Abstract
Purpose
The relationship between diffuse myocardial fibrosis and complex ventricular arrhythmias (ComVA) in patients with non-ischemic dilated cardiomyopathy (NICM) is not well established. We hypothesized that NICM patients with ComVA would have higher native myocardial T1 time, suggesting more extensive myocardial diffuse fibrosis.
Materials and Methods
We prospectively enrolled NICM patients with a history of ComVA (n=50) and age-matched NICM patients without ComVA (n=57). Imaging was performed at 1.5T with a protocol that included cine MR for left ventricular (LV) function, late gadolinium enhancement (LGE) for focal scar, and native T1 mapping for diffuse fibrosis assessment.
Results
Global native T1 time was significantly higher in patients with NICM with ComVA when compared to patients with NICM without ComVA (1131±42 vs. 1107±45 ms, p=0.006), and this finding remained after excluding segments with scar on LGE (1124±36 vs. 1102±44 ms, p=0.006). Native T1 was similar in NICM patients with and without the presence of LGE (1121±39 vs. 1117±48 ms, p=0.68) and mildly correlated with LV end-diastolic volume index (r=0.27, p=0.005), LV end-systolic volume index (r=0.24, p=0.01), and LV ejection fraction (r=−0.28, p=0.003). Native T1 value for each 10ms increment was an independent predictor of ComVA (OR 1.14, 95% CI 1.03–1.25; p=0.008) beyond LV function and LGE.
Conclusion
NICM patients with ComVA have higher native T1 compared to NICM without any documented ComVA. Native myocardial T1 is independently associated with ComVA, after adjusting for LV function and LGE.
Keywords: Native T1 mapping, diffuse myocardial fibrosis, ventricular arrhythmia, non-ischemic dilated cardiomyopathy
INTRODUCTION
Non-ischemic dilated cardiomyopathy (NICM) is associated with significant morbidity and mortality due to the associated risk for progressive heart failure and sudden cardiac death (SCD) [1,2]. SCD due to ventricular arrhythmias accounts for approximately 30% of deaths in NICM [3,4]. Although focal fibrosis evident by the presence and extent of late gadolinium enhancement (LGE) is known to be associated with increased risk of ventricular arrhythmia and adverse arrhythmic outcomes [5–7], the electrophysiologic substrate for ventricular arrhythmias in patients with NICM is not clearly defined. In contrast to ischemic heart disease, interstitial fibrosis, myocyte disarray, and membrane abnormalities likely form the substrate responsible for ventricular tachycardia (VT) in patients with NICM [2]. Prior studies have demonstrated that diffuse myocardial fibrosis alters electrical propagation between myocytes with potential proarrhythmic consequences [8,9].
LGE is present in 40% of patients with NICM with a distinct mid-myocardial presence [10,11]. However, arrhythmia could occur in patients without any evidence of scar in LGE. There is little known about the substrate of arrhythmia in NICM patients with and without scar on LGE. Myocardial tissue characterization using T1 or extracellular volume (ECV) mapping allows for assessment of diffuse myocardial fibrosis [12–15]. Recent studies have shown that native T1 is elevated in patients with NICM compared with healthy volunteers [12,16]. Furthermore, native T1 moderately correlated with cardiac structural makers including LV functional decline and volumetric dilatation [16,17]. In a study by Puntmann et al., increased native T1 was shown to be predictive of heart failure events and all-cause mortality in NICM [18]. Chen et al. recently demonstrated that native T1 was useful for predicting appropriate Implanted Cardioverter Defibrillator (ICD) therapy in heterogeneous patient population including ischemic and non-ischemic cardiomyopathy undergoing ICD implantation [19]. However, the relationship between T1-map-derived diffuse myocardial fibrosis and ventricular arrhythmia in NICM patients remains to be clarified. We hypothesized that NICM patients with ventricular arrhythmias would have higher global native T1 time, suggesting more extensive myocardial diffuse fibrosis that may play an important role in the genesis of VT. Consequently, the aim of this study is to compare the degree of diffuse myocardial fibrosis using T1 mapping in patients with NICM, with and without ventricular arrhythmias.
Materials and Methods
Study Population
We prospectively recruited NICM patients with or without documented complex ventricular arrhythmia (ComVA) and 10 age-matched control subjects with normal LV systolic function, no symptoms of heart failure and no other cardiovascular diseases. The study protocol was approved by our institutional review board, and written informed consent was obtained from all study participants.
Patients were selected from general cardiology as well as cardiac electrophysiology clinics over 3 years who had been referred for a clinical cardiac MR exam. All patients were diagnosed with NICM by their treating cardiologists based on medical history, physical examination, electrocardiography (ECG), and echocardiography [20]. Exclusion criteria were: a) diabetes mellitus, b) history of myocardial infarction or a subendocardial based LGE pattern, c) hypertrophic and infiltrative cardiomyopathies, d) inherited syndromes associated with VT (catecholaminergic, Brugada, or idiopathic QT syndromes, diagnosed on the basis of the family history, ECG and where available, genetic testing for known genes). ComVA was defined as Grade IVb or higher by the Lown and Wolf classification [21]. ComVA was documented on Holter monitor, event monitor, or ECG. The absence of ComVA was determined by a negative Holter or event monitor, or no ECG record of ventricular arrhythmias while being regularly followed by local cardiologists. Patient characteristics were reviewed from the hospital electronic medical records.
