterol Western diet develop more complex atherosclerotic lesions containing fibrous caps and fail to mount Nrf2- mediated antioxidant responses in their vasculature (Collins et al., 2009) indicating a compromised Nrf2 function during the onset of atherosclerosis. Macrophages are central actors in the development and progression of atherosclerosis. Atherosclerosis- prone Ldlr knockout mice receiving bone marrow transplants from Nrf2 knockout mice developed larger and more complex atherosclerotic lesions than mice receiving transplants from wild-type mice, which developed only fatty streaks. The Nrf2 knockout macrophages showed increases in MCP-1- induced migration, LPS- stimulated expression of chemoattractant and proinflammatory genes, and in H2O2- induced apoptosis (Collins et al., 2012). In contrast to the Ldlr knockout mice, ApoE knockout mice (another popular model of atherosclerosis not associated with obesity) rather profit from Nrf2- deficiency, as lack of Nrf2 here attenuated atherosclerotic plaque formation and progression. This may be attributed to decreased macrophage expression of the scavenger receptor CD36 in Nrf2 knockout ApoE knockout mice, which resulted in a decreased uptake of modified low density lipoprotein by macrophages, decreased foam cell formation (Meher et al., 2012; Pedrosa et al., 2010), reduced plasma cholesterol levels (Barajas et al., 2011), or decreased cholesterol crystal- induced inflammasome activity (Freigang et al., 2011). These studies suggest opposing effects of Nrf2 on cholesterol uptake and inflammation and thus pro-or anti-atherosclerotic activity, depending upon the genetic and environmental context of the test model. However, it needs to be noted that rodent models of diabetic atherosclerosis do not fully recapitulate major aspects of human diabetic complications. Large animal models (pigs or non-human primates) in which diabetic cardiovascular disease more closely resembles that in humans, are available (Clarkson et al., 1985; McDonald et al., 2007) and should be employed in future studies in order to fully appreciate the role of Nrf2 in diabetes- associated vascular disease.
7.4.2. Natural Nrf2 activators and diabetes- associated atherosclerosis
Several natural Nrf2 activators have been examined in rodent and in vitro models related to diabetes- associated cardiovascular complications. Sulforaphane and broccoli sprout extract prevented diabetes-induced cardiac dysfunction as well as diabetes-associated cardiac oxidative damage, inflammation, fibrosis, and hypertrophy in STZ-treated mice and db/db knockout diabetic mice, respectively (Bai et al., 2013; Gu et al., 2017; Miao et al., 2012; Z. Xu et al., 2016b; Zhang et al., 2014). The treatment with sulforaphane was associated with an increased Nrf2 signaling as well as an increase in antioxidant defense markers. In one study, the authors also employed Nrf2 knockout counterparts in which the protective influence of sulforaphane was blunted. Thus, the beneficial effect of sulforaphane could be pinned down to Nrf2 activation (Gu et al., 2017). Moreover, sulforaphane protected from type 2 DM- induced aortic pathogenic changes in C57BL/6J mice which were fed with HFD for 3 months, followed by a treatment with STZ. Diabetic and non-diabetic mice were randomly divided into groups with and without 4-month sulforaphane treatment. The aorta of type 2 DM mice exhibited significant increases in the wall thickness and structural derangement, along with increased markers for fibrosis, inflammation, oxidative/nitrative stress, apoptosis, and cell proliferation. These pathological changes were attenuated by sulforaphane treatment which was associated with an upregulation of Nrf2 expression and function (Wang et al., 2014). In cell-based studies, sulforaphane protected endothelial cells from hyperglycemia- triggered dysfunction with an important role for upregulated transketolase (Xue et al., 2008). The studies focusing on the protective effects of sulforaphane against cardiovascular disease are nicely summarized by Bai et al. (2015).
The stilbene resveratrol significantly increased transcriptional activity of Nrf2 in cultured coronary arterial endothelial cells as evident in the upregulated expression of the Nrf2 target genes NQO1, γGCS, and HMOX-1. Resveratrol treatment also attenuated high glucose-induced mitochondrial and cellular oxidative stress. Those effects of resveratrol were mitigated by siRNA- mediated downregulation of Nrf2 or the overexpression of Keap1, establishing a causal link between Nrf2 activation and cytoprotection by resveratrol. In vivo, Nrf2(+/+) mice fed a HFD showed attenuated oxidative stress, improved acetylcholine- induced vasodilation, and inhibited apoptosis in branches of the femoral artery upon resveratrol treatment. Those effects that were blunted in HFD-fed Nrf2 knockout mice, strongly suggesting that resveratrol confers endothelial protection via the activation of Nrf2 in vitro and in vivo (Ungvari et al., 2010).
Magnesium lithospermate B, present e.g. in Salvia miltiorrhiza, inhibited glucose- induced proliferation and migration of aortic vascular smooth muscle cells and prevented neointimal hyperplasia after catheter- induced arterial injury in diabetic rats. siRNA-mediated knockdown of Nrf2 or the Nrf2- regulated antioxidant gene NQO1 blunted the protective effects of magnesium lithospermate B, in cultured aortic smooth muscle cells, strongly indicating a direct role for Nrf2 and its target gene NQO1 expression in the vasoprotective properties of lithospermate (Hur et al., 2010).
In the genetic type 1 diabetic OVE26 mouse model zinc supplementation for 3 months prevented the diabetes- induced increases in aortic oxidative damage, inflammation, and remodeling. Zinc treatment concomitantly upregulated the expression and function of Nrf2 and the expression of metallothioneine, a potent antioxidant (Miao et al., 2013). However, a causal link of Nrf2 activation and vasoprotection was not provided.
7.5. Cancer
DM is also linked with an increased cancer risk (Harding et al., 2015; Steenland et al., 1995; Tsilidis et al., 2015). In 2010, the American Cancer Society and the ADA published a consensus report on DM and cancer recommending a regular cancer screening for DM patients (Giovannucci et al., 2010). The correlation is not surprising as type 2 DM and cancer share common risk factors. Non-modifiable risk factors include age, sex, race and ethnicity, while amendable risk factors are overweight or obesity, smoking, alcohol abuse and physical inactivity. Furthermore, DM might directly or indirectly affect cancer initiation, promotion and/or progression. While the underlying pathways and mechanisms are not fully and unambiguously identified yet, it can be assumed that redox stress, protein stress and inflammation, as met in diabetes, may favor cancer initiation and promotion. The constant promitogenic signals provided by hyperinsulinemia and the facilitated supply of glucose for highly glycolytic cancer cells may further pamper the growth and survival of malignant cells (Grindel et al., 2017; Gristina et al., 2015; Hua et al., 2016; Joshi et al., 2015; Reuter et al., 2010). Of note, metformin, one of the first line medications in type 2 DM has been reported to possess anti-cancer properties (Whitburn et al., 2017; Yu et al., 2017; Zhou et al., 2017) supporting common targets and deregulations in cancer and diabetes.
The role of Nrf2 activation for cancer prevention and therapy has been intensively investigated for years and appears to be far from being solely beneficial or detrimental and is dependent on several parameters, including the duration and strength of Nrf2 activation as well as the type, stage and genetic background of the cancer (Taguchi and Yamamoto, 2017). From the currently available knowledge it may be concluded (not excluding exceptions) that Nrf2 activation and a consequent boost of the cellular detoxification response may provide protection against harmful carcinogenic insults and the onset of cancer. However, activated Nrf2 may deteriorate prognosis and progression as soon as cancer (stem) cells have hijacked Nrf2 signaling for their own advantage in order to optimize fuel supply or drug resistance. The role of activated Nrf2 particularly for diabetic cancer patients is hardly addressed until now and deserves future attention. For a more general insight into Nrf2 and cancer as well as cancer chemoprevention by natural products which is beyond this review, the reader is referred to excellent original studies and reviews (Dinkova-Kostova, 2013; Eggler et al., 2008; Jeong et al., 2006; Karin and Dhar, 2016; Le Marchand et al., 1989; Leinonen et al., 2014; Milkovic et al., 2017; Ryoo et al., 2016; Zhao et al., 2010; Zhu et al., 2016).
7.6. Bottom line
Diabetic neuro-, retino- and nephropathy as well as atherosclerosis possess a clear redox- and stress- dependent etiology and are aggravated by Nrf2 deficiency in respective animal and cell-based studies. For atherosclerosis, the picture is a bit blurred, since different murine models delivered contradicting results for the role of Nrf2, and none of the used animal models actually reflects the situation in humans. For cancer, Nrf2 activation shows a Janus face with a presumable positive effect in prevention, but unwanted protection from therapy with concomitant bad prognosis, when certain cancers have already developed. Diabetic animals or hyperglycemia- stressed cells significantly profited from exogenous activation of Nrf2 in several studies, maybe most convincingly in the context of diabetic nephro- and retinopathy. However, the direct proof that activated Nrf2 accounts for all the beneficial effects observed with the studied multi-target phytochemicals is not always given.
8. Translational outlook: Nrf2 as target in precision medicine and combinatorial therapy for diabetes
8.1. Precision medicine in diabetes
The goal of precision medicine is to customize an individual`s medical treatment according to the specific -omic profile. This implies the collection of phenotypic data and characterization of (epi)genome, transcriptome, proteome, metabolome and microbiome. Such systems biology approach allows accurate deduction of the patients’ disease status at the molecular level and selection of appropriate treatments. The patients’ response is to be closely monitored via validated relevant biomarkers and sensors, and the treatments are adapted accordingly (Lu et al., 2014; Mirnezami et al., 2012; National Research Council (US) Committee on A Framework for Developing a New Taxonomy of Disease, 2011). The ultimate goal is an optimal disease prevention and therapy for each patient.
The model of precision medicine is currently much more advanced in the field of cancer than for diabetes, with monogenic forms of DM clearly leading in front of multifactorial type 2 DM (Hiraizumi et al., 1991; Klonoff, 2015; Pearson, 2014). The current classification of DM into two main forms falls short of the true complexity of the disease and hampers the implementation of individualized care from diagnosis to optimized treatment (Hattersley and Patel, 2017; Pearson, 2014). Type 2 DM mostly results from a mixture of genetic predisposition, environmental cues and life-style habits, complicating the precise definition of discrete clinical subtypes. Such definition would need comprehensive information on individual variations in the different ome-levels and biomarkers. Together with information on outcome, disease progression and treatment from patient records we could then better understand disease etiology and possibly explain or predict the individual bias towards complications or better treatment responses (Adamski, 2016; Mayer, 2016). As a first step into this direction, genome-wide association studies (GWAS) have identified > 100 loci independently contributing to type 2 DM risk (Scott et al., 2017). However, translational implications remained scarce, due to poor knowledge of how these loci influence the pathophysiology of type 2 diabetes. A recent post-GWAS functional analysis has identified new genes and pathways (namely Prc1, Srr, Zfand6, and Zfand3) which are clearly involved in human pancreatic β-cell function and in type 2 DM pathophysiology. (Ndiaye et al., 2017). Staying on this track will step-by step provide a systematic picture of type II DM. Linking it to individual patient data may end up in a precision medicine for diabetes with improved health and quality of life for patients and valuable guidelines to develop and recommend personalized preventive and therapeutic regimens.
8.2. Nrf2 and precision medicine in diabetes
The etiology of DM involves cellular stress which is usually not sufficiently countered by a cellular detoxification program. Therefore, polymorphisms in Nrf2 itself, its target genes or regulators may contribute their share to disease in general (Cho et al., 2015), to susceptibility (Bitarafan et al., 2017) or responsiveness to prevention or treatment strategies (Boettler et al., 2012; Ishikawa, 2014). Knowledge of the genetic status of Nrf2 or other relevant biomarkers for an improper cellular stress response may allow individualized prediction of DM and complications as well as a rational decision for adjuvant therapy with activators of Nrf2. As precision medicine in type 2 DM is still in its infancy, the number of studies addressing single nucleotide polymorphisms (SNPs) of Nrf2 and diabetes/associated complications is relatively low (for summary see table 3).
Table 3.
Known SNPs of Nrf2 with correlation to diabetes and vascular disease.
| SNP of Nrf2 | Correlation with | Cohort |
|---|---|---|
| rs10497511 | Diabetic complications | Han Chinese |
| rs13001694 | Cardiovascular mortality | Dutch cohort |
| rs1806649 | Cardiovascular mortality | Dutch cohort |
| rs1962142 | Diabetic complications | Han Chinese |
| Cerebrovascular disease | Finnish cohort | |
| rs2364723 | Diabetic complication | Han Chinese |
| Cardiovascular mortality | Dutch cohort | |
| rs2706110 | Cerebrovascular disease | Finnish cohort |
| rs35652124 | Cardiovascular mortality | Japanese cohort |
| Forearm blood flow and vascular resistance | African American |
|
| rs672196 | Oxidative stress, insulin resistance, insulin secretion, risk for DM2 diagnosis |
Chinese Mexican men |
| Cerebrovascular disease | Finnish cohort | |
| Forearm vascular resistance | White Americans |
|
| Venous thromboembolism | ESTHER study | |
| rs6726395 | Diabetic complications | Han Chinese |
Figarska et al. examined the relation between Nrf2 and all-cause, cardiovascular, and chronic obstructive pulmonary disease mortality and its associations with triglyceride and cholesterol levels (Figarska et al., 2014). Five single nucleotide polymorphisms (rs4243387, rs2364723, rs13001694, rs1806649, and rs6726395) in Nrf2 were examined in 1,390 subjects from a Dutch cohort. Minor allele carriers of rs13001694, rs2364723, rs1806649 showed a significantly reduced risk of all-cause mortality, of cardiovascular mortality and COPD mortality, respectively. Rs35652124 was positively associated with cardiovascular mortality in a Japanese cohort (Shimoyama et al., 2014). In a Finnish cohort rs6721961, rs1962142, rs2706110 of Nrf2 were associated with cerebrovascular disease (Kunnas et al., 2016). The rs35652124 SNP was associated with lower forearm blood flow and higher vascular resistance in African Americans, and the rs6721961 polymorphism with higher forearm vascular resistance in white Americans (Marczak et al., 2012). Similarly, rs6721961 T allele carriers in the ESTHER study who were given oral estrogen suffered from an increased risk of venous thromboembolism (Bouligand et al., 2011). The rs110857735 polymorphism of Keap1 was associated with an increased incident of cardiovascular events in chronic kidney disease patients (Testa et al., 2016).
With particular reference to diabetes risk, Xu et al. found an association of the Nrf2 SNPs rs2364723, rs10497511, rs1962142 and rs6726395 with complications of type 2 DM, but not with type 2 DM risk in Han Chinese volunteers (X. Xu et al., 2016a). Also in a Chinese population, the Nrf2 rs6721961 polymorphism was significantly associated with oxidative stress, anti-oxidative status, and risk of newly diagnosed type 2 DM. This polymorphism may also contribute to impaired insulin secretory capacity and increased insulin resistance (Xia Wang et al., 2015a). In a Mexican population, the rs6721961 polymorphism was negatively associated with DM in men (Jiménez-Osorio et al., 2016). Concerning polymorphisms in Nrf2 target genes and their association to DM risk, only few should be mentioned here: the rs2071746 SNP in the HMOX-1 promoter is significantly associated with albuminuria development in type 2 DM patients, especially with longer duration and poor glycemic control (Lee et al., 2015). The rs1800566 polymorphism in NQO-1 seems to correlate with increased susceptibility to diabetic nephropathy, as seen in North Indian subjects with type 2 DM (Sharma et al., 2016). Polymorphisms in metallothioneine are also associated with DM and complications: the rs28366003 polymorphism in metallothioneine 2A correlated with diabetic chronic kidney disease in a Japanese population (Hattori et al., 2016), and the rs8052394 of metallothioneine 1A gene was significantly associated with the incidence of type 2 diabetes, while rs10636 and rs11076161 positively related with diabetic neuropathy (Yang et al., 2008). SNPs in SOD and GST were also correlated with altered risks for diabetic complications (Bitarafan et al., 2017). To what extent those observed associations can be translated into causality and generalized from one ethnic group to the entire population needs to be further investigated, though.
8.3. Combination of natural Nrf2 modulators with standard diabetes therapy
Despite many studies in the context of a single Nrf2 activator and T2D, only few studies examined combinations of Nrf2 activators with standard antidiabetic drugs like metformin. The combination of oleanolic acid and metformin, both known to activate Nrf2 (Ashabi et al., 2015; Lu et al., 2015), indicated beneficial additive or synergistic effects of the two drugs. Blood glucose as well as insulin levels had been significantly decreased in db/db mice treated with oleanolic acid or metformin alone. Those levels were even more strongly down-regulated when the mice were treated with both compounds. Also, insulin sensitivity was highly increased and immunoreactivity for gluconeogenetic enzymes in the liver was significantly decreased in the combination group compared to the vehicle group (Xue Wang et al., 2015b). In another study the combination of glyburide (glibenclamide) with curcumin was tested. Also here improved glucose levels together with lower lipid levels in the blood of treated patients could be observed (Neerati et al., 2014). Similarly, a combination of resveratrol with metformin and hydroxymethylbutyrate resulted in potentiated positive effect of metformin in db/db mice (Bruckbauer and Zemel, 2013). Although those studies support combination therapy, there are still a lot of issues which need to be thoroughly addressed before safe recommendations can be given. This includes the questions on the true impact on Nrf2 activation on the observed combinatorial effect or on the long-term outcome of the combination therapy, especially in the context of the cancer risk for diabetics due to the ambiguous role of Nrf2 in cancer onset and treatment (Bai et al., 2016).
9. Final Conclusion
The onset of DM (type 2) and its complications is accompanied by the gradual failure of cells to cope with affluent redox, ER and inflammatory stress. With Nrf2 being a major transcription factor in the cellular stress resistance, it appears as logical target to hit on with exogenous activators in order to counter the chronic metabolic disorder which has meanwhile reached pandemic dimensions. This notion is supported by the facts that Nrf2 is activated in early phases of DM and found less active at later stages, that Nrf2 deficient animals show deteriorated diabetic symptoms and complications in many in vivo models and that the Nrf2 activator CDDO-Me showed very promising renoprotection in clinical trials with patients suffering from diabetic chronic kidney disease. Nonetheless and despite multiple promising correlations, unambiguous causal evidence that Nrf2 activation by exogenous phytochemicals can prevent diabetes and associated complications is still limited. Moreover, depending on cellular context and question of interest, Nrf2 activation can be beneficial or detrimental, which has already been reviewed elsewhere (Chu et al., 2017). Targeting transcription factors generally bears the risk of triggering also the expression of unwanted genes among the respective targets and undesired outcomes. The Nrf2-dependent antioxidant and proteolytic enzymes may be of benefit in the course of diabetes. However, induction of enzymes which quickly detoxify and inactivate a patient´s medication or repression of enzymes for fatty acid synthesis (needed e.g. for β-cell proliferation) may be counterproductive. Continuous bolstering of the antioxidant defense can be detrimental, as insulin requires a transient ROS burst for successful signal transduction and ROS are necessary prerequisites for β-cells to synthesize and secrete insulin, as also indicated by the lacking success of antioxidant supplementation in intervention trials. Once hijacked from malignant cells, activated Nrf2 may do more harm than good. Thus, activation of Nrf2 may hold grand potential to alleviate DM and related complications. However, its activation must be achieved in a timely and highly specific and coordinated manner (based on target genes to be induced, cell types to be affected, history and biomarkers met in the individual patient). The validity of Nrf2 as drug target may be supported by the successful clinical use of dimethylfumarate in multiple sclerosis patients (Bomprezzi, 2015). Moreover, design of novel non-electrophilic selective Nrf2 activators, such as PPI inhibitors, is expected to significantly reduce unwanted side effects.
A plethora of phytochemicals activate Nrf2 signaling and proved beneficial for the relief of insulin resistance, β-cell dysfunction or hyperglycemia- caused complications. Most of the compounds mentioned in this review affect multiple cellular targets and pathways and their investigation often occurred in descriptive and correlative studies, which complicates the clear assignment of their effect to Nrf2 activation. Moreover, some of the compounds would be classified as pan-assay interference substances (PAINS) (Aldrich et al., 2017; Baell and Walters, 2014). If mechanistic detailed investigations were performed, they would occur in in vitro or animal models, which may imperfectly reflect human physiology. Moreover, when taken orally, those compounds are often hardly bioavailable and subject to intense metabolism by oxidation, sulfation, glucuronidation by the cell or microbial conversion in the intestine. Therefore, the concentration of the parent compound at the site of presumable action (β-cell, fat, liver, muscle) may in many cases not exceed pico- to nanomolar concentrations. Low potency, low bioavailability, and low target selectivity do not strongly advocate natural products as the Nrf2 activating principles of choice for adjuvant application against diabetes. However, exactly these features could also be of benefit as they may lead to a repeatedly pulsed low-level activation of Nrf2 which is sufficient to restore cellular homeostasis but not to cause harm in the long run. In addition, by affecting also other synergistic hubs in cellular signaling network (e.g. activation of AMPK or nuclear receptors, inhibition of NF-kB etc.) they may mildly shift the cell to an overall healthier phenotype, as suggested for resveratrol (Plauth et al., 2016). Therefore, it may be hard to pin down the observed bioactivity of a phytochemical to a single molecule-protein interaction. Its action may rather result from a complex interaction of targets in an additive, synergistic or antagonistic manner. Besides experimental and epidemiological data, omics- based technologies (massive parallel sequencing of transcriptomes, mass spectroscopy-based analysis of proteomes and metabolomes) together with fruitful bioinformatics and mathematical concepts, that even allow differentiation between correlation and causality (Sugihara et al., 2012), may be the future to comprehensively grasp the bioactivity of phytochemicals systemically. A recent study connected the hepatic gene expression profile of type II diabetics to elicited signatures of 3800 drugs. The Nrf2 activating sulforaphane was identified as compound to reverse the disease signature and to lower hepatic glucose production and glycated hemoglobin (Axelsson et al., 2017). Such approaches could go hand in hand with the concept of precision medicine and will provide a deep insight into the influence of natural Nrf2 activators on the molecular network of type 2 DM.
Last but not least, the authors would like to mention that in view of the wealth of published studies but limited space and capacity, they were not able to provide an exhaustive review of ALL existing publications on the given topic, and sincerely apologize to those authors whose valuable and substantial contribution to the field may not have been cited.
Acknowledgements
This work was supported by the Austrian Science Fund FWF (P29392-B21) and the Herzfeldeŕsche Familienstiftung.
