Abstract
Objective/Purpose
To evaluate efficacy and safety of monthly intravitreal injections of sirolimus, an immunosuppressive drug, for the treatment of age-related macular degeneration associated geographic atrophy (GA).
Design
Randomized, controlled, single-masked multi-center phase 2 clinical trial of intravitreal sirolimus vs. sham therapy in AREDS2 clinical centers
Subjects
Participants with GA
Methods
Participants eligible in one eye were randomly assigned to a monthly intravitreal injection of sirolimus (20 µL [440 µg]) or sham treatment while participants with two study eyes were assigned to a monthly intravitreal injection in a randomly-selected eye. Best-corrected visual acuities (BVCA), spectral domain optical coherence tomography (OCT), fundus color photography and fundus autofluorescence (FAF) images were obtained at baseline and every 6 months until visit month 24.
Main Outcome Measures
Rate of progression of GA (mm2/year) measured on color fundus photograph from baseline to 24 months. Secondary outcome measures include change in BVCA, worsening of vision by ≥3 lines, and changes in area of GA measured on FAF and OCT.
Results
52 participants (mean age 79 years) were enrolled with 27 study eyes assigned to sirolimus from May 2012 to March 2014. The baseline median area of GA was 4.73 DA (12.01 mm2). The mean (standard deviation) growth rates of GA detected on color fundus photographs were 2.27 (2.17) mm2 and 1.91 (2.27) mm2 at month 12, and 4.94 (2.96) mm2 and 5.72 (3.97) mm2 at month 24, for the sirolimus and sham groups, respectively. There was no statistically significant difference in the GA growth rates between the two treatment groups (P=0.33). Median visual acuity changes and incidence of 15-letter loss from baseline were not different between the 2 treatment groups (p=0.19). The intervention was stopped early because of sterile endophthalmitis that occurred in 3 participants in the sirolimus group. Participants were followed for safety until the study was closed in May 2015 due to lack of efficacy.
Conclusion
Sirolimus did not result in different rates of GA growth in this phase 2 study. Immunosuppression may be important for some stages of the AMD process but may not necessarily be the main pathway for the development of GA.
Introduction
Age-related macular degeneration (AMD) is the leading cause of blindness in the United States (1) and the developed world. Geographic atrophy (GA) associated with AMD is one of the two forms of late AMD characterized often by the development and regression of large drusen, followed by the progressive loss of the outer retinal layers and the retinal pigment epithelium.(2) This process may start at the center of the macula (the fovea) or eventually involve the fovea, leading to central visual loss and GA accounts for approximately 80% of all late AMD. There is no proven effective therapy for preventing the onset or retarding the progression of GA despite numerous agents tested previously. This remains an unmet medical need as the number of individuals affected with AMD is predicted to double by 2020 from numbers estimated in 2004 to be 1.75 million with late AMD.(3)
Although the pathogenesis of AMD is unknown, several pathways have been hypothesized as potentially causal including chronic inflammation.(4–6) Genetic association studies implicated the complement pathway of the immune system in the pathogenesis of late AMD, including GA.(7–9) Dysregulated expression of the complement regulatory proteins as well as the presence of activated microglia (10) and macrophages (11) in the outer retina in eyes with AMD are demonstrated in histopathological studies. Immunoglobins, activated complement factors, and activated microglia are also found in large drusen, the precursors to GA.(12–14) These findings suggest that agents that reduce immune responses may be reasonable to test for the treatment of GA. One such agent is sirolimus, an immunosuppressive drug. (15) It is known as an inhibitor of the mammalian target of rapamycin (m-TOR), a multifunctional serine-threonine kinase. The inhibition of m-TOR can result in a variety of changes in cellular function including cell survival, growth, and proliferation.(16) M-TOR inhibition also results in immunosuppression as T- and B-cell proliferation and antibody production are markedly suppressed.(17) In a study of mice that had postnatal ablation of retinal pigment epithelial (RPE) mitochondrial oxidative phosphorylation, there was gradual dedifferentiation of the RPE which lead to photoreceptor degeneration and reduced electrical responses of the retina to light. (18) Administration of sirolimus (or rapamycin) reduced the RPE dedifferentiation and preserved the photoreceptors. Inhibition of the m-TOR pathway may be a potential therapy for degenerative diseases of the RPE such as GA associated with AMD.
Sirolimus is approved by United States Food and Drugs Administration as an immunosuppressive drug in renal transplants (19) and in stents for balloon angioplasty.(20) A proprietary formulation of a non-aqueous solution of sirolimus has been tested for ocular diseases including diabetic retinopathy, (21) GA, (22, 23)and uveitis.(24) Following success in phase 1 and 2 trials for non-infectious uveitis,(25) sirolimus is currently being evaluated in a phase 3 trial. In this study, we report the results of a multicenter randomized controlled clinical trial of sirolimus for the treatment of GA conducted as an ancillary study of the Age-Related Eye Disease Study 2 (AREDS2).
Methodology
This was an ancillary study of a National Institutes of Health supported study, the Age-Related Eye Disease Study 2 (AREDS2), a clinical trial of oral supplements of omega-3 long-chain polyunsaturated fatty acids and lutein plus zeaxanthin for the treatment of AMD.(26) This ancillary study was a multi-center, randomized, single-masked phase 2 clinical trial conducted in 13 AREDS2 clinical centers. This AREDS2 Ancillary study of sirolimus for GA was reviewed and approved by each of the institutional review boards and written informed consent was obtained from all participants. The research was conducted according to the tenets of the Declaration of Helsinki. This study was registered in clinicaltrials.gov: NCT01675947.
To be eligible, AREDS2 participants had to be at least 55 years or older with sufficiently clear media for quality fundus photographs, and had to have GA area between 0.75 and 8 disc areas (DA) in at least one eye. As part of the AREDS2 ancillary study, the GA had to initially involve the center of the fovea and had to demonstrate a progression rate of at least 2 mm2/year, as determined by the AREDS2 Reading Center. We began enrollment in September 2012. In 2013, when AREDS2 terminated and to increase the pace of recruitment, non-AREDS2 participants with GA not involving the center of the fovea were recruited. These non-AREDS2 participants did not have consistent previous fundus photographs to measure the progression rate of GA.