Cardiac MR Protocol
Cardiac MR images were acquired using a Philips Achieva 1.5 T scanner with a 32-channel cardiac receiver coil. We acquired steady state free precision cine MR (TR/TE=3.3/1.6 ms; flip angle=60°; FOV=320×320 mm2; acquisition matrix=188×188; slice thickness=8 mm; gap=2 mm), and native T1 mapping by slice-interleaved T1 mapping (STONE) sequence with following parameters: diastolic acquisition, 5 slices, TR/TE=2.8/1.4 ms, flip angle=70°, FOV=360×350 mm2, voxel size=2.1×2.1 mm2, slice thickness=8 mm, Turbo Field Echo (TFE) factor=86, parallel imaging SENSE factor=2 [22]. 3D LGE images were acquired 10 to 20 min after injection of 0.1–0.2 mmol/kg of Gd-DTPA (Magnevist; Bayer Schering, Berlin, Germany) or Gd-BOPTA (MultiHance; Bracco Imaging SpA, Milan, Italy) with the following imaging parameters: TR/TE=5.3/2.1 ms; flip angle =70°; FOV= 320×320×125 mm3; acquisition matrix=224×224×23; spatial resolution=1.4×1.4×4 mm3 to 5 mm; reconstruction resolution=0.6×0.6×2 to 2.5 mm3 [23,24]. T1 maps were generated after motion correction using the adaptive registration of varying contrast-weighted images for improved tissue characterization [25]. A 3-parameter fit model was used to estimate voxel-wise T1 values. Native T1 time of 16 myocardial segments from three slices (basal-, mid- and apical-slice) was measured using custom software developed in Matlab (MathWorks, Natick, MA). Since the presence of scar can result in an increase in native T1 values in the corresponding segments, an additional analysis of T1 values was performed after excluding the LGE positive myocardial segments identified by visual assessment. Left ventricular volumes, ejection fraction, and mass were measured using dedicated software (Extend MR WorkSpace, version 2.3.6.3, Philips Healthcare). Left ventricular volumes and mass were indexed to body surface area. The absence or presence and location of LGE were determined visually and if LGE was present, LGE volume was assessed using a custom software developed in Matlab (MathWorks, Natick, MA), which enables manual segmentation of diseased myocardium and normal remote region and quantifies LGE region using thresholding techniques. For each short-axis cross section, after the endocardial and epicardial borders were traced, a region of interest was defined in the normal remote myocardium without any artifact. The software calculated mean and standard deviation (SD) of remote region signal intensity (SI) and thresholds of all pixels with SI greater than mean + 4SD of remote region, and reported a total volume and percentage of total LV mass. In 74 NICM patients (32 with ComVA, 42 without ComVA) post-contrast T1 map and hematocrit assessment on the day of scanning, T1 times of LV myocardium and blood pool were similarly determined, before and after contrast injection. Then extracellular volume fraction was calculated according to the formula as:
Statistical analysis
The characteristics of the participants are presented as mean ± SD for continuous variables, median (quartiles) if not normally distributed, and number (%) of participants for categorical variables. Continuous variables are compared using an unpaired Student`s t-test, non-continuous variables using Mann-Whitney nonparametric test and categorical variables using a chi-square test. Depending on data distribution, either a Pearson or Spearman correlation coefficient was calculated to investigate possible associations of continuous outcome measures. In addition, the cut-off value of native T1 was determined by the area under the receiver operating characteristic (ROC) curve analysis in predicting ComVA. Multivariable logistic regression analyses with six potentially confounding factors (age, male gender, NYHA functional class, LV end-diastolic volume index, LGE volume, and global native T1) were performed with ComVA as the dependent variable, using the rule of thumb of having between 5 and 10 outcomes per predictor. We reported the adjusted odds ratios (OR) and corresponding 95% confidence interval (CI). All tests were 2 sided and p value <0.05 was considered statistically significant. Statistical analyses were performed using SPSS (IBM Inc, Chicago, IL) and MedCalc for Windows (MedCalc Software, Ostend, Belgium).
RESULTS
A total of 107 patients with NICM were recruited including 50 patients with a history of ComVA (35 non-sustained VT, 15 sustained monomorphic VT) and 57 patients without a history of ComVA. The mean age of all patients was 52 years (range 19 to 89). There was no significant difference between the two groups in terms of body surface area, baseline blood pressure, heart rate, history of hypertension, dyslipidemia and smoking. NICM patients with ComVA were frequently male, more likely to be on beta-blockers and angiotensin-converting enzyme inhibitors/ angiotensin-receptor blockers, and have higher New York Heart Association functional classification. Among the cohort with ComVA, 34% (17/50) of the patients underwent an electrophysiologic study (EPS). Eleven of the seventeen had inducible monomorphic VT during EPS and subsequently underwent VT ablation (Table 1).
Table 1.