Abbreviations
- AGE
advanced glycation end products
- AhR
arylhydrocarbon receptor
- AMPK
AMP-activated kinase
- ARE
antioxidant response element
- βTrcP
beta-transducin repeats-containing protein
- CDDO-Im
2-Cyano-3,12-dioxooleana-1,9(11)-dien-28-oic acid imidazolide
- CDDO-Me
Bardoxolone methyl
- Cul
cullin 3
- db/db
homozygous diabetic
- DM
diabetes mellitus
- DNMT
DNA methyltransferase
- ECM
extracellular matrix
- EGCG
epigallocatechin-3-gallate
- eNOS
endothelial nitric oxide synthase
- ER
endoplasmic reticulum
- ERK
extracellular signal regulated kinase
- FGF21
fibroblast growth factor 21
- G6PDH
glucose-6-phosphate dehydrogenase
- γGCS
gamma- glutamyl cysteinyl synthase
- GR
glutathione reductase
- GSK
glycogen synthase kinase
- GST
glutathione-S-transferase
- GWAS
genome-wide association studies
- HFD
high fat diet
- HMOX-1
heme oxygenase 1
- IL
interleukine
- iNOS
inducible nitric oxide synthase
- IRS
insulin receptor substrate
- JNK
jun-N-terminal kinase
- Keap1
Kelch-like ECH-associated protein 1
- KO
knockout
- Ldlr
low density lipoprotein receptor
- LPS
lipopolysaccharide
- Maf
musculoaponeurotic fibrosarcoma oncogene homolog
- MAPK
mitogen-activated kinase
- MG
methylglyoxal
- mTOR
mammalian target of rapamycin
- Neh
Nrf2-ECH-homology
- NF-κB
nuclear factor 'kappa-light-chain-enhancer' of activated B-cells
- NQO1
NAD(P)H quinone dehydrogenase 1
- Nrf2
nuclear factor (erythroid-derived 2)-like 2
- PERK
protein kinase RNA-like endoplasmic reticulum kinase
- PI3K
phosphoinositide-3-kinase
- PK
protein kinase
- PPAR
peroxisome proliferator-activated receptor
- PPI
protein-protein interaction
- PTM
posttranslational modification
- ROS
reactive oxygen species
- RXR
retinoid X receptor
- SNP
single nucleotide polymorphism
- SOD
superoxide dismutase
- STZ
streptozotocin
- TGF
transforming growth factor
- TNF
tumor necrosis factor
- UGT
UDP-glucuronosyltransferase
- UPR
unfolded protein stress response
- UPS
ubiquitin proteasome system
References
- Adamski J. Key elements of metabolomics in the study of biomarkers of diabetes. Diabetologia. 2016;59:2497–2502. doi: 10.1007/s00125-016-4044-y. [DOI] [PubMed] [Google Scholar]
- Agca CA, Tuzcu M, Hayirli A, Sahin K. Taurine ameliorates neuropathy via regulating NF-κB and Nrf2/HO-1 signaling cascades in diabetic rats. Food Chem Toxicol. 2014;71:116–121. doi: 10.1016/j.fct.2014.05.023. [DOI] [PubMed] [Google Scholar]
- Ahmadvand H, Tavafi M, Khosrowbeygi A. Amelioration of altered antioxidant enzymes activity and glomerulosclerosis by coenzyme Q10 in alloxan-induced diabetic rats. J Diabetes Complicat. 2012;26:476–482. doi: 10.1016/j.jdiacomp.2012.06.004. [DOI] [PubMed] [Google Scholar]
- Ahmed SMU, Luo L, Namani A, Wang XJ, Tang X. Nrf2 signaling pathway: pivotal roles in inflammation. Biochim Biophys Acta Mol Basis Dis. 2017;1863:585–597. doi: 10.1016/j.bbadis.2016.11.005. [DOI] [PubMed] [Google Scholar]
- Ahn KS. Transcription factor NF-B: a sensor for smoke and stress signals. Ann N Y Acad Sci. 2005;1056:218–233. doi: 10.1196/annals.1352.026. [DOI] [PubMed] [Google Scholar]
- Akimov NP, Rentería RC. Spatial frequency threshold and contrast sensitivity of an optomotor behavior are impaired in the Ins2Akita mouse model of diabetes. Behav Brain Res. 2012;226:601–605. doi: 10.1016/j.bbr.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers JW, Pop-Busui R. Diabetic neuropathy: mechanisms, emerging treatments, and subtypes. Curr Neurol Neurosci Rep. 2014;14 doi: 10.1007/s11910-014-0473-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldrich C, Bertozzi C, Georg GI, Kiessling L, Lindsley C, Liotta D, Merz KM, Schepartz A, Wang S. The ecstasy and agony of assay interference compounds. J Med Chem. 2017;60:2165–2168. doi: 10.1021/acs.jmedchem.7b00229. [DOI] [PubMed] [Google Scholar]
- Aleksunes LM, Reisman SA, Yeager RL, Goedken MJ, Klaassen CD. Nuclear factor erythroid 2-related factor 2 deletion impairs glucose tolerance and exacerbates hyperglycemia in type 1 diabetic mice. J Pharmacol Exp Ther. 2010;333:140–151. doi: 10.1124/jpet.109.162271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Trad B, Al-Batayneh K, El-Metwally S, Alhazimi A, Ginawi I, Alaraj M, Alkofahi E, Aljumaili O, Kosba A. Nigella sativa oil and thymoquinone ameliorate albuminuria and renal extracellular matrix accumulation in the experimental diabetic rats. Eur Rev Med Pharmacol Sci. 2016;20:2680–2688. [PubMed] [Google Scholar]
- Al-Waili N, Al-Waili H, Al-Waili T, Salom K. Natural antioxidants in the treatment and prevention of diabetic nephropathy; a potential approach that warrants clinical trials. Redox Rep Commun Free Radic Res. 2017;22:99–118. doi: 10.1080/13510002.2017.1297885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RE, Rapp LM, Wiegand RD. Lipid peroxidation and retinal degeneration. Curr Eye Res. 1984;3:223–227. doi: 10.3109/02713688408997203. [DOI] [PubMed] [Google Scholar]
- Andrzejewski S, Gravel S-P, Pollak M, St-Pierre J. Metformin directly acts on mitochondria to alter cellular bioenergetics. Cancer Metab. 2014;2:12. doi: 10.1186/2049-3002-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwar AA, Li FYL, Leake DS, Ishii T, Mann GE, Siow RCM. Induction of heme oxygenase 1 by moderately oxidized low-density lipoproteins in human vascular smooth muscle cells: role of mitogen-activated protein kinases and Nrf2. Free Radic Biol Med. 2005;39:227–236. doi: 10.1016/j.freeradbiomed.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Ariyasu D, Yoshida H, Hasegawa Y. Endoplasmic reticulum (ER) stress and endocrine disorders. Int J Mol Sci. 2017;18:382. doi: 10.3390/ijms18020382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashabi G, Khalaj L, Khodagholi F, Goudarzvand M, Sarkaki A. Pre-treatment with metformin activates Nrf2 antioxidant pathways and inhibits inflammatory responses through induction of AMPK after transient global cerebral ischemia. Metab Brain Dis. 2015;30:747–754. doi: 10.1007/s11011-014-9632-2. [DOI] [PubMed] [Google Scholar]
- Ashcroft FM, Rorsman P. Diabetes mellitus and the β cell: the last ten years. Cell. 2012;148:1160–1171. doi: 10.1016/j.cell.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astrup AS. Cardiovascular morbidity and mortality in diabetes mellitus: prediction and prognosis. Dan Med Bull. 2011;58:B4152. [PubMed] [Google Scholar]
- Aung MH, Kim MK, Olson DE, Thule PM, Pardue MT. Early visual deficits in streptozotocin-induced diabetic long evans rats. Invest Ophthalmol Vis Sci. 2013;54:1370–1377. doi: 10.1167/iovs.12-10927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aung MH, Park HN, Han MK, Obertone TS, Abey J, Aseem F, Thule PM, Iuvone PM, Pardue MT. Dopamine deficiency contributes to early visual dysfunction in a rodent model of type 1 diabetes. J Neurosci. 2014;34:726–736. doi: 10.1523/JNEUROSCI.3483-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelsson AS, Tubbs E, Mecham B, Chacko S, Nenonen HA, Tang Y, Fahey JW, Derry JMJ, Wollheim CB, Wierup N, Haymond MW, et al. Sulforaphane reduces hepatic glucose production and improves glucose control in patients with type 2 diabetes. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aah4477. [DOI] [PubMed] [Google Scholar]
- Ayer A, Zarjou A, Agarwal A, Stocker R. Heme oxygenases in cardiovascular health and disease. Physiol Rev. 2016;96:1449–1508. doi: 10.1152/physrev.00003.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baell J, Walters MA. Chemistry: chemical con artists foil drug discovery. Nature. 2014;513:481–483. doi: 10.1038/513481a. [DOI] [PubMed] [Google Scholar]
- Bahadoran Z, Mirmiran P, Hosseinpanah F, Rajab A, Asghari G, Azizi F. Broccoli sprouts powder could improve serum triglyceride and oxidized LDL/LDL-cholesterol ratio in type 2 diabetic patients: a randomized double-blind placebo-controlled clinical trial. Diabetes Res Clin Pract. 2012a;96:348–354. doi: 10.1016/j.diabres.2012.01.009. [DOI] [PubMed] [Google Scholar]
- Bahadoran Z, Tohidi M, Nazeri P, Mehran M, Azizi F, Mirmiran P. Effect of broccoli sprouts on insulin resistance in type 2 diabetic patients: a randomized double-blind clinical trial. Int J Food Sci Nutr. 2012b;63:767–771. doi: 10.3109/09637486.2012.665043. [DOI] [PubMed] [Google Scholar]
- Bai Y, Cui W, Xin Y, Miao X, Barati MT, Zhang C, Chen Q, Tan Y, Cui T, Zheng Y, Cai L. Prevention by sulforaphane of diabetic cardiomyopathy is associated with up-regulation of Nrf2 expression and transcription activation. J Mol Cell Cardiol. 2013;57:82–95. doi: 10.1016/j.yjmcc.2013.01.008. [DOI] [PubMed] [Google Scholar]
- Bai Y, Wang X, Zhao S, Ma C, Cui J, Zheng Y. Sulforaphane protects against cardiovascular disease via Nrf2 activation. Oxidative Med Cell Longev. 2015;2015:407580. doi: 10.1155/2015/407580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai X, Chen Y, Hou X, Huang M, Jin J. Emerging role of NRF2 in chemoresistance by regulating drug-metabolizing enzymes and efflux transporters. Drug Metab Rev. 2016;48:541–567. doi: 10.1080/03602532.2016.1197239. [DOI] [PubMed] [Google Scholar]
- Baird L, Dinkova-Kostova AT. The cytoprotective role of the Keap1-Nrf2 pathway. Arch Toxicol. 2011;85:241–272. doi: 10.1007/s00204-011-0674-5. [DOI] [PubMed] [Google Scholar]
- Bak MJ, Truong V-L, Ko S-Y, Nguyen XNG, Jun M, Hong S-G, Lee J-W, Jeong W-S. Induction of Nrf2/ARE-mediated cytoprotective genes by red ginseng oil through ASK1–MKK4/7–JNK and p38 MAPK signaling pathways in HepG2 cells. J Ginseng Res. 2016;40:423–430. doi: 10.1016/j.jgr.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakin AV, Stourman NV, Sekhar KR, Rinehart C, Yan X, Meredith MJ, Arteaga CL, Freeman ML. Smad3-ATF3 signaling mediates TGF-beta suppression of genes encoding Phase II detoxifying proteins. Free Radic Biol Med. 2005;38:375–387. doi: 10.1016/j.freeradbiomed.2004.10.033. [DOI] [PubMed] [Google Scholar]
- Balogun E, Hoque M, Gong P, Killeen E, Green CJ, Foresti R, Alam J, Motterlini R. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem J. 2003;371:887–895. doi: 10.1042/bj20021619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao L, Zhang Z, Dai X, Ding Y, Jiang Y, Li Y, Li Y. Effects of grape seed proanthocyanidin extract on renal injury in type 2 diabetic rats. Mol Med Rep. 2015;11:645–652. doi: 10.3892/mmr.2014.2768. [DOI] [PubMed] [Google Scholar]
- Barajas B, Che N, Yin F, Rowshanrad A, Orozco LD, Gong KW, Wang X, Castellani LW, Reue K, Lusis AJ, Araujo JA. NF-E2-related factor 2 promotes atherosclerosis by effects on plasma lipoproteins and cholesterol transport that overshadow antioxidant protection. Arterioscler Thromb Vasc Biol. 2011;31:58–66. doi: 10.1161/ATVBAHA.110.210906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber AJ. A new view of diabetic retinopathy: a neurodegenerative disease of the eye. Prog Neuro-Psychopharmacol Biol Psychiatry. 2003;27:283–290. doi: 10.1016/S0278-5846(03)00023-X. [DOI] [PubMed] [Google Scholar]
- Barber AJ, Lieth E, Khin SA, Antonetti DA, Buchanan AG, Gardner TW. Neural apoptosis in the retina during experimental and human diabetes. Early onset and effect of insulin. J Clin Invest. 1998;102:783–791. doi: 10.1172/JCI2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber AJ, Antonetti DA, Kern TS, Reiter CEN, Soans RS, Krady JK, Levison SW, Gardner TW, Bronson SK. The Ins2Akita mouse as a model of early retinal complications in diabetes. Invest Ophthalmol Vis Sci. 2005;46:2210–2218. doi: 10.1167/iovs.04-1340. [DOI] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer TA, Xu W, Teupser D, auf dem Keller U, Bugnon P, Hildt E, Thiery J, Kan YW, Werner S. Impaired liver regeneration in Nrf2 knockout mice: role of ROS-mediated insulin/IGF-1 resistance. EMBO J. 2008;27:212–223. doi: 10.1038/sj.emboj.7601950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakkiyalakshmi E, Shalini D, Sekar TV, Rajaguru P, Paulmurugan R, Ramkumar KM. Therapeutic potential of pterostilbene against pancreatic beta-cell apoptosis mediated through Nrf2: anti-apoptotic mechanism of pterostilbene. Br J Pharmacol. 2014;171:1747–1757. doi: 10.1111/bph.12577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakkiyalakshmi E, Sireesh D, Rajaguru P, Paulmurugan R, Ramkumar KM. The emerging role of redox-sensitive Nrf2–Keap1 pathway in diabetes. Pharmacol Res. 2015;91:104–114. doi: 10.1016/j.phrs.2014.10.004. [DOI] [PubMed] [Google Scholar]
- Bhakkiyalakshmi E, Dornadula S, Paulmurugan R, Ramkumar KM. Pterostilbene ameliorates streptozotocin-induced diabetes through enhancing anti-oxidant signaling pathways mediated by Nrf2. Chem Res Toxicol. 2016a;29:47–57. doi: 10.1021/acs.chemrestox.5b00378. [DOI] [PubMed] [Google Scholar]
- Bhakkiyalakshmi E, Sireesh D, Sakthivadivel M, Sivasubramanian S, Gunasekaran P, Ramkumar KM. Anti-hyperlipidemic and anti-peroxidative role of pterostilbene via Nrf2 signaling in experimental diabetes. Eur J Pharmacol. 2016b;777:9–16. doi: 10.1016/j.ejphar.2016.02.054. [DOI] [PubMed] [Google Scholar]
- Bitarafan F, Khodaeian M, Tabatabaei-Malazy O, Amoli MM. Influencing of antioxidants' gene variants on risk of diabetes mellitus and its complications: a systematic review. Minerva Endocrinol. 2017 doi: 10.23736/S0391-1977.17.02632-3. [DOI] [PubMed] [Google Scholar]
- Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 2007;297:842. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- Bloom DA, Jaiswal AK. Phosphorylation of Nrf2 at Ser 40 by protein kinase C in RESPONSE to antioxidants leads to the release of Nrf2 from INrf2, but Is not required for Nrf2 stabilization/accumulation in the nucleus and transcriptional activation of antioxidant response element-mediated NAD(P)H:quinone oxidoreductase-1 gene expression. J Biol Chem. 2003;278:44675–44682. doi: 10.1074/jbc.M307633200. [DOI] [PubMed] [Google Scholar]
- Boettler U, Volz N, Teller N, Haupt LM, Bakuradze T, Eisenbrand G, Bytof G, Lantz I, Grifiths LR, Marko D. Induction of antioxidative Nrf2 gene transcription by coffee in humans: depending on genotype? Mol Biol Rep. 2012;39:7155–7162. doi: 10.1007/s11033-012-1547-6. [DOI] [PubMed] [Google Scholar]
- Bomprezzi R. Dimethyl fumarate in the treatment of relapsing–remitting multiple sclerosis: an overview. Ther Adv Neurol Disord. 2015;8:20–30. doi: 10.1177/1756285614564152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Border WA, Ruoslahti E. Transforming growth factor-beta 1 induces extracellular matrix formation in glomerulonephritis. Cell Differ Dev. 1990;32:425–431. doi: 10.1016/0922-3371(90)90059-6. [DOI] [PubMed] [Google Scholar]
- Bouligand J, Cabaret O, Canonico M, Verstuyft C, Dubert L, Becquemont L, Guiochon-Mantel A, Scarabin PY, Estrogen and Thromboembolism Risk (ESTHER) Study Group Effect of NFE2L2 genetic polymorphism on the association between oral estrogen therapy and the risk of venous thromboembolism in post-menopausal women. Clin Pharmacol Ther. 2011;89:60–64. doi: 10.1038/clpt.2010.241. [DOI] [PubMed] [Google Scholar]
- Boulton AJM, Vinik AI, Arezzo JC, Bril V, Feldman EL, Freeman R, Malik RA, Maser RE, Sosenko JM, Ziegler D, American Diabetes Association Diabetic neuropathies: a statement by the American diabetes association. Diabetes Care. 2005;28:956–962. doi: 10.2337/diacare.28.4.956. [DOI] [PubMed] [Google Scholar]
- Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- Bruckbauer A, Zemel MB. Synergistic effects of metformin, resveratrol, and hydroxymethylbutyrate on insulin sensitivity. Diabetes Metab Syndr Obes. 2013;6:93–102. doi: 10.2147/DMSO.S40840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai L, Li Y, Zhang Q, Sun H, Yan X, Hua T, Zhu Q, Xu H, Fu H. Salidroside protects rat liver against ischemia/reperfusion injury by regulating the GSK-3β/Nrf2-dependent antioxidant response and mitochondrial permeability transition. Eur J Pharmacol. 2017;806:32–42. doi: 10.1016/j.ejphar.2017.04.011. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Cornelius C, Mancuso C, Pennisi G, Calafato S, Bellia F, Bates TE, Giuffrida Stella AM, Schapira T, Dinkova Kostova AT, Rizzarelli E. Cellular stress response: a novel target for chemoprevention and nutritional neuroprotection in aging, neurodegenerative disorders and longevity. Neurochem Res. 2008;33:2444–2471. doi: 10.1007/s11064-008-9775-9. [DOI] [PubMed] [Google Scholar]
- Calay D, Mason JC. The multifunctional role and therapeutic potential of HO-1 in the vascular endothelium. Antioxid Redox Signal. 2014;20:1789–1809. doi: 10.1089/ars.2013.5659. [DOI] [PubMed] [Google Scholar]
- Camer D, Yu Y, Szabo A, Dinh CHL, Wang H, Cheng L, Huang X-F. Bardoxolone methyl prevents insulin resistance and the development of hepatic steatosis in mice fed a high-fat diet. Mol Cell Endocrinol. 2015;412:36–43. doi: 10.1016/j.mce.2015.05.018. [DOI] [PubMed] [Google Scholar]
- Carayol N, Chen J, Yang F, Jin T, Jin L, States D, Wang C-Y. A dominant function of IKK/NF-κB signaling in global lipopolysaccharide-induced gene expression. J Biol Chem. 2006;281:31142–31151. doi: 10.1074/jbc.M603417200. [DOI] [PubMed] [Google Scholar]
- Cardozo LFMF, Pedruzzi LM, Stenvinkel P, Stockler-Pinto MB, Daleprane JB, Leite M, Mafra D. Nutritional strategies to modulate inflammation and oxidative stress pathways via activation of the master antioxidant switch Nrf2. Biochimie. 2013;95:1525–1533. doi: 10.1016/j.biochi.2013.04.012. [DOI] [PubMed] [Google Scholar]
- Carmona-Aparicio L, Pérez-Cruz C, Zavala-Tecuapetla C, Granados-Rojas L, Rivera-Espinosa L, Montesinos-Correa H, Hernández-Damián J, Pedraza-Chaverri J, Sampieri A, Coballase-Urrutia E, Cárdenas-Rodríguez N. Overview of Nrf2 as therapeutic target in epilepsy. Int J Mol Sci. 2015;16:18348–18367. doi: 10.3390/ijms160818348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco-Pozo C, Gotteland M, Castillo RL, Chen C. 3,4-dihydroxyphenylacetic acid, a microbiota-derived metabolite of quercetin, protects against pancreatic β-cells dysfunction induced by high cholesterol. Exp Cell Res. 2015;334:270–282. doi: 10.1016/j.yexcr.2015.03.021. [DOI] [PubMed] [Google Scholar]
- Carrasco-Pozo C, Tan KN, Gotteland M, Borges K. Sulforaphane protects against high cholesterol-induced mitochondrial bioenergetics impairments, inflammation, and oxidative stress and preserves pancreatic β-cells function. Oxidative Med Cell Longev. 2017;2017:1–14. doi: 10.1155/2017/3839756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catala A. Lipid peroxidation of membrane phospholipids in the vertebrate retina. Front Biosci Sch Ed. 2011;3:52–60. doi: 10.2741/s131. [DOI] [PubMed] [Google Scholar]
- Ceriello A. New insights on oxidative stress and diabetic complications may lead to a “causal” antioxidant therapy. Diabetes Care. 2003;26:1589–1596. doi: 10.2337/diacare.26.5.1589. [DOI] [PubMed] [Google Scholar]
- Chapple SJ, Siow RCM, Mann GE. Crosstalk between Nrf2 and the proteasome: therapeutic potential of Nrf2 inducers in vascular disease and aging. Int J Biochem Cell Biol. 2012;44:1315–1320. doi: 10.1016/j.biocel.2012.04.021. [DOI] [PubMed] [Google Scholar]
- Chartoumpekis DV, Ziros PG, Psyrogiannis AI, Papavassiliou AG, Kyriazopoulou VE, Sykiotis GP, Habeos IG. Nrf2 represses FGF21 during long-term high-fat diet-induced obesity in mice. Diabetes. 2011;60:2465–2473. doi: 10.2337/db11-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee N, Tian M, Spirohn K, Boutros M, Bohmann D. Keap1-independent regulation of Nrf2 activity by protein acetylation and a BET bromodomain protein. PLoS Genet. 2016;12:e1006072. doi: 10.1371/journal.pgen.1006072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S, Khunti K, Davies MJ. Type 2 diabetes. Lancet. 2017;389:2239–2251. doi: 10.1016/S0140-6736(17)30058-2. [DOI] [PubMed] [Google Scholar]
- Chaudhari N, Talwar P, Parimisetty A, Lefebvre d′Hellencourt C, Ravanan P. A molecular web: endoplasmic reticulum stress, inflammation, and oxidative stress. Front Cell Neurosci. 2014;8 doi: 10.3389/fncel.2014.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C-Y, Jang J-H, Li M-H, Surh Y-J. Resveratrol upregulates heme oxygenase-1 expression via activation of NF-E2-related factor 2 in PC12 cells. Biochem Biophys Res Commun. 2005;331:993–1000. doi: 10.1016/j.bbrc.2005.03.237. [DOI] [PubMed] [Google Scholar]
- Chen W, Sun Z, Wang X-J, Jiang T, Huang Z, Fang D, Zhang DD. Direct Interaction between Nrf2 and p21Cip1/WAF1 Upregulates the Nrf2-Mediated Antioxidant Response. Mol Cell. 2009;34:663–673. doi: 10.1016/j.molcel.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X, Siow RCM, Mann GE. Impaired redox signaling and antioxidant gene expression in endothelial cells in diabetes: a role for mitochondria and the nuclear factor-E2-related factor 2-Kelch-like ECH-associated protein 1 defense pathway. Antioxid Redox Signal. 2011;14:469–487. doi: 10.1089/ars.2010.3283. [DOI] [PubMed] [Google Scholar]
- Cheng A-S, Cheng Y-H, Chiou C-H, Chang T-L. Resveratrol Upregulates Nrf2 Expression To Attenuate Methylglyoxal-Induced Insulin Resistance in Hep G2 Cells. J Agric Food Chem. 2012;60:9180–9187. doi: 10.1021/jf302831d. [DOI] [PubMed] [Google Scholar]