Exclusion criteria included those unlikely to comply with study procedures and/or follow-up visits, and those who had a poor 2-year survival prognosis. Presence of confounding ocular diseases such as glaucoma, significant diabetic retinopathy, uveitis, high myopia, and neovascular AMD requiring therapies such as macular laser photocoagulation, photodynamic therapy, anti-vascular endothelial growth factor agents, and other therapies also rendered the eye ineligible. Prior surgery with vitrectomy ever or cataract surgery or YAG capsulotomy within the past three months also rendered the patient ineligible.
Participants eligible in one eye were randomly assigned to either intravitreal injection of sirolimus (20 µL [440 µg]) or a sham treatment consisting of subconjunctival injection of lidocaine. Participants with both eyes eligible were assigned intravitreal injection of sirolimus in one randomly-selected eye and sham treatment in the fellow eye. Treatments were scheduled monthly for a period of 24 months. Standardized study procedures including best-corrected visual acuity (BCVA), stereoscopic color fundus photography, fundus autofluorescence (FAF) images, and spectral-domain optical coherence tomography (OCT) were performed at baseline and every 6 months following enrollment for the 24-month duration of the study. For safety, BCVA was also measured at study visits month 2 and month 3 and FAF was also added to the month 2 visit.
The imaging was conducted at each clinical site on equipment that were certified by the reading center. Spectral domain OCT images were acquired by either Spectralis (Heidelberg Engineering) or Cirrus (Zeiss Meditec) according to reading center protocols. FAF images were acquired with either blue light scanning laser ophthalmoscopy (Spectralis, Heidelberg Engineering) or optical camera flash photography using the manufacturer’s supplied autofluorescence filters with green light (Zeiss, Topcon).
The study medication (DE-109), a proprietary formulation of a non-aqueous solution of sirolimus, in a vehicle composed of polyethylene glycol 400 (PEG 400) and 4% ethanol (200 proof), was provided as a clear solution by the company (Santen Pharmaceutical Co., Ltd. Osaka, Japan) for the study as a frozen 0.5 mL sterile injectable solution. The dose of 440 ug was chosen based upon prior studies that evaluated other doses. This dose was considered to be safe and bioavailable. The medication was thawed immediately prior to injection, and drawn into a 0.3 mL syringe (Becton Dickinson, Frankling Lakes, NJ) with a 30-gauge,.½inch-long needle. A 20 µL volume (440 ug) injection was given intravitreally after topical anesthesia with 0.5% proparacaine and topical povidone-iodine. Similar preparation of the injection site was performed on the sham treatment group, but subconjunctival injection of lidocaine was given instead of the intravitreal injection.
Investigators who were administering the therapies were not masked to the treatment assignment. The research team including those obtaining BCVA, ocular images and OCT were masked to the treatment group allocation. The certified graders at the fundus photograph reading center were also masked to the treatment assignment and they determined the primary outcome for this study, namely the measurement of the area of GA as documented by fundus photographs over the course of the study.
Outcome Measurements
The primary outcome was the rate of change from baseline over time (24 months) in the area of GA based upon masked gradings of the digital color fundus photos using manual computer planimetry by certified graders at a centralized fundus photograph reading center. Secondary outcomes include change in BCVA, worsening of BCVA of three or more lines (15 or more letters) compared with baseline, change in area of GA as measured on fundus autofluorescence (FAF), change in central subfoveal thickness measured on optical coherence tomography (OCT), and change in drusen area (defined as progression in drusen area of one disk area (DA) or greater) based on masked digital grading of fundus photographs. For participants with AREDS2 information, additional analyses included a comparison of the slope of GA growth measured while enrolled in AREDS2 to the slope of GA growth measured during the sirolimus trial to further assess for any change associated with the intravitreous injections of sirolimus. All secondary analyses used a 0.05 level of significance.
Statistical Analyses
Sample size calculation: A sample size of 50 participants (25 per treatment arm) was considered adequate for this study in order to determine the magnitude of effect between the sirolimus and sham groups for the primary efficacy variable (the rate of change from baseline in area of GA (in mm2 per year) in the study eye). This sample size provided 80% power, assuming at least 3 measures of GA (baseline and months 12 and 24), a difference in the growth rate of at least 1.45 mm2/year between the two arms with within-subject variance of 36 mm2 (estimated using data from AREDS2), correlation among the repeated observations of 0.80, 20% loss to follow-up rate over the course of follow-up, and a 0.05 level of significance.
All analyses were conducted following the intention-to-treat principle. Descriptive statistics were used to characterize the study population and chi-square and Fisher exact tests were used to compare prevalence of baseline characteristics and secondary outcomes between treatment arms. The association between treatment arm and worsening of BCVA of 3 or more lines compared with baseline was determined using a repeated measures logistic model based on a generalized linear model. The association between treatment arm and change in GA from baseline over time at 6, 12, 18 and 24 months was determined using a mixed effects model. SAS (version 9.4) were used for all analyses. The pre-trial and during trial slopes for AREDS2 participants were compared using a mixed effects model and assessing annual change in the square root of GA for the treatment by time pre-trial versus time during trial interaction.
Missing data points during follow-up were not imputed. However, several participants were seen at study closeout between the 12-month and 18-month visit (i.e., at 15 months) or between the 18-month and 24-month visit (i.e., at 21 months). For these participants, the next value forward (either for the 18-month visit or for the 24-month visit, respectively) was imputed based on a linear trajectory of the participant’s data up to the last time point.