Baseline Patient Characteristics
| Characteristics | Control (n=10) |
+ Com VA (n=50) |
− Com VA (n=57) |
p Value |
|---|---|---|---|---|
| Age (years) | 54 ± 6 | 54 ± 16 | 52 ± 14 | 0.54 |
| Male (%) | 7 (70) | 39 (78) | 38 (67) | 0.19 |
| BSA (m2) | 1.71 ± 0.17 | 2.04 ± 0.21 | 1.99 ± 0.21 | 0.20 |
| Hypertension (%) | 1 (10) | 9 (18) | 14 (25) | 0.41 |
| Systolic blood pressure (mmHg) | 124 ± 21 | 119 ± 17 | 116 ± 18 | 0.37 |
| Diastolic blood pressure (mmHg) | 76 ± 13 | 73 ± 11 | 72 ± 16 | 0.90 |
| Diabetes mellitus (%) | 0 (0) | 0 (0) | 0 (0) | - |
| Dyslipidemia (%) | 2 (20) | 9 (18) | 13 (23) | 0.54 |
| Current smoking (%) | 0 (0) | 7 (14) | 13 (23) | 0.24 |
| Medication | ||||
| Beta-blocker | - | 46 (92) | 39 (68) | 0.003 |
| ACEi/ARB | - | 42 (84) | 37 (65) | 0.03 |
| Antiarrhythmic | - | 10 (20) | 6 (11) | 0.17 |
| NYHA functional class | 0.01 | |||
| I-II | - | 29 (58) | 43 (75) | |
| III-IV | - | 21 (42) | 14 (25) | |
| Etiology | 0.09 | |||
| Idiopathic | - | 40 (80) | 53 (93) | |
| Arrhythmia-induced | - | 4 (8) | 1 (2) | |
| LV noncompaction | - | 4 (8) | 0 (0) | |
| Chronic myocarditis | - | 2 (4) | 2 (3) | |
| Peripartum | - | 0(0) | 1 (2) | |
| Electrophysiologic study | - | 17 (34) | - | - |
| Ablation for inducible VA | - | 11 (22) | - | - |
Variables given are mean ± SD or N (%) or median (interquartile range). p-value is for +ComVA versus −ComVA.
ACEi=angiotensin-converting enzyme inhibitors; ARB=angiotensin-receptor blockers; BSA=body surface area, ComVA=complex ventricular arrhythmia NYHA=New York Heart Association; PCI=percutaneous coronary intervention
ComVA (complex ventricular arrhythmia); BSA (body surface area); SBP (systolic blood pressure); DBP (diastolic blood pressure); ACEI (angiotensin-converting enzyme inhibitor)/ARB (angiotensin-receptor blocker); NYHA (New York Heart Association)
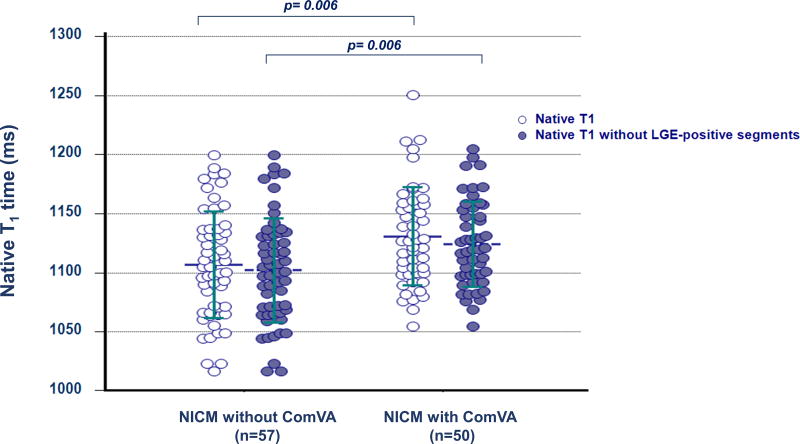
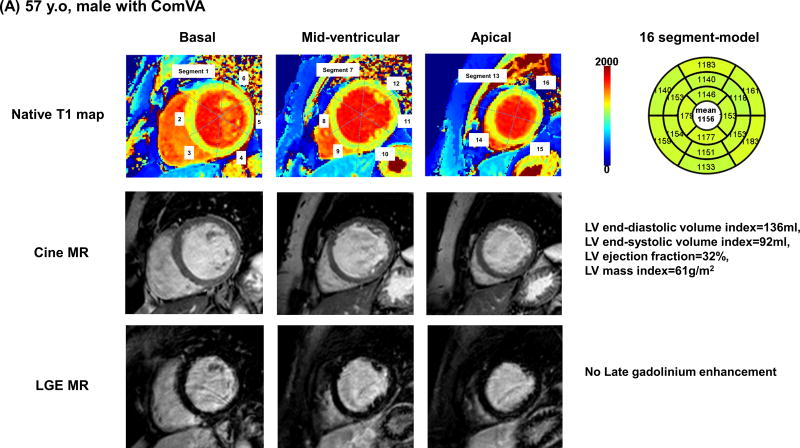
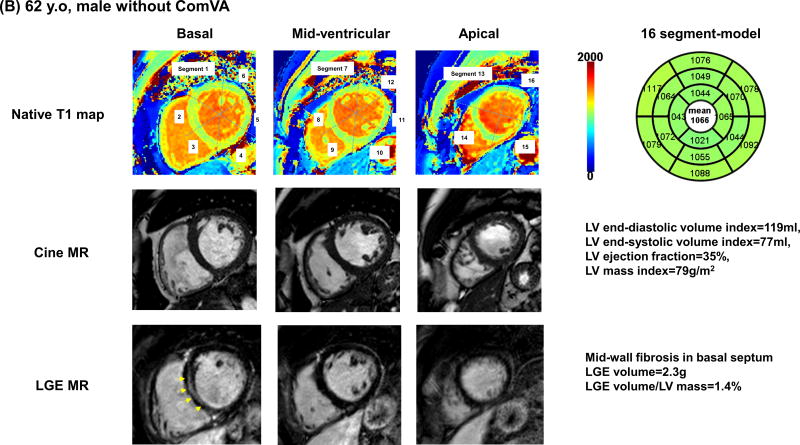
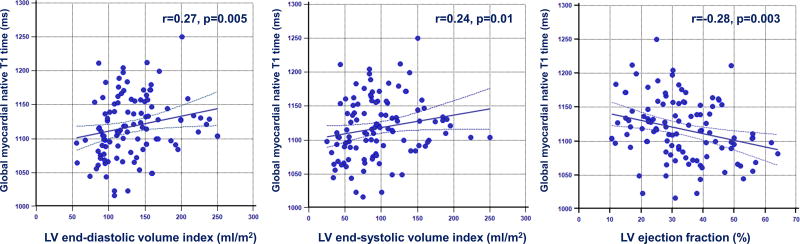
Table 2 summarizes cardiac MR findings. There was a trend for higher LV end-diastolic, end-systolic volume and stroke volume, and lower LV ejection fraction in patients with Com VA. Non-ischemic LGE was observed in 35 patients (33%); mid-wall LGE in 18 patients, focal LGE in 12 patients, and transmural LGE in 5 patients. Patients with ComVA had similar prevalence of LGE (36% vs. 30%, p=0.50), however were more