- Cheng A-S, Cheng Y-H, Lee C-Y, Chung C-Y, Chang W-C. Resveratrol Protects against Methylglyoxal-Induced Hyperglycemia and Pancreatic Damage In Vivo. Nutrients. 2015;7:2850–2865. doi: 10.3390/nu7042850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L-B, Li K, Yi N, Li X, Wang F, Xue B, Pan Y, Yao J, Jiang Q, Wu Z. miRNA-141 attenuates UV-induced oxidative stress via activating Keap1-Nrf2 signaling in human retinal pigment epithelium cells and retinal ganglion cells. Oncotarget. 2017 doi: 10.18632/oncotarget.14489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chennasamudram SP, Kudugunti S, Boreddy PR, Moridani MY, Vasylyeva TL. Renoprotective effects of (+)-catechin in streptozotocin-induced diabetic rat model. Nutr Res N Y N. 2012;32:347–356. doi: 10.1016/j.nutres.2012.03.015. [DOI] [PubMed] [Google Scholar]
- Chiou Y-S, Tsai M-L, Nagabhushanam K, Wang Y-J, Wu C-H, Ho C-T, Pan M-H. Pterostilbene Is More Potent than Resveratrol in Preventing Azoxymethane (AOM)-Induced Colon Tumorigenesis via Activation of the NF-E2-Related Factor 2 (Nrf2)-Mediated Antioxidant Signaling Pathway. J Agric Food Chem. 2011;59:2725–2733. doi: 10.1021/jf2000103. [DOI] [PubMed] [Google Scholar]
- Chiou Y-S, Huang Q, Ho C-T, Wang Y-J, Pan M-H. Directly interact with Keap1 and LPS is involved in the anti-inflammatory mechanisms of (-)-epicatechin-3-gallate in LPS-induced macrophages and endotoxemia. Free Radic Biol Med. 2016;94:1–16. doi: 10.1016/j.freeradbiomed.2016.02.010. [DOI] [PubMed] [Google Scholar]
- Cho H-Y, Marzec J, Kleeberger SR. Functional polymorphisms in Nrf2: implications for human disease. Free Radic Biol Med. 2015;88:362–372. doi: 10.1016/j.freeradbiomed.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H-K, Pokharel YR, Lim SC, Han H-K, Ryu CS, Kim SK, Kwak MK, Kang KW. Inhibition of liver fibrosis by solubilized coenzyme Q10: role of Nrf2 activation in inhibiting transforming growth factor-beta1 expression. Toxicol Appl Pharmacol. 2009;240:377–384. doi: 10.1016/j.taap.2009.07.030. [DOI] [PubMed] [Google Scholar]
- Chorley BN, Campbell MR, Wang X, Karaca M, Sambandan D, Bangura F, Xue P, Pi J, Kleeberger SR, Bell DA. Identification of novel NRF2-regulated genes by ChIP-Seq: influence on retinoid X receptor alpha. Nucleic Acids Res. 2012;40:7416–7429. doi: 10.1093/nar/gks409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury S, Ghosh S, Gupta P, Mukherjee S, Chattopadhyay S. Inflammation-induced ROS generation causes pancreatic cell death through modulation of Nrf2/NF-κB and SAPK/JNK pathway. Free Radic Res. 2015;49:1371–1383. doi: 10.3109/10715762.2015.1075016. [DOI] [PubMed] [Google Scholar]
- Chu X-Y, Liu Y-M, Zhang H-Y. Activating or inhibiting Nrf2? Trends Pharmacol Sci. 2017;38:953–955. doi: 10.1016/j.tips.2017.08.002. [DOI] [PubMed] [Google Scholar]
- Chun K-S, Kundu J, Kundu JK, Surh Y-J. Targeting Nrf2-Keap1 signaling for chemoprevention of skin carcinogenesis with bioactive phytochemicals. Toxicol Lett. 2014;229:73–84. doi: 10.1016/j.toxlet.2014.05.018. [DOI] [PubMed] [Google Scholar]
- Clarkson TB, Koritnik DR, Weingand KW, Miller LC. Nonhuman primate models of atherosclerosis: potential for the study of diabetes mellitus and hyperinsulinemia. Metabolism. 1985;34:51–59. doi: 10.1016/s0026-0495(85)80010-x. [DOI] [PubMed] [Google Scholar]
- Collins AR, Lyon CJ, Xia X, Liu JZ, Tangirala RK, Yin F, Boyadjian R, Bikineyeva A, Praticò D, Harrison DG, Hsueh WA. Age-accelerated atherosclerosis correlates with failure to upregulate antioxidant genes. Circ Res. 2009;104:e42–e54. doi: 10.1161/CIRCRESAHA.108.188771. [DOI] [PubMed] [Google Scholar]
- Collins AR, Gupte AA, Ji R, Ramirez MR, Minze LJ, Liu JZ, Arredondo M, Ren Y, Deng T, Wang J, Lyon CJ, Hsueh WA. Myeloid deletion of nuclear factor erythroid 2-related factor 2 increases atherosclerosis and liver injury. Arterioscler Thromb Vasc Biol. 2012;32:2839–2846. doi: 10.1161/ATVBAHA.112.300345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cominacini L, Mozzini C, Garbin U, Pasini A, Stranieri C, Solani E, Vallerio P, Tinelli IA, Fratta Pasini A. Endoplasmic reticulum stress and Nrf2 signaling in cardiovascular diseases. Free Radic Biol Med. 2015;88:233–242. doi: 10.1016/j.freeradbiomed.2015.05.027. [DOI] [PubMed] [Google Scholar]
- Cooper ME. Pathogenesis, prevention, and treatment of diabetic nephropathy. Lancet Lond Engl. 1998;352:213–219. doi: 10.1016/S0140-6736(98)01346-4. [DOI] [PubMed] [Google Scholar]
- Coskun T, Bina HA, Schneider MA, Dunbar JD, Hu CC, Chen Y, Moller DE, Kharitonenkov A. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology. 2008;149:6018–6027. doi: 10.1210/en.2008-0816. [DOI] [PubMed] [Google Scholar]
- Costa A, Igualá I, Bedini J, Quintó L, Conget I. Uric acid concentration in subjects at risk of type 2 diabetes mellitus: relationship to components of the metabolic syndrome. Metabolism. 2002;51:372–375. doi: 10.1053/meta.2002.30523. [DOI] [PubMed] [Google Scholar]
- Cuadrado A. Structural and functional characterization of Nrf2 degradation by glycogen synthase kinase 3/β-TrCP. Free Radic Biol Med. 2015;88:147–157. doi: 10.1016/j.freeradbiomed.2015.04.029. [DOI] [PubMed] [Google Scholar]
- Cullinan SB, Zhang D, Hannink M, Arvisais E, Kaufman RJ, Diehl JA. Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol Cell Biol. 2003;23:7198–7209. doi: 10.1128/MCB.23.20.7198-7209.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dapito DH, Mencin A, Gwak G-Y, Pradere J-P, Jang M-K, Mederacke I, Caviglia JM, Khiabanian H, Adeyemi A, Bataller R, Lefkowitch JH, et al. Promotion of Hepatocellular Carcinoma by the Intestinal Microbiota and TLR4. Cancer Cell. 2012;21:504–516. doi: 10.1016/j.ccr.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayalan Naidu S, Kostov RV, Dinkova-Kostova AT. Transcription factors Hsf1 and Nrf2 engage in crosstalk for cytoprotection. Trends Pharmacol Sci. 2015;36:6–14. doi: 10.1016/j.tips.2014.10.011. [DOI] [PubMed] [Google Scholar]
- Debnam ES, Unwin RJ. Hyperglycemia and intestinal and renal glucose transport: implications for diabetic renal injury. Kidney Int. 1996;50:1101–1109. doi: 10.1038/ki.1996.416. [DOI] [PubMed] [Google Scholar]
- DeNicola GM, Karreth FA, Humpton TJ, Gopinathan A, Wei C, Frese K, Mangal D, Yu KH, Yeo CJ, Calhoun ES, Scrimieri F, et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011;475:106–109. doi: 10.1038/nature10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick LR, Cruikshank AA, Grenier L, Melandri FD, Nunes SL, Stein RL. Mechanistic studies on the inactivation of the proteasome by lactacystin: a central role for clasto-lactacystin beta-lactone. J Biol Chem. 1996;271:7273–7276. doi: 10.1074/jbc.271.13.7273. [DOI] [PubMed] [Google Scholar]
- Dietz BM, Kang Y-H, Liu G, Eggler AL, Yao P, Chadwick LR, Pauli GF, Farnsworth NR, Mesecar AD, van Breemen RB, Bolton JL. Xanthohumol Isolated from Humulus lupulus Inhibits Menadione-Induced DNA Damage through Induction of Quinone Reductase. Chem Res Toxicol. 2005;18:1296–1305. doi: 10.1021/tx050058x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA. Immunological and Inflammatory Functions of the Interleukin-1 Family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- Dinkova-Kostova AT. Chemoprotection against cancer by isothiocyanates: a focus on the animal models and the protective mechanisms. Top Curr Chem. 2013;329:179–201. doi: 10.1007/128_2012_337. [DOI] [PubMed] [Google Scholar]
- Donath MY, Schumann DM, Faulenbach M, Ellingsgaard H, Perren A, Ehses JA. Islet Inflammation in Type 2 Diabetes: from metabolic stress to therapy. Diabetes Care. 2008;31:S161–S164. doi: 10.2337/dc08-s243. [DOI] [PubMed] [Google Scholar]
- Dong Y, Fernandes C, Liu Y, Wu Y, Wu H, Brophy ML, Deng L, Song K, Wen A, Wong S, Yan D, et al. Role of endoplasmic reticulum stress signalling in diabetic endothelial dysfunction and atherosclerosis. Diab Vasc Dis Res. 2017;14:14–23. doi: 10.1177/1479164116666762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrell C, Schug J, Canaday PS, Russ HA, Tarlow BD, Grompe MT, Horton T, Hebrok M, Streeter PR, Kaestner KH, Grompe M. Human islets contain four distinct subtypes of β cells. Nat Commun. 2016;7:11756. doi: 10.1038/ncomms11756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreger H, Westphal K, Weller A, Baumann G, Stangl V, Meiners S, Stangl K. Nrf2-dependent upregulation of antioxidative enzymes: a novel pathway for proteasome inhibitor-mediated cardioprotection. Cardiovasc Res. 2009;83:354–361. doi: 10.1093/cvr/cvp107. [DOI] [PubMed] [Google Scholar]
- Dronavalli S, Duka I, Bakris GL. The pathogenesis of diabetic nephropathy. Nat Clin Pract Endocrinol Metab. 2008;4:444–452. doi: 10.1038/ncpendmet0894. [DOI] [PubMed] [Google Scholar]
- Eades G, Yang M, Yao Y, Zhang Y, Zhou Q. miR-200a regulates Nrf2 activation by targeting Keap1 mRNA in breast cancer cells. J Biol Chem. 2011;286:40725–40733. doi: 10.1074/jbc.M111.275495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards JL, Vincent AM, Cheng HT, Feldman EL. Diabetic neuropathy: mechanisms to management. Pharmacol Ther. 2008;120:1–34. doi: 10.1016/j.pharmthera.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggler AL, Liu G, Pezzuto JM, van Breemen RB, Mesecar AD. Modifying specific cysteines of the electrophile-sensing human Keap1 protein is insuffcient to disrupt binding to the Nrf2 domain Neh2. Proc Natl Acad Sci. 2005;102:10070–10075. doi: 10.1073/pnas.0502402102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggler AL, Gay KA, Mesecar AD. Molecular mechanisms of natural products in chemoprevention: induction of cytoprotective enzymes by Nrf2. Mol Nutr Food Res. 2008 doi: 10.1002/mnfr.200700249. [DOI] [PubMed] [Google Scholar]
- Ehses JA, Perren A, Eppler E, Ribaux P, Pospisilik JA, Maor-Cahn R, Gueripel X, Ellingsgaard H, Schneider MKJ, Biollaz G, Fontana A, et al. Increased number of islet-associated macrophages in Type 2 diabetes. Diabetes. 2007;56:2356–2370. doi: 10.2337/db06-1650. [DOI] [PubMed] [Google Scholar]
- Eizirik DL, Miani M, Cardozo AK. Signalling danger: endoplasmic reticulum stress and the unfolded protein response in pancreatic islet inflammation. Diabetologia. 2013;56:234–241. doi: 10.1007/s00125-012-2762-3. [DOI] [PubMed] [Google Scholar]
- Ellis EM. Reactive carbonyls and oxidative stress: potential for therapeutic intervention. Pharmacol Ther. 2007;115:13–24. doi: 10.1016/j.pharmthera.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Fallahzadeh MK, Dormanesh B, Sagheb MM, Roozbeh J, Vessal G, Pakfetrat M, Daneshbod Y, Kamali-Sarvestani E, Lankarani KB. Effect of addition of silymarin to renin-angiotensin system inhibitors on proteinuria in type 2 diabetic patients with overt nephropathy: a randomized, double-blind, placebo-controlled trial. Am J Kidney Dis Off J Natl Kidney Found. 2012;60:896–903. doi: 10.1053/j.ajkd.2012.06.005. [DOI] [PubMed] [Google Scholar]
- Fan J, Xu G, Jiang T, Qin Y. Pharmacologic induction of heme oxygenase-1 plays a protective role in diabetic retinopathy in rats. Invest Ophthalmol Vis Sci. 2012;53:6541–6556. doi: 10.1167/iovs.11-9241. [DOI] [PubMed] [Google Scholar]
- Fath El-Bab M, Zaki Nashaat, Mojaddidi M, AL-Barry M, El-Beshbishy H. Diabetic retinopathy is associated with oxidative stress and mitigation of gene expression of antioxidant enzymes. Int J Gen Med. 2013;799 doi: 10.2147/IJGM.S40665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Z, Liu Z, Li X, Jia H, Sun L, Tian C, Jia L, Liu J. α-Tocopherol is an effective Phase II enzyme inducer: protective effects on acrolein-induced oxidative stress and mitochondrial dysfunction in human retinal pigment epithelial cells. J Nutr Biochem. 2010;21:1222–1231. doi: 10.1016/j.jnutbio.2009.10.010. [DOI] [PubMed] [Google Scholar]
- Fenteany G, Standaert RF, Lane WS, Choi S, Corey EJ, Schreiber SL. Inhibition of proteasome activities and subunit-specific amino-terminal threonine modification by lactacystin. Science. 1995;268:726–731. doi: 10.1126/science.7732382. [DOI] [PubMed] [Google Scholar]
- Figarska SM, Vonk JM, Boezen HM. NFE2L2 polymorphisms, mortality, and metabolism in the general population. Physiol Genomics. 2014;46:411–417. doi: 10.1152/physiolgenomics.00178.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentino TV, Prioletta A, Zuo P, Folli F. Hyperglycemia-induced oxidative stress and its role in diabetes mellitus related cardiovascular diseases. Curr Pharm Des. 2013;19:5695–5703. doi: 10.2174/1381612811319320005. [DOI] [PubMed] [Google Scholar]
- Fischer N, Büchter C, Koch K, Albert S, Csuk R, Wätjen W. The resveratrol derivatives trans-3,5-dimethoxy-4-fluoro-4'-hydroxystilbene and trans-2,4',5-trihydroxystilbene decrease oxidative stress and prolong lifespan in Caenorhabditis elegans. J Pharm Pharmacol. 2017;69:73–81. doi: 10.1111/jphp.12657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foresti R, Bucolo C, Platania CMB, Drago F, Dubois-Randé J-L, Motterlini R. Nrf2 activators modulate oxidative stress responses and bioenergetic profiles of human retinal epithelial cells cultured in normal or high glucose conditions. Pharmacol Res. 2015;99:296–307. doi: 10.1016/j.phrs.2015.07.006. [DOI] [PubMed] [Google Scholar]
- Frank RN. Diabetic retinopathy. N Engl J Med. 2004;350:48–58. doi: 10.1056/NEJMra021678. [DOI] [PubMed] [Google Scholar]
- Freigang S, Ampenberger F, Spohn G, Heer S, Shamshiev AT, Kisielow J, Hersberger M, Yamamoto M, Bachmann MF, Kopf M. Nrf2 is essential for cholesterol crystal-induced inflammasome activation and exacerbation of atherosclerosis: innate immunity. Eur J Immunol. 2011;41:2040–2051. doi: 10.1002/eji.201041316. [DOI] [PubMed] [Google Scholar]
- Fridlyand LE, Philipson LH. Oxidative reactive species in cell injury: mechanisms in diabetes mellitus and therapeutic approaches. Ann N Y Acad Sci. 2005;1066:136–151. doi: 10.1196/annals.1363.019. [DOI] [PubMed] [Google Scholar]
- Fu J, Zheng H, Wang H, Yang B, Zhao R, Lu C, Liu Z, Hou Y, Xu Y, Zhang Q, Qu W, et al. Protective Role of Nuclear Factor E2-Related Factor 2 against Acute Oxidative Stress-Induced Pancreatic β -Cell Damage. Oxidative Med Cell Longev. 2015;2015:1–12. doi: 10.1155/2015/639191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Hou Y, Xue P, Wang H, Xu Y, Qu W, Zhang Q, Pi J. Nrf2 in Type 2 diabetes and diabetic complications: Yin and Yang. Curr Opin Toxicol. 2016;1:9–19. doi: 10.1016/j.cotox.2016.08.001. [DOI] [Google Scholar]
- Fujie T, Murakami M, Yoshida E, Tachinami T, Shinkai Y, Fujiwara Y, Yamamoto C, Kumagai Y, Naka H, Kaji T. Copper diethyldithiocarbamate as an activator of Nrf2 in cultured vascular endothelial cells. JBIC J Biol Inorg Chem. 2016;21:263–273. doi: 10.1007/s00775-016-1337-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furusawa Y, Uruno A, Yagishita Y, Higashi C, Yamamoto M. Nrf2 induces fibroblast growth factor 21 in diabetic mice. Genes Cells. 2014;19:864–878. doi: 10.1111/gtc.12186. [DOI] [PubMed] [Google Scholar]
- Gameiro I, Michalska P, Tenti G, Cores Á, Buendia I, Rojo AI, Georgakopoulos ND, Hernández-Guijo JM, Teresa Ramos M, Wells G, López MG, et al. Discovery of the first dual GSK3β inhibitor/Nrf2 inducer. A new multitarget therapeutic strategy for Alzheimer’s disease. Sci Rep. 2017;7:45701. doi: 10.1038/srep45701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Liu C, Wan G, Wang X, Cheng X, Ou Y. Phycocyanin prevents methylglyoxal-induced mitochondrial-dependent apoptosis in INS-1 cells by Nrf2. Food Funct. 2016;7:1129–1137. doi: 10.1039/C5FO01548K. [DOI] [PubMed] [Google Scholar]
- Garstkiewicz M, Strittmatter GE, Grossi S, Sand J, Fenini G, Werner S, French LE, Beer H-D. Opposing effects of Nrf2 and Nrf2-activating compounds on the NLRP3 inflammasome independent of Nrf2-mediated gene expression: innate immunity. Eur J Immunol. 2017;47:806–817. doi: 10.1002/eji.201646665. [DOI] [PubMed] [Google Scholar]
- Gastinger MJ, Singh RSJ, Barber AJ. Loss of cholinergic and dopaminergic amacrine cells in streptozotocin-diabetic rat and Ins2Akita-diabetic mouse retinas. Invest Ophthalmol Vis Sci. 2006;47:3143–3150. doi: 10.1167/iovs.05-1376. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Abdo S, Zhao S, Wu C-H, Shi Y, Lo C-S, Chenier I, Alquier T, Filep JG, Ingelfinger JR, Zhang S-L, et al. Insulin inhibits Nrf2 gene expression via heterogeneous nuclear ribonucleoprotein F/K in diabetic mice. Endocrinology. 2017;158:903–919. doi: 10.1210/en.2016-1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Habel LA, Pollak M, Regensteiner JG, Yee D. Diabetes and cancer: a consensus report. Diabetes Care. 2010;33:1674–1685. doi: 10.2337/dc10-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. J Pathol. 2010;221:3–12. doi: 10.1002/path.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover-Cutter KM, Lin S, Blackwell TK. Integration of the unfolded protein and oxidative stress responses through SKN-1/Nrf. PLoS Genet. 2013;9:e1003701. doi: 10.1371/journal.pgen.1003701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go Y-M, Gipp JJ, Mulcahy RT, Jones DP. H2O 2-dependent activation of GCLC-ARE4 reporter occurs by mitogen-activated protein kinase pathways without oxidation of cellular glutathione or thioredoxin-1. J Biol Chem. 2004;279:5837–5845. doi: 10.1074/jbc.M307547200. [DOI] [PubMed] [Google Scholar]
- Goldfine AB, Fonseca V, Shoelson SE. Therapeutic approaches to target inflammation in type 2 diabetes. Clin Chem. 2011;57:162–167. doi: 10.1373/clinchem.2010.148833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordois A, Scuffham P, Shearer A, Oglesby A, Tobian JA. The health care costs of diabetic peripheral neuropathy in the US. Diabetes Care. 2003;26:1790–1795. doi: 10.2337/diacare.26.6.1790. [DOI] [PubMed] [Google Scholar]
- Gorin Y, Block K. Nox as a target for diabetic complications. Clin Sci. 2013;125:361–382. doi: 10.1042/CS20130065. [DOI] [PubMed] [Google Scholar]
- Goru SK, Kadakol A, Gaikwad AB. Hidden targets of ubiquitin proteasome system: to prevent diabetic nephropathy. Pharmacol Res. 2017;120:170–179. doi: 10.1016/j.phrs.2017.03.024. [DOI] [PubMed] [Google Scholar]
- Grankvist K, Marklund SL, Täljedal IB. CuZn-superoxide dismutase, Mn-superoxide dismutase, catalase and glutathione peroxidase in pancreatic islets and other tissues in the mouse. Biochem J. 1981;199:393–398. doi: 10.1042/bj1990393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grattagliano I, Vendemiale G, Boscia F, Micelli-Ferrari T, Cardia L, Altomare E. Oxidative retinal products and ocular damages in diabetic patients. Free Radic Biol Med. 1998;25:369–372. doi: 10.1016/s0891-5849(98)00059-8. [DOI] [PubMed] [Google Scholar]
- Greaney AJ, Maier NK, Leppla SH, Moayeri M. Sulforaphane inhibits multiple inflammasomes through an Nrf2-independent mechanism. J Leukoc Biol. 2016;99:189–199. doi: 10.1189/jlb.3A0415-155RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg EW, Sattar N, Ali MK. The changing face of diabetes complications. Lancet Diabetes Endocrinol. 2016;4:537–547. doi: 10.1016/S2213-8587(16)30010-9. [DOI] [PubMed] [Google Scholar]
- Grindel A, Brath H, Nersesyan A, Knasmueller S, Wagner K-H. Association of Genomic Instability with HbA1c levels and Medication in Diabetic Patients. Sci Rep. 2017;7:41985. doi: 10.1038/srep41985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisold A, Callaghan BC, Feldman EL. Mediators of diabetic neuropathy: is hyperglycemia the only culprit? Curr Opin Endocrinol Diabetes Obes. 2017;24:103–111. doi: 10.1097/MED.0000000000000320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gristina V, Cupri MG, Torchio M, Mezzogori C, Cacciabue L, Danova M. Diabetes and cancer: a critical appraisal of the pathogenetic and therapeutic links. Biomed Rep. 2015;3:131–136. doi: 10.3892/br.2014.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover JK, Vats V, Yadav SS. Pterocarpus marsupium extract (Vijayasar) prevented the alteration in metabolic patterns induced in the normal rat by feeding an adequate diet containing fructose as sole carbohydrate. Diabetes Obes Metab. 2005;7:414–420. doi: 10.1111/j.1463-1326.2005.00414.x. [DOI] [PubMed] [Google Scholar]
- Gu J, Cheng Y, Wu H, Kong L, Wang S, Xu Z, Zhang Z, Tan Y, Keller BB, Zhou H, Wang Y, et al. Metallothionein is downstream of Nrf2 and partially mediates sulforaphane prevention of diabetic cardiomyopathy. Diabetes. 2017;66:529–542. doi: 10.2337/db15-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Yu S, Zhang C, Kong A-NT. Epigenetic regulation of Keap1-Nrf2 signaling. Free Radic Biol Med. 2015;88:337–349. doi: 10.1016/j.freeradbiomed.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta SK, Kumar B, Nag TC, Agrawal SS, Agrawal R, Agrawal P, Saxena R, Srivastava S. Curcumin prevents experimental diabetic retinopathy in rats through its hypoglycemic, antioxidant, and anti-inflammatory mechanisms. J Ocul Pharmacol Ther Off J Assoc Ocul Pharmacol Ther. 2011;27:123–130. doi: 10.1089/jop.2010.0123. [DOI] [PubMed] [Google Scholar]
- Gupte AA, Lyon CJ, Hsueh WA. Nuclear factor (erythroid-derived 2)-like-2 factor (Nrf2), a key regulator of the antioxidant response to protect against atherosclerosis and nonalcoholic steatohepatitis. Curr Diab Rep. 2013;13:362–371. doi: 10.1007/s11892-013-0372-1. [DOI] [PubMed] [Google Scholar]