Results
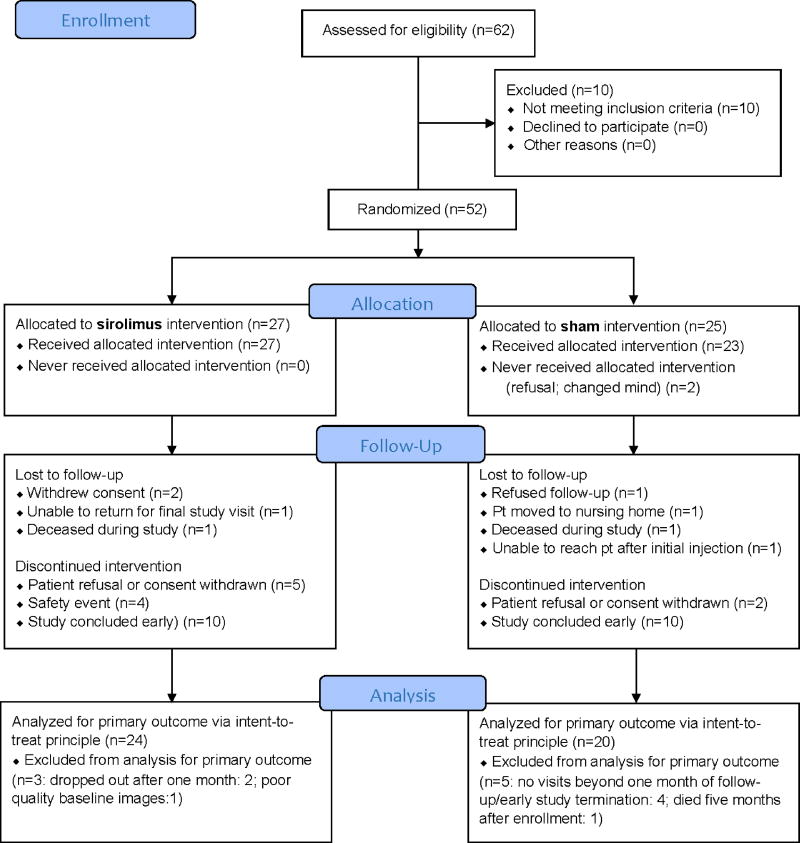
Of the 62 participants screened (CONSORT graph, Figure 1) in 13 AREDS2 participating clinics, 10 were ineligible. Among participants with one eligible eye, 21 participants were randomly assigned to intravitreal sirolimus treatment group while 25 were assigned to sham treatment group between September 2012 and May 2014; an additional six participants with both eyes eligible at the time of enrollment had a randomly-selected eye assigned to inravitreal sirolimus, while the fellow eye was not treated. Due to a gap of no more than two weeks between enrollment and the initiation of treatment, 2 of the 25 participants assigned to the sham treatment group declined to receive their first treatment and withdrew from the study. Among participants who received at least one study injection, 4 participants in each arm were lost to follow-up during the study, not including those who withdrew prior to start of treatment. Additionally, 9 participants in the sirolimus group and 2 participants in the sham group voluntarily withdrew from study injections; they were all followed through the conclusion of the study with the exception of 1 participant. The study injections were stopped early on May 30, 2014 by the Data and Safety Monitoring Committee (DSMC) because of increased risk of sterile endophthalmitis in the sirolimus arm. The study ended by May 2015, again on the recommendation of the DSMC due to lack of efficacy. Consequently, 10 participants in each study group had not reached 24 months of follow-up. This left 24 participants in the sirolimus arm and 20 participants in the sham arm for the analyses of the primary outcome of change in GA area from baseline, using the intent-to-treat principle (Figure 1).
Figure 1.
AREDS2 Sirolimus Study Consort Flow Diagram
Baseline characteristics (Table 1) showed that the mean (SE) age was 79±7.1 years, 56% were female and 96% were white. Half of the participants had some college education. Median baseline BCVA was 63 letters (Snellen equivalent of 20/60), with an interquartile range (IQR) of 22 letters. Median area of GA was 4.73 disk area (DA), with an IQR of 3.08 DA. Thirty of the 52 participants enrolled were not originally enrlled in AREDS2. Four participants who had not participated in AREDS2 and were recruited based upon clinical exam rather than fundus photograph reading center evaluation had GA <0.75 DA, while an additional four who had not participated in AREDS2 had GA > 8 DA; two AREDS2 participants had GA > 8 DA at enrollment. Three participants did not have a baseline grading due to immediate withdrawal from the study or had poor quality photographs.
Table 1.
Baseline Characteristics of the Study Participants
| Study Arm | ||||
|---|---|---|---|---|
|
|
||||
| Sirolimus | Sham | Total | ||
| Total | 27 | 25 | 52 | |
| Age, Mean (standard deviation) | 78.5 (6.9) | 79.8 (7.3) | 79.1 (7.1) | |
| Females, N (%) | 17 (63) | 12 (48) | 29 (56) | |
| Race and Ethnicity, N (%) | ||||
| White | 26 (96) | 24 (96) | 50 (96) | |
| Black or African American | 1 (3) | 1 (4) | 2 (3) | |
| Hispanic | 0 (0) | 0 (0) | 0 (0) | |
| Marital status, N (%) | ||||
| Married | 16 (59) | 14 (56) | 30 (57) | |
| Divorced | 2 (7) | 4 (16) | 6 (11) | |
| Widowed | 8 (29) | 7 (28) | 15 (28) | |
| Never married | 1 (3) | 0 (0) | 1 (1) | |
| Education, N (%) | ||||
| Grade 11 or less | 4 (14) | 5 (20) | 9 (17) | |
| High school graduate | 6 (22) | 11 (44) | 17 (32) | |
| Some college or Associate's degree | 11 (41) | 4 (16) | 15 (28) | |
| Bachelor's degree | 4 (14) | 4 (16) | 8 (15) | |
| Post-graduate work | 2 (7) | 1 (4) | 3 (5) | |
| Medical History, N (%) | ||||
| Cardiovascular disease | 21 (78) | 21 (84) | 42 (81) | |
| Pulmonary diseases | 8 (30) | 10 (40) | 18 (35) | |
| Cancer | 7 (26) | 2 (8) | 9 (17) | |
| Gastrointestinal conditions | 16 (59) | 18 (72) | 34 (65) | |
| Diabetes | 9 (33) | 9 (36) | 18 (35) | |
| Thyroid disease1 | 13 (48) | 2 (8) | 15 (29) | |
| Dermatological disorders | 3 (11) | 6 (24) | 9 (17) | |
| Arthritis | 18 (67) | 14 (56) | 32 (62) | |
| AREDS2 participants, N (%) | ||||
| Yes | 11 (41) | 11 (44) | 22 | |
| No | 16 (59) | 14 (56) | 30 | |
| Baseline visual acuity (study eye) | Minimum | 36 | 36 | 36 |
| Median | 62 | 63 | 63 | |
| Snellen | 20/60 | 20/60 | 20/60 | |
| Maximum | 80 | 83 | 83 | |
| IQR | 21 | 20 | 22 | |
| Mean | 60.5 | 61.1 | 60.8 | |
| Standard deviation | 13.0 | 13.6 | 13.2 | |
| Baseline GA area, DA (study eye) | N | 25 | 24 | 49 |
| Minimum | 0.09 | 0.18 | 0.09 | |
| Median | 4.49 | 4.84 | 4.73 | |
| Maximum | 11.00 | 9.31 | 11.00 | |
| IQR | 2.44 | 4.34 | 3.08 | |
| Mean | 4.64 | 4.19 | 4.42 | |
| Standard deviation | 2.82 | 2.64 | 2.71 | |
| GA area < 0.75 DA, N (%) | 1 (4) | 3 (13) | 4 (8) | |
| GA area > 8 DA, N (%) | 4 (16) | 2 (8) | 6 (12) | |
| GA with Center of Fovea Involved, N (%)2 | ||||
| Yes | 24 (96) | 22 (96) | 46 | |
| No | 1 (4) | 1 (4) | 2 | |
GA: geographic atrophy; DA: disk areas
N: Participants reporting at least one condition in the disease area
Fisher exact test p-value for thyroid disease p= 0.002.