likely to have higher scar volume compared with those without Com VA (6.7% vs. 3.5%, P=0.03). Pertaining to T1 map analysis, 49 segments (3%) were excluded for analysis due to motion artifacts or incomplete registration. NICM patients had significantly higher global native T1 compared with age-matched control (1118 ± 45 vs. 1073 ± 25 ms, p<0.001). Global native T1 time was significantly higher in patients with NICM with ComVA when compared to patients with NICM without ComVA (1131 ± 42 vs. 1107 ± 45 ms, p=0.006). In 74 of 107 NICM patients with extracellular volume fraction data, global extracellular volume fraction appeared significantly higher in patients with ComVA compared to those without ComVA (0.31 ± 0.03 vs. 0.29 ± 0.04, p=0.03). Even after excluding 131 segments with LGE (80 segments in ComVA group and 51 segments in non-ComVA group), native myocardial T1 remains significantly higher in NICM patients with ComVA (1124 ± 36 vs. 1102 ± 44 ms, p=0.006) (Figure 1). Figure 2 shows representative NICM cases with and without ComVA. Global native T1 time over the 16 segments was increased to 1156 ms in a case with ComVA despite of the absence of LGE (Figure 2A), while a case without ComVA showed mid-wall LGE in the basal septum, resulting in relatively high native T1 of 1117 ms in the corresponding segment. However, global native T1 of 1066 ms was in normal range (Figure 2B). No significant difference in native T1 was observed between patients with and without the presence of LGE (1121 ± 39 vs. 1117 ± 48 ms, p=0.68). In the subgroup analysis of patients with ComVA, global native T1 was more likely to be increased in NICM patients with sustained VT vs. non-sustained VT (1147 ± 57 vs. 1125 ± 34 ms, p=0.11). Figure 3 shows the relationship between native T1 and LV volumes and ejection fraction. There was a mild positive correlation with LV end-diastolic volume index (r=0.27, p=0.005), LV end-systolic volume index (r=0.24, p=0.01), and negative correlation with LV ejection fraction (r=−0.28, p=0.003). The ROC curves showed native T1 in the whole myocardium of >1110 ms to be the optimal cut-off points for ComVA, with 64% sensitivity and 56% specificity and area under the ROC curve of 0.64 (95% CI: 0.54 to 0.74). In multivariable analysis, native T1 value for each 10 ms increment was an independent predictor of ComVA (OR 1.14, CI 1.03–1.25; p=0.008) beyond LV function and LGE (Table 3).
Table 2.