- Habib SL, Yadav A, Kidane D, Weiss RH, Liang S. Novel protective mechanism of reducing renal cell damage in diabetes: activation AMPK by AICAR increased NRF2/OGG1 proteins and reduced oxidative DNA damage. Cell Cycle. 2016:1–12. doi: 10.1080/15384101.2016.1231259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han D, Chen W, Gu X, Shan R, Zou J, Liu G, Shahid M, Gao J, Han B. Cytoprotective effect of chlorogenic acid against hydrogen peroxide-induced oxidative stress in MC3T3-E1 cells through PI3K/Akt-mediated Nrf2/HO-1 signaling pathway. Oncotarget. 2017 doi: 10.18632/oncotarget.14747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon N, Coldham N, Gielbert A, Kuhnert N, Sauer MJ, King LJ, Ioannides C. Absolute bioavailability and dose-dependent pharmacokinetic behaviour of dietary doses of the chemopreventive isothiocyanate sulforaphane in rat. Br J Nutr. 2008;99 doi: 10.1017/S0007114507824093. [DOI] [PubMed] [Google Scholar]
- Harding JL, Shaw JE, Peeters A, Cartensen B, Magliano DJ. Cancer risk among people with type 1 and type 2 diabetes: disentangling true associations, detection bias, and reverse causation. Diabetes Care. 2015;38:264–270. doi: 10.2337/dc15-er04a. Diabetes Care 38, 734–735. [DOI] [PubMed] [Google Scholar]
- Harmon JS, Bogdani M, Parazzoli SD, Mak SSM, Oseid EA, Berghmans M, LeBoeuf RC, Robertson RP. β-cell-specific overexpression of glutathione peroxidase preserves intranuclear MafA and reverses diabetes in db/db mice. Endocrinology. 2009;150:4855–4862. doi: 10.1210/en.2009-0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Simmons AN, Kajino-Sakamoto R, Tsuji Y, Ninomiya-Tsuji J. TAK1 Regulates the Nrf2 Antioxidant System Through Modulating p62/SQSTM1. Antioxid Redox Signal. 2016;25:953–964. doi: 10.1089/ars.2016.6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasnain SZ, Prins JB, McGuckin MA. Oxidative and endoplasmic reticulum stress in β-cell dysfunction in diabetes. J Mol Endocrinol. 2016;56:R33–R54. doi: 10.1530/JME-15-0232. [DOI] [PubMed] [Google Scholar]
- Hast BE, Goldfarb D, Mulvaney KM, Hast MA, Siesser PF, Yan F, Hayes DN, Major MB. Proteomic Analysis of Ubiquitin Ligase KEAP1 Reveals Associated Proteins That Inhibit NRF2 Ubiquitination. Cancer Res. 2013;73:2199–2210. doi: 10.1158/0008-5472.CAN-12-4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattersley AT, Patel KA. Precision diabetes: learning from monogenic diabetes. Diabetologia. 2017;60:769–777. doi: 10.1007/s00125-017-4226-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori Y, Naito M, Satoh M, Nakatochi M, Naito H, Kato M, Takagi S, Matsunaga T, Seiki T, Sasakabe T, Suma S, et al. Metallothionein MT2A A-5G polymorphism as a risk factor for chronic kidney disease and diabetes: cross-sectional and cohort studies. Toxicol Sci Off J Soc Toxicol. 2016;152:181–193. doi: 10.1093/toxsci/kfw080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JD, Dinkova-Kostova AT. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem Sci. 2014;39:199–218. doi: 10.1016/j.tibs.2014.02.002. [DOI] [PubMed] [Google Scholar]
- He H-J. Curcumin attenuates Nrf2 signaling defect, oxidative stress in muscle and glucose intolerance in high fat diet-fed mice. World J Diabetes. 2012;3:94. doi: 10.4239/wjd.v3.i5.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M, Siow RCM, Sugden D, Gao L, Cheng X, Mann GE. Induction of HO-1 and redox signaling in endothelial cells by advanced glycation end products: a role for Nrf2 in vascular protection in diabetes. Nutr Metab Cardiovasc Dis NMCD. 2011;21:277–285. doi: 10.1016/j.numecd.2009.12.008. [DOI] [PubMed] [Google Scholar]
- He M, Nitti M, Piras S, Furfaro AL, Traverso N, Pronzato MA, Mann GE. Heme oxygenase-1-derived bilirubin protects endothelial cells against high glucose-induced damage. Free Radic Biol Med. 2015;89:91–98. doi: 10.1016/j.freeradbiomed.2015.07.151. [DOI] [PubMed] [Google Scholar]
- Heiss EH, Schachner D, Werner ER, Dirsch VM. Active NF-E2-related factor (Nrf2) contributes to keep endothelial NO synthase (eNOS) in the coupled state: role of reactive oxygen species (ROS), eNOS, and heme oxygenase (HO-1) levels. J Biol Chem. 2009;284:31579–31586. doi: 10.1074/jbc.M109.009175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiss EH, Liu R, Waltenberger B, Khan S, Schachner D, Kollmann P, Zimmermann K, Cabaravdic M, Uhrin P, Stuppner H, Breuss JM, et al. Plumericin inhibits proliferation of vascular smooth muscle cells by blocking STAT3 signaling via S-glutathionylation. Sci Rep. 2016;6 doi: 10.1038/srep20771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henquin J-C, Dufrane D, Nenquin M. Nutrient control of insulin secretion in isolated normal human islets. Diabetes. 2006;55:3470–3477. doi: 10.2337/db06-0868. [DOI] [PubMed] [Google Scholar]
- Hiraizumi S, Takakasaki S, Shiroki K, Kobata A. Altered protein glycosylation of rat 3Y1 cells induced by activated c-myc gene. Int J Cancer. 1991;48:305–310. doi: 10.1002/ijc.2910480225. [DOI] [PubMed] [Google Scholar]
- Hirotsu Y, Katsuoka F, Funayama R, Nagashima T, Nishida Y, Nakayama K, Douglas Engel J, Yamamoto M. Nrf2–MafG heterodimers contribute globally to antioxidant and metabolic networks. Nucleic Acids Res. 2012;40:10228–10239. doi: 10.1093/nar/gks827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman JM, Ishizuka T, Farese RV. Interrelated effects of insulin and glucose on diacylglycerol-protein kinase-C signalling in rat adipocytes and solei muscle in vitro and in vivo in diabetic rats*. Endocrinology. 1991;128:2937–2948. doi: 10.1210/endo-128-6-2937. [DOI] [PubMed] [Google Scholar]
- Hogan MF, Hull RL. The islet endothelial cell: a novel contributor to beta cell secretory dysfunction in diabetes. Diabetologia. 2017;60:952–959. doi: 10.1007/s00125-017-4272-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höhn A, Weber D, Jung T, Ott C, Hugo M, Kochlik B, Kehm R, König J, Grune T, Castro JP. Happily (n)ever after: aging in the context of oxidative stress, proteostasis loss and cellular senescence. Redox Biol. 2017;11:482–501. doi: 10.1016/j.redox.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder GM, Plummer JL, Ryan AJ. The metabolism and excretion of curcumin (1,7-bis-(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione) in the rat. Xenobiotica Fate Foreign Compd Biol Syst. 1978;8:761–768. doi: 10.3109/00498257809069589. [DOI] [PubMed] [Google Scholar]
- Hong F, Freeman ML, Liebler DC. Identification of sensor cysteines in human keap1 modified by the cancer chemopreventive agent sulforaphane. Chem Res Toxicol. 2005a;18:1917–1926. doi: 10.1021/tx0502138. [DOI] [PubMed] [Google Scholar]
- Hong F, Sekhar KR, Freeman ML, Liebler DC. Specific patterns of electrophile adduction trigger keap1 ubiquitination and Nrf2 activation. J Biol Chem. 2005b;280:31768–31775. doi: 10.1074/jbc.M503346200. [DOI] [PubMed] [Google Scholar]
- Hong YB, Kang HJ, Kwon SY, Kim HJ, Kwon KY, Cho CH, Lee J-M, Kallakury BVS, Bae I. Nuclear factor (erythroid-derived 2)-like 2 regulates drug resistance in pancreatic cancer cells. Pancreas. 2010;39:463–472. doi: 10.1097/MPA.0b013e3181c31314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosker JP, Rudenski AS, Burnett MA, Matthews DR, Turner RC. Similar reduction of first- and second-phase B-cell responses at three different glucose levels in type II diabetes and the effect of gliclazide therapy. Metabolism. 1989;38:767–772. doi: 10.1016/0026-0495(89)90064-4. [DOI] [PubMed] [Google Scholar]
- Hosoya T, Maruyama A, Kang M-I, Kawatani Y, Shibata T, Uchida K, Itoh K, Yamamoto M. Differential responses of the Nrf2-keap1 system to laminar and oscillatory shear stresses in endothelial cells. J Biol Chem. 2005;280:27244–27250. doi: 10.1074/jbc.M502551200. [DOI] [PubMed] [Google Scholar]
- Hsu Y-Y, Tseng Y-T, Lo Y-C. Berberine, a natural antidiabetes drug, attenuates glucose neurotoxicity and promotes Nrf2-related neurite outgrowth. Toxicol Appl Pharmacol. 2013;272:787–796. doi: 10.1016/j.taap.2013.08.008. [DOI] [PubMed] [Google Scholar]
- Hu R, Xu C, Shen G, Jain M, Khor T, Gopalkrishnan A, Lin W, Reddy B, Chan J, Kong A. Gene expression profiles induced by cancer chemopreventive isothiocyanate sulforaphane in the liver of C57BL/6J mice and C57BL/6J/Nrf2 (−/−) mice. Cancer Lett. 2006a;243:170–192. doi: 10.1016/j.canlet.2005.11.050. [DOI] [PubMed] [Google Scholar]
- Hu R, Xu C, Shen G, Jain MR, Khor TO, Gopalkrishnan A, Lin W, Reddy B, Chan JY, Kong A-NT. Identification of Nrf2-regulated genes induced by chemopreventive isothiocyanate PEITC by oligonucleotide microarray. Life Sci. 2006b;79:1944–1955. doi: 10.1016/j.lfs.2006.06.019. [DOI] [PubMed] [Google Scholar]
- Hu C, Eggler AL, Mesecar AD, van Breemen RB. Modification of Keap1 Cysteine Residues by Sulforaphane. Chem Res Toxicol. 2011;24:515–521. doi: 10.1021/tx100389r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua F, Yu J-J, Hu Z-W. Diabetes and cancer, common threads and missing links. Cancer Lett. 2016;374:54–61. doi: 10.1016/j.canlet.2016.02.006. [DOI] [PubMed] [Google Scholar]
- Huang T-C, Chung Y-L, Wu M-L, Chuang S-M. Cinnamaldehyde Enhances Nrf2 Nuclear Translocation to Upregulate Phase II Detoxifying Enzyme Expression in HepG2 Cells. J Agric Food Chem. 2011;59:5164–5171. doi: 10.1021/jf200579h. [DOI] [PubMed] [Google Scholar]
- Huang J, Huang K, Lan T, Xie X, Shen X, Liu P, Huang H. Curcumin ameliorates diabetic nephropathy by inhibiting the activation of the SphK1-S1P signaling pathway. Mol Cell Endocrinol. 2013;365:231–240. doi: 10.1016/j.mce.2012.10.024. [DOI] [PubMed] [Google Scholar]
- Hunter CS, Stein RW. Evidence for loss in identity, de-differentiation, and trans-differentiation of islet β-cells in type 2 diabetes. Front Genet. 2017;8 doi: 10.3389/fgene.2017.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur KY, Kim SH, Choi M-A, Williams DR, Lee Y, Kang SW, Yadav UCS, Srivastava SK, Jung M, Cho JW, Kim SG, et al. Protective effects of magnesium lithospermate B against diabetic atherosclerosis via Nrf2-ARE-NQO1 transcriptional pathway. Atherosclerosis. 2010;211:69–76. doi: 10.1016/j.atherosclerosis.2010.01.035. [DOI] [PubMed] [Google Scholar]
- Hussong M, Börno ST, Kerick M, Wunderlich A, Franz A, Sültmann H, Timmermann B, Lehrach H, Hirsch-Kauffmann M, Schweiger MR. The bromodomain protein BRD4 regulates the KEAP1/NRF2-dependent oxidative stress response. Cell Death Dis. 2014;5:e1195. doi: 10.1038/cddis.2014.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwa Ko J, Yoon S-O, Ju Lee H, Youn Oh J. Rapamycin regulates macrophage activation by inhibiting NLRP3 inflammasome-p38 MAPK-NFκB pathways in autophagy- and p62-dependent manners. Oncotarget. 2017 doi: 10.18632/oncotarget.17256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimura Y, Waguri S, Sou Y, Kageyama S, Hasegawa J, Ishimura R, Saito T, Yang Y, Kouno T, Fukutomi T, Hoshii T, et al. Phosphorylation of p62 activates the Keap1-Nrf2 pathway during selective autophagy. Mol Cell. 2013;51:618–631. doi: 10.1016/j.molcel.2013.08.003. [DOI] [PubMed] [Google Scholar]
- Idris I, Gray S, Donnelly R. Protein kinase C activation: isozyme-specific effects on metabolism and cardiovascular complications in diabetes. Diabetologia. 2001;44:659–673. doi: 10.1007/s001250051675. [DOI] [PubMed] [Google Scholar]
- Inzucchi SE, Majumdar SK. Current therapies for the medical management of diabetes. Obstet Gynecol. 2016;127:780–794. doi: 10.1097/AOG.0000000000001332. [DOI] [PubMed] [Google Scholar]
- Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2012;35:1364–1379. doi: 10.2337/dc12-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa T. Genetic polymorphism in the NRF2 gene as a prognosis marker for cancer chemotherapy. Front Genet. 2014;5:383. doi: 10.3389/fgene.2014.00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadeja RN, Upadhyay KK, Devkar RV, Khurana S. Naturally occurring Nrf2 activators: potential in treatment of liver injury. Oxidative Med Cell Longev. 2016;2016:1–13. doi: 10.1155/2016/3453926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain AB. Vitamin E, Its Beneficial Role in Diabetes Mellitus (DM) and Its Complications. J Clin Diagn Res. 2012 doi: 10.7860/JCDR/2012/4791.2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain M, Khor T, Gopalkrishnan A, Lin W, Hu R, Reddy B, Chan J, Kong A. Gene expression profiles induced by cancer chemopreventive isothiocyanate sulfor-aphane in the liver of C57BL/6J mice and C57BL/6J/Nrf2 (−/−) mice. Cancer Lett. 2006;243:170–192. doi: 10.1016/j.canlet.2005.11.050. [DOI] [PubMed] [Google Scholar]
- Jais A, Einwallner E, Sharif O, Gossens K, Lu TT-H, Soyal SM, Medgyesi D, Neureiter D, Paier-Pourani J, Dalgaard K, Duvigneau JC, et al. Heme Oxygenase-1 Drives Metaflammation and Insulin Resistance in Mouse and Man. Cell. 2014;158:25–40. doi: 10.1016/j.cell.2014.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobs P, Serbulea V, Leitinger N, Eckers A, Haendeler J. Nuclear factor (erythroid-derived 2)-like 2 and thioredoxin-1 in atherosclerosis and ischemia/re-perfusion injury in the heart. Antioxid Redox Signal. 2017;26:630–644. doi: 10.1089/ars.2016.6795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubíková J, Sedlák J, Mithen R, Bao Y. Role of PI3K/Akt and MEK/ERK signaling pathways in sulforaphane- and erucin-induced phase II enzymes and MRP2 transcription, G2/M arrest and cell death in Caco-2 cells. Biochem Pharmacol. 2005;69:1543–1552. doi: 10.1016/j.bcp.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Jeong W-S, Jun M, Kong A-NT. Nrf2: a potential molecular target for cancer chemoprevention by natural compounds. Antioxid Redox Signal. 2006;8:99–106. doi: 10.1089/ars.2006.8.99. [DOI] [PubMed] [Google Scholar]
- Jeong S, Son Y, Lee J, Cheong Y, Park S, Chung H, Pae H. Resveratrol analog piceatannol restores the palmitic acid-induced impairment of insulin signaling and production of endothelial nitric oxide via activation of anti-inflammatory and antioxidative heme oxygenase-1 in human endothelial cells. Mol Med Rep. 2015 doi: 10.3892/mmr.2015.3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha JC, Banal C, Chow BSM, Cooper ME, Jandeleit-Dahm K. Diabetes and kidney disease: role of oxidative stress. Antioxid Redox Signal. 2016;25:657–684. doi: 10.1089/ars.2016.6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji K, Xue L, Cheng J, Bai Y. Preconditioning of H 2 S inhalation protects against cerebral ischemia/reperfusion injury by induction of HSP70 through PI3K/Akt/Nrf2 pathway. Brain Res Bull. 2016;121:68–74. doi: 10.1016/j.brainresbull.2015.12.007. [DOI] [PubMed] [Google Scholar]
- Jiang T, Huang Z, Lin Y, Zhang Z, Fang D, Zhang DD. The protective role of Nrf2 in streptozotocin-induced diabetic nephropathy. Diabetes. 2010;59:850–860. doi: 10.2337/db09-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang T, Harder B, Rojo de la Vega M, Wong PK, Chapman E, Zhang DD. p62 links autophagy and Nrf2 signaling. Free Radic Biol Med. 2015;88:199–204. doi: 10.1016/j.freeradbiomed.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Blasco D, Santofimia-Castaño P, Gonzalez A, Almeida A, Bolaños JP. Astrocyte NMDA receptors’ activity sustains neuronal survival through a Cdk5–Nrf2 pathway. Cell Death Differ. 2015;22:1877–1889. doi: 10.1038/cdd.2015.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez-Osorio A, Picazo A, González-Reyes S, Barrera-Oviedo D, Rodríguez-Arellano M, Pedraza-Chaverri J. Nrf2 and redox status in prediabetic and diabetic patients. Int J Mol Sci. 2014;15:20290–20305. doi: 10.3390/ijms151120290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez-Osorio AS, González-Reyes S, García-Niño WR, Moreno-Macías H, Rodríguez-Arellano ME, Vargas-Alarcón G, Zúñiga J, Barquera R, Pedraza-Chaverri J. Association of Nuclear Factor-Erythroid 2-Related Factor 2, Thioredoxin Interacting Protein, and Heme Oxygenase-1 Gene Polymorphisms with Diabetes and Obesity in Mexican Patients. Oxidative Med Cell Longev. 2016;2016:7367641. doi: 10.1155/2016/7367641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo MS, Kim WD, Kim SG. AMPK regulates nuclear trafficking of Nrf2 through phosphorylation. FASEB J. 2016a;30:864–14. [Google Scholar]
- Joo MS, Kim WD, Lee KY, Kim JH, Koo JH, Kim SG. AMPK Facilitates Nuclear Accumulation of Nrf2 by Phosphorylating at Serine 550. Mol Cell Biol. 2016b;36:1931–1942. doi: 10.1128/MCB.00118-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi S, Liu M, Turner N. Diabetes and its link with cancer: providing the fuel and spark to launch an aggressive growth regime. Biomed Res Int. 2015;2015:390863. doi: 10.1155/2015/390863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung K-A, Kwak M-K. The Nrf2 System as a Potential Target for the Development of Indirect Antioxidants. Molecules. 2010;15:7266–7291. doi: 10.3390/molecules15107266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang KA, Kim JS, Zhang R, Piao MJ, Ko DO, Wang ZH, Maeng YH, Eun SY, Hyun JW. Induction of heme oxygenase-1 by plant extract KIOM-79 via Akt pathway and NF-E2 related factor 2 in pancreatic β-cells. J Toxicol Environ Health A. 2008;71:1392–1399. doi: 10.1080/15287390802271624. [DOI] [PubMed] [Google Scholar]
- Kang M-Y, Park YH, Kim BS, Seo SY, Jeong BC, Kim J-I, Kim HH. Preventive effects of green tea (Camellia sinensis var. assamica) on diabetic nephropathy. Yonsei Med J. 2012;53:138–144. doi: 10.3349/ymj.2012.53.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang KS, Ham J, Kim Y-J, Park JH, Cho E-J, Yamabe N. Heat-processed Panax ginseng and diabetic renal damage: active components and action mechanism. J Ginseng Res. 2013;37:379–388. doi: 10.5142/jgr.2013.37.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanitkar M, Gokhale K, Galande S, Bhonde RR. Novel role of curcumin in the prevention of cytokine-induced islet death in vitro and diabetogenesis in vivo: curcumin prevents islet death in vitro and in vivo. Br J Pharmacol. 2009;155:702–713. doi: 10.1038/bjp.2008.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwar YS, Sun L, Xie P, Liu F-Y, Chen S. A glimpse of various pathogenetic mechanisms of diabetic nephropathy. Annu Rev Pathol. 2011;6:395–423. doi: 10.1146/annurev.pathol.4.110807.092150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M, Dhar D. Liver carcinogenesis: from naughty chemicals to soothing fat and the surprising role of NRF2. Carcinogenesis. 2016;37:541–546. doi: 10.1093/carcin/bgw060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsarou A, Gudbjörnsdottir S, Rawshani A, Dabelea D, Bonifacio E, Anderson BJ, Jacobsen LM, Schatz DA, Lernmark Å. Type 1 diabetes mellitus. Nat Rev Dis Primer. 2017;3:17016. doi: 10.1038/nrdp.2017.16. [DOI] [PubMed] [Google Scholar]
- Kern TS, Barber AJ. Retinal ganglion cells in diabetes. J Physiol. 2008;586:4401–4408. doi: 10.1113/jphysiol.2008.156695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khajehdehi P, Pakfetrat M, Javidnia K, Azad F, Malekmakan L, Nasab MH, Dehghanzadeh G. Oral supplementation of turmeric attenuates proteinuria, transforming growth factor-β and interleukin-8 levels in patients with overt type 2 diabetic nephropathy: a randomized, double-blind and placebo-controlled study. Scand J Urol Nephrol. 2011;45:365–370. doi: 10.3109/00365599.2011.585622. [DOI] [PubMed] [Google Scholar]
- Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, Sandusky GE, Hammond LJ, Moyers JS, Owens RA, Gromada J, et al. FGF-21 as a novel metabolic regulator. J Clin Invest. 2005;115:1627–1635. doi: 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khor TO, Huang Y, Wu T-Y, Shu L, Lee J, Kong A-NT. Pharmacodynamics of curcumin as DNA hypomethylation agent in restoring the expression of Nrf2 via promoter CpGs demethylation. Biochem Pharmacol. 2011;82:1073–1078. doi: 10.1016/j.bcp.2011.07.065. [DOI] [PubMed] [Google Scholar]
- Kim JK. Hyperinsulinemic–euglycemic clamp to assess insulin sensitivity in vivo. In: Stocker C, editor. Type 2 Diabetes. Humana Press; Totowa, NJ: 2009. pp. 221–238. [DOI] [PubMed] [Google Scholar]
- Kim J, Cha Y-N, Surh Y-J. A protective role of nuclear factor-erythroid 2-related factor-2 (Nrf2) in inflammatory disorders. Mutat Res Mol Mech Mutagen. 2010;690:12–23. doi: 10.1016/j.mrfmmm.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Kim J-H, Xu EY, Sacks DB, Lee J, Shu L, Xia B, Kong A-NT. Identification and functional studies of a new Nrf2 partner IQGAP1: a critical role in the stability and transactivation of Nrf2. Antioxid Redox Signal. 2013;19:89–101. doi: 10.1089/ars.2012.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B-R, Lee GY, Yu H, Maeng HJ, Oh TJ, Kim KM, Moon JH, Lim S, Jang HC, Choi SH. Suppression of Nrf2 attenuates adipogenesis and decreases FGF21 expression through PPAR gamma in 3T3-L1 cells. Biochem Biophys Res Commun. 2017 doi: 10.1016/j.bbrc.2017.01.107. [DOI] [PubMed] [Google Scholar]
- Kiritoshi S, Nishikawa T, Sonoda K, Kukidome D, Senokuchi T, Matsuo T, Matsumura T, Tokunaga H, Brownlee M, Araki E. Reactive oxygen species from mitochondria induce cyclooxygenase-2 gene expression in human mesangial cells: potential role in diabetic nephropathy. Diabetes. 2003;52:2570–2577. doi: 10.2337/diabetes.52.10.2570. [DOI] [PubMed] [Google Scholar]