Data not available on 4 of the 52 enrolled participants
Medical history (Table 1) showed high prevalence of comorbidities in this cohort, including cardiovascular disease (81%), gastrointestinal conditions (65%), arthritis (62%), and diabetes (35%). More participants reported having thyroid disease in the sirolimus group than the sham group (13% vs. 2%, Fisher exact-test: p=0.002).
The primary outcome of change in GA area as measured from digitial fundus photographs from baseline is summarized at each time point in Table 2A. There was no statistically significant association in change in GA area over time between treatment groups (p=0.33). The growth of GA measured on FAF, a secondary outcome, did not show a statistically significant association with treatment in change in GA area over time (p=0. 13) (Table 2B, Figure 2). Evaluation of the slopes of GA progression before the trial commenced and during the trial were available in the participants who were originally enrolled in the AREDS2 (n=16). The pre-trial slopes for each participant were compared with the slopes obtained during the trial. In those assigned to intravitreal sirolimus, the slope (SE), calculated by using the square root transformation of GA growth, prior to the trial was 2.814 (0.274) and 2.895 (0.277) during the trial. The results were not statistically significant (p=0.53). For those assigned to sham the pre-trial slope was 3.333 (0.312) and during the trial was 3.383 (0.310). Again this was not statistically significant (p=0.70). Other fundus and FAF secondary measures, including progression of drusen area ≥ 1 DA (p=0.33) and FAF halo (an area of hyperautofluorescence) progression ≥ 50% of the perimeter (p=0.67) were not associated with treatment. Treatment arm also had no effect on change in central subfoveal thickness measured in microns from baseline (p=0.75) (Table 2C).
Table 2.
| A. Change in Geographic Atrophy (GA) from Baseline, by Study Month | |||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Study Arm | |||||||
|
|
|||||||
| Sirolimus | Sham | ||||||
|
|
|||||||
| Study Month |
N | Median # injections |
Mean (SD) change in GA from baseline, mm2 & square root transformation (mm) |
N | Mean (SD) change in GA from baseline, mm2 & square root transformation (mm) |
Mean Difference in change from baseline, mm2 (Sirolimus-Sham) |
Hypothesized mean difference in change from baseline, mm2 (Sirolimus-Sham) |
| 6 | 21 | 4 | mm2: 1.27 (1.27) | 17 | mm2: 0.97 (1.19) | 0.30 | −0.72 |
| mm: 0.22 (0.21) | mm: 0.14 (0.15) | ||||||
| 12 | 20 | 3 | mm2: 2.27 (2.17) | 18 | mm2: 1.91 (2.27) | 0.36 | −1.45 |
| mm: 0.33 (0.29) | mm: 0.30 (0.28) | ||||||
| 18 | 17 | 8 | mm2: 3.36 (3.42) | 14 | mm2: 4.01 (4.41) | −0.65 | −2.17 |
| mm: 0.48 (0.45) | mm: 0.58 (0.50) | ||||||
| 24 | 11 | 10 | mm2: 4.94 (2.96) | 10 | mm2: 5.72 (3.97) | −0.78 | −2.90 |
| mm: 0.68 (0.36) | mm: 0.75 (0.39) | ||||||
| B. Supplemental Fundus and Autofluorescence (AF) Outcomes | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Progression to Drusen Area ≥ 1 DA1 | GA Area (AF) | Fundus Autofluorescence with Halo progression to >= 50% of perimeter2 |
||||||||||
|
|
||||||||||||
| Sirolimus | Sham | Sirolimus | Sham | Sirolimus | Sham | |||||||
|
|
||||||||||||
| Study Month |
N | % progressed | N | % progressed | N | Mean (SD) change in GA area (mm2) from baseline |
N | Mean (SD) change in GA area (mm2) from baseline |
N | % progressed | N | % progressed |
| 6 | 12 | 8% | 9 | 25% | 17 | 1.45 (1.09) | 17 | 2.09 (1.77) | 20 | 30% | 13 | 39% |
| 12 | 10 | 0% | 14 | 21% | 19 | 1.84 (1.30) | 17 | 2.80 (2.33) | 18 | 28% | 13 | 23% |
| 18 | 10 | 10% | 11 | 0% | 15 | 2.68 (3.38) | 13 | 4.29 (2.62) | 14 | 7% | 10 | 30% |
| 24 | 5 | 0% | 8 | 13% | 9 | 5.13 (2.44) | 10 | 5.89 (2.86) | 8 | 25% | 5 | 20% |
| C. Supplemental Optical Coherence Tomography (OCT) Outcomes | ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Center Subfoveal (CSF) Thickness Microns | Volume mm3 | |||||||
|
|
||||||||
| Sirolimus | Sham | Sirolimus | Sham | |||||
|
|
||||||||
| Study Month |
N | Mean (SD) change in CSF thickness microns from baseline |
N | Mean (SD) change in CSF thickness microns from baseline |
N | Mean (SD) change in volume from baseline, mm2 |
N | Mean (SD) change in volume from baseline, mm2 |
| 6 | 20 | −3.8 (38.2) | 17 | −7.9 (13.9) | 11 | 0.03 (0.42) | 4 | 0.00 (0.08) |
| 12 | 20 | −16.7 (20.3) | 20 | −12.8 (20.5) | 8 | −0.24 (0.28) | 9 | 0.62 (1.63) |
| 18 | 18 | −21.0 (25.4) | 14 | −20.9 (24.6) | 8 | 0.09 (0.55) | 6 | −0.14 (0.15) |
| 24 | 11 | −31.8 (32.8) | 10 | −25.0 (25.6) | 5 | 0.20 (0.72) | 3 | −0.33 (0.25) |
p-value for treatment effect (mixed effects model): p=0.33
- Progression to Drusen Area >= 1 DA (repeated measures logistic model): p=0.33
- GA Area (AF) (mixed effects model): p=0.13
- AF Halo Progression (repeated measures logistic model): p=0.67
Excludes 13 participants in the Sirolimus arm and 7 participants in the Sham arm whose GA measured ≥1 DA at enrollment
Excludes 5 participants in the Sirolimus arm and 6 participants in the Sham arm whose Halo progression measured >=50% of the perimeter at enrollment.