Cardiac MR measurements
| Characteristics | Control (n=10) |
+ Com VA (n=50) |
− Com VA (n=57) |
p Value |
|---|---|---|---|---|
| LV EDV (ml) | 112.3 ± 21.3 | 282.6 ± 102.2 | 254.1 ± 94.1 | 0.14 |
| LV EDV index (ml/m2) | 65.5 ± 8.3 | 138.0 ± 44.0 | 127.2 ± 41.4 | 0.19 |
| LV ESV (ml) | 44.6 ± 9.0 | 212.5 ± 107.0 | 179.1 ± 92.2 | 0.09 |
| LV ESV index (ml/m2) | 26.1 ± 4.1 | 104.1 ± 48.7 | 89.3 ± 42.2 | 0.10 |
| LV stroke volume (ml) | 67.7 ± 13.3 | 84.9 ± 22.5 | 75.0 ± 20.8 | 0.02 |
| LV stroke volume index (ml/m2) | 39.4 ± 4.9 | 41.6 ± 10.0 | 37.8 ± 9.9 | 0.05 |
| LV ejection fraction (%) | 60.3 ± 2.7 | 33.1 ± 12.3 | 32.6 ± 12.6 | 0.85 |
| LV mass (g) | 66.5 ± 16.9 | 142.4 ± 60.1 | 138.0 ± 53.8 | 0.69 |
| LV mass index (g/m2) | 38.5 ± 6.7 | 69.5 ± 26.4 | 68.8 ± 23.9 | 0.90 |
| LGE, n (%) | 0 (0) | 18 (36%) | 17 (30%) | 0.50 |
| LGE volume (g) | - | 8.1 (2.8–13.3) | 4.4 (2.8–7.4) | 0.09 |
| LGE volume / LV mass (%) | - | 6.7 (2.8–7.9) | 3.5 (1.8–5.4) | 0.03 |
| Heart rate (beats/min) | 68 ± 10 | 74 ± 18 | 75 ± 14 | 0.71 |
| Native T1 (msec) | 1073 ± 25 | 1131 ± 42 | 1107 ± 45 | 0.006 |
| T1 in basal slice (msec) | 1088 ± 24 | 1124 ± 42 | 1104 ± 50 | 0.03 |
| T1 in mid slice (msec) | 1061 ± 35 | 1130 ± 58 | 1104 ± 50 | 0.02 |
| T1 in apical slice (msec) | 1078 ± 35 | 1144 ± 61 | 1115 ± 49 | 0.009 |
| Native T1 without LGE segments (msec) | - | 1124 ± 36 | 1102 ± 44 | 0.006 |
Variables given are mean ± SD, N (%), or median (interquartile range) p-value is for +ComVA versus −ComVA.
LV=left ventricular; EDV=end-diastolic volume; ESV=end-systolic volume; LGE=late gadolinium enhancement
Figure 1. Comparison of global native T1 time.
Global native T1 time between patients with NICM with and without ComVA (White). Native myocardial T1 time after excluding segments with late gadolinium enhancement between patients with NICM with and without ComVA (Blue). Global native T1 is significantly higher in NICM patients with ComVA (p=0.006 and 0.006, respectively)
ComVA: complex ventricular arrhythmias; NICM: non-ischemic cardiomyopathy
Figure 2. Representative cases with and without ComVA.
(A) 57 years old, non-ischemic dilated cardiomyopathy with ComVA and high global native T1 of 1156 msec. Severe LV systolic dysfunction was documented with severely dilated LV volume and global wall thinning. LGE image by cardiac MR showed no late gadolinium enhancement. (B) 62 years old, non-ischemic dilated cardiomyopathy with mid-wall LGE and no ComVA. Similar to case A, there was severe LV systolic dysfunction with dilated LV cavity size. Although mid-wall fibrosis in LGE images was observed in basal septum (arrows) and native T1 in the corresponding segment was relatively high, global native T1 was in normal range.
ComVA: complex ventricular arrhythmias, LGE: late gadolinium enhancement
Figure 3. Correlation between native T1 time and LV volumes and ejection fraction.
Global native T1 time was mildly correlated with LV a) end-diastolic volume index, b) end-systolic volume index, c) ejection fraction.
Table 3.
Multivariable Logistic Regression Analysis of NICM patients with ComVA as the Dependent Variables
| Odds Ratio | 95% CI | p-value | |
|---|---|---|---|
| Native T1 (each 10ms increment) | 1.14 | 1.03–1.25 | 0.008 |
| NYHA functional class | 2.70 | 1.28–5.71 | 0.009 |
The clinical characteristics that were univariate predictors of ComVA that were also included in the multivariable model were age (p=0.51), male gender (p=0.28), LV EDV index (p=0.87), and LGE volume (%) (p=0.82).
DISCUSSION
In this study of NICM patients with and without ComVA, we found that patients with ComVA had significantly higher global native T1 time compared to NICM patients without ComVA. Native T1 was independently associated with ComVA beyond LV function and LGE, although there was a univariate correlation between native T1 and LV function and a trend for higher native T1 in patients with large-extent (p=0.07) but not presence of LGE.
The pathophysiology of post-infarct VT has been well described, and relates to the propagation of a wave front around ventricular scar sustained by a reentrant mechanism involving a “protected isthmus” [26–29]. However, the mechanism for ventricular arrhythmia in NICM patients is less well understood. Indeed, scar and fibrosis in patients with NICM differ from that in patients with ischemic cardiomyopathy and tend to be smaller, less confluent, less transmural involvement with a predilection for the mid-myocardium and epicardium, and near the base and perivalvular regions [30,31]. In addition, non-ischemic LGE are patchy with fewer fixed boundaries and protected isthmuses, which can change the extent of local conduction slowing [32]. Although the presence and extent of LGE are associated with an increased risk of future cardiac events in NICM patients [5–7], LGE cannot provide absolute quantification of diffuse myocardial fibrosis and many patients with NICM have no focal scar on LGE. The current result is consistent with the recent study by Chen et al. that T1 mapping is useful for the prediction of ventricular arrhythmia in ischemic and non-ischemic patients with implantable cardioverter-defibrillators [19]. However, in their study, native T1 was measured on a single mid-septal myocardium and heterogeneous patient population was included. Diffuse myocardial fibrosis rather than focal fibrosis/scar is an essential process in pathologic remodeling in NICM, and the role of diffuse myocardial fibrosis in the pathophysiology of VT in NICM might be more important. The current study demonstrated that global native T1 values are significantly higher in patients with NICM with ComVA when compared to those without ComVA. Importantly, this highlights that native T1 mapping can be applicable for assessing arrhythmic risk in patients with NICM.