- Klonoff DC. Precision medicine for managing diabetes. J Diabetes Sci Technol. 2015;9:3–7. doi: 10.1177/1932296814563643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knorrwittmann C, Hengstermann A, Gebel S, Alam J, Muller T. Characterization of Nrf2 activation and heme oxygenase-1 expression in NIH3T3 cells exposed to aqueous extracts of cigarette smoke. Free Radic Biol Med. 2005;39:1438–1448. doi: 10.1016/j.freeradbiomed.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Kobayashi A, Kang M-I, Okawa H, Ohtsuji M, Zenke Y, Chiba T, Igarashi K, Yamamoto M. Oxidative Stress Sensor Keap1 Functions as an Adaptor for Cul3-Based E3 Ligase To Regulate Proteasomal Degradation of Nrf2. Mol Cell Biol. 2004;24:7130–7139. doi: 10.1128/MCB.24.16.7130-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Li L, Iwamoto N, Nakajima-Takagi Y, Kaneko H, Nakayama Y, Eguchi M, Wada Y, Kumagai Y, Yamamoto M. The Antioxidant Defense System Keap1-Nrf2 Comprises a Multiple Sensing Mechanism for Responding to a Wide Range of Chemical Compounds. Mol Cell Biol. 2009;29:493–502. doi: 10.1128/MCB.01080-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi EH, Suzuki T, Funayama R, Nagashima T, Hayashi M, Sekine H, Tanaka N, Moriguchi T, Motohashi H, Nakayama K, Yamamoto M. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat Commun. 2016;7:11624. doi: 10.1038/ncomms11624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu M, Kurokawa H, Waguri S, Taguchi K, Kobayashi A, Ichimura Y, Sou Y-S, Ueno I, Sakamoto A, Tong KI, Kim M, et al. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat Cell Biol. 2010 doi: 10.1038/ncb2021. [DOI] [PubMed] [Google Scholar]
- Koriyama Y, Chiba K, Yamazaki M, Suzuki H, Muramoto K, Kato S. Long-acting genipin derivative protects retinal ganglion cells from oxidative stress models in vitro and in vivo through the Nrf2/antioxidant response element signaling pathway. J Neurochem. 2010;115:79–91. doi: 10.1111/j.1471-4159.2010.06903.x. [DOI] [PubMed] [Google Scholar]
- Kowluru RA, Kanwar M. Effects of curcumin on retinal oxidative stress and inflammation in diabetes. Nutr Metab. 2007;4:8. doi: 10.1186/1743-7075-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowluru RA, Mishra M. Epigenetic regulation of redox signaling in diabetic retinopathy: role of Nrf2. Free Radic Biol Med. 2017;103:155–164. doi: 10.1016/j.freeradbiomed.2016.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koya D, Hayashi K, Kitada M, Kashiwagi A, Kikkawa R, Haneda M. Effects of antioxidants in diabetes-induced oxidative stress in the glomeruli of diabetic rats. J Am Soc Nephrol JASN. 2003;14:S250–253. doi: 10.1097/01.asn.0000077412.07578.44. [DOI] [PubMed] [Google Scholar]
- van Kuijk FJ, Buck P. Fatty acid composition of the human macula and peripheral retina. Invest Ophthalmol Vis Sci. 1992;33:3493–3496. [PubMed] [Google Scholar]
- Kulkarni SR, Donepudi AC, Xu J, Wei W, Cheng QC, Driscoll MV, Johnson DA, Johnson JA, Li X, Slitt AL. Fasting induces nuclear factor E2-related factor 2 and ATP-binding cassette transporters via protein kinase A and sirtuin-1 in mouse and human. Antioxid Redox Signal. 2014;20:15–30. doi: 10.1089/ars.2012.5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Mittal R. Nrf2: a potential therapeutic target for diabetic neuropathy. Inflammopharmacology. 2017 doi: 10.1007/s10787-017-0339-y. [DOI] [PubMed] [Google Scholar]
- Kunnas T, Määttä K, Nikkari ST. Genetic polymorphisms of transcription factor NRF2 and of its host gene sulfiredoxin (SRXN1) are associated with cerebrovascular disease in a Finnish Cohort, the TAMRISK Study. Int J Med Sci. 2016;13:325–329. doi: 10.7150/ijms.14849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak M-K, Wakabayashi N, Greenlaw JL, Yamamoto M, Kensler TW. Antioxidants enhance mammalian proteasome expression through the Keap1-Nrf2 signaling pathway. Mol Cell Biol. 2003a;23:8786–8794. doi: 10.1128/MCB.23.23.8786-8794.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak M-K, Wakabayashi N, Itoh K, Motohashi H, Yamamoto M, Kensler TW. Modulation of gene expression by cancer chemopreventive dithiolethiones through the Keap1-Nrf2 pathway: identification of novel gene clusters for cell survival. J Biol Chem. 2003b;278:8135–8145. doi: 10.1074/jbc.M211898200. [DOI] [PubMed] [Google Scholar]
- Kwok BH, Koh B, Ndubuisi MI, Elofsson M, Crews CM. The anti-inflammatory natural product parthenolide from the medicinal herb Feverfew directly binds to and inhibits IkappaB kinase. Chem Biol. 2001;8:759–766. doi: 10.1016/s1074-5521(01)00049-7. [DOI] [PubMed] [Google Scholar]
- Lacy PE. The pancreatic beta cell: structure and function. N Engl J Med. 1967;276:187–195. doi: 10.1056/NEJM196701262760401. [DOI] [PubMed] [Google Scholar]
- Lamark T, Kirkin V, Dikic I, Johansen T. NBR1 and p62 as cargo receptors for selective autophagy of ubiquitinated targets. Cell Cycle. 2009;8:1986–1990. doi: 10.4161/cc.8.13.8892. [DOI] [PubMed] [Google Scholar]
- Landers RA, Bingaman DP. Agents for treatment of diabetic retinopathy and drusen formation in macular degeneration. 2010 20100204244.
- Lazar MA, Birnbaum MJ. De-meaning of metabolism. Science. 2012;336:1651–1652. doi: 10.1126/science.1221834. [DOI] [PubMed] [Google Scholar]
- Le Marchand L, Yoshizawa CN, Kolonel LN, Hankin JH, Goodman MT. Vegetable consumption and lung cancer risk: a population-based case-control study in Hawaii. J Natl Cancer Inst. 1989;81:1158–1164. doi: 10.1093/jnci/81.15.1158. [DOI] [PubMed] [Google Scholar]
- Lee EA, Seo JY, Jiang Z, Yu MR, Kwon MK, Ha H, Lee HB. Reactive oxygen species mediate high glucose-induced plasminogen activator inhibitor-1 up-regulation in mesangial cells and in diabetic kidney. Kidney Int. 2005;67:1762–1771. doi: 10.1111/j.1523-1755.2005.00274.x. [DOI] [PubMed] [Google Scholar]
- Lee H-H, Park S-A, Almazari I, Kim E-H, Na H-K, Surh Y-J. Piceatannol induces heme oxygenase-1 expression in human mammary epithelial cells through activation of ARE-driven Nrf2 signaling. Arch Biochem Biophys. 2010;501:142–150. doi: 10.1016/j.abb.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Lee B-W, Chun SW, Kim SH, Lee Y, Kang ES, Cha B-S, Lee HC. Lithospermic acid B protects beta-cells from cytokine-induced apoptosis by alleviating apoptotic pathways and activating anti-apoptotic pathways of Nrf2–HO-1 and Sirt1. Toxicol Appl Pharmacol. 2011;252:47–54. doi: 10.1016/j.taap.2011.01.018. [DOI] [PubMed] [Google Scholar]
- Lee CA, Li G, Patel MD, Petrash JM, Benetz BA, Veenstra A, Amengual J, von Lintig J, Burant CJ, Tang J, Kern TS. Diabetes-induced impairment in visual function in mice: contributions of p38 MAPK, rage, leukocytes, and aldose reductase. Invest Ophthalmol Vis Sci. 2014a;55:2904–2910. doi: 10.1167/iovs.13-11659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C-C, Lee B-H, Wu S-C. Actinidia callosa peel (kiwi fruit) ethanol extracts protected neural cells apoptosis induced by methylglyoxal through Nrf2 activation. Pharm Biol. 2014b;52:628–636. doi: 10.3109/13880209.2013.860555. [DOI] [PubMed] [Google Scholar]
- Lee HS, Jun J-H, Jung E-H, Koo BA, Kim YS. Epigalloccatechin-3-gallate inhibits ocular neovascularization and vascular permeability in human retinal pigment epithelial and human retinal microvascular endothelial cells via suppression of MMP-9 and VEGF activation. Mol Basel Switz. 2014c;19:12150–12172. doi: 10.3390/molecules190812150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EY, Lee Y-H, Kim SH, Jung KS, Chung KS, Kwon O, Kim BS, Nam CM, Park CS, Lee B-W, Kang ES, et al. Association between heme oxygenase-1 promoter polymorphisms and the development of albuminuria in type 2 diabetes: a case-control study. Medicine (Baltimore) 2015;94:e1825. doi: 10.1097/MD.0000000000001825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DH, Han DH, Nam KT, Park JS, Kim SH, Lee M, Kim G, Min BS, Cha B-S, Lee YS, Sung SH, et al. Ezetimibe, an NPC1L1 inhibitor, is a potent Nrf2 activator that protects mice from diet-induced nonalcoholic steatohepatitis. Free Radic Biol Med. 2016;99:520–532. doi: 10.1016/j.freeradbiomed.2016.09.009. [DOI] [PubMed] [Google Scholar]
- Leinonen HM, Kansanen E, Pölönen P, Heinäniemi M, Levonen A-L. Role of the Keap1-Nrf2 pathway in cancer. Adv Cancer Res. 2014;122:281–320. doi: 10.1016/B978-0-12-420117-0.00008-6. [DOI] [PubMed] [Google Scholar]
- Leloup C, Tourrel-Cuzin C, Magnan C, Karaca M, Castel J, Carneiro L, Colombani A-L, Ktorza A, Casteilla L, Penicaud L. Mitochondrial reactive oxygen species are obligatory signals for glucose-induced insulin secretion. Diabetes. 2009;58:673–681. doi: 10.2337/db07-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenzen S, Drinkgern J, Tiedge M. Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic Biol Med. 1996;20:463–466. doi: 10.1016/0891-5849(96)02051-5. [DOI] [PubMed] [Google Scholar]
- Li M, Fukagawa NK. Age-related changes in redox signaling and VSMC function. Antioxid Redox Signal. 2010;12:641–655. doi: 10.1089/ars.2009.2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J-M, Li Y-C, Kong L-D, Hu Q-H. Curcumin inhibits hepatic protein-tyrosine phosphatase 1B and prevents hypertriglyceridemia and hepatic steatosis in fructose-fed rats. Hepatology. 2010a;51:1555–1566. doi: 10.1002/hep.23524. [DOI] [PubMed] [Google Scholar]
- Li W, Thakor N, Xu EY, Huang Y, Chen C, Yu R, Holcik M, Kong A-N. An internal ribosomal entry site mediates redox-sensitive translation of Nrf2. Nucleic Acids Res. 2010b;38:778–788. doi: 10.1093/nar/gkp1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Horke S, Förstermann U. Vascular oxidative stress, nitric oxide and atherosclerosis. Atherosclerosis. 2014a;237:208–219. doi: 10.1016/j.atherosclerosis.2014.09.001. [DOI] [PubMed] [Google Scholar]
- Li W, Wu W, Song H, Wang F, Li H, Chen L, Lai Y, Janicki JS, Ward KW, Meyer CJ, Wang XL, et al. Targeting Nrf2 by dihydro-CDDO-trifluoroethyl amide enhances autophagic clearance and viability of β-cells in a setting of oxidative stress. FEBS Lett. 2014b;588:2115–2124. doi: 10.1016/j.febslet.2014.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Li Q, Zhao Q, Zhang J, Lin J. Luteolin improves the impaired nerve functions in diabetic neuropathy: behavioral and biochemical evidences. Int J Clin Exp Pathol. 2015;8:10112–10120. [PMC free article] [PubMed] [Google Scholar]
- Li L, Du J, Lian Y, Zhang Y, Li X, Liu Y, Zou L, Wu T. Protective effects of coenzyme Q10 against hydrogen peroxide-induced oxidative stress in PC12 cell: the role of Nrf2 and antioxidant enzymes. Cell Mol Neurobiol. 2016;36:103–111. doi: 10.1007/s10571-015-0224-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M, Zhai X, Wang G, Tian X, Gao D, Shi L, Wu H, Fan Q, Peng J, Liu K, Yao J. Salvianolic acid B protects against acetaminophen hepatotoxicity by inducing Nrf2 and phase II detoxification gene expression via activation of the PI3K and PKC signaling pathways. J Pharmacol Sci. 2015;127:203–210. doi: 10.1016/j.jphs.2014.12.010. [DOI] [PubMed] [Google Scholar]
- Liu G, Eggler AL, Dietz BM, Mesecar AD, Bolton JL, Pezzuto JM, van Breemen RB. Screening method for the discovery of potential cancer chemoprevention agents based on mass spectrometric detection of alkylated Keap1. Anal Chem. 2005;77:6407–6414. doi: 10.1021/ac050892r. [DOI] [PubMed] [Google Scholar]
- Liu Y, Shen X, Yao G. Effects of grape seed proanthocyanidins extracts on experimental diabetic nephropathy in rats. Wei Sheng Yan Jiu. 2006;35:703–705. [PubMed] [Google Scholar]
- Liu H, Cao M, Wang Y, Li L, Zhu L, Xie G, Li Y. Endoplasmic reticulum stress is involved in the connection between inflammation and autophagy in type 2 diabetes. Gen Comp Endocrinol. 2015;210:124–129. doi: 10.1016/j.ygcen.2014.09.006. [DOI] [PubMed] [Google Scholar]
- Liu B, Li C-P, Wang W-Q, Song S-G, Liu X-M. Lignans extracted from eucommia ulmoides oliv. Protects against AGEs-induced retinal endothelial cell injury. Cell Physiol Biochem Int J Exp Cell Physiol Biochem Pharmacol. 2016a;39:2044–2054. doi: 10.1159/000447900. [DOI] [PubMed] [Google Scholar]
- Liu X, Zhou W, Zhang X, Lu P, Du Q, Tao L, Ding Y, Wang Y, Hu R. Dimethyl fumarate ameliorates dextran sulfate sodium-induced murine experimental colitis by activating Nrf2 and suppressing NLRP3 inflammasome activation. Biochem Pharmacol. 2016b;112:37–49. doi: 10.1016/j.bcp.2016.05.002. [DOI] [PubMed] [Google Scholar]
- Liu Z, Dou W, Ni Z, Wen Q, Zhang R, Qin M, Wang X, Tang H, Cao Y, Wang J, Zhao S. Deletion of Nrf2 leads to hepatic insulin resistance via the activation of NF-κB in mice fed a high-fat diet. Mol Med Rep. 2016c doi: 10.3892/mmr.2016.5393. [DOI] [PubMed] [Google Scholar]
- Lu Y-F, Goldstein DB, Angrist M, Cavalleri G. Personalized medicine and human genetic diversity. Cold Spring Harb Perspect Med. 2014;4:a008581. doi: 10.1101/cshperspect.a008581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y-F, Liu J, Wu KC, Klaassen CD. Protection against phalloidin-induced liver injury by oleanolic acid involves Nrf2 activation and suppression of Oatp1b2. Toxicol Lett. 2015;232:326–332. doi: 10.1016/j.toxlet.2014.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M-C, Ji J-A, Jiang Z-Y, You Q-D. The Keap1-Nrf2-Are pathway as a potential preventive and therapeutic target: an update. Med Res Rev. 2016;36:924–963. doi: 10.1002/med.21396. [DOI] [PubMed] [Google Scholar]
- Lucidi P, Rossetti P, Porcellati F, Pampanelli S, Candeloro P, Andreoli AM, Perriello G, Bolli GB, Fanelli CG. Mechanisms of insulin resistance after insulin-induced hypoglycemia in humans: the role of lipolysis. Diabetes. 2010;59:1349–1357. doi: 10.2337/db09-0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z-F, Qi W, Feng B, Mu J, Zeng W, Guo Y-H, Pang Q, Ye Z-L, Liu L, Yuan F-H. Prevention of diabetic nephropathy in rats through enhanced renal antioxidative capacity by inhibition of the proteasome. Life Sci. 2011;88:512–520. doi: 10.1016/j.lfs.2010.12.023. [DOI] [PubMed] [Google Scholar]
- Lupachyk S, Watcho P, Obrosov AA, Stavniichuk R, Obrosova IG. Endoplasmic reticulum stress contributes to prediabetic peripheral neuropathy. Exp Neurol. 2013a;247:342–348. doi: 10.1016/j.expneurol.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Lupachyk S, Watcho P, Stavniichuk R, Shevalye H, Obrosova IG. Endoplasmic reticulum stress plays a key role in the pathogenesis of diabetic peripheral neuropathy. Diabetes. 2013b;62:944–952. doi: 10.2337/db12-0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv H, Liu Q, Wen Z, Feng H, Deng X, Ci X. Xanthohumol ameliorates lipopolysaccharide (LPS)-induced acute lung injury via induction of AMPK/GSK3β-Nrf2 signal axis. Redox Biol. 2017;12:311–324. doi: 10.1016/j.redox.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyss G, Knorre A, Schmidt TJ, Pahl HL, Merfort I. The anti-inflammatory sesquiterpene lactone helenalin inhibits the transcription factor NF-kappaB by directly targeting p65. J Biol Chem. 1998;273:33508–33516. doi: 10.1074/jbc.273.50.33508. [DOI] [PubMed] [Google Scholar]
- Madiraju AK, Erion DM, Rahimi Y, Zhang X-M, Braddock DT, Albright RA, Prigaro BJ, Wood JL, Bhanot S, MacDonald MJ, Jurczak MJ, et al. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature. 2014;510:542–546. doi: 10.1038/nature13270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magesh S, Chen Y, Hu L. Small molecule modulators of Keap1-Nrf2-Are pathway as potential preventive and therapeutic agents: small molecule modulators of KEAP1-NRF2-Are pathway. Med Res Rev. 2012;32:687–726. doi: 10.1002/med.21257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher P, Hanneken A. Flavonoids protect retinal ganglion cells from oxidative stress-induced death. Invest Ophthalmol Vis Sci. 2005;46:4796–4803. doi: 10.1167/iovs.05-0397. [DOI] [PubMed] [Google Scholar]
- Maheshwari RA, Balaraman R, Sen AK, Seth AK. Effect of coenzyme Q10 alone and its combination with metformin on streptozotocin-nicotinamide-induced diabetic nephropathy in rats. Indian J Pharm. 2014;46:627–632. doi: 10.4103/0253-7613.144924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier NK, Leppla SH, Moayeri M. The cyclopentenone prostaglandin 15d-PGJ2 inhibits the NLRP1 and NLRP3 inflammasomes. J Immunol. 2015;194:2776–2785. doi: 10.4049/jimmunol.1401611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra D, Portales-Casamar E, Singh A, Srivastava S, Arenillas D, Happel C, Shyr C, Wakabayashi N, Kensler TW, Wasserman WW, Biswal S. Global mapping of binding sites for Nrf2 identifies novel targets in cell survival response through ChIP-Seq profiling and network analysis. Nucleic Acids Res. 2010;38:5718–5734. doi: 10.1093/nar/gkq212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marczak ED, Marzec J, Zeldin DC, Kleeberger SR, Brown NJ, Pretorius M, Lee CR. Polymorphisms in the transcription factor NRF2 and forearm vasodilator responses in humans. Pharmacogenet Genomics. 2012;22:620–628. doi: 10.1097/FPC.0b013e32835516e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin BC, Warram JH, Krolewski AS, Soeldner JS, Kahn CR, Martin BC, Bergman RN. Role of glucose and insulin resistance in development of type 2 diabetes mellitus: results of a 25-year follow-up study. Lancet. 1992;340:925–929. doi: 10.1016/0140-6736(92)92814-V. [DOI] [PubMed] [Google Scholar]
- Mason JC. Cytoprotective pathways in the vascular endothelium. Do they represent a viable therapeutic target? Vasc Pharmacol. 2016;86:41–52. doi: 10.1016/j.vph.2016.08.002. [DOI] [PubMed] [Google Scholar]
- Masuda Y, Vaziri ND, Li S, Le A, Hajighasemi-Ossareh M, Robles L, Foster CE, Stamos MJ, Al-Abodullah I, Ricordi C, Ichii H. The effect of Nrf2 pathway activation on human pancreatic islet cells. PLoS One. 2015;10:e0131012. doi: 10.1371/journal.pone.0131012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka TA, Artner I, Henderson E, Means A, Sander M, Stein R. The MafA transcription factor appears to be responsible for tissue-specific expression of insulin. Proc Natl Acad Sci. 2004;101:2930–2933. doi: 10.1073/pnas.0306233101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer B. Using systems biology to evaluate targets and mechanism of action of drugs for diabetes comorbidities. Diabetologia. 2016;59:2503–2506. doi: 10.1007/s00125-016-4032-2. [DOI] [PubMed] [Google Scholar]
- McDonald TO, Gerrity RG, Jen C, Chen H-J, Wark K, Wight TN, Chait A, O’Brien KD. Diabetes and arterial extracellular matrix changes in a porcine model of atherosclerosis. J Histochem Cytochem Off J Histochem Soc. 2007;55:1149–1157. doi: 10.1369/jhc.7A7221.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meakin PJ, Chowdhry S, Sharma RS, Ashford FB, Walsh SV, McCrimmon RJ, Dinkova-Kostova AT, Dillon JF, Hayes JD, Ashford MLJ. Susceptibility of Nrf2-null mice to steatohepatitis and cirrhosis upon consumption of a high-fat diet is associated with oxidative stress, perturbation of the unfolded protein response, and disturbance in the expression of metabolic enzymes but not with insulin resistance. Mol Cell Biol. 2014;34:3305–3320. doi: 10.1128/MCB.00677-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meher AK, Sharma PR, Lira VA, Yamamoto M, Kensler TW, Yan Z, Leitinger N. Nrf2 deficiency in myeloid cells is not sufficient to protect mice from high-fat diet-induced adipose tissue inflammation and insulin resistance. Free Radic Biol Med. 2012;52:1708–1715. doi: 10.1016/j.freeradbiomed.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerovich K, Ortis F, Allagnat F, Cardozo AK. Endoplasmic reticulum stress and the unfolded protein response in pancreatic islet inflammation. J Mol Endocrinol. 2016;57:R1–R17. doi: 10.1530/JME-15-0306. [DOI] [PubMed] [Google Scholar]
- Miao X, Bai Y, Sun W, Cui W, Xin Y, Wang Y, Tan Y, Miao L, Fu Y, Su G, Cai L. Sulforaphane prevention of diabetes-induced aortic damage was associated with the up-regulation of Nrf2 and its down-stream antioxidants. Nutr Metab. 2012;9:84. doi: 10.1186/1743-7075-9-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao X, Wang Y, Sun J, Sun W, Tan Y, Cai L, Zheng Y, Su G, Liu Q, Wang Y. Zinc protects against diabetes-induced pathogenic changes in the aorta: roles of metallothionein and nuclear factor (erythroid-derived 2)-like 2. Cardiovasc Diabetol. 2013;12:54. doi: 10.1186/1475-2840-12-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miglio G, Veglia E, Fantozzi R. Fumaric acid esters prevent the NLRP3 inflammasome-mediated and ATP-triggered pyroptosis of differentiated THP-1 cells. Int Immunopharmacol. 2015;28:215–219. doi: 10.1016/j.intimp.2015.06.011. [DOI] [PubMed] [Google Scholar]
- Milkovic L, Zarkovic N, Saso L. Controversy about pharmacological modulation of Nrf2 for cancer therapy. Redox Biol. 2017;12:727–732. doi: 10.1016/j.redox.2017.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirmiran P, Bahadoran Z, Hosseinpanah F, Keyzad A, Azizi F. Effects of broccoli sprout with high sulforaphane concentration on inflammatory markers in type 2 diabetic patients: a randomized double-blind placebo-controlled clinical trial. J Funct Foods. 2012;4:837–841. doi: 10.1016/j.jff.2012.05.012. [DOI] [Google Scholar]
- Mirnezami R, Nicholson J, Darzi A. Preparing for precision medicine. N Engl J Med. 2012;366:489–491. doi: 10.1056/NEJMp1114866. [DOI] [PubMed] [Google Scholar]