- CSF (mixed effects model): p=0.75
- Volume (mixed effects model): Model did not converge due to sparse data
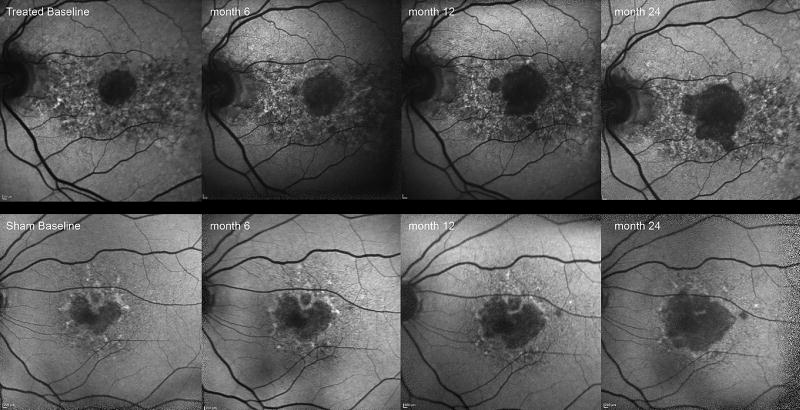
Figure 2.
Autofluorescence images of treated eye (top row) and sham eye (bottom row) at 6 monthly visits over 2 years. Area of GA in the treated eye has increased from 2.28 mm2 at baseline to 6.5 mm2 at the 2 year visit and the eye in sham group increased from 2.03 mm2 to 7.14 mm2
Change in VA from baseline at each study visit that required a standardized BCVA is shown in Table 3. Median vision loss from baseline ranged from 0–6 letters from the 2-month visit through the 24-month visit. and was fairly even between arms, although mean vision loss was generally greater in the sham arm. This difference in means was influenced by 3 participants in the sham group who experienced marked vision loss from baseline starting at just two months after enrollment due to progression of GA. Other reasons for vision loss included sterile endophthalmitis (n=3), iritis following study injection (n= 1), subretinal hemorrhage (n=1) and cataract (n=1). Incidence of 15-letter loss from baseline was somewhat higher for participants in the sham arm, however there was no significant association between treatment arm and the proportion experiencing a 15-letter loss (p=0.19).
Table 3.
Vision Change from Baseline, by Study Month
| Study Month |
Study Arm | |||
|---|---|---|---|---|
|
|
||||
| Sirolimus | Sham | |||
| 2 | Summary | N | 22 | 22 |
| Median | −2.5 | 0.0 | ||
| Mean | −3.7 | −5.4 | ||
| Stdev | 9.2 | 16.5 | ||
| 15-letter loss, N (%) | 2 (9) | 3 (14) | ||
|
| ||||
| 3 | Summary | N | 23 | 19 |
| Median | −3.0 | −2.0 | ||
| Mean | −3.3 | −7.6 | ||
| Stdev | 9.8 | 17.9 | ||
| 15-letter loss, N (%) | 2 (9) | 3 (16) | ||
|
| ||||
| 6 | Summary | N | 23 | 17 |
| Median | −3.0 | −1.0 | ||
| Mean | −4.2 | −10.2 | ||
| Stdev | 12.8 | 20.4 | ||
| 15-letter loss, N (%) | 2 (9) | 5 (29) | ||
|
| ||||
| 9 | Summary | N | 21 | 21 |
| Median | −3.0 | −4.0 | ||
| Mean | −2.2 | −9.2 | ||
| Stdev | 8.9 | 17.4 | ||
| 15-letter loss, N (%) | 2 (10) | 5 (24) | ||
|
| ||||
| 12 | Summary | N | 22 | 20 |
| Median | −3.5 | −3.0 | ||
| Mean | −3.7 | −7.3 | ||
| St dev | 8.7 | 16.8 | ||
| 15-letter loss, N (%) | 1 (5) | 4 (20) | ||
|
| ||||
| 18 | Summary | N | 16 | 11 |
| Median | −4.0 | −3.0 | ||
| Mean | −4.5 | −11.6 | ||
| St dev | 8.8 | 22.4 | ||
| 15-letter loss, N (%) | 3 (19) | 3 (27) | ||
|
| ||||
| 24 | Summary | N | 7 | 5 |
| Median | −4.0 | −6.0 | ||
| Mean | −3.7 | −10.6 | ||
| Stdev | 8.6 | 16.2 | ||
| 15-letter loss, N (%) | 1 (14) | 2 (40) | ||
p-value for treatment effect (repeated measures logistic model): p=0.19
Adverse events (AE) are summarized in Table 4A. More AEs were related to the sirolimus injection compared to sham injection (41% vs. 20%, p=0.0005); this held true for participants reporting at least one AE related to the study injection (63% in the sirolimus arm vs. 20% in the sham arm, p=0.0023). As expected, participants in the sirolimus group reported visualizing drug depot in the vitreous or vitreous floaters related to study injection more frequently than participants in the sham group (44% vs. 0%, p<0.0001) Table 4A. The drug depot was reported to cause visual impairment lasting months following injection that was substantial enough to result in nine participants withdrawing from additional treatments. These participants, however, consented to continued follow-up. One anterior uveitis event and three endophthalmitis events were reported to occur in four sirolimus arm participants. Those affected with endophthalmitis received the standard of care for presumed endophthalmitis but the culture of the vitreous failed to demonstrate growth of any pathogen. The visual acuities from these affected cases returned to the baseline level of visual acuity in all three cases between one and two months after intial report of endophthalmitis. Total adverse events including those from non-ocular adverse events are seen in Table 4B.