While the mechanism of VT in NICM is still unclear, the combination of focal myocardial fibrosis creating regions of conduction block, with nonuniform anisotropy and slow conduction through interstitial fibrosis may promote reentry and result in sustained ventricular arrhythmias. Furthermore, abnormal myocytes within areas of interstitial fibrosis might develop spontaneous diastolic depolarization, resulting in abnormal automaticity and arrhythmia. In addition, chronic volume overloading and LV dilatation associated with NICM could promote enhanced sympathetic tone and contribute to cyclic AMP-mediated triggered sustained ventricular arrhythmias [33]. Diffuse myocardial fibrosis assessed by native T1 mapping can provide incremental value in addition to LGE and LV functional assessments for identifying NICM patients with an underlying substrate for ventricular arrhythmia. LGE enables identification of myocardial fibrosis including myocardial infarction and focal myocardial fibrosis and its patterns can help differentiate between ischemic and non-ischemic cardiomyopathy. Presence of LGE has been shown to have important prognostic information [5–7] and cannot be replaced with native T1 mapping at this point. Native T1 mapping may provide additional information and may be used in NICM patients in whom administration of contrast agent are not desired. Further studies are needed to elucidate the incremental value of native T1 mapping in identifying patients with NICM who are at risk for arrhythmic events and who might benefit from implantable cardioverter defibrillator (ICD) implantation and/or VT ablation.
Although, there were significant difference between NICM patients with and without ComVA, we found that there is substantial overlap in T1 values between them. This observation is similar to other studies in cardiac T1 mapping to discriminate between different patient cohorts based solely on myocardial T1 values [12,13]. This is an important observation which warrants further evaluation. There are many sources in variability that could contribute to this overlap including pulse sequence [34,35] and natural differences among different patients even within the same group. The latter variability is an important one that has clinical implications. While we categorize all these patients into one single large group, i.e. NICM, there may be differences among them. Currently, more precise phenotyping of NICM patients is a major unmet clinical need which is being investigated using different approaches including genetic, proteomics and blood biomarkers [36–38]. Myocardial T1 mapping could also potentially have a role in phenotyping of these patients and warrants further studies. However, this requires a very large sample size which was beyond the scope of this study.
Our study has several limitations. This is a single-center study with a small sample size. Our cohort included patients with non-sustained VT as well as sustained VT. The primary mechanism of these arrhythmias may be different, and a high proportion of NICM have non-sustained VT of unclear clinical significance. However, non-sustained VT and higher burden of ventricular ectopy have been shown to be associated with increased risk for VT/VF and ICD therapies in patients with NICM [39,40]. Myocarditis was excluded based on medical, history, physical examination, ECG, echocardiography, and cardiac MR findings. None of patients had any ongoing symptoms suggesting presence of myocardial edema or inflammation. However, we cannot rule out edema in our patients. There are other mechanisms besides fibrosis that can alter T1. Intramyocardial lipid accumulation and iron overload will change native T1, resulting in the “pseudo normalization” of myocardial native T1 even if diffuse myocardial fibrosis exists. Our T1 mapping sequence cannot separate contribution of these imaging confounders in T1 values.
In conclusion, we found that NICM patients with ComVA have significantly increased native T1-derived diffuse myocardial fibrosis, compared to those without ComVA. Global native T1 was independently associated with ComVA, after adjusting LV function and LGE. Further studies are needed to confirm whether these results provide incremental value to LGE and LV function for risk stratification in patients with NICM at risked for ventricular arrhythmia and SCD.
Acknowledgments
This study is supported in parts by the following grants: NIH R01EB008743, R01HL129185, R21HL127650-01, and AHA 15EIA22710040.