- Mishra M, Zhong Q, Kowluru RA. Epigenetic modifications of Nrf2-mediated glutamate-cysteine ligase: implications for the development of diabetic retinopathy and the metabolic memory phenomenon associated with its continued progression. Free Radic Biol Med. 2014a;75:129–139. doi: 10.1016/j.freeradbiomed.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra M, Zhong Q, Kowluru RA. Epigenetic modifications of Keap1 regulate its interaction with the protective factor Nrf2 in the development of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2014b;55:7256–7265. doi: 10.1167/iovs.14-15193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuishi Y, Taguchi K, Kawatani Y, Shibata T, Nukiwa T, Aburatani H, Yamamoto M, Motohashi H. Nrf2 redirects glucose and glutamine into anabolic pathways in metabolic reprogramming. Cancer Cell. 2012;22:66–79. doi: 10.1016/j.ccr.2012.05.016. [DOI] [PubMed] [Google Scholar]
- Mo C, Wang L, Zhang J, Numazawa S, Tang H, Tang X, Han X, Li J, Yang M, Wang Z, Wei D, et al. The crosstalk between Nrf2 and AMPK signal pathways is important for the anti-inflammatory effect of berberine in LPS-stimulated macrophages and endotoxin-shocked mice. Antioxid Redox Signal. 2014;20:574–588. doi: 10.1089/ars.2012.5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita S, Villalta SA, Feldman HC, Register AC, Rosenthal W, Hoffmann-Petersen IT, Mehdizadeh M, Ghosh R, Wang L, Colon-Negron K, Meza-Acevedo R, et al. Targeting ABL-IRE1α signaling spares ER-stressed pancreatic β cells to reverse autoimmune diabetes. Cell Metab. 2017;25:883–897.e8. doi: 10.1016/j.cmet.2017.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrudula T, Suryanarayana P, Srinivas PNBS, Reddy GB. Effect of curcumin on hyperglycemia-induced vascular endothelial growth factor expression in streptozotocin-induced diabetic rat retina. Biochem Biophys Res Commun. 2007;361:528–532. doi: 10.1016/j.bbrc.2007.07.059. [DOI] [PubMed] [Google Scholar]
- Mukherjee A, Morales-Scheihing D, Salvadores N, Moreno-Gonzalez I, Gonzalez C, Taylor-Presse K, Mendez N, Shahnawaz M, Gaber AO, Sabek OM, Fraga DW, et al. Induction of IAPP amyloid deposition and associated diabetic abnormalities by a prion-like mechanism. J Exp Med. 2017 doi: 10.1084/jem.20161134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mylroie H, Dumont O, Bauer A, Thornton CC, Mackey J, Calay D, Hamdulay SS, Choo JR, Boyle JJ, Samarel AM, Randi AM, et al. PKCε-CREB-Nrf2 signalling induces HO-1 in the vascular endothelium and enhances resistance to inflammation and apoptosis. Cardiovasc Res. 2015;106:509–519. doi: 10.1093/cvr/cvv131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na H-K, Surh Y-J. Modulation of Nrf2-mediated antioxidant and detoxifying enzyme induction by the green tea polyphenol EGCG. Food Chem Toxicol Int J Publ Br Ind Biol Res Assoc. 2008;46:1271–1278. doi: 10.1016/j.fct.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Na L-X, Zhang Y-L, Li Y, Liu L-Y, Li R, Kong T, Sun C-H. Curcumin improves insulin resistance in skeletal muscle of rats. Nutr Metab Cardiovasc Dis. 2011;21:526–533. doi: 10.1016/j.numecd.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Nabavi SF, Habtemariam S, Daglia M, Shafighi N, Barber AJ, Nabavi SM. Anthocyanins as a potential therapy for diabetic retinopathy. Curr Med Chem. 2015;22:51–58. doi: 10.2174/0929867321666140815123852. [DOI] [PubMed] [Google Scholar]
- Nabavi SF, Barber AJ, Spagnuolo C, Russo GL, Daglia M, Nabavi SM, Sobarzo-Sánchez E. Nrf2 as molecular target for polyphenols: a novel therapeutic strategy in diabetic retinopathy. Crit Rev Clin Lab Sci. 2016;53:293–312. doi: 10.3109/10408363.2015.1129530. [DOI] [PubMed] [Google Scholar]
- Nagata N, Xu L, Kohno S, Ushida Y, Aoki Y, Umeda R, Fuke N, Zhuge F, Ni Y, Nagashimada M, Takahashi C, et al. Glucoraphanin ameliorates obesity and insulin resistance through adipose tissue browning and reduction of metabolic endotoxemia in mice. Diabetes. 2017 doi: 10.2337/db16-0662. [DOI] [PubMed] [Google Scholar]
- Nam S, Smith DM, Dou QP. Ester bond-containing tea polyphenols potently inhibit proteasome activity in vitro and in vivo. J Biol Chem. 2001;276:13322–13330. doi: 10.1074/jbc.M004209200. [DOI] [PubMed] [Google Scholar]
- Narasimhan M, Patel D, Vedpathak D, Rathinam M, Henderson G, Mahimainathan L. Identification of novel microRNAs in post-transcriptional control of Nrf2 expression and redox homeostasis in neuronal, SH-SY5Y cells. PLoS One. 2012;7:e51111. doi: 10.1371/journal.pone.0051111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council (US) Committee on A Framework for Developing a New Taxonomy of Disease. The National Academies Collection: Reports funded by National Institutes of Health. National Academies Press (US); Washington (DC): 2011. Toward precision medicine: building a knowledge network for biomedical research and a new taxonomy of disease. [PubMed] [Google Scholar]
- Ndiaye FK, Ortalli A, Canouil M, Huyvaert M, Salazar-Cardozo C, Lecoeur C, Verbanck M, Pawlowski V, Boutry R, Durand E, Rabearivelo I, et al. Expression and functional assessment of candidate type 2 diabetes susceptibility genes identify four new genes contributing to human insulin secretion. Mol Metab. 2017;6:459–470. doi: 10.1016/j.molmet.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neelam K, Goenadi CJ, Lun K, Yip CC, Au Eong K-G. Putative protective role of lutein and zeaxanthin in diabetic retinopathy. Br J Ophthalmol. 2017;101:551–558. doi: 10.1136/bjophthalmol-2016-309814. [DOI] [PubMed] [Google Scholar]
- Neerati P, Devde R, Gangi AK. Evaluation of the effect of curcumin capsules on glyburide therapy in patients with type-2 diabetes mellitus. Phytother Res PTR. 2014;28:1796–1800. doi: 10.1002/ptr.5201. [DOI] [PubMed] [Google Scholar]
- Negi G, Kumar A, Joshi RP, Sharma SS. Oxidative stress and Nrf2 in the pathophysiology of diabetic neuropathy: old perspective with a new angle. Biochem Biophys Res Commun. 2011a;408:1–5. doi: 10.1016/j.bbrc.2011.03.087. [DOI] [PubMed] [Google Scholar]
- Negi G, Kumar A, Sharma SS. Melatonin modulates neuroinflammation and oxidative stress in experimental diabetic neuropathy: effects on NF-κB and Nrf2 cascades. J Pineal Res. 2011b;50:124–131. doi: 10.1111/j.1600-079X.2010.00821.x. [DOI] [PubMed] [Google Scholar]
- Negi G, Kumar A, Sharma SS. Nrf2 and NF-κB modulation by sulforaphane counteracts multiple manifestations of diabetic neuropathy in rats and high glucose-induced changes. Curr Neurovasc Res. 2011c;8:294–304. doi: 10.2174/156720211798120972. [DOI] [PubMed] [Google Scholar]
- Newsholme P, Cruzat VF, Keane KN, Carlessi R, de Bittencourt PIH. Molecular mechanisms of ROS production and oxidative stress in diabetes. Biochem J. 2016;473:4527–4550. doi: 10.1042/BCJ20160503C. [DOI] [PubMed] [Google Scholar]
- Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- Novials A, Montane J, Cadavez-Trigo L. Stress and the inflammatory process: a major cause of pancreatic cell death in type 2 diabetes. Diabetes Metab Syndr Obes Targets Ther. 2014:25. doi: 10.2147/DMSO.S37649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowotny K, Jung T, Höhn A, Weber D, Grune T. Advanced glycation end products and oxidative stress in Type 2 diabetes mellitus. Biomol Ther. 2015;5:194–222. doi: 10.3390/biom5010194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien PD, Hinder LM, Sakowski SA, Feldman EL. ER stress in diabetic peripheral neuropathy: a new therapeutic target. Antioxid Redox Signal. 2014;21:621–633. doi: 10.1089/ars.2013.5807. [DOI] [PubMed] [Google Scholar]
- Obrosova IG, Li F, Abatan OI, Forsell MA, Komjáti K, Pacher P, Szabó C, Stevens MJ. Role of poly(ADP-ribose) polymerase activation in diabetic neuropathy. Diabetes. 2004;53:711–720. doi: 10.2337/diabetes.53.3.711. [DOI] [PubMed] [Google Scholar]
- Oh CJ, Park S, Kim J-Y, Kim H-J, Jeoung NH, Choi Y-K, Go Y, Park K-G, Lee I-K. Dimethylfumarate attenuates restenosis after acute vascular injury by cell-specific and Nrf2-dependent mechanisms. Redox Biol. 2014;2:855–864. doi: 10.1016/j.redox.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlsson H, Karlsson K, Edlund T. IPF1, a homeodomain-containing transactivator of the insulin gene. EMBO J. 1993;12:4251–4259. doi: 10.1002/j.1460-2075.1993.tb06109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onken B, Driscoll M. Metformin induces a dietary restriction-like state and the oxidative stress response to extend C. elegans Healthspan via AMPK, LKB1, and SKN-1. PLoS One. 2010;5(1):e8758. doi: 10.1371/journal.pone.0008758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmeira CM, Rolo AP, Berthiaume J, Bjork JA, Wallace KB. Hyperglycemia decreases mitochondrial function: the regulatory role of mitochondrial biogenesis. Toxicol Appl Pharmacol. 2007;225:214–220. doi: 10.1016/j.taap.2007.07.015. [DOI] [PubMed] [Google Scholar]
- Palsamy P, Subramanian S. Resveratrol protects diabetic kidney by attenuating hyperglycemia-mediated oxidative stress and renal inflammatory cytokines via Nrf2-Keap1 signaling. Biochim Biophys Acta (BBA) - Mol Basis Dis. 2011;1812:719–731. doi: 10.1016/j.bbadis.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Pan Y, Wang Y, Cai L, Cai Y, Hu J, Yu C, Li J, Feng Z, Yang S, Li X, Liang G. Inhibition of high glucose-induced inflammatory response and macrophage infiltration by a novel curcumin derivative prevents renal injury in diabetic rats. Br J Pharmacol. 2012;166:1169–1182. doi: 10.1111/j.1476-5381.2012.01854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J-S, Lee Y-Y, Kim J, Seo H, Kim H-S. β-Lapachone increases phase II antioxidant enzyme expression via NQO1-AMPK/PI3K-Nrf2/ARE signaling in rat primary astrocytes. Free Radic Biol Med. 2016;97:168–178. doi: 10.1016/j.freeradbiomed.2016.05.024. [DOI] [PubMed] [Google Scholar]
- Pearson E. RD Lawrence lecture 2013. Stratified approaches to the management of diabetes. Diabet Med J Br Diabet Assoc. 2014;31:393–398. doi: 10.1111/dme.12391. [DOI] [PubMed] [Google Scholar]
- Pedrosa AMC, Faine LA, Grosso DM, de Las Heras B, Boscá L, Abdalla DSP. Electronegative LDL induction of apoptosis in macrophages: involvement of Nrf2. Biochim Biophys Acta. 2010;1801:430–437. doi: 10.1016/j.bbalip.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Perez-Leal O, Barrero CA, Merali S. Translational control of Nrf2 within the open reading frame. Biochem Biophys Res Commun. 2013;437:134–139. doi: 10.1016/j.bbrc.2013.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Leal O, Barrero CA, Merali S. Pharmacological stimulation of nuclear factor (erythroid-derived 2)-like 2 translation activates antioxidant responses. J Biol Chem. 2017 doi: 10.1074/jbc.M116.770925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pergola PE, Krauth M, Huff JW, Ferguson DA, Ruiz S, Meyer CJ, Warnock DG. Effect of bardoxolone methyl on kidney function in patients with T2D and Stage 3b-4 CKD. Am J Nephrol. 2011a;33:469–476. doi: 10.1159/000327599. [DOI] [PubMed] [Google Scholar]
- Pergola PE, Raskin P, Toto RD, Meyer CJ, Huff JW, Grossman EB, Krauth M, Ruiz S, Audhya P, Christ-Schmidt H, Wittes J, et al. BEAM Study Investigators, Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N Engl J Med. 2011b;365:327–336. doi: 10.1056/NEJMoa1105351. [DOI] [PubMed] [Google Scholar]
- Perla V, Jayanty SS. Biguanide related compounds in traditional antidiabetic functional foods. Food Chem. 2013;138:1574–1580. doi: 10.1016/j.foodchem.2012.09.125. [DOI] [PubMed] [Google Scholar]
- Persson MF, Franzén S, Catrina S-B, Dallner G, Hansell P, Brismar K, Palm F. Coenzyme Q10 prevents GDP-sensitive mitochondrial uncoupling, glomerular hyperfiltration and proteinuria in kidneys from db/db mice as a model of type 2 diabetes. Diabetologia. 2012;55:1535–1543. doi: 10.1007/s00125-012-2469-5. [DOI] [PubMed] [Google Scholar]
- Pi J, Bai Y, Zhang Q, Wong V, Floering LM, Daniel K, Reece JM, Deeney JT, Andersen ME, Corkey BE, Collins S. Reactive oxygen species as a signal in glucose-stimulated insulin secretion. Diabetes. 2007;56:1783–1791. doi: 10.2337/db06-1601. [DOI] [PubMed] [Google Scholar]
- Pi J, Zhang Q, Fu J, Woods CG, Hou Y, Corkey BE, Collins S, Andersen ME. ROS signaling, oxidative stress and Nrf2 in pancreatic beta-cell function. Toxicol Appl Pharmacol. 2010;244:77–83. doi: 10.1016/j.taap.2009.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipeleers D, Kiekens R, Ling Z, Wilikens A, Schuit F. Physiologic relevance of heterogeneity in the pancreatic beta-cell population. Diabetologia. 1994;37(Suppl. 2):S57–64. doi: 10.1007/BF00400827. [DOI] [PubMed] [Google Scholar]
- Pitha-Rowe I, Liby K, Royce D, Sporn M. Synthetic triterpenoids attenuate cytotoxic retinal injury: cross-talk between Nrf2 and PI3K/AKT signaling through inhibition of the lipid phosphatase PTEN. Investig Opthalmology Vis Sci. 2009;50:5339. doi: 10.1167/iovs.09-3648. [DOI] [PubMed] [Google Scholar]
- Plauth A, Geikowski A, Cichon S, Wowro SJ, Liedgens L, Rousseau M, Weidner C, Fuhr L, Kliem M, Jenkins G, Lotito S, et al. Hormetic shifting of redox environment by pro-oxidative resveratrol protects cells against stress. Free Radic Biol Med. 2016;99:608–622. doi: 10.1016/j.freeradbiomed.2016.08.006. [DOI] [PubMed] [Google Scholar]
- Prasad S, Tyagi AK, Aggarwal BB. Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: the golden pigment from golden spice. Cancer Res Treat Off J Korean Cancer Assoc. 2014;46:2–18. doi: 10.4143/crt.2014.46.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad S, Sajja RK, Kaisar MA, Park JH, Villalba H, Liles T, Abbruscato T, Cucullo L. Role of Nrf2 and protective Effects of Metformin against tobacco smoke-induced cerebrovascular toxicity. Redox Biol. 2017;12:58–69. doi: 10.1016/j.redox.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullen TJ, Rutter GA. When less is more: the forbidden fruits of gene repression in the adult β-cell. Diabetes Obes Metab. 2013;15:503–512. doi: 10.1111/dom.12029. [DOI] [PubMed] [Google Scholar]
- Qin S, Hou D-X. Multiple regulations of Keap1/Nrf2 system by dietary phytochemicals. Mol Nutr Food Res. 2016;60:1731–1755. doi: 10.1002/mnfr.201501017. [DOI] [PubMed] [Google Scholar]
- Qiu P, Dong Y, Li B, Kang X, Gu C, Zhu T, Luo Y, Pang M, Du W, Ge W. Dihydromyricetin modulates p62 and autophagy crosstalk with the Keap-1/Nrf2 pathway to alleviate ethanol-induced hepatic injury. Toxicol Lett. 2017;274:31–41. doi: 10.1016/j.toxlet.2017.04.009. [DOI] [PubMed] [Google Scholar]
- Qureshi AA, Guan XQ, Reis JC, Papasian CJ, Jabre S, Morrison DC, Qureshi N. Inhibition of nitric oxide and inflammatory cytokines in LPS-stimulated murine macrophages by resveratrol, a potent proteasome inhibitor. Lipids Health Dis. 2012;11:76. doi: 10.1186/1476-511X-11-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada P, Rojo AI, Chowdhry S, McMahon M, Hayes JD, Cuadrado A. SCF/-TrCP promotes glycogen synthase Kinase 3-dependent degradation of the Nrf2 transcription factor in a Keap1-independent manner. Mol Cell Biol. 2011;31:1121–1133. doi: 10.1128/MCB.01204-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada P, Rojo AI, Evrard-Todeschi N, Innamorato NG, Cotte A, Jaworski T, Tobon-Velasco JC, Devijver H, Garcia-Mayoral MF, Van Leuven F, Hayes JD, et al. Structural and functional characterization of Nrf2 degradation by the glycogen synthase kinase 3/-TrCP axis. Mol Cell Biol. 2012;32:3486–3499. doi: 10.1128/MCB.00180-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid K, Sil PC. Curcumin enhances recovery of pancreatic islets from cellular stress induced inflammation and apoptosis in diabetic rats. Toxicol Appl Pharmacol. 2015;282:297–310. doi: 10.1016/j.taap.2014.12.003. [DOI] [PubMed] [Google Scholar]
- Rastogi R, Geng X, Li F, Ding Y. NOX activation by subunit interaction and underlying mechanisms in disease. Front Cell Neurosci. 2017;10 doi: 10.3389/fncel.2016.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renno WM, Abdeen S, Alkhalaf M, Asfar S. Effect of green tea on kidney tubules of diabetic rats. Br J Nutr. 2008;100:652–659. doi: 10.1017/S0007114508911533. [DOI] [PubMed] [Google Scholar]
- Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 2010;49:1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee SG. Cell signaling: H2O2, a necessary evil for cell signaling. Science. 2006;312:1882–1883. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- Rhee SG, Bae SH. The antioxidant function of sestrins is mediated by promotion of autophagic degradation of Keap1 and Nrf2 activation and by inhibition of mTORC1. Free Radic Biol Med. 2015;88:205–211. doi: 10.1016/j.freeradbiomed.2015.06.007. [DOI] [PubMed] [Google Scholar]
- Rochette L, Zeller M, Cottin Y, Vergely C. Diabetes, oxidative stress and therapeutic strategies. Biochim Biophys Acta Gen Subj. 2014;1840:2709–2729. doi: 10.1016/j.bbagen.2014.05.017. [DOI] [PubMed] [Google Scholar]
- Rojo AI, Medina-Campos ON, Rada P, Zúñiga-Toalá A, López-Gazcón A, Espada S, Pedraza-Chaverri J, Cuadrado A. Signaling pathways activated by the phytochemical nordihydroguaiaretic acid contribute to a Keap1-independent regulation of Nrf2 stability: role of glycogen synthase kinase-3. Free Radic Biol Med. 2012;52:473–487. doi: 10.1016/j.freeradbiomed.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Romeo L, Intrieri M, D’Agata V, Mangano NG, Oriani G, Ontario ML, Scapagnini G. The major green tea polyphenol, (-)-epigallocatechin-3-gallate, induces heme oxygenase in rat neurons and acts as an effective neuroprotective agent against oxidative stress. J Am Coll Nutr. 2009;28(Suppl):492S–499S. doi: 10.1080/07315724.2009.10718116. [DOI] [PubMed] [Google Scholar]
- Romeo GR, Lee J, Shoelson SE. Metabolic syndrome, insulin resistance, and roles of inflammation - mechanisms and therapeutic targets. Arterioscler Thromb Vasc Biol. 2012;32:1771–1776. doi: 10.1161/ATVBAHA.111.241869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa AO, Egea J, Lorrio S, Rojo AI, Cuadrado A, López MG. Nrf2-mediated haeme oxygenase-1 up-regulation induced by cobalt protoporphyrin has anti-nociceptive effects against inflammatory pain in the formalin test in mice. Pain. 2008;137:332–339. doi: 10.1016/j.pain.2007.09.015. [DOI] [PubMed] [Google Scholar]
- Royston KJ, Tollefsbol TO. The epigenetic impact of cruciferous vegetables on cancer prevention. Curr Pharmacol Rep. 2015;1:46–51. doi: 10.1007/s40495-014-0003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth SA, Chen X-L, Mackman N, Ogborne RM, O’Connell MA. Lipopolysaccharide-induced heme oxygenase-1 expression in human monocytic cells is mediated via Nrf2 and protein kinase C. J Immunol. 2005;1950(175):4408–4415. doi: 10.4049/jimmunol.175.7.4408. [DOI] [PubMed] [Google Scholar]
- Rushworth SA, Ogborne RM, Charalambos CA, O’Connell MA. Role of protein kinase C δ in curcumin-induced antioxidant response element-mediated gene expression in human monocytes. Biochem Biophys Res Commun. 2006;341:1007–1016. doi: 10.1016/j.bbrc.2006.01.065. [DOI] [PubMed] [Google Scholar]
- Rutter GA, Pullen TJ, Hodson DJ, Martinez-Sanchez A. Pancreatic β-cell identity, glucose sensing and the control of insulin secretion. Biochem J. 2015;466:203–218. doi: 10.1042/BJ20141384. [DOI] [PubMed] [Google Scholar]
- Ryoo I, Lee S, Kwak M-K. Redox modulating NRF2: a potential mediator of cancer stem cell resistance. Oxidative Med Cell Longev. 2016:2428153. doi: 10.1155/2016/2428153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saaddine JB, Honeycutt AA, Narayan KMV, Zhang X, Klein R, Boyle JP. Projection of diabetic retinopathy and other major eye diseases among people with diabetes mellitus: United States, 2005–2050. Arch Ophthalmol Chic Ill. 2008;1960(126):1740–1747. doi: 10.1001/archopht.126.12.1740. [DOI] [PubMed] [Google Scholar]
- Saha PK, Reddy VT, Konopleva M, Andreeff M, Chan L. The triterpenoid 2-cyano-3,12-dioxooleana-1,9-dien-28-oic-acid methyl ester has potent anti-diabetic effects in diet-induced diabetic mice and Leprdb/db mice. J Biol Chem. 2010;285:40581–40592. doi: 10.1074/jbc.M110.176545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar M, Rojo AI, Velasco D, de Sagarra RM, Cuadrado A. Glycogen synthase kinase-3beta inhibits the xenobiotic and antioxidant cell response by direct phosphorylation and nuclear exclusion of the transcription factor Nrf2. J Biol Chem. 2006;281:14841–14851. doi: 10.1074/jbc.M513737200. [DOI] [PubMed] [Google Scholar]
- Sampath C, Zhu Y, Sang S, Ahmedna M. Bioactive compounds isolated from apple, tea, and ginger protect against dicarbonyl induced stress in cultured human retinal epithelial cells. Phytomedicine Int J Phytother Phytopharm. 2016;23:200–213. doi: 10.1016/j.phymed.2015.12.013. [DOI] [PubMed] [Google Scholar]
- Sandireddy R, Yerra VG, Areti A, Komirishetty P, Kumar A. Neuroinflammation and oxidative stress in diabetic neuropathy: futuristic strategies based on these targets. Int J Endocrinol. 2014;2014 doi: 10.1155/2014/674987. 674987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandireddy R, Yerra VG, Komirishetti P, Areti A, Kumar A. Fisetin imparts neuroprotection in experimental diabetic neuropathy by modulating Nrf2 and NF-κB pathways. Cell Mol Neurobiol. 2016;36:883–892. doi: 10.1007/s10571-015-0272-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SanGiovanni JP, Chew EY. The role of omega-3 long-chain polyunsaturated fatty acids in health and disease of the retina. Prog Retin Eye Res. 2005;24:87–138. doi: 10.1016/j.preteyeres.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Sangokoya C, Telen MJ, Chi J-T. microRNA miR-144 modulates oxidative stress tolerance and associates with anemia severity in sickle cell disease. Blood. 2010;116:4338–4348. doi: 10.1182/blood-2009-04-214817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Yama K, Murao Y, Tatsunami R, Tampo Y. Epalrestat increases intracellular glutathione levels in Schwann cells through transcription regulation. Redox Biol. 2013;2:15–21. doi: 10.1016/j.redox.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer SW, Jong CJ, Mozaffari M. Role of oxidative stress in diabetes-mediated vascular dysfunction: unifying hypothesis of diabetes revisited. Vasc Pharmacol. 2012;57:139–149. doi: 10.1016/j.vph.2012.03.005. [DOI] [PubMed] [Google Scholar]