Table 4.
| A | |||
|---|---|---|---|
|
| |||
| Study Arm | |||
|
|
|||
| Sirolimus (N=27) |
Sham (N=25) |
Total (N=52) |
|
| Number of Events/Participants | |||
| Number of Adverse Events | 111 | 42 | 153 |
| Participants with at least one AE, N (%) | 22 (81) | 16 (64) | 38 (73) |
| Number of SAEs | 12 | 9 | 21 |
| Participants with at least one SAE, N (%) | 7 (26) | 6 (24) | 13 (25) |
| Specific Ocular Events (at least one) Per Participant, N (%) | |||
| Drug depot/floater in vitreous related to injection1 | 12 (44) | 0 (0) | 12 (23) |
| Eye Pain related to injection | 5 (19) | 3 (12) | 8 (15) |
| Anterior Uveitis or Sterile Endophthalmitis2 | 4 (15) | 0 (0) | 4 (10) |
| At least one Reasonable Possibility Study Injection Caused Event, Per Participant, N (%) | |||
| Yes1 | 17 (63) | 5 (20) | 22 (42) |
| Reasonable Possibility Study Injection Caused Event among All AEs, N (%) | |||
| Yes1 | 46 (41) | 5 (20) | 51 (33) |
| Reasonable Possibility Study Injection Caused Event among All SAEs, N (%) | |||
| Yes | 4 (33) | 0 (0) | 4 (19) |
| Deaths | 1 (4) | 1 (4) | 2 (4) |
| B | |||
|---|---|---|---|
|
| |||
| Study Arm | |||
|
|
|||
| Sirolimus (N=27) |
Sham (N=25) |
Total (N=52) |
|
| Number of Events/Participants | |||
| Number of Adverse Events | 111 | 42 | 153 |
| Participants with at least one AE, N (%) | 22 (81) | 16 (64) | 38 (73) |
| Number of SAEs | 12 | 9 | 21 |
| Participants with at least one SAE, N (%) | 7 (26) | 6 (24) | 13 (25) |
| Type of Event among All AEs, N (%) | |||
| Non-ocular1 | 55 (50) | 31 (74) | 86 (56) |
| Non-study eye | 2 (2) | 1 (1) | 3 (2) |
| Study eye1 | 52 (47) | 7 (17) | 59 (39) |
| Both eyes | 2 (2) | 3 (7) | 5 (3) |
| Severity among All AEs, N (%) | |||
| Mild | 76 (68) | 22 (52) | 98 (64) |
| Moderate | 31 (28) | 12 (29) | 43 (28) |
| Severe1 | 4 (4) | 8 (19) | 12 (8) |
| Highest Severity Per Participant, N (%) | |||
| Mild | 10 (37) | 6 (24) | 16 (31) |
| Moderate | 9 (33) | 5 (20) | 14 (27) |
| Severe | 3 (11) | 5 (20) | 8 (15) |
Fisher exact test p<0.05 (Drug depot/floater: p=0.0001; Study injection, pts: p=0.0023; Study injection, all AEs: p=0.0005)
Does not include one case of sterile endophthalmitis event that occurred in a non-study eye that received antivascular endothelial growth factor therapy for neovascular AMD.
Fisher exact test p<0.05 (Non-ocular AEs: p=0.0101; Study eye AEs: p=0.0007; Severe AEs: p=0.0037)
Overall, 111 adverse events were reported in the sirolimus group from 22 (81%) of the 27 randomized participants and 42 adverse events were reported in the sham group from 16 (64%) of the 25 randomized participants (Table 4B). Twelve serious adverse events (SAEs) were reported in the sirolimus group by 7 (26%) participants and 9 SAEs were reported in the sham group by 6 (24%) participants.
Discussion
This phase 2 trial did not demonstrate differences in the rate of growth of GA. No systemic adverse effects were associated with the use of sirolimus. A number of study limitations markedly reduced the ability to demonstrate potential beneficial effect of sirolimus including poor compliance, the inability to complete the study interventions because of severe adverse events, and subsequently limited power to detect a difference. Compliance with treatment was compromised by the visual impairment from the drug depot effect as demonstrated by the high rate of treatment withdrawal (9/27 or 0.33 in the sirolimus group compared to 2/25 or 0.08 in the sham group). The number (3) of sterile endophthalmitis events in this small cohort who received a total number of 154 intravitreal injections was considerably higher than the expected incidence of endophthalmitis found in other intravitreal injections of drugs. A number of studies have published the rates of non-infectious vitritis or sterile endophthalmitis following intravitreal injections of anti-vascular endothelial growth factor (VEGF) agents. Data from a retrospective case series of injections given from 2006 to 2013 indicate the rate of noninfectious vitritis in nearly 100,000 intravitreal injections ranged from 0.02% to 0.16% for the various anti-VEGF agents.(27) For culture positive endophthalmitis in a case series of nearly 500,000 intravitreal injections in 5 retina clinics, the rates ranged from 0.035% to 0.039% for all the anti-VEGF and steroidal formulations administered.(28) Another center found that intravitreal injections of steroid resulted in 6.9 fold increased rate of endophthalmitis when compared with intravitreal injections of anti-VEGF therapies (0.13% vs. 0.019%).(29) The non-infectious vitritis and culture negative cases had better visual acuity outcomes than those that were culture positive.(30) Rates of endophthalmitis reported in a clinical trial were similarly low.(31)
Fifty-two participants were recruited from September 2012 to May 2014. Because of the elevated rate of culture-negative endophthalmitis, the study injections were stopped prior to completing the number of planned intravitreal injections in May 2014. We continued to follow these participants until May 2015 for safety. The ability to evaluate the effect of sirolimus was compromised by these premature terminations.