References
- 1.Jefferies JL, Towbin JA. Dilated cardiomyopathy. Lancet. 2010;375:752–762. doi: 10.1016/S0140-6736(09)62023-7. [DOI] [PubMed] [Google Scholar]
- 2.Felker GM, Thompson RE, Hare JM, et al. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med. 2000;342:1077–1084. doi: 10.1056/NEJM200004133421502. [DOI] [PubMed] [Google Scholar]
- 3.Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 4.Kadish A, Dyer A, Daubert JP, et al. Defibrillators in Non-Ischemic Cardiomyopathy Treatment Evaluation (DEFINITE) Investigators. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004;350:2151–2158. doi: 10.1056/NEJMoa033088. [DOI] [PubMed] [Google Scholar]
- 5.Assomull RG, Prasad SK, Lyne J, et al. Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. J Am Coll Cardiol. 2006;48:1977–1985. doi: 10.1016/j.jacc.2006.07.049. [DOI] [PubMed] [Google Scholar]
- 6.Wu KC, Weiss RG, Thiemann DR, et al. Late gadolinium enhancement by cardiovascular magnetic resonance heralds an adverse prognosis in nonischemic cardiomyopathy. J Am Coll Cardiol. 2008;51:2414–2421. doi: 10.1016/j.jacc.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neilan TG, Coelho-Filho OR, Danik SB, et al. CMR quantification of myocardial scar provides additive prognostic information in nonischemic cardiomyopathy. JACC Cardiovasc Imaging. 2013;6:944–954. doi: 10.1016/j.jcmg.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spach MS, Boineau JP. Microfibrosis produces electrical load variations due to loss of side-to-side cell connections: a major mechanism of structural heart disease arrhythmias. Pacing Clin Electrophysiol. 1997;20:397–413. doi: 10.1111/j.1540-8159.1997.tb06199.x. [DOI] [PubMed] [Google Scholar]
- 9.Massare J, Berry JM, Luo X, et al. Diminished cardiac fibrosis in heart failure is associated with altered ventricular arrhythmia phenotype. J Cardiovasc Electrophysiol. 2010;21:1031–1037. doi: 10.1111/j.1540-8167.2010.01736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCrohon J a, Moon JCC, Prasad SK, et al. Differentiation of heart failure related to dilated cardiomyopathy and coronary artery disease using gadolinium-enhanced cardiovascular magnetic resonance. Circulation. 2003;108:54–59. doi: 10.1161/01.CIR.0000078641.19365.4C. [DOI] [PubMed] [Google Scholar]
- 11.Mahrholdt H, Wagner A, Judd RM, et al. Delayed enhancement cardiovascular magnetic resonance assessment of non-ischaemic cardiomyopathies. Eur Heart J. 2005;26:1461–1474. doi: 10.1093/eurheartj/ehi258. [DOI] [PubMed] [Google Scholar]
- 12.Puntmann VO, Voigt T, Chen Z, et al. Native T1 mapping in differentiation of normal myocardium from diffuse disease in hypertrophic and dilated cardiomyopathy. JACC Cardiovasc Imaging. 2013;6:475–484. doi: 10.1016/j.jcmg.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 13.Hinojar R, Varma N, Child N, et al. T1 Mapping in Discrimination of Hypertrophic Phenotypes: Hypertensive Heart Disease and Hypertrophic Cardiomyopathy: Findings From the International T1 Multicenter Cardiovascular Magnetic Resonance Study. Circ Cardiovasc Imaging. 2015;8 doi: 10.1161/CIRCIMAGING.115.003285. pii: e003285. [DOI] [PubMed] [Google Scholar]
- 14.Bull S, White SK, Piechnik SK, et al. Human non-contrast T1 values and correlation with histology in diffuse fibrosis. Heart. 2013;99:932–937. doi: 10.1136/heartjnl-2012-303052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee S-P, Lee W, Lee JM, et al. Assessment of diffuse myocardial fibrosis by using MR imaging in asymptomatic patients with aortic stenosis. Radiology. 2015;274:359–369. doi: 10.1148/radiol.14141120. [DOI] [PubMed] [Google Scholar]
- 16.Shah RV, Kato S, Roujol S, et al. Native Myocardial T1 as a Biomarker of Cardiac Structure in Non-Ischemic Cardiomyopathy. Am J Cardiol. 2016;117:282–288. doi: 10.1016/j.amjcard.2015.10.046. [DOI] [PubMed] [Google Scholar]
- 17.Puntmann VO, Arroyo Ucar E, Hinojar Baydes R, et al. Aortic stiffness and interstitial myocardial fibrosis by native T1 are independently associated with left ventricular remodeling in patients with dilated cardiomyopathy. Hypertension. 2014;64:762–768. doi: 10.1161/HYPERTENSIONAHA.114.03928. [DOI] [PubMed] [Google Scholar]
- 18.Puntmann VO, Carr-White G, Jabbour A, et al. T1-Mapping and Outcome in Nonischemic Cardiomyopathy. JACC Cardiovasc Imaging. 2016;9:40–50. doi: 10.1016/j.jcmg.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Chen Z, Sohal M, Voigt T, et al. Myocardial tissue characterization by cardiac magnetic resonance imaging using T1 mapping predicts ventricular arrhythmia in ischemic and non-ischemic cardiomyopathy patients with implantable cardioverter-defibrillators. Heart Rhythm. 2015;12:792–801. doi: 10.1016/j.hrthm.2014.12.020. [DOI] [PubMed] [Google Scholar]
- 20.Richardson P, McKenna W, Bristow M, et al. Report of the 1995 World Health Organization/ International Society and Federation of Cardiology Task Force on the Definition and Classification of cardiomyopathies. Circulation. 1996;93:841–842. doi: 10.1161/01.cir.93.5.841. [DOI] [PubMed] [Google Scholar]
- 21.Lown B, Wolf M. Approaches to sudden death from coronary heart disease. Circulation. 1971;44:130–142. doi: 10.1161/01.cir.44.1.130. [DOI] [PubMed] [Google Scholar]
- 22.Weingärtner S, Roujol S, Akçakaya M, et al. Free-breathing multislice native myocardial T1 mapping using the slice-interleaved T1 (STONE) sequence. Magn Reson Med. 2014 Aug 1; doi: 10.1002/mrm.25387. [DOI] [PubMed] [Google Scholar]