- Scott RA, Scott LJ, Mägi R, Marullo L, Gaulton KJ, Kaakinen M, Pervjakova N, Pers TH, Johnson AD, Eicher JD, Jackson AU, et al. An expanded genome-wide association study of type 2 diabetes in Europeans. Diabetes. 2017 doi: 10.2337/db16-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah MS, Brownlee M. Molecular and cellular mechanisms of cardiovascular disorders in diabetes. Circ Res. 2016;118:1808–1829. doi: 10.1161/CIRCRESAHA.116.306923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahzad K, Bock F, Al-Dabet MM, Gadi I, Nazir S, Wang H, Kohli S, Ranjan S, Mertens PR, Nawroth PP, Isermann B. Stabilization of endogenous Nrf2 by minocycline protects against Nlrp3-inflammasome induced diabetic nephropathy. Sci Rep. 2016;6 doi: 10.1038/srep34228. 34228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakeel M. Recent advances in understanding the role of oxidative stress in diabetic neuropathy. Diabetes Metab Syndr Clin Res Rev. 2015;9:373–378. doi: 10.1016/j.dsx.2014.04.029. [DOI] [PubMed] [Google Scholar]
- Shanab AY, Nakazawa T, Ryu M, Tanaka Y, Himori N, Taguchi K, Yasuda M, Watanabe R, Takano J, Saido T, Minegishi N, et al. Metabolic stress response implicated in diabetic retinopathy: the role of calpain, and the therapeutic impact of calpain inhibitor. Neurobiol Dis. 2012;48:556–567. doi: 10.1016/j.nbd.2012.07.025. [DOI] [PubMed] [Google Scholar]
- Shang G, Tang X, Gao P, Guo F, Liu H, Zhao Z, Chen Q, Jiang T, Zhang N, Li H. Sulforaphane attenuation of experimental diabetic nephropathy involves GSK-3 beta/Fyn/Nrf2 signaling pathway. J Nutr Biochem. 2015;26:596–606. doi: 10.1016/j.jnutbio.2014.12.008. [DOI] [PubMed] [Google Scholar]
- Sharma RA, McLelland HR, Hill KA, Ireson CR, Euden SA, Manson MM, Pirmohamed M, Marnett LJ, Gescher AJ, Steward WP. Pharmacodynamic and pharmacokinetic study of oral Curcuma extract in patients with colorectal cancer. Clin Cancer Res Off J Am Assoc Cancer Res. 2001;7:1894–1900. [PubMed] [Google Scholar]
- Sharma M, Mehndiratta M, Gupta S, Kalra OP, Shukla R, Gambhir JK. Genetic association of NAD(P)H quinone oxidoreductase (NQO1*2) polymorphism with NQO1 levels and risk of diabetic nephropathy. Biol Chem. 2016;397:725–730. doi: 10.1515/hsz-2016-0135. [DOI] [PubMed] [Google Scholar]
- Sheetz MJ, King GL. Molecular understanding of hyperglycemia’s adverse effects for diabetic complications. JAMA. 2002;288:2579–2588. doi: 10.1001/jama.288.20.2579. [DOI] [PubMed] [Google Scholar]
- Shen G, Hebbar V, Nair S, Xu C, Li W, Lin W, Keum Y-S, Han J, Gallo MA, Kong A-NT. Regulation of Nrf2 transactivation domain activity: the differential effects of mitogen-activated protein kinase cascades and synergistic stimulatory effect of Raf and creb-binding protein. J Biol Chem. 2004;279:23052–23060. doi: 10.1074/jbc.M401368200. [DOI] [PubMed] [Google Scholar]
- Shimoyama Y, Mitsuda Y, Tsuruta Y, Hamajima N, Niwa T. Polymorphism of Nrf2, an antioxidative gene, is associated with blood pressure and cardiovascular mortality in hemodialysis patients. Int J Med Sci. 2014;11:726–731. doi: 10.7150/ijms.8590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoelson SE. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoelson SE, Lee J, Yuan M. Inflammation and the IKKβ/IκB/NF-κB axis in obesity- and diet-induced insulin resistance. Int J Obes. 2003;27:S49–S52. doi: 10.1038/sj.ijo.0802501. [DOI] [PubMed] [Google Scholar]
- Silva KC, Rosales MAB, Hamassaki DE, Saito KC, Faria AM, Ribeiro PAO, de Faria JBL, de Faria JML. Green tea is neuroprotective in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2013;54:1325–1336. doi: 10.1167/iovs.12-10647. [DOI] [PubMed] [Google Scholar]
- Singh R, Barden A, Mori T, Beilin L. Advanced glycation end-products: a review. Diabetologia. 2001;44:129–146. doi: 10.1007/s001250051591. [DOI] [PubMed] [Google Scholar]
- Sireesh D, Ganesh M-R, Dhamodharan U, Sakthivadivel M, Sivasubramanian S, Gunasekaran P, Ramkumar KM. Role of pterostilbene in attenuating immune mediated devastation of pancreatic beta cells via Nrf2 signaling cascade. J Nutr Biochem. 2017;44:11–21. doi: 10.1016/j.jnutbio.2017.02.015. [DOI] [PubMed] [Google Scholar]
- Skoko JJ, Wakabayashi N, Noda K, Kimura S, Tobita K, Shigemura N, Tsujita T, Yamamoto M, Kensler TW. Loss of Nrf2 in mice evokes a congenital intrahepatic shunt that alters hepatic oxygen and protein expression gradients and toxicity. Toxicol Sci. 2014;141:112–119. doi: 10.1093/toxsci/kfu109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soetikno V, Sari FR, Veeraveedu PT, Thandavarayan RA, Harima M, Sukumaran V, Lakshmanan AP, Suzuki K, Kawachi H, Watanabe K. Curcumin ameliorates macrophage infiltration by inhibiting NF-κB activation and proinflammatory cytokines in streptozotocin induced-diabetic nephropathy. Nutr Metab. 2011;8:35. doi: 10.1186/1743-7075-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soetikno V, Suzuki K, Veeraveedu PT, Arumugam S, Lakshmanan AP, Sone H, Watanabe K. Molecular understanding of curcumin in diabetic nephropathy. Drug Discov Today. 2013;18:756–763. doi: 10.1016/j.drudis.2013.04.009. [DOI] [PubMed] [Google Scholar]
- Solomon SD, Chew E, Duh EJ, Sobrin L, Sun JK, VanderBeek BL, Wykoff CC, Gardner TW. Diabetic retinopathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40:412–418. doi: 10.2337/dc16-2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M-Y, Kim E-K, Moon W-S, Park J-W, Kim H-J, So H-S, Park R, Kwon K-B, Park B-H. Sulforaphane protects against cytokine- and streptozotocin-induced β-cell damage by suppressing the NF-κB pathway. Toxicol Appl Pharmacol. 2009;235:57–67. doi: 10.1016/j.taap.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Song Y, Huang L, Yu J. Effects of blueberry anthocyanins on retinal oxidative stress and Inflammation in diabetes through Nrf2/HO-1 signaling. J Neuroimmunol. 2016;301:1–6. doi: 10.1016/j.jneuroim.2016.11.001. [DOI] [PubMed] [Google Scholar]
- de Souza CG, da Motta LL, de Assis AM, Rech A, Bruch R, Klamt F, Souza DO. Sulforaphane ameliorates the insulin responsiveness and the lipid profile but does not alter the antioxidant response in diabetic rats. Food Funct. 2016;7:2060–2065. doi: 10.1039/C5FO01620G. [DOI] [PubMed] [Google Scholar]
- Sozen E, Ozer NK. Impact of high cholesterol and endoplasmic reticulum stress on metabolic diseases: an updated mini-review. Redox Biol. 2017;12:456–461. doi: 10.1016/j.redox.2017.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenland K, Nowlin S, Palu S. Cancer incidence in the National Health and Nutrition Survey I. Follow-up data: diabetes, cholesterol, pulse and physical activity. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. 1995;4:807–811. [PubMed] [Google Scholar]
- Stefanson A, Bakovic M. Dietary regulation of Keap1/Nrf2/Are pathway: focus on plant-derived compounds and trace minerals. Nutrients. 2014;6:3777–3801. doi: 10.3390/nu6093777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steigerwalt R, Nebbioso M, Appendino G, Belcaro G, Ciammaichella G, Cornelli U, Luzzi R, Togni S, Dugall M, Cesarone MR, Ippolito E, et al. Meriva®, a lecithinized curcumin delivery system, in diabetic microangiopathy and retinopathy. Panminerva Med. 2012;54:11–16. [PubMed] [Google Scholar]
- Stribling D, Armstrong FM, Perkins CM, Smith JC. Aldose reductase in the etiology of diabetic complications. 3. Neuropathy. J Diabet Complicat. 1989;3:139–148. doi: 10.1016/0891-6632(89)90036-6. [DOI] [PubMed] [Google Scholar]
- Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012;481:278–286. doi: 10.1038/nature10759. [DOI] [PubMed] [Google Scholar]
- Stumvoll M, Nurjhan N, Perriello G, Dailey G, Gerich JE. Metabolic effects of metformin in non-insulin-dependent diabetes mellitus. N Engl J Med. 1995;333:550–554. doi: 10.1056/NEJM199508313330903. [DOI] [PubMed] [Google Scholar]
- Su K-H, Dai C. Metabolic control of the proteotoxic stress response: implications in diabetes mellitus and neurodegenerative disorders. Cell Mol Life Sci. 2016;73:4231–4248. doi: 10.1007/s00018-016-2291-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Z-Y, Khor TO, Shu L, Lee JH, Saw CL-L, Wu T-Y, Huang Y, Suh N, Yang CS, Conney AH, Wu Q, et al. Epigenetic reactivation of Nrf2 in murine prostate cancer TRAMP C1 cells by natural phytochemicals Z-ligustilide and radix Angelica Sinensis via promoter CpG demethylation. Chem Res Toxicol. 2013;26:477–485. doi: 10.1021/tx300524p. [DOI] [PubMed] [Google Scholar]
- Sugihara G, May R, Ye H, Hsieh C, Deyle E, Fogarty M, Munch S. Detecting causality in complex ecosystems. Science. 2012;338:496–500. doi: 10.1126/science.1227079. [DOI] [PubMed] [Google Scholar]
- Sun Z, Zhang S, Zhang DD. Keap1 controls postinduction repression of the Nrf2-mediated antioxidant response by escorting nuclear export of Nrf2. Mol Cell Biol. 2007;27:6334–6349. doi: 10.1128/MCB.00630-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Huang Z, Zhang DD. Phosphorylation of Nrf2 at multiple sites by MAP kinases has a limited contribution in modulating the Nrf2-dependent antioxidant response. PLoS One. 2009;4:e6588. doi: 10.1371/journal.pone.0006588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun GY, Chen Z, Jasmer KJ, Chuang DY, Gu Z, Hannink M, Simonyi A. Quercetin attenuates inflammatory responses in BV-2 microglial cells: role of MAPKs on the Nrf2 pathway and induction of heme oxygenase-1. PLoS One. 2015;10:e0141509. doi: 10.1371/journal.pone.0141509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Xiu C, Liu W, Tao Y, Wang J, Qu YI. Grape seed proanthocyanidin extract protects the retina against early diabetic injury by activating the Nrf2 pathway. Exp Ther Med. 2016;11:1253–1258. doi: 10.3892/etm.2016.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Motohashi H, Yamamoto M. Toward clinical application of the Keap1–Nrf2 pathway. Trends Pharmacol Sci. 2013;34:340–346. doi: 10.1016/j.tips.2013.04.005. [DOI] [PubMed] [Google Scholar]
- Sytze Van Dam P, Cotter MA, Bravenboer B, Cameron NE. Pathogenesis of diabetic neuropathy: focus on neurovascular mechanisms. Eur J Pharmacol. 2013;719:180–186. doi: 10.1016/j.ejphar.2013.07.017. [DOI] [PubMed] [Google Scholar]
- Taguchi K, Yamamoto M. The KEAP1-NRF2 system in cancer. Front Oncol. 2017;7(85) doi: 10.3389/fonc.2017.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talalay P, De Long MJ, Prochaska HJ. Identification of a common chemical signal regulating the induction of enzymes that protect against chemical carcinogenesis. Proc Natl Acad Sci U S A. 1988;85:8261–8265. doi: 10.1073/pnas.85.21.8261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan SM, de Haan JB. Combating oxidative stress in diabetic complications with Nrf2 activators: how much is too much? Redox Rep. 2014;19:107–117. doi: 10.1179/1351000214Y.0000000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Aleksunes LM, Yeager RL, Gyamfi MA, Esterly N, Guo GL, Klaassen CD. NF-E2-related factor 2 inhibits lipid accumulation and oxidative stress in mice fed a high-fat diet. J Pharmacol Exp Ther. 2008;325:655–664. doi: 10.1124/jpet.107.135822. [DOI] [PubMed] [Google Scholar]
- Tangvarasittichai S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J Diabetes. 2015;6:456. doi: 10.4239/wjd.v6.i3.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanigawa S, Fujii M, Hou D. Action of Nrf2 and Keap1 in ARE-mediated NQO1 expression by quercetin. Free Radic Biol Med. 2007;42:1690–1703. doi: 10.1016/j.freeradbiomed.2007.02.017. [DOI] [PubMed] [Google Scholar]
- Tanito M, Agbaga M-P, Anderson RE. Upregulation of thioredoxin system via Nrf2-antioxidant responsive element pathway in adaptive-retinal neuroprotection in vivo and in vitro. Free Radic Biol Med. 2007;42:1838–1850. doi: 10.1016/j.freeradbiomed.2007.03.018. [DOI] [PubMed] [Google Scholar]
- Telci A, Çakatay U, Kayali R, Erdoğan C, Orhan Y, Sivas A, Akçay T. Oxidative protein damage in plasma of Type 2 diabetic patients. Horm Metab Res. 2000a;32:40–43. doi: 10.1055/s-2007-978584. [DOI] [PubMed] [Google Scholar]
- Telci A, Cakatay U, Salman S, Satman I, Sivas A. Oxidative protein damage in early stage Type 1 diabetic patients. Diabetes Res Clin Pract. 2000b;50:213–223. doi: 10.1016/s0168-8227(00)00197-2. [DOI] [PubMed] [Google Scholar]
- Testa A, Leonardis D, Spoto B, Sanguedolce MC, Parlongo RM, Pisano A, Tripepi G, Mallamaci F, Zoccali C. A polymorphism in a major antioxidant gene (Kelch-like ECH-associated protein 1) predicts incident cardiovascular events in chronic kidney disease patients: an exploratory study. J Hypertens. 2016;34:928–934. doi: 10.1097/HJH.0000000000000878. [DOI] [PubMed] [Google Scholar]
- Thallas-Bonke V, Thorpe SR, Coughlan MT, Fukami K, Yap FYT, Sourris KC, Penfold SA, Bach LA, Cooper ME, Forbes JM. Inhibition of NADPH oxidase prevents advanced glycation end product-mediated damage in diabetic nephropathy through a protein kinase C-alpha-dependent pathway. Diabetes. 2008;57:460–469. doi: 10.2337/db07-1119. [DOI] [PubMed] [Google Scholar]
- Thimmulappa RK, Mai KH, Srisuma S, Kensler TW, Yamamoto M, Biswal S. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res. 2002;62:5196–5203. [PubMed] [Google Scholar]
- Thomas PK. Diabetic peripheral neuropathies: their cost to patient and society and the value of knowledge of risk factors for development of interventions. Eur Neurol. 1999;41(Suppl. 1):35–43. doi: 10.1159/000052078. https://doi.org/52078. [DOI] [PubMed] [Google Scholar]
- Thomson M, Al-Qattan KK, Divya JS, Ali M. Anti-diabetic and anti-oxidant potential of aged garlic extract (AGE) in streptozotocin-induced diabetic rats. BMC Complement Altern Med. 2016;16:17. doi: 10.1186/s12906-016-0992-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian R, Yang W, Xue Q, Gao L, Huo J, Ren D, Chen X. Rutin ameliorates diabetic neuropathy by lowering plasma glucose and decreasing oxidative stress via Nrf2 signaling pathway in rats. Eur J Pharmacol. 2016;771:84–92. doi: 10.1016/j.ejphar.2015.12.021. [DOI] [PubMed] [Google Scholar]
- Trujillo J, Chirino YI, Molina-Jijón E, Andérica-Romero AC, Tapia E, Pedraza-Chaverrí J. Renoprotective effect of the antioxidant curcumin: recent findings. Redox Biol. 2013;1:448–456. doi: 10.1016/j.redox.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsilidis KK, Kasimis JC, Lopez DS, Ntzani EE, Ioannidis JPA. Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies. BMJ. 2015;350:g7607. doi: 10.1136/bmj.g7607. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Bagi Z, Feher A, Recchia FA, Sonntag WE, Pearson K, de Cabo R, Csiszar A. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am J Physiol Heart Circ Physiol. 2010;299:H18–24. doi: 10.1152/ajpheart.00260.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Bailey-Downs L, Gautam T, Jimenez R, Losonczy G, Zhang C, Ballabh P, Recchia FA, Wilkerson DC, Sonntag WE, Pearson K, et al. Adaptive induction of NF-E2-related factor-2-driven antioxidant genes in endothelial cells in response to hyperglycemia. Am J Physiol Heart Circ Physiol. 2011;300:H1133–1140. doi: 10.1152/ajpheart.00402.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, Ron D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–666. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- Uruno A, Furusawa Y, Yagishita Y, Fukutomi T, Muramatsu H, Negishi T, Sugawara A, Kensler TW, Yamamoto M. The Keap1-Nrf2 system prevents onset of diabetes mellitus. Mol Cell Biol. 2013;33:2996–3010. doi: 10.1128/MCB.00225-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uruno A, Yagishita Y, Yamamoto M. The Keap1–Nrf2 system and diabetes mellitus. Arch Biochem Biophys. 2015;566:76–84. doi: 10.1016/j.abb.2014.12.012. [DOI] [PubMed] [Google Scholar]
- Uruno A, Yagishita Y, Katsuoka F, Kitajima Y, Nunomiya A, Nagatomi R, Pi J, Biswal SS, Yamamoto M. Nrf2-mediated regulation of skeletal muscle glycogen metabolism. Mol Cell Biol. 2016;36:1655–1672. doi: 10.1128/MCB.01095-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanitha P, Senthilkumar S, Dornadula S, Anandhakumar S, Rajaguru P, Ramkumar KM. Morin activates the Nrf2-ARE pathway and reduces oxidative stress-induced DNA damage in pancreatic beta cells. Eur J Pharmacol. 2017;801:9–18. doi: 10.1016/j.ejphar.2017.02.026. [DOI] [PubMed] [Google Scholar]
- Vincent AM, Kato K, McLean LL, Soules ME, Feldman EL. Sensory neurons and schwann cells respond to oxidative stress by increasing antioxidant defense mechanisms. Antioxid Redox Signal. 2009;11:425–438. doi: 10.1089/ars.2008.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi N, Slocum SL, Skoko JJ, Shin S, Kensler TW. When NRF2 talks, Who’s listening? Antioxid Redox Signal. 2010;13:1649–1663. doi: 10.1089/ars.2010.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi N, Skoko JJ, Chartoumpekis DV, Kimura S, Slocum SL, Noda K, Palliyaguru DL, Fujimuro M, Boley PA, Tanaka Y, Shigemura N, et al. Notch-Nrf2 axis: regulation of Nrf2 gene expression and cytoprotection by notch signaling. Mol Cell Biol. 2014;34:653–663. doi: 10.1128/MCB.01408-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walle T. Bioavailability of resveratrol. Ann N Y Acad Sci. 2011;1215:9–15. doi: 10.1111/j.1749-6632.2010.05842.x. [DOI] [PubMed] [Google Scholar]
- Wang G. Singularity analysis of the AKT signaling pathway reveals connections between cancer and metabolic diseases. Phys Biol. 2010;7:046015. doi: 10.1088/1478-3975/7/4/046015. [DOI] [PubMed] [Google Scholar]
- Wang X, Wu H, Chen H, Liu R, Liu J, Zhang T, Yu W, Hai C. Does insulin bolster antioxidant defenses via the extracellular signal–regulated kinases-protein kinase B-nuclear factor erythroid 2 p45-related factor 2 pathway? Antioxid Redox Signal. 2012;16:1061–1070. doi: 10.1089/ars.2011.4460. [DOI] [PubMed] [Google Scholar]
- Wang H, Liu K, Geng M, Gao P, Wu X, Hai Y, Li Y, Li Y, Luo L, Hayes JD, Wang XJ, et al. RXR Inhibits the NRF2-ARE signaling pathway through a direct interaction with the Neh7 domain of NRF2. Cancer Res. 2013;73:3097–3108. doi: 10.1158/0008-5472.CAN-12-3386. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhang Z, Sun W, Tan Y, Liu Y, Zheng Y, Liu Q, Cai L, Sun J. Sulforaphane attenuation of type 2 diabetes-induced aortic damage was associated with the upregulation of Nrf2 expression and function. Oxidative Med Cell Longev. 2014;2014 doi: 10.1155/2014/123963. 123963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Chen H, Liu J, Ouyang Y, Wang D, Bao W, Liu L. Association between the NF-E2 related factor 2 gene polymorphism and oxidative stress, anti-oxidative status, and newly-diagnosed Type 2 diabetes mellitus in a Chinese population. Int J Mol Sci. 2015a;16:16483–16496. doi: 10.3390/ijms160716483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Chen Y, Abdelkader D, Hassan W, Sun H, Liu J. Combination therapy with oleanolic acid and metformin as a synergistic treatment for diabetes. J Diabetes Res. 2015b;2015 doi: 10.1155/2015/973287. 973287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Zhang S, Cheng H, Lv H, Cheng G, Ci X. Nrf2-mediated liver protection by esculentoside A against acetaminophen toxicity through the AMPK/Akt/GSK3β pathway. Free Radic Biol Med. 2016;101:401–412. doi: 10.1016/j.freeradbiomed.2016.11.009. [DOI] [PubMed] [Google Scholar]
- Wardyn JD, Ponsford AH, Sanderson CM. Dissecting molecular cross-talk between Nrf2 and NF-κB response pathways. Biochem Soc Trans. 2015;43:621–626. doi: 10.1042/BST20150014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg SP, Leibel R, Tortoriello DV. Dietary curcumin significantly improves obesity-associated Inflammation and diabetes in mouse models of diabesity. Endocrinology. 2008;149:3549–3558. doi: 10.1210/en.2008-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells G. Peptide and small molecule inhibitors of the Keap1–Nrf2 protein–protein interaction. Biochem Soc Trans. 2015;43:674–679. doi: 10.1042/BST20150051. [DOI] [PubMed] [Google Scholar]
- Whitburn J, Edwards CM, Sooriakumaran P. Metformin and prostate cancer: a new role for an old drug. Curr Urol Rep. 2017;18(46) doi: 10.1007/s11934-017-0693-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White PAS, Araújo JMD, Cercato LM, Souza LA, Barbosa APO, Quintans-Junior LJ, Machado UF, Camargo EA, Brito LC, Santos MRV. Chrysobalanus icaco L. leaves normalizes insulin sensitivity and blood glucose and inhibits weight gain in high-fat diet-induced obese mice. J Med Food. 2016;19:155–160. doi: 10.1089/jmf.2015.0034. [DOI] [PubMed] [Google Scholar]
- WHO. Global report on diabetes. [accessed 7.6.17];2016 [WWW Document]. URL. http://apps.who.int/iris/bitstream/10665/204871/1/9789241565257_eng.pdf.