Two phase 1 studies in which sirolimus was administered subconjunctivally (22)and intravitreally (23) for GA associated with AMD were conducted at the National Eye Institute. These studies enrolled small numbers of patients and the ability to make inferences from such small, non-randomized unmasked studies was limited. It was thought that there may have been more visual acuity loss and more rapid GA progression in those who received sirolimus. This was not confirmed in the current phase 2 randomized trial. The VA loss was not greater in the treated group and the GA growth did not differ by treatment. Even those participants who had the sterile endophthalmitis did not demonstrate vision loss from baseline.
Sirolimus is currently being investigated in a phase 3 trial of non-infectious uveitis following a phase 2 trial which demonstrated efficacy. The rates of sterile or culture-positive endophthalmitis have not been reported to be increased. Since the pathobiology of disease in uveitis is known to be inflammatory, it is not surprising that sirolimus may play an important role in treating this condition. Although inflammation may play an important part in the etiology of AMD, it is possible that the inflammatory process is more important in drusen formation rather than GA formation, as demonstrated by the histopathology. Perhaps additional pathways may be more important in the late stages of AMD, thus suppression of inflammation is inadequate to prevent further progression in established GA. For example, genes associated with extracellular matrix may play a greater role in eyes with neovascular AMD.(32) It is also possible that we did not give sufficient drug or it was administered too late in the course of the disease.
We currently have no effective therapy for GA. Perhaps by the time GA develops, cumulative cellular damage may have occurred so that it may be more difficult to retard or reverse the course of the disease. Cell death is programmed and providing therapy at this stage may be less favorable. Clearly, a better therapeutic strategy would be the prevention of progression at earlier stages of disease. Hopefully, further evaluation of genetic associations with the different stages of AMD may help elucidate different pathways to target in future treatment studies.
Supplementary Material
Acknowledgments
Financial Support: Supported by the intramural program funds and contracts from the National Eye Institute/National Institutes of Health, Department of Health and Human Services, Bethesda Maryland. Santen provided the drugs and the funding for the randomized clinical trial portion of the study. The sponsors and funding organizations did not participate in the design and conduct of the study; data collection, management, analysis and interpretation; and the preparation, review and approval of the manuscript.
Supported in part by an unrestricted grant from Research to Prevent Blindness, Inc. New York, to the Department of Ophthalmology and Visual Sciences, University of Wisconsin-Madison (AD, RPD), and to the Emory Eye Center, Emory University School of Medicine (GBH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest:
Gary Gensler: None
Traci Clemons: None
Amitha Domalpally: None
Ronald P Danis: None
Barbara Blodi: None
Jack Wells
Michael Rauser
John Hoskins
Baker Hubbard: None
Michael Elman
Gary Fish
Alexander Brucker: none
Alan Margherio: none
Emily Y. Chew: none
References
- 1.Congdon N, O'Colmain B, Klaver CC, Klein R, Munoz B, Friedman DS, et al. Causes and prevalence of visual impairment among adults in the United States. Archives of ophthalmology (Chicago, Ill : 1960) 2004;122(4):477–85. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- 2.Klein ML, Ferris FL, 3rd, Armstrong J, Hwang TS, Chew EY, Bressler SB, et al. Retinal precursors and the development of geographic atrophy in age-related macular degeneration. Ophthalmology. 2008;115(6):1026–31. doi: 10.1016/j.ophtha.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 3.Friedman DS, O’Colmain BJ, Munõz B, et al. Eye Diseases Prevalence Research Group. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122(4):465–572. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 4.Penfold PL, Madigan MC, Gillies MC, Provis JM. Immunological and aetiological aspects of macular degeneration. Progress in retinal and eye research. 2001;20(3):385–414. doi: 10.1016/s1350-9462(00)00025-2. [DOI] [PubMed] [Google Scholar]
- 5.Fritsche LG, Fariss RN, Stambolian D, Abecasis GR, Curcio CA, Swaroop A. Age-related macular degeneration: genetics and biology coming together. Annual review of genomics and human genetics. 2014;15:151–71. doi: 10.1146/annurev-genom-090413-025610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ambati J, Atkinson JP, Gelfand BD. Immunology of age-related macular degeneration. Nature reviews Immunology. 2013;13(6):438–51. doi: 10.1038/nri3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haines JL, Hauser MA, Schmidt S, Scott WK, Olson LM, Gallins P, et al. Complement factor H variant increases the risk of age-related macular degeneration. Science (New York, NY) 2005;308(5720):419–21. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- 8.Klein RJ, Zeiss C, Chew EY, Tsai JY, Sackler RS, Haynes C, et al. Complement factor H polymorphism in age-related macular degeneration. Science (New York, NY) 2005;308(5720):385–9. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edwards AO, Ritter R, 3rd, Abel KJ, Manning A, Panhuysen C, Farrer LA. Complement factor H polymorphism and age-related macular degeneration. Science (New York, NY) 2005;308(5720):421–4. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- 10.Gupta N, Brown KE, Milam AH. Activated microglia in human retinitis pigmentosa, late-onset retinal degeneration, and age-related macular degeneration. Experimental eye research. 2003;76(4):463–71. doi: 10.1016/s0014-4835(02)00332-9. [DOI] [PubMed] [Google Scholar]