- 23.Akçakaya M, Rayatzadeh H, Basha TA, et al. Accelerated late gadolinium enhancement cardiac MR imaging with isotropic spatial resolution using compressed sensing: initial experience. Radiology. 2012;264:691–699. doi: 10.1148/radiol.12112489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akçakaya M, Basha TA, Goddu B, et al. Low-dimensional-structure self-learning and thresholding: regularization beyond compressed sensing for MRI reconstruction. Magn Reson Med. 2011;66:756–767. doi: 10.1002/mrm.22841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roujol S, Foppa M, Weingärtner S, et al. Adaptive registration of varying contrast-weighted images for improved tissue characterization (ARCTIC): application to T1 mapping. Magn Reson Med. 2015;73:1469–1482. doi: 10.1002/mrm.25270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.El-Sherif N, Scherlag BJ, Lazzara R, et al. Re-entrant ventricular arrhythmias in the late myocardial infarction period. 1. Conduction characteristics in the infarct zone. Circulation. 1977;55:686–702. doi: 10.1161/01.cir.55.5.686. [DOI] [PubMed] [Google Scholar]
- 27.Klein H, Karp RB, Kouchoukos NT, et al. Intra-operative electrophysiologic mapping of the ventricle during sinus rhythm in patients with a previous myocardial infarction: identification of the electrophysiologic substrate of ventricular arrhythmias. Circulation. 1982;66:847–853. doi: 10.1161/01.cir.66.4.847. [DOI] [PubMed] [Google Scholar]
- 28.de Bakker JM, van Capelle FJ, Janse MJ, et al. Reentry as a cause of ventricular tachycardia in patients with chronic ischemic heart disease: electrophysiologic and anatomic correlation. Circulation. 1988;77:589–606. doi: 10.1161/01.cir.77.3.589. [DOI] [PubMed] [Google Scholar]
- 29.Stevenson WG, Khan H, Sager P, et al. Identification of reentry circuit sites during catheter mapping and radiofrequency ablation of ventricular tachycardia late after myocardial infarction. Circulation. 1993;88:1647–1670. doi: 10.1161/01.cir.88.4.1647. [DOI] [PubMed] [Google Scholar]
- 30.Soejima K, Stevenson WG, Sapp JL, et al. Endocardial and epicardial radiofrequency ablation of ventricular tachycardia associated with dilated cardiomyopathy: the importance of low-voltage scars. J Am Coll Cardiol. 2004;43:1834–1842. doi: 10.1016/j.jacc.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 31.Hsia HH, Callans DJ, Marchlinski FE. Characterization of endocardial electrophysiological substrate in patients with nonischemic cardiomyopathy and monomorphic ventricular tachycardia. Circulation. 2003;108:704–710. doi: 10.1161/01.CIR.0000083725.72693.EA. [DOI] [PubMed] [Google Scholar]
- 32.Nakahara S, Tung R, Ramirez RJ, et al. Characterization of the arrhythmogenic substrate in ischemic and noischemic cardiomyopathy implications for catheter ablation of hemodynamically unstable ventricular tachycardia. J Am Coll Cardiol. 2010;55:2355–2365. doi: 10.1016/j.jacc.2010.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martinez-Rubio A, Schwammenthal Y, Schwammenthal E, et al. Patients with valvular heart disease presenting with sustained ventricular tachyarrhythmias or syncope: results of programmed ventricular stimulation and long-term follow-up. Circulation. 1997;96:500–508. doi: 10.1161/01.cir.96.2.500. [DOI] [PubMed] [Google Scholar]
- 34.Roujol S, Weingärtner S, Foppa M, et al. Accuracy, precision, and reproducibility of four T1 mapping sequences: a head-to-head comparison of MOLLI, ShMOLLI, SASHA, and SAPPHIRE. Radiology. 2014;272:683–689. doi: 10.1148/radiol.14140296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bellm S, Basha TA, Shah RV, et al. Reproducibility of myocardial T1 and T2 relaxation time measurement using slice-interleaved T1 and T2 mapping sequences. J Magn Reson Imaging. 2016;44:1159–1167. doi: 10.1002/jmri.25255. [DOI] [PubMed] [Google Scholar]
- 36.Piran S, Liu P, Morales A, et al. Where genome meets phenome: rationale for integrating genetic and protein biomarkers in the diagnosis and management of dilated cardiomyopathy and heart failure. J Am Coll Cardiol. 2012;60:283–289. doi: 10.1016/j.jacc.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 37.Wu KC, Gerstenblith G, Guallar E, et al. Combined cardiac magnetic resonance imaging and C-reactive protein levels identify a cohort at low risk for defibrillator firings and death. Circ Cardiovasc Imaging. 2012;5(2):178–186. doi: 10.1161/CIRCIMAGING.111.968024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Havmöller R, Chugh SS. Plasma biomarkers for prediction of sudden cardiac death: another piece of the risk stratification puzzle? Circ Arrhythm Electrophysiol. 2012;5:237–243. doi: 10.1161/CIRCEP.111.968057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mittal S, Aktas MK, Moss AJ, et al. The impact of nonsustained ventricular tachycardia on reverse remodeling, heart failure, and treated ventricular tachyarrhythmias in MADIT-CRT. J Cardiovasc Electrophysiol. 2014;25:1082–1087. doi: 10.1111/jce.12456. [DOI] [PubMed] [Google Scholar]
- 40.Dawson DK, Hawlisch K, Prescott G, et al. Prognostic role of CMR in patients presenting with ventricular arrhythmias. JACC Cardiovasc Imaging. 2013;6:335–344. doi: 10.1016/j.jcmg.2012.09.012. [DOI] [PubMed] [Google Scholar]