- Wiernsperger N. Oxidative stress as a therapeutic target in diabetes: revisiting the controversy. Diabete Metab. 2003;29:579–585. doi: 10.1016/S1262-3636(07)70072-1. [DOI] [PubMed] [Google Scholar]
- Wu CC, Hsu MC, Hsieh CW, Lin JB, Lai PH, Wung BS. Upregulation of heme oxygenase-1 by Epigallocatechin-3-gallate via the phosphatidylinositol 3-kinase/Akt and ERK pathways. Life Sci. 2006;78:2889–2897. doi: 10.1016/j.lfs.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Wu KC, Cui JY, Klaassen CD. Beneficial role of Nrf2 in regulating NADPH generation and consumption. Toxicol Sci. 2011;123:590–600. doi: 10.1093/toxsci/kfr183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu KC, Cui JY, Klaassen CD. Effect of graded Nrf2 activation on phase-I and -II drug metabolizing enzymes and transporters in mouse liver. PLoS One. 2012;7:e39006. doi: 10.1371/journal.pone.0039006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K, McDonald P, Liu J, Klaassen C. Screening of natural compounds as activators of the Keap1-Nrf2 pathway. Planta Med. 2013;80:97–104. doi: 10.1055/s-0033-1351097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Wang H, Tang X. Rexinoid inhibits Nrf2-mediated transcription through retinoid X receptor alpha. Biochem Biophys Res Commun. 2014a;452:554–559. doi: 10.1016/j.bbrc.2014.08.111. [DOI] [PubMed] [Google Scholar]
- Wu T, Zhao F, Gao B, Tan C, Yagishita N, Nakajima T, Wong PK, Chapman E, Fang D, Zhang DD. Hrd1 suppresses Nrf2-mediated cellular protection during liver cirrhosis. Genes Dev. 2014b;28:708–722. doi: 10.1101/gad.238246.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Li Y, Wu Y, Zhang Y, Wang Z, Liu X. Lutein suppresses inflammatory responses through Nrf2 activation and NF-κB inactivation in lipopolysaccharide-stimulated BV-2 microglia. Mol Nutr Food Res. 2015;59:1663–1673. doi: 10.1002/mnfr.201500109. [DOI] [PubMed] [Google Scholar]
- Xiao L, Xu X, Zhang F, Wang M, Xu Y, Tang D, Wang J, Qin Y, Liu Y, Tang C, He L, et al. The mitochondria-targeted antioxidant MitoQ ameliorated tubular injury mediated by mitophagy in diabetic kidney disease via Nrf2/PINK1. Redox Biol. 2017;11:297–311. doi: 10.1016/j.redox.2016.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Lloyd DJ, Hale C, Stanislaus S, Chen M, Sivits G, Vonderfecht S, Hecht R, Li Y-S, Lindberg RA, Chen J-L, et al. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes. 2009;58:250–259. doi: 10.2337/db08-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Kulkarni SR, Donepudi AC, More VR, Slitt AL. Enhanced Nrf2 activity worsens insulin resistance, impairs lipid accumulation in adipose tissue, and increases hepatic steatosis in leptin-deficient mice. Diabetes. 2012;61:3208–3218. doi: 10.2337/db11-1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Donepudi AC, Moscovitz JE, Slitt AL. Keap1-knockdown decreases fasting-induced fatty liver via altered lipid metabolism and decreased fatty acid mobilization from adipose tissue. PLoS One. 2013a;8:e79841. doi: 10.1371/journal.pone.0079841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Luo P, Wang Y, Cui Y, Miao L. Nuclear factor (erythroid-derived 2)-like 2 (NFE2L2) is a novel therapeutic target for diabetic complications. J Int Med Res. 2013b;41:13–19. doi: 10.1177/0300060513477004. [DOI] [PubMed] [Google Scholar]
- Xu X, Sun J, Chang X, Wang J, Luo M, Wintergerst KA, Miao L, Cai L. Genetic variants of nuclear factor erythroid-derived 2-like 2 associated with the complications in Han descents with type 2 diabetes mellitus of Northeast China. J Cell Mol Med. 2016a;20:2078–2088. doi: 10.1111/jcmm.12900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Wang S, Ji H, Zhang Z, Chen J, Tan Y, Wintergerst K, Zheng Y, Sun J, Cai L. Broccoli sprout extract prevents diabetic cardiomyopathy via Nrf2 activation in db/db T2DM mice. Sci Rep. 2016b;6(30252) doi: 10.1038/srep30252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue M, Qian Q, Adaikalakoteswari A, Rabbani N, Babaei-Jadidi R, Thornalley PJ. Activation of NF-E2-related factor-2 reverses biochemical dysfunction of endothelial cells induced by hyperglycemia linked to vascular disease. Diabetes. 2008;57:2809–2817. doi: 10.2337/db06-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue M, Rabbani N, Momiji H, Imbasi P, Anwar MM, Kitteringham N, Park BK, Souma T, Moriguchi T, Yamamoto M, Thornalley PJ. Transcriptional control of glyoxalase 1 by Nrf2 provides a stress-responsive defence against dicarbonyl glycation. Biochem J. 2012;443:213–222. doi: 10.1042/BJ20111648. [DOI] [PubMed] [Google Scholar]
- Xue P, Hou Y, Chen Y, Yang B, Fu J, Zheng H, Yarborough K, Woods CG, Liu D, Yamamoto M, Zhang Q, et al. Adipose deficiency of Nrf2 in ob/ob mice results in severe metabolic syndrome. Diabetes. 2013;62:845–854. doi: 10.2337/db12-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagishita Y, Fukutomi T, Sugawara A, Kawamura H, Takahashi T, Pi J, Uruno A, Yamamoto M. Nrf2 protects pancreatic -cells from oxidative and nitrosative stress in diabetic model mice. Diabetes. 2014;63:605–618. doi: 10.2337/db13-0909. [DOI] [PubMed] [Google Scholar]
- Yagishita Y, Uruno A, Fukutomi T, Saito R, Saigusa D, Pi J, Fukamizu A, Sugiyama F, Takahashi S, Yamamoto M. Nrf2 improves leptin and insulin resistance provoked by hypothalamic oxidative stress. Cell Rep. 2017;18:2030–2044. doi: 10.1016/j.celrep.2017.01.064. [DOI] [PubMed] [Google Scholar]
- Yama K, Sato K, Abe N, Murao Y, Tatsunami R, Tampo Y. Epalrestat increases glutathione, thioredoxin, and heme oxygenase-1 by stimulating Nrf2 pathway in endothelial cells. Redox Biol. 2015;4:87–96. doi: 10.1016/j.redox.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yama K, Sato K, Murao Y, Tatsunami R, Tampo Y. Epalrestat upregulates heme oxygenase-1, superoxide dismutase, and catalase in cells of the nervous system. Biol Pharm Bull. 2016;39:1523–1530. doi: 10.1248/bpb.b16-00332. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Nakamura T, Noble NA, Ruoslahti E, Border WA. Expression of transforming growth factor beta is elevated in human and experimental diabetic nephropathy. Proc Natl Acad Sci U S A. 1993;90:1814–1818. doi: 10.1073/pnas.90.5.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K-Y, Lin L-C, Tseng T-Y, Wang S-C, Tsai T-H. Oral bioavailability of curcumin in rat and the herbal analysis from Curcuma longa by LC-MS/MS. J Chromatogr B Anal Technol Biomed Life Sci. 2007;853:183–189. doi: 10.1016/j.jchromb.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Yang L, Li H, Yu T, Zhao H, Cherian MG, Cai L, Liu Y. Polymorphisms in metallothionein-1 and -2 genes associated with the risk of type 2 diabetes mellitus and its complications. Am J Physiol Endocrinol Metab. 2008;294:E987–992. doi: 10.1152/ajpendo.90234.2008. [DOI] [PubMed] [Google Scholar]
- Yang M, Yao Y, Eades G, Zhang Y, Zhou Q. MiR-28 regulates Nrf2 expression through a Keap1-independent mechanism. Breast Cancer Res Treat. 2011;129:983–991. doi: 10.1007/s10549-011-1604-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Fu J, Zheng H, Xue P, Yarborough K, Woods CG, Hou Y, Zhang Q, Andersen ME, Pi J. Deficiency in the nuclear factor E2-related factor 2 renders pancreatic β-cells vulnerable to arsenic-induced cell damage. Toxicol Appl Pharmacol. 2012;264:315–323. doi: 10.1016/j.taap.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Li W, Li Y, Wang Q, Gao L, Zhao J. Dietary Lycium barbarum polysaccharide induces Nrf2/ARE pathway and ameliorates insulin resistance induced by high-fat via activation of PI3K/AKT signaling. Oxidative Med Cell Longev. 2014;2014:1–10. doi: 10.1155/2014/145641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Yao W, Li Q, Liu H, Shi H, Gao Y, Xu L. Mechanism of Tang Luo Ning effect on attenuating of oxidative stress in sciatic nerve of STZ-induced diabetic rats. J Ethnopharmacol. 2015a;174:1–10. doi: 10.1016/j.jep.2015.07.047. [DOI] [PubMed] [Google Scholar]
- Yang Y, Chen G, Cheng X, Teng Z, Cai X, Yang J, Sun X, Lu W, Wang X, Yao Y, Hu C, et al. Therapeutic potential of digitoflavone on diabetic nephropathy: nuclear factor erythroid 2-related factor 2-dependent anti-oxidant and anti-inflammatory effect. Sci Rep. 2015b;5(12377) doi: 10.1038/srep12377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Park S-H, Chang H-C, Shapiro JS, Vassilopoulos A, Sawicki KT, Chen C, Shang M, Burridge PW, Epting CL, Wilsbacher LD, et al. Sirtuin 2 regulates cellular iron homeostasis via deacetylation of transcription factor NRF2. J Clin Invest. 2017;127:1505–1516. doi: 10.1172/JCI88574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao P, Nussler A, Liu L, Hao L, Song F, Schirmeier A, Nussler N. Quercetin protects human hepatocytes from ethanol-derived oxidative stress by inducing heme oxygenase-1 via the MAPK/Nrf2 pathways. J Hepatol. 2007;47:253–261. doi: 10.1016/j.jhep.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Yasuda D, Nakajima M, Yuasa A, Obata R, Takahashi K, Ohe T, Ichimura Y, Komatsu M, Yamamoto M, Imamura R, Kojima H, et al. Synthesis of Keap1-phosphorylated p62 and Keap1-Nrf2 protein-protein interaction inhibitors and their inhibitory activity. Bioorg Med Chem Lett. 2016;26:5956–5959. doi: 10.1016/j.bmcl.2016.10.083. [DOI] [PubMed] [Google Scholar]
- Yokozawa T, Nakagawa T, Oya T, Okubo T, Juneja LR. Green tea polyphenols and dietary fibre protect against kidney damage in rats with diabetic nephropathy. J Pharm Pharmacol. 2005;57:773–780. doi: 10.1211/0022357056154. [DOI] [PubMed] [Google Scholar]
- Yoon SP, Maeng YH, Hong R, Lee BR, Kim CG, Kim HL, Chung JH, Shin BC. Protective effects of epigallocatechin gallate (EGCG) on streptozotocin-induced diabetic nephropathy in mice. Acta Histochem. 2014;116:1210–1215. doi: 10.1016/j.acthis.2014.07.003. [DOI] [PubMed] [Google Scholar]
- Yu R, Chen C, Mo Y-Y, Hebbar V, Owuor ED, Tan T-H, Kong A-NT. Activation of mitogen-activated protein kinase pathways induces antioxidant response element-mediated gene expression via a Nrf2-dependent mechanism. J Biol Chem. 2000;275:39907–39913. doi: 10.1074/jbc.M004037200. [DOI] [PubMed] [Google Scholar]
- Yu S, Khor TO, Cheung K-L, Li W, Wu T-Y, Huang Y, Foster BA, Kan YW, Kong A-N. Nrf2 expression is regulated by epigenetic mechanisms in prostate cancer of TRAMP mice. PLoS One. 2010;5:e8579. doi: 10.1371/journal.pone.0008579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Mao W, Zhai Y, Tong C, Liu M, Ma L, Yu X, Li S. Anti-tumor activity of metformin: from metabolic and epigenetic perspectives. Oncotarget. 2017;8:5619–5628. doi: 10.18632/oncotarget.13639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakkar M, Van der Heiden K, Luong LA, Chaudhury H, Cuhlmann S, Hamdulay SS, Krams R, Edirisinghe I, Rahman I, Carlsen H, Haskard DO, et al. Activation of Nrf2 in endothelial cells protects arteries from exhibiting a proinflammatory state. Arterioscler Thromb Vasc Biol. 2009;29:1851–1857. doi: 10.1161/ATVBAHA.109.193375. [DOI] [PubMed] [Google Scholar]
- Zare Javid A, Hormoznejad R, Yousefimanesh H Allah, Zakerkish M, Haghighizadeh MH, Dehghan P, Ravanbakhsh M. The impact of resveratrol supplementation on blood glucose, insulin, insulin resistance, triglyceride, and periodontal markers in type 2 diabetic patients with chronic periodontitis: resveratrol in diabetic patients with periodontitis. Phytother Res. 2017;31:108–114. doi: 10.1002/ptr.5737. [DOI] [PubMed] [Google Scholar]
- de Zeeuw D, Akizawa T, Agarwal R, Audhya P, Bakris GL, Chin M, Krauth M, Lambers Heerspink HJ, Meyer CJ, McMurray JJ, Parving H-H, et al. Rationale and trial design of Bardoxolone Methyl Evaluation in Patients with Chronic Kidney Disease and Type 2 Diabetes: the Occurrence of Renal Events (BEACON) Am J Nephrol. 2013a;37:212–222. doi: 10.1159/000346948. [DOI] [PubMed] [Google Scholar]
- de Zeeuw D, Akizawa T, Audhya P, Bakris GL, Chin M, Christ-Schmidt H, Goldsberry A, Houser M, Krauth M, Lambers Heerspink HJ, McMurray JJ, et al. Bardoxolone Methyl in Type 2 Diabetes and Stage 4 Chronic Kidney Disease. N Engl J Med. 2013b;369:2492–2503. doi: 10.1056/NEJMoa1306033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai X, Lin M, Zhang F, Hu Y, Xu X, Li Y, Liu K, Ma X, Tian X, Yao J. Dietary flavonoid genistein induces Nrf2 and phase II detoxification gene expression via ERKs and PKC pathways and protects against oxidative stress in Caco-2 cells. Mol Nutr Food Res. 2013;57:249–259. doi: 10.1002/mnfr.201200536. [DOI] [PubMed] [Google Scholar]
- Zhang C, Su Z-Y, Khor TO, Shu L, Kong A-NT. Sulforaphane enhances Nrf2 expression in prostate cancer TRAMP C1 cells through epigenetic regulation. Biochem Pharmacol. 2013;85:1398–1404. doi: 10.1016/j.bcp.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Wang S, Zhou S, Yan X, Wang Y, Chen J, Mellen N, Kong M, Gu J, Tan Y, Zheng Y, et al. Sulforaphane prevents the development of cardiomyopathy in type 2 diabetic mice probably by reversing oxidative stress-induced inhibition of LKB1/AMPK pathway. J Mol Cell Cardiol. 2014;77:42–52. doi: 10.1016/j.yjmcc.2014.09.022. [DOI] [PubMed] [Google Scholar]
- Zhang C, Li Q, Lai S, Yang L, Shi G, Wang Q, Luo Z, Zhao R, Yu Y. Attenuation of diabetic nephropathy by Sanziguben Granule inhibiting EMT through Nrf2-mediated anti-oxidative effects in streptozotocin (STZ)-induced diabetic rats. J Ethnopharmacol. 2017a;205:207–216. doi: 10.1016/j.jep.2017.05.009. [DOI] [PubMed] [Google Scholar]
- Zhang C, Syed TW, Liu R, Yu J. Role of endoplasmic reticulum stress, autophagy, and inflammation in cardiovascular disease. Front Cardiovasc Med. 2017b;4(29) doi: 10.3389/fcvm.2017.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao CR, Gao ZH, Qu XJ. Nrf2-ARE signaling pathway and natural products for cancer chemoprevention. Cancer Epidemiol. 2010;34:523–533. doi: 10.1016/j.canep.2010.06.012. [DOI] [PubMed] [Google Scholar]
- Zhao S-G, Li Q, Liu Z-X, Wang J-J, Wang X-X, Qin M, Wen Q-S. Curcumin attenuates insulin resistance in hepatocytes by inducing Nrf2 nuclear translocation. Hepato-Gastroenterology. 2011a;58 doi: 10.5754/hge11219. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Chen Y, Wang J, Sternberg P, Freeman ML, Grossniklaus HE, Cai J. Age-related retinopathy in NRF2-deficient mice. PLoS One. 2011b;6:e19456. doi: 10.1371/journal.pone.0019456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Gillette DD, Li X, Zhang Z, Wen H. Nuclear factor E2-related factor-2 (Nrf2) is required for NLRP3 and AIM2 inflammasome activation. J Biol Chem. 2014;289:17020–17029. doi: 10.1074/jbc.M114.563114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Whitman SA, Wu W, Wondrak GT, Wong PK, Fang D, Zhang DD. Therapeutic potential of Nrf2 activators in streptozotocin-induced diabetic nephropathy. Diabetes. 2011;60:3055–3066. doi: 10.2337/db11-0807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Q, Mishra M, Kowluru RA. Transcription factor Nrf2-mediated antioxidant defense system in the development of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2013;54:3941–3948. doi: 10.1167/iovs.13-11598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P-T, Li B, Liu F-R, Zhang M-C, Wang Q, Li Y-Y, Xu C, Liu Y-H, Yao Y, Li D. Metformin is associated with survival benefit in pancreatic cancer patients with diabetes: a systematic review and meta-analysis. Oncotarget. 2017;8:25242–25250. doi: 10.18632/oncotarget.15692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Wang H, Chen F, Fu J, Xu Y, Hou Y, Kou HH, Zhai C, Nelson MB, Zhang Q, Andersen ME, et al. An overview of chemical inhibitors of the Nrf2-ARE signaling pathway and their potential applications in cancer therapy. Free Radic Biol Med. 2016;99:544–556. doi: 10.1016/j.freeradbiomed.2016.09.010. [DOI] [PubMed] [Google Scholar]
- Zimmermann M, Nentwig GH. [Survival rate of desmodontal cells in relation to their extraoral dehydration] Schweiz Monatsschrift Zahnmed Rev Mens Suisse Odonto-Stomatol Riv Mens Svizzera Odontol E Stomatol. 1989;99:1007–1010. [PubMed] [Google Scholar]
- Zimmermann K, Baldinger J, Mayerhofer B, Atanasov AG, Dirsch VM, Heiss EH. Activated AMPK boosts the Nrf2/HO-1 signaling axis—a role for the unfolded protein response. Free Radic Biol Med. 2015;88:417–426. doi: 10.1016/j.freeradbiomed.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zochodne DW. Handbook of Clinical Neurology. Elsevier; 2014. Mechanisms of diabetic neuron damage; pp. 379–399. [DOI] [PubMed] [Google Scholar]
- Zou X, Feng Z, Li Y, Wang Y, Wertz K, Weber P, Fu Y, Liu J. Stimulation of GSH synthesis to prevent oxidative stress-induced apoptosis by hydroxytyrosol in human retinal pigment epithelial cells: activation of Nrf2 and JNK-p62/SQSTM1 pathways. J Nutr Biochem. 2012;23:994–1006. doi: 10.1016/j.jnutbio.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Zou X, Gao J, Zheng Y, Wang X, Chen C, Cao K, Xu J, Li Y, Lu W, Liu J, Feng Z. Zeaxanthin induces Nrf2-mediated phase II enzymes in protection of cell death. Cell Death Dis. 2014;5:e1218. doi: 10.1038/cddis.2014.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo Z-F, Zhang Q, Liu X-Z. Protective effects of curcumin on retinal Müller cell in early diabetic rats. Int J Ophthalmol. 2013;6:422–424. doi: 10.3980/j.issn.2222-3959.2013.04.02. [DOI] [PMC free article] [PubMed] [Google Scholar]