- 11.Cherepanoff S, McMenamin P, Gillies MC, Kettle E, Sarks SH. Bruch's membrane and choroidal macrophages in early and advanced age-related macular degeneration. The British journal of ophthalmology. 2010;94(7):918–25. doi: 10.1136/bjo.2009.165563. [DOI] [PubMed] [Google Scholar]
- 12.Johnson LV, Leitner WP, Staples MK, Anderson DH. Complement activation and inflammatory processes in Drusen formation and age related macular degeneration. Experimental eye research. 2001;73(6):887–96. doi: 10.1006/exer.2001.1094. [DOI] [PubMed] [Google Scholar]
- 13.Anderson DH, Mullins RF, Hageman GS, Johnson LV. A role for local inflammation in the formation of drusen in the aging eye. American journal of ophthalmology. 2002;134(3):411–31. doi: 10.1016/s0002-9394(02)01624-0. [DOI] [PubMed] [Google Scholar]
- 14.Hageman GS, Luthert PJ, Victor Chong NH, Johnson LV, Anderson DH, Mullins RF. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch's membrane interface in aging and age-related macular degeneration. Progress in retinal and eye research. 2001;20(6):705–32. doi: 10.1016/s1350-9462(01)00010-6. [DOI] [PubMed] [Google Scholar]
- 15.Mudumba S, Bezwada P, Takanaga H, Hosoi K, Tsuboi T, Ueda K, et al. Tolerability and pharmacokinetics of intravitreal sirolimus. Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics. 2012;28(5):507–14. doi: 10.1089/jop.2011.0226. [DOI] [PubMed] [Google Scholar]
- 16.Sehgal SN. Rapamune (RAPA, rapamycin, sirolimus): mechanism of action immunosuppressive effect results from blockade of signal transduction and inhibition of cell cycle progression. Clinical biochemistry. 1998;31(5):335–40. doi: 10.1016/s0009-9120(98)00045-9. [DOI] [PubMed] [Google Scholar]
- 17.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149(2):274–93. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao C, Yasumura D, Li X, Matthes M, Lloyd M, Nielsen G, Ahern K, et al. mTOR-mediated dedifferentiation of the retinal pigment epithelium initiates photorceptor degeneration in mice. J Clin Invest. 2011;121(1):369–83. doi: 10.1172/JCI44303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Camardo J. The Rapamune era of immunosuppression 2003: the journey from the laboratory to clinical transplantation. Transplantation proceedings. 2003;35(3 Suppl):18s–24s. doi: 10.1016/s0041-1345(03)00356-7. [DOI] [PubMed] [Google Scholar]
- 20.Vishnevetsky D, Patel P, Tijerino H, Gandhi PJ. Sirolimus-eluting coronary stent. American journal of health-system pharmacy : AJHP : official journal of the American Society of Health-System Pharmacists. 2004;61(5):449–56. doi: 10.1093/ajhp/61.5.449. [DOI] [PubMed] [Google Scholar]
- 21.Dugel PU, Blumenkranz MS, Haller JA, Williams GA, Solley WA, Kleinman DM, et al. A randomized, dose-escalation study of subconjunctival and intravitreal injections of sirolimus in patients with diabetic macular edema. Ophthalmology. 2012;119(1):124–31. doi: 10.1016/j.ophtha.2011.07.034. [DOI] [PubMed] [Google Scholar]
- 22.Wong WT, Dresner S, Forooghian F, Glaser T, Doss L, Zhou M, et al. Treatment of geographic atrophy with subconjunctival sirolimus: results of a phase I/II clinical trial. Investigative ophthalmology & visual science. 2013;54(4):2941–50. doi: 10.1167/iovs.13-11650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petrou PA, Cunningham D, Shimel K, Harrington M, Hammel K, Cukras CA, et al. Intravitreal sirolimus for the treatment of geographic atrophy: results of a phase I/II clinical trial. Investigative ophthalmology & visual science. 2015;56(1):330–8. doi: 10.1167/iovs.14-15877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phillips BN, Wroblewski KJ. A retrospective review of oral low-dose sirolimus (rapamycin) for the treatment of active uveitis. Journal of ophthalmic inflammation and infection. 2011;1(1):29–34. doi: 10.1007/s12348-010-0015-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nguyen QD, Ibrahim MA, Watters A, Bittencourt M, Yohannan J, Sepah YJ, et al. Ocular tolerability and efficacy of intravitreal and subconjunctival injections of sirolimus in patients with noninfectious uveitis: primary 6-month results of the SAVE Study. Journal of ophthalmic inflammation and infection. 2013;3(1):32. doi: 10.1186/1869-5760-3-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. Jama. 2013;309(19):2005–15. doi: 10.1001/jama.2013.4997. [DOI] [PubMed] [Google Scholar]
- 27.Williams PD, Chong D, Fuller T, Callanan D. NONINFECTIOUS VITRITIS AFTER INTRAVITREAL INJECTION OF ANTI-VEGF AGENTS: Variations in Rates and Presentation by Medication. Retina (Philadelphia, Pa) 2016;36(5):909–13. doi: 10.1097/IAE.0000000000000801. [DOI] [PubMed] [Google Scholar]
- 28.Rayess N, Rahimy E, Storey P, Shah CP, Wolfe JD, Chen E, et al. Postinjection Endophthalmitis Rates and Characteristics Following Intravitreal Bevacizumab, Ranibizumab, and Aflibercept. American journal of ophthalmology. 2016;165:88–93. doi: 10.1016/j.ajo.2016.02.028. [DOI] [PubMed] [Google Scholar]
- 29.VanderBeek BL, Bonaffini SG, Ma L. The Association between Intravitreal Steroids and Post-Injection Endophthalmitis Rates. Ophthalmology. 2015;122(11):2311–5. e1. doi: 10.1016/j.ophtha.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gregori NZ, Flynn HW, Jr, Schwartz SG, Rosenfeld PJ, Vaziri K, Moshfeghi AA, et al. Current Infectious Endophthalmitis Rates After Intravitreal Injections of Anti-Vascular Endothelial Growth Factor Agents and Outcomes of Treatment. Ophthalmic surgery, lasers & imaging retina. 2015;46(6):643–8. doi: 10.3928/23258160-20150610-08. [DOI] [PubMed] [Google Scholar]
- 31.Meredith TA, McCannel CA, Barr C, Doft BH, Peskin E, Maguire MG, et al. Postinjection endophthalmitis in the comparison of age-related macular degeneration treatments trials (CATT) Ophthalmology. 2015;122(4):817–21. doi: 10.1016/j.ophtha.2014.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fritsche LG, Igl W, Bailey JN. A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. 2016;48(2):134–43. doi: 10.1038/ng.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.